Abstract
Rationale
Propofol is a first-line sedative agent in the intensive care unit (ICU) but may be associated with hypertriglyceridemia and pancreatitis. To date, the relationship between propofol-induced hypertriglyceridemia and pancreatitis, as well as clinician responses to propofol-induced hypertriglyceridemia, have not been comprehensively studied.
Objectives
To assess the incidence of hypertriglyceridemia and pancreatitis in patients receiving continuous propofol infusions in the ICU and to describe the association between hypertriglyceridemia and the use of nonpropofol continuous sedative infusions.
Methods
This was a retrospective observational cohort study conducted at three urban academic hospitals within a single health system. Findings were additionally validated using the Medical Information Mart for Intensive Care-IV (MIMIC-IV) database containing data from a separate tertiary care hospital. Mechanically ventilated adult patients who received a continuous propofol infusion between 2016 and 2021 were included. The primary exposure was serum triglyceride concentration, and hypertriglyceridemia was defined as a triglyceride concentration greater than 400 mg/dl. Outcomes included new-onset pancreatitis as well as receipt of midazolam, dexmedetomidine, or ketamine after the triglyceride measurement. The incidence of pancreatitis was compared between groups using a Fisher’s Exact test. Multivariable logistic regression was used to assess the association between dichotomized triglyceride concentration and alternative sedative use.
Results
In the primary cohort of 7,037 patients, 1,724 (24.5%) had one or more triglyceride concentration measured. Of these, 1,365 (79.2%) had a maximum concentration of less than 400 mg/dl, and 359 (20.8%) had a maximum concentration of greater than 400 mg/dl. Compared with patients with low triglyceride concentrations, patients with high triglyceride concentrations were more likely to receive a continuous infusion of midazolam (37.0% vs. 16.4%; adjusted odds ratio [aOR], 3.1; 95% confidence interval [CI], 2.2–4.4; P < 0.01), ketamine (22.8% vs. 6.9%; aOR, 3.5; 95% CI, 2.3–5.3; P < 0.01), and dexmedetomidine (57.7% vs. 46.6%; aOR, 1.5; 95% CI, 1.1–2.0; P < 0.01). Rates of midazolam infusion increased as triglyceride concentrations exceeded 500 mg/dl. Forty-four (0.6%) patients developed pancreatitis after propofol initiation, of which 4 (9.1%) were considered related to propofol-associated hypertriglyceridemia. Findings were similar in the MIMIC-IV cohort.
Conclusions
Propofol-associated hypertriglyceridemia is relatively common in mechanically ventilated ICU patients who have triglycerides measured. Pancreatitis related to propofol-associated hypertriglyceridemia is rare. Patients who develop hypertriglyceridemia while receiving propofol are more likely to receive continuous infusions of other sedatives.
Keywords: critical care, propofol, deep sedation, triglycerides, pancreatitis
The PADIS (pain, agitation/sedation, delirium, immobility, and sleep disruption) guidelines recommend propofol as a first-line sedative agent for mechanically ventilated patients (1). Because of propofol’s high lipophilicity, propofol must be delivered in a lipid emulsion to be effective as an intravenous agent (most commonly in an emulsion with 10% soybean oil). This formulation is effective but has several drawbacks, including an increased risk of hypertriglyceridemia (2–5). High triglycerides can lead to rare adverse events, such as hypertriglyceridemia-induced pancreatitis (2–4, 6, 7).
Although hypertriglyceridemia is a well-recognized potential adverse effect of propofol infusions, little is known about the incidence of hypertriglyceridemia in critically ill populations, nor the rates of hypertriglyceridemia-associated pancreatitis. Past literature regarding the frequency and significance of propofol-induced hypertriglyceridemia is mixed and predominantly consists of small studies. One study of 20 nonseptic intensive care unit (ICU) patients found that serum lipid concentrations were not significantly influenced by propofol and observed no adverse events (2). Other studies, however, demonstrated significant increases in triglyceride concentrations in patients sedated with propofol (3, 6). One recent retrospective study of 159 adults in the ICU found that 18% developed hypertriglyceridemia, with 10% of those developing pancreatitis (6).
In addition to the above gaps in knowledge, no study has explored the use of alternative sedative agents in response to high triglyceride concentrations. The PADIS guidelines suggest the use of propofol over continuous infusions of benzodiazepines because of increased rates of delirium and longer lengths of stay when patients are sedated with benzodiazepines (1). High triglyceride concentrations, however, may prompt clinicians to switch a patient from the preferred propofol infusion to a benzodiazepine infusion without a clear understanding of the risks and benefits of such a decision.
The present study aims to assess the incidence of propofol-associated hypertriglyceridemia and related pancreatitis. In addition, it looks to determine the association between elevated triglyceride concentrations and the use of alternative sedative infusions. We hypothesized that propofol-associated pancreatitis is rare and that high triglyceride concentrations would be associated with increased use of alternative sedative infusions, including midazolam.
Methods
Research Approach, Setting, and Patient Population
This was a retrospective observational cohort study conducted at three urban academic hospitals within a single health system. Patients were included if they were aged greater than 17 years, admitted to the hospital between January 2016 and April 2021, received invasive mechanical ventilation, and were sedated with a continuous infusion of propofol for any duration. Patients were excluded if they had a known diagnosis of pancreatitis before the start of mechanical ventilation defined by an ICD-10 (International Classification of Diseases, Tenth Revision) code for pancreatitis in addition to an elevated lipase concentration above 180 IU/L (measured before mechanical ventilation start). Only the index ICU stay was included for each patient. Patients without a triglyceride concentration measured are described but are not included in the primary analyses.
