Abstract
Simple Summary
Brain metastasis is a factor of a poor prognosis in patients with non-small-cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) mutations. The standard treatment is systemic therapy combined with intracranial intervention, such as craniotomy or radiotherapy. However, intracranial intervention may result in neurological or cognitive deficiency. We conducted a retrospective study to determine the optimal treatment strategy for EGFR-mutant NSCLC patients with brain metastasis receiving or not receiving intracranial intervention. Intracranial intervention had no statistically significant impact on response rate (RR), progression-free survival (PFS), or overall survival (OS) of patients with EGFR mutations and brain metastasis who received EGFR tyrosine kinase inhibitors (TKIs) as a first-line therapy. Treatment with different EGFR TKIs did not result in significant differences in RR or OS, but PFS differed significantly between the therapies. Afatinib and osimertinib both showed significantly longer PFS than gefitinib in a Cox regression model. The OS of patients with higher graded prognostic assessment (GPA) scores was significantly longer. Physicians should be vigilant about patients who have lower GPA scores at initial diagnosis of NSCLC with brain metastasis.
Abstract
Brain metastasis in patients with non-small-cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) mutations is a factor of poor prognosis. We conducted a retrospective study to determine the optimal treatment strategy for EGFR-mutant NSCLC patients with brain metastasis receiving or not receiving intracranial intervention. A total of 186 patients treated with an EGFR TKI were enrolled in the study, and 79 (42%) received intracranial intervention. Patients who received intracranial intervention and those who did not had a similar treatment response rate (RR), progression-free survival (PFS) (median PFS: 11.0 vs. 10.0 months, p = 0.4842), and overall survival (OS) (median OS: 23.0 vs. 23.2 months, p = 0.2484). Patients treated with gefitinib, erlotinib, afatinib, or osimertinib had a similar RR (63%, 76%, 81%, or 100%, respectively, p = 0.1390), but they had significantly different PFS (median PFS: 7.5, 10.0, 14.8 months, or not reached, respectively, p = 0.0081). In addition, OS tended to be different between different EGFR TKI treatments (median OS of 19.2, 23.7, or 33.0 months for gefitinib, erlotinib, or afatinib treatments, respectively, p = 0.0834). Afatinib and osimertinib both demonstrated significantly longer PFS than gefitinib in a Cox regression model. Graded prognostic assessment (GPA) versions 2017 and 2022 stratified patients with different OS; patients with higher GPA index scores had significantly longer OS (p = 0.0368 and 0.0407 for version 2017 and 2022, respectively).
Keywords: epidermal growth factor receptor, tyrosine kinase inhibitor, brain metastasis, lung cancer, adenocarcinoma
1. Introduction
Non-small-cell lung cancer (NSCLC) is the most common cause of cancer-related mortality worldwide, and the most prevalent cell type of this cancer is adenocarcinoma [1]. Brain metastasis is common in advanced NSCLC. Approximately 10–20% of patients have brain metastasis at initial diagnosis of NSCLC, and about 20–40% develop brain metastasis during treatment [2]. The standard treatment for NSCLC patients with brain metastasis is chemotherapy combined with intracranial intervention, for example, craniotomy or radiotherapy, beforehand [3]. However, not all NSCLC patients with brain metastasis are suitable for surgery due to factors such as poor performance status, tumor occurrence in an inoperable site, and multiple brain metastases [4]. Although the rate of incidence of surgical complications is not high as previously thought, careful, shared decision-making about surgery is still necessary. Some NSCLC patients with brain metastasis are treated with radiotherapy, but previous reports show poor prognoses and a median survival of about 6 months [5]. In addition, NSCLC patients with brain metastasis may show poor responsiveness to chemotherapy, including poor response rates (27–69%) and a short duration of overall survival (OS) (7.4–10 months) [6,7,8].
In the era of precision medicine, the development of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) has changed clinical practice. The rate of incidence of the EGFR mutation in NSCLC patients is approximately 40–60% among Asian and 10% among Western patients [9]. In recent years, randomized trials have further shown that patients with NSCLC harboring susceptible EGFR mutations, such as the exon 21 L858R point mutation and exon 19 deletion, have longer progression-free survival (PFS) when they are treated with EGFR TKIs rather than standard chemotherapy [10,11,12,13,14,15,16]. Previous studies have also reported encouraging results for EGFR TKI treatment of patients with EGFR mutations and brain metastasis [10,13,14,15,17,18,19].
EGFR TKIs have different in vitro sensitivities, plasma drug concentrations, clinical responses, and rates of penetration into the cerebrospinal fluid from the plasma [20,21,22,23,24]. According to previous studies which compared the treatment outcomes of different EGFR TKIs for EGFR-mutant NSCLC patients with brain metastasis, different first-line EGFR TKIs achieved similar treatment outcome [25]. However, the clinical efficacy of different EGFR-TKI-only treatments for patients with brain metastasis is still unknown. In addition, EGFR TKIs combined with brain surgery or radiotherapy to treat metastatic brain tumors is still a popular strategy for patients with EGFR mutations and brain metastasis. However, memory impairment is inevitable in NSCLC patients with brain metastasis after whole-brain radiotherapy [26]. The role of surgery or radiotherapy in treating patients with susceptible EGFR mutations and brain metastasis is still unclear. We conducted this retrospective study with real-world data from three hospitals to determine the optimal treatment strategy for EGFR-mutant NSCLC patients with brain metastasis.
2. Materials and Methods
2.1. Patient Identification
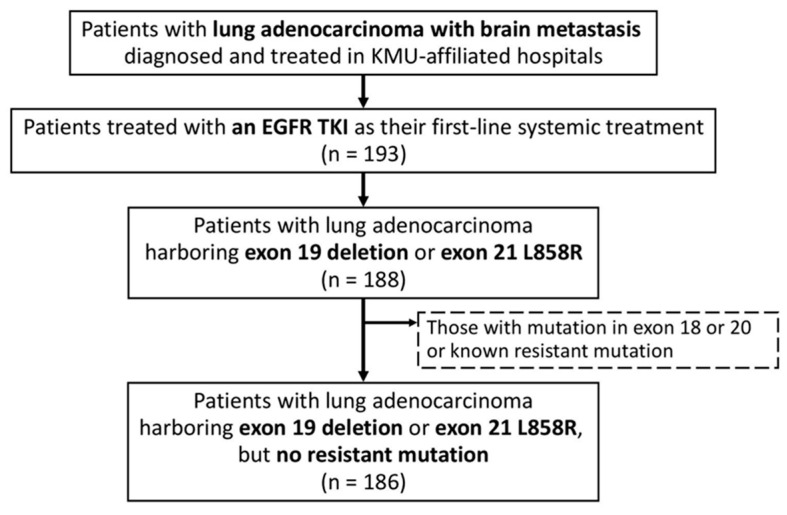
We enrolled patients with lung adenocarcinoma with brain metastasis diagnosed and treated at three hospitals affiliated with Kaohsiung Medical University—Kaohsiung Medical University Hospital, Kaohsiung Municipal Ta-Tung Hospital, and Kaohsiung Municipal Siaogang Hospital (Figure 1). The diagnosis of lung adenocarcinoma was confirmed pathologically according to the World Health Organization pathology classification, and cancer stage was confirmed by a lung cancer team according to the 8th version of the American Joint Committee on Cancer staging system. Formalin-fixed paraffin-embedded tissue blocks were made from tumor specimens obtained from the enrolled patients. Genomic DNA was extracted from tissue blocks for genotyping exons 18 to 21 of the EGFR gene with the cobas® EGFR Mutation Test v2 (Roche Molecular Systems, Inc., Basel, Switzerland). The ready-to-use test kit allows the detection of 42 somatic mutations in the cancer-related EGFR gene with a polymerase chain reaction using a cobas® z480 instrument and a cobas® 4800 analyzer. The examination techniques were the same as those in our previous studies [27,28,29,30]. The Institutional Review Board (IRB) of Kaohsiung Medical University Hospital (KMUH) approved this study (KMUHIRB-E(I)-20210257) and waived the need for written informed consent from all patients.
Figure 1.
Flowchart for identifying the study population. Abbreviations: EGFR, epidermal growth factor receptor; KMU, Kaohsiung Medical University.
In the present study, we enrolled individuals with only the exon 19 deletion or exon 21 L858R point mutation in lung adenocarcinoma. All patients were naïve to systemic treatment and received an EGFR TKI—gefitinib, erlotinib, afatinib, or osimertinib—as their first-line systemic treatment. The patients’ medical records were retrospectively reviewed to determine their baseline clinical characteristics: age at diagnosis, sex, Eastern Cooperative Oncology Group (ECOG) performance status, Karnofsky performance status, EGFR mutation, programmed death ligand 1 (PD-L1), number of metastatic brain nodules, site of extracranial metastasis (ECM), and anti-VEGF treatment. Patients receiving intracranial intervention, i.e., surgery or radiotherapy, for a metastatic brain tumor were identified. Graded prognostic assessment (GPA) index scores (Table A1) were calculated according to three previously published versions—2010 [31], 2017 [32], and 2022 [33].
The response to the initial treatment was classified based on serial imaging studies using the revised Response Evaluation Criteria in Solid Tumors v. 1.1 (RECIST). PFS, intracranial PFS (iPFS), and OS were defined as the time from the start of the initial EGFR TKI treatment to the date of disease progression detected with an imaging examination, the date of disease progression detected on a brain image, and the date of death, respectively.
