Abstract
Interleukin-8 (IL-8), a CXC chemokine, has a central role in leukocyte recruitment to areas of granuloma formation in tuberculosis. In the present studies, we investigated the effect of the TH2-derived cytokines IL-4, IL-10, and IL-13 on Mycobacterium tuberculosis-induced IL-8 secretion from purified human monocytes. Our results demonstrate that IL-4 and IL-10 have a down-regulatory effect on IL-8 secretion and that this effect is dose dependent. IL-10 has a greater effect than IL-4 on secretion, and autologous IL-10 secreted from M. tuberculosis-infected monocytes also down-regulates IL-8 secretion. The down-regulatory effect is partly a result of reduced IL-8 mRNA accumulation analyzed by reverse transcription-PCR. When combined, 1 μM IL-4 and IL-10 had an additive effect in decreasing IL-8 secretion and transcription; there was no synergy of action. IL-13 did not have any significant effect on IL-8 gene expression or secretion. The inhibitory effect of IL-10 but not of IL-4 is associated with decreased nuclear binding of the key activating transcription factor NF-κB. We show for the first time that M. tuberculosis causes up-regulation of nuclear binding of Oct-1 detected by electromobility gel shift assay. However, neither AP-1 nor Oct-1 nuclear binding was altered by IL-4 or IL-10. In summary, this study demonstrates that type 2 responses have an important role in the regulation of M. tuberculosis-induced IL-8 expression but that the mechanisms by which the different cytokines act are distinct.
Mycobacterium tuberculosis is an infectious agent that claims about three million lives each year (64). In part, the clinical and pathological manifestations of tuberculosis (TB) result from unregulated inflammatory responses. The host locally destroys its own tissues while attempting to control the growth of bacilli within macrophages. However, M. tuberculosis infects approximately one in three people worldwide, indicating that the immune response normally contains infection without causing tissue damage. Understanding this successful response is critical for the development of novel approaches to the treatment of and vaccination against TB. The characteristic host tissue response to M. tuberculosis is granuloma formation, which depends on cytokines such as tumor necrosis factor alpha (TNF-α) (4, 26). Granulomas comprise cells of the monocyte lineage together with T cells and in the early stages contain neutrophils (6, 18).
Many chemokines are involved in cellular recruitment to the granuloma (46), but much interest has been focused on interleukin-8 (IL-8). IL-8 is the best characterized of the CXC subfamily of chemokines (2, 39), and novel therapeutic approaches controlling IL-8 secretion during inflammatory responses are the subject of ongoing research (66). IL-8 attracts neutrophils and T cells, both directly and indirectly, to sites of infection and has recently been demonstrated to be involved in monocyte recruitment (14). IL-8 has been identified as a specific attractant for activated human lymphocytes in mononuclear cultures either with anti-CD3 or with purified protein derivative of M. tuberculosis (62). At a cellular level, phagocytosis of M. tuberculosis particularly by tissue monocytes and macrophages is an important stimulus of IL-8 secretion (12, 24, 29, 69). Other cell types, such as respiratory epithelial cells and neutrophils, may also secrete IL-8 in TB (23, 30, 42, 61). Interestingly, IL-8 receptor A and B expression on polymorphonuclear neutrophils from human immunodeficiency virus-seropositive patients is decreased, particularly if they are coinfected with M. tuberculosis (33). In vivo studies have shown that IL-8 is central to normal immune responses to M. tuberculosis and that anti-IL-8 inhibits granuloma formation (28). In TB patients, bronchoalveolar lavage fluid contains IL-8, the concentrations of which correlate with leukocyte numbers (27, 44). IL-8 mRNA has been demonstrated in M. tuberculosis-infected tissue (5). In addition, we and others have found that IL-8 concentrations in plasma were higher in patients who died from TB than in survivors (13, 40).
The regulation of the IL-8 gene in monocytes/macrophages is complex and is stimulus and cell type dependent. In most cells, IL-8 is regulated primarily at the level of gene transcription (43) and is controlled by the transcription regulators NF-κB, AP-1, CCAAT/enhancer binding protein β (C/EBPβ), and Oct-1, all of which have functional binding sites in the IL-8 promoter (21, 37, 53, 65). An extensive number of studies have demonstrated that NF-κB mediates expression of many genes involved in the lipopolysaccharide (LPS)-induced proinflammatory response (3) and activation of NF-κB in monocytes is found in TB (56, 61). In contrast, there are no data on AP-1 or Oct-1 activation in M. tuberculosis-infected monocytes or macrophages. NF-κB has been characterized as belonging to the NF-κB/Rel family of transcription factors, which play important roles in immune responses and in cell differentiation, induced by cytokines, growth factors, and other cell activators. NF-κB proteins are kept in the cytoplasm by association with IκB proteins. After cellular activation, NF-κB dissociates from the inhibitor protein following its phosphorylation, ubiquitination, and degradation and translocates to the nucleus, where it binds to specific consensus DNA sequences (for reviews see references 3 and 15).
Down-regulation of IL-8 secretion is likely to be required so that cell influx to the site of infection by M. tuberculosis is limited. The TH2 lymphocyte-derived cytokines IL-4, IL-10 and IL-13 are potentially important down-regulators of inflammation. Although TB was initially associated with a TH1-type response (16, 31), more recent data indicate that TH2 cytokines may be associated with reactivation of infection in an animal model (20) and with extent of disease in humans (63). IL-4, a cytokine central to driving the development of a TH2 response, is known to inhibit secretion of many inducible cytokines, including IL-8 from LPS-stimulated monocytes (51). However, IL-4 may enhance monocyte function by increasing expression of some proinflammatory molecules, including class 2 major histocompatibility complex and CD23, as well as causing secretion of monocyte-derived chemokine (1, 17). IL-4 has been detected in bronchoalveolar lavage fluid from TB patients (50). However, IL-4 expression has not always been detected in patients with TB (31), and IL-4 knockout mice have normal resistance to infection with M. tuberculosis (38). IL-10 has potent anti-inflammatory properties both in vitro (9) and in vivo (41). In addition to down-regulating proinflammatory cytokine secretion, IL-10 but not IL-4 stimulated release of down-regulatory soluble TNF receptor (22). In contrast, IL-10 activated dendritic cells to become macrophages with increased activity against virulent M. tuberculosis (11). IL-10 not only is secreted from TH2 lymphocytes but also is released from monocytes. Regulation of secretion of monocyte-derived IL-10 after phagocytosis of M. tuberculosis involved multiple signaling pathways (49). IL-13, also detected in TB (48), possesses a variety of immunomodulating properties, which include anti-inflammatory actions on monocytes (8, 35).
