Abstract
Rationale
The role of airway inflammation in disease pathogenesis in children with primary ciliary dyskinesia (PCD) is poorly understood.
Objectives
We investigated relationships between sputum inflammation measurements, age, lung function, bronchiectasis, airway infection, and ultrastructural defects in children with PCD.
Methods
Spontaneously expectorated sputum was collected from clinically stable children and adolescents with PCD ages 6 years and older participating in a multicenter, observational study. Sputum protease and inflammatory cytokine concentrations were correlated with age, lung function, and chest computed tomography measures of structural lung disease, whereas differences in concentrations were compared between ultrastructural defect categories and between those with and without detectable bacterial infection.
Results
Sputum from 77 children with PCD (39 females [51%]; mean [standard deviation] age, 13.9 [4.9] yr; mean [standard deviation] forced expiratory volume in 1 second [FEV1]% predicted, 80.8 [20.5]) was analyzed. Sputum inflammatory marker measurements, including neutrophil elastase activity, IL-1β (interleukin-1β), IL-8, and TNF-α (tumor necrosis factor α) concentrations, correlated positively with age, percentage of bronchiectasis, and percentage of total structural lung disease on computed tomography, and negatively with lung function. Correlations between neutrophil elastase concentrations and FEV1% predicted and percentage of bronchiectasis were −0.32 (95% confidence interval, −0.51 to −0.10) and 0.46 (0.14 to 0.69), respectively. Sputum neutrophil elastase, IL-1β, and TNF-α concentrations were higher in those with detectable bacterial pathogens. Participants with absent inner dynein arm and microtubular disorganization had similar inflammatory profiles compared with participants with outer dynein arm defects.
Conclusions
In this multicenter pediatric PCD cohort, elevated concentrations of sputum proteases and cytokines were associated with impaired lung function and structural damage as determined by chest computed tomography, suggesting that sputum inflammatory measurements could serve as biomarkers in PCD.
Keywords: inflammation, biomarkers, sputum, lung function, bronchiectasis
Primary ciliary dyskinesia (PCD) is a complex, genetically heterogeneous disorder characterized by abnormal motile ciliary structure and function that leads to impaired mucociliary clearance, resulting in recurrent sinopulmonary infections and bronchiectasis (1). Lung disease is manifested as airflow obstruction, atelectasis, and bronchiectasis that often begin in childhood (2–5). Heterogeneity in lung disease is apparent among children with PCD, with lung disease severity and progression related to genotype and ultrastructural defects of the cilia axoneme (5–7).
Airway inflammation is a hallmark feature of lung disease in people with cystic fibrosis (CF) and non-CF bronchiectasis (8, 9). These conditions are characterized by neutrophil-dominated airway inflammation associated with bronchiectasis, lung function decline, and disease progression (10–14). In contrast to CF, airway inflammation in PCD has been far less studied, limited to relatively small cohorts of children with PCD from single centers (15–19). Little is known about the relationships between airway inflammation and lung function, bronchiectasis, and airway infection, and whether inflammation differs among PCD children with different ultrastructural defects.
The primary objectives of this study were to investigate whether measurements of airway inflammation are linked to lung function, bronchiectasis, and airway microbiology in a multicenter cohort of well-phenotyped children with PCD and to evaluate for differences in inflammatory measurements between ultrastructural defect categories that might explain in part previous relationships observed between ciliary ultrastructural defects and lung disease phenotype (5–7). We focused on surrogate measures of neutrophilic airway inflammation, including neutrophil-derived proteases, neutrophil chemoattractants, and proinflammatory cytokines. A secondary objective was to compare sputum inflammatory measurements in these children with PCD to those obtained in a relatively age-matched historical CF cohort.
Methods
Study Design and Population
Data are from a prospective, longitudinal, observational study conducted at multiple clinical research sites of the Genetic Disorders of Mucociliary Clearance Consortium (5, 6). At enrollment, participants were less than 19 years of age. The cohort for the current analysis was limited to those with a confirmed diagnosis of PCD on the basis of hallmark electron microscopy ciliary defects and/or genetic mutations consistent with PCD together with compatible clinical features (20). The evaluation included a comprehensive collection of demographic and clinical information, spirometry, chest computed tomography (CT) scans performed at biennial longitudinal study visits, and the collection of sputum in those who were able to spontaneously expectorate for microbiologic testing and measurement of inflammation. We selected the earliest sputum sample associated with reliable lung function measurements, some of which were collected at the baseline study visit, whereas the remainder were collected at subsequent annual study visits. Sputum quality was assessed by the site research coordinators at the time of collection, and adequacy of samples was defined as a minimum of 0.2 ml, a volume sufficient to measure all inflammatory markers chosen a priori for analysis, with visible mucus plugs or flecks. Prebronchodilator percent predicted forced expiratory volume in 1 second (ppFEV1) values (21) are reported. Respiratory samples were processed for bacterial pathogens commonly found in bronchiectatic airways, including Staphylococcus aureus, Haemophilus influenzae, Streptococcus pneumoniae, Moraxella catarrhalis, and Pseudomonas aeruginosa at each site’s local clinical laboratory. Cultures that grew mixed upper respiratory flora without a predominant bacterial pathogen were deemed negative. CT scans performed at the same baseline study visit during which sputum was collected, using a standardized protocol limiting radiation exposure across study sites (n = 33), were scored to compute the percentage of atelectasis, percentage of bronchiectasis, percentage of airway wall thickening, and percentage of mucus plugging using the MERAGMA-PCD (Melbourne-Rotterdam Annotated Grid Morphometric Analysis for PCD), a grid-based scoring system adapted from the Perth-Rotterdam Annotated Grid Morphometric Analysis for CF (PRAGMA-CF) method (22). We also computed total structural lung disease (percentage of disease) as the sum of the percentage of atelectasis, percentage of bronchiectasis, percentage of airway wall thickening, and percentage of mucus plugging. CT scans performed as part of longitudinal study visits after the baseline study visit did not undergo analysis by the MERAGMA-PCD scoring system, which explains why fewer than half of the subjects have CT scores linked with the sputum inflammation measurements. Participants were clinically stable and free from acute illness for 4 weeks at the time of their study visits. Institutional review boards approved the protocols at each of the research sites. Informed consent and assent were obtained from parents and participants when appropriate.
