Abstract
Background: Tuberculosis (TB) is a major cause of morbidity and mortality in people living with HIV (PLWHIV). Isoniazid preventive therapy (IPT) prevents TB in PLWHIV, but estimates of its effects and actual implementation vary across countries. We reviewed studies that examined the impact of IPT on PLHIV and the factors influencing its implementation in Ethiopia. Methods: We searched PubMed/MEDLINE, Embase, and the Cochrane Central Register of Clinical Controlled Trials from their inception to 1 April 2021 for studies of any design that examined the impact of IPT on PLHIV and the factors influencing its implementation. The protocol was registered in PROSPERO, ID: CRD42021256579. Result: Of the initial 546 studies identified, 13 of which enrolled 12,426 participants, 15,640 PLHIV and 62 HIV clinical care providers were included. PLHIV who were on IPT, independently or simultaneously with ART, were less likely to develop TB than those without IPT. IPT interventions had a significant association with improved CD4 count and reduced all-cause mortality. IPT was less effective in people with advanced HIV infection. The major factors influencing IPT implementation and uptake were stock-outs, fear of developing isoniazid-resistant TB, patient’s refusal and non-adherence, and improper counseling and low commitment of HIV clinical care providers. Conclusion: IPT alone or in combination with ART significantly reduces the incidence of TB and mortality in PLHIV in Ethiopia than those without IPT. More research on safety is needed, especially on women with HIV who receive a combination of IPT and ART. Additionally, studies need to be conducted to investigate the efficacy and safety of the new TPT (3 months combination of isoniazid and rifapentine) in children and people living with HIV.
Keywords: isoniazid prevention therapy (IPT), HIV, tuberculosis, antiretroviral therapy (ART), Ethiopia
1. Introduction
Tuberculosis (TB) remains one of the top 10 causes of death globally and the primary cause of death from a single infectious agent [1]. In 2021, there were 10.6 million TB cases globally and 1.4 million deaths among HIV-negative people, and an additional 187,000 deaths among HIV-positive people [1]. Most people who acquired TB in 2021 were in the regions of South-East Asia (45%), Africa (23%), and the Western Pacific (18%) [1]. In Africa, a widespread scale-up of antiretroviral therapy (ART) strongly declines the incidence of TB [2]. Ethiopia is one of the top 14 triple burden countries for TB, TB/HIV, and MDR-TB [1]. An estimated incidence of all forms of TB in Ethiopia, in 2019, was 140/100,000 population, with 111,039 TB cases notified [1]. TB remains one of the major causes of morbidity and mortality in the country [3].
To prevent and reduce the incidence of TB in people living with HIV (PLHIV), the World Health Organization (WHO) recommended the use of isoniazid preventive therapy (IPT) as a mainstay of the “Three I’s” approach [4]. Per the WHO recommendation, IPT is administered at a daily dose of a maximum of 300 mg daily for 6–9 months in adults and adolescents and 5 mg/kg for children [5,6]. This chemoprophylaxis reduces the risk of an early episode of TB occurrence in people with latent infection or those exposed to infection, and reduces recurrent episodes of TB [5,6]. For patients with latent TB, IPT can be beneficial, with the potential to reduce TB infection irrespective of HIV status [5,6] and protecting communities from [7,8,9]. However, the emergence of drug resistance secondary to IPT administration is a potential risk that is understudied [5].
In Ethiopia, the National TB guideline recommends that IPT should be provided to all HIV-infected individuals who are unlikely to have active TB irrespective of CD4 count, ART status, pregnancy status, or history of treatment for a prior episode of TB before three years. Patients should be supported at home level either by local health extension workers or a family supporter to ensure daily administration of IPT. Patients should be given a one-month supply of isoniazid for six months, with a monthly scheduled follow-up integrated with other treatment services. IPT should also be administered for asymptomatic children under five who were exposed to TB within the past year. However, the current evidence regarding the effects and actual implementation of IPT in Ethiopia remains unclear. This systematic review was, therefore, conducted to examine the outcomes of IPT in PLHIV and factors influencing its implementation in Ethiopia.
2. Methods
The protocol for this systematic review and meta-analysis was registered at the International Prospective Register of Systematic Reviews (PROSPERO) database, ID: CRD42021256579. The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA 2020) guidelines were followed to choose studies to be included in this review.
2.1. Eligibility Critria Studies
Criteria included studies of any design that examined the impact of IPT on PLHIV and the factors influencing its implementation in Ethiopia, published in English language until 1 April 2021.
2.2. Participants
Patients with HIV,
Man or woman of any age,
ART naïve or experienced on their time of enrollment.
2.3. Types of Interventions
2.3.1. For Intervention Studies
Intervention group: isoniazid 300 mg daily for 6–9 months in adults and adolescents and 5 mg/kg for children.
Comparison group: inactive placebo, ART only, or no preventive treatment.
