Abstract
We have constructed a defined acapsular mutant in Pasteurella multocida X-73 (serogroup A:1) by disrupting the hexA gene through the insertion of a tetracycline resistance cassette. The genotype of the hexA::tet(M) strain was confirmed by PCR and Southern hybridization, and the acapsular phenotype of this strain was confirmed by electron microscopy. The hexA::tet(M) strain was attenuated in both mice and chickens. Complementation of the mutant with an intact hexAB fragment restored lethality in mice but not in chickens. In contrast to the results described previously for P. multocida serogroup B (J. D. Boyce and B. Adler, Infect. Immun. 68:3463–3468, 2000), the hexA::tet(M) strain was sensitive to the bactericidal action of chicken serum, whereas the wild-type and complemented strains were both resistant. Following inoculation into chicken muscle, the bacterial count of the hexA::tet(M) strain decreased significantly, while the wild-type and complemented strains both grew rapidly over 4 h. The capsule is thus an essential virulence determinant in the pathogenesis of fowl cholera.
Pasteurella multocida is associated with a wide range of diseases in many species of animals, the major diseases being hemorrhagic septicemia (HS) in ungulates, atrophic rhinitis in swine, and fowl cholera (FC) in wild and domestic birds. Many strains of P. multocida express a capsule on their surfaces. The antigenic specificity of the capsule of P. multocida determines its serogroup, either A, B, D, E, or F (6, 23, 25). Interestingly, the majority of FC, HS, and atrophic rhinitis cases are caused by serogroup A strains, by serogroup B and E strains, and by serogroup D strains, respectively, suggesting that the capsule is related to the pathogenesis of the disease and to host predilection.
Capsules are highly hydrated polysaccharides located external and adherent to the bacterial cell wall (28). The location of extracellular polysaccharides at the outermost surface of the cell is important because they are the first portal of entry and the last barrier to excretion of substances in and out of the cell (7). Various hypotheses have been postulated about the function of the bacterial capsule. These include protection against desiccation in the environment (19), phagocytosis (26), and the bactericidal activity of serum complement (15, 32).
Previous studies of the influence of the capsule on the virulence of P. multocida have used spontaneously derived acapsular variants or enzymatic removal of the capsule (1, 10, 11, 14, 17, 21, 27). These studies suggested that there is a correlation between the capsule and the virulence of P. multocida. However, because these strains were not genetically defined, it is not possible to ascribe definitively their phenotypes to the lack of capsule. Recently, the capsule of an HS strain of P. multocida (serotype B:2) was shown to be involved in virulence for mice by comparing an isogenic acapsular mutant to the wild type and the complemented mutant (5).
We have reported previously the nucleotide sequence of the P. multocida X-73 (serogroup A:1) capsule biosynthetic locus (8). Like many other gram-negative capsule loci, the capsule biosynthetic locus is divided into three regions. Regions 1 and 3 are involved in export and modification of the polysaccharide capsule, while region 2 is involved in the biosynthesis of precursors and polymerization of the polysaccharide capsule. In this paper, we report the construction of an isogenic acapsular mutant of FC-causing P. multocida X-73 and use of this isogenic strain to investigate the role of the capsule in pathogenesis both in the natural host, the chicken, and in mice.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Bacterial strains and plasmids used in this study are shown in Table 1. P. multocida and Escherichia coli DH5α were grown routinely at 37°C in nutrient broth no. 2 (NB; Oxoid, Hampshire, England) and Luria-Bertani broth (2), respectively, or plated on solid media containing 1.5% bacteriological agar (Oxoid). When required, agar or broth was supplemented with ampicillin (100 μg/ml), kanamycin (50 μg/ml), or tetracycline (10 μg/ml for E. coli and 5 μg/ml for P. multocida). P. multocida strains were grown on dextrose starch agar (DSA; Difco, Detroit, Mich.) supplemented with 6% chicken serum for determination of colony morphology.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli DH5α | F−endA1 hsdR17 (rk− mk+) thi-1 λ− recA1 Φ80dlacZΔM15 | 24 |
| P. multocida | ||
| X-73 | Serotype A:1 wild-type strain | 22 |
| PBA930 | X-73 hexA::tet(M) mutant | This study |
| PBA954 | PBA930 containing pPBA1671 | This study |
| AL2 | X-73 containing pPBA1101 | This study |
| AL5 | PBA930 containing pPBA1101 | This study |
| Plasmids | ||
| pPBA1101 | E. coli-P. multocida shuttle vector, Kanr | 12 |
| pPBA1276 | 3-kb EcoRI insert cloned into pWSK29 containing hexAB and part of hexC | 8 |
| pPBA1281 | Cloning vector derived by modifying pWSK29, lacks EcoRV, HindIII, ClaI, AccI, and SacI sites; Ampr | This study |
| pPBA1289 | 3-kb EcoRI insert from pPBA1276 cloned into pPBA1281, Ampr | This study |
| pPBA1622 | pPBA1281 containing the hexA::tet(M) cassette, Ampr, Tetr | This study |
| pPBA1671 | EcoRI-SacI fragment from pPBA1276 containing hexAB cloned into pPBA1101, Kanr | This study |
| pVB101 | tet(M) gene from Tn916 cloned into pBR322, Tetr, Ampr | Vickers Burdetta |
| pWSK29 | Low-copy-number E. coli cloning vector, Ampr | 33 |
Duke University, Durham, N.C.