Sputum Processing and Analysis
Sputum specimens were frozen on-site after collection and shipped on dry ice to the Consortium’s biospecimen repository, located in the Clinical Translational Research Center Core Laboratory at Children's Hospital Colorado and the University of Colorado Anschutz Medical Campus. Samples remained at −70°C before processing. Frozen sputum specimens were thawed and processed in the laboratory usinga standard operating procedure and subsequently analyzed for markers of inflammation, as previously described (11, 23). Cytologic examination (cell counts with differentials) was not performed onthe sputum samples after thawing becauseof the unreliability of sputum cell counts (24, 25). Proinflammatory cytokines IL-1β (interleukin-1β), IL-6, IL-8, and TNF-α (tumor necrosis factor α) were analyzed by multiplex enzyme-linked immunosorbent assay (Luminex Fluorokine Technology, R&D Systems). Free neutrophil elastase (NE) activity was measured using a spectrophotometric assay on the basis of the hydrolysis of the specific substrate MeO-suc-Ala-Ala-Pro-Ala-p-nitroanilide (Sigma Chemical Co.). MMP-9 (matrix metalloprotease-9) activity and LTB4 (leukotriene B4), a neutrophil chemoattractant, were both quantified by commercially available enzyme-linked immunosorbent assay kits (R&D Systems). The lower limits of detection (LLOD) for these assays were as follows: IL-1β, 2.5 pg/ml; IL-6, 2.0 pg/ml; IL-8, 1.0 pg/ml; TNF-α, 2.0 pg/ml; free NE activity, 0.5 μg/ml; MMP-9 activity, 0.25 ng/ml; and LTB4, 19.5 pg/ml.
Statistical Analyses
Descriptive statistics were used to characterize the demographic and baseline characteristics of the study cohort at the time of the first sputum collection. Mean and standard deviation (SD) and median and interquartile range were presented where specified. Values for free NE activity and IL-6 below the limits of detection were randomly imputed using a uniform distribution (0, LLOD); MMP-9 values that exceeded the upper limit were truncated at the upper limit of detection. The sputum inflammation measurements were log-transformed (base 10) and anchored at 1. Wilcoxon nonparametric tests were used to compare sputum inflammation measurements between PCD subjects and historical CF control subjects, PCD subjects separated by ultrastructural defect categories, and those with positive and negative respiratory cultures. As a sensitivity analysis, comparisons of inflammatory measurements across ultrastructural defect categories were performed after matching on the basis of age and ppFEV1. Matches were identified that minimized the differences between the logits (including age and ppFEV1 as covariates) of the propensity scores and used a maximum 0.75 caliper width. All correlations reported are on the basis of Spearman’s nonparametric rho statistic, including corresponding 95% confidence intervals. Strengths of correlations were classified as weak (0.30 to <0.40 and −0.30 to >−0.40), medium (0.40 to <0.50), and strong (⩾0.50). P values were adjusted for multiple comparisons using false discovery rate. Statistical analyses were performed with SAS 9.4 (SAS Institute Inc.).
Results
Baseline Characteristics of Study Participants
The baseline demographics and clinical characteristics of the 77 study participants from six study sites who spontaneously expectorated sputum between 2008 and 2019 are summarized in Table 1. The number of participants that provided sputum samples at each site varied, ranging from 1 to 49. The ciliary ultrastructural defects and PCD-causing genes with two pathogenic or likely pathogenic variants identified in these participants are shown in Table 2. A total of 36 had isolated outer dynein arm (ODA) defects, 6 had combined outer and inner dynein arm (ODA/IDA) defects, 26 had absent IDA and microtubular disorganization (MTD) (IDA/MTD) defects, 4 had normal ciliary ultrastructure, and 5 had other defects (oligocilia [n = 1] and central apparatus defects [n = 4]). One subject had a single X-linked RPGR mutation. The distribution of ciliary ultrastructural defects and PCD-causing genes in our study participants are representative of those in the overall Consortium population (5, 6). Participants with IDA/MTD tended to be younger at the time of initial sputum collection (mean [SD] ages, 13 [4] vs. 15 [5] yr; P = 0.10), tended to have lower growth parameters (mean [SD] weight percentiles, 47 [29] vs. 64 [28]; P = 0.03; mean [SD] body mass index percentiles, 51 [29] vs. 63 [31]; P = 0.16), had significantly lower ppFEV1 (mean, 72 [21] vs. 90 [15]; P < 0.01), and cultured pathogenic bacteria more frequently (70% vs. 31%; P < 0.01) compared with participants with isolated ODA defects (Table 3). There were insufficient numbers of subjects with other ultrastructural defects to make meaningful comparisons.
Table 1.
