Abstract
The sanguis streptococci are primary colonizers of the tooth surface and thus form the foundation for the complex multiple species biofilm known as dental plaque. In addition, these bacteria can colonize native and prosthetic heart valves and are a common cause of endocarditis. Little is known about the molecular mechanisms governing multiple or single species biofilm development within this group of organisms. Using an in vitro assay for biofilm formation, we determined that (i) Streptococcus parasanguis FW213 can form biofilms on inert surfaces such as polystyrene and (ii) environmental and nutritional factors, such as glucose, affect S. parasanguis biofilm formation. Several isogenic mutants of FW213 were tested in the biofilm assay. Strains containing mutations in fap1, a gene encoding a protein required for assembly of fimbriae, were deficient in biofilm formation. Mutants defective in recA, PepO endopeptidase activity, or the production of a fimbriae-associated protein, FimA, were still capable of biofilm formation. Phase-contrast microscopy was used to follow biofilm development by wild-type and fap1 mutant strains on plastic coverslips over time. Wild-type FW213 attached to the surface, formed aggregates of cells, and eventually formed a dense layer of cells that included microcolonies. In contrast, few fap1 mutant cells were observed attached to the surface, and no cell aggregates or microcolonies were formed. These results suggest that the long peritrichous fimbriae of FW213 are critical for the formation of biofilms on solid surfaces.
In the past, the science of microbiology has developed mainly from studies focused on free-floating (planktonic) cells living in batch culture. While a wealth of information about basic microbial growth and physiology has been gained from these studies, it is now widely accepted that in most natural, industrial, and medical environments, the majority of bacteria exist in highly structured, surface-attached communities (5, 6). These structured, microbial communities, known as biofilms, develop on virtually every material that contacts naturally occurring fluids, including those of medical and industrial importance. Mounting evidence indicates that bacterial biofilms are responsible for many persistent and chronic bacterial infections in humans. Biofilm infections may be caused by a single species or by a mixture of species of bacteria and fungi and are associated with such diseases as dental caries, periodontitis, otitis media, musculoskeletal infections, cystic fibrosis pneumonia, native valve endocarditis, and bacterial prostatitis (5, 6, 32). In addition to infections of living tissues, microbial biofilms foul implanted medical devices, such as catheters, artificial cardiac pacemakers, prosthetic heart valves, and orthopedic appliances. Chronic infection of medical devices can lead to incidences of acute sepsis and death, particularly in immunocompromised patients (6), and is the greatest problem affecting the success of biomedical implants within the human body (22).
One of the most common biofilms, dental plaque, is found in the human oral cavity. Total accumulation of microorganisms in plaque on all teeth can range from up to a few milligrams to more than gram (21). The sanguis streptococci are primary colonizers of the tooth surface (2, 25, 36). In addition to comprising a major portion of dental plaque, the sanguis streptococci serve as a substratum for the subsequent adhesion of other plaque bacteria. These later-arriving bacteria include species that are the causative agents of caries and periodontal diseases, some of the most prevalent infections afflicting humans (21).
The sanguis streptococci and related organisms are also a common cause of native heart valve (18, 42) and late prosthetic valve (28) endocarditis. Not all bacteria are capable of causing endocarditis, but these bacteria appear to have specific properties that enable them to colonize a modified valve surface (1, 12, 24, 27). Once the valve surface is colonized, formation of the biofilm vegetation proceeds by a series of complex steps (10, 39). Small surface aggregates of bacteria begin to form, and eventually rounded colonies tightly packed with large numbers of bacteria occur. When the bacterial population reaches a certain size, a layer of fibrin and platelets is deposited over the bacteria. The colonized valve surface is thus transformed into a biofilm vegetation with an organized appearance (10, 23, 40). Because these infections are often persistent and difficult to treat, infective endocarditis is a serious disease resulting in substantial morbidity and mortality despite modern antimicrobial and surgical treatment (9). Since cells grown on surfaces within biofilms are in a physiological state that differs markedly from that of their planktonic counterparts (5, 6, 37), new therapies that target the biofilm phenotype are needed.
Factors involved in the transition from a free-floating, planktonic existence to that of a surface-attached, sessile community are complex, and little is known about the molecular mechanisms of biofilm formation of oral streptococci. Recently, several investigators have demonstrated the power of transposon mutagenesis coupled with a convenient assay for biofilm formation in identifying genes required for bacterial biofilm formation (30, 33, 44, 26). Mutants are tested in 96-well microtiter plates, and biofilm formation within the well is detected by staining with crystal violet. A modification of this assay was used in the present study to begin characterization of biofilm formation by the oral bacterium Streptococcus parasanguis, a primary colonizer of the tooth surface and a known causative agent of bacterial endocarditis (1).
Previous studies with S. parasanguis FW213 have determined that adherence of this organism to an in vitro tooth surface model, saliva-coated hydroxlapatite (SHA), is mediated by long peritrichous surface fimbriae (13, 16). Wild-type, fimbriated FW213 binds to SHA, but isogenic mutants lacking these fimbriae do not. Recent analyses of genetic determinants encoding the biogenesis of these fimbriae revealed that Fap1, a high-molecular-weight glycoprotein, is essential for long fimbrial formation in S. parasanguis FW213 (45, 46). Insertional inactivation of fap1 results in loss of the long peritrichous surface fimbriae and a significant reduction in adhesion of FW213 to SHA (45, 46). In this report, we present data showing that S. parasanguis fap1 mutants are also defective in biofilm development.
