Abstract
Introduction
Brexanolone demonstrates short-term efficacy for the treatment of postpartum depression (PPD). Postpartum depression is linked to infanticide and maternal suicide, and current treatment often fails to adequately control depressive symptoms. The purpose of this analysis is to further understand the experience(s) of women who have received brexanolone for the treatment of PPD.
Methods
Semistructured interviews modeled after the theory of planned behavior (TPB) were conducted to assess women's perceptions of treatment for PPD with brexanolone. Women who received treatment with brexanolone at this inpatient facility were eligible to participate in this study. The TPB is often used to predict intention to perform health-related behaviors. Semistructured interviews were recorded and transcribed, and thematic analysis was conducted to identify common ideas across all interviews. Follow-up assessment of depressive and anxious symptoms was also conducted using the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder-7 (GAD-7), respectively.
Results
Five of the 10 women who received treatment with brexanolone at this facility were interviewed, and common themes related to the TPB were analyzed. Attitudes toward brexanolone were favorable, and having a strong support system was a motivating factor in receiving treatment for PPD. Insurance approval, need for childcare, and poor understanding of symptoms of PPD were barriers to receiving treatment with brexanolone. Symptoms of depression and anxiety were rated as low at the time of the follow-up interview as measured by the PHQ-9 (mean 1.6, range 1 to 3) and GAD-7 (mean 2.8, range 2 to 4), respectively.
Discussion
Brexanolone rapidly and sustainably reduced symptoms of PPD and was well-received by patients. Despite significant barriers to use, women who received treatment with brexanolone advocated for its availability as well as increased awareness of PPD.
Keywords: brexanolone, postpartum depression, suicide, antidepressants, theory of planned behavior
Introduction
Postpartum depression (PPD) affects anywhere from 10% to 30% of mothers,1 most commonly beginning in the last 2 trimesters of pregnancy.2 Nearly half of women experience their first episode of depression during the postpartum period,3 and untreated PPD increases the risk of depression sixfold later in life.4 To date, first-line treatment options include antidepressants, which often fail to address the acuity of PPD, taking 4 to 6 weeks for maximum efficacy.5 PPD is a strong predictor of suicidality,6 and 1 study7 found that women who experienced either a minor or major episode of depression during pregnancy had a 18.4% to 30.6% prevalence of suicidal ideation postpartum. Women who experience suicidal ideation postpartum have a poorer response to their infants' cues,8 and maternal depression is associated with a variety of adverse child development outcomes.9 Therefore, rapid elimination of depressive symptoms postpartum could have a protective influence on child development.
In March 2019, the FDA approved the first medication solely for the treatment of PPD: brexanolone. Brexanolone is an aqueous form of allopregnanolone and works to improve depressive symptoms after childbirth by modulating neuronal excitability through positive allosteric modulation on the GABA type-A receptor.10 This novel mechanism of action addresses the rapid decrease in allopregnanolone that occurs immediately after childbirth.11 An increased level of allopregnanolone is thought to protect against the development of depressive symptoms during pregnancy although the etiology of PPD remains unknown.12 However, due to poor oral bioavailability, brexanolone requires intravenous infusion over 60 hours. Further, brexanolone carries a warning for excessive sedation that can result in loss of consciousness and is classified as a schedule IV controlled substance with an FDA mandated Risk Evaluation and Mitigation Strategy.13
These factors prevent widespread use of brexanolone for PPD despite studies that demonstrate a rapid improvement in depressive symptoms. A meta-analysis14 of 3 randomized controlled trials (n = 267) concludes that brexanolone is significantly superior to placebo in reducing depressive symptoms at 72 hours postinfusion in women with moderate-to-severe PPD with a number needed to treat for remission of 3 to 8. Brexanolone is well-tolerated with rates of adverse drug reactions and discontinuation due to intolerability not statistically significantly different between brexanolone and placebo.
Currently, there is very limited real-world data exploring the use of brexanolone outside of clinical trials. A recent study by Patterson et al15 followed 16 women who received brexanolone and projected their Hamilton Depression Rating Scale score 90 days postinfusion. The results of their study demonstrate a dramatic decrease in depressive symptoms following the 60-hour infusion, which was maintained at follow-up visits.15 The authors highlight significant challenges and barriers faced in administering brexanolone treatment, including the need for prior authorization, a medically supervised setting, and overall costs involved.
