Abstract
Progressive granuloma formation is a hallmark of chronic mycobacterial infection. Granulomas are localized, protective inflammatory reactions initiated by CD4+ T cells, which contribute to control of bacterial growth and blockade of bacterial dissemination. In order to understand the costimulatory requirements that allow CD4+ T cells to directly or indirectly induce granulomas, we studied granuloma formation after 6 weeks in Mycobacterium bovis BCG-infected CD28- and CD40 ligand (CD40L)-deficient mice and compared it to granuloma formation in infected wild-type inbred mice and infected cytokine-deficient mice. We characterized granulomas morphologically in liver sections, analyzed granuloma infiltrating cells by flow cytometry, and measured cytokine production by cultured granuloma cells. CD28-deficient mice have no defect at the local inflammatory site, inasmuch as they form protective granulomas and control bacterial growth. However, there are fewer activated T cells in the spleen compared to infected wild-type animals, and quantitative differences in the cellular composition of the granuloma are observed by flow cytometry. In CD40L-deficient mice, the granuloma phenotype is very similar to the phenotype in gamma interferon (IFN-γ)-deficient mice. Both IFN-γ-deficient and CD40L-deficient mice form granulomas which prevent bacterial dissemination, but control of bacterial growth is significantly impaired. The relative proportion of CD4+ T cells in granulomas from both CD28−/− and CD40L−/− mice is significantly decreased compared with wild-type animals. Both models demonstrate that the phenotype and activation stage of systemic T cells do not always correlate with the phenotype and activation stage of the localized granulomatous response.
Granuloma formation around infected macrophage is a defining cellular response to mycobacterium infections. Layers of extracellular matrix enclose a microenvironment of infected cells and an intense inflammatory infiltrate. Granulomas eliminate bacteria and also protect surrounding host tissue from destructive inflammatory responses. Without granuloma formation, mycobacterial infections can become widely disseminated and frequently lethal, as occurs in human AIDS-associated tuberculosis or in the infection of SCID or recombinase-activating gene-deficient mice (22, 27, 33, 34). The involvement of T lymphocytes in initiation, regulation, and resolution of granuloma formation has been well documented for both human and murine infections (18, 22).
Murine models of Mycobacterium tuberculosis and Mycobacterium bovis infection have been used to study the role of cytokine regulatory networks in T lymphocyte-macrophage interactions (11). Mycobacterial infections induce a Th1-type T-cell response in which gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) play crucial roles in granuloma formation and function (6, 9, 21, 23, 30, 39). In contrast to the extensive characterization of cytokine requirements for protective granuloma formation, the study of essential T-cell costimulatory signaling is relatively new. The role of CD28 has not been studied, and the role of CD40-CD40 ligand (CD40L) interactions in protection against mycobacterial infection is controversial. One recent study reported that the CD40L-mediated pathway of T-cell activation is dispensable for resistance to M. tuberculosis infection (4). This was in contrast to the enhanced susceptibility to infection observed clinically in patients with a defective CD40L gene (2, 45) and to other experimental infections with intracellular pathogens, including Leishmania (3, 20, 43).
In this study, we examined the role of CD28 and the role of CD40-CD40L for M. bovis bacille Calmette-Guérin (BCG)-induced protective granuloma formation. We took advantage of the availability of both CD28 and CD40L gene knockout mice to compare their roles in generating the cytokine milieu required for effective granulomatous immune responses. Additionally, we focused on characterizing the effector T-lymphocyte activation phenotype and function in the granuloma in the absence of specific costimulatory molecules and correlated that with control of bacterial dissemination and formation of effective granulomas as assessed by quantitative histopathology. Here we report that there is a striking change in the granuloma phenotype of CD40L knockout mice after intraperitoneal infection with BCG. Our data suggest that CD40L costimulatory function, but not CD28 function, is essential for the generation of IFN-γ levels adequate for the control of mycobacterial replication at the local inflammatory site. This is consistent with a model in which CD40L-mediated interactions are essential for the induction of a strong Th1 immune response to intracellular pathogens. We show that both CD40L-CD40 and CD28-B7 interactions influence the cellular composition of granulomas and demonstrate that the systemic T-cell response does not closely correlate with the T-cell response detected in the local inflammatory lesion.
MATERIALS AND METHODS
Animals.
In these studies we used C57BL/6, IFN-γ gene-deficient, TNF-α gene deficient, CD28 gene-deficient, and CD40L gene-deficient strains of mice purchased from Jackson Labs. Animals were housed at animal facilities at both the University of Wisconsin Medical School and the William S. Middleton Memorial Veterans Administration Hospital. Both facilities are accredited and meet Public Health Service policy.
M. bovis BCG infections.
BCG (substrain Pasteur, from G. Fennelly) was grown in Middlebrook 7H9 (Difco Laboratories, Detroit, Mich.) with 0.05% Tween 80 and 10% oleic acid-dextrose-catalase (OADC) (Difco) supplement and stored in frozen aliquots at −70°C. For infections, ampoules were thawed, and the inoculum was diluted in saline plus 0.05% Tween 80 and briefly exposed to sonic oscillation in order to obtain a single-cell suspension. Mice were infected intraperitoneally with 107 BOG in 100 μl (12) in order to maximize the production of liver granulomas. The dose injected is not lethal and induces a disease that is cleared with time. Infection was verified by histology of liver tissue samples. We chose liver granulomas for analysis because they can be isolated in larger numbers free of surrounding tissue than lung granulomas.
Histology.
