Abstract
Midazolam is a drug with actions towards the central nervous system producing sedative and anticonvulsants effects, used for sedation and seizures treatments. A better understanding about its effects in the different scenarios presented in the literature could be helpful to gather information regarding its clinical indications, pharmacological interactions, and adverse events. From this perspective, the aim of this study was to analyze the global research about midazolam mapping, specifically the knowledge of the 100 most-cited papers about this research field. For this, a search was executed on the Web of Science-Core Collection database using bibliometric methodological tools. The search strategy retrieved 34,799 articles. A total of 170 articles were evaluated, with 70 articles being excluded for not meeting the inclusion criteria. The 100 most-cited articles rendered 42,480 citations on WoS-CC, ranging from 253 to 1744. Non-systematic review was the most published study type, mainly from North America, during the period of 1992 to 2002. The most frequent keywords were midazolam and pharmacokinetics. Regarding the authors, Thummel and Kunze were the ones with the greatest number of papers included. Our findings showed the global research trends about midazolam, mainly related to its different effects and uses throughout the time.
Keywords: midazolam, sedation, bibliometrics
1. Introduction
Midazolam is a drug from the benzodiazepine class with anxiolytic, sedative, myorelaxant, anticonvulsant, and amnesic pharmacological properties [1]. It enhances the neural-inhibitory actions of the amino-acid neurotransmitter gamma-aminobutyric acid (GABA) on GABAA receptors [2]. It can be administered by several routes, and has a short elimination period and a small period of action, making it the first option for sedation and analgesia, compared to other drugs with the same pharmacological actions, such as other benzodiazepines [3].
These drug indications are mainly related to the reduction of anxiety, sedation, and analgesic action [4]. It is important to remember that benzodiazepines can be administered in combination with other drugs, especially in surgical procedures [5]. However, considering the rapid effects after administration and its already-cited short duration of action, Midazolam has been used in non-surgical procedures, such as outpatient examinations and in dentistry [6].
In addition, this drug has also been playing important roles in the treatment of seizures and epileptic events [7,8]. A meta-analysis showed that midazolam is one of the most successful drugs in achieving seizure cessation [9]. In face of this, it is necessary to compile data about the use of midazolam and its clinical applicability.
The mapping of the existent knowledge on the midazolam field of study, using bibliometrics tools, could retrieve important data metrics with information about the main evidence and networks, offering a fully global panoramic view about its clinical recommendations, treatment protocols, side effects, and other important data about the drug. Thus, the objective of this article was to identify and analyze the 100 most-cited articles on midazolam to map knowledge and trace the metric parameters regarding its effects and use.
2. Materials and Methods
2.1. Study Search and Selection Strategy
This study used bibliometric-analysis tools as a method to retrieve the most-cited articles on the subject, considering that they have a relevant impact on the production and dissemination of scientific knowledge [10].
The search was performed on May 2022 using the following strategy: TS = (Midazolam or “Midazolam hydrochloride” or doronicum or versed or “Midazolam maleate”) on the Web of Science-Core Collection (WoS-CC), without restrictions on language, year of publication, and type of study. Studies were retrieved and evaluated by two independent researchers. Disagreements were solved by a third researcher.
2.2. Eligibility Criteria
For the study to be included in this knowledge mapping, it should present midazo-lam as the object of study, such as in a molecular approach, in pre-clinical and clinical investigations, as well as studies that compare it with other drugs or review studies. Editorial articles, commentaries, conference papers, and open letter were not selected.
2.3. Data Extraction
The articles selected were fully reviewed and the main information was extracted, such as number of citations, titles, authors, year of publication, study design, URL/DOI, keywords, country and continent of the corresponding author, and article aims. The density of citation was a parameter retrieved with the calculation of the number of citations divided by the time of publication in years (considering the first year after publication until 2021).
Study designs were categorized into systematic or non-systematic reviews, cross-sectional, case-control, cohort, randomized or non-randomized clinical trials, in vitro studies, in vivo studies, in situ studies, clinical or case reports, and guidelines [11]. The articles selected on WoS-CC were also searched on Scopus and Google Scholar databases, and compared with the number of citations on WoS-CC. Journals with published articles present in the ranking were also compiled and their impact factors (2021–2022 years) were assessed using the Journal Citation Reports tool by ClarivateTM on 17 November 2022 (https://jcr.clarivate.com/jcr/home (accessed on 17 November 2022)).
To begin compiling the information present in each included study, it was necessary to read all the studies completely and, where it was possible, to extract the main uses of midazolam, its mechanisms of action, as well as possible implications on organisms. The ranking of the articles was determined by the density of citations and number of citations on WoS-CC with the total number of citations as the tie-breaking criterion in case of ties.
2.4. Worldwide Distribution Analysis and Network Visualization
MapChart (https://mapchart.net/ (accessed on 30 July 2022)) was used to illustrate the global distribution of publications selected for this study using country and continent information from the corresponding author. The Visualization of Similarities Viewer software (VOSviewer version 1.6.17, CWTS, Leiden University, Leiden, The Netherlands) was used to create an illustration of co-authorship networks and keywords occurrence.
Author names and keywords were introduced in the software as units of analysis which were expressed as nodes connected to clusters in VOSviewer. The interpretation of clusters is given by the size of the cluster and the proximity to other clusters. In this way, nodes with more expressive interaction/appearance were represented by larger circles and the proximity to each other indicated strongly related nodes. The thickness of the lines connected to the clusters showed the strength of the interaction. The cluster number was determined by a resolution parameter [12]. Authors who appeared in at least 1 of the 100 selected articles were included. Co-authoring groupings were represented by a different color. In addition, keywords used by the authors were inserted into the software as a unit of analysis to visualize overlapping co-occurrence networks.
3. Results
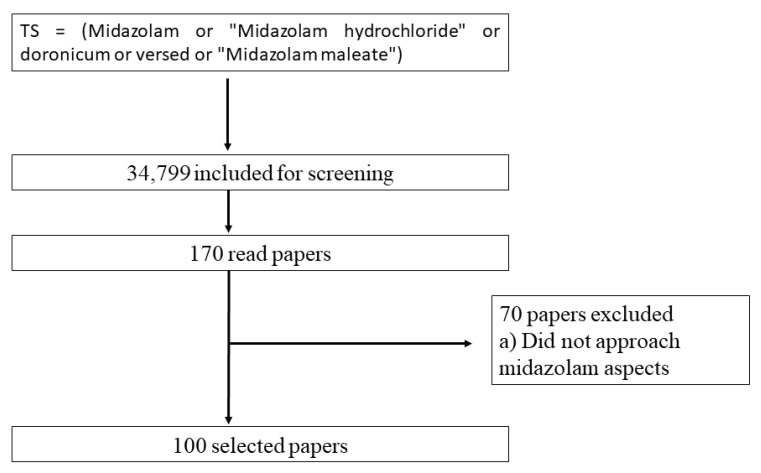
The search strategy retrieved 34,799 articles from WoS-CC. A total of 170 articles were evaluated by eligibility criteria. A total of 70 articles were excluded for not meeting these criteria (Supplementary Table S1) and 100 articles were selected. (Figure 1).
Figure 1.
Flowchart of the literature and article screening.
In Table 1, it is possible to observe the retrieved studies according to the search string used. We categorized them in descending order of citation density. The study with the highest citation density on WoS-CC was Glauser et al. [13] with 95.20 (citations per year) and the lowest was Dundee et al. [7] with 8.08. However, when we consider the total number of citations on WoS-CC, the study conducted by Kress et al. [14] was the most cited when compared to the other studies, while the least-cited paper had 1744 citations.
Table 1.
The 100 most-cited articles about midazolam considering the citation density.
| Rankings Density of Citation/No Citation, WoS-CC a |
Author/Year | Density of Citation | No Citation, WoS-CC a | No. Citation, Scopus | No. Citation, Google Scholar | Study Object | DOI/URL |
|---|---|---|---|---|---|---|---|
| 01/24 | Glauser et al., 2016 [13] | 92.20 | 476 | 519 | 837 | Epilepsy treatment | https://doi.org/10.5698/1535-7597-16.1.48 |
| 02/10 | Murrough et al., 2013 [15] | 84.75 | 678 | 727 | 1031 | Depression use | https://doi.org/10.1176/appi.ajp.2013.13030392 |
| 03/01 | Kress et al., 2000 [14] | 83.05 | 1744 | 311 | 3494 | Sedative effects | https://doi.org/10.1056/NEJM200005183422002 |
| 04/04 | Riker et al., 2009 [16] | 80.83 | 970 | 1163 | 1720 | Sedation aspects | https://doi.org/10.1001/jama.2009.56 |
| 05/02 | Jevtovic-Todorovic et al., 2003 [17] | 75.94 | 1367 | 1553 | 2147 | Anesthesia effects | https://doi.org/10.1523/jneurosci.23-03-00876.2003 |
| 06/20 | Ferguson et al., 2013 [18] | 64.38 | 515 | 595 | 890 | Sedation aspects | https://doi.org/10.1056/NEJMoa1215554 |
| 07/22 | Casali et al., 2013 [19] | 60.88 | 487 | 532 | 787 | Sedation aspects | https://doi.org/10.1126/scitranslmed.3006294 |
| 08/03 | Jacobi et al., 2002 [20] | 59.95 | 1139 | 1483 | 2321 | Sedation and analgesia aspects | https://doi.org/10.1097/00003246-200201000-00020 |
| 09/19 | Jakob et al., 2012 [21] | 57.67 | 519 | 582 | 958 | Sedation aspects | https://doi.org/10.1001/jama.2012.304 |
| 10/17 | Strøm et al., 2010 [22] | 49.27 | 542 | 610 | 1006 | Sedation aspects | https://doi.org/10.1016/S0140-6736(09)62072-9 |
| 11/39 | Silbergleit et al., 2012 [23] | 43.00 | 387 | 460 | 674 | Epilepsy treatment | https://doi.org/10.1056/NEJMoa1107494 |
| 12/28 | Needham et al., 2010 [24] | 40.91 | 450 | 476 | 817 | Sedation aspects | https://doi.org/10.1016/j.apmr.2010.01.002 |
| 13/07 | Lamba et al., 2002 [25] | 38.84 | 738 | 806 | 1152 | Cytochrome P450 approach | https://doi.org/10.1016/S0169-409X(02)00066-2 |
| 14/06 | Glass et al., 1997 [26] | 36.86 | 884 | 1085 | 1850 | Sedation aspects | https://doi.org/10.1097/00000542-199704000-00014 |
| 15/31 | Klotz, 2009 [27] | 35.17 | 422 | 466 | 761 | Historical aspects | https://doi.org/10.1080/03602530902722679 |
| 16/37 | Izzo & Ernst, 2009 [28] | 32.75 | 393 | 460 | 982 | Drug interactions | https://doi.org/10.2165/11317010-000000000-00000 |
| 17/79 | Mehta et al., 2012 [29] | 32.44 | 292 | 330 | 519 | Sedation aspects | https://doi.org/10.1001/jama.2012.13872 |
| 18/64 | Zhao et al., 2011 [30] | 32.30 | 323 | 341 | 451 | Pharmacology | https://doi.org/10.1038/clpt.2010.298 |
| 19/11 | Dresser et al., 2000 [31] | 31.33 | 658 | 807 | 1115 | Cytochrome P450 | https://doi.org/10.2165/00003088-200038010-00003 |