2.2. Statistical Analysis
In this retrospective study, we adopted the statistical approaches used in our previous studies [27,28,29,30] and in other similar studies [25]. Categorical variables and continuous variables were compared using a chi-squared test and analysis of variance, respectively. Survival times were estimated using the Kaplan–Meier method, and differences between groups were compared with the log-rank test. A Cox regression analysis was used to determine the predictive factors of PFS, iPFS, and OS. Both univariate and multivariable analyses were performed. To avoid overadjustment, we used a backward variable selection method, retaining only variables with a p-value of < 0.15, so as to develop reduced multivariate models. Hazard ratios (HR) with 95% confidence intervals (CIs) are presented for the predictors. All statistical analyses were performed with SAS software (version 9.4 for Windows, SAS Institute Inc., Cary, NC, USA). A two-tailed p-value of < 0.05 was set as the critical value for statistical significance.
3. Results
3.1. Study Characteristics
We identified 193 patients with lung adenocarcinoma and brain metastasis treated with an EGFR TKI as their first-line systemic treatment (Figure 1). After excluding those with the exon 18 or 20 mutation or a known resistant mutation, the remaining 186 patients with the exon 19 deletion or exon 21 L858R point mutation were enrolled in the study (Table 1 and Table A1). The mean (±standard deviation) age of the enrolled patients was 65.5 (±11.2) years, and 63 (34%) patients were male. Most patients’ performance status upon diagnosis was good. (A total of 85% had an ECOG performance status of ≤1, and 84% had a Karnofsky performance status of ≥80.) The exon 19 deletion and exon 21 L858R were detected in the tumors of 85 (46%) and 102 (55%) of the patients, respectively (one patient’s tumor had both mutations). A total of 63 (34%) patients had a single brain metastasis, while 68 (37%) had more than four metastatic brain nodules.
Table 1.
Baseline characteristics of the study cohort.
| Variables | All Patients | Gefitinib | Erlotinib | Afatinib | Osimertinib | p-Value |
|---|---|---|---|---|---|---|
| n | 186 | 27 | 104 | 48 | 7 | |
| Sex | ||||||
| Female | 123 (66%) | 16 (59%) | 71 (68%) | 30 (63%) | 6 (86%) | 0.5197 |
| Male | 63 (34%) | 11 (41%) | 33 (32%) | 18 (38%) | 1 (14%) | |
| Age (years) | 65.5 ± 11.2 | 64.9 ± 13.2 | 66.5 ± 10.7 | 63.3 ± 10.6 | 68.9 ± 13.6 | 0.3470 |
| <65 | 92 (49%) | 16 (59%) | 47 (45%) | 26 (54%) | 3 (43%) | 0.5044 |
| ≥65 | 94 (51%) | 11 (41%) | 57 (55%) | 22 (46%) | 4 (57%) | |
| ECOG performance status | ||||||
| 0–1 | 159 (85%) | 23 (85%) | 90 (87%) | 42 (88%) | 4 (57%) | 0.1884 |
| ≥2 | 27 (15%) | 4 (15%) | 14 (13%) | 6 (13%) | 3 (43%) | |
| Karnofsky performance status | ||||||
| 100 | 12 (6%) | 3 (11%) | 7 (7%) | 1 (2%) | 1 (14%) | 0.2980 |
| 90 | 21 (11%) | 4 (15%) | 9 (9%) | 8 (17%) | 0 (0%) | |
| 80 | 125 (67%) | 16 (59%) | 74 (71%) | 32 (67%) | 3 (43%) | |
| 70 | 27 (15%) | 4 (15%) | 14 (13%) | 6 (13%) | 3 (43%) | |
| ≤60 | 1 (1%) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | |
| EGFR mutation | ||||||
| Exon 19 deletion | 85 (46%) | 10 (37%) | 46 (44%) | 25 (52%) | 4 (57%) | 0.5591 |
| Exon 21 L858R | 102 (55%) | 17 (63%) | 59 (57%) | 23 (48%) | 3 (43%) | 0.5311 |
| PD-L1 † | ||||||
| <1% | 29 (29%) | 1 (33%) | 16 (24%) | 12 (43%) | 0 (0%) | 0.1701 |
| ≥1% | 72 (71%) | 2 (67%) | 50 (76%) | 16 (57%) | 4 (100%) | |
| Number of brain metastases | ||||||
| 1 | 63 (34%) | 15 (56%) | 31 (30%) | 15 (31%) | 2 (29%) | 0.0378 |
| 2–4 | 55 (30%) | 10 (37%) | 30 (29%) | 14 (29%) | 1 (14%) | |
| ≥5 | 68 (37%) | 2 (7%) | 43 (41%) | 19 (40%) | 4 (57%) | |
| Number of ECM sites | ||||||
| 0 | 25 (13%) | 4 (15%) | 15 (14%) | 6 (13%) | 0 (0%) | 0.6122 |
| 1–2 | 91 (49%) | 14 (52%) | 45 (43%) | 28 (58%) | 4 (57%) | |
| ≥3 | 70 (38%) | 9 (33%) | 44 (42%) | 14 (29%) | 3 (43%) | |
| Site of ECMs | ||||||
| Lung metastasis | 99 (53%) | 12 (44%) | 58 (56%) | 25 (52%) | 4 (57%) | 0.7590 |
| Pleural metastasis/effusion | 81 (44%) | 12 (44%) | 49 (47%) | 17 (35%) | 3 (43%) | 0.6064 |
| Bone metastasis | 116 (62%) | 18 (67%) | 63 (61%) | 30 (63%) | 5 (71%) | 0.8965 |
| Liver metastasis | 26 (14%) | 1 (4%) | 16 (15%) | 7 (15%) | 2 (29%) | 0.2844 |
| Pericardial metastasis/effusion | 51 (27%) | 6 (22%) | 31 (30%) | 11 (23%) | 3 (43%) | 0.5741 |
| Adrenal metastasis | 23 (12%) | 2 (7%) | 15 (14%) | 5 (10%) | 1 (14%) | 0.7504 |
| Other metastasis | 12 (6%) | 4 (15%) | 6 (6%) | 1 (2%) | 1 (14%) | 0.1424 |
| GPA (version 2010) score | ||||||
| ≥2.5 | 26 (14%) | 7 (26%) | 11 (11%) | 8 (17%) | 0 (0%) | 0.2945 |
| 1.5–2.0 | 66 (35%) | 11 (41%) | 37 (36%) | 15 (31%) | 3 (43%) | |
| ≤1.0 | 94 (51%) | 9 (33%) | 56 (54%) | 25 (52%) | 4 (57%) | |
| GPA (version 2017) score | ||||||
| ≥2.5 | 36 (19%) | 8 (30%) | 18 (17%) | 10 (21%) | 0 (0%) | 0.0552 |
| 1.5–2.0 | 120 (65%) | 19 (70%) | 65 (63%) | 32 (67%) | 4 (57%) | |
| ≤1.0 | 30 (16%) | 0 (0%) | 21 (20%) | 6 (13%) | 3 (43%) | |
| GPA (version 2022) score | ||||||
| ≥3.0 | 17 (9%) | 3 (11%) | 10 (10%) | 4 (8%) | 0 (0%) | 0.1905 |
| 1.5–2.5 | 149 (80%) | 24 (89%) | 79 (76%) | 41 (85%) | 5 (71%) | |
| ≤1.0 | 20 (11%) | 0 (0%) | 15 (14%) | 3 (6%) | 2 (29%) | |
| Anti-VEGF treatment | ||||||
| No | 175 (94%) | 27 (100%) | 95 (91%) | 47 (98%) | 6 (86%) | 0.1545 |
| Yes | 11 (6%) | 0 (0%) | 9 (9%) | 1 (2%) | 1 (14%) | |
| Brain OP (operation for brain tumor) | ||||||
| No | 136 (73%) | 16 (59%) | 80 (77%) | 33 (69%) | 7 (100%) | 0.0919 |
| Yes | 50 (27%) | 11 (41%) | 24 (23%) | 15 (31%) | 0 (0%) | |
| Brain RT (radiotherapy for brain tumor) | ||||||
| No | 125 (67%) | 14 (52%) | 80 (77%) | 24 (50%) | 7 (100%) | 0.0006 |
| Yes | 61 (33%) | 13 (48%) | 24 (23%) | 24 (50%) | 0 (0%) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; ECM, extracranial metastasis; GPA, graded prognostic assessment; OP, surgery for brain tumor; PD-L1, programmed death ligand 1; RT, radiotherapy for brain tumor; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor. Data are presented as n (%) or mean ± standard deviation. p-values are presented for the results of the chi-squared test or the analysis of variance. † PD-L1 was examined in 101 patients.
