Abstract
Rationale
Periextubation corticosteroids are commonly used in children to prevent upper airway obstruction (UAO). However, the best timing and dose combination of corticosteroids is unknown.
Objectives
To compare effectiveness of different corticosteroid regimens in preventing UAO and reintubation.
Methods
MEDLINE, CINAHL, and Embase search identified randomized trials in children using corticosteroids to prevent UAO. All studies used dexamethasone. The studies were categorized based on timing of initiation of dexamethasone (early use: >12 h before extubation) and the dose (high dose: ⩾0.5 mg/kg/dose). We performed Bayesian network meta-analysis with studies grouped into four regimens: high dose, early use (HE); low dose, early use (LE); high dose, late use (HL); and low dose, late use.
Results
Eight trials (n = 903) were included in the analysis. For preventing UAO (odds ratio; 95% credible interval), HE (0.13; 0.04–0.36), HL (0.39; 0.19–0.74), and LE (0.15; 0.04–0.58) regimens appear to be more effective than no dexamethasone (low certainty). HE and LE had the highest probability of being the top-ranked regimens for preventing UAO (surface under the cumulative ranking curve 0.901 and 0.808, respectively). For preventing reintubation, the effect estimate was imprecise for all four dexamethasone regimens compared with no dexamethasone (very low certainty). HE and LE were the top-ranked regimens (surface under the cumulative ranking curve 0.803 and 0.720, respectively) for preventing reintubation. Sensitivity analysis showed that regimens that started >12 hours before extubation were likely more effective than regimens started >6 hours before extubation.
Conclusions
Periextubation dexamethasone can prevent postextubation UAO in children, but effectiveness is highly dependent on timing and dosing regimen. Early initiation (ideally >12 h before extubation) appears to be more important than the dose of dexamethasone. Ultimately, the specific steroid strategy should be personalized, considering the potential for adverse events associated with dexamethasone and the individual risk of UAO and reintubation.
Keywords: dexamethasone, upper airway obstruction, extubation, meta-analysis
Postextubation upper airway obstruction (UAO) is a common complication of pediatric endotracheal intubation. Although the causes and anatomic locations are multiple, edema in the subglottic space is among the most common etiologies for postextubation UAO, which can lead to increased respiratory load after extubation and extubation failure (1). Postextubation UAO is reported to contribute to reintubation in 37% of children undergoing elective extubation (2).
Preextubation corticosteroids have been used for decades to prevent postextubation UAO and extubation failure (3). However, corticosteroid treatment regimens vary substantially based on the medication used, dose, timing, and the number of doses administered. The optimal combination of dose and timing of corticosteroids to prevent postextubation UAO in children is unknown, despite numerous randomized controlled trials (RCT). Standard meta-analyses with statistical pooling have been conducted, but they are not able to determine if one dosing regimen is superior to another (4). Network meta-analysis (NMA) can distinguish the relative efficacy of different regimens of corticosteroids in preventing postextubation UAO (5).
The objective of this study is to perform a standard pairwise meta-analysis and a network meta-analysis of all the pediatric trials of preextubation corticosteroids to determine 1) whether corticosteroids are effective in preventing or reducing the severity of postextubation UAO and reintubation; and 2) what combination of corticosteroid dose and timing is most effective in preventing postextubation UAO and reintubation.
Methods
We used the Preferred Reporting Items for Systematic Reviews and Metanalyses (PRISMA) checklist for network metanalyses to prepare this report (see Table E1 in the online supplement) (6). This review was conducted as part of a project to develop clinical practice guidelines for ventilator liberation in children. The protocol for the systematic review was submitted to the international prospective register for systematic reviews, PROSPERO, at the University of York, United Kingdom, and the application was accepted in January 2021 (registration number CRD42021228702). Details of the protocol for the systematic review can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42021228702.
Review Question
In acutely hospitalized children receiving invasive mechanical ventilation (IMV) for >24 hours, should systemic corticosteroids be administered before extubation to prevent postextubation UAO?
Outcomes were selected before the literature search. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to rate outcomes into three categories based on their importance for decision making: 1) critical; 2) important but not critical; and 3) outcomes of limited importance (7). Using this process, the panel of experts categorized the outcomes as following:
-
1.
Critical outcomes: mortality, failure to liberate from IMV (i.e., reintubation), total duration of IMV, pediatric intensive care unit (PICU) length of stay (LOS), postextubation UAO.
-
2.
Important outcomes: liberation from noninvasive respiratory support, ventilator-free days, new tracheostomy rate, total duration of noninvasive respiratory support, hospital LOS, pressure injuries, effort of breathing, crossover to other treatments.