In addition to this primary cohort, a similar cohort was abstracted from the Medical Information Mart for Intensive Care-IV (MIMIC-IV) database (8). MIMIC-IV is a large database of deidentified patient records from Beth Israel Deaconess Medical Center. Patients included in the database from 2017 to 2019 were included. Other inclusion and exclusion criteria mirrored those of the primary cohort.
Primary Exposure and Outcomes
The primary exposure for this study was hypertriglyceridemia. The serum triglyceride concentration was dichotomized as has been done in prior studies into high (>400 mg/dl) or low (⩽400 mg/dl) concentrations (3, 6). The authors can confirm that there is no current protocol in place at either the hospitals for the primary cohort or for the MIMIC cohort with respect to when triglyceride concentrations should be checked for patients receiving propofol or at what threshold propofol should be discontinued. The primary analysis explored the highest triglyceride concentration measured (through day 28 after the start of mechanical ventilation), and a sensitivity analysis used the first triglyceride concentration measured. The primary outcome was the receipt of a continuous infusion of midazolam after the maximum triglyceride measurement. Additional outcomes with respect to sedative choice were receipt of dexmedetomidine and receipt of ketamine. Patient-centered outcomes included survival to ICU discharge, length of ICU stay in survivors to ICU discharge, ventilator days during the index ICU stay among ICU survivors, and survival to hospital discharge.
Adverse Effects of Propofol-induced Hypertriglyceridemia
The primary adverse effect related to propofol-induced hypertriglyceridemia is pancreatitis. For this study, pancreatitis was defined by an ICD-10 code for pancreatitis (K85.xx for acute pancreatitis or K86.xx for chronic pancreatitis) in addition to a lipase concentration above 180 IU/L. In a sensitivity analysis, each patient in the primary cohort with an ICD-10 code for pancreatitis was additionally hand-reviewed by two physician authors (P.P. and A.M.) to ascertain whether pancreatitis was thought by the clinical team to be potentially related to propofol. Attributability was categorized as follows: “likely” related to propofol if the clinical team documented a suspected relationship to propofol and no other clear alternative etiology existed, “uncertain” if the clinical team documented uncertainty regarding the etiology of pancreatitis, and “unlikely” if there was a clear alternative etiology (e.g., gallstone) or pancreatitis onset was before ICU admission. As a sensitivity analysis, pancreatitis was defined only using the lipase criteria.
Statistical Analysis Plan
Descriptive statistics were used to describe the study population. Categorical variables are presented as counts with percentages and are compared using Fisher’s exact tests. Continuous variables are presented as means with standard deviations or medians with interquartile ranges and are compared using t tests or rank-sum tests. Logistic regression was used to assess the relationship between the primary independent variable/outcome of the use of midazolam and the primary exposure/dependent variable of dichotomized triglyceride concentration. Additional covariates included in the model were age, biological sex, ICU type (dichotomized as primary medical vs. primary surgical), and the physiology component of the Acute Physiology and Chronic Health Evaluation (APACHE) III score. The same model was used to assess other binary outcomes as described above. As a sensitivity analysis, triglyceride concentrations were broken into categories by 100 mg/dl (0–100 mg/dl, 101–200 mg/dl, etc.) in the primary logistic regression. Additional sensitivity analyses were performed 1) using the first triglyceride concentration measured after the start of mechanical ventilation in place of the maximum triglyceride concentration; 2) limiting the timing of alternative sedative agents to the first 3 days after the triglyceride concentration; 3) controlling for coronavirus disease (COVID-19) status in the regression model; and 4) excluding patients from the primary cohort admitted after March 1, 2020 (9–11). Finally, cumulative doses of sedative agents were calculated in the 48 hours before and after the maximum triglyceride concentration and displayed graphically by exposure group.
All analyses were repeated in the MIMIC-IV patient cohort to verify the findings in an external sample.
This study was approved by the Institutional Review Board at Albert Einstein College of Medicine. All statistics were performed using STATA, version 16 (StataCorp LP).
Results
Primary Cohort Characteristics
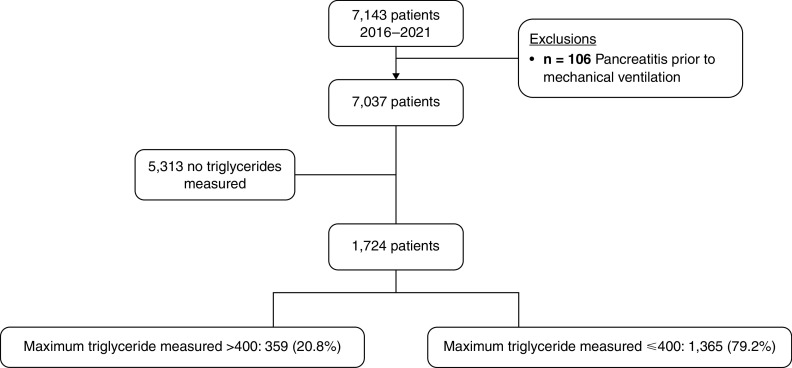
A total of 7,143 patients met initial inclusion criteria, and 106 were excluded for pancreatitis before mechanical ventilation, leaving a total of 7,037 patients in the final cohort, of which 5,313 (75.5%) had no triglyceride concentration measured. Of those with at least one triglyceride concentration measured, 1,365 (79.2%) had at least one triglyceride concentration measured with a maximum concentration ⩽400 mg/dl, and 359 (20.8%) had a maximum triglyceride concentration >400 mg/dl (see Figure 1). Characteristics of the final cohort can be found in Table 1, both for the overall cohort and divided on the basis of no triglyceride concentration, low maximum triglyceride concentration, and high maximum triglyceride concentration.
