Abstract
Aims:
The athlete's heart in power training is characterized by physiologic concentric remodeling. Our aim was to analyze left ventricular (LV) myocardial deformation and contractile reserve (CR) in top-level power athletes (PA) at rest and during exercise and their possible correlations with functional capacity.
Methods:
Standard echo, lung ultrasound, and LV 2D speckle-tracking strain were performed at rest and during exercise in PA and in age- and sex-comparable healthy controls.
Results:
250 PA (male: 62%; 33.6 ± 4.8 years) and 180 age- and sex-comparable healthy controls were enrolled. LV ejection fraction (EF) at baseline was comparable between the two groups, while LV global longitudinal strain (GLS) was reduced in PA (GLS: −17.8 ± 2.4 in PA vs. −21.9 ± 3.8 in controls; P < 0.01). Conversely, myocardial work efficiency (MWE) did not show significant difference between the two groups (94.4 ± 3.2 in PA vs. 95.9 ± 4.6% in controls; P NS). At peak exertion during exercise stress echocardiography (ESE), PA showed better exercise capacity and peak VO2 consumption (51.6 ± 10.2 in EA vs. 39.8 ± 8.2 mL/Kg/min in controls, P < 0.0001), associated with augmented pulmonary artery systolic pressure (PASP). By multivariable analysis, MWE at rest was the most predictive factor of maximal watts (P < 0.0001), peak VO2, (P < 0.0001), PASP (P < 0.001), and number of B-lines (P < 0.001), all measured at peak effort.
Conclusions:
In power athletes, MWE showed less load dependency than GLS. Normal resting values of MWE in PA suggest a physiological LV remodeling, associated with a better exercise capacity and preserved CR during physical stress.
Keywords: Contractile reserve, myocardial work, power athletes, stress echocardiography, two-dimensional strain
INTRODUCTION
Cardiac remodeling is a common finding in elite athletes. Power training is characterized by anaerobic performance with mainly static–isometric muscular involvement. These activities can cause structural and electrical changes in the heart that are physiological responses to the gradual increase in systemic arterial resistance, with a predominant pressure overload, responsible of left ventricular (LV) remodeling with increased LV wall thickness and mass, and unchanged LV chamber size (concentric hypertrophy).[1,2,3]
In general, physiological cardiac remodeling in athletes is associated with normal LV diastolic function, normal or enhanced LV ejection fraction (EF), and optimal contractile reserve (CR),[4] but recent studies have documented decrements in LV function during intense exercise of uncertain significance.[3] In some cases, it is important to differentiate the physiological adaptations from pathological conditions, and exercise stress echocardiography (ESE) could be useful in clinical management, providing LV CR assessment.[5,6]
In addition, 2D speckle-tracking echocardiography (2DSTE) technique is a useful tool to study myocardial deformation and subclinical LV dysfunction.[7] In some studies, 2D-derived global longitudinal strain (GLS) of endurance athletes (EA), LV appeared to be reduced at rest, compared with healthy sedentary controls,[8,9,10,11] probably for the increased afterload, the cardiac hypertrophy and the sinus bradycardia. Data about the LV GLS in power athletes (PA) are lacking.
Recently, myocardial work (MW) was introduced as a new noninvasive index for the evaluation of LV myocardial deformation, by LV pressure–strain analysis. In this way, MW provides incremental information to LVEF and strain in different LV-loading conditions, like in athletes.[12,13]
Previously, our group analyzed the role of MW in a group of EA, showing an association between the normal resting values of MW efficiency (MWE) with physiological cardiac remodeling and as a predictor of preserved CR.[13]
We hypothesize that MW may be a more reliable cardiac function index than GLS also in PA, due to its lower dependence on overload, to differentiate physiological from pathological remodeling. Therefore, we aimed to analyze LV systolic function in PA, at rest, and effort using standard and new echocardiographic tools and to study a possible relation of baseline and stress echocardiographic parameters with functional capacity during physical activity.
METHODS
Study population
A total of 250 PA (weight-lifting, body building, boxing, and rugby) were prospectively enrolled in two cardiologic centers, Monaldi Hospital (Naples) and Umberto I Hospital (Nocera Inferiore – Salerno), from May 2019 to February 2020.