The aim of this study was first to investigate the effects of IL-4, -10, and -13 on regulation of M. tuberculosis-induced IL-8 gene expression and secretion. Secondly, we examined the role of monocyte-derived IL-10 in autologous IL-8 secretion. We show that IL-8 gene expression and secretion in M. tuberculosis-stimulated human monocytes are down-regulated by IL-4 and IL-10 but not by IL-13. The data show the presence of a autocrine regulatory pathway. The mechanism by which IL-10 (but not IL-4) down-regulates IL-8 transcription is in part via reduced nuclear binding of NF-κB.
MATERIALS AND METHODS
Culture of M. tuberculosis.
M. tuberculosis strain H37-Rv (National Collection of Type Cultures, Colindale, United Kingdom) was maintained in Dubos medium enriched with albumin Cohn fraction V plus dextrose and sodium chloride at 37°C. Single-cell suspensions were obtained by sonicating cultures for 1 to 2 min to disperse clumped bacilli. The viable multiplicity of infection used to stimulate cell cultures was calculated in triplicate by plating serial dilutions of M. tuberculosis suspensions on Middlebrook 7H10 plates and counting colonies 3 to 4 weeks later.
Isolation of peripheral blood monocytes.
Peripheral blood mononuclear cells were obtained from human blood (pooled buffy coats purchased from the North London Blood Transfusion Service, Colindale, United Kingdom). Cells were isolated by density gradient centrifugation over Ficoll-Hypaque (Amersham Pharmacia Biotech AB, Uppsala, Sweden). Monocyte separation was performed by adhesion purification for 1.5 h. Nonadherent cells were removed by three washes with phosphate-buffered saline. Monocytes were cultured in RPMI 1640 medium (Life Technologies, Paisley, United Kingdom) supplemented with 10% fetal calf serum, 2 mM glutamine, and 50 U of penicillin/ml. Cells were maintained at 37°C in a humidified 5% CO2 incubator for up to 48 h during experiments. Based on trypan blue exclusion, cells were at least 95% viable.
Experimental protocols.
For each experiment, adherence-purified monocytes were seeded in triplicate in flat-bottomed culture plates at 106 cells/ml and were left overnight at 37°C and 5% CO2. Cells were then either challenged with M. tuberculosis, left unstimulated (negative control), or exposed to LPS (Escherichia coli O111:B4; Sigma-Aldrich, Poole, United Kingdom), the positive control, at 1 μg/ml. In specific experiments, cultures were pretreated for 1 h and throughout the duration of the study with either anti-IL-10 antibody (a generous gift from R. de Waal Malefyt, DNAX, Palo Alto, Calif.) or with the cytokines IL-4, IL-10, and IL-13 (used at concentrations of 0.1, 1, and 10 μM). At specific time points, tissue culture supernatants were harvested and IL-8 concentrations were quantitated by specific enzyme-linked immunosorbent assay (ELISA). Cells were harvested concurrently for extraction of either RNA or nuclear proteins. Experiments were performed at least three times.
Semiquantitative RT-PCR.
At specific times, monocytes were washed with phosphate-buffered saline prior to addition of Tri-Reagent (Sigma-Aldrich). The samples were homogenized by vortexing vigorously for 15 s and stored at −70°C until processed for extraction of total RNA according to the manufacturer's instructions. RNA was dissolved in sterile, RNase-free water and quantitated spectrophotometrically at 260 nm. Integrity of RNA was assessed by gel electrophoresis on a 2% agarose gel. RNA samples were then reverse transcribed and amplified by PCR (RT-PCR) in accordance with the manufacturer's instructions using the Superscript one-step RT-PCR system (Life Technologies), in which RT and PCR steps are performed in a single tube. For each experiment, equivalent amounts of intact RNA (0.1 to 0.2 μg) were used in a 25-μl reaction mixture, and the reaction was performed using a TouchDown thermal cycler (Hybaid Limited, Teddington, United Kingdom). Preliminary experiments established optimal RT-PCR conditions for the primer pairs used. cDNA synthesis was achieved in a 45-min incubation at 50°C and was followed immediately with 35 cycles of PCR amplification: denaturation (94°C) for 30 s, annealing (55°C for the IL-8 gene and 65°C for the β-actin gene) for 1 min, and extension (72°C) for 2 min. There was a final extension step at 72°C for 10 min. Primer sequences were as follows: for the IL-8 gene, 5′-CTCCATAAGGCACAAACTTTC-3′ (sense) and 5′-ATCACTCTCAGTTCTTTGATA-3′ (antisense), and for the β-actin gene, 5′-GTGGGGCGCCCCAGGCACCA-3′ (sense) and 5′-CTTTAGCACGCACTGTAATTCCTC-3′ (antisense). Omitting the RT/TaqMix and substituting 2 U of Taq DNA polymerase in the reaction mixture verified absence of genomic DNA in RNA preparation for β-actin gene. Primers for IL-8 gene amplification spanned the first two exons, and therefore size differences between genomic DNA and cDNA were readily identifiable; amplification of genomic DNA would result in a 1.13-kb fragment, in contrast with the 265-bp band that would be obtained after intron slicing (37). Positive cDNA controls and negative controls (reverse-transcribed diethyl pyrocarbonate-treated water) were included in all experiments. Ten microliters of each reaction product was loaded on a 1.5% agarose gel in Tris-acetate-EDTA buffer, and PCR products were visualized by ethidium bromide staining.
Preparation of nuclear extract.
At specific time points, nuclear extracts were prepared after adherent cells were washed once with cold phosphate-buffered saline. Cells were lysed immediately with the addition of cold extraction buffer A, consisting of 10 mM HEPES (pH 7.9), 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol (DTT), and 0.2% NP-40. The culture dish was scraped, and the lysate was centrifuged at 1,850 × g for 1 min at 4°C. Supernatants were discarded, and the nuclear pellets were resuspended in 60 μl of buffer C, consisting of 20 mM HEPES (pH 7.9), 25% glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 0.5 mM DTT, and 0.2 mM EDTA. A protease inhibitor cocktail tablet (Roche, Lewes, United Kingdom) was added to buffers A and C. Samples were then left on ice for 10 min. The solution was centrifuged at 1,850 × g for 2 min at 4°C, and soluble nuclear extracts were recovered, aliquoted, and stored at −70°C. Ten microliters of each extract was kept for protein concentration measurements. Protein concentrations were determined with a Bio-Rad Protein Assay II kit (Bio-Rad, Hempstead, United Kingdom).