The aim of this study is to identify motivating factors and barriers toward receiving treatment with brexanolone by applying behavior change theory and qualitative methodology. This approach nicely complements quantitative measures and reveals insights into the subjective experience of individuals who receive novel treatments.16
Methods
Design and Participants
This was a prospective, explorative, qualitative study consisting of semistructured interviews with patients with PPD who received treatment with brexanolone. Our institution, an acute care hospital located in New Jersey, began offering treatment with brexanolone at the end of 2019. Treatments were halted in March 2020 due to the COVID-19 pandemic. All patients who received treatment with brexanolone at this inpatient facility and were under the care of the hospital's Center for Perinatal Mood and Anxiety Disorders (PMAD) program were eligible for this study. This program provides support from a multidisciplinary team of experts in psychiatry, nursing, and social work. Patients have access to individual and group counseling, mother–infant bonding sessions, and medication management.
Recruitment
Prior to the interview, eligible patients received a letter in the mail detailing the study objectives and procedures with the opportunity to opt out via telephone call. If the participant did not decline to participate, a member of the research team contacted the participant by phone to schedule a video interview. Before proceeding, study details were further explained, and informed consent was obtained. Interviews were conducted virtually and were approximately 50 to 75 minutes in length. Additionally, patients received a $20 gift card for their participation. Demographic characteristics, past medical history, and baseline Edinburgh Postnatal Depression Scale (EPDS) score assessed at the time of brexanolone infusion were obtained via retrospective review of the patients' charts. This study was approved by the institution's institutional review board and was funded by the College of Psychiatric and Neurologic Pharmacists Foundation Defining the Future Research Grant.
Interview Structure
All interviews began by asking the participant to describe their symptoms of PPD and what influenced them to receive brexanolone. All interviews were conducted by the same member of the research team who was not involved in making treatment decisions.
Interview questions were framed by constructs of the theory of planned behavior (TPB).17 The TPB is used to measure intention to perform a health behavior based on behavioral beliefs, social norms, and perceived behavioral control. The TPB is frequently utilized to measure intention to perform a health behavior during the peripartum period.18-20 Participants were asked to describe what influenced them to manage their depression symptoms with brexanolone (Figure). Interview questions were deliberately open-ended, and probes were used when additional information was required (Table 1). Participants were encouraged to share their experiences with PPD, previous treatments for depression, involvement in group therapy, and overall interaction with the healthcare system in the perinatal period. Changes in health and daily functioning were volunteered by participants. At the end of each interview, the interviewer conducted a screening for current symptoms of depression and anxiety using the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder-7 (GAD-7).
FIGURE.
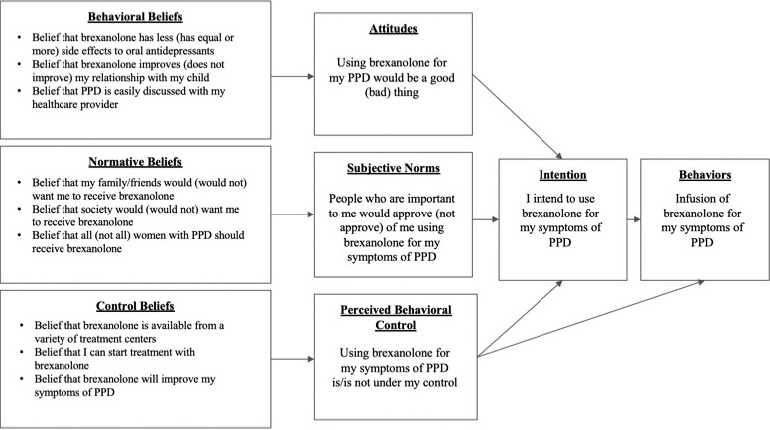
Theory of planned behavior model (PPD = postpartum depression)
TABLE 1.
