Abstract
Rationale
Sex-based differences in pulmonary arterial hypertension (PAH) are known, but the contribution to disease measures is understudied.
Objectives
We examined whether sex was associated with baseline 6-minute-walk distance (6MWD), hemodynamics, and functional class.
Methods
We conducted a secondary analysis of participant-level data from randomized clinical trials of investigational PAH therapies conducted between 1998 and 2014 and provided by the U.S. Food and Drug Administration. Outcomes were modeled as a function of an interaction between sex and age or sex and body mass index (BMI), respectively, with generalized mixed modeling.
Results
We included a total of 6,633 participants from 18 randomized clinical trials. A total of 5,197 (78%) were female, with a mean age of 49.1 years and a mean BMI of 27.0 kg/m2. Among 1,436 males, the mean age was 49.7 years, and the mean BMI was 26.4 kg/m2. The most common etiology of PAH was idiopathic. Females had shorter 6MWD. For every 1 kg/m2 increase in BMI for females, 6MWD decreased 2.3 (1.6–3.0) meters (P < 0.001), whereas 6MWD did not significantly change with BMI in males (0.31 m [−0.30 to 0.92]; P = 0.32). Females had lower right atrial pressure (RAP) and mean pulmonary artery pressure, and higher cardiac index than males (all P < 0.03). Age significantly modified the sex by RAP and mean pulmonary artery pressure relationships. For every 10-year increase in age, RAP was lower in males (0.5 mm Hg [0.3–0.7]; P < 0.001), but not in females (0.13 [−0.03 to 0.28]; P = 0.10). There was a significant decrease in pulmonary vascular resistance (PVR) with increasing age regardless of sex (P < 0.001). For every 1 kg/m2 increase in BMI, there was a 3% decrease in PVR for males (P < 0.001), compared with a 2% decrease in PVR in females (P < 0.001).
Conclusions
Sexual dimorphism in subjects enrolled in clinical trials extends to 6MWD and hemodynamics; these relationships are modified by age and BMI. Sex, age, and body size should be considered in the evaluation and interpretation of surrogate outcomes in PAH.
Keywords: sex, sexual dimorphism, pulmonary arterial hypertension, body mass index
Most patients with pulmonary arterial hypertension (PAH) are female, and women with PAH have better survival compared with men. Sex and sexual dimorphism in pulmonary vascular disease have therefore become a focus of clinical and experimental studies in recent years. There are several ongoing clinical trials to modulate sex hormone signaling as a treatment strategy in PAH (1–4) (NCT 03648385, NCT03229499, and NCT03528902). However, we do not fully understand if biological sex influences the severity of pulmonary vascular disease.
Six-minute-walk distance (6MWD), pulmonary hemodynamics, and functional class are widely held clinical measures incorporated into modern risk assessment scores and are intermediate endpoints in PAH trials (5–7). Sex-specific reference equations derived from healthy adults exist for 6MWD (8, 9), but whether sex directly impacts 6MWD (and functional class assessment) in PAH is not known (10–12). We and others have demonstrated that there are differential sex-specific responses in 6MWD to PAH therapies in clinical trials (12, 13) and that males with PAH have worse hemodynamics than females at baseline, especially at a younger age (<45 yr old) (14). Registry data have suggested that older males have worse survival, and U.S.-based risk calculators (Registry to Evaluate Early and Long-term PAH Disease Management [REVEAL] 1.0 and 2.0) include males greater than 60 years old as a high-risk variable (6, 15–17). Age and body size may confound the relationships between sex and PAH assessments because sex steroids vary with life cycle events and adiposity (18–21).
We aimed to examine whether sex, as reported on case report forms (CRFs), was associated with baseline values of 6MWD, hemodynamics, and functional class in clinical trial participants with PAH and, if present, whether these associations were modified by age and body mass index (BMI) using data from 18 randomized clinical trials in PAH. Our research questions were not a priori hypotheses of the original studies. We hypothesized that, among clinical trial participants with PAH, female sex would be associated with more favorable baseline indices (higher 6MWD and cardiac index [CI], lower right atrial pressure [RAP], mean pulmonary artery pressure [PAP], pulmonary vascular resistance [PVR], and functional class) but that these differences would attenuate with older age and increasing BMI.
Methods
Study Selection and Data Harmonization
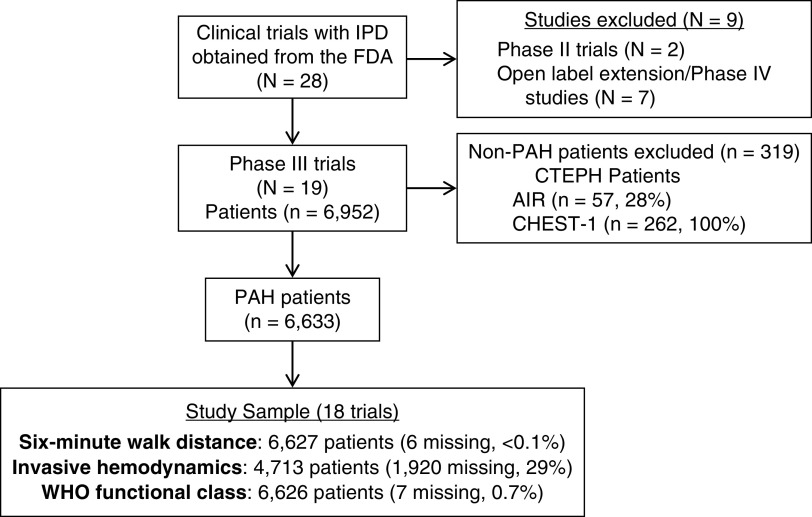
Patient-level data from 18 randomized clinical trials (RCTs) of investigational PAH therapies conducted between 1998 and 2013 were provided by the FDA (U.S. Food and Drug Administration). Phase III trials of currently approved PAH therapies were included as well as the sitaxentan (STRIDE) trials, which did not receive FDA approval. Table E1 in the data supplement provides trial-level characteristics. Of the 18 included studies, 10 required assessment of hemodynamics during screening or at baseline for study enrollment, whereas the remainder allowed historical hemodynamic data for inclusion. All participants previously provided informed consent, and all RCTs were performed with institutional review board approval at local sites.