Baseline demographic and clinical characteristics of the study participants
| All Subjects N = 77 |
|
|---|---|
| Age at initial diagnosis (yr), mean (SD) | 4.3 (3.9) |
| Age at specimen collection (yr), mean (SD) | 13.9 (4.9) |
| Female sex, n (%) | 39 (51) |
| Race, n (%) | |
| White | 63 (82) |
| Asian | 9 (12) |
| Other/unknown | 5 (6) |
| Growth parameters, mean (SD) | n = 70* |
| Height percentile | 47.5 (29.7) |
| Weight percentile | 55.3 (30.2) |
| Body mass index percentile | 56.4 (31.8) |
| Clinical features, n (%) | |
| Neonatal respiratory distress | 63 (82) |
| Situs inversus/laterality defect | 38 (49) |
| Chronic otitis media | 73 (95) |
| Chronic nasal congestion | 75 (97) |
| Chronic cough | 75 (97) |
| FEV1% predicted, mean (SD) | 80.8 (20.5) |
| Respiratory culture results, n (%) | |
| Haemophilus influenzae | 14 (18) |
| Staphylococcus aureus | 22 (29) |
| Streptococcus pneumoniae | 9 (12) |
| Moraxella catarrhalis | 2 (3) |
| Pseudomonas aeruginosa | 3 (4) |
| No pathogenic bacteria detected | 39 (51) |
| One pathogenic bacterium detected | 27 (35) |
| Two or more pathogenic bacteria detected | 11 (14) |
| Medications, n (%) | |
| Azithromycin | 18 (23) |
| Hypertonic saline | 18 (23) |
| Dornase alfa | 9 (12) |
| Inhaled corticosteroids | 42 (55) |
Definition of abbreviations: FEV1 = forced expiratory volume in 1 second; SD = standard deviation.
Percentiles are provided for subjects less than 20 yr old at their study visit.
Table 2.
Primary ciliary dyskinesia-causing genes and cilia ultrastructure defects of the study cohort
| PCD-causing Gene | Cilia Ultrastructure | N |
|---|---|---|
| DNAH5 | ODA | 22 |
| CCDC40 | IDA/MTD | 13 |
| CCDC39 | IDA/MTD | 10 |
| DNAI1 | ODA | 5 |
| DNAI2 | ODA | 4 |
| None identified | ODA | 4 |
| None identified | IDA/MTD | 3 |
| DNAH11 | Normal EM | 3 |
| SPAG1 | ODA/IDA | 2 |
| RSPH4A | CA | 2 |
| CCDC103 | ODA/IDA | 1 |
| CCDC114 | ODA | 1 |
| DNAAF1 (LRRC50) | ODA/IDA | 1 |
| HEATR2 | ODA/IDA | 1 |
| LLRC6 | ODA/IDA | 1 |
| RPGR (X-linked) | Normal EM | 1 |
| RSPH1 | CA | 1 |
| RSPH9 | CA | 1 |
| CCNO | Oligocilia | 1 |
Definition of abbreviations: CA = central apparatus; EM = electron micrographs; IDA = inner dynein arm; MTD = microtubular disorganization; ODA = outer dynein arm; PCD = primary ciliary dyskinesia.
Table 3.
Clinical characteristics (mean values and standard deviations) and sputum inflammatory measurement concentrations (logarithmically transformed median values and interquartile range) in the subjects with isolated outer dynein arm defects and inner dynein arm/microtubular disorganizations defects
| ODA (n = 36) | IDA/MTD (n = 26) | P Value | |
|---|---|---|---|
| Clinical characteristics | |||
| Age, yr | 14.9 (4.8) | 13.0 (4.4) | 0.10 |
| FEV1% predicted | 89.5 (14.8) | 71.5 (21.0) | <0.01 |
| Weight percentile | 64.4 (27.7), n = 32 | 47.3 (28.7), n = 24 | 0.03 |
| Body mass index percentile | 62.5 (31.0), n = 32 | 50.8 (29.1), n = 24 | 0.16 |
| Negative culture, n (%) | 25 (69) | 7 (30) | <0.01 |
| Haemophilus influenzae, n (%) | 3 (8) | 8 (31) | 0.04 |
| Staphylococcus aureus, n (%) | 7 (19) | 10 (38) | 0.15 |
| Streptococcus pneumoniae, n (%) | 3 (8) | 4 (15) | 0.44 |
| Moraxella catarrhalis, n (%) | 0 (0) | 2 (8) | 0.17 |
| Pseudomonas aeruginosa, n (%) | 2 (6) | 1 (4) | 0.99 |
| Protein analyte concentrations, median (IQR) | |||
| NE log μg/ml | 1.6 (1.0–1.9) | 1.6 (1.3–2.0) | 0.60 |
| MMP-9 log ng/ml | 4.0 (3.6–4.3) | 3.8 (3.5–4.1) | 0.27 |
| IL-1β log pg/ml | 3.9 (3.0–4.1) | 3.6 (2.7–4.1) | 0.61 |
| IL-6 log pg/ml | 1.8 (1.5–2.2) | 1.8 (1.5–2.2) | 0.93 |
| IL-8 log pg/ml | 5.3 (4.8–5.5) | 5.1 (4.9–5.6) | 0.66 |
| TNF-α log pg/ml | 2.4 (1.9–2.9) | 2.4 (1.7–3.1) | 0.94 |
| LTB4 log pg/ml | 3.6 (3.5–3.9) | 3.5 (3.4–3.6) | 0.06 |
Definition of abbreviations: FEV1 = forced expiratory volume in 1 second; IDA = inner dynein arm; IL = interleukin; IQR = interquartile range; LTB4 = leukotriene B4; MMP = matrix metalloproteinase; MTD = microtubular disorganization; NE = neutrophil elastase; ODA = outer dynein arm; TNF = tumor necrosis factor.
