Abstract
Background: The aim of the study is to analyze the prevalence of using patients’ reported outcomes measures and experiences (PROMs and PREMs) in relation to integrated care (IC). Material and methods: To select eligible studies (<10 years, full-text), PubMed was used. The general subject of the articles referring to the type of disease was indicated on the basis of a review of all full-text publications discussing the effectiveness of IC (N = 6518). The final search included MeSH headings related to outcomes measures and IC. Full-text screening resulted in including 73 articles (23 on COPD, 40 on diabetes/obesity and 10 on depression) with 93.391 participants. Results: Analysis indicated that authors used multiple outcome measures, with 54.8% of studies including at least one patient reported. PROMs were more often used than PREMs. Specific (disease or condition/dimension) outcome measures were reported more often than general, especially those dedicated to self-assessment of health in COPD and depression. PROMs and PREMs were most commonly used in studies from the USA and Netherlands. Conclusion: Using PROMS/PREMS is becoming more popular, although it is varied, both due to the place of research and type of disease.
Keywords: chronic obstructive pulmonary disease (COPD), depression/mood disorder, managed care, multidisciplinary care, obesity, patient adherence
1. Introduction
Analysis of the effectiveness of care has traditionally focused on objective clinical indicators. According to international HTA (health technology assessment) guidelines, to conduct research on the effectiveness of treatment, hard endpoints, followed by clinically relevant surrogate points, must be used [1]. Today, the discussion on the role of other outcomes is open and ongoing. Using Patient-reported outcome measures (PROMs) and patient-reported experience measures (PREMs) seems to be increasingly relevant for assuring a good quality of care and supporting gathering knowledge considering disease course. It is believed that their role is particularly important in patient-focused care models, i.e., in integrated care and integrating treatment methods from various areas [2,3]. Integrated care is currently accepted as a form of care around the world; however, its understanding and definition may vary depending on the perspective or purpose for which such a definition is built [4]. A health system-based definition provides that it should be understood as: “health services that are managed and delivered so that people receive a continuum of health promotion, disease prevention, diagnosis, treatment, disease-management, rehabilitation and palliative care services, coordinated across the different levels and sites of care within and beyond the health sector, and according to their needs throughout the life course” [5]. Integrated care is provided through integrated care programs that proactively organize and coordinate the comprehensive delivery of both health and social care services, aiming to improve patient outcomes and reduce healthcare expenses [6].
PROMs are questionnaires or scales that allow for the measurement of the results of treatment from the patient’s perspective [7]. They can be divided into generic, disease-specific and condition/dimension-specific [8,9,10]. PREMs bring information on how patients conceive medical care. Relational PREMs deal with relations between patients and medical personnel, patients’ expectations and preferences. Functional PREMs are related to basic expectations about technical issues related to delivering healthcare [11]. Information obtained from tools such as PROMs and PREMs are used in many ways: in scientific research, projects improve the quality of care and audits and are conducted for pharmacoeconomic purposes [12]. They allow the management of healthcare in a patient-centric manner and gain feedback from healthcare providers that can be used in building quality improvement strategies [13]. Moreover, they allow clinicians to better understand patients and identify those health outcomes that are crucial from the patient’s point of view [14]. The collection of this type of data is supported by researchers who indicate that attachment to routinely used outcome measures can lead to a marginalization of patient needs and implementation of treatment that will be characterized by low compliance [15,16]. Although the importance of patient-centered outcomes is unquestionable, some doubts are raised considering the reliability of data. The quality of measurement is determined by how data is obtained and with the use of research tools [17]. It was found that there are statistically significant differences in assessing the relevance of symptoms by a doctor and a patient [16,18]. Information collected through PROMs and PREMs can be used in many areas: providing individual medical care of good quality or supporting the decision-making process in managing healthcare.
The relevance of this study for understanding the idea and role of patient-centric care is significant as it provides a comprehensive set of basic knowledge about research using data coming directly from patients, identifies areas in which their role is already proven, and where there is still a need to work on increasing the dissemination of usage of such indicators. Moreover, the collection of data based on patient-reported measures might result in numerous positive results, among others: support for communication between medical staff and patients, increase in patient satisfaction and compliance, and enabling patients to control their own health condition better. These elements are crucial for providing effective integrated care and, in a broader aspect, a successful healthcare system based on a value-based approach.
2. Material and Methods
The main research question in this scoping review was what is the prevalence of using outcomes measures reported by patients remaining under the support of integrated care (IC). The paper also sets specific questions:
What types of PROMs and PREMs are used to describe the effectiveness of IC programs? Are there differences in the use of PROMs and PREMs depending on the type of disease and the country?
The studies included in the analysis concerned:
-
2.
The patients suffering from one of three chronic diseases: COPD, obesity/diabetes or depression, who received integrated care.
-
3.
The intervention discussed was integrated care, which was assessed by researchers in terms of its effectiveness.
The comparison included an analysis of type, time and place of outcomes measures reported by patients receiving integrated care. Additionally, all indicators that were used by researchers to describe the IC used were subject to a preliminary quantitative assessment. Outcomes measures were a basic element of the analysis, along with an assessment of the frequency of using PROMs and PREMs, their type, the place of conducting the research and the date of publishing.
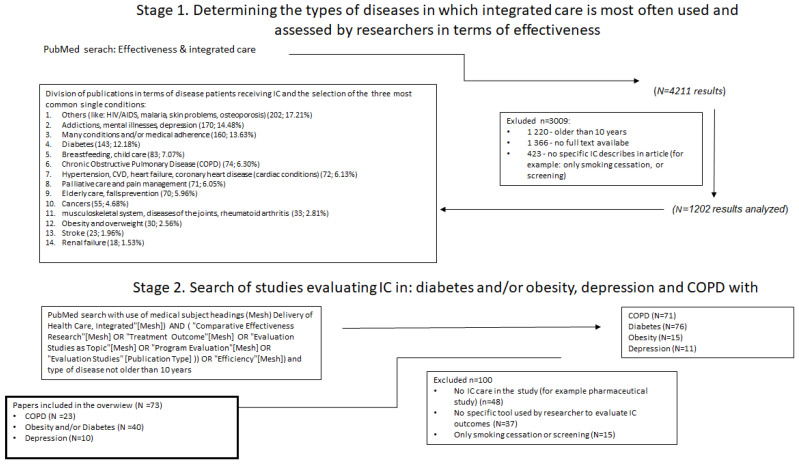
The study design included two stages (Figure 1).
Figure 1.
Search strategy.
Due to the fact that many PROMs and PREMs are dedicated to a specific disease, PubMed first searched to determine which chronic conditions research on the effectiveness of integrated care is carried out most often. Analysis of 6518 available full-text articles indicated that in the first place, most often, these are mental diseases and addiction to psychoactive substances 14.48% (mostly depression), many coexisting conditions (13.63%), diabetes and/or obesity (12.18%), breastfeeding and childcare (7.07%), chronic obstructive pulmonary disease—COPD (6.30%), palliative care and chronic pain (6.05%) and others (including among others: malaria, HIV, bedsores, infectious diseases, osteoporosis, allergies, psoriasis, multiple sclerosis)—17.21%. As a result, the overview was performed on the three most common diseases—diabetes and/or obesity, depression and COPD.
Stage two of the search included medical subject headings (“Delivery of Health Care, Integrated” [Mesh]) and selected chronic conditions: “Pulmonary Disease, Chronic Obstructive”, “Diabetes Mellitus”, “Obesity”, “Depression” limiting the search strategy to “Comparative Effectiveness Research” [Mesh], “Treatment Outcome” [Mesh] or “Program Evaluation” [Mesh]). The inclusion criteria were: articles not older than 10 years, full text available and English language. The exclusion criteria were all those that did not comply with the inclusion criteria, studies not using specific research tools to assess the effect or did not assess IC and studies evaluating pharmaceutical and/or surgical procedures. Protocols of currently planned studies were enrolled in the analysis.
