Introduction:
Prevalence estimates of SARS-CoV-2 infection in Africa are limited, particularly among pregnant women and in those living with HIV. This study assessed the seroprevalence of SARS-CoV-2 antibodies among Mozambican HIV-infected pregnant women during the first year of the pandemic, before COVID-19 vaccines were deployed in the country.
Setting:
The study was conducted in Manhiça district, a semirural area in southern Mozambique.
Methods:
A prospective cohort study including pregnant women living with HIV was conducted from November 2019 to June 2021. Women were enrolled at the first antenatal care clinic visit and followed until postpartum. HIV viral load and IgM/IgG antibodies against SARS-CoV-2 were determined in blood samples at first antenatal care clinic visit and at delivery. Associations between SARS-CoV-2 serostatus and maternal characteristics at enrolment were analyzed.
Results:
A total of 397 women were enrolled. SARS-CoV-2 IgG/IgM antibodies were detected in 7.1% of women at enrolment and in 8.5% of women at delivery. Overall, SARS-CoV-2 antibodies were detected in 45 women (11.3%; 95% confidence interval 8.4 to 14.9%) during the study period; the first seropositive sample was identified in September 2020. Having undetectable HIV viral load was associated with seropositivity of SARS-CoV-2 IgG/IgM [odds ratio 3.35 (1.10 to 11.29); P = 0.039].
Conclusion:
Seroprevalence of SARS-CoV-2 antibodies in this cohort of Mozambican unvaccinated pregnant women was similar to reported global estimates of approximately 10% in pregnancy for 2021. The findings also suggest that pregnant women with high HIV viral load may have an impaired immune response against SARS-CoV-2 and might need to be carefully managed in case of COVID-19.
Key Words: SARS-CoV-2, seroprevalence, HIV, pregnancy, Africa
INTRODUCTION
Since the first case of coronavirus disease 2019 (COVID-19) was reported in Africa in February 2020, more than 8.8 million COVID-19 confirmed cases and over 171,000 related deaths have been notified in the region as of May 2022.1 A significant number of infected individuals may remain undetected given testing capacity constraints of using reverse transcription polymerase chain reaction (RT-PCR)—considered the gold standard of SARS-CoV-2 infection diagnosis, in low-income settings.2 Thus, seroprevalence studies have been more widely conducted to provide information on the extent of the population or community exposure to SARS-CoV-2 infection in low-middle-income countries. Using this methodology, it has been shown that the virus has circulated extensively in sub-Saharan Africa (SSA) with an overall seroprevalence of 22%, ranging from 2% in Sierra Leone to 63% in South Africa.3–5 A recent meta-analysis including data from 151 distinct seroprevalence studies conducted in Africa reported that SARS-CoV-2 seroprevalence rose from 3.0% [95% CI: 1.0%–9.2%] in Q2 2020% to 65.1% [95% CI: 56.3%–73.0%] in Q3 2021.6
Although preliminary data from some western countries suggested that the incidence of COVID-19 was not higher in HIV-infected individuals, recent studies have shown that HIV infection is associated with a significant increased risk of contracting SARS-CoV-2.7–9 In addition, immunosuppressed HIV-infected individuals have been shown to have higher incidence of severe COVID-19 and death than non–HIV-infected individuals.10,11 The latter is quite relevant for SSA where the highest HIV incidence worldwide still concentrates.12
Pregnant women have an increased susceptibility to suffer severe SARS-CoV-2 infection, mostly explained by pregnancy-associated physiologic changes, including a decreased lung volume, an increased risk for thromboembolic disease, and some pregnancy-related immunological alterations.13,14 In addition, SARS-CoV-2 infection during pregnancy has been associated with increased risk of adverse maternal outcomes, preeclampsia, and preterm birth.15–18
Reports on the prevalence of SARS-CoV-2 infection in pregnant women in SSA countries are scarce. A health facility-based study conducted between April 2020 and March 2021 on the prevalence of anti–SARS-CoV-2 antibodies in Ethiopian pregnant women found a seroprevalence of 5.4%.19 A study conducted among 100 pregnant women from South Africa has reported increased maternal mortality risk attributed to COVID-19 compared with baseline rates.20 However, no information about the frequency of SARS-CoV-2 infection in HIV-infected pregnant women from SSA is yet available. Given the increased severity of SARS-CoV-2 infection among both pregnant women and HIV-infected individuals, it is important to assess the burden of the infection in these populations to prioritize detection of the infection in these groups.