MATERIALS AND METHODS
Bacterial strains and media.
Bacterial strains used in this study are listed in Table 1. The wild-type S. parasanguis strain used was FW213 (4). All other strains are derivatives of FW213 and were generated by gene replacement techniques. Strains were grown statically in the presence of 5% CO2 at 37°C in Todd-Hewitt broth (TH; Difco Laboratories, Detroit, Mich.). Biofilm formation of S. parasanguis FW213 was assessed in each of the following media: TH, TH supplemented with 0.2% yeast extract (TH+YE), TH supplemented with glucose to give a final concentration of 1% (TH+Glu), brain heart infusion (BHI; Difco Laboratories), chemically defined medium (CDM [41]), Trypticase soy broth (TSB; Becton Dickinson and Company, Sparks, Md.), and TSB supplemented with 0.2% yeast extract (TSB+YE). The effect of glucose concentration on FW213 biofilm formation was examined by supplementing TH, BHI, CDM, or TSB with glucose to a final concentration of 1% (wt/vol).
TABLE 1.
S. parasanguis strains used in this study
| Strain | Relevant characteristics | Reference |
|---|---|---|
| FW213 | Wild type | 4 |
| VT930 | FW213 fimA::aphA-3; Kmr | 15 |
| VT1393 | FW213 fap1::aphA-3 in XbaI site of 5′ non-repeat region; Kmr | 45 |
| VT1428 | FW213 fap1::ermAM in 3′ anchor region; Emr | 46 |
| VT1346 | FW213 pepO::aphA-3; Kmr | 19 |
| VT1354 | FW213 recA::aphA-3; Kmr | 20 |
Biofilm formation assay.
The biofilm formation assay used in this study was adapted from the method of O'Toole and Kolter (31) and is based on the ability of bacteria to form biofilms on solid surfaces, such as polystyrene or polyvinyl chloride. S. parasanguis strains grown overnight in TH were washed once in sterile distilled (dH2O) and diluted 1:200 in fresh medium; 200 μl was inoculated into wells of a non-tissue culture-treated polystyrene flat-bottom 96-well microtiter plate (Nunc 269787; Krackeler Scientific, Inc., Albany, N.Y.). Non-tissue culture-treated polyvinyl chloride round-bottom 96-well plates (Falcon 3911; Becton Dickinson Labware, Franklin Lakes, N.J.) were also used in initial studies. Wells filled with only growth media were included as negative controls. Plates were incubated at 37°C for 16 h either aerobically in 5% CO2 or anaerobically. Before biofilm quantification, growth of wild-type and mutant strains was assessed by measuring the absorbance of cultures in the wells at 490 nm with an EL311 automated microplate reader (BIO-TEK Instruments, Inc., Winooski, Vt.). Media, including any unattached bacteria, were then decanted from the wells, and any remaining planktonic cells were removed by rinsing with dH2O. Wells were air dried, and adherent bacteria were stained for 15 min with a 0.5% (wt/vol) solution of crystal violet (Fisher Scientific, Pittsburgh, Pa.). After rinsing with dH2O, bound dye was released from stained cells using either 95% ethanol, ethanol-acetone (80:20), or 30% glacial acetic acid. This allowed indirect measurement of biofilms formed on both the bottom and sides of the well. Biofilm formation was quantified by measuring absorbance of the solution at 562 nm with a microplate reader (BIO-TEK Instruments).
Time course assay.
Early-stationary-phase cultures of S. parasanguis were diluted 1:50 in TH+Glu, and 200 μl of subculture was inoculated into the wells of a polystyrene microtiter plate and allowed to incubate at 37°C, 5% CO2 from 1 to 14 h. Before biofilm quantification, bacterial growth at each time point was assessed by measuring the absorbance of cultures in the wells at 490 nm with a microplate reader. Wells were subsequently rinsed and stained with crystal violet, and biofilm formation was quantified as described above.
Phase-contrast microscopy.
Direct visualization of S. parasanguis biofilm formation on vinyl coverslips (Structure Probe, Inc., West Chester, Pa.) over time was achieved using phase-contrast microscopy. For earlier time points (1 to 6 h), 12 ml of a culture, grown to mid-logarithmic phase in TH+Glu, was transferred to a 50-ml Corning centrifuge tube containing a vinyl coverslip. Incubation was continued without shaking at 37°C, 5% CO2. At chosen time points, planktonic cells were removed from the coverslip by rinsing, and attached cells were examined by phase-contrast microscopy using a Nikon Eclipse E400 microscope. Digitized images were captured using a SpotRT monochrome camera driven by Spot version 3.0.1 (AppleEvent) software (Diagnostic Instruments, Inc., Sterling Heights, Mich.). For later time points (15 to 18 h), late-logarithmic-phase cultures of S. parasanguis were diluted 1:200 in TH+Glu. Twelve milliliters of each subculture was added to a 50-ml Corning centrifuge tube containing a plastic coverslip and incubated at 37°C, 5% CO2 for 15 to 18 h. Plastic coverslips were then removed and rinsed, and remaining cells were visualized as described above.
