Abstract
Multifunctional nanoparticles are being formulated to overcome the side effects associated with anticancer drugs as well as conventional drug delivery systems. Cancer therapy has gained the advancement due to various pragmatic approaches with better treatment outcomes. The metal nanostructures such as gold and silver nanoparticles accessible via eco-friendly method provide amazing characteristics in the field of diagnosis and therapy towards cancer diseases. The environmental friendly approach has been proposed as a substitute to minimize the use of hazardous compounds associated in chemical synthesis of nanoparticles. In this attempt, researchers have used various microbes, and plant-based agents as reducing agents. In the last 2 decades various papers have been published emphasizing the benefits of the eco-friendly approach and advantages over the traditional method in the cancer therapy. Despite of various reports and published research papers, eco-based nanoparticles do not seem to find a way to clinical translation for cancer treatment. Present review enumerates the bibliometric data on biogenic silver and gold nanoparticles from Clarivate Analytics Web of Science (WoS) and Scopus for the duration 2010 to 2022 for cancer treatment with a special emphasis on breast, ovarian and cervical cancer. Furthermore, this review covers the recent advances in this area of research and also highlights the obstacles in the journey of biogenic nanodrug from clinic to market.
Keywords: biogenic, silver nanoparticles, gold nanoparticles, cancer therapy, bibliometric analysis
1 Introduction
Cancer in general is a highly complex disease prevalent globally and incidents of breast cancer in women are on the rise, 30% new cases are reported every year (Holliday and Spiers, 2011). Chemotherapy, radiotherapy, immunotherapy, surgery, vaccinations for cancer, stem cell therapy, photo dynamic therapy and combinational methods are applied for cancer treatment which pose their own risk as toxicity, limited bioavailability, non-specific action, etc. (Raza et al., 2016; Chahal et al., 2018).
Recent advances in nanotechnology may pose an alternative approach to treat cancer as high surface area, shape and charge on the nanoparticles help them to interact with biological membrane, indicate increase in permeability, and thus enhance their activity (Klochkov et al., 2021). Gold nanoparticles (Au@NP)and silver nanoparticles (Ag@NP) have revealed their remarkable possibilities (Sarkar et al., 2011 and Sarkar et al., 2012) for diagnosis and treatment of cancer and as drug delivery in both in vivo, in vitro systems (Cai et al., 2008) and could minimize the chemotherapeutic drug-induced antagonistic effects. Biological synthesis of nanomaterials is prominently acquiring importance as physical and chemical methods employed for synthesis of nanoparticles demonstrate few limitations as high cost, low-productivity and toxicity (Acharya and Sarkar 2014; Mollick et al., 2014; Jadhav et al., 2018). Nano biomaterials have been reported to be approved by FDA for use as anticancer drugs, and diagnostic agents (Anand et al., 2021).
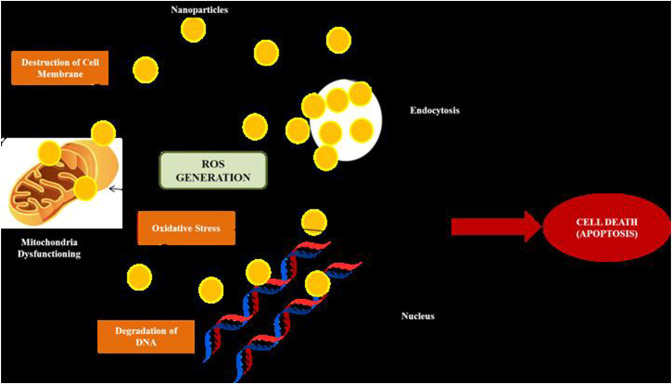
Bioactive phytochemicals such as polyphenols, phenolic acids, flavonoids, terpenes, and alkaloids etc. present in plants have been shown to exhibit anticancerous properties (Ren et al., 2003; Balunas and Kinghorn, 2005; Hu, 2011). Generally phytoconstituents present in the plants are converted to nanoparticles by reaction with metal salts which behave as reducing and capping agents (Singh P et al., 2018) Figure 1, but researchers also used other extended methods of preparation of such nanoparticles for better yield. Nano particles act as tug boats of research to overcome the above listed challenges (Hira et al., 2018). Since different plants possess different phytoconstituents, having variable chemical structure, display variable actions against different types of cancers. The action of nanoparticles on target cancer cell is represented in Figure 2.
FIGURE 1.
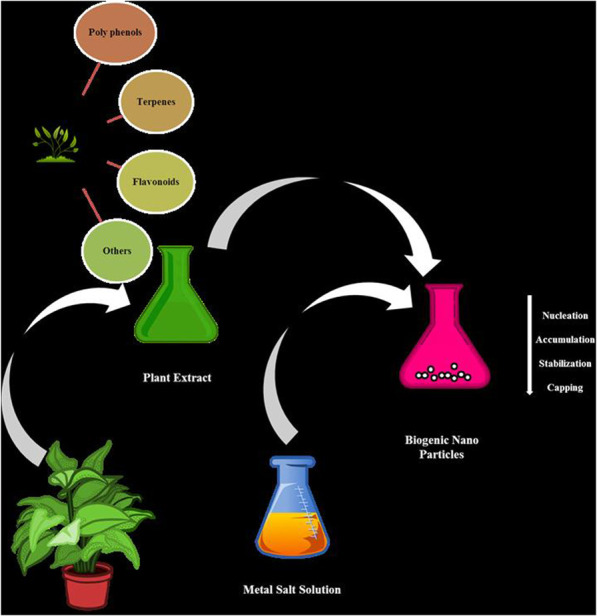
General green synthesis of nanoparticles by using plant extracts and metal salt solutions.
FIGURE 2.
Mechanism of action of nanoparticles on cancer cell.
The combination of nanotechnology and pharmaceutical sciences creates a boom in the medical field due to a variety of applications. Nanoparticles are less toxic, possess better optical, photothermal properties and can be used as biomedicine (Raza et al., 2016; Yazdi et al., 2016), assay for diagnostic (Liu et al., 2015), drug delivery or gene delivery (Yang et al., 2015) and expansion of radiotherapy. AuNPs have shown efficacy for treatment of prostate (Barabadi et al., 2019), breast (Anand et al., 2021), and colorectal cancer (Khan A et al., 2021). Nowadays, biogenic nanoparticles, prepared by natural extracts like plant extracts are in the frame for use as treatment for cancer as a sustainable approach. Some people used olive oil, licorice root (Glycyrrhiza glabra) and coconut oil for preparation of Gold coated Iron nanoparticles (Al-Radadi, 2022). Not only in cancer, the role of biogenic Au@NP and Ag@NP is magnificent in other fields as well. Studies on mice show in vivo anti-diabetic and anticancer effects as well anti-hypoglycemia and hepatic-protective potential when biogenic Au@NP and Ag@NP prepared from Ajuga bracteosa, aqueous extract were used (Nazer et al., 2022).
Though the utilization of natural resources or molecules to treat human malignancies, illness, diseases, is expanding (Tuasha et al., 2018; Kashyap et al., 2021) as they play an important role as chemo-protective or radio-protective and therapeutic agents to treat cancer (Khan F. A et al., 2021; Raj et al., 2022) but it is also observed that because of chemical and physical changes in the process, the alteration in the molecular structure of phytochemicals may alter their efficacy against cancer (Seca and Pinto, 2018). Such challenges emphasise the requirement for different approaches to overcome these constraints. Consequently, creating innovative formulations and nanotechnology-based methods for delivering drugs are considered to be the promising approach.
2 Quantitative approach and strategy of bibliometric analysis
Apart from obstacles, nanoparticles are still winning on other methods of cancer therapy. Among various applications of nanoparticles, the large quantity of drug delivery with large surface area of nanoparticle is inspiring (Faraji and Wipf 2009).
This paper emphasizes on bibliometric analysis of researches undergoing in the field of use of biogenic nanoparticles in cancer treatment, specifically breast, ovarian and cervical cancer. Such analysis focuses on publication trend in biogenic nanoparticle cancer therapy.
Bibliometric is the application of statistical techniques for assessing the research output of nations, institutions, and people, as well as quantifying academic success (Darroudi et al., 2021). However, bibliometric analysis is insufficient in evaluating a study area’s outputs; therefore, it needs to be taken in to consideration other types of inputs, including a literature review to learn about trends in publishing. This study examines the state of research in this area of study through bibliometric and qualitative literature reviews from the past to the present. This section provides an overview of the current status and future directions in this field.