The FDA provided raw datasets from each study as SAS transport files with an overall goal to improve clinical trial planning, conduct, and efficiency in PAH. Analyses were not prespecified with the FDA. We converted the transport files to SAS datasets. The FDA provided data dictionaries and blank CRFs for most studies. We followed the Study Data Tabulation Model (Version 1.4) for the harmonization process described in Study Data Tabulation Model Implementation Guide: Human Clinical Trials, Version 3.2, adding nonstandard variables when necessary. We assigned a four-digit numeric StudyID to each study and a six-digit numeric USUBJID to each randomized participant. We assumed all individuals were unique in the randomized studies, except for the sitaxentan trials in which subjects rolled over across Study of Sitaxsentan Sodium Treatment in Patients with Pulmonary Arterial Hypertension (STRIDE) trials were tracked. The randomization date was assigned as the primary reference point. Participant-level data, including demographics, anthropometrics, 6MWD, hemodynamics, and functional class, were harmonized across all studies. Although CRFs varied by trial in querying about sex versus gender, all trials coded sex as female or male in the data to the FDA, and as such, we report sex here (22). PAH subtype was characterized on the basis of the 2018 World Symposium on Pulmonary Hypertension consensus definitions (23). We included only participants with Group 1 PAH (idiopathic PAH, heritable PAH, drug- and toxin-induced PAH, PAH associated with connective tissue disease [CTD], human immunodeficiency virus infection, congenital heart disease, and portal hypertension) and excluded participants with chronic thromboembolic pulmonary hypertension (i.e., Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase-Stimulator Trial 1 [CHEST-1] trial and 57 of 203 [28%] participants with chronic thromboembolic pulmonary hypertension enrolled in the Aerosolized Iloprost Randomized [AIR] trial) (Figure 1). The harmonization and data analysis was deemed exempt from review by the University of Pennsylvania Institutional Review Board. Baseline characteristics by treatment group assignment were reproduced and compared against published results to confirm the accuracy of the harmonization process.
Figure 1.
Study sample. AIR = aerosolized iloprost randomized; CHEST-1 = Chronic Thromboembolic Pulmonary Hypertension Soluble Guanylate Cyclase-Stimulator Trial 1; CTEPH = chronic thromboembolic pulmonary hypertension; FDA = U.S. Food and Drug Administration; IPD = individual participant data; PAH = pulmonary arterial hypertension; WHO = World Health Organization.
Statistical Analysis
All modeling was accomplished using SAS Software 9.4 (SAS Inc.) with the GLIMMIX procedure. Outcomes (6MWD, individual hemodynamic indices, and functional class) were modeled as a function of an interaction between sex and age or sex and BMI, respectively. In a one-stage participant-level analysis, data were modeled using generalized linear mixed modeling with a random intercept and slope with sandwich estimation, in which observations were nested by the study, given that only preintervention baseline data and interactions were examined (24). Outcomes including 6MWD, RAP, mean PAP, cardiac output (CO), and pulmonary capillary wedge pressure (PCWP) were modeled with a normal distribution; PVR and CI were modeled with a lognormal (ln) distribution and functional class a binomial distribution. Pulmonary artery (PA) compliance (CO / heart rate) / (systolic PAP − diastolic PAP) was calculated when available (systolic and diastolic PAPs were reported in seven trials; n = 2,511) and included as an exploratory endpoint. Sensitivity analyses were conducted to investigate whether there was evidence of effect modification by PAH diagnosis (idiopathic vs. CTD) in models including two- (sex × PAH diagnosis) and three-way (sex × age × PAH diagnosis; sex × BMI × PAH diagnosis) interactions as well as background PAH therapy (sex × background therapy, with two- and three-way interactions) for the main outcomes of interest. Modeling results with BMI were confirmed with both imputed data for missing BMI (n = 852 of 6,633, using hierarchical multiple imputation) and weight alone. α was established a priori at 0.05, and all estimates were calculated for 95% confidence.
Results
We included a total of 6,633 participants from 18 RCTs (Figure 1). A total of 5,197 (78%) were female, with a mean age of 49.1 years and a mean BMI of 27.0 kg/m2 (Table 1). Among 1,436 males, the mean age was 49.7 years, and the mean BMI was 26.4 kg/m2. The most common etiology of PAH was idiopathic; however, females were less likely to have idiopathic PAH and more likely to have CTD–PAH than males. The prevalence of diabetes and hypertension was similar across females and males. Table 1 includes the average values of 6MWD, hemodynamics, and functional class proportions for females and males, respectively. A total of 2,491 (1,971 [38%] females and 520 [36%] males) participants were on background PAH therapy at enrollment.
Table 1.
Subject characteristics by sex
| Variable | Total n = 6,633 |
Female n = 5,197 |
Male n = 1,436 |
|---|---|---|---|
| Age, yr | 49.2 ± 15.4 | 49.1 ± 15.2 | 49.7 ± 16.1 |
| Race, n (%) | |||
| White | 4,571 (68.9) | 3,514 (67.6) | 1,057 (73.6) |
| Black | 266 (4.0) | 227 (4.4) | 39 (2.7) |
| Asian | 956 (14.4) | 744 (14.3) | 212 (14.8) |
| Multiple/other | 109 (1.6) | 93 (1.8) | 16 (1.1) |
| Unknown | 731 (11.0) | 619 (11.9) | 112 (7.8) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 693 (10.5) | 600 (11.6) | 93 (6.5) |
| Not Hispanic or Latino | 5,702 (86.0) | 4,404 (84.7) | 1,298 (90.4) |
| Unknown | 238 (3.6) | 193 (3.7) | 45 (3.1) |
| Body mass index, kg/m2 (n = 5,781) | 26.9 ± 6.4 | 27.0 ± 6.6 | 26.4 ± 5.3 |
| Etiology, n (%) | |||
| Idiopathic | 3,986 (60.2) | 2,937 (56.6) | 1,049 (73.1) |
| Heritable | 70 (1.1) | 52 (1.0) | 18 (1.25) |
| Drug and/or toxin | 120 (1.8) | 103 (2.0) | 17 (1.2) |
| Connective tissue disease | 1,847 (27.9) | 1,640 (31.6) | 207 (14.4) |
| Congenital heart disease | 519 (7.8) | 407 (7.8) | 112 (7.8) |
| HIV infection | 65 (1.0) | 36 (0.7) | 29 (2.0) |
| Portopulmonary hypertension | 13 (0.2) | 10 (0.2) | 3 (0.2) |
| Other | 6 (0.1) | 5 (0.1) | 1 (0.1) |
| Hypertension, n (%) | 2,097 (31.6) | 1,628 (31.3) | 469 (32.7) |
| Diabetes, n (%) | 353 (5.3) | 258 (5.0) | 95 (6.6) |
| Asthma, n (%) | 420 (6.3) | 366 (7.0) | 54 (3.8) |
| Background PAH therapy, n (%) | 2,491 (37.6) | 1,971 (37.9) | 520 (36.2) |
| Hemodynamics | |||
| Right atrial pressure, mm Hg (n = 4,382) | 8 (5–11) | 8 (5–11) | 8 (5–12) |
| Mean PAP, mm Hg (n = 4,713) | 51 (42–61) | 51 (41–61) | 52 (43–63) |
| PCWP, mm Hg (n = 4,524) | 9 (7–12) | 9 (7–12) | 9 (7–12) |
| Cardiac output, L/min (n = 3,302) | 4.1 (3.2–5.0) | 4.0 (3.2–4.9) | 4.2 (3.4–5.1) |
| Cardiac index, L/min/m2 (n = 3,845) | 2.3 (1.9–2.8) | 2.3 (1.9–2.9) | 2.2 (1.9–2.7) |
| PVR, dynes/sec/cm−5 (n = 4,573) | 818 (545–1,167) | 823 (544–1,184) | 785 (552–1,103) |
| PA compliance, ml/mm Hg (n = 2,511) | 1.1 (0.7–1.6) | 1.0 (0.7–1.5) | 1.1 (0.8–1.6) |
| Baseline 6-min-walk distance, meters (n = 6,627) | 347 ± 84 | 344 ± 83 | 358 ± 86 |
| WHO functional class, n (%) | |||
| I | 49 (0.7) | 33 (0.6) | 16 (1.1) |
| II | 2,228 (33.6) | 1,743 (33.6) | 485 (33.8) |
| III | 4,140 (62.5) | 3,261 (62.8) | 879 (61.2) |
| IV | 209 (3.2) | 156 (3.0) | 53 (3.7) |
| Assigned to treatment arm in the trial, n (%) | 3,978 (60.0) | 3,115 (59.9) | 863 (60.0) |
Definition of abbreviations: HIV = human immunodeficiency virus; PA = pulmonary artery; PAH = pulmonary arterial hypertension; PAP = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; WHO = World Health Organization.
