Abstract
Oxidative stress occurs in animals challenged with bacterial endotoxin and can affect the expression of important host inflammatory genes. However, much less is known about the effects of oxidative stress on responses to gram-negative bacteria. The current study compared the effects of redox imbalance on hepatic responses of mice to Escherichia coli bacteria versus purified endotoxic lipopolysaccharide (LPS). Oxidative stress induced by glutathione depletion virtually eliminated hepatic tumor necrosis factor alpha responses to both E. coli and LPS. Inducible NO synthase (iNOS) and intercellular adhesion molecule-1 (ICAM-1) expression was also markedly inhibited by glutathione depletion in LPS-challenged mice, but was unaffected in E. coli-infected animals. Three findings suggested that gamma interferon (IFN-γ) production explained the differences between LPS and bacterial challenge. Glutathione depletion completely inhibited the IFN-γ response to LPS, but only partially inhibited IFN-γ production in infected mice. Exogenous IFN-γ restored iNOS and ICAM-1 responses to LPS in stressed mice. Conversely, IFN-γ-deficient, glutathione-depleted mice showed a marked decrease in iNOS and ICAM-1 expression when challenged with E. coli. These findings indicate that both the nature of the microbial challenge and the production of IFN-γ can be important in determining the effects of redox imbalance during gram-negative bacterial infections.
Sepsis is a response to infection characterized by the production of inflammatory cytokines, such as tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and gamma interferon (IFN-γ), and the release of highly reactive oxygen and nitrogen intermediates. These oxidizing species are thought to contribute to much of the end-stage tissue damage seen in this disease. Considerable evidence indicates that oxygen and nitrogen metabolites can also regulate the expression of genes that are induced by bacterial components in vitro (7, 16, 20, 35, 38) by causing oxidative or reductive/oxidative (redox) stress within cells (7, 16, 20, 35). Similarly, Nathens et al. (29) showed that the depletion of the antioxidant glutathione in vivo inhibited the expression of intercellular adhesion molecule-1 (ICAM-1) in the lungs of rats challenged with bacterial lipopolysaccharide (LPS). Significant tissue redox imbalance occurs in both endotoxemia and gram-negative bacterial infections (32, 36, 43), suggesting that oxidative stress may be an important regulator of inflammatory mediator production during sepsis. Consistent with this prediction, Matushak and his colleagues (28, 47) reported that oxidative stress resulting from hypoxia-reoxygenation of perfused rat livers inhibited the in vivo production of TNF-α and IL-1β in response to challenge with live Escherichia coli bacteria.
Glutathione is the most plentiful nonprotein thiol found in most cells and as such serves as a major scavenger of intracellular oxidants (19). Both the depletion of total intracellular glutathione and the oxidation of the glutathione sulfhydryl (GSH) to form glutathione disulfide significantly alters the redox state of cells. For example, oxidative stress occurs when cells are exposed to electrophiles, such as diethyl maleate (DEM), that conjugate directly with GSH and cause the export of the resulting glutathione adduct from the cell (19). Likewise, buthionine sulfoximine (BSO) depletes GSH in vitro and in vivo by specifically inhibiting γ-glutamylcysteine synthase, a rate-limiting enzyme in GSH biosynthesis (18). In turn, glutathione redox imbalance can lead to the expression of a number of “stress-responsive” genes, including those coding for heat shock proteins (15).
Animal models in which endotoxic LPS has served as a surrogate for infection have proven valuable for defining the basic pathophysiological responses to gram-negative bacteria and the important inflammatory cells and soluble mediators of sepsis. However, animal endotoxicosis models have not always predicted the nature of host inflammatory responses to gram-negative bacterial challenge (6, 8, 12, 21, 48). For this reason, we have compared the effects of tissue oxidative stress on the expression of several inflammatory genes in LPS-challenged and E. coli-infected mice. The mouse liver was chosen for this purpose because many important inflammatory mediators, including TNF-α, IFN-γ, CD14, inducible NO synthase (iNOS), and ICAM-1, are expressed by hepatic cells following either LPS or bacterial challenge. The organ also suffers substantial damage in sepsis that is mediated, in part, by the expression of these genes (23, 33, 41). Importantly, oxidative stress occurs in the liver when mice are given glutathione-depleting agents (46).
IFN-γ is an important mediator of endotoxemia and gram-negative bacterial sepsis in a number of mammalian species. The cytokine enhances LPS lethality in rodents, and antibody to IFN-γ can diminish LPS-induced inflammatory responses and lethality (2, 22, 25, 40). Likewise, the targeted disruption of the genes for either IFN-γ or its receptor subunits has been shown to modify responses to LPS, including lethality in several rodent models (3, 24, 37). Kamijo et al. (24) showed that IFN-γ mediated the ability of Corynebacterium parvum to enhance LPS-induced cytokine production, iNOS expression, and lethality in mice. Salkowski et al. (37) used IFN-γ-null mice to show that the sustained induction of mouse hepatic iNOS mRNA by LPS required IFN-γ gene expression. Similar conclusions regarding the importance of IFN-γ have been made with many but not all gram-negative infection models (13, 26, 27). Kohler et al. (26) reported that IFN-γ enhanced lethality in mice challenged with E. coli bacteria and that neutralizing antibody to the cytokine improved survival. Despite these findings, very little is known about the effects of oxidative stress on the production of IFN-γ by animals challenged with gram-negative bacteria or bacterial LPS.
Because mice that have been injected with LPS or infected with E. coli bacteria show significantly decreased levels of hepatic glutathione as early as 6 h postchallenge (M. J. Parmely, F. Wang, and D. Wright, unpublished data), we have asked to what extent tissue redox imbalance alters hepatic responses to LPS or infection. The present study will show that glutathione depletion inhibits LPS-induced iNOS and ICAM-1 expression in the mouse liver but has no apparent effect on these responses in E. coli-infected mice. The ability of glutathione-depleted, infected mice to produce IFN-γ appears to prevent many of the inhibitory effects of oxidative stress on these responses, suggesting that IFN-γ-dependent signaling pathways are utilized during infection that are not activated following LPS challenge. These findings predict that individuals in redox imbalance, including patients whose glutathione levels are intentionally manipulated to enhance the action of certain cancer chemotherapeutic agents (1), have the potential to show substantially altered responses to bacterial challenge.
MATERIALS AND METHODS
Reagents.
E. coli O111:B4 LPS, DEM, and BSO were purchased from Sigma Chemical Co. (St. Louis, Mo.). Recombinant mouse IFN-γ was provided by Genentech (South San Francisco, Calif.). The following antibodies were used in the present study: rat anti-mouse TNF-α (MP6-XT22; PharMingen, San Diego, Calif.), rabbit anti-mouse iNOS (SC-650; Santa Cruz Biotechnology, Santa Cruz, Calif.), goat anti-mouse ICAM-1 (SC-1511; Santa Cruz), rat anti-mouse macrophage F4/80 (a gift of Joan Hunt, University of Kansas Medical Center), rat anti-mouse CD14 (rmC5-3; PharMingen), rabbit anti-rat heat shock protein-32 (HSP-32) (SPA-895; StressGen, Victoria, BC, Canada), biotinylated goat anti-rabbit immunoglobulin G (IgG; BioGenex; San Ramon, Calif.), biotinylated rabbit anti-rat IgG (Vector Laboratories; Burlingame, Calif.), and biotinylated donkey anti-goat IgG (Santa Cruz).