In Mozambique, the first SARS-CoV-2–infected individual was diagnosed on March 22, 2020, and as of September 19, 2022, a total of 230,219 COVID-19 cases and 2222 deaths had been reported in the country.21 This study aimed to determine the seroprevalence of SARS-CoV-2 antibodies in HIV-infected pregnant women from southern Mozambique during the first year of the pandemic, before COVID-19 vaccines were rolled out.
METHODS
Study Area and Population
This study was conducted in the Manhiça district, Maputo province, where the Centro de Investigação em Saúde de Manhiça (CISM) is situated. Pregnant women living with HIV and attending the antenatal care (ANC) clinics of the Manhiça district Hospital and Palmeira Health Post were included in this study. The CISM is running a Demographic Surveillance System (DSS) in the district since 1996.22 The community prevalence of HIV was estimated to be 36.6% in 2015 and 25% in pregnant women in 2021 (Nhampossa et al, unpublished).23,24 Pregnant women attending the first ANC clinic visit were routinely tested for HIV and syphilis infection using rapid diagnostic tests (Determine, Abbott Laboratories-USA, and Uni-Gold HIV, Trinity Biotech-Ireland for HIV, and SD Bioline Syphilis 3.0, Standard Diagnostics-Korea for syphilis). Following national guidelines, after diagnosis all HIV-infected women are scheduled to receive life-long antiretroviral therapy (ART). At the time of this study, first-line ART was based on efavirenz (EFZ) regimens until January 2020, when it changed to dolutegravir (DTG)-based regimens. A middle-upper arm circumference (MUAC) lower than 22.0 cm was used as a proxy for malnutrition following current SPHERE guidelines and literature.25,26 The WHO cutoff points on hemoglobin were used to characterize maternal hemoglobin status as normal or anemia.27
Study Design
This is a prospective cohort study to determine the prevalence of SARS-CoV-2 antibodies in pregnant women living with HIV, nested into an ongoing clinical trial (NCT03671109) aiming to evaluate dihydroartemisinin–piperaquine as intermittent preventive treatment for malaria prevention.28 All women attending the ANC clinic for the first time in their pregnancy were invited to participate if meeting the following inclusion criteria: confirmed HIV infection, residents in the study area, gestational age 28 weeks or older, and agree to deliver in the study site's maternity ward. A signed written informed consent was obtained from all the participants or from the legal guardian in case of minors. Women's demographic and clinical data were recorded. Blood samples were collected at enrolment and at delivery to determine HIV viral load (HIV-VL) and IgM/IgG antibodies against SARS-CoV-2.
Laboratory Analyses
Qualitative determination of total anti–SARS-CoV-2 antibodies was performed using a chemiluminescent immunoassay (Atellica IM SARS-CoV-2 Total) presenting SARS-CoV-2 spike protein RBD (S1RBD) antigens. Total immunoglobulin (Ig) titers were considered negative with a result <1 U/mL (nonreactive). The manufacturer reported specificities of 99.8% and sensitivities of 100% (14 or more days since PCR testing).29 A quality control of the Atellica assays was undertaken with 60 prepandemic sera samples collected between 2010 and 2013 from pregnant women of the same study area; all samples yielded negative SARS-CoV-2 Ig, confirming the high specificity of the techniques.
IgG and IgM were analyzed individually for women who were positive for total Ig. Atellica IM SARS-CoV-2 IgG was used for the detection of IgG.29,30 The manufacturer reported specificities of 99.9% and sensitivities of 91.1% (14–20 days since PCR testing). Quantitative suspension array technology (qSAT) assays based on the xMAP Luminex platform were used for IgM detection. This is a qSAT assay against membrane protein (M) SARS-CoV-2 antigen.31 Prior validation of this assay has shown that it demonstrates specificities of 100% and sensitivities of 95.78%.31 In women with positive total Ig, the levels of IgM and IgG between 0.95 and 0.99 U/mL were considered positive.
Quantitative PCR HIV-VL was determined using COBAS AMPLICOR, an in vitro reverse transcriptase polymerase chain reaction (RT-PCR) assay for the detection and quantification of HIV type 1 (HIV-1).