The athletes were excluded for (1) evidence of coronary heart disease; (2) valvular and congenital heart disease; (3) cardiomyopathies; (4) congestive heart failure; (5) diabetes mellitus; (6) sinus tachycardia and arrhythmogenic conditions; (7) uncontrolled hypertension; (8) severe chronic obstructive pulmonary disease; and (9) echocardiograms of inadequate quality. PA did not take alcohol, coffee, and any energy drink 24 h before the protocol.
Training protocol
All the athletes had trained intensively for 15–20 h/week for more than 5 years. They underwent anaerobic isometric static exercise at incremental workloads of 40%–60% of maximal heart rate and their training protocol included 3 h/day of weightlifting at high workload. Body builders and weight lifters (n = 119) performed full range of motion barbell exercises including power clean, power snatch, bench press, squat, and deadlift. Maximum self-reported one-repetition squat results were 178 kg (36). The training of boxing athletes (n = 71) focused on weight lifting and on the development of technical and tactical skills, specific to the sport of boxing. Rugby players (n = 60) had six training sessions and a match weekly. To increment muscle strength their exercise program consists in 3 h/day of weight lifting. Further to the strength training, all PA performed about 2 h/week of low-intensity endurance training (“fat burning”). All athletes were regularly investigated to control the strict adherence to training protocol and to competition and to test the abstinence of illicit substances assumption.
Control group
PA athletes were compared with a group of 180 asymptomatic subjects referred to our divisions. They trained for <2 h/week for the last 5 years. Controls were excluded if they had (1) arterial systemic hypertension; (2) coronary artery disease; (3) primary cardiomyopathy and/or genetic cardiovascular disease; (4) congenital heart disease; (4) mitral or aortic valvular insufficiency of higher degree than trivial, valvular stenosis of any degree; (5) any previous cardiac or vascular surgery or interventional procedure; (6) any kind of cardiac therapy; and (7) previous cardioembolic stroke and transient ischemic attacks.
Study protocol
A clinical and laboratory evaluation with clinical history, resting 12 derivation electrocardiogram, standard transthoracic echocardiography, supine bicycle ESE and LV 2DSTE with MW analysis were performed in all athletes and controls.
Transthoracic echocardiography was performed with a commercially available ultrasound system (Vivid E9; GE Ultrasound, Milwaukee, WI, USA). Standard echocardiographic parameters, such as LVEF, LV mass, left atrial volume index, LV stroke volume, cardiac valves evaluation, and the study of diastolic function, were assessed as previously reported.[13]
Furthermore, we assessed B-lines with lung ultrasound, at rest and at peak of effort, by the 4-site simplified scan.
LV GLS was assessed from the apical four, two, and long-axis views to obtain the average of local strains of all 16 segments expressed as bull's eye.[13]
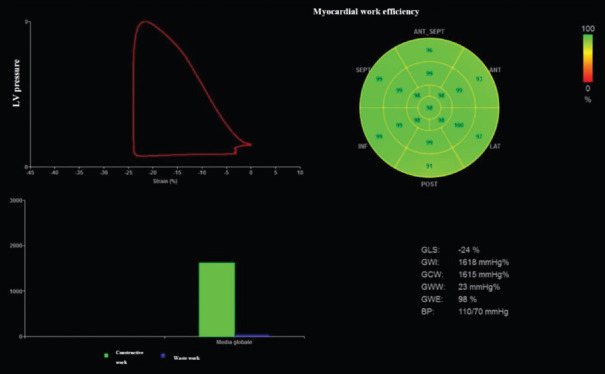
MW was calculated using a specific software with a combination of LV strain and a noninvasively estimated LV pressure with systolic cuff pressure. Along with segmental and global values for MW, a set of additional indices are also provided: constructive work, wasted work (WW), and MWE [Figure 1].[13] All subjects enrolled underwent a semisupine bicycle ESE after the resting echocardiogram, using a standard protocol (incremental steps of 25 W for 2 min) as previously reported.[13] Heart rate (HR), systolic blood pressure, workload (number of Watts achieved by supine bicycle test), and rate–pressure product were measured at rest and at peak of effort.[2,5] Peak oxygen consumption (peak VO2) was assessed by cardiopulmonary test during the ESE study. Echocardiographic parameters measured at baseline and at peak effort included LVEF, LV GLS, LV E/e’, tricuspid annular plane systolic excursion (TAPSE), and pulmonary arterial systolic pressure (PASP). Pulmonary B-lines with the 4-site simplified scan were assessed: each site scored from 0 = A-lines to 10 = white lung. The positivity criterion for B-lines was a stress score higher than rest for ≥2 points.[14]
Figure 1.