Electromobility gel shift assays (EMSAs).
A double-stranded oligonucleotide containing the NF-κB consensus sequence (Promega, Southampton, United Kingdom) was end labeled by using [γ-32P]ATP and T4 polynucleotide kinase (Promega). Probes were purified using a Centri-sep spin column (Sigma) following the manufacturer's protocols. Nuclear extract protein (2 to 3 μg) and identical amounts of labeled oligonucleotide (approximately 2 × 104 counts · min−1) were mixed in the presence of incubation buffer [1 mM MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 50 mM NaCl, 10 mM Tris-HCl (pH 7.5), 0.05 μg of poly(dI-dC)/μl, 4% glycerol] for 10 min at room temperature. For supershift analysis, 1 μg of NF-κB p65 antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.) was added to the incubation reaction. Probe binding specificity was confirmed in competition experiments using a 100-fold excess of cold, unlabeled probe. Protein-DNA complexes were resolved in 5% polyacrylamide gels, electrophoresed for 1 h at room temperature in 0.5× TBE (45 mM Tris-borate, 1 mM EDTA [pH 8.0]). Gels were exposed to X-ray film overnight at −80°C.
Measurement of IL-8 concentrations.
Monocyte culture supernatants were analyzed for IL-8 concentrations by sandwich ELISA using matched pairs of antibodies (R&D Systems, Minneapolis, Minn.). Samples were run with serial dilutions of recombinant human IL-8 as standards. The lower limit of sensitivity of the IL-8 ELISA was 15 pg/ml. All results are expressed as the means ± standard deviations of triplicate cultures.
Data analysis.
All statistical analyses were performed using GraphPad Prism (GraphPad Software Inc., San Diego, Calif.). Data are presented as means from at least three separate experiments with standard errors of the means (SEM). Student's t test was used to assess differences between experimental conditions. A probability (P) value of <0.05 was taken as significant. Densitometric analyses were performed using NIH Image 1.58.
RESULTS
Effects of IL-4, IL-10, and IL-13 on M. tuberculosis-induced IL-8 secretion.
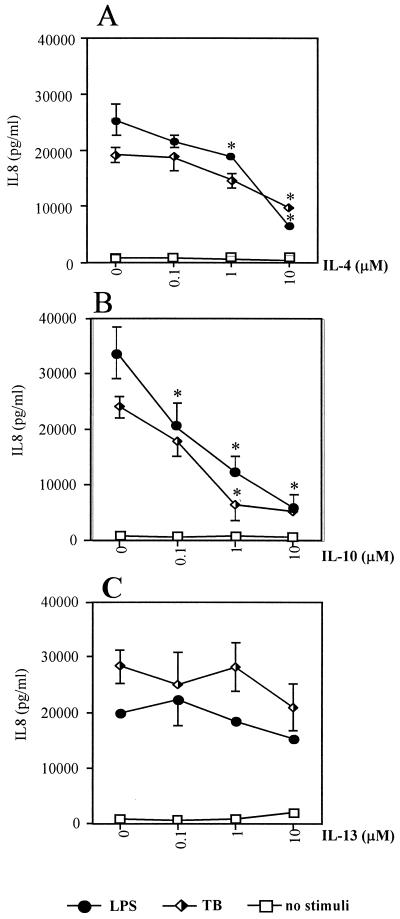
We first investigated the effects of IL-4, -10, and -13 on IL-8 secretion from human primary monocyte cultures pretreated with these cytokines for 1 h before stimulating cells with M. tuberculosis or appropriate controls. IL-4 and IL-10 significantly down-regulated IL-8 secretion in a dose-dependent manner (Fig. 1A and B). IL-10 was more potent than IL-4: 1 μM IL-10 caused approximately an 80% down-regulation of IL-8 secretion, whereas IL-4 at this concentration caused about a 40% decrease in IL-8 secretion. As expected, LPS-induced IL-8 secretion was down-regulated by IL-4 and IL-10. In contrast, IL-13 did not have any significant effect on either LPS- or M. tuberculosis-induced IL-8 secretion (Fig. 1C).
FIG. 1.
IL-8 secretion from primary by human monocytes stimulated with either LPS or M. tuberculosis (TB) or left unstimulated under control conditions or cultured in the presence of 0.1, 1, and 10 μM IL-4 (A), IL-10 (B), or IL-13 (C) . Data are means ± SEM for at least three experiments. ∗, P < 0.05 versus control without TH2 cytokine.
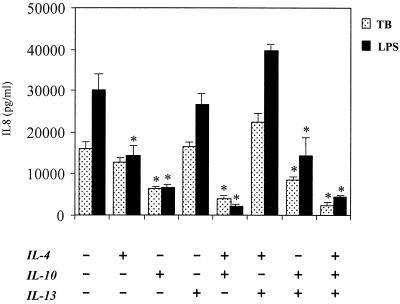
To investigate the possibility of synergistic interactions between the different TH2 cytokines, we next examined the effects that combinations of 1 μM IL-4, -10, and -13 had on IL-8 secretion (Fig. 2). The effects of IL-4 and IL-10 on M. tuberculosis-induced IL-8 secretion were simply additive, with the effects of IL-4 and IL-10 combined being equal to the sum of their individual actions. The presence of IL-13 inhibited the action of IL-4 on M. tuberculosis-induced IL-8 secretion but did not affect inhibition of IL-8 secretion by IL-10.
FIG. 2.
Effects of combined 1 μM IL-14, IL-10, or IL-13 on production of IL-8 by human monocytes stimulated with LPS or M. tuberculosis (TB) or left under control conditions. Data are means ± SEM for at least three experiments and were obtained by ELISA . ∗, P < 0.05 compared to control cultures not pretreated with cytokines (column 1).
Autologous regulation of M. tuberculosis-induced IL-8 secretion by IL-10.