Recombinant DNA techniques.
Genomic DNA was prepared by the cetyltrimethylammonium bromide (CTAB) precipitation method (2) while plasmid DNA was prepared by the alkaline lysis method (4). Standard restriction, dephosphorylation, and ligation of DNA were performed by using DNA-modifying enzymes supplied either by New England Biolabs (Beverley, Mass.) or by Roche Molecular Biochemicals (Basel, Switzerland) and used according to the manufacturers' instructions. Plasmids were introduced into E. coli by electroporation as described previously (2). Electrocompetent cells of P. multocida X-73 were prepared by essentially the same method, except that when cultures reached an A600 of ≈0.6, they were treated with 37 U of hyaluronidase (Roche Molecular Biochemicals) per ml of culture for 1 h at 37°C. Electroporation of P. multocida X-73 cells was carried out at 2.5 kV, 25 μF, and 600 Ω with ∼5 μg of plasmid DNA.
Electron microscopy techniques.
P. multocida cells cultured on DSA were prepared for electron microscopy as described by Jacques and Graham (13), with slight modifications. Cells were fixed in 2.5% glutaraldehyde–75 mM lysine in 0.1 M cacodylate buffer for 10 min, recovered by centrifugation, and further fixed in glutaradehyde for 30 min, followed by postfixation in 1% osmium tetroxide for 2 h. Samples were then dehydrated in an ascending series of acetone, embedded in Epon, and polymerized at 50°C overnight. Sections (70-nm thickness) were cut with a Ultracut UCT ultramicrotome (Leica Microsystems, Wetzlar, Germany), poststained with uranyl citrate and lead citrate, and examined with a Jeol (Peabody, Mass.) model 1010 transmission electron microscope at an accelerating voltage of 80 kV.
Extraction of capsular hyaluronic acid.
Crude capsular polysaccharides were prepared by the method of Gentry et al. (9), with slight modifications to ensure minimal cell lysis and loss of viability. Overnight cultures of P. multocida AL2, AL5, and PBA954 grown in NB with 50 μg of kanamycin/ml were diluted in 20 ml of fresh NB with 50 μg of kanamycin/ml to an A600 of ∼0.1 and incubated at 37°C with aeration until mid-exponential phase (A600 of ∼0.5). Cells were harvested from 5 ml of culture by centrifugation at 7,600 × g for 15 min, washed once in 1 ml of phosphate-buffered saline (PBS), resuspended in 1 ml of PBS, and incubated at 42°C for 1 h to extract the capsule. Viable counts were determined before and after the incubation at 42°C, after which the cells were pelleted by centrifugation at 7,600 × g for 15 min and the supernatant containing the capsular polysaccharide was transferred to a new tube.
