Abstract
Pseudomonas aeruginosa is one of the most virulent opportunistic Gram-negative bacterial pathogens in humans. It causes many acute and chronic infections with morbidity and mortality rates as high as 40%. P. aeruginosa owes its pathogenic versatility to a large arsenal of cell-associated and secreted virulence factors which enable this pathogen to colonize various niches within hosts and protect it from host innate immune defenses. Induction of cytotoxicity in target host cells is a major virulence strategy for P. aeruginosa during the course of infection. P. aeruginosa has invested heavily in this strategy, as manifested by a plethora of cytotoxins that can induce various forms of cell death in target host cells. In this review, we provide an in-depth review of P. aeruginosa cytotoxins based on their mechanisms of cytotoxicity and the possible consequences of their cytotoxicity on host immune responses.
Keywords: Pseudomonas aeruginosa, infection, virulence factors, cytotoxins, apoptosis, pyroptosis, necroptosis, necrosis, innate immunity, inflammatory responses
1. Introduction
Pseudomonas aeruginosa is one of the most versatile and virulent opportunistic bacterial pathogens described to date [1]. It is a leading cause of bacteremia and sepsis in patients receiving cancer drugs, the most common cause of nosocomial pneumonia, a frequent cause of infections in diabetic ulcers, burn wounds, surgical wounds, and corneal ulcers, and a deadly cause of chronic infection in cystic fibrosis patients [1,2,3,4,5,6,7,8,9,10,11]. Despite aggressive antibiotic therapy, the fatality rate amongst individuals with P. aeruginosa infections can reach as high as 40% [1,12,13,14]. These figures have not improved in decades due to the high degree of intrinsic and acquired resistance of P. aeruginosa to many antibiotics, as well as the emergence of multi-drug resistant strains [15,16,17,18].
P. aeruginosa derives its versatility to cause infections in a broad host range from the large array of virulence factors it possesses. We define virulence factors as factors that are not required for growth in culture media per se. Rather, in their absence, P. aeruginosa becomes impaired in its ability to colonize and cause infection in a manner that benefits its survival and/or persistence in vivo. These factors aid the bacterium in colonization and dissemination within the host, protect it against host immune defenses, and/or exacerbate epithelial injury to prevent wound healing, thus maintaining P. aeruginosa’s preferred niche, the wound itself [19,20,21].
Induction of cytotoxicity in target host cells is a major virulence strategy that P. aeruginosa employs during the course of infection. P. aeruginosa has invested heavily in this strategy, as manifested by a plethora of cytotoxins that can induce various forms of programmed cell death in target host cells. Before we categorize these virulence cytotoxins based on their mechanisms of cytotoxicity and the consequences of their cytotoxicity on host immune responses, we will briefly discuss major types of programmed cell deaths.
1.1. Apoptosis
In 1965, Lockshin and Williams et al. discovered that during the metamorphosis of the silkworm, specific cells died [22]. They designated this type of death as “programmed” because the same cells died each time. In 1972, Kerr et al. observed a specific type of cell death in human tissues in which the cells exhibited specific morphological characteristics including chromatin condensation (karyorrhexis), nuclear condensation (pyknosis), and fragmentation, cellular shrinkage and fragmentation [23]. They coined this cell death process “Apoptosis,” which means “falling off” like the leaves falling from a tree in autumn. Today, our knowledge of apoptosis has vastly improved. Apoptosis plays fundamental roles in development [24], immune system maturation [25], genome maintenance [26], wound healing [27], protection against autoimmunity and cancer [28,29], and innate immune defenses against pathogens [30]. It has been estimated that out of approximately 37.2 trillion cells in an adult human, 50–70 billion cells (~0.2%) die each day by apoptosis [31], highlighting the importance of apoptosis in human physiology and health. As important as apoptosis is for health, its dysregulation can have dire consequences, leading to many pathological conditions including but not limited to neurodegenerative diseases, autoimmune disorders, impaired infection control, and cancer (reviewed in [32,33]).
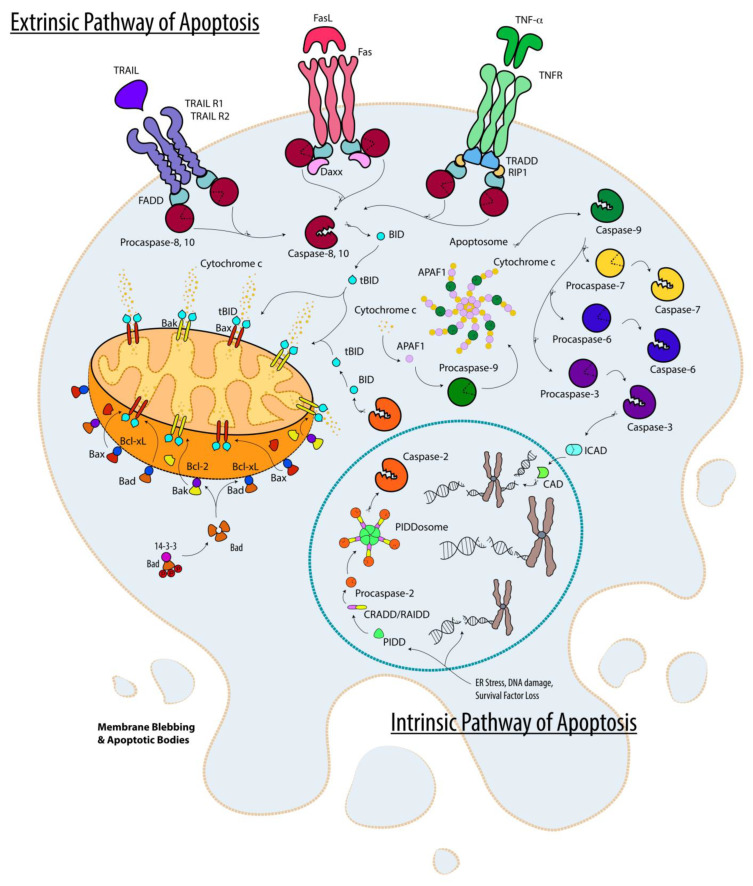
At the molecular level, apoptosis is mediated by cysteine aspartate proteases (caspases) which are subdivided into initiator Caspases 2, 8, 9 (mouse and human) and Caspase-10 (human), as well as effector (a.k.a., executioner) Caspases 3, 6, and 7 (mouse and human) [34]. Multiple apoptotic programmed cell deaths (PCD) have been described, but intrinsic (a.k.a., mitochondrial) apoptosis and extrinsic (death receptor-mediated) apoptosis are the most well-characterized apoptotic PCDs [35,36,37] and summarized in Figure 1).
Figure 1.
Apoptosis. The intrinsic (mitochondrial) and the extrinsic (death receptor-mediated) pathways of apoptotic cell deaths are depicted. These pathways are described in the text.
Internal stimuli such as DNA damage trigger intrinsic apoptosis by activating the pro-apoptotic BH3-only subgroup of pro-apoptotic Bcl-2 family proteins (Bim, Bid, Bad, and Puma), which in turn disrupt interactions between the pro-apoptotic Bcl-2 family proteins (Bax and/or Bak) and anti-apoptotic Bcl-2 family proteins (Bcl-2 and BCL-xL, BCL-W, MCL1, and A1/BFL-1), thus freeing Bax and Bak to mobilize to the mitochondrial outer membrane where their oligomerization is presumed to disrupt the mitochondrial outer membrane, finally resulting in the release of cytochrome c [38]. Cytosolic cytochrome c then combines with APAF1 and pro-Caspase-9 to form the “Apoptosome,” which activates the initiator pro-Caspase-9 into mature caspase-9 via autoproteolysis, in turn activating effector Caspases 3, 6, and 7 (Caspase-3 is the primary effector caspase) that carry out cell death. Endoplasmic reticulum (ER) and genotoxic stresses can also lead to the activation of the initiator Caspase-2 through the PIDDosome multicomplex (containing PIDD, CRADD/RAID, and pro-Caspase-2) which in turn activates Bid into tBid (truncated Bid) by cleavage, initiates Bax-mediated mitochondrial outer membrane disruption, and eventually leads to Caspase-9 dependent intrinsic apoptosis [39,40,41].
Extrinsic apoptosis involves the activation of so-called death receptors (TNFR1, FasR, DR3, DR4, and DR5) by their cognate external ligands (TNF-α, FasL, TRAIL (Apo2L), and Apo3L) [37,42]. Depending on the specific receptor and ligand, death receptor/ligand interaction results in the recruitment of TRADD, FADD, or DAXX adaptor proteins, leading to the formation of a polymeric activation complex known as DISC (death-inducing signaling complex), which in turn activates initiator Caspases 8 (mouse and human) and/or 10 (human only), subsequently activating the effector Caspases 3, 6, and 7, that carry out the task of cellular demise. Activated Caspases 8 and 10 can further amplify cell death by cross-feeding into intrinsic apoptosis via cleavage of Bid into tBid [43,44].
Apoptotic PCDs are generally believed to be anti-inflammatory in nature for the following reasons. First, the degradation of cytosolic proteins by activated caspases during apoptosis reduces the so-called danger-associated molecular pattern molecules (DAMPs) which are highly pro-inflammatory in nature [45]. Second, DAMPs are not directly released into the environment during apoptosis, thus, they are not available to activate pattern recognition receptors (PRRs) and trigger inflammatory responses in neighboring cells. Rather, they are packaged in membrane-bound apoptotic bodies which are then rapidly cleared by macrophages via phagocytosis [46,47]. Third, uptake of apoptotic bodies suppresses inflammation in macrophages by inhibiting the production of inflammatory cytokines by anti-inflammatory factors such as TGF-β and prostaglandin E2, thus transitioning macrophages into so-called M2 anti-inflammatory phenotype [48,49]. The anti-inflammatory nature of apoptosis is perhaps an important reason why the majority of cytotoxins produced by pathogens employ this mechanism to induce cell death in their target host cells. Apoptosis-inducing cytotoxins in P. aeruginosa will be discussed later.
1.2. Pyroptosis
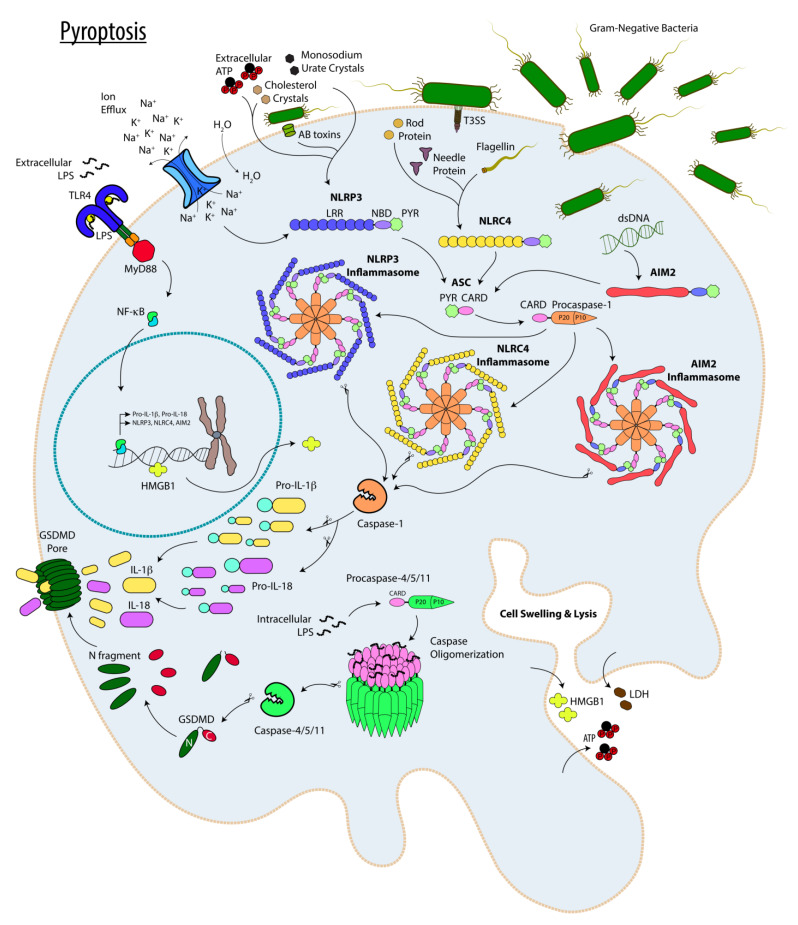
Pyroptosis is a form of inflammatory PCD which involves either Caspase-1-dependent canonical inflammasomes or non-canonical inflammasomes driven by Caspase-11 (in mice) or Caspase-4 and Caspase-5 (in humans) [50,51] and summarized in Figure 2. Canonical inflammasomes are multiprotein oligomeric structures formed by ASC adaptor proteins bridging the interaction between NLRP1b, NLRP3, NLRC4, AIM2, or Pyrin (canonical inflammasome subtypes) with pro-Caspase-1 in response to external or internal stimuli (e.g., extracellular adenosine triphosphate (ATP), microbial products, or DAMPs), ultimately culminating in the activation of Caspase-1 though autocleavage and processing [52,53]. In contrast to Caspase-1, which requires the assembly of the aforementioned multiprotein inflammasome complexes for its activation, Caspases 11, 4, and 5 do not require multiprotein complexes for self-processing and activation. Rather, it appears that their activation results from the direct interaction between their CARD domains with intracellular LPS through its lipid A moiety [54,55,56]. Activated Caspases 1, 11, 4, and 5 then drive the cleavage of the pro-pyroptotic factor Gasdermin D (GSDMD), which then oligomerizes to form pores in the plasma membrane causing cell death [55,56,57]. It is worth noting that in contrast to pro-Caspase-1 which is expressed in resting cells, pro-Caspase-11 is not expressed in resting cells and its expression requires priming by LPS which triggers a signaling pathway involving Toll-like receptor 4 (TLR4)/MyD88/NF-κB [58].
Figure 2.
Pyroptosis. The main players involved in pyroptotic programmed cell death are depicted. The pathways are described in the text.
Pyroptotic cell death is highly pro-inflammatory in nature not only because cellular demise by this mechanism is associated with the release of cellular contents and cytosolic DAMPs through GSDMD-generated pores in the plasma membrane [55,56], but also because of the processing and release of pro-inflammatory cytokines IL-1β and IL-18 by active Caspase-1 [52,53]. Noncanonical Caspases 11, 4, and 5 do not directly process pro-IL-1 and pro-IL-18 pro-inflammatory cytokines into their active forms (IL-1β and IL-18) by themselves, but they do so indirectly via NLRP3 canonical inflammasome subtype activation by promoting K+ efflux in a manner that is dependent on the TRIF adaptor protein [59,60,61,62,63,64].
Because of its inflammatory nature, pyroptosis may be viewed as an altruistic form of cellular sacrifice that is intended to limit infection and spare uninfected neighboring cells through alert signals and inflammatory mediators (IL-1β, IL-18, and DAMPs). In line with this notion, CIAS1 7 unit repeat genetic polymorphism in NLRP3 has been associated with decreased IL-1β levels and increased occurrence of vaginal Candida and Mycoplasma infections [65,66]. Conversely, in cases where the invading pathogen infects and resides within host immune leukocytes, pyroptosis may be beneficial to the pathogen. For instance, polymorphisms in CARD8 (C10X) and NLRP3 (Q705K), both of which are associated with increased NLRP3 inflammasome activity, have shown significant association with increased extrapulmonary Mycobacterium tuberculosis infection [67].
It is worth noting that exuberant inflammasome activity has been associated with many pathological conditions including several immunological disorders (such as familial cold auto-inflammatory syndrome (FCAS), cryopyrin-associated periodic syndromes (CAPS), Crohn’s disease, and ulcerative colitis [68,69,70,71,72]); neurological disorders (such as, progressive multiple sclerosis, age-related macular degeneration, Alzheimer’s disease) [73,74,75]; cardiovascular diseases (such as, atherosclerosis, atrial fibrillation) [76,77,78], cancer [79,80,81]; and insulin resistance and type 2 diabetes [82,83,84].
1.3. Necroptosis
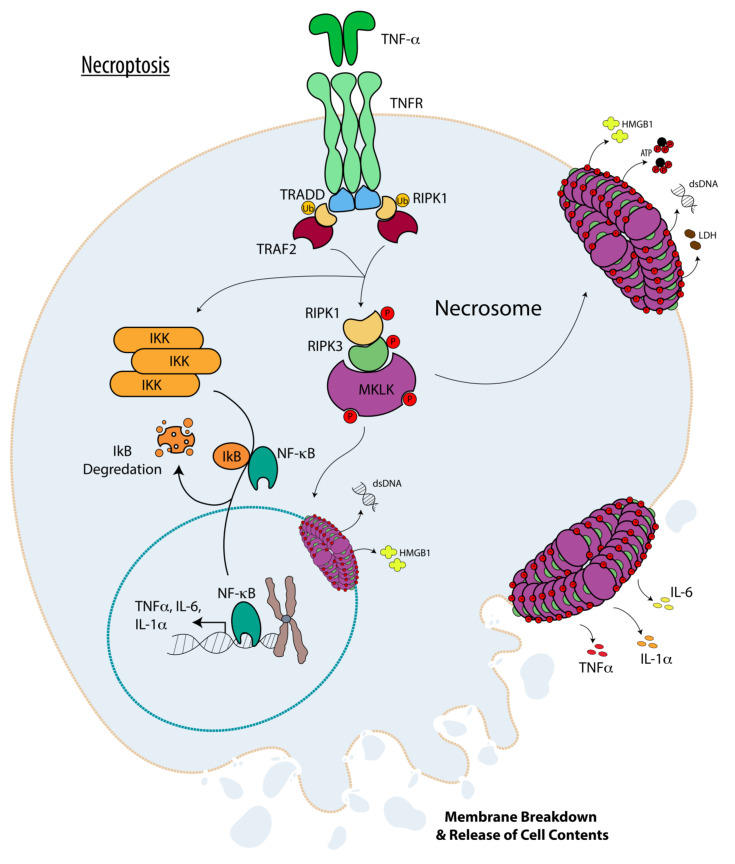
For decades, necrosis was mostly viewed as an “accidental cell death” that occurred as a result of extreme physicochemical insults such as heat or membrane damage, but this view began to change with the seminal reports published by Holler et al. [85] and Degterev et al. [86], in which they described an alternative Caspase-8-independent non-apoptotic pathway of cell death triggered by Fas ligand (FasL), mediated by Receptor-Interacting Protein Kinase (RIPK), and inhibited by necrostatin-1. Today, necrotic cell death is divided into 2 subcategories, namely “necrosis (a.k.a., oncosis)” which is unregulated, instantaneous accidental cell death as the direct result of massive cellular and/or membrane damage caused by extreme physicochemical conditions, and “necroptosis” a form of regulated, protein-based cell death occurring in response to signaling cues and milder physicochemical conditions [87,88].
Morphologically, necrosis (oncosis) and necroptosis manifest similar features including loss of plasma membrane integrity, cellular and organelle swelling, and the release of cytoplasmic contents into the surrounding environment [89,90]. Mechanistically, necroptosis is initiated by TNF family receptors [85,86,91,92]; by pattern recognition receptors (PRRs) including Toll-like receptors (e.g., TLR3 and TLR4), and by cytosolic viral sensory pathways including retinoic acid-inducible gene-I-like (RIG-I), stimulator of interferon gene (STING), and DNA-dependent activator of IFN-regulatory factors (DAI) [93,94,95,96,97,98] and summarized in Figure 3. Necroptosis is ultimately executed by mixed lineage kinase domain-like (MLKL) protein, which upon phosphorylation at multiple sites by RIPK3 [99,100,101], is mobilized to the plasma membrane through interaction between its exposed N-terminal 4-helix Bundle (NB) domain with phosphatidylinositol phosphates (PIP) within the inner leaflet of the plasma membrane, where it carries out cellular demise by homotypic oligomerization and pore-formation within the plasma membrane in a manner that is assisted by the cytosolic chaperone Hsp90 (heat shock protein 90kDa) [101,102,103,104,105,106]. RIPK3 itself is recruited to a pro-cell death multiprotein cytosolic complex known as “necrosome” via its RIPK homology interaction motif (RHIM) domain interacting with either RIPK1 (in case of TNF family receptors [107]), TRIF (in case of TLRs [108,109]), or directly with DAI cytosolic viral sensor itself through its RHIM domain [96].
Figure 3.
Necroptosis. The main players involved in necroptotic programmed cell death are depicted. The pathways are described in the text.
It is worth noting that upon recruitment to TNF family receptors (TNFRs), RIPK1 can exist in either “closed” or “open” conformations, which can drastically affect cellular survival and responses to TNF-α. In the “closed” conformation, RIPK1 is ubiquitinated by cIAP1/2 at TNFR which keeps RIPK1 locked in association with TNFR and suppresses its kinase activity [110], allowing for the recruitment of NEMO, IKKα, and IKKβ, collectively known as IkB kinase complex [111]. These interactions then culminate in NF-kB and MAPK activation which in turn drive the cell toward “pro-inflammatory” and “survival” phenotypes, due to NF-kB and MAPK activities, respectively [112,113,114]. In its kinase active “open” conformation, RIPK1 is not ubiquitinated and dissociates from TNFR and recruits and activates RIPK3 in necrosome, which in turn drives the cell toward pro-inflammatory necroptosis cell death, mediated by MLKL [115,116]. It should further be noted that necroptosis cannot occur unless extrinsic apoptosis is inhibited because Caspase-8 in association with cFLIP and FADD (occurring during the extrinsic apoptosis initiation by death receptors engagement with their ligands as discussed above) can recruit both RIPK1 and RIPK3 to a complex dubbed as “Ripoptosome” where they are degraded by Caspase8 [117,118]. Caspase-8 also targets the deubiquitinase cylindromatosis CYLD which further prevents RIPK1 initiation of necroptosis [119].