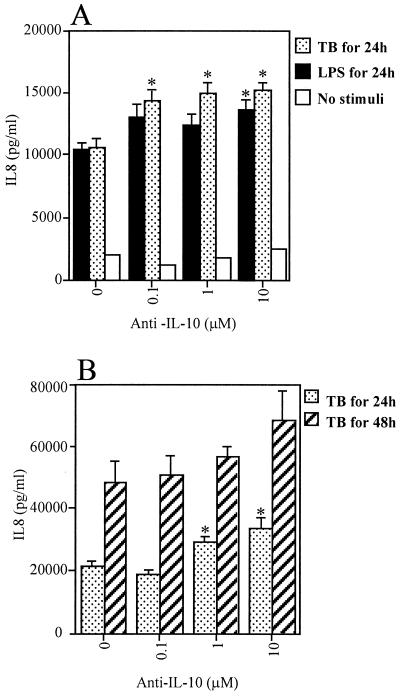
Since IL-10 may be secreted by human monocytes (9), we next investigated the possibility that monocyte-derived cytokines may have an autoregulatory role in the immune response to M. tuberculosis. Cells were cultured in the absence or presence of anti-IL-10 antibody for 1 h before and then during infection with M. tuberculosis or stimulation with LPS. A dose-dependent effect was observed, with increasing concentrations of anti-IL-10 antibody resulting in a significant increase in IL-8 secretion (Fig. 3A). The effect of anti-IL-10 on M. tuberculosis-induced IL-8 was observed using significantly lower concentrations of antibody than was required to inhibit LPS-induced IL-8 secretion. This effect was observed after both 24 and 48 h of culture, although it was statistically significant only at 24 h, when the standard deviations were smaller. These data indicate that an autologous negative feedback loop down-regulating IL-8 secretion exists in M. tuberculosis-infected monocytes (Fig. 3B).
FIG. 3.
Effect of 0.1, 1, and 10 μM anti-IL-10 human antibody on IL-8 secretion from human monocytes. (A) IL-8 secretion from monocytes exposed for 24 h to LPS or M. tuberculosis (TB) or cultured without a stimulus; (B) IL-8 secretion from anti-IL-10 treated monocytes infected with M. tuberculosis (TB) for 48 h. Data are means ± SEM.
Effects of TH2-derived cytokines on M. tuberculosis-induced IL-8 gene expression.
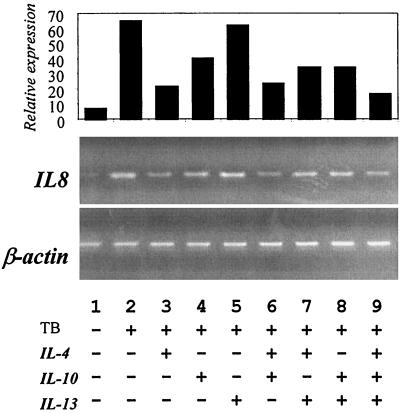
To investigate the mechanism by which IL-4 or IL-10 either alone or in combination resulted in inhibition of IL-8 production after exposure to M. tuberculosis, we used the semiquantitative technique of RT-PCR to examine IL-8 mRNA accumulation in monocytes as described in Materials and Methods. As we have previously found, IL-8 mRNA accumulation in monocytes is increased upon M. tuberculosis infection (Fig. 4). In the presence of TH2-derived cytokines, we observed that IL-4 (Fig. 4, lane 3) and to a lesser extent IL-10 (lane 4) caused a reduction in IL-8 mRNA accumulation. There were no additional changes when the inhibitory cytokines were used in combination (Fig. 4, lane 7), indicating that their additive inhibitory effects on secretion are predominately due to posttranscriptional regulation. IL-13 consistently had a negligible effect on IL-8 mRNA levels (Fig. 4, lane 5).
FIG. 4.
IL-8 mRNA expression in human monocytes stimulated with M. tuberculosis (TB) in the presence or absence of 10 μM IL-4, IL-10, and IL-13 alone or in combination. Expression was analyzed by RT-PCR as described in Materials and Methods 24 h after infection with TB. The graph shows relative IL-8 mRNA expression corrected for total mRNA using the housekeeping β-actin gene. mRNA bands were quantified by densitometry using NIH Image 1.58. Data are representative of at least three independent experiments.
IL-4, IL-10, and NF-κB nuclear binding in M. tuberculosis-infected monocytes.
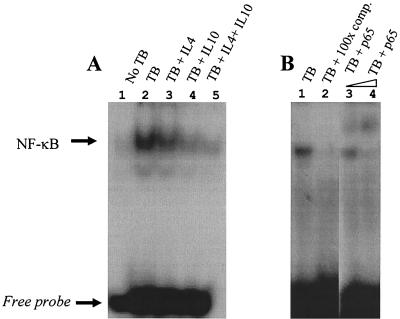
IL-8 gene expression is critically regulated by the activity of transcription factor NF-κB (43, 53). Nuclear binding of NF-κB in M. tuberculosis-infected human monocytes has been described (56), and in our preliminary studies we found that this is maximal at about 90 min after infection. We therefore examined NF-κB binding activity by EMSA in cultures that had been preincubated with either IL-4 or IL-10. Figure 5A shows representative data from one of at least three independent experiments, which demonstrate that nuclear binding of NF-κB in M. tuberculosis-infected monocytes is decreased by preincubation with 10 μM IL-10 (lanes 2, 4, and 5). In contrast, IL-4 had negligible effects on NF-κB binding (lane 3). We confirmed that the observed effects were specific at 90 min by competition experiments using 100-fold excess unlabeled probe (Fig. 5B, lanes 1 and 2). We further demonstrated that the active form of NF-κB was being translocated to the nucleus by supershifting the majority of the M. tuberculosis-induced complex by preincubating nuclear extracts with specific antibody that binds the active p65 component of NF-κB (Fig. 6, lanes 3 and 4). We are currently investigating in more detail the nature of the complete NF-κB complex.
FIG. 5.
Effect of 10 μM IL-4 and IL-10 alone or in combination on the nuclear binding of the transcription factor NF-κ B in human monocytes infected with M. tuberculosis (TB) or left uninfected (A). Specificity of binding is demonstrated with the presence of a molar excess of unlabeled probe competing out the nuclear signal. A supershift assay to further confirm translocation of the active form of NF-κ B to the nucleus was performed with antibody against the p65 NF-κB subunit (B). Data are representative of at least three independent experiments. comp., competitor.