Small pieces of liver were fixed in 10% formalin prior to being imbedded in paraffin for thin sectioning (8 to 10 μm). Hematoxylin and eosin staining and Ziehl-Neelsen staining for acid-fast bacteria were done by the Department of Pathology's Histopathology Service, University of Wisconsin. Quantitative studies were performed by direct microscopic examination using a Olympus reticular eyepiece containing a 10 by 10 grid. Liver granuloma burden is the number of granulomas per grid in a field under 100× total magnification. Granuloma size is the number of grid squares covered at 400× total magnification. Bacteria per lesion is the number of acid-fast rods visible per granuloma at 1,000× total magnification using an oil immersion lens. Data are presented as the mean ± standard error of the mean for a minimum of 30 counts per mouse liver section. The number of individual mice is indicated in the figure legends.
Isolation of splenocytes and granuloma-infiltrating cells.
Isolation of granulomas is described in references 29, 36, and 38. Spleens were removed aseptically from 8- to 16-week-old mice, and viable cells were separated by centrifugation through Lympholyte M solution (Cedarlane Laboratories, Hornsby, Ontario, Canada) as described (35).
Flow cytometry and antibodies.
Splenocytes or granuloma cell suspensions were incubated for 30 min at 4°C with different labeled antibodies at saturation and then washed and analyzed. Unlabeled anti-Fc receptor antibody 2.4G2 (50 μg/ml) was used to block nonspecific binding of Fc receptors. Cell surface staining on 10,000 events was measured by fluorescence-activated cell sorting (FACS) on a FACS Calibur (Becton Dickinson) and analyzed using the Cellquest computer program (Power Macintosh version 3.0; Becton Dickinson).
Monoclonal antibodies used were purified from hybridoma cell lines obtained from the American Type Culture Collection (Rockville, Md.) as indicated. Hybridoma cells were cultured in HB-101 serum-free medium, and the antibodies were precipitated from supernatants by 45% saturated ammonium sulfate. The purified antibodies were labeled with biotin, fluorescein isothiocyanate, or Cy5 (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.). Antibodies used included those specific for murine CD4 (GK1.5), CD8 (53-6.7), MAC-1 (MI/70.15), CD44 (Pgp-1) (IM 7.8.1), L-selectin (CD62L) (Mel-14), and NK cells (DX5). Monoclonal antibodies against murine T-cell receptor α/β (α/β TCR) and CD4 were from Sigma Chemical Co. (St. Louis, Mo.) and against murine major histocompatibility complex (MHC) class II (αI-Ab) and NK cells were from Pharmingen (San Diego, Calif.).
Cytokine measurements.
Samples for cytokine analysis were collected from 106 liver granuloma cells or spleen cells (live by trypan blue exclusion) seeded into 96-well plates in 0.2 ml of complete medium and stimulated with 10 μg of α-CD3 antibody per ml. After 72 h, cell culture supernatants were harvested and stored at −70°C until testing. Measurement of secreted IFN-γ was made by enzyme-linked immunosorbent assay (ELISA) using standard methods. Briefly, plates were coated with anti-IFN-γ capture antibody (Pharmingen). Serial twofold dilutions of either murine recombinant IFN-γ (rIFN-γ) (Genzyme, Cambridge, Mass.) or test supernatants were added to triplicate wells. Bound cytokine was detected with biotinylated anti-mouse IFN-γ (Pharmingen) followed by avidin phosphatase (Molecular Probes, Eugene, Oreg.). Wells were developed with 50 μM 4-methylumbelliferyl phosphate (Molecular Probes), and fluorescence intensity was measured with an HTS7000 Bioassay reader (Perkin Elmer, Foster City, Calif.). Units of IFN-γ were calculated with reference to the rIFNγ standard. All data are expressed as the amount of secreted cytokine per 106 cells.
Organ load.
Bacterial colony formation was determined by plating serial dilutions of liver homogenates on Middlebrook 7H10 agar plates (Difco) supplemented with 10% OADC and cycloheximide (10 μg/ml). Colonies were counted after 3 weeks of incubation at 37°C.
RESULTS
Liver histopathology.
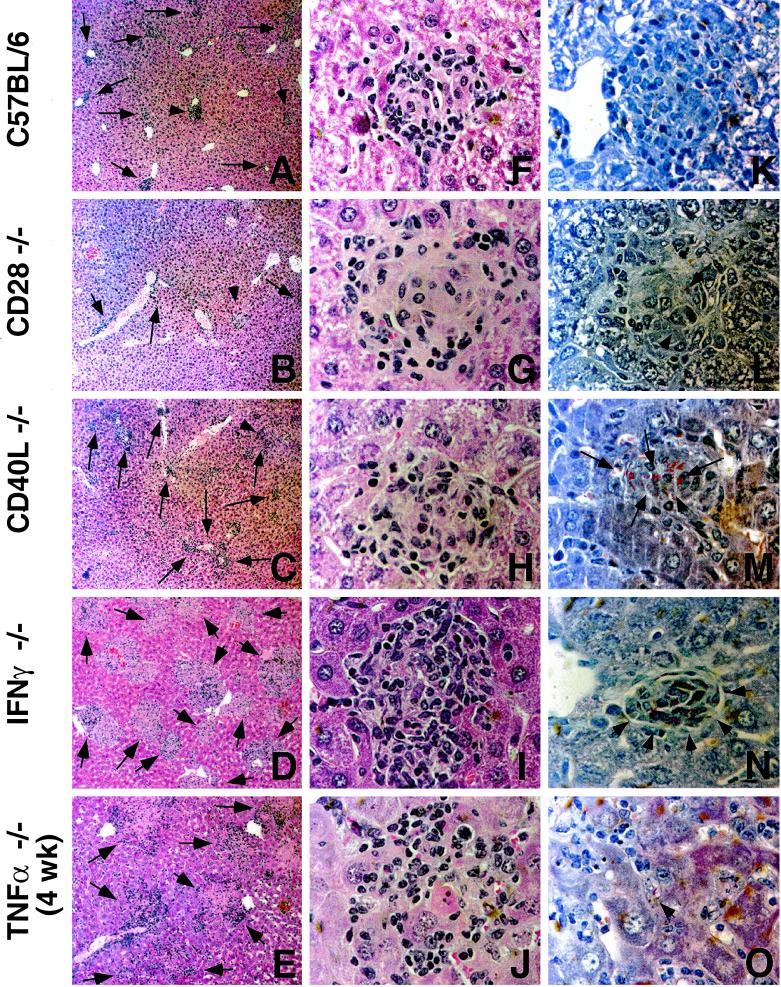