Quantification of Fap1.
Surface expression of Fap1 was determined by a whole-bacterial-cell enzyme-linked immunosorbent assay (bactELISA [11]) as described previously (45). Incubation with mouse monoclonal antibody F51 (13), which is specific to the Fap1 subunit of S. parasanguis fimbriae, was used as the primary antibody in the assay. Primary antibody was detected using anti-mouse horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove, Pa.). Color development was quantified by measurement of absorbance at 490 nm with an automated microplate reader (BIO-TEK Instruments).
RESULTS AND DISCUSSION
S. parasanguis biofilm formation on polystyrene.
A number of recent studies have used molecular and genetic approaches to identify genes important for biofilm formation (reviewed in reference 34). One genetic approach that has been particularly fruitful in identifying genes critical for biofilm formation uses a convenient macroscopic assay to screen for biofilm-defective mutants (31). This assay is based on the ability of bacteria to form biofilms on surfaces such as polystyrene (16). Bacteria are cultured in the wells of plastic tissue culture plates, and the presence or absence of a biofilm is detected by staining the wells with crystal violet. In the present study, characterization of S. parasanguis FW213 biofilm formation was initiated by investigating the ability FW213 to form biofilms on plastic surfaces.
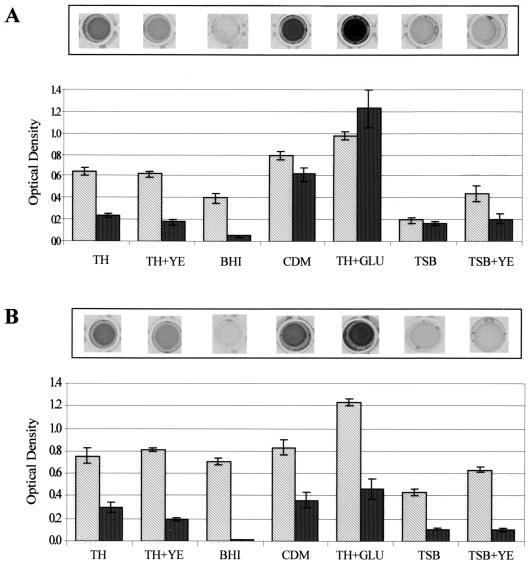
Initial experiments were conducted to determine the optimal conditions for FW213 biofilm formation. Using a standard streptococcal growth medium, TH, we detected biofilms on both polystyrene (Fig. 1) and polyvinyl chloride (data not shown) microtiter plates under either aerobic (Fig. 1A) or anaerobic (Fig. 1B) conditions. Although growth and biofilm formation was somewhat greater in TH under anaerobic conditions, biofilm formation was more convenient to quantify under aerobic conditions. Aerobically grown cells formed biofilms on the bottom and lower sides of the well. By contrast, anaerobically grown cells formed biofilms throughout the well, including on the side of the well above the growth medium, making it more difficult to solubilize the crystal violet-stained biofilm. Acetic acid was more effective than ethanol or ethanol-acetone (80:20) at releasing bound dye, especially from heavier biofilms (compare wells shown in Fig. 1A to those shown in Fig. 2.).
FIG. 1.
Bacterial growth and biofilm formation of S. parasanguis FW213 under different growth conditions. Cells grown in TH to early stationary growth phase were washed once in sterile water and subcultured (1:200) into either TH, TH+YE, BHI broth, CDM, TH+Glu, TSB, or TSB+YE. These cultures were then grown in polystyrene microtiter dishes at 37°C for 16 h either aerobically in 5% CO2 (A) or anaerobically (B). Growth (light bars) and biofilm formation (dark bars) were quantified as optical density at 490 and 562 nm, respectively. Assays were performed in quadruplicate; mean values and standard deviations are shown. A representative row of crystal violet-stained microtiter plate wells is shown above each graph.
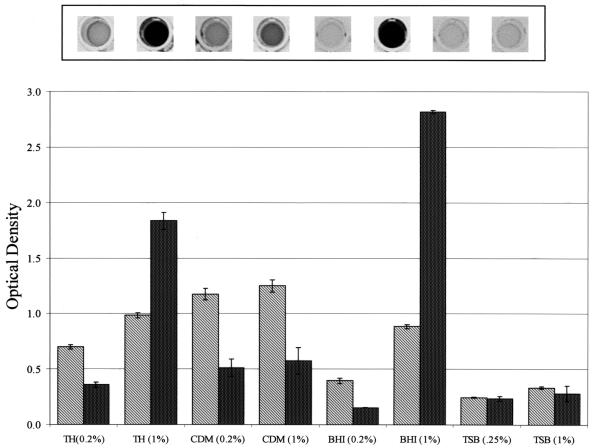
FIG. 2.