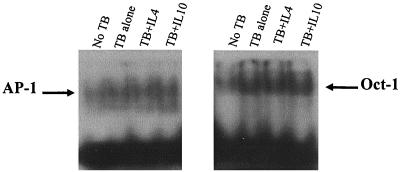
FIG. 6.
Nuclear binding of transcription factors AP-1 (left panel) and Oct-1 (right panel) in human monocytes under control conditions or exposed to M. tuberculosis (TB) together with the effect of pretreatment with 10 μM IL-4 or IL-10. Data are representative of at least three independent experiments.
AP-1 and Oct-1 nuclear binding in M. tuberculosis-infected monocytes.
In addition to NF-κB, AP-1 has also been implicated as an important control step of IL-8 secretion (43). AP-1 binding is constitutively observed in nuclear extracts from adhesion-purified primary human monocytes and does not significantly alter following infection with M. tuberculosis (Fig. 6, left panel). Furthermore, AP-1 binding is not influenced by pretreatment of cultures with either IL-4 or IL-10. Oct-1 is a ubiquitously expressed transcription factor and has been shown to repress IL-8 promoter activity (68). However, although Oct-1 binding was increased by infection with M. tuberculosis (Fig. 6, right panel), nuclear binding was not altered by the presence of IL-4 or IL-10 in the culture medium.
DISCUSSION
We have investigated the mechanisms by which the TH2-derived, down-regulatory cytokines IL-4, -10, and -13 may affect IL-8 secretion from human monocytes infected by M. tuberculosis. Our results demonstrate that IL-4 and IL-10 but not IL-13 inhibit IL-8 secretion and that these cytokines act in an additive fashion. Furthermore, autologous IL-10 secreted from M. tuberculosis-infected monocytes also down-regulated IL-8 secretion, although the biological importance of this relatively modest effect is uncertain. The actions of TH2-derived cytokines are in part mediated by reduced IL-8 gene expression. The inhibitory effect of IL-10 but not of IL-4 is associated with decreased nuclear binding of the key activating transcription factor NF-κB. In contrast, neither AP-1 nor Oct-1 nuclear binding was altered by IL-4 or IL-10. Thus, the effects and mechanisms of action of IL-4 and IL-10 on M. tuberculosis-induced IL-8 secretion from human monocytes are entirely distinct.
IL-10 has the greatest effect on down-regulating IL-8 secretion. Both exogenous cytokine and autologous monocyte-derived IL-10 regulated IL-8 secretion in M. tuberculosis-infected cells. In a analogous manner, autologous TNF-α has been shown to up-regulate IL-8 secretion (7). However, IL-10 inhibits IL-8 mRNA to a lesser extent than IL-4. This suggests the possibility of a posttranscriptional down-regulatory control of IL-8 by IL-4 enhancing mRNA degradation as observed previously (52, 58) rather than transcriptional control reported to regulate IL-6 secretion (55). Although, IL-13 can substitute for IL-4 in several physiological responses, our results show that this cytokine does not have any effect on IL-8 secretion in M. tuberculosis-infected human monocytes. IL-4 and IL-13 are well recognized as activating distinct signaling cascades (25, 57, 60), although they can act in a manner similar to that observed in down-regulation of TNF-α in LPS-stimulated murine macrophages (34).
Our results showed a marked reduction in NF-κB nuclear binding by IL-10 (Fig. 5), which is in agreement with inhibition of this transcription factor by IL-10 as observed in LPS-stimulated monocytes (58). In contrast, IL-4 did not affect NF-κB binding in this study, which is consistent with the fact that this cytokine showed little inhibitory effect on LPS-induced NF-κB activation in human monocytes (32), although IL-4 has been shown in other cell types to influence NF-κB activity as a result of formation of DNA–STAT6–NF-κB complexes (54). Recent data suggest that IL-10 probably acts both by suppressing IκB kinase activity to reduce NF-κB translocation and by directly altering the binding of NF-κB (47). If IL-4 does not repress IL-8 secretion via regulation of NF-κB or Oct-1, it is possible that the SOCS/Jab/CIS families of negative regulators, which are expressed rapidly following cellular stimulation, have a role in such down-regulation (67).
DNA binding of Oct-1 was also up-regulated in TB-activated monocytes. IL-4 and IL-10 did not alter such Oct-1 binding, which demonstrates that the down-regulation of IL-8 secretion by these TH2 cytokines is unlikely to involve Oct-1. One possible confounding factor is that Oct-1 binds to a motif overlapping that of the C/EBP element acting as a transcriptional repressor, which may help to prevent expression of the IL-8 promoter in the uninduced state (65). The binding of C/EBP upon cellular activation by TB would therefore release Oct-1 and allow its detection by binding Oct-1 probe in an EMSA.
The biological actions of IL-10 are mediated through the cell surface receptor IL-10R and are complex (19). Although we have shown an effect on NF-κB and IL-8 transcription, it is likely that other signaling pathways will mediate some IL-10 actions. For example, IL-10 induces activation of members of the JAK-STAT kinase family (10, 59). Activation of mitogen-activated kinases may be involved in the anti-inflammatory effects of IL-10 (45), and these signaling pathways potentially affect both NF-κB and AP-1, although we did not observe any significant changes in AP-1 in this system.
In summary, our data indicate that type 2 response and monocyte-derived IL-10 play an important role in the regulation of M. tuberculosis-induced IL-8 expression in human monocytes. IL-4 and IL-10 have divergent mechanisms of action which in part reflect distinct effects of these cytokines on IL-8 mRNA accumulation and on NF-κB binding. The role of Oct-1 activation in M. tuberculosis-infected monocytes, which we have shown for the first time, remains to be determined. Despite the fact that IL-4 and IL-13 have a shared receptor chain, in the immune response to this important intracellular pathogen, there is a new example of their divergent action.
ACKNOWLEDGMENT
This work was generously supported by a grant from the British Lung Foundation (BLF).