To examine the T-cell costimulatory requirements for protective granuloma formation in the liver, we infected CD40L and CD28 gene-deficient mice with BCG. At 6 weeks, when a chronic infection had been established, we sacrificed the mice and examined thin liver sections for granuloma formation. Figure 1 shows that CD28-deficient mice can form adequate numbers of well-formed lesions similar to those found in C57BL/6 mice (compare Fig. 1B and 1A). Staining for acid-fast bacteria illustrates that the extent of infection found within individual granulomas is also very similar to that seen in the wild-type mice (compare 1L with 1K). This is in stark contrast to the CD40L-deficient mice, which have substantially more bacteria present per lesion (Fig. 1M). These sections were compared to the histopathology observed after infection of IFN-γ- and TNF-α-deficient mice (Fig. 1D, E, I, J, N, and O). Both IFN-γ and TNF-α are known to be essential for protective immunity against mycobacterial infection. IFN-γ-deficient mice have elevated numbers of bacteria present within lesions, but there are well-formed granulomas and the surrounding tissue is healthy (Fig. 1D, I, and N). In contrast, TNF-α-deficient mice form very large, poorly organized lesions which do not effectively contain the bacterial infection (Fig. 1E, J, and O). Bacteria and infected macrophage are observed which have no surrounding inflammation. The extensive bacterial dissemination and organ damage associated with this response was repeatedly lethal before 6 weeks: the TNF-α section illustrated in Fig. 1 is from a 4-week infection. The tissue pathology that we observed with the CD40L-deficient mice is hence very similar to that of the IFN-γ-deficient mice (compare Fig. 1H to I and 1M to N). Both have good granuloma structures but poor control of bacteria, suggesting that CD40L−/− mice may be deficient in IFN-γ but provide sufficient TNF-α. Histopathology in CD28−/− mice is comparable to that in wild-type mice.
FIG. 1.
Histopathology of liver sections from BCG-infected mice sacrificed after 6 weeks of infection. Liver sections were stained with hematoxylin and eosin (A to J) or using the Ziehl-Neelsen method for acid-fast bacteria (K to O). (A to E) Typical frequency of granulomas in the host liver. Total magnification, ×100. Arrows indicate the presence of inflammatory lesions. (F to J) Epitheloid granulomas which are representative for wild-type and genetically deficient mice. Total magnification, ×1,000. (K to O) Quantity and localization of mycobacteria, marked with arrows, within the liver tissue. Magnification, ×1,000.
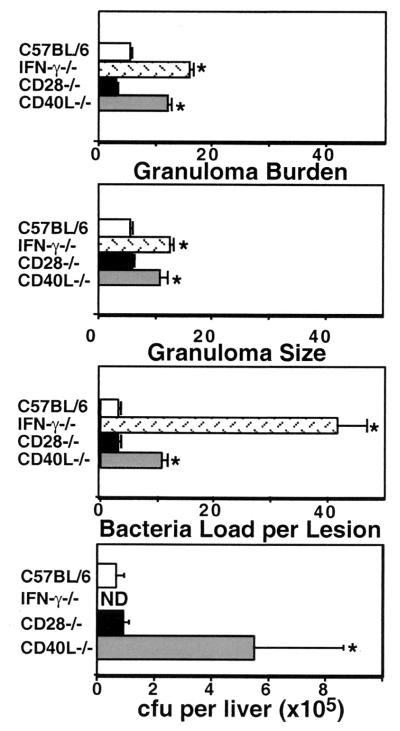
We rigidly quantified our observations by directly counting granulomas, number of bacteria per granuloma, and average size of granulomas. Figure 2 shows that, on average, the granulomas and the number of bacteria per lesion from CD28-deficient mice were the same as from C57BL/6 mice. Granulomas in CD40L-deficient mice are significantly larger and more numerous than in the wild-type mice (P < 0.05, student's t test). Additionally, the number of bacteria per lesion shows a threefold increase in CD40L-deficient mice compared to wild-type and CD28-deficient mice. Granulomas from IFN-γ-deficient mice are larger and more numerous than wild-type granulomas and are very comparable to the granulomas of CD40L-deficient mice. The number of bacteria per lesion in IFN-γ-deficient mice is almost 13 times higher than the number of bacteria in wild-type lesions. The data suggest that IFN-γ and CD40L but not CD28 are crucial for effective granuloma formation and bacterial killing. Data from TNF-α-deficient mice are not presented due both to the significantly smaller sample size and to the fact that the lesions are so disorganized as to make the comparison questionable. Identical trends were seen in bacterial load measurements made by plating liver homogenates and calculating CFU per liver (Fig. 2, last panel).
FIG. 2.
Quantitative analysis of liver granuloma formation 6 weeks after infection. Data in the top three panels are the means ± standard error of the mean of microscopic counts derived from liver sections from 12 wild-type, 12 CD28-deficient, 5 CD40L-deficient, and 5 IFN-γ-deficient mice and represent a minimum of 30 counts per mouse. The last panel shows the mean CFU per liver ± standard error of the mean after growth of serial dilutions of organ homogenates on 7H10, medium for 3 weeks at 37°C. Statistical significance was analyzed by student's t test. ∗, P < 0.05.