The hyaluronic acid content in the capsular extracts was measured by the method of Moses et al. (18), with slight modifications. Hyaluronic acid standards from 0.5 to 5 μg were prepared in a volume of 100 μl as recommended by Benchetrit et al. (3). To each sample, 0.9 ml of staining solution {0.2 mg of 1-ethyl-2-[3-(1-ethyl-naphthol[1,2-d-]thiazolin-2-ylidene)-2-methylpropenyl] naptho-[1,2-d]thiazolium bromide [Stains-all; Kodak, Rochester, N.Y.] per ml and 0.06% glacial acetic acid in 50% formamide} was added. The A640 of the standards and samples was measured immediately, and the amount of hyaluronic acid in each sample was determined by comparison to the standard curve.
Assessment of virulence of P. multocida for mice and chickens.
P. multocida strains were grown to an A600 of 0.8 and diluted to obtain cultures of 102 to 109 CFU/ml. The viable counts were confirmed by plating duplicate samples onto nutrient agar (supplemented with antibiotics when appropriate). Groups of female BALB/c mice (6 to 8 weeks old; weight, ∼20 g) were injected intraperitoneally with aliquots of 0.1 ml of the appropriate dilution. Mice were monitored for clinical symptoms and were killed by cervical dislocation if considered moribund, according to Australian animal ethics requirements.
Commercial Leghorn layers (15 weeks old) were used to assess the virulence of P. multocida strains for chickens. They were infected by intramuscular injection with doses of bacteria, observed for clinical symptoms, and killed if the infection was considered terminal.
Serum sensitivity assays.
The sensitivity of P. multocida strains and E. coli DH5α to the bactericidal complement activity of chicken serum was determined by the addition of ∼105 CFU to chicken serum and subsequent incubation for 3 h at 37°C with aeration. Viable counts were determined at 0 and 3 h. Complement activity was inactivated in control samples by heating at 60°C for 30 min. Tests were performed in duplicate for E. coli strains and in triplicate for P. multocida strains.
Growth of P. multocida in chicken muscle.
Colonies of P. multocida strains, grown overnight on sheep blood agar, were resuspended in 2 ml of sterile PBS to a concentration of 106 CFU/ml (determined by viable count). Sterile India ink was added to the suspension, and 0.1 ml was inoculated into defined zones of the deep pectoral muscle of each of five chickens. The chickens were killed at 4 h postinoculation, and the muscle surrounding each inoculation site (visualized due to the India ink) was excised, weighed, and macerated in a stomacher with an equivalent amount of PBS for 5 min. Viable counts of the bacteria recovered from each muscle portion were determined.
Statistics.
Statistical analyses of serum sensitivity data, production of extracellular hyaluronic acid, and bacterial growth in chicken muscle were performed by using Welch's t test, and approximate probability values were determined by using Instat version 2.03 (GraphPad Software).
RESULTS
Disruption of hexA.
The EcoRI fragment in pPBA1276 (Table 1) contained a unique ClaI site within the hexA gene. To allow the use of this site for mutagenesis, vector pPBA1281 was constructed by excising the EcoRV-to-HincII fragment from pWSK29, thereby removing the EcoRV, HindIII, ClaI, AccI, and SacI sites. The 3-kb EcoRI fragment from pBPA1276 was then cloned into pPBA1281 to generate pPBA1289, which contained a unique ClaI site within hexA. The tet(M) cassette from pVB101 was excised as a 3-kb fragment, end filled with T4 polymerase, and ligated into the end-filled, unique ClaI site to form plasmid pPBA1622.
Plasmid pPBA1622 was introduced into P. multocida X-73 by electroporation, and allelic recombinants were selected on nutrient agar supplemented with 5 μg of tetracycline/ml. Construction of a genotypic hexA::tet(M) mutant requires a homologous double crossover, and the hexA::tet(M) mutant genotype was confirmed by PCR and Southern hybridization (data not shown). The hexA::tet(M) mutant was designated PBA930.
Complementation of acapsular mutant PBA930.
The hexAB genes were cloned into the E. coli-P. multocida shuttle vector pPBA1101 (12), generating plasmid pPBA1671, which was introduced into PBA930 by electroporation to produce strain PBA954. In addition, strains AL2 and AL5 were constructed by the introduction of plasmid pPBA1101 into strains X-73 and PBA930, respectively, to allow a direct comparison of the three isogenic strains. Growth of the acapsular mutant strains, PBA930 and AL5, was not reduced compared to that of the wild-type strains, AL2 and X-73, at 37 or 42°C with or without kanamycin selection (data not shown). The growth profile of PBA954 suggested a reduction in growth rate at 42°C compared to those of strains AL2 and AL5 without selection and showed only limited growth at 42°C in NB supplemented with 50 μg of kanamycin/ml.
