Abstract
A murine model was used to characterize the local immune and inflammatory response during ocular toxoplasmosis. Major histocompatibility complex (MHC) class I, normally expressed at low levels in immune-privileged sites such as the eye, was up-regulated during infection as determined by competitive reverse transcriptase (RT)-PCR and immunocytochemistry for both β2-microglobulin and the MHC class I heavy chain. However, the eyes of chronically infected mice also had increased levels of mRNA transcripts for transforming growth factor β, a cytokine associated with immune privilege and constitutively expressed in normal eyes. Transcripts for a number of inflammatory mediators, including interleukin-6 (IL-6), were increased during chronic infection. The role of IL-6 was further investigated by comparing disease progression and the development of the local immune response in wild-type (WT) and IL-6-deficient mice (IL-6−/− mice). Following infection, IL-6−/− mice developed more severe inflammation in the retina and vitreous humor compared with WT mice. This increased severity of disease was associated with reduced ocular IL-1α and increased tumor necrosis factor α mRNA production compared with WT mice. Moreover, the increased severity of disease in IL-6−/− mice correlated with increased eye parasite burden as determined by RT-PCR for the Toxoplasma gondii bradyzoite-specific LDH2 gene. These results demonstrate alterations to components of immune privilege as a result of ocular toxoplasmosis and a role for IL-6 in controlling parasite numbers and inflammation in the eye.
Ocular toxoplasmosis (OT) is associated with inflammation of the retina and uveal tract. It is a recurring and progressive disease that can result in destruction of the retina and loss of sight (6). The relative contribution of the host inflammatory response versus parasite proliferation in causing destruction of the retina has not been defined (29). The eye is an immune-privileged site and inflammatory products such as tumor necrosis factor α (TNF-α), gamma interferon (IFN-γ), and nitric oxide production have been linked to experimental autoimmune uveitis (25, 39). Under normal circumstances, the eye constitutively expresses transforming growth factor β (TGF-β) and has low levels of major histocompatibility complex (MHC) class I expression in order to down-regulate inflammatory mediators and the immune response (33). In addition, high constitutive expression of Fas ligand on ocular cells would serve to induce apoptosis and down-regulate the function of T cells and NK cells (33, 34). Together these factors would generally serve to prevent damage or destruction of the eye as a result of inflammation. However, these anti-inflammatory processes may also contribute to an immunological environment that is conducive to the survival and multiplication of Toxoplasma gondii. For example, not only can TGF-β down-regulate the ability of NK cells to respond to T. gondii products (15), but also TGF-β can antagonize the activation of macrophages by reducing their ability to produce nitric oxide, which is an important anti-T. gondii effector mechanism in murine and human cells (13). In addition, TGF-β can prevent the induction of the tryptophan-degrading enzyme indolamine, which has been shown to prevent parasite multiplication in human cells (21). Furthermore, the relatively low expression of MHC class I molecules in the eye (34) would serve to reduce the potential activities of anti-T. gondii cytolytic CD8+ T cells, which are known to play a significant role in killing T. gondii-infected cells (7, 10, 24). Immunological control of T. gondii in the eye is therefore complex, because many of the mechanisms required to control parasite numbers may also adversely influence immune privilege and cause tissue damage.
The present study was undertaken to characterize further the immune response during OT. To achieve this we employed a combination of competitive reverse transcription-PCR (cRT-PCR) and immunocytochemistry (ICC). Although it cannot be assumed that the mRNA levels measured through cRT-PCR correlate absolutely with protein production, many studies have found cRT-PCR to be a useful method for evaluating ongoing immune responses. Infection was found to significantly alter the expression of immune system components associated with immune privilege in the eye; i.e., there was increased expression of β2-microglobulin and TGF-β. As previously described, infection was found to induce or increase levels of mRNA transcripts for a number of inflammatory mediators, including TNF-α and inducible nitric oxide synthase (iNOS), in ocular tissue. Both of these mediators are known to have a protective role for the host during OT (9, 14, 30). Previous studies have found that infection of human retinal pigment epithelial (HRPE) cells with T. gondii induces interleukin-6 (IL-6) (22). Accordingly, we also found transcripts for IL-6 in the eyes of mice infected with T. gondii. The role of IL-6 was therefore investigated by comparing disease progression and the development of the local immune response in wild-type (WT) and IL-6-deficient (IL-6−/−) mice. Following infection with T. gondii, IL-6−/− mice developed more severe inflammation in their retinas, vitreous humor, and uveas than did WT mice. The increased severity of disease in IL-6−/− mice compared with WT mice was associated with increased numbers of parasites.
MATERIALS AND METHODS
Mice and T. gondii infections.
Female mice of the 129/SVJ strain (WT) and IL-6−/− mice of the same genetic background, as previously described (17), were bred and maintained at the University of Strathclyde. Mice were infected with 10 T. gondii tissue cysts (Beverley strain) by the intraperitoneal route at 4 to 6 weeks of age, as previously described (28). Four weeks after infection, eyes were removed from both infected and age-matched uninfected control animals for histopathological examination, ICC, or RNA extraction. This time point was chosen because preliminary studies found that OT was not clearly evident at 3 weeks postinfection, and it was also chosen to precede the mortality evident after 4 weeks postinfection.
Tissue processing and histopathology.