Comparison of T-cell accumulation and T-cell phenotype between local inflammatory site and spleen.
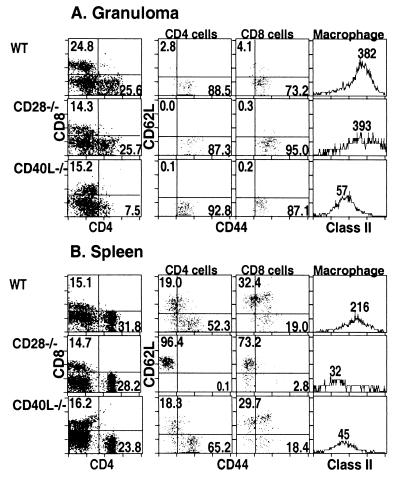
Flow cytometry was used to measure the phenotype and cell surface marker expression of cell populations found in spleen and in liver granulomas after 6 weeks of BCG infection. Figure 3A shows that CD40L-deficient mice have a significant reduction in the relative proportion of CD4+ T cells present in granuloma-infiltrating cells compared to both C57BL/6 and CD28-deficient mice (third row compared to first and second rows). Combined CD44 and CD62L (L-selectin) antibody staining indicates that activation (CD44 high, CD62L low) of both CD4+ and CD8+ T cells is unimpaired. The absolute number of CD4+ T cells on a per-liver basis might be unchanged or even larger, since Fig. 2 showed that CD40L-deficient mice have more and larger granulomas, but the CD4+ T cells are proportionally fewer in the lesions. The lower level of MHC class II molecules on macrophage populations likely indicates a lower local concentration of IFN-γ in the granulomatous lesion. That correlates well with the abolished bacterial control shown in Fig. 1 and 2. We conclude that in the absence of CD40L expression on T cells, insufficient numbers of activated CD4+ T cells capable of secreting IFN-γ home to local inflammatory sites. The phenotype of CD28-deficient granuloma cells is almost indistinguishable from that of C57BL/6 granuloma cells with the exception that a smaller percentage of CD8+ T cells is observed in both CD28-deficient mice and CD40L-deficient mice (first column, 24.8% versus 14.3% and 15.2%, respectively).
FIG. 3.
FACS analysis of cell populations and activation phenotype of T cells from mice chronically infected with BCG. Infected mice were sacrificed after 6 weeks, and granuloma cells (A) and splenocytes (B) were analyzed for CD4+ T-cell, CD8+ T-cell, and macrophage cell marker expression. The first columns of plots represent CD4+ and CD8+ marker expression on cells from a lymphocyte gate, where numbers indicate the percent of gated cells. The middle two columns showing CD44 and CD62L expression are gated as indicated on the CD4+ and CD8+ T-cell populations shown in the left-hand column; numbers indicate the percentage of gated cells in the given quadrant. The right-hand column of histograms represents MHC class II expression on a macrophage gate (Mac-1+); numbers indicate the geometric mean of the staining intensity.
A very different pattern of activation was observed when we measured the same parameters on splenocytes isolated from the different mouse strains infected with BCG. Splenocytes from infected CD40L-deficient mice have a modest decrease in the fraction of CD4+ T cells present, comparable activation of CD4+ and CD8+ T cells relative to infected C57BL/6 splenocytes, and lowered MHC class II expression on macrophages. In contrast, splenocytes from infected CD28-deficient mice have an indistinguishable CD4+-CD8+ T-cell ratio but are profoundly lacking in either CD4+ or CD8+ T-cell activation and have low MHC class II expression on macrophages relative to splenocytes from infected C57BL/6 mice. The lack of activated T cells found in splenocytes from CD28-deficient mice is very different from the infected wild-type mice, in which a large proportion of T cells are activated. Thus, although CD28-deficient mice are able to form good protective granulomas and control bacteria locally, systemic activation of T cells is largely impaired. These data clearly demonstrate that the study of T-cell activation in a systemic site is not always predictive of activation at local lesions.
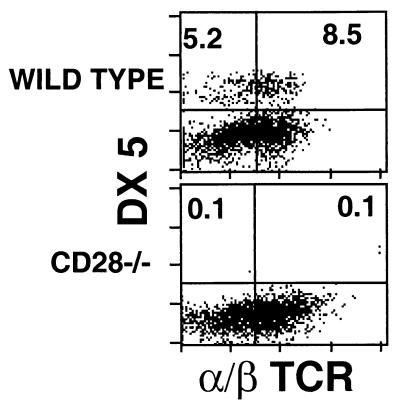
Interestingly, there is a lack of granuloma-recruited NK cells and NK T cells in BCG-infected CD28-deficient mice, as measured by flow cytometry using DX5- and α/β TCR-specific antibodies. Figure 4 shows the relative accumulation of these cell types in C57BL/6 and CD28-deficient mouse granulomas. NK cell numbers in the spleen of CD28-deficient mice were just slightly lower than in wild-type and CD40L−/− mice (data not shown). This suggests that although NK cells and NK T cells may play a role in antimycobacterial control in granulomas, other mechanisms are able to compensate adequately for their absence.
FIG. 4.
FACS analysis of NK cell populations from granuloma T cells in mice chronically infected with BCG. Infected mice were sacrificed after 6 weeks, and granuloma cells from either C57BL/6 or CD28-deficient mice were analyzed for DX5 and α/β TCR cell marker expression. Staining analysis is shown from a lymphocyte gate where numbers indicate the percentage of gated cells in the given quadrant.
IFN-γ measurements.
