Abstract
The clinically important serogroups B, C, Y, and W-135 of Neisseria meningitidis produce sialic acid capsules that are critical in pathogenesis. In each of these serogroups, the capsule transport (ctrABCD) and capsule biosynthesis (synABCD) operons are divergently transcribed from putative promoters located in a 134-bp intergenic region (J. S. Swartley, J. H. Ahn, L. J. Liu, C. M. Kahler, and D. S. Stephens, J. Bacteriol. 178:4052–4059, 1996). In this study we further assessed the role of the intergenic sequence in the transcriptional regulation of the sialic acid capsules of N. meningitidis. Insertional mutagenesis or deletions of the 134-bp sequence in the serogroup B meningococcal strain NMB resulted in a marked reduction or elimination of ctrABCD and synABCD transcription, with a concomitant loss of encapsulation. Chromosomal transcriptional lacZ-ermC reporter fusions of syn and ctr promoters were constructed through allelic exchange. Using these constructs, both operons were found to be constitutively transcribed in meningococci, the biosynthesis operon about fourfold higher than the transport operon. Both promoters showed increased activity during stationary-phase growth. In addition to the promoters, a 70-bp 5′ untranslated region (UTR) upstream of synA was found to have a direct repeat and an inverted repeat that overlapped three putative integration host factor binding sites. Mutation of this 70-bp UTR and of the direct repeat upregulated both syn and ctr transcription. Regulation through the synA UTR was absent in a K1 Escherichia coli strain that produces identical capsular polysaccharide, implicating species-specific regulation. Meningococcal sialic acid capsule expression is initiated by divergent promoters in a 134-bp intergenic region, is repressed at the transcriptional level by the 5′ UTR of synA, is increased during stationary-phase growth, and shows species-specific regulation. Transcriptional regulation is another important control point for sialic capsule expression in N. meningitidis.
Capsular polysaccharide is a major virulence factor of Neisseria meningitidis. Of the 12 different meningococcal capsular polysaccharides so far defined, 5 (serogroups A, B, C, Y, and W-135) are most often associated with invasive disease. With the exception of serogroup A, these capsules are polymers of or contain sialic acid. Capsular polysaccharides protect the meningococcus from a variety of cellular and humoral host immune defenses, including phagocytosis, opsonization, and complement-mediated killing (16, 17), and allow survival during invasive meningococcal disease. In addition, the (α2→8)-linked polysialic acid capsule of serogroup B meningococci is a poor immunogen due to structural identity with surface antigens of human tissues such as the neural cell adhesion molecule N-CAM (34). Capsules also have an important role in meningococcal transmission by facilitating loss of meningococci from human mucosal surfaces and protecting the organism from environmental stress. During other events in human pathogenesis (e.g., nasopharyngeal colonization and attachment to epithelial and endothelial cells), meningococci that have downregulated or switched off capsule are selected (27, 35–37).
The genetic basis for the control of expression of meningococcal capsule has been partially elucidated. Frosch et al. (9) identified a 24-kb cps gene complex that encodes factors necessary for the expression of serogroup B capsule when cloned into Escherichia coli. We also defined this region in N. meningitidis by Tn916 mutagenesis (28, 30, 33). Subsequent work has identified the genetic basis for the different meningococcal capsular polysaccharides (5, 32) and shown that regions A and C of the cps complex are critically involved in expression of the serogroups A, B, C, Y, and W-135 capsules (30, 32). For the sialic acid capsule-producing meningococci, region A consists of four polycistronic capsule biosynthetic genes, synABCD, while region C contains ctrABCD, responsible for capsule transport across the inner and outer membranes (Fig. 1A). We have shown that synABCD and ctrABCD are operons separated by a 134-bp intergenic region that contains putative promoters that initiate divergent transcription from adjacent start sites (Fig. 1B). Examination of the nucleotide sequences surrounding the transcriptional start sites (30) of both operons showed that the synA promoter had identity with the ς70 class of constitutive promoters, while ctrA was preceded by a perfect −10 extended promoter (19). Transcriptional activity of the putative promoters was confirmed when they were cloned in front of a lacZ reporter in E. coli, with the synA promoter exhibiting higher activity (30).
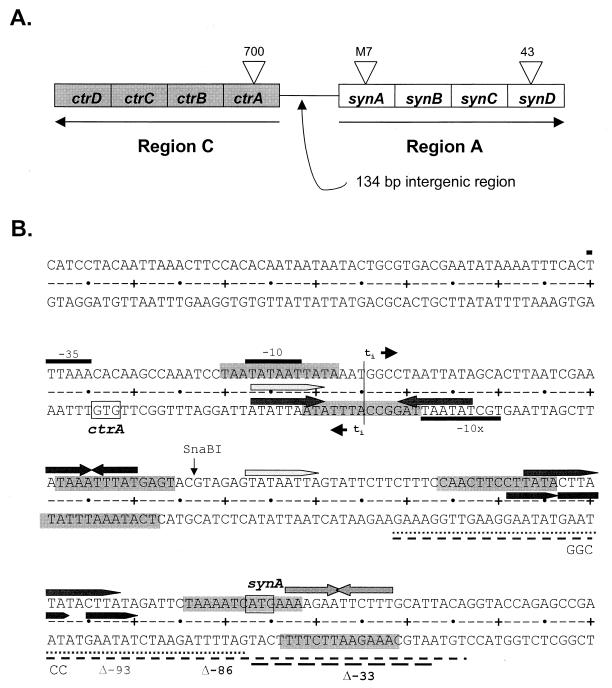
FIG. 1.
(A) Schematic diagram of region A (biosynthesis operon, synA/B/C/D) and region C (capsule transport operon, ctrA/B/C/D) of the capsule locus in N. meningitidis with the locations of Tn916 mutations (700, M7, and 43) noted (30). Arrows indicate the directions of transcription and the start codons of synA and ctrA separated by a 134-bp intergenic region. (B) Nucleotide sequence of the intergenic region from −95 to +145 of synA is shown. The −10 and −35 elements of the synA promoter are indicated above the sequence, while the extended −10 (−10x) sequence of ctrA is indicated below the sequence. The start codons for synA (ATG) and ctrA (GTG) are boxed. A vertical line marks the adjacent transcription initiation sites. The deletions of this region in mutants S(C)A286(Δ−86), S(C)A293(Δ−93), and S(C)A233(Δ−33) are indicated by broken lines underneath the sequence, and the base substitutions (GGCCC) in mutant S(C)A247 are specified below the sequence. Solid arrows above the sequence show the locations of inverted (IR) and direct (DR) repeats. Putative IHF binding sites are shaded in gray.