| 20/05 | Chernik et al., 1990 [32] | 30.77 | 954 | 1071 | 1741 | Sedation aspects | https://doi.org/10.1097/00004714-199008000-00003 |
| 21/36 | Greicius et al., 2008 [33] | 30.54 | 397 | 421 | 600 | Sedation aspects | https://doi.org/10.1002/hbm.20537 |
| 22/08 | Thummel & Wilkinson, 1998 [34] | 30.26 | 696 | 128 | 1071 | Cytochrome P450 approach | https://doi.org/10.1146/annurev.pharmtox.38.1.389 |
| 23/95 | Shorvon & Ferlisi, 2012 [35] | 29.33 | 264 | 310 | 451 | Epilepsy treatment | https://doi.org/10.1093/brain/aws091 |
| 24/42 | Verbeeck, 2008 [36] | 28.46 | 370 | 466 | 599 | Liver evaluation | https://doi.org/10.1007/s00228-008-0553-z |
| 25/43 | Pan-dharipande et al., 2008 [37] | 28.31 | 368 | 406 | 668 | Risk factors | https://doi.org/10.1097/TA.0b013e31814b2c4d |
| 26/81 | Ferrarelli et al., 2010 [38] | 27.73 | 305 | 334 | 493 | Anesthesia aspects | https://doi.org/10.1073/pnas.0913008107 |
| 27/29 | Hu et al., 2005 [39] | 27.50 | 440 | 769 | 283 | Pharmacology | https://doi.org/10.2165/00003495-200565090-00005 |
| 28/26 | Bowles et al., 2004 [40] | 27.29 | 464 | 558 | 863 | Sedation aspects | https://doi.org/10.1136/gut.2003.016436 |
| 29/27 | Li et al., 2004 [41] | 27.29 | 464 | 508 | 775 | Cytochrome P450 approach | https://doi.org/10.1124/dmd.32.8.821 |
| 30/12 | Paine et al., 1997 [42] | 27.00 | 648 | 694 | 974 | Cytochrome P450 approach | https://jpet.aspetjour-nals.org/content/283/3/1552.long |
| 31/25 | Niemi et al., 2003 [43] | 26.33 | 474 | 530 | 761 | Pharmacology | https://doi.org/10.2165/00003088-200342090-00003 |
| 32/82 | Shehabi et al., 2010 [44] | 26.27 | 289 | 327 | 445 | Cytochrome P450 approach | https://doi.org/10.1097/CCM.0b013e3181f85759 |
| 33/58 | McQuaid & Laine, 2008 [45] | 25.92 | 337 | 348 | 524 | Sedation aspects | https://doi.org/10.1016/j.gie.2007.12.046 |
| 34/49 | Stevens et al., 2007 [46] | 25.57 | 358 | 417 | 692 | Risk factors | https://doi.org/10.1007/s00134-007-0772-2 |
| 35/53 | Payen et al., 2007 [47] | 24.93 | 349 | 402 | 689 | Analgesia and sedation aspects | https://doi.org/10.1097/01.anes.0000264747.09017.da |
| 36/90 | Meierkord et al., 2010 [48] | 24.64 | 271 | 327 | 488 | Epilepsy treatment | https://doi.org/10.1111/j.1468-1331.2009.02917.x |
| 37/15 | Kollef et al., 1998 [49] | 24.00 | 552 | 713 | 1165 | Sedation aspects | https://doi.org/10.1378/chest.114.2.541 |
| 38/13 | Cruz et al., 1994 [50] | 23.93 | 646 | 676 | 929 | Anxiety use | https://doi.org/10.1016/0091-3057(94)90472-3 |
| 39/50 | Johnson et al., 2006 [51] | 23.73 | 356 | 380 | 509 | Cytochrome P450 approach | https://doi.org/10.2165/00003088-200645090-00005 |
| 40/18 | Bailey et al., 1998 [52] | 23.26 | 535 | 658 | 938 | Food interaction | https://doi.org/10.1046/j.1365-2125.1998.00764.x |
| 41/74 | Riss et al., 2008 [53] | 23.00 | 299 | 345 | 541 | Pharmacology | https://doi.org/10.1111/j.1600-0404.2008.01004.x |
| 42/38 | Walsky & Obach, 2004 [54] | 22.88 | 389 | 429 | 561 | Cytochrome P450 approach | https://doi.org/10.1124/dmd.32.6.647 |
| 43/48 | Zhou et al., 2005 [55] | 22.44 | 359 | 424 | 545 | Cytochrome P450 approach | https://doi.org/10.2165/00003088-200544030-00005 |
| 44/84 | Lichtenstein et al., 2008 [56] | 21.85 | 284 | 373 | 538 | Guideline | https://doi.org/10.1016/j.gie.2008.09.029 |
| 45/96 | Maldonado et al., 2009 [57] | 21.83 | 262 | 309 | 560 | Sedation aspects | https://doi.org/10.1176/appi.psy.50.3.206 |
| 46/57 | Willoughby et al., 2005 [58] | 21.50 | 344 | 432 | 675 | Sedation aspects | https://doi.org/10.1056/NEJMoa050382 |
| 47/23 | Lowenstein & Alldredge, 1998 [59] | 21.09 | 485 | 20 | 35 | Epilepsy treatment | https://doi.org/10.1056/NEJM199804023381407 |
| 48/68 | Krauss & Green, 2006 [60] | 21.07 | 316 | 365 | 607 | Sedation and analgesia aspects | https://doi.org/10.1016/S0140-6736(06)68230-5 |
| 49/60 | Yon et al., 2005 [61] | 20.63 | 330 | 378 | 508 | Apoptotic pathways investigation | https://doi.org/10.1016/j.neuroscience.2005.03.064 |
| 50/85 | De Graeff & Dean, 2007 [62] | 20.21 | 283 | 327 | 555 | Clinical use | https://doi.org/10.1089/jpm.2006.0139 |
| 51/41 | Claassen et al., 2002 [63] | 19.95 | 379 | 466 | 717 | Epilepsy treatment | https://doi.org/10.1046/j.1528-1157.2002.28501.x |
| 52/32 | Venkata-krishnan et al., 2000 [64] | 19.90 | 418 | 474 | 642 | Cytochrome P450 approach | https://doi.org/10.2165/00003088-200038020-00002 |
| 53/67 | Young et al., 2005 [65] | 19.88 | 318 | 371 | 519 | Neurodegeneration | https://doi.org/10.1038/sj.bjp.0706301 |
| 54/21 | Thummel et al., 1996 [66] | 19.84 | 496 | 550 | 703 | Cytochrome P450 approach | https://doi.org/10.1016/S0009-9236(96)90177-0 |
| 55/09 | Reves et al., 1985 [67] | 19.00 | 684 | 750 | 1243 | Midazolam pharmacology | https://doi.org/10.1097/00000542-198503000-00017 |
| 56/16 | Kauffman et al., 1992 [68] | 19.72 | 543 | 501 | 645 | Sedation aspects | https://pennstate.pure.elsevier.com/en/publications/guidelines-for-monitoring-and-management-of-pediatric-patients-du-2 |
| 57/51 | Lin et al., 2002 [69] | 19.68 | 355 | 377 | 537 | Cytochrome P450 approach | https://doi.org/10.1124/mol.62.1.162 |
| 58/34 | Kim et al., 1999 [70] | 18.36 | 404 | 452 | 599 | Cytochrome P450 approach | https://doi.org/10.1023/a:1018877803319 |
| 59/44 | Putensen et al., 2001 [71] | 18.30 | 366 | 442 | 692 | Acute respiratory | https://doi.org/10.1164/ajrccm.164.1.2001078 |
| 60/14 | Kronbach et al., 1989 [72] | 17.47 | 559 | 529 | 688 | Cytochrome P450 approach | https://citeseerx.ist.psu.edu/view-doc/download?doi=10.1.1.989.1642&rep=rep1&type=pdf |
| 61/30 | Schuetz et al., 1996 [73] | 17.36 | 434 | 495 | 641 | Cytochrome P450 approach | https://molpharm.aspetjournals.org/content/49/2/311.long |
| 62/45 | Streetman et al., 2000 [74] | 17.29 | 363 | 404 | 536 | Cytochrome P450 approach | https://doi.org/10.1097/00008571-200004000-00001 |
| 63/88 | McIntyre et al., 2005 [75] | 17.06 | 273 | 330 | 505 | Epilepsy treatment | https://doi.org/10.1016/S0140-6736(05)66909-7 |
| 64/61 | Dielenberg & McGregor, 2001 [76] | 16.40 | 328 | 338 | 481 | Behavioral and anxiety evaluation | https://doi.org/10.1016/S0149-7634(01)00044-6 |
| 65/35 | Paine et al., 1996 [77] | 16.12 | 403 | 437 | 591 | Cytochrome P450 approach | https://doi.org/10.1016/S0009-9236(96)90162-9 |
| 66/66 | Özdemir et al., 2000 [78] | 15.19 | 319 | 350 | 487 | Cytochrome P450 approach | https://doi.org/10.1097/00008571-200007000-00001 |
| 67/54 | Gorski et al., 1998 [79] | 15.17 | 349 | 381 | 471 | Cytochrome P450 approach | https://doi.org/10.1016/S0009-9236(98)90146-1 |
| 68/83 | Andrew Williams et al., 2002 [80] | 14.95 | 284 | 322 | 551 | Sedation aspects | https://doi.org/10.1124/dmd.30.8.883 |
| 69/63 | Venn et al., 1999 [81] | 14.68 | 323 | 417 | 702 | Comparison with other drugs | https://doi.org/10.1046/j.1365-2044.1999.01114.x |
| 70/80 | Bai et al., 2001 [82] | 14.55 | 291 | 303 | 445 | GABA receptor | https://doi.org/10.1124/mol.59.4.814 |
| 71/93 | Yuan et al., 2002 [83] | 14.11 | 268 | 303 | 429 | Cytochrome P450 approach | https://doi.org/10.1124/dmd.30.12.1311 |
| 72/77 | Lang et al., 2000 [84] | 14.10 | 296 | 390 | 660 | Anesthesia and sedation aspects | https://doi.org/10.1016/S0140-6736(00)02162-0 |
| 73/94 | Gurley et al., 2002 [85] | 13.89 | 264 | 293 | 397 | Cytochrome P450 approach | https://doi.org/10.1067/mcp.2002.126913 |
| 74/59 | Schmiedlin-Ren et al., 1997 [86] | 13.83 | 332 | 366 | 466 | Cytochrome P450 approach | https://molpharm.aspetjournals.org/content/51/5/741.long |
| 75/87 | Olkkola et al., 1994 [87] | 13.33 | 360 | 498 | 786 | Cytochrome P450 approach | https://doi.org/10.1038/clpt.1994.60 |
| 76/71 | Pelkonen et al., 1998 [88] | 13.22 | 304 | 312 | 393 | Cytochrome P450 approach | https://doi.org/10.1080/004982598238886 |
| 77/13,18 | Kenworthy et al., 1999 [89] | 13.18 | 290 | 309 | 415 | Cytochrome P450 approach | https://doi.org/10.1046/j.1365-2125.1999.00073.x |
| 78/81 | Wang et al., 2001 [90] | 13.10 | 262 | 328 | 452 | Cytochrome P450 approach | https://doi.org/10.1067/mcp.2001.118522 |
| 79/89 | Krauss & Green, 2000 [91] | 12.95 | 272 | 333 | 481 | Sedation and analgesia aspects | https://doi.org/10.1056/NEJM200003303421306 |
| 80/55 | Christopher Gorski et al., 1994 [92] | 12.81 | 346 | 368 | 479 | Cytochrome P450 approach | https://doi.org/10.1016/0006-2952(94)90543-6 |
| 81/40 | Arrowsmith et al., 1991 [93] | 12.77 | 383 | 450 | 595 | Adverse effects | https://doi.org/10.1016/S0016-5107(91)70773-6 |
| 82/52 | Parke et al., 1992 [94] | 12.14 | 352 | 475 | 697 | Sedation aspects | https://doi.org/10.1136/bmj.305.6854.613 |
| 83/100 | Ostermann et al., 2000 [95] | 12.05 | 253 | 323 | 552 | Sedation aspects | https://doi.org/10.1001/jama.283.11.1451 |
| 84/62 | Lown et al., 1994 [96] | 12.00 | 324 | 353 | 452 | Cytochrome P450 approach | https://dmd.aspetjour-nals.org/content/22/6/947.long |
| 85/73 | Kup-ferschmidt et al., 1995 [97] | 11.65 | 303 | 345 | 439 | Pharmacology | https://doi.org/10.1016/0009-9236(95)90068-3 |
| 86/98 | Scott et al., 1999 [3] | 11.64 | 256 | 307 | 463 | Epilepsy treatment | https://doi.org/10.1016/S0140-6736(98)06425-3 |
| 87/47 | Bailey et al., 1990 [98] | 11.61 | 360 | 382 | 443 | Respiratory and adverse effects | https://doi.org/10.1097/00000542-199011000-00005 |
| 88/33 | Greenblatt et al., 1984 [99] | 11.27 | 417 | 448 | 653 | Pharmacology | https://doi.org/10.1097/00000542-198461010-00006 |
| 89/78 | Quine et al., 1995 [100] | 11.27 | 293 | 303 | 464 | Sedation aspects | https://doi.org/10.1136/gut.36.3.462 |
| 90/91 | Schmiedlin-Ren et al., 1997 [101] | 11.25 | 270 | 295 | 398 | Cytochrome P450 approach | https://doi.org/10.1124/mol.51.5.741 |
| 91/72 | Thummel et al., 1994 [102] | 11.22 | 303 | 314 | 405 | Cytochrome P450 approach | https://jpet.aspetjournals.orgcontent/271/1549.long |
| 92/86 | Cheng et al., 1996 [103] | 11.04 | 276 | 300 | 446 | Sedation aspects | https://doi.org/10.1016/S0022-5223(96)70062-4 |
| 93/65 | Hunt et al., 1992 [104] | 11.03 | 320 | 355 | 528 | Cytochrome P450 approach | https://doi.org/10.1016/0006-2952(92)90010-G |
| 94/76 | Ashton, 1994 [105] | 11.00 | 297 | 358 | 563 | Advantages and disadvantages | https://doi.org/10.2165/00003495-199448010-00004 |
| 95/70 | Heinrichs et al., 1992 [106] | 10.48 | 304 | 338 | 498 | Behavioral evaluation | https://doi.org/10.1016/0006-8993(92)90708-H |
| 96/87 | Olkkola et al., 1993 [107] | 9.82 | 275 | 310 | 383 | Pharmacology | https://doi.org/10.1038/clpt.1993.25 |
| 97/92 | Magorian et al., 1993 [108] | 9.61 | 269 | 323 | 602 | Anesthesia aspects | https://doi.org/10.1097/00000542-199311000-00007 |
| 98/99 | Mangano et al., 1992 [109] | 8.83 | 256 | 311 | 489 | Analgesia aspects | https://doi.org/10.1097/00000542-199203000-00004 |
| 99/56 | Allonen et al., 1981 [110] | 8.63 | 345 | 300 | 433 | Effects on the body | https://doi.org/10.1038/clpt.1981.217 |
| 100/75 | Dundee et al., 1984 [7] | 8.08 | 299 | 296 | 337 | Pharmacology | https://doi.org/10.2165/00003495-198428060-00002 |
a Web of Science Core Collection.
