Abstract
Objective: Small intestinal bacterial overgrowth (SIBO) syndrome is associated with depression and anxiety. This study aimed to examine for the first time the correlation between personality traits, situational anxiety, and stress in Polish patients with SIBO. Methodology: This study included 26 patients with SIBO aged 20–35 years and 24 non-SIBO patients aged 20–35 years. The following instruments were used: NEO-FFI Personality Inventory, KPS Sense of Stress Questionnaire, and the anxiety-state subscale from the State-Trait Anxiety Inventory (STAI). Results: Compared to the non-SIBO subgroup, SIBO patients expressed specific patterns of personality traits: higher neuroticism, lower extroversion, and a higher state of anxiety and stress. Unlike the non-SIBO subgroup, stress (total emotional tension, external, and intrapsychic) correlated negatively only with extroversion. Conclusions: Personality is the primary regulator of experience and behavior. The specificity captured in the research is a premise for an in-depth study considering various psychological variables to determine cause-effect relationships.
Keywords: SIBO, IBS, personality traits, stress, anxiety
1. Introduction
Increasing evidence of somatic and mental symptoms in the general population, and the strong need for adequate diagnosis and treatment, has drawn attention to the association of gut microbiota with both gastrointestinal and extra-gastrointestinal diseases. Dysbiosis and inflammation of the gut have been linked to several mental health issues, including anxiety and depression, in children [1] and adults [2,3]. A dysbiotic state of the gut microbiota is becoming recognized as an environmental factor that interacts with a host’s metabolism and has a role in pathological conditions, both systemic—obesity, diabetes, and atopy—and gut-related IBS and IBD, although the specific contribution of the gut microbiota to these diseases is unclear [4]. The heterogeneous etiology of metabolic and gastrointestinal diseases has been associated with different microbes, although little information exists about the causal direction of the association [5]. The composition of human microbiota is influenced by host genotype, environment, and diet. Signaling molecules and metabolic products of the microbiota influence several intestinal functions: visceral-sensing, motility, digestion, permeability secretion, energy harvest, mucosal immunity, and barrier effect [6]. One condition could be small intestinal bacterial overgrowth (SIBO) occurring when there is an abnormal increase in the overall bacterial population in the small intestine. However, when diagnosing SIBO, the main criterion is the number of bacteria, not their type. Overgrowth can affect both bacteria that are generally either physiologic or pathogenic. Most often, there is an overgrowth of Streptococcus, Escherichia coli, Lactobacillus and Bacteroides bacteria [7].
The functioning of the gastrointestinal tract can be impaired by the effect of stress. It is often a trigger for disease, causing relapse or a more severe course of the disease [8], and is strongly related to the (chronic) activation of neurological paths between the hypothalamus, pituitary, and adrenal glands, to produce a cascade of stress hormones and neurotransmitters [9].
Experimental studies indicate that psychological stress can negatively affect the transit time of the small intestine, promote SIBO syndrome, and significantly disrupt the balance of the intestinal barrier [10,11]. Stress, anxiety, and depression can come from many other physical disturbances, such as systemic inflammation or leaky gut and might be related to hormonal changes. The altered microbiome can be one of the most significant contributors to anxiety and/or depression [12].
Studies on stress and anxiety in patients with SIBO are limited and inconclusive. Some studies indicate a strong association with depression, anxiety somatisation, and catastrophising compared to controls with irritable bowel syndrome (IBS) [13,14]. However, others studies show that SIBO patients, compared to IBS subgroups, present lower anxiety, depression, and everyday stress [15].
The chronic activation of the hypothalamic-pituitary-adrenal (HPA) axis may play a crucial role in developing SIBO because the stress response is closely linked to the gut microbiome.
The relation between psychological stress and SIBO based on the neurobiological transfer might be affected by the growth and virulence of bacteria mechanism [16,17,18]. On the other hand, stress might also be the secondary reaction of patients suffering from serious SIBO symptoms. Arguably, a vicious circle of mechanisms is at work here. To predetermine whether there is a primary psychological basis for the occurrence of SIBO, the reference to personality should be made because the Big Five personality model [19] is a strong predictor of illness behavior [20]. The specificity of personality and affective state profile in patients with inflammatory bowel disease [21] was the background to look for specificity in SIBO patients.
Although SIBO patients experience many psychosomatic problems, stress might have also have a significant impact on their mental health.
The present article aimed to describe the peculiar personality pattern and its relationship with stress indicators and the situational state of anxiety in Polish patients with SIBO. The following hypothesis was proposed:
Patients with SIBO experience higher levels of neuroticism and lower levels of extroversion than those without SIBO.
Patients with SIBO experience a higher situational anxiety-state than those without SIBO.
Patients with SIBO experienced higher stress levels than those in the non-SIBO subgroup.
The personality and stress correlation in patients with SIBO are significantly different from those in the non-SIBO subgroup.