Effect of glucose on S. parasanguis growth and biofilm formation. Cells grown in TH to early stationary growth phase were washed once in sterile water and then subcultured (1:200) into the media indicated. The percentage of glucose in each medium is given in parentheses. Cultures were grown aerobically (5% CO2) at 37°C in polystyrene microtiter dishes for 16 h. Representative crystal violet-stained wells are shown above the graph. Growth (light bars) and biofilm formation (dark bars) are quantified below as optical density at 490 and 562 nm, respectively. Assays were performed in quadruplicate; mean values and standard deviations are shown.
Environmental factors affect S. parasanguis biofilm formation.
Previous studies indicate that the nutritional content of the growth medium can regulate biofilm development (5, 26, 31). In general, bacteria tend to adhere to available surfaces and form mature biofilms in environments that provide sufficient nutrients but will not adhere to surfaces in environments that are nutrient deficient. S. parasanguis FW213 biofilm formation was tested in a variety of growth media under aerobic (Fig. 1A) and anaerobic (Fig. 1B) conditions. Under both aerobic and anaerobic conditions, growth was about 1.2- and 1.6-fold greater in CDM and TH+Glu, respectively, than in TH. By contrast, biofilm formation increased significantly, about 2.6-fold in CDM and 5.1-fold in TH+Glu, under aerobic conditions but by a much smaller increment under anaerobic conditions. The final concentration of glucose in both CDM and TH+Glu is 1.0%, about four to five times greater than that in TH, BHI, or TSB.
The apparent effect of glucose on biofilm formation prompted us to compare biofilm formation of cells grown in the other types of media with and without glucose supplementation (Fig. 2). In all cases, glucose enhanced biofilm formation; the most dramatic effect was with TH and BHI. This result suggests that the increased glucose promoted biofilm formation in S. parasanguis, which is in agreement with results from previous studies with other genera of bacteria (3, 31). It also supports the premise that bacteria will form biofilms under favorable nutrient conditions (5). However, there clearly are exceptions to this latter generalization. For, example, the addition of YE to TH and TSB enhanced growth of FW213 under aerobic and anaerobic conditions, but biofilm formation was not promoted by the addition of YE, a highly nutrient rich extract (Fig. 1). When added to TH, YE in fact appears to suppress biofilm formation. Biofilm formation is also suppressed in BHI, another nutrient-rich medium used to promote growth of fastidious microorganisms, including the streptococci. Other investigators have also reported that there are environmental conditions that enhance cell growth but do not promote significant biofilm formation (26, 31).
Analysis of S. parasanguis mutants.
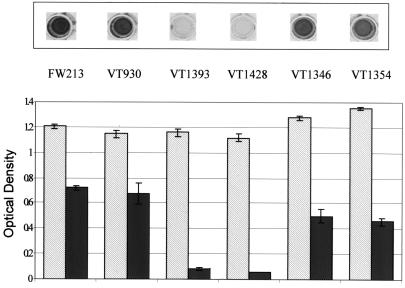
A number of mutants of FW213 available in our laboratory (Table 1) were examined in the above assay to determine their ability to form a biofilm (Fig. 3). In the biofilm assay, all of the mutants grew to the same extent as wild-type FW213 (Fig. 3). However, two of the mutants, VT1393 (45) and VT1428 (46), displayed severe defects in biofilm formation (Fig. 3). Both mutant strains had a growth rate indistinguishable from that of wild type in liquid medium (data not shown). Both VT1393 and VT1428 contain an inactivated copy of fap1. VT1393 has an insertion mutation in the 5′ non repetitive region of fap1 (45), while VT1428 has an insertion mutation at a site just upstream of the 3′ cell wall sorting signal (46). Previous studies in our laboratory have demonstrated that Fap1 is essential for formation of long fimbriae by S. parasanguis FW213 and that Fap1 is a structural subunit of the long fimbriae (45, 46). Long fimbriae are absent on the surface of Fap1-deficient strains, while monoclonal antibodies localize Fap1 to the long fimbriae and detect Fap1 as a major component of purified fimbriae (45, 46).
FIG. 3.
Growth and biofilm formation of S. parasanguis FW213 and its isogenic mutant strains. S. parasanguis strains FW213 (wild type), VT930 (fimA), VT1393 (fap1), VT1428 (fap1), VT1346 (pepO), and VT1354 (recA) were grown in TH to early stationary growth phase, washed once in sterile water, and then subcultured (1:200) into TH+Glu. Cultures were incubated in polystyrene microtiter dishes at 37°C, 5% CO2 for 17 h. Crystal violet-stained wells are shown above the graph. Growth (light bars) and biofilm formation (dark bars) are quantified below as optical density at 490 and 562 nm, respectively. Assays were performed in quadruplicate; mean values and standard deviations are shown.
Our current findings, using two independent mutant alleles of fap1, indicate that Fap1 plays an important role in S. parasanguis FW213 biofilm formation. Thus, the long peritrichous fimbriae, comprised of Fap1, may not be limited to the mediation of cell-to-surface interactions on the tooth surface but may be involved in the mediation of cell-to-surface interactions on solid surfaces in general. It has been demonstrated in other bacterial species that fimbriae (or pili) are required for biofilm formation (30, 33, 43, 44).