Interviewer guide for postpartum depression (PPD)
| Interviewer guide based on the theory of planned behavior | |
| Construct | Open-ended probes from interviewer guide |
| Behavioral beliefs | 1. What do you believe are the advantages or disadvantages of receiving treatment with brexanolone? |
| 2. How comfortable are you in talking to your healthcare provider about postpartum depression? | |
| 3. How important do you believe brexanolone was in treating your PPD? | |
| 4. Can you describe how treatment with brexanolone impacted your relationship with your child? | |
| Normative beliefs | 1. What people in your life approved of you receiving treatment with brexanolone? |
| 2. What people in your life disapproved of you receiving treatment with brexanolone? | |
| 3. What individual would benefit most from receiving brexanolone? | |
| Control beliefs | 1. What circumstances enabled you to receive treatment with brexanolone? |
| 2. What circumstances made it difficult to receive treatment with brexanolone? | |
| 3. How confident were you that brexanolone would improve your PPD symptoms? | |
Data Analysis
Semistructured interviews were videorecorded on a HIPAA-compliant platform, and no personal identifiers were linked with the recordings. Interviews were then transcribed using NVivo 12 qualitative analysis software to perform thematic analysis as described by Braun and Clarke.21,22 Thematic analysis is a method for identifying and reporting patterns within a text. As the interview questions were derived from the TPB, a deductive analysis strategy was followed. First, the interview transcripts were read multiple times by the researcher who conducted the interviews for familiarity. Next, initial codes were generated by separating meaningful statements into groups. These codes were then visually represented with a thematic map and applied to the theoretical framework. The member of the research team who conducted the interviews developed preliminary themes. For interpretative rigor and credibility, interview transcripts were then shared with another member of the research team who developed another list of potential themes. To establish internal validity, preliminary themes were discussed among the authors until all agreed that the final themes accurately represented the data. Quotes included in the current paper were selected by the research team as a best representation of themes that emerged from the interviews. Descriptive statistics are reported when appropriate.
Results
Of the 10 women who received treatment with brexanolone at this institution, 5 women agreed to a follow-up interview. Interviews were conducted 16 to 20 months postinfusion. Demographic information is outlined in Table 2. The mean age of patients who participated in the interviews was 36.6 years (range 31 to 49), and average body mass index was 27.2 (SD 6.7). The average amount of time between childbirth and brexanolone infusion was 20.4 weeks (SD 4.1 weeks). All patients were concurrently taking medications to treat anxiety and/or depression at the time of brexanolone administration (SSRIs, SNRIs). Although all patients had been treated with benzodiazepines prior to brexanolone treatment, benzodiazepines were not coadministered with brexanolone. Cooccurring medical diagnoses included hypothyroidism, gestational hypertension, and preeclampsia. All women interviewed scored <13 on the EPDS scale immediately following the 60-hour infusion, indicating none or minimal symptoms of depression. Notably, all women interviewed had GAD-7 and PHQ-9 scores indicating an absence of anxiety and depression at the time of the follow-up interview (Table 3), and none reported symptomatic relapse prior to the interview. The women reported mild sedation related to brexanolone, but none experienced loss of consciousness or any infusion-related reactions.
TABLE 2.
Baseline demographics and characteristics of the study
| Subject |
Age (mean = 36.6) |
Race |
BMI (mean = 27.2) |
Medical History |
Concurrent Treatment for Depression/Anxiety |
Obstetric History |
Time Until Brexanolone Treatment, Weeks Postpartum (mean = 20.4) |
| 1 | 31 | White | 22.3 | Gestational hypertension, preterm labor, gestational thrombocytopenia | Clonazepam | G3P1 | 20 |
| 2 | 33 | White | 26.4 | Hypothyroidism | Sertraline, Clonazepam | G2P1 | 16 |
| 3 | 34 | White | 20 | Preeclampsia, scoliosis | Sertraline, Clonazepam | G2P2 | 26 |
| 4 | 36 | White | 39.2 | MDD, gestational hypertension | Venlafaxine XR, Alprazolam | G1P1 | 24 |
| 5 | 49 | White | 28.1 | Infertility, hypertension | Sertraline, Clonazepam | G5P2 | 16 |
TABLE 3.