2. Methods
2.1. Participants
Fifty people participated in the study, of which 26 were diagnosed with SIBO and 24 without SIBO (non-SIBO control subgroup).
The SIBO subgroup (13 females, 13 males) aged 21 to 35 years (M = 27.58; SD = 3.56) varied in their education levels (high school: 38.5%, college: 61.5%) as well as place of residence (big city: 76.9%, medium: 11.5% small: 3.8%, rural areas: 7.7%), marital status (married: 50%, unmarried: 50%), and employment status (full-time: 76.9%, student: 19.2%, self-employed: 3.8%).
The non-SIBO subgroup (females: 12, male: 12), aged 21 to 34 years (M = 27.96; SD = 3.81), too, varied in their education levels (high school: 62.5%, college: 33.3%) as well as place of residence (big city: 75%, medium: 12.5% small: 4.2%, rural areas: 4.2%), marital status (married: 50%, unmarried: 41.7%, divorced: 8.3%), and employment status (full-time: 62.5%, student: 25%, self-employed: 12.5%).
2.2. Instruments
The following tools were used in the study:
Polish adaptation of NEO-FFI [22], which consists of 60 items, evaluated on a 5-point Likert scale from strongly disagree to strongly agree. Cronbach’s reliability of measurement using the Polish version of NEO-FFI scales ranges from 0.68 to 0.82.
The subscale X-1 of Polish adaptation of STAI [23], The State-Trait Anxiety Inventory [24], to measure anxiety-state.
The Sense of Stress Questionnaire [25] was used to measure the stress experience’s structure, consisting of 27 statements evaluated on a five-point Likert scale. The questionnaire consists of three subscales: emotional tension, resulting from anxiety and excessive nervousness, with Cronbach’s alpha = 0.708; external stress with Cronbach’s alpha = 0.584; and intrapsychic stress, linked to one’s inability to cope with one’s inner experiences with Cronbach’s alpha = 0.606.
2.3. Procedure
This study was conducted in Krakow dietary centres that treat gastrointestinal disorders, including SIBO, within the period since January till March 2022. A hydrogen-methane breath test with lactulose was performed to confirm or exclude the presence of SIBO. Those with a positive breath test result, showing characteristic symptoms of the disorder, such as abdominal pain, bloating, diarrhoea, or constipation, qualified for the SIBO subgroup, and those with a negative breath test result regardless the somatic symptoms were included in the non-SIBO subgroup. Participants did not declare any abdominal surgery, weight loss, nor antibiotic treatment during last two weeks. Participation in this study was voluntary, anonymous, and unpaid.
3. Results
Basic descriptive statistics of the studied quantitative variables are presented in Table 1.
Table 1.
Basic descriptive statistics of the quantitative variables.
| M | Me | SD | Sk. | Kurt. | Min. | Max. | D | p | |
|---|---|---|---|---|---|---|---|---|---|
| Openness | 24.26 | 24.00 | 5.87 | 0.03 | 0.06 | 9.00 | 37.00 | 0.99 | 0.943 |
| Neuroticism | 26.76 | 27.50 | 9.91 | −0.13 | −1.20 | 9.00 | 42.00 | 0.95 | 0.022 |
| Agreeableness | 31.58 | 32.00 | 5.89 | −0.13 | −0.16 | 17.00 | 44.00 | 0.99 | 0.961 |
| Extroversion | 22.06 | 22.50 | 8.96 | 0.08 | −0.71 | 6.00 | 44.00 | 0.97 | 0.348 |
| Conscientiousness | 31.00 | 31.00 | 7.08 | −0.31 | 0.25 | 11.00 | 43.00 | 0.97 | 0.344 |
| Anxiety state | 45.62 | 46.00 | 7.56 | −0.18 | −0.88 | 30.00 | 59.00 | 0.97 | 0.274 |
| Emotional tension | 21.82 | 23.00 | 6.15 | −0.25 | −1.23 | 11.00 | 32.00 | 0.94 | 0.010 |
| External stress | 16.92 | 17.00 | 4.88 | 0.27 | −0.29 | 8.00 | 29.00 | 0.98 | 0.468 |
| Intrapsychic stress | 19.90 | 20.00 | 6.58 | 0.27 | −0.80 | 8.00 | 34.00 | 0.97 | 0.154 |
| Total stress | 58.64 | 60.00 | 15.92 | −0.13 | −1.03 | 30.00 | 85.00 | 0.96 | 0.087 |
Note: M, mean; Me, median; SD, standard deviation; Sk, standard deviation—Skewness; Kurt—curvature; Min and Max—the lowest and highest values of the distribution; D—the result of the Shapiro-Wilk test; p—significance.