For bibliometric analysis, data is collected on biogenic silver and gold nanoparticles from Clarivate Analytics Web of Science (WoS) and Scopus for the duration 2010 to 2022 for cancer treatment with a special emphasis on breast, ovarian and cervical cancer. The core collected data was 341 papers, after searching the keywords as “Biogenic gold nanoparticles + breast cancer + clinical trials”, “Biogenic gold nanoparticles + ovarian cancer + clinical trials”, “Biogenic gold nanoparticles+ cervical cancer + clinical trials”, “Biogenic gold nanoparticles+ cancer therapy + clinical trials”, “Biogenic silver nanoparticles + breast cancer + clinical trials”, “Biogenic silver nanoparticles + ovarian cancer +clinical trials”, “Biogenic silver nanoparticles + cervical cancer + clinical trials” and “Biogenic silver nanoparticles + cancer therapy + clinical trials”. The duplicity of publication was found in each keyword search; therefore after removing duplicity, 141 papers were left. These 141 articles were analysed on the parameters indicated:
• General publication trend
• Most cited papers
• Most Contributing authors
• Top Journals
• Articles on Clinical trials
• Plant extract for Biogenic Au@NP and Ag@NP

3 Result and discussion
It was observed that out of 341, 141 publications related to biogenic gold and silver nanoparticles for identified cancer therapies. This bibliographic analysis provides the idea general publication trends in this field, the most contributing authors with citation levels, top groups which are working in collaborations, different plant extracts used to formulate biogenic nanoparticles, applications of nanoparticles on different cell lines and obstacles in the journey of Nanoparticles from clinic to market.
3.1 General publication trend
According to Table 1, the maximum 42 publications were reported in 2021 but the maximum citations were observed for papers published in about 2018 though publications number is very less i.e., 13.
TABLE 1.
Details of number of publications in each year.
| Year | Number of publications | Average number of authors for each document |
|---|---|---|
| 2013 | 2 | 7.00 |
| 2014 | 2 | 3.50 |
| 2015 | 3 | 9.33 |
| 2016 | 9 | 3.78 |
| 2017 | 7 | 4.14 |
| 2018 | 13 | 5.69 |
| 2019 | 17 | 4.06 |
| 2020 | 19 | 5.37 |
| 2021 | 42 | 5.88 |
| 2022 | 27 | 6.85 |
The publications illustrate a variety of applications of biogenic Ag@NP and Au@NP with reference to cancer therapy. Currently, nanoparticles are used to improve impact of blue light therapy for cancer treatment. Blue Light therapy is a therapy to heal deep wounds (Akasov et al., 2022). As previously discussed, ecological and green synthetic approach to the synthesis of metal nanoparticles has gained a lot of importance in the recent era, like gold coated iron (Fe@Au) nanoparticles have been synthesized using an extract solution of olive oil, licorice root and coconut oil (Al-Radadi, 2022). It was suggested that Au@NP synthesized from biocompatible plants play vital role in cancer therapy. Researchers used Fluorescent plant based markers with biogenic Au@NP for cancer detection as well (Sargazi et al., 2022). In some studies, biogenic Au@NP was used for neurological controls as well (Zare et al., 2022). As flavonoglycone hesperidin, an anticancer agent has poor biocompatibility i.e. hesperidin loaded Au@NP were used due to their bioactive potential and compatibility (Sulaiman et al., 2022).
Specifically, biogenic Ag@NP and Au@NP have attracted attention for their antineoplastic activity toward leukaemia attributable to their unique physico-chemical properties (Mostafavi et al., 2022). Biogenic Ag@NP can be used in dental therapy due to its antibacterial and antifouling properties, the scope of such work also relates to mouth cancer (Ahmed et al., 2022). Researchers used biogenic Ag@NP which displayed potent toxic effects against both cancer cell lines and pathogens, and also exhibited antioxidant activity (Wei et al., 2021). Recently Ag@NP were also tested with spirulina on rats to treat prostate cancer, and it was found that a class of rats showed good result in the context of treatment with stability in hormone imbalance (El-Magid et al., 2022).
In 2021, maximum publications were reported, but most of them were review articles related to cancer therapy Au@NP synthesized using Argemone mexicana L. aqueous extract have been used on cell line, HCT-15. It was found that these biogenic nanoparticles were effective in cell growth suppression and induction of apoptosis (Datkhile et al., 2021). As an anti-leukaemia agent, Au@NPs prepared by using Tribulus terrestris extract, were used and it was observed that the Au@NPs exhibited steady reduction in % cell viability with an increase in its concentration (Zhao et al., 2021). It was reported that cetuximab-targeted gold nanorods (CTX-AuNR) is an attractive therapeutic strategy for triple negative breast cancer (TNBC). CTX-AuNR with near infrared (NIR) irradiation, can serve as a potent photoimmunotherapy strategy for handling epidermal growth factor receptor overexpressing TNBC cells (Emami et al., 2021). The Nutraceuticals present in marine algae is also found to show spectacular algal-mediated synthesis of nanotheranostics. Such algae used to prepare thermodynamically stable NPs (Menaa et al., 2021). The phytochemicals present in Pomegranate (Punica granatum L.) has demonstrated tremendous potential for preventing cancer. The chemical components of pomegranates, the outcomes of preclinical (in vitro, ex vivo, and in vivo) and clinical investigations on the anticancer impact of pomegranate phytochemicals and molecular targets in a variety of malignancies, such as breast cancer, gastrointestinal tract cancer, uterine and ovarian cancer, leukemia, lung cancer, neurological (glioma) etc. have been published (Wong et al., 2021).
Recent developments in determining the half-maximal inhibitory concentration, a crucial factor for advancing clinical trials, have mostly focused on the use of standard 2D cell culture and in vivo murine models to assess the anticancer effect of green biogenic Au and Ag nanoparticles (Tinajero-Díaz et al., 2021).
According to bibliographic analysis the most cited papers were reported in 2018. There are two most cited research articles, one is from “International Journal of Molecular Sciences” and other is from “Frontiers in Microbiology”. The first paper emphasized on the supreme role of Au@NPs in the diagnosis of a variety of cancers and for drug delivery applications. Gold nanoparticles non-toxic and non-immunogenic properties, as well as their high permeability and retention, impact, offer further advantages by making it simple for medications to accumulate at tumour locations (Singh J et al., 2018). The advantages of biogenic nanoparticles over other antibacterial or antimicrobial therapies were also discussed, which revealed their potential and non-toxic behaviour (Baptista et al., 2018).
3.2 Most cited papers
Bibliographic analysis of required data emphasize on applications of Au@NP in diagnosis and as therapeutic agent for human cancer due to its spectacular physical properties. The other published articles deals with the introduction, reviews and applications of Nanoparticles (Table 2). The usage of biogenic nanoparticles (Ag and Au) offers a current approach which not only leads to green synthesis or use of plant extract, but also overcome the toxic effects of nanomaterials as well. Researchers used such nanoparticles in vivo, in vitro and ex vitro strategies and confirmed the benefits of such biogenic nanoparticles in cancer therapy.
TABLE 2.
Details of most cited papers till date.