Relationships Between Sputum Inflammation Measures and Clinical Variables
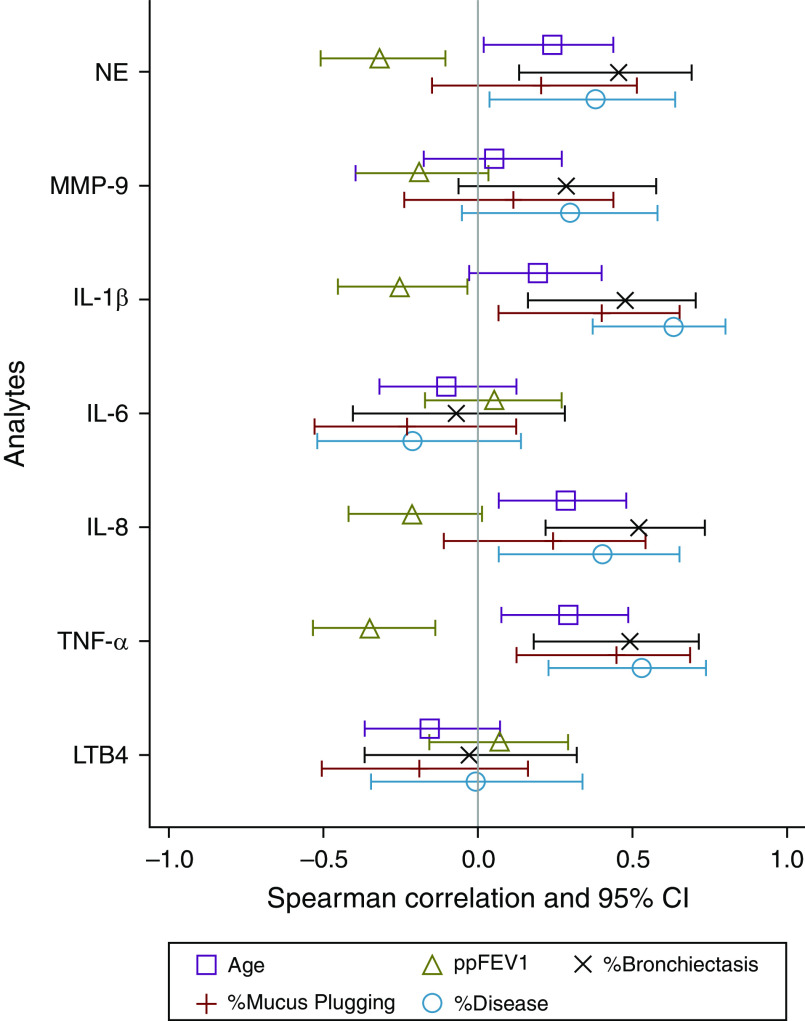
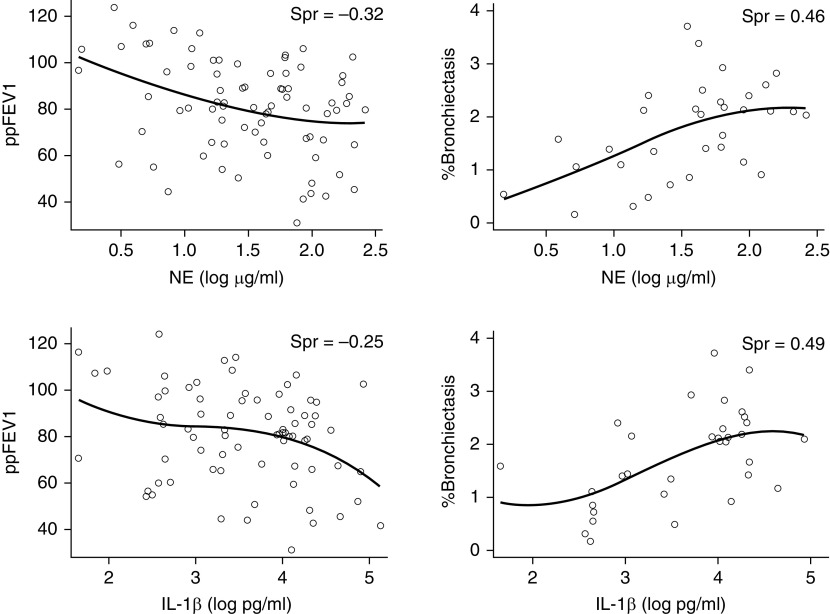
Correlations between the sputum inflammatory measurement concentrations and age, ppFEV1, and the CT measures of the percentage of bronchiectasis, percentage of mucus plugging, and percentage of disease are shown in Figure 1 and Table E1 in the online supplement. Notably, values for free NE activity from five sputum samples and IL-6 from six samples were below the limits of detection of their respective assays, whereas MMP-9 values in five samples exceeded the upper limits of quantitation. Several inflammatory measures correlated positively with age: sputum NE, IL-8, and TNF-α concentrations increased with advancing age. In addition, sputum NE, IL-1β, and TNF-α concentrations correlated negatively with lung function with increased inflammatory measurements associated with lower ppFEV1. Also, sputum NE, IL-1β, IL-8, and TNF-α concentrations positively correlated with two CT measures of structural lung injury, the percentage of bronchiectasis and percentage of disease with increased inflammation associated with greater structural lung injury. Individual correlation plots between sputum NE and IL-1β concentrations and ppFEV1 and the percentage of bronchiectasis are displayed in Figure 2. Sputum IL-1β and TNF-α also correlated positively with the CT measure of the percentage of mucus plugging. Finally, sputum NE, IL-1β, and TNF-α concentrations were higher in those with detectable bacterial pathogens in their sputa compared with those with negative aerobic cultures (Table E2). Sputum MMP-9, IL-6, and LTB4 concentrations were not associated with any of the clinical variables and were not higher in participants who had detectable pathogens.