Bacterial challenge.
E. coli O111:B4 bacteria were provided by David Morrison (University of Missouri-Kansas City) and grown overnight in Trypticase soy broth (TSB) at 37°C with shaking (100 rpm). An aliquot of this culture was then inoculated into TSB, and the bacteria were grown to a density of approximately 5 × 108 bacteria/ml. The bacteria were then washed with phosphate-buffered saline (PBS) and injected by the intraperitoneal (i.p.) route. No antibiotics were given to infected animals. Actual numbers of CFU were determined for each innoculum by growing serially diluted bacteria on Trypticase soy agar at 37°C overnight.
Mice.
LPS-responsive female mice (8 to 12 weeks old) of the C57BL/6 and C3HeB/FeJ strains were purchased from Jackson Laboratories (Bar Harbor, Maine), and CF1 strain mice were obtained from Charles River (Wilmington, Mass.). Wild-type BALB/c (IFN-γ+/+) and IFN-γ-deficient mice of the BALB/c background (BALB/c-Ifngtm/Ts) (IFN-γ−/−) were obtained from Jackson Laboratories. They were housed in sterilized cages on autoclaved bedding and provided sterile food and water ad libitum. All mice were maintained in positive-pressure microisolator chambers under a 12-h light–12-h dark cycle. Animal care and use protocols were approved by an institutional review board at the University of Kansas Medical Center.
Depletion and measurement of tissue glutathione.
Tissue glutathione was depleted by the i.p. injection of DEM prepared in sesame oil (5.3 mmol of DEM/kg of body weight) (46). Alternatively, mice were injected i.p. with BSO (5 mmol/kg) in 0.15 M NaCl (vehicle) 6 h and again 3 h prior to bacterial or LPS challenge. Total hepatic glutathione concentrations (reduced plus oxidized) were measured as previously described (46) using the kinetic recycling assay of Tietze (44) and are expressed here as nanomoles per milligram of protein.
Immunohistology.
Sections (5-μm thick) of paraformaldehyde-fixed liver tissues were prepared, and immunohistochemical staining was performed by the indirect peroxidase-conjugated streptavidin procedure as previously described (46). Following deparaffinization and rehydration, the sections were incubated for 15 min with a blocking solution consisting of PBS containing 0.5% IgG- and protease-free bovine serum albumin (Jackson ImmunoResearch Laboratories, West Grove, Pa.), 0.5% coldwater fish skin gelatin (Sigma Chemical Co.), and either 5% normal goat serum (for iNOS and HSP-32 staining), 5% normal rabbit serum (for TNF-α, CD14, and F4/80 staining), or 5% normal donkey serum (for ICAM-1 staining). Then an additional avidin-biotin blocking reagent (Vector Laboratories) was applied according to the manufacturer's instructions. The sections were incubated for 2 h with the primary antibodies, washed in PBS, and incubated for 30 min with biotinylated forms of either goat anti- rabbit IgG, rabbit anti-rat IgG, or donkey anti-goat IgG. After washing in PBS, the sections were treated for 15 min with 1% H2O2 (Sigma Chemical) in methanol to inactivate endogenous tissue peroxidases. The bound secondary antibodies were then detected with a peroxidase-streptavidin conjugate (BioGenex), and reaction sites were visualized using diaminobenzidine (DAB kit; Vector Laboratories). The sections were counterstained with Gill II hematoxylin (Shandon, Pittsburgh, Pa.).
Controls included liver sections from infected or LPS-stimulated mice incubated with normal rat, rabbit, or goat IgG instead of the corresponding primary antibodies. In addition, preabsorbing the TNF-α and iNOS antibodies for 60 min with a 10-fold molar excess of recombinant mouse TNF-α (Genentech, South San Francisco, Calif.) or iNOS peptide (Santa Cruz), respectively, eliminated all reactivity. The iNOS antibody also failed to react with liver tissue from LPS-stimulated iNOS-null mutant mice (NOS2tm/Lau; Jackson Laboratories).
All slides were read independently by two observers, and the densities of positive cells were determined by microscopic examination of at least five high-power fields (HPF) (magnification, ×400; HPF = 0.11 mm2) per tissue section. The staining of hepatic sinusoidal endothelial cells was scored in a semiquantitative fashion, with 0, 1, 2, and 3 corresponding to negative, faint, moderate, and strong staining, respectively. The identity of Kupffer cells was established in serial sections by F4/80 staining. Intrasinusoidal leukocytes were identified by staining for α-naphthol-ASD-chloroacetate esterase and their absence of F4/80 staining. Sinusoidal endothelial cells were F4/80-negative, esterase-negative cells apposing the sinusoidal spaces.
Measurement of serum TNF-α and IFN-γ.
Serum TNF levels were measured by the L929 cell bioassay as previously described (34). Serum IFN-γ concentrations were measured by enzyme-linked immunosorbent assay (ELISA) (Pharmingen). Because the samples were first diluted, the detection limits vary with the assay and are specified in each case.
Statistical analysis.
Each experiment was performed at least twice, and representative results are shown. Student's two-tailed t test was used to determine statistical significance (P < 0.01). Serum cytokine concentrations that were undetectable were assigned the value of the detection limit of the assay for the purposes of calculating group means.
RESULTS
Glutathione depletion and oxidative stress responses in the mouse liver.
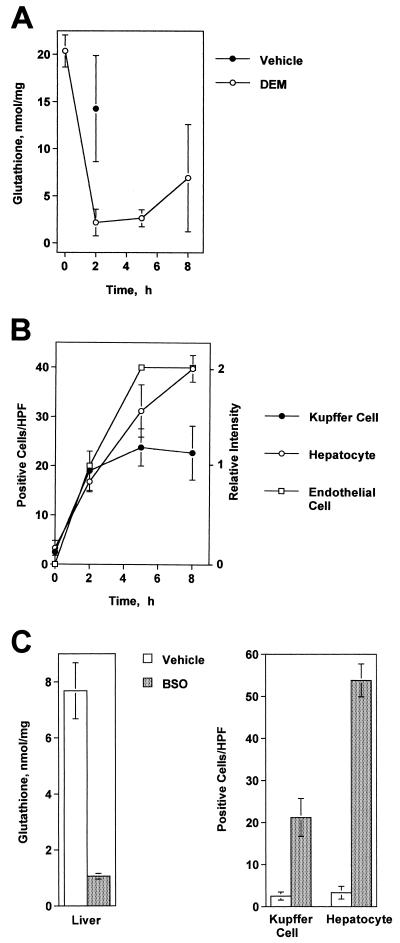
A single i.p. injection of DEM (5.3 mmol/kg) depleted hepatic glutathione levels in CF1 mice by greater than 90%, a condition which persisted for several hours (Fig. 1A). This effect was accompanied by the elevated expression of HSP-32, an oxidative stress-responsive gene, by liver Kupffer cells, hepatocytes, and hepatic sinusoidal endothelial cells (Fig. 1B and 2A). Similarly, injecting mice twice with BSO (5 mmol/kg) significantly decreased the glutathione levels in their livers and induced a comparable pattern of HSP-32 expression (Fig. 1C). Thus, the treatment of mice with DEM or BSO caused significant redox imbalance in their livers.