Figure 1.
Cohort selection.
Table 1.
Cohort characteristics
| Variable | Overall (N = 7,037) |
No Triglycerides Measured (n = 5,313) |
Low Triglyceride* (n = 1,365) |
High Triglyceride* (n = 359) |
|---|---|---|---|---|
| Age (yr), mean (SD) | 61.5 (15.2) | 61.5 (15.2) | 61.52 (14.3) | 55.54 (13.7) |
| Sex (female), n (%) | 3,040 (43.2) | 2,328 (43.8) | 579 (42.4) | 133 (37.0) |
| Race (Black), n (%) | 2,192 (31.1) | 1,709 (32.2) | 380 (27.8) | 103 (28.7) |
| Ethnicity (not Hispanic), n (%) | 3,747 (53.2) | 2,847 (53.6) | 729 (53.4) | 171 (47.6) |
| Body mass index, mean (SD) | 30.0 (9.3) | 29.6 (9.3) | 30.5 (9.1) | 33.4 (9.1) |
| ICU type (medical), n (%) | 3,647 (51.8) | 2,728 (51.3) | 694 (50.8) | 225 (62.7) |
| Sars-CoV-2–positive, n (%) | 672 (9.5) | 314 (5.9) | 217 (15.9) | 141 (39.3) |
| Propofol days before first triglyceride measurement, median (IQR) | 1.78 (0.5–4.8) | n/a | 1.58 (0.4–4.7) | 2.32 (0.8–5.4) |
| Propofol days before highest triglyceride measurement, median (IQR) | 2.49 (0.7–6.5) | n/a | 2.02 (0.59–5.7) | 4.77 (2.0–9.1) |
| Cumulative propofol dose (g), median (IQR) | 12.9 (5.8–29.0) | 10.5 (4.9–22.5) | 22.4 (10.3–46.1) | 49.8 (27.6–86.3) |
| Receipt of continuous paralytics before triglyceride measurement, n (%) | 1,120 (15.9) | 0 (0.0) | 837 (61.3) | 283 (78.8) |
| Days hospitalized before mechanical ventilation, median (IQR) | 0.8 (0.2–4.0) | 0.8 (0.3–4.0) | 0.8 (0.2–3.9) | 0.9 (0.2–4.3) |
| APACHE-III score, mean (SD) | 63.9 (23.5) | 62.8 (23.8) | 66.3 (22.2) | 70.4 (22.8) |
| Ventilator d in survivors | 2.44 (0.78–6.95) | 1.97 (0.67–5.42) | 5.35 (1.63–12.67) | 11.35 (5.87–21.09) |
| ICU length of stay in hospital survivors (d), median (IQR) | 6.1 (3.3–11.7) | 5.3 (3.0–9.9) | 9.4 (5.0–16.7) | 15.8 (9.8–24.0) |
| Hospital length of stay in hospital survivors (d), median (IQR) | 18.4 (10.4–32.7) | 16.6 (9.5–29.1) | 25.1 (15.0–43.7) | 35.9 (21.2–55.8) |
| Hospital mortality, n (%) | 2,279 (32.4) | 1,569 (29.5) | 513 (37.6) | 197 (54.9) |
Definition of abbreviations: APACHE = Acute Physiology and Chronic Health Evaluation; ICU = intensive care unit; IQR = interquartile range; Sars-CoV-2 = severe acute respiratory syndrome coronavirus 2; SD = standard deviation.
Reflects maximum triglyceride measurement.
Triglyceride Concentrations
For the 1,724 patients who had at least one triglyceride concentration measured, the median first measured triglyceride concentration was 171 mg/dl (interquartile range [IQR], 109–296), and the median maximum triglyceride concentration was 186 mg/dl (IQR, 115–351). The first triglyceride concentration was measured at a median of 1.7 (IQR, 0.5–5.1) days after the initiation of mechanical ventilation. Most patients had only one triglyceride concentration measured, but 273 (15.8%) had 3 or more measurements, and 127 (7.4%) had 5 or more measurements. Patients with high as compared with low maximum triglyceride measurements had higher median APACHE III physiology scores at ICU admission (70.4 ± 22.8 vs. 66.3 ± 22.2; P < 0.01) and were more likely to be hospitalized in a medical ICU (62.7% vs. 50.8%; P < 0.01). Patients with high maximum triglyceride measurements had a median of 4.8 (IQR, 2.0–9.1) propofol days before their maximum measurement as compared with 2.0 (IQR, 0.5–5.7) propofol days in patients with low maximum triglyceride measurements (P < 0.01). Patients with high maximum triglyceride concentrations also had a higher maximum propofol dose as compared with patients with lower maximum triglyceride concentrations (median, 50.0 mcg/kg/min [IQR, 47.5–60.0] vs. 40.0 mcg/kg/min [IQR, 25.0–50.0]); P < 0.01) and higher cumulative doses of propofol during the ICU stay (median, 22.4 g [10.3–46.1] vs. 49.8 g [27.6–86.3]).