Statistical Analysis
The primary outcome measure was the prevalence of total antibodies (defined as the presence of IgG and/or IgM) against SARS-CoV-2 detected in blood samples at enrolment and/or at delivery. Seroprevalence of total Ig, IgG, and IgM against SARS-CoV-2 was calculated as proportions with 95% Confidence Intervals (95 CI). Participant's sociodemographic and clinical characteristics at baseline are presented as proportions (for categorical variables) and mean and SD (for continuous quantitative variables). Bivariate logistic regression was used to test associations between presence of SARS-CoV-2 antibodies and baseline maternal characteristics and to calculate odds ratio (ORs) and 95 CIs in a subsample of women tested at enrolment after March 2020 (when the first cases of COVID-19 were reported in the country). Multivariate analysis was also performed by logistic regression and adjusting for variables selected based on the previous literature and if the univariate analysis yielded a P value < 0.2. Given that the number of events was low in some variables, the penalized likelihood method was used to estimate logistic regression models to avoid sparse data bias. Undetectable HIV-VL was defined as having less than 50 copies of HIV RNA per milliliter of blood and malnutrition having a middle-upper arm circumference (MUAC) ≤22 centimeters (cm). The analysis was performed using the statistical software Stata v16.0 (College Station TX: StataCorp LLC).
Ethical Statement
The study protocol and informed consent form were reviewed and approved by the National Committee on Health Bioethics of Mozambique and the Hospital Clinic of Barcelona Ethics Committee (Spain).
RESULTS
Characteristics of Study Participants
A total of 397 women were enrolled in this study between November 2019 and June 2021. The study profile is shown in Figure 1. There were 395 and 236 blood samples available from the first ANC clinic visit and delivery, respectively.
FIGURE 1.
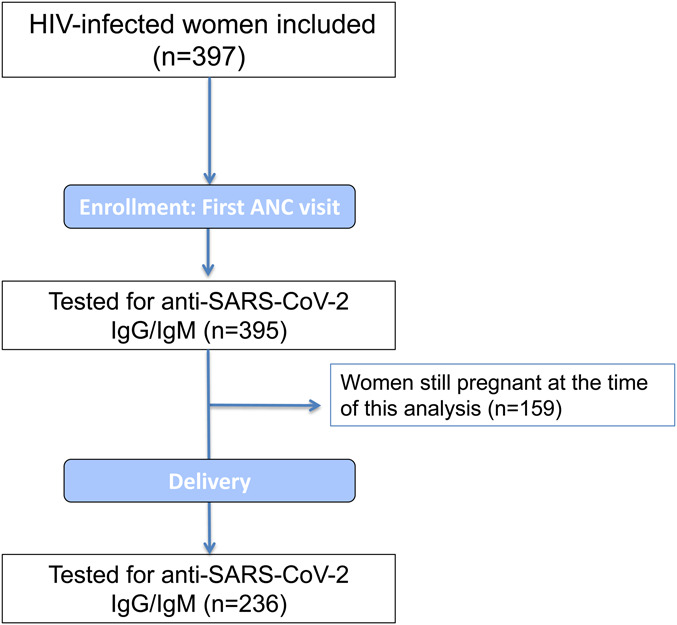
Study profile.
Sociodemographic and clinical characteristics of participants at enrolment are presented in Table 1. The mean age of participants ranged from 13 to 47 years. A total of 92 study participants (23.4%) were diagnosed with HIV infection at the first ANC clinic visit and were thus ART-naïve.
TABLE 1.
Characteristics of Study Participants at Enrolment (N = 397)
| N (%) | |
| Age (yrs) | |
| Mean (SD) | 28.2 (6.6) |
| Median (IQR) | 27 (17; 37) |
| Gestational age (wk) | |
| Mean (SD) | 18.8 (4.6) |
| Literate* | 371 (79.7) |
| Gravidity | |
| Primigravidae | 48 (12.1) |
| Multigravidae | 349 (87.9) |
| Anemia (<11 g/dL) | 195 (49.1) |
| MUAC (cm)† | |
| ≤22.0 | 8 (2.0) |
| >22.0 | 386 (97.2) |
| NI | 3 (0.8) |
| HIV VL load (copies/mm) | |
| <50 (undetectable) | 252 (63.5) |
| ≥50 | 145 (36.5) |
| ARV regimen | |
| Treatment naïve | 92 (23.4) |
| TDF + 3TC + EFV | 88 (22.4) |
| TDF + 3TC + DTG | 209 (53.2) |
| Another regimen | 4 (1.0) |
| NI | 4 (1.0) |
| Time on ARV therapy (yrs) | |
| Median (IQR) | 2.9 (−2.6; −8.4) |
| Rapid syphilis test positive | 13 (3.3) |
Being able to read and/or write.
MUAC, middle-upper arm circumference.
SD, standard deviation; IQR, interquartile range; NI, no information; TDF, tenofovir; 3TC, lamivudine; EFV, efavirenz; DTG, dolutegravir.
