Abstract
Laparoscopic cholecystectomy is a standard treatment for patients with gallstones in the gallbladder. However, multiple risk factors affect the probability of conversion from laparoscopic cholecystectomy to open surgery. A greater understanding of the preoperative factors related to conversion is crucial to improve patient safety. In the present systematic review, we summarized the current knowledge about the main factors associated with conversion. Next, we carried out several meta-analyses to evaluate the impact of independent clinical risk factors on conversion rate. Male gender (OR = 1.907; 95%CI = 1.254–2.901), age > 60 years (OR = 4.324; 95%CI = 3.396–5.506), acute cholecystitis (OR = 5.475; 95%CI = 2.959–10.130), diabetes (OR = 2.576; 95%CI = 1.687–3.934), hypertension (OR = 1.931; 95%CI = 1.018–3.662), heart diseases (OR = 2.947; 95%CI = 1.047–8.296), obesity (OR = 2.228; 95%CI = 1.162–4.271), and previous upper abdominal surgery (OR = 3.301; 95%CI = 1.965–5.543) increased the probability of conversion. Our analysis of clinical factors suggested the presence of different preoperative conditions, which are non-modifiable but could be useful for planning the surgical scenario and improving the post-operatory phase.
Keywords: conversion, laparoscopic cholecystectomy, open surgery, risk factors
1. Introduction
The laparoscopic cholecystectomy (LC)—firstly introduced in the early 1990s—is a routine surgical procedure used for the treatment of gallstones in the gallbladder, usually characterized by cholesterol or bilirubin undissolved in bile [1]. Currently, LC is considered the gold standard, with many advantages over traditional open cholecystectomy (OC), such as related decreased postoperative complications and shorter hospitalization [2]. The consensus for LC, a mini-invasive technique with a great cosmetic result and significant cost reductions, is still growing [2,3,4]. However, OC still has a place in the treatment of gallstones in the gallbladder. Specifically, OC is principally preserved for the challenging cases in which laparoscopy fails [2]. The term “conversion” refers only to cases in which cholecystectomy starts in laparoscopic and finishes in a laparotomic way [5]. Thus, the well-selected cases with specific indications for the open technique (e.g., suspected or confirmed gallbladder cancer) are not considered as cases of conversion [2]. Current literature suggests that the rate of intraoperative conversion from LC to OC ranges from 1 to 15% [6]. Conversion rates vary widely, depending on several risk factors—including old age [7], male gender [8,9], obesity [10,11], previous abdominal surgery [12,13], diabetes [3] and acute cholecystitis [14]. Despite that patient-specific factors associated with a higher likelihood of conversion have been thoroughly studied, previous reviews agreed on only two important risk factors—male gender and old age [15,16]. For this reason, further efforts are needed to avoid the potential complications brought through an intraoperative conversion from LC to OC. The preoperative factors that predict conversion could help to stratify patients and to improve decision making [4,17]. The time elapses to decide whether to change from LC to OC is related to complications during the surgery and higher rates of mortality [1,3,5,17].
A greater understanding of these factors related to conversion is crucial to allow safer procedures and better surgical planning.
In this scenario, the main aim of the present systematic review was to summarize the current knowledge about the main factors associated with conversion from LC to OC in patients with gallstone disease in the gallbladder. Next, we carried out several meta-analyses to evaluate the impact of independent risk factors on conversion rate.
2. Materials and Methods
2.1. Literature Search
The current systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) statements and the Cochrane Handbook’s guidelines (PRISMA checklist available in Table S1). A systematic literature search in the PubMed, Medline and Web of Science databases was carried out for observational studies investigating risk factors for conversion from laparoscopic cholecystectomy to open surgery in patients with gallstone disease in the gallbladder. Literature search was conducted independently by two authors using the following keywords: (predicting factor OR prediction OR preoperative risk factor) AND (laparoscopic cholecystectomy OR cholecystectomy) AND (gall-bladder OR gallbladder OR gallbladder diseases) AND (conversion to open surgery OR conversion). The databases were searched from September 2021 to May 2022 without language restriction; abstracts and unpublished studies were not included. Moreover, the reference lists from selected articles, including relevant review papers, were searched through to identify all relevant studies. The PRISMA guidelines were followed.
2.2. Selection Criteria
Studies were selected only if they satisfied the following criteria: (i) observational studies; (ii) patients with gallstone disease in gallbladder; (iii) treated by laparoscopic cholecystectomy; (iv) consideration of conversion from video-laparoscopic to open cholecystectomy; (v) focus on which factors could increase the probability to have a conversion. Conversely, the following criteria of exclusion were used: (i) studies on patients with other gallstone diseases (e.g., choledocholithiasis); (ii) where cholecystectomy was part of another procedure (e.g., acute cholecystitis, gallbladder cancer, gangrenous cholecystitis); or (iii) where cholecystectomy was carried out exclusively as an emergency treatment; (iv) patients that had indication for cholecystectomy in other principal disease (e.g., symptomatic gallstone disease in cirrhotic patients; cholecystectomy in acute biliary pancreatitis); (v) patients receiving other treatments (e.g., percutaneous cholecystostomy, partial cholecystectomy); (vi) studies recruiting patients < 16 years old; (vi) letters, case reports and case series, clinical trials, previous systematic reviews and meta-analyses, non-English articles and abstracts without full text.
2.3. Study Selection and Data Extraction
Two of the authors independently assessed for inclusion all the references identified through the literature search. From all the eligible studies, two authors independently extracted the following information in a standard format: first author’s last name, year of publication, country, period of study (years), study design, number of participants, and conversion rate. Where available, OR and 95%CI of the following risk factors for conversion from LC to OC were also extracted: (i) clinical factors: age, gender, anatomical ambiguity, ASA score, presence of inflammation, admission status, smoking, acute cholecystitis, diabetes mellitus, obesity, hypertension, heart disease, neurological disease, renal disease, lung disease, obstructive jaundice, previous upper abdominal. surgery, and gallstone pancreatitis; (ii) laboratory factors: WBC, AST, ALT, ALP, bilirubin, hemoglobin, LDH, γ-GTP, lipase, albumin, PCR, platelet count, neutrophil ratio; (iii) radiological factors: cholecystitis, gallbladder polyps, gallbladder wall thickness, pericholecystic fluid, impacted stone at the neck, intrahepatic duct dilation, common bile duct diameter, multiple stone, pericholecystic edema. Cross-checked data were entered into STATA software (version 17) by the first author and checked for accuracy by the second author. During study selection and data extraction, any disagreements were resolved by discussion and consensus with a third author.
2.4. Risk of Bias and Quality Assessment
The methodological quality of the studies included in the meta-analyses was assessed using the Newcastle–Ottawa scale (NOS), as recommended by the Cochrane Collaboration [18]. The NOS scale rates the studies on selection, comparability, and outcome with a score from 0 to 9. A score of 0 stars indicates the highest possible degree of bias, whereas 9 stars indicate the lowest degree of bias.