2.3.2. For Non-Intervention Studies
Effectiveness, barriers, or opportunities implementing IPT program in Ethiopia based on experiences from PLHIV or HIV clinical service providers.
2.4. Outcome Measures
2.4.1. Primary Outcome Measure
Incidence of definite or probable TB. Definite TB was defined by a microbiological, chest X-ray, or histological identification of TB.
2.4.2. Secondary Outcome Measures
Incidence of death.
Factors associated with TB incidence.
Incidence of adverse drug reactions leading treatment discontinuation.
Barriers to implementation of IPT.
2.5. Search Strategy
A computerized systematic search method was used to search for articles from online databases PubMed/MEDLINE, Embase, and Cochrane Central Register of Clinical controlled Trials (CENTRAL) databases from inception to 1 April 2021. The search was based on the Cochrane Handbook for Systematic Reviews of Interventions [10], with a combination of the words (tuberculosis) AND (isoniazid preventive therapy) considered the major search term (Table 1).
Table 1.
Search term for MEDLINE.
| Search | Most Reset Queries |
|---|---|
| #1 | Search HIV infections[MeSH] OR HIV[TW] OR HIV-1*[MeSH] OR HIV-2*[TW] OR HIV-1[TW] OR HIV-2[TW] OR HIV infec* [TW] OR Human immunodeficiency virus [TW] OR Human immune-deficiency virus [TW] OR ((Human immune* [TW]) AND (deficiency virus [TW])) OR Acquired immune deficiency syndrome [TW] OR AIDS [MeSH] OR ((acquired immune* [TW]) AND (deficiency syndrome [TW])) OR sexual transmitted diseases, viral [MeSH: No Exp] |
| #2 | Search Tuberculosis [MeSH] OR TB [MeSH] |
| #3 | Search preventive therapy [MeSH] OR Chemoprevention [MeSH] OR Prophylaxis [MeSH] |
| #4 | Search #1 AND #2 AND #3 |
| #5 | Search #1 AND #2 AND #3 Limits: Publication date from 1980 to 2021 |
2.6. Study Selection
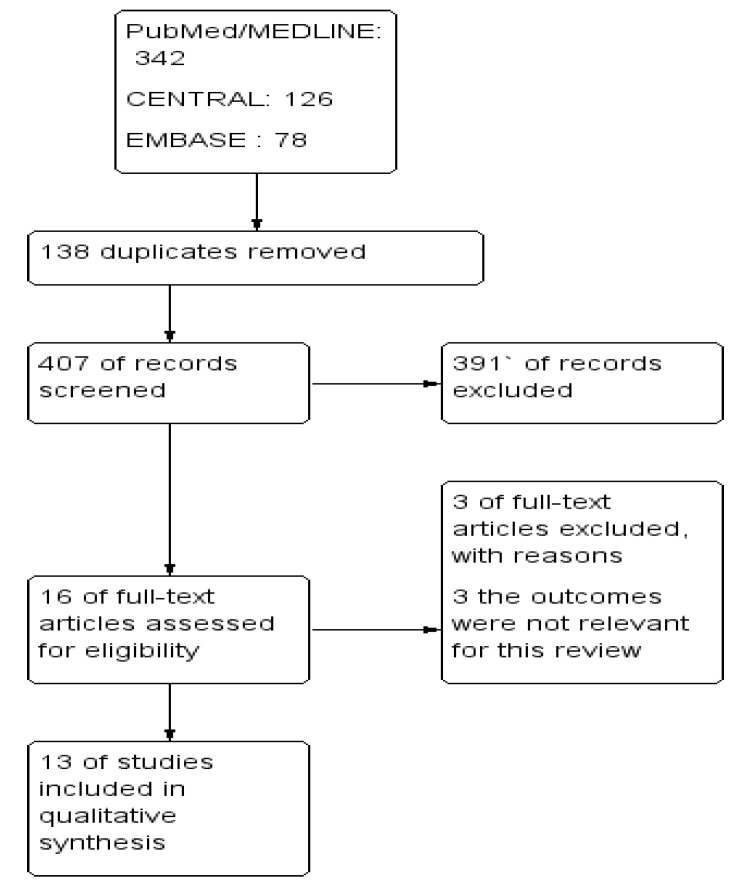
The Cochrane Handbook for Systematic Reviews of Interventions [11] was followed. To import the research articles from the electronic databases and remove duplicates, ENDNOTE software version X7 was used. Two authors independently reviewed the results of the literature search and obtained full-text copies of all potentially relevant studies. Disagreements were resolved through discussion. When clarification was necessary, the trial authors were contacted for further information. The screening and selection process was reported in a PRISMA flow chart (Figure 1).
Figure 1.
PRISMA study flow diagram.