Similar to pyroptosis, necroptotic programmed cell death is also highly pro-inflammatory in nature because MLKL-mediated pore formation in the plasma membrane leads to the release of cytoplasmic DAMPs [120]. Moreover, MLKL, RIP1, and/or RIP3 have also been shown to activate Caspase-1-dependent canonical inflammasomes, (particularly NLRP3 inflammasome subtype), leading to the processing and release of pro-inflammatory cytokines IL-1β and IL-18 [121,122,123]. Because of its pro-inflammatory nature, necroptosis for the most part benefits the host and is detrimental to invading pathogens. For example, necroptosis has been shown to play an important role against many invading pathogens including but not limited to, Staphylococcus aureus, Yersinia pestis, human herpes simplex virus (HSV), and vaccinia virus [124,125,126].
Ironically, necroptosis can also benefit pathogens in some cases and be detrimental to the host. For example, necroptosis has been shown to enhance infection in the case of the Salmonella Typhimurium [127] and S. aureus in the airway lung infection models [128]. As is the case for pyroptosis, exuberant necroptosis can also have dire pathological consequences. Necroptosis has been shown to cause lethal lung damage, while its inhibition has been demonstrated to protect against lung injury and improve survival in a neonatal sepsis mouse model [129]. Necroptosis has also been shown to drive Listeria monocytogenes septicemia-associated acute hepatic injury, while its inhibition by RIPK1 deletion protects against this pathology [130]. Necroptosis has been reported to drive systemic inflammatory response syndrome (SIRS) [131], while necroptosis inhibition by genetic means (e.g., deletion in RIPK3 gene) or by pharmacological means (RIPK1 inhibition by necrostatin-1) protects against SIRS [131]. In addition, necroptosis has been implicated in various inflammatory disorders such as Crohn’s and ulcerative colitis [132,133], rheumatoid arthritis [134], multiple sclerosis [135], and chronic obstructive pulmonary disease (COPD), [136] to name a few.
Having described the main PCD mechanisms implicated in P. aeruginosa cytotoxins, we now focus on various cytotoxins in P. aeruginosa and their modes of cytotoxicity.
2. Apoptosis-Inducing Cytotoxins in Pseudomonas aeruginosa
P. aeruginosa possesses many cytotoxins that induce various forms of apoptotic programmed cell death. Although, it is not clear why P. aeruginosa prefers to kill its target host cells by apoptosis, it can be postulated that apoptosis would benefit P. aeruginosa during infection in the host because of its anti-inflammatory and immunosuppressive nature [137,138,139]. Below, we will discuss the apoptosis-inducing cytotoxins in P. aeruginosa cytotoxins and their mechanisms of action.
2.1. Toxin A (ToxA)
Toxin A (ToxA)–A.K.A., Exotoxin A (ExoA), or Pseudomonas Exotoxin (PE)—is an AB toxin secreted by the T2SS [140,141]. AB toxins are composed of A and B components where the A component encodes the active enzymatic domain and the B component is responsible for the transport of the A component across the cytoplasmic membrane of target host cells [142]. Once internalized in host cells, ToxA ADP-ribosylates eukaryotic elongation factor-2 (eEF-2) resulting in the inhibition of protein synthesis and causing apoptotic cell death [143]. ToxA-induced apoptosis exhibits features of both intrinsic and extrinsic apoptosis [144,145]. In mouse embryonic fibroblasts, ToxA induces intrinsic (mitochondrial) apoptosis manifested by rapid degradation of Mcl-1 pro-survival protein and loss of mitochondrial membrane potential [144]. ToxA-induced apoptosis in this cell line was shown to be dependent on BAK (not BAX) oligomerization in the mitochondrial outer membrane, and ToxA-induced apoptosis was completely abolished in cells where Mcl-1 or Bcl-XL were overexpressed [144]. In human mast cells, ToxA induces extrinsic apoptosis [145]. ToxA-intoxicated mast cells manifest extrinsic apoptosis features including activation of initiator Caspase-8 and down-regulation of FLIPs (FLICE-like inhibitory proteins) [145]. Moreover, ToxA-induced apoptosis in this cell line was shown to be dependent on Caspase-8 and Caspase-3 [145]. ToxA deficient strains were shown to be significantly less virulent than the wild-type strain in a mouse model of infection [146]. Although ToxA-induced apoptosis would be expected to be anti-inflammatory, ToxA impacts on immune responses have not been directly investigated. In one study involving the keratitis model of infection, it was shown that ToxA deficient mutant bacteria were rapidly cleared from the eye, with a reduced sign of inflammation at the site of infection [147]. Whether reduced inflammatory responses in the eye were due to reduced bacterial burden or the absence of ToxA remains unknown. Due to its potent cytotoxicity, ToxA has also been extensively evaluated as a potential anti-cancer immunotoxin therapy [148,149].
2.2. N-3-Oxododecanoyl Homoserine Lactone (C12-HSL)
N-3-oxododecanoyl homoserine lactone (C12-HSL) is a pheromone that functions as the autoinducer for the Las quorum-sensing in P. aeruginosa [150]. In addition to its role in the Las quorum-sensing, C12-HSL has been shown to induce various forms of apoptosis, depending on the cell line studied. For example, exposure to C12-HSL has been shown to result in the activation of initiator Caspase-8 and the effector Caspase-3 in macrophages and neutrophils [151]. In Jurkat T lymphocytes, C12-HSL causes mitochondrial outer membrane damage leading to intrinsic (mitochondrial) apoptosis, mediated by the initiator Caspase-9 [152]. Overexpression of mitochondrial membrane stabilizer Bcl-2 completely abrogated C12-HSL-induced apoptosis in Jurkat T lymphocytes [152]. C12-HSL has also been shown to induce intrinsic apoptosis by downregulating the STAT3 survival/proliferation pathway in breast carcinoma cells [153]. STAT3 is a known anti-apoptosis transcription factor that protects against intrinsic apoptosis by increasing the expression of anti-apoptotic proteins (i.e., Bcl-2 and Bcl-xL) which stabilize the mitochondrial outer membrane [154]. Similarly, C12-HSL causes mitochondrial dysfunction and induces intrinsic apoptosis by attenuating the expression of PGC-1α and its downstream effector BEAS-2B in primary lung epithelial cells [155]. PGC-1α is a master regulator of mitochondrial biogenesis and cellular respiration [156]. Yet, in airway epithelial cells, exposure to C12-HSL has been shown to lead to both intrinsic apoptosis—manifested by cytochrome c release and Caspase-9 activation—and extrinsic apoptosis, as shown by Caspase-8 activation [157]. Interestingly, N-butyryl-L-homoserine lactone (C4-HSL), (a closely related autoinducer that activates the Rhl quorum sensing in P. aeruginosa [150]), lacks the ability to induce apoptosis likely because of its relatively shorter fatty acid chain [151,152]. Interestingly, at sub-lethal doses C12-HSL has been shown to dampen NF-κB activation and suppress TNF-α and IL-12 pro-inflammatory cytokine expression in the RAW264.7 mouse macrophage cell line by triggering the unfolded protein response (UPR) [158]. In contrast, C12-HSL has also been shown to induce IL-8 pro-inflammatory cytokine production in human epithelial and fibroblast cells through the activation of NF-κB and AP-2 [159]. Moreover, C12-HSL injection into the skin of mice led to the production of inflammatory mediators (IL-1α, IL-6, and MIP-2) in vivo [160].
2.3. Azurin
Azurin is a cupredoxin-type electron transfer protein that is involved in electron transfer during denitrification in P. aeruginosa [161]. Azurin has also been shown to induce apoptosis in J774 macrophages and many cancer cell lines by stabilizing tumor suppressor p53 and enhancing its activity, which in turn leads to increased levels of ROS and cell demise [162,163,164]. Notably, P53 has been shown to induce intrinsic apoptosis, involving mitochondrial outer membrane damage, cytochrome c release into the cytosol, and activation of Caspase-9 through the Apoptosome [165]. It also targets the non-receptor tyrosine kinases (NRTKs) signaling network [166]. Azurin expression has been shown to be elevated in P. aeruginosa isolates of the lung in CF patients [167]. This finding likely reflects the need for azurin’s redox function in sustaining P. aeruginosa within the anaerobic or microaerobic environment in CF airways [167,168]. Whether azurin’s function as a cytotoxin plays any physiological role for P. aeruginosa in vivo remains to be determined.
2.4. Pyocyanin
Pyocyanin is another important virulence factor secreted by the T2SS [169,170,171]. Pyocyanin is a water-soluble blue–green, non-fluorescent phenazine-derived pigment metabolite that is capable of oxidizing and reducing molecules and generating reactive oxygen species (ROS) [172,173]. In the environment, pyocyanin has been shown to have bactericidal activity against many bacteria, particularly Gram-positive bacteria such as Staphylococcus aureus [174]. The mechanism underlying pyocyanin-induced cytotoxicity in S. aureus was shown to involve ROS production [175]. Pyocyanin has also been shown to induce apoptosis in neutrophils by mitochondrial damage and increased ROS [176,177,178,179]. Pyocyanin also impacts immune responses in the host. In mammalian hosts, pyocyanin exposure leads to increased IL-8 via activation of MAPK and NF-kB pathways [180,181]. Despite this pro-inflammatory effect, which could be counterproductive to pathogenicity, pyocyanin plays an important virulence function for P. aeruginosa in vivo. Pyocyanin has been shown to be crucial for P. aeruginosa in establishing chronic infection and in inducing lung damage in mice [182,183]. Consistent with these reports, pyocyanin is detected in large quantities in the sputum of cystic fibrosis (CF) patients infected with P. aeruginosa, enough to inhibit ciliary beating and cause toxicity in the respiratory epithelium in vitro [184].
There are also other apoptosis-inducing cytotoxins that are discussed below under the categories of membrane-associated cytotoxins and Type III Secretion System (T3SS or TTSS) Exotoxins.
3. Membrane-Associated Cytotoxins
3.1. Lipopolysaccharide (LPS)
Lipopolysaccharide (LPS) is another cell-bound major virulence factor of P. aeruginosa [185,186]. LPS is found in the outer membrane of the bacterium and is composed of a hydrophobic domain called Lipid A which is anchored onto a core polysaccharide and a hydrophilic tail made of O-specific polysaccharide [185]. The O-specific polysaccharide is variable and is useful for serotyping strains of P. aeruginosa [187]. LPS is an integral part of the outer membrane of Gram-negative bacteria, providing structural support for the bacteria, serving as an adhesin, and well as protection from the environment [185,188]. LPS has also been shown to contribute to P. aeruginosa pathogenesis by facilitating host cell adhesion through binding to the ganglioside asialo-GM1 found on epithelial cells [189]. LPS recognition by the NLRC4 (a.k.a. IPAF) canonical or Caspase-11 non-canonical inflammasomes has also been shown to result in Caspase-1 or Caspase-11-dependent pyroptotic cell death [190,191]. In addition, LPS has also been shown to induce apoptosis in A549-transformed lung cells by enhancing ROS production via downregulation of the anti-apoptotic Sirtuin1 (SIRT1) [192].
As for its impact on inflammatory responses, LPS is massively immunogenic and can trigger inflammatory responses through TLR4 recognition [193]. LPS-triggered TLR4 signaling results in the production of pro-inflammatory cytokines which also contribute to the pathology associated with both sepsis and bacteremia [194,195,196]. In addition, cytoplasmic recognition of LPS can trigger Caspase-11-dependent pyroptotic cell death which is highly pro-inflammatory in nature [197]. Given the immunogenicity of LPS, it has been a target for developing anti P. aeruginosa vaccines; however, there has been limited success thus far due to the variability of the O-specific polysaccharide [198].
3.2. Flagella
Flagella are membrane associated appendages that perform many virulence functions for P. aeruginosa. Flagella mediate adhesion to biotic and abiotic surfaces [199,200], mediate swimming motility [201] and function in biofilm formation and maturation [202,203]. Numerous animal models have shown that motility enhances bacterial dissemination and virulence in the host. For example, in neonate mice, flagellation has been demonstrated to enhance virulence in bacteremia and pneumonia models of infection [204]. Similarly, flagellated strains have been shown to cause more damage and exacerbate burn wounds than non-flagellated isogenic strains [205,206]. In addition, P. aeruginosa flagellin recognition by NLRC4/IPAF inflammasome can trigger Caspas-1 dependent pyroptosis [190].
Having flagella is beneficial in increasing dissemination and enhancing infection; however, flagella can also be a source of vulnerability for P. aeruginosa, as monomeric flagellin detection by Toll-like receptor 5 (TLR5) has been shown to trigger robust inflammatory responses in various immune leukocytes, resulting in diminished bacterial survival in an acute lung infection model [207,208]. In addition, cytoplasmic flagellin recognition by Naip5 and Naip6 [209,210] can also lead to activation of NLRC4 canonical inflammasome which further amplifies inflammatory responses against P. aeruginosa infection [211]. As a result, P. aeruginosa strains often downregulate the expression of flagellar components after the establishment of infection and during chronic infection to evade host innate immune responses [212,213,214]. Downregulation of immunogenic virulence factors and/or structures (i.e, flagellum) is a common theme among P. aeruginosa infections, especially in CF patients, where expression of virulence factors could hinder bacterial survival rather than aid in its dissemination [215,216].
3.3. Porins
P. aeruginosa also possesses over 20 porins in its outer membrane that serve many crucial physiological functions essential for virulence including nutrient uptake, adhesion, decreasing permeability to antibiotics, and signaling ([217,218]). For example, OprF, one of the major porins in the P. aeruginosa outer membrane, has been shown to be required for full virulence of P. aeruginosa [219]. Deletion in the oprF gene impairs many virulence-associated functions including colonization, quorum sensing (QS), and secretion through the T3SS [219]. In addition to these virulence functions, purified porins have also been shown to induce intrinsic apoptosis in epithelial target host cells, as manifested by reductions in the bcl-2 gene expression [220].
As crucial as they are to the pathophysiology of P. aeruginosa, porin recognition by pattern recognition receptors (PRRs) can also lead to the production of pro-inflammatory cytokines, inflammatory responses, and complement activation, thus interfering with P. aeruginosa’s ability to colonize and cause infection [221,222]. Interestingly, adoptive transfer of dendritic cells immunized with wild-type or recombinant OprF ex vivo has been shown to be protective against P. aeruginosa lung infection in mice [223].
3.4. Rhamnolipids
P. aeruginosa can produce about 30 different congeners of surface-active rhamnolipids, which are glycolipid biosurfactants composed of mono- or di-rhamnose linked to 3-hydroxy-fatty acids of different lengths [224,225,226]. Rhamnolipids have been detected in large quantities (range: 8–65 µg/mL) in the sputum of CF patients, and their presence has been associated with CF lung pathology [227,228]. Because of their high potential for use in various biotechnological applications P. aeruginosa rhamnolipids have been investigated extensively [226,229,230].
Rhamnolipids play several important virulence functions for P. aeruginosa. First, rhamnolipid-expressing P. aeruginosa strains, as well as purified rhamnolipids, have been shown to compromise the barrier function of human respiratory epithelium by disrupting the tight junctions, thus facilitating the paracellular invasion by P. aeruginosa [231]. Second, exposure to rhamnolipids has been shown to interfere with ciliary beating and mucociliary clearance of P. aeruginosa in tracheal rings of guinea pig animal models [227]. Third, rhamnolipids have also been shown to aid P. aeruginosa in swarming motility [232]; in turn, swarming motility has been demonstrated to regulate the expression of virulence genes and antibiotic resistance in P. aeruginosa [233]. Forth, rhamnolipids have also been demonstrated to play a role in structural biofilm development and in maintaining channels between multicellular structures in biofilms [234,235]. Fifth, rhamnolipids appear to modulate P. aeruginosa membrane composition by reducing LPS and porin membrane proteins [236], thus potentially contributing to their well-known intrinsic resistance towards antibiotics [237]. Rhamnolipids can also induce cytotoxicity in target epithelial cells. MCF7 breast cancer cells intoxicated with mono- and di-rhamnolipids from P. aeruginosa undergo apoptotic cell death, manifested by nuclear condensation and fragmentation, p53 activation, mitochondrial damage, and appearance of sub-G1 (apoptotic) subpopulations [238,239]. Rhamnolipids have been shown to cause potent necrotic cell death in human and murine neutrophils and macrophages [240,241].
As is the case for many virulence factors and virulence structures in P. aeruginosa, rhamnolipids can both trigger and combat host innate immune responses. For instance, rhamnolipids are required for the expression of flagellin-induced psoriasin (S100A7) antimicrobial peptides and chemokines in human skin [242]. In this context, the production of rhamnolipids may be detrimental to P. aeruginosa pathogenesis in vivo. In contrast, rhamnolipids have been shown to cause potent necrotic cell death in humans and murine neutrophils and macrophages, therefore protecting P. aeruginosa against clearance in the lungs [240,241].
4. Type III Secretion System (T3SS or TTSS) Exotoxins
T3SS is a highly conserved virulence structure that is found in several other important pathogenic Gram-negative bacteria, including P. aeruginosa [243,244,245]. T3SS arguably plays the most significant role in the pathogenesis of T3SS-expressing P. aeruginosa and without it, T3SS-expressing P. aeruginosa becomes severely attenuated in its ability to cause infection [243,246,247]. Six effector proteins, referred to as exotoxins (Exo) have been shown to be secreted through the T3SS of P. aeruginosa but only four of these effector proteins (ExoS, ExoU, ExoY, and ExoT) have been demonstrated to possess virulence functions, including cytotoxicity [243,248]. Due to the narrow channel of the needle complex, the effector proteins must be secreted in their unfolded state [249], and because of this, they all require specific chaperones which can help maintain the effector toxins in an unfolded state while in the bacterial cytosol and during translocation and can also aid targeting the effector to the T3SS apparatus [250,251,252]. In addition, all exotoxins require host cofactors for their activation within the host cell [253,254,255]. The dependence of activity on host cofactors ensures that P. aeruginosa is protected from the harmful activities of these virulence factors. We will now discuss the T3SS and its effector proteins with demonstrated virulence functions including cytotoxicity.
4.1. T3SS Apparatus
A growing body of data has demonstrated that insertion of the T3SS apparatus of Gram-negative pathogens, including P. aeruginosa in the host plasma membrane results in cell death [256,257,258]. It is assumed that the damage caused by the T3SS pore-forming activity is the cause of passive necrotic cell death due to trauma and membrane leakage. However, the T3SS-induced necrosis has been shown to be completely blocked by ExoT [259], suggesting that the T3SS-induced cell death is a form of programmed cell death and not accidental necrosis, occurring as a consequence of T3SS-induced massive trauma to the membrane. Similarly, the T3SS in Yersinia has also been shown to cause pore formation and induce cytotoxicity in target host cells and the Yersinia T3SS-induced cytotoxicity was also shown to be blocked by YopE effector toxin [257], which is a homolog of the GAP domain of ExoT [260,261]. Two recent reports have indicated that the cell death induced by the T3SS of P. aeruginosa is pyroptosis [262,263]. As discussed above, pyroptosis is mediated by Caspases 1 and 11 (in mice) and 4 and 5 (in human), which are inhibited by the pancaspase inhibitor z-VAD. However, the T3SS-induced cytotoxicity was not appreciably affected by z-VAD [259], suggesting that T3SS-induced cytotoxicity is not pyroptosis. Given that the T3SS-induced cytotoxicity is completely abrogated by a toxin (ExoT) that induces potent apoptosis (discussed below) suggests that the T3SS-induced cytotoxicity is necroptosis because it is prevented under apoptotic conditions, as discussed above. Whether necroptosis and/or pyroptosis is the underlying mechanism of T3SS-induced cytotoxicity remains to be further investigated.
As for the impact of the T3SS on the host’s innate immune responses, this virulence structure is perhaps the most prominent trigger of inflammatory responses in the host during infection. Various inflammasome subtypes (e.g., NLRP3 and/or NLRC4) have been implicated in the recognition of and in responses to T3SS and P. aeruginosa infection, although NLRC4 canonical inflammasome appears to be the primary mode of T3SS recognition in BMDMs and in host tissues [19,190,264,265,266,267]. There also appears to be some contradictory reports regarding the impact of T3SS-triggered inflammatory responses on the outcome of infection, in that the same inflammasome (NLRC4) has been shown to be either crucial in P. aeruginosa clearance, thus benefiting the host; or paradoxically facilitating bacterial colonization and enhancing P. aeruginosa pathogenesis, thus benefiting the pathogen. For example, Franchi et al. demonstrated that recognition of T3SS-expressing P. aeruginosa by NLRC4 inflammasome triggers the production of IL-1β in intestinal phagocytes that are crucial in limiting P. aeruginosa gastric infections [268]. Similarly, NLRC4 was found to contribute to the recognition and clearance of T3SS-expressing P. aeruginosa in a wound model [19], and in a cystic fibrosis (CF) lung model of infection [269]. In contrast, Faur et al. demonstrated that Nlrc4-deficient mice showed enhanced bacterial clearance and decreased lung injury contributing to increased animal survival following pleural infection with a T3SS-expressing P. aeruginosa strain [270]. These reports suggest that specific organs and/or sites within a host may have evolved distinct mechanism(s) to detect and respond to T3SS and its effectors during P. aeruginosa infection.