3.2. Intracranial Intervention
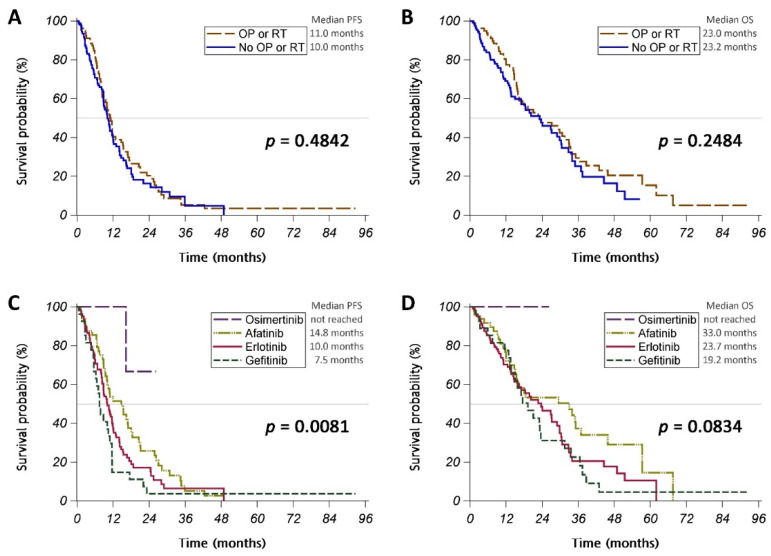
Of the 186 patients enrolled in this study, 79 (42%) received intracranial intervention for a metastatic brain tumor. (A total of 50 patients underwent surgery, and 61 underwent radiotherapy) (Table A2). Patients receiving intracranial intervention tended to have fewer brain metastases (p = 0.0600) and significantly fewer ECM (p = 0.0174) than those who did not receive intracranial intervention, while the two groups’ performance statuses were similar. The patients receiving intracranial intervention and those who did not receive the intervention showed a similar response to the initial treatment (Table A3), and the PFS (median PFS: 11.0 vs. 10.0 months; p = 0.4842) and OS (median OS: 23.0 vs. 23.2 months; p = 0.2484) of the two groups were also similar (Figure 2A,B and Figure A1A,B).
Figure 2.
(A,C) Progression-free survival (PFS) and (B,D) overall survival (OS) of patients classified according to (A,B) those undergoing or not undergoing surgery (OP) or radiotherapy (RT) for brain tumor or (C,D) those receiving different epidermal growth factor receptor tyrosine kinase inhibitors.
3.3. Different EGFR TKIs
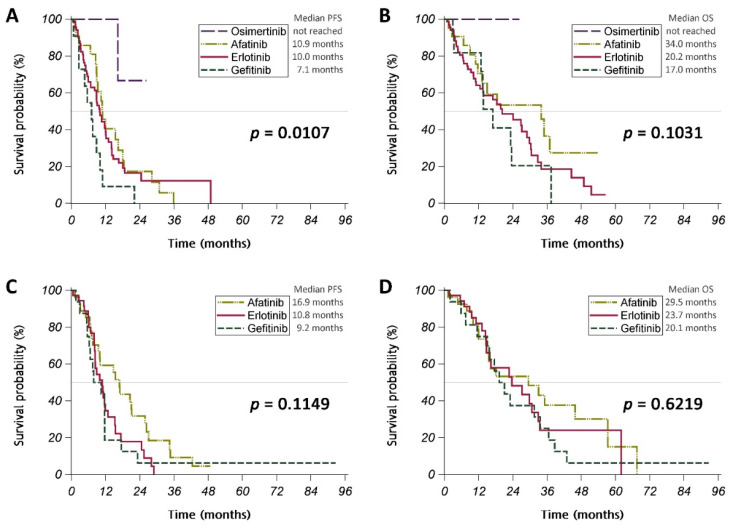
EGFR TKIs taken as the first-line systemic treatment were gefitinib (27 patients), erlotinib (104 patients), afatinib (48 patients), and osimertinib (7 patients) (Table 1). Patients receiving different EGFR TKIs were similar in age, performance status, and number of ECM. Patients with a higher number of metastatic brain nodules were less likely to be treated with gefitinib. The initial treatment response rate was similar in patients treated with gefitinib, erlotinib, afatinib, and osimertinib (63%, 76%, 81%, and 100%, respectively; p = 0.1390) (Table 2). The PFS of patients taking gefitinib, erlotinib, afatinib, or osimertinib was significantly different (median PFS: 7.5 months, 10.0 months, 14.8 months, or not reached, respectively; p = 0.0081) (Figure 2C). A similar result was noted for patients without intracranial intervention (Figure A2A), but not for those with intracranial intervention (Figure A2C). The OS of patients taking gefitinib, erlotinib, afatinib, or osimertinib tended to be different (median OS: 19.2 months, 23.7 months, and 33.0 months, or not reached, respectively; p = 0.0834) (Figure 2D), while these results were not repeated after the patients were stratified by whether or not they received intracranial intervention (Figure A2B,D).
Table 2.
Initial treatment response and site of disease progression.
| Variables | All Patients | Gefitinib | Erlotinib | Afatinib | Osimertinib | p-Value |
|---|---|---|---|---|---|---|
| Initial treatment response | ||||||
| Progressive disease (PD) | 18 (10%) | 4 (15%) | 9 (9%) | 5 (10%) | 0 (0%) | 0.6378 |
| Stable disease (SD) | 26 (14%) | 6 (22%) | 16 (15%) | 4 (8%) | 0 (0%) | |
| Partial response (PR) | 139 (75%) | 17 (63%) | 77 (74%) | 38 (79%) | 7 (100%) | |
| Complete response (CR) | 3 (2%) | 0 (0%) | 2 (2%) | 1 (2%) | 0 (0%) | |
| Disease control rate | 168 (90%) | 23 (85%) | 95 (91%) | 43 (90%) | 7 (100%) | 0.6325 |
| Response rate | 142 (76%) | 17 (63%) | 79 (76%) | 39 (81%) | 7 (100%) | 0.1390 |
| Initial intracranial treatment response † | ||||||
| Intracranial progressive disease (iPD) | 9 (6%) | 1 (5%) | 3 (3%) | 5 (11%) | 0 (0%) | 0.6033 |
| Intracranial stable disease (iSD) | 28 (18%) | 4 (20%) | 19 (22%) | 4 (9%) | 1 (14%) | |
| Intracranial partial response (iPR) | 76 (48%) | 9 (45%) | 43 (49%) | 20 (45%) | 4 (57%) | |
| Intracranial complete response (iCR) | 46 (29%) | 6 (30%) | 23 (26%) | 15 (34%) | 2 (29%) | |
| Intracranial disease control rate | 150 (94%) | 19 (95%) | 85 (97%) | 39 (89%) | 7 (100%) | 0.2667 |
| Intracranial response rate | 122 (77%) | 15 (75%) | 66 (75%) | 35 (80%) | 6 (86%) | 0.8749 |
| Site of disease progression | ||||||
| Intracranial progression | 48 (26%) | 5 (19%) | 24 (23%) | 19 (40%) | 0 (0%) | 0.0394 |
| Extracranial progression | 21 (11%) | 5 (19%) | 10 (10%) | 6 (13%) | 0 (0%) | 0.4469 |
| Lung | 5 (3%) | 1 (4%) | 3 (3%) | 1 (2%) | 0 (0%) | 0.9439 |
| Pleural nodule/effusion | 7 (4%) | 3 (11%) | 2 (2%) | 2 (4%) | 0 (0%) | 0.1516 |
| Bone | 9 (5%) | 0 (0%) | 6 (6%) | 3 (6%) | 0 (0%) | 0.5455 |
| Liver | 2 (1%) | 0 (0%) | 1 (1%) | 1 (2%) | 0 (0%) | 0.8397 |
| Adrenal gland | 1 (1%) | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.1155 |
| Other | 1 (1%) | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 0.1155 |
† Initial intracranial treatment response was assessed in 159 patients. Data are presented as n (%). p-values are presented for the results of the chi-squared test.
3.4. Prognostic Factors for PFS and OS
Univariate Cox regression analyses showed that treatment with afatinib or osimertinib was significantly associated with better PFS (HR [95% CI] = 0.54 [0.33–0.88], p = 0.0133 for afatinib; HR [95% CI] = 0.10 [0.01–0.75], p = 0.0248 for osimertinib) (Table 3), whereas male sex (HR [95% CI] = 1.54 [1.09–2.17], p = 0.0133) and bone metastasis (HR [95% CI] = 1.57 [1.12–2.21], p = 0.0091) were significantly associated with poorer PFS. Multivariable analyses with backward variable selection confirmed that afatinib and osimertinib were independent prognostic factors of better PFS, while male sex and bone metastasis were independent prognostic factors of poorer PFS (model 2R in Table 3). Univariate Cox regression analyses or multivariable analyses did not find any factors significantly associated with iPFS (Table A4).
Table 3.