Due to the obtained indices of the Shapiro-Wilk test, the values of the skewness of the distribution of the studied variables in the range of +/− 2 (see Table 1), it was assumed that the distribution of the studied variable is not significantly asymmetric with respect to the mean [26]. Such a value was noted for all the studied variables. Therefore, in this chapter, statistical analyses will be performed using parametric tests.
3.1. Personality Traits, Situational State of Anxiety and Stress in People with SIBO
Personality characteristics, anxiety, and stress in patients with SIBO is presented in Table 2.
Table 2.
Personality, anxiety, and stress in people with SIBO.
| SIBO Group | Non-SIBO Group | 95% CI | |||||||
|---|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | t | p | LL | UL | d Cohena | |
| Openness | 25.23 | 6.66 | 23.21 | 4.79 | 1.22 | 0.227 | −1.30 | 5.35 | 0.35 |
| Neuroticism | 34.58 | 5.49 | 18.29 | 5.70 | 10.29 | <0.001 | 13.10 | 19.47 | 2.91 |
| Agreeableness | 31.35 | 5.10 | 31.83 | 6.75 | −0.29 | 0.773 | −3.87 | 2.90 | 0.08 |
| Extroversion | 16.85 | 7.99 | 27.71 | 6.14 | −5.36 | <0.001 | −14.94 | −6.78 | 1.52 |
| Conscientiousness | 30.69 | 5.36 | 31.33 | 8.67 | −0.31 | 0.757 | −4.81 | 3.53 | 0.09 |
| Anxiety state | 49.58 | 6.22 | 41.33 | 6.55 | 4.57 | <0.001 | 4.61 | 11.87 | 1.29 |
| Emotional tension | 25.54 | 4.36 | 17.79 | 5.23 | 5.71 | <0.001 | 5.02 | 10.47 | 1.62 |
| External stress | 18.27 | 4.50 | 15.46 | 4.94 | 2.10 | 0.041 | 0.12 | 5.50 | 0.60 |
| Intrapsychic stress | 23.85 | 5.48 | 15.63 | 4.80 | 5.62 | <0.001 | 5.28 | 11.16 | 1.59 |
| Total stress | 67.65 | 11.89 | 48.88 | 13.98 | 5.13 | <0.001 | 11.42 | 26.14 | 1.45 |
Note: M, mean; SD, standard deviation; t, results of Student’s t-test; p, significance; CI [LL, UL], confidence intervals, lower limit and upper limits of the interval; d Cohen size effect coefficient.
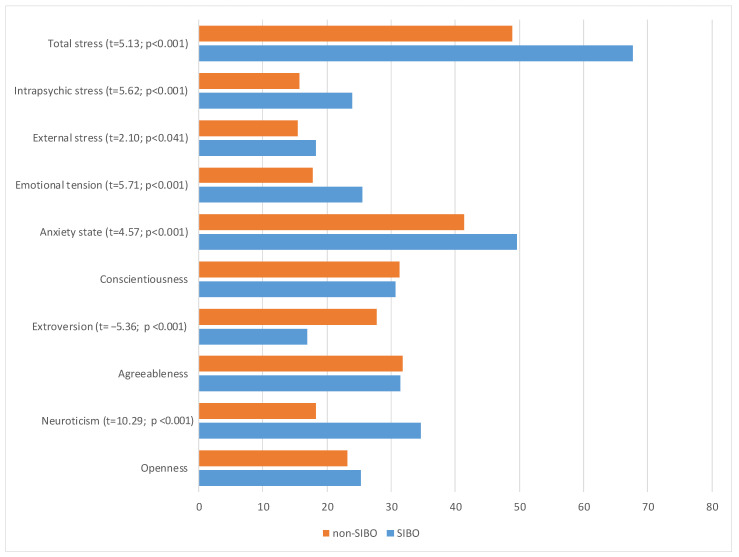
In the SIBO subgroup, in comparison to the non-SIBO subgroup, significant differences in personality traits (see Table 2 and Figure 1) were found: higher neuroticism (t = 10.29; p < 0.001), and lower extroversion (t = −5.36; p < 0.001). The strength of the recorded effects, as measured by Cohen's d coefficient, was very high. The higher anxiety-state was also discovered (t = 4.57; p < 0.001) as well as all stress indicators: emotional tension (t = 5.71; p < 0.001), external stress (t = 2.10; p < 0.050), intrapsychic stress (t = 5.62; p < 0.001), and total stress (t = 5.13; p < 0.001). The strength of the observed effect for the level of external stress was moderately high, while it was very high for the other three variables.
Figure 1.
Subgroup differences in personality, anxiety, and stress expressed in means indicators.
3.2. Personality Stress and Anxiety—State Relationships in SIBO Context
Anxiety state. The relationships between stress and personality traits in the SIBO context are presented in Table 3.
Table 3.
