Abstract
Formation of transmembrane pores by staphylococcal alpha-toxin can provoke a spectrum of events depending on target cell species and toxin dose, and in certain cases, repair of the lesions has been observed. Here, we report that transcriptional processes are activated as a response of cells to low toxin doses. Exposure of monocytic (THP-1) or epithelial (ECV304) cells to 40 to 160 ng/ml alpha-toxin provoked a drop in cellular ATP level that was followed by secretion of substantial amounts of interleukin-8 (IL-8). Cells transfected with constructs comprising the proximal IL-8 promoter fused to luciferase or to green fluorescent protein cDNA exhibited enhanced reporter gene expression following toxin treatment. Electrophoretic mobility shift and immunofluorescence assays demonstrated that IL-8 secretion was preceded by activation of NF-κB. Transfection experiments conducted with p65/p50 double-deficient cells showed that activation of the IL-8 promoter/reporter by toxin was absolutely dependent on NF-κB. In contrast, this transcription factor was not required for lesion repair. Attack of cells by low doses of a pore-forming toxin can lead to transcriptional gene activation, which is followed by production of mediators that may contribute to the initiation and propagation of inflammatory lesions.
More than 300 publications dealing with pore-forming bacterial toxins have been published during the past decade, the majority focusing on structural aspects and mechanisms of pore formation (3, 6, 7). Biological consequences other than cell lysis have been described, e.g., secretory responses (4, 5), production of lipid mediators (11), interleukin-1β (IL-1β) maturation (27), and programmed cell death (12). Killing of human keratinocytes by alpha-toxin is due to enhanced permeability for monovalent ions (27). Certain cells can repair a limited number of lesions (24). These findings all indicate that cells attacked by pore-forming toxins are not inevitably and instantly paralyzed and that they retain the capacity to mount active responses to membrane damage. In this investigation, we questioned whether cell damage by alpha-toxin might result in the activation and exploitation of transcriptional mechanisms. NF-κB transcription factors are involved in the response to many types of stress. Proteins encoded by NF-κB-regulated genes are devoted to intercellular signaling, cell adhesion, and other defense-related functions (9, 10). NF-κB has also been implicated in the regulation of apoptosis (1, 8). Biochemical features of NF-κB include constitutive expression in the cytosol, rapid activation, and translocation into the nucleus.
We report that alpha-toxin causes rapid activation of NF-κB, which leads to the expression of IL-8 in monocytic THP-1 cells and in ECV304 cells. Transcriptional activation was also shown in 3T3 fibroblasts, where it was found that recovery to sublethal toxin attack was not dependent on activation of this transcription factor.
MATERIALS AND METHODS
Wild type alpha-toxin and the nonlytic toxin mutant H35R were prepared as previously described (13, 21). Treatment with toxin or nonlytic mutant was transient; cells were incubated for 15 min at room temperature (RT) in tissue culture media containing the respective proteins and were subsequently washed twice in phosphate-buffered saline (PBS) before continuation of the culture.
Cell lines and culture conditions.
Human myelomonocytic THP-1 leukemia cells and ECV304 cells (identical to the T24 bladder carcinoma cell line [W. G. Dirks, R. A. MacLeod, and H. G. Drexler, Letter, In vitro Cell. Dev. Biol. Anim. 35:558–559]) were obtained from the American Type Culture Collection and cultured in a humidified incubator with 5% CO2 in RPMI medium with 8% fetal calf serum (FCS)–20 mM HEPES or in Dulbecco's modified Eagle's medium (DMEM) with 10% FCS, respectively. NF-κB p50/p65 double knockout and wild-type fibroblasts were derived from E12.5 mouse embryos harvested from time-mated females that are heterozygous for gene-targeted knockout alleles established for NF-κB1 (23) and RelA (2) and are immortalized by the standard 3T3 procedure. Molecular characterization of the p50/p65 double knockout cells has revealed a complete absence of κB-binding activity and NF-κB function and will be presented elsewhere (A. Hoffmann and D. Baltimore, unpublished data). The cells were cultured in DMEM with 10% FCS.
Plasmids.