Factors associated with progression-free survival.
| Variables | Univariate | Multivariable Model 1 | Multivariable Model 1R | Multivariable Model 2 | Multivariable Model 2R |
|---|---|---|---|---|---|
| OP (vs. no OP) | 0.90 [0.63–1.28] | ||||
| RT (vs. no RT) | 0.93 [0.66–1.30] | ||||
| OP or RT (vs. no OP or RT) | 0.89 [0.65–1.23] | 0.81 [0.56–1.15] | 0.79 [0.55–1.16] | ||
| EGFR TKI: (vs. gefitinib) | |||||
| Erlotinib | 0.75 [0.48–1.16] | 0.75 [0.44–1.27] | 0.79 [0.50–1.23] | 0.73 [0.42–1.27] | 0.76 [0.49–1.19] |
| Afatinib | 0.54 [0.33–0.88] * | 0.52 [0.31–0.89] * | 0.53 [0.32–0.87] * | 0.51 [0.30–0.88] * | 0.51 [0.31–0.84] ** |
| Osimertinib | 0.10 [0.01–0.75] * | 0.11 [0.01–0.83] * | 0.11 [0.01–0.81] * | 0.10 [0.01–0.77] * | 0.11 [0.02–0.83] * |
| Anti-VEGF treatment (vs. no anti-VEGF) | 0.81 [0.36–1.83] | 0.86 [0.37–2.00] | 0.94 [0.40–2.22] | ||
| Male (vs. female) | 1.54 [1.09–2.17] * | 1.52 [1.07–2.17] * | 1.50 [1.06–2.11] * | 1.64 [1.13–2.39] ** | 1.57 [1.11–2.23] * |
| Age (≥65 vs. <65) | 0.83 [0.60–1.15] | 0.82 [0.57–1.18] | 0.87 [0.60–1.27] | ||
| ECOG PS (≥2 vs. ≤1) | 0.74 [0.46–1.20] | 0.81 [0.48–1.35] | 0.86 [0.51–1.45] | ||
| Exon 19 deletion vs. L858R | 0.99 [0.72–1.36] | 0.87 [0.61–1.23] | 0.94 [0.67–1.34] | ||
| PD–L1 ≥ 1% vs. PD-L1 < 1% or not tested | 1.00 [0.72–1.40] | 1.04 [0.71–1.51] | 1.14 [0.77–1.68] | ||
| Number of brain metastases (≥5 vs. <5) | 0.87 [0.62–1.22] | 1.00 [0.70–1.43] | 1.02 [0.70–1.48] | ||
| Extracranial metastasis (vs. no) | 1.26 [0.80–1.99] | 1.47 [0.91–2.38] | 1.40 [0.89–2.22] | ||
| Lung metastasis (vs. no) | 1.12 [0.81–1.54] | 0.97 [0.67–1.39] | |||
| Pleural metastasis/effusion (vs. no) | 1.29 [0.93–1.79] | 1.48 [0.98–2.23] | 1.38 [0.99–1.92] | ||
| Bone metastasis (vs. no) | 1.57 [1.12–2.21] ** | 1.66 [1.14–2.42] ** | 1.59 [1.13–2.23] ** | ||
| Liver metastasis (vs. no) | 0.99 [0.61–1.61] | 0.94 [0.55–1.60] | |||
| Pericardial metastasis/effusion (vs. no) | 1.16 [0.80–1.67] | 0.93 [0.58–1.49] | |||
| Adrenal metastasis (vs. no) | 0.99 [0.59–1.64] | 0.87 [0.49–1.55] | |||
| Other site metastasis (vs. no) | 0.97 [0.48–1.99] | 1.35 [0.61–2.98] |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; OP, surgery for brain tumor; PD-L1, programmed death ligand 1; PS, performance status; RT, radiotherapy for brain tumor; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor. A patient with cancer harboring both the exon 19 deletion and L858R was included in the exon 19 deletion group. Data are presented as hazard ratio (HR) with 95% confidence interval. After building the maximal models of multivariable Cox regression (models 1 and 2), the corresponding reduced multivariable Cox regression models (models 1R and 2R, respectively) were built using backward variable selection, retaining only variables with p-values < 0.15. * p-value < 0.05; ** p-value < 0.01.
Univariate Cox regression analyses revealed that the male sex was associated with significantly poorer OS (HR [95% CI] = 1.52 [1.03–2.26], p = 0.0368) (Table 4). Multivariate analyses with backward variable selection showed that independent prognostic factors of poorer OS included male sex (HR [95% CI] = 1.63 [1.09–2.44], p = 0.0169) and extracranial metastasis (HR [95% CI] = 1.99 [1.14–3.49], p = 0.0160) (model 3R in Table 4).
Table 4.
Factors associated with overall survival.
| Variables | Univariate | Multivariable Model 3 | Multivariable Model 3R | Multivariable Model 4 | Multivariable Model 4R |
|---|---|---|---|---|---|
| OP (vs. no OP) | 0.80 [0.53–1.21] | ||||
| RT (vs. no RT) | 0.81 [0.55–1.19] | ||||
| OP or RT (vs. no OP or RT) | 0.80 [0.55–1.17] | 0.89 [0.60–1.33] | 0.87 [0.57–1.33] | ||
| EGFR TKI: (vs. gefitinib) | |||||
| Erlotinib | 0.91 [0.56–1.46] | 0.83 [0.48–1.43] | 0.88 [0.54–1.42] | 0.87 [0.48–1.56] | 0.85 [0.52–1.39] |
| Afatinib | 0.63 [0.37–1.08] | 0.61 [0.34–1.10] | 0.63 [0.37–1.08] | 0.65 [0.36–1.19] | 0.63 [0.37–1.08] |
| Osimertinib | † | † | † | † | † |
| Anti-VEGF treatment (vs. no anti-VEGF) | 0.28 [0.04–1.99] | 0.35 [0.05–2.53] | 0.30 [0.04–2.24] | ||
| Male (vs. female) | 1.52 [1.03–2.26] * | 1.57 [1.04–2.36] * | 1.63 [1.09–2.44] * | 1.53 [1.00–2.34] * | 1.54 [1.03–2.29] * |
| Age (≥65 vs. <65) | 1.32 [0.91–1.91] | 1.43 [0.95–2.14] | 1.45 [0.99–2.12] | 1.48 [0.98–2.24] | 1.42 [0.97–2.08] |
| ECOG PS (≥2 vs. ≤1) | 0.72 [0.38–1.34] | 0.70 [0.36–1.37] | 0.65 [0.34–1.22] | 0.81 [0.41–1.59] | |
| Exon 19 deletion vs. L858R | 1.10 [0.76–1.59] | 1.10 [0.74–1.64] | 1.18 [0.79–1.77] | ||
| PD–L1 ≥ 1% vs. PD-L1 < 1% or not tested | 1.17 [0.79–1.72] | 1.20 [0.78–1.84] | 1.20 [0.77–1.88] | ||
| Number of brain metastases (≥5 vs. <5) | 0.88 [0.60–1.30] | 0.97 [0.63–1.47] | 0.91 [0.59–1.39] | ||
| Extracranial metastasis (vs. no) | 1.69 [0.97–2.93] | 1.92 [1.08–3.43] * | 1.99 [1.14–3.49] * | ||
| Lung metastasis (vs. no) | 1.36 [0.94–1.98] | 1.23 [0.81–1.88] | |||
| Pleural metastasis/effusion (vs. no) | 1.13 [0.78–1.64] | 1.23 [0.77–1.97] | |||
| Bone metastasis (vs. no) | 1.38 [0.94–2.03] | 1.52 [0.98–2.37] | 1.43 [0.97–2.11] | ||
| Liver metastasis (vs. no) | 0.82 [0.45–1.49] | 0.66 [0.35–1.28] | |||
| Pericardial metastasis/effusion (vs. no) | 0.86 [0.56–1.32] | 0.64 [0.37–1.10] | |||
| Adrenal metastasis (vs. no) | 1.24 [0.70–2.17] | 1.19 [0.61–2.31] | |||
| Other site metastasis (vs. no) | 1.24 [0.57–2.67] | 1.07 [0.43–2.66] |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; OP, surgery for brain tumor; PD-L1, programmed death ligand 1; PS, performance status; RT, radiotherapy for brain tumor; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor. A patient with cancer harboring both the exon 19 deletion and L858R was included in the exon 19 deletion group. Data are presented as hazard ratio (HR) with 95% confidence interval. After building the maximal models of multivariable Cox regression (models 3 and 4), the corresponding reduced multivariable Cox regression models (models 3R and 4R, respectively) were built using backward variable selection, retaining only variables with p-values < 0.15. † HR could not be assessed due to no event in a group. * p-value < 0.05.
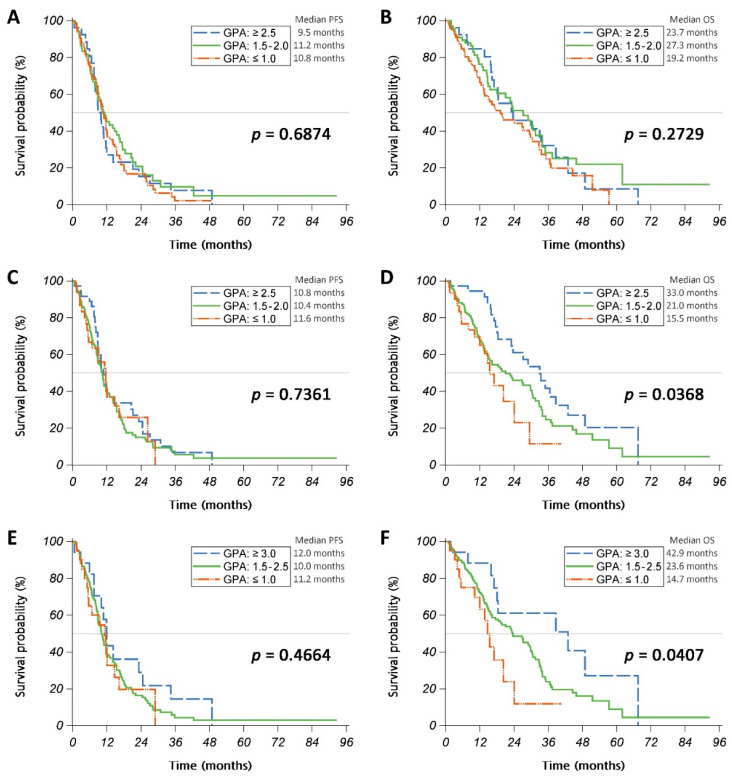
We then examined whether different versions of the GPA (Table A1) could properly stratify patients’ risk. Patients stratified with the 2010, 2017, or 2022 version of the GPA showed a similar PFS (Figure A3A,C,E). Patients stratified with GPA version 2010 had similar OS (Figure A3B). GPA versions 2017 and 2022 appropriately stratified patients with different OS, while patients with higher GPA scores had significantly longer OS (p = 0.0368 and p = 0.0407 for GPA versions 2017 and 2022, respectively) (Figure A3D,F).