| Authors | Title | Year | Source title | References |
|---|---|---|---|---|
| Singh P., Pandit S., Mokkapati V.R.S.S., Garg A., Ravikumar V., Mijakovic I | Gold nanoparticles in diagnostics and therapeutics for human cancer | 2018 | International Journal of Molecular Sciences | Singh J et al. (2018) |
| Baptista P.V., McCusker M.P., Carvalho A., Ferreira D.A., Mohan N.M., Martins M., Fernandes A.R | Nano-strategies to fight multidrug resistant bacteria-"A Battle of the Titans" | 2018 | Frontiers in Microbiology | Baptista et al. (2018) |
| Rafique M., Sadaf I., Rafique M.S., Tahir M.B | A review on green synthesis of silver nanoparticles and their applications | 2017 | Artificial Cells, Nanomedicine and Biotechnology | Rafique et al. (2017) |
| Jeyaraj M., Sathishkumar G., Sivanandhan G., MubarakAli D., Rajesh M., Arun R., Kapildev G., Manickavasagam M., Thajuddin N., Premkumar K., Ganapathi A | Biogenic silver nanoparticles for cancer treatment: An experimental report | 2013 | Colloids and Surfaces B: Biointerfaces | Jeyaraj et al. (2013) |
| Austin L.A., MacKey M.A., Dreaden E.C., El-Sayed M.A | The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery | 2014 | Archives of Toxicology | Austin et al. (2014) |
| Bahrami B., Hojjat-Farsangi M., Mohammadi H., Anvari E., Ghalamfarsa G., Yousefi M., Jadidi-Niaragh F | Nanoparticles and targeted drug delivery in cancer therapy | 2017 | Immunology Letters | Bahrami et al. (2017) |
| Navya P.N., Kaphle A., Srinivas S.P., Bhargava S.K., Rotello V.M., Daima H.K | Current trends and challenges in cancer management and therapy using designer nanomaterials | 2019 | Nano Convergence | Navya et al. (2019) |
| Prasad M., Lambe U.P., Brar B., Shah I., J M, Ranjan K., Rao R., Kumar S., Mahant S., Khurana S.K., Iqbal H.M.N., Dhama K., Misri J., Prasad G | Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world | 2018 | Biomedicine and Pharmacotherapy | Prasad et al. (2018) |
| Carvalho P.M., Felício M.R., Santos N.C., Gonçalves S., Domingues M.M | Application of light scattering techniques to nanoparticle characterization and development | 2018 | Frontiers in Chemistry | Carvalho et al. (2018) |
| Padovani G.C., Feitosa V.P., Sauro S., Tay F.R., Durán G., Paula A.J., Durán N | Advances in Dental Materials through Nanotechnology: Facts, Perspectives and Toxicological Aspects | 2015 | Trends in Biotechnology | Padovani et al. (2015) |
3.3 Most contributing author
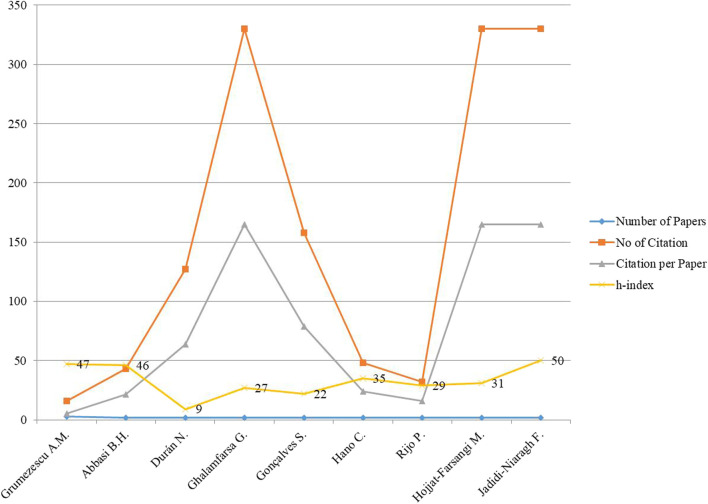
The bibliographic analysis showed the Dr Alexandru Mihai Grumezescu, Romania, contributed the maximum on applications nanomaterials as anticancer and antimicrobial activities. Figure 3 is shows that though numbers of publications in this field are less but the citation factor is high. Researchers who are working in these fields have good h-index as well, in the range of 20–50.
FIGURE 3.
Author details in cancer therapy with nanoparticles.
3.4 Top journals
As per data we have identified six top Journals in the required key words projected for bibliographic analysis are Pharmaceutics, Advanced Drug Delivery Reviews, Nanomaterials, International Journal of Molecular Sciences, International Journal of Nanomedicine and Nanoscale consecutively.
3.5 Application of Au@NPs and Ag@NPs on variety of cell line
Several anti-cancer drugs such as Myocet, DaunoXome, ONPATTRO Patisiran ALN-TTR02, VYXEOS CPX-351 based on nanotechnology have been approved by US FDA and EMA (Bhardwaj et al., 2021).
The efficiency of silver and gold nanoparticles derived from Acalypha. indica leaves were tested for their cytotoxicity against MDA-MB-231, human breast cancer cells and they illustrated 40% cell inhibition and exhibited prominent cytotoxic and apoptotic effect through DNA destruction (Krishnaraj et al., 2014).
The gold nanoparticles biosynthesized using Argemone mexicana L. aqueous extract have been found to show genotoxic effects on human cervical adenocarcinoma (HeLa), human breast adenocarcinoma (MCF-7) and human colon adenocarcinoma (HCT-15) cells by stopping growth of cell and initiation of caspase-mediated apoptosis by stimulating p53 and caspase-3 genes generation (Datkhile et al., 2021). Gold nanoparticles (AuNPs) prepared from Mimosa pudica leaves extract exhibited anticancer properties for breast cancer cell lines (MDA-MB-231 & MCF-7) without creating any toxicity. The cytotoxicity test was done by fluorescence microscopy on cancer cells and Human mammary epithelial cells, it was found that there is no toxic effect on normal cells (Suganya et al., 2016). In most of the cases, different breast cancer cell line like MDA-MB-468, MCF-7, MDA-MB and MDA-MB-231 were used to evaluate the anti-cancer efficacy of silver and gold nanoparticles. AuNPs from Commiphora wightii leaf extract were prepared which displayed anticancer properties on MCF-7 cells by killing them (Uzma et al., 2020). SPE-AuNPs were prepared from the aqueous peel extract of Spondias dulcis (SPE) which displayed substantial cytotoxicity to MCF-7 human breast cancer cells through the production of reactive oxygen species (Pechyen et al., 2022).
Curcumin-coated silver nanoparticles (cAgNPs) along with cisplastin showed better efficacy through programmed cell death in A2780 cells as compared to when cAgNPs or cisplastin are used alone (Ramezani et al., 2019). The viability of MCF-7 cells was reduced to 50% with AgNPs synthesized from Dendrophthoe falcata (Sathishkumar et al., 2014). AgNPs synthesized from Datura inoxia impedes 50% propagation of human breast cancer cell line MCF7 (Gajendran et al., 2014). Cell viability of MCF-7 cells effectively decreased when treated with Rhizophora mucronata mediated Ag NPs (Rajivgandhi et al., 2022a). Efficacy of Silver nanoparticles, synthesized from the extracts of Geodorum densiflorum rhizome, Kaempferia rotunda and Zizyphus mauritiana was determined against GSC cells along with MCF-7 and BxPC-3 cells and in vivo anticancer properties were studied against EAC cells (Kabir et al., 2022) and G. desiflorum nanoparticles were found to inhibit growth of EAC cells by 90%.
Cytotoxicity studies of Ag/AgCl-NPs obtained from the Z. mauritiana fruit extract were monitored against human breast cancer cell line (MCF-7) and mice Ehrlich ascites carcinoma (EAC) cells and it was observed that apoptosis is induced through the Fas-mediated pathway (Kabir et al., 2020).
Biogenic AuNPs derived from Anacardium occidentale leaves showed IC50 of 6 and 600 μg/ml, respectively towards MCF-7 cells and peripheral blood mononuclear cells (PBMCs) using an MTT assay (Sunderam et al., 2019). Biogenic AuNPs prepared from C. papaya and Catharanthus roseus (C. roseus) exhibited cytotoxicity against MCF-7 (Muthukumar et al., 2016). Cytotoxic impact of AuNPs prepared from Aegle marmelos, Eugenia jambolana, and Soursop were evaluated against MCF-7 cells (Vijayakumar, 2019) having IC 50 values of 172 ± 4, 163 ± 4, and 98 ± 4 µg/ml, respectively. Au@NPs synthesized from Dragon fruit were found to exhibit cytotoxicity having IC50 value of around 500 μg/ml against MCF-7 breast cancer cells using an alamarBlue® assay (Divakaran et al., 2019).
Nerium oleander-conjugated gold nanoparticles prepared from stem bark of the plant showed cytotoxicity against MCF-7 breast cancer cell. It exhibited efficacy for the apoptosis of tumor selectively (Barai et al., 2018). The Lycium chinense AgNPs synthesized from fruit extract of the plant showed appreciable cytotoxic impact against human breast cancer MCF7 cell line (Chokkalingam et al., 2019). Green AuNPs prepared from Camellia sinensis, Coriandrum sativum, Mentha arvensis, Phyl-lanthus amarus, Artabotrys hexapetalus, Mimusops elengi, Syzygium aromaticum exhibited anticancer effectiveness against MCF7 with minimum concentration of 2 μg/ml (Kamla Priya and Iyer, 2015).