Data not defined as n (%) are displayed as mean ± standard deviation or median (interquartile range).
Six-Minute-Walk Distance Varies by Sex, and BMI Modifies this Relationship
Females had shorter 6MWD than males regardless of age (P = 0.02). As age increased, walk distance decreased in both females and males (P for interaction = 0.52) (Figure 2A). For every year increase in age, walk distance decreased by 1.5 (1.3–1.7) meters in females and 1.6 [1.2–1.9] meters in males (both P < 0.001).
Figure 2.
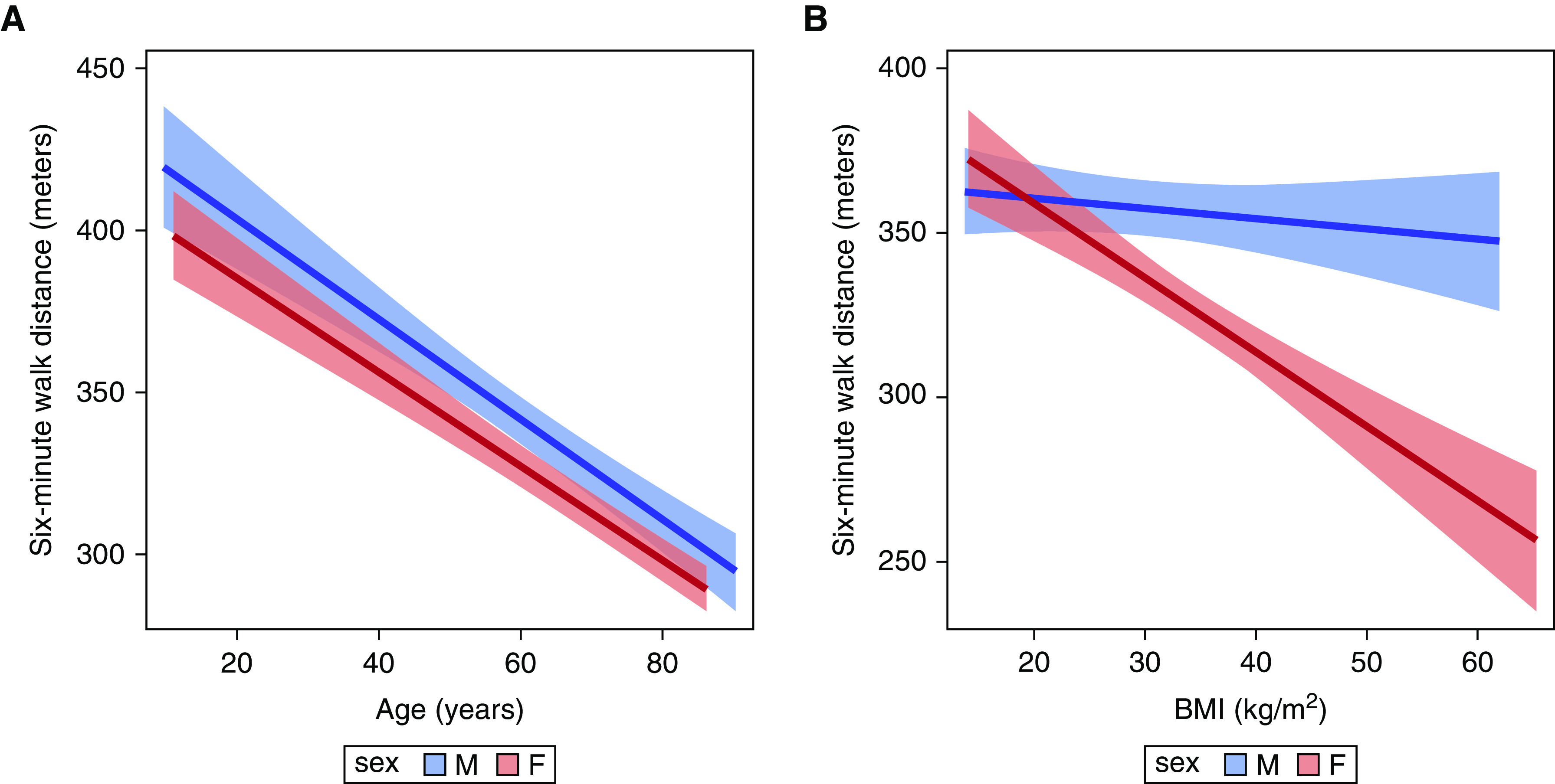
Associations between six-minute-walk distance and (A) age and (B) BMI stratified by sex. Red = females; blue = males. The solid line is the effect estimate; lighter bands are the 95% confidence band. BMI = body mass index.
The relationship between BMI and 6MWD was different for females and males (P for interaction < 0.001) (Figure 2B). For every 1 kg/m2 increase in BMI for females, walk distance decreased by 2.3 (1.6–3.0) meters (P < 0.001), whereas walk distance did not significantly change with BMI in males (0.31 m [−0.30 to 0.92]; P = 0.32). This was confirmed with both imputed BMI and using weight alone (data not shown).