-
3.
Outcome of limited importance: gastrointestinal (GI) bleeding, transient hypertension.
Literature Search
Comprehensive search strategies were composed and conducted by two medical librarians in MEDLINE (Ovid), Embase (Elsevier), and CINAHL Complete (EBSCO) on March 10, 2021 and rerun again on January 18, 2022 for all human studies that include children 18 years or younger. There were no language or date limitations. The complete search strategy is provided in Table E2. Pairs of reviewers independently screened the title and abstracts and performed full-text review. Any conflicts were resolved by a third reviewer. Title, abstract screening, and full-text review were performed using the systematic review software Covidence (Veritas Health Innovation; https://www.covidence.org/). We used the following eligibility criteria:
-
1.
Patients: We included studies conducted in the PICU or the pediatric cardiac intensive care unit that were performed on critically ill children up to age 18 years, receiving IMV for >24 hours, who underwent or were scheduled for planned ventilator liberation. We excluded studies involving preterm infants or where extubation occurred outside the intensive care units (e.g., operating rooms).
-
2.
Study type: We included randomized trials evaluating the use of corticosteroids before extubation.
Data Collection
Data abstraction was done by a pair of independent reviewers using standardized data extraction forms in Redcap (8).
Risk of Bias within Individual Studies
Risk of bias of included studies was assessed using the Cochrane tool for the assessment of risk of bias in randomized trials (RoB 2.0) (9).
Data Synthesis
We planned two meta-analyses: a standard pairwise meta-analysis with different corticosteroid regimens pooled as one and an NMA where we lumped studies based on the dose used and the timing of initiation of corticosteroids relative to the time of extubation.
Pairwise meta-analysis
Based on a previously published meta-analysis, we expected most studies to compare corticosteroids with a placebo or no corticosteroids (4). Expecting different dosing regimens, we planned a random effects model for the analysis.
Network meta-analysis
Nodes (interventions in a network plot) were determined by the dose of systemic corticosteroid used and the timing of the first dose in relation to the extubation. The only corticosteroid used in the included studies was dexamethasone. Therefore, nodes were determined based on dexamethasone dose and timing. Intravenous dexamethasone ⩾0.5 mg/kg/dose was considered high dose. Initiation of systemic corticosteroids ⩾12 hours before extubation was considered early corticosteroid use (12-h model). These criteria test the hypotheses that the effectiveness of dexamethasone in reducing upper airway edema may be dependent on the dose of corticosteroid used as well as the number and timing of preextubation doses administered. This classification of interventions led to four nodes: early use of high-dose corticosteroids (HE), early use of low-dose corticosteroids (LE), late use of high-dose corticosteroids (HL), and late use of low-dose corticosteroids (LL). The arm with no corticosteroid or placebo constituted the fifth node. A sensitivity analysis was planned, with early use being defined as >6 hours before extubation (6-h model). The 6-hour duration was tested based on the notion that initiation of corticosteroids 12 hours before extubation is more likely to delay extubation by 1 day than corticosteroid initiation 6 hours before extubation.
Statistical Analysis
We performed the NMA using a Bayesian analytic framework. A Bayesian approach has been preferred for network meta-analysis because it is better able to handle studies with very few or zero events and produce probability and ranking outputs that are intuitive to end users (5). The effect of the intervention for dichotomous outcomes was summarized as odds ratio and 95% credible interval (CrI); for continuous measures, data were summarized as mean difference and 95% CrI. A Bayesian random effects model for network meta-analysis was adopted because it assumes and accounts for unexplained heterogeneity across studies.
Because of relatively sparse data, imposing a random effects model generally requires the adoption of Bayesian methods with informative priors on between-trials heterogeneity. An empirical study conducted by Turner and colleagues provides the basis for choosing a plausible prior for the between-studies variance parameter (in our analysis a log normal distribution [−3.02 to 1.852]), which is assumed to be equal across comparisons (10).
The analysis was conducted with the Markov chain Monte Carlo methods (11). Four Markov chains, yielding 400,000 iterations (100,000 iterations per chain after an initial burn-in of 10,000 and a thinning of 10) generating the posterior distributions of the model parameters, were performed.
Convergence was checked by using the Brooks-Gelman-Rubin diagnostic (12). The goodness of fit of the model was assessed with residual deviance (11). The I2 statistic was used to assess statistical heterogeneity. We used the node-splitting approach to calculate the Bayesian P value to determine inconsistency (13).