Personality traits and stress correlations in two subgroups.
| Variables | Anxiety State | Emotional Tension | External Stress | Intrapsychic Stress | Total Stress | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SIBO (n = 26) |
Non-SIBO (n = 24) | Z | SIBO (n = 26) |
Non-SIBO (n = 24) |
Z | SIBO (n = 26) |
Non-SIBO (n = 24) |
Z | SIBO (n = 26) |
Non-SIBO (n = 24) |
Z | SIBO (n = 26) |
Non-SIBO (n = 24) |
Z | |
| Openness | −0.307 | −0.088 | Z = −0.76 | −0.113 | 0.090 | Z = −0.67 | 0.309 | 0.100 | Z = 0.73 | 0.083 | 0.047 | Z = 0.12 | 0.114 | 0.085 | Z = 0.10 |
| Neuroticism | −0.015 | 0.285 | Z = −1.02 | 0.119 | 0.561 ** | Z = −1.71 | −0.076 | 0.636 *** | Z = −2.74 ** | 0.366 | 0.511 * | Z = −0.60 | 0.183 | 0.610 ** | Z = −1.74 |
| Agreeableness | −0.274 | −0.605 ** | Z = 1.39 | 0.024 | −0.547 ** | Z = 2.11 * | 0.212 | −0.640 *** | Z = 3.23 *** | 0.169 | −0.565 ** | Z = 2.69 ** | 0.167 | −0.625 *** | Z = 2.99 ** |
| Extroversion | −0.622 *** | −0.428 * | Z = −0.90 | −0.578 ** | −0.476 * | Z = −0.47 | −0.157 | −0.332 | Z = 0.62 | −0.291 | −0.374 | Z = 0.31 | −0.405 * | −0.424 * | Z = 0.08 |
| Conscientiousness | −0.276 | −0.369 * | Z = 0.34 | 0.067 | −0.496 * | Z = 2.02 * | 0.091 | −0.379* | Z = 1.62 | 0.064 | −0.306 | Z = 1.26 | 0.089 | −0.425 * | Z = 1.80 |
Note; * p < 0.05; ** p < 0.001; *** p < 0.0001.
Fisher’s Z tests were performed to verify if the correlations between anxiety state and personality traits met the level of significant differentiation in the subgroups. Pearson’s correlation coefficient was calculated to find the relationship between situational anxiety state and personality traits in the context of SIBO background (Table 3). In non-SIBO subgroup anxiety-state correlated negatively with agreeableness (r = −0.605; p < 0.010), extroversion (r = −0.428; p < 0.001) and conscientiousness (r = −0.369; p < 0.050). This means that increase of situational state anxiety occurred in specific pattern of personality traits (lower agreeableness, extroversion and conscientiousness), however, in SIBO subgroup the increase in situational state anxiety was related only to lower extroversion (r = 0.622; p < 0.001).
In the SIBO subgroup, only one personality trait was found to be related to stress. There was a negative correlation between extroversion and total stress (r = −0.405; p < 0.050) as well as emotional tension (r = −0.578. p < 0.001), and no other relationship between personality and external or intrapsychic stress were found. This means that in SIBO patients, if the level of introversion increases, the total stress, and emotional tension also increases.
In the non-SIBO subgroup, total stress and emotional tension were found to have multidimensional relationship with four personality traits: positively with neuroticism (r = 0.610; p < 0.001; r = 0.561; p < 0.001) and negatively with agreeableness (r = −0.625; p < 0.0001; r = −0.547; p < 0.0001), extroversion (r = −0.424; p < 0.050; r = −0.568; p < 0.001), and conscientiousness (r = −0.425; p < 0.05; r = −0.496; p < 0.05). External stress positively correlated with neuroticism (r = 0.636; p < 0.0001) and negatively correlated with agreeableness (r = −0.640; p < 0.0001) and conscientiousness (r = −0.379; p < 0.050), but intrapsychic stress was correlated with neuroticism (r = 0.511; p < 0.050) and agreeableness (r = −0.565; p < 0.01).
The results described above for the SIBO subgroup are interesting; however, only a few subgroup differentiations (SIBO vs. non-SIBO) were statistically significant based on Fisher’s Z test indicators. Significant differences were found in terms of dependency agreeableness and total stress (Z = 2.99; p < 0.001), emotional tension (Z = 2.11; p < 0.050), and intrapsychic stress (Z = 2.69; p < 0.001) as well as in terms of dependency between external stress and neuroticism (Z = −2.74; p < 0.001) and agreeableness (Z = 3.23; p < 0.0001).
4. Discussion
The intestinal microbial ecosystem balance, called eubiosis, is a fundamental concept. The eubiosis/dysbiosis condition of the gut microbiota strongly influences our healthy and disease status [27]. There is microbial imbalance (dysbiosis) in patients with chronic intestinal inflammation and colorectal cancer. A complex interplay between the host, bacteria, and bacterial genes is implicated in the development of these intestinal diseases [28].