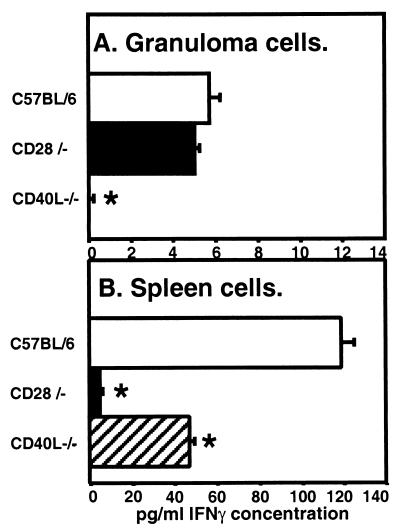
The capacity of BCG-induced granuloma-infiltrating cells to produce IFN-γ was measured directly by ELISA of cell culture supernatants. As expected, the amount of IFN-γ produced by granuloma cells from CD40L-deficient mice after 3 days of in vitro stimulation by α-CD3 antibody was below the assay sensitivity and hence significantly lower (P < 0.05, Student's t test) than levels produced by granuloma cells from C57BL/6 or CD28-deficient mice (Fig. 5A). IFN-γ levels produced by CD28-deficient mouse splenocytes similarly stimulated with α-CD3 antibody were 5- to 10-fold lower than those from CD40L-deficient or C57BL/6 mouse stimulated splenocytes, respectively (Fig. 5B). The overall level of IFN-γ detected in granuloma cell culture supernatants was less than in splenocyte culture supernatants. The comparable expression of MHC class II molecules on macrophages from C57BL/6 infected animals in either compartment makes it unlikely that the difference reflects differences in vivo (Fig. 3A and B). We must consider that although we used similar cell numbers in culture, there may be a greater fraction of T cells present in total splenocytes than in granuloma cell preparations (∼10 to 15%). Additionally, the viability of granuloma cells in culture may be lower than the viability of spleen cells.
FIG. 5.
IFN-γ secreted by granuloma cells or spleen cells after in vitro culture in the presence of α-CD3 antibody. (A) Granuloma cells or (B) spleen cells isolated after 6 weeks of BCG infection by the indicated strains were cultured in vitro for 3 days in the presence of α-CD3 (145-2C11) (5 μg/ml) to activate TCR-mediated signaling. IFN-γ secreted into culture supernatants was measured by ELISA. Values shown are the averages of quadruplicate measurements from one experiment, and error bars represent the standard error of the mean. Statistical significance was analyzed by student's t test. ∗, P < 0.05. Results shown are representative of three independent experiments.
DISCUSSION
The essential role of CD4+ T cells in granuloma formation in response to intracellular and extracellular mycobacteria is undisputed. BCG infection of class II-deficient mice showed that without CD4+ T cells, infected and dying macrophages recruit a damaging neutrophilic inflammatory response that is unable to control bacterial replication (26). T cells, their directly elaborated cytokines, and cytokines from activated macrophage are essential both for forming a protective structure to contain the inflammatory response and for eradicating the sequestered bacteria. Mice deficient in IFN-γ or cytokines that regulate IFN-γ levels, including Eta-1 (osteopontin) (1, 31), and interleukin-12 (IL-12) (7, 19), cannot control bacterial growth. In the absence of TNF-α, inflammation is uncontrolled and tissue-damaging abscesses are formed (10, 23, 39).
In this study we examined the costimulatory signal requirements for activation of CD4+ T cells to regulate sufficient production of IFN-γ and TNF-α to form protective granulomas in response to chronic BCG infection. To our knowledge, this is the only study to directly examine the phenotype of cells that accumulate in BCG-induced granulomas and correlate that phenotype to protective histopathology. In the absence of costimulatory molecules, our data demonstrated that there can be substantial differences between splenic responses and local responses at the granulomatous inflammatory site.
CD40L upregulation after TCR engagement is linked to a cascade of events, including production of cytokines and chemokines, significantly IFN-γ and TNF-α in the case of mycobacteria, and increased expression of accessory molecules on antigen-presenting cells. Recent studies have suggested that CD28-mediated signals are not always required for resistance to various infections, but instead are important for the optimal production of IL-2 and IFN-γ and/or good memory responses (13, 25, 44). Autocrine production of IL-2 after initial CD28 costimulation can sustain CD40L expression in the absence of CD28 or TCR signaling (41). This may represent an important mechanism for reactivating previously stimulated T cells in the absence of antigen. Since our work has shown that CD28 and IL-2 are not essential for protective granuloma formation (data not shown), it seems likely that the requirement for CD40L expression in BCG-induced granuloma formation is not dependent upon CD28 engagement and IL-2 signaling. Our data suggest an alternative model in which CD40L-mediated costimulation is required to produce sufficient IFN-γ locally. The evidence for this includes the qualitative and quantitative similarity of histopathology between CD40L-deficient and IFN-γ-deficient mice (Fig. 1 and 2). Additionally, IFN-γ levels are greatly depressed in granuloma culture supernatants from CD40L-deficient mice (Fig. 5), and MHC class II expression is reduced on granuloma-infiltrating macrophages from these mice (Fig. 3), suggesting that the amount of IFN3-γ in the granuloma is not sufficient for normal macrophage activation. A correlation between CD40L expression and IFN-γ production after mycobacterial infection has also been shown by clinical studies of peripheral blood mononuclear cells (PBMCs) from tuberculosis patients and healthy tuberculin reactors (37). It is also clear from our data that sufficient TNF-α is available without CD40L or CD28, as the extensive and damaging inflammation that results in early death in TNF-α-deficient animals is not seen (Fig. 1).
Our flow cytometric data also showed that in the absence of CD40L, CD4+ T-cell recruitment to the local inflammatory site is substantially reduced (Fig. 3). However, there are activated T cells in the spleens of BCG-infected CD40L-deficient mice. Since activated T cells are capable of homing to local inflammatory sites, it is likely that CD4+ T-cell accumulation in the granuloma is regulated not only at the level of T-cell activation, but also by other factors such as motility, extravasation, recruitment or chemotactic sensitivity. The cellular composition of granulomas in CD40L−/− mice indicated that recruitment of both CD4+ and CD8+ T cells is altered.