Left panel (a) LV pressure–strain loop showing relationship between timing of cardiac events to change in LV pressure and GLS in a power athlete. Right panel (b) 17-segment bull's-eye representation of MW efficiency showing homogeneous areas of high efficiency coded in green. LV: Left ventricular, GLS: Global longitudinal strain, MW: myocardial work
Statistical analysis
Analysis results were presented as means ± standard deviation and median (I, III quartile) for continuous variables. To study differences intragroup and between groups, paired and unpaired t-tests were performed.
Change of EF and LV GLS (%) between baseline and effort measurements was calculated as following: (Peak value – baseline value)/baseline value × 100. Linear regression analyses and multivariable linear regression analysis were performed to identify independent factors of functional capacity, associated with any clinical/echocardiographic variables.
The variables in the study were clinical data (age, sex, body mass index, and mean blood pressure), standard echocardiographic indices (LV volumes, LV mass index, Doppler mitral inflow measurements, and LA volume), B-lines, and strain measurements (LV GLS-MWE). Variable selection was performed in the multivariable linear regression with interactive stepwise backward elimination method, according to Wald statistics. The tests were significant as two-sided P < 0.01. Intraobserver variability and interobserver variability were expressed with coefficient of variation (COV) and by Bland–Altman analysis. All the analyses were performed by SPSS for Windows release 21.0 (Chicago, IL, USA).
RESULTS
We selected 250 PA (33.6 ± 4.8 years; 150 males – 62%) and 180 age- and sex-comparable healthy controls (32.6 ± 4.3 years; 102 males – 57%) (P = NS). As expected, athletes showed higher body surface area (BSA) (2.01 ± 0.4 vs. 1.89 ± 0.7 m2; P < 0.05), systolic blood pressure (135.3 ± 9.1 vs. 125.3 ± 8.4 mmHg; P < 0.01), and reduced HR (69.1 ± 7.3 vs. 79.5 ± 9.3 beats/min; P < 0.01) compared with control group.
By echocardiography, LV wall thickness and mass index were significantly increased in PA, whereas LVEF and right ventricular (RV) functional indexes at rest did not show significant differences. Regarding the diastolic function analysis, athletes showed raised mitral E velocity and E/A ratio, with no difference in E/e’ ratio compared to controls [Table 1]. LV GLS and regional peak myocardial strains were significantly reduced in PA, whereas MWE and WW were similar between the two groups.
Table 1.