In this study we further investigated the hypothesis that the intergenic region separating the biosynthetic and capsule transport operons is critical for transcriptional regulation of serogroup B meningococcal capsule expression. Deletion, insertion, and site-directed mutagenesis of the intergenic region was performed, and transcriptional reporter gene fusions were constructed in order to define the role of the region in transcriptional regulation of meningococcal capsule expression.
MATERIALS AND METHODS
Strains and growth conditions.
Strains used in this study are listed in Table 1. Meningococcal strains were grown on GC base agar (Difco Laboratories) supplemented with glucose and iron at 37°C with 3.5% carbon dioxide. Liquid cultures were vigorously aerated in GC broth with the same supplements and 4.3% sodium bicarbonate at 37°C. β-Galactosidase expression in reporter constructs was assayed using the Miller method (21). Meningococcal transformants containing the lacZ-ermC constructs were selected and maintained in the presence of erythromycin (3 μg/ml) (Sigma). E. coli strains were grown in Luria-Bertani (LB) broth (Bethesda Research Laboratories) at 37°C with appropriate antibiotic selection (erythromycin, 300 μg/ml; spectinomycin, 100 μg/ml; kanamycin, 50 μg/ml; and ampicillin, 100 μg/ml).
TABLE 1.
Strains used in this study
| Strain | Relevant characteristicsa | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | Cloning host | 25 |
| CAB1 | K1 E. coli clinical isolate | Laboratory collection |
| N. meningitidis | ||
| NMB | B:2B:P1.2,5:L2 (CDC8201085), parent strain for all following constructs | 28 |
| 43 | synD::Tn916, class I insertion | 30 |
| M7 | synA::Tn916, class II insertion | 33 |
| 700 | ctrA::Tn916, class II insertion | 30 |
| ΩINT1 | Ω(Spr) cassette inserted into SnaBI site within intergenic region | This study |
| ΔΩINT1 | Same as ΩINT1 but with sequence between JS75 and JS74 primers deleted | This study |
| ΩINT2 | Ω(Spr) cassette inserted into EcoRI site 10 bp downstream of synA ATG start codon | This study |
| ΔΩINT2 | Same as ΩINT2 but with sequence between JS94 and JS93 primers deleted | This study |
| SA1 | lacZ-erm cassette inserted into SnaBI site within intergenic region | This study |
| SA2 | lacZ-erm cassette inserted into SnaBI site created within synA 339 bp from TTS | This study |
| CA2 | lacZ-ermC cassette inserted into SspI site within ctrA, 285 bp from TTS | This study |
| SA286 | SA2 strain with 70 bp between primers JS56 and JS86 deleted, removing both DR and IR | This study |
| CA286 | CA2 strain with same deletion as SA286 | This study |
| SA293 | SA2 strain with 46 bp between primers JS56 and JS93 deleted, removing only DR | This study |
| CA293 | CA2 strain with same deletion as SA293 | This study |
| SA233 | SA2 strain with 20 bp downstream of synA ATG start codon deleted, removing only IR | This study |
| CA233 | CA2 strain with same deletion as SA233 | This study |
| SA247 | SSA2 strain with 5 bp (TTATA) mutated to CCGGG within DR | This study |
| CA247 | CA2 strain with the same mutation as SA247 | This study |
| 988 | lacZ-ermC cassette inserted into chromosomal locus 120A1 | This study |
| SA295 | syn promoter fragment containing 95 bp upstream of TTS fused to lacZ-ermC cassette and integrated into 120A1 locus | This study |
TTS, transcriptional start site; DR, direct repeat; IR, inverted repeat.
Transformation.
Meningococci were transformed with DNA by the technique of Janik et al. (15). E. coli transformation was performed using the chemical transformation method described by Chung and Miller (4) or electroporation with a GenePulser (Bio-Rad).
DNA constructs.
To create mutations in the 134-bp intergenic region, a 900-bp PCR product of primers LJ4 and JS44 (Table 2), containing the intergenic region and the 5′ ends of both the ctrA and synA genes, was cloned into pCR2.1 (Invitrogen) or pGEM-T (Promega), yielding pTINT and pGINT, respectively. An Ω-spectinomycin cassette with strong transcriptional and translational terminators on either side was obtained from pHP45Ω (23). A SmaI fragment of Ω was inserted into the SnaBI site of pTINT and the EcoRI-Ω fragment was inserted into the EcoRI site of pGINT to generate pΩINT1 and pΩINT2, respectively. These plasmids were used to transform N. meningitidis strain NMB, and the spectinomycin-resistant transformants NMBΩINT1 and NMBΩINT2, respectively, were isolated. To create the NMBΔΩINT1 mutant, a ligation of the LJ4-JS75 and JS74-JS44 PCR products was used as a template and reamplified with external primers LJ4 and JS44. The resulting PCR product of the expected size was cloned into pCR2.1, and the Ω cassette was subsequently inserted into the SnaBI site to give pΔΩINT1. pΔΩINT1 was then used to transform strain NMB as described above. Analogously, the NMBΔΩINT2 mutant was generated by the LJ4-JS94 and JS93-JS73 pairs and cloned into pGEM, followed by the insertion of the Ω cassette into the EcoRI site. The mutations were verified by PCR amplification, Southern hybridization, and sequencing analysis.
TABLE 2.
Primers used in this study
| Primer | Sequence |
|---|---|
| JS41 | GGCCACTTAATTCTAATGCTTTG |
| JS44 | GCTTGTTCATTTGCTACCAAGTGG |
| JS56 | GAATACTAATTATACTCTACGTACTC |
| JS73 | GAGAGATAAACGTAATGGTATTGC |
| JS74 | GAGTACGTAGAGTATAATTAGTATTC |
| JS75 | GTGTGGAAGTTTAATTGTAGGATG |
| JS86 | GGTACCAGAGCCGACTTCGG |
| JS93 | ATGAAAAGAATTCTTTGCATTACAGG |
| JS94 | GTGTTTAAACTGAAATTTTATATTCGTC |
| LJ4 | CCACCACCAAACAARACTGCCG |
| RN3 | CAATACCATTACGTATATCTCTCG |
| RN7 | CCAGCCGAAGCATAACCATCG |
| RN8 | GAGAGATATACGTAATGGTATTGCC |
| YT01 | CGGCTCTGGTACCTGTAGATTTTAGAATCT |
| YT03 | CGATTGGGACGATATGACGG |
| YT05 | GCCTGAGTGCCATCGCGCAT |
| YT29 | TTATTTGTCGTCGTCGTCTTTGTAGTCATTAGTTAAATTATTAATACTGTTCGCGCC |
| YT30 | GAGTAATTAAGAAGCTTGAGCAACTTCCTG |
| YT33 | AGATTCTAAAATCTACAGGTACCAGAGCCG |
| YT47 | GAATCTATAAGTACCCGGGTATAAGGAAGTTGG |
To create the ctrA::lacZ (CA2) and synA::lacZ (SA1) reporter constructs, a PCR product of JS99-JS56 primers containing a unique SspI site within ctrA gene and a PCR product of LJ4-JS73 primers with a unique SnaBI site downstream of the synA transcriptional start site was cloned into pCR2.1. The appropriate transformants were digested with SspI or SnaBI and ligated with the BamHI-blunted lacZ-ermC cassette, which contains a transcriptional terminator downstream of ermC, from pAErmC′G (22, 39). The constructs were transformed into E. coli strain DH5α, and transformants were selected on LB agar containing 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) and erythromycin. The colonies were further tested for correct orientation of the lacZ gene relative to the transcription. The lacZ-ermC cassette was then integrated into wild-type meningococcal strain NMB via transformation and allelic exchange, with selection for erythromycin-resistant NMB transformants.