4.2. ExoS
ExoS is a bifunctional protein consisting of an N-terminal GTPase Activating Protein (GAP) domain and a C-terminal ADP-ribosyltransferase (ADPRT) domain that is directly translocated into host cytoplasm through the T3SS [271] using SpcS chaperone protein [272]. Upstream of the GAP domain is a membrane localization domain (MLD) which targets the toxin to the mammalian cytoplasmic membrane [273]. Deletion of the MLD was found to not affect ExoS translocation; however, ADP-ribosylation of Ras, a known target of ExoS, was lost, thus demonstrating the importance of this sequence in mediating ExoS interaction with some of its targets [274].
The GAP domain of ExoS targets RhoA, Rac1, and CDC42 [275,276]. These small Ras-like GTPases are active when bound to GTP and inactive when bound to GDP [277]. ExoS inactivates RhoA, Rac1, and Cdc42 through allosteric interaction of a conserved arginine-finger in its GAP domain with the aforementioned GTPases, stimulating them to hydrolyze their bound GTP to GDP [275,276,277,278]. These small GTPases play important roles in coordinating and maintaining the actin cytoskeleton; thus, inactivating their signaling affects processes, such as cell migration and cell division and leads to cell rounding [279,280].
The ADPRT domain of ExoS has many targets in mammalian cells and requires the host 14-3-3 protein as the cofactor for its activity within host cells [254,281]. Targets include Ras, Rab and Rho family of proteins, as well as ezrin, radixin, meosin, vimentin, and cychlophilin A [282]. ADP ribosylation of these proteins by ExoS/ADPRT can lead to disruption of the cytoskeleton, endocytosis, and cell–cell binding, as well as inhibition of DNA synthesis and ultimately apoptotic cell death [279,282,283,284,285].
ExoS-intoxicated cells display signs of both Caspase-9 dependent intrinsic apoptosis and death receptor-mediated Caspase-8 dependent extrinsic apoptosis and both domains of ExoS contribute to ExoS-induced apoptosis [285,286,287,288,289]. Intoxication with ExoS/GAP has been shown to lead to enrichment of Bax and Bim into the mitochondrial outer membrane; disruption of mitochondrial membrane and release of cytochrome c into the cytosol; and activation of initiator Caspase-9 and executioner Caspase-3 caspases, leading to intrinsic/mitochondrial apoptosis in target host cell [285]. ExoS/ADPRT intoxication has been shown to result in the activation of initiator Caspase-8 in a manner that is dependent on the FADD adaptor protein, although ExoS-induced apoptosis was independent of Fas death receptor and Caspase-8 activities [290].
As for ExoS’s impact on host immune responses, ExoS has been shown to either dampen or trigger immune responses during infection. Intoxication of PBMCs, monocytes, and T cells, with ExoS, or recombinant ExoS (rExoS), strongly induced transcription of pro-inflammatory cytokines and chemokines, namely, IL-1α, IL-1β, IL-6, IL-8, MIP-1α, MIP-1β, MCP-1, RANTES [291,292]. The same group further showed that the induction of pro-inflammatory cytokines in ExoS-treated monocytes was due to the activation of TLR4 signaling by the ExoS/GAP domain and TLR2 signaling by ExoS/ADPRT domain activities [293]. Adding to the confusion in the field, Galle et al. reported that ExoS dampened Caspase-1 mediated IL-1β production in macrophages in a manner that was dependent on its ADPRT domain activity [294]. More recently, it was reported that ExoS had no impact on inflammatory responses in a wound model for infection with P. aeruginosa, although ExoS was required for full colonization of bacteria in the wound [19]. These discrepancies are likely due to technical differences in these studies.
4.3. ExoT
ExoT is the only T3SS effector that is expressed in all T3SS-expressing P. aeruginosa clinical strains [295], indicating a more fundamental role for this virulence factor in P. aeruginosa pathogenesis. The importance of ExoT to P. aeruginosa pathogenesis is further highlighted by the observation that it is actively targeted for degradation by host defenses in epithelial cells. ExoT becomes complexed with Crk and a Crk binding partner Cbl-b, an E3 ubiquitin ligase [296]. As a result, ExoT becomes polyubiquitinated and is targeted for proteasomal degradation. In their study, Balachandran et al. showed that mice lacking Cbl-b were significantly more susceptible to infection by strains expressing ExoT.
ExoT shares 76% protein homology with ExoS and possesses an N-terminal GAP domain and a C-terminal ADPRT domain [271,297,298]. ExoT also contains sequence homology with the MLD of ExoS, although this sequence has not been experimentally mapped [282]. Nevertheless, support for ExoT MLD is demonstrated by similar intracellular fractionation patterns between ExoS and ExoT [278] as well as experiments showing how ADPRT domain switching in ExoS and ExoT maintains their substrate specificities in both chimeras [299].
Similar to ExoS/GAP, the ExoT/GAP domain also targets RhoA, Rac1, and CDC42 [279,280]. In contrast to the ExoS/ADPRT domain, the ADPRT domain of ExoT targets only three non-overlapping substrates; namely, CrkI and CrklI isoforms of Crk adapter protein and phosphoglycerate kinase 1 (PGK1) glycolytic enzyme [300]. Similar to ExoS, the ADPRT domain of ExoT also requires the host 14-3-3 protein as the cofactor for its activity [254]. ExoT has been shown to inhibit bacterial phagocytosis by macrophages, cell migration, and cause cell rounding in a manner that is primarily dependent on its GAP domain activity, although the ADPRT domain also contributes [21,298,300,301,302]. ExoT also exerts potent anti-proliferative effects in its target host cells [303]. The GAP domain of ExoT has been shown to inhibit cell division in epithelial cells by inhibiting the early stage of cytokinesis at the cleavage furrowing step, likely through its inhibitory effect on RhoA; whereas the ADPRT domain blocks the late stage of cytokinesis at the abscission step by targeting CrkI [303]. In addition, both domains of ExoT have been shown to cause cell cycle arrest in G1 interphase in melanoma cells by dampening the expression of G1/S checkpoint proteins ERK1/2, cyclin D1, and cyclin E1 [304].
ExoT is also a potent inducer of apoptotic cell death in its target hosts and both domains contribute to this virulence activity [259,305]. The ExoT/ADPRT was shown to be necessary and sufficient to induce anoikis apoptosis by transforming Crk adaptor protein into a cytotoxin which interfered with the integrin survival signaling by destabilizing the focal adhesion sites through persistent activation of the anoikis mediator, p38β [306]. The ExoT/GAP was shown to be necessary and sufficient to induce intrinsic/mitochondrial apoptosis by activating the initiator Caspase-9 and the effector Caspase-3 through upregulation of the expression and subcellular mobilization of Bax, Bid, and Bim—pro-apoptotic Bcl2 family of proteins—into mitochondrial outer membrane [307]. Interestingly, the ExoT/ADPRT-induced anoikis apoptosis has faster kinetics occurring within 5.5 ± 1.3 h, whereas the ExoT/GAP-induced mitochondrial apoptosis shows slower kinetics occurring within 16.2 ± 1.3 h in intoxicated cells [306,307].
It is important to note that while pre-treatment with the pancaspase inhibitor z-VAD effectively protects eukaryotic cells from ExoT-induced apoptosis, it does not protect the host cells from ExoT-induced disruption of actin cytoskeleton [307], ExoT-induced focal adhesion site disassembly [306], or ExoT-mediated anti-proliferative effects on cytokinesis [303], or ExoT-mediated induction of G1 cell cycle arrest in target host cells [304], indicating that ExoT-induced apoptosis can be uncoupled from ExoT’s other virulence functions.
As for ExoT’s impact on host immune responses, Mohamed et al. recently demonstrated that ExoT inhibits IL-1β and IL-18 pro-inflammatory cytokines production in primary macrophages by inhibiting the phosphorylation cascade through Abl→PKCδ→NLRC4 by targeting CrkII, which they further showed to be required for Abl transactivation and NLRC4 canonical inflammasome activation in response to T3SS and P. aeruginosa infection [19]. They corroborated these in vitro data in an animal model of wound infection, showing that recognition of T3SS leads to the phosphorylation cascade through Abl→PKCδ→NLRC4, culminating in the activation of NLRC4 inflammasome in response to P. aeruginosa infection. Interestingly, they showed that in the wound infection model, ExoT was the primary anti-inflammatory agent for P. aeruginosa, and other T3SS effector proteins (ExoU and ExoS) had no impact on inflammatory responses in wound tissues [19].
4.4. ExoU
ExoU is a potent inducer of rapid necrotic cytotoxicity in target eukaryotic host cells [243,255,308,309]. ExoU has a patatin-like domain that contains phospholipase A2 activity and can target phospholipids, lysophospholipids, and neutral lipids [255,308,310]. ExoU utilizes the chaperone protein called SpcU for secretion through the T3SS [311]. ExoU also requires host DNAJC5 chaperone and ubiquitin as the cofactor for its activity within the target host cell [253,254,255,312,313,314]. The MLD (membrane localization domain) of ExoU has been mapped to residues 550–687 in its C-terminal domain [315]. This allows ExoU to target the plasma membrane where it can carry out its phospholipase activity [316].
The necrotic nature of ExoU-induced cytotoxicity would suggest a pro-inflammatory consequence for this toxin in the host environment. Consistent with this notion, excessive inflammatory responses due to ExoU-induced endothelial barrier disruption have been shown to culminate in the acute respiratory distress syndrome (ARDs) in a pneumonia animal model of infection [317]. Intriguingly, ExoU has also been shown to function as an anti-inflammatory agent for P. aeruginosa. In a pneumonia model of infection, ExoU was shown to create a localized immunosuppressed zone in the vicinity of bacteria by directly killing phagocytic leukocytes (neutrophils and macrophages), albeit there were more inflammatory mediators in the lungs of mice infected with ExoU-expressing P. aeruginosa strain [318,319]. In another report, ExoU was shown to dampen IL-1β pro-inflammatory cytokine production by inhibiting Caspase-1-dependent NLRC4 (a.k.a., IPAF) activation in macrophages [320]. In the same report, ExoU was shown to reduce serum IL-1β and enhance bacterial fitness in a systemic model of infection in mice. To add to the confusion, in a wound model of infection in mice, it was recently demonstrated that ExoU had no impact on pro-inflammatory cytokines production and inflammatory leukocyte responses in a murine wound model of infection [19].
4.5. ExoY
ExoY is an adenylyl and guanylyl cyclase that shares sequence homology with Bordetella pertussis CyaA and Bacillus anthracis edema factor [321,322]. In a recent study, ExoY was detected in 93% of clinical isolates in critically ill pneumonia patients who tested positive for P. aeruginosa, and its presence was associated with end-organ dysfunction in this patient cohort [323]. ExoY requires binding to filamentous actin (F-actin) for its activity [324]. The primary activity of ExoY on mammalian cells appears to be as an edema factor, increasing vascular permeability [322,325]. However, ExoY possesses other virulence activities including disruption of actin cytoskeleton [321,326], inhibition of phagocytic uptake by the host immune system [327], and inhibition of endothelial repair after injury [328]. ExoY drives vascular permeability through its adenylyl cyclase activity which causes Tau hyperphosphorylation and insolubility [322].
In one report, ExoY was also shown to induce cell lysis in Madin–Darby canine kidney (MDCK) epithelial cells, as determined by the release of lactate dehydrogenase (LDH) into the culture supernatant [329]. In another report, infection with ExoY-expressing P. aeruginosa was associated with increased apoptosis in the lung of infected mice [330]. However, in a recent report, ExoY was found to cause an accumulation of active Caspase-7 without causing cell death in pulmonary microvascular endothelial cells (PMVECs). Whether or not ExoY is a bona fide cytotoxin requires further investigation.
As for ExoY’s impact on host immune responses, Kloth et al. recently reported that infection with ExoY-expressing P. aeruginosa was associated with elevated levels of pro-inflammatory cytokines in the sera and the bronchoalveolar lavage fluids (BALFs) and increased infiltration of neutrophilic granulocytes in the perivascular space in an acute airway infection model in mice [330]. Interestingly, these effects were also observed when ExoY was catalytically inactive, suggesting that at least initial inflammatory responses to ExoY are independent of its catalytic activity. Since in these studies, the control infections with the ExoY-deficient and the T3SS mutant strains were not included, it remains unclear whether these pro-inflammatory responses in host tissue were directed at ExoY or whether they were in response to the functional T3SS which itself is a potent inducer of inflammatory responses [19].
5. Other Pore-Forming Cytotoxins
We discussed the T3SS-mediated pore-forming cytotoxicity above. Here, we discuss other known pore-forming cytotoxins in P. aeruginosa.
5.1. CTX
CTX is a cytotoxin contained within the øCTX pro-phage in lysogenized P. aeruginosa bacterial strains such as PA158 [331,332]. CTX is expressed as a pro-cytotoxin of 286 amino acids, with both its activation and secretion requiring the removal of 20 amino acids from its C-terminus [333]. CTX has been shown to cause cytotoxicity in leukocytes through its pore-forming activity [332,334]. CTX inactivation has been demonstrated to result in reduced virulence in a systemic infection model in leukopenic mice, indicating that it is required for full P. aeruginosa pathogenesis in this model [335]. The impact of CTX cytotoxicity on immune responses has not been directly investigated. However, given that killing by pore formation would result in the release of DAMPs [336], CTX-mediated cytotoxicity is likely pro-inflammatory in nature.
5.2. Exolysin (ExlA)
Phylogenetic analyses based on whole-genome sequencing of various P. aeruginosa strains have recently revealed a new clade (PA7-like clade) of highly cytotoxic P. aeruginosa strains that lack the genes for the T3SS and its effectors [337,338,339]. The cytotoxicity associated with the PA7-like clade is attributed to ExolysinA (ExlA) cytotoxin which causes potent cytotoxicity in eukaryotic cells by pore formation [339,340]. ExlA export across the P. aeruginosa membrane is dependent on an outer membrane protein, ExlB, and together ExlA and ExlB define a new active two-partner secretion (TPS) system in P. aeruginosa [339,340]. ExlA/B-expressing P. aeruginosa strains are highly virulent in mice, causing lung hemorrhage and septicemia in a manner that is dependent on the ExlA/B expression [339]. ExlA was also found to be essential for bacterial dissemination to the liver and spleen [339]. These strains have also been known to cause necrotic lesions in pneumocytes and endothelial cells, resulting in alveolo-vascular barrier breakdown in mice [341]. Interestingly, exposure to ExlA results in Caspase-1/Caspase-11--dependent cytotoxicity in BMDMs [342], suggesting that pyroptosis may be the underlying mechanism of ExlA-induced cytotoxicity [51]. However, the same group also reported that ExlA-induced cytotoxicity in BMDMs is also blocked by RIP1 kinase inhibitors, necrostatin-1, and necrostatin-5 [342], suggesting that necroptosis may also be involved [343,344]. Clearly, more work is needed to elucidate the mechanisms underlying ExlA-induced cytotoxicity in various cell lines and in vivo.
Regardless, ExlA cytotoxicity has been shown to be highly inflammatory in nature. ExlA has been demonstrated to promote the maturation of IL-1β through the NLRP3 inflammasome leading to alveolar tissue damage and inflammation in the lungs of mice infected with ExlA-expressing P. aeruginosa [341]. The authors further showed that deficiency in Caspase-1/11 protected against alveolar tissue damage and improve survival in infected mice, by partially reducing the inflammatory environment. Interestingly, the bacterial burden was significantly lower in the lungs of Capase-1/11 knockout mice as compared to wild-type mice, indicating that Caspase-1/11 function may enhance bacterial fitness in this environment and at this time point. Even more surprisingly, the levels of IL-1β and IL-18 were similarly elevated in the lungs of wild-type and Caspase-1/11 knockout mice infected with ExlA-proficient strain, suggesting an inflammasome-independent mechanism for IL-1β and IL-18 processing and activation under these conditions. It is worth noting that despite higher initial inoculum, the infection titers of the ExlA-expressing P. aeruginosa strain were 3–4 log order lower than the T3SS-expressing P. aeruginosa strain that was used in these studies, suggesting a fitness disadvantage for the PA7-like clade in the mammalian host.
6. Cytotoxins as Potential Therapeutic Targets
Pseudomonas aeruginosa infections are very difficult to treat because this pathogen has evolved a plethora of intrinsic and acquired resistance mechanisms to current antibiotics [345,346,347,348,349,350]. Since P. aeruginosa cytotoxins play crucial roles in its pathogenesis in vivo, targeting these toxins, (as novel strategies to reduce P. aeruginosa virulence and render this pathogen incapable of causing the disease), has been gaining momentum. For example, T3SS and its effectors play a pivotal role in P. aeruginosa pathogenesis and without it, P. aeruginosa becomes avirulent and cannot cause disease [20,243,246]. In a recent report, the T3SS inhibitors (salicylidene acylhydrazide INP0341 and of hydroxyquinoline INP1750) were shown to reduce cell death and inflammasome activation by clinical isolates expressing T3SS toxins [351]. INP0341 has also been shown to prevent corneal infection by P. aeruginosa in an experimental model of murine keratitis [352]. Other strategies to block the T3SS (e.g., anti-PcrV antibody, phenylacetic acid), or inhibit its effectors, (e.g., ExoU inhibition by Phospholipase A2 inhibitors or ExoS inhibition by small molecules), have also been shown to protect eukaryotic cells and tissues from cytotoxicity and P. aeruginosa infection [255,353,354,355]. Similarly, inhibitors of quorum sensing, rhamnolipids, and pyocyanin have also shown promising results in protecting against cellular cytotoxicity and tissue damage during P. aeruginosa infection [240,356,357]. Clinical trials are needed to evaluate these strategies as viable therapeutics for P. aeruginosa infections.
7. Concluding Remarks
P. aeruginosa is a highly successful bacterial pathogen capable of infecting numerous hosts, as diverse as plants and mammals [358,359,360]. The induction of cell death in target host cells appears to be a major virulence strategy that is fundamental to P. aeruginosa pathogenesis during infection, as manifested by many virulence factors that serve this purpose for P. aeruginosa, as discussed in this review and summarized in Table 1. It is unlikely that P. aeruginosa evolved all these cytotoxins as redundant means to merely kill its target host cells without consideration for the consequences associated with their different modes of cytotoxicity on the host immune system. It is more likely that depending on host and environmental factors, P. aeruginosa may prefer to use a subset of these virulence cytotoxins to fulfill its needs. For example, early after infection where bacteria numbers are few, P. aeruginosa is vulnerable, thus it may prefer to deploy apoptotic-inducing cytotoxins as a way to remain stealth, given the anti-inflammatory nature of apoptosis. However, the caveat with apoptotic cell deaths may be the slower kinetics of cell death due to the dependence of apoptosis on the elaborate protein-based complexes mediating this form of cell death as discussed above. Conversely, as the bacteria numbers increase during infection, it is inevitable for the host to recognize and mount effective immune responses against P. aeruginosa. Under such conditions, P. aeruginosa may prefer to use potent and fast-acting necrotic and pore-forming toxins, such as ExoU, to eliminate the threat posed by host immune phagocytic leukocytes. In addition, it remains unclear which mode of cytotoxicity may prevail in response to these cytotoxins. For example, apoptosis and necroptosis are mutually exclusive, as discussed above, so the mode of cytotoxicity may depend on the level of the competing cytotoxins and their kinetics of cytotoxicity. Moreover, other chemical and/or physical factors, (e.g., aerobic vs. anaerobic environment, tissue vs. blood, presence or absence of cofactors, pH, etc.), may also influence the choice of cytotoxins for P. aeruginosa. Clearly, more work is needed to tease out these possibilities.
Table 1.