2.5. Statistical Analysis
Strength of association between each clinical factor and probability of conversion from LC to OC was estimated as ORs (95% CIs). The significance of pooled OR was determined by the Z test. Heterogeneity across studies was measured using the Q test, considering significant statistical heterogeneity as p < 0.1. As the Q test only indicates the presence of heterogeneity and not its magnitude, we also reported the I2 statistic, which estimates the percentage of outcome variability that can be attributed to heterogeneity across studies. An I2 value of 0% denotes no observed heterogeneity, whereas 25% is “low”, 50% is “moderate” and 75% is “high” heterogeneity [19]. According to heterogeneity across studies, we used the fixed-effects model (Mantel–Haenszel method) in case of negligible heterogeneity, or the random-effects models (DerSimonian–Laird method) when heterogeneity was significant. All statistical analyses were performed using STATA 17 (STATA Corp, College Station, TX, USA).
3. Results
3.1. Study Characteristics
The detailed steps of the study selection are given as a PRISMA flow diagram in Figure 1. A total of 427 articles were retrieved from the databases, and 99 duplicates were removed. From 328 articles, 187 were excluded after reading titles and/or abstracts. From these, 53 were excluded according to selection criteria, whereas 35 studies were included in the systematic review.
Figure 1.
PRISMA flow diagram of study selection [20].
Table 1 summarizes the main characteristics of observational studies included in the systematic review. In general, studies were conducted between 1990 and 2019 and published from 1997 to 2021. The majority of studies were retrospective [2,3,4,5,17,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40], while only nine were prospective [1,41,42,43,44,45,46,47,48]. The study conducted by Goonawardena and colleagues reported both retrospective and prospective data [49]. A total of four studies were conducted in Turkey [30,31,37,41], four in in United States [17,33,36,43], three studies in India [45,47,48], two in Japan [21,29], two in Australia [26,49], two in the United Kingdom [40,44], two in Poland [3,42], and two in Ireland [32,35]. Only one study was conducted in Mexico [5], Lebanon [4], Greece [23], Pakistan [25], Egypt [2], Thailand [1], Netherlands [27], China [28], Singapore [34], Iraq [46], Pakistan [38], Saudi-Arabia [39], and Iran [24]. Similarly, only one study was conducted in Italy [22]. Overall, sample sizes ranged from 73 to 11,669, with a mean age from 35 to 68.35. Instead, the percentage of men ranged from 23.3 to 95.1%.
Table 1.
Characteristics of studies included in the systematic review [1,2,3,4,5,17,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49].
| First Author and Publication Year | Country | Study Period | Study Design | Number of Patients | Age (Mean and SD) | Male (%) | Conversion Rate (%) |
|---|---|---|---|---|---|---|---|
| Warchałowski et al., 2020 [3] | Poland | 2008–2018 | Retrospective | 3213 | 65.7 ± 1.4 | 120 (45.6) | 263 (8.2) |
| Goonawardena et al., 2015 [49] | Australia | 2010–2012 | Prospective and retrospective | 732 | 56 ± 18 | 20(43) | 47 (6.4) |
| Ishizuka et al., 2016 [21] | Japan | 2000–2010 | Retrospective | 461 | NA | 231 (50.1) | 25 (5.4) |
| Osuch et al., 2020 [42] | Poland | 2013–2018 | Prospective | 504 | 54.8 ± 16.9 | 22 (62.9) | 35 (6.9) |
| Chávez et al., 2017 [5] | Mexico | 2009–2013 | Retrospective | 1386 | 42 ± 15 | 5 (35.7) | 14 (1) |
| Al Masri et al., 2018 [4] | Lebanon | 2000–2015 | Retrospective | 4668 | 68.35 ± 13.7 | 37 (80.4) | 48 (1.03) |
| Licciardello et al., 2014 [22] | Italy | 2011–2013 | Retrospective | 414 | 65.9 ± 13.9 | 14 (42.5) | 33 (7.9) |
| Simopoulos et al., 2005 [23] | Greece | 1992–2004 | Retrospective | 1804 | 52.66 ± 14.7 | 31 (33.0) | 94 (5.2) |
| Beksac et al., 2016 [37] | Turkey | 2006–2011 | Retrospective | 1335 | 61.9 ± 12.5 | 57 (54.8) | 104 (7.8) |
| Lipman et al., 2007 [17] | Ohio (USA) | 2000–2005 | Retrospective | 1377 | NA | 57 (50.9) | 112 (8.1) |
| Tayeb et al., 2005 [25] | Pakistan | 1997–2001 | Retrospective | 1249 | 44.2 ± 12.4 | 17 (23.3) | 94 (23.3) |
| El Nakeeb et al., 2017 [2] | Egypt | 2011–2016 | Retrospective | 3269 | 54 | 47 (56.6) | 83 (2.5) |
| Ishizaki et al., 2006 [29] | Japan | 1993–2004 | Retrospective | 1339 | 58 ± 13 | 57 (64) | 89 (7.5) |
| Ercan et al., 2010 [30] | Turkey | 2002–2007 | Retrospective | 2015 | 57.8 ± 13.0 | 60 (59.4) | 101 (5.0) |
| Sutcliffe et al., 2016 [44] | UK | 2014 | Prospective | 8820 | 51 ± 17 | 110 (49.8) | 297 (3.4) |
| Genc et al., 2010 [31] | Turkey | 1999–2010 | Retrospective | 5382 | 49.34 ± 9.9 | 84 (51.5) | 163 (3.2) |
| Hu et al., 2018 [26] | Australia | 2013–2016 | Retrospective | 1035 | 61 ± 15 | 24 (55.8) | 43 (4.2) |
| Kaafarani et al., 2009 [43] | USA | 2005–2008 | Prospective | 11,669 | 63.9 ± 12.4 | 903 (95.1) | 949 (8.1) |
| Tongyoo et al., 2021 [1] | Thailand | 2018–2019 | Prospective | 152 | 57.61 ± 13.8 | 9 (56.2) | 16 (10.5) |
| Van der Velden et al., 1998 [27] | Netherlands | 1993–1996 | Retrospective | 134 | 50.7 | NA | 23 (17.2) |
| Kama et al., 2001 [41] | Turkey | 1992–1999 | Prospective | 1000 | 43.8 | 22 (45.8) | 48 (4.8) |
| Zhang et al., 2008 [28] | China | 2005–2006 | Retrospective | 1265 | 60.0 ± 11.9 | 50 (53.2) | 94 (7.4) |
| Lal et al., 2002 [45] | India | 1999–2000 | Prospective | 73 | 35 | NA | 17 (23.3) |
| O’Leary et al., 2013 [32] | Ireland | 2000–2006 | Retrospective | 1061 | 43.3 | 31 (53.4) | 58 (5.5) |
| Rosen et al., 2002 [33] | USA | 1996–2000 | Retrospective | 1347 | 59.9 | 29 (41) | 71 (5.3) |
| Alponat et al., 1997 [34] | Singapore | 1990–1995 | Retrospective | 783 | NA | 22 (38) | 58 (7.4) |
| Chandio et al., 2009 [35] | Ireland | 2004–2006 | Retrospective | 324 | 61 | NA | 39 (12) |
| Faraj et al., 2020 [46] | Iraq | 2019 | Prospective | 485 | 52 ± 17.3 | 11 (50) | 22 (4.5) |
| Zulfiqar et al., 2019 [38] | Pakistan | 2017–2018 | Retrospective | 283 | NA | NA | 10 (3.5) |
| Kumar et al., 2016 [47] | India | 2013–2015 | Prospective | 112 | 43.4 | 5 (41.7) | 12 (10.7) |
| Nouh et al., 2019 [39] | Saudi Arabia | 2014–2015 | Retrospective | 589 | NA | 2 (32.2) | 9 (1.5) |