A mutant defective in the production of FimA, another protein associated with FW213 fimbriae, was capable of biofilm formation (Fig. 3). The fimA gene encodes the lipoprotein portion of an ATP binding cassette transporter (15) that belongs to a family of transporters involved with the uptake of metal cations (8). While FimA is a virulence factor of native valve endocarditis and facilitates binding to fibrin (1), it does not appear to play a role in biofilm formation under the environmental conditions used in this study. FimA is not required for the assembly of FW213 fimbriae or for adhesion to SHA (14).
An isogenic strain of FW213 defective in RecA function (20) was capable of biofilm formation (Fig. 3) as might be expected. Likewise, a strain defective in PepO endopeptidase activity (19) formed a biofilm in the above assay. S. parasanguis PepO is similar in structure and activity to mammalian enzymes that play essential roles in events such as inflammatory response phenomena, pain, and cardiovascular regulation.
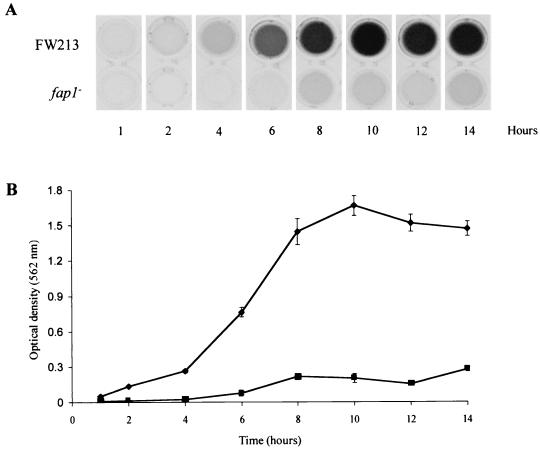
Time course assay of biofilm development of wild-type FW213 and of a fap1 mutant.
The difference in biofilm formation between wild-type FW213 and the fap1 mutants prompted us to study biofilm development in these strains in more detail. Biofilm development by both wild-type FW213 and the fap1 mutant VT1393 was studied over a period of 14 h (Fig. 4). The wild-type strain displayed a time-dependent increase in biofilm formation that reached a plateau, as detected by crystal violet staining after about 10 h. By contrast, FW213 cells lacking Fap1, and hence the long peritrichous fimbriae, were severely hindered in the ability to form biofilms and displayed only low levels of crystal violet staining over the course of the experiment. The apparent differences in biofilm-forming competency could not be explained by unequal growth, as growth of the fap1 mutant was equivalent to that of the wild type at each time point (data not shown).
FIG. 4.
Biofilm development over 14 h for S. parasanguis FW213 and fap1 mutant VT1393. Cells were grown in TH to early stationary growth phase, washed once in sterile water, and diluted 1:50 in TH+Glu. Cells were then grown in polystyrene microtiter dishes, and at the times indicated, biofilm formation was assayed. (A) Representative crystal violet-stained wells at each time point for FW213 and the fap1 mutant. (B) Quantification of crystal violet staining. Assays were performed in quadruplicate; mean values and standard deviations are shown.
Microscopic analysis of wild-type and fap1 mutant biofilms.
The detection of biofilm formation in the above studies was done indirectly by determining the amount of crystal violet-stained cells that attach to wells of polystyrene microtiter dishes. Biofilm formation, however, is considered a complex developmental process (5, 7, 30, 31, 44) that can be broken down into a series of observable steps (5, 44). These steps are likely to include attachment and immobilization on a surface, cell-to-cell attachment, microcolony formation, formation of a confluent biofilm, and formation of a characteristic three-dimensional biofilm structure. To outline steps involved in biofilm formation by S. parasanguis FW213, we used an experimental system for the development of three-dimensional biofilms as reported by Watnick and Kolter (44). We also used this system to help identify the role(s) of Fap1 and fimbriae during FW213 biofilm formation.
Biofilm development on vinyl coverslips by wild-type FW213 or the fap1 mutant VT1393 was observed microscopically at 1, 2, 3, 6, 15, and 18 h after inoculation. Images taken at 1, 3, and 18 h after inoculation are presented in Fig. 5. Each image is a representative sample of what was observed in multiple fields. After 1 h of incubation (Fig. 5A), numerous cells of the wild-type strain had attached to the plastic coverslip and several small clusters had formed. Fewer of the fap1 mutant cells had attached to the coverslip after 1 h of incubation, and no small cell clusters were seen (Fig. 5D). After 3 and 18 h of incubation, the patterns of attachment seen for wild-type FW213 (Fig. 5B and C) and the fap1 mutant (Fig. 5E and F) were quite different. Many more wild-type S. parasanguis FW213 cells had attached to the coverslip, and larger clusters of cells had formed. By 18 h, the wild-type biofilm consisted of multiple layers of cells and displayed dark clusters of cells (microcolonies) interspersed with areas of less densely packed cells. In contrast, the fap1 mutant strain displayed broad empty areas of plastic surface with only a few cells scattered throughout. No cell clusters or microcolonies were evident. These data are consistent with the indirect analyses of biofilm formation using crystal violet staining shown in Fig. 3 and 4. They demonstrate that Fap1 and fimbriae play a critical role in FW213 biofilm formation.