It resulted in identifying 173 articles. Full-text screening resulted in excluding 100 papers based on inclusion and exclusion criteria. Among the remaining 73 articles, 23 considered COPD, 40 diabetes or obesity and 10 depression.
For each of the identified studies, general characteristics and data on elements used in IC were extracted. The analysis included only those articles in which authors indicated specified outcome measures for assessment of clinical end-point, PROMs and/or PREMs or utilization of healthcare resources. The frequency of using patient-reported outcomes measures and experiences (divided into the following groups: generic, disease-specific, condition/dimension-specific) was analyzed in relation to the country, year and type of disease.
3. Results
3.1. General Characteristics of the Studies
The search identified 73 studies from the years 2009–2019 that were eligible for the analysis. This search enrolled a total of over 93.391 thousand patients. The median number of patients within the study group was 263 (ranging from 20 to 17.142). Most of the studies were original studies on the evaluation of integrated care models, and the newest studies (mostly from the years 2017–2019) also studied protocols planned for realization. The eligible studies described models of IC offering various forms of support for patients, usually more than one (85%) (Table 1 and Table 2).
Table 1.
Elements of integrated care.
| Elements of IC | N | Percent |
|---|---|---|
| Education (incl. education for staff members), self-management, support after discharge | 56 | 76.7 |
| Community-based or home-based care, also a hospital community-based type of care | 35 | 47.9 |
| Support of additional specialists, multispecialty team | 35 | 47.9 |
| Treatment or action plan, treatment coordination, adherence | 35 | 47.9 |
| Others (for example, IT services, home oxygen service, smoking cessation) | 33 | 45.2 |
| Pulmonary rehabilitation | 12 | 52.2 * |
* of studies dealing with COPD.
Table 2.
Total number of assessed outcomes [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90].
| Total Number of Instruments Used | N | Percent |
|---|---|---|
| <5 | 21 | 28.8 |
| 6 to 10 | 26 | 35.6 |
| 11 to 15 | 13 | 17.8 |
| 15 to 20 | 2 | 2.7 |
| >20 | 5 | 6.8 |
| n/a | 6 | 8.2 |
| Total | 73 | 100.0 |
3.2. Outcomes Measures Used in Studies
The assessment of care was reported in all studies, but not all of them used PROMs or PREMs for assessing effectiveness (54.8% used at least one PROM or PREM). The most common approach was to analyze from 6 to 10 different outcomes.
3.3. PROMs and PREMs in Evaluation of IC
PROMs or PREMs were reported in 39 studies, in 24 of which outcomes were described with PROMs, in 11 both PROMs and PREMs and in 4 with exclusive use of PREMs. Specific (disease or condition/dimension) outcomes were reported more often than general (22 vs. 8). The most commonly used PROM was the Medical Research Council scale (mMRC) and St. George’s Respiratory Questionnaire (SGRQ) (both N = 8). For details see Table 3.
Table 3.
Main characteristics reporting PROMs and PREMs in evaluation of integrated care.
| Author (First) | Year | Country (ISO 3166-1) | Population | Disease | PROMS | PREMS | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Generic | Disease Specific | Condition or Dimension Specific | Generic | Disease or Condition Specific | ||||||
| HRQoL | Other than HRQoL | |||||||||
| Afolabi [19] | 2013 | GBR | 199 | COPD | CRQ | |||||
| CAT | ||||||||||
| Carron [22] | 2017 | CHE | 57 | COPD | SF-36 | CAT | mMRC | PACIC | ||
| Number of COPD exacerbations reported by patients | ||||||||||
| CRQ | ||||||||||
| SEM-CD | ||||||||||
| Henoch [23] | 2016 | CHE | 7810 | COPD | Exercise capacity (Likert scale) | CCQ | mMRC | |||
| Davis [24] | 2016 | CAN | 140 | COPD | MMAS-8 | SGRQ | ||||
| Esteban [25] | 2016 | ESP | 119 | COPD | HADS | SGRQ | mMRC | |||
| LCADL | ||||||||||
| Gillis [26] | 2017 | CAN | 174 | COPD | CTM-3 | |||||
| Expectations (single open-ended question) | ||||||||||
| “Helpfulness” of care (Likert scale) | ||||||||||
| Garner [27] | 2017 | GBR | n/a | COPD | CAT | Place of death | ||||
| Hernandez [28] | 2015 | ESP | 155 | COPD | HADS | mMRC | ||||
| IADL | ||||||||||
| Hogg [29] | 2012 | GBR | 1114 | COPD | HADS | CRQ-SR | ||||
| Jarab [31] | 2012 | JOR | 106 | COPD | CSES | |||||
| SGRQ | ||||||||||
| Ko [32] | 2014 | HKG | 185 | COPD | SGRQ | mMRC | ||||
| Ko [33] | 2017 | HKG | 180 | COPD | SGRQ | mMRC | ||||
| Koolen [34] | 2018 | NLD | n/a | COPD | PAM | CCQ | CQIAC | |||
| MSQ | NCSI | CSPAM | ||||||||
| PCRS | ||||||||||
| CPSET | ||||||||||
| PACIC | ||||||||||
| Kruis [35] | 2014 | NLD | 1086 | COPD | EQ-5L | IPAQ | CCQ | mMRC | PACIC | |
| SF-36 | SMAS-30 | SGRQ | ||||||||
| Kruis [36] | 2010 | NLD | 1086 | COPD | EQ-5L | IPAQ | CCQ | mMRC | PACIC | |
| SF-36 | SMAS-30 | SGRQ | ||||||||
| Pinnock [38] | 2013 | GBR | 128 | COPD | HADS | SGRQ | ||||
| SECD-6 | ||||||||||
| LINQ | ||||||||||
| Wu [40] | 2015 | SGP | 62 | COPD | CAT | PACIC | ||||
| Aponte [41] | 2017 | USA | 180 | Diab and Obes | DKQ | |||||
| Beauregard [43] | 2018 | CAN | 1185 | Diab and Obes | Enquête québécoise sur l’activité physique et la santé |
|||||
| Chwastiak [46] | 2017 | USA | 151 | Diab and Obes | PHQ-9 | |||||
| Ciccone [47] | 2010 | ITA | 1160 | Diab and Obes | SF-12 | |||||
| Fottrell [49] | 2016 | BGD | Diab and Obes | EQ-5L | SRQ | |||||