Eyes were fixed in 0.1 M phosphate buffer (pH 7.4) containing 4% formaldehyde. Sections were cut from paraffin-embedded tissues and stained with hematoxylin and eosin stain. All sections were examined and assessed without knowledge of the group from which they originated. The number of inflammatory cells in the vitreous humor, retina, and choroid were graded from 1 to 3 as follows: 1, mild (less than 10 inflammatory cells per field at ×400 magnification); 2, moderate (between 11 and 20 inflammatory cells per field at ×400 magnification); and 3, severe (between 21 and 30 inflammatory cells per field at ×400 magnification). The score at each site was compared using the Mann-Whitney U test. In addition, the cysts in three consecutive sections were counted.
Immunocytochemistry.
Sections of the eyes processed for histology were dewaxed, and the endogenous peroxidase was blocked prior to pressure cooking for 90 s. Serial sections were then blocked with 1% bovine serum albumin in Tris buffer and incubated with either goat anti-β2-microglobulin (Santa Cruz Biotechnology, Inc.), mouse anti-BAG1 (bradyzoite specific antibody [4a]) or fluorescein isothiocyanate-conjugated mouse anti-mouse H-2Db clone no. KH95 (Pharmingen, Oxford, United Kingdom) in Tris buffer. After washing, sections were stained with the appropriate second antibody conjugated to peroxidase, and the reaction product was developed using diaminobenzidine and hydrogen peroxide as a chromogen. Alternatively, sections stained for H-2Db were examined by fluorescence microscopy. Slides were counterstained with hematoxylin prior to examination. To identify any possible quantitative difference, sections of the eyes from infected and uninfected controls of both WT and IL-6−/− mice were stained in parallel using identical reagents and incubation times.
RNA extraction.
Four weeks after infection, eyes from infected or uninfected control SVJ or IL-6−/− mice were carefully removed and homogenized in 1 ml of TRIzol reagent (Gibco BRL Life Technologies, Paisley, United Kingdom). Total RNA was isolated following a protocol based on the single-step acid guanidinium thiocyanate-phenol-chloroform RNA isolation method (8).
Reverse transcription.
cDNA was produced from total RNA using Moloney murine leukemia virus RT (Gibco BRL Life Technologies) according to the manufacturer's specifications. In a 90-μl reaction volume, 7 μg of RNA was combined with 18 μl of 5× first-strand buffer (250 mM Tris-HCl [pH 8.3], 375 mM KCl, and 15 mM MgCl2)–18 μl of deoxynucleoside triphosphate (dNTP) mix (10 mM)–9 μl of 0.1 M dithiothreitol–80 U of RNasin ribonuclease inhibitor (Promega)–500 ng of random hexamer primers (Promega)–1,200 U of Moloney murine leukemia virus RT. Following a 10-min preincubation at 27°C, the mixture was incubated at 42°C for 60 min, and then the reaction was terminated by heating at 95°C for 5 min. All cDNA was stored at −20°C until used in PCR.
cRT-PCR for cytokine transcripts.
The level of cytokine transcripts was assessed by cRT-PCR as described previously (5, 11, 30) using multispecific competitor plasmids pMUS (32), pNIL (18), and pQRS (27). cRT-PCR is a flexible system that easily assesses differences in multiple immune mediators even in small amounts of nonlymphoid tissue such as the eye. Although it cannot be assumed that mRNA levels measured through cRT-PCR correlate absolutely with protein production, many studies have found this to be a useful method to evaluate ongoing immune responses (5, 11, 18, 27, 30, 32). To ensure that equal quantities of cDNA were used in PCR for each sample, levels of transcript were first normalized against hypoxanthine-guanosine phosphoribosyl transferase (HPRT). To achieve this, PCR was first performed for HPRT in the absence of competitor (pQRS), and samples were adjusted until similar band intensities were observed. To confirm normalization, PCR for HPRT was then performed in the presence of a constant amount of competitor. Where necessary, samples were adjusted until the resulting amplification resulted in equal intensities of specific and competitor products for all of the samples (30). All PCR mixtures were made to 25 μl and contained a final concentration of 1× PCR buffer (50 mM KCl, 10 mM Tris-HCl [pH 9.0], and 0.1% Triton X-100)–1.5 mM MgCl2–200 μM for each dNTP–0.5 μM for each primer–0.25 U of Taq DNA polymerase (Promega). Cycling conditions included an initial denaturation at 94°C for 3 min followed by 35 cycles of 94°C for 30 s, 58°C for 30 s, and 72°C for 1 min. Reactions were finished with a final extension period for 10 min at 72°C. Primers utilized in these experiments are as follows: HPRT forward and reverse primers (5′-GGATTTGAAATTCCAGAG-3′ and 5′-GAGGGTAGGCTGGCCTATGGCT-3′, respectively) for use with pQRS; β2-microglobulin forward and reverse primers (5′-TGACCGGCTTGTATGCTATC-3′ and 5′-CAGTGTGAGCCAGGATATAG-3′) for pMUS; TGF-β forward and reverse primers (5′-ACCGCAACAACGCCATCTAT-3′ and 5′-GTAACGCCAGGAATTGTTGC-3′) for pMUS; IL-1α forward and reverse primers (5′-CAGTTCTGCCATTGACCATC-3′ and 5′-TCTCACTGAAACTCAGCCGT-3′) for pMUS; TNF-α forward and reverse primers (5′-TCTCATCAGTTCTATGGCCC-3′ and 5′-GGGAGTAGACAAGGTACAAC-3′) for pMUS; IL-6 forward and reverse primers (5′-GTTCTCTGGGAAATCGTGGA-3′ and 5′-TGTACTCCAGGTAGCTATGG-3′) for pMUS; and iNOS forward and reverse primers (5′-AGCTCCTCCCAG GACCACAC-3′ and 5′-ACGCTGAGTACCTCATTGGC-3′) for use with pNIL. IFN-γ forward and reverse primers (5′-GCTCTGAGACAATGAACGCT-3′ and 5′-AAAGAGATAATCTGGCTCTGC-3′) and IL-10 forward and reverse primers (5′-AGCCGGGAAGACAATAACTG-3′ and 5′-CATTTCCGATAAGGCTTGG-3′) were also used in the absence of competitor. The amounts of specific and competitor product of each reaction were assessed from scanned images of ethidium bromide-stained gels using NIH Image. The ratios of the total volume (density times area) of competitor to specific products were determined, and statistical analyses were performed using the Mann-Whitney U test.