Although previous immunohistochemical studies have shown that human tuberculoid granulomatous lesions contain readily detectable levels of B7-1 and B7-2 on epithelioid cells and of CD28 on T cells (42), our data indicated that CD28 is dispensable for protective granulomatous responses against BCG in a murine model. T cells from CD28-deficient mice have reduced expression of IL-2 receptor α(IL-2Rα), reduced T-helper cell function, and reduced proliferation to concanavalin A but are still able to mount an effective anti-lymphocytic choriomeningitis virus cytolytic immune response in vivo (40). Although resistance to primary infection by Schistosoma mansoni is impaired in CD28-deficient mice (24), and Th2 protective responses are diminished, egg-induced granulomatous reactions in the livers of these mice are unaffected. Similarly, B7-1/2−/− mice also form intact granulomas around schistosome eggs despite limited T-cell proliferative capacity and a skew towards Th1-type cytokine production (15).
The results of our study suggested that costimulation mediated by either CD40L or other accessory molecules can compensate for the lack of CD28 during the local response to BCG. In the microenvironment of the granuloma, local concentrations of IL-15 and IL-12 may be sufficient to maintain CD40L expression (41) and drive IFN-γ production (44) independent of CD28. Bacteria within granulomas may also alter costimulatory requirements relative to the periphery due to higher local concentrations of antigen (25). Although CD28-mediated signaling was not required at the local inflammatory site, the systemic response to BCG infection was much lower, perhaps reflecting either reduced systemic T-cell activation or complete recruitment of the few systemically activated T cells to the local inflammatory site.
The mechanisms responsible for the somewhat lower proportions of CD8+ T cells and the absence of NK cells and NK T cells found in granuloma cell populations from BCG-infected CD28−/− mice are not understood. Since NK cells are activated by CD28 (5, 17), one explanation is that activation is required for NK cell recruitment to the granuloma. Our data suggest that NK and NK T cells are not absolutely essential for protective granuloma formation. Additionally, our study makes it clear that the peripheral spleen cell phenotype is not a sure predictor of either granuloma formation or granuloma-infiltrating cell phenotype and underscores the importance of examining the granuloma cells directly.
Our data regarding the susceptibility of CD40L-deficient mice to BCG infection is contrary to the published reports of Campos-Neto using intravenous infection with M. tuberculosis (4), but consistent with the overall agreement among infection studies regarding the role of CD40L (3, 8, 20, 43, 46, 48). The results of their study were surprising given that cell lines transfected with CD40L genes gain the ability to inhibit the growth of M. avium in human monocyte-derived macrophages in vitro (14). Samten et al. also presented data that CD40L disregulation contributes to reduced IFN-γ production in PBMCs from tuberculosis patients (37). It has been shown that BCG requires CD40L-CD40 interactions for the IL-12-regulated production of IFN-γ from human macrophages (28). Skin lesions from resistant tuberculoid leprosy patients contain elevated levels of CD40 and CD40L mRNA and protein compared to those from susceptible lepromatous patients (47).
The experimental differences between our work and the previous study include differences in the bacteria, the dosage, and the route of infection. Our experimental mice were infected at high dose intraperitoneally with BCG for the rapid induction of a chronic immune response and maximum liver granuloma formation. Villegas and coworkers (44) have reported differences in the requirement for CD40L between acute (CD40L independent) and chronic (CD40L dependent) stages of infection with Toxoplasma gondii. This difference might be mediated by differences in the initially predominant antigen-presenting cell population encountered via alternative infection routes, given that recent reports have demonstrated variable requirements for CD40-CD40L interactions in different subpopulations of antigen-presenting cells (32).
Delineating the requirement for CD40L-mediated costimulation for granuloma formation has important consequences for our approach to managing pathologic granulomatous diseases without an infectious etiology. In sarcoidosis and Crohn's disease, targeted immunotherapies to downregulate T-cell function may be beneficial. This type of approach is already being taken using anti-CD40L antibodies to treat autoimmune demyelinating disease models in mice and has shown promising results (16). Our data suggest that granuloma formation in response to BCG can compensate for the loss of CD28 function and that CD40L expression plays an antimycobacterial protective role via the regulation of IFN-γ. Additionally, the models strikingly show that the status of systemic responses may not be reflected at local inflammatory sites. Hence, the study of immune responses at local lesions, like granulomas, deserves significantly more attention.
ACKNOWLEDGMENTS
This work was supported by an R01 award (AI 48087-01) from the National Institutes of Health, an American Lung Association award, and an institutional Howard Hughes award to M.S.
We thank Satoshi Kinoshita for his excellent histopathology services, Diane Sewell and Dominic Co for careful reading of the manuscript, and members of our laboratory for many helpful discussions.