| Gucciardi [50] | 2012 | CAN | 1200 | Diab and Obes | Patients’ experiences and views (in-depth interviews) | |||||
| Husted [54] | 2014 | DNK | 71 | Diab and Obes | TSRQ-21 | PCD | HCCQ | PAID-20 | ||
| WHO5 | ||||||||||
| Jansink [55] | 2013 | NLD | 940 | Diab and Obes | VAS scale | |||||
| Vermunt [68] | 2012 | NLD | 925 | Diab and Obes | Satisfaction about program (Likert scale) | |||||
| Zhang [72] | 2013 | AUS | 456 | Diab and Obes | SF-12 | HADS | DQoL-brief | CSQ-8 | ||
| SMAS-30 | ||||||||||
| van Eeghen [75] | 2018 | USA | 20 | Diab and Obes | PHQ-9 | Satisfaction about program (Likert scale) | ||||
| Hoffman [77] | 2018 | USA | 97 | Diab and Obes | Sizing Me Up |
FFQ | ||||
| IPAQ | ||||||||||
| PMI | ||||||||||
| PAQ | ||||||||||
| Wake [80] | 2012 | AUS | 120 | Diab and Obes | PedsQL | PCSC | BPQ | |||
| SDQ | ||||||||||
| Unützer [82] | 2012 | USA | 7977 | Dep | PHQ-9 | |||||
| Hepner [83] | 2011 | USA | 113 | Dep | Satisfaction about program (Likert scale) | |||||
| Murphy [84] | 2017 | VNM | n/a | Dep | WHODAS | SRQ-20 | ||||
| CAGE | ||||||||||
| Poulsen [85] | 2017 | DNK | n/a | Dep | EQ-5L | WSAS | BDI-II | PSS | CSQ-8 | |
| Flanagan QOLS | IPQ | BAI | KES | |||||||
| GSS | 4DSQ | RTW-SE | ||||||||
| SPS | ||||||||||
| Salisbury [86] | 2016 | GBR | 609 | Dep | EQ-5L | HeiQ | PHQ-9 | GAD-7 | Care coordination (Haggerty) | |
| MMAS-8 | ||||||||||
| eHEALs | ||||||||||
| Sanchez [87] | 2017 | USA | 11895 | Dep | PAQ | PHQ-9 | GAD-7 | |||
| DKM | SCMHC | |||||||||
| SD | LSAS | |||||||||
| Von Korff [88] | 2011 | USA | 214 | Dep | Quality of life (Likert scale) | WHODAS | SLC-20 | SDS | ||
| Wagner [89] | 2014 | USA | n/a | Dep | PHQ-9 | |||||
| MOS-HIV | ||||||||||
| Wu [90] | 2014 | USA | 964 | Dep | SF-12 | PHQ-9 | SDS | Satisfaction about program (Likert scale) | ||
Abbreviations: AUS—Australia; BAI—Beck Anxiety Inventory; BDI-II—Beck Depression Inventory-II; BGD—Bangladesh; BPQ—Body figure perception questionnaire; CAGE—Cut-down, Annoyed, Guilty, Eye-opener Questionnaire; CAN—Canada; CAT—COPD assessment test; CCQ—Clinical COPD Questionnaire; CHE—Switzerland; CPSET—Care Process Self Evaluation Tool; CQIAC—Consumer Quality Index Asthma and COPD; CRQ—Chronic Respiratory Diseases Questionnaire; CRQ-SR—Chronic Respiratory Questionnaire self-report dyspnea scale; CSES—COPD Self-Efficacy Scale; CSPAM—Clinician Support for Patient Activation Measure; CSQ-8—Client Satisfaction Questionnaire; CTM-3—Care Transitions Measure-3; Dep—depression; Diab and Obes—diabetes and obesity; DKM—Depression Knowledge Measure; DKQ—Diabetes Knowledge Questionnaire; DNK—Denmark; DQoL-brief—Diabetes Quality of Life Scale; eHEALs—eHealth Literacy Scale; 4DSQ—Four-Dimensional Symptom Questionnaire; EQ-5L—Euro Qol-5D-5L; ESP—Spain; FFQ—Food Frequency Questionnaire; GAD-7—Generalized anxiety; GBR—United Kingdom; GSS—General Self-Efficacy Scale; HADS—Hospital Anxiety and Depression Scale; HCCQ—Health Care Climate Questionnaire; HeiQ—Health Education Impact Questionnaire; HKG—Hong Kong; IADL—Lawton Instrumental Activities of Daily Living Scale; IPAQ—International Physical Activity Questionnaire; IPQ—Illness Perception Questionnaire; ITA—Italy; JOR—Jordan; KES—Karolinska Exhaustion Scale; LCADL—London Chest Activity of Daily Living; LINQ—Lung information needs questionnaire; LSAS—Latino Scale for Antidepressant Stigma; MMAS-8—Morisky Medication Adherence Scale; mMRC—Medical Research Council scale; MOS-HIV—Medical Outcomes Study HIV Health Survey; MOS-HIV—Medical Outcomes Study HIV Health Survey; MSQ—Marshall Sitting Questionnaire; NCSI—Nijmegen Clinical Screening Instrument; NLD—Netherlands; PACIC—Patient Assessment of Chronic Illness Care Questionnaire; PAID-20- Problem Areas In Diabetes (20 item); PAM—Patient Activation Measure; PAQ—Patient Adherence Questionnaire; PAQ—Patient Adherence Questionnaire; PCD—Perceived Competence in Diabetes Scale; PCRS—Primary Care Recourses and Supports for Chronic Disease Self-Management; PCSC—Harter’s perceived Competence scale for Children; PedsQL—Pediatric Enuresis Module to Assess Quality of Life; PHQ-9—Patient Health Questionnaire-9); PMI—Parent Motivation Inventory; PSS—Perceived Stress Scale; RTW-SE—Return to Work Self-Efficacy; SCMHC—Stigma Concerns about Mental Health Care; SD—Social Distance Scale; SDQ—Strengths and difficulties Questionnaire; SDS—Sheehan Disability Scale; SECD-6—Self-efficacy for managing chronic disease 6 item scale; SEM-CD -Self-Efficacy for Managing Chronic Disease 6-Item Scale; SF-12—Short Form Health Survey; SF-36—Short Form 36; SGP—Singapore; SLC-20—20-item Symptom Checklist Depression Scale; SMAS-30—Self-Management Ability Scale-30; SPS—Stanford Presenteeism Scale); SRQ-20—Self-Reporting Questionnaire; TSRQ -21—21-item Treatment Self-Regulation Questionnaire; USA—United States of America; WHO5—World Health Organization-5 scale; WHODAS—World Health Organization Disability Assessment; WSAS—Work and Social Adjustment Scale.
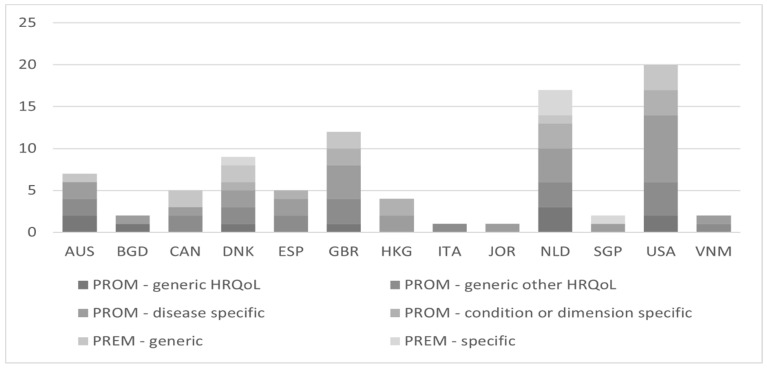
As shown in Figure 2, PROMs and PREMs were most commonly used in studies from the USA, followed by the Netherlands. In ten studies from the USA, twelve different tools were used to assess patients’ outcomes or experiences, out of which PHQ-9 was the most popular (N = 7). In six studies from the Netherlands, eight different tools were used—most often CCQ (N = 5). All types of patient-reported indicators were present in studies from Denmark and the Netherlands.
Figure 2.
Number of PROMs and PREMs used in studies by country.