FIG. 1.
DEM and BSO cause oxidative stress in the livers of C3HeB/FeJ mice. (A) Time course of glutathione depletion in the mouse liver following an i.p. injection of DEM (5.3 mmol/kg). The glutathione levels at 2 and 5 h differed significantly from those of the vehicle controls (P < 0.01). (B) The expression of HSP-32 in the liver following DEM treatment as determined by immunohistology. Shown here are the frequencies of HSP-32-positive Kupffer cells and hepatocytes per HPF (magnification, X400) and the relative intensity of HSP-32 staining in sinusoidal endothelial cells. (C) Glutathione depletion and HSP-32 expression in the livers of mice injected twice with BSO (5 mmol/kg) or saline. Control and BSO-treated groups differed significantly in each case (P < 0.01). These experiments were repeated with similar results, and the effects were also seen in CF1 mice.
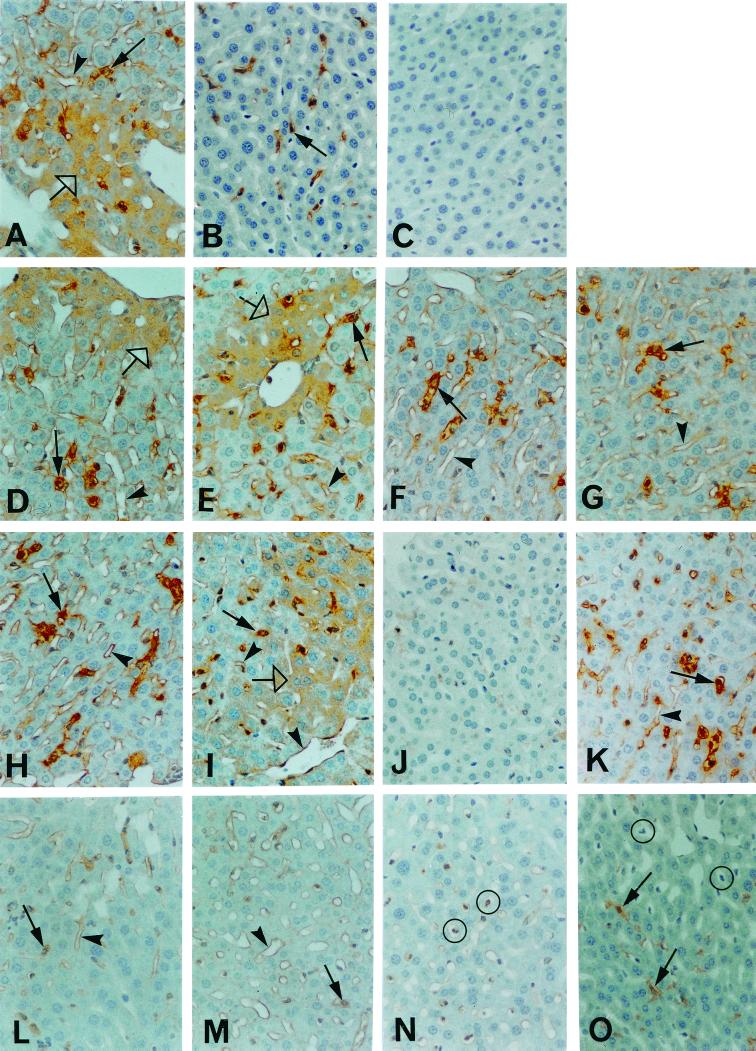
FIG. 2.
Glutathione depletion differentially inhibits hepatic inflammatory responses in mice challenged with 5 × 107 E. coli bacteria. All injections were by the i.p. route. Immunohistology was used to detect the expression of specific proteins in the livers of the following groups of mice. (A) HSP-32 staining in mice 5 h after the injection of DEM (see Fig. 1A). Note the expression of HSP-32 by Kupffer cells (solid arrow), hepatocytes (open arrow) and hepatic sinusoidal endothelial cells (arrowhead). (B) TNF-α staining in Kupffer cells (solid arrow) 1.5 h after vehicle-treated mice were challenged with E. coli bacteria. (C) TNF-α staining 1.5 h after DEM-treated mice were challenged with E. coli. Note the absence of TNF-α expression. (D) iNOS staining of Kupffer cells (solid arrow), hepatocytes (open arrow), and hepatic sinusoidal endothelial cells (arrowhead) 6 h after challenging vehicle-treated mice with E. coli. (E) iNOS staining in DEM-treated mice challenged with E. coli. (F) ICAM-1 staining of Kupffer cells (solid arrow) and endothelial cells (arrowhead) 6 h after challenge of the vehicle control group with E. coli. (G) ICAM-1 staining in DEM-treated, E. coli-challenged mice. (H) iNOS staining in DEM-treated mice that were challenged with 4 mg of LPS/kg of body weight showing positive Kupffer cells (solid arrow) and sinusoidal endothelial cells (arrowhead). (I) iNOS staining in DEM-treated mice that were challenged with 4 mg LPS plus 80 μg of recombinant IFN-γ per kg of body weight. Note the staining of all three hepatic cell types, which is similar to that seen in infected mice (panel E above). (J) ICAM-1 staining in DEM-treated mice that were challenged with LPS. (K) ICAM-1 staining in DEM-treated mice challenged with LPS and recombinant IFN-γ. The intense staining is similar that seen in infected mice (panel G above). (L) ICAM-1 staining of Kupffer cells (solid arrow) and endothelial cells (arrowhead) in wild-type BALB/c mice infected with E. coli. (M) ICAM-1 staining in wild-type BALB/c mice treated with DEM and then infected with E. coli. Note the similar staining pattern as in panel L. (N) ICAM-1 staining in IFN-γ-null mice treated with DEM and challenged with E. coli. Note the presence of sinusoidal leukocytes expressing ICAM-1 (circled). (O) F4/80 staining of Kupffer cells (solid arrow) in IFN-γ-null mice treated with DEM and challenged with E. coli. Sinusoidal leukocytes (circled) are negative. Original magnification, ×250.
Effects of glutathione depletion on host responses to live E. coli bacteria.