A second synA::lacZ fusion (SA2) was generated downstream of the 20-bp inverted repeat. A unique SnaBI site was created by means of the PCR-ligation mutagenesis method of Ali and Steinkasserer (1). First, two halves of the synA-specific PCR products were amplified using special internal primers (JS74-RN8 and RN3-JS41) designed to introduce a new SnaBI restriction site into the synA sequence. These products were purified and mixed together (60 ng of each) to serve as the template for a secondary PCR amplification using nested primers (JS86-JS44). This product was ligated into pGEM and then transformed into E. coli. Colonies with the appropriate size insert were selected and digested with SnaBI to determine which insert had the new restriction site. Next, a blunt copy of the lacZ-ermC cassette was inserted into this new SnaBI site. This construct was transformed into NMB as described above.
Specific deletions of the intergenic region were created using similar PCR-ligation mutagenesis procedures. The sequence between primers JS56 and JS86 was removed in strains SA286 and CA286. The sequence between primers JS56 and JS93 was deleted in strains SA293 and CA293. Mutagenic primers YT01 and YT33 were used for removing the 20-bp palindromic sequence in strains SA233 and CA233, while the primer YT47, which contains a 5-base substitution to disrupt the direct repeat, was used for generating mutants SA247 and CA247.
Two promoter fragments, one between RN7 and JS73 and the other between LJ8 and JS56, were obtained by PCR and cloned into pBlue-Topo vector (Invitrogen) to generate transcriptional lacZ reporters. The promoter and lacZ fusions were released by SpeI-XbaI digestion and subcloned into pHP45 (SmaI) to give pYT140S and pYT141S. The resulting plasmids, which have the same promoter fragment as SA2 and SA1, were transformed into E. coli K1 strain CAB1. An ∼5-kb PCR product that contains the synA promoter lacZ-erm fusion was amplified by Taq polymerase (Perkin-Elmer) and Taq Extender (Stratagene) from strain SA2 using primers LJ6 and JS44. This PCR fragment was cloned into an integration plasmid, which contained ∼1 kb of intergenic meningococcal sequence. Incorporation of the PCR product within the HincII site of this sequence allowed homologous recombination into a chromosomal location that is distant from the cps locus. The double-crossover recombination into this locus and the intactness of the cps locus were confirmed by PCR.
RNA isolation and slot blots.
Total RNA was purified according to the published procedure (30). RNA samples were denatured in 500 μl of cold denaturing buffer (10 mM NaOH, 1 mM EDTA) and immediately transferred to a Zeta-Probe GT membrane with a PR648 slot blot filtration manifold (Hoefer Scientific). The wells were rinsed once with 500 μl of denaturing buffer. The blotted membrane was rinsed in 2X SSC (1x SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) before prehybridization for 5 min at 65°C in P buffer, composed of 0.5 M NaHPO4 (pH 7.2), 1 mM EDTA, and 7% SDS. The membrane was hybridized with denatured DNA probe in P buffer overnight at 65°C. After hybridization, the membrane was washed twice in E buffer (40 mM NaHPO4 [pH 7.2], 1 mM EDTA) containing 5% SDS and then twice in E buffer with 1% SDS, all at 65°C. The membrane was then subjected to autoradiography. The probe for synA transcription was PCR amplified from chromosomal DNA using JS44 and JS86 and labeled by random-primed labeling (Boehringer Mannheim) with [32P]dATP (NEN DuPont).
Colony immunoblots.
Colony immunoblots were performed essentially as described by Swartley et al. (30). Wild-type strain NMB served as the positive control for encapsulation, while the capsule-negative mutant strain M7 (28) was the negative control. The primary anti-serogroup B capsular monoclonal antibody 2-2-B was generously supplied by Wendell Zollinger (Walter Reed Army Institute of Research). The secondary antibody was goat anti-mouse immunoglobulin M (IgM)-IgG-alkaline phosphatase conjugate (Jackson Immunochemicals). The membranes were developed with NBT-BCIP (nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate).
Whole-cell ELISA.
Serogroup B capsule-specific monoclonal antibody 2-2-B was employed in the whole-cell enzyme-linked immunosorbent assay (ELISA) following the published protocol (31) with minor modifications; 100 μl of a 1:3 dilution of the cell suspension (optical density at 650 nm [OD650] = 0.1) was added to the microtiter plates. Incubation was done at 37°C instead of 33°C.
Statistical analysis.
Student's t test with a two-tailed hypothesis was used to determine the significant difference (P ≤ 0.05) between two variables in these studies.
RESULTS
Insertion and deletion mutagenesis confirmed that the ctrA-synA intergenic region is required for production and expression of serogroup B meningococcal capsular polysaccharide.
A series of controlled deletion and Ω-spectinomycin cassette insertions in the 134-bp ctrA-synA intergenic region were created as described in Materials and Methods and are shown schematically in Fig. 2A. The production and expression of capsule were quantified by whole-cell ELISAs using monoclonal antibody 2-2-B, and the results are shown in Fig. 2B. The mutations within the intergenic region eliminated or dramatically reduced capsular polysaccharide expression (P < 0.006 for each mutant), indicating the essential role of this region in controlling the production and transport of capsule.
FIG. 2.