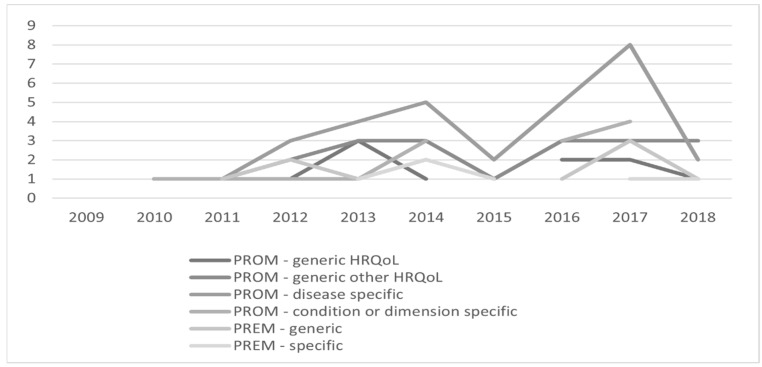
As shown in Figure 3, the first studies using specified questionnaires for assessing PROMs and PREMs were published within the analyzed period of time in 2010, and these two studies used only condition- or dimension-specific PROMs. The highest number of PROMs and PREMs was noted in 2017, mostly due to the widespread use of disease-specific PROMs.
Figure 3.
Number of PROMs and PREMS in studies by year of publication.
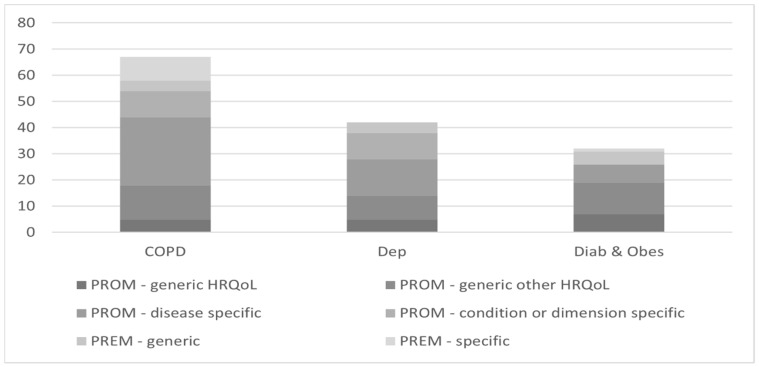
As shown in Figure 4, all types of PROMs and PREMs were reported as measures only in studies dealing with COPD. Likewise, PROMS were used most frequently in the assessment of IC dedicated to this disease (18 of 23 studies, 78.2%). Studies related to depression did not mention specific PREMs, and those related to diabetes did not use specific PREMs nor condition/dimension PROMs.
Figure 4.
Number of PROMs and PREMS in studies by type of disease.
4. Discussion
Reliable assessment of care, including integrated care, requires a multi-criterial approach. There is an increased interest in using the information provided by patients considering their health and experience related to healthcare [91,92]. Contemporary understanding of healthcare goes far beyond just providing health services. It is increasingly indicated that the health system must be designed to achieve health goals that are important to patients [91,93,94], which requires using PROMs and PREMs. The presented article can be considered a compendium of knowledge about the available indicators and the desired direction of further research aimed at the improvement of the effectiveness of integrated care. It identifies areas where research activities are particularly needed, which can be an inspiration for further research. It also allows you to familiarize yourself with a wide range of PROMs and PREMS questionnaires, indicating the added value of their use in healthcare management without overlooking them [12]. It is clear that various integrated care programs have been and are being evaluated in terms of clinical and economic effectiveness for many years, and the effectiveness of such interventions in these aspects has been repeatedly demonstrated [95,96,97]. However, the presented overview of practice in the assessment of IC indicates a different approach to this aspect, directing attention to the perspective and subjective feelings of patients, which resulted in some interesting observations. First of all, using PROMs is more popular than using PREMs. The use of patient-reported data is diverse both in terms of the type of disease and the country in which the research is carried out. According to the results of the presented overview, using PROMs and PREMs is most popular in the USA and Netherlands. In the United States, work has been ongoing since 2017 to incorporate additional incentive payments into the Medicare system to achieve desired health goals [98]. In 2004 USA initiated PROMIS (Patient-Reported Outcome Measurement Information System) to improve standards of data collection [99]. The Netherlands is commonly considered a European leader in national registry collection. Some registries include PROMs—for example National Quality Registry for Parkinson’s disease or the low back pain registry. Back in 2007, the country used for the first time value-based payments and introduced a successful bundled system payment for COPD and type-2 diabetes, which included PROMs [8]. Moreover, OECD undertakes numerous initiatives in this subject; for example, it is monitoring the collection of PREMs data in member countries [100]. Based on available data, disease-specific PROMs seem to give the broadest and most specific information on the condition of health of patients (both physiological and psychological) and combine the positive features of generic and state-specific indicators—on the one hand, relative versatility, and on the other, sufficient accuracy [101,102]. According to the presented overview, disease-specific PROMs are most commonly used—especially in the assessment of IC dedicated to COPD and depression. This is due to the availability of recognized research tools such as CAT or SGRQ in COPD or PHQ-9 in depression. PREMs were used less frequently, and the history of their usage is shorter, especially as a condition-specific tool [103] and in specific groups of patients; for example, children. The most significant limitation of the publication is a potential bias resulting from a limited to 10 years period in which eligible articles were published and the inclusion of only full-text articles indexed by PubMed. By deciding on such a research method, it is impossible to determine whether unpublished studies have adopted another form of reporting PROMs and PREMs. However, it seems that the analysis of nearly 7.000 publications allows for an overview of contemporary reporting practice outcomes measures reported by patients. Available studies seem to prove there is a correlation between experience and the effectiveness of procedures [104,105]. Nevertheless, PREMs should be collected and analyzed together with PROMs as some disparities might occur depending on clinical outcomes [7,106].
5. Conclusions
Using outcomes measures reported by patients remaining under the support of integrated care is varied, both due to the place of research and the type of disease. Interest in these indicators seems to be increasing, especially over the last few years. Nevertheless, it seems necessary to continue work on building research tools for reliable data acquisition, especially in the field of specific PREMs.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.EUnetHTA Endpoints Used for Relative Effectiveness Assessment of Pharmaceuticals: Clinical Endpoints. [(accessed on 30 April 2021)]. Available online: https://www.eunethta.eu/wp-content/uploads/2018/01/Clinical-endpoints.pdf2015.
- 2.Field J., Holmes M.M., Newell D. PROMs data: Can it be used to make decisions for individual patients? A narrative review. Patient Relat. Outcome Meas. 2019;10:233–241. doi: 10.2147/PROM.S156291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organisation for Economic Co-Operation and Development Measuring What Matters: The Patient-Reported Indicator Surveys. [(accessed on 14 December 2022)]. Available online: https://www.oecd.org/health/health-systems/Measuring-what-matters-the-Patient-Reported-Indicator-Surveys.pdf2019.
- 4.Goodwin N. Understanding Integrated Care. Int. J. Integr. Care. 2016;16:6. doi: 10.5334/ijic.2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Contandriapoulos A.P., Denis J.L., Touati N., Rodriguez C. Groupe de Recherche Interdisciplinaire en Santé. Université de Montréal; Montréal, QC, Canada: 2003. The integration of health care: Dimensions and implementation. Working Paper N04–01. [Google Scholar]
- 6.Lemmens L.C., Molema C.C., Versnel N., Baan C.A., de Bruin S.R. Integrated care programs for patients with psychological comorbidity: A systematic review and meta-analysis. J. Psychosom. Res. 2015;79:580–594. doi: 10.1016/j.jpsychores.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Weldring T., Smith S.M. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs) Health Serv. Insights. 2013;6:61–68. doi: 10.4137/HSI.S11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams K., Sansoni J., Morris D., Grootemaat P., Thompson C. Patient-reported outcome measures. Literature review. [(accessed on 1 December 2022)];Aust. Comm. Saf. Qual. Health Care. 2016 Available online: https://www.safetyandquality.gov.au/sites/default/files/migrated/PROMs-Literature-Review-December-2016.pdf. [Google Scholar]
- 9.Desomer A., van den Heede K., Triemstra M., Paget J., De Boer R., Kohn L., Cleemput I. Use of Patient-Reported Outcome and Experience Measures in Patient Care and Policy–Short report. Health Services Research (HSR) Brussels: Belgian Health Care Knowledge Centre (KCE) 2018. [(accessed on 15 April 2021)]. Available online: https://kce.fgov.be/en/use-of-patient-reported-outcome-and-experience-measures-in-patient-care-and-policy.