3.6 Plant extract for Biogenic Au@NP (gold nanoparticles) and Ag@NP (silver nanoparticles)
Table 3 represents the plants which have been utilized for the preparation of nanoparticles for cancer therapy. According to the studies Licorice root (Glycyrrhiza glabra), Medicago sativa, Olax Scandens, Hubertia ambavilla, Hypericum lanceolatum, Argemone mexicana L., Tribulus terrestris, Solanaceae family, Marine algae, Carissa carandas leaf, Leucophyllum frutescens and Russelia equisetiformis leaves, Eucalyptus tereticornis leaves, Artemisia Sieberi Besser leaves, Gelidiella acerosaon, Ajuga bracteosa, Floral extracts of Callistemon viminalis, Caesalpinia pulcherrima stem, Garcinia mangostana, Cinnamomum zeylanicum, Salvia officinalis leaf, Tilia cordata flowers, Aegle marmelos, Curcumae kwangsiensis leaf, Spondias dulcis (Anacardiaceae) peel, Vigna radiate and many more, plant part extracts were used to prepare biogenic gold and silver nanoparticles for different applications. It implies that the scope of biogenic nanoparticles is better in the field of cancer therapy.
TABLE 3.
Plant used to form gold and silver nanoparticles along with their applications.
| S.No. | Name of plants to prepare biogenic nanoparticles | Type of nanoparticles | Applications | Reference |
|---|---|---|---|---|
| 1 | Olive oil, Licorice root (Glycyrrhiza glabra) and Coconut oil | Gold coated iron (Fe@Au) nanoparticles | Against Helicobacter pylori (H. pylori) and ulcer | Al-Radadi, (2022) |
| 2 | Medicago sativa, Olax Scandens, H. ambavilla, and H. lanceolatum | Fluorescent-plant-based markers, including Au@NPs | Cancer therapy | Sargazi et al. (2022) |
| 3 | Argemone mexicana L | Argemone mexicana –Au@NPs | Human colon cancer cell line, HCT-15 | Datkhile et al. (2021) |
| 4 | Tribulus terrestris | Au@NPs-Tribulus | Potential anti-Leukemia drug | Zhao et al. (2021) |
| 5 | Solanaceae family | Nanoparticles | Chemotherapeutic agents in various in vitro and in vivo cancer models | Kowalczyk et al. (2022) |
| 6 | Spirulina algae to AgNPs as a combination | Ag@NPs and Spirulina algae (Sp) | Prostate cancer | El-Magid et al. (2022) |
| 7 | Marine algae | Nanoparticles | Nanotheranostic purposes | Menaa et al. (2021) |
| 8 | Carissa carandas leaf | Ag@NP | Antimicrobial activity and Antibiofilm activity | Rahuman et al. (2021) |
| 9 | Leucophyllum frutescens and Russelia equisetiformis leaves | Ag@NP | Potential cytotoxic and Antibacterial capacity | Mohammed and Al-mergin, (2021) |
| 10 | Eucalyptus tereticornis leaves | Ag@NP | Potential anticancer activity | Kiran et al. (2020) |
| 11 | Artemisia Sieberi Besser leaves | Ag@NP | Stability checking of Nanoparticles at different parameters | Rousta and Ghasemi, (2019) |
| 12 | Red algae Gelidiella acerosaon | - | Anticancer properties | SM et al. (2018) |
| 13 | Ajuga bracteosa | AB-Ag@NP | Anti-diabetic and hepato-protective phytoconstituents | Nazer et al. (2022) |
| 14 | Floral extracts of Callistemon viminalis | Nickel oxide Nanoparticles (NiO-NP) | Diverse biomedical applications | Sani et al. (2021) |
| 15 | Marine Algae Chaetomorpha linum | Ag@NP | Human Colon Cancer Cell HCT-116 | Acharya et al. (2021) |
| 16 | Caesalpinia pulcherrima stem | Ag@NP | Breast cancer cells through in vitro approaches | Rajivgandhi et al., (2022b) |
| 17 | Garcinia mangostana | Ag@NP | Against human breast cancer | Alobaid et al. (2022) |
| 18 | Cinnamomum zeylanicum | Ag@NP | Improve the fertility status of rats with polycystic ovarian syndrome | Alwan and Al-saeed,, (2021) |
| 19 | Salvia officinalis leaf | Ag@NP | Anti-human ovarian cancer activity | Luo et al. (2022) |
| 20 | Tilia cordata flowers | Ag@NP | Antioxidant and anti-tumor activities | Saygi and Cacan, (2021) |
| 21 | Aegle marmelos | Chitosan entrapped Ag@NP | Human cervical cancer cells-HeLa | Sukumar et al. (2022) |
| 22 | Curcumae kwangsiensis leaf | Au@NP | Anti-human ovarian cancer | Chen et al. (2021) |
| 23 | Spondias dulcis (Anacardiaceae) peel | Au@NP | Breast cancer | Pechyen et al. (2022) |
| 24 | Vigna radiata | Au@NP | Inhibits colony formation in breast cancer | Singh et al. (2021) |
3.7 Top groups working on nanoparticles for cancer therapy
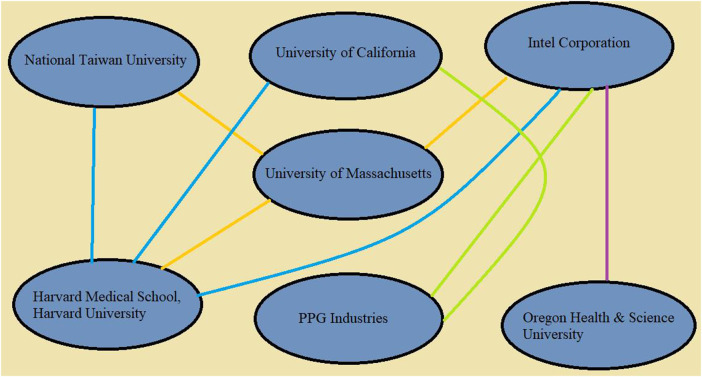
Globally there are many potential research groups are working on nanoparticle cancer therapy. Table 4 shows the collaboration among top groups/institutes/research labs working on gold nanoparticles/silver nanoparticles/nanoparticles for treatment of cancer. Figure 4 represents the interconnection or collaborations between these groups. Such data enhanced the scope of bibliographic analysis. These findings reflect that researchers are working on nanoparticles for cancer therapy since long, but to overcome their toxic effect, now researchers are moving ahead towards use of biogenic nanoparticles.
TABLE 4.
Details of groups working on cancer therapy using nanoparticles.
| S.No. | Author | Affiliation | Collaborated with | Affiliation |
|---|---|---|---|---|
| 1 | Baoshan Xing | University of Massachusetts | Jian Zhao | Ocean University of China |
| 2 | Vincent Rotello | University of Massachusetts | Yi-Cheun Yeh | National Taiwan University |
| 3 | Yi-Cheun Yeh | National Taiwan University | Rubul Mout | Harvard Medical School, Harvard University |
| 4 | Yi-Cheun Yeh | National Taiwan University | Bradley Duncan | MIT Lincoln Laboratory, Harvard University |
| 5 | Yi-Cheun Yeh | National Taiwan University | Gulen Yesilbag Tonga | Harvard Medical School, Harvard University |
| 6 | Gulen Yesilbag Tonga | Harvard Medical School, Harvard University | Krishnendu Saha | Intel Corporation |
| 7 | Krishnendu Saha | Intel Corporation | Xiaoning Li | Oregon Health & Science University |
| 8 | Krishnendu Saha | Intel Corporation | Sung Tae Kim | Inje University |
| 9 | Krishnendu Saha | Intel Corporation | Daniel F. Moyano | PPG Industries |
| 10 | Krishnendu Saha | Intel Corporation | Sukru Gokhan Elci | University of Massachusetts |
| 11 | Ziwen Jiang | University of California | Daniel F. Moyano | PPG Industries |
| 12 | Ziwen Jiang | University of California | Gulen Yesilbag Tonga | Harvard Medical School, Harvard University |
FIGURE 4.
Interconnection between organizations for cancer therapy through use of nanoparticles.
4 Obstacles in the journey of nanoparticles from clinic to market
Though first nano chemo drug Doxil entered the market in 1995, even after 27 years, number of nanomedicines approved by FDA are still inconsequential. 16 nano-based cancer drugs have been recommended by FDA whereas nearly 75 nano formulations are in clinical trials now (He et al., 2019). Still the gap between research and actual marketability of the drug molecule is huge. Some of the drugs even after approval are withdrawn from the market due to various reasons.