| AbdelDayen et al., 2019 [40] | UK | 2013–2014 | Retrospective | 280 | 49.9 | NA | 11 (3.9) |
| Naik et al., 2018 [48] | India | 2016–2017 | Prospective | 86 | 48.92 ± 11.9 | 2 (33.3) | 6 (6.9) |
| Raman et al., 2012 [36] | USA | 2006–2009 | Retrospective | 874 | NA | NA | 68 (7.8) |
| Gholipour et al., 2009 [24] | Iran | 1997–2004 | Retrospective | 793 | 48.9 ± 15 | NA | 71 (9) |
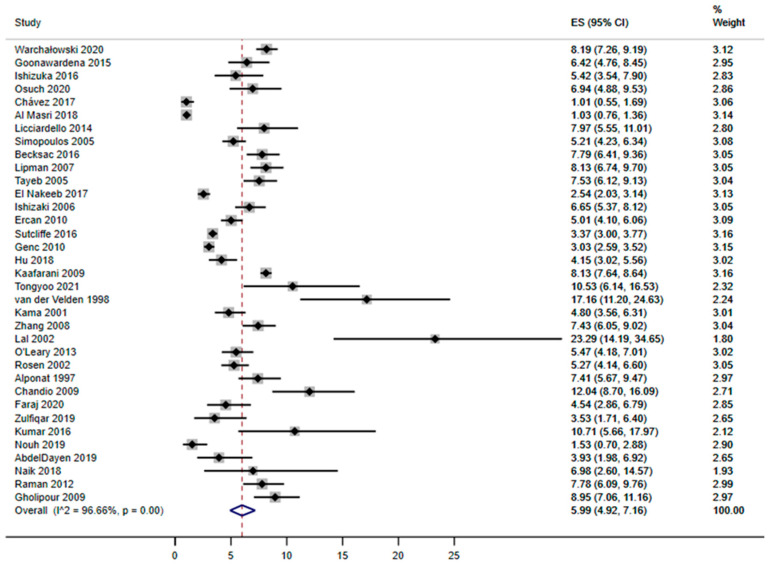
All studies included in the systematic review reported conversion rate from laparoscopy to open surgery during cholecystectomy. Single values from all studies ranged from 1% to 23.3%, while the estimated pooled proportion of conversion rate was 5.99% (95%CI = 4.92–7.16%; Figure 2).
Figure 2.
Forest plot of the pooled conversion rate [1,2,3,4,5,17,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. The graph shows individual and pooled conversion rates (expressed in terms of percentage).
3.2. Risk Factors for Conversion from Laparoscopy to Open Surgery during Cholecystectomy
Studies included in the systematic review also evaluated the main risk factors associated with the probability of conversion from LC to OC. Increasing age has been reported as a risk factor for conversion by the difficult dissection secondary to a repeated number of attacks of inflammation and ensuing thickening and fibrosis of the gallbladder [3,22,23,25,28]. In addition, male gender has been considered as a risk factor for conversion, probably due to the increased severity of gallstone disease and the more frequent adhesions and inflammations observed in men [4,17,31]. However, results reported by the included studies were controversial. In particular, only the study conducted by Kaafarani et al. estimated that, among the population of Veteran American soldiers, conversion rate was 95.1% in male participants [42]. Van der Velden and colleagues, instead, did not report the exact proportion of men and women with cholecystectomy conversion [27].
Beyond age and gender, additional risk factors (e.g., clinical, laboratory and radiological factors) could lead to changes from laparoscopy to open surgery during cholecystectomy. For this reason, the studies included in the systematic review investigated the impact of these risk factors—individually or as a cumulative effect—on conversion rate.
All patients included in the systematic review received a diagnosis of gallstones in the gallbladder by imaging and were subjected to planned laparoscopic cholecystectomy. Among the clinical factors, admission status, acute cholecystitis, diabetes, obesity, hypertension, heart disease and previous abdominal surgery were frequently investigated. With respect to the admission status, patients treated in emergency showed higher probability of conversion than those with elective treatment [3,4,5,24,27,28,31,34,43,44,49]. Similarly, acute cholecystitis—defined as onset due to cholecystolithiasis just before surgery—was considered as a risk factor for conversion [1,2,3,17,21,22,23,25,27,29,31,33,34,35,37,44,47,49]. In this context, previous comorbidities (i.e., hypertension, diabetes, obesity and heart diseases) [2,3,4,5,17,23,30,31,39,43,46,47,49], cirrhosis [2,34,38] and upper abdominal surgery [2,4,23,24,26,28,30,33,35,37,41,43,46] also increased the probability of conversion during the cholecystectomy due to physiological and anatomical difficulties. Three studies considered neurological diseases as risk factor for conversion [3,4,43], with Al Masri and colleagues also assessing renal disease [4].
Moreover, six studies identified the preoperative endoscopic retrograde cholangial-pancreatography (ERCP) as a significant factor to predict conversion [2,4,30,31,33,44]. The obstructive jaundice has been found in some studies as a factor related to conversion, although it is mainly due to the association with a form of cholangitis [2,41,49]. Pancreatitis is reported in three studies [23,44,46].
Regarding the main laboratory tests, some authors did not report any parameters. On the other hand, several studies evaluated the main values that could be associated with conversion, even if the evidence is still controversial. For instance, white blood cell (WBC) count, albumin, and glomerular filtration rate were significantly associated with conversion in several studies [17,24,25,28,30,33,34,37,41,43,49]. The liver function test (LFT) with the measurement of AST [22,30,43], ALT [5,17,22,30,34], ALP [4,17,22,24,30,33,34,37,39,49], GGT [4,21,22], bilirubin total [4,17,24,30,33,39,49] and direct [4,21,39] were the most frequent flags measured in these patients. Few studies reported a significant association of conversion rate with fibrinogen [22], platelet count and INR [43]. Moreover, three studies considered the C-reactive protein as significative as the risk factors for conversion [21,22,28].
Some studies investigated the impact of gallbladder (GB) thickness > 4 mm in conversion [1,2,17,24,25,26,27,28,29,30,32,33,34,38,40,41,44,45,47,48,49], while Raman et al. analyzed the different sizes of thickness in relation to conversion rate [36]. It has been well-established that the analysis of the various dimensions of GB at ultrasound is crucial during the dissection of GB. For this reason, a methodical approach is required: (i) the measure of GB major axis [4,5,27] and (ii) the echogenicity of the GB’s content, in which it is possible to find a single stone or multiple stones [2,27,30,47], but also biliary sand and biliary sludge. Moreover, the presence of stones in the Hourtman’s pouch—corresponding to the neck of the gallbladder—and the contracted GB were also considered as significant elements involved in conversion [1,32]. Dilated common bile duct (CBD) and choledocholithiasis were also considered in several studies [2,4,5,17,21,24,25,26,27,28,30,32,33,35,41,44,45,46,49], as well as the intra-hepatic ducts (IHDs), because the ultrasound diagnostic method leads to assessment of the real state of the intra- and extra-hepatic bile ducts for planning the most appropriate procedure [5,49]. In this context, US Murphy Sign [25,28,37,49] and hepatomegaly [5] were factors significantly related with conversion.