REFERENCES
- 1.Ashkar S, Weber G F, Panoutsakopoulou V, Sanchirico M E, Jansson M, Zawaideh S, Rittling S R, Denhardt D T, Glimcher M J, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 2.Callard R E, Armitage R J, Fanslow W C, Spriggs M K. CD40 ligand and its role in X-linked hyper-IgM syndrome. Immunol Today. 1993;14:559–564. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 3.Campbell K A, Ovendale P J, Kennedy M K, Fanslow W C, Reed S G, Maliszewski C R. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:283–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 4.Campos-Neto A, Ovendale P, Bement T, Koppi T A, Fanslow W C, Rossi M A, Alderson M R. CD40 ligand is not essential for the development of cell-mediated immunity and resistance to Mycobacterium tuberculosis. J Immunol. 1998;160:2037–2041. [PubMed] [Google Scholar]
- 5.Chambers B J, Salcedo M, Ljunggren H G. Triggering of natural killer cells by the costimulatory molecule CD80 (B7–1) Immunity. 1996;5:311–317. doi: 10.1016/s1074-7613(00)80257-5. [DOI] [PubMed] [Google Scholar]
- 6.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper A M, Magram J, Ferrante J, Orme I M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosyns M, Tsirkin S, Jones M, Flavell R, Kikutani H, Hayward A R. Requirement of CD40-CD40 ligand interaction for elimination of Cryptosporidium parvum from mice. Infect Immun. 1998;66:603–607. doi: 10.1128/iai.66.2.603-607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 10.Ehlers S, Benini J, Kutsch S, Endres R, Rietschel E T, Pfeffer K. Fatal granuloma necrosis without exacerbated mycobacterial growth in tumor necrosis factor receptor p55 gene-deficient mice intravenously infected with Mycobacterium avium. Infect Immun. 1999;67:3571–3579. doi: 10.1128/iai.67.7.3571-3579.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flesch I E, Kaufmann S H. Role of cytokines in tuberculosis. Immunobiology. 1993;189:316–339. doi: 10.1016/S0171-2985(11)80364-5. [DOI] [PubMed] [Google Scholar]
- 12.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-α is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 13.Gause W C, Chen S J, Greenwald R J, Halvorson M J, Lu P, Zhou X D, Morris S C, Lee K P, June C H, Finkelman F D, Urban J F, Abe R. CD28 dependence of T cell differentiation to IL-4 production varies with the particular type 2 immune response. J Immunol. 1997;158:4082–4087. [PubMed] [Google Scholar]
- 14.Hayashi T, Rao S P, Meylan P R, Kornbluth R S, Catanzaro A. Role of CD40 ligand in Mycobacterium avium infection. Infect Immun. 1999;67:3558–3565. doi: 10.1128/iai.67.7.3558-3565.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez H J, Sharpe A H, Stadecker M J. Experimental murine schistosomiasis in the absence of B7 costimulatory molecules: reversal of elicited T cell cytokine profile and partial inhibition of egg granuloma formation. J Immunol. 1999;162:2884–2889. [PubMed] [Google Scholar]
- 16.Howard L M, Miga A J, Vanderlugt C L, Dal Canto M C, Laman J D, Noelle R J, Miller S D. Mechanisms of immunotherapeutic intervention by anti-CD40L (CD154) antibody in an animal model of multiple sclerosis. J Clin Investig. 1999;103:281–290. doi: 10.1172/JCI5388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hunter C A, Ellis-Neyer L, Gabriel K E, Kennedy M K, Grabstein K H, Linsley P S, Remington J S. The role of the CD28/B7 interaction in the regulation of NK cell responses during infection with Toxoplasma gondii. J Immunol. 1997;158:2285–2293. [PubMed] [Google Scholar]
- 18.Infante-Duarte C, Kamradt T. Th1/Th2 balance in infection. Springer Semin Immunopathol. 1999;21:317–338. doi: 10.1007/BF00812260. [DOI] [PubMed] [Google Scholar]
- 19.Jouanguy E, Doffinger R, Dupuis S, Pallier A, Altare F, Casanova J L. IL-12 and IFN-gamma in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol. 1999;11:346–351. doi: 10.1016/s0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]
- 20.Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 21.Kamijo R, Le J, Shapiro D, Havell E A, Huang S, Aguet M, Bosland M, Vilcek J. Mice that lack the interferon-γ receptor have profoundly altered responses to infection with bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993;178:1435–1440. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaufmann S H, Ladel C H. Role of T cell subsets in immunity against intracellular bacteria: experimental infections of knock-out mice with Listeria monocytogenes and Mycobacterium bovis BCG. Immunobiology. 1994;191:509–519. doi: 10.1016/S0171-2985(11)80457-2. [DOI] [PubMed] [Google Scholar]
- 23.Kindler V, Sappino A P, Grau G E, Piguet P F, Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989;56:731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- 24.King C L, Xianli J, June C H, Abe R, Lee K P. CD28-deficient mice generate an impaired Th2 response to Schistosoma mansoni infection. Eur J Immunol. 1996;26:2448–2455. doi: 10.1002/eji.1830261027. [DOI] [PubMed] [Google Scholar]
- 25.Kundig T M, Shahinian A, Kawai K, Mittrucker H W, Sebzda E, Bachmann M F, Mak T W, Ohashi P S. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity. 1996;5:41–52. doi: 10.1016/s1074-7613(00)80308-8. [DOI] [PubMed] [Google Scholar]
- 26.Ladel C H, Daugelat S, Kaufmann S H. Immune response to Mycobacterium bovis bacille Calmette-Guerin infection in major histocompatibility complex class I- and II-deficient knock-out mice: contribution of CD4 and CD8 T cells to acquired resistance. Eur J Immunol. 1995;25:377–384. doi: 10.1002/eji.1830250211. [DOI] [PubMed] [Google Scholar]