- 10.Fitzpatrick R., Davey C., Buxton M., Jones D. Evaluating patient-based outcome measures for use in clinical trials. Health Technol. Asses. 1998;14:74. doi: 10.3310/hta2140. [DOI] [PubMed] [Google Scholar]
- 11.Doyle C., Lennox L., Bell D. A systematic review of evidenceon the links between patient experienceand clinical safety and effectiveness. BMJ Open. 2013;3:21–29. doi: 10.1136/bmjopen-2012-001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bandurska E., Ciećko W., Zarzeczna-Baran M. Wykorzystanie wskaźników efektywności pochodzących od pacjentów w opiece zdrowotnej. [Use of patient-reported outcomes measures in healthcare] Bezpieczeństwo Pacjentów I Pers. Med. 2019;3:226–231. [Google Scholar]
- 13.Valderas J.M., Kotzeva A., Espallargues M., Guyatt G., Ferrans C., Halyard M.Y., Revicki D.A., Symonds T., Parada A., Alonso J., et al. The impact of measuring patient-reported outcomes in clinical practice: A systematic review of the literature. Qual. Life Res. 2008;17:179–193. doi: 10.1007/s11136-007-9295-0. [DOI] [PubMed] [Google Scholar]
- 14.Griggs C., Schneider J., Kazis L., Ryan C. Patient-reported outcome measures a stethoscope for the patient history. Ann. Surg. 2017;265:1066–1067. doi: 10.1097/SLA.0000000000002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Steuten L., Vrijhoef B., Severens H., van Merode F., Spreeuwenberg C. Are we measuring what matters in health technology assessment of disease management? Systematic literature review. Int. J. Technol. Assess. 2006;22:47–57. doi: 10.1017/S0266462306050835. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Gutierrez R., McCoy R. Measuring What Matters in Diabetes. JAMA. 2019;21:1865–1866. doi: 10.1001/jama.2019.4310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenhalgh J., Meadows K. The effectiveness of the use of patient-based measures of health in routine practice in improving the process and outcomes of patient care: A literature review. J. Eval. Clin. Pr. 1999;5:401–416. doi: 10.1046/j.1365-2753.1999.00209.x. [DOI] [PubMed] [Google Scholar]
- 18.Solberg L., Asche S., Butler J., Carrell D., Norton C., Jarvik J., Smith-Bindman R., Tillema J., Whi R. It Is Time to Ask Patients What Outcomes Are Important to Them. Am. J. Acc. Care. 2015;4:48–54. [Google Scholar]
- 19.Afolabi G., Stevens R., Turner M., Harvey M., Norman L., Dogan S., Gray W. Development of a Pulmonary Rehabilitation Service for People With COPD. A tiered model of integrated care. J. Cardiopulm. Rehabil. Prev. 2013;13:323–327. doi: 10.1097/HCR.0b013e31829c2004. [DOI] [PubMed] [Google Scholar]
- 20.Alshabanat A., Otterstatter M., Sin D., Rempel C., van Eeden S., FitzGerald J. Impact of a COPD comprehensive case management program on hospital length of stay and readmission rates. Int. J. Chronic Obstr. Pulm. Dis. 2017;21:961–971. doi: 10.2147/COPD.S124385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balaban R., Zhang F., Vialle-Valentin C., Galbraith A., Burns M., Larochelle M., Ross-Degan D. Impact of a Patient Navigator Program on Hospital-Based and Outpatient Utilization Over 180 Days in a Safety-Net Health System. J. Gen. Intern. Med. 2017;32:981–989. doi: 10.1007/s11606-017-4074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carron T., Bridevaux P., Lörvall K., Parmentier R., Moix J., Beytrison V., Pernet R., Rey C., Roberfroid P., Chhajed P., et al. Feasibility, acceptability and effectiven ess of integrated care for COPD patients: A mixed methods evaluation of a pilot community-based programme. Swiss Med. Wkly. 2017;147:w14567. doi: 10.4414/smw.2017.14567. [DOI] [PubMed] [Google Scholar]
- 23.Henoch I., Strang S., Löfdahl C., Ekberg-Jansson A. Management of COPD, equal treatment across age, gender, and social situation? A register study. Int. J. Chronic Obs. 2016;11:2681–2690. doi: 10.2147/COPD.S115238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis E., Marra C., Gamble J., Farrell J., Lockyer J., FitzGerald M., Abu-Ashour W., Gillis C., Hawboldt J. Effectiveness of a pharmacist-driven intervention in COPD (EPIC): Study protocol for a randomized controlled trial. Trials. 2016;17:1–8. doi: 10.1186/s13063-016-1623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Esteban C., Moraza J., Iriberri M., Aguirre U., Goiria B., Quintana J., Aburto M., Capelastegiu A. Outcomes of a telemonitoring-based program (telEPOC) in frequently hospitalized COPD patients. Int. J. Chronic Obstr. 2016;11:2919–2930. doi: 10.2147/COPD.S115350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gillis D., Demmons J., Rocker G. Expanding the INSPIRED COPD Outreach Program™ to the emergency department: A feasibility assessment. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:1597–1604. doi: 10.2147/COPD.S136183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garner A., Hodson M., Ketsetzis G., Pulle L., Yorek J., Bhowmik A. An analysis of the economic and patient o utcome impact of an integrated COPD service in east London. Int. J. Chronic Obstr. Pulm. Dis. 2017;12:1653–1662. doi: 10.2147/COPD.S127843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernandez C., Alonso A., Garcia-Aymerich J., Serra I., Marti D., Rodriguez-Roisin R., Narsavage G., Gomez M., Roca J. Effectiveness of community-based integrated care in frail COPD patients: A randomised controlled trial. Int. J. Chronic Obstr. Pulm. Dis. 2015;25:1–6. doi: 10.1038/npjpcrm.2015.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogg L., Garrod R., Thorton H., McDonnell L., Bellas H., White P. Effectiveness, Attendance, and Completion of an Integrated, System-Wide Pulmonary Rehabilitation Service for COPD: Prospective Observational Study. COPD: J. Chronic Obstr. Pulm. Dis. 2012;9:546–554. doi: 10.3109/15412555.2012.707258. [DOI] [PubMed] [Google Scholar]
- 30.Jain V., Allison R., Beck S., Jain R., Mills P., McCurley S. Van Gundy, K.; Peterson, M. Impact of an integrated disease management program in reducing exacerbations in patients with severe asthma and COPD. Respir. Med. 2014;108:1794–1800. doi: 10.1016/j.rmed.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 31.Jarab A., AlQudah S., Khdour M., Shamssain M., Mukattash T. Impact of pharmaceutical care on health outcomes in pati ents with COPD. Int. J. Clin. Pharm. 2012;34:53–62. doi: 10.1007/s11096-011-9585-z. [DOI] [PubMed] [Google Scholar]
- 32.Ko F., Ngai J., Ng S., Chang K., Cheung R., Leung M., Pun M., Hui D. COPD care programme can reduce readmissions and in-patient bed days. Respir. Med. 2014;108:1771–1778. doi: 10.1016/j.rmed.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 33.Ko F., Cheung R., Rainer T., Lum C., Wong I., Hui D. Comprehensive care programme for patients with chronic obstructive pulmonary disease:a randomised controlled trial. Thorax. 2017;72:122–128. doi: 10.1136/thoraxjnl-2016-208396. [DOI] [PubMed] [Google Scholar]