With respect to operative findings, anatomical ambiguity was considered a risk factor for higher probability of conversion [2,3,4,5,17,21,22,23,25,27,28,29,30,31,34,35,36,37,41,42,46,47,49]. In addition, inflamed GB (i.e., complication of acute cholecystitis) [3,17,21,22,23,25,30,31,34,39,42], peritoneal adhesion [2,5,17,21,22,25,27,28,29,30,31,32,33,34,36,38,39,45,46] and fistula of GB (i.e., consequences of chronic cholecystitis) [2,22,23,30,31,33,34,36,42,46,49] were indicated as risk factors for conversion. During laparoscopic cholecystectomy, some studies reported that bleeding [2,4,5,21,22,23,27,29,30,31,32,33,36,39,41,42,44,45,46,47,49], bile duct injury [2,5,17,21,22,23,25,28,29,30,31,32,33,41,42,44,46,47] and bowel injury [31,36,41,44,46] were the main complications leading to conversion. Moreover, grade of difficultly [44], inability to create pneumoperitoneum [2,23,34,36] and anesthesia complications [2] were considered as risk factors for conversion. Mirizzi’s Syndrome and cancer of gallbladder were significantly associated with conversion in several studies [5,22,23,33,34,38,45], but their diagnosis was accidental and occurred during GB extraction.
3.3. The Association of Clinical Factors with the Probability of Conversion
Among studies included in the systematic review, 11 with available data on the association between clinical factors and the risk of conversion were included in the meta-analyses (Table 2). We performed individual meta-analyses for the following clinical factors analyzed by at least three studies: gender, age, acute cholecystitis, admission status, diabetes, hypertension, heart diseases, obesity, and previous upper abdominal surgery.
Table 2.
| First Author and Publication Year | Risk Factors for Conversion |
|---|---|
| Warchałowski et al., 2020 [3] | Gender, Age, Anatomical ambiguity, Admission Status, Acute Cholecystitis, Choledocholithiasis, Diabetes Mellitus, Hypertension, Heart Disease, Neurological Disease |
| Goonawardena et al., 2015 [49] | Gender, Age, Ethnicity, ASA > III, Acute Cholecystitis, Admission Status, BMI > 30, Obstructive Jaundice, Previous Upper Abdominal Surgery, Gallstone pancreatitis, Diabetes Mellitus, WBC, ALP, Bilirubin, Lipase, PCR, Choledocholithiasis, Cholecystitis, Gallbladder Wall width, Pericholecystic fluid, Impacted stone at the neck, Intrahepatic Duct dilatation |
| Ishizuka et al., 2016 [21] | Gender, Obesity, WBC, AST, ALT, ALP, Bilirubin, LDH, γ-GT, Albumin, PCR, Platelet count, Neutrophil ratio |
| Al Masri et al., 2018 [4] | Gender, Age, Admission Status, Obesity, Smoke, Previous Upper Abdominal Surgery, Diabetes Mellitus, Hypertension, Heart Disease, Dyslipidemia, Neurological Disease, Renal Disease, Lung Disease, WBC, AST, ALT, ALP, Bilirubin, Hemoglobin, γ-GT, Lipase, Platelet count, Choledocholithiasis, Gallbladder Wall width, Pericholecystic fluid, Impacted stone at the neck |
| Licciardello et al., 2014 [22] | Age, Acute Cholecystitis |
| Simopoulos et al., 2005 [23] | Gender, Age, Acute Cholecystitis, Presence of inflammation, Obesity, Previous Upper Abdominal Surgery, Diabetes Mellitus, Hypertension, Heart Disease |
| Lipman et al., 2007 [17] | Gender, Diabetes Mellitus, WBC, Bilirubin, Albumin, Pericholecystic fluid |
| Tayeb et al., 2005 [25] | Age, Acute Cholecystitis, Obesity, Obstructive jaundice, Previous Upper Abdominal Surgery, Gallstone pancreatitis, Tenderness in RUQ, Palpable Gallbladder, Diabetes Mellitus, Hypertension, WBC, ALP, Bilirubin, Gallbladder Wall with, Pericholecystic fluid, Dilatation of CBD, Multiple stones, US-Murphy sign |
| Kaafarani et al., 2009 [43] | Gender, Admission status, Previous Upper Abdominal Surgery, Hypertension, WBC, Albumin |
| Zhang et al., 2008 [28] | Gender, Age, Acute Cholecystitis, Previous Upper Abdominal Surgery, Diabetes Mellitus, Obesity, WBC, Gallbladder Wall width |
| Gholipour et al., 2009 [24] | Gender, Age, Admission Status, Smoke, Previous Upper Abdominal Surgery, WBC, ALP, Bilirubin, Gallbladder Wall width, Dilatation of CBD, Pericholecystic edema |
Overall, 10 studies estimated the probability of conversion associated with male gender. The Q test and I2 statistics showed significant heterogeneity between studies (p < 0.1; I2 = 85.9%). Using the random effect model, the meta-analysis showed that male gender increased the probability of conversion (OR = 1.907; 95%CI = 1.254–2.901; Figure 3A).
Figure 3.
Forest plots of the associations of male gender (A) [3,4,17,21,23,24,25,28,43,49], age over 60 years (B) [3,22,23,25,28] and admission status (C) [3,4,24,43,49] with the probability of conversion. The graph (A) shows the ORs for the comparison between male and female genders (reference group). The graph (B) shows the ORs for the comparison between age > 60 years and ≤ 60 years (reference group). The graph (C) shows the ORs for the comparison between emergency and elective treatment (reference group).
Overall, five studies estimated the probability of conversion associated with age over 60 years. The Q test and I2 statistics showed no significant heterogeneity across studies (p > 0.1; I2 = 0.0%). Using the fixed effect model, the meta-analysis showed that age over 60 years increased the probability of conversion (OR = 4.324; 95%CI = 3.396–5.506; Figure 3B).
Overall, five studies estimated the probability of conversion associated with admission status. The Q test and I2 statistics showed significant heterogeneity across studies (p < 0.1; I2 = 93.9%). Using the random effect model, however, no association was evident (OR = 2.350; 95%CI = 0.991–5.574; Figure 3C).
Overall, six studies estimated the probability of conversion associated with acute cholecystitis. The Q test and I2 statistics showed significant heterogeneity across studies (p < 0.1; I2 = 84.4%). Using the random effect model, the meta-analysis showed that acute cholecystitis increased the probability of conversion (OR = 5.475; 95%CI = 2.959–10.130; Figure 4A).
Figure 4.