Stress has a negative impact on the function of the enteric nervous system, which can consequently lead to the development of dysbiosis, inflammation, or IBS. The gut microbiome is particularly susceptible to stress factors, and the restoration of eubiosis is associated with long term treatment [29]. Gut inflammation is triggered by an imbalanced microbiome, and its effect can be found in various CNS disorders, including, but not limited to, anxiety, depressive disorders, schizophrenia, and autism [30,31]. Because of the excessive translocation of bacteria under stress, it can be inferred that this is one of the factors that may contribute to SIBO. This study aimed to examine the relationships between personality traits, the state of anxiety, and stress in Polish patients with SIBO.
Higher neuroticism was found in the SIBO subgroup, which is related to the overstimulation of the sympathetic nervous system responsible for proper digestion as well as greater vulnerability to stress [32]. Based on the Big Five model, similar conclusions can be drawn because there is a positive correlation between neuroticism and gastrointestinal diseases [33,34,35]. The lower extroversion in the SIBO subgroup may be due to different experiences of positive events, which translates into a different perception of one's dysfunction and experience of somatic complaints. In Polish studies, neuroticism and negative emotionality have been shown to be strong predictors of, among other things, peptic ulcer disease [36], but also other autoimmune diseases such as psoriasis [37].
The correlation between negative emotional states, higher anxiety, and the functioning of the gastrointestinal tract, as well as changes at the level of the gut microbiota in humans and animals, prompted us to consider the occurrence of similar states in patients with SIBO. The hypothesis of a higher state of anxiety and stress in the SIBO subgroup compared to non-SIBO was confirmed. Although the sample size was small, the results were consistent with those of a large group of patients [13]. Recent systematic review studies had shown that medical history and chronic illness are associated with increased anxiety and distress caused by the external situation [38].
Stress defined as a disruption in homeostasis due to external or internal stressors, including environmental or physical and psychological stimuli, may affect the gut microbiota, leading to changes in the host physiology and further increasing disease risk [39]. The stress level in the SIBO subgroup compared with the non-SIBO group was significantly higher, both as total scores and at the level of three indicators: emotional tension, external stress, and intrapsychic stress.
Intestinal complaints caused by the presence of SIBO had a negative impact on the comfort of the subjects' lives; therefore, negative emotional states, tension, or discomfort associated with the symptoms was observed. Other authors also found that stress has an impact on the composition of the microbiota and affects the functioning of the digestive system [40]. They also found a correlation between IBS and increased feelings of stress, anxiety, and the occurrence of sleep problems or worse functioning in daily life, as well as the quality of life [41].
SIBO’s effect of painful sensations, and individual personality characteristics are significant factors that can influence patients’ perception of painful stimuli and their reaction to them [20,42]. Some specific personality traits, such as a negative worldview, might predispose patients to develop psychological comorbidities such as depression, anxiety, and stress. Several studies conducted on adults with gastrointestinal disorders have reported dysfunctional personality characteristics [43,44,45]. Individuals with gastrointestinal disorders exhibit high levels of anxiety and tension, as well as neuroticism, and in stressful situations, they may tend to focus on themselves and their own symptoms, and this promotes increased feelings of negative emotional states [46].
5. Conclusions
In conclusion, it can be said that the findings of the study on Polish patients with SIBO is consistent with foreign studies on the correlation of personality traits with gastrointestinal disorders. The present study indicates that patients with bacterial overgrowth in the small intestine showed higher levels of neuroticism and lower levels of extroversion than those without this disorder. The SIBO subgroup had higher situational anxiety and stress, both overall and at the level of the three indicators: emotional tension, external stress, and intrapsychic stress. It was also shown that, in the SIBO subgroup, extroversion was lower than that in the non-SIBO subgroup and correlated with higher stress (particularly emotional tension). Knowledge of the gut-brain axis effects on SIBO pathophysiology and personality can help identify medical and psychological CBT psychotherapy treatments for stress reduction under these conditions [47,48,49].
5.1. Limitations of the Study
The study conducted on SIBO patients, implemented on a Polish research sample for the first time, based on purposive sampling, required sampling based on a positive hydrogen test result. Therefore, among the large group reporting to the facility for diagnostic purposes, the subgroup size was significantly limited.
Participants completed psychological questionnaires while waiting for the next hydrogen test result; therefore, they may have experienced situational anxiety in the waiting situation. This procedure may have affected the severity of anxiety, which may have translated into the results of the study.
5.2. Future Studies
In future studies, the possibility of interfering factors should be kept at an absolute minimum or subjected to strict control. Such factors as education levels, which may be associated with a higher income and overall socioeconomic status, should be control as a covariate.