Summary of the cytotoxic in P. aeruginosa strains and their mode of cytotoxicity and impacts on immune responses.
| Toxin | Function | Toxin Type | Host Target | Cytotoxicity Type | Host Immune Impact |
|---|---|---|---|---|---|
| ToxA | ADP-ribosylation [140,141] | AB Toxin [140,141] | eEF-2 [143] | Intrinsic and extrinsic apoptosis [144,145] | Anti-inflammatory [147] |
| C12-HSL | Autoinducer for the Rhl qourum-sensing [150] | Apoptotic cytotoxin [153,155] | Dampens expression of STAT3 and BEAS-2B [153,155] | Intrinsic and extrinsic apoptosis [151,152,153] | - Pro-inflammatory [159,160] - Anti-inflammatory [158] |
| Azurin | Cuperedoxin protein; Redox modulation [161] | Apoptotic cytotoxin [162,163,164,166] | p53 and non-receptor tyrosine kinases (NRTKs) [162,163,164,166] | Intrinsic apoptosis [162,163,164,165,166] | Unknown |
| Pyocyanin | - Pigment molecule [169,170,171] - Redox modulation [172,173] |
Apoptotic cytotoxin [176,177,178,179] |
Causes mitochondrial dysfunction [176,177,178,179] | ROS and intrinsic apoptosis features [176,177,178,179] | Pro-inflammatory [180,181] |
| Lipopolysaccharide (LPS) | Cell wall stabilization and adhesin [185,188] | Pyroptotic cytotoxin and possibly apoptosis [190,191,192] | - TLR4, Caspase-11, and SIRT1 [190,191,192] | - Pyroptotic cell death via Caspases 1 and 11 [190,191] - ROS-induced apoptosis [192] |
Highly pro-inflammatory via several mechanisms [193,194,195,196,197] |
| Flagella | Motility, biofilm [201] | Membrane disruption [199,200] | - TLR5 [207,208] - Naip5 and Naip 6 [209,210] |
Pyroptotic cell death via Caspase-1 inflammasome [190]. | Highly pro-inflammatory via several mechanisms [207,208,209,210,211] |
| Porins | Nutrient uptake; adhesin; signaling [217,218] | Causes mytochondrial dysfunction [220] | Dampens expression of BCL-2 [220] | Intrinsic apoptosis [220] | Pro-inflammatory via activation of PRRs [221,222] |
| Rhamnolipids | Glycolipid biosurfactants; involved in swarming motility, biofilm development, membrane composition, and antibiotic resistance [232,234,235,236,237] | - Apoptotic cytotoxin [238,239] - Necrotic cytotoxin [240,241] |
Mitochondrial outer membrane and p53 [238,239]. | - Features of intrinsic apoptosis [238,239] - Necrotic cell death feature as well [240,241] |
- Pro-inflammatory [240,241,242] |
| T3SS | Translocation machinery essential for P.aeruginosa pathogenesis [243,246,247] | - Pyroptotic [262,263] - Potentially necroptotic [181,182,183,184] |
Host membrane [256,257,258] | - Pyroptosis [262,263] - Necroptosis [181,182,183,184] |
Pro-inflammatory [19,188,189,190,191,192,193,194] |
| ExoS | Bifunctional exotoxin (GAP/ADPRT) toxin involved in many virulence functions [271,275,276,277,278,279,280] | Apoptotic cytotoxin [285,290] |
GAP: RhoA, Rac1, CDC42 [278,284] ADPRT: Ras, Rab, Rho, Ezrin, Radixin, Meosin, Vimentin, Cyclophilin A [279,282,283,284,285] |
GAP: Intrinsic and extrinsic apoptosis [285] ADPRT: Features of extrinsic apoptosis [290] |
- Pro-inflammatory [291,292,293] - Anti-inflammatory [294] - No effect on immune responses in wound tissues [19] |
| ExoT | Bifunctional exotoxin (GAP/ADPRT) toxin involved in many virulence functions [21,298,300,301,302,303,304] | Apoptotic cytotoxin [259,305,306,307] |
GAP: RhoA, Rac1, CDC42 [279,280] ADPRT: CrkI, CrkII, PGK1 [300] |
GAP: Intrinsic apoptosis [259,305,307] ADPRT: Anoikis apoptosis [306] |
Anti-inflammatory by blocking NLRC4 inflammasome [19] |
| ExoU | Patatin-like phospholipase Exotoxin [255,308,310] | Necrotic cytotoxin [255,308,309,361] | Phospholipids, lysophospholipids, neutral lipids [255,308,310] | Necrosis [180,233,234,235] | - Pro-inflammatory due to necrosis [317,318,319] - Anti-inflammatory [320] - Local immunosuppressant due to killing leukocytes [318,319] - No effect on immune responses in wound tissues [19] |
| ExoY | Adenylyl and guanylyl cyclase Exotoxin involves in several virulence functions [321,322,325,327,328] | Exotoxin | Filamentous actin and Tau [322,324] | Cell lysis [255], apoptosis [256] | - Pro-inflammatory [330] - No effect on immune responses in wound tissues [19] |
| CTX | øCTX pro-phage cytotoxin [331,332] | Pore-forming cytotoxin [332,334] | Host membrane [332,334] | Cell lysis [258,260] | Unknown but likely pro-inflammatory due to release of DAMPs [336] |
| ExlA | AB cytotoxin required for pathogenesis of PA7-like clade of P. aeruginosa strains [339] | Pore-forming [339,340] | Host membrane translocation mediated by ExlB [339,340] | - Pyroptosis, mediated by Caspase-1/11 [342] - Necroptosis, mediated by RIP1 kinase [342] |
Highly pro-inflammatory [341] |
It is worth noting that the impacts of some of the cytotoxins on host immune responses were at times confusing (in that they had been implicated in both pro-inflammatory or anti-inflammatory responses), or not congruent with the nature of the cell death induced by these toxins as discussed above and summarized in Table 1. These seeming discrepancies may be due to differences in experimental procedures that in most studies primarily were reliant on in vitro cell culture-based assays. Or, they may reflect the possibility that specific sites within a host may have evolved different mechanism(s) to detect and/or respond to P. aeruginosa and its cytotoxins during infection. It is therefore prudent to assess immune responses in appropriate in vivo models as a way to evaluate the net impact of P. aeruginosa cytotoxins on host immune responses.
Author Contributions
S.J.W., J.W.G., M.Y.S., A.H.D. and S.H.S. wrote the paper. J.W.G. created the figures. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Institutes of Health (NIH) grants R01AI150668, RO1DK107713, and R21AI110685-01 (all to S.H.S).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Engel J.N. Molecular pathogenesis of acute Pseudomonas aeruginosa infections. In: Hauser A., Rello J., editors. Severe Infections Caused by Pseudomonas aeruginosa. Kluwer Academic/Plenum Press; New York, NY, USA: 2003. pp. 201–230. [Google Scholar]
- 2.Yamaguchi T., Yamada H. Role of mechanical injury on airway surface in the pathogenesis of Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 1991;144:1147–1152. doi: 10.1164/ajrccm/144.5.1147. [DOI] [PubMed] [Google Scholar]
- 3.Zahm J.-M., Chevillard M., Puchelle E. Wound repair of human surface respiratory epithelium. Am. J. Respir. Cell Mol. Biol. 1991;5:242–248. doi: 10.1165/ajrcmb/5.3.242. [DOI] [PubMed] [Google Scholar]
- 4.Tsang K., Rutman A., Tanaka E., Lund V., Dewar A., Cole P., Wilson R. Interaction of Pseudomonas aeruginosa with human respiratory mucosa in vitro. Eur. Respir. J. 1994;7:1746–1753. doi: 10.1183/09031936.94.07101746. [DOI] [PubMed] [Google Scholar]
- 5.de Bentzmann S., Plotkowski C., Puchelle E. Receptors in the Pseudomonas aeruginosa adherence to injured and repairing airway epithelium. Am. J. Respir. Crit. Care Med. 1996;154:S155–S162. doi: 10.1164/ajrccm/154.4_Pt_2.S155. [DOI] [PubMed] [Google Scholar]
- 6.Madsen S.M., Westh H., Danielsen L., Rosdahl V.T. Bacterial colonization and healing of venous leg ulcers. APMIS. 1996;104:895–899. doi: 10.1111/j.1699-0463.1996.tb04955.x. [DOI] [PubMed] [Google Scholar]
- 7.Halbert A.R., Stacey M.C., Rohr J.B., Jopp-McKay A. The effect of bacterial colonization on venous ulcer healing. Australas. J. Dermatol. 1992;33:75–80. doi: 10.1111/j.1440-0960.1992.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 8.Gjødsbøl K., Christensen J.J., Karlsmark T., Jørgensen B., Klein B.M., A Krogfelt K. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006;3:225–231. doi: 10.1111/j.1742-481X.2006.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winstanley C., Kaye S.B., Neal T.J., Chilton H.J., Miksch S., Hart C.A. Genotypic and phenotypic characteristics of Pseudomonas aeruginosa isolates associated with ulcerative keratitis. Pt 6J. Med. Microbiol. 2005;54:519–526. doi: 10.1099/jmm.0.46005-0. [DOI] [PubMed] [Google Scholar]
- 10.Waters C.M., Goldberg J.B. Pseudomonas aeruginosa in cystic fibrosis: A chronic cheater. Proc. Natl. Acad. Sci. USA. 2019;116:6525–6527. doi: 10.1073/pnas.1902734116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emerson J., Rosenfeld M., McNamara S., Ramsey B., Gibson R.L. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 12.Koch C. Early infection and progression of cystic fibrosis lung disease. Pediatr. Pulmonol. 2002;34:232–236. doi: 10.1002/ppul.10135. [DOI] [PubMed] [Google Scholar]
- 13.Harbarth S., Ferrière K., Hugonnet S., Ricou B., Suter P., Pittet D. Epidemiology and prognostic determinants of bloodstream infections in surgical intensive care. Arch. Surg. 2002;137:1353–1359. doi: 10.1001/archsurg.137.12.1353. [DOI] [PubMed] [Google Scholar]
- 14.Osmon S., Ward S., Fraser V., Kollef M.H. Hospital mortality for patients with bacteremia due to Staphylococcus aureus or Pseudomonas aeruginosa. Chest. 2004;125:607–616. doi: 10.1378/chest.125.2.607. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee M., Anju C., Biswas L., Kumar V.A., Mohan C.G., Biswas R. Antibiotic resistance in Pseudomonas aeruginosa and alternative therapeutic options. Int. J. Med. Microbiol. 2016;306:48–58. doi: 10.1016/j.ijmm.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Nathwani D., Raman G., Sulham K., Gavaghan M., Menon V. Clinical and economic consequences of hospital-acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control. 2014;3:32. doi: 10.1186/2047-2994-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Breidenstein E.B., de la Fuente-Núñez C., Hancock R.E. Pseudomonas aeruginosa: All roads lead to resistance. Trends Microbiol. 2011;19:419–426. doi: 10.1016/j.tim.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Bachta K., Mekalanos J. Open Forum Infectious Diseases. Infectious Diseases Society of America; Arlington, VA, USA: 2015. Tackling Multidrug Resistance by Targeting Efflux in Pseudomonas aeruginosa. [Google Scholar]
- 19.Mohamed M.F., Gupta K., Goldufsky J.W., Roy R., Callaghan L.T., Wetzel D.M., Kuzel T.M., Reiser J., Shafikhani S.H. CrkII/Abl phosphorylation cascade is critical for NLRC4 inflammasome activity and is blocked by Pseudomonas aeruginosa ExoT. Nat. Commun. 2022;13:1295. doi: 10.1038/s41467-022-28967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldufsky J., Wood S.J., Jayaraman V., Majdobeh O., Chen L., Qin S., Zhang C., DiPietro L.A., Shafikhani S.H. Pseudomonas aeruginosa uses T3SS to inhibit diabetic wound healing. Wound Repair Regen. 2015;23:557–564. doi: 10.1111/wrr.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garrity-Ryan L., Shafikhani S., Balachandran P., Nguyen L., Oza J., Jakobsen T., Sargent J., Fang X., Cordwell S., Matthay M.A., et al. The ADP ribosyltransferase domain of Pseudomonas aeruginosa ExoT contributes to its biological activities. Infect. Immun. 2004;72:546–558. doi: 10.1128/IAI.72.1.546-558.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lockshin R.A., Williams C.M. Programmed cell death—I. Cytology of degeneration in the intersegmental muscles of the Pernyi silkmoth. J. Insect Physiol. 1965;11:123–133. doi: 10.1016/0022-1910(65)90099-5. [DOI] [PubMed] [Google Scholar]
- 23.Kerr J.F.R., Wyllie A.H., Currie A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bandyopadhyay A., Tsuji K., Cox K., Harfe B.D., Rosen V., Tabin C.J. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Opferman J. Apoptosis in the development of the immune system. Cell Death Differ. 2008;15:234–242. doi: 10.1038/sj.cdd.4402182. [DOI] [PubMed] [Google Scholar]
- 26.Zhivotovsky B., Kroemer G. Apoptosis and genomic instability. Nat. Rev. Mol. Cell Biol. 2004;5:752–762. doi: 10.1038/nrm1443. [DOI] [PubMed] [Google Scholar]
- 27.Greenhalgh D.G. The role of apoptosis in wound healing. Int. J. Biochem. Cell Biol. 1998;30:1019–1030. doi: 10.1016/S1357-2725(98)00058-2. [DOI] [PubMed] [Google Scholar]
- 28.Gallucci S., Caricchio R., Cohen P.L. The Autoimmune Diseases. Elsevier; Amsterdam, The Netherlands: 2020. Cell Death and Autoimmune Disease; pp. 291–303. [Google Scholar]
- 29.Lowe W.S., Lin A.W. Apoptosis in cancer. Carcinogenesis. 2000;21:485–495. doi: 10.1093/carcin/21.3.485. [DOI] [PubMed] [Google Scholar]
- 30.Jorgensen I., Rayamajhi M., Miao E.A. Programmed cell death as a defence against infection. Nat. Rev. Immunol. 2017;17:151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elliott M.R., Ravichandran K.S. Clearance of apoptotic cells: Implications in health and disease. J. Cell Biol. 2010;189:1059–1070. doi: 10.1083/jcb.201004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh R., Letai A., Sarosiek K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019;20:175–193. doi: 10.1038/s41580-018-0089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien M.A., Kirby R. Apoptosis: A review of pro-apoptotic and anti-apoptotic pathways and dysregulation in disease. J. Vet. Emerg. Crit. Care. 2008;18:572–585. doi: 10.1111/j.1476-4431.2008.00363.x. [DOI] [Google Scholar]
- 34.Shalini S., Dorstyn L., Dawar S., Kumar S. Old, new and emerging functions of caspases. Cell Death Differ. 2015;22:526–539. doi: 10.1038/cdd.2014.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang A., Wu Y., Lai H.W., Yew D.T. Apoptosis–A brief review. Neuroembryol. Aging. 2004;3:47–59. doi: 10.1159/000085404. [DOI] [Google Scholar]
- 36.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hongmei Z. Apoptosis and Medicine. InTechOpen; London, UK: 2012. Extrinsic and intrinsic apoptosis signal pathway review. [Google Scholar]
- 38.Lomonosova E., Chinnadurai G. BH3-only proteins in apoptosis and beyond: An overview. Oncogene. 2008;27((Suppl. S1)):S2–S19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tinel A., Tschopp J. The PIDDosome, a protein complex implicated in activation of caspase-2 in response to genotoxic stress. Science. 2004;304:843–846. doi: 10.1126/science.1095432. [DOI] [PubMed] [Google Scholar]
- 40.Fava L.L., Bock F.J., Geley S., Villunger A. Caspase-2 at a glance. J. Cell Sci. 2012;125:5911–5915. doi: 10.1242/jcs.115105. [DOI] [PubMed] [Google Scholar]
- 41.Jang T.-H., Park H.H. PIDD mediates and stabilizes the interaction between RAIDD and caspase-2 for the PIDDosome assembly. BMB Rep. 2013;46:471–476. doi: 10.5483/BMBRep.2013.46.9.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wachmann K., Pop C., van Raam B.J., Drag M., Mace P.D., Snipas S.J., Zmasek C., Schwarzenbacher R., Salvesen G.S., Riedl S.J. Activation and specificity of human caspase-10. Biochemistry. 2010;49:8307–8315. doi: 10.1021/bi100968m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schug Z.T., Gonzalvez F., Houtkooper R.H., Vaz F.M., Gottlieb E. BID is cleaved by caspase-8 within a native complex on the mitochondrial membrane. Cell Death Differ. 2011;18:538–548. doi: 10.1038/cdd.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mühlethaler-Mottet A., Flahaut M., Bourloud K.B., Nardou K., Coulon A., Liberman J., Thome M., Gross N. Individual caspase-10 isoforms play distinct and opposing roles in the initiation of death receptor-mediated tumour cell apoptosis. Cell Death Dis. 2011;2:e125. doi: 10.1038/cddis.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roh J.S., Sohn D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018;18:e27. doi: 10.4110/in.2018.18.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maderna P., Godson C. Phagocytosis of apoptotic cells and the resolution of inflammation. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2003;1639:141–151. doi: 10.1016/j.bbadis.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 47.Maderna P., Godson C. Clearance of apoptotic cells by phagocytes. Cell Death Differ. 2008;15:243–250. doi: 10.1038/sj.cdd.4402184. [DOI] [PubMed] [Google Scholar]
- 48.Fadok V.A., Bratton D.L., Konowal A., Freed P.W., Westcott J.Y., Henson P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Henson P.M., Bratton D.L. Antiinflammatory effects of apoptotic cells. J. Clin. Investig. 2013;123:2773–2774. doi: 10.1172/JCI69344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Man S.M., Karki R., Kanneganti T.-D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017;277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bergsbaken T., Fink S.L., Cookson B.T. Pyroptosis: Host cell death and inflammation. Nat. Rev. Microbiol. 2009;7:99–109. doi: 10.1038/nrmicro2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schroder K., Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 53.Place D.E., Kanneganti T.D. Recent advances in inflammasome biology. Curr. Opin. Immunol. 2018;50:32–38. doi: 10.1016/j.coi.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi J., Zhao Y., Wang Y., Gao W., Ding J., Li P., Hu L., Shao F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature. 2014;514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 55.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 56.Shi J., Gao W., Shao F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 2017;42:245–254. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 57.Ding J., Wang K., Liu W., She Y., Sun Q., Shi J., Sun H., Wang D.C., Shao F. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 58.Yang J., Zhao Y., Shao F. Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Curr. Opin. Immunol. 2015;32:78–83. doi: 10.1016/j.coi.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 59.Kayagaki N., Warming S., Lamkanfi M., Walle L.V., Louie S., Dong J., Newton K., Qu Y., Liu J., Heldens S., et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 60.Viganò E., Diamond C.E., Spreafico R., Balachander A., Sobota R.M., Mortellaro A. Human caspase-4 and caspase-5 regulate the one-step non-canonical inflammasome activation in monocytes. Nat. Commun. 2015;6:8761. doi: 10.1038/ncomms9761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baker P.J., Boucher D., Bierschenk D., Tebartz C., Whitney P.G., D’Silva D.B., Tanzer M.C., Monteleone M., Robertson A.A., Cooper M., et al. NLRP3 inflammasome activation downstream of cytoplasmic LPS recognition by both caspase-4 and caspase-5. Eur. J. Immunol. 2015;45:2918–2926. doi: 10.1002/eji.201545655. [DOI] [PubMed] [Google Scholar]
- 62.Schmid-Burgk J.L., Gaidt M.M., Schmidt T., Ebert T.S., Bartok E., Hornung V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur. J. Immunol. 2015;45:2911–2917. doi: 10.1002/eji.201545523. [DOI] [PubMed] [Google Scholar]
- 63.Rühl S., Broz P. Caspase-11 activates a canonical NLRP3 inflammasome by promoting K+ efflux. Eur. J. Immunol. 2015;45:2927–2936. doi: 10.1002/eji.201545772. [DOI] [PubMed] [Google Scholar]
- 64.Rathinam V.A.K., Vanaja S.K., A Fitzgerald K. Fitzgerald, Regulation of inflammasome signaling. Nat. Immunol. 2012;13:333. doi: 10.1038/ni.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lev-Sagie A., Prus D., Linhares I., Lavy Y., Ledger W.J., Witkin S.S. Polymorphism in a gene coding for the inflammasome component NALP3 and recurrent vulvovaginal candidiasis in women with vulvar vestibulitis syndrome. Am. J. Obstet. Gynecol. 2009;200:303.e1–303.e6. doi: 10.1016/j.ajog.2008.10.039. [DOI] [PubMed] [Google Scholar]
- 66.Witkin S.S., Bierhals K., Linhares I., Normand N., Dieterle S., Neuer A. Genetic polymorphism in an inflammasome component, cervical mycoplasma detection and female infertility in women undergoing in vitro fertilization. J. Reprod. Immunol. 2010;84:171–175. doi: 10.1016/j.jri.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 67.Asfaw Idosa B. Ph.D. Thesis. Örebro University; Örebro, Sweden: 2016. Inflammasome Polymorphisms and the Inflammatory Response to Bacterial Infections. [Google Scholar]