Different interventions were ranked using the surface under the cumulative ranking curve (SUCRA) and the rank probabilities generated by the Bayesian approach. SUCRA is expressed as a percentage and provides the relative probability of an intervention being the best among all the options (14). SUCRA of 1 for an intervention indicates that the intervention is certain to be the best among all the interventions tested, whereas a SUCRA of 0 indicates that the intervention is certain to be the worst among the treatments tested. It is recommended that the ranks be interpreted in the context of the certainty of evidence and the absolute risk reduction (ARR) of the pairwise comparisons (15, 16).
The standard pairwise meta-analysis with all systemic corticosteroid regimens pooled as one was performed using RevMan 5.4 (The Cochrane Collaboration, 2020). The network meta-analysis was conducted using the GeMTC package of R version 3.5.3 (RStudio) (17).
Assessment of Certainty of the Evidence
We assessed certainty of evidence using recently published guidance by the GRADE working group (18–20). Thresholds for ARR were determined by a survey of authors. The authors considered a difference of >3% (>30 per 1,000 ARR) in reintubations, a difference of >10% (>100 per 1,000 ARR) in UAO (assuming a third of patients with UAO get reintubated), a difference in PICU LOS of >24 hours, and a difference in length of IMV difference of >12 hours as clinically significant.
Results
A total of 11,235 records were screened, of which 11,107 were excluded. The full texts of 128 records were assessed for eligibility. A total of eight randomized trials fulfilled the eligibility criteria and were included in the analysis (21–28). All the included studies used dexamethasone as the corticosteroid. Thus, results and conclusions of this review are limited to the use of dexamethasone for the prevention of postextubation UAO. Figure 1 shows the reason for exclusion of records during the full-text review (29, 30).
Figure 1.

Preferred reporting items for systematic reviews and meta-analyses flow diagram showing flow of information through the different phases of the systematic review. NICU = neonatal intensive care unit.
The eight trials had a total sample size of 903 subjects. Three studies performed per-protocol analysis (21, 26, 27), and we included 835 total subjects in our meta-analysis. One randomized trial was not included in our analysis because stridor was reported as a continuous outcome rather than a dichotomous outcome, it included two neonates, and three participants in the placebo group received dexamethasone (31). Details of the study characteristics are provided in Table E3. Six trials compared dexamethasone to either isotonic saline or no placebo (21–25, 28). Two studies compared different dosing regimens of dexamethasone to each other (26, 27). The dexamethasone dose used in the trials ranged from 0.15 mg/kg/dose to 1 mg/kg/dose, with a maximum dose of 10 mg. Time of initiation of dexamethasone ranged from 1 to 24 hours before extubation. Total number of doses ranged from three to six doses, with some doses given after extubation. All studies used a 6-hour dosing interval. All studies reported reintubation and UAO, five studies reported length of IMV, three studies reported PICU LOS, four studies reported GI bleeding, and three studies reported the incidence of hypertension. Only data for reintubation and UAO were available for all the nodes in the network analysis.
There was some risk of bias across the studies included in the NMA for both reintubation and UAO (Figures E1A and E1B). One study had a high risk for bias in the assessment of UAO due to lack of blinding (23). In addition, in two studies the concealment of allocation was not clear (22, 24), and one study performed per-protocol analysis without sufficient reason to do so (21).
Effects of the Interventions
In the standard pairwise meta-analysis, we combined the six studies (n = 473) that compared intravenous dexamethasone to no dexamethasone. Data for reintubation, UAO, length of IMV, PICU LOS, GI bleeding, and hypertension were pooled across studies (Figure 2). Dexamethasone was associated with a trend for a lower rate of reintubation (odds ratio, 0.55; 95% Confidence Interval (CI), 0.21–1.46; low certainty). Dexamethasone use was associated with a significantly lower rate of UAO (odds ratio, 0.40; 95% CI, 0.21–0.73; moderate certainty). Three studies (n = 298) reported a decrease in length of IMV (mean difference, −0.27 days; 95% CI, −0.89 to 0.35 days) with dexamethasone use (23, 24, 28), although this did not meet the threshold for either clinical or statistical significance. Dexamethasone was associated with (two studies, n = 145) a modest and statistically nonsignificant increase in PICU LOS (mean difference, 0.44 days; 95% CI, −0.66 to 1.55 days; very low certainty) (23, 28). There were very few adverse events reported: two studies (n = 146) reported one subject with GI bleeding (in the dexamethasone group) (21, 25), and three studies (n = 235) reported two subjects with hypertension (one each in dexamethasone and placebo groups) (21, 23, 25).
Figure 2.