- 34.Koolen E., van der Wees G., Dekhuijzen R., Heijdra Y., van Hul A. Evaluation of the COPDnet integrated care model in patients with COPD: The study protocol. Int. J. Chronic Obstr. Pulm. Dis. 2018;13:2237–2244. doi: 10.2147/COPD.S153992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kruis A., Boland M., Assendelft W., Gussekloo J., Tsiachristas A., Stijnen T., Blom C., Sont J., Rutten-van Mölken M., Chavannes N. Effectiveness of integrated disease management for primary care chronic obstructive pulmonary disease patients: Results of cluster randomised trial. BMJ. 2014;349:g5392. doi: 10.1136/bmj.g5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kruis A., von Adrichem J., Erkelens M., Scheepers H., Veen H., Muris J., Chavannes N. Sustained effects of integrated COPD management on health status and exercise capacity in primary care patients. Int. J. Chronic Obstr. Pulm. Dis. 2010;5:407–413. doi: 10.2147/COPD.S9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luk E., Hutchinson A., Tacey M., Irving L., Khan F. COPD: Health Care Utilisation Patterns with Different Disease Management Interventions. Lung. 2017;195:455–461. doi: 10.1007/s00408-017-0010-9. [DOI] [PubMed] [Google Scholar]
- 38.Pinnock H., Hanley J., McCloughan L., Todd A., Krishan A., Lewis S., Stoddart A., van del Pol M., MacNee A., Sheikh A., et al. Effectiveness of telemonitoring integrated into existing clinical services on hospital admission for exacerbation of chronic obstructive pulmonary disease: Researcher blind, multicentre, randomised controlled trial. BMJ. 2013;347:f6070. doi: 10.1136/bmj.f6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Titova E., Steinshamn S., Indredavik B., Henriksen A. Long term effects of an integrated care intervention on hospital utilization in patients with severe COPD: A single centre controlled study. Respir. Res. 2015;16:1–10. doi: 10.1186/s12931-015-0170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu C., Tan W., See R., Yu W., Kwek L., Toh M., Chee T., Chua S. A matched-group study protocol to evaluate the implementation of an Integrated Care Pathway programme for chronic obstructive pulmonary disease in Singapore. BMJ Open. 2015;5:e005655. doi: 10.1136/bmjopen-2014-005655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aponte J., Jackson T., Wyka K., Ikechi C. Health effectiveness of community health workers as a diabetes self-management intervention. Diabetes Vasc. Dis. Res. 2017;14:316–326. doi: 10.1177/1479164117696229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barcelo A., Cafiero E., de Boer M., Mesa A., Lopez M., Jimenez R., Esqueda A., Martinez J., Holgiun E., Meiners M., et al. Using collaborative learning to improve diabetes care and outcomes: The VIDA project. Prim. Care Diabetes. 2010;4:145–153. doi: 10.1016/j.pcd.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Beauregard M., Provost S., Pineault R., Grimard D., Perez J., Fournier M. Effects on patients of variations in the implementation of a cardiometabolic risk intervention program in Montréal. Health Promot. Chronic Dis. Prev. Can. Res. 2018;2:64–77. doi: 10.24095/hpcdp.38.2.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benedict A., Spence M., Sie J., Chin H., Ngo C., Salmingo J., Vidaurreta A., Rashid N. Evaluation of a Pharmacist-Managed Diabetes Program in a Primary Care Setting Within an Integrated Health Care System. J. Manag. Care Spec. Pharm. 2018;24:114–122. doi: 10.18553/jmcp.2018.24.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen S., Hou X., Sun Y., Hu G., Zhou X., Xue H., Chen P., Wu J., Bao Y., Jia W. A seven-year study on an integrated hospital-community diabetes management program in Chinese patients with diabetes. Prim. Care Diabetes. 2018;12:231–237. doi: 10.1016/j.pcd.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 46.Chwastiak J., Jackson S., Russo J., Kiefer M., Belyeu B., Mertens K., Chew L., Lin E. A collaborative care team to integrate behavioral health care and treatment of poorly-controlled type 2 diabetes in an urban safety net primary care clinic. Gen. Hosp. Psychiatry. 2017;44:10–15. doi: 10.1016/j.genhosppsych.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Ciccone M., Aquilino A., Cortese F., Scicchitano P., Sassara P., Mola E., Rollo R., Caldarola P., Giorgino F., Pomo V., et al. Feasibility and effectiveness of a disease and care management model in the primary health care system for patients with heart failure and diabetes (Project Leonardo) Vasc. Health Risk Manag. 2010;6:297–305. doi: 10.2147/VHRM.S9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.da Silva Marinhoa M., Fontbonneb A., Mary J., Barbosaa V., de Melo Rodriguesc H., de Carvalhoa E., de Souza W.V., Cesse E.A. The impact of an intervention to improve diabetes management in primary healthcare professionals’ practices in Brazil. Prim. Care Diabetes. 2017;11:538–545. doi: 10.1016/j.pcd.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Fottrell E., Jennings H., Kuddus A., Ahmed N., Morrison J., Akter K., Shaha S., Nahar B., Nahar T., Haghparast-Bidgoli H., et al. The effect of community groups and mobile phone messages on the prevention and control of diabetes in rural Bangladesh: Study protocol for a three-arm cluster randomised controlled trial. Trials. 2016;17:1–15. doi: 10.1186/s13063-016-1738-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gucciardi E., Fortugno M., Horodezny S., Lou W., Sidani S., Espin S., Webster F., Shah B. Will Mobile Diabetes Education Teams (MDETs) in primary care improve patient care processes and health outcomes? Study pprotocol for a randomized controlled trial. Trials. 2012;13:1–10. doi: 10.1186/1745-6215-13-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harris M., Breaves F., Patterson S., Jones J., Pappas Y., Majeed A., Car J. The North West London Integrated Care Pilot Innovative Srategies to Improve Care Coordination for Older Adults and People With Diabetes. J. Ambul. Care Manag. 2012;35:216–225. doi: 10.1097/JAC.0b013e31824d15c7. [DOI] [PubMed] [Google Scholar]
- 52.Huckfeldt P., Meeker D., Peters A., Guterman J., Diaz G., Goldman D. Diabetes Management for Low-Income Patients In Los Angeles: Two Strategies Improved Disease Control In The Short Term. Health Aff. 2012;31:168–176. doi: 10.1377/hlthaff.2011.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huque R., Nasreen S., Ahmed F., Hicks J., Walley J., Newell J., Elsey H. Integrating a diabetes and hypertension case management package within primary health care: A mixed methods feasibility study in Bangladesh. BMC Health Serv. Res. 2018;18:1–10. doi: 10.1186/s12913-018-3601-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Husted G., Thorsteinsson B., Esbensen B., Gluud C., Winkel P., Hommel E., Elsey H. Effect of guided self-determination youth intervention integrated into outpatient visits versus treatment as usual on glycemic control and life skills: A randomized clinical trial in adolescents with type 1 diabetes. Trials. 2014;15:1–12. doi: 10.1186/1745-6215-15-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jansink R., Braspenning J., Keizer E., Van der Weijden T., Elwyn G., Grol R. No identifiable Hb1Ac or lifestyle chang e after a comprehensive diabetes programme including motivational interviewing: A cluster randomised trial. Scand. J. Prim. Health. 2013;31:119–127. doi: 10.3109/02813432.2013.797178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Katz I., Pirabhahar S., Williamson P., Raghunath V., Brennan F., O'Sullivan A., Youssef G., Lane C., Jacobson G., Feldman P., et al. Connect CKD-virtual medical consulting: A web-based chronic kidney disease, hypertension and diabetes integrated care program. Nephrology. 2018;23:646–652. doi: 10.1111/nep.13070. [DOI] [PubMed] [Google Scholar]