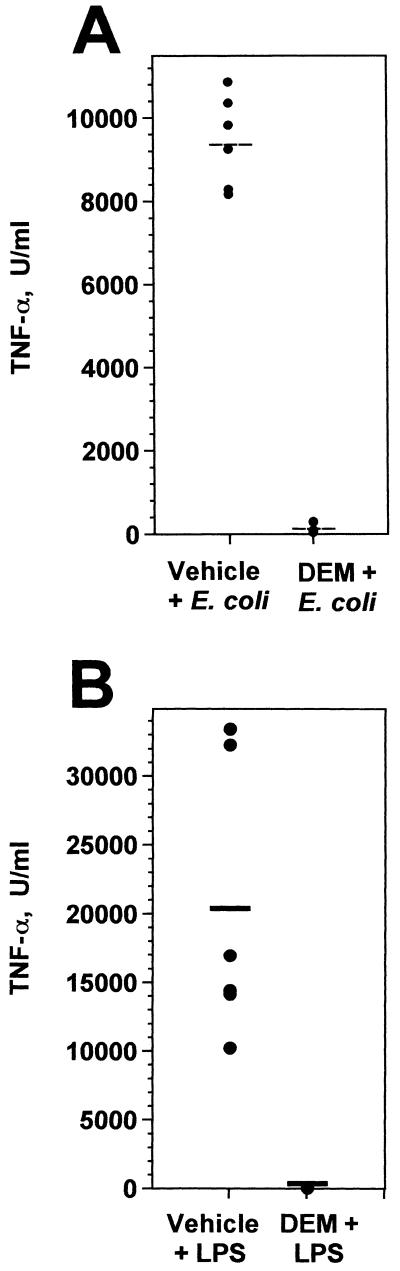
To determine the effects of oxidative stress on hepatic inflammatory responses to E. coli, groups of mice were treated with either vehicle (sesame oil) or DEM and challenged i.p. with 4 × 107 to 5 × 107 CFU of E. coli O111:B4 bacteria 2 h later. This dose represents 3 to 4 50% lethal doses for this bacterial strain when injected by the i.p. route. Sera were collected 90 min later, and circulating TNF-α concentrations were measured. While mice that had been treated with vehicle and challenged with bacteria showed high circulating levels of TNF-α, DEM treatment almost completely blocked this response to bacterial challenge (Fig. 3A). This inhibitory effect was due to a marked decrease in TNF-α synthesis, as determined by enumerating TNF-α-producing Kupffer cells in the liver by immunohistology (Fig. 2B and C).
FIG. 3.
DEM inhibits the serum TNF-α response to bacterial or LPS challenge. Groups of C3HeB/FeJ mice (per group) were treated with either vehicle or DEM and then challenged i.p. 2 h later with either 5 × 107 viable E. coli bacteria (A) or 100 μg of E. coli LPS (B). Blood was collected 1.5 h later, and serum TNF-α was determined by bioassay, which had a detection limit of 50 U/ml. Horizontal lines represent group means. In both panels A and B, the DEM-treated and control groups differed significantly (P < 0.01). Comparable results were obtained with CF1 mice.
The effects of glutathione depletion on macrophage activation in the liver were selective in the sense that E. coli-induced Kupffer cell iNOS expression was unaffected by DEM treatment (Fig. 2D and E). Hepatocytes and sinusoidal endothelial cells also expressed iNOS 6 h after an i.p. challenge with E. coli, but neither of these responses was affected by DEM treatment. Similarly, the induction of ICAM-1 expression by endothelial cells and Kupffer cells was comparable in the vehicle and DEM-treated groups (Fig. 2F and G). Thus, unlike the TNF-α response in the liver, hepatic iNOS and ICAM-1 responses to bacterial infection were unaffected by glutathione depletion. Importantly, depleting hepatic glutathione with BSO produced an identical pattern of responses to E. coli challenge (Table 1).
TABLE 1.
Effects of BSO on E. coli-induced inflammatory responses in the mouse livera
| Antigen | Treatment | Positive cellsb
|
||
|---|---|---|---|---|
| KC (cells/HPF) | Hep (cells/HPF) | EC (intensity score) | ||
| TNF-α | Vehicle | 17.5 ± 1.9 | — | 0 |
| BSO | 0.6 ± 0.8∗ | — | 0 | |
| iNOS | Vehicle | 16.7 ± 2.0 | 41.8 ± 3.9 | 1 |
| BSO | 15.0 ± 1.9 | 38.3 ± 5.3 | 1 | |
| ICAM-1 | Vehicle | 23.0 ± 3.0 | — | 3 |
| BSO | 19.7 ± 3.1 | — | 3 | |
Female C3HeB/FeJ mice (six per group) were challenged i.p. with 4 × 107 CFU of E. coli O111:B4 bacteria, and their livers were recovered either 1.5 h (for TNF-α staining) or 6 h (for iNOS and ICAM-1 staining) later. Mice were treated with either 0.15 M NaCl (vehicle) or 5 mmol of BSO/kg at 6 h and again at 3 h prior to challenge with bacteria. At least 5 randomly selected HPF (×400; HPF = 0.11 mm2) were scored for each tissue section.
Values are means ± SD. KC, Kupffer cell; Hep, hepatocyte; EC, sinusoidal endothelial cell. Semiquantitative scoring of endothelial cell staining used an intensity range from 0 to 3 (see Materials and Methods). ∗, this group differed significantly from the vehicle control group (P < 0.01). —, none detected.
Responses to E. coli bacteria versus purified E. coli LPS.
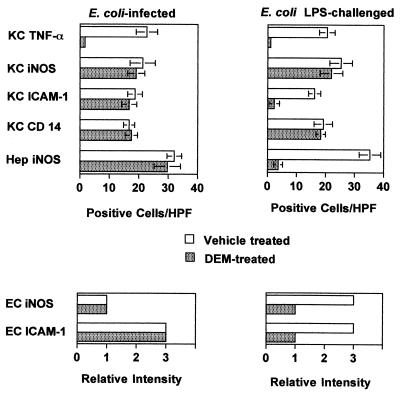
The effects of DEM on the TNF-α, iNOS, ICAM-1, and CD14 responses to E. coli O111:B4 bacteria were then compared to its effects on responses to chemically purified LPS of the same E. coli serotype. For this purpose, mice were injected i.p. with 100 μg of LPS, a dose which elicited inflammatory responses comparable to those elicited by live bacterial challenge. The semiquantitative data that are summarized in Fig. 4 were derived from immunohistology experiments of the type shown in Fig. 2, in which the densities or staining intensities of positive cells were recorded. It is clear from this analysis that Kupffer cell TNF-α responses to both E. coli and LPS challenge were significantly inhibited by DEM treatment, and this effect was confirmed when serum TNF-α responses to bacterial and LPS challenge were measured (Fig. 3A and B). Although all other responses to E. coli challenge were unaffected by DEM treatment (Fig. 4, left panels), the effects of glutathione depletion on hepatic responses to LPS were entirely different. Kupffer cell ICAM-1, hepatocyte, and endothelial cell iNOS and endothelial cell ICAM-1 responses to LPS were all markedly inhibited by DEM (Fig. 4, right panels). Thus, the ability of redox imbalance to regulate hepatic inflammatory responses to live gram-negative bacteria was fundamentally different from its effects on the same responses to LPS.
FIG. 4.
Comparison of the effects of glutathione depletion on hepatic inflammatory responses to E. coli bacteria and LPS. C3HeB/FeJ mice (six per group) were treated with vehicle or DEM and then challenged i.p. with either 5 × 107 CFU of E. coli bacteria or 100 μg of E. coli LPS. Liver tissues were recovered either 1.5 h (for TNF-α) or 6 h (for iNOS, CD14 or ICAM-1) later. Kupffer cell (KC) and hepatocyte (Hep) responses are expressed as positive cells per HPF (magnification, ×400) (mean ± standard deviation), whereas sinusoidal endothelial cell (EC) responses are expressed as relative staining intensity. Comparable results were obtained with CF1 mice.