Forest plot of effect estimates and 95% CIs of the pairwise meta-analysis. CI = confidence intervals; GI = gastrointestinal; IMV = invasive mechanical ventilation; IV = inverse variance; M-H = mantel-haenszel; PICU = pediatric intensive care unit; SD = standard deviation.
In the NMA, we grouped the eight trials (n = 835) into five nodes evaluating outcomes of UAO and reintubation. In the 12-hour model (early use defined as dexamethasone initiation ≥12 h before extubation), three studies were included in the HE node (n = 447) (23, 26, 27), four studies in the HL node (n = 400) (21, 24, 26, 28), one study in the LE node (n = 238) (27), two studies in the LL node (n = 112) (22, 25), and six studies were included in the no-corticosteroid node (n = 473) (21–25, 28). Tables 1 and 2 describe the relative effect estimates and absolute estimates for UAO and reintubation of the nodes with dexamethasone compared with the no-dexamethasone node. For UAO, the largest absolute risk reduction (34–36% reduction), with the baseline risk of 46%, was seen with early dosing regimens (number needed to treat of 2.8). For preventing UAO, HE, HL, and LE all appear to be effective. HE had the highest probability of being the first-ranked intervention, with a SUCRA of 0.901. The effect estimate for reintubation was imprecise for all four intervention groups when compared with no dexamethasone. Among the interventions, HE had the highest probability of being the first rank for preventing reintubation, with a SUCRA of 0.803. The summary effects of all the comparisons together with the GRADE certainty of evidence estimates is provided in Figures 4 and 5. Analysis for both outcomes reached convergence using the Brooks-Gelman-Rubin diagnostic, with the overall potential scale reduction factor <1.005. Closed network loops for both UAO and reintubation did not show any inconsistency.
Table 1.
Summary of findings for 12-hour model

|
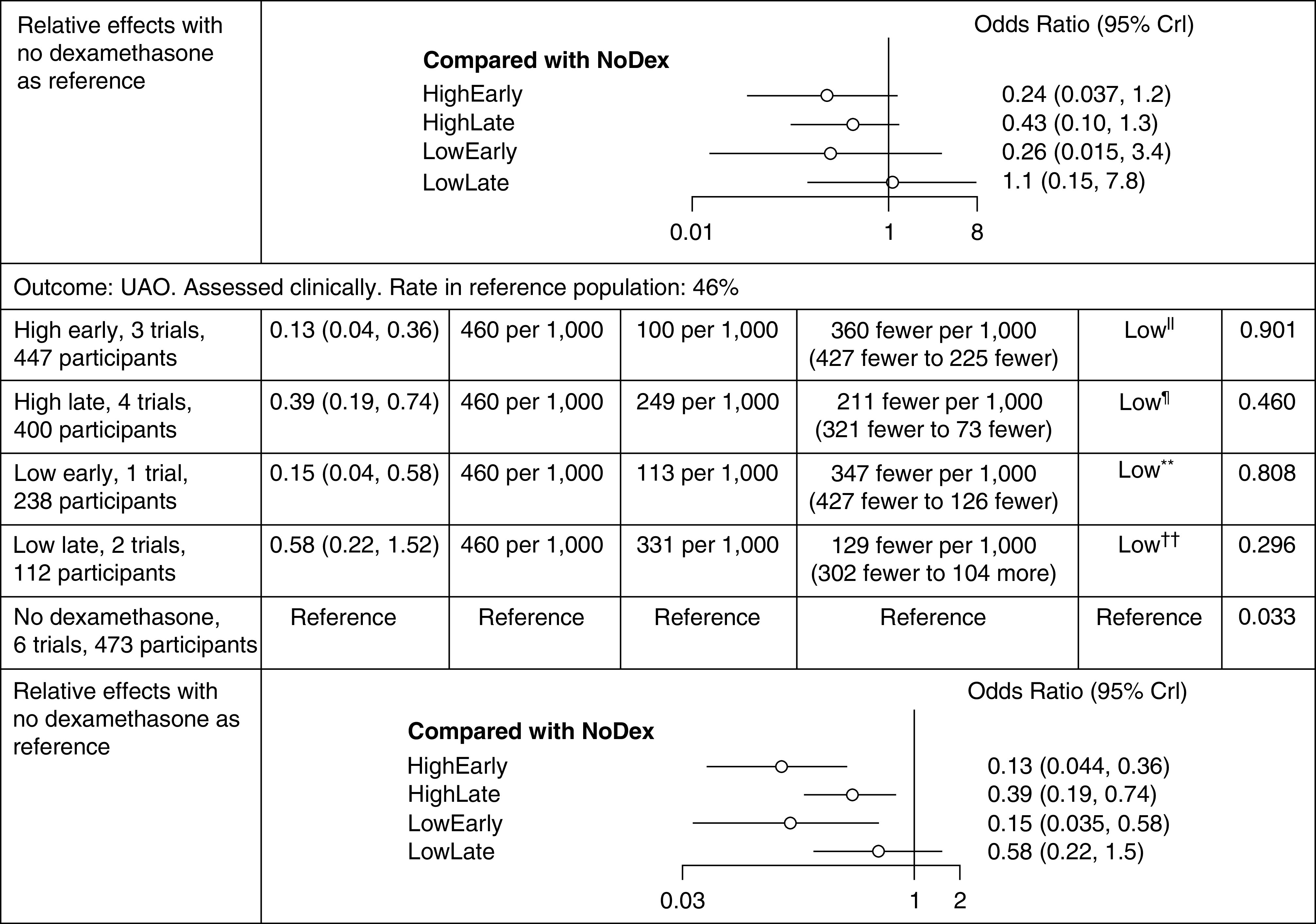
|
Definition of abbreviations: CICU = cardiac intensive care unit; Crl = credible interval; PICU = pediatric intensive care unit; SUCRA = surface under the cumulative ranking curve; UAO = upper airway obstruction.