Females Have Less Hemodynamic Impairment Than Males, and Age and BMI Modifies These Relationships RAP
Overall, females had lower RAP than males (P < 0.001) (Figure 3A). Age significantly modified this relationship (P for interaction < 0.01). For every 10-year increase in age, RAP was slightly lower in males (0.5 [0.3–0.7] mm Hg; P < 0.001), but not in females (0.13, [−0.03 to 0.28]; P = 0.10). For both females and males, higher BMI was associated with higher RAP, and there was no evidence of effect modification by sex (P for interaction = 0.47) (Figure 4B).
Figure 3.
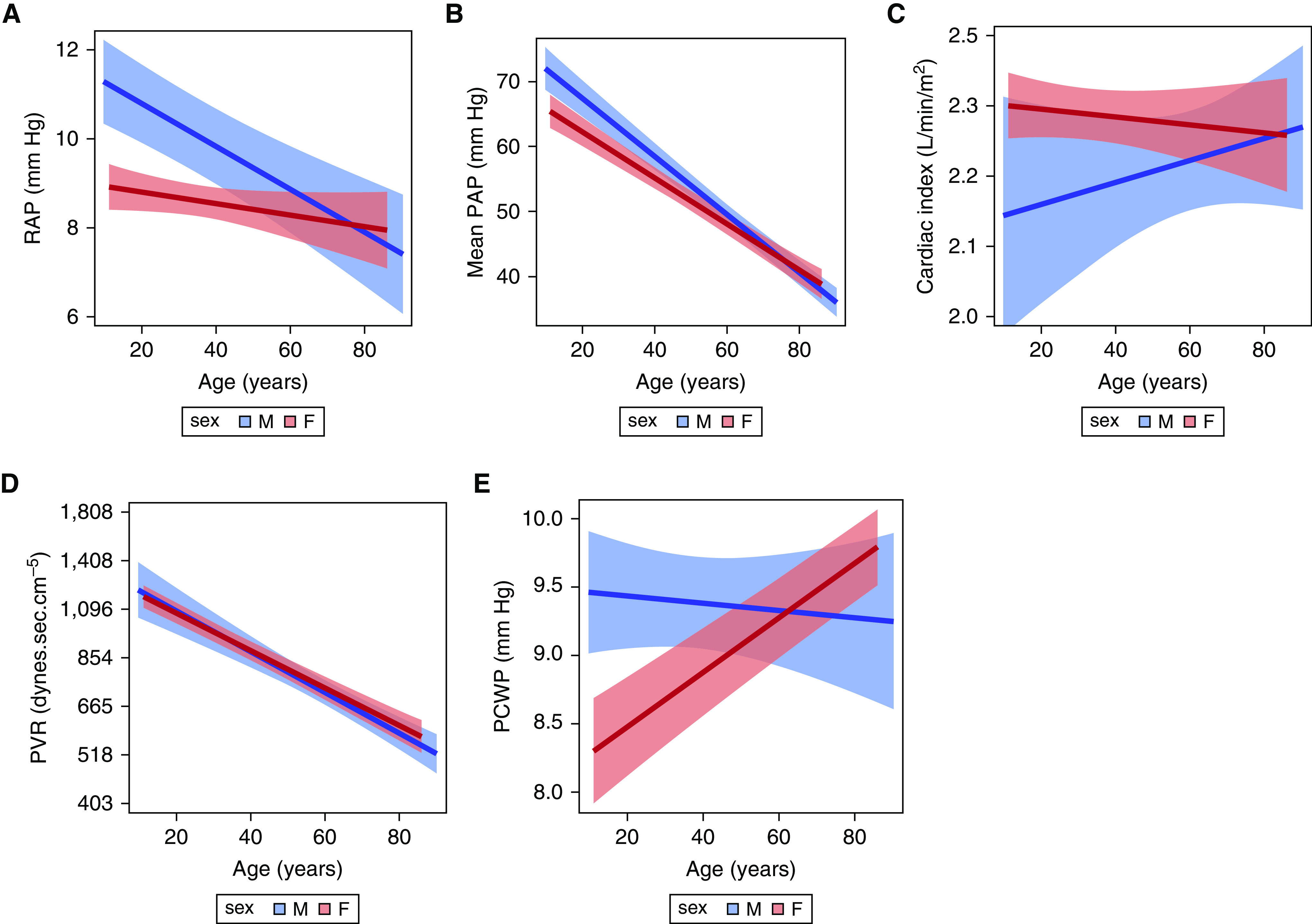
Associations between age and (A) RAP, (B) mean PAP, (C) cardiac index (y-axis values are back-transformed but remain in ln scale), (D) PVR (y-axis values are back-transformed but remain in ln scale), and (E ) PCWP, stratified by sex. Red = females; blue = males. The solid line is the effect estimate; lighter bands are the 95% confidence band. PAP = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RAP = right atrial pressure.
Figure 4.