The most-cited article was “Daily Interruption of Sedative Infusions in Critically ill patients undergoing mechanical ventilations” Kress et al. [14], published by The New England Journal of Medicine. The article had 1744 citations. The aim of this article was to correlate early withdrawal of sedatives with the length of hospital stay. The period of years with the most publications is 1992–2002 (n = 52 papers and 22,125 citations), followed by the years 2003–2013 (n = 39 papers and 16,308 citations) (Table 2).
Table 2.
Characteristics of the 100 most-cited studies about Midazolam.
| Characteristics | Number of Papers | Number of Citations in WoS-CC a | Citation Ratio b |
|---|---|---|---|
| Publication period | |||
| 1981–1991 | 8 | 4001 | 500, 13 |
| 1992–2002 | 52 | 22,125 | 425, 48 |
| 2003–2013 | 39 | 16,308 | 418, 15 |
| 2014–2016 | 1 | 476 | 476, 00 |
| Journal (Impact factor c) | |||
| Clinical Pharmacology & Therapeutics (7.051) | 10 | 3380 | 338, 00 |
| Anesthesiology (9.198) | 7 | 3219 | 459, 86 |
| New England Journal of Medicine (176.082) | 6 | 3747 | 624, 50 |
| Drug Metabolism and Disposition (3.579) | 6 | 2061 | 343, 50 |
| Clinical Pharmacokinetics (5.577) | 5 | 2265 | 453, 00 |
| Molecular Pharmacology (4.058) | 5 | 1909 | 381, 80 |
| Lancet (202.731) | 5 | 1683 | 336, 60 |
| Drugs (11.431) | 4 | 1429 | 357, 25 |
| Jama-Journal of the American Medical Association (157.375) | 4 | 2034 | 508, 50 |
| Gastrointestinal Endoscopy (10.396) | 3 | 1004 | 334, 67 |
| Critical Care Medicine (9296) | 2 | 1428 | 714, 00 |
| Journal of Pharmacology and Experimental Therapeutics (4404) | 2 | 951 | 475, 50 |
| British Journal of Clinical Pharmacology (3716) | 2 | 825 | 412, 5 |
| Gut (31,795) | 2 | 757 | 378, 5 |
| Pharmacogenetics (7221) d | 2 | 682 | 341 |
| Biochemical Pharmacology (6100) | 2 | 666 | 333 |
| Other journals with only one article | 33 | 1487 | 450, 60 |
| Experimental design | |||
| Non-systematic review | 21 | 7898 | 376, 09 |
| Cohort | 14 | 6261 | 447, 21 |
| Randomized clinical trial | 13 | 7148 | 549, 84 |
| Non-randomized clinical trial | 13 | 5154 | 396, 46 |
| In-vitro study | 10 | 4156 | 415, 60 |
| Clinical report | 5 | 1585 | 317, 00 |
| In vivo study | 9 | 4831 | 536, 77 |
| Guideline | 7 | 3111 | 444, 4 |
| Systematic review | 5 | 1750 | 350, 00 |
| Case report | 2 | 696 | 348, 00 |
| Cross-sectional | 1 | 320 | 320, 00 |
| Number of authors | |||
| 1–2 | 13 | 4852 | 373, 23 |
| 3–4 | 24 | 11,365 | 473, 54 |
| 5–6 | 20 | 7609 | 380, 45 |
| >6 | 44 | 19,084 | 433, 73 |
a WoS-CC: Web of Science Core Collection; b Citation ratio: number of citations per number of articles; c Impact factor (2021–2022 years) retrieved on https://jcr.clarivate.com/jcr/home on 17 November 2022; d This journal title was changed; thus, it was considered the journal’s impact factor from the previous year with the original title and ISSN (2006–2022 years).
The oldest article on the list was published in 1981 by Allonen et al. [110], entitled “Midazolam Kinetics”, in Clinical Pharmacology & Therapeutics. The aim of the article was to review its pharmacodynamics and pharmacokinectics. The most recent article on the list was published in 2016 by Glauser et al. [13], titled “Evidence-Based Guidelines: Treatment of Epileptic Status in Children and Adults: American Epilepsy Society Guidelines Committee Report by Epilepsy Current “. The aim of the article was to compare drugs (diazepam, phenobarbital, midazolam, and lorazepam) in terms of efficacy, tolerability, and safety for the treatment of epileptic episodes in children and adults. The most frequent type of study among the 100 articles was non-systematic reviews (n = 21), followed by cohort (n = 14), randomized (n = 13) and non-randomized (n = 13) clinical trial studies (Table 2).
The articles selected in this study were published in 49 different journals. The top seven journals were: Clinical Pharmacology & Therapeutics (n = 10), Anesthesiology (n = 7), New England Journal of Medicine (n = 6), Drug Metabolism and Disposition (n = 6), Clinical Pharmacokinetics (n = 5), Molecular Pharmacology (n = 5), and Lancet (n = 5). In addition to having the highest number of publications, they are among the journals with the highest number of citations. A total of 33% of journals had only one article published and 15% of the articles were published in journals with high impact factors in health sciences, such as New England Journal of Medicine (n = 6; 3747 citations), Lancet (n = 5; 1683 citations), and Drugs (n = 4; 1429 citations). The Journal of Neuroscience had the highest citation ratio, despite having only one article published out of 100 selected in this study (Table 2).
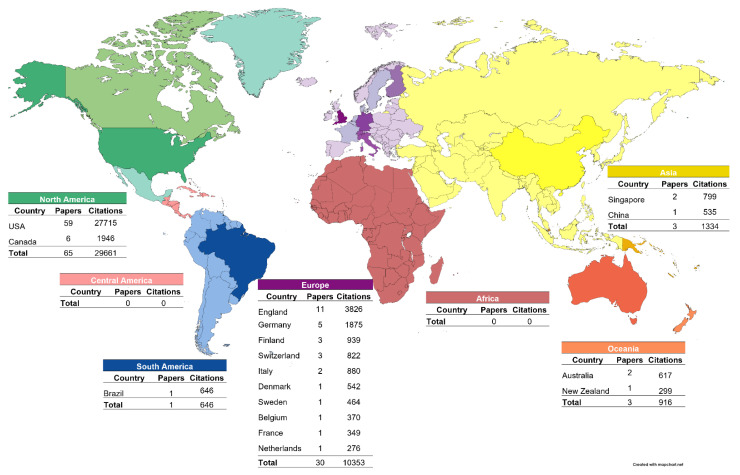
The continent with the most articles on the list was North America (n = 65), followed by the European continent (n = 30), while Asia and Oceania both presented the same number of articles (n = 3). South America only published one, while Africa and Central America did not contribute to any article on the list. Specifically, USA was the country with the most appearances on the list (n = 59), followed by England (n = 11), and Canada (n = 6) (Figure 2).
Figure 2.
Distribution of the 100 most-cited articles about Midazolam by continents and countries. Mapchart created with mapchart.net and adapted by the authors.
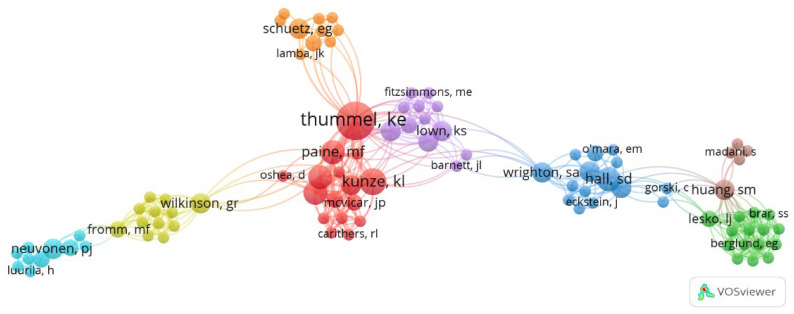
The list of the 100 most-cited articles on Midazolam composed of 556 authors. Based on the ranking of most-cited articles, it was observed that almost 50% of the papers had more than six authors (n = 44), while 24 papers with 3–4 authors appeared on the list, followed by 20 articles with 5–6 authors and 13 articles with 1–2 authors (Table 2). The major contribution as first author was Thummel, K.E. (n = 3), followed by Gorski, J.C.; Krauss, B.; Olkkota, K.T.; Paine, M.F.; and Schmiedlin-Ren, P. (n = 2). The author with the most articles on the list is Thummel, K.E. (n = 10; 4565 citation), followed by Kunze, K.L. (n = 5; 2174 citations), Perkins J.D. (n = 4; 1850 citations), Shen, D.D. (n = 4; 1850 citation), Paine, M.F. (n = 4; 1817 citations), and Hall, S.D. (n = 4; 1241). The other authors appeared in less than four articles on the list: a total of 16 authors contributed in three articles, 37 authors contributed in two articles, and 497 authors contributed in only one article. Figure 3 shows the clusters created with the network between the authors on the publication of papers on midazolam-related topics.