(A) Schematic diagram of the capsule biosynthesis and transport operon intergenic region. The locations of various primers (half-arrows) used in this study are noted below the diagram. The shaded bar is the 134-bp intergenic region between the two start codons of synA and ctrA. Base numbering is from the corresponding transcriptional start sites, which are indicated by bent arrows. The repeats examined in mutagenesis studies are also shown as arrows. The Ω cassette was inserted into the SnaBI site within the 5′ UTR of synA in the two INT1 constructs, whereas the two INT2 constructs were created with the Ω cassette inserted at the start of the coding sequence. In addition, the sequence between primers JS75 and JS74 was deleted in the ΔΩINT1 strain, while the sequence between JS94 and JS93 was removed in the ΔΩINT2 construct. (B) Capsule whole-cell ELISA of wild-type strain NMB and meningococcal mutants ΩINT1, ΩINT2, ΔΩINT1, and ΔΩINT2. An unencapsulated synA::Tn916 mutant (28) was used as the negative control. A ctrA::Tn916 mutant, 700 (30), is included for comparison. Results are shown as the average values from three independent experiments.
Transcription of ctr and syn operons is initiated from the intergenic region.
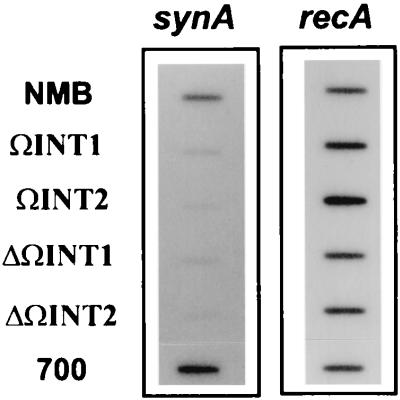
The capsule-specific ELISA results suggested that capsule production is initiated within the intergenic region through transcriptional activation of the syn and ctr operons. To further confirm this hypothesis, changes in synA transcriptional level were examined by RNA slot blots. As shown in Fig. 3, the mutations within the 134-bp intergenic region reduced synA RNA expression. However, the synA mRNA signal from the ctrA mutant 700 (ctrA::Tn916), in which the transposon was at the bp 285 location of ctrA (−285 bp relative to the transcriptional start site of synA), yielded a synA mRNA signal similar to that of the wild-type strain. This result indicated that the 285-bp sequence upstream of the synA transcriptional start site was sufficient to control synA expression and the inactivation of ctrA had no significant influence on synA transcription.
FIG. 3.
RNA slot blots of synA transcription. Total-cell RNA was purified from parental strain NMB and mutants ΩINT1, ΩINT2, ΔΩINT1, and ΔΩINT2, as described in Materials and Methods. The mRNA level of recA was also measured and used as a control for RNA loading.
syn promoter is constitutively more active than ctr promoter, and transcription from both promoters is growth phase dependent.
To further investigate the relative strength and possible mechanisms of regulation of the syn and ctr promoters, a lacZ-ermC cassette was inserted downstream of both promoters, creating transcriptional reporter strains. Two syn reporter strains were constructed, one with lacZ inserted in the synA coding region (SA2), and the other with lacZ in the 5′ untranslated region (UTR) of synA (SA1) (Fig. 2A). The ctr promoter strain (CA2) contained lacZ in the coding region. Overnight cultures of the meningococcal reporter strains were diluted into fresh GC broth and grown with aeration at 37°C, and the expression of β-galactosidase activity was monitored (Fig. 4A). The ctr promoter reporter strain (CA2) produced low levels of activity that increased moderately (∼2-fold) when entering the stationary phase. The SA2 syn construct gave an expression pattern similar to that of CA2, but was about fourfold higher (P < 0.05). The SA1 syn construct consistently yielded ∼2-fold higher activity than that of the SA2 reporter strain throughout growth (P < 0.05). These results suggested that the sequence between the insertion sites of SA1 and SA2 had a negative or downregulating role in syn transcription, yet the growth phase-dependent regulation was not located in this region, since SA1 still produced an ∼2-fold increase in the stationary phase.
FIG. 4.
(A) Expression of a lacZ reporter located in synA in SA1 (diamonds) or SA2 (squares) or in ctrA in CA2 (triangles) during growth. Each time point shows the average value of three experimental measurements, and data are representative of three independent assays. (B) Expression of synA::lacZ reporters during mid-log growth phase (OD550 ≈ 0.5) in different meningococcal backgrounds: 1, SA2, wild type; 2, SA2 in a ctrA::Ω background; 3, SA295, synA::lacZ with −95 to +339 promoter fragment of synA fused with lacZ-ermC and integrated into a noncoding chromosomal locus and retaining an intact capsule A and C region; 4, SA295 in a synA::Tn916 background; 5, lacZ-ermC cassette without promoter integrated into the same locus as SA295.
Determination of minimal promoter region required for syn transcription.
As described above, a Tn916 insertion (ctrA mutant 700) at bp −285 of the synA transcription start site did not alter the synA mRNA level in RNA slot blot experiments (Fig. 3). This result was also confirmed by the transcriptional reporter assays. As shown in Fig. 4B, the SA2 syn reporter produced similar activity in either the wild-type or ctrA::Ω background, indicating that no cis element was present beyond −285 bp. To confirm these results, a lacZ transcriptional fusion construct of synA was also generated at a distant heterologous chromosomal locus, site 120A1, which has no intrinsic promoter activity. This construct, SA295, has an intact capsule locus and contains at the distant site the lacZ reporter and the −95 bp upstream sequence of the synA transcriptional start site. The SA295 construct had ∼80% of the transcriptional activity of the SA2 strain. These results suggested that most of the required cis transcriptional determinants for the syn operon resided in the 95 bp upstream of the synA transcriptional start site.
syn and ctr operons do not appear to be influenced by product inhibition or feedback regulation.
Many biosynthesis pathways utilize product inhibition or feedback regulation to control overall transcription and product synthesis. For example, the expression of capsule transport proteins could be regulated by the biosynthesis operon substrates (e.g., expression of the ctrABCD transport operon may be influenced by the amount of sialic acid or capsule polymer synthesized by the synABCD gene products). To test whether feedback regulation influenced the meningococcal capsule region, ctr transcriptional fusions were generated in backgrounds with biosynthesis operon mutations, synA::Tn916, synC::Ω or synD::Tn916, and the corresponding transcriptional activity was compared to that in constructs without these mutations. No significant difference in transcriptional activities was noted in these backgrounds (data not shown). Similarly, disruption of ctrA with insertion of an Ω cassette did not affect syn operon expression (Fig. 4B). In addition, the production and expression of capsule did not influence syn operon expression, since the distant-site SA295 syn construct had similar activity in encapsulated and unencapsulated (synA::Tn916) backgrounds (Fig. 4B). Finally, the inactivation of polysialyltransferase (synD::Tn916) did not affect the transcription of the syn (SA2) reporter construct (data not shown). Overall, these results indicated that, at least under in vitro growth conditions, the ctr and syn operons did not exhibit evidence of product or feedback regulation.