Baseline standard Doppler echo and strain measurements in power athletes compared with controls
| Variable | Power athletes (n=250) | Controls (n=180) | P |
|---|---|---|---|
| IVSd (mm) | 12.4±2.1 | 8.3±3.1 | <0.001 |
| PWd (mm) | 12.1±1.9 | 7.7±3.3 | <0.001 |
| LVEDV (ml) | 118.4±12.3 | 112.4±15.6 | NS |
| LVESV (ml) | 36.9±8.1 | 35.4±6.2 | NS |
| LV mass index (g/m2) | 171.3±25.4 | 88.6±18.6 | <0.0001 |
| RWT | 0.44±0.05 | 0.32±0.05 | <0.001 |
| Biplane LVEF (%) | 68.3±8.5 | 68.7±6.3 | NS |
| LV GLS (%) | −17.8±2.4 | −21.9±3.8 | <0.01 |
| MWE | 94.4±3.2 | 95.9±4.6 | NS |
| Myocardial wasted work (%) | 5.6±3.3 | 4.8±3.8 | NS |
| LV stroke volume (ml) | 82.3±23.8 | 76.3±17.9 | <0.01 |
| Mitral E velocity (cm/s) | 0.84±0.7 | 0.76±0.5 | <0.01 |
| Mitral A velocity (cm/s) | 0.51±0.6 | 0.50±0.3 | NS |
| E/A ratio | 1.6±0.5 | 1.48±0.8 | <0.01 |
| Mitral septal E’ velocity (cm/s) | 0.19±0.3 | 0.18±0.6 | NS |
| Mitral lateral E’ velocity (cm/s) | 0.20±0.3 | 0.19±0.7 | NS |
| E/e’ratio | 4.5±3.1 | 4.3±3.3 | NS |
| Aortic root diameter (mm) | 33.3±3.2 | 29.8±3.7 | NS |
| LAVI (ml/m²) | 29.4±4.1 | 26.9±5.4 | <0.01 |
| PASP (mmHg) | 28.5±5.8 | 20.5±6.3 | <0.01 |
| TAPSE (mm) | 22.4±3.4 | 23.8±4.4 | NS |
| Tricuspid S’ velocity (cm/s) | 14.8±3.3 | 15.9±4.9 | NS |
| B-lines (median and IQR) | 0.70 (0-15) | 0.62 (0-15) | NS |
IVSd=Interventricular septum thickness at end diastole, PWd=Posterior wall thickness at end diastole, RWT=Relative wall thickness, LV=Left ventricle, LVEDD=LV end diastolic diameter, LVEF=LV ejection fraction, LV GLS=LV global longitudinal strain, LAVI=Left atrial volume index, PASP=Systolic pulmonary artery pressure, TAPSE=Tricuspid annular plane systolic excursion, LVEDV=LV end-diastolic volume, LVESV=LV end-systolic volume, IQR=Interquartile range, NS=Not significant, MWE=Myocardial work efficiency, LVESD=LV end systolic diameter
During ESE, athletes showed longer exercise time, higher exercise capacity, and VO2 peak compared to the control group. Furthermore, both athletes and controls showed increase in LV EF, LV GLS, MWE, TAPSE, and RV tissue Doppler Sm peak velocity during exertion, and changes of these parameters (Delta %) between baseline and effort measurements were comparable between the two groups [Table 2]. At peak of effort, there are no differences of echocardiographic parameters regarding LV EF between the two groups [Table 2]. Moreover, during physical activity, athletes showed higher PASP with enhanced pulmonary B-lines compared to the control group (P < 0.01).
Table 2.
Functional measurements and echocardiographic parameters at peak exertion in the overall study population
| Variable | Power athletes (n=250) | Controls (n=180) | P |
|---|---|---|---|
| Functional measurements | |||
| Exercise time (min) | 19.5±4.8 | 14.9±5.4 | <0.0001 |
| Exercise capacity (watts) | 270.3±35.3 | 148.7±24.9 | <0.0001 |
| Peak heart rate (bpm) | 171.4±15.3 | 159.7±17.9 | <0.001 |
| Peak systolic blood pressure (mmHg) | 186.4±12.3 | 173.6±15.1 | <0.01 |
| Rate-pressure product (bpm × mmHg) | 31,200±3322 | 27,600±5382 | <0.001 |
| Peak VO2 (mL/kg/min) | 51.6±10.2 | 39.8±8.2 | <0.0001 |
| Echocardiographic parameters | |||
| Biplane LVEF (%) | 73.3±4.6 | 75.7±6.1 | NS |
| PASP (mmHg) | 38.4±5.1 | 28.2±5.8 | <0.001 |
| E/e’ ratio | 7.3±2.9 | 6.9±4.4 | NS |
| LV GLS (%) | −20.3±3.8 | −25.1±3.2 | <0.01 |
| MWE (%) | 96.8±3.6 | 97.3±4.6 | NS |
| TAPSE (mm) | 28.4±2.9 | 29.4±4.2 | NS |
| TDI RV peak systolic velocity Sm (cm/s) | 17.1±3.1 | 18.6±4.2 | NS |
| B-lines (median and IQR) | 2.8 (0–40) | 1.31 (0–25) | <0.01 |
| Delta EF (%) | 7.6±4.8 | 9.4±5.1 | NS |
| Delta GLS (%) | 14.2±2.7 | 14.6±3.8 | NS |
| Delta MWE (%) | 2.4±2.2 | 1.8±2.8 | NS |
| Delta TAPSE (%) | 26.7±3.4 | 23.9±4.4 | NS |
| Delta TDI RV Sm (%) | 19.5±3.3 | 17.5±4.4 | NS |
LV=Left ventricle, PASP=Systolic pulmonary artery pressure, TAPSE=Tricuspid annular plane systolic excursion, TDI RV=Tissue Doppler right ventricle, imaging, LV GLS=LV global longitudinal strain, Peak VO2=Peak oxygen consumption, LVEF=LV ejection fraction, MWE=Myocardial work efficiency, IQR=Interquartile range, NS=Not significant
At univariate analysis, independent associations of baseline GLS and MWE with maximal workload (watts reached), peak V02, PASP, and B-lines during effort, were found, more significant than the relation of LVEF with the same parameters. LV MWE at rest had the best correlation with functional parameters during ESE [Table 3].
