Abstract
Coxiella burnetii, the agent of Q fever, is an obligate intracellular microorganism that grows in monocytes/macrophages. The internalization of virulent organisms by monocytes is lower than that of avirulent variants and is associated with actin cytoskeleton reorganization. We studied the activation of protein tyrosine kinases (PTKs) by C. burnetii in THP-1 monocytes. Virulent organisms induced early PTK activation and the tyrosine phosphorylation of several endogenous substrates, including Hck and Lyn, two Src-related kinases. PTK activation reflects C. burnetii virulence since avirulent variants were unable to stimulate PTK. We also investigated the role of PTK activation in C. burnetii-stimulated F-actin reorganization. Tyrosine-phosphorylated proteins were colocalized with F-actin inside cell protrusions induced by C. burnetii, and PTK activity was increased in Triton X-100-insoluble fractions. In addition, lavendustin A, a PTK inhibitor, and PP1, a Src kinase inhibitor, prevented C. burnetii-induced cell protrusions and F-actin reorganization. We finally assessed the role of PTK activation in bacterial phagocytosis. Pretreatment of THP-1 cells with lavendustin A and PP1 upregulated the uptake of virulent C. burnetii but had no effect on the phagocytosis of avirulent organisms. Thus, it is likely that PTK activation by C. burnetii negatively regulates bacterial uptake by interfering with cytoskeleton organization.
Coxiella burnetii, the cause of Q fever, is an obligate intracellular microorganism that inhabits monocytes/macrophages (23). This gram-negative bacterium is classified in gamma subdivision of class Proteobacteria (33). The virulence of C. burnetii affects its entry into macrophages (3). Indeed, virulent organisms are poorly internalized and survive in monocytes, whereas avirulent variants are efficiently phagocytozed but are eliminated. The uptake of virulent C. burnetii depends on αvβ3 integrin, whereas that of avirulent bacteria requires αvβ3 integrin and CR3 (αMβ2 integrin, CD11b/CD18). The coengagement of αvβ3 integrin and CR3 is responsible for the efficient phagocytosis of avirulent C. burnetii by monocytes, and the restricted phagocytosis of virulent organisms is related to the impairment of CR3 activity. In addition, only virulent organisms induce cell protrusions rich in F-actin in monocytes (22), suggesting that the actin cytoskeleton is involved in the control of C. burnetii phagocytosis.
The phagocytosis of particles by macrophages depends primarily on the reorganization of actin cytoskeleton underlying the region of plasma membrane that is in contact with the particle. F-actin assembly in this region is initiated by signals arising from the interaction between ligand and phagocyte receptors (13). The phagocytosis mediated by immunoglobulin Fc receptors (FcγR) depends on the activation of protein tyrosine kinases (PTK), as demonstrated by the use of PTK inhibitors or the replacement of tyrosine residues in tyrosine activation motifs of FcγR (28). Although the mechanism of integrin-mediated phagocytosis is less understood, it may also involve cytoskeleton reorganization and PTK activation. Hence, the engagement of β1 and β2 integrins on neutrophils and macrophages leads to the phosphorylation of cytoskeleton-associated proteins and the redistribution of integrins into cytoskeleton (20, 36). In nonphagocytic cells, the activation of PTK also provides an uptake signal for several invasive pathogens such as Yersinia species (27), Listeria monocytogenes (17), enteropathogenic Escherichia coli (7), Helicobacter pylori (29), and Campylobacter species (35). In addition to its effect on phagocytosis, the activation of PTK favors the microbicidal activity of phagocytic cells. To prevent PTK-mediated microbicidal responses, some pathogens down-modulate the PTK pathway. Hence, YopH, the plasmid product of Yersinia species that contains C-terminal tyrosine phosphatase domain, mediates the dephosphorylation of tyrosine phosphoproteins such as p130cas (1), and it inhibits bacterial internalization by macrophages (2). Salmonella enterica serovar Typhimurium possesses a tyrosine phosphatase, SptP, which once injected into target cells induces the disruption of actin cytoskeleton and thus may regulate bacterial uptake by phagocytes (11). Alternatively, Mycobacterium tuberculosis and/or its lipoarabinomannan down-regulates macrophage activation by stimulating the activity of SHP-1, a cytosolic protein tyrosine phosphatase (25).
In this study, we examined whether C. burnetii stimulates PTK activity in THP-1 monocytes and if PTK activation is related to bacterial uptake through cytoskeleton reorganization. We showed that virulent C. burnetii organisms, but not avirulent organisms, induced an increase in PTK activity and the tyrosine phosphorylation of several endogenous substrates including myeloid Src-related kinases, Hck and Lyn. The tyrosine phosphoproteins stimulated by C. burnetii were redistributed in cell protrusions, and PTK activity was increased in Triton X-100-insoluble fraction, showing that PTK activation is related to cytoskeleton rearrangement. In addition, the uptake of virulent C. burnetii was increased by PTK and Src kinase inhibitors, suggesting that PTK activation is critical for the phagocytosis of virulent C. burnetii through cytoskeleton modulation.
MATERIALS AND METHODS
Cells and bacteria.