Prevalence of Anti–SARS-CoV-2 Antibodies at Enrolment and at Delivery
At enrolment, total Ig against SARS-CoV-2 were detected in 28 of 395 women (7.1%, 95 CI: 4.8%–10.1%). Among these 28 women with positive SARS-CoV-2 Ig: 13 women had only detectable IgM; 13 women had both IgM and IgG; and in 2 women, only IgG was detected (Table 2). At delivery, 20 of 236 women tested positive for total Ig (8.5%, 95 CI: 5.3–12.8, Table 2): 7 with only IgM detected, 12 had both IgM and IgG detected, and 1 woman with only IgG detected.
TABLE 2.
Seroprevalence of Anti–SARS-CoV-2 Immunoglobulins at Recruitment and at Delivery
| First ANC Clinic (n = 395) | Delivery (n = 236) | |||
| Positive | Positive | |||
| n | % (95 CI) | N | % (95 CI) | |
| Total Ig | 28 | 7.1 (4.8–10.1) | 20 | 8.5 (5.3–12.8) |
| By isotype | ||||
| IgM | 13 | 3.2 (1.9–5.6) | 7 | 2.9 (1.5–6.1) |
| IgM + IgG | 13 | 3.2 (1.9–5.6) | 12 | 5.1 (2.9–8.8) |
| IgG | 2 | 0.5 (0.1–2) | 1 | 0.4 (0.05–3) |
ANC, antenatal care; 95 CI, 95% confidence interval; study period, November 2019 to June 2021.
Four (14.3%) of the 28 women who tested positive at enrolment had delivered at the time of this analysis. Three of these 4 women were still positive at delivery (average of 26.1 weeks [SD: 24.6] – 6 months between both tests) whereas one tested negative for total Ig at that moment.
In total, 45 of the 397 study participants had SARS-CoV-2 Ig either at enrolment or at delivery resulting in an overall anti–SARS-CoV-2 total Ig seroprevalence of 11.3% (95% CI: 8.4%–14.9%) in this cohort of pregnant women living with HIV.
Figure 2 shows the proportion of seropositive samples tested from all the study period: November 2019 to May 2021. The first seropositive sample was identified on September 16, 2020, after 6 months of the first COVID-19 cases were reported in the country. The proportion of SARS-COV-2–positive samples among study participants rose progressively from December 2020, reaching a peak of 57% of tested samples on April 2021.
FIGURE 2.
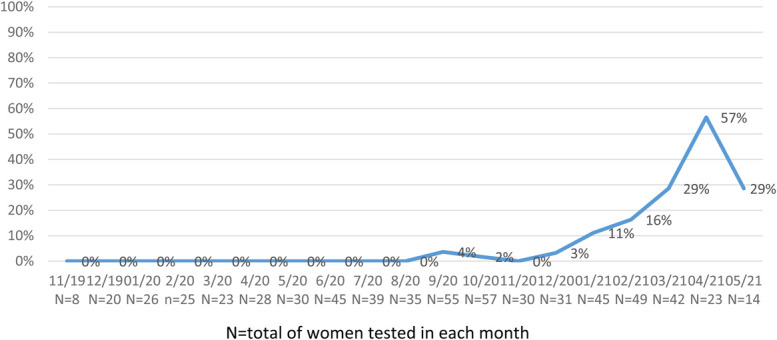
Proportion of women with SARS-CoV-2 antibodies from November 2019 to May 2021 (by month).
Footnote: N, total number of women tested in each month.
Maternal Factors at Enrolment Associated With Detected Anti–SARS-CoV-2 Antibodies
Table 3 summarizes the results of the analysis on the association between maternal factors at enrolment and detection of anti–SARS-CoV-2 total Ig in the subsample of women tested after March 2020, when the first cases of COVID-19 were reported in the country. In the univariate analysis, having an undetectable HIV-VL (<50 copies/mm) at enrolment was associated with the presence of anti–SARS-CoV-2 total Ig (P = 0.023); this association remained statistically significant in the multivariate analysis (P = 0.039). Similarly, being malnourished was associated with the presence of SARS-CoV-2 total Ig both in the univariate (P = 0.029) and multivariate (P = 0.047) analyses.
TABLE 3.