5′ UTR of synA inhibits both syn and ctr operon transcription.
A transcription-regulatory role of the 5′ UTR sequence of synA was suggested because of the difference in transcription between the SA1 and SA2 syn constructs (Fig. 4A). Additional mutations were generated in this region to further characterize important elements. A 16-bp direct repeat (+78 to +93 relative to the synA transcriptional start site) and a 20-bp inverted repeat (+108 to +127) were identified within the sequence between the SnaBI and the EcoRI sites (Fig. 1B). In addition, several putative integration host factor (IHF) binding sites were identified in the intergenic region using the E. coli IHF consensus sequence 5′-YAANNNNTTGATW-3′ (where Y is C/T, W is A/T, and N is G/A/T/C), three of these overlapped either the direct or inverted repeat. Specific deletions that removed either the direct (mutants SA293 and CA293) or inverted (mutants SA233 and CA233) repeat or both repeats (mutants SA286 and CA286) were made in both syn and ctr reporter strains (Fig. 1B and 5). In addition, a 5-base substitution disrupting the symmetry of the direct repeat (mutants SA247 and CA247) was also generated in the reporter strains.
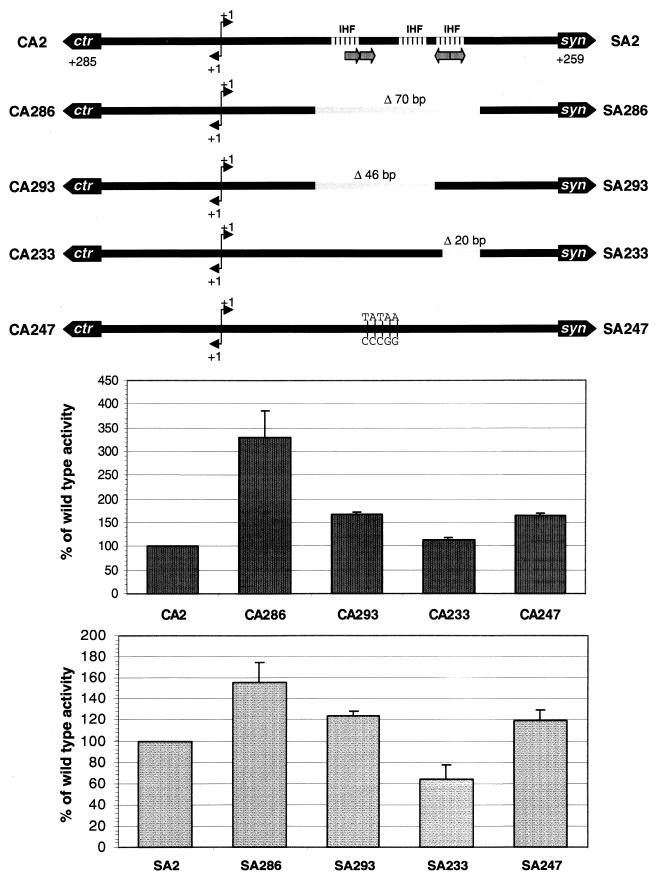
FIG. 5.
Transcriptional activities of synA::lacZ (SA2) and ctrA::lacZ (CA2) reporters in N. meningitidis containing mutations within 5′ UTR of synA. The mutations are shown in the diagram at the top and also noted in Fig. 1B. Each strain was grown in GC medium to mid-exponential phase, and β-galactosidase activities were assayed by the method of Miller (21). The values are presented as the percentage of the wild-type activity (SA2, 929.2 ± 158.0 U; CA2, 227.0 ± 29.8 U). Data shown are the averages of at least three independent experiments. Two-tailed unpaired Student's t test indicated that values for all mutants except SA293 and CA233 are statistically significantly different from that of the wild-type parent strain (P < 0.05).
Figure 5 shows the activities of these mutants relative to those of the wild-type parent strain. Deletion of the 20-bp inverted repeat (mutants SA233 and CA233) modestly decreased syn transcription but had no effect on ctr expression. In contrast, deletion of the direct repeat, which precedes the ribosome-binding site, increased ctr (mutants CA293 and CA247) and, to a lesser degree, syn expression (mutants SA293 and SA247). Removing both repeats (mutant CA286) enhanced ctr expression ∼3-fold, while syn expression increased 50% (mutant SA286). Furthermore, the increase in transcription seen with deletions of both repeats (mutants SA286 and CA286) was more significant than with the individual deletion of the repeats, implying a synergistic effect between these structural elements.
No inhibition of synA and ctrA transcription by the 5′ UTR of synA in an E. coli K1 background.
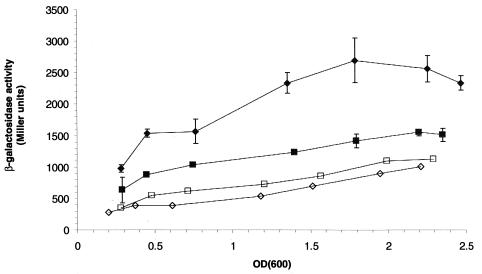
The identical capsule [(α2→8)-linked polysialic acid] expressed by serogroup B meningococci is expressed in E. coli K1 strains. The E. coli K1 strain is the etiologic agent of human neonatal meningitis, and the K1 capsule locus has been extensively investigated (38). Meningococcal capsular biosynthesis and transport gene homologues have been identified in the K1 E. coli strain. In order to examine if the meningococcal regulation machinery was present in this genetic background, the intergenic region was fused with a promoterless lacZ gene (pBlue-Topo), subcloned into a pBR322-derived plasmid (pHP45), and transformed into a clinical isolate of the K1 strain, CAB1. The 5′ end of the promoters contained the sequence downstream of the SspI site in ctrA, defined above as being of sufficient promoter length for controlling syn expression. The 3′ end of the promoters corresponded to either the SA1 or SA2 meningococcal construct, respectively. The SA1 construct should have higher activity than the SA2 construct if analogous regulation is present in the K1 strain. However, SA1 and SA2 produced similar activity in the E. coli K1 background (Fig. 6), indicating that the regulatory mechanisms that yield higher activity in the SA1 construct were absent in the E. coli K1 strain.
FIG. 6.