The human myelomonocytic cell line THP-1 was cultured as previously described (22). Cells were propagated at an initial density of 4 × 105 cells per ml in RPMI 1640 containing 20 mM HEPES, 10% fetal bovine serum, 2 mM l-glutamine, penicillin (100 U ml−1), and streptomycin (100 μg ml−1) (Gibco-BRL, Life Technologies, Eragny, France) by biweekly passages. THP-1 cells were maintained in Hanks' balanced salt solution (HBSS) for 4 h at 37°C before stimulation. C. burnetii organisms (Nine Mile strain) were injected into mice as previously described (3). They were recovered from spleens after 10 days and were cultured in mouse L929 fibroblasts maintained in antibiotic-free Eagle minimal essential medium (Gibco-BRL) supplemented with 4% fetal bovine serum and 2 mM l-glutamine for two passages. Avirulent variants of C. burnetii were cultured in L929 cells by repeated passages (21). After 1 week, L929 cells were sonicated, and the homogenates were centrifuged at 8,000 × g for 10 min. Bacteria were layered on 25 to 45% linear Renografin gradient. Then the gradients were spun down, and the bacteria were collected, washed, and suspended in serum-free HBSS before being stored at −80°C. The concentration of C. burnetii was determined by Gimenez staining. Bacterial viability was determined as previously described (6). Briefly, monolayers of HEL cells were infected in shell vials. After 10 days, cells were fixed and intracellular C. burnetii organisms were revealed by indirect immunofluorescence. Viable organisms were assessed by measuring the number of fluorescent vacuoles per shell vial.
Tyrosine kinase assay.
THP-1 cells were stimulated with C. burnetii (bacterium-to-cell ratio of 200:1) in HBSS containing 2 mM sodium orthovanadate for different periods at 37°C. In some experiments, they were preincubated with cytochalasin D (1 μg ml−1; Sigma Chemical Co., St. Louis, Mo.) for 10 min before bacterial stimulation. Thereafter, THP-1 cells were homogenized in the presence of protease inhibitors as previously described (37). For cytoskeletal preparations, 1% Triton X-100 was added to cells for 10 min at 4°C, and the lysates were spun down at 15,800 × g for 30 min. The supernatant (Triton-soluble fraction) was saved, and the pellet (Triton-insoluble fraction) was resuspended in the same volume of lysis buffer. Twenty microliters of lysate or fractions (corresponding to 100 μg of proteins ml−1) was added to a 30-μl reaction mixture consisting of p-nitrophenylphosphate (0.5 μg ml−1) and glutamine-tyrosine copolymer [poly(Glu, Tyr); (1 μg ml−1) Sigma]. The reaction was started by adding 1 μCi of [γ-32P]ATP (10 Ci mmol−1; NEN, Paris, France) in the presence of 10 μM ATP. After 10 min at 30°C, 40 μl of solution was spotted on filter paper (P81; Whatman), and radioactivity was counted with a model 2100TR Packard scintillation counter.
Western blotting.
THP-1 cells (106 per assay) were incubated with C. burnetii for different periods at 37°C. The reaction was stopped by centrifugation at 4°C, and the experiment was performed as previously described (37). Cell pellets were suspended in ice-cold lysis solution containing orthovanadate and protease inhibitors (Roche Diagnostics, Meylan, France). After 30 min of incubation, Laemmli buffer was added to cell homogenates. The mixture was boiled, and about 30 μg of proteins was loaded on sodium dodecyl sulfate (SDS)–7.5% polyacrylamide gels. The proteins were transferred to nitrocellulose sheets, and unreacted sites were blocked by incubating sheets in a solution containing 0.05% Tween 20 and 5% milk for 2 h. Blots were washed and incubated with a 1:3,000 dilution of monoclonal antibody (MAb) directed against phosphotyrosine (PY-99; Santa-Cruz Biotechnology, Tebu, Perray-en-Yvelines, France) for 60 min. After washing, nitrocellulose sheets were incubated with a 1:3,000 dilution of peroxidase-conjugated F(ab′)2 anti-mouse immunoglobulin G (IgG; Amersham, Orsay, France) for 60 min. Blots were revealed using an enhanced chemiluminescence detection kit as specified by the manufacturer (Amersham). The molecular weight of tyrosine-phosphorylated proteins was determined with kaleidoscope standards (Bio-Rad Laboratories, Marnes-la-Coquette, France). Autoradiographs were quantitated by scanning densitometry and absorbance curves integrated using Image Master software (Pharmacia Biotech, St. Quentin-en-Yvelines, France). Densitometric analyses were performed on gels with different exposure times, and the ones giving linear absorbance curves were used to obtain semiquantitative assessment.
Immunoprecipitation assay.
THP-1 cells (4 × 106 per assay) were stimulated with C. burnetii for different periods, and cell homogenates were incubated overnight with 1 μg of rabbit affinity-purified antibody (Ab) (Santa Cruz Biotechnology) directed against Hck, Lyn, or Fgr in lysis buffer. Protein G-agarose beads (Roche Diagnostics) were added to cell preparations for 45 min. The beads were then washed three times with buffer consisting of 200 mM NaCl, 20 mM Tris-HCl, 1 mM EDTA, and 1% Triton X-100 and again washed with the same solution containing 500 mM NaCl. After a final washing with phosphate-buffered saline, the immunoprecipitates were spun down and suspended in 30 μl of Laemmli buffer. Samples were loaded on SDS–10% polyacrylamide gels, electrophoresed, and transferred onto nitrocellulose sheets. Tyrosine-phosphorylated proteins were revealed using MAb PY-99 as described above for Western blotting. Membranes were stripped and reprobed with anti-Hck, anti-Lyn, or anti-Fgr Ab.
Laser scanning confocal fluorescence microscopy.
The colocalization between tyrosine phosphoproteins and F-actin was determined as follows. THP-1 cells were incubated with C. burnetii in HBSS containing sodium orthovanadate for different periods and fixed with 3.7% formaldehyde. After permeabilization with lysophosphatidylcholine (LPC; 0.1 mg ml−1; Sigma) in HBSS, cells were incubated with antiphosphotyrosine MAb (1:20 dilution) for 30 min, rhodamine-conjugated F(ab′)2 anti-mouse IgG (Immunotech, Marseille, France) and 10 bodipy phallacidin (10 U ml−1; Molecular Probes, Eugene, Oreg.) for 20 min. The specimens were mounted in slow-fade solution (Molecular Probes) and examined with a laser scanning confocal fluorescence microscope (Leica, Lyon, France) equipped with a 60× (numerical aperture, 1.4) oil immersion lens as previously described (4). Serial optical sections of images were collected at 0.5-μm intervals and analyzed with Adobe Photoshop 3.0. Bodipy and rhodamine images were converted into green and red images and merged to synthesize a yellow color.