One study with high risk of bias, indirectness (single direct study), imprecision.
All studies with some risk of bias, serious inconsistency in direct comparison, imprecision.
No direct comparison, imprecision.
Some risk of bias, very serious imprecision in direct comparison.
One study with high risk of bias, indirectness (single direct study)
All studies with some risk of bias, serious inconsistency in direct comparison.
No direct comparison.
Some risk of bias, serious imprecision in direct comparison.
Table 2.
Summary of findings for 6-h model

|

|
Definition of abbreviations: CICU = cardiac intensive care unit; Crl = credible interval; PICU = pediatric intensive care unit; SUCRA = surface under the cumulative ranking curve; UAO = upper airway obstruction.
Multiple studies with some risk of bias, serious inconsistency due in direct comparison, imprecision.
Indirectness (single direct study), very serious imprecision in direct comparison.
Multiple studies with some risk of bias, serious inconsistency due in direct comparison.
Indirectness (single direct study), serious imprecision in direct comparison.
Figure 4.

Effect estimates (95% Crl) and GRADE certainty of effect estimate for all comparisons in the 12-hour model. Crl = credible interval; OR = odds ratio; UAO = upper airway obstruction.
Figure 5.

Effect estimates (95% Crl) and GRADE certainty of effect estimate for all comparisons in the 6-hour model. Crl = credible interval; OR = odds ratio; UAO = upper airway obstruction.
Sensitivity analysis that used 6 hours instead of 12 hours as the cutoff for early initiation of corticosteroids showed similar results, although the benefits of early use of steroids were less clear, with odds ratios closer to 1 (Table 2) compared with the 12-hour model (Table 1). In this 6-hour model, HE and HL appeared to be associated with lower rates of reintubation, whereas HE and LE were the most effective regimens for preventing UAO. The cumulative rankings in the 12-hour model and the 6-hour model for reintubation and UAO (Figure 3) give another perspective of the relative efficacy of the different regimens.
Figure 3.

Cumulative probability curves and surface under the cumulative ranking curve (SUCRA) values for different dexamethasone regimens. For each regimen, the cumulative probability of being ranked first through fifth is displayed. The more the curve for a certain regimen is located toward the upper left corner, the higher its SUCRA value and the better its effectiveness. UAO = upper airway obstruction.
Two participants developed GI bleeding in the four trials (n = 512) included in the NMA. Because only two events were reported, it was not feasible to statistically pool the outcome of GI bleeding in the NMA.
Discussion
Systemic corticosteroids have been used across the age spectrum for the prevention of upper airway edema after endotracheal intubation (4). In this review we used a Bayesian NMA framework to study the relative efficacy of different regimens of systemic corticosteroids in preventing postextubation UAO and reintubation. Our analysis suggests that earlier administration of dexamethasone (at least 6–12 h before extubation) is perhaps more important than the dose of administration (0.5 mg/kg/dose vs. <0.5 mg/kg/dose), with high-dose (0.5 mg/kg/dose) dexamethasone administered early (>12 h before extubation) likely to be the most effective strategy. These findings regarding the use of multiple repeated doses administered >12 hours before extubation are consistent with previous systematic reviews conducted in adults (4).