Analysis of Maternal Factors Associated With SARS-CoV-2 Ig Positivity at First ANC Clinic Visit
| SARS-CoV-2 Total Ig Negative | SARS-CoV-2 Total Ig Positive | Unadjusted OR (95 CI) | Unadjusted P | Adjusted OR (95 CI) | Adjusted P | |
| Age* | 28.5 (6.6) [276] | 27.1 (7.0) [28] | 0.97 (0.91–1.03) | 0.329 | ||
| Primigravidae | 32/276 (11.6) | 5/28 (17.9) | 1.76 (0.65–4.78) | 0.267 | ||
| Gestational age* | 18.8 (4.5) [276] | 18.9 (5.1) [28] | 1.00 (0.92–1.10) | 0.871 | ||
| Literate† | 220 (79.7) | 23 (82.1) | 1.09 (0.41–2.90) | 0.855 | ||
| Malnourished (MUAC < 22.0 cm) | 4/275 (1.4) | 2/27 (7.4) | 5.92 (1.20–29.25) | 0.029 | 5.51 (1.02–29.81) | 0.047 |
| Anemia (<11 g/dL) | 134/275 (48.7) | 12/28 (42.9) | 0.80 (0.37–1.72) | 0.565 | 0.85 (0.38–1.89) | 0.695 |
| Undetectable HIV VL (<50 copies/mm) | 175/276 (63.4) | 24/28 (85.5) | 3.14 (1.12–8.86) | 0.023 | 3.35 (1.10–11.29) | 0.039 |
| ARV treatment | ||||||
| No treatment | 65/275 (23.6) | 6/28 (21.4) | Ref | 0.731 | Ref | 0.418 |
| TDF/3TC/EFV | 31/275 (11.3) | 1/28 (3.6) | 0.48 (0.08–2.98) | 0.21 (0.02–1.72) | ||
| TDF/3TC/DTG | 176/275 (64.0) | 21/28 (10.8) | 1.23 (0.49–3.09) | 0.76 (0.22–2.62) | ||
| Other treatments | 3/275 (1.1) | 0/28 (0.0) | 1.44 (0.07–31.03) | 4.49 (0.13–74.05) | ||
| Time on ARV regime (d, median/IQR)‡ | 1205 (2066) [276] | 682 (2198) [28] | 0.99 (0.99–1.00) | 0.803 | 0.99 (0.99–1.00) | 0.591 |
| Positive syphilis test | 7/276 (2.5) | 2/28 (7.1) | 0.34 (0.08–1.42) | 0.139 | 0.29 (0.08–1.72) | 0.206 |
Values are number n/N (%) unless indicated otherwise. Subanalysis performed in tested women after March 2020, when first COVID-19 cases were reported in the country.
Mean (SD) [N].
Can read and/or write.
Median (IQR) [N]. MUAC, middle-upper arm circumference.
TDF, tenofovir; 3TC, lamivudine; EFV, efavirenz; DTG, dolutegravir; SD, standard deviation; IQR, interquartile range.
DISCUSSION
To the best of our knowledge, this is the first study reporting on the frequency of exposure to SARS-CoV-2 infection in pregnant women living with HIV from SSA during the first year of the pandemic, and before COVID-19 vaccines were rolled out. Overall, SARS-CoV-2 seroprevalence in this population group was 11.3%, which is in line with the global SARS-CoV-2 prevalence of infection in pregnant women in 2021 (ranging from 7% to 10%).32 The study findings also indicate the likely absence of SARS-CoV-2 circulation in this rural area of the country during the first 6 months of the pandemic.
Although the periods are different, the SARS-CoV-2 seroprevalence observed in this study is nearly 2-fold higher than that of 5.7% reported among Ethiopian pregnant women tested between April 2020 and March 2021, indicating a higher circulation of the virus in this area of southern Mozambique.19 Another study conducted in parturient women from Angola between January and April 2021, found a prevalence of 0.4% of SARS-CoV-2 infection using a rapid antigenic testing and RT-PCR.33 By contrast, a study conducted in Kenyan pregnant women from May 2020 to February 2021 reported a prevalence of 31% of clinical COVID-19.34 However, none of these studies provided seroprevalence data, making it difficult to compare their results with this study. Besides differences in the type of diagnostic tests and definitions of infection used, these disparities in the prevalence of maternal SARS-CoV-2 infection reported in SSA suggest variability in viral circulation in the region.
A recent study on the prevalence of SARS-CoV-2 infection in 239 women giving birth at the Maputo Central Hospital (HCM), in Mozambique, reported a prevalence of 9.2% PCR-confirmed cases between October 2020 to July 2021, which is slightly lower than the SARS-CoV-2 seroprevalence found in our study (11.3%).35 Overall, the present findings seem to be representative and a reflect of the epidemic in the country.