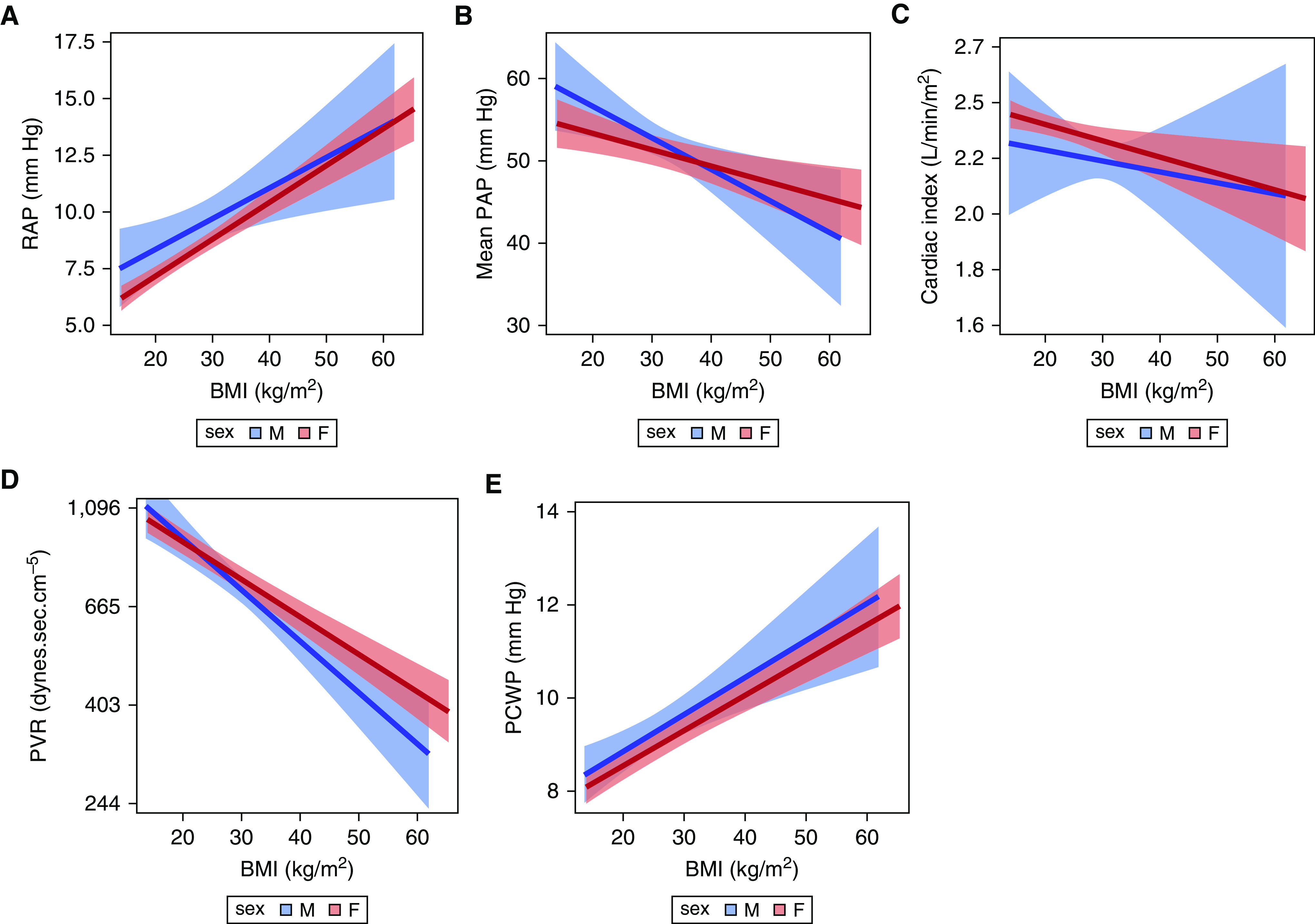
Associations between BMI and (A) RAP, (B) mean PAP, (C) cardiac index (y-axis values are back-transformed but remain in ln scale), (D) PVR (y-axis values are back-transformed but remain in ln scale), and (E ) PCWP. Red = females; blue = males. The solid line is the effect estimate; lighter bands are the 95% confidence band. BMI = body mass index; PAP = pulmonary artery pressure; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RAP = right atrial pressure.
Mean PAP
Females had lower mean PAP as compared with males (P < 0.001) (Figure 3B). The relationship between age and mean PAP differed by sex (P for interaction < 0.001), in which males had a steeper decline in mean PAP with increasing age as compared to females. For every 10-year increase in age for males, mean PAP decreased by 4.5 (3.9–5.0) mm Hg (P < 0.001) versus a decrease of 3.6 (3.1–4.1) mm Hg in females (P < 0.001). As with RAP, there was no evidence of a relationship between BMI and mean PAP by sex (P = 0.09) (Figure 4B).
CO and CI
Males had higher CO as compared with females (4.4 [4.2–4.5] vs. 4.1 [4.0–4.3]; P < 0.001). There were no significant differences observed between age and CO by sex (P for interaction = 0.73). As expected (25), as BMI increased, CO increased (P < 0.001), but these relationships did not differ by sex (P for interaction = 0.48). For every 10 kg/m2 increase in BMI, CO increased 0.1 (0.0–0.1) L/min in females and males.
Females had higher CI than males (P = 0.03) (Figure 3C). This difference attenuated with older age (on the ln scale) but did not reach statistical significance (P for interaction = 0.07). There was no effect modification observed for BMI and CI by sex (P for interaction = 0.75) (Figure 4C).
PVR
Overall, PVR did not significantly vary by sex. As seen in Figure 3D, the relationship between age and PVR (on the ln scale) was not significantly different for females and males (P for interaction = 0.35), but there was a significant overall decrease in PVR with increasing age (P < 0.001). The relationship between BMI and PVR was different for females and males (P for interaction = 0.02) (Figure 4D). For every 1 kg/m2 increase in BMI, there was a 3% decrease in PVR for males (P < 0.001), compared with a 2% decrease in PVR in females (P < 0.001).
PCWP
The relationship between age and PCWP was significantly different for females and males (P for interaction < 0.01) (Figure 3E). Whereas there was no relationship observed between age and PCWP in males, increasing age was strongly associated with increasing PCWP in females (P < 0.001). Increasing BMI was associated with increasing PCWP (P < 0.001), but this did not vary by sex (P for interaction = 0.77) (Figure 4E).
Please see the data supplement for a discussion of results for PA compliance and functional class.
Effect Modification by Idiopathic versus CTD-associated PAH and Background PAH Therapy
Because patients with CTD-associated PAH tend to be older, have more comorbid illness and different hemodynamic profiles than patients with idiopathic PAH (26, 27), we conducted an additional sensitivity analysis to determine whether PAH diagnosis may have impacted our results, particularly with respect to age and BMI. There was no evidence of interaction by idiopathic versus CTD-associated PAH diagnosis in any of the models with sex, age, and BMI (all P values for two- and three-way interactions = 0.08–0.98; data not shown).
Finally, we assessed whether there was evidence of interaction between sex and any background PAH therapy. Females had stronger evidence of effect modification by background treatment than males with respect to 6MWD. At baseline, females on background therapy had higher 6MWD (by roughly 20 m) than treatment-naive females, a difference that was not noted in males (P for interaction, sex × background therapy < 0.0001). For all hemodynamics (except PVR) and functional class, there was no evidence of effect modification by background PAH therapy (all P values for two- and three-way interactions = 0.07–0.99; data not shown). Males on background therapy had about 0.1 Wood unit higher PVR than untreated males, whereas PVR in females did not differ by background therapy (P for interaction, sex × background therapy = 0.03).
Discussion
We have demonstrated, in a large secondary analysis of well-characterized clinical trial subjects, sex-based differences in PAH disease metrics at enrollment. These relationships were modified by age and BMI. Although female trial participants tended to have more favorable hemodynamics than male participants, the interactions among sex, age, and body size were complex and variable for other endpoints, including 6MWD and functional class. Although some of the observed effect sizes were small, cross-sectional differences at baseline may accrue to large differences over the lifespan and with changes in the body size of a patient with PAH. Very small differences in short-term hemodynamics with active treatment in clinical trials translate to reduced odds of important clinical events, including death (28).