Forest plots of the associations of acute cholecystitis (A) [3,22,23,25,28,49], heart disease (B) [3,4,23] and previous upper abdominal surgery (C) [4,23,24,25,28,43,49] with the probability of conversion. The graph (A) shows the ORs for the comparison between acute and non-acute cholecystitis (reference group). The graph (B) shows the ORs for the comparison between patients with heart disease and those without it (reference group). The graph (C) shows the ORs for the comparison between patients with previous upper abdominal surgery and those without it (reference group).
Overall, three studies estimated the probability of conversion associated with heart diseases. The Q test and I2 statistics showed significant heterogeneity across studies (p < 0.1; I2 = 74.1%). The pooled OR, obtained through the random effect model, was 2.947 (95%CI = 1.047–8.296), showing that heart diseases increased the probability of conversion (Figure 4B).
Overall, seven studies estimated the probability of conversion associated with previous upper abdominal surgery. The Q test and I2 statistics showed significant heterogeneity across studies (p < 0.1; I2 = 77.3%). The pooled OR, obtained through the random effect model, was 3.301 (95%CI = 1.965–5.543), showing that previous upper abdominal surgery increased the probability of conversion (Figure 4C).
Overall, seven studies estimated the probability of conversion associated with diabetes. The Q test and I2 statistics showed significant heterogeneity across studies (p < 0.1; I2 = 73.2%). Using the random effect model, the meta-analysis showed that diabetes increased the probability of conversion (OR = 2.576; 95%CI = 1.687–3.934; Figure 5A).
Figure 5.
Forest plots of the associations of diabetes (A) [3,4,17,23,25,28,49], hypertension (B) [3,4,23,25,43] and obesity (C) [4,23,25,28,49] with the probability of conversion. The graph (A) shows the ORs for the comparison between patients with diabetes and those without it (reference group). The graph (B) shows the ORs for the comparison between patients with hypertension and those without it (reference group). The graph (C) shows the ORs for the comparison between obese and non-obese patients (reference group).
Overall, five studies estimated the probability of conversion associated with hypertension. The Q test and I2 statistics showed significant heterogeneity across studies (p < 0.1; I2 = 90%). Using the random effect model, the meta-analysis showed that hypertension increased the probability of conversion (OR = 1.931; 95%CI = 1.018–3.662; Figure 5B).
Overall, five studies estimated the probability of conversion associated with obesity (BMI ≥ 30 Kg/m2). The Q test and I2 statistics showed significant heterogeneity across studies (p < 0.1; I2 = 84.8%). The pooled OR, obtained through the random effect model, was 2.228 (95%CI = 1.162–4.271), showing that obesity increased the probability of conversion (Figure 5C).
3.4. Quality of Studies
As reported, the methodological quality of the studies included in the meta-analyses were assessed using the Newcastle–Ottawa scale (NOS). The quality of case-control studies ranged from seven to nine stars, with the definition of cases and ascertainment of exposure being the items most affecting the quality of studies. With respect to prospective studies, the quality assessed was eight stars, with the representativeness of the exposed cohort as the item most affecting the quality of studies.
4. Discussion
In the present systematic review and meta-analysis, we aimed to identify the main preoperative patient-related risk factors for conversion from LC to OC in patients with gallstone disease in the gallbladder. The analyses of these factors could help to redefine the surgical strategy, improving patient safety. In some cases, conversion may be necessary to prevent injury and treat intraoperative complications [3]. Overall, the studies included have investigated the association of several risk factors—male sex, older age, BMI > 30 kg/m2, thickness of gallbladder wall > 3 mm, previous abdominal surgery and comorbidities—with the risk of conversion. However, findings were controversial, and the available data were heterogeneous, making it difficult to summarize the evidence in a systematic way. Moreover, not all studies have analyzed the same risk factors affecting conversion. For this reason, we carried out a systematic review of 35 studies to estimate conversion rate and, after, to investigate the main risk factors for conversion. Although conversion rate reported by each study ranged from 1% to 23.3%, our results showed a pooled conversion rate of 6.0%.
Among studies included in the systematic review, 11 with available data on the association between clinical factors and the risk of conversion were included in individual meta-analyses. Firstly, 10 studies evaluated the relationship between male gender and the probability of conversion from LC to OC [3,4,17,21,23,24,25,28,43,49]. Several studies reported male gender as a risk factor for conversion, with ORs ranging from 1.6 to 5.9 [3,4,17,23,28,43,49]. However, there were also studies that did not demonstrate any association between male gender and conversion [21,24,25]. When we pooled all studies together, we noted 1.91 increased odds of conversion in male patients than in females. The underlying reason is unclear, but it may be attributable to body fat distribution, which is different in males and females, making the laparoscopic approach generally more difficult in men than women. Moreover, it has been proposed that men are less likely than women to seek medical advises [50,51]. Inflammation and dense adhesions were also frequently cited as reasons for conversion in men [17]. Among the main characteristics, five studies evaluated the relationship between age and the probability of conversion from LC to OC [3,22,23,25,28]. All studies demonstrated the positive association between age over 60 years and conversion, with ORs ranging from 3.0 to 6.2. This finding was confirmed by our meta-analysis, showing that age over 60 years increased the probability of conversion, with an OR equal to 4.3. It is plausible that older people have a longer history of gallstones and an increased number of cholecystitis attacks [52,53,54].
Acute cholecystitis can also affect conversion rate, as investigated by six studies included in our meta-analysis [3,22,23,25,28,49]. Only one study did not find any statistical association between acute cholecystitis and conversion [23]. When we pooled all studies together, we noted 5.5 increased odds of conversion in patients with acute cholecystitis versus in their counterpart. The high rates of conversion from LC to OC for acute cholecystitis could depend on a technical difficulty of managing severe inflammatory adhesions around the inflamed gallbladder, making the dissection of Calot’s triangle and the recognition of the anatomy more difficult.
In this context, the comorbidities such as diabetes and hypertension were associated with a higher conversion rate, probably because both complications could determine a higher risk of infections and a general poor condition of the patient. Seven studies evaluated the relationship between diabetes and the probability of conversion from LC to OC [3,4,17,23,25,28,49]. Several studies reported diabetes as a risk factor for conversion, with ORs ranging from 1.9 to 5.5 [3,4,17,23,49]. However, there were also studies that did not demonstrate any association between diabetes and conversion [25,28]. When we pooled all studies together, we demonstrated that diabetes increased the probability of conversion, with an OR equal to 2.6, confirming that such a relationship is due to the presence of acute inflammation or changes in the wall from microvascular diseases. Moreover, some diabetic patients may not develop symptoms of gallbladder disease early due to autonomic and peripheral neuropathy, leading to delayed diagnosis and a greater risk for conversion. Similarly, three studies suggested the association between hypertension and higher probability of conversion from LC to OC, with ORs ranging from 1.2 to 7.0 [3,4,43]. This finding was confirmed by our meta-analysis, reporting an OR equal to 1.9.