- 57.Kornelius E., Chiou J., Yang Y., Lu Y., Peng C., Huang C. The Diabetes Shared Care Program and Risks of Cardiovascular Events in Type 2 Diabetes. Am. J. Med. 2015;128:977–985. doi: 10.1016/j.amjmed.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 58.Ku G., Kegels G. Integrating chronic care with primary care activities: Enriching healthcare staff knowledge and skills and improving glycemic control of a cohort of people with diabetes through the First Line Diabetes Care Project in the Philippines. Glob. Health Action. 2014;7:25286. doi: 10.3402/gha.v7.25286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Labhardt N., Balo J., Ndam M. Task shifting to non-physician clinicians for integrated management of hypertension and diabetes in rural Cameroon: A programme assessment at two years. BMC Health Serv. Res. 2010;10:1–10. doi: 10.1186/1472-6963-10-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liddy C., Johnston S., Nash K., Ward N., Irving H. Health coaching in primary care: A feasibility model for diabetes care. BMC Prim. Care. 2014;15:1–8. doi: 10.1186/1471-2296-15-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lien A., Jiang Y., Mou C., Sun M., Gau B., Yen H. Integrative traditional Chinese medicine therapy reduces the risk of diabetic ketoacidosis in patients with type 1 diabetes mellitus. J. Ethnopharmacol. 2016;191:324–330. doi: 10.1016/j.jep.2016.06.051. [DOI] [PubMed] [Google Scholar]
- 62.MacRury S., Stephen K., Main F., Gorman J., Jones S., Macfarlane D. Reducing Amputations in People with Diabetes (RAPID): Evaluation of a New Care Pathway. Int. J. Environ. Res. Public Health. 2018;15:999. doi: 10.3390/ijerph15050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newlyn N., MaGrath R., Fulcher G. Evaluation of the performance and outcomes for the first year of a diabetes rapid access clinic. Med. J. Aust. 2016;205:172. doi: 10.5694/mja16.00460. [DOI] [PubMed] [Google Scholar]
- 64.Salant T., Slavin S., Baumrin E., Bordeu M., Rowley M., Brackett E., Severin P., Behforouz H. Lessons in Translation Insights From a Collaboration Integrating Community Health Workers Into Diabetes Care. J. Ambul. Care Manag. 2013;36:156–165. doi: 10.1097/JAC.0b013e31827fb325. [DOI] [PubMed] [Google Scholar]
- 65.Seidu S., Bodicoat D., Davies M., Daly H., Stribling B., Farooqi A., Brady E., Khunti K. Evaluating the impact of an enhanced primary care diabetes service on diabetes outcomes: A before–after study. Prim. Care Diabetes. 2017;11:171–177. doi: 10.1016/j.pcd.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 66.Turnacilar M., Sancar M., Apikoglu-Rabus S., Hursitoglu M., Izzettin F. Improvement of diabetes indices of care by a short pharmaceutical care program. Pharm. World Sci. 2009;31:689–695. doi: 10.1007/s11096-009-9333-9. [DOI] [PubMed] [Google Scholar]
- 67.Rümenapf G., Geiger S., Schneider B., Amendt K., Wilhelm N., Morbach S., Nagel N. Readmissions of patients with diabetes mellitus and foot ulcers after infra-popliteal bypass surgery–attacking the problem by an integrated case management model. Vasa. 2013;42:56–57. doi: 10.1024/0301-1526/a000235. [DOI] [PubMed] [Google Scholar]
- 68.Vermunt P., Milder I., Wielaard F., Baan C., Schelfhout J., Westert G., van Oers H. Implementation of a lifestyle intervention for type 2 diabetes prevention in Dutch primary care: Opportunities for intervention delivery. BMC Fam. Pract. 2012;13:1–10. doi: 10.1186/1471-2296-13-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walsh J., Harris B., Roberts A. Evaluation of a community diabetes initiative: Integrating diabetes. Prim. Care Diabetes. 2015;9:203–210. doi: 10.1016/j.pcd.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 70.Webb E., Rheeder P. A cluster-randomized trial to estimate the effect of mobile screening and treatment feedback on HbA1c and diabetes-related complications in Tshwane primary health care clinics, South Africa. Prim. Care Diabetes. 2017;11:546–554. doi: 10.1016/j.pcd.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 71.Yang G., Yuan S., Fu H., Wan G., Zhu L., Yuan M., Lv Y., Zhang J., Du X., Li Y., et al. Influence of educational attainments on long term glucose control and morbid events in patients with type 2 diabetes receiving integrated care from 15 China urban communities: The Beijing Community Diabetes Study 11. Prim. Care Diabetes. 2015;9:473–481. doi: 10.1016/j.pcd.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 72.Zhang J., Burridge L., Baxter K., Donald M., Foster M., Hollingworth S., Ware R., Russell A., Lackson C. A new model of integrated p rimary-secondary care for complex diabetes in the community: Study protocol for a randomised controlled trial. Trials. 2013;14:1–9. doi: 10.1186/1745-6215-14-382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bennett W., Gadzune K., Appel L., Clark J. Insights from the POWER Practice-Based Weight Loss Trial: A Focus Group Study on the PCP’s Role in Weight Management. J. Gen. Intern. Med. 2013;29:50–58. doi: 10.1007/s11606-013-2562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brunisholz K., Joy E., Hashibe M., Gren L., Savitz L., Hamilton S., Cannon W., Huynh K., Schafer T., Newman L., et al. Stepping Back to Move Forward: Evaluating the Effectiveness of a Diabetes Prevention Program Within a Large Integrated Healthcare Delivery System. J. Healthc. Qual. 2017;39:278–293. doi: 10.1097/JHQ.0000000000000103. [DOI] [PubMed] [Google Scholar]
- 75.Van Eeghen C., Littenberg B., Kessler R. Chronic care coordination by integrating care through a team-based, population-driven approach: A case study. Transl. Behav. Med. 2018;8:468–480. doi: 10.1093/tbm/ibx073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gross S., Augustyn M., Henderson J., Baig K., Williams C., Ajao B., Bell-Waddy P., Paige D. Integrating Obstetrical Care and WIC Nutritional Services to Address Maternal Obesity and Postpartum Weight Retention. Matern. Child. Health J. 2018;22:794–802. doi: 10.1007/s10995-018-2449-6. [DOI] [PubMed] [Google Scholar]
- 77.Hoffman J., Frerichs L., Story M., Jones J., Gaskin K., Apple A., Skinner A., Armstrong S. An Integrated Clinic-Community Partnership for Child Obesity Treatment: A Randomized Pilot Trial. Pediatrics. 2018;141:1. doi: 10.1542/peds.2017-1444. [DOI] [PubMed] [Google Scholar]
- 78.Johnson M., Jastrzab R., Tate J., Johnson K., Hall-Lipsy E., Martin R., Taylor A., Warholak T. Evaluation of an Academic-Community Partnership to Implement MTM Services in Rural Communities to Improve Pharmaceutical Care for Patients with Diabetes and/or Hypertension. J. Manag. Care Spec. Pharm. 2018;24:132–141. doi: 10.18553/jmcp.2018.24.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prestes M., Gayarre M., Elgart J., Gonzalez L., Rucci E., Gagliardino J. Multistrategic approach to improve quality of care of people with diabetes at the primary care level: Study design and baseline data. Prim. Care Diabetes. 2017;11:193–200. doi: 10.1016/j.pcd.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 80.Wake M., Lycett K., Sabin M., Gunn J., Gibbons K., Hutton C., McCallum Z., York E., Stringer M., Wittert G. A shared-care model of obesity treatment for 3–10 year old children: Protocol for the HopSCOTCH randomised controlled trial. BMC Pediatr. 2012;12:1–9. doi: 10.1186/1471-2431-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Angstman K., Doganer Y., Dejesus R., Rohrer J. Increased medical cost metrics for patients 50 years of age and older in the collaborate care model of treatment for depression. Psychogeriatrics. 2016;16:102–106. doi: 10.1111/psyg.12129. [DOI] [PubMed] [Google Scholar]