Outcomes
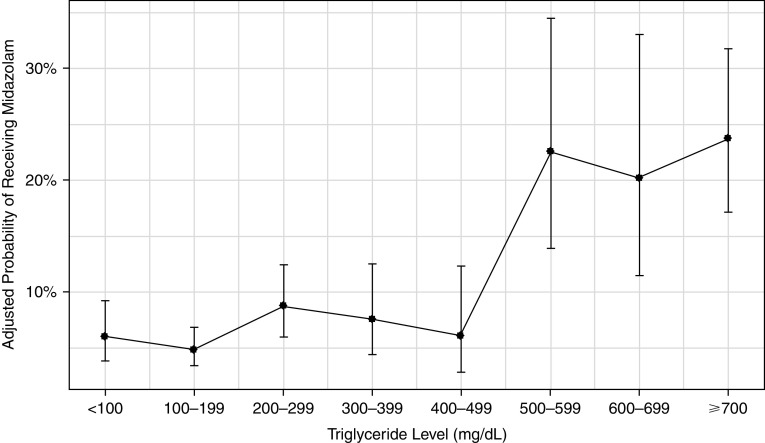
As compared with patients with low triglyceride concentrations on the basis of maximum concentration during the ICU stay, patients with high triglycerides were more likely to receive a continuous infusion of midazolam (37.0% vs. 16.4%; adjusted odds ratio [aOR], 3.1; 95% confidence interval [CI], 2.2–4.4; P < 0.01), ketamine (22.8% vs. 6.9%; aOR, 3.5; 95% CI, 2.3–5.3; P < 0.01), and dexmedetomidine (57.7% vs. 46.6%; aOR, 1.5; 95% CI, 1.1–2.0; P < 0.01). In the predefined sensitivity analysis, rates of midazolam infusion appear to increase once triglyceride concentrations exceed 500 mg/dl (see Figure 2). Compared with patients with maximum triglyceride concentrations <100 mg/dl, patients with a maximum triglyceride concentration between 500 and 599 g/dl had a 4.6-times higher odds of receiving midazolam (aOR, 4.6; 95% CI, 2.1–9.9; P < 0.01). Stepwise increases in triglyceride concentration also resulted in higher rates of ketamine and dexmedetomidine use, as well as falling propofol use (see Figures E1–E3 in the data supplement).
Figure 2.
Adjusted probability of receiving midazolam infusion by triglyceride concentration.
Similar findings were demonstrated when the first triglyceride measured in the ICU was used in place of the maximum triglyceride concentration when the assessment of alternative sedatives was limited to the 3 days after maximum triglyceride measurement, and when controlling for COVID-19 status in the regression model, with the exception of dexmedetomidine in the latter two sensitivity analyses (see Table E1). In the primary cohort, excluding the time period after February 2020, there was no longer a significant association between high triglycerides and the use of midazolam (aOR, 1.5; 95% CI, 0.8–2.6; P = 0.21) or ketamine (aOR, 1.4; 95% CI, 0.4–4.3; P = 0.6), but the relationship with dexmedetomidine remained (aOR, 1.8; 95% CI, 1.3–2.7; P < 0.01); however, the point estimates and overall trend toward higher alternative sedative use with increasing triglyceride concentration remained. Patients with high triglyceride concentrations were less likely to receive additional propofol after their maximum triglyceride measurement (28.1% vs. 37.6%; P = 0.01, assessment started the day following triglyceride concentration measurement). Comparing the cumulative sedative doses in the 48 hours before the maximum triglyceride concentration to the 48 hours after, patients in the high-triglyceride group had a decline in propofol received but an increase in midazolam. Patients in the low triglyceride group had a slight increase in propofol received over the subsequent 24 hours, then a decrease over the full 48 hours in combination with a decrease in midazolam received (see Figure 3 for the full cohort and Figures E4 and E5 for cumulative dose changes by 100 mg/dl triglyceride concentration increments, as well as Tables E2–E4).
Figure 3.