In our prior study, which included 11 randomized trials and 1,700 subjects with idiopathic and CTD-associated PAH, we demonstrated that males had worse hemodynamics than females, especially at a younger age, but the effect estimates were generally larger (14). The current study includes additional trials, a substantially larger sample size, and a more heterogeneous group of PAH subtypes, which may have decreased the magnitude of the associations observed. As previously demonstrated, males had lesser hemodynamic derangements (lower RAP and mean PAP and higher CI) with increasing age, which was not seen in females except for PA compliance. The scope of inference for this study extends only to clinical trial participants, but a similar observation has been made in experimental models of pulmonary hypertension, as male and younger animals have a more severe pulmonary hypertensive phenotype (29–31). We have previously demonstrated sex-based differences in hemodynamics outside of clinical trials among armed service veterans with (largely non-World Health Organization Group 1) pulmonary hypertension (32), which adds external validity to our observations. In both females and males, PVR decreased with age, which has also been reported in observational studies and PAH registries (33–35). This may be because of detrimental effects of sex hormones in earlier life (e.g., estrogen), survival, or a type of volunteer- or self-selection bias, in which older or more overweight individuals with PAH feel worse despite lower disease burden, making them more inclined to enroll in trials. The more modest associations seen here may be because of spectrum bias, the exclusion of patients with more extreme severity of illness (both healthy and sick) on the basis of trial eligibility, such as young males with severe disease or long-term female survivors. Although 28% of participants had CTD-associated PAH, findings were similar between participants with idiopathic PAH and CTD-associated PAH in sensitivity analyses. Although background therapy may have sex-specific interactions with some disease metrics (specifically 6MWD and PVR), it is not clear that these differences would be clinically meaningful. Further, potentially eligible subjects were on stable background therapy before enrollment, and nesting by study would account for temporal changes in treatment regimens across trials. Whether our observations about hemodynamics and other disease metrics would hold in more “real world” PAH registries remains to be seen.
Sexual dimorphism in PAH may be explained by differential right ventricular (RV) adaptation to pulmonary afterload. RV function is a critical determinant of outcome in PAH, and women with variable types of pulmonary vascular disease are known to have better RV function than men with pulmonary hypertension (36–40). Although the trials included here did not include RV imaging on the full study samples, female trial participants had lower RAP and higher CI than male participants, which indirectly supports this concept. In a subset of participants in which PA compliance could be calculated, older females had significantly higher PA compliance than younger females but overall lower PA compliance than males. These findings are somewhat discordant with a recent report by Tello and colleagues that demonstrated better PA–RV coupling using more robust measures in females with PAH irrespective of age (40). We and others have reported sex-based differences in hemodynamic measures and RV function and morphology (14, 36) previously, but the inclusion of 6MWD and functional class here is novel. Although 6MWD has been the basis for most drug approvals in PAH and 6MWD and functional class are integral to risk assessment and therapeutic escalation (5, 7, 15, 41), to our knowledge, no studies have examined how key demographic and anthropometric features influence these outcomes in PAH. To this point, age, sex, and BMI were among the components that contributed to unique PAH phenotypes with varied treatment responses and survival in a recent cluster analysis from the Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension (COMPERA) registry (17).
Females had significantly shorter 6MWD than males, and higher BMI was associated with shorter 6MWD. This may be because of differences in stride length influenced by height, unaccounted-for comorbidities including deconditioning or differences in systemic musculature, or occult heart failure with preserved ejection fraction. Males had sharper decreases in PVR and increases in PA compliance with increasing BMI. Although this is beyond the scope of the primary data collected in PAH trials, higher degrees of insulin resistance in females could explain our findings, as insulin resistance has been associated with more severe PAH, particularly in females (42, 43). The prevalence of diabetes mellitus was similar across males and females in the clinical trials we included, however.
If sex, age, and BMI influence major endpoints in PAH trials, failure to recognize these relationships from the outset may lead to erroneous conclusions or apparent treatment heterogeneity in terms of therapeutic efficacy. This would also apply to subgroup analyses of trials that examine treatment effects by sex, age, and BMI in isolation, without considering their interactions. The purpose of our study was to examine the degree to which there are sex-, age-, and BMI-based interactions at baseline in clinical trials and not treatment-based interactions. We conducted a one-stage analysis with primary, patient-level data, for this reason, the preferable way to minimize the risk of ecological fallacy (24). The pooling of results at the study level could be subject to the same ecological fallacy, known as Simpson’s paradox (44).
Effect estimates for currently approved PAH therapies may be larger or smaller than originally reported. Knowledge of how baseline demographic and anthropometric characteristics impact potential endpoints is important for planning efficient trials. In clinical trials in a rare disease like PAH, treatment allocation may vary by sex or age (because of random imbalance at baseline in trials of relatively small sample size, and especially given the sex bias that exists in PAH) and could translate into bias in key primary and secondary endpoints like 6MWD. As we move toward more precision-based approaches in PAH (45), these key characteristics and their interrelationships should be considered and may inform adaptive trial design in the future. A stratified randomization trial (e.g., permuted block, urn, or urn block) would balance the main analysis (placebo vs. active treatment) by demographic features that affect 6MWD (the primary endpoint and the basis for drug approval for most PAH therapies), thus optimizing the detection of any difference by treatment that exists. In addition, this design would allow for interaction effects (moderators such as sex, age, and BMI) using balanced subgroup analysis. Thus, our results suggest the very real need for this type of random allocation design to be implemented in RCTs in PAH.
Limitations
Although this analysis used the largest study of individual data from PAH clinical trials to our knowledge, there were limitations. Our conclusions only extend to PAH subjects who would have been eligible for enrollment in these clinical trials. We are unable to make inferences about patients who would have been excluded (e.g., patients with 6MWDs above or below threshold values and functional class IV patients) and have almost no data on screen failures. Most participants (67% of females and 74% of males) were white, and we were underpowered to examine ethnicity-based interactions. Future studies should enrich underrepresented individuals and should expand beyond biological sex to study gender and intersectionality in persons living with PAH. This is a likely biased sample, only comprised of patients in clinical trials submitted to the FDA for drug approval. All the trials included were efficacy (not effectiveness) trials, and as such, it is impossible to quantify the possible effect this selection bias had on the results. As our sample contains nearly all phase III trials conducted in PAH, there is less of a risk of publication bias (i.e., P values are not dictating what is reported); however, studies of ineffective drugs would not be submitted to the FDA, and, therefore would not be included.
Some of the observed associations with hemodynamics were inconsistent (e.g., sex-based differences in mean PAP and CI but not PVR). Measurement error, which is inflated with calculated values like PVR and PA compliance, may have biased toward the null. Although our analyses were conducted at the level of the individual participant, it would have to be the case that most female participants had the same pattern of higher CI, lower mean PAP, and lower PVR (and the inverse pattern for nearly all males) for example, which is unlikely. Whether hemodynamic measures (CO and PVR) should be routinely indexed to body surface area is controversial, but our data suggest consideration of body size is important (46). We have included baseline observations only. Comparative effectiveness studies considering sex, age and body size, and the response to PAH treatment(s) are beyond the scope of the current study and will be the focus of future studies. As there is emerging evidence that the response to PAH treatments may vary by sex (12, 13, 17, 36), varied background treatments may have contributed to our findings. Treatment-induced changes in these endpoints should be investigated with sex-, age-, and anthropomorphic-based interactions in mind.