Owing to the lack of research on personality traits and the correlation between personality traits and anxiety or stress in patients diagnosed with SIBO, further research in this area is necessary to deepen the topic in question and to draw concrete conclusions. Future studies should explore the activation of the immune response in the mucosa of SIBO-positive IBS patients and healthy controls. There is scope to examine inflammation and immune activation status by determining the imbalance of cytokines and immune cell changes from intestinal biopsies in patients with IBS and SIBO. Therefore, it will be valuable to develop a better understanding of the roles of SIBO and IBS in the pathogenesis of psychological disorders.
Acknowledgments
The authors would like to thank Angela Winstanley from ShipCon Limassol Ltd., Cyprus, for her proofreading the first version of this article.
Author Contributions
Conceptualization, K.B.; Methodology, J.K.; Formal analysis, J.K.; Resources, K.B.; Data curation, K.B.; Writing—original draft, K.B. and V.T.; Writing—review & editing, J.K.; Supervision, J.K. and V.T.; Project administration, J.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki under relevant local legislation. Permission (No 01/11/2021) to carry out the research was provided by the Ethical Committees of Pedagogical University of Krakow for studies involving humans.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical reason.
Conflicts of Interest
All authors declare that they have no conflict of interest.
Funding Statement
This research received no external funding however the publishing charge was carried out by the Pedagogical University of Krakow.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Avelar R.D., Ryan P.M., Toro Monjaraz E.M., Ramirez Mayans J.A., Quigley E.M. Small Intestinal Bacterial Overgrowth in Children: A State-Of-The-Art. Review. Front. Pediatr. 2019;7:363. doi: 10.3389/fped.2019.00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Foster J.A., McVey Neufeld K.A. Gutbrain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305–312. doi: 10.1016/j.tins.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Clapp M., Aurora N., Herrera L., Bhatia M., Wilen E., Wakefield S. Gut microbiota's effect on mental health: The gut-brain axis. Clin. Pract. 2017;15:987. doi: 10.4081/cp.2017.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takakura W., Pimentel M. Small Intestinal Bacterial Overgrowth and Irritable Bowel Syndrome–An Update. Front. Psychiatry. 2020;11:664. doi: 10.3389/fpsyt.2020.00664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bull M.J., Plummer N.T. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014;13:17–22. [PMC free article] [PubMed] [Google Scholar]
- 6.Montalto M., D’Onofrio F., Gallo A., Cazzato A., Gasbarrini G. Intestinal microbiota and its functions. Dig. Liver Dis. 2009;2((Suppl. 3)):30–34. doi: 10.1016/S1594-5804(09)60016-4. [DOI] [Google Scholar]
- 7.Bures J., Cyrany J., Kohoutova D., Förstl M., Rejchrt S., Kvetina J., Vorisek V., Kopacova M. Small intestinal bacterial overgrowth syndrome. World J. Gastroenterol. 2010;16:2978. doi: 10.3748/wjg.v16.i24.2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bharwani A., Mian M.F., Foster J.A., Surette M.G., Bienensttock J., Forsythe P. Structural and functional consequences of chronic psychosocial stress on the microbiome and host. Psychoneuroendocrinology. 2016;63:217–227. doi: 10.1016/j.psyneuen.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Sudo N. Microbial Endocrinology: The Microbiota-Gut-Brain Axis in Health and Disease. Springer; New York, NY, USA: 2014. Microbiome, HPA axis and production of endocrine hormones in the gut; pp. 177–194. [DOI] [PubMed] [Google Scholar]
- 10.Wang S.X., Wu W.C. Effects of psychological stress on small intestinal motility and bacteria and mucosa in mice. World J. Gastroenerology. 2005;11:2016–2021. doi: 10.3748/wjg.v11.i13.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maslennikov R., Pavlov C., Ivashkin V. Small intestinal bacterial overgrowth in cirrhosis: Systematic review and metaanalysis. Hepatol. Int. 2018;6:567–576. doi: 10.1007/s12072-018-9898-2. [DOI] [PubMed] [Google Scholar]
- 12.Madison A., Kiecolt-Glaser J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019;28:105–110. doi: 10.1016/j.cobeha.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Addolorato G., Mirijello A., D’Angelo C., Leggio L., Ferrulli A., Abenavoli L., Gasbarrini G. State and trait anxiety and depression in patients affected by gastrointestinal diseases: Psychometric evaluation of 1641 patients referred to an internal medicine outpatient setting. Int. J. Clin. Pract. 2008;62:1063–1069. doi: 10.1111/j.1742-1241.2008.01763.x. [DOI] [PubMed] [Google Scholar]