PTK inhibitors and bacterial phagocytosis.
THP-1 cells (5 × 105 per assay) were pretreated with 10 μM lavendustin A (BioMol, Tebu), 10 μM PP1 (Alexis Biochemicals, Coger, Paris), or dimethyl sulfoxide as vehicle for 30 min and then incubated with C. burnetii in 0.5 ml of HBSS at 37°C. In some experiments, they were pretreated with 2 μM calphostin C, 0.1 μM KT5926, or 5 μM ML-7 (BioMol), inhibitors of protein kinase C, calmodulin kinase II, and myosin light chain kinase, respectively. After 2 h, cells were washed to remove free bacteria and centrifuged at 800 × g. Then they were fixed with 1% formaldehyde, and bacteria were revealed by immunofluorescence as previously described (3). Briefly, cell preparations were incubated with rabbit Ab directed against C. burnetii at 1:250 in the presence or the absence of LPC (0.1 mg ml−1) washed, and incubated with a 1:200 dilution of fluorescein isothiocyanate-conjugated F(ab′)2 anti-rabbit IgG (Immunotech). Without LPC, only cell-bound organisms were revealed; bound and ingested organisms were revealed in the presence of LPC. The association index was quantified as follows: (number of bacteria per positive cell) × (percentage of positive cells) × 100. The difference of indexes in the presence and the absence of LPC quantified the uptake of C. burnetii (phagocytosis index).
Data analysis.
Results are given as mean ± standard error (SE). The statistical analysis was conducted with paired Student's t test. Differences were considered as significant if P < 0.05.
RESULTS
C. burnetii-induced PTK activity and tyrosine phosphorylations.
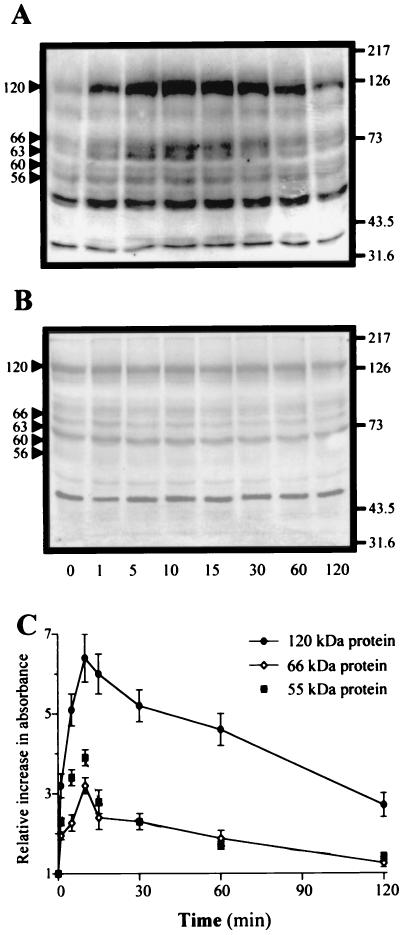
THP-1 cells were incubated with C. burnetii at a bacterium-to-cell ratio of 200:1 for different periods, and PTK activation was determined using an assay based on the incorporation of 32P into poly(Glu,Tyr) (Table 1). The addition of virulent C. burnetii to THP-1 monocytes elicited PTK activation, which was increased fourfold after 10 min. Then, the PTK activity steadily decreased, reaching baseline values between 30 and 60 min. In contrast, avirulent variants of C. burnetii were unable to elicit an increase in PTK activity whatever the duration of the experiment. We also determined the molecular masses of endogenous substrates that were tyrosine phosphorylated by Western blotting. C. burnetii stimulated the tyrosine phosphorylation of proteins migrating at 55 to 56, 60, 63 to 66, 90, and 120 kDa (Fig. 1A). Their phosphorylation levels increased after 1 min of stimulation, reached maximum values between 10 and 15 min, and then steadily decreased to resting values at 120 min. Avirulent variants of C. burnetii did not elicit a significant increase in tyrosine phosphorylations whatever the time of stimulation (Fig. 1B). Phosphorylation levels of the major substrates were quantified by densitometric scanning (Fig. 1C). The phosphorylation of 55- and 66-kDa proteins at 10 min was threefold higher than unstimulated values. Phosphorylation of the 120-kDa protein was increased threefold after 1 min and reached a maximum (sixfold increase) after 10 min. Hence, C. burnetii virulence was related to PTK activation and tyrosine phosphorylation of cellular substrates.
TABLE 1.
C. burnetti-stimulated kinase activitiesa
| C. burnetti | Mean cpm after stimulation/cpm before stimulationb ± SE (n = 5)
|
||
|---|---|---|---|
| 10 min | 30 min | 60 min | |
| Virulent | 3.85 ± 1.22c | 1.94 ± 0.45 | 1.10 ± 0.07 |
| Avirulent | 1.14 ± 0.10 | 1.10 ± 0.11 | 1.05 ± 0.03 |
THP-1 cells were incubated with C. burnetii at a bacterium-to-cell ratio of 200:1 for 10, 30, and 60 min and then homogenized in lysis buffer. The reaction was started by incubating cell homogenates with poly(Glu,Tyr) and 1 μCi [γ-32P]ATP; radioactivity was measured with a scintillation counter.
59,580 ± 7,475 cpm.
P < 0.05.
FIG. 1.