Conclusions
There are sex-based differences in common disease surrogates in PAH, including 6MWD, functional class, and hemodynamics among clinical trial participants. These relationships are modified by age and BMI. Sex, age, and body size should be considered in the evaluation and interpretation of surrogates and therapeutic efficacy in PAH.
Acknowledgments
Acknowledgment
The authors thank all patients with PAH who have participated in clinical trials.
Footnotes
Supported by the Cardiovascular Medical Research and Education Fund (S.M.K.) and the National Institutes of Health/National Heart, Lung, and Blood Institute (R01 HL141268 [C.E.V.]; K24 HL103844 [S.M.K.]; and T32-HL007891 [J. Minhas and J. Min]).
Author Contributions: C.E.V.: study concept and design, results interpretation, and drafting and revision of the manuscript. G.L.B.: data analysis, results interpretation, and drafting and revision of the manuscript. J. Moutchia, D.H.A., R.L.M., J. Minhas, J. Min, J.H.H., R.J.U., and N.A.-N.: data cleaning and harmonization, analytic approach, and revision of the manuscript. S.M.K.: data procurement, study design, and revision of the manuscript.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Kawut SM, Archer-Chicko CL, DeMichele A, Fritz JS, Klinger JR, Ky B, et al. Anastrozole in pulmonary arterial hypertension. A randomized, double-blind, placebo-controlled trial. Am J Respir Crit Care Med . 2017;195:360–368. doi: 10.1164/rccm.201605-1024OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hester J, Ventetuolo C, Lahm T. Sex, gender, and sex hormones in pulmonary hypertension and right ventricular failure. Compr Physiol . 2019;10:125–170. doi: 10.1002/cphy.c190011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walsh TP, Baird GL, Atalay MK, Agarwal S, Arcuri D, Klinger JR, et al. Experimental design of the effects of dehydroepiandrosterone in pulmonary hypertension (EDIPHY) trial. Pulm Circ . 2021;11:2045894021989554. doi: 10.1177/2045894021989554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kawut SM, Pinder D, Al-Naamani N, McCormick A, Palevsky HI, Fritz J, et al. Fulvestrant for the treatment of pulmonary arterial hypertension. Ann Am Thorac Soc . 2019;16:1456–1459. doi: 10.1513/AnnalsATS.201904-328RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boucly A, Weatherald J, Savale L, Jais X, Cottin V, Prevot G, et al. Risk assessment, prognosis and guideline implementation in pulmonary arterial hypertension. Eur Respir J . 2017;50:1700889. doi: 10.1183/13993003.00889-2017. [DOI] [PubMed] [Google Scholar]
- 6. Benza RL, Gomberg-Maitland M, Elliott CG, Farber HW, Foreman AJ, Frost AE, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest . 2019;156:323–337. doi: 10.1016/j.chest.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 7. Hoeper MM, Kramer T, Pan Z, Eichstaedt CA, Spiesshoefer J, Benjamin N, et al. Mortality in pulmonary arterial hypertension: prediction by the 2015 European pulmonary hypertension guidelines risk stratification model. Eur Respir J . 2017;50:1700740. doi: 10.1183/13993003.00740-2017. [DOI] [PubMed] [Google Scholar]
- 8. Enright PL, Sherrill DL. Reference equations for the six-minute walk in healthy adults. Am J Respir Crit Care Med . 1998;158:1384–1387. doi: 10.1164/ajrccm.158.5.9710086. [DOI] [PubMed] [Google Scholar]
- 9. Britto RR, Probst VS, de Andrade AF, Samora GA, Hernandes NA, Marinho PE, et al. Reference equations for the six-minute walk distance based on a Brazilian multicenter study. Braz J Phys Ther . 2013;17:556–563. doi: 10.1590/S1413-35552012005000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miyamoto S, Nagaya N, Satoh T, Kyotani S, Sakamaki F, Fujita M, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med . 2000;161:487–492. doi: 10.1164/ajrccm.161.2.9906015. [DOI] [PubMed] [Google Scholar]
- 11. Gabler NB, French B, Strom BL, Palevsky HI, Taichman DB, Kawut SM, et al. Validation of 6-minute walk distance as a surrogate end point in pulmonary arterial hypertension trials. Circulation . 2012;126:349–356. doi: 10.1161/CIRCULATIONAHA.112.105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gabler NB, French B, Strom BL, Liu Z, Palevsky HI, Taichman DB, et al. Race and sex differences in response to endothelin receptor antagonists for pulmonary arterial hypertension. Chest . 2012;141:20–26. doi: 10.1378/chest.11-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mathai SC, Hassoun PM, Puhan MA, Zhou Y, Wise RA. Gender differences in response to tadalafil in pulmonary arterial hypertension. Chest . 2014;147:188–197. doi: 10.1378/chest.14-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ventetuolo CE, Praestgaard A, Palevsky HI, Klinger JR, Halpern SD, Kawut SM. Sex and haemodynamics in pulmonary arterial hypertension. Eur Respir J . 2014;43:523–530. doi: 10.1183/09031936.00027613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benza RL, Gomberg-Maitland M, Miller DP, Frost A, Frantz RP, Foreman AJ, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest . 2012;141:354–362. doi: 10.1378/chest.11-0676. [DOI] [PubMed] [Google Scholar]
- 16. Shapiro S, Traiger GL, Turner M, McGoon MD, Wason P, Barst RJ. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest . 2012;141:363–373. doi: 10.1378/chest.10-3114. [DOI] [PubMed] [Google Scholar]
- 17. Hoeper MM, Pausch C, Grünig E, Klose H, Staehler G, Huscher D, et al. Idiopathic pulmonary arterial hypertension phenotypes determined by cluster analysis from the COMPERA registry. J Heart Lung Transplant . 2020;39:1435–1444. doi: 10.1016/j.healun.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 18. Mair KM, Harvey KY, Henry AD, Hillyard DZ, Nilsen M, MacLean MR. Obesity alters oestrogen metabolism and contributes to pulmonary arterial hypertension. Eur Respir J . 2019;53:1801524. doi: 10.1183/13993003.01524-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ventetuolo CE, Baird GL, Barr RG, Bluemke DA, Fritz JS, Hill NS, et al. Higher estradiol and lower dehydroepiandrosterone-sulfate levels are associated with pulmonary arterial hypertension in men. Am J Respir Crit Care Med . 2015;193:1168–1175. doi: 10.1164/rccm.201509-1785OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baird GL, Archer-Chicko C, Barr RG, Bluemke DA, Foderaro AE, Fritz JS, et al. Lower DHEA-S levels predict disease and worse outcomes in post-menopausal women with idiopathic, connective tissue disease- and congenital heart disease-associated pulmonary arterial hypertension. Eur Respir J . 2018;51:1800467. doi: 10.1183/13993003.00467-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baird GL, Walsh T, Aliotta J, Allahua M, Andrew R, Bourjeily G, et al. Insights from the menstrual cycle in pulmonary arterial hypertension. Ann Am Thorac Soc . 2020;18:218–228. doi: 10.1513/AnnalsATS.202006-671OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Clayton JA, Tannenbaum C. Reporting sex, gender, or both in clinical research? JAMA . 2016;316:1863–1864. doi: 10.1001/jama.2016.16405. [DOI] [PubMed] [Google Scholar]