IFN-γ prevents the inhibitory effects of glutathione depletion in the liver.
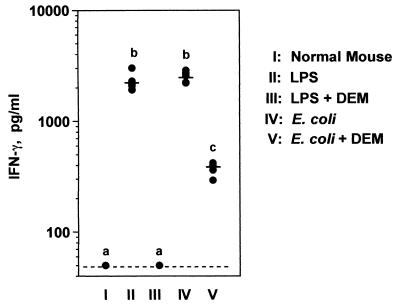
We next asked whether the production of IFN-γ was inhibited by glutathione depletion and whether the ability of the different groups of mice to produce IFN-γ might explain the findings described above. Control and DEM-treated CF1 mice were challenged with either LPS or live bacteria, and 6 h later their sera were collected for the measurement of IFN-γ. LPS and E. coli organisms stimulated comparable IFN-γ responses (Fig. 5). Of interest, DEM-treated mice did not produce detectable IFN-γ when challenged with LPS. However, when challenged with bacteria, the serum IFN-γ levels of DEM-treated mice, although decreased, were substantial (i.e., at least eight times those of unchallenged mice). This effect was also seen in mice of the C57BL/6 and BALB/c strains and indicates that infected animals maintained the ability to produce significant quantities of IFN-γ despite glutathione depletion.
FIG. 5.
Effects of DEM on IFN-γ production in response to LPS or E. coli bacteria. CF1 mice (six per group) were treated with vehicle or DEM for 2 h and then challenged i.p. with either 100 μg of E. coli LPS or 5 × 107 CFU of E. coli bacteria. Serum was collected 6 h later, and IFN-γ concentrations were measured by ELISA. The dashed line represents the detection limit of this particular assay (50 pg/ml). Solid lines represent group means. Groups designated by different lowercase letters are significantly different from each other (P < 0.01). This experiment was performed a total of five times in CF1, C57BL/6, and BALB/c mice with similar results.
To ascertain whether IFN-γ produced in response to infection provided an alternative signal for iNOS and ICAM-1 expression in DEM-treated mice that was not induced by LPS, animals were challenged with either 100 μg of LPS or LPS plus 2 μg of mouse recombinant IFN-γ. This dose of IFN-γ by itself did not stimulate iNOS or ICAM-1 expression, but has been shown by others to alter inflammatory responses to LPS (22, 25, 26). Six hours after challenge, liver samples were obtained, and hepatic iNOS and ICAM-1 responses were measured. The results, shown in Fig. 2H to K and summarized in Table 2, indicate that exogenous IFN-γ significantly altered host responses in the livers of glutathione-depleted, LPS-challenged mice. While the TNF-α response was not restored by IFN-γ, hepatic iNOS and ICAM-1 expression was significantly enhanced when IFN-γ was coinjected with LPS. Thus, the combination of LPS and IFN-γ induced the same responses in DEM-treated mice (i.e., hepatocyte iNOS [Fig. 2I] and Kupffer cell and endothelial cell ICAM-1 [Fig. 2K]) that were seen after E. coli challenge. Recombinant IFN-γ did not increase the frequency of iNOS-positive Kupffer cells in DEM-treated, LPS-stimulated mice (Table 2), indicating that the cytokine did not simply prime cells for all LPS-induced responses. Because the frequency of F4/80-positive Kupffer cells was equivalent in the two treatment groups, it is also unlikely that IFN-γ increased Kupffer cell ICAM-1 expression by recruiting mononuclear phagocytes to the liver. Rather, the results indicate that exogenous IFN-γ converted several stress-sensitive, LPS-induced responses to stress-resistant responses that mimicked what was seen in infected animals.
TABLE 2.
IFN-γ restores the hepatic iNOS and ICAM-1 responses to LPS in DEM-treated CF1 micea
| Antigen | Challenge | Positive cellsb
|
||
|---|---|---|---|---|
| KC (cells/HPF) | Hep (cells/HPF) | EC (intensity score) | ||
| TNF-α | LPS | <0.2 | — | 0 |
| LPS + IFN-γ | <0.2 | — | 0 | |
| iNOS | LPS | 15.4 ± 2.3 | 0.6 ± 0.9 | 1 |
| LPS + IFN-γ | 17.6 ± 2.3 | 28.8 ± 4.6∗ | 2 | |
| ICAM-1 | LPS | 4.0 ± 1.6 | — | 1 |
| LPS + IFN-γ | 14.0 ± 4.1∗ | — | 2 | |
| F4/80 | LPS | 24.6 ± 4.3 | — | 0 |
| LPS + IFN-γ | 26.4 ± 3.8 | — | 0 | |
CF1 mice (five per group) were injected i.p. with DEM (5.3 mmol/kg) 2 h prior to challenge. Mice were challenged i.p. with either 100 μg of E. coli O111:B4 LPS or LPS plus 2 μg of recombinant IFN-γ. Liver samples were recovered 6 h later for immunohistology and read at ×400 magnification.
See Table 1, footnotes a and b. ∗, these groups differed significantly from their controls (LPS only) (P < 0.01).
To determine whether IFN-γ mediated these inflammatory responses to E. coli challenge in glutathione-depleted mice, wild-type BALB/c (IFN-γ+/+) and homozygous IFN-γ-null (IFN-γ−/−) mutant mice were treated with either vehicle or DEM and then challenged with E. coli bacteria. Glutathione-depleted wild-type mice retained the ability to express both hepatic ICAM-1 and iNOS when challenged with bacteria (Fig. 2L and M and Table 3), despite a significant decrease in their IFN-γ responses. In contrast, IFN-γ-null mice that had been treated with DEM showed markedly reduced hepatic iNOS and ICAM-1 expression (i.e., hepatocyte iNOS, Kupffer cell ICAM-1, and sinusoidal endothelial cell iNOS and ICAM-1) (Fig. 2N and Table 3). This was also seen in IFN-γ-deficient mice that had been treated with vehicle and then challenged with bacteria. Therefore, the production of IFN-γ was essential for these responses in infected animals. Numerous ICAM-1-positive leukocytes were present within the hepatic sinusoids of IFN-γ-null mice 6 h after infection (Fig. 2N), but these intrasinusoidal leukocytes did not express the F4/80 monocyte-macrophage marker (Fig. 2O).
TABLE 3.