However, most of these findings are driven by the outcome of postextubation UAO. Reintubation rates were not statistically different between the dexamethasone and placebo groups. Reintubation rates specifically attributable to UAO were not reported separately in the studies, although this is difficult to surmise because reintubation, even when UAO is present, is often multifactorial (i.e., UAO plus muscle weakness, poor respiratory drive, etc.) (1). Hence, a larger sample size may be required to show a benefit on the outcome of reintubation.
This analysis showed a large UAO prevention effect for early dosing (at least 6–12 h before extubation) of dexamethasone. However, the effect sizes may have been influenced by the high incidence of UAO (46%) in the control groups of the trials. It is possible that the trial population was somehow at higher risk of developing postextubation UAO than standard patients or that the assessment tools used were very sensitive for the diagnosis of UAO. Two studies described high proportions of intubations taking place in uncontrolled environments with a subsequent higher likelihood for postextubation UAO (26, 27), and one study included only children at high risk for extubation failure (23, 26, 27). In other studies in which objective assessment tools to diagnose subglottic UAO were used, the incidence of UAO was reported to be 12% (1). Nevertheless, even with a 12% UAO incidence, the absolute effect of dexamethasone use would be a 10.3% UAO reduction, a clinically significant effect based on our a priori threshold of effect sizes. Dexamethasone dosing within 6 hours of planned extubation appeared to be less effective than earlier dosing. Comparing the effect sizes of dexamethasone timing (>12 h vs. >6 h vs. ⩽6 h), a time–response relationship is observed, with dosing >12 hours being the most effective and dosing <6 hours of extubation being the least effective in preventing UAO. If steroids are started within 6 hours of a planned extubation, a higher dose of 0.5 mg/kg/dose may be more effective than 0.25 mg/kg/dose, with an absolute effect of 21% less UAO. This would be clinically significant if the baseline incidence of UAO is high (i.e., close to 46%, similar to what is seen in the placebo arm of the RCTs), but the absolute effect may not be clinically significant with lower baseline rates of UAO.
A pairwise meta-analysis of trials in adults using preextubation systemic corticosteroids reported clinically important effects only in participants who failed a cuff-leak test (suggesting higher likelihood of postextubation UAO) (32). In children, the air leak test is probably predictive of postextubation UAO only in children with cuffed endotracheal tubes (1). Although none of the pediatric trials restricted inclusion based on cuff-leak test, it is likely dexamethasone will have the greatest benefit in a group of children at high risk for developing postextubation UAO. Potential risk factors for the development of postextubation UAO in children include abnormal cuff-leak test in children with cuffed endotracheal tubes, multiple airway instrumentations, excessive positive fluid balance, sedation level before extubation, and previous history of stridor (1), although there is some inconsistency in these variables in the literature.
Use of preextubation dexamethasone involves some trade-offs. In our review, there were very few major adverse events reported. This is like the meta-analysis in adults (n = 2,472), where no cases of hyperglycemia or GI bleeding were reported (32). The trade-offs for using dexamethasone, therefore, mostly depend on the risk of prolonging IMV (to administer corticosteroid) versus a patient’s risk of reintubation due to postextubation UAO.
Limitations
Our pairwise analysis showed moderate heterogeneity for reintubation and UAO. An important source of heterogeneity is the variability in the rates of UAO. Some studies included in our review had high rates of UAO in the no-dexamethasone arm, with one study having a UAO rate of 87.5% (21). Similarly, the reintubation rates were highly variable, ranging from 5% to 63%. These differences may be attributed to the subjectivity in diagnosing UAO, the wide age range for subjects included in the studies, as well as the multifactorial nature of extubation failure in children. Nevertheless, the NMA did not show any inconsistency, and it offered more precise effect estimates. Our ability to describe the trade-offs of benefits and harms associated with dexamethasone was limited by the rarity of adverse effects in the included studies. The lack of adverse effects could be due to inadequate reporting, as has been suggested recently, but adult studies have reported similarly low rates of adverse effects (33). Our review includes trials that span nearly 30 years. Recently, there has been a suggestion that trials >20 years old may overestimate effect size (34). In our review, two studies are >20 years old; one showed large effect size for both reintubation and UAO in favor of dexamethasone (21), and the other did not find any difference between dexamethasone and placebo. Therefore, we do not believe the age of trials on their own influenced our results (24).