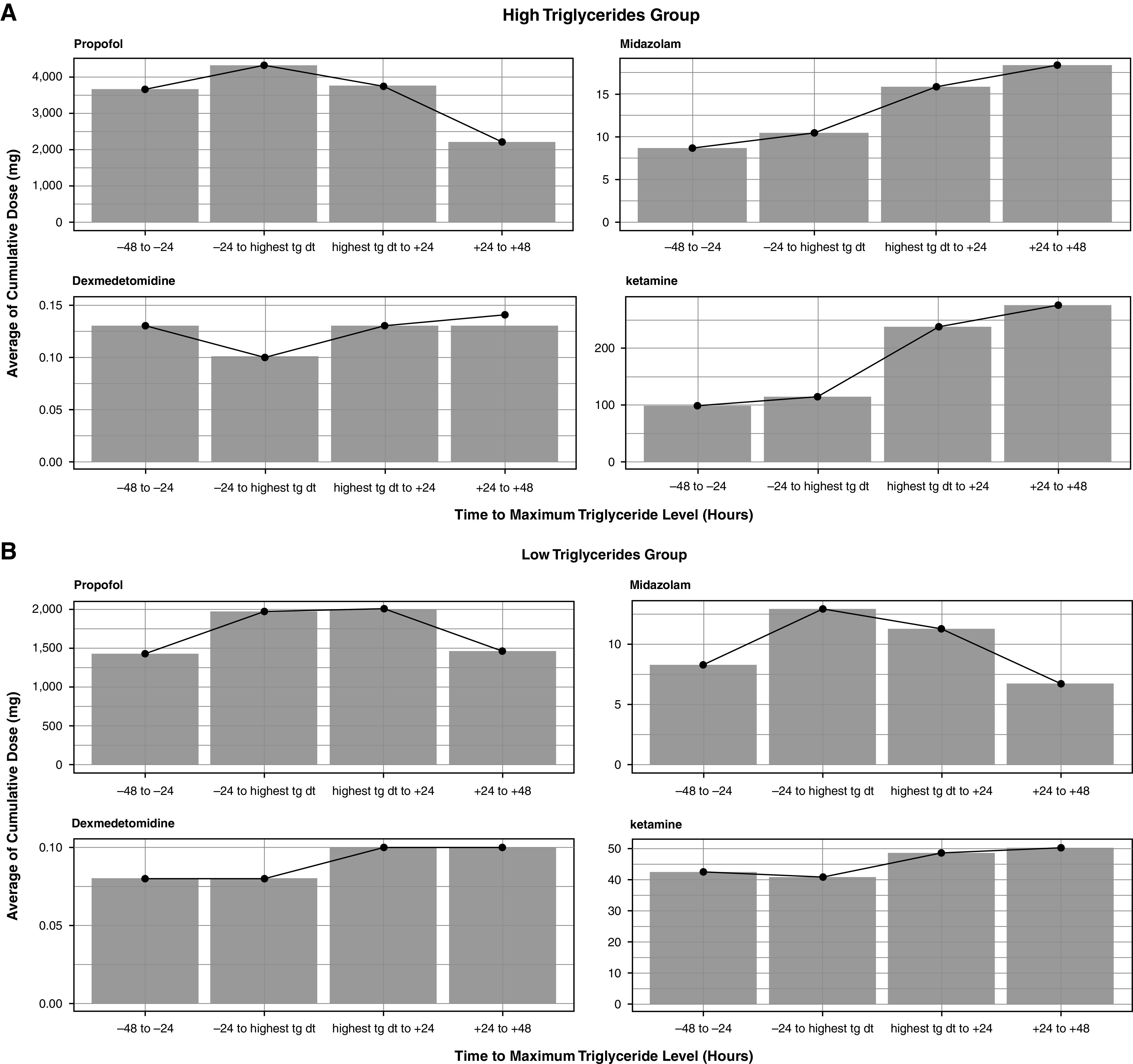
Cumulative sedative dose before and after maximum triglyceride concentration. Cumulative dose of propofol (top-left corner), midazolam (top-right corner), dexmedetomidine (bottom-left corner), and ketamine (bottom-right corner) by 24-hour block starting 48 hours before the maximum triglyceride concentration and ending 48 hours after the maximum. Doses represent the mean number of milligrams received. (A) The high triglyceride group. (B) The low triglyceride group. tg dt = triglyceride date.
Patients with high triglyceride concentrations were more likely to die in the ICU (54.9% vs. 37.6%; P < 0.01), and this relationship persisted after multivariable adjustment (aOR for mortality, 2.1; 95% CI, 1.6–2.6; P < 0.01). They also had longer lengths of stay in the ICU and the hospital and had more ventilator days in survivors (see Table 1).
Adverse Effects of Propofol-induced Hypertriglyceridemia
There were 44 (0.6%) patients who met the criteria for pancreatitis. Of patients without triglyceride concentrations measured, 18 (0.3%) developed pancreatitis, as compared with 14 (1.0%) patients in the low triglyceride group and 12 (3.3%) patients in the high triglyceride group (P < 0.01). Overall, 4 (0.06% of the full cohort) pancreatitis cases were adjudicated as likely related to the propofol infusion, 12 (0.2%) were assessed as uncertain, and 28 (0.4%) were unlikely related. In an analysis defining pancreatitis on the basis of elevated lipase alone, there were 165 total pancreatitis cases (2.3% of the cohort). In patients with no triglycerides measured, 83 (1.6%) patients developed pancreatitis by the lipase-only definition as compared with 48 (3.5%) patients with a low maximum triglyceride concentration and 34 (9.5%) with high maximum triglyceride concentrations. Patients in the high maximum triglyceride group were more likely to receive gemfibrozil (17.3% vs. 0.2%; P < 0.01) and insulin infusions (30.4% vs. 17.1%; P < 0.01) compared with those in the low triglyceride group.