In this study, after the first seropositive case was identified in September 16, 2020, the seroprevalence raised from November 2020, when the African region was experiencing the second wave of the COVID-19 pandemic.1,36 This finding supports global estimates suggesting that the burden of the second wave of SARS-CoV-2 was higher than the first wave in the region (mean of weekly cases 18,273 in July 2020 versus 23,790 weekly cases reported in December 2020).36 On the other hand, in this study, we included data collected until June 2021 just before the third wave of SARS-CoV-2 started in the country in July 2021; thus, it is likely that SARS-CoV-2 seroprevalence continued rising thereafter. In fact, community-based household sero-surveys conducted in the same area from April to June 2021 and from October to November 2021, reported SARS-CoV-2 seroprevalences of 27.7% and 63%, respectively (Mandomando et al, unpublished).
Three of the 4 women with detected total anti–SARS-CoV-2 Ig at enrolment who delivered at the time of this analysis were still positive at delivery (average of more than 6 months between both tests). High rates of SARS-CoV-2 seropositivity up to 6 months postinfection have been reported previously in the general population.37 Notably, the proportion of women in this study with recent SARS-CoV-2 infection (IgM positive and IgG negative) was similar at enrolment (3.2%) and at delivery (2.9%), suggesting a continuous circulation of the virus, and of the risk of infection (and reinfection) during the study period. IgM antibodies typically peak within the first few weeks after infection, and then fall below detectable limits 2–3 months after infection.38,39 The study findings indicate that there were recent infections during pregnancy among study participants. The potential effects of these infections on maternal and infant health will need to be studied considering the reported association between maternal SARS-CoV-2 infection and the risk of adverse pregnancy outcomes.15,16
HIV infection has been associated with a significantly higher risk of contracting SARS-CoV-2.9 Early reports during the pandemic suggested a delayed antibody response against SARS-CoV-2 in individuals living with HIV.40 It has also been reported that SARS-CoV-2–infected immunocompromised individuals (due to HIV infection or other conditions) had lower median antibody titers or remained seronegative against SARS-CoV-2.41–44 In agreement with these findings, in this study, we found that HIV viral suppression was associated with the presence of SARS-CoV-2 antibodies in pregnant women. It could be hypothesized that women with undetectable HIV-VL may be more capable of generating a better immune response against SARS-CoV-2 than those with detectable HIV-VL, as already reported in vaccine-induced immune responses to yellow fever and hepatitis B.45,46 The findings from a community-based SARS-CoV-2 sero-survey conducted in South Africa has recently reported that individuals living with HIV with high viral load were less likely to be SARS-CoV-2 seropositive compared with HIV-uninfected individuals, which is in agreement with our findings.47 In another note, in a study including 28 pregnant women living with HIV from South Africa, virally suppressed HIV infection was not associated with worse COVID-19 outcomes in pregnancy.20
Maternal malnutrition (estimated by MUAC assessment) was associated with the presence of SARS-CoV-2 total Ig, suggesting that poor nutritional status might increase the risk of infection.48 On the other hand, malnutrition can impair immunological responses.49 In this study, only 6 women presented with malnutrition, and therefore, findings on this regard should be taken with caution. Another limitation of this study is the fact that some participants were still pregnant at the time of this analysis, and thus, we have only been able to assess the presence of SARS-CoV-2 antibodies at the time of delivery in 236 of 397 study participants (59.4%).
In conclusion, seroprevalence of SARS-CoV-2 antibodies in the first year of the COVID-19 pandemic in this cohort of pregnant women living with HIV from southern Mozambique is in agreement with global estimates for 2021. Women with HIV viral suppression may be able to mount a better immune response against SARS-CoV-2 than those with detectable HIV-VL. Detection of infection and measures of protection should both be prioritized in pregnant women living with HIV and especially in those with detectable or high HIV-VL to prevent complications.
Footnotes
The clinical trial in which the study is nested and the SARS-CoV-2 serology analyses are part of the EDCTP2 programme supported by the European Union (Project Grants Number RIA2016MC-1613-MAMAH and RIA2020EF-2956-MA-CoV). This study is sponsored by the Barcelona Institute for Global Health (ISGlobal). ISGlobal is supported by the Spanish Ministry of Science and Innovation through the 'Centro de Excelencia Severo Ochoa 2019–2023' Program (CEX2018-000806-S), and it is a member of the CERCA Programme, Generalitat de Catalunya. The project funders do not have any role on study design, data collection, and analysis.
All authors declare no conflict of interest to disclose.
Patient and public involvement: Patients and/or the public were not involved in the design, conduct, reporting, or dissemination plans of this research.
Ethical Approval statement: The study protocol and informed consent form were reviewed and approved by the National Committee on Health Bioethics of Mozambique and the Hospital Clinic of Barcelona Ethics Committee (Spain).
T.N. and A.F.-R. Contributed equally.