Conclusions
Evidence from this network meta-analysis suggests early initiation of dexamethasone (12 h before extubation) using a high dose (0.5 mg/kg/dose) is the most effective strategy to prevent postextubation UAO and possibly reintubation due to UAO. Early initiation with doses <0.5 mg/kg/dose is probably as effective as early initiation of high-dose dexamethasone in preventing UAO. Given the complex nature of trade-offs with each patient, the decision to use a specific strategy of dexamethasone should be personalized, taking into consideration the risk of postextubation UAO, risk factors for extubation failure (such as respiratory muscle weakness), the potential for adverse effects (such as GI bleeding and hypertension), and the time available before planned extubation.
Acknowledgments
Acknowledgment
The authors thank Dr. Christopher Newth, Professor of Pediatrics, University of Southern California, Children’s Hospital Los Angeles, for critically reviewing the final draft of the manuscript.
Footnotes
Supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development grant number R13HD102137.
Author Contributions: N.P.I., R.G.K., and S.A.-S. were responsible for the study design, data analysis, data interpretation, and preparing the first draft of the manuscript. H.J.C. and E.C.W. were responsible for literature search and acquiring full texts. Y.M.L.-F., S.G.-D., A.K.B., and J.C.H. were responsible for abstract and full-text screening and data extraction. N.P.I., M.Z., and Y.Z. performed statistical analysis. N.P.I., S.A.-S., and R.G.K. are responsible for study data integrity. All authors reviewed the manuscript and approved its final submitted version.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Khemani RG, Hotz J, Morzov R, Flink R, Kamerkar A, Ross PA, et al. Evaluating risk factors for pediatric post-extubation upper airway obstruction using a physiology-based tool. Am J Respir Crit Care Med . 2016;193:198–209. doi: 10.1164/rccm.201506-1064OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kurachek SC, Newth CJ, Quasney MW, Rice T, Sachdeva RC, Patel NR, et al. Extubation failure in pediatric intensive care: a multiple-center study of risk factors and outcomes. Crit Care Med . 2003;31:2657–2664. doi: 10.1097/01.CCM.0000094228.90557.85. [DOI] [PubMed] [Google Scholar]
- 3. Hawkins DB, Crockett DM, Shum TK. Corticosteroids in airway management. Otolaryngol Head Neck Surg . 1983;91:593–596. doi: 10.1177/019459988309100601. [DOI] [PubMed] [Google Scholar]
- 4. Khemani RG, Randolph A, Markovitz B. Corticosteroids for the prevention and treatment of post-extubation stridor in neonates, children and adults. Cochrane Database Syst Rev . 2009:CD001000. doi: 10.1002/14651858.CD001000.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dias S, Caldwell DM. Network meta-analysis explained. Arch Dis Child Fetal Neonatal Ed . 2019;104:F8–F12. doi: 10.1136/archdischild-2018-315224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med . 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 7.Schunemann HJ, Brozek J, Guyatt G, Oxman AD, editors. GRADE handbook for grading quality of evidence and strength of recommendations. 2013. https://gdt.gradepro.org/app/handbook/handbook.html [Google Scholar]
- 8. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform . 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ . 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 10. Turner RM, Jackson D, Wei Y, Thompson SG, Higgins JP. Predictive distributions for between-study heterogeneity and simple methods for their application in Bayesian meta-analysis. Stat Med . 2015;34:984–998. doi: 10.1002/sim.6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Making . 2013;33:607–617. doi: 10.1177/0272989X12458724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat . 1998;7:434–455. [Google Scholar]
- 13. Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials. Med Decis Making . 2013;33:641–656. doi: 10.1177/0272989X12455847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol . 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 15. Mbuagbaw L, Rochwerg B, Jaeschke R, Heels-Andsell D, Alhazzani W, Thabane L, et al. Approaches to interpreting and choosing the best treatments in network meta-analyses. Syst Rev . 2017;6:79. doi: 10.1186/s13643-017-0473-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murad MH, Montori VM, Ioannidis JP, Jaeschke R, Devereaux PJ, Prasad K, et al. How to read a systematic review and meta-analysis and apply the results to patient care: users’ guides to the medical literature. JAMA . 2014;312:171–179. doi: 10.1001/jama.2014.5559. [DOI] [PubMed] [Google Scholar]
- 17. van Valkenhoef G, Lu G, de Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta-analysis. Res Synth Methods . 2012;3:285–299. doi: 10.1002/jrsm.1054. [DOI] [PubMed] [Google Scholar]