- 23. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J . 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kontopantelis E. A comparison of one-stage vs two-stage individual patient data meta-analysis methods: a simulation study. Res Synth Methods . 2018;9:417–430. doi: 10.1002/jrsm.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. de Divitiis O, Fazio S, Petitto M, Maddalena G, Contaldo F, Mancini M. Obesity and cardiac function. Circulation . 1981;64:477–482. doi: 10.1161/01.cir.64.3.477. [DOI] [PubMed] [Google Scholar]
- 26. Lefèvre G, Dauchet L, Hachulla E, Montani D, Sobanski V, Lambert M, et al. Survival and prognostic factors in systemic sclerosis-associated pulmonary hypertension: a systematic review and meta-analysis. Arthritis Rheum . 2013;65:2412–2423. doi: 10.1002/art.38029. [DOI] [PubMed] [Google Scholar]
- 27. Fisher MR, Mathai SC, Champion HC, Girgis RE, Housten-Harris T, Hummers L, et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis Rheum . 2006;54:3043–3050. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 28. Ventetuolo CE, Gabler NB, Fritz JS, Smith KA, Palevsky HI, Klinger JR, et al. Are hemodynamics surrogate end points in pulmonary arterial hypertension? Circulation . 2014;130:768–775. doi: 10.1161/CIRCULATIONAHA.114.009690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rabinovitch M, Gamble WJ, Miettinen OS, Reid L. Age and sex influence on pulmonary hypertension of chronic hypoxia and on recovery. Am J Physiol . 1981;240:H62–H72. doi: 10.1152/ajpheart.1981.240.1.H62. [DOI] [PubMed] [Google Scholar]
- 30. McMurtry IF, Frith CH, Will DH. Cardiopulmonary responses of male and female swine to simulated high altitude. J Appl Physiol . 1973;35:459–462. doi: 10.1152/jappl.1973.35.4.459. [DOI] [PubMed] [Google Scholar]
- 31. Resta TC, Kanagy NL, Walker BR. Estradiol-induced attenuation of pulmonary hypertension is not associated with altered eNOS expression. Am J Physiol Lung Cell Mol Physiol . 2001;280:L88–L97. doi: 10.1152/ajplung.2001.280.1.L88. [DOI] [PubMed] [Google Scholar]
- 32. Ventetuolo CE, Hess E, Austin ED, Barón AE, Klinger JR, Lahm T, et al. Sex-based differences in veterans with pulmonary hypertension: results from the Veterans Affairs Clinical Assessment Reporting and Tracking database. PLoS One . 2017;12:e0187734. doi: 10.1371/journal.pone.0187734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med . 2012;186:790–796. doi: 10.1164/rccm.201203-0383OC. [DOI] [PubMed] [Google Scholar]
- 34. Hoeper MMHD, Huscher D, Ghofrani HA, Delcroix M, Distler O, Schweiger C, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol . 2013;168:871–880. doi: 10.1016/j.ijcard.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 35. Badagliacca R, D'Alto M, Ghio S, Argiento P, Bellomo V, Brunetti ND, et al. Risk reduction and hemodynamics with initial combination therapy in pulmonary arterial hypertension. Am J Respir Crit Care Med . 2021;203:484–492. doi: 10.1164/rccm.202004-1006OC. [DOI] [PubMed] [Google Scholar]
- 36. Jacobs W, van de Veerdonk MC, Trip P, de Man F, Heymans MW, Marcus JT, et al. The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest . 2014;145:1230–1236. doi: 10.1378/chest.13-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kawut SM, Al-Naamani N, Agerstrand C, Berman Rosenzweig E, Rowan C, Barst RJ, et al. Determinants of right ventricular ejection fraction in pulmonary arterial hypertension. Chest . 2009;135:752–759. doi: 10.1378/chest.08-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J . 2014;35:3452–3462. doi: 10.1093/eurheartj/ehu193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prins KW, Rose L, Archer SL, Prtizker M, Weir EK, Olson MD, et al. Clinical determinants and prognostic implications of right ventricular dysfunction in pulmonary hypertension caused by chronic lung disease. J Am Heart Assoc . 2019;8:e011464. doi: 10.1161/JAHA.118.011464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tello K, Richter MJ, Yogeswaran A, Ghofrani HA, Naeije R, Vanderpool R, et al. Sex differences in right ventricular-pulmonary arterial coupling in pulmonary arterial hypertension. Am J Respir Crit Care Med . 2020;202:1042–1046. doi: 10.1164/rccm.202003-0807LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Galie N, Hoeper M, Humbert M, Torbicki A, Vachiery J-L, Barbera JA, et al. ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) Eur Heart J . 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 42. Zamanian RT, Hansmann G, Snook S, Lilienfeld D, Rappaport KM, Reaven GM, et al. Insulin resistance in pulmonary arterial hypertension. Eur Respir J . 2009;33:318–324. doi: 10.1183/09031936.00000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brunner NW, Skhiri M, Fortenko O, Hsi A, Haddad F, Khazeni N, et al. Impact of insulin resistance on ventricular function in pulmonary arterial hypertension. J Heart Lung Transplant . 2014;33:721–726. doi: 10.1016/j.healun.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 44. Baduashvili A. Simpson’s paradox: meta-analysis comparing torsemide versus furosemide in patients with heart failure. Am J Cardiol . 2020;125:1285. doi: 10.1016/j.amjcard.2020.01.031. [DOI] [PubMed] [Google Scholar]
- 45. Austin ED, Loyd JE. Toward precision medicine in pulmonary arterial hypertension. Am J Respir Crit Care Med . 2015;192:1272–1274. doi: 10.1164/rccm.201508-1607ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Weatherald J, Boucly A, Lau E, Godinas L, Savale L, Jaïs X, et al. Are indexed values better for defining exercise pulmonary hypertension? Eur Respir J . 2017;50:1700240. doi: 10.1183/13993003.00240-2017. [DOI] [PubMed] [Google Scholar]