Tyrosine phosphorylations stimulated by C. burnetii. THP-1 cells were incubated with virulent (A) or avirulent C. burnetii (B) at a bacterium-to-cell ratio of 200:1 for different periods at 37°C. Tyrosine phosphorylations were revealed by using antiphosphotyrosine MAb, peroxidase-conjugated secondary Ab, and an enhanced chemiluminescence kit. The molecular masses of phosphoproteins were determined using standards of known molecular mass (right margin, in kilodaltons). Arrowheads at the left indicate positions of phosphorylated proteins. Panels A and B are representative of five distinct experiments. (C) Tyrosine phosphorylation levels of 55-, 66-, and 120-kDa proteins were assessed by densitometric scanning. Results are expressed as relative increase over the unstimulated value and are the mean ± SE of five distinct experiments.
C. burnetii-stimulated tyrosine phosphorylation of Src-related kinases.
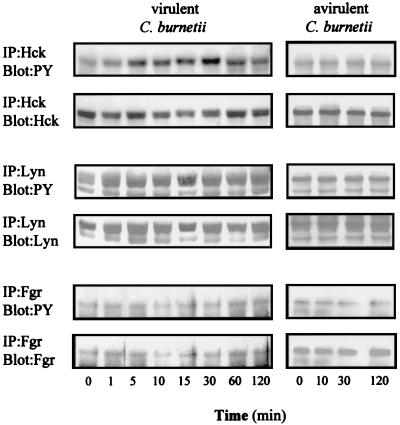
C. burnetii-stimulated phosphoproteins migrating from 55 to 66 kDa may be Src-related kinases. The three main monocyte PTKs, Hck, Lyn, and Fgr (37), were immunoprecipitated from whole cell extract, and their phosphorylation levels were assessed (Fig. 2). Hck migrated as a 60-kDa protein. Its tyrosine phosphorylation was increased 2.7 ± 0.2-fold after 5 min of stimulation with virulent C. burnetii and became maximum (3.9 ± 0.3-fold increase) at 30 min, before returning to baseline by 120 min. The amounts of immunoprecipitated Hck remained constant during the 120 min of stimulation. Lyn migrated as a doublet of 53- and 55-kDa proteins; their phosphorylation was increased 2.2 ± 0.2-fold after 1 min and remained constant during 120 min. Fgr was immunoprecipitated as a 58-kDa protein but was not tyrosine phosphorylated in response to C. burnetii. Again, avirulent variants of C. burnetii were unable to stimulate the tyrosine phosphorylation of Hck and Lyn (Fig. 2). Thus, virulent C. burnetii specifically stimulated the tyrosine phosphorylation of Hck and Lyn.
FIG. 2.
Src-related kinases in C. burnetii-stimulated cells. THP-1 cells were stimulated with virulent or avirulent C. burnetii for different periods, homogenized, and incubated with 1 μg of anti-Hck, -Lyn, or -Fgr MAb. Proteins were immunoprecipitated (IP), electrophoresed, and transferred onto nitrocellulose sheets. Immunoblotting was performed with antiphosphotyrosine MAb (PY). Membranes were stripped and reprobed with anti-Hck, -Lyn, and -Fgr MAbs, respectively. Each blot is representative of three distinct experiments.
Association between tyrosine phosphoproteins and actin cytoskeleton.
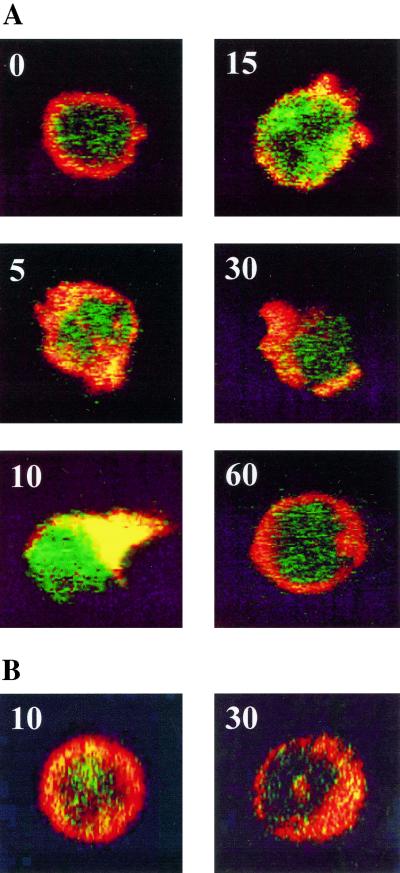
As virulent C. burnetii induces the early reorganization of F-actin inside polarized protrusions in THP-1 cells (22), we wondered if the stimulation of PTK and the reorganization of actin cytoskeleton are related. First, we investigated the localization of tyrosine phosphoproteins and F-actin in C. burnetii-stimulated THP-1 cells (Fig. 3). Only virulent organisms induced a maximum reorganization of F-actin at 10 min, according to previous findings (22). Tyrosine phosphoproteins were detected in areas rich in F-actin after 5 min and reached maximal concentrations in cell protrusions at 10 min. After 15 min of stimulation, phosphotyrosine staining declined with the recovery of cell shape. Second, C. burnetii-stimulated THP-1 cells were treated with 1% Triton X-100 and spun down, leading to Triton-soluble and Triton-insoluble fractions. Virulent C. burnetii organisms significantly (P < 0.05) enhanced PTK activity in the Triton-insoluble fraction but elicited only a moderate increase in PTK activity in the Triton-soluble fraction (Fig. 4A). Avirulent variants of C. burnetii did not stimulate PTK activity in Triton-insoluble and -soluble fractions (Fig. 4B). Third, THP-1 cells were pretreated by cytochalasin D and then stimulated by C. burnetii. Cytochalasin D at 1 μg ml−1 markedly impaired the activation of PTK induced by virulent organisms in Triton-insoluble fraction (Fig. 4). Taken together, these results showed that phosphoproteins and F-actin reorganization stimulated by virulent C. burnetii were associated.