- 68.Mao L., Kitani A., Similuk M., Oler A.J., Albenberg L., Kelsen J., Aktay A., Quezado M., Yao M., Montgomery-Recht K., et al. Loss-of-function CARD8 mutation causes NLRP3 inflammasome activation and Crohn’s disease. J. Clin. Investig. 2018;128:1793–1806. doi: 10.1172/JCI98642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Theodoropoulou K., Wittkowski H., Busso N., Von Scheven-Gête A., Moix I., Vanoni F., Hengten V., Horneff G., Haas J.-P., Fischer N., et al. Increased Prevalence of NLRP3 Q703K Variant Among Patients With Autoinflammatory Diseases: An International Multicentric Study. Front. Immunol. 2020;11:877. doi: 10.3389/fimmu.2020.00877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhen Y., Zhang H. NLRP3 inflammasome and inflammatory bowel disease. Front. Immunol. 2019;10:276. doi: 10.3389/fimmu.2019.00276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hoffman H.M., Kuemmerle-Deschner J.B., Goldbach-Mansky R. Textbook of Autoinflammation. Springer; Berlin/Heidelberg, Germany: 2019. Cryopyrin-Associated Periodic Syndromes (CAPS) pp. 347–365. [Google Scholar]
- 72.Mangan M.S.J., Olhava E.J., Roush W.R., Seidel H.M., Glick G.D., Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018;17:588. doi: 10.1038/nrd.2018.97. [DOI] [PubMed] [Google Scholar]
- 73.Malhotra S., Costa C., Eixarch H., Keller C.W., Amman L., Martínez-Banaclocha H., Midaglia L., Sarró E., Machín-Díaz I., Villar L.M., et al. NLRP3 inflammasome as prognostic factor and therapeutic target in primary progressive multiple sclerosis patients. Brain A J. Neurol. 2020;143:1414–1430. doi: 10.1093/brain/awaa084. [DOI] [PubMed] [Google Scholar]
- 74.Gao J., Liu R.T., Cao S., Cui J.Z., Wang A., To E., Matsubara J.A. NLRP3 inflammasome: Activation and regulation in age-related macular degeneration. Mediat. Inflamm. 2015;2015:690243. doi: 10.1155/2015/690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duan Y., Kelley N., He Y. Role of the NLRP3 inflammasome in neurodegenerative diseases and therapeutic implications. Neural Regen. Res. 2020;15:1249. doi: 10.4103/1673-5374.272576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu D., Zeng X., Li X., Mehta J.L., Wang X. Role of NLRP3 inflammasome in the pathogenesis of cardiovascular diseases. Basic Res. Cardiol. 2018;113:5. doi: 10.1007/s00395-017-0663-9. [DOI] [PubMed] [Google Scholar]
- 77.Yao C., Veleva T., Scott L., Jr., Cao S., Li L., Chen G., Jeyabal P., Pan X., Alsina K.M., Abu-Taha I., et al. Enhanced cardiomyocyte NLRP3 inflammasome signaling promotes atrial fibrillation. Circulation. 2018;138:2227–2242. doi: 10.1161/CIRCULATIONAHA.118.035202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.An N., Gao Y., Si Z., Zhang H., Wang L., Tian C., Yuan M., Yang X., Li X., Shang H., et al. Regulatory mechanisms of the NLRP3 inflammasome, a novel immune-inflammatory marker in cardiovascular diseases. Front. Immunol. 2019;10:1592. doi: 10.3389/fimmu.2019.01592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hu B., Elinav E., Huber S., Strowig T., Hao L., Hafemann A., Jin C., Wunderlich C., Wunderlich T., Eisenbarth S.C., et al. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc. Natl. Acad. Sci. USA. 2013;110:9862–9867. doi: 10.1073/pnas.1307575110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei Q., Mu K., Li T., Zhang Y., Yang Z., Jia X., Zhao W., Huai W., Guo P., Han L. Deregulation of the NLRP3 inflammasome in hepatic parenchymal cells during liver cancer progression. Lab. Investig. 2014;94:52–62. doi: 10.1038/labinvest.2013.126. [DOI] [PubMed] [Google Scholar]
- 81.Guo B., Fu S., Zhang J., Liu B., Li Z. Targeting inflammasome/IL-1 pathways for cancer immunotherapy. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep36107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jourdan T., Godlewski G., Cinar R., Bertola A., Szanda G., Liu J., Tam J., Han T., Mukhopadhyay B., Skarulis M.C., et al. Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes. Nat. Med. 2013;19:1132. doi: 10.1038/nm.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shin J.J., Lee E.K., Park T.J., Kim W. Damage-associated molecular patterns and their pathological relevance in diabetes mellitus. Ageing Res. Rev. 2015;24:66–76. doi: 10.1016/j.arr.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 84.Donath M.Y. Targeting inflammation in the treatment of type 2 diabetes: Time to start. Nat. Rev. Drug Discov. 2014;13:465–476. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- 85.Holler N., Zaru R., Micheau O., Thome M., Attinger A., Valitutti S., Bodmer J.L., Schneider P., Seed B., Tschopp J. Fas triggers an alternative, caspase-8–independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 86.Degterev A., Huang Z., Boyce M., Li Y., Jagtap P., Mizushima N., Cuny G.D., Mitchison T.J., Moskowitz M.A., Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat. Chem. Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 87.Galluzzi L., Kepp O., Chan F.K.-M., Kroemer G. Necroptosis: Mechanisms and Relevance to Disease. Annu. Rev. Pathol. 2017;12:103–130. doi: 10.1146/annurev-pathol-052016-100247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Grootjans S., Vanden Berghe T., Vandenabeele P. Initiation and execution mechanisms of necroptosis: An overview. Cell Death Differ. 2017;24:1184–1195. doi: 10.1038/cdd.2017.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Proskuryakov S.Y., Konoplyannikov A.G., Gabai V.L. Necrosis: A specific form of programmed cell death? Exp. Cell Res. 2003;283:1–16. doi: 10.1016/S0014-4827(02)00027-7. [DOI] [PubMed] [Google Scholar]
- 90.Nirmala J.G., Lopus M. Cell death mechanisms in eukaryotes. Cell Biol. Toxicol. 2020;36:145–164. doi: 10.1007/s10565-019-09496-2. [DOI] [PubMed] [Google Scholar]
- 91.Vercammen D., Vandenabeele P., Beyaert R., Declercq W., Fiers W. Tumour necrosis factor-induced necrosis versus anti-Fas-induced apoptosis in L929 cells. Cytokine. 1997;9:801–808. doi: 10.1006/cyto.1997.0252. [DOI] [PubMed] [Google Scholar]
- 92.Jouan-Lanhouet S., I Arshad M., Piquet-Pellorce C., Martin-Chouly C., Le Moigne-Muller G., Van Herreweghe F., Takahashi N., Sergent O., Lagadic-Gossmann D., Vandenabeele P., et al. TRAIL induces necroptosis involving RIPK1/RIPK3-dependent PARP-1 activation. Cell Death Differ. 2012;19:2003–2014. doi: 10.1038/cdd.2012.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Laster S.M., Wood J.G., Gooding L.R. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J. Immunol. 1988;141:2629–2634. doi: 10.4049/jimmunol.141.8.2629. [DOI] [PubMed] [Google Scholar]
- 94.Seya T., Shime H., Takaki H., Azuma M., Oshiumi H., Matsumoto M. TLR3/TICAM-1 signaling in tumor cell RIP3-dependent necroptosis. Oncoimmunology. 2012;1:917–923. doi: 10.4161/onci.21244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chan F.K.-M., Shisler J., Bixby J.G., Felices M., Zheng L., Appel M., Orenstein J., Moss B., Lenardo M.J. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. JBC. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 96.Kuriakose T., Man S.M., Subbarao Malireddi R.K., Karki R., Kesavardhana S., Place D.E., Neale G., Vogel P., Kanneganti T.D. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 2016;1:aag2045. doi: 10.1126/sciimmunol.aag2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schock S.N., Chandra N.V., Sun Y., Irie T., Kitagawa Y., Gotoh B., Coscoy L., Winoto A. Induction of necroptotic cell death by viral activation of the RIG-I or STING pathway. Cell Death Differ. 2017;24:615–625. doi: 10.1038/cdd.2016.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maelfait J., Liverpool L., Bridgeman A., Ragan K.B., Upton J.W., Rehwinkel J. Sensing of viral and endogenous RNA by ZBP1/DAI induces necroptosis. EMBO J. 2017;36:2529–2543. doi: 10.15252/embj.201796476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang D.-W., Shao J., Lin J., Zhang N., Lu B.-J., Lin S.-C., Dong M.-Q., Han J. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 100.He S., Wang L., Miao L., Wang T., Du F., Zhao L., Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 101.Wang H., Sun L., Su L., Rizo J., Liu L., Wang L.-F., Wang F.-S., Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol. Cell. 2014;54:133–146. doi: 10.1016/j.molcel.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 102.Dondelinger Y., Declercq W., Montessuit S., Roelandt R., Goncalves A., Bruggeman I., Hulpiau P., Weber K., Sehon C.A., Marquis R.W., et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell Rep. 2014;7:971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 103.Cai Z., Jitkaew S., Zhao J., Chiang H.-C., Choksi S., Liu J., Ward Y., Wu L.-G., Liu Z.-G. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell Biol. 2014;16:55–65. doi: 10.1038/ncb2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Murphy J.M., Czabotar P.E., Hildebrand J.M., Lucet I.S., Zhang J.-G., Alvarez-Diaz S., Lewis R., Lalaoui N., Metcalf D., Webb A.I., et al. The pseudokinase MLKL mediates necroptosis via a molecular switch mechanism. Immunity. 2013;39:443–453. doi: 10.1016/j.immuni.2013.06.018. [DOI] [PubMed] [Google Scholar]
- 105.Su L., Quade B., Wang H., Sun L., Wang X., Rizo J. A plug release mechanism for membrane permeation by MLKL. Structure. 2014;22:1489–1500. doi: 10.1016/j.str.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jacobsen A.V., Lowes K., Tanzer M.C., Lucet I.S., Hildebrand J.M., Petrie E., van Delft M., Liu Z., A Conos S., Zhang J.-G., et al. HSP90 activity is required for MLKL oligomerisation and membrane translocation and the induction of necroptotic cell death. Cell Death Dis. 2016;7:e2051. doi: 10.1038/cddis.2015.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cho Y.S., Challa S., Moquin D., Genga R., Ray T.D., Guildford M., Chan F.K.-M. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.He S., Liang Y., Shao F., Wang X. Toll-like receptors activate programmed necrosis in macrophages through a receptor-interacting kinase-3–mediated pathway. Proc. Natl. Acad. Sci. USA. 2011;108:20054–20059. doi: 10.1073/pnas.1116302108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kaiser W.J., Sridharan H., Huang C., Mandal P., Upton J.W., Gough P.J., Sehon C.A., Marquis R.W., Bertin J., Mocarski E.S. Toll-like receptor 3-mediated necrosis via TRIF, RIP3, and MLKL. JBC. 2013;288:31268–31279. doi: 10.1074/jbc.M113.462341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Annibaldi A., John S.W., Berghe T.V., Swatek K.N., Ruan J., Liccardi G., Bianchi K., Elliott P.R., Choi S.M., Van Coillie S., et al. Ubiquitin-Mediated Regulation of RIPK1 Kinase Activity Independent of IKK and MK2. Mol. Cell. 2018;69:566–580.e5. doi: 10.1016/j.molcel.2018.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Häcker H., Karin M. Regulation and function of IKK and IKK-related kinases. Sci. STKE. 2006;2006 doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 112.Kelliher M.A., Grimm S., Ishida Y., Kuo F., Stanger B.Z., Leder P. The death domain kinase RIP mediates the TNF-induced NF-κB signal. Immunity. 1998;8:297–303. doi: 10.1016/S1074-7613(00)80535-X. [DOI] [PubMed] [Google Scholar]
- 113.Lee T.H., Shank J., Cusson N., Kelliher M.A. The kinase activity of Rip1 is not required for tumor necrosis factor-α-induced IκB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. JBC. 2004;279:33185–33191. doi: 10.1074/jbc.M404206200. [DOI] [PubMed] [Google Scholar]
- 114.Dondelinger Y., Jouan-Lanhouet S., Divert T., Theatre E., Bertin J., Gough P.J., Giansanti P., Heck A.J., Dejardin E., Vandenabeele P., et al. NF-κB-independent role of IKKα/IKKβ in preventing RIPK1 kinase-dependent apoptotic and necroptotic cell death during TNF signaling. Mol. Cell. 2015;60:63–76. doi: 10.1016/j.molcel.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 115.Micheau O., Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/S0092-8674(03)00521-X. [DOI] [PubMed] [Google Scholar]
- 116.Pasparakis M., Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 117.Lin Y., Devin A., Rodriguez Y., Liu Z.-G. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Feng S., Yang Y., Mei Y., Ma L., Zhu D.-E., Hoti N., Castanares M., Wu M. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell. Signal. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 119.Legarda D., Justus S.J., Ang R.L., Rikhi N., Li W., Moran T.M., Zhang J., Mizoguchi E., Zelic M., Kelliher M.A., et al. CYLD proteolysis protects macrophages from TNF-mediated auto-necroptosis induced by LPS and licensed by type I IFN. Cell Rep. 2016;15:2449–2461. doi: 10.1016/j.celrep.2016.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaczmarek A., Vandenabeele P., Krysko D.V. Necroptosis: The release of damage-associated molecular patterns and its physiological relevance. Immunity. 2013;38:209–223. doi: 10.1016/j.immuni.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 121.Lawlor K.E., Khan N., Mildenhall A., Gerlic M., Croker B.A., D’Cruz A.A., Hall C., Spall S.K., Anderton H., Masters S.L., et al. RIPK3 promotes cell death and NLRP3 inflammasome activation in the absence of MLKL. Nat. Commun. 2015;6:6282. doi: 10.1038/ncomms7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lukens J.R., Vogel P., Johnson G.R., Kelliher M.A., Iwakura Y., Lamkanfi M., Kanneganti T.-D. RIP1-driven autoinflammation targets IL-1α independently of inflammasomes and RIP3. Nature. 2013;498:224–227. doi: 10.1038/nature12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kang S., Fernandes-Alnemri T., Rogers C., Mayes L., Wang Y., Dillon C.P., Roback L., Kaiser W.J., Oberst A., Sagara J., et al. Caspase-8 scaffolding function and MLKL regulate NLRP3 inflammasome activation downstream of TLR3. Nat. Commun. 2015;6:7515. doi: 10.1038/ncomms8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kitur K., Wachtel S., Brown A., Wickersham M., Paulino F., Penaloza H., Soong G., Bueno S.M., Parker D., Prince A. Necroptosis promotes Staphylococcus aureus clearance by inhibiting excessive inflammatory signaling. Cell Rep. 2016;16:2219–2230. doi: 10.1016/j.celrep.2016.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Huang Z., Wu S.Q., Liang Y., Zhou X., Chen W., Li L., Wu J., Zhuang Q., Chen C.A., Li J., et al. RIP1/RIP3 binding to HSV-1 ICP6 initiates necroptosis to restrict virus propagation in mice. Cell Host Microbe. 2015;17:229–242. doi: 10.1016/j.chom.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 126.Weng D., Marty-Roix R., Ganesan S., Proulx M.K., Vladimer G.I., Kaiser W.J., Mocarski E.S., Pouliot K., Chan F.K.-M., Kelliher M.A., et al. Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc. Natl. Acad. Sci. USA. 2014;111:7391–7396. doi: 10.1073/pnas.1403477111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Robinson N., McComb S., Mulligan R., Dudani R., Krishnan L., Sad S. Type I interferon induces necroptosis in macrophages during infection with Salmonella enterica serovar Typhimurium. Nat. Immunol. 2012;13:954. doi: 10.1038/ni.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kitur K., Parker D., Nieto P., Ahn D.S., Cohen T., Chung S., Wachtel S., Bueno S.M., Prince A. Toxin-induced necroptosis is a major mechanism of Staphylococcus aureus lung damage. PLoS Pathog. 2015;11:e1004820. doi: 10.1371/journal.ppat.1004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bolognese A.C., Yang W.-L., Hansen L.W., Denning N.-L., Nicastro J.M., Coppa G.F., Wang P. Inhibition of necroptosis attenuates lung injury and improves survival in neonatal sepsis. Surgery. 2018;164:110–116. doi: 10.1016/j.surg.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Qian Z., Shuying W., Ranran D. Inhibitory effects of JQ1 on listeria monocytogenes-induced acute liver injury by blocking BRD4/RIPK1 axis. Biomed. Pharmacother. 2020;125:109818. doi: 10.1016/j.biopha.2020.109818. [DOI] [PubMed] [Google Scholar]
- 131.Duprez L., Takahashi N., Van Hauwermeiren F., Vandendriessche B., Goossens V., Vanden Berghe T., Declercq W., Libert C., Cauwels A., Vandenabeele P. RIP kinase-dependent necrosis drives lethal systemic inflammatory response syndrome. Immunity. 2011;35:908–918. doi: 10.1016/j.immuni.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 132.Günther C., Martini E., Wittkopf N., Amann K., Weigmann B., Neumann H., Waldner M.J., Hedrick S.M., Tenzer S., Neurath M.F., et al. Caspase-8 regulates TNF-α-induced epithelial necroptosis and terminal ileitis. Nature. 2011;477:335–339. doi: 10.1038/nature10400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pierdomenico M., Negroni A., Stronati L., Vitali R., Prete E., Bertin J., Gough P.J., Aloi M., Cucchiara S. Necroptosis is active in children with inflammatory bowel disease and contributes to heighten intestinal inflammation. Am. J. Gastroenterol. 2014;109:279–287. doi: 10.1038/ajg.2013.403. [DOI] [PubMed] [Google Scholar]
- 134.Polykratis A., Martens A., Eren R.O., Shirasaki Y., Yamagishi M., Yamaguchi Y., Uemura S., Miura M., Holzmann B., Kollias G., et al. A20 prevents inflammasome-dependent arthritis by inhibiting macrophage necroptosis through its ZnF7 ubiquitin-binding domain. Nat. Cell Biol. 2019;21:731–742. doi: 10.1038/s41556-019-0324-3. [DOI] [PubMed] [Google Scholar]
- 135.Ofengeim D., Ito Y., Najafov A., Zhang Y., Shan B., DeWitt J.P., Ye J., Zhang X., Chang A., Vakifahmetoglu-Norberg H., et al. Activation of necroptosis in multiple sclerosis. Cell Rep. 2015;10:1836–1849. doi: 10.1016/j.celrep.2015.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mizumura K., Cloonan S., Nakahira K., Bhashyam A.R., Cervo M., Kitada T., Glass K., Owen C.A., Mahmood A., Washko G.R., et al. Mitophagy-dependent necroptosis contributes to the pathogenesis of COPD. J. Clin. Investig. 2014;124:3987–4003. doi: 10.1172/JCI74985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Perry J.S.A., Morioka S., Medina C.B., Etchegaray J.I., Barron B., Raymond M.H., Lucas C.D., Onengut-Gumuscu S., Delpire E., Ravichandran K.S. Interpreting an apoptotic corpse as anti-inflammatory involves a chloride sensing pathway. Nat. Cell Biol. 2019;21:1532–1543. doi: 10.1038/s41556-019-0431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Voll R.E., Herrmann M., Roth E.A., Stach C., Kalden J.R., Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 139.Nakahashi-Oda C., Tahara-Hanaoka S., Shoji M., Okoshi Y., Nakano-Yokomizo T., Ohkohchi N., Yasui T., Kikutani H., Honda S.-I., Shibuya K., et al. Apoptotic cells suppress mast cell inflammatory responses via the CD300a immunoreceptor. JEM. 2012;209:1493–1503. doi: 10.1084/jem.20120096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Allured V.S., Collier R.J., Carroll S.F., McKay D.B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc. Natl. Acad. Sci. USA. 1986;83:1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Leppla S.H. Large-scale purification and characterization of the exotoxin of Pseudomonas aeruginosa. Infect. Immun. 1976;14:1077–1086. doi: 10.1128/iai.14.4.1077-1086.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Odumosu O., Nicholas D., Yano H., Langridge W. AB toxins: A paradigm switch from deadly to desirable. Toxins. 2010;2:1612–1645. doi: 10.3390/toxins2071612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jørgensen R., Wang Y., Visschedyk D., Merrill A.R. The nature and character of the transition state for the ADP-ribosyltransferase reaction. EMBO Rep. 2008;9:802–809. doi: 10.1038/embor.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Du X., Youle R.J., FitzGerald D.J., Pastan I. Pseudomonas Exotoxin A-mediated apoptosis is Bak dependent and preceded by the degradation of Mcl-1. Mol. Cell. Biol. 2010;30:3444–3452. doi: 10.1128/MCB.00813-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jenkins C.E., Swiatoniowski A., Issekutz A.C., Lin T.-J. Pseudomonas aeruginosa exotoxin A induces human mast cell apoptosis by a caspase-8 and-3-dependent mechanism. JBC. 2004;279:37201–37207. doi: 10.1074/jbc.M405594200. [DOI] [PubMed] [Google Scholar]