Figure 3.
Network visualization of co-authorship cluster of Midazolam studies. Clusters with the same color and with more proximity indicate a strong relationship between each other, and co-citation is represented by the lines between clusters.
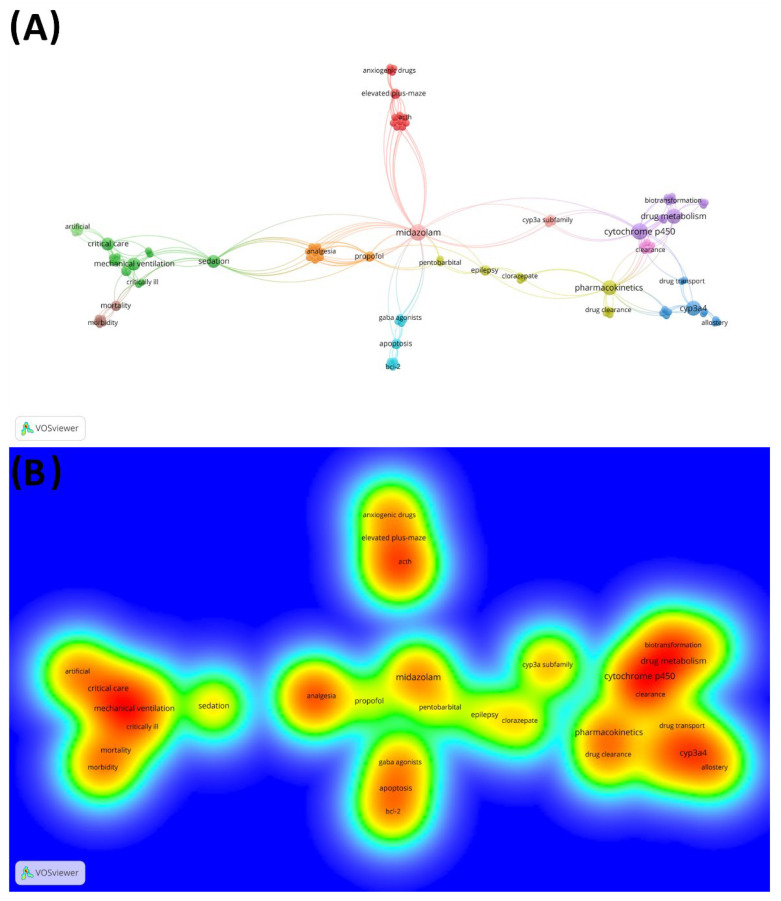
Among a total of 145 author-keywords, grouped into 17 clusters, the keywords with the highest occurrence in the 100 most-cited articles were midazolam (n = 5), cytochrome p450 (n = 5), pharmacokinetics (n = 4), drug metabolism (n = 4), CYP3A4 (n = 4), sedation (n = 3), critical care (n = 3), mechanical ventilation (n = 3), and anesthesia (n = 3) (Figure 3). The first three words were also the ones that had the greatest binding strength with a total link strength of 33, 23, and 20, respectively. The words had a diverse frequency in the periods of time, with the words ‘midazolam’ and ‘cytochrome p450′ being frequently featured in the period of 1992–2002. Not all studies had author-keywords (Table 3).
Table 3.
Most frequent author-keywords (at least two occurrences) of the 100 most-cited studies about Midazolam.
| Rank | Keyword | Frequency Total (Percent) |
Total Link Strength | Frequency in 1981–1991 |
Frequency in 1992–2002 |
Frequency in 2003–2013 |
Frequency in 2014–2016 |
|---|---|---|---|---|---|---|---|
| 1 | midazolam | 5 (3.45%) | 33 | 0 | 4 | 1 | 0 |
| 2 | cytochrome p450 | 5 (3.45%) | 23 | 0 | 4 | 1 | 0 |
| 3 | pharmacokinetics | 4 (2.76%) | 20 | 0 | 1 | 3 | 0 |
| 4 | drug metabolism | 4 (2.76%) | 18 | 0 | 3 | 1 | 0 |
| 5 | cyp3a4 | 4 (2.76%) | 13 | 0 | 4 | 0 | 0 |
| 6 | sedation | 3 (2.07%) | 19 | 0 | 2 | 1 | 0 |
| 7 | critical care | 3 (2.07%) | 15 | 0 | 1 | 1 | 0 |
| 8 | mechanical ventilation | 3 (2.07%) | 15 | 0 | 1 | 2 | 0 |
| 9 | anesthesia | 3 (2.07%) | 8 | 0 | 1 | 2 | 0 |
| 10 | propofol | 2 (1.38%) | 14 | 0 | 2 | 0 | 0 |
| 11 | elevated plus-maze | 2 (1.38%) | 13 | 0 | 2 | 0 | 0 |
| 12 | delirium | 2 (1.38%) | 11 | 0 | 0 | 2 | 0 |
| 13 | apoptosis | 2 (1.38%) | 10 | 0 | 0 | 2 | 0 |
| 14 | mortality | 2 (1.38%) | 10 | 0 | 0 | 2 | 0 |
| 15 | epilepsy | 2 (1.38%) | 8 | 0 | 1 | 1 | 0 |
From 1981 to 1991, eight studies were published, among them three articles addressed the clinical use of midazolam for sedative purposes, two articles investigated pharmacovigilance parameters (one evaluated the safety and efficacy of midazolam, and the other analyzed side effects), two studies were related to pharmacokinetics and pharmacodynamics of this drug, and one study presented the pharmacological properties and therapeutic use of midazolam.
In the mapping period from 1992 to 2002, 52 published articles were found: a total of 30 studies showed the pharmacodynamics and pharmacokinetics of midazolam; 13 articles published, as its main objective, discussed the sedative effect of this drug; and 9 articles highlighted its analgesic, anxiolytic, and antiepileptic effects.
During the period of 2003 to 2013, 39 articles were published: a total of 12 studies addressed the sedative effects of midazolam, five evaluated its use as a therapeutic alternative for epilepsy treatments, four articles evaluated pharmacovigilance parameters investigating the side effects from the use of midazolam, four studies evaluated the anesthetic effect (in which two studies evaluated the level of neurodegeneration), three studies showed results of its effects as analgesic, three studies presented pharmacodynamic steps, such as drug interactions with other drugs, seven articles addressed its pharmacokinetics, showing its mechanism of action through CYP-isoenzymes-promoting chemical modification of several exogenous molecules, and lastly, one article evaluated midazolam for severe-condition treatments.
Finally, in the last period mapped, during the years 2014 to 2016, one article on the use of midazolam for epilepsy treatments was published in which the authors evaluated the efficacy, tolerability, and safety data for anticonvulsant treatment of children and adults with convulsive epilepsy, and used this analysis to develop an evidence-based treatment algorithm to prescribe midazolam.
4. Discussion
This review mapped the produced knowledge found on the most cited-studies about the use of midazolam. A total of 100 studies with different levels of evidence were analyzed, and they included many experimental designs with a predomination of non-systematic reviews, randomized clinical trials, and non-randomized clinical trials. Because of the bibliometric approach performance, it was possible to gather metric information about these articles and extract the main findings considering the follow-up of trends in the use of midazolam.
Experimental designs determine evidence-based practice that supports the work of health professionals [95]. The designs with the highest levels of evidence are systematic reviews and meta-analyses, followed by randomized clinical trials and cohort studies [111]. The experimental design with the highest appearance in the list of the 100 most-cited articles were non-systematic reviews with the main objective of discuss the effects of midazolam, including sedation, analgesia, metabolism, and mechanisms of action, compared to other drugs. Non-systematic reviews provide readers with a current and ordered view of the literature on a specific topic. This type of study can be a very important explanatory space for beginning readers. However, there are positive and negative points that arise from the methods chosen for its development, for example, the selection of articles with low scientific evidence [112].
Among the journals with the highest number of publications, Clinical Pharmacology & Therapeutics (n = 10) and Anesthesiology (n = 7) were the ones with the most articles. The first journal addresses the clinical application of drugs while the second is a journal that approaches the main clinical application associating the sedation-anesthesia aspects. In addition, a prevalence of journals that essentially address molecular aspects of drugs and medications, with papers that evidence midazolam mechanism of actions, pharmacokinetics, and pharmacodynamics.
Midazolam is a hypnotic-sedative drug with anxiolytic properties capable of inducing amnesia and reliable hypnosis in the benzodiazepine group, more specifically the subfamily of imidazobenzodiazepines, with properties distinct from other benzodiazepines; Midazolam has rapid onset (2 to 3 min), is short duration (45 to 60 min), and has high hepatic metabolic clearance, when compared to other drugs in the group [7,60,67].
This drug is used clinically in the treatment of convulsive-epileptic status in children and adults [13,23,48], and as a sedative in colonoscopy [40], endoscopy [45,93] intubation, and extubation of mechanically ventilated patients [3]. In addition, it is used in palliative-sedation therapy to relieve refractory symptoms by reducing consciousness in terminally ill patients [62].
Among the 100 most-cited studies retrieved by our search, the most-cited non-systematic review addressed the relationship between the interaction of drugs, like midazolam, with systematic effects mediated by the consumption of grapefruits, such as modulation on the expression of cytochrome P450 [52]. Midazolam is metabolized by cytochrome P450 3A isoenzymes and the inhibition of this molecule is associated with an increase in midazolam bioavailability [66,102]. Thus, the consumption of grapefruits inhibits cytochrome P450 34A molecules, delaying absorption, reducing first-pass effect of the drug, and increasing blood plasma levels of midazolam, resulting in possible excessive levels of its effects, mainly related to sedation [112].
In addition to this part of the debate, our metrics revealed that Kenneth Thummel is the author with the most publications and citations on the 100 most-cited ranking. He obtained his PhD in pharmaceutical sciences at the University of Washington in 1987, became a postdoctoral fellow in pharmacology at the Center for Health Sciences at the University of Connecticut (USA), and later a professor at the University of Washington’s School of Pharmacy. He investigates the kinetic of drug metabolism, first-pass intestinal metabolism mediated by cytochrome P4503A, mechanism of metabolic clearance of drugs, and genetic modifiers of the responses to drug specializations in drug metabolism. All of his work presented in our ranking built a solid consensus about midazolam metabolization by cytochrome P450 3A, demonstrating to the scientific community the importance of pharmaco-interactions with this molecule and midazolam administration [25,42,69,77].
His evidences are the ones that elucidate the intestinal and hepatic metabolism in the oral first-pass elimination of a CYP3A substrate using midazolam [66]. Thummel and his co-workers created links with other authors, such as Wrighton, S.A., in order to investigate even more about this important enzyme of midazolam elimination [96]. With this, a network was created with other groups of authors (Hall, S.D. and Gorski C.), which investigated the influence of on CYP expression/inhibition, making the drug a standard substrate to the CYP3A subunit. Their work even recognized other interactors on CYP expression using midazolam as a positive control, highlighting the inhibition of CYP subunits by midazolam hydroxylation [90]. Furthermore, our analysis showed that this group of authors collaborated with Huang, S.M., Lesko, L.J., and Madani, S., investigating the potential effects between midazolam interaction and other drugs. The data shown in Figure 3 infer that the research field on midazolam has been related to the understanding of the molecules involved in drug pharmacokinetics, especially, the correlation between specific enzymes in the process and drugs interaction, bringing to the readership a broader perspective on the simultaneous use of midazolam with other substances, guiding new perspectives and raising questions about the topic. In this way, midazolam is an important player to systemic metabolism [25,66]. In this sense, the elderly need special attention, given the reduction of liver mass and/or reduction of perfusion, and therefore, have a lower ability to metabolize drugs [27].