FIG. 3.
Colocalization of tyrosine phosphoproteins and F-actin. THP-1 cells were stimulated with virulent (A) or avirulent (B) C. burnetii for the times (minutes) indicated. Tyrosine phosphoproteins and F-actin were labeled with antiphosphotyrosine MAb and rhodamine-conjugated F(ab′)2 anti-mouse IgG and with bodipy phallacidin, respectively. Cells were examined with a laser scanning confocal fluorescence microscope, and representative cells are shown. The colocalization of tyrosine phosphoproteins and F-actin appears in yellow.
FIG. 4.
Distribution of PTK activity in cell fractions. THP-1 cells were pretreated with cytochalasin D (1 μg ml−1) or not pretreated and stimulated by virulent (A) or avirulent (B) C. burnetii for 10 min. Cells were then lysed by 1% Triton X-100. Triton-soluble and -insoluble fractions were incubated with poly(Glu, Tyr) and 1 μCi of [γ-32P]ATP. Radioactivity was measured with a scintillation counter; cpm in Triton-soluble fraction before stimulation = 29,500 ± 5,870; cpm in Triton-insoluble fraction before stimulation = 25,660 ± 5,110. Results are expressed as the ratio of counts after stimulation to counts before stimulation and represent the mean ± SE of five distinct experiments. ∗, P < 0.05.
Effect of PTK inhibitors on the F-actin reorganization and C. burnetii phagocytosis.
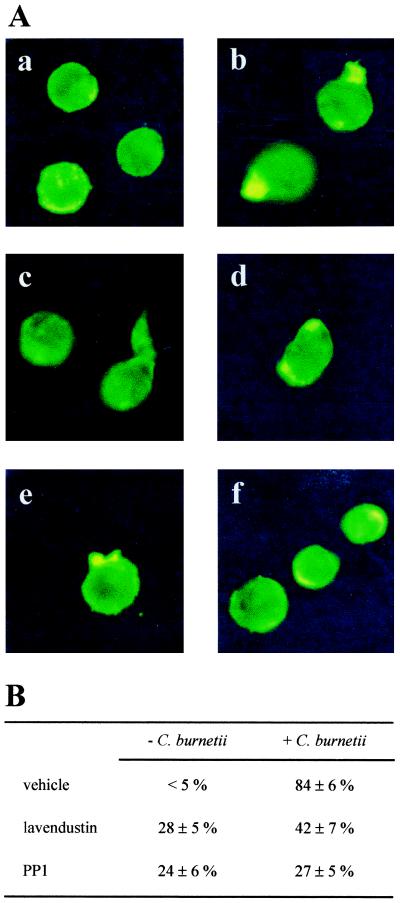
The role of PTK in the reorganization of F-actin cytoskeleton was assessed by using lavendustin A, an inhibitor of PTK, and PP1, an inhibitor of Src-related kinases (32). The doses of inhibitors were determined as those inhibiting tyrosine phosphorylations induced by virulent C. burnetii (data not shown). THP-1 cells were pretreated with 10 μM lavendustin A or PP1 for 30 min before stimulation with C. burnetii for 10 min at 37°C. In unstimulated cells or cells incubated with avirulent variants of C. burnetii, lavendustin A, and PP1 to a lesser extent, induced aberrant reorganization of F-actin in 25 to 30% of cells; the changes included concentrations of F-actin inside the cells or at their periphery, as well as long and narrow protrusions at the cell surface (Fig. 5A. images c and d). Virulent C. burnetii induced polarized protrusions in 84% ± 6% of cells after 10 min, as previously reported (22). Cell pretreatment with lavendustin A and PP1 inhibited C. burnetii-stimulated polarized protrusions rich in F-actin in 50% (P < 0.01) and 68% (P < 0.001) of cells, respectively (Fig. 5). Using the same inhibitors, i.e., lavendustin A and PP1, we also investigated the role of PTK in the uptake of C. burnetii by THP-1 cells (Table 2). Pretreatment of THP-1 cells with 10 μM lavendustin A increased the phagocytosis of virulent C. burnetii 1.9-fold. PP1 at 10 μM increased the phagocytosis of virulent organisms in a similar way. In contrast, the two inhibitors had no effect on the phagocytosis of avirulent variants of C. burnetii. To rule out a bias caused by the high amount of phagocytosis of avirulent bacteria (3), THP-1 cells were incubated with a lower concentration of bacteria (bacterium-to-cell ratio of 50:1). Under these conditions, lavendustin A and PP1 at 10 μM were unable to increase the phagocytosis of avirulent variants of C. burnetii (Table 2). The effect of lavendustin A and PP1 was specific since nonrelated kinase inhibitors including inhibitors of protein kinase C (calphostin C), calmodulin kinase II (KT 5926), and myosin light chain kinase (ML-7) did not significantly affect the phagocytosis of virulent and avirulent C. burnetii by THP-1 cells (data not shown). These results suggest that Src-related kinases are involved in F-actin reorganization and bacterial phagocytosis stimulated by C. burnetii.
FIG. 5.
Effect of PTK inhibitors on F-actin organization. (A) THP-1 cells were pretreated with 10 μM PTK inhibitors for 30 min before stimulation with virulent C. burnetii for 10 min. F-actin was labeled with bodipy phallacidin, and its reorganization was examined with fluorescence microscopy. a, control cells; b, C. burnetii-stimulated cells; c, cells preincubated with lavendustin A; d, cells preincubated with lavendustin A and stimulated by C. burnetii; e, cells preincubated with PP1; f, cells preincubated with PP1 and stimulated by C. burnetii. (B) Percentage of cells with protrusions rich in F-actin.