Major challenges associated with the use of NPs as drug for cancer are as follows:
4.1 Biological challenges
Nanoparticles are injected intravenously in to blood and they do not get enough time to interact with target site or we can say they lack time due to route followed for administration of drug (Lv et al., 2012). As a result, they are required in higher concentration to show the desirable impact. As people are using biologically safe NP’s but still in some cases it leads to organ failure such as lungs, liver etc., due to difference in solubility, particle size, surface area and other associated factors (Jia et al., 2018). According to some findings the utilization of controlled Ag@NPs prepared from extract of Gongronema Latifoliumis are beneficial as compared to conventional drugs, AgNPs have a larger inhibitory zone against bacterial cells (Aisida et al.,2019).
4.2 Technological challenges
Technological issues associated with nanoparticles are optimization and scale up of synthesis, as most of the synthesis are done at smaller scale and testing is mostly done in vitro level and sometimes in vivo. Clinical translation of NPs as drug for cancer therapy will be more feasible if in vitro and in vivo studies are also combined with computational and modelling studies based on understanding of mechanism of metastasis in cancer. (Gavas et al., 2021).
Cellular internalization of nano chemotherapeutic drug poses problem for its clinical development. Drug comes in to market once complete cellular internalization is defined. Also, response of each patient to the same drug is different due to change of genetic history and environmental factors. Furthermore, it takes very long span of time from drug development to pre-clinical and clinical trials on higher animals and humans (Mundekkad and Cho, 2022). Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) may offer an alternative for cancer cure and many nanoparticles have been used to produce targeted CRISPR/Cas9 to stop the growth of tumor cells (Shi et al., 2020; Duan et al., 2021).
5 Conclusion and future scope
Bibliographic analysis provides the record of publications with respect to input of keywords. This bibliometric analysis was done on biogenic silver and gold nanoparticles utilized in breast, ovarian and cervical cancer treatment from Clarivate Analytics Web of Science (WoS) and Scopus for the duration 2010 to 2022. The findings of bibliographic analysis are providing the information regarding the top publications, top journals in which maximum publications of this field are available and articles details which are having experiments on cell line. This review also provides the details of variety of plant sources which are utilized for the preparation of biogenic Au@NPs and Ag@NPs in different applications including cancer therapy, which is an additional support for bibliographic analysis. All such studies revealed the benefits of biogenic nanoparticles in various applications. The discussion on applications of NPs on cell lines in cancer therapy was also elaborate the efficacy of biogenic NPs against cancer. These reports reveal that though nanoparticles have been studied in vitro for anti-proliferative activity against cell line still in vivo research applications are very less and clinical translation of nanoparticles as medicines for cancer treatment is almost negligible. To design NPs which possess good solubility, specificity, non-toxicity, flexibility, binding capacity and can be utilized for target drug delivery systems, it is important to understand mechanism of formation of Protein Crown, enhanced permeability and retention effect, cellular and physiological factors that control effectiveness of NPs based drug delivery systems. After understanding biological and technical obstacles in the journey of Nanoparticles from clinic to market, it is a high time to intensify and further explore experimental studies on green nanoparticles to understand their mechanism of action at molecular stage. This article may help many researchers understand the flow of experiments on utilization of biogenic Au@NPs and Ag@NPs in cancer therapy, the gaps and the publication trends, to start off further experimentation that might lead to the drugs for the cancer treatment and also overcome the obstacles in this field.
Acknowledgments
The authors acknowledge the support received from the leadership and management of K.R. Mangalam University, Gurugram, Haryana, India.
Author contributions
SR, MB, AK and DK contributed to conception and design of the study. SR, MB, AK and DK wrote sections of the manuscript and prepared tables and figures. All authors equally contributed to manuscript revision, read, and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Acharya D., Satapathy S., Somu P., Parida U. K., Mishra G. (2021). Apoptotic effect and anticancer activity of biosynthesized silver nanoparticles from marine algae Chaetomorpha linum extract against human colon cancer cell HCT-116. Biol. Trace Elem. Res. 199 (5), 1812–1822. 10.1007/s12011-020-02304-7 [DOI] [PubMed] [Google Scholar]
- Acharya K., Sarkar J. (2014). Bryo-synthesis of gold nanoparticles. Int. J. Pharm. Sci. Rev. Res. 29 (1), 82–86. [Google Scholar]
- Ahmed O., Sibuyi N. R. S., Fadaka A. O., Madiehe M. A., Maboza E., Meyer M., et al. (2022). Plant extract-synthesized silver nanoparticles for application in dental therapy. Pharmaceutics 14 (2), 380. 10.3390/pharmaceutics14020380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aisida S. O., Ugwu K., Akpa P. A., Nwanya A. C., Ejikeme P. M., Botha S., et al. (2019). Biogenic synthesis and antibacterial activity of controlled silver nanoparticles using an extract of Gongronema Latifolium. Mater. Chem. Phys. 237, 121859. 10.1016/j.matchemphys.2019.121859 [DOI] [Google Scholar]
- Akasov R., Khaydukov E. V., Yamada M., Zvyagin A. V., Leelahavanichkul A., Leanse L. G., et al. (2022). Nanoparticle enhanced blue light therapy. Adv. Drug Deliv. Rev. 184, 114198. 10.1016/j.addr.2022.114198 [DOI] [PubMed] [Google Scholar]
- Al-Radadi N. S. (2022). Microwave assisted green synthesis of Fe@ Au core–shell NPs magnetic to enhance olive oil efficiency on eradication of helicobacter pylori (life preserver). Arabian J. Chem. 15 (5), 103685. 10.1016/j.arabjc.2022.103685 [DOI] [Google Scholar]
- Alobaid H. M., Alzhrani A. H., Majrashi N. A., Alkhuriji A. F., Alajmi R. A., Yehia H. M., et al. (2022). Effect of biosynthesized silver nanoparticles by Garcinia mangostana extract against human breast cancer cell line MCF-7. Food Sci. Technol. 42, 42. 10.1590/fst.41622 [DOI] [Google Scholar]
- Alwan S. H., Al-Saeed M. H. (2021). Biosynthesized silver nanoparticles (using Cinnamomum zeylanicum bark extract) improve the fertility status of rats with polycystic ovarian syndrome. Biocatal. Agric. Biotechnol. 38, 102217. 10.1016/j.bcab.2021.102217 [DOI] [Google Scholar]
- Anand K., Saravanan M., Chandrasekaran B., Kanchi S., Panchu S. J., Chen Q. S. (Editors) (2021). Handbook on nanobiomaterials for therapeutics and diagnostic applications (Elsevier; ), Netherlands. [Google Scholar]
- Austin L. A., Mackey M. A., Dreaden E. C., El-Sayed M. A. (2014). The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery. Arch. Toxicol. 88 (7), 1391–1417. 10.1007/s00204-014-1245-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahrami B., Hojjat-Farsangi M., Mohammadi H., Anvari E., Ghalamfarsa G., Yousefi M., et al. (2017). Nanoparticles and targeted drug delivery in cancer therapy. Immunol. Lett. 190, 64–83. 10.1016/j.imlet.2017.07.015 [DOI] [PubMed] [Google Scholar]
- Balunas M. J., Kinghorn A. D. (2005). Drug discovery from medicinal plants. Life Sci. 78 (5), 431–441. 10.1016/j.lfs.2005.09.012 [DOI] [PubMed] [Google Scholar]