Detection of parasites by RT-PCR.
To increase sensitivity for the detection of parasite-specific transcripts in the eyes of infected mice, a modified PCR followed by enhanced chemiluminescence (ECL) was performed. RT-PCR was performed as outlined above using biotin-labeled primers specific to the bradyzoite stage (LDH2) or the tachyzoite stage (SAG2). Primers utilized were LDH2for (5′-ATGACGGGTACCGTTAGC-3′), LDH2rev (5′-GCTCGCTTCATGAAAGCA-3′), SAG2for (5′-TGATGCATGCTCCAGTGGTTC-3′), and SAG2rev (5′-ACAAGCATGCGAGACCGG-3′). Following electrophoresis, the PCR products were transferred onto a Hybond-N+ nylon membrane (Amersham Pharmacia Biotech) by capillary transfer, as described by Sambrook et al. (31). Following heat fixation, the membrane was blocked overnight and incubated at room temperature in a 1:32,000 dilution of streptavidin-horseradish peroxidase (Amersham Pharmacia Biotech) for 1 h. After washing, the membrane was flooded with ECL detection reagent (Amersham Pharmacia Biotech) and exposed to film.
RESULTS
Cytokine expression in the eyes of infected WT mice.
Preliminary investigation of mRNA expression for selected cytokines in the eyes of WT mice infected with T. gondii was carried out. Samples were normalized against HPRT in the presence of pQRS as described in Materials and Methods. Similar intensities of the lower bands (specific product) and the corresponding upper bands (competitor product) indicated that all samples were normalized relative to the competitor and each other. For all subsequent PCRs, sample volumes remained unchanged.
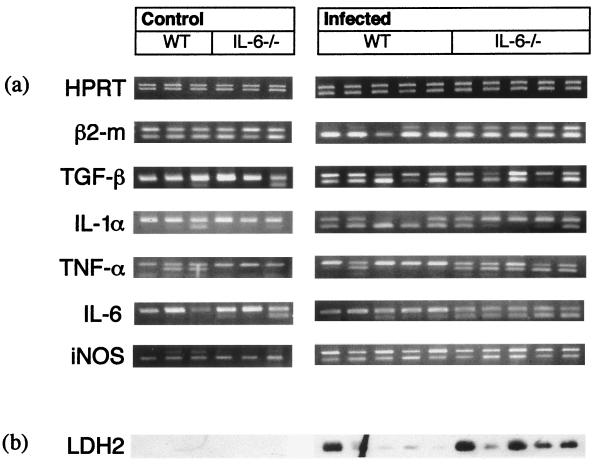
To investigate the effect of infection on immune privilege in the eye, PCR was next carried out for β2-microglobulin and TGF-β. Transcripts for both of these molecules were present in the eyes of normal control mice. Following infection, an increase in the level of transcript for β2-microglobulin in the eyes of infected mice was demonstrated by an increase in the intensity of specific product relative to the pMUS competitor construct. TGF-β transcript was also increased in the eyes of T. gondii-infected mice. IL-6 transcript was absent from the eyes of normal control mice, but it was present in the eyes of all infected mice. These results are not shown, because these results are also evident in subsequent experiments that compare WT and IL-6−/− mice (Fig. 1a).
FIG. 1.
The effect of T. gondii infection on intraocular expression of β2-microglobulin, TGF-β, IL-1α, TNF-α, IL-6, and iNOS, as measured by cRT-PCR. (a) Samples were first normalized for HPRT expression (note similar intensities of upper band [competitor product] with lower band [specific product]). The quantity of sample used to achieve this normalization was used in subsequent PCR. β2-Microglobulin expression was increased in infected mice compared with uninfected control mice (note increased intensities of lower band [specific product] compared with upper band [competitor product] in infected mice, but equal intensity of both bands in control samples). TGF-β was present in control samples but was up-regulated during infection (note low levels or absence of lower band [specific product] in control samples due to competition with competitor [upper band]). However, in samples from infected mice, both competitor and specific products are present, indicating increased expression. IL-1α transcripts were absent or present at low levels in control samples (lower band). Expression of IL-1α was increased in WT mice following infection (note presence of lower band in all samples) but not to the same extent as in IL-6−/− mice (note increased intensity of upper band [competitor product] in IL-6−/− samples compared with lower band [specific product]). TNF-α expression was increased in IL-6−/− samples following infection but not in WT mice (note presence of lower band [specific product] in all samples compared with low intensity or absence in WT samples). IL-6 expression (albeit nonfunctional) was increased in IL-6−/− samples following infection but not in WT mice (note presence of lower band [specific product] in all samples compared with low intensity or absence in WT samples). Expression of iNOS was low or absent in control mice but increased during infection (note increased intensity of upper bands [specific product] relative to lower bands [competitor product] in infected mice compared with control mice). IFN-γ and IL-10 transcripts were present in the eyes of both control and infected mice of each strain but at low levels which precluded comparison using a competitive construct (results not shown). These results are representative of two replicate experiments. (b) Transcripts for T. gondii LDH2 were absent from control mice but were present at low levels in four of five WT mice and at high levels in four of five IL-6−/− mice (note artifact in lane 2 for WT mice). These results are representative of two replicate experiments.