Figure 1.
Forest plot of correlations between the sputum inflammatory measurement concentrations and age, ppFEV1, and the computed tomography measures of the percentage of bronchiectasis, percentage of mucus plugging, and percentage of disease. Correlations are significant if the CIs (represented by the horizontal lines) do not cross zero. CI = confidence interval; IL = interleukin; LTB4 = leukotriene B4; MMP = matrix metalloproteinase; NE = neutrophil elastase; ppFEV1 = forced expiratory volume in 1 second percent predicted; TNF = tumor necrosis factor.
Figure 2.
Associations between sputum neutrophil elastase (NE) activity and IL-1β (interleukin-1β) concentrations (log10 transformed) and lung function (ppFEV1) and bronchiectasis. ppFEV1 = forced expiratory volume in 1 second percent predicted; Spr = Spearman’s rank correlation coefficient.
Comparisons of Sputum Inflammation Measures Between Groups
On the basis of data that children with IDA/MTD ciliary defects have lower lung function and a greater rate of lung function decline compared with those with isolated ODA defects (5–7), we postulated that the IDA/MTD cohort would have increased airway inflammation compared with the ODA cohort. However, the sputum inflammatory measurement concentrations were not different between these two groups (Table 3). After matching subjects in these two groups on the basis of age and lung function, only sputum LTB4 concentrations differed and were higher in the ODA defect cohort (Table E3).
The distributions of ages, ppFEV1, and sputum NE and IL-8 concentrations for the study participants across the six study sites are shown in Figure E1. There were no differences in these parameters across the study sites. Moreover, we did not identify any differences in sputum inflammatory measures between those routinely treated with azithromycin, hypertonic saline, dornase alfa, or inhaled corticosteroids compared with those who were not (data not shown).
To provide context to the degree of airway inflammation observed in these children with PCD, expectorated sputum measurements of inflammation performed in the same laboratory in historically published relatively age-matched CF individuals (26, 27) are shown in Table E4. Sputum IL-8 and TNF-α concentrations were significantly higher in the PCD cohort compared with those measured in CF subjects, whereas NE, IL-1β, and IL-6 concentrations were similar between the two groups.
Discussion
In this multicenter cohort of children and adolescents with confirmed PCD, several sputum inflammatory measures, including NE activity, IL-1β, IL-8, and TNF-α concentrations correlated positively with age, bronchiectasis, and total structural lung disease on chest CT and negatively with pulmonary function. Sputum NE, IL-1β, and TNF-α concentrations were also higher in subjects with detectable bacterial pathogens. Although PCD individuals with IDA/MTD defects have greater lung disease, sputum inflammatory markers did not differ between those with IDA/MTD and isolated ODA defects. Furthermore, sputum inflammatory markers in our PCD cohort were comparable to or even exceeded those measured in a relatively age-matched CF cohort.
Airway inflammation has long been recognized as a hallmark feature of bronchiectasis and CF lung disease, but the role of inflammation in PCD lung disease has been less clear. In PCD, it is believed that ciliary dysfunction leads to impaired mucociliary clearance and retention of mucus secretions that disrupt normal host defenses, rendering the airways more vulnerable to infection. Airway inflammation is a downstream consequence of impaired mucociliary clearance, mucus stasis, mucus hyperconcentration, and persistent infection (28–31). The inflammatory response, in turn, results in injury and abnormal airway remodeling leading to bronchiectasis. Rather than proceeding in a stepwise fashion, the pathophysiology of PCD lung disease likely evolves from a “vicious vortex” in which airway dysfunction, inflammation, infection, and structural damage are linked (32).
Results from our study shed new insights on the relationships between airway inflammation and lung function, airway infection, and structural lung injury in PCD. On the basis of a few single-center studies which demonstrated neutrophil-dominated airway inflammation (15, 18, 19), we measured surrogate markers of neutrophilic airway inflammation, including neutrophil-derived proteases, neutrophil chemoattractants, and proinflammatory cytokines. In contrast to a smaller study, which did not find a correlation between sputum inflammatory measures and lung function in a PCD cohort (18), we observed inverse correlations between sputum NE activity and cytokine concentrations with ppFEV1. Our larger, multicenter study population had a broader range of pulmonary function measures, which may explain why we were able to detect relationships between airway inflammation and airflow obstruction. The most common bacteria isolated in our study cohort were H. influenzae and S. aureus, and we found greater inflammation in individuals with detectable bacterial pathogens in sputa compared with those without. P. aeruginosa is associated with increased airway inflammation in CF (33, 34), but this organism was uncommon in our PCD cohort. Furthermore, we found that inflammation was associated with CT-derived measures of bronchiectasis, mucus plugging, and total structural lung disease.