- 146.Miyazaki S., Matsumoto T., Tateda K., Ohno A., Yamaguchi K. Role of exotoxin A in inducing severe Pseudomonas aeruginosa infections in mice. J. Med. Microbiol. 1995;43:169–175. doi: 10.1099/00222615-43-3-169. [DOI] [PubMed] [Google Scholar]
- 147.Pillar C.M., Hobden J.A. Pseudomonas aeruginosa exotoxin A and keratitis in mice. IOVS. 2002;43:1437–1444. [PubMed] [Google Scholar]
- 148.Pastan I., Beers R., Bera T.K. Recombinant immunotoxins in the treatment of cancer. Methods Mol. Biol. 2004;248:503–518. doi: 10.1385/1-59259-666-5:503. [DOI] [PubMed] [Google Scholar]
- 149.Du X., Ho M., Pastan I. New immunotoxins targeting CD123, a stem cell antigen on acute myeloid leukemia cells. J. Immunother. 2007;30:607–613. doi: 10.1097/CJI.0b013e318053ed8e. [DOI] [PubMed] [Google Scholar]
- 150.Duan K., Surette M.G. Surette, Environmental regulation of Pseudomonas aeruginosa PAO1 Las and Rhl quorum-sensing systems. J. Bacteriol. 2007;189:4827–4836. doi: 10.1128/JB.00043-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tateda K., Ishii Y., Horikawa M., Matsumoto T., Miyairi S., Pechere J.C., Standiford T.J., Ishiguro M., Yamaguchi K. The Pseudomonas aeruginosa autoinducer N-3-oxododecanoyl homoserine lactone accelerates apoptosis in macrophages and neutrophils. Infect. Immun. 2003;71:5785–5793. doi: 10.1128/IAI.71.10.5785-5793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jacobi C.A., Schiffner F., Henkel M., Waibel M., Stork B., Daubrawa M., Eberl L., Gregor M., Wesselborg S. Effects of bacterial N-acyl homoserine lactones on human Jurkat T lymphocytes-OdDHL induces apoptosis via the mitochondrial pathway. Int. J. Med. Microbiol. 2009;299:509–519. doi: 10.1016/j.ijmm.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 153.Li L., Hooi D., Chhabra S.R., Pritchard D., Shaw P.E. Bacterial N-acylhomoserine lactone-induced apoptosis in breast carcinoma cells correlated with down-modulation of STAT3. Oncogene. 2004;23:4894–4902. doi: 10.1038/sj.onc.1207612. [DOI] [PubMed] [Google Scholar]
- 154.Bhardwaj A., Sethi G., Vadhan-Raj S., Bueso-Ramos C., Takada Y., Gaur U., Nair A.S., Shishodia S., Aggarwal B.B. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-κB–regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2006;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 155.Maurice N.M., Bedi B., Yuan Z., Goldberg J.B., Koval M., Hart C.M., Sadikot R.T. Pseudomonas aeruginosa induced host epithelial cell mitochondrial dysfunction. Sci. Rep. 2019;9:11929. doi: 10.1038/s41598-019-47457-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brown G.C., Murphy M.P., Jornayvaz F.R., Shulman G.I. Regulation of mitochondrial biogenesis. Essays Biochem. 2010;47:69–84. doi: 10.1042/bse0470069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schwarzer C., Fu Z., Patanwala M., Hum L., Lopez-Guzman M., Illek B., Kong W., Lynch S.V., Machen T.E. Pseudomonas aeruginosa biofilm-associated homoserine lactone C12 rapidly activates apoptosis in airway epithelia. Cell Microbiol. 2012;14:698–709. doi: 10.1111/j.1462-5822.2012.01753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang J., Gong F., Li L., Zhao M., Song J. Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl) homoserine lactone attenuates lipopolysaccharide-induced inflammation by activating the unfolded protein response. Biomed. Rep. 2014;2:233–238. doi: 10.3892/br.2014.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Smith R.S., Fedyk E.R., Springer T.A., Mukaida N., Iglewski B.H., Phipps R.P. IL-8 production in human lung fibroblasts and epithelial cells activated by the Pseudomonas autoinducer N-3-oxododecanoyl homoserine lactone is transcriptionally regulated by NF-κB and activator protein-2. J. Immunol. 2001;167:366–374. doi: 10.4049/jimmunol.167.1.366. [DOI] [PubMed] [Google Scholar]
- 160.Smith R.S., Harris S.G., Phipps R., Iglewski B. The Pseudomonas aeruginosa quorum-sensing molecule N-(3-oxododecanoyl) homoserine lactone contributes to virulence and induces inflammation in vivo. J. Bacteriol. 2002;184:1132–1139. doi: 10.1128/jb.184.4.1132-1139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vijgenboom E., Busch J.E., Canters G.W. In vivo studies disprove an obligatory role of azurin in denitrification in Pseudomonas aeruginosa and show that azu expression is under control of RpoS and ANR. Microbiology. 1997;143:2853–2863. doi: 10.1099/00221287-143-9-2853. [DOI] [PubMed] [Google Scholar]
- 162.Yamada T., Goto M., Punj V., Zaborina O., Kimbara K., Das Gupta T.K., Chakrabarty A.M. The bacterial redox protein azurin induces apoptosis in J774 macrophages through complex formation and stabilization of the tumor suppressor protein p53. Infect. Immun. 2002;70:7054–7062. doi: 10.1128/IAI.70.12.7054-7062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Punj V., Bhattacharyya S., Saint-Dic D., Vasu C., Cunningham E.A., Graves J., Yamada T., Constantinou A.I., Christov K., White B., et al. Bacterial cupredoxin azurin as an inducer of apoptosis and regression in human breast cancer. Oncogene. 2004;23:2367–2378. doi: 10.1038/sj.onc.1207376. [DOI] [PubMed] [Google Scholar]
- 164.Yang D.-S., Miao X.-D., Ye Z.-M., Feng J., Xu R.-Z., Huang X., Ge F.-F. Bacterial redox protein azurin induce apoptosis in human osteosarcoma U2OS cells. Pharmacol. Res. 2005;52:413–421. doi: 10.1016/j.phrs.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 165.Schuler M., Green D.R. Mechanisms of p53-dependent apoptosis. Biochem. Soc. Trans. 2001;29:684–688. doi: 10.1042/bst0290684. [DOI] [PubMed] [Google Scholar]
- 166.Gao M., Zhou J., Su Z., Huang Y. Bacterial cupredoxin azurin hijacks cellular signaling networks: Protein–protein interactions and cancer therapy. Protein Sci. 2017;26:2334–2341. doi: 10.1002/pro.3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hogardt M., Heesemann J. Adaptation of Pseudomonas aeruginosa during persistence in the cystic fibrosis lung. Int. J. Med. Microbiol. 2010;300:557–562. doi: 10.1016/j.ijmm.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 168.Yoon S.S., Hennigan R.F., Hilliard G.M., Ochsner U.A., Parvatiyar K., Kamani M.C., Allen H.L., DeKievit T.R., Gardner P.R., Schwab U., et al. Pseudomonas aeruginosa anaerobic respiration in biofilms: Relationships to cystic fibrosis pathogenesis. Dev. Cell. 2002;3:593–603. doi: 10.1016/S1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]
- 169.Turner J.M., Messenger A. Advances in Microbial Physiology. Elsevier; Amsterdam, The Netherlands: 1986. Occurrence, biochemistry and physiology of phenazine pigment production; pp. 211–275. [DOI] [PubMed] [Google Scholar]
- 170.Lau G.W., Hassett D.J., Ran H., Kong F. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004;10:599–606. doi: 10.1016/j.molmed.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 171.Meyer J.M., Neely A., Stintzi A., Georges C., A Holder I. Pyoverdin is essential for virulence of Pseudomonas aeruginosa. Infect. Immun. 1996;64:518–523. doi: 10.1128/iai.64.2.518-523.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hall S., McDermott C., Anoopkumar-Dukie S., McFarland A.J., Forbes A., Perkins A.V., Davey A.K., Chess-Williams R., Kiefel M.J., Arora D., et al. Cellular effects of pyocyanin, a secreted virulence factor of Pseudomonas aeruginosa. Toxins. 2016;8:236. doi: 10.3390/toxins8080236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ran H., Hassett D.J., Lau G.W. Human targets of Pseudomonas aeruginosa pyocyanin. Proc. Natl. Acad. Sci. USA. 2003;100:14315–14320. doi: 10.1073/pnas.2332354100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Baron S.S., Rowe J.J. Antibiotic action of pyocyanin. Antimicrob. Agents Chemother. 1981;20:814–820. doi: 10.1128/AAC.20.6.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Noto M.J., Burns W.J., Beavers W.N., Skaar E.P. Mechanisms of pyocyanin toxicity and genetic determinants of resistance in Staphylococcus aureus. J. Bacteriol. 2017;199:e00221-17. doi: 10.1128/JB.00221-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Usher L.R., Lawson R.A., Geary I., Taylor C.J., Bingle C.D., Taylor G.W., Whyte M.K.B. Induction of neutrophil apoptosis by the Pseudomonas aeruginosa exotoxin pyocyanin: A potential mechanism of persistent infection. J. Immunol. 2002;168:1861–1868. doi: 10.4049/jimmunol.168.4.1861. [DOI] [PubMed] [Google Scholar]
- 177.Leidal K.G., Munson K.L., Denning G.M. Small molecular weight secretory factors from Pseudomonas aeruginosa have opposite effects on IL-8 and RANTES expression by human airway epithelial cells. Am. J. Respir. Cell Mol. 2001;25:186–195. doi: 10.1165/ajrcmb.25.2.4273. [DOI] [PubMed] [Google Scholar]
- 178.Denning G.M., A Wollenweber L., A Railsback M., Cox C.D., Stoll L.L., Britigan E.B. Pseudomonas pyocyanin increases interleukin-8 expression by human airway epithelial cells. Infect. Immun. 1998;66:5777–5784. doi: 10.1128/IAI.66.12.5777-5784.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Manago A., Becker K.A., Carpinteiro A., Wilker B., Soddemann M., Seitz A.P., Edwards M.J., Grassmé H., Szabo I., Gulbins E. Pseudomonas aeruginosa pyocyanin induces neutrophil death via mitochondrial reactive oxygen species and mitochondrial acid sphingomyelinase. Antioxid. Redox Signal. 2015;22:1097–1110. doi: 10.1089/ars.2014.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dias S., Boyd R., Balkwill F. IL-12 regulates VEGF and MMPs in a murine breast cancer model. Int. J. Cancer. 1998;78:361–365. doi: 10.1002/(SICI)1097-0215(19981029)78:3<361::AID-IJC17>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 181.Chai W., Zhang J., Duan Y., Pan D., Liu W., Li Y., Yan X., Chen B. Pseudomonas pyocyanin stimulates IL-8 expression through MAPK and NF-κB pathways in differentiated U937 cells. BMC Microbiol. 2014;14:26. doi: 10.1186/1471-2180-14-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lau G.W., Ran H., Kong F., Hassett D.J., Mavrodi D. Pseudomonas aeruginosa pyocyanin is critical for lung infection in mice. Infect. Immun. 2004;72:4275–4278. doi: 10.1128/IAI.72.7.4275-4278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Caldwell C.C., Chen Y., Goetzmann H.S., Hao Y., Borchers M.T., Hassett D.J., Young L.R., Mavrodi D., Thomashow L., Lau G.W. Pseudomonas aeruginosa exotoxin pyocyanin causes cystic fibrosis airway pathogenesis. AJP. 2009;175:2473–2488. doi: 10.2353/ajpath.2009.090166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wilson R., Sykes D.A., Watson D., Rutman A., Taylor G.W., Cole P. Measurement of Pseudomonas aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect. Immun. 1988;56:2515–2517. doi: 10.1128/iai.56.9.2515-2517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pier G.B. Pseudomonas aeruginosa lipopolysaccharide: A major virulence factor, initiator of inflammation and target for effective immunity. Int. J. Med. Microbiol. 2007;297:277–295. doi: 10.1016/j.ijmm.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cryz S.J., Pitt T.L., Fürer E., Germanier R. Role of lipopolysaccharide in virulence of Pseudomonas aeruginosa. Infect. Immun. 1984;44:508–513. doi: 10.1128/iai.44.2.508-513.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kipnis E., Sawa T., Wiener-Kronish J. Targeting mechanisms of Pseudomonas aeruginosa pathogenesis. Med. Mal. Infect. 2006;36:78–91. doi: 10.1016/j.medmal.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 188.Ivanov I.E., Kintz E.N., Porter L.A., Goldberg J.B., Burnham N.A., Camesano T.A. Relating the physical properties of Pseudomonas aeruginosa lipopolysaccharides to virulence by atomic force microscopy. J. Bacteriol. 2011;193:1259–1266. doi: 10.1128/JB.01308-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Gupta S.K., Berk R.S., Masinick S., Hazlett L.D. Pili and lipopolysaccharide of Pseudomonas aeruginosa bind to the glycolipid asialo GM1. Infect. Immun. 1994;62:4572–4579. doi: 10.1128/iai.62.10.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Miao E.A., Ernst R.K., Dors M., Mao D.P., Aderem A. Pseudomonas aeruginosa activates caspase 1 through Ipaf. Proc. Natl. Acad. Sci. USA. 2008;105:2562–2567. doi: 10.1073/pnas.0712183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hagar J.A., Powell D.A., Aachoui Y., Ernst R.K., Miao E.A. Cytoplasmic LPS activates caspase-11: Implications in TLR4-independent endotoxic shock. Science. 2013;341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu X., Shao K., Sun T. SIRT1 regulates the human alveolar epithelial A549 cell apoptosis induced by Pseudomonas aeruginosa lipopolysaccharide. Cell. Physiol. Biochem. 2013;31:92–101. doi: 10.1159/000343352. [DOI] [PubMed] [Google Scholar]
- 193.Schoeniger A., Fuhrmann H., Schumann J. LPS- or Pseudomonas aeruginosa-mediated activation of the macrophage TLR4 signaling cascade depends on membrane lipid composition. PeerJ. 2016;4:e1663. doi: 10.7717/peerj.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nakamoto K., Watanabe M., Sada M., Inui T., Nakamura M., Honda K., Wada H., Ishii H., Takizawa H. Pseudomonas aeruginosa-derived flagellin stimulates IL-6 and IL-8 production in human bronchial epithelial cells: A potential mechanism for progression and exacerbation of COPD. Exp. Lung Res. 2019;45:255–266. doi: 10.1080/01902148.2019.1665147. [DOI] [PubMed] [Google Scholar]
- 195.Lin C.K., Kazmierczak B.I. Inflammation: A double-edged sword in the response to Pseudomonas aeruginosa infection. J. Innate Immun. 2017;9:250–261. doi: 10.1159/000455857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Brandl K., Glück T., Huber C., Salzberger B., Falk W., Hartmann P. TLR-4 surface display on human monocytes is increased in septic patients. Eur. J. Med. Res. 2005;10:319. [PubMed] [Google Scholar]
- 197.Huang X., Feng Y., Xiong G., Whyte S., Duan J., Yang Y., Wang K., Yang S., Geng Y., Ou Y., et al. Caspase-11, a specific sensor for intracellular lipopolysaccharide recognition, mediates the non-canonical inflammatory pathway of pyroptosis. Cell Biosci. 2019;9:31. doi: 10.1186/s13578-019-0292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pier G.B. Promises and pitfalls of Pseudomonas aeruginosa lipopolysaccharide as a vaccine antigen. Carbohydr. Res. 2003;338:2549–2556. doi: 10.1016/S0008-6215(03)00312-4. [DOI] [PubMed] [Google Scholar]
- 199.Lillehoj E.P., Kim B.T., Kim K.C. Identification of Pseudomonas aeruginosa flagellin as an adhesin for Muc1 mucin. Am. J. Physiol. 2002;282:L751–L756. doi: 10.1152/ajplung.00383.2001. [DOI] [PubMed] [Google Scholar]
- 200.Lindhout T., Lau P.C.Y., Brewer D., Lam J.S. Truncation in the core oligosaccharide of lipopolysaccharide affects flagella-mediated motility in Pseudomonas aeruginosa PAO1 via modulation of cell surface attachment. Microbiology. 2009;155:3449–3460. doi: 10.1099/mic.0.030510-0. [DOI] [PubMed] [Google Scholar]
- 201.Dasgupta N., Wolfgang M.C., Goodman A.L., Arora S.K., Jyot J., Lory S., Ramphal R. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 2003;50:809–824. doi: 10.1046/j.1365-2958.2003.03740.x. [DOI] [PubMed] [Google Scholar]
- 202.Rasamiravaka T., Labtani Q., Duez P., El Jaziri M. The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. Biomed Res. Int. 2015;2015:759348. doi: 10.1155/2015/759348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Baker A.E., Webster S.S., Diepold A., Kuchma S.L., Bordeleau E., Armitage J.P., O’Toole G.A. Flagellar stators stimulate c-di-GMP production by Pseudomonas aeruginosa. J. Bacteriol. 2019;201:JB. 00741-18. doi: 10.1128/JB.00741-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Feldman M., Bryan R., Rajan S., Scheffler L., Brunnert S., Tang H., Prince A. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 1998;66:43–51. doi: 10.1128/IAI.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Drake D., Montie T.C. Flagella, motility and invasive virulence of Pseudomonas aeruginosa. Pt 1J. Gen. Microbiol. 1988;134:43–52. doi: 10.1099/00221287-134-1-43. [DOI] [PubMed] [Google Scholar]
- 206.Arora S.K., Neely A.N., Blair B., Lory S., Ramphal R. Role of motility and flagellin glycosylation in the pathogenesis of Pseudomonas aeruginosa burn wound infections. Infect. Immun. 2005;73:4395–4398. doi: 10.1128/IAI.73.7.4395-4398.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Feuillet V., Medjane S., Mondor I., Demaria O., Pagni P.P., Galán J.E., Flavell R.A., Alexopoulou L. Involvement of Toll-like receptor 5 in the recognition of flagellated bacteria. Proc. Natl. Acad. Sci. USA. 2006;103:12487–12492. doi: 10.1073/pnas.0605200103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Balloy V., Verma A., Kuravi S., Si-Tahar M., Chignard M., Ramphal R. The role of flagellin versus motility in acute lung disease caused by Pseudomonas aeruginosa. J. Infect. Dis. 2007;196:289–296. doi: 10.1086/518610. [DOI] [PubMed] [Google Scholar]
- 209.Lightfield K.L., Persson J.J., Brubaker S.W., E Witte C., Von Moltke J., A Dunipace E., Henry T., Sun Y.-H., Cado D., Dietrich W.F., et al. Critical function for Naip5 in inflammasome activation by a conserved carboxy-terminal domain of flagellin. Nat. Immunol. 2008;9:1171–1178. doi: 10.1038/ni.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kofoed E.M., Vance R.E. Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature. 2011;477:592–595. doi: 10.1038/nature10394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Zhao Y., Shao F. The NAIP–NLRC 4 inflammasome in innate immune detection of bacterial flagellin and type III secretion apparatus. Immunol. Rev. 2015;265:85–102. doi: 10.1111/imr.12293. [DOI] [PubMed] [Google Scholar]
- 212.Faure E., Kwong K., Nguyen D. Pseudomonas aeruginosa in chronic lung infections: How to adapt within the host? Front. Immunol. 2018;9:2416. doi: 10.3389/fimmu.2018.02416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Evans T.J. Small colony variants of Pseudomonas aeruginosa in chronic bacterial infection of the lung in cystic fibrosis. Future Microbiol. 2015;10:231–239. doi: 10.2217/fmb.14.107. [DOI] [PubMed] [Google Scholar]
- 214.Cohen T., Parker D., Prince A. Pseudomonas. Springer; Berlin/Heidelberg, Germany: 2015. Pseudomonas aeruginosa host immune evasion; pp. 3–23. [Google Scholar]
- 215.Pier G.B., Meluleni G., Neuger E. A murine model of chronic mucosal colonization by Pseudomonas aeruginosa. Infect. Immun. 1992;60:4768–4776. doi: 10.1128/iai.60.11.4768-4776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mahenthiralingam E., E Campbell M., Speert D.P. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect. Immun. 1994;62:596–605. doi: 10.1128/iai.62.2.596-605.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Beceiro A., Tomás M., Bou G. Antimicrobial resistance and virulence: A successful or deleterious association in the bacterial world? CMR. 2013;26:185–230. doi: 10.1128/CMR.00059-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Chevalier S., Bouffartigues E., Bodilis J., Maillot O., Lesouhaitier O., Feuilloley M.G.J., Orange N., Dufour A., Cornelis P. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol. Rev. 2017;41:698–722. doi: 10.1093/femsre/fux020. [DOI] [PubMed] [Google Scholar]