- 82.Unützer J., Chan Y., Hafer E., Knaster J., Shields A., Powers D., Veith R. Quality Improvement with Pay-for-Performance Incentives in In tegrated Behavioral Health Care. Am. J. Public Health. 2012;102:e41–e45. doi: 10.2105/AJPH.2011.300555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hepner K., Hunter S., Paddock S., Zhou A., Watkins K. Training Addiction Counselors to Implement CBT for Depression. Adm. Policy Ment. Health Ment. Health Serv. Res. 2011;38:313–323. doi: 10.1007/s10488-011-0359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Murphy J., Goldsmith C., Jones W., Oanh P., Nguyen V. The effectiveness of a Supported Self-management task-shifting intervention for adult depression in Vietnam communities: Study protocol for a randomized controlled trial. Trials. 2017;18:1–12. doi: 10.1186/s13063-017-1924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poulsen R., Hoff A., Fisker J., Hjorthøj C., Eplov L. Integrated mental health care and vocational rehabilitation to improve retu rn to work rates for people on sick leave because of depression and anxiety (the Danish IBB IS trial): Study protocol for a randomized controlled trial. Trials. 2017;18:1–15. doi: 10.1186/s13063-017-2272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Salisbury C., O’Cathain A., Edwards L., Thomas C., Gaunt D., Hollinghurst S., Nicholl J., Large S., Yardley L., Lewis G., et al. Effectiveness of an integrated telehealth service for patients with depression: A pragmatic randomised controlled trial of a complex intervention. Lancet Psychiatry. 2016;3:515–525. doi: 10.1016/S2215-0366(16)00083-3. [DOI] [PubMed] [Google Scholar]
- 87.Sanchez K., Eghaneyan B., Killian M., Cabassa L., Trivedi M. Measurement, Education and Tracking in Integrated Care (METRIC): Use of a culturally adapted education tool versus standard education to increase engagement in depression treatment among Hispanic patients: Study protocol for a randomized control trial. Trials. 2017;18:1–13. doi: 10.1186/s13063-017-2109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Von Korff M., Katon W., Lin E., Ciechanowski P., Peterson D., Ludman E., Young B., Rutter C. Functional outcomes of multi-condition collaborative care and successful ageing: Results of randomised trial. BMJ. 2014;343 doi: 10.1136/bmj.d6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wagner G., Ngo V., Glick P., Obuku E., Musisi S., Akena D. INtegration of DEPression Treatment into HIV Care i n Uganda (INDEPTH-U ganda): Study protocol for a randomized controlled trial. Trials. 2014;15:343. doi: 10.1186/1745-6215-15-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wu B., Haomiao J., Vidyanti I., Lee P., Eli K., Wu S. Collaborative Depression Care Among Latino Patients in Diabetes Disease Management, Los Angeles, 2011–2013. Prev. Chronic Dis. 2014;11:E148. doi: 10.5888/pcd11.140081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Porter M. Value-based health care delivery. Ann. Surg. 2008;248:503–509. doi: 10.1097/SLA.0b013e31818a43af. [DOI] [PubMed] [Google Scholar]
- 92.Morris M., Atkinson V., Woods J., Myles P., Hodge A., Jones C., Lloyd D., Rovtar V., Clifford A., Brusco N. Patient Judgement of Change with Elective Surgery Correlates with Patient Reported Outcomes and Quality of Life. Healthcare. 2022;10:999. doi: 10.3390/healthcare10060999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gentry S., Badrinath P. Defining Health in the Era of Value-based Care: Lessons from England of Relevance to Other Health Systems. Cureus. 2017;9:e1079. doi: 10.7759/cureus.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zipfel N., van der Nat P., Rensing B., Daeter E., Westert G., Groenewoud A. The implementation of change model adds value to value-based healthcare: A qualitative study. BMC Health Serv. Res. 2019;19:1–12. doi: 10.1186/s12913-019-4498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baxter S., Johnson M., Chambers D., Sutton A., Goyder E., Booth A. The effects of integrated care: A systematic review of UK and international evidence. BMC Health Serv. Res. 2018;18:1–13. doi: 10.1186/s12913-018-3161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Flanagan S., Damery S., Combes G. The effectiveness of integrated care interventions in improving patient quality of life (QoL) for patients with chronic conditions. An overview of the systematic review evidence. Health Qual. Life Outcomes. 2017;15:1–11. doi: 10.1186/s12955-017-0765-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bandurska E., Damps-Konstańska I., Popowski P., Jedrzejczyk T., Janowiak P., Swietnicka K., Zarzeczna-Baran M., Jassem E. Cost-Effectiveness Analysis of Integrated Care in Management of Advanced Chronic Obstructive Pulmonary Disease (COPD) Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019;25:2879–2885. doi: 10.12659/MSM.913358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lavallee D., Chenok K., Love R., Petersen C., Holve E., Segal C., Franklin P. Incorporating Patient-Reported Outcomes into Health Care To Engage Patients And Enhance Care. Health Aff. 2016;35:575–582. doi: 10.1377/hlthaff.2015.1362. [DOI] [PubMed] [Google Scholar]
- 99.De Faoite D. The advantages of electronic patient-reported measures and an example digital platform to collect ePROs after total knee arthroplasty. Med. Access Point Care. 2018;2 doi: 10.1177/2399202618813463. [DOI] [Google Scholar]
- 100.OECD Putting People at the Centre of Health Care. PaRIS Survey of Patients with Chronic Conditions. 2019. [(accessed on 20 April 2022)]. Available online: https://www.oecd.org/health/health-systems/PaRIS-survey-Patients-with-Chronic-Conditions-June-2019.pdf.
- 101.Miedany Y. Adopting patient-centered care in standard practice: PROMs moving toward disease-specific era. Clin. Exp. Rheumat. 2014;32:S40–S46. [PubMed] [Google Scholar]
- 102.Coelho A., de Bienassis K., Klazinga N., Santo S., Frade P., Costa A., Gaspar T. Mental Health Patient-Reported Outcomes and Experiences Assessment in Portugal. Int. J. Env. Res. Public Health. 2022;19:11153. doi: 10.3390/ijerph191811153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hodson M., Andrew S., Roberts C. Towards an understanding of PREMs and PROMs in COPD. Breathe. 2013;9:358–364. doi: 10.1183/20734735.006813. [DOI] [Google Scholar]
- 104.Jayakumar P., Phil D., Teunis T., Vranceanu A., Lamb S., Ring D., Gwilym S. Relationship Between Magnitude of Limitations and Patient Experience During Recovery from Upper-Extremity Fracture. JB JS Open Access. 2019;4:1–7. doi: 10.2106/JBJS.OA.19.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Black N., Varaganum M., Hutchings A. Relationship between patient reported experience (PREMs) and patient reported outcomes (PROMs) in elective surgery. BMJ Qual. Saf. 2014;23:534–542. doi: 10.1136/bmjqs-2013-002707. [DOI] [PubMed] [Google Scholar]
- 106.Kingsley C., Patel S. Patient-reported outcome measures and patient-reported experience measures. BJA Educ. 2017;17:137–144. doi: 10.1093/bjaed/mkw060. [DOI] [Google Scholar]