Our data help to establish the value of sputum measurements of neutrophilic inflammation as correlates of disease severity (i.e., lung function) and structural injury (i.e., bronchiectasis) in PCD. Considering two neutrophil-derived proteases, higher NE concentrations correlated with impaired lung function and structural lung injury, whereas MMP-9 did not. Of the proinflammatory cytokines, IL-1β, IL-8, and TNF-α, which act as neutrophil chemoattractants and activators, were associated with poorer lung function and structural injury. Another known chemoattractant, LTB4, however, was not associated with clinical outcomes in our PCD cohort. Although identifying relationships between inflammation and clinical outcomes, including lung function and bronchiectasis, does not prove causation, NE, IL-1β, IL-8, and TNF-α measurements provide useful insights into PCD lung disease, which defines them as biomarkers. This study represents an important step in the clinical validation of sputum inflammatory biomarkers as correlates of disease severity and activity, similar to what has been shown in CF (35). Indeed, airway inflammation may be a specific therapeutic target in people with PCD, as it is in CF and other forms of bronchiectasis (36, 37). Our results provide justification for interventions that reduce or neutralize free NE activity and proinflammatory cytokines to prevent their deleterious effects on the diseased PCD airway. Inflammatory biomarkers could be useful outcome measures in studies evaluating the efficacy of mucoactive and antiinflammatory drugs for PCD.
Interestingly, previous comparisons between PCD and CF have not revealed significant differences in sputum biophysical properties (16) or most inflammatory measures (17, 18), except for one study that reported higher concentrations of sputum IL-8 in the PCD cohort (16). Similarly, we found that sputum IL-8 and TNF-α concentrations were higher in our PCD cohort compared with measurements in relatively age-matched CF subjects performed in the same laboratory. Although there has been a long-standing debate in the CF community about whether inflammation is intrinsic to cystic fibrosis transmembrane conductance regulator protein dysfunction on the basis of exaggerated inflammatory responses to infectious stimuli and impaired resolution of inflammation (38), our data suggest that increased inflammation is not unique to CF.
Strengths and Limitations
Strengths of this study include the assessment of airway inflammation in a multicenter pediatric cohort with diverse ciliary ultrastructural defects and PCD-causing genetic defects representative of the larger PCD population and examination of relationships between inflammation and CT measures of structural lung injury. There are several limitations as well. Selection bias is possible on the basis of the referral pattern of study participants to our research sites and which subjects were able to spontaneously expectorate sputum during their study visits. The relatively small sample size for each ciliary ultrastructure defect group limited our ability to investigate differences in inflammation between all PCD cohorts. Although we compared inflammatory measures to provide context regarding the magnitude of airway inflammation in children with PCD, we used a historical CF group that had fewer subjects and could be a weakness. There is also the inherent drawback that sputum likely originates from the endobronchial space of localized damaged central airways, which may not be representative of inflammation in other lung compartments, such as intramural or peribronchial spaces, that could affect lung function and lead to structural lung injury. It is possible that delayed processing and thawing of sputum may induce cell lysis and release of inflammatory mediators that may affect sputum inflammation biochemical measurements. One publication found that storage at −70°C prevented the degradation of sputum biomarkers (39). In a recent study, there were no differences in paired measurements of neutrophil elastase, IL-1β, and myeloperoxidase concentrations in sputum aliquots that underwent immediate processing compared with those that underwent delayed processing after freezing and shipping (23). We examined a targeted panel of proteases and cytokines because of their established role in neutrophilic airway inflammation, but alternative candidate markers of inflammation, including calprotectin (40), high mobility group box protein-1 (41), neutrophil extracellular traps (42), and other novel mediators, could be considered in future investigations.
Conclusions
This study adds to our knowledge of the role of lower airway inflammation in the pathogenesis of PCD lung disease, demonstrating that elevated concentrations of sputum NE and cytokines were associated with impaired lung function and structural lung damage. Although our data do not prove a causal association, they do highlight relationships between airway inflammation and important pulmonary outcomes in PCD, suggesting that sputum inflammatory measurements could serve as biomarkers to monitor disease activity and useful endpoints for clinical drug trials. More comprehensive longitudinal analyses are needed to determine whether airway and possibly systemic (blood-based) inflammatory measures correlate with or are predictive of disease progression, including lung function decline and worsening structural lung injury.
Acknowledgments
Acknowledgment
The authors thank Rebecca Baldermann and the laboratory technicians in the Pediatric Clinical Translational Research Center Core Laboratory at Children's Hospital Colorado for their outstanding technical assistance in this work, Federico Mollica and Mariette Kemner-van de Corput of the Erasmus MC Lung Analysis core laboratory for the computed tomography image analysis, and all of the study participants and their families.
Footnotes
Supported by the National Heart, Lung, and Blood Institute (NHLBI)/National Institutes of Health (NIH) (U54 HL09640958), funded by the Office of Rare Diseases Research (ORDR, National Center for Advancing Translational Sciences [NCATS]), and by NIH/NCATS Colorado Clinical and Translational Science Award (CTSA) grant UL1 TR002535. The Genetic Disorders of Mucociliary Clearance Consortium (U54HL096458) is part of the NCATS Rare Diseases Clinical Research Network (RDCRN) and supported by the RDCRN Data Management and Coordinating Center (DMCC) (U2CTR002818). RDCRN is an initiative of the ORDR funded through a collaboration between NCATS and NHLBI.