Table 3.
Univariable analysis: Correlations between resting left ventricle echo indexes and functional parameters during effort in power athletes
| Variable | R | P |
|---|---|---|
| LVEF | ||
| Watts (at peak effort) | 0.22 | 0.1 |
| VO2 peak | 0.25 | 0.1 |
| PASP during ESE | −0.32 | <0.05 |
| B-lines during ESE | −0.32 | <0.05 |
| LV GLS | ||
| Watts (at peak effort) | −0.38 | <0.01 |
| VO2 peak | −0.44 | <0.01 |
| PASP during ESE | 0.52 | <0.01 |
| B-lines during ESE | 0.43 | <0.01 |
| LV MWE | ||
| Watts (at peak effort) | 0.55 | <0.0001 |
| VO2 peak | 0.67 | <0.0001 |
| PASP during ESE | −0.54 | <0.001 |
| B lines during ESE | −0.5 | <0.001 |
LV=Left ventricular, LVEF: LV ejection fraction, LV GLS=LV global longitudinal strain, LV MWE=LV myocardial work, PASP=Systolic pulmonary artery pressure efficiency, ESE=Echocardiography, VO2 peak=Oxygen consumption peak
At multivariable analysis, LV MWE at rest was related to peak effort parameters such as maximal watts reached (beta coefficient: 0.41; P < 0.01), peak VO2 (beta: 0.51; P < 0.001), PASP (beta: 0.40, P < 0.01), and number of B-lines during effort (beta: −0.38; P < 0.01). LV GLS (beta: −0.45; P < 0.01) and MWE (beta: 0.52; P < 0.001) were strong independent predictors for the presence of significant CR during ESE.
Intraobserver variability: COV: LV MWE: 5.37 (intraclass correlation coefficients [ICC] 0.73); Bland–Altman analysis: LV MWE (95% confidence interval [CI] ± 1.5; percent error 3.2%).
Interobserver variability: COV: LV MWE: 7.22 (ICC 0.77); Bland–Altman analysis: LV MWE (95% CI ± 1.7; percent error 3.6%).
DISCUSSION
MW is a novel index of LV function, less load dependent than GLS.[12,13] In different physiologic and pathologic conditions, an increase in preload or afterload may lead to reduced strain, while MW may be preserved. In fact, MW allows the quantification of global function corrected by systolic blood pressure. This may be of some importance in athletes both EA than PA with variable loading conditions and in different phases of the training program.
In our analysis, PA showed a LV adaptation at rest and a load dependency of strain measurement as demonstrated by lower values of GLS, associated with normal results of MWE and myocardial WW.
PA showed optimal exercise capacity, with an augmented peak VO2 during ESE. During effort, PA showed lower increase of LV GLS compared with healthy controls, mainly related to afterload increase. This finding is similar in EA,[13] but in this case, the lower GLS can be explained by the increased of preload and a larger LV that needs less deformation to maintain stroke volume during resting conditions.