FIG. 5.
Phase-contrast micrographs comparing biofilm formation of wild-type S. parasanguis FW213 and the fap1 mutant VT1393 on plastic coverslips over time. (A to C) FW213 biofilms taken at 1, 3, and 18 h after inoculation, respectively; (D to F) fap1 mutant biofilms taken at comparable times. The arrow indicates one of many microcolonies that form over the course of the experiment. Bar = 30 μm.
Effect of glucose on surface expression of Fap1.
Given our findings that Fap1 and fimbriae play a role in FW213 biofilm formation and that glucose promotes biofilm formation in FW213, we asked whether glucose concentration in the growth medium affected the surface expression of Fap1 and fimbriae. Utilizing a bactELISA with a monoclonal antibody specific to Fap1, we determined that an increase in glucose concentration did not increase surface expression of Fap1. This result suggests that factors other than Fap1 and fimbriae were responding to the increased glucose concentration and were contributing to FW213 biofilm formation in these experiments.
Although environmental signals such as carbon source availability play a role in regulating biofilm formation, little is known about the molecular mechanisms that link environmental signals and biofilm development. However, a recent study has demonstrated that the global carbon metabolism regulator Crc of Pseudomonas aeruginosa is a component of a signal transduction pathway required for biofilm formation (29). Crc senses carbon source availability and subsequently affects expression of the type IV pilA gene. Since type IV pilus-mediated twitching motility is required for P. aeruginosa biofilm formation, Crc appears to tie nutritional cues to the regulation of biofilm development.
S. parasanguis FW213 belongs to a group of gram-positive bacteria with low GC content. In this group of bacteria, carbon catabolite repression is mediated by CcpA (38). It would be interesting to determine whether S. parasanguis FW213 possesses CcpA and, if so, whether CcpA serves as a regulator that links carbon availability to biofilm formation in this organism.
While glucose may serve as a nutritional signal for biofilm formation in S. parasanguis FW213, an increase in glucose concentration may possibly signal a change in the osmolarity of the environment. Previous studies suggest that bacteria encounter conditions of higher osmolarity both as they approach a solid surface and as they exist within a biofilm. These changes in osmolarity result in the expression of surface- and biofilm-specific genes (31, 35). FW213 may perceive the increase in glucose concentration as an increase in osmolarity, thus triggering the expression of additional genes required for biofilm formation. An increase in sucrose concentration also promoted FW213 biofilm formation (data not shown), giving additional support to the hypothesis that osmolarity may be affecting biofilm formation. At some point, however, increases in osmolarity begin to inhibit biofilm formation (26, 31).
An increase of glucose or sucrose concentration in TH broth affects the final pH of the medium after bacterial growth (data not shown). Thus, localized changes in pH maybe also be important in biofilm formation. However, in other organisms, no effect on biofilm formation is seen over a range of pH. With Pseudomonas fluorescens, no effect on biofilm formation was seen in media ranging from pH 5 to 8.5 (31). Similarly, Streptococcus gordonii biofilm formation was unaffected in media ranging from pH 6 to 10.5 (26). Additional studies are required to elucidate the relationship of carbon source availability, osmolarity, and pH to biofilm formation in S. parasanguis FW213.
Conclusions.
This study was undertaken to investigate whether S. parasanguis FW213 can form biofilms in vitro and to determine some of the environmental parameters that affect biofilm formation. It was determined that S. parasanguis FW213 can form biofilms on solid surfaces, such as polystyrene and polyvinyl-chloride, under a range of growth conditions. Glucose promoted biofilm formation in FW213, suggesting that glucose is an environmental signal regulating FW213 biofilm development. This result supports the idea that bacteria form biofilms under favorable nutrient conditions. It does not rule out, however, the possibility that other factors associated with an increase in glucose concentration, such as pH and/or osmolarity, play a role in biofilm formation.
We demonstrated that those FW213 strains deficient in the production of Fap1, and hence in the production of fimbriae, are defective in the ability to form biofilms on a solid surface. Discrete stages of biofilm formation of both the wild type and the fap1 mutant on polystyrene were examined via phase-contrast microscopy. Differences in initial attachment and in the progression of biofilm formation over time indicate that Fap1 and fimbriae play a role in early cell-to-surface interactions during biofilm formation. The few fap1 mutant cells attached to the surface never formed cell aggregates or microcolonies. Further studies are needed to determine whether Fap1 and fimbriae also play a role in cell-to-cell interactions.
Currently the biofilm assay is being used in studies aimed at identification of additional genes necessary for biofilm initiation and/or development in S. parasanguis FW213. Understanding critical steps involved in biofilm formation and metabolism may suggest new therapies for treatment or prevention of biofilm-related infections.
ACKNOWLEDGMENTS
We thank Diane Meyer, Carlene Raper, Tom Lewis, and Joyce Oetjen for helpful comments and review of the manuscript and Gary Ward for help with microscopy.