Colony and cellular morphology of P. multocida strains.
P. multocida strains X-73, PBA930, and PBA954 were grown on DSA supplemented with chicken serum. Wild-type strain X-73 produced mucoid colonies (20), consistent with the presence of a capsule, whereas both the hexA::tet(M) mutant PBA930 and the complemented strain PBA954 produced nonmucoid colonies.
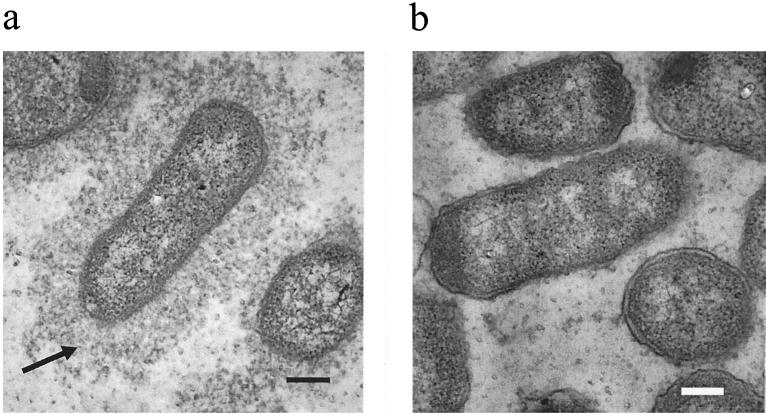
Cells of P. multocida strains X-73, PBA930, and PBA954 were observed by electron microscopy. Wild-type P. multocida strain X-73 exhibited a clearly visible capsule, which extended beyond the outer membrane by at least one cell diameter, whereas the hexA::tet(M) mutant PBA930 had no visible capsule (Fig. 1). The complemented strain PBA954 showed at best a very slight and patchy production of capsule on some cells only (Fig. 1).
FIG. 1.
Electron micrographs of P. multocida strains X-73 (a), PBA930 (b), and PBA954 (c). The capsule in strain X-73 is indicated by the arrow. Bars, 200 nm.
Hyaluronic acid capsule production.
The production of extracellular polysaccharide was determined by direct chemical assay for hyaluronic acid (Table 2). The viability of the cells was determined after the capsule extraction procedure and ranged from 70 to 90% of that prior to extraction. Acapsular strain AL5 produced significantly less hyaluronic acid than did strains AL2 and PBA954 (P < 0.01). No significant difference between the hyaluronic acid produced by strains AL2 and PBA954 was observed (P > 0.05). These data indicate that PBA954 had restored the ability to transport extracellular hyaluronic acid. However, the lack of a discernible capsule by electron microscopy indicates some deficiency in surface presentation.
TABLE 2.
Extracellular hyaluronic acid produced by P. multocida strains AL2, AL5, and PBA954
| P. multocida strain | Amt of hyaluronic acid (10−9 μg/CFU)a | Viabilityb |
|---|---|---|
| AL2 | 2.8 ± 0.5 | 0.7 ± 0.1 |
| AL5 | 0.6 ± 0.3 | 0.7 ± 0.2 |
| PBA954 | 3.4 ± 0.9 | 0.9 ± 0.2 |
The levels of hyaluronic acid produced by strains AL2 and PBA954 compared to that of AL5 were significantly different (P = 0.002 and P = 0.006, respectively) while the difference in the levels of hyaluronic acid produced by strains AL2 and PBA954 were not significant (P < 0.05).
Viability is expressed as the ratio of bacterial count (CFU per milliliter) after treatment at 42°C to that before treatment at 42°C.
Virulence for mice and chickens.
To assess the influence of the capsule on virulence, various doses of P. multocida X-73, PBA930, AL5, and PBA954 were injected intraperitoneally into groups of mice. Inactivation of the hexA gene resulted in at least a 106-fold decrease in virulence of PBA930 and AL5. Virulence was restored to wild-type levels by the introduction of the plasmid carrying the intact hexAB genes (PBA954) (Table 3). P. multocida strains X-73 and PBA954 recovered from the blood of infected mice were genotypically and phenotypically identical to the original strains (data not shown). Due to the lack of virulence, strains PBA930 and AL5 could not be recovered from mice.