Role of IFN-γ in regulating E. coli-induced hepatic inflammatory mediator expression in DEM-treated micea
| Treatmentb | Mouse strain | Serum IFN-γ (pg/ml) | Antigen (cell type)-positive cells/HPFc
|
Intensity score
|
|||
|---|---|---|---|---|---|---|---|
| iNOS (Hep) | iNOS (KC) | ICAM-1 (KC) | iNOS (EC) | ICAM-1 (EC) | |||
| Vehicle | IFN-γ+/+ | 3,360 | 38.4 ± 9.9 | 20.3 ± 3.2 | 20.0 ± 3.5 | 3 | 3 |
| DEM | IFN-γ+/+ | 275 | 33.6 ± 9.8 | 18.7 ± 5.5 | 23.3 ± 7.4 | 3 | 3 |
| Vehicle | IFN-γ−/− | <33 | 4.1 ± 6.2∗ | 16.4 ± 1.0 | 1.0 ± 1.1∗ | 1 | 1 |
| DEM | IFN-γ−/− | <33 | 1.7 ± 1.4∗ | 13.4 ± 3.4 | 1.9 ± 0.5∗ | 1 | 1 |
BALB/c wild-type or mutant IFN-γ-null mice (five per group) were treated with vehicle or DEM and challenged with E. coli 2 h later. Liver tissue and blood samples were recovered 6 h later. Shown are the average values from two experiments. The detection limit of the IFN-γ assay was 33 pg/ml. ∗, this group differed significantly from the corresponding wild-type group (P < 0.01). Also see Table 1, footnote a.
See Fig. 1.
Values are means ± SD.
DISCUSSION
Previously we reported that hepatic TNF-α and iNOS responses to Salmonella enteritidis LPS were selectively inhibited in C3HeB/FeJ mice by glutathione depletion (46). The present study sought to extend these findings to mice with gram-negative bacterial infections and has yielded a number of unexpected findings regarding the effects of tissue redox imbalance. We selected a monomicrobial gram-negative infection model to enable a direct comparison between bacterial infection and challenge with LPS purified from the same species and serotype (i.e., E. coli O111:B4). Although the magnitude and cell type-specific expression of several different inflammatory mediators were comparable in LPS-challenged and E. coli-infected mice (Fig. 3, 4, and 5), the effects of oxidative stress on responses to these two microbial stimuli were fundamentally different. Glutathione depletion significantly inhibited hepatic TNF-α, iNOS, and ICAM-1 responses to LPS and eliminated IFN-γ production. By contrast, only one of these responses (TNF-α production) was inhibited to a similar extent in mice that were challenged with live E. coli bacteria. The fact that DEM and BSO treatment produced essentially identical inhibitory effects supports the conclusion that glutathione depletion per se was responsible for these changes. Because hepatocytes, Kupffer cells, and sinusoidal endothelial cells each expressed HSP-32 following DEM and BSO treatment and showed specific changes in their responses to LPS challenge, it is likely that glutathione depletion directly affected each of these cell types.
We cannot presently exclude the possibility that the differences between the effects of oxidative stress on host responses to LPS versus live bacteria resulted from differences in the tissue distribution, physical-chemical composition, and/or dose of endotoxic LPS delivered under these two conditions of microbial challenge. Indeed, Ge et al. (17) have reported that LPS localizes to different hepatic cell types in rats injected with chemically purified LPS versus animals infected with live E. coli bacteria. We have recently reproduced these findings in C3H mice (M. J. Parmely and F. Wang, unpublished data). In LPS-challenged animals, immunoreactive LPS was found in both hepatocytes and Kupffer cells, whereas antigenic LPS was not detected in hepatocytes following E. coli challenge. Kupffer cells accumulated LPS under both conditions. In the present study, LPS-induced but not E. coli-induced Kupffer cell ICAM-1 expression was inhibited by oxidative stress. Thus, glutathione depletion had remarkably different effects on the Kupffer cell ICAM-1 responses to LPS and E. coli, despite similar LPS localization to this cell type following these two forms of microbial challenge.
Responsiveness to LPS is not necessary for the induction of many host responses to viable E. coli bacteria (5,14), indicating that non-LPS bacterial components (e.g., bacterial DNA) also activate inflammatory mediator expression in infected animals. If these responses are ultimately shown to arise from novel signaling pathways that are activated in infected but not endotoxemic mice, the present study will have established that at least some LPS-independent pathways are relatively resistant to changes in cellular redox state.
The expression of ICAM-1 and iNOS in the rodent liver is induced by endotoxin (10, 37, 45) and ischemia-reperfusion (4), and TNF-α appears to provide an important proximal signal for the induction of these genes (4, 11, 37). For this reason, we were not surprised that LPS-induced hepatic ICAM-1 and iNOS expression declined in parallel with the loss of TNF-α production in glutathione-depleted, LPS-challenged mice. However, the hepatic ICAM-1 and iNOS responses to E. coli were undiminished by DEM or BSO treatment, despite a comparable inhibition of TNF-α production in infected animals. These findings strongly suggest that ICAM-1 and iNOS induction during gram-negative bacterial infections is not entirely dependent upon TNF-α production and that alternative signaling pathways exist for eliciting these responses.
A number of findings reported here suggest that the production of IFN-γ is important in determining the ultimate effects of redox imbalance on hepatic inflammatory responses to gram-negative bacteria in the mouse. Whereas serum IFN-γ was undetectable in glutathione-depleted mice that had been challenged with LPS, DEM treatment failed to completely block IFN-γ responses to bacterial challenge. When IFN-γ was coadministered with LPS to DEM-treated animals, their hepatic inflammatory responses were indistinguishable from those of glutathione-depleted, E. coli-infected mice. In other words, hepatic iNOS and ICAM-1 responses to LPS were completely restored by cochallenge with exogenous IFN-γ. Conversely, the ability of glutathione-depleted mice to produce IFN-γ was essential for hepatic iNOS and ICAM-1 responses to E. coli challenge, because the responses were absent from DEM-treated IFN-γ-null mice. The finding that infected IFN-γ-deficient mice with normal hepatic glutathione also failed to express iNOS or ICAM-1 strongly suggests that DEM acts by inhibiting IFN-γ production rather than its action. Overall, these results indicate that IFN-γ is an important signal for the induction of hepatic iNOS and ICAM-1 responses to gram-negative bacterial infection during oxidative stress. Conversely, the lack of iNOS and ICAM-1 responses in glutathione-depleted, LPS-challenged mice appears to result from their failure to produce this cytokine.
The production of IFN-γ in mice challenged with LPS is mediated almost entirely by activated NK-1+ natural killer cells and NK T cells (9, 30, 31, 39, 42). Non-LPS microbial components often induce more complex patterns of cell activation and IFN-γ production (9, 30, 39). For example, Nguyen and Biron (30) showed that C57BL/6 mice infected with lymphocytic choriomeningitis virus produced greatly elevated levels of circulating IFN-γ when challenged with LPS compared to uninfected, LPS-challenged mice. Mice challenged only with virus produced very little IFN-γ. The heightened response to cochallenge was due to the production of IFN-γ by CD4+ and CD8+ T cells in addition to NK cells. Likewise, Seki et al. (39) have reported that polymicrobial peritonitis in BALB/c and C57BL/6 mice resulting from cecal ligation and puncture activated large numbers of IFN-γ-producing cells in the liver. Approximately 20% of these cells lacked the NK-1 marker. These reports raise the interesting possibility that undefined components of gram-negative bacteria induce novel IFN-γ responses by cells not activated in LPS-challenged animals. The present study would predict that this latter portion of the IFN-γ response to infection is not inhibited by tissue redox imbalance and mediates important host responses to infection.