This work was supported by Public Health Service grant R37-DE11000 from the National Institutes of Health to P.F.-T. and an award from the University of Vermont Committee on Research and Scholarship to E.H.F.
REFERENCES
- 1.Burnette-Curley D, Wells V, Viscount H, Munro C L, Fenno J C, Fives-Taylor P, Macrina F L. FimA, a major virulence factor associated with Streptococcus parasanguis endocarditis. Infect Immun. 1995;63:4669–4674. doi: 10.1128/iai.63.12.4669-4674.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carlsson J, Grahnen M, Johnson G, Wikner S. Establishment of Streptococcus sanguis in the mouths of infants. Arch Oral Biol. 1970;15:1143–1148. doi: 10.1016/0003-9969(70)90005-1. [DOI] [PubMed] [Google Scholar]
- 3.Christensen G D, Simson W A, Younger J J, Baddour L M, Barrrett F F, Melton D M, Beachy E H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole R M, Calandra G B, Huff E, Nugent K M. Attributes of potential utility in differentiating among ‘group H’ streptococci or Streptococcus sanguis. J Dental Res. 1976;55:A142–153. doi: 10.1177/002203457605500106011. [DOI] [PubMed] [Google Scholar]
- 5.Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 6.Costerton J W, Stewart S S, Greenberg E P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 7.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 8.Dintilhac A, Claverys J-P. The adc locus, which affects competence for genetic transformation in Streptococcus pneumoniae, encodes an ABC transporter with a putative lipoprotein homologous to a family of streptococcal adhesins. Res Microbiol. 1997;148:119–131. doi: 10.1016/S0923-2508(97)87643-7. [DOI] [PubMed] [Google Scholar]
- 9.Durack D. Prevention of infective endocarditis. N Engl J Med. 1995;332:38–44. doi: 10.1056/NEJM199501053320107. [DOI] [PubMed] [Google Scholar]
- 10.Durack D T, Beeson P B. Experimental bacterial endocarditis. I. Colonization of a sterile vegetation. Br J Exp Pathol. 1972;53:44–49. [PMC free article] [PubMed] [Google Scholar]
- 11.Elder B L, Boraker D K, Fives-Taylor P M. Whole bacteria cell enzyme-linked immunosorbent assay for Streptococcus sanguis fimbrial antigens. J Clin Microbiol. 1982;16:141–144. doi: 10.1128/jcm.16.1.141-144.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erickson P R, Herzberg M C. Purification and partial characterization of a 65-kDa platelet aggregation-associated antigen from the surface of Streptococcus sanguis. J Biol Chem. 1990;265:14080–14087. [PubMed] [Google Scholar]
- 13.Fachon-Kalweit S, Elder B L, Fives-Taylor P. Antibodies that bind to fimbriae block adhesion of Streptococcus sanguis to saliva-coated hydroxyapatite. Infect Immun. 1985;48:617–624. doi: 10.1128/iai.48.3.617-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fenno J C. Genetic analysis of a chromosomal locus of Streptococcus parasanguis encoding a fimbrial adhesin and membrane transport system. Ph.D. thesis. Burlington: University of Vermont; 1993. [Google Scholar]
- 15.Fenno J C, Shaikh A, Spatafora G, Fives-Taylor P. The fimA locus of Streptococcus parasanguis encodes an ATP-binding membrane transport system. Mol Microbiol. 1995;15:849–863. doi: 10.1111/j.1365-2958.1995.tb02355.x. [DOI] [PubMed] [Google Scholar]
- 16.Fives-Taylor P M, Thompson D W. Surface properties of Streptococcus sanguis FW213 mutants nonadherent to saliva-coated hydroxyapatite. Infect Immun. 1985;47:752–759. doi: 10.1128/iai.47.3.752-759.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fletcher M. The effects of culture concentration and age, time, and temperature on bacterial attachment to polystyrene. Can J Microbiol. 1977;23:1–6. [Google Scholar]
- 18.Franciolo P. Antibiotic treatment of streptococcal and enterococcal endocarditis: an overview. Eur Heart J. 1995;16(Suppl. B):75–79. doi: 10.1093/eurheartj/16.suppl_b.75. [DOI] [PubMed] [Google Scholar]
- 19.Froeliger E H, Oetjen J, Bond J P, Fives-Taylor P. Streptococcus parasanguis pepO encodes an endopeptidase with structure and activity similar to those of enzymes that modulate peptide receptor signaling in eukaryotic cells. Infect Immun. 1999;67:5206–5214. doi: 10.1128/iai.67.10.5206-5214.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Froeliger E H, Tomich M, Fives-Taylor P. Construction and analysis of a Streptococcus parasanguis recA mutant: homologous recombination is not required for adhesion in an in vitro tooth surface model. J Bacteriol. 1999;181:63–67. doi: 10.1128/jb.181.1.63-67.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbons R J, VanHoute J. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- 22.Habash M, Reid G. Microbial biofilms: their development and significance for medical device-related infections. J Clin Pharmacol. 1999;39:887–898. doi: 10.1177/00912709922008506. [DOI] [PubMed] [Google Scholar]