Cytochrome P450, drug metabolism, and pharmacokinetics are connected keywords revealed by our bibliometric analysis (Figure 4). Keywords work as codes for indexing articles in databases and, when used in search of articles, they present more significant results in comparison to phrases. In addition, keywords can illustrate the field of research in a given subject [112]. Other keywords that occurred frequently among the 100 most-cited articles are midazolam, cytochrome p450, pharmacokinetics, drug metabolism, cyp3a4, sedation, critical care, and mechanical ventilation, suggesting that fields of investigation associated with these keywords are an important part of the discussion related to the use of this drug, since most of them reported on the importance of cytochrome p450 protein in the metabolization of midazolam. Furthermore, it is possible to relate it to its clinical applicability both in outpatient and intensive-care settings.
Figure 4.
Network of co-occurrence and density-citation view of keywords used by the authors on the 100 most-cited studies about Midazolam. (A) Keywords network, in which clusters are represented by different colors and the lines indicate co-citation links between keywords; (B) Keywords- density visualization ranging from blue (lowest density) to red (highest density).
A direct link found in the analysis of the keywords was the relation between midazolam and epilepsy. Status epilepticus is a condition where the patient has continuous or rapidly repeating seizures [113]. Benzodiazepines remain the first-choice drugs for the management of epilepsy and seizures, and from this class, midazolam is the only water-soluble drug available [53]. For status-epilepticus treatment, midazolam can be administered via intramuscular, intravenous, and buccally and nasally [114,115]. Clinical trials comparing midazolam and diazepam efficacy in children showed better results in the cases using midazolam [3,75,115]. Buccal midazolam was able to reduce the number of seizure episodes and the time to stop seizing in treated children with a decrease of respiratory depression requiring interventional need [75].
Furthermore, a systematic review performed in 2002 suggested that the treatment of refractory-status epilepticus using pentobarbital was better than using midazolam or propofol [63]. However, after 10 years, a study reviewing the outcome data reported by 121 studies published since 1981, indicated that midazolam presented better results in comparison to thiopental/pentobarbital and propofol in the control of seizures [85]. These findings point to the fact that science is continuous, and the interpretation of the literature data must occur with caution to details and related factors, such as methodology and year of publication. Most recent articles are essential for updating protocols, advancing diagnosis and knowledge of the current and scientific clinical scenario, and helping researchers to study, investigate, and advance future issues [10].
The most recent article on our ranking is ‘Evidence-based guideline: treatment of epileptic convulsive status in children and adults: Report of the Guideline Committee of the American Epilepsy Society’, published in 2016 [13]. It was a randomized clinical trial that analyzed data on the efficacy, tolerability, and safety of Midazolam IM, Fenobarbital IV, Diazepam IV, and Lorazepam IV drugs for seizure treatments in children and adults to propose a possible algorithm-based treatment guideline. In this study, it was observed that in adult patients, Midazolam administered via intramuscular, presented superior efficacy, when compared to Lorazepam. However, in the infant population, there were not satisfactory analyses to reach a more reliable conclusion.
Another important scientific metric is the number of citation of an article. Articles with 100 or more citations can be considered classic, depending on the research area. Classical articles influence the development of scientific knowledge and clinical practices [116]. For a better evaluation about citations, the WoS-CC database was chosen for our bibliometric analysis. This database allows the retrieval of publications since 1945 and has high-quality journals indexed from all over the world [117,118]. However, we also performed searches on Scopus and Google Scholar databases to compare this citation index. We chose Scopus because of its high recognition, credibility, and innovative scope in the field of bibliometrics, and Google Scholar because of its accessibility in many countries [119,120]. Still reflecting on citations, the density of citation, i.e., the number of citations per year after publication, can provide insights into the scientific knowledge presented in the studies. Our bibliometric review revealed that although [13] is the most recent study in the top 100 most-cited articles about midazolam, it has already obtained the highest citation density in this field, reflecting that the guidelines about the treatment of epilepticus status is possibly an emerging trend related this drug, as this article accumulates a considerable number of citations in a short period of time.
The most-cited article found in our bibliometric analysis was ‘Daily Interruption of Sedatives in critically ill patients’ mechanical ventilation’ [14], which had 1744 citations. It was a randomized clinical study that evaluated the impact of continuously sedative administration in patients hospitalized in intensive care, and its possible adverse events, such as changes in mental status and the length of stay of patients in hospitals.
Guidelines for sedation of patients hospitalized in intensive-care units have recommended the use of γ-aminobutyric acid (GABA) receptor agonists, such as midazolam, despite the risks associated to its prolonged use [16]. Regarding adverse effects, it was demonstrated in a randomized controlled trial that continuous infusion of midazolam in patients undergoing mechanical ventilation had a longer length of stay in the intensive- care unit and a longer need for mechanical ventilation when compared to patients who had a daily interruption of midazolam infusions [3,14]. This fact was also observed in a prospective-observational cohort study [49], where patients with continuous sedation had 13.5 ± 33.7 days of hospitalization, compared to 4.8 ± 4.1 days of patients with an interruption of sedation.
In another prospective cohort study [44], the safety and efficacy of dexmedetomidine were compared to midazolam’s. An association between time of delirium and death of patients admitted to the intensive-care unit on mechanical ventilation was observed. Patients with one day of delirium had a risk of death of 1.70, while two days of delirium had a rate of 2.69 and three days or more of delirium had a rate of 3.37. In addition, ventilation time and hospital stay were relevant variables even after dose adjustment.
Anesthesia and sedation were the most popular clinical applications of midazolam [3]. Out of the 100 most-cited studies about the drug, more than a quarter of the articles (n = 37) had these as study objects, which indicates the importance of this field of investigation. Midazolam can be effectively used for mild and deep sedation. It was preferred over other benzodiazepines because of its short duration and fewer emergency complications [60]. In comparison to other drugs, such as propofol, midazolam showed worse performance in parameters evaluated on the moderate-sedation-of-routine-endoscopic procedures. A systematic review and meta-analysis retrieved in our bibliometric search evaluating the efficacy, safety, and efficiency of sedative agents revealed that midazolam had a longer sedation and recovery time than propofol. In addition, the data of the study showed that 34% of patients had at least some memories of the procedure [45]. Besides, another systematic review evidenced that dexmedetomidine provided more comfort to the patient and clinician during procedural sedation than midazolam, even though the safety profiles were similar [121].
Perhaps, midazolam use in the sedation of patients in the intense-care unit is the commonest use of the drug. Sedation in this case is employed to achieve comfort and analgesia at the invasive-mechanical ventilation; it also relieves anxiety and reduces stress [122]. Midazolam is widely used in this case, even though the Clinical Practice Guidelines for The Management of Pain, Agitation, and Delirium in Adult Patients in The Intensive Care Unit recommends sedation with non-benzodiazepines in association with analgesic agents [123]. However, it is important to note that patients admitted to the intensive-care unit have different reasons to be there. For example, patients undergoing elective surgeries who will need mechanical ventilation for a short period and critically ill patients who will stay intubated for longer periods. The protocols for each case must be considered individually.
Midazolam showed good results in short-period sedations [124]. However, studies correlated the continuous use of midazolam to depression of the respiratory system, oversedation, neurological impairment, and delayed extubation [125]. With this in mind, many studies proposed interruption protocols of sedative infusions in critical patients undergoing mechanical ventilation, attempting to verify if sedative drugs were associated with longer mechanical-ventilation needs [14,29].
Moreover, the literature reported other adverse side events of midazolam use. Those effects were mainly related to patients undergoing longer use of the drug, as the ones in the intensive-care unit. Three studies on our 100 most-cited list in the field associated midazolam with delirium development. Two of them evidenced potential risk of transition of patients with mechanical ventilation to delirium [37,44], while the other one showed that the incidence of patients who developed postoperative delirium experiences after undergoing cardiac-valve procedures was higher in patients who used midazolam in the sedative protocol than patients who used dexmedetomidine [35]. Unfortunately, none of them elucidated the possible mechanisms related with this apparent relationship.
North America directs important financial resources to research and are home of many major research centers for the diagnosis, treatment, and follow-up of clinical cases [10]. Our bibliometric analysis revealed that the USA is the country with most articles among the 100 most-cited studies in the field of Midazolam research. A great number of the studies investigates drug metabolism; this growing interest in USA in investigating midazolam may be due to the clarification of the mechanism of action and its adverse events with interaction associated with other substances.
Through this set of information extracted from these 100 studies, it is possible to see the main authors, countries, and keywords with the search strategy developed. However, it is important to emphasize that only the study ranking on the WOS database was considered, i.e., the positions of the studies may change in other databases. Another point is that the elaboration of the search strategy aims to retrieve a greater number of studies on the subject, but this recovery depends directly on the keywords used in the search strategy and that this process may present weaknesses, such as the non-retrieval of some studies.
5. Conclusions
The present study identified the 100 most-cited articles on midazolam, and highlighted the quantity and quality of research and the evolution of scientific knowledge about midazolam. The bibliometric results highlighted the knowledge presented by older articles, such as the pharmacokinetics, metabolism, and bioavailability of midazolam, while the most recent ones indicated the use of midazolam for the treatment of seizures in adults and children. The USA stood out with the largest number of articles and also as the headquarters of journals with the largest number of publications on the list. It indicated the large number of researchers from the USA working in the area of pharmacokinetics and clinical pharmacology involving this drug, which has different therapeutic actions, such as anesthetic, sedative, and anxiolytic, demonstrated mainly in the non-systematic review articles that are part of the list of the 100 most-cited articles.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare11010096/s1. Table S1: Titles of the excluded studies.