Results from the MIMIC-IV Dataset
In the MIMIC-IV cohort, there were 9,194 total patients, of whom 7,548 (82.1%) had no triglycerides measured, 1,386 (15.1%) had at least one triglyceride concentration measured with a maximum concentration ⩽400 mg/dl, and 260 (2.8%) had a maximum triglyceride concentration >400 mg/dl. See Table E5 for the characteristics of this cohort. Similar to the primary cohort, patients with higher triglyceride concentrations were more likely to receive midazolam infusions (55.0% vs. 27.2%; aOR, 3.6; 95% CI, 2.4–5.5; P < 0.01). Patients in the MIMIC-IV cohort with high triglyceride concentrations were also more likely to receive dexmedetomidine (72.3% vs. 51.8%; aOR, 1.3; 95% CI, 0.9–1.9; P = 0.17) but not ketamine, although few patients overall received ketamine (5.0% vs. 2.2%; aOR, 0.11; 95% CI, 0.0–0.8; P = 0.50). See Table 2 for a comparison to the primary cohort. When examined as a categorical variable, there was a substantial increase in the probability of receiving a midazolam infusion for patients with maximum triglyceride concentrations above 400 mg/dl (aOR for patients with triglyceride concentrations 400–599 mg/dl, 3.1; 95% CI, 1.2–8.1; P = 0.02 compared with reference of <100 mg/dl) (Figure E6). Patients with high triglyceride concentrations had higher mortality rates and longer lengths of stay compared with patients with low triglyceride concentrations (see Table E5). Changes in cumulative propofol dose can be found in Figure E7.
Table 2.
Rate and adjusted odds of receiving midazolam, dexmedetomidine, and ketamine in the primary and MIMIC-IV cohorts
| Sedative Agent | Primary Cohort |
MIMIC-IV |
||
|---|---|---|---|---|
| % | aOR (95% CI) | % | aOR (95% CI) | |
| Midazolam infusion | ||||
| High triglyceride (>400 mg/dl) | 37.0 | 3.1 (2.2–4.4) | 55.0 | 3.6 (2.4–5.5) |
| Low triglyceride (⩽400 mg/dl) | 16.4 | 27.2 | ||
| Dexmedetomidine | ||||
| High triglyceride (>400 mg/dl) | 57.7 | 1.5 (1.1–1.9) | 72.3 | 1.3 (0.9–1.9) |
| Low triglyceride (⩽400 mg/dl) | 46.6 | 51.8 | ||
| Ketamine infusion | ||||
| High triglyceride (>400 mg/dl) | 22.8 | 2.3 (2.3–5.3) | 5.0 | 0.1 (0.0–0.8) |
| Low triglyceride (⩽400 mg/dl) | 6.9 | 2.2 | ||
Definition of abbreviations: aOR = adjusted odds ratio; CI = confidence interval; MIMIC-IV = Medical Information Mart for Intensive Care-IV.
In the full cohort, there were 153 (1.7%) patients with an ICD-10 code for pancreatitis and a lipase concentration above 180 IU/L first measured after initiation of mechanical ventilation. Of patients without triglyceride concentrations measured, 89 (1.2%) developed pancreatitis, compared with 41 patients (3.0%) in the low triglyceride group and 23 (8.8%) in the high triglyceride group. Using the lipase-only definition of pancreatitis, 182 (2.4%) patients without triglyceride measures, 98 (7.1%) patients with low maximum triglyceride concentrations, and 43 (16.5%) patients with high maximum triglyceride concentrations developed pancreatitis. Gemfibrozil prescriptions were rare in all groups but slightly higher in patients with high triglyceride concentrations (1.5% vs. 0.2%) (see Table E3).
Discussion
In this large, retrospective cohort of critically ill and mechanically ventilated patients who received a continuous infusion of propofol, a minority developed hypertriglyceridemia with concentrations over 400 mg/dl. These patients were more likely to be found in the medical ICU setting, had increased severity of illness with elevated APACHE III physiology scores at ICU admission, had longer lengths of stay, and were more likely to die in the ICU and hospital. Patients with elevated triglyceride concentrations were more likely to receive a continuous infusion of alternative sedative agents, including midazolam, ketamine, and dexmedetomidine. Patients with high triglyceride concentrations, compared with those with low triglyceride concentrations, were more likely to develop pancreatitis, although overall, this complication was uncommon.
Our findings are in line with those of similar but substantially smaller studies by Devlin and colleagues and Corrado and colleagues, which found an incidence of hypertriglyceridemia of 18% and 28%, respectively (3, 6). In those studies, the incidence of pancreatitis was slightly more common than in the main cohort of the present study and similar to that in the MIMIC-IV cohort. In the study by Devlin and colleagues, 10% of patients in the hypertriglyceridemia group developed pancreatitis, as did 5.3% of the patients in the study by Corrado and colleagues. The lower incidence of pancreatitis in the primary cohort herein may be because of the exclusion of patients with pancreatitis preceding the ICU admission and potentially to the relatively high rate of gemfibrozil prescription in the high triglyceride group, although the association between gemfibrozil and pancreatitis was not assessed and is beyond the scope of the present study. This latter possibility is hypothesis-generating only but notable in comparison to prescriptions of gemfibrozil in the MIMIC-IV database and an interesting area of practice variation.