REFERENCES
- 1.Andrew D P, Chang M S, McNinch J, Wathen S T, Rihanek M, Tseng J, Spellberg J P, Elias C G., III STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J Immunol. 1998;161:5027–5038. [PubMed] [Google Scholar]
- 2.Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998;392:565–568. doi: 10.1038/33340. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin A S. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 4.Bean A G D, Roach D R, Briscoe H, France M P, Korner H, Sedgwick J D, Britton W J. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin 1. J Immunol. 1999;162:3504–3511. [PubMed] [Google Scholar]
- 5.Bergeron A, Bonay M, Kambouchner M, Lecossier D, Riquet M, Soler P, Hance A, Tazi A. Cytokine patterns in tuberculous and sarcoid granulomas: correlations with histopathologic features of the granulomatous response. J Immunol. 1997;159:3034–3043. [PubMed] [Google Scholar]
- 6.Dannenberg A M, Rook G A W. Pathogenesis of pulmonary tuberculosis: an interplay of tissue-damaging and macrophage-activating immune responses. In: Bloom B R, editor. Tuberculosis—pathogenesis, protection, and control. Washington, D.C.: ASM Press; 1994. pp. 459–483. [Google Scholar]
- 7.DeForge L E, Kenney J S, Jones M L, Warren J S, Remick D G. Biphasic production of IL-8 in lipopolysaccharide (LPS)-stimulated human whole blood. Separation of LPS-and cytokine-stimulated components using anti-tumor necrosis factor and anti-IL-1 antibodies. J Immunol. 1992;148:2133–2141. [PubMed] [Google Scholar]
- 8.de Waal Malefyt R, Figdor C G, Huijbens R, Mohan-Peterson S, Bennett B, Culpepper J, Dang W, Zurawski G, de Vries J E. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-γ or IL-10. J Immunol. 1993;151:6370–6381. [PubMed] [Google Scholar]
- 9.de Waal Malefyt R, Abrams J, Bennett B, Figdor C G, de Vries J E. Interleukin 10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finbloom D S, Winestock K D. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1α and STAT3 complexes in human T cells and monocytes. J Immunol. 1995;155:1079–1090. [PubMed] [Google Scholar]
- 11.Fortsch D, Rollinghoff M, Stenger S. IL-10 converts human dendritic cells into macrophage-like cells with increased antibacterial activity against virulent Mycobacterium tuberculosis. J Immunol. 2000;165:978–987. doi: 10.4049/jimmunol.165.2.978. [DOI] [PubMed] [Google Scholar]
- 12.Friedland J S, Remick D G, Shattock R, Griffin G E. Secretion of interleukin-8 following phagocytosis of Mycobacterium tuberculosis by human monocyte cell line. Eur J Immunol. 1992;22:1373–1378. doi: 10.1002/eji.1830220607. [DOI] [PubMed] [Google Scholar]
- 13.Friedland J S, Hartley J C, Hartley C G, Shattock R J, Griffin G E. Inhibition of ex vivo proinflammatory cytokine secretion in fatal Mycobacterium tuberculosis infection. Clin Exp Immunol. 1995;100:233–238. doi: 10.1111/j.1365-2249.1995.tb03659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerszten R E, Garcia-Zepeda E A, Lim Y C, Yoshida M, Ding H A, Gimbrone M A, Jr, Luster A D, Luscinskas F W, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–723. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh S, May M J, Kopp E B. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 16.Haanen J B, de Waal Malefijt R, Res P C, Kraakman E M, Ottenhoff T H, de Vries R R, Spits H. Selection of a human T helper type 1-like T cell subset by mycobacteria. J Exp Med. 1991;174:583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart P H, Bonder C S, Balogh J, Dickensheets H L, Donnelly R P, Finlay-Jones J J. Differential responses of human monocytes and macrophages to IL-4 and IL-13. J Leukoc Biol. 1999;66:575–578. [PubMed] [Google Scholar]
- 18.Hernandez-Pando R, Orozco H, Mancilla R. T-cell lung granulomas induced by Sepharose-coupled Mycobacterium tuberculosis protein antigens: immunosuppressive phenomena reversed with cyclophosphamide and indomethacin. Immunology. 1995;86:506–511. [PMC free article] [PubMed] [Google Scholar]
- 19.Ho A S Y, Liu Y, Khan T A, Hsu D H, Bazan J F, Moore K W. A receptor for interleukin 10 is related to interferon receptors. Proc Natl Acad Sci USA. 1993;90:11267–11271. doi: 10.1073/pnas.90.23.11267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard A D, Zwilling B S. Cytokine production by CD4 and CD8 T cells during the growth of Mycobacterium tuberculosis in mice. Clin Exp Immunol. 1998;113:443–449. doi: 10.1046/j.1365-2249.1998.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isshiki H, Akira S, Tanabe O, Nakajima T, Shimamoto T, Hirano T, Kishimoto T. Constitutive and interleukin-1 (IL-1)-inducible factors interact with the IL-1-responsive element in the IL-6 gene. Mol Cell Biol. 1990;10:2757–2764. doi: 10.1128/mcb.10.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joyce D A, Gibbons D P, Green P, Steer J H, Feldmann M, Brennan F M. Two inhibitors of pro-inflammatory cytokine release, interleukin-10 and interleukin-4, have contrasting effects on release of soluble p75 tumor necrosis factor receptor by cultured monocytes. Eur J Immunol. 1994;24:2699–2705. doi: 10.1002/eji.1830241119. [DOI] [PubMed] [Google Scholar]
- 23.Kasahara K, Sato I, Ogura K, Takeuchi H, Kobayashi K, Adachi M. Expression of chemokines and induction of rapid cell death in human blood neutrophils by Mycobacterium tuberculosis. J Infect Dis. 1998;178:127–137. doi: 10.1086/515585. [DOI] [PubMed] [Google Scholar]
- 24.Kasahara K, Tobe T, Tomita M, Mukaida N, Shao-Bo S, Matsushima K, Yoshida T, Sugihara S, Kobayashi K. Selective expression of monocyte chemotactic and activating factor/monocyte chemoattractant protein 1 in human blood monocytes by Mycobacterium tuberculosis. J Infect Dis. 1994;170:1238–1247. doi: 10.1093/infdis/170.5.1238. [DOI] [PubMed] [Google Scholar]