TABLE 3.
Survival of BALB/c mice infected with P. multocida strains
| Strain | Dose (CFU) | No. of survivors/group size |
|---|---|---|
| X-73 | 55 | 0/5 |
| 3.2 × 102 | 0/5 | |
| PBA930 | 1.3 × 106 | 5/5 |
| 1.3 × 107 | 5/5 | |
| 1.3 × 108 | 5/5 | |
| AL5 | 8.3 × 107 | 3/3 |
| PBA954 | 31 | 0/5 |
| 1.2 × 103 | 0/5 |
Strains AL2, AL5, and PBA954 were injected intramuscularly into chickens. Strain AL5 showed a decrease in virulence compared to that of wild-type strain AL2, with no clinical symptoms of FC being observed even in chickens injected with 2.6 × 108 CFU of AL5 (Table 4). However, in contrast to the results obtained for mice, the complemented strain, PBA954, was incapable of killing chickens (Table 4).
TABLE 4.
Survival of chickens infected with P. multocida strains
| Strain | Dose (CFU) | No. of survivors/group size |
|---|---|---|
| AL2 | 6.5 × 104 | 0/5 |
| AL5 | 2.6 × 104 | 5/5 |
| 2.6 × 108 | 4/4 | |
| PBA954 | 3.4 × 104 | 5/5 |
| 3.4 × 107 | 5/5 |
Sensitivity to chicken serum.
P. multocida strains X-73, PBA930, and PBA954 were incubated in 90% chicken serum to determine their sensitivity to complement-mediated killing (Table 5). Control strain E. coli DH5α multiplied approximately 10-fold in heat-treated serum, but its viability decreased to undetectable levels in untreated serum, thereby confirming complement bactericidal activity. Both the wild-type strain P. multocida X-73 and the complemented mutant PBA954 were serum resistant (no significant difference in the survival ratios was observed for heated versus unheated serum). However, the acapsular mutant PBA930 was considered to be serum sensitive as the viability was reduced by 50% after incubation in untreated serum (29–31). The growth of all strains in heated serum differed only slightly. The growth of wild-type X-73 and the acapsular mutant PBA930 was around seven generations, while the complemented mutant PBA954 grew to around six generations.
TABLE 5.
Resistance of E. coli DH5α and P. multocida strains X-73, PBA930, and PBA954 to chicken serum
| Strain | Serum heat treatment | Survival ratioa |
|---|---|---|
| E. coli DH5α | − | 0b |
| + | 10.4b | |
| P. multocida | ||
| X-73 | − | 200 ± 82c |
| + | 200 ± 90 | |
| PBA930 | − | 0.50 ± 0.22bc |
| + | 123 ± 32b | |
| PBA954 | − | 28.3 ± 5.8c |
| + | 65 ± 28 |
Survival rate = (CFU/ml at t = 3h)/(CFU/ml at t = 0h).
Survival ratios for serum and heated serum were significantly different (P = 0.003).
The P values for the difference in the survival ratios for both P. multocida X-73 and PBA954 compared with that of PBA930 were both significant (0.02 and 0.001, respectively).
Growth of P. multocida in chicken muscle.
The ability of strains to grow in chicken breast muscle was assessed. Wild-type strain X-73 was observed to multiply more than 25-fold in 4 h, while the acapsular mutant strain PBA930 decreased to less than 2% of the injected dose (Table 6). The complemented strain PBA954 showed restored growth ability but not to the level of the wild-type parent.
TABLE 6.
Growth of P. multocida strains in chicken muscle
| Strain | Injected dose (CFU) | Survival ratioa |
|---|---|---|
| X-73 | 8.5 × 105 | 25 ± 14 |
| PBA930 | 6.0 × 105 | 0.016 ± 0.007 |
| PBA954 | 2.6 × 105 | 6.5 ± 3.8 |
Survival ratio = (CFU/ml at t = 4)/CFU injected. The survival ratios for all three strains in chicken muscle were significantly different (P < 0.05).