Reporter plasmids pIL8LUC and pIL8EGFP were constructed using standard procedures. A fragment of the IL-8 promoter region comprising positions −420 to +102 was generated from human genomic DNA by PCR amplification with primers 5′-GGATCCATTGGCTGGCTTATCTTCACC-3′ (forward) and 5′-GGATCCTTTACACACAGTGAGAATGGT-3′ (reverse). The PCR product was cloned into pCR3.1 (Invitrogen, De Schelp, The Netherlands), and the insert was subcloned via restriction sites incorporated into the primers. The IL-8 promoter fragment was inserted into the BamHI site of the multiple cloning site in the promoterless pGL2 basic plasmid carrying the firefly luciferase gene (Promega Deutschland GmbH, Heidelberg, Germany). pIL8EGFP was made accordingly by subcloning the insert into the BgIII site of the promoterless pEGFP-1 plasmid (Clontech Laboratories GmbH, Heidelberg, Germany), which was harboring a green fluorescent protein (GFP) derivative. Final constructs were verified by Taq dye terminator sequencing using an Applied Biosystems 373A automated sequencer. Plasmids for transfection were prepared by two rounds of cesium chloride density centrifugation.
EMSA.
Nuclear extracts for the electrophoretic mobility shift assay (EMSA) were prepared from 107 cells per sample by using a hypoosmolaric NP-40-mediated lysis procedure followed by a high-salt extraction of the nuclei, as described earlier (18). A double-standard synthetic oligonucleotide of the sequence 5′-CTCTCGGAAAGTCCCCTCTG-3′, comprising the NF-κB site of the murine immunoglobulin kappa chain gene (in bold) was labeled with [γ-32P]ATP by using T4 kinase and purified by nondenaturing polyacrylamide gel electrophoresis (PAGE) or gel permeation chromatography. Binding reaction mixtures contained 10 μg of total protein from the extracts. Preincubation of the lysates with 3 μg of poly(dI-dC) (Roche) was followed by addition of probe (40,000 cpm) and a further incubation for 15 min at RT. Complexes were resolved on a 5% nondenaturing PAGE gel and visualized by autoradiography of the vacuum-dried gel for 6 to 16 h using a reflection screen. In competition experiments, a 200-fold excess of unlabeled double-stranded oligonucleotide was added to the binding reaction mixture. Supershifts were performed with antibodies from Santa Cruz Biotechnology Inc. according to the protocols of the supplier.
Indirect immunofluorescence detection of NF-κB translocation.
Confluent ECV304 cells in eight-well glass chamber slides were fixed with cold 70% ethanol for 20 min on ice, washed twice with PBS, and stained for NF-κB p65 by using a polyclonal rabbit antibody (Santa Cruz Biotechnology Inc.) at a final concentration of 5 μg/ml for 1 h at RT. Cells were washed three times with PBS and then were incubated with Cyt3-conjugated donkey anti-rabbit immunoglobulin G (Dianova) at a concentration of 3.75 μg/ml for 30 min at RT. After three washes, the cells were covered with mounting fluid and visualized under a fluorescence microscope (Axiophot, Zeiss, Germany).
Measurement of cytokines.
Supernatants of cells grown in 24-well plates were assayed for cytokines with enzyme-linked immunosorbent assay (ELISA) kits for tumor necrosis factor alpha (TNF-α), IL-8, and RANTES from R&D (Wiesbaden, Germany) according to the manufacture's protocols. For dose-response experiments, 5 × 105 THP cells and 105 ECV304 cells were pretreated with toxin for 15 min at RT, washed with PBS, and cultured for 24 h at 37°C in a humidified incubator with 5% CO2. Kinetics of IL-8 release were determined with supernatants of THP-1 cells treated for 15 min with 100 ng of alpha-toxin/ml.
Measurement of ATP.
Measurements of cellular ATP levels were performed with the luciferase-based CSL II kit from Roche and a 9500 luminometer from Berthold (Bad Wildbad, Germany).
Transfection and reporter assays.
For transient transfections of ECV304 and 3T3 cells, 80,000 cells per well were seeded into 24-well tissue culture plates 1 day prior to transfection. Transfection was achieved with Lipofectin reagent (Life Technologies) according to the protocol of the manufacturer. Generally, 0.4 μg of LUC-reporter plasmid and, where indicated, 0.05 μg of the cytomegalovirus promoter-based expression plasmids were transfected per well. Cells were treated with alpha-toxin 24 after transfection; treatment protocol was as described in the ELISA section. Reporter activity was determined 48 h after transfection using commercial luciferase reporter reagent and lysis reagent (Promega). Measurements were performed in a Berthold 9500 luminometer.
Stably transfected ECV304 clones harboring pIL8EGFP were obtained by limiting dilution and three rounds of geneticin selection after transfection. Screening of transfected clones was done by FACScan. Clone G7 was selected for these experiments since it exhibited appreciable basal level expression of GFP while still being inducible by phorbol myristate acetate (PMA).
RESULTS
Recovery of ECV304 cells from attack by low doses of alpha-toxin.