2.7. Data Extraction and Management
The title and abstract were produced from the electronic search and were independently screened by two authors based on studies and the selection criteria. The information collected were study characteristics, including study design, study setting, age and number of participants enrolled, interventions, study title, journal, year of publication, publication status, follow-up period, funding of the trial or sources of support, baseline characteristics of study subjects, incidence of TB, factors associated with IPT, and IPT implementation barriers. One author independently extracted data, and these were cross-checked by another author. Missing data were requested from the authors whenever necessary.
3. Result
A total of 546 studies through the databases were searched, of which, 16 full-text studies were assessed further for eligibility and 13 of them fulfilled the inclusion criteria for further analysis (Table 2).
3.1. Characteristics of Included Studies
In this review, 13 studies that enrolled a total of 15,640 PLHIV and 62 health professionals working on HIV care were included (Table 2). Many of the patients were aged greater than 14 years [12,13,14,15,16] and female [12,13,14,15,17,18]. The CD4 count was > 200 cell count/μL in most of the participants in one study [13] and less than 199 cells/mm3 in another study [14], and most of the patients were in HIV stage III followed by stage II [13,14,19].
3.2. Incidence of TB
Among PLHIV, the incidence of TB after administration of IPT independently or simultaneously with ART shows a significant reduction in the incidence of TB as compared to patients who were on ‘ART only’ and ‘No intervention’ [12,13,15,16,17,18,19,20,21]. Simultaneous administration of both IPT and ART reduced the incidence of TB by 80% [12], 93.7% [13], and 65% [14], respectively. Completion of IPT showed a significant protective effect against the occurrence of active TB for 3 years when compared to IPT non-exposed patients [13]. IPT was associated with a significant change in CD4 count [15,19,20] and reduced all-cause mortality [14,17,19,20].
3.3. Factors Associated with TB Incidence among PLWHIV Who Took IPT
The risk of developing TB or dying was significantly higher in PLHIV on WHO stage III and above at baseline [12,13,14,16,17,18,22], male [12,13,14,22], with a CD4 count of less than a 350 cell count/μL and those with opportunistic infections [15,16,17,18,21,22], children with delayed motor development [21] who did not take cotrimoxazole preventive therapy [18,21,22], use anti-pain [22], and have a hemoglobin level less than 10 mg/dL [16,21,22]. The risk of TB infection and death was lower in those who held good body weight [14,18,21,22] and referred to the hospital from other health facilities [14]. In some of the studies, the effects of age [14,21] and baseline CD4 count had a suboptimal effect on TB incidence or death [14].
3.4. Barriers in the Implementation of IPT
A significant number of HIV clinical care providers reported that several barriers hinder IPT coverage and its effective implementation, including isoniazid stock-outs, fear of developing isoniazid resistance, patient’s refusal and non-adherence, and improper counseling and low commitment of HIV clinical care providers [23]. Lack of patient empowerment and proper counseling on IPT, weak patient/healthcare provider communication, information gaps, low commitments from health administrators and other stakeholders to effectively run the IPT program, and underlying mental health issues resulting in missed or irregular patient adherence to IPT were also reported as barriers for effective implementation of IPT in Ethiopia [24]. Additionally, clinician impressions that ruling out active TB among HIV patients is difficult was found to be a significant barrier to IPT uptake [25].
Table 2.
Characteristics of included studies.
| S. No | Study ID | Design | Setting | Age | Follow Up | Subjects | Patient Important Outcome | ||
|---|---|---|---|---|---|---|---|---|---|
| 1. | Mindachew et al., 2014 [24] | Qualitative | Hospital | N/A | N/A | 12 heath professional | barriers | Lack of patient empowerment and proper counseling on IPT, weak patient/healthcare provider relationship, lack of patient information, low reinforcement by health officials and stakeholders to strengthen IPT uptake and adherence forgetfulness, patient IPT non-adherence, and non-disclosure of HIV zero-status. | |
| 2. | Yirdaw et al., 2014 [12] | Retrospective cohort | Hospitals (n = 5) | Mean (30 years) | 2 years | 5407 patients | IPT before ART | aHR = 0.18, 95% CI = 0.08–0.42 | |
| IPT before ART’ | HR = 0.25, 95% CI = 0.11–0.59 | ||||||||