Patients in the present study who developed hypertriglyceridemia had higher illness severity, older age, longer lengths of stay, and increased mortality. The reason for this is not clear and may reflect that elevated triglycerides are an inflammatory marker (12, 13), that multiorgan dysfunction leads to increased production/decreased clearance of excess triglycerides (14), that higher triglyceride concentrations reflect increased depth/duration of sedation needed, that actions in response to elevated triglycerides may worsen outcomes, or that triglycerides themselves promote inflammation and are causal of increased illness severity. The present study’s findings are in line with prior work, which has also shown an association between hypertriglyceridemia and both illness severity at ICU admission and worse outcomes (3, 6). Kenes and colleagues studied patients with COVID-19 and found that patients with acute respiratory distress syndrome from COVID-19 had higher rates of hypertriglyceridemia than those without COVID-19, even after adjusting for differences in cumulative propofol doses. This stipulates that certain diseases and risk factors may increase the risk of propofol-induced hypertriglyceridemia independently (11). In the present study, the MIMIC-IV cohort was drawn from a period before COVID-19, which suggests that the present findings are not limited to practices during the COVID-19 pandemic. The sensitivity analyses for the primary cohort 1) controlling for COVID-19 status; and 2) excluding the COVID-19 period had some different findings from the primary analysis. Although overall point estimates were in the same direction for all models, some associations were no longer statistically significant. This may be because of either/both 1) loss of power because of a smaller sample size; or 2) practice variation during the COVID-19 pandemic, such as changes in sedation practice (15, 16).
The 2018 PADIS guidelines do not provide guidance regarding monitoring of triglyceride concentrations in patients receiving propofol (1). Interestingly, this contrasts with the 2002 guidelines by the American College of Critical Care Medicine, which initially recommended monitoring triglyceride concentrations starting 2 days after infusion (17). In our cohort, 73% of patients did not have triglyceride concentrations measured, although 15% had at least three measurements. Analysis of the MIMIC-IV cohort demonstrated a larger proportion of patients (84.9%) without triglyceride concentrations measured. There remains no clear evidence-based practice recommendation to guide the monitoring of triglyceride concentrations. There was a cumulative dose relationship between propofol infusion and higher triglyceride concentrations, which is in line with findings by Corrado and colleagues (3) and Witenko and colleagues (9).
To date, no prior study has systematically explored the use of nonpropofol sedative agents in patients with propofol-associated hypertriglyceridemia. In our analysis, the clinician response to hypertriglyceridemia is clear, with a strong association between high triglyceride concentrations and the use of alternative sedative agents, especially midazolam. This finding was robust in multivariate analysis and two distinct patient cohorts. The 2018 PADIS guidelines recommend the use of nonbenzodiazepine sedatives, as benzodiazepines are associated with increased length of stay, duration of mechanical ventilation, and delirium (1). The use of inferior sedative agents is an important balancing measure to consider when addressing hypertriglyceridemia in the ICU. Given that high propofol doses are associated with an increased risk of hypertriglyceridemia, it is important to prevent the problem through targeting lower sedation targets, daily awakening trials, the early use of antipsychotic therapies, and perhaps the prescription of fibrates to patients at highest risk.
Multiple pathways to acute pancreatitis in the ICU exist (7, 18, 19). Overall, only 0.5% of patients developed pancreatitis after the initiation of mechanical ventilation. Although it is clear from both cohorts studied that pancreatitis is more common in patients with hypertriglyceridemia, the event is rare, and it is imperative for clinicians to understand the risks and benefits of using alternative sedative agents, especially benzodiazepine infusions, in response to high triglyceride concentrations.
Strengths and Limitations
The present study has a number of important strengths. First, it is the largest study to date investigating propofol-associated hypertriglyceridemia. Second, the present study evaluates clinician response to elevations in triglycerides, which has not been previously explored. Finally, the present study provides adjudication as to the likelihood that pancreatitis is related to hypertriglyceridemia. There are also limitations of our study. First, our data is derived from three academic hospitals, and the results may not be generalizable. There is no defined protocol for triglyceride measurement at the study sites, and some sedation practices may be unique to the study hospitals. Nevertheless, we incorporated data from the MIMIC-IV database to offset potential institutional biases. Another limitation is the retrospective nature of our study and the dependence on clinical documentation. Finally, other medications which can potentially increase triglycerides (e.g., clevidipine and niacin) were not assessed.
Conclusions
Among critically ill patients receiving mechanical ventilation and a continuous infusion of propofol, hypertriglyceridemia is common among those with triglycerides measured. Patients who develop hypertriglyceridemia are more likely to receive a continuous infusion of an alternative sedative agent, such as midazolam. The incidence of propofol-associated pancreatitis is low but higher in patients with hypertriglyceridemia. Clinicians must balance the low risk of propofol-associated pancreatitis against the potential adverse effects of less ideal sedative agents.
Footnotes
Supported by the National Institute of General Medical Sciences (K23GM128005 [A.M.])