- 23.Hyde J A J, Darouiche R O, Costerton J W. Strategies for prophylaxis against prosthetic value endocarditis: a review article. J Heart Valve Dis. 1998;7:316–326. [PubMed] [Google Scholar]
- 24.Johnson C M. Adherence events in the pathogenesis of infective endocarditis. Infect Dis Clin North Am. 1993;7:21–36. [PubMed] [Google Scholar]
- 25.Kolenbrander P E, London J. Adhere today, here tomorrow: oral bacterial adherence. J Bacteriol. 1993;175:3247–3252. doi: 10.1128/jb.175.11.3247-3252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loo C Y, Corliss D A, Ganeshkumar N. Streptococcus gordonii biofilm formation: identification of genes that encode for biofilm phenotypes. J Bacteriol. 2000;182:1374–1382. doi: 10.1128/jb.182.5.1374-1382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowrance J H, Baddour L M, Simpson W A. The role of fibronectin binding in the rat model of experimental endocarditis caused by Streptococcus sanguis. J Clin Investig. 1990;86:7–13. doi: 10.1172/JCI114717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lutwick L I, Vaghjimal A, Connolly M W. Postcardiac surgery infections. Crit Care Clin. 1998;14:221–250. doi: 10.1016/s0749-0704(05)70393-6. [DOI] [PubMed] [Google Scholar]
- 29.O'Toole G A, Gibbs K A, Hager P W, Phibbs P V, Jr, Kolter R. The global carbon metabolism regulator Crc is a component of a signal transduction pathway required for biofilm development by Pseudomonas aeruginosa. J Bacteriol. 2000;182:425–431. doi: 10.1128/jb.182.2.425-431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 31.O'Toole G A, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signaling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 32.Potera C. Foraging a link between biofilms and disease. Science. 1999;283:1837–1839. doi: 10.1126/science.283.5409.1837. [DOI] [PubMed] [Google Scholar]
- 33.Pratt L A, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 34.Pratt L A, Kolter R. Genetic analyses of bacterial biofilm formation. Curr Opin Microbiol. 1999;2:598–603. doi: 10.1016/s1369-5274(99)00028-4. [DOI] [PubMed] [Google Scholar]
- 35.Prigent-Combaret C, Vidal O, Dorel C, Lejeune P. Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli. J Bacteriol. 1999;181:5993–6002. doi: 10.1128/jb.181.19.5993-6002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Socransky S S, Manganiello S A, Propas D, Oram Y, van Houte J. Bacteriological studies of developing supragingival plaque. J Periodont Res. 1977;12:90–106. doi: 10.1111/j.1600-0765.1977.tb00112.x. [DOI] [PubMed] [Google Scholar]
- 37.Stickler D. Biofilms. Curr Opin Microbiol. 1999;2:270–275. doi: 10.1016/S1369-5274(99)80047-2. [DOI] [PubMed] [Google Scholar]
- 38.Stulke J, Hillen W. Carbon catabolite repression in bacteria. Curr Opin Microbiol. 1999;2:195–201. doi: 10.1016/S1369-5274(99)80034-4. [DOI] [PubMed] [Google Scholar]
- 39.Sullam P M, Drake T A, Sande M A. Pathogenesis of endocarditis. Am J Med. 1985;78(Suppl. 6B):110–115. doi: 10.1016/0002-9343(85)90373-0. [DOI] [PubMed] [Google Scholar]
- 40.Sullam P M, Frank U, Yeaman M R, Tauber M G, Bayer A S, Chambers H F. Effect of thrombocytopenia on the early course of streptococcal endocarditis. J Infect Dis. 1993;168:910–914. doi: 10.1093/infdis/168.4.910. [DOI] [PubMed] [Google Scholar]
- 41.Terleckyj B, Willett N P, Shockman G D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975;11:649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tunkell A R, Mandell G L. Infecting microorganisms. In: Kaye D, editor. Infectious endocarditis. New York, N.Y: Raven Press; 1992. pp. 85–97. [Google Scholar]
- 43.Vidal O, Longin R, Prigent-Combaret C, Dorel C, Hooreman M, Lejeune P. Isolation of an Escherichia coli K-12 mutant strain able to form biofilms on inert surfaces: involvement of a new ompR allele that increases curli expression. J Bacteriol. 1998;180:2442–2449. doi: 10.1128/jb.180.9.2442-2449.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watnick P I, Kolter R. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol Microbiol. 1999;34:586–595. doi: 10.1046/j.1365-2958.1999.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu H, Mintz K P, Ladha M, Fives-Taylor P M. Isolation and characterization of Fap1, a fimbriae-associated adhesin of Streptococcus parasanguis FW213. Mol Microbiol. 1998;28:487–500. doi: 10.1046/j.1365-2958.1998.00805.x. [DOI] [PubMed] [Google Scholar]
- 46.Wu H, Fives-Taylor P M. Identification of dipeptide repeats and a cell wall sorting signal in the fimbriae-associated adhesin, Fap1 of Streptococcus parasanguis. Mol Microbiol. 1999;34:1070–1081. doi: 10.1046/j.1365-2958.1999.01670.x. [DOI] [PubMed] [Google Scholar]