Baseline and follow-up clinical assessment
| Subject |
Brexanolone Treatment Period (72 h) |
Day of Qualitative Interview Depression and Anxiety Score |
||
| Preinfusion EPDSa (20.4 ± 5.6) |
Postinfusion EPDSa (2.8 ± 3.6) |
GAD-7b (2.8 ± 0.8) |
PHQ-9c (1.6 ± 0.9) |
|
| 1 | 22 | 9 | 2 | 2 |
| 2 | 15 | 0 | 2 | 1 |
| 3 | 26 | 2 | 3 | 1 |
| 4 | 25 | 1 | 3 | 3 |
| 5 | 14 | 2 | 4 | 1 |
EPDS = Edinburgh Postnatal Depression Scale; GAD-7 = Generalized Anxiety Disorder-7; HQ-9 = Patient Health Questionnaire-9.
Range 0 to 30; none or minimal depression (0 to 6), mild depression (7 to 13), moderate depression (14 to 19), severe depression (19 to 30). Reduction of EPDS score by 4 points demonstrates a clinically significant improvement.
Range 0 to 27; none or minimal depression (0 to 4), mild depression (5 to 9), moderately severe depression (15 to 19), severe depression (20 to 27).
Range 0 to 21; minimal anxiety (0 to 4), mild anxiety (5 to 9), moderate anxiety (10 to 14), severe anxiety (15 to 21).
Overall, participants were grateful to have received treatment with brexanolone but acknowledged that significant barriers exist to accessing treatment for PPD.
More detailed findings from each of the TPB constructs are described below.
Behavioral Beliefs Toward Brexanolone
Rapid-Acting Agent
The women interviewed experienced a rapid improvement in depressive symptoms after treatment with brexanolone. One woman further described the importance of this rapid response as an antisuicidal agent compared with conventional antidepressants:
You know, that takes time. And when it comes to a situation like…postpartum depression, postpartum psychosis, whatever it may be, time is very critical…that long period of waiting to see if this is going to work…4 to 6 weeks…just the mom that gets a little too fed up, that just loses that little glimmer of hope. And, you know, then they're gone forever.
Alternative to Available Antidepressants
Most women expressed concerns related to traditional antidepressant side effects, including increased anxiety and gastrointestinal issues:
Standard antidepressants tend to come with a plethora of side effects from weight gain, irritability, increased anxiety…you can have increased suicidal thoughts or actions, all of those things…and you have to find the right dosage.
Every woman interviewed felt she had tried all other available treatments unsuccessfully; thus, having brexanolone as another option was meaningful: “I had hope. I actually had hope at the opportunity of doing this.” One woman said this in describing the shortcomings of her oral antidepressant treatment:
I was feeling more like me…having much better moments throughout the day compared to the difficult ones. But I still I had a great fear of being alone with my baby because it was just like I was still trying to avoid this scary thought. And even though I was learning so much in therapy, it was still so hard to use those coping skills.
Stigma
Most women were apprehensive to discuss their symptoms of depression with their medical providers. They were concerned that they would lose custody of their children or be admitted to a psychiatric hospital:
I had had that intrusive thought toward my baby…I was so scared, like I can't admit this to him…people are so afraid of like, what are they gonna do to me? What are they gonna do to my children?
A Tool in Depression Treatment
Women reported that brexanolone helped them return to their usual selves. As they reported continuous improvements in the days following brexanolone, all women stressed the importance of the nonpharmacologic treatment options combined with brexanolone:
I feel what brexanolone did for me personally was it allowed me to access all of these coping strategies that I had been taught for 2 months and use them. It completely lowered the volume on these intrusive thoughts.
Improved Relationships
Brexanolone had a positive influence on the mother–child relationship, particularly in allowing the mother to feel present in the moment with her child. Several women reported that when their child was born, they did not feel a strong emotional connection to their baby. After treatment with brexanolone, 1 woman described a drastic change in feeling toward her child: “The second day when I woke up, the curtains were open. And the only thing that I wanted was my daughter.”
Normative Beliefs
Initial Apprehension From Family Members
Despite relatives providing support and childcare, several women spoke of initial disapproval from family members regarding seeking treatment for PPD:
She kind of has that old school mentality of you know, you don't need antidepressants, you're fine. Go take a walk, go exercise and you'll feel fine…you need more vegetables, and you'll feel fine.
Comradery From Women With Lived Experience
Support from other women suffering from PPD was meaningful in the improvement of depressive symptoms:
They also had group therapy there, which was so lifesaving to be in a room with other women so vulnerable and talking about these things and then having others be like, I'm having scary thoughts too or I'm experiencing this horrible rage or whatever. And then it just kind of like opens up the doors for people to be very vulnerable and honest and you feel less alone.