Expression of synA::lacZ transcriptional fusions in genetic backgrounds of meningococcal strain NMB (solid symbols) and E. coli K1 strain CAB1 (open symbols). Meningococcal reporter strains SA2 (solid squares) and SA1 (solid diamonds) have a lacZ-ermC cassette recombined in the synA locus on the chromosome, while E. coli strains contain a low-copy-number plasmid with the promoter fusion of lacZ corresponding to that of SA2 (open squares) and SA1 (open diamonds). Each time point was measured in triplicate, and the results of one representative experiment are presented.
DISCUSSION
Recent studies have genetically and biochemically characterized the capsule transport ctrABCD and biosynthesis synABCD(E, F) operons of the cps gene complex in serogroups B, C, Y, and W-135 N. meningitidis (5, 9, 30–33). Insertional mutagenesis of genes required for capsule biosynthesis and capsule transport produces unencapsulated phenotypes, confirming the necessity of these genes (30). A number of studies have indicated that the expression of meningococcal capsules is closely associated with virulence (16–18) but that capsule may not be constitutively expressed on the meningococcal surface (27, 35–37). These data indicate that expression of capsule is subject to regulation.
Capsule expression in N. meningitidis is known to be controlled through genetic on-off switch mechanisms and possibly through posttranscriptional modification. Two kinds of on-off switch mechanisms of meningococcal capsule expression have been defined. Reversible insertion and the excision of a naturally occurring insertion element, IS1301, into synA have been shown in a serogroup B strain, B1940 (11). Other IS1301 insertions in the capsule biosynthesis operon eliminate capsule expression (32; J. Dolan-Livengood and D. S. Stephens, unpublished data). In addition, a poly(C) track within synD, the polysialyltransferase, of serogroup B strains alters capsule expression at a frequency of 10−3 through a slipped-strand mispairing mechanism (12). Finally, enzymatic activity of a CMP-sialic acid hydrolase has been detected in meningococcal cell extracts and is proposed to be a posttranscriptional regulatory mechanism for meningococcal capsular polysaccharide (20).
We found that transcriptional regulation is also a pathway for control of sialic acid capsule expression in N. meningitidis. We have previously shown that the first genes of the biosynthesis and transport operons, synA and ctrA, respectively, are transcribed divergently from abutting start sites located in a 134-bp intergenic region. Furthermore, the sequence of this intergenic region is identical among all serogroups synthesizing capsules containing sialic acid (30). The data presented in this report confirm that the 134-bp intergenic region contains the promoters of these operons and identifies other transcriptional regulatory elements of serogroup B meningococcal capsule expression. Coordinate transcriptional regulation of divergent promoters in bacterial pathogens is well described. In Vibrio cholerae, for example, two virulence genes, acfA and acfD, are transcribed in opposite directions from a 173-bp intergenic region (8). Expression of these genes is under the coordinate control of ToxR, which activates the promoter of acfD and represses acfA transcription.
The syn and ctr promoters present in the 134-bp intergenic region were both capable of actively transcribing lacZ in the meningococcus. The ctr promoter produced lower levels of lacZ transcription than the syn promoter in a meningococcal background, confirming previous work with these promoters in E. coli (30). The syn promoter is a near consensus ς70-type promoter, while the ctr promoter is similar to the −10 extended promoter class, originally identified in work with lambdoid phages in E. coli, and lacks a consensus −35 recognition sequence (19). Under most conditions, RNA polymerase would be predicted to bind tightly to the consensus ς70 syn promoter, instead of the −10 extended ctr promoter, and thus transcribe the biosynthesis operon more efficiently. Sialic acid, which is synthesized by SynA/B/C proteins of the biosynthesis operon, is used for lipooligosaccharide (LOS) sialylation as well as capsule polymerization. This might explain why the syn promoter is constitutively expressed at a high level. Indeed, a mutation that inactivates the serogroup B meningococcal polysialyltransferase shifts the partially sialylated LOS of the wild-type parent strain to a fully sialylated LOS (18) and indicates shunting of sialic acid into the LOS pathway when capsule expression is blocked. Since the only known function of the proteins encoded by the capsule transport operon appears to be transport of the assembled capsule polymers, these proteins may not need to be expressed in high quantity.
A relatively small (<200 bp) upstream region controls the syn operon. This mechanism is not uncommon. The production of an exopolysaccharide by the phytopathogen Pseudomonas solanacearum is accomplished by an 18-kb gene cluster that is controlled by a 140-bp region upstream of the transcription start site and a multicomponent regulatory network (14). The regulation of bundle-forming pili in enteropathogenic E. coli is controlled by the sequence within −95 bp upstream of the transcriptional start point (24).
In addition to the promoters, we identified within the 134-bp region a 70-bp sequence of the 5′ UTR of synA that influences both ctr and syn promoter activity. A 20-bp inverted repeat of this region, which includes the synA start codon, was suggested as a possible regulatory element for transcription of syn and ctr operons (6). In Neisseria gonorrhoeae and N. meningitidis, a 16-bp inverted repeat encompassing the putative ribosome-binding site of the rfaC gene, encoding the LOS α1,5-heptosyltransferase I, is involved in the transcriptional regulation of this gene (40). Deletion of the 20-bp inverted repeat sequence moderately decreased syn transcription but had no effect on ctr expression. In contrast, deletion of a 16-bp direct repeat which precedes the ribosome-binding site enhanced both ctr and syn expression. Interestingly, our laboratory had previously shown that clinical meningococcal isolates occur with deletions in the direct repeat. For example, a serogroup W-135 strain was found to have an 8-bp deletion and 2-nucleotide substitution in the direct repeat (30). The elimination of the direct repeat element in vivo would be predicted to upregulate syn and ctr promoter activities and capsule expression in meningococci.
A 70-bp deletion that removed both the inverted and direct repeats produced the most significant increase in transcription, suggesting that the combination of the direct and inverted repeats facilitated the greatest repression of transcription of the syn and ctr operons. An arrangement similar to this direct-inverted repeat motif is found downstream of the vrg promoters in Bordetella pertussis and is thought to be the target for a transcriptional repressor that binds to this region and prevents transcription (2). This direct-inverted repeat sequence may also assume a topology that interferes with RNA polymerase binding, and the removal of this region enhances the efficiency of the transcription complexes. Alternatively, the sequence within the 5′ UTR of synA may influence the stability of mRNA and therefore modulate the transcriptional level of syn genes. Interestingly, three putative IHF binding motifs were also identified within the 70-bp region. IHF is known to bend DNA within the promoter region and modulate transcription (10). The involvement of IHF in the regulation of K5 capsule gene clusters in pathogenic E. coli has recently been reported (26).