Previously, Szauder et al.[11] have studied LV mechanics in endurance and PA, with 2STE analysis. They found that LV GLS was lower in EA and in PA (but in the latter group did not reach the statistical significance) compared to healthy controls. Furthermore, PA showed a reduced circumferential strain, related to the increased LV mass.[11] Conventional echocardiographic parameters failed to differentiate the two groups; 2D-STE indices showed a different pattern of deformation compared to each other and to healthy controls as well.[11] Nowadays, there are no data in literature about MW differences between endurance and power sports.
We did not find any difference between PA and controls groups in terms of RV function and LV diastolic pressure, while PASP increases and B-lines were mildly higher in athletes. Similar to EA,[13] elevated PASP may be induced by strenuous exercise. A previous study[15] identified higher PASP in 12.9% of athlete population at peak of effort, associated with RV enlargement and preserved RV function. In athletes involved in strenuous exercise, pulmonary B-lines can be detected at echocardiography in the absence of respiratory symptoms.[13,14]
We found a strong relationship between MWE and functional capacity and indices of pulmonary and hemodynamic congestion such as B-lines and PASP. Previously, our group showed that in EA functional capacity indices, pulmonary B-lines and E/e’ were related to MWE, probably due to physical activity volume overload.[13]
Concentric myocardial remodeling in PA is a well-tolerated physiological adaptation to pressure overload, with preserved CR and optimal functional capacity. MWE could be able to differentiate pathological and physiological remodeling and its value at rest may be a good early predictor of cardiac function during strenuous exercise, both in PA than in EA.[13]
2-D STE and MW analysis at rest and during ESE could have a key role to support clinicians for decision-making for sports eligibility of PA in the “gray area” of LV hypertrophy.
The athlete's heart represents an optimal model of myocardial deformation, for the structural and functional adaptation to the different load conditions and load-dependency of strain measurement. Previously, in EA has been reported mild impairment of GLS, lower apical radial strain and lower twisting at rest than in sedentary controls, together with an increase of basal and middle radial and circumferential strain, probably related to volume-overload condition.[16,17,18]
As for the effects of strength training and the load conditions, STE analysis showed a different pattern of myocardial deformation in EA and in PA: while global radial strain was similar, GLS was lower in runners and global circumferential strain was lower in bodybuilders.[8,9,10] EA and PA seem to be similar regarding features provided by 2D-STE and MW,[13] except for the stronger relation between MWE and PASP in the PA, while in the EA, MWE is mainly associated with E/e’: these findings may be explicated by the different loading conditions of the two groups. As a result, a direct comparison between these groups should be performed.
2D-STE and MW may improve the sensitivity and specificity for the differential diagnosis of cardiomyopathies and athlete's heart, identifying early structural–functional myocardial alterations.[18,19,20] A value of GLS <−15% may be indicative of myocardial disease.[21]
The analysis of cardiac longitudinal deformation during effort may add important information to heart contractility and physiology: An isometric activity determines improvement of the GLS, in particular in medium-apical LV segments, suggesting the presence of a higher regional CR.[20,22] What's more, ESE, identifying SV increase and normal LV CR during exercise has a key role to differentiate physiological from pathological remodeling in athletes.
Nowadays, only few reports have analyzed the role of MWE in different clinical scenarios,[23,24] and data about MWE in athletes are lacking. For this reason, larger studies are needed to confirm the role of MW analysis in athletes for the differential diagnosis between pathological and physiological conditions and to analyze the different cardiac compensative mechanisms in the various types of sport.
Study limitations
This study has several limitations. First, the technical limitations of 2DSTE that is dependent on the image resolution and as well as frame rate. Furthermore, the small sample size of our population which does not allow to reach definitive conclusions about myocardial deformation analysis in PA.
Changes in preload and afterload may reduce the myocardial deformation and consequently the GLS, with some impact to our results.[13] In PA, LV mass and wall thickness were higher compared to healthy controls, as an adaptation to higher afterload. For this reason, we also performed MW analysis, which is less load-dependent measure of LV function than GLS.[12,13] We have indexed LVM using BSA; data about fat-free mass in our population were not available. Finally, our results are similar to those previously published about EA;[13] unfortunately, we did not perform a direct comparison between PA and EA. Larger studies are needed to better define any differences between these groups and the physiological adaptations to the different loading conditions.