Women who had benefited from brexanolone encouraged others to go forward with the treatment:
I had met a mom at the group, and she had gotten this treatment. So, I became close with her, and we were talking about it. And she had such a great experience. Our stories were very similar in the sense that, like, her intrusive thoughts…although it was different than mine…was her worst symptom as well...and she said that it helped so much with that.
Need for Increased Availability
When asked to describe an individual who would benefit most from brexanolone, all participants suggested that brexanolone should be available as an option for everyone. However, they indicated that women with severe suicidality and an intolerance to oral antidepressants would be the best candidates to receive brexanolone:
So, it's taking that person who is like, oh my God, I'm going to kill myself and giving them the treatment and then providing them the tools afterwards. But it's the others that …I don't want to tell people to wait.
Perceived Behavioral Control
Access Difficulties
Participants reported that the time for insurance approval, cost, and the required hospital stay were barriers to receiving brexanolone. Participants also felt that the need for childcare during admission could be a barrier to receiving brexanolone, and 1 recommended that the hospital provide childcare services during their admission. Although the 3-day hospitalization created inconveniences in childcare, most women viewed the treatment as a chance to rest, “I looked at it as a retreat that might be a miracle.” During the infusion, women were allowed to have visitors if they wished. Additionally, 1 of the women interviewed expressed her frustration with the current approval criteria to receive brexanolone:
The biggest obstacle to get through is that they don't really like you to have the treatment past 6 months postpartum when postpartum depression can go on for years and years…to have a woman, have a mother, have to suffer for it.
Factors that facilitated treatment acceptance with brexanolone were the advocacy and efforts of the perinatal healthcare team combined with encouragement from other women who had received treatment with brexanolone:
But she said she was like; it works like you just have to trust me…like this is going to work and you are going to feel like the blinds are open.
Women also mentioned the importance of having a supportive partner during the 3-day infusion, acknowledging that not all women are fortunate to have assistance with childcare during the required hospital stay:
Being a single mom, I am the sole provider… If I'm not there, and I can't really count on my family, how am I gonna do this…in my situation I ended up having to hire a sitter for 3 or 4 days…she brought my son to the hospital so he could visit, which was great.
Poor Symptom Literacy
Several women were unsure that the symptoms they were experiencing were symptoms of PPD, and all women expressed dissatisfaction with current PPD screening measures:
I truly believe that if I was educated about these mood disorders prior to having my babies…and I knew the facts and I knew that these things called intrusive thoughts were a very common symptom…I would have been able to acknowledge that that is the scary thought that people refer to…and I would have gotten help.
We're handed a piece of paper after we have a baby…call if any of these arise…we fill out the Edinburgh scale…that Edinburgh does not speak to a postpartum mom, it can be so triggering for a mom…I remember throwing that out.
Low Expectations
Confidence that brexanolone was going to improve depressive symptoms prior to initiating treatment was generally low across the group. Several women stated that they went into the treatment with nothing to lose, and in the worst case scenario they would spend 3 days in the hospital being cared for:
I don't think I had all my eggs in that basket to begin with, though, like, I was kind of like…Alright, let's see. I wasn't convinced, but I wasn't thinking like it was all hocus pocus either
Discussion and Limitations
Conceptualized using the TPB, this study helped gain an understanding of the motivating factors and barriers faced by women with PPD seeking treatment with brexanolone. Overall, patients reported positive attitudes toward treating their depression with brexanolone. Motivating factors associated with receiving treatment with brexanolone were a strong sense of support from family and other women with lived experience and its rapid onset of action. Barriers to receiving treatment with brexanolone were predominantly logistical, financial, and insurance-related. However, limited knowledge of PPD further delayed treatment.
Women who participated in this study noted a marked improvement soon after receiving treatment with brexanolone, contrary to treatment with oral antidepressants. Even the possibility that brexanolone could help to improve symptoms of PPD was encouraging despite low confidence among the group. Along with having another pharmacologic option for their depression, all women interviewed praised the nonpharmacologic treatment services that they were provided at the PMAD clinic.