TABLE 2.
Effects of PTK inhibitors on C. burnetii phagocytosisa
| C. burnetii | Phagocytosis index (mean ± SE (n = 3)
|
||
|---|---|---|---|
| No pretreatment | Lavendustin A | PP1 | |
| Virulent (200:1) | 130 ± 37 | 287 ± 29b | 262 ± 41b |
| Avirulent | |||
| 200:1 | 479 ± 69 | 422 ± 35 | 454 ± 51 |
| 50:1 | 218 ± 91 | 180 ± 19 | 195 ± 25 |
THP-1 cells were pretreated with 10 μM lavendustin or PP1 for 30 min before stimulation with C. burnetii at the bacterium-to-cell ratios indicated for 2 h at 37°C. Bacterial phagocytosis with determined by indirect immunofluorescence.
P < 0.02.
DISCUSSION
We show here that virulent C. burnetii organisms, but not avirulent variants, induced PTK activation in THP-1 monocytes. Bacterium-mediated activation of PTK has already been reported for nonphagocytic cells (8). In macrophages, the activation of PTK would create hostile environment for pathogens. Hence, the survival of microorganisms in macrophages has been rather associated with the limitation of PTK activation (2, 25). This study describes a novel strategy of PTK activation by an intracellular pathogen to establish an ecological niche in microbicidal cells.
PTK activation stimulated by virulent C. burnetii is associated with the tyrosine phosphorylation of three major endogenous substrates migrating as 55, 63 to 66, and 120 kDa. The 55-kDa substrate may correspond to the tyrosine phosphorylation of some Src-related kinases. Myeloid cells including THP-1 cells express mainly Hck, Lyn, and Fgr kinases (32, 37). Using immunoprecipitation assays, we showed that Hck was phosphorylated on tyrosine residues in response to C. burnetii, but the increase in Hck phosphorylation was delayed compared to whole tyrosine phosphorylations. Lyn was also tyrosine phosphorylated in response to virulent organisms. The increase in tyrosine phosphorylation levels of Lyn was lower than that of Hck but occurred earlier after the addition of C. burnetii. Thus, Lyn may be the initial Src-related PTK activated after the engagement of monocyte receptors by C. burnetii, and Hck may be a downstream target. Otherwise, the activation of Lyn may be required for early PTK activity whereas Hck may be involved in sustained PTK activation. The phosphorylation of Lyn and Hck was specific since Fgr was not tyrosine phosphorylated in response to virulent C. burnetii. This pattern of tyrosine phosphorylation of Src-related kinases is distinct from that caused by other pathogens (5, 15, 16, 30). We also showed that a 120-kDa protein was a major substrate of C. burnetii-stimulated PTK. Some cytoskeleton-associated PTK have been reported to migrate with similar molecular masses. Focal adhesion kinase may be a candidate but it was not expressed by THP-1 cells, and p130cas was not tyrosine phosphorylated in response to C. burnetii (data not shown). Recently, it has been reported that Pyk2, a PTK related to focal adhesion kinase, is expressed in myeloid cells and is tyrosine phosphorylated in response to lipopolysaccharide (34). Although Pyk2 was expressed in THP-1 cells, it was not tyrosine phosphorylated in response to virulent C. burnetii (data not shown). This 120-kDa protein remains to be identified.
We previously found that virulent C. burnetii organisms, but not avirulent variants, stimulate early reorganization of the actin cytoskeleton inside cell protrusions in THP-1 monocytes (22). Since the kinetics of tyrosine phosphorylations and F-actin rearrangement were superimposable, we studied the relationship between the two events. First, virulent C. burnetii induced the colocalization of tyrosine phosphoproteins and F-actin inside the protrusions. The association of PTK with F-actin is required for Shigella internalization (9). Second, PTK activity was increased in Triton-insoluble fraction of stimulated cells. This increase corresponded to an association with actin cytoskeleton rather than to lipid domains of the plasma membrane since it was inhibited by cytochalasin D. This finding may be related to enteropathogenic E. coli infection, in which the translocation of PTK substrates to the Triton-insoluble fraction is prevented by cytochalasin D (19). Third, PTK activation is involved in the induction of cell protrusions and F-actin reorganization by virulent C. burnetii. The inhibitors of PTK and Src kinases, lavendustin A and PP1, respectively, prevented C. burnetii-stimulated cell protrusions and F-actin rearrangement. These inhibitors also induced various changes in cell morphology, consisting of F-actin concentrations and filopodial structures, as elsewhere described (12). It is likely that the activation of Lyn and/or Hck by virulent C. burnetii results in the tyrosine phosphorylation of several substrates, including actin-associated proteins and subsequently in the reorganization of F-actin, both events leading to the constitution of actin-PTK complex inside cell protrusions.