| IPT and ART | aHR = 0.20,95% CI = 0.10–0.42 | ||||||||
| IPT and ART | HR = 0.36; 95% CI = 0.17–0.74 | ||||||||
| IPT only | HR = 0.24, 95% CI = 0.13–0.44 | ||||||||
| IPT after ART | HR = 0.19, 95% CI = 0.11–0.34 | ||||||||
| TB incidence | 295 | ||||||||
| 3. | Assebe et al., 2015 [17] | Retrospective cohort study | Hospital | N/A | Mean 24.1 months | 588 | Overall TB incidence | 49 | |
| IPT 294 | No IPT 294 | Overall TB incidence | 3.78 cases per 100 PY (95% CI: 2.85, 4.99 cases per 100 PY) | ||||||
| Incidence of TB among IPT Plus ART | 2.22 cases per 100 PY (95% CI: 1.29, 3.82 cases per 100 PY) | ||||||||
| Incidence of TB among ART alone | 5.06 cases per 100 PY (95% CI: 3.65, 7.02 cases per100 PY) | ||||||||
| Incidence of TB among IPT Plus ART | aHR 2.02 (95% CI: 1.04–3.92) | ||||||||
| 4. | Nigusse et al., 2015 [16] | Retrospective follow up study | Hospital | Median 38 (IQR: 31.2–42) | 5 years | 480 | Overall TB incidence | 70 | |
| Overall TB incidence | 3.59 per 100 PY | ||||||||
| TB incidence among IPT | aHR = 0.49, 95% CI = 0.26–0.94 | ||||||||
| 5. | Ayele, 2015 [14] | Retrospective cohort study | Hospital | Range 15–99 years | 839 days | 1922 (374 received IPT) | Overall TB incidence | 258 | |
| Incidence of TB/death among IPT plus ART | HR = 0.35; 95% CI (0.16, 0.77) | ||||||||
| Incidence of TB/death among ART alone | HR = 1.22; 95% CI (0.45, 3.28) | ||||||||
| Incidence of TB/death among IPT plus ART | aHR = 0.40; 95% CI (0.18, 0.87) | ||||||||
| Incidence of TB among IPT plus ART | 5.20 per 100 PYs | ||||||||
| Incidence of TB among ART alone | 8.05 per 100 PYs | ||||||||
| 6. | Alemu et al., 2016 [21] | Retrospective cohort study | Hospitals (n = 2), Health centers (n = 6) |
Median (IQR) 6 (3.5–9.00) years |
N/A | 645 | Overall TB incidence | 79 | |
| Overall TB incidence | 4.2: 95% CI (3.4, 5.3) PY | ||||||||
| 7. | Teklay et al., 2016 [23] | Qualitative study | Hospitals (n = 11) | Mean (±SD) 30 (±6) years |
N/A | 50 health providers | Barriers | Isoniazid stock out | |
| Fear of creating isoniazid resistance Problems in patient acceptance Lack of commitment of health managers |
|||||||||
| 8. | Abossie et al., 2017 [15] | Hospital-based retrospective study | Hospital | Mean (±SD) 31.27 (+12.0) |
271 | Incidence of TB among IPT Plus ART | 12 (8.7%) | ||
| IPT 138 | No IPT 133 | Incidence of TB among ART alone | 37 (27.8%) | ||||||
| Incidence of TB among IPT Plus ART | RR 0.31 (95% CI 0.122, 0.49) | ||||||||
| 9. | Semu et al., 2017 [13] | Retrospective cohort | Public health institutions | Mean (±SD) 34.9 (±9.1) years |
5 years | 2524 patients | TB Incidence Rate among IPT | 0.21/100 PY | |
| TB-incidence Rate among at IPT completion | aIRR 0.037 (95% CI, 0.016–0.072) | ||||||||
| overall TB incidence | 6.7/100 PY | ||||||||
| TB incidence | 277 | ||||||||
| Incidence of TB among IPT-with-HAART | 0.42/100 PY | ||||||||
| Incidence of TB among IPT-with-HAART | aIRR = 0.063 (95% CI 0.035–0.104) | ||||||||
| Incidence of TB among alone HAART | 7.83 cases/100 PY | ||||||||
| 10. | Tiruneh et al., 2019 [18] | Retrospective cohort study | Hospital and health center | Mean (±SD) 33 years (±9) years |
Median 26 months | 600 | |||
| IPT 200 | No IPT 400 | Overall TB Incidence | 53 (8.8%) | ||||||
| Overall TB Incidence | 57 cases per 100 PY | ||||||||
| Incidence of TB among IPT group | 1.98 per 100 PY | ||||||||
| Incidence of TB among non-IPT group | 4.52 per 100 PY | ||||||||
| Incidence of TB among IPT group | aHR 0.45, 95% CI 0.219–0.920 | ||||||||
| Incidence of TB among IPT group | HR 0.397, 95% CI 0.203–0.774 | ||||||||
| 11. | Gebremariam et al., 2020 [20] | Retrospective cohort study | Hospitals (n = 2) | N/A | 5 years | 968 patients | Incidence of TB among ART plus IPT | 8 (0.5 cases/100 PY) | |
| IPT 484 | No IPT 484 | Incidence of TB among ART plus IPT | aHR 0.17; 95% CI 0.08–0.35 | ||||||
| Incidence of TB among ART alone | 49 (3 cases/100 PY) | ||||||||
| Deaths on ART plus IPT | 12 (0.5 cases/100 PYs) | ||||||||
| Deaths on ART alone | 35 (2.1 cases/100 PYs) | ||||||||
| Death reduction among ART plus IPT | aHR 0.48; 95% CI 0.24–0.97 | ||||||||
| 12. | Atey et al., 2020 [19] | Retrospective Cohort Study | Hospitals (n = 5) | N/A | N/A | 1863 | Incidence of TB among IPT Plus ART | 28 | |
| IPT 621 | No IPT 1242 | Incidence of TB among ART alone | 272 | ||||||
| Overall incidence | 300 | ||||||||
| Incidence rate of mortality among IPT Plus ART | 440 per 100,000 PYs | ||||||||
| Incidence rate of mortality among ART alone | 1490 per 100,000 PYs | ||||||||
| 13. | Legese et al., 2020 [22] | Institutional based cross-sectional | Hospital | Mean (±SD) 37.94 (±12.15) | 6 months | 372 (231 on IPT) | Overall incidence of TB among IPT group | 13 (3.5%) | |