An interesting finding that expands on prior research and deserves further exploration is the positive change in relationship between mother and child after treatment with brexanolone. A strong bond between mother and baby is not only important for emotional development, but also the infant's physical health. A systematic review23 assessed the impact of PPD on maternal and infant outcomes and identified a reduction of infant quality of life and even a threefold increased risk of mortality in infants up to 6 months of age. Brexanolone's ability to reverse depression symptoms quickly after childbirth and maintain remission for an extended period of time may protect against negative consequences in childhood development.
Our study highlights the limitations with current screening methods, including worsening stigma and an inability to recognize the signs and symptoms of PPD.24 A report25 issued by the CDC identified that 1 in 5 women are not asked about depression during a prenatal visit despite guidelines from the American College of Obstetricians and Gynecologists that recommend women be screened at least once for depressive symptoms during the perinatal period.26 Not only does PPD often go undiagnosed, women who are experiencing PPD are infrequently linked with the appropriate treatment. One study27 found that only 25% of positively screened women with PPD received treatment. Financial barriers are largely responsible for women not being able to access care for PPD, and this factor may be unmodifiable.28 However, another significant barrier to accessing treatment that can be addressed is a lack of awareness and education on PPD among patients and providers. Our findings highlight an opportunity for improving the way clinicians screen for PPD; perhaps by supplementing use of a questionnaire with resources that provide support and education on symptoms that women may encounter during the postpartum period.
Our study has several limitations that must be addressed. We recognize that our cohort is small and our participants' report as to the efficacy of brexanolone is subject to selection bias. All 10 women who received brexanolone were contacted, but only 5 women returned phone calls. Additionally, women from ethnic minority groups were not represented in our study, reducing the generalizability of our findings. The coronavirus pandemic limited treatment availability, further limiting our sample size. Despite limited participants, this study's qualitative methods modeled after a behavior change theory uncovered insights into the real-life experiences and feelings of patients. Understanding lived experience of individuals who receive novel treatments may be useful in future health system integration. PHQ-9 and GAD-7 scores were not available at baseline; however, EPDS scores were documented every 12 hours during the 60-hour brexanolone infusion. Last, the strong support from the PMAD clinic and the services provided may have had a significant influence on our participants' improvement.
Conclusion
This study demonstrated that women affected by PPD positively perceived brexanolone's ability to alleviate depressive symptoms and improve their relationship with their newborn child. Our results were consistent with previous studies and confirm that brexanolone adjunctive to traditional antidepressants is beneficial to women with severe PPD. Individuals' behavioral, normative, and control beliefs regarding treatment for PPD provided important information on what motivated them to pursue treatment with brexanolone. Our study may serve as a foundation for clinicians to utilize to motivate patients to accept treatment with brexanolone and successfully integrate it into health care systems. Future studies that address longitudinal outcomes of women who receive brexanolone are warranted as well as studies that assess maternal–infant bonding and child development. This information is important to health care providers working with women as they are positioned to educate them on PPD, improve their self-efficacy in finding care, and empower them to reduce stigma associated with PPD. Furthermore, brexanolone's efficacy appears to be sustained over a period of 16 to 20 months as evidenced by low depression and anxiety symptom scores at follow-up.
Funding Statement
Disclosures: The authors of this paper do not have any financial disclosures.