- 219.Fito-Boncompte L., Chapalain A., Bouffartigues E., Chaker H., Lesouhaitier O., Gicquel G., Bazire A., Madi A., Connil N., Véron W., et al. Full virulence of Pseudomonas aeruginosa requires OprF. Infect. Immun. 2011;79:1176–1186. doi: 10.1128/IAI.00850-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Buommino E., Morelli F., Metafora S., Rossano F., Perfetto B., Baroni A., Tufano M.A. Porin from Pseudomonas aeruginosa induces apoptosis in an epithelial cell line derived from rat seminal vesicles. Infect. Immun. 1999;67:4794–4800. doi: 10.1128/IAI.67.9.4794-4800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cusumano V., A Tufano M., Mancuso G., Carbone M., Rossano F., Fera M.T., A Ciliberti F., Ruocco E., A Merendino R., Teti G. Porins of Pseudomonas aeruginosa induce release of tumor necrosis factor alpha and interleukin-6 by human leukocytes. Infect. Immun. 1997;65:1683–1687. doi: 10.1128/iai.65.5.1683-1687.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mishra M., Ressler A., Schlesinger L.S., Wozniak D.J. Identification of OprF as a complement component C3 binding acceptor molecule on the surface of Pseudomonas aeruginosa. Infect. Immun. 2015;83:3006–3014. doi: 10.1128/IAI.00081-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Peluso L., De Luca C., Bozza S., Leonardi A., Giovannini G., Lavorgna A., De Rosa G., Mascolo M., De Luna L.O., Catania M.R., et al. Protection against Pseudomonas aeruginosa lung infection in mice by recombinant OprF-pulsed dendritic cell immunization. BMC Microbiol. 2010;10:9. doi: 10.1186/1471-2180-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ochsner U.A., Fiechter A., Reiser J. Isolation, characterization, and expression in Escherichia coli of the Pseudomonas aeruginosa rhlAB genes encoding a rhamnosyltransferase involved in rhamnolipid biosurfactant synthesis. JBC. 1994;269:19787–19795. doi: 10.1016/S0021-9258(17)32089-6. [DOI] [PubMed] [Google Scholar]
- 225.Cabrera-Valladares N., Richardson A.-P., Olvera C., Treviño L.G., Déziel E., Lépine F., Soberón-Chávez G. Monorhamnolipids and 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs) production using Escherichia coli as a heterologous host. Appl. Microbiol. Biotechnol. 2006;73:187–194. doi: 10.1007/s00253-006-0468-5. [DOI] [PubMed] [Google Scholar]
- 226.Maier R., Soberón-Chávez G. Pseudomonas aeruginosa rhamnolipids: Biosynthesis and potential applications. Appl. Microbiol. Biotechnol. 2000;54:625–633. doi: 10.1007/s002530000443. [DOI] [PubMed] [Google Scholar]
- 227.Read R., Roberts P., Munro N., Rutman A., Hastie A., Shryock T., Hall R., McDonald-Gibson W., Lund V., Taylor G., et al. Effect of Pseudomonas aeruginosa rhamnolipids on mucociliary transport and ciliary beating. J. Appl. Physiol. 1992;72:2271–2277. doi: 10.1152/jappl.1992.72.6.2271. [DOI] [PubMed] [Google Scholar]
- 228.Kownatzki R., Tümmler B., Döring G. Rhamnolipid of Pseudomonas aeruginosa in sputum of cystic fibrosis patients. Lancet. 1987;1:1026–1027. doi: 10.1016/S0140-6736(87)92286-0. [DOI] [PubMed] [Google Scholar]
- 229.Sharma D., Kanneganti T.-D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. J. Cell Biol. 2016;213:617–629. doi: 10.1083/jcb.201602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Varjani S.J., Upasani V.N. Core flood study for enhanced oil recovery through ex-situ bioaugmentation with thermo-and halo-tolerant rhamnolipid produced by Pseudomonas aeruginosa NCIM 5514. Bioresour. Technol. 2016;220:175–182. doi: 10.1016/j.biortech.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 231.Zulianello L., Canard C., Köhler T., Caille D., Lacroix J.-S., Meda P. Rhamnolipids are virulence factors that promote early infiltration of primary human airway epithelia by Pseudomonas aeruginosa. Infect. Immun. 2006;74:3134–3147. doi: 10.1128/IAI.01772-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang S., Yu S., Zhang Z., Wei Q., Yan L., Ai G., Liu H., Ma L.Z. Coordination of swarming motility, biosurfactant synthesis, and biofilm matrix exopolysaccharide production in Pseudomonas aeruginosa. Appl. Environ. Microbiol. 2014;80:6724–6732. doi: 10.1128/AEM.01237-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Overhage J., Bains M., Brazas M.D., Hancock R.E.W. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J. Bacteriol. 2008;190:2671–2679. doi: 10.1128/JB.01659-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pamp S.J., Tolker-Nielsen T. Multiple roles of biosurfactants in structural biofilm development by Pseudomonas aeruginosa. J. Bacteriol. 2007;189:2531–2539. doi: 10.1128/JB.01515-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Davey M.E., Caiazza N.C., O’Toole G.A. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2003;185:1027–1036. doi: 10.1128/JB.185.3.1027-1036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Sotirova A., Spasova D., Vasileva-Tonkova E., Galabova D. Effects of rhamnolipid-biosurfactant on cell surface of Pseudomonas aeruginosa. Microbiol. Res. 2009;164:297–303. doi: 10.1016/j.micres.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 237.Okamoto K., Gotoh N., Nishino T. Pseudomonas aeruginosa reveals high intrinsic resistance to penem antibiotics: Penem resistance mechanisms and their interplay. Antimicrob. Agents Chemother. 2001;45:1964–1971. doi: 10.1128/AAC.45.7.1964-1971.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Rahimi K., Lotfabad T.B., Jabeen F., Ganji S.M. Cytotoxic effects of mono-and di-rhamnolipids from Pseudomonas aeruginosa MR01 on MCF-7 human breast cancer cells. Colloids Surf. B. 2019;181:943–952. doi: 10.1016/j.colsurfb.2019.06.058. [DOI] [PubMed] [Google Scholar]
- 239.Dwivedi S., Saquib Q., Al-Khedhairy A.A., Ahmad J., Siddiqui M.A., Musarrat J. Rhamnolipids functionalized AgNPs-induced oxidative stress and modulation of toxicity pathway genes in cultured MCF-7 cells. Colloids Surf. B. 2015;132:290–298. doi: 10.1016/j.colsurfb.2015.05.034. [DOI] [PubMed] [Google Scholar]
- 240.Jensen P., Bjarnsholt T., Phipps R., Rasmussen T.B., Calum H., Christoffersen L., Moser C., Williams P., Pressler T., Givskov M., et al. Rapid necrotic killing of polymorphonuclear leukocytes is caused by quorum-sensing-controlled production of rhamnolipid by Pseudomonas aeruginosa. Microbiology. 2007;153:1329–1338. doi: 10.1099/mic.0.2006/003863-0. [DOI] [PubMed] [Google Scholar]
- 241.Van Gennip M., Christensen L.D., Alhede M., Phipps R., Jensen P., Christophersen L., Pamp S.J., Moser C., Mikkelsen P.J., Koh A.Y., et al. Inactivation of the rhlA gene in Pseudomonas aeruginosa prevents rhamnolipid production, disabling the protection against polymorphonuclear leukocytes. APMIS. 2009;117:537–546. doi: 10.1111/j.1600-0463.2009.02466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Meyer-Hoffert U., Zimmermann A., Czapp M., Bartels J., Koblyakova Y., Gläser R., Schröder J.-M., Gerstel U. Flagellin delivery by Pseudomonas aeruginosa rhamnolipids induces the antimicrobial protein psoriasin in human skin. PLoS ONE. 2011;6:e16433. doi: 10.1371/journal.pone.0016433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Hauser A.R. The type III secretion system of Pseudomonas aeruginosa: Infection by injection. Nat. Rev. Microbiol. 2009;7:654–665. doi: 10.1038/nrmicro2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Hoiczyk E., Blobel G. Polymerization of a single protein of the pathogen Yersinia enterocolitica into needles punctures eukaryotic cells. Proc. Natl. Acad. Sci. USA. 2001;98:4669–4674. doi: 10.1073/pnas.071065798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kubori T., Matsushima Y., Nakamura D., Uralil J., Lara-Tejero M., Sukhan A., Galán J.E., Aizawa S.-I. Supramolecular structure of the Salmonella typhimurium type III protein secretion system. Science. 1998;280:602–605. doi: 10.1126/science.280.5363.602. [DOI] [PubMed] [Google Scholar]
- 246.Engel J., Balachandran P. Role of Pseudomonas aeruginosa type III effectors in disease. Curr. Opin. Microbiol. 2009;12:61–66. doi: 10.1016/j.mib.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 247.Coburn B., Sekirov I., Finlay B.B. Type III secretion systems and disease. CMR. 2007;20:535–549. doi: 10.1128/CMR.00013-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Burstein D., Satanower S., Simovitch M., Belnik Y., Zehavi M., Yerushalmi G., Ben-Aroya S., Pupko T., Banin E. Novel type III effectors in Pseudomonas aeruginosa. MBio. 2015;6:e00161-15. doi: 10.1128/mBio.00161-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Feldman M.F., Müller S., Wüest E., Cornelis G.R. SycE allows secretion of YopE-DHFR hybrids by the Yersinia enterocolitica type III Ysc system. Mol. Microbiol. 2002;46:1183–1197. doi: 10.1046/j.1365-2958.2002.03241.x. [DOI] [PubMed] [Google Scholar]
- 250.Parsot C., Hamiaux C., Page A.L. The various and varying roles of specific chaperones in type III secretion systems. Curr. Opin. Microbiol. 2003;6:7–14. doi: 10.1016/S1369-5274(02)00002-4. [DOI] [PubMed] [Google Scholar]
- 251.Cornelis G.R. The type III secretion injectisome. Nat. Rev. Microbiol. 2006;4:811–825. doi: 10.1038/nrmicro1526. [DOI] [PubMed] [Google Scholar]
- 252.Letzelter M., Sorg I., Mota L.J., Meyer S., Stalder J., Feldman M., Kuhn M., Callebaut I., Cornelis G.R. The discovery of SycO highlights a new function for type III secretion effector chaperones. EMBO J. 2006;25:3223–3233. doi: 10.1038/sj.emboj.7601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Ottmann C., Yasmin L., Weyand M., Veesenmeyer J.L., Diaz M.H., Palmer R.H., Francis M., Hauser A.R., Wittinghofer A., Hallberg B. Phosphorylation-independent interaction between 14-3-3 and exoenzyme S: From structure to pathogenesis. EMBO J. 2007;26:902–913. doi: 10.1038/sj.emboj.7601530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Liu S., Yahr T., Frank D.W., Barbieri J.T. Biochemical relationships between the 53-kilodalton (Exo53) and 49-kilodalton (ExoS) forms of exoenzyme S of Pseudomonas aeruginosa. J. Bacteriol. 1997;179:1609–1613. doi: 10.1128/jb.179.5.1609-1613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Phillips R.M., Six D., Dennis E.A., Ghosh P., Matsumoto K., Shionyu M., Go M., Shimizu K., Shinomura T., Kimata K., et al. In vivo phospholipase activity of the Pseudomonas aeruginosa cytotoxin ExoU and protection of mammalian cells with phospholipase A2 inhibitors. JBC. 2003;278:41326–41332. doi: 10.1074/jbc.M302472200. [DOI] [PubMed] [Google Scholar]
- 256.Stockbauer K.E., Foreman-Wykert A.K., Miller J.F. Bordetella type III secretion induces caspase 1-independent necrosis. Cell Microbiol. 2003;5:123–132. doi: 10.1046/j.1462-5822.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- 257.Viboud G.I., Bliska J.B. A bacterial type III secretion system inhibits actin polymerization to prevent pore formation in host cell membranes. EMBO J. 2001;20:5373–5382. doi: 10.1093/emboj/20.19.5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Lee V.T., Smith R.S., Tümmler B., Lory S. Activities of Pseudomonas aeruginosa effectors secreted by the Type III secretion system in vitro and during infection. Infect. Immun. 2005;73:1695–1705. doi: 10.1128/IAI.73.3.1695-1705.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Shafikhani S.H., Morales C., Engel J. The Pseudomonas aeruginosa type III secreted toxin ExoT is necessary and sufficient to induce apoptosis in epithelial cells. Cell Microbiol. 2008;10:994–1007. doi: 10.1111/j.1462-5822.2007.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Andor A., Trulzsch K., Essler M., Roggenkamp A., Wiedemann A., Heesemann J., Aepfelbacher M. YopE of Yersinia, a GAP for Rho GTPases, selectively modulates Rac-dependent actin structures in endothelial cells. Cell Microbiol. 2001;3:301–310. doi: 10.1046/j.1462-5822.2001.00114.x. [DOI] [PubMed] [Google Scholar]
- 261.Von Pawel-Rammingen U., Telepnev M.V., Schmidt G., Aktories K., Wolf-Watz H., Rosqvist R. GAP activity of the Yersinia YopE cytotoxin specifically targets the Rho pathway: A mechanism for disruption of actin microfilament structure. Mol. Microbiol. 2000;36:737–748. doi: 10.1046/j.1365-2958.2000.01898.x. [DOI] [PubMed] [Google Scholar]
- 262.Eren E., Planès R., Buyck J., Bordignon P.J., Colom A., Cunrath O., Dreier R.F., Santos J.C., Duplan-Eche V., Näser E., et al. Type-3 Secretion System–induced pyroptosis protects Pseudomonas against cell-autonomous immunity. bioRxiv. 2019 doi: 10.1101/650333. [DOI] [Google Scholar]
- 263.Balakrishnan A., Karki R., Berwin B., Yamamoto M., Kanneganti T.-D. Guanylate binding proteins facilitate caspase-11-dependent pyroptosis in response to type 3 secretion system-negative Pseudomonas aeruginosa. Cell Death Discov. 2018;4:66. doi: 10.1038/s41420-018-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Franchi L., Stoolman J., Kanneganti T.-D., Verma A., Ramphal R., Núñez G. Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur. J. Immunol. 2007;37:3030–3039. doi: 10.1002/eji.200737532. [DOI] [PubMed] [Google Scholar]
- 265.Rimessi A., Bezzerri V., Patergnani S., Marchi S., Cabrini G., Pinton P. Mitochondrial Ca2+-dependent NLRP3 activation exacerbates the Pseudomonas aeruginosa-driven inflammatory response in cystic fibrosis. Nat. Commun. 2015;6:1–16. doi: 10.1038/ncomms7201. [DOI] [PubMed] [Google Scholar]
- 266.Deng Q., Wang Y., Zhang Y., Li M., Li D., Huang X., Wu Y., Pu J., Wu M. Pseudomonas aeruginosa triggers macrophage autophagy to escape intracellular killing by activation of the NLRP3 inflammasome. Infect. Immun. 2016;84:56–66. doi: 10.1128/IAI.00945-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Gugliandolo E., Fusco R., Ginestra G., D’amico R., Bisignano C., Mandalari G., Cuzzocrea S., Di Paola R. Involvement of TLR4 and PPAR-α receptors in host response and NLRP3 inflammasome activation, against pulmonary infection with Pseudomonas aeruginosa. Shock. 2019;51:221–227. doi: 10.1097/SHK.0000000000001137. [DOI] [PubMed] [Google Scholar]
- 268.Franchi L., Kamada N., Nakamura Y., Burberry A., Kuffa P., Suzuki S., Shaw M.H., Kim Y.G., Núñez G. NLRC4-driven production of IL-1beta discriminates between pathogenic and commensal bacteria and promotes host intestinal defense. Nat. Immunol. 2012;13:449–456. doi: 10.1038/ni.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Iannitti R.G., Napolioni V., Oikonomou V., De Luca A., Galosi C., Pariano M., Massi-Benedetti C., Borghi M., Puccetti M., Lucidi V., et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat. Commun. 2016;7:10791. doi: 10.1038/ncomms10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Faure E., Mear J.-B., Faure K., Normand S., Couturier-Maillard A., Grandjean T., Balloy V., Ryffel B., Dessein R., Chignard M., et al. Pseudomonas aeruginosa type-3 secretion system dampens host defense by exploiting the NLRC4-coupled inflammasome. Am. J. Respir. Crit. Care Med. 2014;189:799–811. doi: 10.1164/rccm.201307-1358OC. [DOI] [PubMed] [Google Scholar]
- 271.Barbieri J.T. Pseudomonas aeruginosa exoenzyme S, a bifunctional type-III secreted cytotoxin. Int. J. Med. Microbiol. 2000;290:381–387. doi: 10.1016/S1438-4221(00)80047-8. [DOI] [PubMed] [Google Scholar]
- 272.Da-Kang S.H., Quenee L., Bonnet M., Lauriane K.U., Derouazi M., Lamotte D., Toussaint B., Polack B. Orf1/SpcS chaperones ExoS for type three secretion by Pseudomonas aeruginosa. Biomed. Environ. Sci. 2008;21:103–109. doi: 10.1016/S0895-3988(08)60014-8. [DOI] [PubMed] [Google Scholar]
- 273.Pederson K.J., Krall R., Riese M.J., Barbieri J.T. Intracellular localization modulates targeting of ExoS, a type III cytotoxin, to eukaryotic signalling proteins. Mol. Microbiol. 2002;46:1381–1390. doi: 10.1046/j.1365-2958.2002.03256.x. [DOI] [PubMed] [Google Scholar]
- 274.Riese M.J., Barbieri J.T. Membrane localization contributes to the in vivo ADP-ribosylation of Ras by Pseudomonas aeruginosa ExoS. Infect. Immun. 2002;70:2230–2232. doi: 10.1128/IAI.70.4.2230-2232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Henriksson M.L., Sundin C., Jansson A.L., Forsberg Å., Palmer R.H., Hallberg B. Exoenzyme S shows selective ADP-ribosylation and GTPase-activating protein (GAP) activities towards small GTPases in vivo. Pt 3Biochem. J. 2002;367:617–628. doi: 10.1042/bj20020714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Katzmann D.J., Odorizzi G., Emr S.D. Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 2002;3:893–905. doi: 10.1038/nrm973. [DOI] [PubMed] [Google Scholar]
- 277.Wittinghofer A., Würtele M., Wolf E., Pederson K.J., Buchwald G., Ahmadian M.R., Barbieri J.T. How the Pseudomonas aeruginosa ExoS toxin downregulates Rac. Nat. Struct. Biol. 2001;8:23–26. doi: 10.1038/83007. [DOI] [PubMed] [Google Scholar]
- 278.Krall R., Sun J., Pederson K.J., Barbieri J.T. In vivo Rho GTPase-activating protein activity of Pseudomonas aeruginosa cytotoxin ExoS. Infect. Immun. 2002;70:360–367. doi: 10.1128/IAI.70.1.360-367.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Krall R., Schmidt G., Aktories K., Barbieri J.T. Pseudomonas aeruginosa ExoT is a Rho GTPase-activating protein. Infect. Immun. 2000;68:6066–6068. doi: 10.1128/IAI.68.10.6066-6068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Kazmierczak B.I., Engel J.N. Pseudomonas aeruginosa ExoT acts in vivo as a GTPase-activating protein for RhoA, Rac1, and Cdc42. Infect. Immun. 2002;70:2198–2205. doi: 10.1128/IAI.70.4.2198-2205.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Maresso A.W., Deng Q., Pereckas M.S., Wakim B.T., Barbieri J.T. Pseudomonas aeruginosa ExoS ADP-ribosyltransferase inhibits ERM phosphorylation. Cell Microbiol. 2007;9:97–105. doi: 10.1111/j.1462-5822.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 282.Barbieri J.T., Sun J. Pseudomonas aeruginosa ExoS and ExoT. Rev. Physiol. Biochem. Pharmacol. 2004;152:79–92. doi: 10.1007/s10254-004-0031-7. [DOI] [PubMed] [Google Scholar]
- 283.Jia J., Wang Y., Zhou L., Jin S. Expression of Pseudomonas aeruginosa Toxin ExoS Effectively Induces Apoptosis in Host Cells. Infect. Immun. 2006;74:6557–6570. doi: 10.1128/IAI.00591-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Goehring U.-M., Schmidt G., Pederson K.J., Aktories K., Barbieri J.T. The N-terminal domain of Pseudomonas aeruginosa exoenzyme S is a GTPase-activating protein for Rho GTPases. JBC. 1999;274:36369–36372. doi: 10.1074/jbc.274.51.36369. [DOI] [PubMed] [Google Scholar]
- 285.Kaminski A., Gupta K.H., Goldufsky J.W., Lee H.W., Gupta V., Shafikhani S.H. Pseudomonas aeruginosa ExoS Induces Intrinsic Apoptosis in Target Host Cells in a Manner That is Dependent on its GAP Domain Activity. Sci. Rep. 2018;8:14047. doi: 10.1038/s41598-018-32491-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Fuchs Y., Steller H. Live to die another way: Modes of programmed cell death and the signals emanating from dying cells. Nat. Rev. Mol. Cell Biol. 2015;16:329–344. doi: 10.1038/nrm3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Jia J., Alaoui-El-Azher M., Chow M., Chambers T.C., Baker H., Jin S. c-Jun NH2-terminal kinase-mediated signaling is essential for Pseudomonas aeruginosa ExoS-induced apoptosis. Infect. Immun. 2003;71:3361–3370. doi: 10.1128/IAI.71.6.3361-3370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Kaufman M.R., Jia J., Zeng L., Ha U., Chow M., Jin S. Pseudomonas aeruginosa mediated apoptosis requires the ADP-ribosylating activity of ExoS. Microbiology. 2000;146:2531–2541. doi: 10.1099/00221287-146-10-2531. [DOI] [PubMed] [Google Scholar]
- 289.Peter M.E. Programmed cell death: Apoptosis meets necrosis. Nature. 2011;471:310–312. doi: 10.1038/471310a. [DOI] [PubMed] [Google Scholar]
- 290.Alaoui-El-Azher M., Jia J., Lian W., Jin S. ExoS of Pseudomonas aeruginosa induces apoptosis through a Fas receptor/caspase 8-independent pathway in HeLa cells. Cell. Microbiol. 2006;8:326–338. doi: 10.1111/j.1462-5822.2005.00624.x. [DOI] [PubMed] [Google Scholar]