Comparison of ocular pathology in infected WT and IL-6−/− mice.
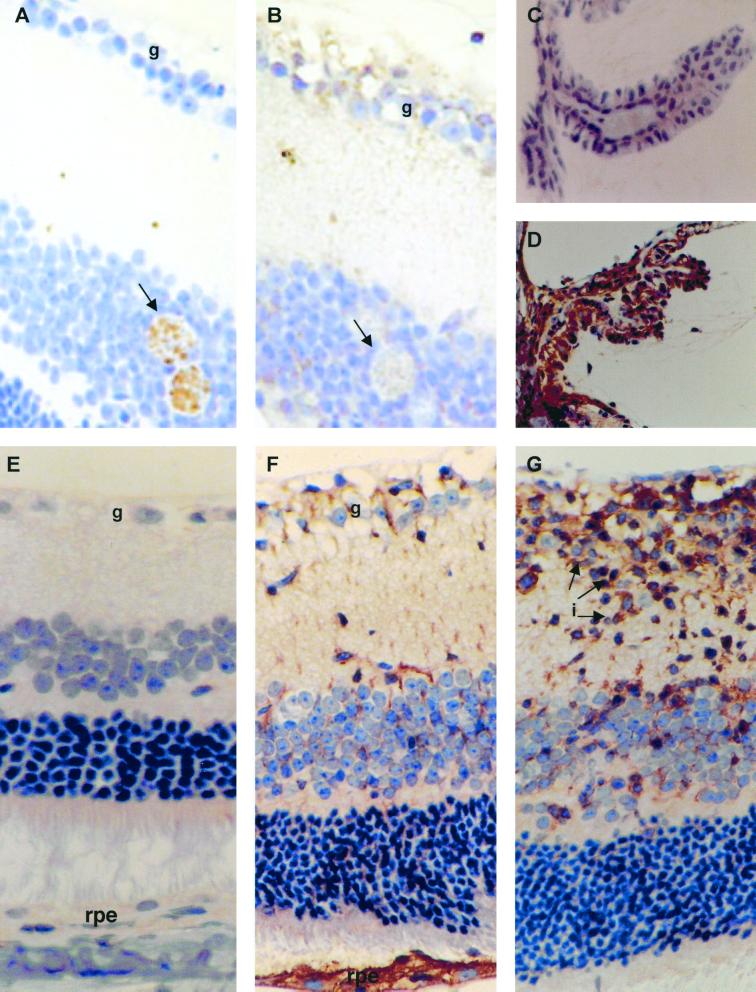
The relevance of our preliminary finding that IL-6 transcripts are present in the eyes of mice infected with T. gondii was tested by comparing the severity of OT in WT and IL-6−/− mice. The eyes of both WT and IL-6−/− mice infected with T. gondii had inflammation in their retinas, vitreous humor, and choroid (Table 1). Inflammatory cells were also present within the ciliary body and anterior chamber. Retinal inflammation was most marked in the inner retinal layers. When the inflammation in each site was compared using the Mann-Whitney U test, the score for inflammation in the retina and vitreous humor in IL-6−/− mice was significantly greater than in WT mice (retina, P = 0.027; vitreous humor, P = 0.026). Similar results were obtained in four experiments using male or female mice. Female mice generally had more severe inflammation than male mice (results not shown). In some of the eyes from IL-6−/− mice, there were clusters of several cysts. Although there were no significant differences in the overall cyst burdens, IL-6−/− mice generally had increased numbers of cysts in their eyes compared with WT mice, and cysts were often found in clusters rather than as individual cysts as in the WT mice. T. gondii tissue cysts stained positive for BAG1 but, as expected, were negative for β2-microglobulin (Fig. 2A and B). Eyes from uninfected control mice were normal.
TABLE 1.
Comparison of histopathology in the eyes of female WT and IL-6−/− mice following infection with T. gondiia
| Mouse strain | Level of inflammationb by eye location
|
No. of cysts | ||
|---|---|---|---|---|
| Retina | Choroid | Vitreous humor | ||
| WT | 2 | 0 | 1 | 0 |
| 2 | 1 | 1 | 0 | |
| 1 | 1 | 0 | 1 | |
| 2 | 1 | 1 | 1 | |
| 0 | 1 | 0 | 0 | |
| IL-6−/− | 3 | 0 | 2 | 0 |
| 3 | 2 | 2 | 5 | |
| 3 | 0 | 2 | 2 | |
| 2 | 1 | 1 | 0 | |
Mice were infected intraperitoneally with 15 cysts of the Beverley strain, and their eyes were examined histologically 4 weeks after infection. Eyes from IL-6−/− mice (n = 4) had significantly increased inflammation in their retinas (P = 0.027) and vitreous humor (P = 0.026) compared with WT mice (n = 5). Similar results were obtained in four experiments using male or female mice, although female mice generally had more severe inflammation than male mice.