Author Contributions: S.D.Davis, S.D.Dell, T.W.F., M.R., M.R.K., M.W.L., and S.D.S. designed the study. S.D.Davis, S.D.Dell, T.W.F., M.R., M.W.L., and S.D.S. were the lead site investigators, recruited and studied patients at their sites, and reviewed the manuscript. B.D.W. performed statistical analyses and reviewed the manuscript. K.M.S. provided the clinical data linked with the sputum specimens and reviewed the manuscript. H.A.W.M.T. supervised the image analysis and reviewed the manuscript. O.K., J.E.H., and S.D.S. prepared the initial draft of the manuscript and incorporated comments and edits from the other study authors. S.D.S. serves as the guarantor of this manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med . 2013;188:913–922. doi: 10.1164/rccm.201301-0059CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown DE, Pittman JE, Leigh MW, Fordham L, Davis SD. Early lung disease in young children with primary ciliary dyskinesia. Pediatr Pulmonol . 2008;43:514–516. doi: 10.1002/ppul.20792. [DOI] [PubMed] [Google Scholar]
- 3. Marthin JK, Petersen N, Skovgaard LT, Nielsen KG. Lung function in patients with primary ciliary dyskinesia: a cross-sectional and 3-decade longitudinal study. Am J Respir Crit Care Med . 2010;181:1262–1268. doi: 10.1164/rccm.200811-1731OC. [DOI] [PubMed] [Google Scholar]
- 4. Green K, Buchvald FF, Marthin JK, Hanel B, Gustafsson PM, Nielsen KG. Ventilation inhomogeneity in children with primary ciliary dyskinesia. Thorax . 2012;67:49–53. doi: 10.1136/thoraxjnl-2011-200726. [DOI] [PubMed] [Google Scholar]
- 5. Davis SD, Ferkol TW, Rosenfeld M, Lee HS, Dell SD, Sagel SD, et al. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am J Respir Crit Care Med . 2015;191:316–324. doi: 10.1164/rccm.201409-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Davis SD, Rosenfeld M, Lee HS, Ferkol TW, Sagel SD, Dell SD, et al. Primary ciliary dyskinesia: longitudinal study of lung disease by ultrastructure defect and genotype. Am J Respir Crit Care Med . 2019;199:190–198. doi: 10.1164/rccm.201803-0548OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shoemark A, Rubbo B, Legendre M, Fassad MR, Haarman EG, Best S, et al. Topological data analysis reveals genotype-phenotype relationships in primary ciliary dyskinesia. Eur Respir J . 2021;58:2002359. doi: 10.1183/13993003.02359-2020. [DOI] [PubMed] [Google Scholar]
- 8. Roesch EA, Nichols DP, Chmiel JF. Inflammation in cystic fibrosis: an update. Pediatr Pulmonol . 2018;53:S30–S50. doi: 10.1002/ppul.24129. [DOI] [PubMed] [Google Scholar]
- 9. Chalmers JD, Chotirmall SH. Bronchiectasis: new therapies and new perspectives. Lancet Respir Med . 2018;6:715–726. doi: 10.1016/S2213-2600(18)30053-5. [DOI] [PubMed] [Google Scholar]
- 10. Mayer-Hamblett N, Aitken ML, Accurso FJ, Kronmal RA, Konstan MW, Burns JL, et al. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am J Respir Crit Care Med . 2007;175:822–828. doi: 10.1164/rccm.200609-1354OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sagel SD, Wagner BD, Anthony MM, Emmett P, Zemanick ET. Sputum biomarkers of inflammation and lung function decline in children with cystic fibrosis. Am J Respir Crit Care Med . 2012;186:857–865. doi: 10.1164/rccm.201203-0507OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, et al. AREST CF Investigators Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med . 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 13. DeBoer EM, Swiercz W, Heltshe SL, Anthony MM, Szefler P, Klein R, et al. Automated CT scan scores of bronchiectasis and air trapping in cystic fibrosis. Chest . 2014;145:593–603. doi: 10.1378/chest.13-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chalmers JD, Moffitt KL, Suarez-Cuartin G, Sibila O, Finch S, Furrie E, et al. Neutrophil elastase activity is associated with exacerbations and lung function decline in bronchiectasis. Am J Respir Crit Care Med . 2017;195:1384–1393. doi: 10.1164/rccm.201605-1027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zihlif N, Paraskakis E, Lex C, Van de Pohl LA, Bush A. Correlation between cough frequency and airway inflammation in children with primary ciliary dyskinesia. Pediatr Pulmonol . 2005;39:551–557. doi: 10.1002/ppul.20202. [DOI] [PubMed] [Google Scholar]
- 16. Bush A, Payne D, Pike S, Jenkins G, Henke MO, Rubin BK. Mucus properties in children with primary ciliary dyskinesia: comparison with cystic fibrosis. Chest . 2006;129:118–123. doi: 10.1378/chest.129.1.118. [DOI] [PubMed] [Google Scholar]
- 17. Hilliard TN, Regamey N, Shute JK, Nicholson AG, Alton EW, Bush A, et al. Airway remodelling in children with cystic fibrosis. Thorax . 2007;62:1074–1080. doi: 10.1136/thx.2006.074641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ratjen F, Waters V, Klingel M, McDonald N, Dell S, Leahy TR, et al. Changes in airway inflammation during pulmonary exacerbations in patients with cystic fibrosis and primary ciliary dyskinesia. Eur Respir J . 2016;47:829–836. doi: 10.1183/13993003.01390-2015. [DOI] [PubMed] [Google Scholar]
- 19. Koucký V, Uhlík J, Hoňková L, Koucký M, Doušová T, Pohunek P. Ventilation inhomogeneity and bronchial basement membrane changes in chronic neutrophilic airway inflammation. Chest . 2020;157:779–789. doi: 10.1016/j.chest.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 20.Zariwala MA, Knowles MR, Leigh MW, National Institutes of Health https://www.ncbi.nlm.nih.gov/books/NBK1122/