- 291.Epelman S., Bruno T.F., Neely G.G., Woods D.E., Mody C.H. Pseudomonas aeruginosa exoenzyme S induces transcriptional expression of proinflammatory cytokines and chemokines. Infect. Immun. 2000;68:4811–4814. doi: 10.1128/IAI.68.8.4811-4814.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Epelman S., Neely G.G., Ma L.L., Gjomarkaj M., Pace E., Melis M., Woods D.E., Mody C.H. Distinct fates of monocytes and T cells directly activated by Pseudomonas aeruginosa exoenzyme S. J. Leukoc. Biol. 2002;71:458–468. doi: 10.1189/jlb.71.3.458. [DOI] [PubMed] [Google Scholar]
- 293.Epelman S., Stack D., Bell C., Wong E., Neely G.G., Krutzik S., Miyake K., Kubes P., Zbytnuik L.D., Ma L.L., et al. Different domains of Pseudomonas aeruginosa Exoenzyme S activate distinct TLRs. J. Immunol. 2004;173:2031–2040. doi: 10.4049/jimmunol.173.3.2031. [DOI] [PubMed] [Google Scholar]
- 294.Galle M., Schotte P., Haegman M., Wullaert A., Yang H.J., Jin S., Beyaert R. The Pseudomonas aeruginosa Type III secretion system plays a dual role in the regulation of caspase-1 mediated IL-1beta maturation. J. Cell. Mol. Med. 2008;12:1767–1776. doi: 10.1111/j.1582-4934.2007.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Feltman H., Schulert G., Khan S., Jain M., Peterson L., Hauser A.R. Prevalence of type III secretion genes in clinical and environmental isolates of Pseudomonas aeruginosa. Microbiology. 2001;147:2659–2669. doi: 10.1099/00221287-147-10-2659. [DOI] [PubMed] [Google Scholar]
- 296.Balachandran P., Dragone L., Garrity-Ryan L., Lemus A., Weiss A., Engel J. The ubiquitin ligase Cbl-b limits Pseudomonas aeruginosa exotoxin T-mediated virulence. J. Clin. Investig. 2007;117:419–427. doi: 10.1172/JCI28792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Yahr T., Mende-Mueller L.M., Friese M., Frank D.W. Identification of type III secreted products of the Pseudomonas aeruginosa exoenzyme S regulon. J. Bacteriol. 1997;179:7165–7168. doi: 10.1128/jb.179.22.7165-7168.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sun J., Barbieri J.T. Pseudomonas aeruginosa ExoT ADP-ribosylates CT10 regulator of kinase (Crk) proteins. JBC. 2003;278:32794–32800. doi: 10.1074/jbc.M304290200. [DOI] [PubMed] [Google Scholar]
- 299.Sun J., Maresso A., Kim J.J., Barbieri J.T. How bacterial ADP-ribosylating toxins recognize substrates. Nat Struct Mol Biol. 2004;11:868–876. doi: 10.1038/nsmb818. [DOI] [PubMed] [Google Scholar]
- 300.Deng Q., Sun J., Barbieri J.T. Uncoupling Crk signal transduction by Pseudomonas exoenzyme T. JBC. 2005;280:35953–35960. doi: 10.1074/jbc.M504901200. [DOI] [PubMed] [Google Scholar]
- 301.Garrity-Ryan L., Kazmierczak B., Kowal R., Comolli J., Hauser A., Engel J.N. The arginine finger domain of ExoT is required for actin cytoskeleton disruption and inhibition of internalization of Pseudomonas aeruginosa by epithelial cells and macrophages. Infect. Immun. 2000;68:7100–7113. doi: 10.1128/IAI.68.12.7100-7113.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Cowell B.A., Chen D.Y., Frank D.W., Vallis A.J., Fleiszig S.M.J. ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect. Immun. 2000;68:403–406. doi: 10.1128/IAI.68.1.403-406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shafikhani S.H., Engel J. Pseudomonas aeruginosa type III-secreted toxin ExoT inhibits host-cell division by targeting cytokinesis at multiple steps. Proc. Natl. Acad. Sci. USA. 2006;103:15605–15610. doi: 10.1073/pnas.0605949103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Mohamed M.F., Wood S.J., Roy R., Reiser J., Kuzel T.M., Shafikhani S.H. Pseudomonas aeruginosa ExoT induces G1 cell cycle arrest in melanoma cells. Cell Microbiol. 2021;23:e13339. doi: 10.1111/cmi.13339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Goldufsky J., Wood S., Hajihossainlou B., Rehman T., Majdobeh O., Kaufman H.L., Ruby C.E., Shafikhani S.H. Pseudomonas aeruginosa exotoxin T induces potent cytotoxicity against a variety of murine and human cancer cell lines. Pt 2J. Med. Microbiol. 2015;64:164–173. doi: 10.1099/jmm.0.000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wood S., Goldufsky J., Shafikhani S.H. Pseudomonas aeruginosa ExoT Induces Atypical Anoikis Apoptosis in Target Host Cells by Transforming Crk Adaptor Protein into a Cytotoxin. PLoS Pathog. 2015;11:e1004934. doi: 10.1371/journal.ppat.1004934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Wood S.J., Goldufsky J.W., Bello D., Masood S., Shafikhani S.H. Pseudomonas aeruginosa ExoT induces mitochondrial apoptosis in target host cells in a manner that depends on its GTPase-activating protein (GAP) domain activity. JBC. 2015;290:29063–29073. doi: 10.1074/jbc.M115.689950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Sato H., Frank D.W. ExoU is a potent intracellular phospholipase. Mol. Microbiol. 2004;53:1279–1290. doi: 10.1111/j.1365-2958.2004.04194.x. [DOI] [PubMed] [Google Scholar]
- 309.Finck-Barbançon V., Goranson J., Zhu L., Sawa T., Wiener-Kronish J.P., Fleiszig S.M.J., Wu C., Mende-Mueller L., Frank D.W. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol. Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 310.Tamura M., Ajayi T., Allmond L.R., Moriyama K., Wiener-Kronish J.P., Sawa T. Lysophospholipase A activity of Pseudomonas aeruginosa type III secretory toxin ExoU. Biochem. Biophys Res. Commun. 2004;316:323–331. doi: 10.1016/j.bbrc.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 311.Finck-Barbançon V., Yahr T.L., Frank D.W. Identification and characterization of SpcU, a chaperone required for efficient secretion of the ExoU cytotoxin. J. Bacteriol. 1998;180:6224–6231. doi: 10.1128/JB.180.23.6224-6231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Deruelle V., Bouillot S., Job V., Taillebourg E., Fauvarque M.-O., Attrée I., Huber P. The bacterial toxin ExoU requires a host trafficking chaperone for transportation and to induce necrosis. Nat. Commun. 2021;12:1–14. doi: 10.1038/s41467-021-24337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Anderson D.M., Schmalzer K.M., Sato H., Casey M., Terhune S.S., Haas A.L., Feix J.B., Frank D.W. Ubiquitin and ubiquitin-modified proteins activate the Pseudomonas aeruginosa T3SS cytotoxin, ExoU. Mol. Microbiol. 2011;82:1454–1467. doi: 10.1111/j.1365-2958.2011.07904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Anderson D.M., Feix J.B., Monroe A.L., Peterson F.C., Volkman B.F., Haas A.L., Frank D.W. Identification of the major ubiquitin-binding domain of the Pseudomonas aeruginosa ExoU A2 phospholipase. JBC. 2013;288:26741–26752. doi: 10.1074/jbc.M113.478529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Rabin S.D.P., Veesenmeyer J.L., Bieging K.T., Hauser A.R. A C-terminal domain targets the Pseudomonas aeruginosa cytotoxin ExoU to the plasma membrane of host cells. Infect. Immun. 2006;74:2552–2561. doi: 10.1128/IAI.74.5.2552-2561.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Gendrin C., Contreras-Martel C., Bouillot S., Elsen S., Lemaire D., Skoufias D., Huber P., Attree I., Dessen A. Structural basis of cytotoxicity mediated by the type III secretion toxin ExoU from Pseudomonas aeruginosa. PLoS Pathog. 2012;8:e1002637. doi: 10.1371/journal.ppat.1002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Lindsey A.S., Sullivan L.M., Housley N.A., Koloteva A., King J.A., Audia J.P., Alvarez D.F. Analysis of pulmonary vascular injury and repair during Pseudomonas aeruginosa infection-induced pneumonia and acute respiratory distress syndrome. Pulm. Circ. 2019;9:2045894019826941. doi: 10.1177/2045894019826941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Diaz M.H., Shaver C.M., King J.D., Musunuri S., Kazzaz J.A., Hauser A.R. Pseudomonas aeruginosa induces localized immunosuppression during pneumonia. Infect. Immun. 2008;76:4414–4421. doi: 10.1128/IAI.00012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Diaz M.H., Hauser A.R. Pseudomonas aeruginosa cytotoxin ExoU is injected into phagocytic cells during acute pneumonia. Infect. Immun. 2010;78:1447–1456. doi: 10.1128/IAI.01134-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Sutterwala F.S., Mijares L.A., Li L., Ogura Y., Kazmierczak B.I., Flavell R.A. Immune recognition of Pseudomonas aeruginosa mediated by the IPAF/NLRC4 inflammasome. JEM. 2007;204:3235–3245. doi: 10.1084/jem.20071239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Yahr T.L., Vallis A.J., Hancock M.K., Barbieri J.T., Frank D.W. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc. Natl. Acad. Sci. USA. 1998;95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Ochoa C.D., Alexeyev M., Pastukh V., Balczon R., Stevens T. Pseudomonas aeruginosa exotoxin Y is a promiscuous cyclase that increases endothelial tau phosphorylation and permeability. JBC. 2012;287:25407–25418. doi: 10.1074/jbc.M111.301440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Wagener B.M., Anjum N., Christiaans S.C., Banks M.E., Parker J.C., Threet A.T., Walker R.R., Isbell K.D., Moser S.A., Stevens T., et al. Exoenzyme Y contributes to end-organ dysfunction caused by Pseudomonas aeruginosa pneumonia in critically ill patients: An exploratory study. Toxins. 2020;12:369. doi: 10.3390/toxins12060369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Belyy A., Raoux-Barbot D., Saveanu C., Namane A., Ogryzko V., Worpenberg L., David V., Henriot V., Fellous S., Merrifield C., et al. Actin activates Pseudomonas aeruginosa ExoY nucleotidyl cyclase toxin and ExoY-like effector domains from MARTX toxins. Nat. Commun. 2016;7:1–14. doi: 10.1038/ncomms13582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Sayner S.L., Frank D.W., King J., Chen H., VandeWaa J., Stevens T. Paradoxical cAMP-induced lung endothelial hyperpermeability revealed by Pseudomonas aeruginosa ExoY. Circ Res. 2004;95:196–203. doi: 10.1161/01.RES.0000134922.25721.d9. [DOI] [PubMed] [Google Scholar]
- 326.Sayner S.L., Frank D.W., King J., Chen H., VandeWaa J., Stevens T. The amino-terminal domain of Pseudomonas aeruginosa ExoS disrupts actin filaments via small-molecular-weight GTP-binding proteins. Mol. Microbiol. 1999;32:393–401. doi: 10.1046/j.1365-2958.1999.01359.x. [DOI] [PubMed] [Google Scholar]
- 327.Cowell B.A., Evans D.J., Fleiszig S.M. Actin cytoskeleton disruption by ExoY and its effects on Pseudomonas aeruginosa invasion. FEMS Microbiol. Lett. 2005;250:71–76. doi: 10.1016/j.femsle.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 328.Stevens T.C., Ochoa C.D., Morrow K.A., Robson M.J., Prasain N., Zhou C., Alvarez D.F., Frank D.W., Balczon R., Stevens T. The Pseudomonas aeruginosa exoenzyme Y impairs endothelial cell proliferation and vascular repair following lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;306:L915–L924. doi: 10.1152/ajplung.00135.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Lin H.-H., Huang S.-P., Teng H.-C., Ji D.-D., Chen Y.-S., Chen Y.-L. Presence of the exoU gene of Pseudomonas aeruginosa is correlated with cytotoxicity in MDCK cells but not with colonization in BALB/c mice. JCM. 2006;44:4596–4597. doi: 10.1128/JCM.01531-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Kloth C., Schirmer B., Munder A., Stelzer T., Rothschuh J., Seifert R. The role of Pseudomonas aeruginosa ExoY in an acute mouse lung infection model. Toxins. 2018;10:185. doi: 10.3390/toxins10050185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Hayashi T., Baba T., Matsumoto H., Terawaki Y. Phage-conversion of cytotoxin production in Pseudomonas aeruginosa. Mol. Microbiol. 1990;4:1703–1709. doi: 10.1111/j.1365-2958.1990.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 332.Xiong G., Struckmeier M., Lutz F. Pore-forming Pseudomonas aeruginosa cytotoxin. Toxicology. 1994;87:69–83. doi: 10.1016/0300-483X(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 333.Hayashi T., Kamio Y., Hishinuma F., Usami Y., Titani K., Terawaki Y. Pseudomonas aeruginosa cytotoxin: The nucleotide sequence of the gene and the mechanism of activation of the protoxin. Mol. Microbiol. 1989;3:861–868. doi: 10.1111/j.1365-2958.1989.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 334.Ohnishi M., Hayashi T., Tomita T., Terawaki Y. Mechanism of the cytolytic action of Pseudomonas aeruginosa cytotoxin: Oligomerization of the cytotoxin on target membranes. FEBS Lett. 1994;356:357–360. doi: 10.1016/0014-5793(94)01311-X. [DOI] [PubMed] [Google Scholar]
- 335.Baltch A., Smith R., Franke M., Ritz W., Michelsen P., Bopp L., Lutz F. Pseudomonas aeruginosa cytotoxin as a pathogenicity factor in a systemic infection of leukopenic mice. Toxicon Off. J. Int. Soc. Toxinol. 1994;32:27–34. doi: 10.1016/0041-0101(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 336.Rock K.L., Kono H. The inflammatory response to cell death. Annu. Rev. Pathmechdis. Mech. Dis. 2008;3:99–126. doi: 10.1146/annurev.pathmechdis.3.121806.151456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Winsor G.L., Griffiths E.J., Lo R., Dhillon B.K., Shay J.A., Brinkman F.S.L. Enhanced annotations and features for comparing thousands of Pseudomonas genomes in the Pseudomonas genome database. Nucleic Acids Res. 2015;44:D646–D653. doi: 10.1093/nar/gkv1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Boukerb A.M., Marti R., Cournoyer B. Genome sequences of three strains of the Pseudomonas aeruginosa PA7 clade. Genome Announc. 2015;3:e01366-15. doi: 10.1128/genomeA.01366-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Elsen S., Huber P., Bouillot S., Couté Y., Fournier P., Dubois Y., Timsit J.-F., Maurin M., Attrée I. A type III secretion negative clinical strain of Pseudomonas aeruginosa employs a two-partner secreted exolysin to induce hemorrhagic pneumonia. Cell Host Microbe. 2014;15:164–176. doi: 10.1016/j.chom.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 340.Basso P., Ragno M., Elsen S., Reboud E., Golovkine G., Bouillot S., Huber P., Lory S., Faudry E., Attrée I. Pseudomonas aeruginosa pore-forming exolysin and type IV pili cooperate to induce host cell lysis. MBio. 2017;8:e02250-16. doi: 10.1128/mBio.02250-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Bouillot S., Munro P., Gallet B., Reboud E., Cretin F., Golovkine G., Schoehn G., Attrée I., Lemichez E., Huber P. Pseudomonas aeruginosa Exolysin promotes bacterial growth in lungs, alveolar damage and bacterial dissemination. Sci. Rep. 2017;7:2120. doi: 10.1038/s41598-017-02349-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Basso P., Wallet P., Elsen S., Soleilhac E., Henry T., Faudry E., Attrée I. Multiple Pseudomonas species secrete exolysin-like toxins and provoke Caspase-1-dependent macrophage death. Environ. Microbiol. 2017;19:4045–4064. doi: 10.1111/1462-2920.13841. [DOI] [PubMed] [Google Scholar]
- 343.Zhang Y.-F., He W., Zhang C., Liu X.-J., Lu Y., Wang H., Zhang Z.-H., Chen X., Xu D.-X. Role of receptor interacting protein (RIP)1 on apoptosis-inducing factor-mediated necroptosis during acetaminophen-evoked acute liver failure in mice. Toxicol Lett. 2014;225:445–453. doi: 10.1016/j.toxlet.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 344.Dhuriya Y.K., Sharma D. Necroptosis: A regulated inflammatory mode of cell death. J. Neuroinflammation. 2018;15:199. doi: 10.1186/s12974-018-1235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Pang Z., Raudonis R., Glick B.R., Lin T.J., Cheng Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019;37:177–192. doi: 10.1016/j.biotechadv.2018.11.013. [DOI] [PubMed] [Google Scholar]
- 346.Prevention, CDC Antibiotic Resistance Threats in the United States. [(accessed on 10 September 2022)];2019 Available online: https://www.cdc.gov/drugresistance/biggest-threats.html.
- 347.Lynch J.P., III, Zhanel G.G., Clark N.M. Seminars in Respiratory and Critical Care Medicine. Thieme Medical Publishers; New York, NY, USA: 2017. Emergence of Antimicrobial Resistance among Pseudomonas aeruginosa: Implications for Therapy. [DOI] [PubMed] [Google Scholar]
- 348.Potron A., Poirel L., Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: Mechanisms and epidemiology. Int. J. Antimicrob. Agents. 2015;45:568–585. doi: 10.1016/j.ijantimicag.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 349.McCarthy K. Seminars in Respiratory and Critical Care Medicine. Thieme Medical Publishers; New York, NY, USA: 2015. Pseudomonas aeruginosa: Evolution of antimicrobial resistance and implications for therapy. [DOI] [PubMed] [Google Scholar]
- 350.Oliver A., Mulet X., López-Causapé C., Juan C. The increasing threat of Pseudomonas aeruginosa high-risk clones. Drug Resist. Updates. 2015;21:41–59. doi: 10.1016/j.drup.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 351.Anantharajah A., Buyck J.M., Sundin C., Tulkens P.M., Mingeot-Leclercq M.-P., Van Bambeke F. Salicylidene acylhydrazides and hydroxyquinolines act as inhibitors of type three secretion systems in Pseudomonas aeruginosa by distinct mechanisms. Antimicrob. Agents Chemother. 2017;61:e02566-16. doi: 10.1128/AAC.02566-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Sharma P., Elofsson M., Roy S. Attenuation of Pseudomonas aeruginosa infection by INP0341, a salicylidene acylhydrazide, in a murine model of keratitis. Virulence. 2020;11:795–804. doi: 10.1080/21505594.2020.1776979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Warrener P., Varkey R., Bonnell J.C., DiGiandomenico A., Camara M., Cook K., Peng L., Zha J., Chowdury P., Sellman B., et al. A novel anti-PcrV antibody providing enhanced protection against Pseudomonas aeruginosa in multiple animal infection models. Antimicrob. Agents Chemother. 2014;58:4384–4391. doi: 10.1128/AAC.02643-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Lee V.T., Pukatzki S., Sato H., Kikawada E., Kazimirova A.A., Huang J., Li X., Arm J.P., Frank D.W., Lory S. Pseudolipasin A is a specific inhibitor for phospholipase A2 activity of Pseudomonas aeruginosa cytotoxin ExoU. Infect. Immun. 2007;75:1089–1098. doi: 10.1128/IAI.01184-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Arnoldo A., Curak J., Kittanakom S., Chevelev I., Lee V.T., Sahebol-Amri M., Koscik B., Ljuma L., Roy P.J., Bedalov A., et al. Identification of small molecule inhibitors of Pseudomonas aeruginosa exoenzyme S using a yeast phenotypic screen. PLoS Genet. 2008;4:e1000005. doi: 10.1371/annotation/76d35829-07a2-479f-bbc1-cce6755b6d8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Kaufmann G.F., Park J., Mee J.M., Ulevitch R.J., Janda K.D. The quorum quenching antibody RS2-1G9 protects macrophages from the cytotoxic effects of the Pseudomonas aeruginosa quorum sensing signalling molecule N-3-oxo-dodecanoyl-homoserine lactone. Mol. Immunol. 2008;45:2710–2714. doi: 10.1016/j.molimm.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Zhu F., Xiong F., He J., Liu K., You Y., Xu Q., Miao J., Du Y., Zhang L., Ren H., et al. Brd4 inhibition ameliorates Pyocyanin-mediated macrophage dysfunction via transcriptional repression of reactive oxygen and nitrogen free radical pathways. Cell Death Dis. 2020;11:1–16. doi: 10.1038/s41419-020-2672-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Starkey M., Rahme L.G. Modeling Pseudomonas aeruginosa pathogenesis in plant hosts. Nat. Protoc. 2009;4:117–124. doi: 10.1038/nprot.2008.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Tan M.-W., Mahajan-Miklos S., Ausubel F.M. Ausubel, Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA. 1999;96:715–720. doi: 10.1073/pnas.96.2.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Bassetti M., Vena A., Croxatto A., Righi E., Guery B. How to manage Pseudomonas aeruginosa infections. Drugs Context. 2018;7:212527. doi: 10.7573/dic.212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Hauser A.R., Kang P.J., Engel J.N. PepA, a secreted protein of Pseudomonas aeruginosa, is necessary for cytotoxicity and virulence. Mol. Microbiol. 1998;27:807–818. doi: 10.1046/j.1365-2958.1998.00727.x. [DOI] [PubMed] [Google Scholar]