DISCUSSION
Previous studies have suggested that the capsule is an important virulence factor in P. multocida. However, these studies all used spontaneously arising acapsular mutants (10, 14, 17, 21, 27) or enzymatic depolymerization of the capsule polysaccharides (1, 10, 11, 17, 21). We have constructed an isogenic hexA knockout mutant in P. multocida X-73, designated PBA930. The serogroup A capsule in P. multocida is composed primarily of hyaluronic acid and confers a highly mucoid colony morphology (20). In contrast, strain PBA930 was observed to be nonmucoid, and cells of PBA930 appeared acapsular by electron microscopy compared to the wild-type strain, which showed a prominent capsule. In addition, the amount of extracellular hyaluronic acid produced by AL5 was reduced fivefold. The cexA::tet(M) mutant was reported previously to be acapsular due to lack of capsule export (5), and we propose that the hexA::tet(M) strain is also acapsular because of a deficiency in capsule export.
Recently, we reported the capsule as a virulence factor for mice in an HS-causing P. multocida serogroup B strain by using an isogenic acapsular mutant (5). In the present study, the capsule was shown to be a virulence factor for P. multocida serogroup A in mice as well as in the natural host, chickens, by using a similar isogenic acapsular mutant. Acapsular P. multocida strain AL5 was avirulent in both mice and chickens compared to the wild-type strain. The in vitro growth rates of the acapsular strains at 37 and 42°C were similar to, or higher than, those of the capsulated strains X-73 and AL2, indicating that the reduction in virulence was not due to decreased growth rate per se.
An intact copy of hexAB in the P. multocida-E. coli shuttle vector pPBA1101 was introduced into PBA930 to complement the disrupted hexA::tet(M), with the resultant strain designated PBA954. PBA954 produced wild-type levels of extracellular hyaluronic acid, indicating restored ability to export hyaluronic acid. However, the lack of an observable capsule by electron microscopy and the nonmucoid colony morphology indicated that a normal capsule was not produced. We suggest that the amount of extracellular capsule produced may not reflect its distribution on the surface of the cell, accounting for the disparity seen in the colony and cellular morphologies. However, the partial restoration of surface capsule was clearly sufficient to restore full virulence for mice, serum resistance, and growth in chicken muscle in vivo (see below).
When the intact hexAB genes were restored in the complemented strain PBA954, the ability to cause lethal infection was restored to wild-type levels in BALB/c mice but not in chickens. The construction of an isogenic acapsular mutant by disruption of a single gene with the concomitant loss of virulence for both mice and chickens and the restoration of virulence for mice with the reintroduction of the intact gene argue for a key role for the capsule in the virulence of P. multocida. Clearly, the amount of capsule produced by PBA954 was sufficient to allow lethal infection in mice but not in chickens. A plausible hypothesis relates to the higher body temperature of chickens (41 to 42°C) than that of mice (37°C). We have observed that PBA954 was unable to grow in the presence of kanamycin at 42°C and grew slowly at 42°C even without selection, suggesting that overexpression of hexAB was deleterious to the cells. The reduced growth of PBA954 in vivo in chicken muscle and in heated serum provides further support for the lowered growth rate of PBA954. Thus, the inability of PBA954 to establish a lethal infection in chickens is possibly due to both reduced growth rate in vivo and abnormal or incomplete presentation of the capsule, which may be sufficient to allow the strain to evade phagocytosis in mice but not in chickens, perhaps due to the higher body temperature of the latter. The low level of complement activity in mouse serum (16) may also be a factor in allowing establishment of a lethal infection by PBA954.
The results of serum resistance and muscle growth assays were consistent with a role for the type A capsule in survival in vivo. Wild-type strain X-73 was resistant to the bactericidal action of chicken serum, while acapsular strain PBA930 was sensitive. This agrees with previous findings obtained by using spontaneously derived acapsular mutants and enzymatic removal of capsule (10). The complemented strain PBA954 regained serum resistance. Therefore, a major function of the type A capsule appears to be its ability to protect against the bactericidal activity of complement. Interestingly, serum sensitivity was not significantly linked to capsule for P. multocida serogroup B:2 (5).