ACKNOWLEDGMENTS
Financial support for this work was provided by the American Heart Association (grants KS-97-GS-62 and 51345Z) and the Margaret Jane Harley Fund.
We thank David Morrison for providing E. coli strain O111:B4 bacteria and Fred Samson for reviewing the manuscript.
REFERENCES
- 1.Bailey H H, Ripple G, Tutsch K D, Arzoomanian R Z, Alberti D, Feierabend C, Mahvi D, Schink J, Pomplun M, Mulcahy R T, Wilding G. Phase I study of continuous-infusion l-S,R-buthionine sulfoximine with intravenous melphalan. J Natl Cancer Inst. 1997;89:1789–1796. doi: 10.1093/jnci/89.23.1789. [DOI] [PubMed] [Google Scholar]
- 2.Billiau A, Heremans H, Vanderkerckhove F, Dillen C. Anti-interferon-γ antibody protects mice against the generalized Shwartzman reaction. Eur J Immunol. 1987;17:1851–1854. doi: 10.1002/eji.1830171228. [DOI] [PubMed] [Google Scholar]
- 3.Car B C, Eng V M, Schnyder B, Ozmen L, Huang S, Gallay P, Heumann D, Aguet M, Ryffel B. Interferon γ receptor deficient mice are resistant to endotoxin shock. J Exp Med. 1994;179:1437–1444. doi: 10.1084/jem.179.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colletti M M, Cortis A, Lukacs N, Kunkel S L, Green M, Strieter R M. Tumor necrosis factor up-regulates intercellular adhesion molecule 1, which is important in the neutrophil-dependent lung and liver injury associated with hepatic ischemia and reperfusion in the rat. Shock. 1998;10:182–191. doi: 10.1097/00024382-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Cross A S, Sadoff J C, Bernton N K, Gemski P. Pretreatment with recombinant murine tumor necrosis factor and murine interleukin-1 protects mice from lethal infection. J Exp Med. 1989;169:2021–2027. doi: 10.1084/jem.169.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cross A S, Opal S M, Sadoff J C, Gemski P. Choice of bacteria in animal models of sepsis. Infect Immun. 1993;61:2741–2747. doi: 10.1128/iai.61.7.2741-2747.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeForge L E, Preston A M, Takeuchi E, Kenney J, Boxer L A, Remick D G. Regulation of interleukin 8 gene expression by oxidant stress. J Biol Chem. 1993;268:25568–25576. [PubMed] [Google Scholar]
- 8.Deitch E A. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Dobashi H, Seki S, Habu Y, Ohkawa T, Takeshita S, Hiraide H, Sekine I. Activation of mouse liver natural killer cells and NK1.1+ T cells by bacterial superantigen-primed Kupffer cells. Hepatology. 1999;30:430–436. doi: 10.1002/hep.510300209. [DOI] [PubMed] [Google Scholar]
- 10.Essani N A, McGuire G M, Manning A M, Jaeschke H. Differential induction of mRNA for ICAM-1 and selectins in hepatocytes, Kupffer cells and endothelial cells during endotoxemia. Biochem Biophys Res Commun. 1995;211:74–82. doi: 10.1006/bbrc.1995.1780. [DOI] [PubMed] [Google Scholar]
- 11.Essani N A, Fisher M A, Farhood A, Manning A M, Smith C W, Jaeschke H. Cytokine-induced upregulation of hepatic intercellular adhesion molecule-1 messenger RNA expression and its role in the pathophysiology of murine endotoxin shock and acute liver failure. Hepatology. 1995;21:1632–1639. [PubMed] [Google Scholar]
- 12.Evans G F, Snyder Y M, Butler L D, Zuckerman S H. Differential expression of interluekin-1 and tumor necrosis factor in murine septic shock models. Circ Shock. 1989;29:279–290. [PubMed] [Google Scholar]
- 13.Evans T, Carpenter A, Silva A, Cohen J. Differential effects of monoclonal antibodies to tumor necrosis factor alpha and gamma interferon on induction of hepatic nitric oxide synthase in experimental gram-negative sepsis. Infect Immun. 1992;60:4133–4139. doi: 10.1128/iai.60.10.4133-4139.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evans T J, Strivens E, Carpenter A, Cohen J. Differences in cytokine response and induction of nitric oxide synthase in endotoxin-resistant and endotoxin-sensitive mice after intravenous Gram-negative infection. J Immunol. 1993;150:5033–5040. [PubMed] [Google Scholar]
- 15.Ewing J F, Maines M D. Glutathione depletion induces heme oxygenase-1 (HSP32) mRNA and protein in rat brain. J Neurochem. 1993;60:1512–1519. doi: 10.1111/j.1471-4159.1993.tb03315.x. [DOI] [PubMed] [Google Scholar]
- 16.Feng L, Xia Y, Garcia G E, Hwang D, Wilson C G. Involvement of reactive oxygen intermediates in cyclooxygenase-2 expression induced by interleukin-1, tumor necrosis factor-α, and lipopolysaccharide. J Clin Investig. 1995;95:1669–1675. doi: 10.1172/JCI117842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge Y, Ezzell R M, Clark B D, Loiselle P M, Amato S F, Warren H S. Relationship of tissue and cellular interleukin-1 and lipopolysaccharide after endotoxemia and bacteremia. J Infect Dis. 1997;176:1313–1321. doi: 10.1086/514127. [DOI] [PubMed] [Google Scholar]
- 18.Griffith O W, Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine) J Biol Chem. 1979;254:7558–7560. [PubMed] [Google Scholar]
- 19.Griffith O W. Biologic and pharmacologic regulation of mammalian gluthathione synthesis. Free Radical Biol Med. 1999;27:922–935. doi: 10.1016/s0891-5849(99)00176-8. [DOI] [PubMed] [Google Scholar]
- 20.Griscavage J M, Rogers N E, Sherman M P, Ignarro L J. Inducible nitric oxide synthase from a rat alveolar macrophage cell line is inhibited by nitric oxide. J Immunol. 1993;151:6329–6337. [PubMed] [Google Scholar]
- 21.Gutierrez-Ramos J, Bluethmann H. Molecules and mechanisms operating in septic shock: lessons from knockout mice. Immunol Today. 1997;18:329–334. doi: 10.1016/s0167-5699(97)01085-2. [DOI] [PubMed] [Google Scholar]
- 22.Heinzel F P. The role of IFN-γ in the pathology of experimental endotoxemia. J Immunol. 1990;145:2920–2924. [PubMed] [Google Scholar]
- 23.Jaeschke H. Cellular adhesion molecules: regulation and functional significance in the pathogenesis of liver diseases. Am J Physiol. 1997;273:G602–G611. doi: 10.1152/ajpgi.1997.273.3.G602. [DOI] [PubMed] [Google Scholar]