REFERENCES
- 1.Li R, Pei S, Chen B, et al. Substantial undocumented infection facilitates the rapid dissemination of novel coronavirus (SARS-CoV2). Sci (NY). 2020;368:489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan JF, Yip CC, To KK, et al. Improved molecular diagnosis of COVID-19 by the novel, highly sensitive and specific COVID-19-RdRp/hel real-time reverse transcription-PCR assay validated in vitro and with clinical specimens. J Clin Microbiol. 2020;58:e00310-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sykes W, Mhlanga L, Swanevelder R, et al. Prevalence of anti-SARS-CoV-2 antibodies among blood donors in Northern Cape, KwaZulu-Natal, Eastern Cape, and free state provinces of South Africa in January 2021. Res Sq [Preprint]. 2021. [Google Scholar]
- 4.Barrie MB, Lakoh S, Kelly JD, et al. SARS-CoV-2 antibody prevalence in Sierra Leone, March 2021: a cross-sectional, nationally representative, age-stratified serosurvey. BMJ Glob Health. 2021;6:e007271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chisale MRO, Ramazanu S, Mwale SE, et al. Seroprevalence of anti-SARS-CoV-2 antibodies in Africa: a systematic review and meta-analysis. Rev Med Virol. 2022;32:e2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis HC, Ware H, Whelan M, et al. SARS-CoV-2 infection in Africa: a systematic review and meta-analysis of standardised seroprevalence studies, from January 2020 to December 2021. MedRxiv [preprint]. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with Covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ssentongo P, Heilbrunn ES, Ssentongo AE, et al. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Sci Rep. 2021;11:6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffmann C, Casado JL, Harter G, et al. Immune deficiency is a risk factor for severe COVID-19 in people living with HIV. HIV Med. 2021;22:372–378. [DOI] [PubMed] [Google Scholar]
- 11.Western Cape Department of Health in collaboration with the National Institute for Communicable Diseases SA. Risk factors for coronavirus disease 2019 (COVID-19) death in a population cohort study from the western Cape Province, South Africa. Clin Infect Dis. 2021;73:e2005–e2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FIND. SARS-COV-2 DIAGNOSTIC PIPELINE. [Internet]; 2020. Available at: https://www.finddx.org/covid-19/pipeline/. Accessed April 15, 2020.
- 13.Ellington S, Strid P, Tong VT, et al. Characteristics of women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status – United States, January 22–June 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:769–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wastnedge EAN, Reynolds RM, van Boeckel SR, et al. Pregnancy and COVID-19. Physiol Rev. 2021;101:303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McClymont E, Albert AY, Alton GD, et al. Association of SARS-CoV-2 infection during pregnancy with maternal and perinatal outcomes. Jama. 2022;327:1983–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newton SM, Reeves EL, O'Malley Olsen E, et al. Preterm birth among pregnant persons with severe acute respiratory syndrome Coronavirus 2 infection. J Perinatol. 2022;42:1328–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papageorghiou AT, Deruelle P, Gunier RB, et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225:289.e1–289.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175:817–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Assefa N, Regassa LD, Teklemariam Z, et al. Seroprevalence of anti-SARS-CoV-2 antibodies in women attending antenatal care in eastern Ethiopia: a facility-based surveillance. BMJ Open. 2021;11:e055834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Waard L, Langenegger E, Erasmus K, et al. Maternal and neonatal outcomes of COVID-19 in a high-risk pregnant cohort with and without HIV. S Afr Med J. 2021;111:1174–1180. [DOI] [PubMed] [Google Scholar]
- 21.WHO. Advice on the use of point-of-care immunodiagnostic tests for COVID-19. WHO [Internet]; 2020. Available at: https://www.who.int/news-room/commentaries/detail/advice-on-the-use-of-point-of-care-immunodiagnostic-tests-for-covid-19. Accessed April 15, 2020.