Author Contributions: A.M., P.P., and N.J.Q. conceived of and designed the study. A.M., J.W., S.L., J.B., and P.P. contributed to data acquisition, analysis, and interpretation. A.M., P.P., and M.N.G. interpreted the data and drafted the first manuscript version. All authors reviewed the final manuscript and revised it for important intellectual content. All authors approved the final version of the manuscript and agree to be accountable for all aspects of the work.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med . 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 2. Gottardis M, Khünl-Brady KS, Koller W, Sigl G, Hackl JM. Effect of prolonged sedation with propofol on serum triglyceride and cholesterol concentrations. Br J Anaesth . 1989;62:393–396. doi: 10.1093/bja/62.4.393. [DOI] [PubMed] [Google Scholar]
- 3. Corrado MJ, Kovacevic MP, Dube KM, Lupi KE, Szumita PM, DeGrado JR. The incidence of propofol-induced hypertriglyceridemia and identification of associated risk factors. Crit Care Explor . 2020;2:e0282. doi: 10.1097/CCE.0000000000000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gottschling S, Meyer S, Krenn T, Kleinschmidt S, Reinhard H, Graf N, et al. Effects of short-term propofol administration on pancreatic enzymes and triglyceride levels in children. Anaesthesia. 2005;60:660–663. doi: 10.1111/j.1365-2044.2005.04231.x. [DOI] [PubMed] [Google Scholar]
- 5.Devaud JC, Berger MM, Pannatier A, Marques-Vidal P, Tappy L, Rodondi N, et al. Hypertriglyceridemia: a potential side effect of propofol sedation in critical illness. Intensive Care Med. 2012;38:1990–8. doi: 10.1007/s00134-012-2688-8. [DOI] [PubMed] [Google Scholar]
- 6. Devlin JW, Lau AK, Tanios MA. Propofol-associated hypertriglyceridemia and pancreatitis in the intensive care unit: an analysis of frequency and risk factors. Pharmacotherapy . 2005;25:1348–1352. doi: 10.1592/phco.2005.25.10.1348. [DOI] [PubMed] [Google Scholar]
- 7. Asghar MU, Cheema HA, Tanveer K, Leinwand J. Propofol infusion and acute pancreatitis: a review. Am J Ther . 2020;27:e371–e374. doi: 10.1097/MJT.0000000000001021. [DOI] [PubMed] [Google Scholar]
- 8. Goldberger A, Amaral L, Glass L, Hausdorff J, Ivanov PC, Mark RG. PhysioBank, PhysioToolkit, and PhysioNet: components of a new research resource for complex physiologic signals. Circulation . 2020;101:e215–e20. doi: 10.1161/01.cir.101.23.e215. [DOI] [PubMed] [Google Scholar]
- 9. Witenko CJ, Littlefield AJ, Abedian S, An A, Barie PS, Berger K. The safety of continuous infusion propofol in mechanically ventilated adults with coronavirus disease 2019. Ann Pharmacother . 2022;56:5–15. doi: 10.1177/10600280211017315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kovacevic MP, Dube KM, Lupi KE, Szumita PM, DeGrado JR. Evaluation of hypertriglyceridemia in critically ill patients with coronavirus disease 2019 receiving propofol. Crit Care Explor . 2021;3:e0330. doi: 10.1097/CCE.0000000000000330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kenes MT, McSparron JI, Marshall VD, Renius K, Hyzy RC. Propofol-associated hypertriglyceridemia in coronavirus disease 2019 versus noncoronavirus disease 2019 acute respiratory distress syndrome. Crit Care Explor . 2020;2:e0303. doi: 10.1097/CCE.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cetinkaya A, Erden A, Avci D, Karagoz H, Karahan S, Basak M, et al. Is hypertriglyceridemia a prognostic factor in sepsis? Ther Clin Risk Manag . 2014;10:147–50. doi: 10.2147/TCRM.S57791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jun JC, Shin MK, Yao Q, Bevans-Fonti S, Poole J, Drager LF, et al. Acute hypoxia induces hypertriglyceridemia by decreasing plasma triglyceride clearance in mice. Am J Physiol Endocrinol Metab . 2012;303:E377–88. doi: 10.1152/ajpendo.00641.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spitzer JJ, Bagby GJ, Mészáros K, Lang CH. Alterations in lipid and carbohydrate metabolism in sepsis. JPEN J Parenter Enteral Nutr . 1988;12:53S–58S. doi: 10.1177/014860718801200604. [DOI] [PubMed] [Google Scholar]
- 15. Stephens RJ, Evans EM, Pajor MJ, Pappal RD, Egan HM, Wei M, et al. A dual-center cohort study on the association between early deep sedation and clinical outcomes in mechanically ventilated patients during the COVID-19 pandemic: the COVID-SED study. Crit Care . 2022;26:179. doi: 10.1186/s13054-022-04042-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ammar MA, Sacha GL, Welch SC, Bass SN, Kane-Gill SL, Duggal A, et al. Sedation, analgesia, and paralysis in COVID-19 patients in the setting of drug shortages. J Intensive Care Med . 2021;36:157–174. doi: 10.1177/0885066620951426. [DOI] [PubMed] [Google Scholar]
- 17. Barr J, Fraser GL, Puntillo K, Ely EW, Gélinas C, Dasta JF, et al. American College of Critical Care Medicine Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med . 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 18. Muniraj T, Aslanian HR. Hypertriglyceridemia independent propofol-induced pancreatitis. JOP . 2012;13:451–3. doi: 10.6092/1590-8577/822. [DOI] [PubMed] [Google Scholar]
- 19. Gottschling S, Larsen R, Meyer S, Graf N, Reinhard H. Acute pancreatitis induced by short-term propofol administration. Paediatr Anaesth . 2005;15:1006–8. doi: 10.1111/j.1460-9592.2004.01562.x. [DOI] [PubMed] [Google Scholar]