Among coexisting diseases in patients undergoing cholecystectomy; in addition, obesity and heart diseases could be considered as risk factors for conversion from LC to OC. Obesity has been previously described as an important factor for conversion, probably for causes related to excessive intra-abdominal fat, difficult mobilization of the liver, placing trocars and moving the instruments due to the thickness of the abdominal wall [5]. Previous studies investigated the relationship between obesity and conversion; however, the results were controversial probably due to few obese patients [4,23,25,28,49]. Our meta-analysis showed 2.2 increased odds of conversion in diabetic patients than in their counterpart. Similarly, when we pooled all studies investigating the role of heart diseases as risk factors for conversion [3,4,23], we demonstrated 2.9 increased probability of conversion for patients with previous heart diseases.
In this scenario, previous abdominal surgery has been considered as a risk factor for conversion, due to existing peritoneal adhesions, which make it difficult to perform gallbladder dissection. Overall, seven studies estimated the probability of conversion associated with previous upper abdominal surgery, with ORs ranging from 1.6 to 15.5 [4,23,24,25,28,43,49]. After applying the meta-analysis, the pooled OR was 3.3, confirming that a history of upper abdominal surgery increased the probability of conversion.
Overall, our findings confirmed what was evident in a previous meta-analysis, where the preoperative predictive factors reported were age > 65 years, male gender, acute cholecystitis, history of diabetes, thickened gallbladder walls and previous upper abdominal surgery [55]. In addition, the meta-analysis by Rothman and colleagues demonstrated that gallbladder wall > 4–5 mm, age> 60 years, male gender, and acute cholecystitis were risk factors for conversion from LC to OC [16]. The analysis of clinical factors suggested the presence of different preoperative variables, which are non-modifiable but could be useful for planning the surgical scenario and improving the post-operatory phase. Our systematic review and meta-analysis provided robust implications for systematic quality improvement efforts. The present analysis could improve clinical practice, promoting appropriate patient counseling throughout accurate risk factor assessment. A better understanding of preoperative risk factors for conversion from LC to OC might help prevent conversion by optimizing patient clinical condition. Alternatively, the preoperative optimization of patients with comorbidities could set a more realistic evaluation of conversion risk and subsequent recovery time. For this reason, it should be necessary to improve the management of these patients before surgery. A key strategy may be represented by the validation of specific recommendations for clinicians and their subsequent training. Next, ad hoc surveys could be developed for evaluating the adherence of clinicians to the guidelines for the management of these patients. In this scenario, it is well known that radiological and laboratory factors should also be studied for their potential impact on the risk of conversion from LC to OC.
Our work had some limitations to be considered. Firstly, for the large period considered, the technologies and knowledge regarding laparoscopic cholecystectomy suffered great changes during the years, as well as conversion rate, which did not always remain the same. Included studies were heterogeneous in terms of study period. We recognize that excluding studies conducted before a certain year or applying a meta-regression to evaluate the impact of study period could be important. However, the majority of studies were conducted during a wide range of time. For this reason, we cannot apply subgroup analyses and/or meta-regressions. Another limitation is the retrospective nature of the data collection, which may lead to a limited ability to correctly classify the preoperative diagnosis and make the analyzed group very heterogeneous. Although we excluded all epidemiological studies in which cholecystectomy was carried out exclusively as an emergency treatment, the meta-analysis included studies in which patients were treated in elective surgery and/or both in elective and in emergency surgery. To evaluate the impact of admission status on conversion rate, we performed a specific meta-analysis that, however, did not point out a significant association. Moreover, the lack of data (e.g., ORs and 95%CI) did not allow us to carry out meta-analyses for other important risk factors for conversion (e.g., radiological and laboratory factors).
5. Conclusions
In conclusion, the present systematic review and meta-analysis identified the most frequent factors associated with conversion from LC to OC. Our results demonstrated that an accurately derived estimation of the risk for conversion could be obtained from readily available preoperative data. However, further studies with larger sample size, more accurate information, and standardized definitions and protocols are needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph20010408/s1, Table S1: PRISMA 2020 checklist.
Author Contributions
Conceptualization, G.B. and A.A.; formal analysis, R.M.S.L. and A.M.; data curation, R.M.S.L., M.B., A.M., S.Q. and G.B.; writing—original draft preparation, R.M.S.L., M.B. and A.M.; writing—review and editing, R.M.S.L., M.B., A.M., S.Q., G.B. and A.A.; supervision, G.B. and A.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tongyoo A., Chotiyasilp P., Sriussadaporn E., Limpavitayaporn P., Mingmalairak C. The pre-operative predictive model for difficult elective laparoscopic cholecystectomy: A modification. Asian J. Surg. 2021;44:656–661. doi: 10.1016/j.asjsur.2020.11.018. [DOI] [PubMed] [Google Scholar]
- 2.El Nakeeb A., Mahdy Y., Salem A., El Sorogy M., El Rafea A.A., El Dosoky M., Said R., Ellatif M.A., Alsayed M.M.A. Open Cholecystectomy Has a Place in the Laparoscopic Era: A Retrospective Cohort Study. Indian J. Surg. 2017;79:437–443. doi: 10.1007/s12262-017-1622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warchałowski Ł., Łuszczki E., Bartosiewicz A., Dereń K., Warchałowska M., Oleksy Ł., Stolarczyk A., Podlasek R. The Analysis of Risk Factors in the Conversion from Laparoscopic to Open Cholecystectomy. Int. J. Environ. Res. Public Health. 2020;17:7571. doi: 10.3390/ijerph17207571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al Masri S., Shaib Y., Edelbi M., Tamim H., Jamali F., Batley N., Faraj W., Hallal A. Predicting Conversion from Laparoscopic to Open Cholecystectomy: A Single Institution Retrospective Study. World J. Surg. 2018;42:2373–2382. doi: 10.1007/s00268-018-4513-1. [DOI] [PubMed] [Google Scholar]