0, none; 1, mild; 2, moderate; 3, severe.
FIG. 2.
Immunohistochemical staining for BAG-1 and β2-microglobulin. (A) Serial section of retina from an infected IL-6−/− mouse showing positive staining of Toxoplasma cysts for BAG-1 (arrow) (magnification, ×360). (B) Serial section of retina from an infected IL-6−/− mouse showing positive staining of ganglion cells (g) for β2-microglobulin. Toxoplasma cysts are indicated with an arrow. (C) Ciliary body from an uninfected WT mouse which shows no immunostaining for β2-microglobulin (magnification, ×250). (D) Ciliary body from an infected WT mouse showing positive staining of the ciliary body epithelium for β2-microglobulin (magnification, ×225). (E) Retina from uninfected WT mouse which shows no immunostaining for β2-microglobulin (magnification, ×90) (note absence of staining in retinal pigment epithelium [rpe]). (F) Retina from an infected mouse showing positive staining of the ganglion cells and retinal pigment epithelium for β2-microglobulin (magnification, ×90). (G) Retina from an infected IL-6−/− mouse showing positive staining of inflammatory cells (i) in the inner retinal layers for β2-microglobulin.
Comparison of cytokine expression and parasite numbers in the eyes of infected WT and IL-6−/− mice.
The ocular immune response was compared in WT and IL-6−/− mice infected with T. gondii to determine whether the increased severity in IL-6−/− mice was due to an increased inflammatory response or to greater parasite burdens. Samples from eyes of three uninfected and five infected mice each for the WT and IL-6−/− strains were again normalized by performing competitive RT-PCR for HPRT (Fig. 1a).
Significant increases in the transcription of genes encoding β2-microglobulin (P = 0.0245), TGF-β (P = 0.0245), iNOS (P = 0.0245), and IL-6 (P = 0.0245) were observed in infected WT and IL-6−/− mice (Fig. 1a) (IL-6 transcripts in IL-6−/− mice are nonfunctional). IL-1α transcript was also increased during T. gondii infection (P = 0.0245). However, this increase was greater in WT than in IL-6−/− mice (P = 0.0163). Conversely, although transcripts for TNF-α were expressed in uninfected control eyes, TNF-α was induced to a greater extent in IL-6−/− mice than in WT mice (P = 0.0163) (Fig. 1a). IFN-γ and IL-10 transcripts were present in the eyes of both control and infected mice of each strain but at low levels which precluded comparison using a competitive construct (results not shown).
Finally, we investigated the amount of transcript of bradyzoite-specific LDH2 gene and tachyzoite-specific SAG2 gene as a semiquantitative analysis of the number of parasites in the eyes of infected mice. Sensitivity of the PCR was increased at least 10-fold by using biotin-labeled primers and subsequent transfer to a nylon membrane and ECL. Despite this, SAG2 transcript could only be detected in a sample from one mouse, indicating that very few tachyzoites were present in the eyes of infected mice (data not shown). LDH2 transcript was observed in the eyes of both WT and IL-6−/− mice, indicating the presence of the bradyzoite cyst stages. Levels of LDH2 transcripts were generally higher in the eyes of infected IL-6−/− mice compared to WT mice (Fig. 1b) in two experiments (P = 0.374).
Comparison of staining for β2-microglobulin and MHC class I heavy chain in the eyes of uninfected and infected WT and IL-6−/− mice.
In the eyes from infected WT and IL-6−/− mice, there was membranous staining for β2-microglobulin in the ciliary body epithelium (Fig. 2D), ganglion cell layer of the retina (Fig. 2F), and inflammatory cells within the retina (Fig. 2G). Cytoplasmic staining was also identified in the ciliary body epithelium and in the retinal pigment epithelium (Fig. 2D and F). Similar staining was observed for MHC class I (results not shown). There was no difference in the pattern or intensity of staining between WT and IL-6−/− mice (data not shown). Eyes from uninfected control mice were negative for β2-microglobulin (Fig. 2E) and MHC class I (results not shown).
DISCUSSION
The pathogenesis of murine OT was investigated. Of particular interest was the extent to which factors associated with the immune privilege nature of the eye were compromised. A hallmark of immune privilege in the eye is constitutive expression of TGF-β (33). We also found constitutive expression of TGF-β in the eye, but in addition we found that OT significantly increases levels of TGF-β transcripts. While this increase in levels of TGF-β may serve to maintain immune privilege and to reduce damage due to inflammation, it may also have beneficial effects on the multiplication and survival of T. gondii. For example, TGF-β has been shown not only to reduce the ability of NK cells, a major source of IFN-γ, to respond to T. gondii but also to markedly down-regulate macrophage functions, including their ability to produce nitric oxide and indolamine (13, 15, 21). IFN-γ is the major mediator of resistance against T. gondii, and treatment of a number of nonphagocytic human cells has been shown to prevent the multiplication of T. gondii by inducing indolamine, which degrades tryptophan and starves the parasite of this amino acid (26, 36). Following stimulation with IFN-γ, nitric oxide has been shown to mediate killing of T. gondii in a number of murine and human cell types, and, significantly, nitric oxide has been shown to play a protective role during murine OT (19).