- 21. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J . 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenow T, Oudraad MC, Murray CP, Turkovic L, Kuo W, de Bruijne M, et al. Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST CF) PRAGMA-CF. A quantitative structural lung disease computed tomography outcome in young children with cystic fibrosis. Am J Respir Crit Care Med . 2015;191:1158–1165. doi: 10.1164/rccm.201501-0061OC. [DOI] [PubMed] [Google Scholar]
- 23. Jain R, Baines A, Khan U, Wagner BD, Sagel SD. Evaluation of airway and circulating inflammatory biomarkers for cystic fibrosis drug development. J Cyst Fibros . 2021;20:50–56. doi: 10.1016/j.jcf.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kips JC, Fahy JV, Hargreave FE, Ind PW, in’t Veen JC. Methods for sputum induction and analysis of induced sputum: a method for assessing airway inflammation in asthma. Eur Respir J Suppl . 1998;26:9S–12S. [PubMed] [Google Scholar]
- 25. Efthimiadis A, Spanevello A, Hamid Q, Kelly MM, Linden M, Louis R, et al. Methods of sputum processing for cell counts, immunocytochemistry and in situ hybridisation. Eur Respir J Suppl . 2002;37:19s–23s. doi: 10.1183/09031936.02.00001902. [DOI] [PubMed] [Google Scholar]
- 26. Zemanick ET, Wagner BD, Robertson CE, Stevens MJ, Szefler SJ, Accurso FJ, et al. Assessment of airway microbiota and inflammation in cystic fibrosis using multiple sampling methods. Ann Am Thorac Soc . 2015;12:221–229. doi: 10.1513/AnnalsATS.201407-310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harris JK, Wagner BD, Zemanick ET, Robertson CE, Stevens MJ, Heltshe SL, et al. Changes in airway microbiome and inflammation with ivacaftor treatment in patients with cystic fibrosis and the G551D mutation. Ann Am Thorac Soc . 2020;17:212–220. doi: 10.1513/AnnalsATS.201907-493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med . 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 29. Livraghi-Butrico A, Kelly EJ, Klem ER, Dang H, Wolfgang MC, Boucher RC, et al. Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucosal Immunol . 2012;5:397–408. doi: 10.1038/mi.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rosen BH, Evans TIA, Moll SR, Gray JS, Liang B, Sun X, et al. Infection is not required for mucoinflammatory lung disease in CFTR-knockout ferrets. Am J Respir Crit Care Med . 2018;197:1308–1318. doi: 10.1164/rccm.201708-1616OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boucher RC. Muco-obstructive lung diseases. N Engl J Med . 2019;380:1941–1953. doi: 10.1056/NEJMra1813799. [DOI] [PubMed] [Google Scholar]
- 32. Flume PA, Chalmers JD, Olivier KN. Advances in bronchiectasis: endotyping, genetics, microbiome, and disease heterogeneity. Lancet . 2018;392:880–890. doi: 10.1016/S0140-6736(18)31767-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sagel SD, Gibson RL, Emerson J, McNamara S, Burns JL, Wagener JS, et al. Inhaled Tobramycin in Young Children Study Group Cystic Fibrosis Foundation Therapeutics Development Network. Impact of Pseudomonas and Staphylococcus infection on inflammation and clinical status in young children with cystic fibrosis. J Pediatr . 2009;154:183–188. doi: 10.1016/j.jpeds.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. LaFayette SL, Houle D, Beaudoin T, Wojewodka G, Radzioch D, Hoffman LR, et al. Cystic fibrosis-adapted Pseudomonas aeruginosa quorum sensing lasR mutants cause hyperinflammatory responses. Sci Adv . 2015;1:e1500199. doi: 10.1126/sciadv.1500199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sagel SD, Chmiel JF, Konstan MW. Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc . 2007;4:406–417. doi: 10.1513/pats.200703-044BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chalmers JD, Haworth CS, Metersky ML, Loebinger MR, Blasi F, Sibila O, et al. WILLOW Investigators Phase 2 trial of the DPP-1 inhibitor brensocatib in bronchiectasis. N Engl J Med . 2020;383:2127–2137. doi: 10.1056/NEJMoa2021713. [DOI] [PubMed] [Google Scholar]
- 37. Crichton ML, Aliberti S, Chalmers JD. A systematic review of pharmacotherapeutic clinical trial end-points for bronchiectasis in adults. Eur Respir Rev . 2019;28:180108. doi: 10.1183/16000617.0108-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nichols DP, Chmiel JF. Inflammation and its genesis in cystic fibrosis. Pediatr Pulmonol . 2015;50:S39–S56. doi: 10.1002/ppul.23242. [DOI] [PubMed] [Google Scholar]
- 39. Charman J, Reid L. The effect of freezing, storing and thawing on the viscosity of sputum. Biorheology . 1973;10:295–301. doi: 10.3233/bir-1973-10302. [DOI] [PubMed] [Google Scholar]
- 40. Gray RD, Imrie M, Boyd AC, Porteous D, Innes JA, Greening AP. Sputum and serum calprotectin are useful biomarkers during CF exacerbation. J Cyst Fibros . 2010;9:193–198. doi: 10.1016/j.jcf.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 41. Liou TG, Adler FR, Keogh RH, Li Y, Jensen JL, Walsh W, et al. Sputum biomarkers and the prediction of clinical outcomes in patients with cystic fibrosis. PLoS One . 2012;7:e42748. doi: 10.1371/journal.pone.0042748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Keir HR, Shoemark A, Dicker AJ, Perea L, Pollock J, Giam YH, et al. Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. Lancet Respir Med . 2021;9:873–884. doi: 10.1016/S2213-2600(20)30504-X. [DOI] [PubMed] [Google Scholar]