4. Discussion
This study made several important findings. First, the patients who received intracranial intervention and those who did not showed a similar response to the initial treatment; these groups were also similar in PFS and OS. Second, patients responded in a similar way to gefitinib, erlotinib, afatinib, and osimertinib as the initial treatment. Third, afatinib and osimertinib were independent prognostic factors of a better PFS, while male sex and bone metastasis were independent prognostic factors of a poorer PFS. The independent prognostic factors of a poorer OS were male sex and extracranial metastasis. Finally, GPA versions 2017 and 2022 appropriately stratified patients with different OS, while patients with higher GPA scores had significantly longer OS.
Brain metastasis is associated with poor prognosis of NSCLC patients [34]. Systemic therapy combined with surgery or radiotherapy is the standard treatment. A previous retrospective study of 296 patients evaluated the benefits of craniotomy for patients with NSCLC and brain metastases. Most of the patients had ≤3 brain lesions, and about 90% of the patients received whole-brain radiotherapy for their brain metastasis. However, the overall survival of the relatively few patients (less than 10%) who underwent craniotomy was significantly longer. Regardless of EGFR mutation status, craniotomy remains a strong prognostic factor of better survival. Some patients may develop complications after craniotomy, the most serious being intracranial hemorrhage, which could cause mortality [35]. In our study, 27% of the patients underwent craniotomy for brain metastasis, but their PFS and OS were similar to those of patients who did not have these interventions.
Radiotherapy for brain lesions is the standard of care for brain metastasis in patients who are not suitable for craniotomy. In a previous retrospective study, which compared the efficacy of first-line EGFR TKIs in combination with radiotherapy vs. EGFR TKI only for patients with EGFR-mutant lung adenocarcinoma with brain metastasis, treatment of symptomatic brain metastasis with the combined therapy achieved a higher response rate and significant improvement in iPFS than did EGFR TKI alone [36]. However, in patients with asymptomatic brain metastasis, the combined EGFR TKI and radiotherapy treatment and EGFR-TKI-only treatment achieved similar iPFS, but the former may result in memory or cognition impairment months later [36]. Another study that evaluated patients with metastatic brain cancer receiving whole-brain radiotherapy showed significant memory impairment after brain radiotherapy. These results suggest that memory impairment may be an early marker of cognitive impairment in patients with brain metastasis who undergo brain radiotherapy [26]. In the present study, 33% of the patients received radiotherapy, which, in almost all cases, was whole-brain radiotherapy. We found that the rate of response to systemic treatment, PFS, and OS of patients receiving radiotherapy were similar to those of patients who did not receive radiotherapy. The intracranial intervention had no statistically significant impact on the survival of the patients with EGFR mutations who received only EGFR TKIs as the first-line therapy.
Although NSCLC progression depends on driver mutations, it is also affected by the extracellular matrix interaction which is mediated by integrins [37,38]. Integrins are cell adhesions and play a key role in the regulation of the process of tumor angiogenesis, which consists of basement membrane degradation and endothelial cell migration, proliferation, and stabilization [39,40]. EGFR regulates integrin activation and cell adhesion, providing control over cellular responses to the environment [41]. Deciphering the molecular mechanisms underlying EGFR and extracellular matrix interactions might provide a better understanding of disease pathobiology and aid in developing therapeutic strategies. According to previous studies, EGFR TKIs are still the standard first-line treatment for EGFR-mutant NSCLC patients. Gefitinib, a first-generation EGFR TKI, has been reported to have low efficacy in treating brain metastasis and poor rates of penetration of the cerebrospinal fluid from the plasma [42]. In contrast, erlotinib, another first-generation EGFR TKI, is effective in treating brain metastasis, is able to cross the blood–brain barrier, and shows a relatively high concentration in the cerebrospinal fluid [43]. Therefore, physicians frequently prescribe erlotinib instead of gefitinib for patients with brain metastasis. Furthermore, the LUX-Lung 3 and LUX-Lung 6 trials showed that afatinib is effective against metastatic brain tumors and yields significantly improved treatment outcomes [13,14]. The FLAURA trial demonstrated that, compared to first-generation TKIs, osimertinib causes a statistically significant improvement in the treatment outcomes of patients with brain metastasis [15,16]. In the subgroup analysis of the central nervous system response in the FLAURA study, osimertinib had significantly better central nervous system efficacy, and patients had a lower intracranial progression rate compared to those who received first-generation EGFR TKIS [44]. The results of the BLOOM study also showed an excellent treatment effect of osimertinib in patients with leptomeningeal carcinomatosis [24]. Chen et al. compared first- and second-generation EGFR TKIs for the treatment of brain metastasis and found no significant differences in PFS, OS, or iPFS in real-world practice between patients who received different generations of EGFR TKIs [25]. In our study, we compared the efficacy between first-, second- and third-generation EGFR TKIs for the treatment of mutant-EGFR lung cancer with brain metastasis. All four EGFR TKIs had similar response rates, but significantly different PFS. Their clinical efficacy was similar in patients without intracranial intervention for brain metastasis. The OS of patients taking different EGFR TKIs was also different. Patients treated with osimertinib had the longest OS compared to those treated with gefitinib, erlotinib, or afatinib. In addition, the OS of patients treated with afatinib was longer than that of patients receiving first-generation EGFR TKIs.
Older age, poor performance status, and extracranial metastasis are regarded as independent factors of poor prognosis of patients with brain metastasis [45]. Chen et al. found that NSCLC patients with uncommon mutations, multiple brain metastases, and concomitant liver metastases tended to have a shorter OS [25]. In our study, multivariable analyses with backward variable selection confirmed that afatinib and osimertinib are independent prognostic factors of better PFS, while male sex and bone metastasis are independent prognostic factors of poorer PFS. Independent prognostic factors of poorer OS included male sex and extracranial metastasis.
GPA has previously been used in decision-making to treat NSCLC patients with brain metastasis [31]. The DS-GPA has been upgraded to the lung-molGPA, which includes the EGFR and ALK mutation status and PD-L1 expression [33]. The lung-molGPA has six factors, with total scores ranging from 0 to 4. Cheng et al. examined the effects of lung-molGPA and different treatment strategies on survival of EGFR-mutant NSCLC patients with brain metastasis; a lung-molGPA of ≥3 was associated with improved OS [46]. In our study, GPA versions 2017 and 2022 appropriately stratified patients with different OS, while patients with higher GPA scores (≥2.5 by version 2017 and ≥3 by version 2022) had significantly longer OS.
Our study has several limitations. First, the number of patients receiving osimertinib was relatively small compared to the number receiving other EGFR TKIs under Taiwan’s National Health Insurance system rules. Taiwan’s National Health Insurance (NHI) is a government-run, single-payer program introduced in 1995 that now covers more than 99% of all Taiwanese citizens. Only gefitinib, erlotinib, and afatinib were allowed by Taiwan’s NHI before 2020. Osimertinib was relatively expensive, and only some patients could afford osimertinib. Osimertinib has been allowed for lung adenocarcinoma with brain metastasis in the deletion 19 subgroup by Taiwan’s NHI since April 2022. This is the reason why only small numbers of patients with lung cancer with brain metastasis were treated with osimertinib in our study. Second, the choice of EGFR TKI for the patient may depend on clinicians’ decisions, which may be influenced by previous studies. In our study, the erlotinib group had significantly more patients than the other groups. Third, the type of radiotherapy for brain tumors in our study was almost always whole-brain radiotherapy. Only a few patients received stereotactic radiosurgery for brain metastasis. Furthermore, iPFS was difficult to evaluate in our study because of its retrospective nature and the check-up interval of the brain image not being clearly defined, which may influence the accuracy of the evaluation of iPFS.
5. Conclusions
In conclusion, our study demonstrated that intracranial intervention had no statistically significant impact on the survival of patients with EGFR mutations and brain metastasis receiving EGFR TKIs as the first-line therapy. In daily practice, many lung cancers with brain metastasis receive intracranial intervention, such as craniotomy and radiotherapy. Our study suggests that intracranial intervention is not necessary for these patients. Patients treated with osimertinib or afatinib had better clinical outcomes compared to those treated with first-generation EGFR TKIs. Afatinib and osimertinib were independent prognostic factors of better PFS. Male sex and bone metastasis were independent prognostic factors of poorer PFS and OS. Patients with higher GPA scores had significantly longer OS. Therefore, physicians should be vigilant about patients with lower GPA scores at the initial diagnosis of NSCLC with brain metastasis.
Acknowledgments
The authors thank the staff members of the Cancer Center, Kaohsiung Medical University Hospital, Kaohsiung Municipal Ta-Tung Hospital, and Kaohsiung Municipal Siaogang Hospital for their assistance.
Appendix A
Figure A1.
(A) Progression-free survival (PFS) and (B) overall survival (OS) of patients classified according to those undergoing or not undergoing surgery (OP) or radiotherapy (RT) for a brain tumor.
Figure A2.
(A,C) Progression-free survival (PFS) and (B,D) overall survival (OS) of patients receiving different epidermal growth factor receptor tyrosine kinase inhibitors and (A,B) undergoing or (C,D) not undergoing surgery (OP) or radiotherapy (RT) for a brain tumor.