The wild-type strain grew rapidly within chicken muscle, whereas the acapsular mutant survived poorly (Table 6). Although the complemented strain was unable to establish a lethal infection, it showed some restoration of in vivo survival and growth, consistent with its partially restored capsule and serum resistance phenotype. Previous work in our laboratory suggested that the reduction in virulence of acapsular bacteria was due to an increased susceptibility to phagocytosis (5). It is reasonable to suggest that part of the reason for the decrease in bacterial numbers of the acapsular strain in chicken muscle could also similarly be attributed to increased phagocytic clearance. Thus, the data presented here indicate that the capsule of serogroup A is an essential virulence factor in mice and also in the natural host, the chicken.
ACKNOWLEDGMENTS
We thank Ian McPherson and Vicki Vallance for invaluable technical assistance.
This work was funded in part by project grants from the Australian Research Council, the Rural Industries Research and Development Corporation, and the Australian Centre for International Agricultural Research, Canberra, Australia.
REFERENCES
- 1.Anderson L C, Rush H G, Glorioso J C. Strain differences in the susceptibility and resistance of Pasteurella multocida to phagocytosis and killing by rabbit polymorphonuclear neutrophils. Am J Vet Res. 1984;45:1193–1198. [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing and Wiley Interscience; 1990. [Google Scholar]
- 3.Benchetrit L C, Pahuja S L, Gray E D, Edstrom R D. A sensitive method for the assay of hyaluronidase activity. Anal Biochem. 1977;79:431–437. doi: 10.1016/0003-2697(77)90418-3. [DOI] [PubMed] [Google Scholar]
- 4.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce J D, Adler B. The capsule is a virulence determinant in the pathogenesis of Pasteurella multocida M1404 (B:2) Infect Immun. 2000;68:3463–3468. doi: 10.1128/iai.68.6.3463-3468.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter G A. Pasteurellosis: Pasteurella multocida and Pasteurella haemolytica. Adv Vet Sci Comp Med. 1967;11:321–379. [PubMed] [Google Scholar]
- 7.Cheng K J, Costerton J W. Ultrastructure of cell envelopes of bacteria of the bovine rumen. Appl Microbiol. 1975;29:841–849. doi: 10.1128/am.29.6.841-849.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chung J Y, Zhang Y M, Adler B. The capsule biosynthetic locus of Pasteurella multocida A:1. FEMS Microbiol Lett. 1998;166:289–296. doi: 10.1111/j.1574-6968.1998.tb13903.x. [DOI] [PubMed] [Google Scholar]
- 9.Gentry J M, Corstvet R E, Panciera R J. Extraction of capsular material from Pasteurella haemolytica. Am J Vet Res. 1982;43:2070–2073. [PubMed] [Google Scholar]
- 10.Hansen L M, Hirsh D W. Serum resistance is correlated with encapsulation of avian strains of Pasteurella multocida. Vet Microbiol. 1989;21:177–184. doi: 10.1016/0378-1135(89)90030-8. [DOI] [PubMed] [Google Scholar]
- 11.Harmon B G, Glisson J R, Latimer K S, Steffens W L, Nunnally J C. Resistance of Pasteurella multocida A:3,4 to phagocytosis by turkey macrophages and heterophils. Am J Vet Res. 1991;52:1507–1511. [PubMed] [Google Scholar]
- 12.Homchampa P, Strugnell R A, Adler B. Cross protective immunity conferred by a marker-free aroA mutant of Pasteurella multocida. Vaccine. 1997;15:203–208. doi: 10.1016/s0264-410x(96)00139-9. [DOI] [PubMed] [Google Scholar]
- 13.Jacques M, Graham L. Improved preservation of bacterial capsule for electron microscopy. J Electron Microsc Tech. 1989;11:167–169. doi: 10.1002/jemt.1060110212. [DOI] [PubMed] [Google Scholar]
- 14.Jacques M, Kobisch M, Belanger M, Dugal F. Virulence of capsulated and noncapsulated isolates of Pasteurella multocida and their adherence to porcine respiratory tract cells and mucus. Infect Immun. 1993;61:4785–4792. doi: 10.1128/iai.61.11.4785-4792.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahler C M, Martin L E, Shih G C, Rahman M M, Carlson R W, Stephens D S. The (α2→8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect Immun. 1998;66:5939–5947. doi: 10.1128/iai.66.12.5939-5947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim K S, Kang J H, Cross A S. The role of capsular antigens in serum resistance and in vivo virulence of Escherichia coli. FEMS Microbiol Lett. 1986;35:275–278. [Google Scholar]