- 5.Chávez K.V., Márquez-González H., Aguirre I., Orellana J.C. Prognostic risk factors for conversion in laparoscopic cholecystectomy. Updates Surg. 2018;70:67–72. doi: 10.1007/s13304-017-0494-0. [DOI] [PubMed] [Google Scholar]
- 6.Hu A.S.Y., Menon R., Gunnarsson R., de Costa A. Risk factors for conversion of laparoscopic cholecystectomy to open surgery–A systematic literature review of 30 studies. Am. J. Surg. 2017;214:920–930. doi: 10.1016/j.amjsurg.2017.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Kama N.A., Kologlu M., Doganay M., Reis E., Atli M., Dolapci M. A risk score for conversion from laparoscopic to open cholecystectomy. Am. J. Surg. 2001;181:520–525. doi: 10.1016/S0002-9610(01)00633-X. [DOI] [PubMed] [Google Scholar]
- 8.Thesbjerg S.E., Harboe K.M., Bardram L., Rosenberg J. Sex differences in laparoscopic cholecystectomy. Surg. Endosc. 2010;24:3068–3072. doi: 10.1007/s00464-010-1091-1. [DOI] [PubMed] [Google Scholar]
- 9.van der Steeg H.J., Alexander S., Houterman S., Slooter G.D., Roumen R.M. Risk factors for conversion during laparoscopic cholecystectomy–experiences from a general teaching hospital. Scand. J. Surg. 2011;100:169–173. doi: 10.1177/145749691110000306. [DOI] [PubMed] [Google Scholar]
- 10.Angrisani L., Lorenzo M., De Palma G., Sivero L., Catanzano C., Tesauro B., Persico G. Laparoscopic cholecystectomy in obese patients compared with nonobese patients. Surg. Laparosc. Endosc. 1995;5:197–201. [PubMed] [Google Scholar]
- 11.Ammori B.J., Vezakis A., Davides D., Martin I.G., Larvin M., McMahon M.J. Laparoscopic cholecystectomy in morbidly obese patients. Surg. Endosc. 2001;15:1336–1339. doi: 10.1007/s004640000019. [DOI] [PubMed] [Google Scholar]
- 12.Diez J., Delbene R., Ferreres A. The feasibility of laparoscopic cholecystectomy in patients with previous abdominal surgery. HPB Surg. 1998;10:353–356. doi: 10.1155/1998/35456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karayiannakis A.J., Polychronidis A., Perente S., Botaitis S., Simopoulos C. Laparoscopic cholecystectomy in patients with previous upper or lower abdominal surgery. Surg. Endosc. 2004;18:97–101. doi: 10.1007/s00464-003-9001-4. [DOI] [PubMed] [Google Scholar]
- 14.Utsumi M., Aoki H., Kunitomo T., Mushiake Y., Yasuhara I., Taniguchi F., Arata T., Katsuda K., Tanakaya K., Takeuchi H. Preoperative Risk Factors for Conversion of Laparoscopic Cholecystectomy to Open Cholecystectomy and the Usefulness of the 2013 Tokyo Guidelines. Acta Med. Okayama. 2017;71:419–425. doi: 10.18926/AMO/55440. [DOI] [PubMed] [Google Scholar]
- 15.Tang B., Cuschieri A. Conversions during laparoscopic cholecystectomy: Risk factors and effects on patient outcome. J. Gastrointest. Surg. 2006;10:1081–1091. doi: 10.1016/j.gassur.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Philip Rothman J., Burcharth J., Pommergaard H.C., Viereck S., Rosenberg J. Preoperative Risk Factors for Conversion of Laparoscopic Cholecystectomy to Open Surgery–A Systematic Review and Meta-Analysis of Observational Studies. Dig. Surg. 2016;33:414–423. doi: 10.1159/000445505. [DOI] [PubMed] [Google Scholar]
- 17.Lipman J.M., Claridge J.A., Haridas M., Martin M.D., Yao D.C., Grimes K.L., Malangoni M.A. Preoperative findings predict conversion from laparoscopic to open cholecystectomy. Surgery. 2007;142:556–563. doi: 10.1016/j.surg.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Wells G.A., O’Connell D., Peterson J., Welch V., Losos M. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality If Nonrandomized Studies in Meta-Analyses. 2009. [(accessed on 1 November 2022)]. Available online: https://www.scienceopen.com/document?vid=54b48470-4655-4081-b5d4-e8ebe8d1792e.
- 19.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishizuka M., Shibuya N., Shimoda M., Kato M., Aoki T., Kubota K. Preoperative hypoalbuminemia is an independent risk factor for conversion from laparoscopic to open cholecystectomy in patients with cholecystolithiasis. Asian J. Endosc. Surg. 2016;9:275–280. doi: 10.1111/ases.12301. [DOI] [PubMed] [Google Scholar]
- 22.Licciardello A., Arena M., Nicosia A., Di Stefano B., Calì G., Arena G., Minutolo V. Preoperative risk factors for conversion from laparoscopic to open cholecystectomy. Eur. Rev. Med. Pharmacol. Sci. 2014;18:60–68. [PubMed] [Google Scholar]
- 23.Simopoulos C., Botaitis S., Polychronidis A., Tripsianis G., Karayiannakis A.J. Risk factors for conversion of laparoscopic cholecystectomy to open cholecystectomy. Surg. Endosc. 2005;19:905–909. doi: 10.1007/s00464-004-2197-0. [DOI] [PubMed] [Google Scholar]
- 24.Gholipour C., Fakhree M.B., Shalchi R.A., Abbasi M. Prediction of conversion of laparoscopic cholecystectomy to open surgery with artificial neural networks. BMC Surg. 2009;9:13. doi: 10.1186/1471-2482-9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tayeb M., Raza S.A., Khan M.R., Azami R. Conversion from laparoscopic to open cholecystectomy: Multivariate analysis of preoperative risk factors. J. Postgrad. Med. 2005;51:17–20. [PubMed] [Google Scholar]
- 26.Hu A.S.Y., Donohue P.O., Gunnarsson R.K., de Costa A. External validation of the Cairns Prediction Model (CPM) to predict conversion from laparoscopic to open cholecystectomy. Am. J. Surg. 2018;216:949–954. doi: 10.1016/j.amjsurg.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 27.Van der Velden J.J., Berger M.Y., Bonjer H.J., Brakel K., Laméris J.S. Can sonographic signs predict conversion of laparoscopic to open cholecystectomy? Surg. Endosc. 1998;12:1232–1235. doi: 10.1007/s004649900826. [DOI] [PubMed] [Google Scholar]
- 28.Zhang W.J., Li J.M., Wu G.Z., Luo K.L., Dong Z.T. Risk factors affecting conversion in patients undergoing laparoscopic cholecystectomy. ANZ J. Surg. 2008;78:973–976. doi: 10.1111/j.1445-2197.2008.04714.x. [DOI] [PubMed] [Google Scholar]
- 29.Ishizaki Y., Miwa K., Yoshimoto J., Sugo H., Kawasaki S. Conversion of elective laparoscopic to open cholecystectomy between 1993 and 2004. Br. J. Surg. 2006;93:987–991. doi: 10.1002/bjs.5406. [DOI] [PubMed] [Google Scholar]
- 30.Ercan M., Bostanci E.B., Teke Z., Karaman K., Dalgic T., Ulas M., Ozer I., Ozogul Y.B., Atalay F., Akoglu M. Predictive factors for conversion to open surgery in patients undergoing elective laparoscopic cholecystectomy. J. Laparoendosc. Adv. Surg. Tech. A. 2010;20:427–434. doi: 10.1089/lap.2009.0457. [DOI] [PubMed] [Google Scholar]