- 18. Brignardello-Petersen R, Bonner A, Alexander PE, Siemieniuk RA, Furukawa TA, Rochwerg B, et al. GRADE Working Group Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol . 2018;93:36–44. doi: 10.1016/j.jclinepi.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 19. Brignardello-Petersen R, Mustafa RA, Siemieniuk RAC, Murad MH, Agoritsas T, Izcovich A, et al. GRADE Working Group GRADE approach to rate the certainty from a network meta-analysis: addressing incoherence. J Clin Epidemiol . 2019;108:77–85. doi: 10.1016/j.jclinepi.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 20. Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, et al. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol . 2017;87:4–13. doi: 10.1016/j.jclinepi.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anene O, Meert KL, Uy H, Simpson P, Sarnaik AP. Dexamethasone for the prevention of postextubation airway obstruction: a prospective, randomized, double-blind, placebo-controlled trial. Crit Care Med . 1996;24:1666–1669. doi: 10.1097/00003246-199610000-00011. [DOI] [PubMed] [Google Scholar]
- 22. Cesar RG, de Carvalho WB. L-epinephrine and dexamethasone in postextubation airway obstruction: a prospective, randomized, double-blind placebo-controlled study. Int J Pediatr Otorhinolaryngol . 2009;73:1639–1643. doi: 10.1016/j.ijporl.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 23. de Carvalho HT, Fioretto JR, Bonatto RC, Ribeiro CF, Martin JG, Carpi MF. Use of dexamethasone to prevent extubation failure in pediatric intensive care unit: a randomized controlled clinical trial. J Pediatr Intensive Care . 2022;11:41–47. doi: 10.1055/s-0040-1719044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tellez DW, Galvis AG, Storgion SA, Amer HN, Hoseyni M, Deakers TW. Dexamethasone in the prevention of postextubation stridor in children. J Pediatr . 1991;118:289–294. doi: 10.1016/s0022-3476(05)80505-0. [DOI] [PubMed] [Google Scholar]
- 25. Ritu JU, Jhamb U. Dexamethasone in prevention of postextubation stridor in ventilated children: a randomized, double-blinded, placebo-controlled trial. Indian J Crit Care Med . 2020;24:1230–1235. doi: 10.5005/jp-journals-10071-23679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baranwal AK, Meena JP, Singhi SC, Muralidharan J. Dexamethasone pretreatment for 24 h versus 6 h for prevention of postextubation airway obstruction in children: a randomized double-blind trial. Intensive Care Med . 2014;40:1285–1294. doi: 10.1007/s00134-014-3358-9. [DOI] [PubMed] [Google Scholar]
- 27. Parajuli B, Baranwal AK, Kumar-M P, Jayashree M, Takia L. Twenty-four-hour pretreatment with low dose (0.25 mg/kg/dose) versus high dose (0.5 mg/kg/dose) dexamethasone in reducing the risk of postextubation airway obstruction in children: a randomized open-label noninferiority trial. Pediatr Pulmonol . 2021;56:2292–2301. doi: 10.1002/ppul.25388. [DOI] [PubMed] [Google Scholar]
- 28. Malhotra D, Gurcoo S, Qazi S, Gupta S. Randomized comparative efficacy of dexamethasone to prevent postextubation upper airway complications in children and adults in ICU. Indian J Anaesth . 2009;53:442–449. [PMC free article] [PubMed] [Google Scholar]
- 29. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed) . 2021;74:790–799. doi: 10.1016/j.rec.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Haddaway NR, Page MJ, Pritchard CC, McGuinness LA. PRISMA2020: an R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and open synthesis. Campbell Syst Rev 2022;18:e1230. [DOI] [PMC free article] [PubMed]
- 31. Harel Y, Vardi A, Quigley R, Brink LW, Manning SC, Carmody TJ, et al. Extubation failure due to post-extubation stridor is better correlated with neurologic impairment than with upper airway lesions in critically ill pediatric patients. Int J Pediatr Otorhinolaryngol . 1997;39:147–158. doi: 10.1016/s0165-5876(97)01488-2. [DOI] [PubMed] [Google Scholar]
- 32. Kuriyama A, Umakoshi N, Sun R. Prophylactic corticosteroids for prevention of postextubation stridor and reintubation in adults: a systematic review and meta-analysis. Chest . 2017;151:1002–1010. doi: 10.1016/j.chest.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 33. Qureshi R, Mayo-Wilson E, Li T. Harms in systematic reviews paper 1: an introduction to research on harms. J Clin Epidemiol . 2022;143:186–196. doi: 10.1016/j.jclinepi.2021.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smail-Faugeron V, Tan A, Caille A, Yordanov Y, Hajage D, Tubach F, et al. Meta-analyses frequently include old trials that are associated with a larger intervention effect: a meta-epidemiological study. J Clin Epidemiol . 2022;145:144–153. doi: 10.1016/j.jclinepi.2022.01.023. [DOI] [PubMed] [Google Scholar]