CONCLUSIONS
LV concentric remodeling is the physiological cardiac adaptation in PA, related to pressure overload. According to previous studies,[11,13] LV GLS at rest is slightly reduced, probably for the afterload dependency of strain measurements. MW is an innovative index of LV myocardial deformation, with a less load dependency than GLS. Moreover, we showed that MW is related to exercise capacity and LV CR in PA, with a potential role to differentiate physiological and pathological conditions in athletes.
Larger studies, about myocardial deformation and MW analyses, are needed to better understand the physiological mechanisms of cardiac adaptation and the possible long-term impact of such changes in elite athletes.
Ethical clearance
The study was approved by the institutional Ethics Committee o ASL SALERNO (Approval No: AQ 136/2021).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Galderisi M, Cardim N, D’Andrea A, Bruder O, Cosyns B, Davin L, et al. The multi-modality cardiac imaging approach to the Athlete's heart: An expert consensus of the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:353. doi: 10.1093/ehjci/jeu323. [DOI] [PubMed] [Google Scholar]
- 2.Lancellotti P, Magne J. Stress echocardiography in regurgitant valve disease. Circ Cardiovasc Imaging. 2013;6:840–9. doi: 10.1161/CIRCIMAGING.113.000474. [DOI] [PubMed] [Google Scholar]
- 3.Carbone A, D’Andrea A, Riegler L, Scarafile R, Pezzullo E, Martone F, et al. Cardiac damage in athlete's heart: When the “supernormal” heart fails! World J Cardiol. 2017;9:470–80. doi: 10.4330/wjc.v9.i6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo Iudice F, Petitto M, Ferrone M, Esposito R, Vaccaro A, Buonauro A, et al. Determinants of myocardial mechanics in top-level endurance athletes: Three-dimensional speckle tracking evaluation. Eur Heart J Cardiovasc Imaging. 2017;18:549–55. doi: 10.1093/ehjci/jew122. [DOI] [PubMed] [Google Scholar]
- 5.Cotrim C, Almeida AR, Miranda R, Almeida AG, Cotrim H, Picano E, et al. Stress-induced intraventricular gradients in symptomatic athletes during upright exercise continuous wave Doppler echocardiography. Am J Cardiol. 2010;106:1808–12. doi: 10.1016/j.amjcard.2010.07.053. [DOI] [PubMed] [Google Scholar]
- 6.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–70. doi: 10.1093/ehjci/jev014. [DOI] [PubMed] [Google Scholar]
- 7.D’Andrea A, Bossone E, Radmilovic J, Caso P, Calabrò R, Russo MG, et al. The role of new echocardiographic techniques in athlete's heart. F1000Res. 2015;4:289. doi: 10.12688/f1000research.6745.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santoro A, Alvino F, Antonelli G, Caputo M, Padeletti M, Lisi M, et al. Endurance and strength athlete's heart: Analysis of myocardial deformation by speckle tracking echocardiography. J Cardiovasc Ultrasound. 2014;22:196–204. doi: 10.4250/jcu.2014.22.4.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’Andrea A, Limongelli G, Caso P, Sarubbi B, Della Pietra A, Brancaccio P, et al. Association between left ventricular structure and cardiac performance during effort in two morphological forms of athlete's heart. Int J Cardiol. 2002;86:177–84. doi: 10.1016/s0167-5273(02)00194-8. [DOI] [PubMed] [Google Scholar]
- 10.Richand V, Lafitte S, Reant P, Serri K, Lafitte M, Brette S, et al. An ultrasound speckle tracking (two-dimensional strain) analysis of myocardial deformation in professional soccer players compared with healthy subjects and hypertrophic cardiomyopathy. Am J Cardiol. 2007;100:128–32. doi: 10.1016/j.amjcard.2007.02.063. [DOI] [PubMed] [Google Scholar]