Figure A3.
(A,C,E) Progression-free survival (PFS) and (B,D,F) overall survival (OS) of patients classified by the (A,B) 2010, (C,D) 2017, and (E,F) 2022 versions of the graded prognostic assessment (GPA).
Table A1.
Graded prognostic assessment (GPA) index of patients with newly diagnosed lung adenocarcinoma with brain metastases.
| Version | Factors | Score | ||
|---|---|---|---|---|
| 0 | 0.5 | 1 | ||
| 2010 | KPS | ≤60 | 70–80 | 90–100 |
| Age | >60 | 50–60 | <50 | |
| Number of BM | ≥4 | 2–3 | 1 | |
| ECM | Present | Absent | ||
| 2017 | KPS | ≤70 | 80 | 90–100 |
| Age | ≥70 | <70 | ||
| Number of BM | ≥5 | 1–4 | ||
| ECM | Present | Absent | ||
| EGFR and ALK | Both negative or unknown | EGFR- or ALK-positive | ||
| 2022 | KPS | ≤70 | 80 | 90–100 |
| Age | ≥70 | <70 | ||
| Number of BM | ≥5 | 1–4 | ||
| ECM | Present | Absent | ||
| EGFR and ALK | Both negative or unknown | EGFR- or ALK-positive | ||
| PD–L1 | Negative or unknown | Positive | ||
Abbreviations: ALK, anaplastic lymphoma kinase; BM, brain metastasis; ECM, extracranial metastasis; EGFR, epidermal growth factor receptor; KPS, Karnofsky performance status; PD-L1, programmed death ligand 1.
Table A2.
Baseline characteristics of the study cohort.
| Variables | All Patients | No OP/RT | OP or RT | p-Value |
|---|---|---|---|---|
| n | 186 | 107 | 79 | |
| Brain OP (operation for brain tumor) | 0 | 50 | ||
| Brain RT (radiotherapy for brain tumor) | 0 | 61 | ||
| Sex | ||||
| Female | 123 (66%) | 74 (69%) | 49 (62%) | 0.3096 |
| Male | 63 (34%) | 33 (31%) | 30 (38%) | |
| Age (year) | 65.5 ± 11.2 | 66.8 ± 11.0 | 63.8 ± 11.3 | 0.0688 |
| <65 | 92 (49%) | 49 (46%) | 43 (54%) | 0.2442 |
| ≥65 | 94 (51%) | 58 (54%) | 36 (46%) | |
| ECOG performance status | ||||
| 0–1 | 159 (85%) | 94 (88%) | 65 (82%) | 0.2863 |
| ≥2 | 27 (15%) | 13 (12%) | 14 (18%) | |
| Karnofsky performance status | ||||
| 100 | 12 (6%) | 8 (7%) | 4 (5%) | 0.2026 |
| 90 | 21 (11%) | 11 (10%) | 10 (13%) | |
| 80 | 125 (67%) | 77 (72%) | 48 (61%) | |
| 70 | 27 (15%) | 11 (10%) | 16 (20%) | |
| ≤60 | 1 (1%) | 0 (0%) | 1 (1%) | |
| EGFR mutation | ||||
| Exon 19 deletion | 85 (46%) | 48 (45%) | 37 (47%) | 0.7892 |
| Exon 21 L858R | 102 (55%) | 60 (56%) | 42 (53%) | 0.6934 |
| PD-L1 † | ||||
| <1% | 29 (29%) | 17 (27%) | 12 (32%) | 0.5299 |
| ≥1% | 72 (71%) | 47 (73%) | 25 (68%) | |
| Number of brain metastases | ||||
| 1 | 63 (34%) | 29 (27%) | 34 (43%) | 0.0600 |
| 2–4 | 55 (30%) | 33 (31%) | 22 (28%) | |
| ≥5 | 68 (37%) | 45 (42%) | 23 (29%) | |
| Number of ECM sites | ||||
| 0 | 25 (13%) | 8 (7%) | 17 (22%) | 0.0174 |
| 1–2 | 91 (49%) | 54 (50%) | 37 (47%) | |
| ≥3 | 70 (38%) | 45 (42%) | 25 (32%) | |
| Site of ECM | ||||
| Lung metastasis | 99 (53%) | 65 (61%) | 34 (43%) | 0.0167 |
| Pleural metastasis/effusion | 81 (44%) | 53 (50%) | 28 (35%) | 0.0554 |
| Bone metastasis | 116 (62%) | 72 (67%) | 44 (56%) | 0.1067 |
| Liver metastasis | 26 (14%) | 20 (19%) | 6 (8%) | 0.0310 |
| Pericardial metastasis/effusion | 51 (27%) | 32 (30%) | 19 (24%) | 0.3762 |
| Adrenal metastasis | 23 (12%) | 13 (12%) | 10 (13%) | 0.9170 |
| Other metastasis | 12 (6%) | 8 (7%) | 4 (5%) | 0.5078 |
| GPA (version 2010) score | ||||
| ≥2.5 | 26 (14%) | 9 (8%) | 17 (22%) | 0.0021 |
| 1.5–2.0 | 66 (35%) | 33 (31%) | 33 (42%) | |
| ≤1.0 | 94 (51%) | 65 (61%) | 29 (37%) | |
| GPA (version 2017) score | ||||
| ≥2.5 | 36 (19%) | 12 (11%) | 24 (30%) | 0.0048 |
| 1.5–2.0 | 120 (65%) | 76 (71%) | 44 (56%) | |
| ≤1.0 | 30 (16%) | 19 (18%) | 11 (14%) | |
| GPA (version 2022) score | ||||
| ≥3.0 | 17 (9%) | 6 (6%) | 11 (14%) | 0.1327 |
| 1.5–2.5 | 149 (80%) | 88 (82%) | 61 (77%) | |
| ≤1.0 | 20 (11%) | 13 (12%) | 7 (9%) | |
| EGFR TKI | ||||
| Gefitinib | 27 (15%) | 11 (10%) | 16 (20%) | 0.0022 |
| Erlotinib | 104 (56%) | 68 (64%) | 36 (46%) | |
| Afatinib | 48 (26%) | 21 (20%) | 27 (34%) | |
| Osimertinib | 7 (4%) | 7 (7%) | 0 (0%) | |
| Anti-VEGF treatment | ||||
| No | 175 (94%) | 101 (94%) | 74 (94%) | 0.8366 |
| Yes | 11 (6%) | 6 (6%) | 5 (6%) |
Abbreviations: ECM, extracranial metastasis; ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; GPA, graded prognostic assessment; OP, surgery for brain tumor; PD-L1, programmed death ligand 1; RT, radiotherapy for brain tumor; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor. Data are presented as n (%) or mean ± standard deviation. p-values are presented for results of the chi-squared test or analysis of variance. † PD-L1 was examined in 101 patients.
Table A3.
Initial treatment response and site of disease progression.
| Variables | All Patients | No OP/RT | OP or RT | p-Value |
|---|---|---|---|---|
| Initial treatment response | ||||
| Progressive disease (PD) | 18 (10%) | 11 (10%) | 7 (9%) | 0.9521 |
| Stable disease (SD) | 26 (14%) | 14 (13%) | 12 (15%) | |
| Partial response (PR) | 139 (75%) | 80 (75%) | 59 (75%) | |
| Complete response (CR) | 3 (2%) | 2 (2%) | 1 (1%) | |
| Disease control rate | 168 (90%) | 96 (90%) | 72 (91%) | 0.7462 |
| Response rate | 142 (76%) | 82 (77%) | 60 (76%) | 0.9133 |
| Initial intracranial treatment response † | ||||
| Intracranial progressive disease (iPD) | 9 (6%) | 3 (3%) | 6 (8%) | 0.1141 |
| Intracranial stable disease (iSD) | 28 (18%) | 11 (13%) | 17 (24%) | |
| Intracranial partial response (iPR) | 76 (48%) | 44 (51%) | 32 (44%) | |
| Intracranial complete response (iCR) | 46 (29%) | 29 (33%) | 17 (24%) | |
| Intracranial disease control rate | 150 (94%) | 84 (97%) | 66 (92%) | 0.1846 |
| Intracranial response rate | 122 (77%) | 73 (84%) | 49 (68%) | 0.0185 |
| Site of disease progression | ||||
| Intracranial progression | 48 (26%) | 25 (23%) | 23 (29%) | 0.3757 |
| Extracranial progression | 21 (11%) | 12 (11%) | 9 (11%) | 0.9698 |
| Lung | 5 (3%) | 4 (4%) | 1 (1%) | 0.3027 |
| Pleural nodule/effusion | 7 (4%) | 3 (3%) | 4 (5%) | 0.4235 |
| Bone | 9 (5%) | 5 (5%) | 4 (5%) | 0.9024 |
| Liver | 2 (1%) | 1 (1%) | 1 (1%) | 0.8286 |
| Adrenal gland | 1 (1%) | 0 (0%) | 1 (1%) | 0.2432 |
| Other | 1 (1%) | 1 (1%) | 0 (0%) | 0.3889 |
† Initial intracranial treatment response was assessed for 159 patients. Data are presented as n (%). p-values are presented for results of the chi-squared test.
Table A4.