- 24.Kamijo R, Le J, Shapiro D, Havell E A, Huang S, Aguet M, Bosland M, Vilcek J. Mice that lack the interferon-γ receptor have profoundly altered responses to infection with bacillus Calmette-Guerin and subsequent challenge with lipopolysaccharide. J Exp Med. 1993;178:1435–1440. doi: 10.1084/jem.178.4.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katschinski T, Galanos C, Coumbos A, Freudenberg M A. Gamma interferon mediates Proprionibacterium acnes-induced hypersensitivity to lipopolysaccharide in mice. Infect Immun. 1992;60:1994–2001. doi: 10.1128/iai.60.5.1994-2001.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohler J, Heumann D, Garotta G, LeRoy D, Bailat S, Barras C, Baumgartner J-D, Glauser M P. IFN-γ involvement in the severity of Gram-negative infections in mice. J Immunol. 1993;151:916–921. [PubMed] [Google Scholar]
- 27.Le Roy D, Heumann D, Glauser M-P, Mauel J, Smith J, Betz Corradin S. Nitric oxide production in experimental Gram-negative infections: studies with cytokine receptor-deficient mice. Shock. 1998;10:37–42. doi: 10.1097/00024382-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Matuschak G M, Johanns C A, Chen Z, Gaynor J, Lechner A J. Brief hypoxic stress downregulates E. coli-induced IL-1α and IL-1β gene expression in perfused liver. Am J Physiol. 1996;271:R1311–R1318. doi: 10.1152/ajpregu.1996.271.5.R1311. [DOI] [PubMed] [Google Scholar]
- 29.Nathens A B, Biaar R, Watson R W G, Issekutz T B, Marshall J C, Dackiw A P B, Rotstein O D. Thiol-mediated regulation of ICAM-1 expression in endotoxin-induced acute lung injury. J Immunol. 1998;160:2959–2966. [PubMed] [Google Scholar]
- 30.Nguyen K B, Biron C A. Synergism for cytokine-mediated disease during concurrent endotoxin and viral challenges: roles for NK and T cell IFN-γ production. J Immunol. 1999;162:5238–5246. [PubMed] [Google Scholar]
- 31.Ogasawara K, Takeda K, Hashimoto W, Tatoh M, Okuyama R, Yanai N, Obinata M, Kumagai K, Takada H, Hiraide H, Seki S. Involvement of NK1+ T cells and their IFN-γ production in the generalized Shwartzman reaction. J Immunol. 1998;160:3522–3527. [PubMed] [Google Scholar]
- 32.Pacht E R, Timerman A P, Lykens M G, Merola A J. Deficiency of alveolar fluid glutathione in patients with sepsis and ARDS. Chest. 1991;100:1397–1403. doi: 10.1378/chest.100.5.1397. [DOI] [PubMed] [Google Scholar]
- 33.Parent C, Eichacker P Q. Neutrophil and endothelial cell interactions in sepsis: the role of adhesion molecules. Infect Dis Clin North Am. 1999;13:427–447. doi: 10.1016/s0891-5520(05)70084-2. [DOI] [PubMed] [Google Scholar]
- 34.Parmely M J, Gale A, Clabaugh M, Horvat R, Zhou W-W. Proteolytic inactivation of cytokines by Pseudomonas aeruginosa. Infect Immun. 1990;58:3009–3014. doi: 10.1128/iai.58.9.3009-3014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parmely M J. Nitric oxide as a signaling molecule in the systemic inflammatory response to LPS. In: Brade H, Morrison D, Opal S, Vogel S, editors. Endotoxin in health and disease. New York, N. Y: Marcel Dekker; 1999. pp. 591–604. [Google Scholar]
- 36.Peavy D L, Fairchild E J., II Evidence for lipid peroxidation in endotoxin-poisoned mice. Infect Immun. 1986;52:613–616. doi: 10.1128/iai.52.2.613-616.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salkowski S A, Detore G, McNally R, van Rooijen R, Vogel S N. Regulation of inducible nitric oxide synthase messenger RNA expression and nitric oxide production by lipopolysaccharide in vivo. J Immunol. 1997;158:905–912. [PubMed] [Google Scholar]
- 38.Scheffler L A, Wink D A, Milillo G, Cox G W. Exogenous nitric oxide regulates IFN-γ plus lipopolysaccharide-induced nitric oxide synthase expression in mouse macrophages. J Immunol. 1995;155:886–894. [PubMed] [Google Scholar]
- 39.Seki S, Osada S I, Ono S, Aosasa S, Habu Y, Nishikage T, Homchizuki M, Hiraide H. Role of liver NK cells and peritoneal macrophages in gamma intereferon and interleukin-10 production in experimental bacterial peritonitis in mice. Infect Immun. 1998;66:5286–5294. doi: 10.1128/iai.66.11.5286-5294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith S R, Terminelli C, Kenworthy-Bott L, Calzetta A, Donkin J. The cooperative effects of TNF-α and IFN-γ are determining factors in the ability of IL-10 to protect mice from lethal endotoxemia. J Leukocyte Biol. 1994;55:711–718. doi: 10.1002/jlb.55.6.711. [DOI] [PubMed] [Google Scholar]
- 41.Symeonides S, Balk R A. Nitric oxide in the pathogenesis of sepsis. Infect Dis Clin North Am. 1999;13:449–463. doi: 10.1016/s0891-5520(05)70085-4. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi M, Ogasawara K, Taakeda K, Hashimoto W, Sakihara H, Kumagai K, Anzai R, Satoh M, Seki S. LPS induces NK-1.1+αβ T cells with potent cytotoxicity in the liver of mice via production of IL-12 from Kupffer cells. J Immunol. 1996;156:2436–2442. [PubMed] [Google Scholar]
- 43.Taylor D E, Ghio A J, Piantadosi C A. Reactive oxygen species produced by liver mitochondria of rats in sepsis. Arch Biochem Biophys. 1995;316:70–76. doi: 10.1006/abbi.1995.1011. [DOI] [PubMed] [Google Scholar]
- 44.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 45.Vos T A, van Goor H, Tuyt L, de Jager-Krikken A, Leuvenink R, Kuipers F, Jansen P L M, Moshage H. Expression of inducible nitric oxide synthase in endotoxemic rat hepatocytes is dependent on the cellular glutathione status. Hepatology. 1999;29:421–426. doi: 10.1002/hep.510290231. [DOI] [PubMed] [Google Scholar]
- 46.Wang F, Wang L Y, Wright D, Parmely M J. Redox imbalance differentially inhibits lipopolysaccharide-induced macrophage activation in the mouse liver. Infect Immun. 1999;67:5409–5416. doi: 10.1128/iai.67.10.5409-5416.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wibbenmeyer L A, Lechner A J, Munoz C F, Matuschak G M. Downregulation of E. coli-induced TNF-α expression in perfused liver by hypoxia-reoxygenation. Am J Physiol. 1995;268:G311–G319. doi: 10.1152/ajpgi.1995.268.2.G311. [DOI] [PubMed] [Google Scholar]
- 48.Zanetti G, Heumann D, Gerain J, Kohler J, Abbet P, Barras C, Lucas R, Glauser M-P, Baumgartner J-D. Cytokine production after intravenous or peritoneal Gram-negative bacterial challenge in mice. Comparative protective efficacy of antibodies to tumor necrosis factor-α and to lipopolysaccharide. J Immunol. 1992;148:1890–1897. [PubMed] [Google Scholar]