- Baptista P. V., McCusker M. P., Carvalho A., Ferreira D. A., Mohan N. M., Martins M., et al. (2018). Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front. Microbiol. 9, 1441. 10.3389/fmicb.2018.01441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barabadi H., Damavandi Kamali K., Jazayeri Shoushtari F., Tajani B., Mahjoub M. A., Alizadeh A., et al. (2019). Emerging theranostic silver and gold nanomaterials to combat prostate cancer: A systematic review. J. Clust. Sci. 30 (6), 1375–1382. 10.1007/s10876-019-01588-7 [DOI] [Google Scholar]
- Barai A. C., Paul K., Dey A., Manna S., Roy S., Bag B. G., et al. (2018). Green synthesis of Nerium oleander-conjugated gold nanoparticles and study of its in vitro anticancer activity on MCF-7 cell lines and catalytic activity. Nano Converg. 5 (1), 10–19. 10.1186/s40580-018-0142-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj K. K., Rabha B., Pati S., Sarkar T., Choudhury B. K., Barman A., et al. (2021). Green synthesis of gold nanoparticles using plant extracts as beneficial prospect for cancer theranostics. Molecules 26 (21), 6389. 10.3390/molecules26216389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai W., Gao T., Hong H., Sun J. (2008). Applications of gold nanoparticles in cancer nanotechnology. Nanotechnol. Sci. Appl. 1, 17–32. 10.2147/nsa.s3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho P. M., Felício M. R., Santos N. C., Gonçalves S., Domingues M. M. (2018). Application of light scattering techniques to nanoparticle characterization and development. Front. Chem. 6, 237. 10.3389/fchem.2018.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahal A., Saini A. K., Chhillar A. K., Saini R. V. (2018). Natural antioxidants as defense system against cancer. Asian J. Pharm. Clin. Res. 11, 38–44. 10.22159/ajpcr.2018.v11i5.24119 [DOI] [Google Scholar]
- Chen J., Li Y., Fang G., Cao Z., Shang Y., Alfarraj S., et al. (2021). Green synthesis, characterization, cytotoxicity, antioxidant, and anti-human ovarian cancer activities of Curcumae kwangsiensis leaf aqueous extract green-synthesized gold nanoparticles. Arabian J. Chem. 14 (3), 103000. 10.1016/j.arabjc.2021.103000 [DOI] [Google Scholar]
- Chokkalingam M., Singh P., Huo Y., Soshnikova V., Ahn S., Kang J., et al. (2019). Facile synthesis of Au and Ag nanoparticles using fruit extract of Lycium chinense and their anticancer activity. J. Drug Deliv. Sci. Technol. 49, 308–315. 10.1016/j.jddst.2018.11.025 [DOI] [Google Scholar]
- Darroudi M., Gholami M., Rezayi M., Khazaei M. (2021). An overview and bibliometric analysis on the colorectal cancer therapy by magnetic functionalized nanoparticles for the responsive and targeted drug delivery. J. Nanobiotechnology 19 (1), 399–420. 10.1186/s12951-021-01150-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datkhile K. D., Patil S. R., Durgawale P. P., Patil M. N., Hinge D. D., Jagdale N. J., et al. (2021). Biogenic synthesis of gold nanoparticles using Argemone mexicana L. and their cytotoxic and genotoxic effects on human colon cancer cell line (HCT-15). J. Genet. Eng. Biotechnol. 19 (1), 9–11. 10.1186/s43141-020-00113-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divakaran D., Lakkakula J. R., Thakur M., Kumawat M. K., Srivastava R. (2019). Dragon fruit extract capped gold nanoparticles: Synthesis and their differential cytotoxicity effect on breast cancer cells. Mater. Lett. 236, 498–502. 10.1016/j.matlet.2018.10.156 [DOI] [Google Scholar]
- Duan L., Ouyang K., Xu X., Xu L., Wen C., Zhou X., et al. (2021). Nanoparticle delivery of CRISPR/Cas9 for genome editing. Front. Genet. 12, 673286. 10.3389/fgene.2021.673286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Magid A. D. A., AbdEl-Hamid O. M., Younes M. A. (2022). The biochemical effects of silver nanoparticles and spirulina extract on experimentally induced prostatic cancer in rats. Biol. Trace Elem. Res., 1–11. 10.1007/s12011-022-03298-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami F., Pathak S., Nguyen T. T., Shrestha P., Maharjan S., Kim J. O., et al. (2021). Photoimmunotherapy with cetuximab-conjugated gold nanorods reduces drug resistance in triple negative breast cancer spheroids with enhanced infiltration of tumor-associated macrophages. J. Control. Release 329, 645–664. 10.1016/j.jconrel.2020.10.001 [DOI] [PubMed] [Google Scholar]
- Faraji A. H., Wipf P. (2009). Nanoparticles in cellular drug delivery. Bioorg. Med. Chem. 17 (8), 2950–2962. 10.1016/j.bmc.2009.02.043 [DOI] [PubMed] [Google Scholar]
- Gajendran B., Chinnasamy A., Durai P., Raman J., Ramar M. (2014). Biosynthesis and characterization of silver nanoparticles from Datura inoxia and its apoptotic effect on human breast cancer cell line MCF7. Mater. Lett. 122, 98–102. 10.1016/j.matlet.2014.02.003 [DOI] [Google Scholar]
- Gavas S., Quazi S., Karpiński T. M. (2021). Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res. Lett. 16 (1), 173–221. 10.1186/s11671-021-03628-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He H., Liu L., Morin E. E., Liu M., Schwendeman A. (2019). Survey of clinical translation of cancer nanomedicines-Lessons learned from successes and failures. Acc. Chem. Res. 52, 2445–2461. 10.1021/acs.accounts.9b00228 [DOI] [PubMed] [Google Scholar]
- Hira I., Kumar A., Kumari R., Saini A. K., Saini R. V. (2018). Pectin-guar gum-zinc oxide nanocomposite enhances human lymphocytes cytotoxicity towards lung and breast carcinomas. Mat. Sci. Eng. C Mat. Biol. Appl. 90, 494–503. 10.1016/j.msec.2018.04.085 [DOI] [PubMed] [Google Scholar]
- Holliday D. L., Speirs V. (2011). Choosing the right cell line for breast cancer research. Breast Cancer Res. 13 (4), 215–217. 10.1186/bcr2889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M. L. (2011). Dietary polyphenols as antioxidants and anticancer agents: More questions than answers. Chang. Gung Med. J. 34 (5), 449–460. [PubMed] [Google Scholar]
- Jadhav K., Deore S., Dhamecha D., Hr R., Jagwani S., Jalalpure S., et al. (2018). Phytosynthesis of silver nanoparticles: Characterization, biocompatibility studies, and anticancer activity. ACS Biomater. Sci. Eng. 4 (3), 892–899. 10.1021/acsbiomaterials.7b00707 [DOI] [PubMed] [Google Scholar]
- Jeyaraj M., Sathishkumar G., Sivanandhan G., MubarakAli D., Rajesh M., Arun R., et al. (2013). Biogenic silver nanoparticles for cancer treatment: An experimental report. Colloids Surf. B Biointerfaces 106, 86–92. 10.1016/j.colsurfb.2013.01.027 [DOI] [PubMed] [Google Scholar]
- Jia G., Han Y., An Y., Ding Y., He C., Wang X., et al. (2018). NRP-1 targeted and cargo-loaded exosomes facilitate simultaneous imaging and therapy of glioma in vitro and in vivo . Biomaterials 178, 302–316. 10.1016/j.biomaterials.2018.06.029 [DOI] [PubMed] [Google Scholar]
- Kabir S. R., Dai Z., Nurujjaman M., Cui X., Asaduzzaman A. K. M., Sun B., et al. (2020). Biogenic silver/silver chloride nanoparticles inhibit human glioblastoma stem cells growth in vitro and Ehrlich ascites carcinoma cell growth in vivo . J. Cell. Mol. Med. 24 (22), 13223–13234. 10.1111/jcmm.15934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir S. R., Islam F., Asaduzzaman A. K. M. (2022). Biogenic silver/silver chloride nanoparticles inhibit human cancer cells proliferation in vitro and Ehrlich ascites carcinoma cells growth in vivo . Sci. Rep. 12 (1), 8909–8914. 10.1038/s41598-022-12974-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamala Priya M. R., Iyer P. R. (2015). Anticancer studies of the synthesized gold nanoparticles against MCF 7 breast cancer cell lines. Appl. Nanosci. 5 (4), 443–448. 10.1007/s13204-014-0336-z [DOI] [Google Scholar]