4. Discussion
In this study, IPT shows a significant reduction in the risk of TB and dying as compared to that of ART only and non-intervention. It also shows an effect on the improvement of CD4 count. Several studies conducted elsewhere also reported that IPT reduces the incidence of TB [26,27,28,29,30]. Recent studies reported that IPT is effective in the reduction in TB disease on pregnant women living with HIV and with their CD4 count ≤ 350 cells/μL [31,32]. In West Africa, the early initiation of ART and 6 months of IPT showed a significant reduction in HIV-related illness by 44% and the risk of mortality from any cause by 35% as compared to the risks with deferred initiation of ART and no IPT [33]. There were several factors, such as being male, low baseline CD4 count, and hemoglobin level less than 10 mg/dl, that negatively influenced the effectiveness of IPT. In agreement with our finding, a study from Malawi reported that male PLHIV had low adherence to IPT as compared to female [34]. This might be a reason for the difference in the effectiveness of IPT between male and female patients. Additionally, the protective effect of INH was more extreme in contacts exposed to drug-sensitive tuberculosis (adjusted hazard ratio, 0.30; 95% confidence interval, 0.18–0.48) and to multidrug-resistant tuberculosis (adjusted hazard ratio, 0.19; 95% confidence interval, 0.05–0.66) compared with those exposed to mono-INH-resistant tuberculosis (adjusted hazard ratio, 0.80; 95% confidence interval, 0.23–2.80) [35].
Implementation of the IPT program in Ethiopia is facing several challenges, including stock-out that may question sustainability of the program and may provoke drug resistance. In concurrence to our finding, studies from India reported the lack of awareness on the role and way of taking IPT, risk perception among patients’ parents, cumbersome screening process, isoniazid stock-outs, inadequate knowledge among healthcare providers, and poor programmatic monitoring as main barriers to IPT implementation [36,37]. Adequate supply and availability of isoniazid at the health facilities, preparing unambiguous treatment guideline, contact tracing, provision of IPT for children, community-based intervention, and provision of adequate training for health care providers on IPT enhanced the reduction in TB incidence and patient’s adherence to IPT [38,39,40,41]. Thus, for effective implementation and outcomes of IPT program in Ethiopia, there is a need to enhance patient adherence and effectiveness of IPT through effective communication, build the capacity of the healthcare providers through training and motivation packages, sustainably increase isoniazid supply, and strengthen program partnership and collaboration.
5. Conclusions
IPT alone or in combination with ART significantly reduces the incidence of TB and mortality in PLHIV in Ethiopia than those without IPT. More research on safety is needed, especially on women with HIV who receive a combination of IPT and ART. Additionally, studies need to be conducted to investigate the efficacy and safety of the new TPT (3-month combination of isoniazid and rifapentine) in children and people living with HIV.
Acknowledgments
We would like to express our gratitude to the Center for Innovative Drug Development and Therapeutic Trials for Africa (CDT-Africa), College of Health Sciences, Addis Ababa University, for supporting the study.
Abbreviations
| ART | Anti-Retroviral Therapy |
| aHR | Adjusted Hazard Ratio |
| aIRR | Adjusted incidence rate ratio |
| CENTRAL | Cochrane Center for Clinical Trial |
| CI | Confidence Interval |
| IPT | Isoniazid Preventive Therapy |
| HAART | Highly active antiretroviral therapy |
| HR | Hazard Ratio |
| HIV | Human Immune Virus |
| IR | Incidence ratio |
| PLWHIV | People Living with Human Immunodeficiency Virus |
| PY | Person per Year |
| SD | Standard Deviation |
| TB | Tuberculosis |
| TST | Tuberculin Skin Test |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| WHO | World Health Organization |
Author Contributions
D.G.A., E.D.Z. and W.M. developed the protocol, reviewed the reference list, extracted data, and conducted the analyses. D.G.A., E.D.Z., W.M., D.A.E., D.B., T.T.W., A.A., M.A., A.M. and T.M. assessed the quality of the data and reviewed the analysis. DGA developed the draft manuscript and T.M. critically reviewed it. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. The Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA 2020) guidelines were followed to choose studies to be included in this review.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are within the manuscript.