In contrast to previously reported effects of PTK activation on bacterial phagocytosis (8), PTK activation negatively controlled C. burnetii uptake. Indeed, lavendustin A increased the uptake of virulent C. burnetii by THP-1 cells. This effect was specific to PTK since other kinase inhibitors had no effect. It was also specific to C. burnetii virulence since the phagocytosis of avirulent variants was not affected. The inhibitor of Src kinases, PP1, increased bacterial uptake as did lavendustin A, suggesting that Src kinases such as Hck and Lyn mediate the signal that restricts C. burnetii phagocytosis. Although Src-related kinases are known to be involved in FcγR-dependent phagocytosis (10, 24), they may also provide an inhibitory signal for FcγR- and CR3-mediated phagocytosis (14). As Src kinases affect cytoskeleton-dependent functions such as spreading and phagocytosis (9, 10, 31), it is likely that Hck and Lyn limit the phagocytosis of virulent C. burnetii through their effect on the actin cytoskeleton. Reorganization of the actin cytoskeleton may in turn affect the dynamics of receptors involved in the internalization of C. burnetii. We previously showed that the interaction of virulent C. burnetii with monocytes through αvβ3 integrin elicits a signal that restricts the availability of CR3 (3), thus accounting for the low efficiency of bacterial phagocytosis. Recent results showed that CR3 was not recruited in cell protrusions stimulated by virulent C. burnetii, which thus prevented functional interaction of αvβ3 integrin with CR3 (unpublished data). Hence, PTK activation may result in the formation of membrane ruffles that limit the redistribution of CR3 in contact zones between αvβ3 integrin and C. burnetii. Alternatively, αvβ3 integrin may be the target of PTK, thus interfering with the cross-talk between αvβ3 integrin, CR3, and cytoskeleton. For instance, it has been reported that the ligation of αvβ3 integrin leads to the tyrosine phosphorylation of the cytoplasmic domain of β3 chain and to its physical linkage to cytoskeleton (18, 26).
To conclude, we describe a novel mechanism of control of bacterial entry into phagocytic cells. The virulence of C. burnetii is associated with PTK activation including myeloid Src-related kinases, Hck and Lyn, and cytoskeleton reorganization. Both events control C. burnetii phagocytosis since specific inhibitors of PTK and Src kinases prevented F-actin reorganization and upregulated the uptake of C. burnetii. The control of C. burnetii phagocytosis likely impairs the mechanism by which macrophages eradicate the infection.
ACKNOWLEDGMENT
We thank Ivan Dikic for his generous gift of the anti-Pyk2 serum.
REFERENCES
- 1.Black D S, Bliska J B. Identification of p130cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 1997;16:2730–2744. doi: 10.1093/emboj/16.10.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bliska J B, Clemens J C, Dixon J E, Falkow S. The Yersinia tyrosine phosphatase: specificity of a bacterial virulence determinant for phosphoproteins in the J774A.1 macrophage. J Exp Med. 1992;176:1625–1630. doi: 10.1084/jem.176.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Capo C, Lindberg F P, Meconi S, Zaffran Y, Tardei G, Brown E J, Raoult D, Mege J L. Subversion of monocyte functions by Coxiella burnetii: impairment of the cross-talk between αvβ3 integrin and CR3. J Immunol. 1999;163:6078–6085. [PubMed] [Google Scholar]
- 4.Capo C, Meconi S, Sanguedolce M V, Bardin N, Flatau G, Boquet P, Mege J L. Effect of cytotoxic necrotizing factor-1 on actin cytoskeleton: role in the regulation of integrin-dependent phagocytosis. J Immunol. 1998;161:4301–4308. [PubMed] [Google Scholar]
- 5.Dehio C, Prevost M C, Sansonetti P J. Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp60c-src-mediated signalling pathway. EMBO J. 1995;14:2471–2482. doi: 10.1002/j.1460-2075.1995.tb07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dellacasagrande J, Capo C, Raoult D, Mege J L. IFN-γ-mediated control of Coxiella burnetii survival in monocytes: the role of cell apoptosis and TNF. J Immunol. 1999;162:2259–2265. [PubMed] [Google Scholar]
- 7.Donnenberg M S, Kaper J B, Finlay B B. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 8.Dramsi S, Cossart P. Intracellular pathogens and the actin cytoskeleton. Annu Rev Cell Dev Biol. 1998;14:137–166. doi: 10.1146/annurev.cellbio.14.1.137. [DOI] [PubMed] [Google Scholar]
- 9.Dumenil G, Sansonetti P, Tran Van Nhieu G. Src tyrosine kinase activity down-regulates Rho-dependent responses during Shigella entry into epithelial cells and stress fiber formation. J Cell Sci. 2000;113:71–80. doi: 10.1242/jcs.113.1.71. [DOI] [PubMed] [Google Scholar]
- 10.Fitzer-Attas C J, Lowry M, Crowley M T, Finn A J, Meng F, DeFranco A L, Lowell C A. Fcγ receptor-mediated phagocytosis in macrophages lacking the src family tyrosine kinases Hck, Fgr, and Lyn. J Exp Med. 2000;191:669–681. doi: 10.1084/jem.191.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu Y, Galan J E. The Salmonella typhimurium tyrosine phosphatase SptP is translocated into host cells and disrupts the actin cytoskeleton. Mol Microbiol. 1998;27:359–368. doi: 10.1046/j.1365-2958.1998.00684.x. [DOI] [PubMed] [Google Scholar]
- 12.Fuortes M, Melchior M, Han H, Lyon E J, Nathan C. Role of the tyrosine kinase pyk2 in the integrin-dependent activation of human neutrophils by TNF. J Clin Investig. 1999;104:322–327. doi: 10.1172/JCI6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg S. Signal transduction in phagocytosis. Trends Cell Biol. 1995;5:93–99. doi: 10.1016/s0962-8924(00)88957-6. [DOI] [PubMed] [Google Scholar]