- 27.Ledru E, Ledru S, Zoubga A. Granuloma formation and tuberculosis transmission in HIV-infected patients. Immunol Today. 1999;20:336–337. doi: 10.1016/s0167-5699(98)01436-4. [DOI] [PubMed] [Google Scholar]
- 28.Mendez-Samperio P, Ayala-Verdin H E, Trejo-Echeverria A. Interleukin-12 regulates the production of Bacille Calmette-Guerin-induced interferon-gamma from human cells in a CD40-dependent manner. Scand J Immunol. 1999;50:61–67. [PubMed] [Google Scholar]
- 29.Metwali A, Elliott D, Blum A M, Li J, Sandor M, Lynch R G, Noben-Trauth N, Weinstock J V. The granulomatous response in murine schistomiasis mansoni does not switch to TH1 in IL-4-deficient C57BL/6 mice. J Immunol. 1996;157:4546–4553. [PubMed] [Google Scholar]
- 30.Murray P J, Young R A, Daley G Q. Hematopoietic remodeling in interferon-gamma-deficient mice infected with mycobacteria. Blood. 1998;91:2914–2924. [PubMed] [Google Scholar]
- 31.Nau G J, Guilfoile P, Chupp G L, Berman J S, Kim S J, Kornfeld H, Young R A. A chemoattractant cytokine associated with granulomas in tuberculosis and silicosis. Proc Natl Acad Sci USA. 1997;94:6414–6419. doi: 10.1073/pnas.94.12.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozaki M E, Coren B A, Huynh T N, Redondo D J, Kikutani H, Webb S R. CD4+ T cell responses to CD40-deficient APCs: defects in proliferation and negative selection apply only with B cells as APCs. J Immunol. 1999;163:5250–5256. [PubMed] [Google Scholar]
- 33.Palmieri F, Pellicelli A M, Girardi E, De Felici A P, De Mori P, Petrosillo N, Ippolito G. Negative predictors of survival in HIV-infected patients with culture-confirmed pulmonary tuberculosis. Infection. 1999;27:331–334. doi: 10.1007/s150100050038. [DOI] [PubMed] [Google Scholar]
- 34.Perlman D C, El-Helou P, Salomon N. Tuberculosis in patients with human immunodeficiency virus infection. Semin Respir Infect. 1999;14:344–352. [PubMed] [Google Scholar]
- 35.Reiner S L, Wang Z E, Hatam F, Scott P, Locksley R M. TH1 and TH2 cell antigen receptors in experimental leishmaniasis. Science. 1993;259:1457–1460. doi: 10.1126/science.8451641. [DOI] [PubMed] [Google Scholar]
- 36.Sacco R E, Jensen R J, Thoen C O, Sandor M, Weinstock J, Lynch R G, Dailey M O. Cytokine secretion and adhesion molecule expression by granuloma T lymphocytes in mycobacterium avium infection. AmJ Pathol. 1996;148:1935–1948. [PMC free article] [PubMed] [Google Scholar]
- 37.Samten B, Thomas E K, Gong J, Barnes P F. Depressed CD40 ligand expression contributes to reduced gamma interferon production in human tuberculosis. Infect Immun. 2000;68:3002–3006. doi: 10.1128/iai.68.5.3002-3006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandor M, Sperling A I, Cook G A, Weinstock J V, Lynch R G, Bluestone J A. Two waves of gamma delta T cells expressing different V delta genes are recruited into schistosome-induced liver granulomas. J Immunol. 1995;155:275–284. [PubMed] [Google Scholar]
- 39.Senaldi G, Yin S, Shaklee C L, Piguet P, Mak T W, Ulich T R. Corynebacterium parvum- and Mycobacterium bovis bacillus Calmette-Guerin-induced granuloma formation is inhibited in TNF receptor 1 (TNF-R1) knockout mice and by treatment with soluble TNF-R1. J Immunol. 1996;157:5022–5026. [PubMed] [Google Scholar]
- 40.Shahinian A, Pfeffer K, Lee K P, Kundig T M, Kishihara K, Wakeham A, Kawai K, Ohashi P S, Thompson C B, Mak T W. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 41.Skov S, Bonyhadi M, Odum N, Ledbetter J A. IL-2 and IL-15 regulate CD154 expression on activated CD4 T cells. J Immunol. 2000;164:3500–3505. doi: 10.4049/jimmunol.164.7.3500. [DOI] [PubMed] [Google Scholar]
- 42.Soler P, Boussaud V, Moreau J, Bergeron A, Bonnette P, Hance A J, Tazi A. In situ expression of B7 and CD40 costimulatory molecules by normal human lung macrophages and epithelioid cells in tuberculoid granulomas. Clin Exp Immunol. 1999;116:332–339. doi: 10.1046/j.1365-2249.1999.00887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soong L, Xu J C, Grewal I S, Kima P, Sun J, Longley Jr B J, Ruddle N H, McMahonpratt D, Flavell R A. Disruption of CD40-CD40 ligand interactions results in an enhanced susceptibility to Leishmania amazonensis. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 44.Villegas E N, Elloso M M, Reichmann G, Peach R, Hunter C A. Role of CD28 in the generation of effector and memory responses required for resistance to Toxoplasma gondii. J Immunol. 1999;163:3344–3353. [PubMed] [Google Scholar]
- 45.Vogel L A, Noelle R J. CD40 and its crucial role as a member of the TNFR family. Semin Immunol. 1998;10:435–442. doi: 10.1006/smim.1998.0145. [DOI] [PubMed] [Google Scholar]
- 46.Wiley J A, Harmsen A G. CD40 ligand is required for resolution of Pneumocystis carinii pneumonia in mice. J Immunol. 1995;155:3525–3529. [PubMed] [Google Scholar]
- 47.Yamauchi P S, Bleharski J R, Uyemura K, Kim J, Sieling P A, Miller A, Brightbill H, Schlienger K, Rea T H, Modlin R L. A role for CD40-CD40 ligand interactions in the generation of type 1 cytokine responses in human leprosy. J Immunol. 2000;165:1506–1512. doi: 10.4049/jimmunol.165.3.1506. [DOI] [PubMed] [Google Scholar]
- 48.Zhou P, Seder R A. CD40 ligand is not essential for induction of type 1 cytokine responses or protective immunity after primary or secondary infection with Histoplasma capsulatum. J Exp Med. 1998;187:1315–1324. doi: 10.1084/jem.187.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]