Author Contributions
Conceptualization, M.C.P.C. and R.R.L.; methodology, M.C.P.C., P.F.S.M., D.C.B.-d.-S., T.V.D., G.L.C.B. and J.M.P.; software and formal analysis M.C.P.C.; investigation, data curation and writing—original draft preparation, M.C.P.C., P.F.S.M., D.C.B.-d.-S., D.S.-M., M.K.M.F., T.V.D., G.L.C.B. and J.M.P.; writing—review and editing, M.C.P.C., D.C.B.-d.-S., M.C.P.C., P.F.S.M., D.C.B.-d.-S., D.S.-M., M.K.M.F., T.V.D., G.L.C.B. and J.M.P.; supervision, R.R.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil), Finance Code 001; and Research Pro-Rectory of the Federal University of Pará (PROPESP, UFPA, Brazil). R.R.L. is a researcher from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and received grant under number 312275/2021-8. The APC was funded by Pró-Reitoria de Pesquisa e Pós-graduação from Federal University of Pará (PROPESP-UFPA).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Nordt S.P., Clark R.F. Midazolam: A review of therapeutic uses and toxicity. J. Emerg. Med. 1997;15:357–365. doi: 10.1016/S0736-4679(97)00022-X. [DOI] [PubMed] [Google Scholar]
- 2.Garnock-Jones K.P. Oromucosal midazolam: A review of its use in pediatric patients with prolonged acute convulsive seizures. Pediatr. Drugs. 2012;14:251–261. doi: 10.2165/11209320-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Scott R.C., Besag F.M., Neville B.G. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: A randomised trial. Lancet. 1999;353:623–626. doi: 10.1016/S0140-6736(98)06425-3. [DOI] [PubMed] [Google Scholar]
- 4.Tomlin S. Medicines tailored for children—The introduction of buccal midazolam. Pharm. J. 2011;287:161–162. [Google Scholar]
- 5.Olkkola K.T., Ahonen J. Midazolam and other benzodiazepines. Handb. Exp. Pharmacol. 2008;182:335–360. doi: 10.1007/978-3-540-74806-9_16. [DOI] [PubMed] [Google Scholar]
- 6.Gorski J.C., Vannaprasaht S., Hamman M.A., Ambrosius W.T., Bruce M.A., Haehner-Daniels B., Hall S.D. The effect of age, sex, and rifampin administration on intestinal and hepatic cytochrome P450 3A activity. Clin. Pharmacol. Ther. 2003;74:275–287. doi: 10.1016/S0009-9236(03)00187-5. [DOI] [PubMed] [Google Scholar]
- 7.Dundee J.W., Halliday N.J., Harper K.W., Brogden R.N. Midazolam: A Review of its Pharmacological Properties and Therapeutic Use. Drugs. 1984;28:519–543. doi: 10.2165/00003495-198428060-00002. [DOI] [PubMed] [Google Scholar]
- 8.Armijo J.A., Herranz J.L., Pardo M.A.P., Adin J. Intranasal and buccal midazolam in the treatment of acute seizures. Rev. Neurol. 2004;38:458–468. [PubMed] [Google Scholar]
- 9.Zhao Z.Y., Wang H., Wen B., Yang Z., Feng K., Fan J. A Comparison of Midazolam, Lorazepam, and Diazepam for the Treatment of Status Epilepticus in Children: A Network Meta-analysis. J. Child. Neurol. 2016;31:1093–1107. doi: 10.1177/0883073816638757. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad P., Dummer P.M.H., Noorani T.Y., Asif J.A. The top 50 most-cited articles published in the International Endodon-tic Journal. Int. Endod. J. 2019;52:803–818. doi: 10.1111/iej.13083. [DOI] [PubMed] [Google Scholar]
- 11.Alderson P., Green S., Higgins J. Glossary of Terms in the Cochrane Collaboration. [(accessed on 11 June 2022)];Cochrane Handb. Syst. Rev. 2005 Available online: http://community-archive.cochrane.org/sites/default/files/uploads/glossary.pdf%0Ahttp://scholar.google.com/scholar?http://community.cochrane.org/sites/default/files/uploads/glossary.pdfhl=en&btnG=Search&q=intitle:Glossary+of+Terms+in+the+Cochrane+Collabor. [Google Scholar]
- 12.Van Eck N.J., Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84:523–538. doi: 10.1007/s11192-009-0146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glauser T., Shinnar S., Gloss D., Alldredge B., Arya R., Bainbridge J., Bare M., Bleck T., Dodson W.E., Garrity L., et al. Evidence-based guideline: Treatment of convulsive status epilepticus in children and adults: Report of the guideline com-mittee of the American epilepsy society. Epilepsy Curr. 2016;16:48–61. doi: 10.5698/1535-7597-16.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kress J.P., Pohlman A.S., O′Connor M.F., Hall J.B. Daily interruption of sedative infusions in critically ill patients under-going mechanical ventilation. N. Engl. J. Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 15.Murrough J.W., Iosifescu D.V., Chang L.C., Al Jurdi R.K., Green C.E., Perez A.M., Iqbal S., Pillemer S., Foulkes A., Shah A., et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: A two-site randomized controlled trial. Am. J. Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riker R.R., Shehabi Y., Bokesch P.M. Dexmedetomidine vs midazolam for sedation of critically Ill patients A randomized trial. J. Am. Med. Assoc. 2009;301:489–499. doi: 10.1001/jama.2009.56. [DOI] [PubMed] [Google Scholar]
- 17.Jevtovic-Todorovic V., Hartman R.E., Izumi Y., Benshoff N.D., Dikranian K., Zorumski C.F., Olney J.W., Wozniak D.F. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persis-tent learning deficits. J. Neurosci. 2003;23:876–882. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferguson N.D., Cook D.J., Guyatt G.H., Mehta S., Hand L., Austin P., Zhou Q., Matte A., Walter S.D., Lamontagne F., et al. High-frequency oscillation in early acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 19.Casali A.G., Gosseries O., Rosanova M., Boly M., Sarasso S., Casali K.R., Casarotto S., Bruno M.-A., Laureys S., Tononi G., et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci. Transl. Med. 2013;5:3006294. doi: 10.1126/scitranslmed.3006294. [DOI] [PubMed] [Google Scholar]
- 20.Jacobi J., Fraser G.L., Coursin D.B., Riker R.R., Fontaine D., Wittbrodt E.T., Chalfin D.B., Masica M.F., Bjerke H.S., Co-plin W.M., et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit. Care Med. 2002;30:119–141. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Jakob S.M., Ruokonen E., Grounds R.M., Sarapohja T., Garratt C., Pocock S.J., Bratty J.R., Takala J. Dexmedetomidine vs. midazolam or propofol for sedation during prolonged mechanical ventilation: Two randomized controlled trials. J. Am. Med. Assoc. 2012;307:1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 22.Strøm T., Martinussen T., Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: A randomised trial. Lancet. 2010;375:475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 23.Silbergleit R., Durkalski V., Lowenstein D., Conwit R., Pancioli A., Palesch Y., Barsan W. Intramuscular versus Intrave-nous Therapy for Prehospital Status Epilepticus. N. Engl. J. Med. 2012;366:591–600. doi: 10.1056/NEJMoa1107494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Needham D.M., Korupolu R., Zanni J.M., Pradhan P., Colantuoni E., Palmer J.B., Brower R.G., Fan E. Early Physical Medicine and Rehabilitation for Patients with Acute Respiratory Failure: A Quality Improvement Project. Arch. Phys. Med. Rehabil. 2010;91:536–542. doi: 10.1016/j.apmr.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 25.Lamba J.K., Lin Y.S., Schuetz E.G., Thummel K. Genetic contribution to variable human CYP3A-mediated metabolism. Adv. Drug. Deliv. Rev. 2002;54:1271–1294. doi: 10.1016/S0169-409X(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 26.Glass P.S., Bloom M., Kearse L., Rosow C., Sebel P., Manberg P. Bispectral analysis measures sedation and memory ef-fects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86:836–847. doi: 10.1097/00000542-199704000-00014. [DOI] [PubMed] [Google Scholar]
- 27.Klotz U. Pharmacokinetics and drug metabolism in the elderly. Drug Metab. Rev. 2000;41:67–76. doi: 10.1080/03602530902722679. [DOI] [PubMed] [Google Scholar]
- 28.Izzo A.A., Ernst E. Interactions between herbal medicines and prescribed drugs: An updated systematic review. Drugs. 2009;69:1777–1798. doi: 10.2165/11317010-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29.Mehta S., Burry L., Cook D., Fergusson D., Steinberg M., Granton J., Herridge M., Ferguson N., Devlin J., Tanios M., et al. Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: A randomized controlled trial. J. Am. Med. Assoc. 2012;308:1985–1992. doi: 10.1001/jama.2012.13872. [DOI] [PubMed] [Google Scholar]
- 30.Zhao P., Zhang L.A., Grillo J., Liu Q., Bullock J.M., Moon Y.J., Song P., Brar S.S., Madabushi R., Wu T.C., et al. Applica-tions of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin. Pharmacol. Ther. 2011;89:259–267. doi: 10.1038/clpt.2010.298. [DOI] [PubMed] [Google Scholar]
- 31.Dresser G.K. Pharmacokinetic-pharmacodynamic consequences and clinical relevance of cytochrome P450 3A4 inhibition. Clin. Pharmacokinet. 2000;38:41–57. doi: 10.2165/00003088-200038010-00003. [DOI] [PubMed] [Google Scholar]
- 32.Cherenik D.A., Gillings D., Laine H., Hendler J., Silver J.M., Davidson A.B., Schwam E.M., Siegel J.L. Validity and Reli-ability of the Observers. J. Clin. Psychopharmacol. 1990;10:244–251. doi: 10.1097/00004714-199008000-00003. [DOI] [PubMed] [Google Scholar]
- 33.Greicius M.D., Kiviniemi V., Tervonen O., Vainionpää V., Alahuhta S., Reiss A.L., Menon V. Persistent default-mode network connectivity during light sedation. Hum. Brain Mapp. 2008;29:839–847. doi: 10.1002/hbm.20537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thummel K.E., Wilkinson G.R. In vitro and in vivo drug interactions involving human CYP3A. Annu. Rev. Pharmacol. Toxicol. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- 35.Shorvon S., Ferlisi M. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and rec-ommendations for therapy. Brain. 2012;135:2314–2328. doi: 10.1093/brain/aws091. [DOI] [PubMed] [Google Scholar]
- 36.Verbeeck R.K. Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur. J. Clin. Pharmacol. 2008;64:1147–1161. doi: 10.1007/s00228-008-0553-z. [DOI] [PubMed] [Google Scholar]
- 37.Pandharipande P., Cotton B.A., Shintani A., Thompson J., Pun B.T., Morris J.A., Dittus R., Ely E.W. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J. Trauma Inj. Infect. Crit. Care. 2008;65:34–41. doi: 10.1097/TA.0b013e31814b2c4d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrarelli F., Massimini M., Sarasso S., Casali A., Riedner B.A., Angelini G., Tononi G., Pearce R.A. Breakdown in corti-cal effective connectivity during midazolam-induced loss of consciousness. Proc. Natl. Acad. Sci. USA. 2010;107:2681–2686. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Z., Yang X., Ho P.C.L., Chan S.Y., Heng P.W.S., Chan E., Duan W., Koh H.L., Zhou S. Herb-drug interactions: A literature review. Drugs. 2005;65:1239–1282. doi: 10.2165/00003495-200565090-00005. [DOI] [PubMed] [Google Scholar]