- 17.Maheswaran S K, Thies E S. Influence of encapsulation on phagocytosis of Pasteurella multocida by bovine neutrophils. Infect Immun. 1979;26:76–81. doi: 10.1128/iai.26.1.76-81.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moses A E, Wessels M R, Zalcman K, Alberti S, Natanson-Yaron S, Menes T, Hanski E. Relative contributions of hyaluronic aid capsule and M protein to virulence in a mucoid strain of the group A Streptococcus. Infect Immun. 1997;65:64–71. doi: 10.1128/iai.65.1.64-71.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ophir T, Gutnick D L. A role for exopolysaccharides in the protection of microorganisms from desiccation. Appl Environ Microbiol. 1994;60:740–745. doi: 10.1128/aem.60.2.740-745.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pandit K K, Smith J E. Capsular hyaluronic acid in Pasteurella multocida type A and its counterparts in type D. Res Vet Sci. 1993;54:20–24. doi: 10.1016/0034-5288(93)90005-z. [DOI] [PubMed] [Google Scholar]
- 21.Pruimboom I M, Rimler R B, Ackermann M R, Brogden K A. Capsular hyaluronic acid-mediated adhesion of P. multocida to turkey air sac macrophages. Avian Dis. 1996;40:887–893. [PubMed] [Google Scholar]
- 22.Rimler R B. Comparisons of Pasteurella multocida lipopolysaccharides by sodium dodecyl sulfate-polyacrylamide gel electrophoresis to determine relationship between group B and E hemorrhagic septicemia strains and serologically related group A strains. J Clin Microbiol. 1990;28:654–659. doi: 10.1128/jcm.28.4.654-659.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rimler R B, Rhoades K R. Serogroup F, a new capsule serogroup of Pasteurella multocida. J Clin Microbiol. 1987;25:615–618. doi: 10.1128/jcm.25.4.615-618.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Sawada T, Rimler R B, Rhoades K R. Indirect hemagglutination test that uses glutaraldehyde-fixed sheep erythrocytes sensitized with extract antigens for detection of Pasteurella antibody. J Clin Microbiol. 1982;15:752–756. doi: 10.1128/jcm.15.5.752-756.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith H E, Damman M, van der Velde J, Wagenaar F, Wisselink H J, Stockhofe-Zurwieden N, Smits M A. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect Immun. 1999;67:1750–1756. doi: 10.1128/iai.67.4.1750-1756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Snipes K P, Hirsh D C. Association of complement sensitivity with virulence of Pasteurella multocida isolated from turkeys. Avian Dis. 1986;30:500–504. [PubMed] [Google Scholar]
- 28.Sutherland I W. Surface carbohydrates of the prokaryotic cell. London, United Kingdom: Academic Press Inc.; 1977. [Google Scholar]
- 29.Taylor P W. Genetical studies of serum resistance in Escherichia coli. J Gen Microbiol. 1975;89:57–66. doi: 10.1099/00221287-89-1-57. [DOI] [PubMed] [Google Scholar]
- 30.Taylor P W. Bactericidal and bacteriolytic activity of serum against gram-negative bacteria. Microbiol Rev. 1983;47:46–83. doi: 10.1128/mr.47.1.46-83.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor P W, Roberts A P, Gower P E. Evaluation of a technique for the estimation of serum bactericidal activity against Gram-negative organisms. Med Lab Technol. 1972;29:272–279. [PubMed] [Google Scholar]
- 32.Vogel U, Weinberger A, Frank R, Muller A, Kohl J, Atkinson J P, Frosch M. Complement factor C3 deposition and serum resistance in isogenic capsule and lipooligosaccharide sialic acid mutants of serogroup B Neisseria meningitidis. Infect Immun. 1997;65:4022–4029. doi: 10.1128/iai.65.10.4022-4029.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang R F, Kusher S R. Construction of versatile low-copy-number vectors for cloning, sequencing and gene expression in Escherichia coli. Gene. 1991;100:195–199. [PubMed] [Google Scholar]