- 11.Szauder I, Kovács A, Pavlik G. Comparison of left ventricular mechanics in runners versus bodybuilders using speckle tracking echocardiography. Cardiovasc Ultrasound. 2015;13:7. doi: 10.1186/s12947-015-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Andrea A, Sperlongano S, Formisano T, Tocci G, Cameli M, Tusa M, et al. Stress Echocardiography and Strain in Aortic Regurgitation (SESAR protocol): Left ventricular contractile reserve and myocardial work in asymptomatic patients with severe aortic regurgitation. Echocardiography. 2020;37:1213–21. doi: 10.1111/echo.14804. [DOI] [PubMed] [Google Scholar]
- 13.D’Andrea A, Radmilovic J, Carbone A, Mandoli GE, Santoro C, Evola V, et al. Speckle tracking evaluation in endurance athletes: The “optimal” myocardial work. Int J Cardiovasc Imaging. 2020;36:1679–88. doi: 10.1007/s10554-020-01871-z. [DOI] [PubMed] [Google Scholar]
- 14.Fagenholz PJ, Gutman JA, Murray AF, Noble VE, Thomas SH, Harris NS. Chest ultrasonography for the diagnosis and monitoring of high-altitude pulmonary edema. Chest. 2007;131:1013–8. doi: 10.1378/chest.06-1864. [DOI] [PubMed] [Google Scholar]
- 15.Mirea O, Corîci OM, Istrătoaie O, Donoiu I, Iancău M, Militaru C. Left and right ventricular morphology and function in athletes with elevated pulmonary systolic arterial pressure. Echocardiography. 2018;35:769–76. doi: 10.1111/echo.14016. [DOI] [PubMed] [Google Scholar]
- 16.D’Andrea A, Cocchia R, Riegler L, Scarafile R, Salerno G, Gravino R, et al. Left ventricular myocardial velocities and deformation indexes in top-level athletes. J Am Soc Echocardiogr. 2010;23:1281–8. doi: 10.1016/j.echo.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Caselli S, Di Paolo FM, Pisicchio C, Pandian NG, Pelliccia A. Patterns of left ventricular diastolic function in Olympic athletes. J Am Soc Echocardiogr. 2015;28:236–44. doi: 10.1016/j.echo.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 18.D’Andrea A, Radmilovic J, Ballo P, Mele D, Agricola E, Cameli M, et al. Left ventricular hypertrophy or storage disease. The incremental value of speckle tracking strain bull's-eye? Echocardiography. 2017;34:746–59. doi: 10.1111/echo.13506. [DOI] [PubMed] [Google Scholar]
- 19.Forsythe L, George K, Oxborough D. Speckle tracking echocardiography for the assessment of the athlete's heart: Is it ready for daily practice? Curr Treat Options Cardiovasc Med. 2018;20:83. doi: 10.1007/s11936-018-0677-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millar LM, Fanton Z, Finocchiaro G, Sanchez-Fernandez G, Dhutia H, Malhotra A, et al. Differentiation between athlete's heart and dilated cardiomyopathy in athletic individuals. Heart. 2020;106:1059–65. doi: 10.1136/heartjnl-2019-316147. [DOI] [PubMed] [Google Scholar]
- 21.Pelliccia A, Caselli S, Sharma S, Basso C, Bax JJ, Corrado D, et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: Recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete's heart. Eur Heart J. 2018;39:1949–69. doi: 10.1093/eurheartj/ehx532. [DOI] [PubMed] [Google Scholar]
- 22.Stefani L, Toncelli L, Di Tante V, Vono MC, Cappelli B, Pedrizzetti G, et al. Supernormal functional reserve of apical segments in elite soccer players: An ultrasound speckle tracking handgrip stress study. Cardiovasc Ultrasound. 2008;6:14. doi: 10.1186/1476-7120-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edwards NF, Scalia GM, Shiino K, Sabapathy S, Anderson B, Chamberlain R, et al. Global myocardial work is superior to global longitudinal strain to predict significant coronary artery disease in patients with normal left ventricular function and wall motion. J Am Soc Echocardiogr. 2019;32:947–57. doi: 10.1016/j.echo.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Monge García MI, Jian Z, Settels JJ, Hunley C, Cecconi M, Hatib F, et al. Determinants of left ventricular ejection fraction and a novel method to improve its assessment of myocardial contractility. Ann Intensive Care. 2019;9:48. doi: 10.1186/s13613-019-0526-7. [DOI] [PMC free article] [PubMed] [Google Scholar]