Low levels of MHC class I expression are another hallmark of immune privilege in the eye (34). This study found that MHC class I is up-regulated in the eyes of mice with OT, as demonstrated by increased levels of β2-microglobulin mRNA transcripts and up-regulation of cell surface MHC class I. Expression of MHC class I has been shown to be increased by IFN-γ, and accordingly we did find transcripts for IFN-γ in the eyes of infected but not uninfected mice (3). Up-regulation of MHC class I in the cells of the eye during infection would generally facilitate CD8+ cytotoxic T lymphocyte (CTL) killing of T. gondii-infected cells and may serve to limit parasite multiplication. Notably, CD8+ CTLs have been demonstrated in vitro to lyse T. gondii-infected cells (12, 35). In addition, adoptive transfer of CD8+ T cells from chronically infected mice to naïve mice has also been shown to mediate protection against infection with T. gondii (24). The accentuation of one aspect of immune privilege, namely TGF-β production, coupled with the increased expression of MHC class I, which represents an ablation of immune privilege during OT, requires explanation. We suggest that the up-regulation of MHC class I may confer ability in the immune system to efficiently and specifically recognize and lyse infected cells, while the up-regulation of TGF-β may protect uninfected cells from nonspecific inflammatory responses.
During the course of these studies a number of inflammatory mediators were up-regulated in the eyes of mice during infection, including TNF-α, iNOS, IL-1, and IL-6. While TNF-α and iNOS have previously been shown to be increased and to play a beneficial role during OT, the roles of IL-1 and IL-6 have not been investigated (9, 14, 30). IL-6 has, however, been previously detected in the eyes of cats with OT and in the vitreous humor of infected human eyes (20, 22). Furthermore, a recent study found that in vitro infection of HRPE cells with T. gondii induced the transcription of a number of inflammatory mediators, including IL-6 (23). We therefore investigated the role of IL-6 during OT by comparing disease progression and the local immune response in WT and IL-6−/− mice.
Following infection with T. gondii, IL-6−/− mice developed a more severe inflammation in their retina vitreous than WT mice. Previous studies have provided conflicting data concerning the role of IL-6 during toxoplasmosis. Thus, while in vitro studies have found that IL-6 inhibits IFN-γ-mediated T. gondii killing (4), further studies suggest that IL-6 may reduce parasite multiplication by promoting encystment (40). Murine models of disease again provide conflicting data regarding the role of IL-6 (16). In support of a disease-promoting role, neutralization of IL-6 has been shown to reduce cyst burdens and the severity of the inflammatory process in the brains of infected animals (37). In contrast, a host-protective role is supported by the increased mortality and severity of neurological disease observed in IL-6−/− mice compared with WT mice after infection with T. gondii (16). Nevertheless, IL-6 has been shown to have a role in down-regulating the inflammatory response by inhibiting the production of IL-1 and TNF-α (1, 2, 38). This would suggest that IL-6 could promote parasite growth while reducing pathology. Further studies were therefore performed to determine whether the increased severity of ocular inflammation observed in IL-6−/− mice was due to increased parasite multiplication and associated destruction of host cells or was a direct consequence of a failure to control inflammation in the absence of IL-6. In agreement with our preliminary studies, cRT-PCR revealed that β2-microglobulin and TGF-β were increased in infected WT and IL-6−/− mice compared with uninfected controls. The inflammatory mediators IL-1α, TNF-α, and iNOS were also up-regulated in the eyes of both WT and IL-6−/− mice following infection, as were those of IL-6. Furthermore, IL-6−/− mice had increased levels of IL-6 transcripts, albeit nonfunctional, compared with WT mice. This is consistent with previous studies of these mice and suggests a role for IL-6 in regulation of its transcription (17).
Whereas there was no significant difference in the levels of iNOS transcript between mouse strains, IL-6−/− mice had reduced ocular IL-1α. This finding indicates that the increased susceptibility of the IL-6−/− mice over WT mice is not due to reduced nitric oxide production. Consistent with previous studies, which found that mice deficient in IL-6 produced more TNF-α in response to lipopolysaccharide challenge than did WT mice (17) following infection with T. gondii, the eyes of IL-6−/− mice had increased levels of transcript for TNF-α compared with WT mice. While previous studies demonstrated a protective role for TNF-α during OT (9), the IL-6−/− mice had more severe ocular inflammation despite producing more TNF-α than WT mice. Furthermore, in a series of experiments, IL-6−/− mice generally had increased parasite burdens in their eyes compared with WT mice, as determined by RT-PCR for the T. gondii bradyzoite-specific LDH2 gene. The inability of histological examination to detect this difference is undoubtedly due to the relative rarity of tissue cysts in the eye and the fact that this type of analysis examines only a few cross sections of the eye. In comparison, the RT-PCR method assesses parasite number in the entire eye and in this respect provides a clear advantage over histological examination alone. It is interesting that transcripts for SAG2 were generally not detectable in the eyes of either WT or IL-6−/− mice by RT-PCR, indicating that the form of the parasite present was predominately the bradyzoite and not the tachyzoite. It is unlikely that this is a limitation of the method used, because we can detect as few as 10 tachyzoites in a sample.