- 25.Keegan A D, Johnston J A, Tortolani P J, McReynolds L J, Kinzer C, O'Shea J J, Paul W E. Similarities and differences in signal transduction by interleukin 4 and interleukin 13: analysis of Janus kinase activation. Proc Natl Acad Sci USA. 1995;92:7681–7685. doi: 10.1073/pnas.92.17.7681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kindler V, Sappino A P, Grau G E, Piguet P F, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 27.Kurashima K, Mukaida N, Fujimura M, Yasui M, Nakazumi Y, Matsuda T. Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am J Respir Crit Care Med. 1997;155:1474–1477. doi: 10.1164/ajrccm.155.4.9105097. [DOI] [PubMed] [Google Scholar]
- 28.Larsen C G, Thomsen K M, Gesser B, Thomsen P D, Deleuran B W, Nowak J, Skodt V, Thomsen H K, Deleuran M, Thestrup-Pedersen K, et al. The delayed-type hypersensitivity reaction is dependent on IL-8. Inhibition of a tuberculin skin reaction by an anti-IL-8 monoclonal antibody. J Immunol. 1995;155:2151–2157. [PubMed] [Google Scholar]
- 29.Law K F, Jagirdar J, Weiden M D, Bodkin M, Rom W N. Tuberculosis in HIV-positive patients: cellular response and immune activation in the lung. Am J Respir Crit Care Med. 1996;153:1377–1384. doi: 10.1164/ajrccm.153.4.8616569. [DOI] [PubMed] [Google Scholar]
- 30.Lin Y, Zhang M, Barnes P F. Chemokine production by a human alveolar epithelial cell line in response to Mycobacterium tuberculosis. Infect Immun. 1998;66:1121–1126. doi: 10.1128/iai.66.3.1121-1126.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin Y, Zhang M, Hofman F M, Gong J, Barnes P F. Absence of a prominent Th2 cytokine response in human tuberculosis. Infect Immun. 1996;64:1351–1356. doi: 10.1128/iai.64.4.1351-1356.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lischke A, Moriggl R, Brandlein S, Berchtold S, Kammer W, Sebald W, Groner B, Liu X, Hennighausen L, Friedrich K. The interleukin-4 receptor activates STAT5 by a mechanism that relies upon common gamma-chain. J Biol Chem. 1998;273:31222–31229. doi: 10.1074/jbc.273.47.31222. [DOI] [PubMed] [Google Scholar]
- 33.Meddows-Taylor S, Martin D J, Tiemessen C T. Dysregulated production of interleukin-8 in individuals infected with human immunodeficiency virus type 1 and Mycobacterium tuberculosis. Infect Immun. 1999;67:1251–1260. doi: 10.1128/iai.67.3.1251-1260.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mijatovic T, Kruys V, Caput D, Defrance P, Huez G. Interleukin-4 and -13 inhibit tumor necrosis factor-α mRNA translational activation in lipopolysaccharide-induced mouse macrophages. J Biol Chem. 1997;272:14394–14398. doi: 10.1074/jbc.272.22.14394. [DOI] [PubMed] [Google Scholar]
- 35.Minty A, Chalon P, Derocq J-M, Dumont X, Guillemot J-C, Kaghad M, Labit C, Leplatois P, Liauzun P, Miloux B, Minty C, Casellas P, Loison G, Lupker J, Shire D, Ferrara P, Caput D. Interleukin-13 is a new human lymphokine regulating inflammatory and immune responses. Nature. 1993;362:248–250. doi: 10.1038/362248a0. [DOI] [PubMed] [Google Scholar]
- 36.Mukaida N, Shiroo M, Matsushima K. Genomic structure of the human monocyte-derived neutrophil chemotactic factor IL-8. J Immunol. 1989;143:1366–1371. [PubMed] [Google Scholar]
- 37.Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of nuclear factor κB and cis-regulatory binding protein-like factor binding elements in activating the interleukin-8 gene by pro-inflammatory cytokines. J Biol Chem. 1990;265:21128–21133. [PubMed] [Google Scholar]
- 38.North R J. Mice incapable of making IL-4 or IL-10 display normal resistance to infection with Mycobacterium tuberculosis. Clin Exp Immunol. 1998;113:55–58. doi: 10.1046/j.1365-2249.1998.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oppenheim J J, Zachariae C O, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- 40.Pace E, Gjomarkaj M, Melis M, Profita M, Spatafora M, Vignola A M, Bonsignore G, Mody C H. Interleukin-8 induces lymphocyte chemotaxis into the pleural space. Role of pleural macrophages. Am J Respir Crit Care Med. 1999;159:1592–1599. doi: 10.1164/ajrccm.159.5.9806001. [DOI] [PubMed] [Google Scholar]
- 41.Rennick D M, Fort M M, Davidson N J. Studies with IL-10−/− mice: an overview. J Leukoc Biol. 1997;61:389–396. doi: 10.1002/jlb.61.4.389. [DOI] [PubMed] [Google Scholar]
- 42.Riedel D D, Kaufmann S H. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect Immun. 1997;65:4620–4623. doi: 10.1128/iai.65.11.4620-4623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roebuck K A. Regulation of interleukin-8 gene expression. J Interferon Cytokine Res. 1999;19:429–438. doi: 10.1089/107999099313866. [DOI] [PubMed] [Google Scholar]
- 44.Sadek I M, Sada E, Toossi Z, Schwander S K, Rich E A. Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am J Respir Cell Mol Biol. 1998;19:513–521. doi: 10.1165/ajrcmb.19.3.2815. [DOI] [PubMed] [Google Scholar]
- 45.Sato K, Nagayama H, Tadokoro K, Judi T, Takahashi T A. Extracellular signal-regulated kinase, stress-activated protein kinase/c-Jun N-terminal kinase, and p38 mapk are involved in IL-10-mediated selective repression of TNF-γ-induced activation and maturation of human peripheral blood monocyte-derived dendritic cells. J Immunol. 1999;162:3865–3872. [PubMed] [Google Scholar]
- 46.Schluger N W, Rom W N. The host immune response to tuberculosis. Am J Respir Crit Care Med. 1998;157:679–691. doi: 10.1164/ajrccm.157.3.9708002. [DOI] [PubMed] [Google Scholar]