- 14.Grover M., Kanazawa M., Palsson O.S., Chitkara D.K., Gangarosa L.M., Drossman D.A., Whitehead W.E. Small Intestinal Bacterial Overgrowth in Irritable Bowel Syndrome: Association with Colon Motility, Bowel Symptoms and Psychological Distress. Neurogastroenterol. Motil. 2008;20:998–1008. doi: 10.1111/j.1365-2982.2008.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu H., Fox M., Zheng X., Deng Y., Long Y., Huang Z., Dai N. Small Intestinal Bacterial Overgrowth in Patients with Irritable Bowel Syndrome: Clinical Characteristics, Psychological Factors, and Peripheral Cytokines. Gastroenterol. Res. Pract. 2016;2016:3230859. doi: 10.1155/2016/3230859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarkodie E.K., Zhou S., Baidoo S.A., Chu W. Influences of stress hormones on microbial infections. Microb Pathog. 2019;131:270–276. doi: 10.1016/j.micpath.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Neuman H., Debelius J.W., Knight R., Koren O. Microbial endocrinology: The interplay between the microbiota and the endocrine system. FEMS Microbiol Rev. 2015;39:509–521. doi: 10.1093/femsre/fuu010. [DOI] [PubMed] [Google Scholar]
- 18.Freestone P.P., Sandrini S.M., Haigh R.D., Lyte M. Microbial endocrinology: How stress influences susceptibility to infection. Trends Microbiol. 2008;16:55–64. doi: 10.1016/j.tim.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 19.McCrae R.R., Costa P.T., Jr. A Five-Factor theory of personality. In: Pervin L.A., John O.P., editors. Handbook of Personality: Theory and Research. Guilford Press; New York, NY, USA: 1999. pp. 139–153. [Google Scholar]
- 20.Williams P.G. Personality and Illness Behavior. In: Vollrath M.E., editor. Handbook of Personality and Health. John Wiley & Sons Ltd; Hoboken, NJ, USA: 2006. pp. 157–173. [DOI] [Google Scholar]
- 21.Robertson D.A., Ray J., Diamond I., Edwards J.G. Personality profile and affective state of patients with inflammatory bowel disease. Gut. 1989;30:623–626. doi: 10.1136/gut.30.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zawadzki B., Strelau J., Szczepaniak P., Śliwińska M. Inwentarz Osobowości NEO-FFI Paula T. Costy Jr i Roberta R. McCrae. Adaptacja Polska. Pracownia Testów Psychologicznych; Warszawa, Poland: 2007. [Google Scholar]
- 23.Spielberger C.D., Strelau J., Tysarczyk T., Wrześniewski K. STAI-Inwentarz Stanu i Cechy Lęku STAI. Podręcznik. Pracownia Testów Psychologicznych Polskiego Towarzystwa Psychologicznego; Warszawa, Poland: 2011. [Google Scholar]
- 24.Spielberger C.D., Gorsuch R.L., Lushene R., Vagg P.R., Jacobs G.A. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press; Palo Alto, CA, USA: 1983. [Google Scholar]
- 25.Plopa M., Makarowski R. Kwestionariusz Poczucia Stresu. Podręcznik. Vizja Press & IT; Warszawa, Poland: 2010. [Google Scholar]
- 26.George D., Mallery P. IBM SPSS Statistics 26 Step by Step: A Simple Guide and Reference. 16th ed. Routledge; London, UK: 2019. [DOI] [Google Scholar]
- 27.Iebba V., Totino V., Gagliardi A., Santangelo F., Cacciotti F., Trancassini M., Mancini C., Cicerone C., Corazziari E., Pantanella F., et al. Eubiosis and dysbiosis: The two sides of the microbiota. New Microbiol. 2016;39:1–12. [PubMed] [Google Scholar]
- 28.Yang Y., Jobin C. Microbial imbalance and intestinal pathologies: Connections and contributions. Dis. Model. Mech. 2014;7:1131–1142. doi: 10.1242/dmm.016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilchmann-Diounou H., Menard S. Psychological Stress, Intestinal Barrier Dysfunctions, and Autoimmune Disorders: An Overview. Front. Immunol. 2020;11:1823. doi: 10.3389/fimmu.2020.01823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trajkovski V. The Gut-Brain Link with Autism Spectrum Disorders; Proceedings of the Conference in Krakow Pedagogical University; Krakow, Poland. 8–26 June 2020; [(accessed on 20 June 2021)]. Available online: https://www.youtube.com/watch?v=4DXbixpo5Jc. [Google Scholar]
- 31.Dinan T.G., Cryan J.F. Melancholic microbes: A link between gut microbiota and depression? Neurogastroenterol. Motil. 2013;25:713–719. doi: 10.1111/nmo.12198. [DOI] [PubMed] [Google Scholar]
- 32.Gunthert K.C., Cohen L.H., Armeli S. The role of neuroticism in daily stress and coping. J. Personal. Soc. Psychol. 1999;77:1087–1100. doi: 10.1037/0022-3514.77.5.1087. [DOI] [PubMed] [Google Scholar]