- 31.Genc V., Sulaimanov M., Cipe G., Basceken S.I., Erverdi N., Gurel M., Aras N., Hazinedaroglu S.M. What necessitates the conversion to open cholecystectomy? A retrospective analysis of 5164 consecutive laparoscopic operations. Clinics. 2011;66:417–420. doi: 10.1590/S1807-59322011000300009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Leary D.P., Myers E., Waldron D., Coffey J.C. Beware the contracted gallbladder–Ultrasonic predictors of conversion. Surgeon. 2013;11:187–190. doi: 10.1016/j.surge.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Rosen M., Brody F., Ponsky J. Predictive factors for conversion of laparoscopic cholecystectomy. Am. J. Surg. 2002;184:254–258. doi: 10.1016/S0002-9610(02)00934-0. [DOI] [PubMed] [Google Scholar]
- 34.Alponat A., Kum C.K., Koh B.C., Rajnakova A., Goh P.M. Predictive factors for conversion of laparoscopic cholecystectomy. World J. Surg. 1997;21:629–633. doi: 10.1007/PL00012288. [DOI] [PubMed] [Google Scholar]
- 35.Chandio A., Timmons S., Majeed A., Twomey A., Aftab F. Factors influencing the successful completion of laparoscopic cholecystectomy. JSLS. 2009;13:581–586. doi: 10.4293/108680809X1258998404560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Raman S.R., Moradi D., Samaan B.M., Chaudhry U.S., Nagpal K., Cosgrove J.M., Farkas D.T. The degree of gallbladder wall thickness and its impact on outcomes after laparoscopic cholecystectomy. Surg. Endosc. 2012;26:3174–3179. doi: 10.1007/s00464-012-2310-8. [DOI] [PubMed] [Google Scholar]
- 37.Beksac K., Turhan N., Karaagaoglu E., Abbasoglu O. Risk Factors for Conversion of Laparoscopic Cholecystectomy to Open Surgery: A New Predictive Statistical Model. J. Laparoendosc. Adv. Surg. Tech. A. 2016;26:693–696. doi: 10.1089/lap.2016.0008. [DOI] [PubMed] [Google Scholar]
- 38.Zulfiqar M.R., Wasim E. A retrospective analysis of incidence of transformation of laproscopic cholecystectomy into open cholecystectomy. Indo Am. J. Pharm. Sci. 2019;6:5231–5234. [Google Scholar]
- 39.Nouh T.A., Abunayan F., Alsaadoun A.S., Alsehly A.F., Almanie M.A., Alhawassi S.A. Factors Affecting Conversion from Laparoscopic Cholecystectomy to Open Cholecystectomy at a Tertiary Care Facility in Saudi Arabia: A Cross-Sectional Study. Int. Surg. 2019;131:98–101. doi: 10.9738/INTSURG-D-19-00025.1. [DOI] [Google Scholar]
- 40.AbdelDayem M., Osgood L., Escofet X., Farag M. A New Preoperative Scoring System to Predict Difficulty of Laparoscopic Cholecystectomy and Risk of Conversion to Open Surgery. Indian J. Surg. 2019;82:501–506. doi: 10.1007/s12262-019-02033-9. [DOI] [Google Scholar]
- 41.Kama N.A., Doganay M., Dolapci M., Reis E., Atli M., Kologlu M. Risk factors resulting in conversion of laparoscopic cholecystectomy to open surgery. Surg. Endosc. 2001;15:965–968. doi: 10.1007/s00464-001-0008-4. [DOI] [PubMed] [Google Scholar]
- 42.Osuch C., Dolecki M., Rogula W.P., Łapiak A., Matyja M., Czerwińska A., Rubinkiewicz M., Matyja A. Gender as a predictive factor in cholecystectomy–is it true or false? Folia Med. Cracov. 2020;60:97–107. [PubMed] [Google Scholar]
- 43.Kaafarani H.M., Smith T.S., Neumayer L., Berger D.H., Depalma R.G., Itani K.M. Trends, outcomes, and predictors of open and conversion to open cholecystectomy in Veterans Health Administration hospitals. Am. J. Surg. 2010;200:32–40. doi: 10.1016/j.amjsurg.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 44.Sutcliffe R.P., Hollyman M., Hodson J., Bonney G., Vohra R.S., Griffiths E.A., the CholeS study group, West Midlands Research Collaborative Preoperative risk factors for conversion from laparoscopic to open cholecystectomy: A validated risk score derived from a prospective U.K. database of 8820 patients. HPB. 2016;18:922–928. doi: 10.1016/j.hpb.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lal P., Agarwal P.N., Malik V.K., Chakravarti A.L. A difficult laparoscopic cholecystectomy that requires conversion to open procedure can be predicted by preoperative ultrasonography. J. Soc. Laparoendosc. Surg. 2002;6:59–63. [PMC free article] [PubMed] [Google Scholar]
- 46.Faraj F.I., Da A., Hoaf F., Faruk H.I., Deari A., Halkawt O. Laparoscopic Cholecystectomy to Open Cholecystectomy in Sulaymaniyah Teaching Hospital, Incidence and Risk Factors Assessment. Pak. J. Med. Health Sci. 2020;14:1244–1248. [Google Scholar]
- 47.Kumar N.S.B., Zakkaria M. Factors affecting conversion of laparoscopic cholecystectomy to open surgery in a tertiary hospital in south india. J. Evol. Med. Dent. Sci. 2016;5:256–261. doi: 10.14260/jemds/2016/54. [DOI] [Google Scholar]
- 48.Naik M.B., Naik M.G., Sadhanala N. Preoperative clinical and radiological assessment in predicting difficult laparoscopic cholecystectomy- a study at government general hospital, guntur. J. Evol. Med. Dent. Sci. 2018;7:2694–2698. doi: 10.14260/jemds/2018/607. [DOI] [Google Scholar]
- 49.Goonawardena J., Gunnarsson R., de Costa A. Predicting conversion from laparoscopic to open cholecystectomy presented as a probability nomogram based on preoperative patient risk factors. Am. J. Surg. 2015;210:492–500. doi: 10.1016/j.amjsurg.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Bickel A., Rappaport A., Kanievski V., Vaksman I., Haj M., Geron N., Eitan A. Laparoscopic management of acute cholecystitis. Prognostic factors for success. Surg. Endosc. 1996;10:1045–1049. doi: 10.1007/s004649900237. [DOI] [PubMed] [Google Scholar]
- 51.Zisman A., Gold-Deutch R., Zisman E., Negri M., Halpern Z., Lin G., Halevy A. Is male gender a risk factor for conversion of laparoscopic into open cholecystectomy? Surg. Endosc. 1996;10:892–894. doi: 10.1007/BF00188477. [DOI] [PubMed] [Google Scholar]
- 52.Fried G.M., Barkun J.S., Sigman H.H., Joseph L., Clas D., Garzon J., Hinchey E.J., Meakins J.L. Factors determining conversion to laparotomy in patients undergoing laparoscopic cholecystectomy. Am. J. Surg. 1994;167:35–39. doi: 10.1016/0002-9610(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 53.Peters J.H., Krailadsiri W., Incarbone R., Bremner C.G., Froes E., Ireland A.P., Crookes P., Ortega A.E., Anthone G.A., Stain S.A. Reasons for conversion from laparoscopic to open cholecystectomy in an urban teaching hospital. Am. J. Surg. 1994;168:555–558. doi: 10.1016/S0002-9610(05)80121-7. [DOI] [PubMed] [Google Scholar]
- 54.Sanabria J.R., Gallinger S., Croxford R., Strasberg S.M. Risk factors in elective laparoscopic cholecystectomy for conversion to open cholecystectomy. J. Am. Coll. Surg. 1994;179:696–704. [PubMed] [Google Scholar]
- 55.Yang T.F., Guo L., Wang Q. Evaluation of Preoperative Risk Factor for Converting Laparoscopic to Open Cholecystectomy: A Meta-Analysis. Hepatogastroenterology. 2014;61:958–965. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.