Conflicts of Interest
We declare that they have no competing interest.
Funding Statement
This review did not receive specific funding. T.M. was supported in part by the Fogarty International Center and National Institute of Allergy and Infectious Diseases of the US National Institutes of Health under Award Number D43TW009127.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Global Tuberculosis Report 2022. World Health Organization; Geneva, Switzerland: 2022. [(accessed on 10 November 2022)]. Available online: http://apps.who.int/iris. [Google Scholar]
- 2.Global Tuberculosis Report 2018. World Health Organization; Geneva, Switzerland: 2018. [(accessed on 10 November 2022)]. Available online: http://apps.who.int/iris. [Google Scholar]
- 3.Ethiopia National Strategic Plan Tuberculosis and Leprosy Control 2013–2020. Addis Ababa: Federal Democratic Republic of Ethiopia Ministry of Health. 2017. 2017. [(accessed on 16 February 2022)]. Available online: https://www.afro.who.int/sites/default/files/2019-04/Ethiopia%20-%20National%20Strategic%20Plan%20Tuberculosis%20and%20Leprosy%20Control%202013-2020.pdf.
- 4.WHO . Isoniazid Preventive Terapy (IPT) and TB Infection Control (IC) for People Living with HIV, Report of a JointWorld Health Organization HIV/Aids and TB Department Meeting. WHO; Geneva, Switzerland: 2008. [(accessed on 12 December 2021)]. Three I’s Meeting Intensified Case Finding (ICF) Available online: http://apps.who.int/iris. [Google Scholar]
- 5.WHO . Implementing the WHO Stop TB Strategy: A Handbook for National Tuberculosis Control Programmes. Volume 7. World Health Organization; Geneva, Switzerland: 2008. [(accessed on 12 December 2021)]. Isoniazid Preventive Therapy. Available online: https://www.ncbi.nlm.nih.gov/books/NBK310749/ [PubMed] [Google Scholar]
- 6.Isoniazid Preventive Therapy (IPT) for People Living with HIV. Consensus Statement of the Core Group of the TB/HIV Working Group of the Stop TB Partnership. [(accessed on 12 December 2021)]. Available online: http://www.stoptb.org/wg/tb_hiv/assets/documents/IPT%20Consensus%20Statement%20TB%20HIV%20Core%20Group.pdf.
- 7.Rangaka M.X., Wilkinson R.J., Boulle A., Glynn J.R., Fielding K., van Cutsem G., Wilkinson K.A., Goliath R., Mathee S., Goemaere E., et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: A randomised double-blind, placebo-controlled trial. Lancet. 2014;384:682–690. doi: 10.1016/S0140-6736(14)60162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samandari T., Agizew T.B., Nyirenda S., Tedla Z., Sibanda T., Mosimaneotsile B., Motsamai O.I., Shang N., Rose C.E., Shepherd J. Tuberculosis incidence after 36 months’ isoniazid prophylaxis in HIV-infected adults in Botswana: A posttrial observational analysis. AIDS. 2015;29:351–359. doi: 10.1097/QAD.0000000000000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zar H.J., Cotton M.F., Strauss S., Karpakis J., Hussey G., Schaaf H.S., Rabie H., Lombard C.J. Effect of isoniazid prophylaxis on mortality and incidence of tuberculosis in children with HIV: Randomised controlled trial. BMJ. 2007;334:136. doi: 10.1136/bmj.39000.486400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shaweno T., Kura Z. Determinants of modern contraceptive use among sexually active men in Ethiopia; using EDHS 2016 national survey. Contracept. Reprod. Med. 2020;5:5. doi: 10.1186/s40834-020-00108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tura A.K., Scherjon S., van Roosmalen J., Zwart J., Stekelenburg J., van den Akker T. Surviving mothers and lost babies—Burden of stillbirths and neonatal deaths among women with maternal near miss in eastern Ethiopia: A prospective cohort study. J. Glob. Health. 2020;10:01041310. doi: 10.7189/jogh.10.010413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yirdaw K.D., Jerene D., Gashu Z., Edginton M.E., Kumar A.M.V., Letamo Y., Feleke B., Teklu A.M., Zewdu S., Weiss B., et al. Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PLoS ONE. 2014;9:e104557. doi: 10.1371/journal.pone.0104557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semu M., Fenta T.G., Medhin G., Assefa D. Effectiveness of isoniazid preventative therapy in reducing incidence of active tuberculosis among people living with HIV/AIDS in public health facilities of Addis Ababa, Ethiopia: A historical cohort study. BMC Infect. Dis. 2017;17:1–8. doi: 10.1186/s12879-016-2109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayele H.T., van Mourik M.S.M., Bonten M.J.M. Effect of isoniazid preventive therapy on tuberculosis or death in persons with HIV: A retrospective cohort study. BMC Infect. Dis. 2015;15:1–8. doi: 10.1186/s12879-015-1089-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abossie A., Yohanes T. Assessment of isoniazid preventive therapy in the reduction of tuberculosis among art patients in Arba Minch Hospital, Ethiopia. Ther. Clin. Risk Manag. 2017;13:361–366. doi: 10.2147/TCRM.S127765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahlet N. Ph.D. Thesis. Addis Ababa University; Addis Ababa, Ethiopia: 2015. Incidence and Factors Predicting Active TB Occourance among Patients Enrolled in Art, Zewditu Hospital, Addis Ababa, Retrospective Cohort Study. [Google Scholar]