Factors associated with intracranial progression-free survival.
| Variables | Univariate | Multivariable Model 5 | Multivariable Model 6 |
|---|---|---|---|
| OP (vs. no OP) | 0.97 [0.53–1.79] | ||
| RT (vs. no RT) | 0.76 [0.42–1.36] | ||
| OP or RT (vs. no OP or RT) | 1.05 [0.60–1.83] | 0.95 [0.51–1.77] | 1.02 [0.52–2.02] |
| EGFR TKI: (vs. gefitinib) | |||
| Erlotinib | 1.41 [0.54–3.70] | 1.36 [0.47–3.95] | 1.34 [0.44–4.11] |
| Afatinib | 1.59 [0.60–4.23] | 1.59 [0.57–4.45] | 1.58 [0.55–4.52] |
| Osimertinib | † | † | † |
| Anti-VEGF treatment (vs. no anti-VEGF) |
2.04 [0.63–6.65] | 1.81 [0.52–6.29] | 1.44 [0.39–5.39] |
| Male (vs. female) | 1.70 [0.94–3.07] | 1.51 [0.82–2.80] | 1.38 [0.74–2.58] |
| Age (≥65 vs. <65) | 0.71 [0.40–1.28] | 0.69 [0.37–1.29] | 0.65 [0.33–1.27] |
| ECOG PS (≥2 vs. ≤1) | 0.77 [0.31–1.96] | 0.94 [0.33–2.63] | 1.17 [0.40–3.41] |
| Exon 19 deletion vs. L858R | 0.76 [0.44–1.34] | 0.72 [0.39–1.32] | 0.73 [0.39–1.36] |
| PD-L1 ≥ 1% vs. PD-L1 < 1% or not tested |
1.43 [0.81–2.54] | 1.39 [0.75–2.56] | 1.50 [0.79–2.84] |
| Number of brain metastases (≥5 vs. <5) | 0.69 [0.38–1.27] | 0.66 [0.35–1.26] | 0.58 [0.29–1.14] |
| Extracranial metastasis (vs. no) | 1.12 [0.52–2.39] | 1.18 [0.53–2.63] | |
| Lung metastasis (vs. no) | 1.20 [0.69–2.09] | 1.37 [0.70–2.67] | |
| Pleural metastasis/effusion (vs. no) | 0.87 [0.49–1.55] | 0.95 [0.47–1.95] | |
| Bone metastasis (vs. no) | 1.43 [0.79–2.60] | 1.32 [0.66–2.64] | |
| Liver metastasis (vs. no) | 1.16 [0.55–2.48] | 1.10 [0.46–2.63] | |
| Pericardial metastasis/effusion (vs. no) | 0.86 [0.45–1.65] | 0.72 [0.32–1.60] | |
| Adrenal metastasis (vs. no) | 1.59 [0.62–4.07] | 2.30 [0.79–6.70] | |
| Other metastasis (vs. no) | 0.56 [0.08–4.10] | 0.52 [0.06–4.47] |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor; OP, surgery for brain tumor; PD-L1, programmed death ligand 1; PS, performance status; RT, radiotherapy for brain tumor; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor. A patient with cancer harboring both the exon 19 deletion and L858R was included in the exon 19 deletion group. Data are presented as hazard ratio (HR) with a 95% confidence interval. † HR could not be assessed due to no event in a group.
Author Contributions
Conceptualization, C.-Y.K., M.-J.T. and C.-J.Y.; Methodology, M.-J.T. and C.-J.Y.; Formal analysis, M.-J.T.; Investigation, C.-Y.K., M.-J.T., Y.-M.T., Y.-C.T., C.-J.Y., J.-Y.H. and I.-W.C.; Data curation, C.-Y.K., T.-H.L., K.-L.W., Y.-M.T., Y.-C.T., C.-H.C. and H.-C.C.; Writing—original draft preparation, C.-Y.K., M.-J.T. and C.-J.Y.; Writing—review and editing, C.-Y.K., M.-J.T., J.-Y.H., C.-J.Y. and I.-W.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUH) (KMUHIRB-E(I)-20210257).
Informed Consent Statement
The Institutional Review Board (IRB) of Kaohsiung Medical University Hospital (KMUH) approved this study (KMUHIRB-E(I)-20210257) and waived the need for written informed consent from all patients.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Shi A.A., Digumarthy S.R., Temel J.S., Halpern E.F., Kuester L.B., Aquino S.L. Does initial staging or tumor histology better identify asymptomatic brain metastases in patients with non-small cell lung cancer? J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2006;1:205–210. doi: 10.1016/S1556-0864(15)31569-0. [DOI] [PubMed] [Google Scholar]
- 2.Arrieta O., Saavedra-Perez D., Kuri R., Aviles-Salas A., Martinez L., Mendoza-Posada D., Castillo P., Astorga A., Guzman E., De la Garza J. Brain metastasis development and poor survival associated with carcinoembryonic antigen (CEA) level in advanced non-small cell lung cancer: A prospective analysis. BMC Cancer. 2009;9:119. doi: 10.1186/1471-2407-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taimur S., Edelman M.J. Treatment options for brain metastases in patients with non-small-cell lung cancer. Curr. Oncol. Rep. 2003;5:342–346. doi: 10.1007/s11912-003-0077-8. [DOI] [PubMed] [Google Scholar]
- 4.Antuña A.R., Vega M.A., Sanchez C.R., Fernandez V.M. Brain Metastases of Non-Small Cell Lung Cancer: Prognostic Factors in Patients with Surgical Resection. J. Neurol. Surg. A. Cent. Eur. Neurosurg. 2018;79:101–107. doi: 10.1055/s-0037-1601874. [DOI] [PubMed] [Google Scholar]
- 5.Gaspar L., Scott C., Rotman M., Asbell S., Phillips T., Wasserman T., McKenna W.G., Byhardt R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997;37:745–751. doi: 10.1016/S0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 6.Moscetti L., Nelli F., Felici A., Rinaldi M., De Santis S., D’Auria G., Mansueto G., Tonini G., Sperduti I., Pollera F.C. Up-front chemotherapy and radiation treatment in newly diagnosed nonsmall cell lung cancer with brain metastases: Survey by Outcome Research Network for Evaluation of Treatment Results in Oncology. Cancer. 2007;109:274–281. doi: 10.1002/cncr.22399. [DOI] [PubMed] [Google Scholar]
- 7.Bearz A., Garassino I., Tiseo M., Caffo O., Soto-Parra H., Boccalon M., Talamini R., Santoro A., Bartolotti M., Murgia V., et al. Activity of Pemetrexed on brain metastases from Non-Small Cell Lung Cancer. Lung Cancer. 2010;68:264–268. doi: 10.1016/j.lungcan.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 8.Barlesi F., Gervais R., Lena H., Hureaux J., Berard H., Paillotin D., Bota S., Monnet I., Chajara A., Robinet G. Pemetrexed and cisplatin as first-line chemotherapy for advanced non-small-cell lung cancer (NSCLC) with asymptomatic inoperable brain metastases: A multicenter phase II trial (GFPC 07-01) Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2011;22:2466–2470. doi: 10.1093/annonc/mdr003. [DOI] [PubMed] [Google Scholar]
- 9.Pao W., Girard N. New driver mutations in non-small-cell lung cancer. Lancet. Oncol. 2011;12:175–180. doi: 10.1016/S1470-2045(10)70087-5. [DOI] [PubMed] [Google Scholar]
- 10.Mok T.S., Wu Y.L., Thongprasert S., Yang C.H., Chu D.T., Saijo N., Sunpaweravong P., Han B., Margono B., Ichinose Y., et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 11.Mitsudomi T., Morita S., Yatabe Y., Negoro S., Okamoto I., Tsurutani J., Seto T., Satouchi M., Tada H., Hirashima T., et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet. Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 12.Zhou C., Wu Y.L., Chen G., Feng J., Liu X.Q., Wang C., Zhang S., Wang J., Zhou S., Ren S., et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet. Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 13.Wu Y.L., Zhou C., Hu C.P., Feng J., Lu S., Huang Y., Li W., Hou M., Shi J.H., Lee K.Y., et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet. Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 14.Yang J.C., Wu Y.L., Schuler M., Sebastian M., Popat S., Yamamoto N., Zhou C., Hu C.P., O’Byrne K., Feng J., et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet. Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 15.Ramalingam S.S., Vansteenkiste J., Planchard D., Cho B.C., Gray J.E., Ohe Y., Zhou C., Reungwetwattana T., Cheng Y., Chewaskulyong B., et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 16.Soria J.C., Ohe Y., Vansteenkiste J., Reungwetwattana T., Chewaskulyong B., Lee K.H., Dechaphunkul A., Imamura F., Nogami N., Kurata T., et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 17.Wu Y.L., Zhou C., Cheng Y., Lu S., Chen G.Y., Huang C., Huang Y.S., Yan H.H., Ren S., Liu Y., et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: A phase II study (CTONG-0803) Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013;24:993–999. doi: 10.1093/annonc/mds529. [DOI] [PubMed] [Google Scholar]
- 18.Park S.J., Kim H.T., Lee D.H., Kim K.P., Kim S.W., Suh C., Lee J.S. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77:556–560. doi: 10.1016/j.lungcan.2012.05.092. [DOI] [PubMed] [Google Scholar]
- 19.Metro G., Chiari R., Ricciuti B., Rebonato A., Lupattelli M., Gori S., Bennati C., Castrioto C., Floridi P., Minotti V., et al. Pharmacotherapeutic options for treating brain metastases in non-small cell lung cancer. Expert Opin. Pharmacother. 2015;16:2601–2613. doi: 10.1517/14656566.2015.1094056. [DOI] [PubMed] [Google Scholar]