- 40.Bowles C.J.A., Leicester R., Romaya C., Swarbrick E., Williams C.B., Epstein O. A prospective study of colonoscopy prac-tice in the UK today: Are we adequately prepared for national colorectal cancer screening tomorrow? Gut. 2004;53:277–283. doi: 10.1136/gut.2003.016436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X.Q., Andersson T.B., Ahlström M., Weidolf L. Comparison of inhibitory effects of the proton pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole, pantoprazole, and rabeprazole on human cytochrome P450 activities. Drug Metab. Dispos. 2004;32:821–827. doi: 10.1124/dmd.32.8.821. [DOI] [PubMed] [Google Scholar]
- 42.Paine M.F., Khalighi M., Fisher J.M., Shen D.D., Kunze K.L., Marsh C., Perkins J.D.E., Thummel K. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J. Pharmacol. Exp. Ther. 1997;283:1552–1562. [PubMed] [Google Scholar]
- 43.Niemi M., Backman J.T., Fromm M.F., Neuvonen P.J., Kivistö K.T. Pharmacokinetic interactions with rifampicin: Clinical relevance. Clin. Pharmacokinet. 2003;42:819–850. doi: 10.2165/00003088-200342090-00003. [DOI] [PubMed] [Google Scholar]
- 44.Shehabi Y. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit. Care Med. 2010;38:2311–2318. doi: 10.1097/CCM.0b013e3181f85759. [DOI] [PubMed] [Google Scholar]
- 45.McQuaid K.R. A systematic review and meta-analysis of randomized, controlled trials of moderate sedation for routine endoscopic procedures. Gastrointest. Endosc. 2008;67:910–923. doi: 10.1016/j.gie.2007.12.046. [DOI] [PubMed] [Google Scholar]
- 46.Stevens R.D., Dowdy D.W., Michaels R.K., Mendez-Tellez P.A., Pronovost P.J., Needham D.M. Neuromuscular dysfunc-tion acquired in critical illness: A systematic review. Intensive Care Med. 2007;33:1876–1891. doi: 10.1007/s00134-007-0772-2. [DOI] [PubMed] [Google Scholar]
- 47.Payen F., Chanques G., Mantz J., Hercule C., Auriant I., Leguillou J.-L., Binhas M., Genty C., Rolland C., Bosson J.-L., et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: A prospective multicenter patient-based study. Anesthesiology. 2007;106:687–695. doi: 10.1097/01.anes.0000264747.09017.da. [DOI] [PubMed] [Google Scholar]
- 48.Meierkord H., Boon P., Engelsen B., Göcke K., Shorvon S., Tinuper P., Holtkamp M. EFNS guideline on the management of status epilepticus in adults. Eur. J. Neurol. 2010;17:348–355. doi: 10.1111/j.1468-1331.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 49.Kollef M.H., Levy N.T., Ahrens T.S., Schaiff R., Prentice D., Sherman G. The use of continuous IV sedation is associated with prolongation of mechanical ventilation. Chest. 1998;114:541–548. doi: 10.1378/chest.114.2.541. [DOI] [PubMed] [Google Scholar]
- 50.Cruz A.P.M., Frei F., Graeff F. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol. Biochem. Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 51.Johnson T.N., Rostami-Hodjegan A., Tucker G.T. Prediction of the clearance of eleven drugs and associated variability in neonates, infants and children. Clin. Pharmacokinet. 2006;45:931–956. doi: 10.2165/00003088-200645090-00005. [DOI] [PubMed] [Google Scholar]
- 52.Bailey D.G., Malcolm J., Arnold O., Spence J.D. Grapefruit juice-drug interactions. Br. J. Clin. Pharmacol. 1998;46:101–110. doi: 10.1046/j.1365-2125.1998.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riss J., Cloyd J., Gates J., Collins S. Benzodiazepines in epilepsy: Pharmacology and pharmacokinetics. Acta Neurol. Scand. 2008;118:69–86. doi: 10.1111/j.1600-0404.2008.01004.x. [DOI] [PubMed] [Google Scholar]
- 54.Walsky R.L., Obach R.S. Validated assays for human cytochrome P450 activities. Drug Metab. Dispos. 2004;32:647–660. doi: 10.1124/dmd.32.6.647. [DOI] [PubMed] [Google Scholar]
- 55.Zhou S., Chan S.Y., Goh B.C., Chan E., Duan W., Huang M., McLeod H.L. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin. Pharmacokinet. 2005;44:279–304. doi: 10.2165/00003088-200544030-00005. [DOI] [PubMed] [Google Scholar]
- 56.Lichtenstein D.R., Jagannath S., Baron T.H., Anderson M.A., Banerjee S., Dominitz J.A., Fanelli R.D., Gan S.I., Harrison M.E., Ikenberry S.O., et al. Sedation and anesthesia in GI endoscopy. Gastrointest. Endosc. 2008;68:815–826. doi: 10.1016/j.gie.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 57.Maldonado J.R., Wysong A., van der Starre P.J., Block T., Miller C., Reitz B.A. Dexmedetomidine and the reduction of postoperative delirium after cardiac surgery. Psychosomatics. 2009;50:206–217. doi: 10.1176/appi.psy.50.3.206. [DOI] [PubMed] [Google Scholar]
- 58.Willoughby R.E., Tieves K.S., Hoffman G.M., Ghanayem N.S., Amlie-Lefond C.M., Schwabe M.J., Chusid M.J., Rupprecht C.E. Survival after Treatment of Rabies with Induction of Coma. N. Engl. J. Med. 2005;352:2508–2514. doi: 10.1056/NEJMoa050382. [DOI] [PubMed] [Google Scholar]
- 59.Lowenstein D.H., Alldredge B.K. Status epilepticus. N. Engl. J. Med. 1998;338:970–976. doi: 10.1056/NEJM199804023381407. [DOI] [PubMed] [Google Scholar]
- 60.Krauss B., Green S.M. Procedural sedation and analgesia in children. Lancet. 2006;367:766–780. doi: 10.1016/S0140-6736(06)68230-5. [DOI] [PubMed] [Google Scholar]
- 61.Yon J.H., Daniel-Johnson J., Carter L., Jevtovic-Todorovic V. Anesthesia induces neuronal cell death in the developing rat brain via the intrinsic and extrinsic apoptotic pathways. Neuroscience. 2005;135:815–827. doi: 10.1016/j.neuroscience.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 62.De Graeff A., Dean M. Palliative sedation therapy in the last weeks of life: A literature review and recommendations for standards. J. Palliat. Med. 2006;10:67–85. doi: 10.1089/jpm.2006.0139. [DOI] [PubMed] [Google Scholar]
- 63.Claassen J. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: A systematic review. Epilepsia. 2002;43:146–153. doi: 10.1046/j.1528-1157.2002.28501.x. [DOI] [PubMed] [Google Scholar]
- 64.Venkatakrishnan K. Effects of the antifungal agents on oxidative drug metabolism. Clinical relevance. Clin. Pharmacokinet. 2000;38:111–180. doi: 10.2165/00003088-200038020-00002. [DOI] [PubMed] [Google Scholar]
- 65.Young C., Jevtovic-Todorovic V., Qin Y.-Q., Tenkova T., Wang H., Labruyere J., Olney J.W. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Br. J. Pharmacol. 2005;146:189–197. doi: 10.1038/sj.bjp.0706301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thummel K.E., O′Shea D., Paine M.F., Shen D.D., Kunze K.L., Perkins J.D., Wilkinson G.R. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin. Pharmacol. Ther. 1996;59:491–502. doi: 10.1016/S0009-9236(96)90177-0. [DOI] [PubMed] [Google Scholar]
- 67.Reves J.G., Fragen R.J., Vinik H.R., Greenblatt D.J. Midazolam: Pharmacology and uses. Anesthesiology. 1985;62:310–324. doi: 10.1097/00000542-198503000-00017. [DOI] [PubMed] [Google Scholar]
- 68.Kauffman R.E., Banner W., Berlin C.M., Blumer J.L., Gorman R.L., Lambert G.H., Wilson G.S., Bennett D.R., Cordero J.F., Cote C.J., et al. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Pediatrics. 1992:1110–1115. [Google Scholar]
- 69.Lin Y.S., Dowling A.L.S., Quigley S.D., Farin F.M., Zhang J., Lamba J., Schuetz E.G., Thummel K.E. Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Mol. Pharmacol. 2002;62:162–172. doi: 10.1124/mol.62.1.162. [DOI] [PubMed] [Google Scholar]
- 70.Kim R.B., Wandel C., Leake B., Cvetkovic M., Fromm M.F., Dempsey P.J., Roden M.M., Belas F., Chaudhary A.K., Ro-den D.M., et al. Interrelationship between substrates and inhibitors of human CYP3A and P-glycoprotein. Pharm. Res. 1999;16:408–414. doi: 10.1023/a:1018877803319. [DOI] [PubMed] [Google Scholar]
- 71.Putensen C., Zech S., Wrigge H., Zinserling J., Stüber F., VON Spiegel T., Mutz N. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am. J. Respir. Crit. Care Med. 2001;164:43–49. doi: 10.1164/ajrccm.164.1.2001078. [DOI] [PubMed] [Google Scholar]
- 72.Kronbach T., Mathys D., Umeno M., Gonzalez F.J.A., Meyer U. Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol. Pharmacol. 1989;36:89–96. [PubMed] [Google Scholar]
- 73.Schuetz E.G. Modulators and substrates of P-glycoprotein and cytochrome P4503A coordinately up-regulate these proteins in human colon carcinoma cells. Mol. Pharmacol. 1996;49:311–318. [PubMed] [Google Scholar]
- 74.Streetman D.S., Bertino J.S., Nafziger A.N. Phenotyping of drug-metabolizing enzymes in adults: A review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10:187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 75.McIntyre J., Robertson S., Norris E., Appleton R., Whitehouse W.P., Phillips B., Martland T., Berry K., Collier J., Smith S., et al. Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: A randomised controlled trial. Lancet. 2005;366:205–210. doi: 10.1016/S0140-6736(05)66909-7. [DOI] [PubMed] [Google Scholar]
- 76.Dielenberg R.A., McGregor I.S. Defensive behavior in rats towards predatory odors: A review. Neurosci. Biobehav. Rev. 2001;25:597–609. doi: 10.1016/S0149-7634(01)00044-6. [DOI] [PubMed] [Google Scholar]
- 77.Paine M.F., Shen D.D., Kunze K.L., Perkins J.D., Marsh C., McVicar J.P., Barr D.M., Gillies B.S., Thummel K.E. First-pass metabolism of midazolam by the human intestine. Clin. Pharmacol. Ther. 1996;60:14–24. doi: 10.1016/S0009-9236(96)90162-9. [DOI] [PubMed] [Google Scholar]
- 78.Özdemir V., Kalow W., Tang B.K., Paterson A.D., Walker S.E., Endrenyi L., Kashuba A.D. Evaluation of the genetic component of variability in CYP3A4 activity: A repeated drug administration method. Pharmacogenetics. 2000;10:373–388. doi: 10.1097/00008571-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 79.Gorski J.C., Jones D.R., Haehner-Daniels B.D., Hamman M.A., O’Mara E.M., Jr., Hall S.D. The contribution of intestinal and hepatic CYP3A to the interaction between midazolam and clarithromycin. Clin. Pharmacol. Ther. 1998;64:133–143. doi: 10.1016/S0009-9236(98)90146-1. [DOI] [PubMed] [Google Scholar]
- 80.Andrew Williams J., Ring B.J., Cantrell V.E., Jones D.R., Eckstein J., Ruterbories K., Hamman M.A., Hall S.D., Wrighton S.A. Comparative metabolic capabilities of CYP3A4, CYP3A5, and CYP3A7. Drug Metab. Dispos. 2002;30:883–891. doi: 10.1124/dmd.30.8.883. [DOI] [PubMed] [Google Scholar]
- 81.Venn R.M., Bradshaw C.J., Spencer R., Brealey D., Caudwell E., Naughton C., Vedio A., Singer M., Feneck R., Treacher D., et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 2011;54:1136–1142. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 82.Bai D., Zhu G., Pennefather P., Jackson M.F., MacDonald J.F., Orser B.A. Distinct functional and pharmacological proper-ties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol. Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- 83.Yuan R., Madani S., Wei X.-X., Reynolds K., Huang S.-M. Evaluation of cytochrome p450 probe substrates commonly used by the pharmaceutical industry to study in vitro drug interactions. Drug Metab. Dispos. 2002;30:1311–1319. doi: 10.1124/dmd.30.12.1311. [DOI] [PubMed] [Google Scholar]
- 84.Lang E.V., Benotsch E.G., Fick L.J., Lutgendorf S., Berbaum M.L., Berbaum K., Logan H., Spiegel D. Adjunctive non-pharmacological analgesia for invasive medical procedures: A randomised trial. Lancet. 1999;355:1486–1490. doi: 10.1016/S0140-6736(00)02162-0. [DOI] [PubMed] [Google Scholar]