- Kashyap D., Tuli H. S., Yerer M. B., Sharma A., Sak K., Srivastava S., et al. (2021. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges, Seminars cancer Biol., 69, 5–23. [DOI] [PubMed] [Google Scholar]
- Khan A., Ali S., Murad W., Hayat K., Siraj S., Jawad M., et al. (2021). Phytochemical and pharmacological uses of medicinal plants to treat cancer: A case study from khyber pakhtunkhwa, north Pakistan. J. Ethnopharmacol. 281, 114437. 10.1016/j.jep.2021.114437 [DOI] [PubMed] [Google Scholar]
- Khan F. A., Aldahhan R., Almohazey D. (2021). Impact of gold nanoparticles on colon cancer treatment and diagnosis. Nanomedicine 16 (10), 779–782. 10.2217/nnm-2021-0043 [DOI] [PubMed] [Google Scholar]
- Kiran M. S., Betageri V. S., Kumar C. R., Vinay S. P., Latha M. S. (2020). In-vitro antibacterial, antioxidant and cytotoxic potential of silver nanoparticles synthesized using novel Eucalyptus tereticornis leaves extract. J. Inorg. Organomet. Polym. Mat. 30 (8), 2916–2925. 10.1007/s10904-020-01443-7 [DOI] [Google Scholar]
- Klochkov S. G., Neganova M. E., Nikolenko V. N., Chen K., Somasundaram S. G., Kirkland C. E., et al. (2021). Implications of nanotechnology for the treatment of cancer: Recent advances, Seminars cancer Biol., 69, 190–199. 10.1016/j.semcancer.2019.08.028 [DOI] [PubMed] [Google Scholar]
- Kowalczyk T., Merecz-Sadowska A., Rijo P., Mori M., Hatziantoniou S., Górski K., et al. (2022). Hidden in plants—a review of the anticancer potential of the Solanaceae family in in vitro and in vivo studies. Cancers 14 (6), 1455. 10.3390/cancers14061455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnaraj C., Muthukumaran P., Ramachandran R., Balakumaran M. D., Kalaichelvan P. T. (2014). Acalypha indica linn: Biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol. Rep. 4, 42–49. 10.1016/j.btre.2014.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Xu M., Chen Q., Guan G., Hu W., Zhao X., et al. (2015). Gold nanorods/mesoporous silica-based nanocomposite as theranostic agents for targeting near-infrared imaging and photothermal therapy induced with laser. Int. J. Nanomedicine 10, 4747–4761. 10.2147/IJN.S82940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo D., Chinnathambi A., Alahmadi T. A., Prabakaran D. S., Zhang G. (2022). Preparation, characterization and investigation of the anti-human ovarian cancer activity of silver nanoparticles green-formulated by Salvia officinalis leaf aqueous extract. Archives Med. Sci. 10.5114/aoms/145111 [DOI] [Google Scholar]
- Lv Y., Zou Y., Yang L. (2012). Uncertainty and sensitivity analysis of properties of phase change micro/nanoparticles for thermal protection during cryosurgery. Forsch. Ingenieurwes. 76 (1), 41–50. 10.1007/s10010-012-0153-z [DOI] [Google Scholar]
- Menaa F., Wijesinghe U., Thiripuranathar G., Althobaiti N. A., Albalawi A. E., Khan B. A., et al. (2021). Marine algae-derived bioactive compounds: A new Wave of nanodrugs? Mar. Drugs 19 (9), 484. 10.3390/md19090484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed A. E., Al-Megrin W. A. (2021). Biological potential of silver nanoparticles mediated by Leucophyllum frutescens and Russelia equisetiformis extracts. Nanomaterials 11 (8), 2098. 10.3390/nano11082098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollick M., Rahaman M., Bhowmick B., Maity D., Mondal D., Roy I., et al. (2014). Green synthesis of silver nanoparticles-based nanofluids and investigation of their antimicrobial activities. Microfluid. Nanofluidics 16 (3), 541–551. 10.1007/s10404-013-1252-3 [DOI] [Google Scholar]
- Mostafavi E., Zarepour A., Barabadi H., Zarrabi A., Truong L. B., Medina-Cruz D. (2022). Antineoplastic activity of biogenic silver and gold nanoparticles to combat leukemia: Beginning a new era in cancer theragnostic. Biotechnol. Rep. 34, e00714. 10.1016/j.btre.2022.e00714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundekkad D., Cho W. C. (2022). Nanoparticles in clinical translation for cancer therapy. Int. J. Mol. Sci. 23 (3), 1685. 10.3390/ijms23031685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumar T., Sambandam B., Aravinthan A., Sastry T. P., Kim J. H. (2016). Green synthesis of gold nanoparticles and their enhanced synergistic antitumor activity using HepG2 and MCF7 cells and its antibacterial effects. Process Biochem. 51 (3), 384–391. 10.1016/j.procbio.2015.12.017 [DOI] [Google Scholar]
- Navya P. N., Kaphle A., Srinivas S. P., Bhargava S. K., Rotello V. M., Daima H. K. (2019). Current trends and challenges in cancer management and therapy using designer nanomaterials. Nano Converg. 6 (1), 23–30. 10.1186/s40580-019-0193-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazer S., Andleeb S., Ali S., Gulzar N., Raza A., Khan H., et al. (2022). Cytotoxicity, anti-diabetic, and hepato-protective potential of Ajuga bracteosa-conjugated silver nanoparticles in balb/c mice. Curr. Pharm. Biotechnol. 23 (3), 318–336. 10.2174/1389201022666210421101837 [DOI] [PubMed] [Google Scholar]
- Padovani G. C., Feitosa V. P., Sauro S., Tay F. R., Durán G., Paula A. J., et al. (2015). Advances in dental materials through nanotechnology: Facts, perspectives and toxicological aspects. Trends Biotechnol. 33 (11), 621–636. 10.1016/j.tibtech.2015.09.005 [DOI] [PubMed] [Google Scholar]
- Pechyen C., Ponsanti K., Tangnorawich B., Ngernyuang N. (2022). Biogenic synthesis of gold nanoparticles mediated by Spondias dulcis (Anacardiaceae) peel extract and its cytotoxic activity in human breast cancer cell. Toxicol. Rep. 9, 1092–1098. 10.1016/j.toxrep.2022.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M., Lambe U. P., Brar B., Shah I., Manimegalai J., Ranjan K., et al. (2018). Nanotherapeutics: An insight into healthcare and multi-dimensional applications in medical sector of the modern world. Biomed. Pharmacother. 97, 1521–1537. 10.1016/j.biopha.2017.11.026 [DOI] [PubMed] [Google Scholar]
- Rafique M., Sadaf I., Rafique M. S., Tahir M. B. (2017). A review on green synthesis of silver nanoparticles and their applications. Artif. Cells Nanomed. Biotechnol. 45 (7), 1272–1291. 10.1080/21691401.2016.1241792 [DOI] [PubMed] [Google Scholar]
- Rahuman H. B. H., Dhandapani R., Palanivel V., Thangavelu S., Paramasivam R., Muthupandian S. (2021). Bioengineered phytomolecules-capped silver nanoparticles using Carissa carandas leaf extract to embed on to urinary catheter to combat UTI pathogens. PloS one 16 (9), e0256748. 10.1371/journal.pone.0256748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj S., Manchanda R., Bhandari M., Alam M. (2022). Review on natural bioactive products as radioprotective therapeutics: Present and past perspective. Curr. Pharm. Biotechnol. 23, 1721–1738. 10.2174/1389201023666220110104645 [DOI] [PubMed] [Google Scholar]
- Rajivgandhi G. N., Chackaravarthi G., Ramachandran G., Chelliah C. K., Maruthupandy M., Alharbi M. S., et al. (2022a). Morphological damage and increased ROS production of biosynthesized silver nanoparticle against MCF-7 breast cancer cells through in vitro approaches. J. King Saud Univ. - Sci. 34 (2), 101795. 10.1016/j.jksus.2021.101795 [DOI] [Google Scholar]
- Rajivgandhi G. N., Ramachandran G., Kannan M. R., Velanganni A. A. J., Siddiqi M. Z., Alharbi N. S., et al. (2022b). Photocatalytic degradation and anti-cancer activity of biologically synthesized Ag NPs for inhibit the MCF-7 breast cancer cells. J. King Saud Univ. - Sci. 34 (1), 101725. 10.1016/j.jksus.2021.101725 [DOI] [Google Scholar]
- Ramezani T., Nabiuni M., Baharara J., Parivar K., Namvar F. (2019). Sensitization of resistance ovarian cancer cells to cisplatin by biogenic synthesized silver nanoparticles through p53 activation. Iran. J. Pharm. Res. 18 (1), 222–231. [PMC free article] [PubMed] [Google Scholar]