Two-component regulatory systems have been identified that control capsular polysaccharide production and expression in bacterial pathogens. For example, the colanic acid capsule of E. coli is controlled by the RcsB-RcsC two-component system (29), while the AlgQ-AlgR system controls the alginate capsule in Pseudomonas aeruginosa (7) and the CsrR-CsrS system is involved in hyaluronic acid capsule production in Streptococcus pyogenes (13). In preliminary studies, we have not found that transcription of the sialic acid-containing meningococcal capsules is influenced by environmental conditions that act as triggers for two-component regulatory systems. Changes in temperature, pH, osmolarity, iron, carbon source, and serum exposure have not affected syn or ctr transcription. In support of these observations, only four putative response regulators in the serogroup A and serogroup B meningococcal genomes have been noted. In contrast, E. coli and Bacillus subtilis have 34 and 35 response regulator proteins, respectively. Mutations in three of the putative meningococcal two-component regulatory systems showed no effect in syn or ctr transcription (Y.-L. Tzeng and D. S. Stephens, unpublished data).
We did find that transcription of the syn operon occurs at the highest levels during the stationary phase of the meningococcal growth curve. The increase in synthesis of sialic acid for incorporation into the LOS or the polysialic acid capsule pathway appears greatest during the later stages of the bacterial growth curve. In the plant pathogen Erwinia stewartii, a homoserine lactone autoinducer is required for the induction of capsule biosynthesis (3). The possibility of a quorum-sensing mechanism regulating capsule expression in N. meningitidis will require further study.
In summary, expression of the sialic acid capsule of N. meningitidis is initiated by divergent promoters located within a 134-bp intergenic region separating the biosynthesis and transport operons. Transcription of these operons is repressed by a 70-bp 5′ UTR of synA, is increased during stationary growth, and shows species-specific regulation. The 134-bp synABCD-ctrABCD intergenic region is an important control point for the transcriptional regulation of sialic acid capsule expression.
ACKNOWLEDGMENTS
We thank Lane Pucko for administrative assistance.
This work was supported by Public Health Service grant AI/40247 (to D.S.S.) from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Ali S A, Steinkasserer A. PCR-ligation-PCR mutagenesis: a protocol for creating gene fusions and mutations. Biotechniques. 1995;18:746–750. [PubMed] [Google Scholar]
- 2.Beattie D T, Knapp S, Mekalanos J J. Evidence that modulation requires sequences downstream of the promoters of two vir-repressed genes of Bordetella pertussis. J Bacteriol. 1990;172:6997–7004. doi: 10.1128/jb.172.12.6997-7004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beck von Bodman S, Farrand S K. Capsular polysaccharide biosynthesis and pathogenicity in Erwinia stewartii require induction by an N-acylhomoserine lactone autoinducer. J Bacteriol. 1995;177:5000–5008. doi: 10.1128/jb.177.17.5000-5008.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung C T, Miller R H. A rapid and convenient method for the preparation and storage of competent bacterial cells. Nucleic Acids Res. 1988;16:3580. doi: 10.1093/nar/16.8.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claus H, Vogel U, Muhlenhoff M, Gerardy-Schahn R, Frosch M. Molecular divergence of the sia locus in different serogroups of Neisseria meningitidis expressing polysialic acid capsules. Mol Gen Genet. 1997;257:28–34. doi: 10.1007/pl00008618. [DOI] [PubMed] [Google Scholar]
- 6.Collado-Vides J, Magasanik B, Gralla J D. Control site location and transcriptional regulation in Escherichia coli. Microbiol Rev. 1991;55:371–394. doi: 10.1128/mr.55.3.371-394.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deretic V, Konyecsni W M. Control of mucoidy in Pseudomonas aeruginosa: transcriptional regulation of algR and identification of the second regulatory gene, algQ. J Bacteriol. 1989;171:3680–3688. doi: 10.1128/jb.171.7.3680-3688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiRita V J, Parsot C, Jander G, Mekalanos J J. Regulatory cascade controls virulence in Vibrio cholerae. Proc Natl Acad Sci USA. 1991;88:5403–5407. doi: 10.1073/pnas.88.12.5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frosch M, Weisgerber C, Meyer T F. Molecular characterization and expression in Escherichia coli of the gene complex encoding the polysaccharide capsule of Neisseria meningitidis group B. Proc Natl Acad Sci USA. 1989;86:1669–1673. doi: 10.1073/pnas.86.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goosen N, van de Putte P. The regulation of transcription initiation by integration host factor. Mol Microbiol. 1995;16:1–7. doi: 10.1111/j.1365-2958.1995.tb02386.x. [DOI] [PubMed] [Google Scholar]
- 11.Hammerschmidt S, Hilse R, van Putten J P, Gerardy-Schahn R, Unkmeir A, Frosch M. Modulation of cell surface sialic acid expression in Neisseria meningitidis via a transposable genetic element. EMBO J. 1996;15:192–198. [PMC free article] [PubMed] [Google Scholar]
- 12.Hammerschmidt S, Muller A, Sillmann H, Muhlenhoff M, Borrow R, Fox A, van Putten J, Zollinger W D, Gerardy-Schahn R, Frosch M. Capsule phase variation in Neisseria meningitidis serogroup B by slipped-strand mispairing in the polysialyltransferase gene (siaD): correlation with bacterial invasion and the outbreak of meningococcal disease. Mol Microbiol. 1996;20:1211–1220. doi: 10.1111/j.1365-2958.1996.tb02641.x. [DOI] [PubMed] [Google Scholar]
- 13.Heath A, DiRita V J, Barg N L, Engleberg N C. A two-component regulatory system, CsrR-CsrS, represses expression of three Streptococcus pyogenes virulence factors, hyaluronic acid capsule, streptolysin S, and pyrogenic exotoxin B. Infect Immun. 1999;67:5298–5305. doi: 10.1128/iai.67.10.5298-5305.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang J, Schell M. Molecular characterization of the eps gene cluster of Pseudomonas solanacearum and its transcriptional regulation at a single promoter. Mol Microbiol. 1995;16:977–989. doi: 10.1111/j.1365-2958.1995.tb02323.x. [DOI] [PubMed] [Google Scholar]
- 15.Janik A, Juni E, Heym G A. Genetic transformation as a tool for detection of Neisseria gonorrhoeae. J Clin Microbiol. 1976;4:71–81. doi: 10.1128/jcm.4.1.71-81.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarvis G A. Recognition and control of neisserial infection by antibody and complement. Trends Microbiol. 1995;3:198–201. doi: 10.1016/s0966-842x(00)88921-0. [DOI] [PubMed] [Google Scholar]