- 20.Kelly W.J., Shah N.J., Subramaniam D.S. Management of Brain Metastases in Epidermal Growth Factor Receptor Mutant Non-Small-Cell Lung Cancer. Front Oncol. 2018;8:208. doi: 10.3389/fonc.2018.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pareek V., Welch M., Ravera E., Zampolin R.L., Sequist L.V., Halmos B. Marked Differences in CNS Activity among EGFR Inhibitors: Case Report and Mini-Review. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer. 2016;11:e135–e139. doi: 10.1016/j.jtho.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 22.Togashi Y., Masago K., Masuda S., Mizuno T., Fukudo M., Ikemi Y., Sakamori Y., Nagai H., Kim Y.H., Katsura T., et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother. Pharmacol. 2012;70:399–405. doi: 10.1007/s00280-012-1929-4. [DOI] [PubMed] [Google Scholar]
- 23.Tamiya A., Tamiya M., Nishihara T., Shiroyama T., Nakao K., Tsuji T., Takeuchi N., Isa S.I., Omachi N., Okamoto N., et al. Cerebrospinal Fluid Penetration Rate and Efficacy of Afatinib in Patients with EGFR Mutation-positive Non-small Cell Lung Cancer with Leptomeningeal Carcinomatosis: A Multicenter Prospective Study. Anticancer. Res. 2017;37:4177–4182. doi: 10.21873/anticanres.11806. [DOI] [PubMed] [Google Scholar]
- 24.Yang J.C.H., Kim S.W., Kim D.W., Lee J.S., Cho B.C., Ahn J.S., Lee D.H., Kim T.M., Goldman J.W., Natale R.B., et al. Osimertinib in Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer and Leptomeningeal Metastases: The BLOOM Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020;38:538–547. doi: 10.1200/JCO.19.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y.H., Chen Y.F., Chen C.Y., Shih J.Y., Yu C.J. Clinical factors associated with treatment outcomes in EGFR mutant non-small cell lung cancer patients with brain metastases: A case-control observational study. BMC Cancer. 2019;19:1006. doi: 10.1186/s12885-019-6140-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng H., Chen H., Lv Y., Chen Z., Li C.R. Prospective memory impairment following whole brain radiotherapy in patients with metastatic brain cancer. Cancer Med. 2018;7:5315–5321. doi: 10.1002/cam4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuo C.Y., Lee M.H., Tsai M.J., Yang C.J., Hung J.Y., Chong I.W. The Factors Predicting Concordant Epidermal Growth Factor Receptor (EGFR) Mutation Detected in Liquid/Tissue Biopsy and the Related Clinical Outcomes in Patients of Advanced Lung Adenocarcinoma with EGFR Mutations. J. Clin. Med. 2019;8:1758. doi: 10.3390/jcm8111758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y.C., Tsai M.J., Lee M.H., Kuo C.Y., Shen M.C., Tsai Y.M., Chen H.C., Hung J.Y., Huang M.S., Chong I.W., et al. Lower starting dose of afatinib for the treatment of metastatic lung adenocarcinoma harboring exon 21 and exon 19 mutations. BMC Cancer. 2021;21:495. doi: 10.1186/s12885-021-08235-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang C.J., Hung J.Y., Tsai M.J., Wu K.L., Liu T.C., Chou S.H., Lee J.Y., Hsu J.S., Huang M.S., Chong I.W. The salvage therapy in lung adenocarcinoma initially harbored susceptible EGFR mutation and acquired resistance occurred to the first-line gefitinib and second-line cytotoxic chemotherapy. BMC Pharm. Toxicol. 2017;18:21. doi: 10.1186/s40360-017-0130-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang C.J., Tsai M.J., Hung J.Y., Lee M.H., Tsai Y.M., Tsai Y.C., Hsu J.F., Liu T.C., Huang M.S., Chong I.W. The clinical efficacy of Afatinib 30 mg daily as starting dose may not be inferior to Afatinib 40 mg daily in patients with stage IV lung Adenocarcinoma harboring exon 19 or exon 21 mutations. BMC Pharm. Toxicol. 2017;18:82. doi: 10.1186/s40360-017-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sperduto P.W., Chao S.T., Sneed P.K., Luo X., Suh J., Roberge D., Bhatt A., Jensen A.W., Brown P.D., Shih H., et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: A multi-institutional analysis of 4259 patients. Int. J. Radiat. Oncol. Biol. Phys. 2010;77:655–661. doi: 10.1016/j.ijrobp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 32.Sperduto P.W., Yang T.J., Beal K., Pan H., Brown P.D., Bangdiwala A., Shanley R., Yeh N., Gaspar L.E., Braunstein S., et al. Estimating Survival in Patients With Lung Cancer and Brain Metastases: An Update of the Graded Prognostic Assessment for Lung Cancer Using Molecular Markers (Lung-molGPA) JAMA Oncol. 2017;3:827–831. doi: 10.1001/jamaoncol.2016.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sperduto P.W., De B., Li J., Carpenter D., Kirkpatrick J., Milligan M., Shih H.A., Kutuk T., Kotecha R., Higaki H., et al. Graded Prognostic Assessment (GPA) for Patients With Lung Cancer and Brain Metastases: Initial Report of the Small Cell Lung Cancer GPA and Update of the Non-Small Cell Lung Cancer GPA Including the Effect of Programmed Death Ligand 1 and Other Prognostic Factors. Int. J. Radiat. Oncol. Biol. Phys. 2022;114:60–74. doi: 10.1016/j.ijrobp.2022.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Getman V., Devyatko E., Dunkler D., Eckersberger F., End A., Klepetko W., Marta G., Mueller M.R. Prognosis of patients with non-small cell lung cancer with isolated brain metastases undergoing combined surgical treatment. Eur. J. Cardiothorac. Surg. 2004;25:1107–1113. doi: 10.1016/j.ejcts.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Yen C.T., Wu W.J., Chen Y.T., Chang W.C., Yang S.H., Shen S.Y., Su J., Chen H.Y. Surgical resection of brain metastases prolongs overall survival in non-small-cell lung cancer. Am. J. Cancer. Res. 2021;11:6160–6172. [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y., Yang J., Li X., Hao D., Wu X., Yang Y., He C., Wang W., Wang J. First-line epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitor alone or with whole-brain radiotherapy for brain metastases in patients with EGFR-mutated lung adenocarcinoma. Cancer Sci. 2016;107:1800–1805. doi: 10.1111/cas.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergonzini C., Kroese K., Zweemer A.J.M., Danen E.H.J. Targeting Integrins for Cancer Therapy—Disappointments and Opportunities. Front Cell Dev. Biol. 2022;10:863850. doi: 10.3389/fcell.2022.863850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hassanein S.S., Abdel-Mawgood A.L., Ibrahim S.A. EGFR-Dependent Extracellular Matrix Protein Interactions Might Light a Candle in Cell Behavior of Non-Small Cell Lung Cancer. Front Oncol. 2021;11:766659. doi: 10.3389/fonc.2021.766659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slack R.J., Macdonald S.J.F., Roper J.A., Jenkins R.G., Hatley R.J.D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022;21:60–78. doi: 10.1038/s41573-021-00284-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y., Fu L., Wang B., Li Z., Wei D., Wang H., Zhang C., Ma Z., Zhu T., Yu G. Construction of a prognostic risk assessment model for lung adenocarcinoma based on Integrin β family-related genes. J. Clin. Lab. Anal. 2022;36:e24419. doi: 10.1002/jcla.24419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao T.C., Ma V.P., Blanchard A., Urner T.M., Grandhi S., Salaita K., Mattheyses A.L. EGFR activation attenuates the mechanical threshold for integrin tension and focal adhesion formation. J. Cell Sci. 2020;133:jcs238840. doi: 10.1242/jcs.238840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng H., Perez-Soler R. Leptomeningeal metastases in non-small-cell lung cancer. Lancet. Oncol. 2018;19:e43–e55. doi: 10.1016/S1470-2045(17)30689-7. [DOI] [PubMed] [Google Scholar]
- 43.Deng Y., Feng W., Wu J., Chen Z., Tang Y., Zhang H., Liang J., Xian H., Zhang S. The concentration of erlotinib in the cerebrospinal fluid of patients with brain metastasis from non-small-cell lung cancer. Mol. Clin. Oncol. 2014;2:116–120. doi: 10.3892/mco.2013.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reungwetwattana T., Nakagawa K., Cho B.C., Cobo M., Cho E.K., Bertolini A., Bohnet S., Zhou C., Lee K.H., Nogami N., et al. CNS Response to Osimertinib Versus Standard Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors in Patients With Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018;33:3290–3297. doi: 10.1200/JCO.2018.78.3118. [DOI] [PubMed] [Google Scholar]
- 45.Sperduto P.W., Kased N., Roberge D., Xu Z., Shanley R., Luo X., Sneed P.K., Chao S.T., Weil R.J., Suh J., et al. Summary report on the graded prognostic assessment: An accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng W.C., Shen Y.C., Chien C.R., Liao W.C., Chen C.H., Hsia T.C., Tu C.Y., Chen H.J. The optimal therapy strategy for epidermal growth factor receptor-mutated non-small cell lung cancer patients with brain metastasis: A real-world study from Taiwan. Thorac. Cancer. 2022;13:1505–1512. doi: 10.1111/1759-7714.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.