- 47.Schottelius A J, Mayo M W, Sartor R B, Baldwin A S., Jr Interleukin-10 signaling blocks inhibitor of κB kinase activity and nuclear factor κB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- 48.Seah G T, Scott G M, Rook G A. Type 2 cytokine gene activation and its relationship to extent of disease in patients with tuberculosis. J Infect Dis. 2000;181:385–389. doi: 10.1086/315200. [DOI] [PubMed] [Google Scholar]
- 49.Shaw T C, Thomas L H, Friedland J S. Regulation of IL-10 secretion after phagocytosis of Mycobacterium tuberculosis by human monocytic cells. Cytokine. 2000;12:483–486. doi: 10.1006/cyto.1999.0586. [DOI] [PubMed] [Google Scholar]
- 50.Somoskovi A, Zissel G, Zipfel P F, Ziegenhagen M W, Klaucke J, Haas H, Schlaak M, Muller-Quernheim J. Different cytokine patterns correlate with the extension of disease in pulmonary tuberculosis. Eur Cytokine Netw. 1999;10:135–142. [PubMed] [Google Scholar]
- 51.Standiford T J, Strieter R M, Chensue S W, Westwick J, Kasahara K, Kunkel S L. IL-4 inhibits the expression of IL-8 from stimulated human monocytes. J Immunol. 1990;145:1435–1439. [PubMed] [Google Scholar]
- 52.Standiford T J, Kunkel S L, Liebler J M, Burdick M D, Gilbert A R, Strieter R M. Gene expression of macrophage inflammatory protein-1 α from human blood monocytes and alveolar macrophages is inhibited by IL-4. Am J Respir Cell Mol Biol. 1993;9:192–198. doi: 10.1165/ajrcmb/9.2.192. [DOI] [PubMed] [Google Scholar]
- 53.Stein B, Baldwin A S., Jr Distinct mechanisms for regulation of the interleukin-8 gene involve synergism and cooperativity between C/EBP and NF-κB. Mol Cell Biol. 1993;13:7191–7198. doi: 10.1128/mcb.13.11.7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 55.Takeshita S, Gage J R, Kishimoto T, Vredevoe D L, Martinez-Maza O. Differential regulation of IL-6 gene transcription and expression by IL-4 and IL-10 in human monocytic cell lines. J Immunol. 1996;156:2591–2598. [PubMed] [Google Scholar]
- 56.Toossi Z, Hamilton B D, Phillips M H, Averill L E, Ellner J J, Salvekar A. Regulation of nuclear factor-kappa B and its inhibitor I kappa B-alpha/MAD-3 in monocytes by Mycobacterium tuberculosis and during human tuberculosis. J Immunol. 1997;159:4109–4116. [PubMed] [Google Scholar]
- 57.Wang L-M, Keegan A D, Paul W E, Heidaran M A, Gutkind J S, Pierce J H. IL-4 activates a distinct signal transduction cascade from IL-3 in factor-dependent myeloid cells. EMBO J. 1992;11:4899–4908. doi: 10.1002/j.1460-2075.1992.tb05596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang P, Wu P, Siegel M I, Egan R W, Billah M M. Interleukin (IL)-10 inhibits nuclear factor κB (NF-κB) activation in human monocytes: IL-10 and IL-4 suppress cytokine synthesis by different mechanism. J Biol Chem. 1995;270:9558–9563. doi: 10.1074/jbc.270.16.9558. [DOI] [PubMed] [Google Scholar]
- 59.Weber-Nordt R M, Riley J K, Greenlund A C, Moore K W, Darnell J E, Schreiber R D. Stat3 recruitment by two distinct ligand-induced, tyrosine-phosphorylated docking sites in the interleukin-10 receptor intracellular domain. J Biol Chem. 1996;271:27954–27961. doi: 10.1074/jbc.271.44.27954. [DOI] [PubMed] [Google Scholar]
- 60.Welham J M, Learmonth L, Bone H, Schrader J W. Interleukin-13 signal transduction in lymphohemopoietic cells. Similarities and differences in signal transduction with interleukin-4 and insulin. J Biol Chem. 1995;270:12286–12296. doi: 10.1074/jbc.270.20.12286. [DOI] [PubMed] [Google Scholar]
- 61.Wickremasinghe M, Thomas L H, Friedland J S. Pulmonary epithelial cells are a source of IL-8 in the response to Mycobacterium tuberculosis: essential role of IL-1 from infected monocytes in a NF-κB-dependent network. J Immunol. 1999;163:3936–3947. [PubMed] [Google Scholar]
- 62.Wilkinson P C, Newman I. Identification of IL-8 as a locomotor attractant for activated human lymphocytes in mononuclear cell cultures with anti-CD3 or purified protein derivative of Mycobacterium tuberculosis. J Immunol. 1992;149:2689–2694. [PubMed] [Google Scholar]
- 63.Wilsher L M, Hagan C, Prestidge R, Wells A U, Murison G. Human in vitro immune responses to Mycobacterium tuberculosis. Tuber Lung Dis. 1999;79:371–377. doi: 10.1054/tuld.1999.0223. [DOI] [PubMed] [Google Scholar]
- 64.World Health Organization. Global tuberculosis control. W. H. O. report 2000. W. H. O./CDS/TB/2000.275. Geneva, Switzerland: World Health Organization; 2000. [Google Scholar]
- 65.Wu G D, Lai E J, Huang N, Wen X. Oct-1 and CCAAT/enhancer-binding protein (C/EBP) bind to overlapping elements within the interleukin-8 promoter. The role of Oct-1 as a transcriptional repressor. J Biol Chem. 1997;272:2396–2403. [PubMed] [Google Scholar]
- 66.Yang X D, Corvalan J R, Wang P, Roy C M, Davis C G. Fully human anti-interleukin-8 monoclonal antibodies: potential therapeutics for the treatment of inflammatory disease stages. J Leukoc Biol. 1999;66:401–410. doi: 10.1002/jlb.66.3.401. [DOI] [PubMed] [Google Scholar]
- 67.Yasukawa H, Sasaki A, Yoshuimura A. Negative regulation of cytokine signalling pathways. Annu Rev Immunol. 2000;18:143–164. doi: 10.1146/annurev.immunol.18.1.143. [DOI] [PubMed] [Google Scholar]
- 68.Zhang H, Shepherd A T, Eason D D, Wei S, Diaz J I, Djeu J Y, Wu G D, Blanck G. Retinoblastoma protein expression leads to reduced Oct-1 DNA binding activity and enhances interleukin-8 expression. Cell Growth Differ. 1999;10:457–465. [PubMed] [Google Scholar]
- 69.Zhang Y, Broser M, Cohen H, Bodkin M, Law K, Reibman J, Rom W N. Enhanced interleukin-8 release and gene expression in macrophages after exposure to Mycobacterium tuberculosis and its components. J Clin Investig. 1995;95:586–592. doi: 10.1172/JCI117702. [DOI] [PMC free article] [PubMed] [Google Scholar]