- 33.Farnam A., Somi M.H., Sarami F., Farhang S. Five personality dimensions in patients with irritable bowel syndrome. Neuropsychiatr. Dis. Treat. 2008;4:959–962. doi: 10.2147/ndt.s3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tayama J., Nakaya N., Hamaguchi T., Tomiie T., Shinozaki M., Saigo T., Shirabe S., Fukudo S. Effects of personality traits on the manifestations of irritable bowel syndrome. BioPsychoSocial Med. 2012;6:20. doi: 10.1186/1751-0759-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ranasinghe N., Devanarayana N.M., Rajindrajith S., Perera M.S., Nishanthinie S., Warnakulasuriya T., de Zoysa P.T. Functional gastrointestinal diseases and psychological maladjustment, personality traits and quality of life. BMC Gastroenterol. 2018;18:33. doi: 10.1186/s12876-018-0760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ogińska-Bulik N., Juczyński Z. Właściwości osobowości sprzyjające chorobom somatycznym–rola typu D. Psychoonkologia. 2007;11:1–7. [Google Scholar]
- 37.Woźniewicz A., Basińska M. Cechy osobowości według modelu Wielkiej Piątki jako wyznaczniki osobowości stresowej u chorych na łuszczycę. In: Liberska H., Malina A., Suwalska-Barancewicz D., editors. Funkcjonowanie Współczesnych Młodych Ludzi w Zmieniającym się Świecie. Dyfin; Warszawa, Poland: 2012. pp. 312–324. [Google Scholar]
- 38.Lebel S., Mutsaers B., Tomei C., Leclair C.S., Jones G., Petricone-Westwood D., Rutkowski N., Ta V., Trudel G., Laflamme S.Z., et al. Health anxiety and illness-related fears across diverse chronic illnesses: A systematic review on conceptualization, measurement, prevalence, course, and correlates. PLoS ONE. 2020;15:e0234124. doi: 10.1371/journal.pone.0234124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lobionda S., Sittipo P., Kwon H.Y., Lee Y.K. The Role of Gut Microbiota in Intestinal Inflammation with Respect to Diet and Extrinsic Stressors. Microorgisms. 2019;19:271. doi: 10.3390/microorganisms7080271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foster J.A., Rinaman L., Cryan J.F. Stress and the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blagden S., Kingstone T., Soundy A., Lee R., Singh S., Roberts L. A Comparative Study of Quality of Life in Persons With Irritable Bowel Syndrome and Inflammatory Bowel Disease. Gastroenterol. Nurs. 2015;38:268–278. doi: 10.1097/SGA.0000000000000124. [DOI] [PubMed] [Google Scholar]
- 42.Wciórka J. Miejsce lęku w psychopatologii. Postępy Psychiatr. I Neurol. 1994;3:37–52. [Google Scholar]
- 43.Jonsson B.H., Theorell T., Gotthard R. Symptoms and personality in patients with chronic functional dyspepsia. J. Psychosom. Res. 1995;39:93–102. doi: 10.1016/0022-3999(94)00086-K. [DOI] [PubMed] [Google Scholar]
- 44.Tanum L., Malt U.F. Personality and physical symptoms in nonpsychiatric patients with functional gastrointestinal disorder. J. Psychosom. Res. 2001;50:139–146. doi: 10.1016/S0022-3999(00)00219-1. [DOI] [PubMed] [Google Scholar]
- 45.Muscatello M.R.A., Bruno A., Mento C., Pandolfo G., Zoccali R.A. Personality traits and emotional patterns in irritable bowel Syndrome. World J. Gastroenterol. 2016;22:6402–6415. doi: 10.3748/wjg.v22.i28.6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Denys K., Orzechowska A., Gałecki P. Kiedy podejrzewać podłoże psychiczne w zaburzeniach układu pokarmowego? [(accessed on 20 June 2021)];Med. Dyplomie Psychiatr. 2015 :72–80. Available online: https://www.researchgate.net/publication/283343970_Kiedy_podejrzewac_podloze_psychiczne_w_zaburzeniach_ukladu_pokarmowego. [Google Scholar]
- 47.Gałęcka M., Basińska A.M., Bartnicka A. Znaczenie mikrobioty jelitowej w kształtowaniu zdrowia człowieka-implikacje w praktyce lekarza rodzinnego. Forum Med. Rodz. 2018;12:50–59. [Google Scholar]
- 48.Jacobs J., Gupta A., Bhatt R., Brawer J., Gao K., Tillisch K., Lagishetty V., Firth R., Gudleski G., Ellingson B., et al. Cognitive behavioral therapy for irritable bowel syndrome induces bidirectional alterations in the brain-gut-microbiome axis associated with gastrointestinal symptom improvement. Microbiome. 2021;9:1–14. doi: 10.1186/s40168-021-01188-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melehin A.I. Cognitive Behavioral Psychotherapy of Interoceptive Influence in the Treatment of Irritable Bowel Syndrome. Clin. Psychol. Spec. Educ. 2020;9:1–33. doi: 10.17759/cpse.2020090201. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to ethical reason.