References
- 1.Shorey S, Chee CYI, Ng ED, Chan YH, Tam WWS, Chong YS. Prevalence and incidence of postpartum depression among healthy mothers: a systematic review and meta-analysis. J Psychiatr Res. 2018;104:235–48. doi: 10.1016/j.jpsychires.2018.08.001. https://doi.org/10.1016/j.jpsychires.2018.08.001 PubMed PMID: 30114665. [DOI] [PubMed] [Google Scholar]
- 2.Bennett HA, Einarson A, Taddio A, Koren G, Einarson TR. Prevalence of depression during pregnancy: systematic review. Obstet Gynecol. 2004;103(4):698–709. doi: 10.1097/01.AOG.0000116689.75396.5f. https://doi.org/10.1097/01.aog.0000116689.75396.5f PubMed PMID: 15051562. [DOI] [PubMed] [Google Scholar]
- 3.Wisner KL, Sit DK, Mcshea MC, Rizzo D, Zoretich R, Hughes C, et al. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70(5):490–8. doi: 10.1001/jamapsychiatry.2013.87. https://doi.org/10.1001/jamapsychiatry.2013.87 PubMed PMID: 23487258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Josefsson A, Sydsjö G. A follow-up study of postpartum depressed women: recurrent maternal depressive symptoms and child behavior after four years. Arch Womens Ment Health. 2007;10(4):141–5. doi: 10.1007/s00737-007-0185-9. https://doi.org/10.1007/s00737-007-0185-9 PubMed PMID: 17533557. [DOI] [PubMed] [Google Scholar]
- 5.Stewart DE, Vigod SN. Postpartum depression: pathophysiology, treatment, and emerging therapeutics. Annu Rev Med. 2019;70(1):183–96. doi: 10.1146/annurev-med-041217-011106. https://doi.org/10.1146/annurev-med-041217-011106 PubMed PMID: 30691372. [DOI] [PubMed] [Google Scholar]
- 6.Do T, Hu Z, Otto J, Rohrbeck P. Depression and suicidality during the postpartum period after first time deliveries active component service women and dependent spouses US Armed Forces 20072012 MSMR. 2013;20(9):2–7. PubMed PMID: 24093957. [PubMed] [Google Scholar]
- 7.Mauri M, Oppo A, Borri C, Banti S. Suicidality in the perinatal period: comparison of two self-report instruments. Results from PND-ReScU. Arch Womens Ment Health. 2012;15(1):39–47. doi: 10.1007/s00737-011-0246-y. https://doi.org/10.1007/s00737-011-0246-y PubMed PMID: 22215284. [DOI] [PubMed] [Google Scholar]
- 8.Paris R, Bolton RE, Weinberg MK. Postpartum depression, suicidality, and mother-infant interactions. Arch Womens Ment Health. 2009;12(5):309–21. doi: 10.1007/s00737-009-0105-2. https://doi.org/10.1007/s00737-009-0105-2 PubMed PMID: 19728036. [DOI] [PubMed] [Google Scholar]
- 9.Kingston D, Tough S. Prenatal and postnatal maternal mental health and school-age child development: a systematic review. Matern Child Health J. 2014;18(7):1728–41. doi: 10.1007/s10995-013-1418-3. https://doi.org/10.1007/s10995-013-1418-3 PubMed PMID: 24352625. [DOI] [PubMed] [Google Scholar]
- 10.Kanes SJ, Colquhoun H, Doherty J, Raines S, Hoffmann E, Rubinow DR, et al. Open-label, proof-of-concept study of brexanolone in the treatment of severe postpartum depression. Hum Psychopharmacol. 2017;32(2):e2576. doi: 10.1002/hup.2576. https://doi.org/10.1002/hup.2576 PubMed PMID: 28370307 PubMed Central PMCID: PMC5396368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maguire J, Mody I. GABAAR plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59(2):207–13. doi: 10.1016/j.neuron.2008.06.019. https://doi.org/10.1016/j.neuron.2008.06.019 PubMed PMID: 18667149 PubMed Central PMCID: PMC2875248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellgren C, Åkerud H, Skalkidou A, Bäckström T, Sundström-Poromaa I. Low serum allopregnanolone is associated with symptoms of depression in late pregnancy. Neuropsychobiology. 2014;69(3):147–53. doi: 10.1159/000358838. https://doi.org/10.1159/000358838 PubMed PMID: 24776841. [DOI] [PubMed] [Google Scholar]
- 13.Brexanolone [package insert] Cambridge (MA) Sage Therapeutics. 2019.