- 85.Gurley B.J., Gardner S.F., Hubbard M.A., Williams D.K., Gentry W.B., Cui Y., Ang C.Y.W. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin. Pharmacol. Ther. 2002;72:276–287. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 86.Schmiedlin-Ren P., Edwards D.J., Fitzsimmons M.E., He K., Lown K.S., Woster P.M., Rahman A., Thummel K.E., Fisher J.M., Hollenberg P.F., et al. Mechanisms of enhanced oral availability of CYP3A4 substrates by grapefruit constituents - Decreased enterocyte CYP3A4 concentration and mechanism-based inactivation by furanocoumarins. Mol. Pharmacol. 1997;51:741–754. doi: 10.1124/mol.51.5.741. [DOI] [PubMed] [Google Scholar]
- 87.Olkkola K.T., Backman J.T., Neuvonen P.J. Midazolam should be avoided in patients receiving the systemic antimycotics ketoconazole or itraconazole. Clin. Pharmacol. Ther. 1994;55:481–485. doi: 10.1038/clpt.1994.60. [DOI] [PubMed] [Google Scholar]
- 88.Pelkonen O., Mäeenpäeä J., Taavitsainen P., Rautio A., Raunio H. Inhibition and induction of human cytochrome P450 (CYP) enzymes. Xenobiotica. 1998;28:1203–1253. doi: 10.1080/004982598238886. [DOI] [PubMed] [Google Scholar]
- 89.Kenworthy K.E. CYP3A4 drug interactions: Correlation of 10 in vitro probe substrates. Br. J. Clin. Pharmacol. 1999;48:716–727. doi: 10.1046/j.1365-2125.1999.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang Z., Gorski J.C., Hamman M.A., Huang S., Lesko L.J., Hall S.D. The effects of St John’s wort (Hypericum perforatum) on human cytochrome P450 activity. Clin. Pharmacol. Ther. 2001;70:317–326. doi: 10.1016/S0009-9236(01)17221-8. [DOI] [PubMed] [Google Scholar]
- 91.Krauss B., Green S.M. Sedation and Analgesia for Procedures in Children. N. Engl. J. Med. 2000;342:938–945. doi: 10.1056/NEJM200003303421306. [DOI] [PubMed] [Google Scholar]
- 92.Christopher Gorski J., Hall S.D., Jones D.R., VandenBranden M. Regioselective biotransformation of midazolam by members of the human cytochrome P450 3A (CYP3A) subfamily. Biochem. Pharmacol. 1994;47:1643–1653. doi: 10.1016/0006-2952(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 93.Arrowsmith J.B., Gerstman B.B., Fleischer D.E., Benjamin S.B. Results from the American Society for Gastrointestinal En-doscopy/U.S. Food and Drug Administration collaborative study on complication rates and drug use during gastrointestinal endoscopy. Gastrointest. Endosc. 1991;37:421–427. doi: 10.1016/S0016-5107(91)70773-6. [DOI] [PubMed] [Google Scholar]
- 94.Parke T.J., Stevens J.E., Rice A.S., Greenaway C.L., Bray R.J., Smith P.J., Waldmann C.S., Verghese C. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: Five case reports. Br. Med. J. 1992;305:613–616. doi: 10.1136/bmj.305.6854.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ostermann M.E., Keenan S.P., Seiferling R.A., Sibbald W.J. Sedation in the intensive care unit: A systematic review. J. Am. Med. Assoc. 2000;283:1451–1459. doi: 10.1001/jama.283.11.1451. [DOI] [PubMed] [Google Scholar]
- 96.Lown K.S., Kolars J.C.E., Thummel K., Barnett J.L., Kunze K.L.A., Wrighton S., Watkins P.B. Interpatient heterogeneity in expression of CYP3A4 and CYP3A5 in small bowel: Lack of prediction by the erythromycin breath test. Drug Metab. Dispos. 1994;22:947–955. [PubMed] [Google Scholar]
- 97.Kupferschmidt H.R., Ha H.R., Ziegler W.H., Meier P.J., Krähenbühl S. Interaction between grapefruit juice and midazo-lam in humans. Clin. Pharmacol. Ther. 1995;58:20–28. doi: 10.1016/0009-9236(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 98.Bailey P.L., Pace N.L., Ashburn M.A., Moll J.W.B., East K.A., Stanley T.H. Frequent hypoxemia and apnea after sedation with midazolam and fentanyl. Anesthesiology. 1990;73:826–830. doi: 10.1097/00000542-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 99.Greenblatt D.J., Abernethy D.R., Locniskar A., Harmatz J.S., Limjuco R.A., Shader R.I. Effect of age, gender, and obesity on midazolam kinetics. Anesthesiology. 1984;61:27–35. doi: 10.1097/00000542-198461010-00006. [DOI] [PubMed] [Google Scholar]
- 100.Quine M.A. Prospective audit of upper gastrointestinal endoscopy in two regions of England: Safety, staffing, and sedation methods. Gut. 1995;36:462–467. doi: 10.1136/gut.36.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schmiedlin-Ren P., Thummel K.E., Fisher J.M., Paine M.F., Lown K.S., Watkins P.B. Expression of enzymatically active CYP3A4 by Caco-2 cells grown on extracellular matrix-coated permeable supports in the presence of 1 al-pha,25-dihydroxyvitamin D-3. Mol. Pharmacol. 1997;51:741–754. doi: 10.1124/mol.51.5.741. [DOI] [PubMed] [Google Scholar]
- 102.Thummel K.E., Shen D.D., Podoll T.D., Kunze K.L., Trager W.F., Hartwell P.S.A., Raisys V., Marsh C., McVicar J.P., Barr D.M. Use of midazolam as a human cytochrome P450 3A probe: I. In vitro-in vivo correlations in liver transplant patients. [(accessed on 11 June 2022)];J. Pharmacol. Exp. Ther. 1994 271:549–556. Available online: https://pubmed.ncbi.nlm.nih.gov/7965755/ [PubMed] [Google Scholar]
- 103.Cheng D.C.H., Karski J., Peniston C., Asokumar B., Raveendran G., Carroll J., Nierenberg H., Roger S., Mickle D., Tong J., et al. Morbidity outcome in early versus conventional tracheal extubation after coronary artery bypass grafting: A pro-spective randomized controlled trial. J. Thorac. Cardiovasc. Surg. 1996;112:755–764. doi: 10.1016/S0022-5223(96)70062-4. [DOI] [PubMed] [Google Scholar]
- 104.Hunt C.M., Westerkam W.R., Stave G.M. Effect of age and gender on the activity of human hepatic CYP3A. Biochem. Pharmacol. 1992;44:275–283. doi: 10.1016/0006-2952(92)90010-G. [DOI] [PubMed] [Google Scholar]
- 105.Ashton H. Guidelines for the Rational Use of Benzodiazepines: When and What to Use. Drugs. 1994;48:25–40. doi: 10.2165/00003495-199448010-00004. [DOI] [PubMed] [Google Scholar]
- 106.Heinrichs S.C., Pich E.M., Miczek K.A., Britton K.T., Koob G.F. Corticotropin-releasing factor antagonist reduces emo-tionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;581:190–197. doi: 10.1016/0006-8993(92)90708-H. [DOI] [PubMed] [Google Scholar]
- 107.Olkkola K.T., Aranko K., Luurila H., Hiller A., Saarnivaara L., Himberg J.-J., Neuvonen P.J. A potentially hazardous interaction between erythromycin and midazolam. Clin. Pharmacol. Ther. 1993;53:298–305. doi: 10.1038/clpt.1993.25. [DOI] [PubMed] [Google Scholar]
- 108.Magorian T., Flannery K.B., Miller R.D. Comparison of rocuronium, succinylcholine, and vecuronium for rapid- sequence induction of anesthesia in adult patients. Anesthesiology. 1993;79:913–918. doi: 10.1097/00000542-199311000-00007. [DOI] [PubMed] [Google Scholar]
- 109.Mangano D.T., Siliciano D., Hollenberg M., Leung J.M., Browner W.S., Goehner P., Merrick S., Verrier E. Postoperative myocardial ischemia: Therapeutic trials using intensive analgesia following surgery. Anesthesiology. 1992;76:342–353. doi: 10.1097/00000542-199203000-00004. [DOI] [PubMed] [Google Scholar]
- 110.Allonen H., Ziegler G., Klotz U. Midazolam kinetics. Clin. Pharmacol. Ther. 1981;30:653–661. doi: 10.1038/clpt.1981.217. [DOI] [PubMed] [Google Scholar]
- 111.Moura L.K.B., De Mesquita R.F., Mobin M., Matos F.T.C., Monte T.L., Lago E.C., Falcão C.A.M., Ferraz M.D.A.L., Santos T.C., Sousa L.R.M. Uses of bibliometric techniques in public health research. [(accessed on 11 June 2022)];Iran. J. Public Health. 2017 46:1435–1436. Available online: https://pubmed.ncbi.nlm.nih.gov/29308389/ [PMC free article] [PubMed] [Google Scholar]
- 112.Ullah R., Ullah R. Top-cited Articles in Regenerative Endodontics: A Bibliometric Analysis. J. Endod. 2018;44:1650–1664. doi: 10.1016/j.joen.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 113.Goho C. Oral midazolam-grapefruit juice drug interaction. Pediatr. Dent. 2001;23:365–366. [PubMed] [Google Scholar]
- 114.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Moher D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021;134:103–112. doi: 10.1016/j.jclinepi.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 115.Hayashi K., Osawa M., Aihara M., Izumi T., Ohtsuka Y., Haginoya K., Kato I., Kaneko K., Sugai K., Takahashi T., et al. Efficacy of Intravenous Midazolam for Status Epilepticus in Childhood. Pediatr. Neurol. 2007;36:366–372. doi: 10.1016/j.pediatrneurol.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 116.Clementino L.C., Souza K.S.C., Castelo-Branco M., Perazzo M.F., Ramos-Jorge M.L., Mattos F.F., Paiva S.M., Mar-tins-Júnior P.A. Top 100 most-cited oral health-related quality of life papers: Bibliometric analysis. Community Dent. Oral Epidemiol. 2022;50:199–205. doi: 10.1111/cdoe.12652. [DOI] [PubMed] [Google Scholar]
- 117.Lahat E., Goldman M., Barr J., Bistritzer T., Berkovitch M. Comparison of intranasal midazolam with intravenous diaze-pam for treating febrile seizures in children: Prospective randomised study. Br. Med. J. 2000;321:83–86. doi: 10.1136/bmj.321.7253.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bakkalbasi N., Bauer K., Glover J., Wang L. Three options for citation tracking: Google Scholar, Scopus and Web of Science. Biomed. Digit. Libr. 2006;3:7. doi: 10.1186/1742-5581-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Falagas M., Pitsouni E.I., Malietzis G., Pappas G. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: Strengths and weaknesses. FASEB J. 2008;22:338–342. doi: 10.1096/fj.07-9492LSF. [DOI] [PubMed] [Google Scholar]
- 120.Keighobadi M., Nakhaei M., Sharifpour A., Khasseh A.A., Safanavaei S., Tabaripour R., Fakhar M. A Bibliometric Analysis of Global Research on Lophomonas Sin Scopus (1933–2019) Infect. Disord. Drug Targets. 2020;21:230–237. doi: 10.2174/1871526520666200727153142. [DOI] [PubMed] [Google Scholar]
- 121.Alryalat S.A.S., Malkawi L.W., Momani S.M. Comparing bibliometric analysis using pubmed, scopus, and web of science databases. J. Vis. Exp. 2019;2019:58494. doi: 10.3791/58494. [DOI] [PubMed] [Google Scholar]
- 122.Barends C.R.M. Dexmedetomidine versus midazolam in procedural sedation. A systematic review of efficacy and safety. PLoS ONE. 2017;12:e0169525. doi: 10.1371/journal.pone.0169525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Garcia R., Salluh J.I., Andrade T.R., Farah D., da Silva P.S., Bastos D.F., Fonseca M.C. A systematic review and me-ta-analysis of propofol versus midazolam sedation in adult intensive care (ICU) patients. J. Crit. Care. 2021;64:91–99. doi: 10.1016/j.jcrc.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 124.Barr J., Fraser G., Puntillo K., Ely E.W., Gélinas C., Dasta J.F., Davidson J.E., Devlin J.W., Kress J.P., Joffe A.M., et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit. Care Med. 2013;41:263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 125.Swart E.L., Plotz F.B. Growing up with Midazolam in the Neonatal and Pediatric Intensive Care. Curr. Drug Metab. 2012;13:760–766. doi: 10.2174/138920012800840347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available within the article.