- 14.Gresham H D, Dale B M, Porter J W, Chang P W, Vines C M, Lowell C A, Langenaur C F, Willman C L. Negative regulation of phagocytosis in murine macrophages by the src kinase member, Fgr. J Exp Med. 2000;191:515–528. doi: 10.1084/jem.191.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauck C R, Gulbins E, Lang F, Meyer T F. Tyrosine phosphatase SHP-1 is involved in CD66-mediated phagocytosis of Opa52-expressing Neisseria gonorrhoeae. Infect Immun. 1999;67:5490–5494. doi: 10.1128/iai.67.10.5490-5494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrera-Velit P, Reiner N E. Bacterial lipopolysaccharide induces the association and coordinate activation of p53/56lyn and phosphatidylinositol-3-kinase in human monocytes. J Immunol. 1996;156:1157–1165. [PubMed] [Google Scholar]
- 17.Ireton K, Payrastre B, Chap H, Ogawa W, Sakave H, Kasuga M, Cossart P. A role for phosphoinositide-3-kinase in bacterial invasion. Science. 1996;274:780–782. doi: 10.1126/science.274.5288.780. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins A L, Nannizzi-Alaimo L, Silver D, Sellers J R, Ginsberg M H, Law D A, Phillipps D R. Tyrosine phosphorylation of the β3 cytoplasmic domain mediates integrin-cytoskeletal interactions. J Biol Chem. 1998;273:13878–13885. doi: 10.1074/jbc.273.22.13878. [DOI] [PubMed] [Google Scholar]
- 19.Kenny B, Finlay B B. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-γ1. Infect Immun. 1997;65:2528–2536. doi: 10.1128/iai.65.7.2528-2536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lowell C A, Berton G. Integrin signal transduction in myeloid leukocytes. J Leukoc Biol. 1999;65:313–320. doi: 10.1002/jlb.65.3.313. [DOI] [PubMed] [Google Scholar]
- 21.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meconi S, Jacomo V, Boquet P, Raoult D, Mege J L, Capo C. Coxiella burnetii induces reorganization of the actin cytoskeleton in human monocytes. Infect Immun. 1998;66:5527–5533. doi: 10.1128/iai.66.11.5527-5533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mege J L, Maurin M, Capo C, Raoult D. Coxiella burnetii: the “query” fever bacterium. A model of immune subversion by a strictly intracellular microorganism. FEMS Microbiol Rev. 1997;19:209–217. doi: 10.1111/j.1574-6976.1997.tb00298.x. [DOI] [PubMed] [Google Scholar]
- 24.Meng F, Lowell C A. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of src-family kinase Hck, Fgr and Lyn. J Exp Med. 1997;185:1661–1670. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nandan D, Knutson K L, Lo R, Reiner N E. Exploitation of host cell signaling machinery: activation of macrophage phosphotyrosine phosphatases as a novel mechanism of molecular microbial pathogenesis. J Leukoc Biol. 2000;67:464–470. doi: 10.1002/jlb.67.4.464. [DOI] [PubMed] [Google Scholar]
- 26.Patil S, Jedsadayanmata A, Wencel-Drake J D, Wang W, Knezevic I, Lam S C. Identification of a talin-binding site in the integrin β3 subunit distinct from the NPLY regulatory motif of post-ligand binding functions. The talin N-terminal head domain interacts with the membrane proximal region of the β3 cytoplasmic tail. J Biol Chem. 1999;274:28575–28583. doi: 10.1074/jbc.274.40.28575. [DOI] [PubMed] [Google Scholar]
- 27.Rosenshine I, Duronio V, Finlay B B. Tyrosine protein kinase inhibitors block invasion-promoted bacterial uptake by epithelial cells. Infect Immun. 1992;60:2211–2217. doi: 10.1128/iai.60.6.2211-2217.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez-Mejorada G, Rosales C. Signal transduction by immunoglobulin Fc receptors. J Leukoc Biol. 1998;63:521–533. doi: 10.1002/jlb.63.5.521. [DOI] [PubMed] [Google Scholar]
- 29.Segal E D, Falkow S, Tompkins C S. Helicobacter pylori attachment to gastric cells induces cytoskeletal rearrangements and tyrosine phosphorylation of host cell proteins. Proc Natl Acad Sci USA. 1996;93:1259–1264. doi: 10.1073/pnas.93.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stefanova I, Corcoran M L, Horak E M, Wahl L M, Bolen J B, Horak I D. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J Biol Chem. 1993;268:20725–20728. [PubMed] [Google Scholar]
- 31.Suen P W, Ilic D, Caveggion E, Berton G, Damsky C H, Lowell C A. Impaired integrin-mediated signal transduction, altered cytoskeletal structure and reduced motility in Hck/Fgr deficient macrophages. J Cell Sci. 1999;112:4067–4078. doi: 10.1242/jcs.112.22.4067. [DOI] [PubMed] [Google Scholar]
- 32.Thomas S M, Brugge J S. Cellular functions regulated by src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 33.Weisburg W G, Dobson M E, Samuel J E, Dash G A, Mallavia L P, Baca O, Mandelco L, Sechrest J E, Weiss E, Woese C R. Phylogenetic diversity of Rickettsiae. J Bacteriol. 1989;171:4202–4206. doi: 10.1128/jb.171.8.4202-4206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams L M, Ridley A J. Lipopolysaccharide induces actin reorganization and tyrosine phosphorylation of Pyk2 and paxillin in monocytes and macrophages. J Immunol. 2000;164:2028–2036. doi: 10.4049/jimmunol.164.4.2028. [DOI] [PubMed] [Google Scholar]
- 35.Wooldridge K G, Ketley J M. Campylobacter-host cell interactions. Trends Microbiol. 1997;5:96–102. doi: 10.1016/S0966-842X(97)01004-4. [DOI] [PubMed] [Google Scholar]
- 36.Yan S R, Berton G. Antibody-induced engagement of β2 integrins in human neutrophils causes a rapid redistribution of cytoskeletal proteins, src-family tyrosine kinases, and p72syk that precedes de novo actin polymerization. J Leukoc Biol. 1998;64:401–408. doi: 10.1002/jlb.64.3.401. [DOI] [PubMed] [Google Scholar]
- 37.Zaffran Y, Escallier J C, Ruta S, Capo C, Mege J L. Zymosan-triggered association of tyrosine phosphoproteins and lyn kinase with the cytoskeleton in human monocytes. J Immunol. 1995;154:3488–3497. [PubMed] [Google Scholar]