Whether the protective role of IL-6 during OT is due to ocular or systemic immunity remains to be determined. However, a role for IL-6 in the ocular site is suggested, as transcripts for IL-6 are found only in the eyes of infected mice. Furthermore, in IL-6−/− mice, IL-1α production is increased while TNF-α production is decreased compared with that in WT animals. Significantly, therefore, these studies do demonstrate that intraocularly produced IL-6 does not have a proinflammatory or disease-potentiating effect during OT.
Together these results demonstrate that OT selectively induces changes to immune privilege in the eye that may favor MHC class I-mediated killing of infected cells while limiting inflammation by increasing TGF-β production. In addition, IL-6 plays a beneficial role in limiting parasite burden and inflammation in the eyes of mice infected with T. gondii.
REFERENCES
- 1.Aderka D, Le J, Vilvek J. IL-6 inhibits lipopolysaccharide-induced tumor necrosis factor production in cultured human monocytes, U937 cells, and in mice. J Immunol. 1989;143:3517–3523. [PubMed] [Google Scholar]
- 2.Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL-6 and related molecules (IL-1 and TNF) FASEB J. 1990;4:2860–2867. [PubMed] [Google Scholar]
- 3.Barez S, Boumpas D T, Percopo C M, Anastassiou E D, Hooks J J, Detrick B. Modulation of major histocompatibility complex class 1 genes in human retinoblastoma cells by interferons. Investig Ophthalmol Vis Sci. 1993;34:2613–2621. [PubMed] [Google Scholar]
- 4.Beaman M H, Hunter C A, Remington J S. Enhancement of intracellular replication of Toxoplasma gondii by IL-6: interactions with IFN-gamma and TNF-alpha. J Immunol. 1994;153:4583–4587. [PubMed] [Google Scholar]
- 4a.Bohne W, Gross V, Ferguson D J, Heesemann J. Cloning and characterization of a bradyzoite-specifically expressed gene (hsp30/bag1) of Toxoplasma gondii, related to genes encoding small heat-shock proteins of plants. Mol Microbiol. 1995;16:1221–1230. doi: 10.1111/j.1365-2958.1995.tb02344.x. [DOI] [PubMed] [Google Scholar]
- 5.Bouaboula M, Legoux P, Pessegue B, Delpech B, Dumont X, Piechaczyk M, Casellas P, Shire D. Standardization of mRNA titration using a polymerase chain reaction method involving co-amplification with a multispecific internal control. J Biol Chem. 1992;267:21830–21838. [PubMed] [Google Scholar]
- 6.Boyer K M. Diagnosis and treatment of congenital toxoplasmosis. Adv Pediatr Infect Dis. 1996;11:449–467. [PubMed] [Google Scholar]
- 7.Brown C R, McLeod R. Class I MHC genes and CD8+ T cells determine cyst number in Toxoplasma gondii infection. J Immunol. 1990;145:3438–3441. [PubMed] [Google Scholar]
- 8.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 9.Gazzinelli R T, Brezin A, Li Q, Nussenblatt R B, Chan C C. Toxoplasma gondii: acquired ocular toxoplasmosis in the murine model, protective role of TNF-alpha and IFN-gamma. Exp Parasitol. 1994;78:217–229. doi: 10.1006/expr.1994.1022. [DOI] [PubMed] [Google Scholar]
- 10.Gazzinelli R T, Hakim F T, Hieny S, Shearer G M, Sher A. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J Immunol. 1991;146:286–292. [PubMed] [Google Scholar]
- 11.Gilliland G, Perrin S, Blanchard K, Bunn H F. Analysis of cytokine mRNA and DNA: detection and quantitation by competitive polymerase chain reaction. Proc Natl Acad Sci USA. 1990;87:2725–2729. doi: 10.1073/pnas.87.7.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakim F T, Gazzinelli R T, Denkers E, Hieny S, Shearer G M, Sher A. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J Immunol. 1991;147:2310–2316. [PubMed] [Google Scholar]
- 13.Hausmann E H, Hao S Y, Pace J L, Parmely M J. Transforming growth factor β1 and gamma interferon provide opposing signals to lipopolysaccharide-activated mouse macrophages. Infect Immun. 1994;62:3625–3632. doi: 10.1128/iai.62.9.3625-3632.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi S, Chan C C, Gazzinelli R T, Pham N T, Cheung M K, Roberge F G. Protective role of nitric oxide in ocular toxoplasmosis. Br J Ophthalmol. 1996;80:644–648. doi: 10.1136/bjo.80.7.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter C A, Bermudez L, Beernink H, Waegell W, Remington J S. Transforming growth factor-beta inhibits interleukin-12-induced production of interferon-gamma by natural killer cells: a role for transforming growth factor-beta in the regulation of T cell-independent resistance to Toxoplasma gondii. Eur J Immunol. 1995;25:994–1000. doi: 10.1002/eji.1830250420. [DOI] [PubMed] [Google Scholar]
- 16.Jebbari H, Roberts C W, Ferguson D J P, Bluethmann H, Alexander J. A protective role for IL-6 during early infection with Toxoplasma gondii. Parasite Immunol. 1998;20:231–239. doi: 10.1046/j.1365-3024.1998.00152.x. [DOI] [PubMed] [Google Scholar]