- Raza M. A., Kanwal Z., Rauf A., Sabri A. N., Riaz S., Naseem S. (2016). Size-and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials 6 (4), 74. 10.3390/nano6040074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren W., Qiao Z., Wang H., Zhu L., Zhang L. (2003). Flavonoids: Promising anticancer agents. Med. Res. Rev. 23 (4), 519–534. 10.1002/med.10033 [DOI] [PubMed] [Google Scholar]
- Rousta M. H., Ghasemi N. (2019). Green synthesis of silver nanoparticles using a mountain plant extract. Rev. Roum. Chim. 64 (2), 143–152. 10.33224/rrch/2019.64.2.04 [DOI] [Google Scholar]
- Sani A., Hassan D., Khalil A. T., Mughal A., El-Mallul A., Ayaz M., et al. (2021). Floral extracts-mediated green synthesis of NiO nanoparticles and their diverse pharmacological evaluations. J. Biomol. Struct. Dyn. 39 (11), 4133–4147. 10.1080/07391102.2020.1775120 [DOI] [PubMed] [Google Scholar]
- Sargazi S., Laraib U., Er S., Rahdar A., Hassanisaadi M., Zafar M. N., et al. (2022). Application of green gold nanoparticles in cancer therapy and diagnosis. Nanomaterials 12 (7), 1102. 10.3390/nano12071102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar J., Ray S., Chattopadhyay D., Laskar A., Acharya K. (2012). Mycogenesis of gold nanoparticles using a phytopathogen Alternaria alternata . Bioprocess Biosyst. Eng. 35 (4), 637–643. 10.1007/s00449-011-0646-4 [DOI] [PubMed] [Google Scholar]
- Sarkar J., Saha S., Chattopadhyay D., Patra S., Acharya K. (2011). Mycosynthesis of silver nanoparticles and investigation of their antimicrobial activity. J. Nanosci. Nanoeng. App 1, 17–26. [Google Scholar]
- Sathishkumar G., Gobinath C., Wilson A., Sivaramakrishnan S. (2014). Dendrophthoe falcata (lf) ettingsh (neem mistletoe): A potent bioresource to fabricate silver nanoparticles for anticancer effect against human breast cancer cells (MCF-7). Spectrochim. Acta. A Mol. Biomol. Spectrosc. 128, 285–290. 10.1016/j.saa.2014.02.096 [DOI] [PubMed] [Google Scholar]
- Saygi K. O., Cacan E. (2021). Antioxidant and cytotoxic activities of silver nanoparticles synthesized using Tilia cordata flowers extract. Mater. Today Commun. 27, 102316. 10.1016/j.mtcomm.2021.102316 [DOI] [Google Scholar]
- Seca A. M., Pinto D. C. (2018). Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic applicationPlant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci.International J. Mol. Sci. 1919 (11), 263263. 10.3390/ijms19010263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Yang X., Li Y., Wang D., Liu W., Zhang Z., et al. (2020). MicroRNA-responsive release of Cas9/sgRNA from DNA nanoflower for cytosolic protein delivery and enhanced genome editing. Biomaterials 256, 120221–221. 10.1016/j.biomaterials.2020.120221 [DOI] [PubMed] [Google Scholar]
- Singh J., Dutta T., Kim K. H., Rawat M., Samddar P., Kumar P. (2018). ‘Green’synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnology 16 (1), 84–24. 10.1186/s12951-018-0408-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Das M. K., Ansari A., Mohanta D., Rajamani P. (2021). Biogenic nanosized gold particles: Physico-chemical characterization and its anticancer response against breast cancer. Biotechnol. Rep. 30, e00612. 10.1016/j.btre.2021.e00612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Pandit S., Mokkapati V. R. S. S., Garg A., Ravikumar V., Mijakovic I. (2018). Gold nanoparticles in diagnostics and therapeutics for human cancer. Int. J. Mol. Sci. 19 (7), 1979. 10.3390/ijms19071979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sm F. M. B., Chitra K., Joseph B., Sundararajan R. (2018). Gelidiella acerosa inhibits lung cancer proliferation. BMC Complement. Altern. Med. 18 (1), 104–114. 10.1186/s12906-018-2165-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganya U. S., Govindaraju K., Prabhu D., Arulvasu C., Karthick V., Changmai N., et al. (2016). Anti-proliferative effect of biogenic gold nanoparticles against breast cancer cell lines (MDA-MB-231 & MCF-7). Appl. Surf. Sci. 371, 415–424. 10.1016/j.apsusc.2016.03.004 [DOI] [Google Scholar]
- Sukumar D. T., Gunasangkaran G., Arumugam V. A., Muthukrishnan S. (2022). Effects of biogenic synthesis of chitosan entrapped silver nanoparticle from Aegle marmelos on human cervical cancer cells (HeLa). J. Drug Deliv. Sci. Technol. 70, 103189. 10.1016/j.jddst.2022.103189 [DOI] [Google Scholar]
- Sulaiman G. M., Waheeba H. M., Al-Shmgani H., Eassa H. A., Al-Amiery A. A., Jabir M. S., et al. (2022). Synthesis, molecular modeling, DNA damage interaction, and antioxidant potential of hesperidin loaded on gold nanoparticles. J. Biomimetics, Biomaterials Biomed. Eng. 54, 17–29. 10.4028/www.scientific.net/jbbbe.54.17 [DOI] [Google Scholar]
- Sunderam V., Thiyagarajan D., Lawrence A. V., Mohammed S. S. S., Selvaraj A. (2019). In-vitro antimicrobial and anticancer properties of green synthesized gold nanoparticles using Anacardium occidentale leaves extract. Saudi J. Biol. Sci. 26 (3), 455–459. 10.1016/j.sjbs.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinajero-Díaz E., Salado-Leza D., Gonzalez C., Martínez Velázquez M., López Z., Bravo-Madrigal J., et al. (2021). Green metallic nanoparticles for cancer therapy: Evaluation models and cancer applications. Pharmaceutics 13 (10), 1719. 10.3390/pharmaceutics13101719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuasha N., Petros B., Asfaw Z. (2018). Medicinal plants used by traditional healers to treat malignancies and other human ailments in Dalle District, Sidama Zone, Ethiopia. J. Ethnobiol. Ethnomed. 14 (1), 15–21. 10.1186/s13002-018-0213-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzma M., Sunayana N., Raghavendra V. B., Madhu C. S., Shanmuganathan R., Brindhadevi K. (2020). Biogenic synthesis of gold nanoparticles using Commiphora wightii and their cytotoxic effects on breast cancer cell line (MCF-7). Process Biochem. 92, 269–276. 10.1016/j.procbio.2020.01.019 [DOI] [Google Scholar]
- Vijayakumar S. (2019). Eco-friendly synthesis of gold nanoparticles using fruit extracts and in vitro anticancer studies. J. Saudi Chem. Soc. 23 (6), 753–761. 10.1016/j.jscs.2018.12.002 [DOI] [Google Scholar]
- Wei S., Wang Y., Tang Z., Xu H., Wang Z., Yang T., et al. (2021). A novel green synthesis of silver nanoparticles by the residues of Chinese herbal medicine and their biological activities. RSC Adv. 11 (3), 1411–1419. 10.1039/d0ra08287b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. L., Strandberg K. R., Croley C. R., Fraser S. E., Venkata K. C. N., Fimognari C., et al. (2021). Pomegranate bioactive constituents target multiple oncogenic and oncosuppressive signaling for cancer prevention and intervention. Semin. Cancer Biol. 73, 265–293. 10.1016/j.semcancer.2021.01.006 [DOI] [PubMed] [Google Scholar]
- Yang Z., Gao D., Cao Z., Zhang C., Cheng D., Liu J., et al. (2015). Drug and gene co-delivery systems for cancer treatment. Biomater. Sci. 3 (7), 1035–1049. 10.1039/c4bm00369a [DOI] [PubMed] [Google Scholar]
- Yazdi M. H., Sepehrizadeh Z., Mahdavi M., Shahverdi A. R., Faramarzi M. A. (2016). Metal, metalloid, and oxide nanoparticles for therapeutic and diagnostic oncology. Nano Biomed. Eng. 8 (4), 246–267. 10.5101/nbe.v8i4.p246-267 [DOI] [Google Scholar]
- Zare I., Yaraki M. T., Speranza G., Najafabadi A. H., Haghighi A. S., Nik A. B., et al. (2022). Gold nanostructures: Synthesis, properties, and neurological applications. Chemical society reviews, Royal Society of Chemistry, United Kingdom, [DOI] [PubMed] [Google Scholar]
- Zhao P., El-kott A., Ahmed A. E., Khames A., Zein M. A. (2021). Green synthesis of gold nanoparticles (Au NPs) using Tribulus terrestris extract: Investigation of its catalytic activity in the oxidation of sulfides to sulfoxides and study of its anti-acute leukemia activity. Inorg. Chem. Commun. 131, 108781. 10.1016/j.inoche.2021.108781 [DOI] [Google Scholar]