- 17.Assebe L.F., Reda H.L., Wubeneh A.D., Lerebo W.T., Lambert S.M. The effect of isoniazid preventive therapy on incidence of tuberculosis among HIV-infected clients under pre-ART care, Jimma, Ethiopia: A retrospective cohort study. BMC Public Health. 2015;15:346. doi: 10.1186/s12889-015-1719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tiruneh G., Getahun A., Adeba E. Assessing the Impact of Isoniazid Preventive Therapy (IPT) on Tuberculosis Incidence and Predictors of Tuberculosis among Adult Patients Enrolled on ART in Nekemte Town, Western Ethiopia: A Retrospective Cohort Study. Interdiscip. Perspect. Infect Dis. 2019;2019:1413427. doi: 10.1155/2019/1413427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Atey T.M., Bitew H., Asgedom S.W., Endrias A., Berhe D.F. Does Isoniazid Preventive Therapy Provide Better Treatment Outcomes in HIV-Infected Individuals in Northern Ethiopia? A Retrospective Cohort Study. AIDS Res. Treat. 2020;2020:7025738. doi: 10.1155/2020/7025738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gebremariam T.H., Shewamare A., Deyessa N., Haile L., Blumberg H.M., Auld S., Degu W.A. Effect of isoniazid preventive therapy on prevention of tuberculosis and reduction of all-cause mortality among HIV patients on antiretroviral therapy. Am. J. Respir. Crit. Care Med. 2020;201:A2692. doi: 10.1164/ajrccm-conference.2020.201.1_MeetingAbstracts.A2692. [DOI] [Google Scholar]
- 21.Alemu Y.M., Andargie G., Gebeye E. High incidence of tuberculosis in the absence of isoniazid and cotrimoxazole preventive therapy in children living with HIV in Northern Ethiopia: A retrospective follow-up study. PLoS ONE. 2016;11:e0152941. doi: 10.1371/journal.pone.0152941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legese H., Degefa H., Gebrewahd A., Gebremedhin H. Utilization of isoniazid prophylaxis therapy and its associated factors among HIV positive clients taking antiretroviral therapy at Fre Semaetat primary hospital, Hawzien districts, Tigrai, Northern Ethiopia. Trop. Dis. Travel Med. Vaccines. 2020;6:11. doi: 10.1186/s40794-020-00106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teklay G., Teklu T., Legesse B., Tedla K., Klinkenberg E. Barriers in the implementation of isoniazid preventive therapy for people living with HIV in Northern Ethiopia: A mixed quantitative and qualitative study. BMC Public Health. 2016;16:840. doi: 10.1186/s12889-016-3525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mindachew M., Deribew A., Memiah P., Biadgilign S. Perceived barriers to the implementation of Isoniazid preventive therapy for people living with HIV in resource constrained settings: A qualitative study. Pan Afr. Med. J. 2014;17:26. doi: 10.11604/pamj.2014.17.26.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai J., Dememew Z., Jerene D., Abashawl A., Feleke B., Teklu A.M., Ruff A. Provider barriers to the uptake of isoniazid preventive therapy among people living with HIV in Ethiopia. Int. J. Tuberc. Lung Dis. 2019;23:371–377. doi: 10.5588/ijtld.18.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Churchyard G.J., Fielding K.L., Lewis J.J., Coetzee L., Corbett E.L., Godfrey-Faussett P., Hayes R.J., Chaisson R.E., Grant A.D. A trial of mass isoniazid preventive therapy for tuberculosis control. N. Engl. J. Med. 2014;370:301–310. doi: 10.1056/NEJMoa1214289. [DOI] [PubMed] [Google Scholar]