- 17.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 18.Kopf M, Brombacher F, Kohler G, Kienzle G, Widmann K H, Lefrang K, Humborg C, Ledermann B, Solbach W. IL-4-deficient Balb/c mice resist infection with Leishmania major. J Exp Med. 1996;184:1127–1136. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Langermans J A M, Van D H M E B, Nibbering P H, Hiemstra P S, Fransen L, Van F R. IFN-gamma-induced l-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J Immunol. 1992;148:568–574. [PubMed] [Google Scholar]
- 20.Lappin M R, Dow S W, Reif J S, Chavkin M J. Elevated interleukin 6 activity in aqueous humor of cats with uveitis. Vet Immunol Immunopathol. 1997;58:17–26. doi: 10.1016/S0165-2427(96)05766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacKenzie C R, Gonzalez R G, Kniep E, Roch S, Daubener W. Cytokine mediated regulation of interferon-gamma-induced IDO activation. Adv Exp Med Biol. 1999;467:533–539. doi: 10.1007/978-1-4615-4709-9_66. [DOI] [PubMed] [Google Scholar]
- 22.Murray P I, Hoekzema R, Van H M A C, De H F D, Kijlstra A. Aqueous humor interleukin-6 levels in uveitis. Investig Ophthalmol Vis Sci. 1990;31:917–920. [PubMed] [Google Scholar]
- 23.Nagineni C N, Detrick B, Hooks J J. Toxoplasma gondii infection induces gene expression and secretion of interleukin 1 (IL-1), IL-6, granulocyte-macrophage colony-stimulating factor, and intercellular adhesion molecule 1 by human retinal pigment epithelial cells. Infect Immun. 2000;68:407–410. doi: 10.1128/iai.68.1.407-410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parker S J, Roberts C W, Alexander J. CD8+ T cells are the major lymphocyte subpopulation involved in the protective immune response to Toxoplasma gondii in mice. Clin Exp Immunol. 1991;84:207–212. doi: 10.1111/j.1365-2249.1991.tb08150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parks D J, Cheung M K, Chan C C, Roberge F G. The role of nitric oxide in uveitis. Arch Ophthalmol. 1994;112:544–546. doi: 10.1001/archopht.1994.01090160124032. [DOI] [PubMed] [Google Scholar]
- 26.Pfefferkorn E R. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA. 1984;81:908–912. doi: 10.1073/pnas.81.3.908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiner S L, Zheng S, Corry D B, Locksley R M. Constructing polycompetitor cDNAs for quantitative PCR. J Immunol Methods. 1993;165:37–46. doi: 10.1016/0022-1759(93)90104-f. [DOI] [PubMed] [Google Scholar]
- 28.Roberts C W, Cruickshank S M, Alexander J. Sex-determined resistance to Toxoplasma gondii is associated with temporal differences in cytokine production. Infect Immun. 1995;63:2549–2555. doi: 10.1128/iai.63.7.2549-2555.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roberts F, McLeod R. Pathogenesis of toxoplasmic retinochoroiditis. Parasitol Today. 1999;15:51–57. doi: 10.1016/s0169-4758(98)01377-5. [DOI] [PubMed] [Google Scholar]
- 30.Roberts F, Roberts C W, Ferguson D J, McLeod R. Inhibition of nitric oxide production exacerbates chronic ocular toxoplasmosis. Parasite Immunol. 2000;22:1–5. doi: 10.1046/j.1365-3024.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Shire D. Open exchange of reagents and information useful for the measurement of cytokine mRNA levels by PCR. Eur Cytokine Netw. 1993;4:161. [Google Scholar]
- 33.Streilein J W, Ksander B R, Taylor A W. Immune deviation in relation to ocular immune privilege. J Immunol. 1997;158:3557–3560. [PubMed] [Google Scholar]
- 34.Streilein J W, Stein-Streilein J. Does innate immune privilege exist? J Leukoc Biol. 2000;67:479–487. doi: 10.1002/jlb.67.4.479. [DOI] [PubMed] [Google Scholar]
- 35.Subauste C S, Koniaris A H, Remington J S. Murine CD8+ cytotoxic T lymphocytes lyse Toxoplasma gondii-infected cells. J Immunol. 1991;147:3955–3959. [PubMed] [Google Scholar]
- 36.Suzuki Y, Orellana M A, Schreiber R D, Remington J S. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki Y, Yang Q, Conley F K, Abrams J S, Remington J S. Antibody against interleukin-6 reduces inflammation and numbers of cysts in brains of mice with toxoplasmic encephalitis. Infect Immun. 1994;62:2773–2778. doi: 10.1128/iai.62.7.2773-2778.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulich T R, Yin S, Guo K, Yi E S, Remick D, Del Castillo J. Intratracheal injection of endotoxin and cytokines. II. Interleukin 6 and transforming growth factor beta inhibits acute inflammation. Am J Pathol. 1991;138:1097–1101. [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Z Y, Hakanson R. Role of nitric oxide (NO) in ocular inflammation. Br J Pharmacol. 1995;116:2447–2450. doi: 10.1111/j.1476-5381.1995.tb15094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weiss L M, Laplace D, Takvorian P M, Tanowitz H P, Cali A, Wittner M. A cell culture system for the study of the development of bradyzoites. J Eukaryot Microbiol. 1995;42:150–157. doi: 10.1111/j.1550-7408.1995.tb01556.x. [DOI] [PubMed] [Google Scholar]