- 17.Jarvis G A, Vedros N A. Sialic acid of group B Neisseria meningitidis regulates alternative complement pathway activation. Infect Immun. 1987;55:174–180. doi: 10.1128/iai.55.1.174-180.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahler C M, Martin L E, Shih G C, Rahman M M, Carlson R W, Stephens D S. The (α2→8)-linked polysialic acid capsule and lipooligosaccharide structure both contribute to the ability of serogroup B Neisseria meningitidis to resist the bactericidal activity of normal human serum. Infect Immun. 1998;66:5939–5947. doi: 10.1128/iai.66.12.5939-5947.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A, Malloch R A, Fujita N, Smillie D A, Ishihama A, Hayward R S. The minus 35-recognition region of Escherichia coli sigma 70 is inessential for initiation of transcription at an “extended minus 10” promoter. J Mol Biol. 1993;232:406–418. doi: 10.1006/jmbi.1993.1400. [DOI] [PubMed] [Google Scholar]
- 20.Masson L, Holbein B E. Physiology of sialic acid capsular polysaccharide synthesis in serogroup B Neisseria meningitidis. J Bacteriol. 1983;154:728–736. doi: 10.1128/jb.154.2.728-736.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 22.Monod M, Denoya C, Dubnau D. Sequence and properties of pIM13, a macrolide-lincosamide-streptogramin B resistance plasmid from Bacillus subtilis. J Bacteriol. 1986;167:138–147. doi: 10.1128/jb.167.1.138-147.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 24.Puente J L, Bieber D, Ramer S W, Murray W, Schoolnik G K. The bundle-forming pili of enteropathogenic Escherichia coli: transcriptional regulation by environmental signals. Mol Microbiol. 1996;20:87–100. doi: 10.1111/j.1365-2958.1996.tb02491.x. [DOI] [PubMed] [Google Scholar]
- 25.Raleigh E A, Lech K, Brent R. Selected topics from classical bacterial genetics. In: Ausubel F M, et al., editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1995. p. 1.4. [DOI] [PubMed] [Google Scholar]
- 26.Rowe S, Hodson N, Griffiths G, Roberts I S. Regulation of the Escherichia coli K5 capsule gene clusters: evidence for the roles of H-NS, BipA, and integration host factor in regulation of group 2 capsule gene clusters in pathogenic E. coli. J Bacteriol. 2000;182:2741–2745. doi: 10.1128/jb.182.10.2741-2745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens D S, Spellman P A, Swartley J S. Effect of the (α 2→8)-linked polysialic acid capsule on adherence of Neisseria meningitidis to human mucosal cells. J Infect Dis. 1993;167:475–479. doi: 10.1093/infdis/167.2.475. [DOI] [PubMed] [Google Scholar]
- 28.Stephens D S, Swartley J S, Kathariou S, Morse S A. Insertion of Tn916 in Neisseria meningitidis resulting in loss of group B capsular polysaccharide. Infect Immun. 1991;59:4097–4102. doi: 10.1128/iai.59.11.4097-4102.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stout V, Gottesman S. RcsB and RcsC: a two-component regulator of capsule synthesis in Escherichia coli. J Bacteriol. 1990;172:659–669. doi: 10.1128/jb.172.2.659-669.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swartley J S, Ahn J H, Liu L J, Kahler C M, Stephens D S. Expression of sialic acid and polysialic acid in serogroup B Neisseria meningitidis: divergent transcription of biosynthesis and transport operons through a common promoter region. J Bacteriol. 1996;178:4052–4059. doi: 10.1128/jb.178.14.4052-4059.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swartley J S, Liu L J, Miller Y K, Martin L E, Edupuganti S, Stephens D S. Characterization of the gene cassette required for biosynthesis of the (α1→6)-linked N-acetyl-d-mannosamine-1-phosphate capsule of serogroup A Neisseria meningitidis. J Bacteriol. 1998;180:1533–1539. doi: 10.1128/jb.180.6.1533-1539.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swartley J S, Marfin A A, Edupuganti S, Liu L J, Cieslak P, Perkins B, Wenger J D, Stephens D S. Capsule switching of Neisseria meningitidis. Proc Natl Acad Sci USA. 1997;94:271–276. doi: 10.1073/pnas.94.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swartley J S, Stephens D S. Identification of a genetic locus involved in the biosynthesis of N-acetyl-d-mannosamine, a precursor of the (α2→8)-linked polysialic acid capsule of serogroup B Neisseria meningitidis. J Bacteriol. 1994;176:1530–1534. doi: 10.1128/jb.176.5.1530-1534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Troy F A. Polysialylation: from bacteria to brains. Glycobiology. 1992;2:5–23. doi: 10.1093/glycob/2.1.5. [DOI] [PubMed] [Google Scholar]
- 35.Virji M, Makepeace K, Ferguson D J, Achtman M, Moxon E R. Meningococcal Opa and Opc proteins: their role in colonization and invasion of human epithelial and endothelial cells. Mol Microbiol. 1993;10:499–510. doi: 10.1111/j.1365-2958.1993.tb00922.x. [DOI] [PubMed] [Google Scholar]
- 36.Virji M, Makepeace K, Ferguson D J, Achtman M, Sarkari J, Moxon E R. Expression of the Opc protein correlates with invasion of epithelial and endothelial cells by Neisseria meningitidis. Mol Microbiol. 1992;6:2785–2795. doi: 10.1111/j.1365-2958.1992.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 37.Virji M, Makepeace K, Moxon E R. Distinct mechanisms of interactions of Opc-expressing meningococci at apical and basolateral surfaces of human endothelial cells: the role of integrins in apical interactions. Mol Microbiol. 1994;14:173–184. doi: 10.1111/j.1365-2958.1994.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 38.Whitfield C, Roberts I S. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol Microbiol. 1999;31:1307–1319. doi: 10.1046/j.1365-2958.1999.01276.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhou D, Apicella M A. Plasmids with erythromycin resistance and catechol 2,3-dioxygenase- or beta-galactosidase-encoding gene cassettes for use in Neisseria spp. Gene. 1996;171:133–134. doi: 10.1016/0378-1119(96)00103-5. [DOI] [PubMed] [Google Scholar]
- 40.Zhou D, Zaleski A, Buscher B, Preston A, Apicella M A. A sixteen bp palindrome sequence encompassing the putative ribosomal binding site is conserved in pathogenic Neisseria rfaC genes and is involved in the regulation of expression of meningococcal rfaC. In: Zollinger W D, Frasch C E, Deal C D, editors. Tenth International Pathogenic Neisseria Conference, 8 to 13 September 1996. 1996. pp. 356–357. Baltimore, Md. [Google Scholar]