- 27.Golub J.E., Pronyk P., Mohapi L., Thsabangu N., Moshabela M., Struthers H., Gray G.E., McLntyre J.A., Chaisson R.E., Martinson N.A. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV- infected adults in South Africa: A prospective cohort. AIDS. 2009;23:631–636. doi: 10.1097/QAD.0b013e328327964f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomes V.F., Andersen A., Lemvik G., Wejse C., Oliveira I., Vieira F.J., Carlos L.J., Da Silva Vieira C., Aaby P., Gustafson P. Impact of isoniazid preventive therapy on mortality among children less than 5 years old following exposure to tuberculosis at home in Guinea-Bissau: A prospective cohort study. BMJ Open. 2013;3:e001545. doi: 10.1136/bmjopen-2012-001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta A., Sun X., Krishnan S., Matoga M., Pierre S., McIntire K., Koech L., Faesen S., Kityo C., Dadabhai S.S., et al. Isoniazid Adherence Reduces Mortality and Incident Tuberculosis at 96 Weeks among Adults Initiating Antiretroviral Therapy With Advanced Human Immunodeficiency Virus in Multiple High-Burden Settings. Open Forum Infect. Dis. 2022;9:ofac325. doi: 10.1093/ofid/ofac325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabasaba A., Mwambi H., Somi G., Ramadhani A., Mahande M.J. Effect of isoniazid preventive therapy on tuberculosis incidence and associated risk factors among HIV infected adults in Tanzania: A retrospective cohort study. BMC Infect. Dis. 2019;19:62. doi: 10.1186/s12879-019-3696-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalk E., Heekes A., Mehta U., De Waal R., Jacob N., Cohen K., Myer L., Davies M.A., Maartens G., Boulle A. Safety and Effectiveness of Isoniazid Preventive Therapy in Pregnant Women Living with Human Immunodeficiency Virus on Antiretroviral Therapy: An Observational Study Using Linked Population Data. Clin. Infect. Dis. 2020;71:E351–E358. doi: 10.1093/cid/ciz1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Badje A., Moh R., Gabillard D., Guéhi C., Kabran M., Ntakpé J.B., Carrou J.L., Kouame G.M., Ouattara E., Messou E., et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: Long-term follow-up of the Temprano ANRS 12136 trial. Lancet Glob. Health. 2017;5:e1080–e1089. doi: 10.1016/S2214-109X(17)30372-8. [DOI] [PubMed] [Google Scholar]
- 33.Danel C., Moh R., Gabillard D., Badje A., Le Carrou J., Ouassa T., Ouattara E., Anzian A., Ntakpé J.B., Minga A., et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N. Engl. J. Med. 2015;373:808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 34.Little K.M., Khundi M., Barnes G.L., Ngwira L.G., Nkhoma A., Makombe S., Corbett E.L., Chaisson R.E., Dowdy D.W. Predictors of isoniazid preventive therapy completion among adults newly diagnosed with HIV in rural Malawi. Int. J. Tuberc. Lung Dis. 2018;22:371–377. doi: 10.5588/ijtld.16.0836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang C.-C., Becerra M.C., Calderon R., Contreras C., Galea J., Grandjean L., Lecca L., Yataco R., Zhang Z., Megan M. Isoniazid Preventive Therapy in Contacts of Multidrug-Resistant Tuberculosis. Am. J. Respir. Crit. Care Med. 2020;202:1159–1168. doi: 10.1164/rccm.201908-1576OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Singh A.R., Kharate A., Bhat P., Kokane A.M., Bali S., Sahu S., Verma M., Nagar M., Kumar A.M.V. Isoniazid preventive therapy among children living with tuberculosis patients: Is it working? A mixed-method study from Bhopal, India. J. Trop. Pediatr. 2017;63:274–285. doi: 10.1093/tropej/fmw086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makanjuola T., Taddese H.B., Booth A. Factors associated with adherence to treatment with isoniazid for the prevention of tuberculosis amongst people living with HIV/AIDS: A systematic review of qualitative data. PLoS ONE. 2014;9:e87166. doi: 10.1371/journal.pone.0087166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takarinda K.C., Choto R.C., Harries A.D., Mutasa-Apollo T., Chakanyuka-Musanhu C. Routine implementation of isoniazid preventive therapy in HIV-infected patients in seven pilot sites in Zimbabwe. Public Health Action. 2017;7:55–60. doi: 10.5588/pha.16.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picone C.M., Freitas A.C., Gutierrez E.B., Avelino-Silva V.I. Access and adherence to isoniazid preventive therapy and occurrence of active TB in a cohort of people living with HIV: A retrospective cohort study in Sao Paulo, Brazil. Rev. Inst. Med. Trop. Sao Paulo. 2020:62. doi: 10.1590/s1678-9946202062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarrett B.A., Woznica D.M., Tilchin C., Mpungose N., Motlhaoleng K., Golub J.E., Martinson N.A., Hanrahan C.F. Promoting Tuberculosis Preventive Therapy for People Living with HIV in South Africa: Interventions Hindered by Complicated Clinical Guidelines and Imbalanced Patient- Provider Dynamics. AIDS Behav. 2020;24:1106–1117. doi: 10.1007/s10461-019-02675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Datiko D.G., Yassin M.A., Theobald S.J., Cuevas L.E. A community-based isoniazid preventive therapy for the prevention of childhood tuberculosis in Ethiopia. Int. J. Tuberc. Lung Dis. 2017;21:1002–1007. doi: 10.5588/ijtld.16.0471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.