- 22.Sacoor C, Nhacolo A, Nhalungo D, et al. Profile: Manhica Health Research Centre (Manhica HDSS). Int J Epidemiol. 2013;42:1309–1318. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez R, Munguambe K, Aponte J, et al. High HIV prevalence in a southern semi-rural area of Mozambique: a community-based survey. HIV Med. 2012;13:581–588. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Varela E, Augusto O, Fuente-Soro L, et al. Quantifying the gender gap in the HIV care cascade in southern Mozambique: we are missing the men. PLoS One. 2021;16:e0245461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sphere guidelines: humanitarian charter and minimum standards in humanitarian response. 2018. [Google Scholar]
- 26.Ververs MT, Antierens A, Sackl A, et al. Which anthropometric indicators identify a pregnant woman as acutely malnourished and predict adverse birth outcomes in the humanitarian context? PLoS Curr. 2013;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; Geneva, Switzerland: Worl Health Organization; 2011. Available at: https://apps.who.int/iris/handle/10665/85839. Accessed May 15, 2022. [Google Scholar]
- 28.Gonzalez R, Nhampossa T, Mombo-Ngoma G, et al. Evaluation of the safety and efficacy of dihydroartemisinin-piperaquine for intermittent preventive treatment of malaria in HIV-infected pregnant women: protocol of a multicentre, two-arm, randomised, placebo-controlled, superiority clinical trial (MAMAH project). BMJ Open. 2021;11:e053197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottfredsson M. [The Spanish flu in Iceland 1918. Lessons in medicine and history]. Laeknabladid. 2008;94:737–745. [PubMed] [Google Scholar]
- 30.Jamieson DJ, Honein MA, Rasmussen SA, et al. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet (London, England). 2009;374:451–458. [DOI] [PubMed] [Google Scholar]
- 31.Dobano C, Vidal M, Santano R, et al. Highly sensitive and specific multiplex antibody assays to quantify immunoglobulins M, A, and G against SARS-CoV-2 antigens. J Clin Microbiol. 2021;59:e01731-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birmingham Uo. Prevalence of COVID-19 in pregnant and postnatal women. https://www.birmingham.ac.uk/research/who-collaborating-centre/pregcov/about/prevalence.aspx. Accessed May 15, 2022.
- 33.Sebastiao CS, Parimbelli P, Mendes M, et al. Prevalence and risk factors of SARS-CoV-2 infection among parturients and newborns from Luanda, Angola. Pathogens. 2021;10:1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Otieno NA, Azziz-Baumgartner E, Nyawanda BO, et al. SARS-CoV-2 infection among pregnant and postpartum women, Kenya, 2020–2021. Emerg Infect Dis. 2021;27:2497–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.M'poca Charles C, Osman NB, Arijama D, et al. Clinical and Epidemiological aspects of SARS-CoV-2 infection among pregnant and postpartum women in Mozambique: a prospective cohort study. Res Sq [Preprint]. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salyer SJ, Maeda J, Sembuche S, et al. The first and second waves of the COVID-19 pandemic in Africa: a cross-sectional study. Lancet. 2021;397:1265–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Z, Ren L, Yang J, et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet. 2021;397:1075–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020;383:1724–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Post N, Eddy D, Huntley C, et al. Antibody response to SARS-CoV-2 infection in humans: a systematic review. PLoS One. 2020;15:e0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao J, Liao X, Wang H, et al. Early virus clearance and delayed antibody response in a case of coronavirus disease 2019 (COVID-19) with a history of coinfection with human immunodeficiency virus type 1 and hepatitis C virus. Clin Infect Dis. 2020;71:2233–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383:2586–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meiring S, Tempia S, Bhiman JN, et al. Prolonged shedding of SARS-CoV-2 at high viral loads amongst hospitalised immunocompromised persons living with HIV, South Africa. Clin Infect Dis. 2022;75:e144–e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tarhini H, Recoing A, Bridier-Nahmias A, et al. Long-term severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infectiousness among three immunocompromised patients: from prolonged viral shedding to SARS-CoV-2 superinfection. J Infect Dis. 2021;223:1522–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spinelli MA, Lynch KL, Yun C, et al. SARS-CoV-2 seroprevalence, and IgG concentration and pseudovirus neutralising antibody titres after infection, compared by HIV status: a matched case-control observational study. Lancet HIV. 2021;8:e334–e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin C, Florence E, Domingo C, et al. Seroconversion and antibody persistence after yellow fever vaccination in people living with HIV: impact of baseline HIV viral load and yellow fever seropositivity. J Trav Med. 2022:taac024. [DOI] [PubMed] [Google Scholar]
- 46.Overton ET, Sungkanuparph S, Powderly WG, et al. Undetectable plasma HIV RNA load predicts success after hepatitis B vaccination in HIV-infected persons. Clin Infect Dis. 2005;41:1045–1048. [DOI] [PubMed] [Google Scholar]
- 47.Wolter N, Tempia S, von Gottberg A, et al. Seroprevalence of SARS-CoV-2 after the second wave in South Africa in HIV-infected and uninfected persons: a cross-sectional household survey. Clin Infect Dis. 2022;75:e57–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calder PC. Nutrition and immunity: lessons for COVID-19. Nutr Diabetes. 2021;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]