- 14.Zheng W, Cai D-B, Zheng W, Sim K, Ungvari GS, Peng X-J, et al. Brexanolone for postpartum depression: a meta-analysis of randomized controlled studies. Psychiatry Res. 2019;279(7454):83–9. doi: 10.1016/j.psychres.2019.07.006. https://doi.org/10.1016/j.psychres.2019.07.006 PubMed PMID: 31323375. [DOI] [PubMed] [Google Scholar]
- 15.Patterson R, Krohn H, Richardson E, Kimmel M, Meltzer-Brody S. A brexanolone treatment program at an academic medical center: patient selection, 90-day posttreatment outcomes, and lessons learned. J Acad Consult Liaison Psychiatry. 2022;63(1):14–22. doi: 10.1016/j.jaclp.2021.08.001. https://doi.org/10.1016/j.jaclp.2021.08.001 PubMed PMID: 34438099. [DOI] [PubMed] [Google Scholar]
- 16.Dugdale S, Elison S, Davies G, Ward J. Applying behavior change theories and qualitative methods in substance misuse implementation research: conceptualizing the adoption of breaking free online in real-world clinical practice. Qual Health Res. 2017;27(7):1049–59. doi: 10.1177/1049732316683379. https://doi.org/10.1177/1049732316683379 PubMed PMID: 28818021. [DOI] [PubMed] [Google Scholar]
- 17.Ajzen I. The theory of planned behavior. Organizational Behav Hum Decis Process. 1991;50(2):179–211. doi: 10.1016/0749-5978(91)90020-t. [DOI] [Google Scholar]
- 18.Tengku Ismail TA, Wan Muda WAM, Bakar MI. The extended theory of planned behavior in explaining exclusive breastfeeding intention and behavior among women in Kelantan, Malaysia. Nutr Res Pract. 2016;10(1):49–55. doi: 10.4162/nrp.2016.10.1.49. https://doi.org/10.4162/nrp.2016.10.1.49 PubMed PMID: 26865916 PubMed Central PMCID: PMC4742311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hales D. Postpartum physical activity: measuring theory of planned behavior constructs. Am J Health Behav. 2010;34(4):387–401. doi: 10.5993/ajhb.34.4.1. https://doi.org/10.5993/ajhb.34.4.1 PubMed PMID: 20218751 PubMed Central PMCID: PMC3327138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Downe S, Finlayson K, Tunçalp Ö, Gülmezoglu AM. Provision and uptake of routine antenatal services a qualitative evidence synthesis Cochrane Database Syst Rev. 2019;6(6):CD012392. doi: 10.1002/14651858.CD012392.pub2. https://doi.org/10.1002/14651858.CD012392.pub2 PubMed PMID: 31194903 PubMed Central PMCID: PMC6564082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychology. 2006;3(2):77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 22.NVivo Melbourne QSR International. 2020.
- 23.Slomian J, Honvo G, Emonts P, Reginster J-Y, Bruyère O. Consequences of maternal postpartum depression: a systematic review of maternal and infant outcomes. Womens Health (Lond) 2019;15(1780):174550651984404. doi: 10.1177/1745506519844044. https://doi.org/10.1177/1745506519844044 PubMed PMID: 31035856 PubMed Central PMCID: PMC6492376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute for Health and Care Excellence Clinical Guidelines Antenatal and postnatal mental health clinical management and service guidance Leicester National Institute for Health and Care Excellence. 2014.
- 25.Bauman BL, Ko JY, Cox S, D'Angelo DV, MPH, Warner L, Folger S, et al. Vital signs: postpartum depressive symptoms and provider discussions about perinatal depression—United States, 2018. MMWR Morb Mortal Wkly Rep. 2020;69(19):575–81. doi: 10.15585/mmwr.mm6919a2. https://doi.org/10.15585/mmwr.mm6919a2 PubMed PMID: 32407302 PubMed Central PMCID: PMC7238954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.ACOG Committee Opinion No. 757: Screening for perinatal depression. Obstet Gynecol. 2018;132(5):e208–12. doi: 10.1097/AOG.0000000000002927. https://doi.org/10.1097/aog.0000000000002927 PubMed PMID: 30629567. [DOI] [PubMed] [Google Scholar]
- 27.Goodman JH, Tyer-Viola L. Detection, treatment, and referral of perinatal depression and anxiety by obstetrical providers. J Womens Health. 2010;19(3):477–90. doi: 10.1089/jwh.2008.1352. https://doi.org/10.1089/jwh.2008.1352 PubMed PMID: 20156110. [DOI] [PubMed] [Google Scholar]
- 28.Hansotte E, Payne SI, Babich SM. Positive postpartum depression screening practices and subsequent mental health treatment for low-income women in Western countries: a systematic literature review. Public Health Rev. 2017;38:3. doi: 10.1186/s40985-017-0050-y. https://doi.org/10.1186/s40985-017-0050-y PubMed PMID: 29450075 PubMed Central PMCID: PMC5809911. [DOI] [PMC free article] [PubMed] [Google Scholar]