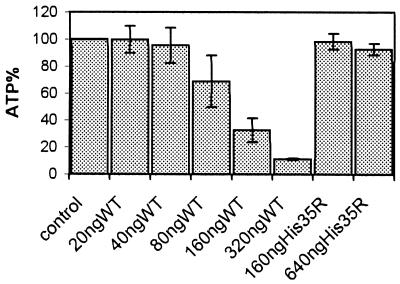
Membrane permeabilization by alpha-toxin is accompanied by a drop in cellular ATP levels (24). As shown in Fig. 1, ATP depletion in ECV304 cells commenced at toxin concentrations of approximately 40 ng/ml. While an ATP loss of approximately 5% was discernable at this concentration, the finding was not of statistical significance (P > 0.5). In contrast, at 160 ng/ml, a significant drop of cellular ATP was seen (70% when employing the wild-type toxin versus 2% with the H35R mutant; P < 0.001). ATP loss exceeded 90% after incubation with 320 ng of alpha-toxin/ml. The alpha-toxin H35R mutant, which binds to cells but fails to form functional channels, did not provoke significant ATP depletion, even when applied at 640 ng/ml.
FIG. 1.
Measurement of ATP in lysates of ECV304 cells following a 2-h treatment with wild-type(WT) alpha-toxin or the nonlytic mutant H35R. The data are means from three independent experiments, with error bars indicating standard deviation.
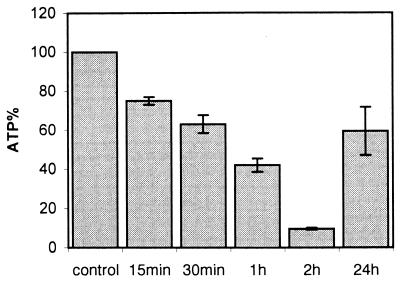
In the next experiment, ECV304 cells were exposed to 160 ng of alpha-toxin/ml and ATP measurements were conducted over an extended time period. As shown in Fig. 2, cellular ATP dropped progressively during the first 2 h down to about 10% of the values of untreated controls. At 24 h, however, levels had returned to approximately 60% of those of the controls (P < 0.001 for 2 h versus 24 h). Staining with propidium iodide (12, 25) revealed approximately 40% of the cells to be dead (not shown).
FIG. 2.
Replenishment of cellular ATP levels after alpha-toxin treatment. ECV304 cells were exposed to 160 ng of alpha-toxin/ml, and cellular ATP was determined at the depicted times. Values are the means of three independent experiments; error bars indicate standard deviation.
When toxin was applied at 40 to 80 ng/ml, initial ATP depletion was 40 to 70% after 2 h and 70 to 80% of the cells were viable after 4 h, as judged from total cellular ATP and their ability to exclude propidium iodide (not shown).
Activation of NF-κB in alpha-toxin-treated cells.
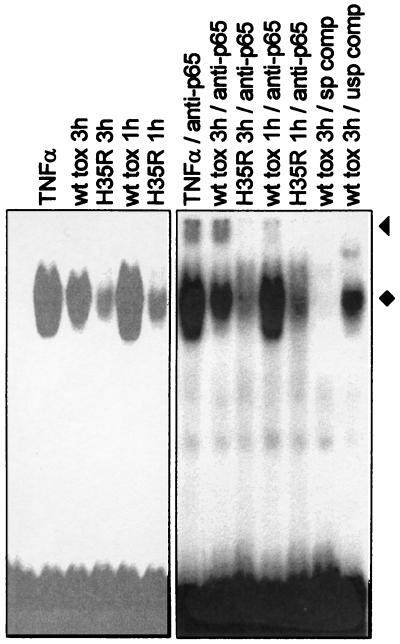
We proceeded to test whether toxin might elicit a prototypic stress-related response and performed mobility shift assays with a probe for NF-κB. A striking induction of NF-κB binding to a consensus site was seen within 1 h after treatment with 160 ng of alpha-toxin/ml (Fig. 3). The nonfunctional H35R mutant did not produce this effect, which showed that NF-κB activation was not due to potential impurities in toxin preparations and that activation required membrane permeabilization. The specificity of the induced complexes was confirmed by using cold competitor oligonucleotides. The presence of p65, the major activating species of NF-κB, was demonstrated by supershift experiments.
FIG. 3.
Toxin treatment activates NF-κB. EMSAs were performed with extracts from THP-1 cells treated for 1 or 3 h with wild-type (wt) or H35R toxin. The specificity of complex formation was assessed by the addition of specific (sp) or unspecific (usp) unlabeled competitor oligonucleotides; an oligonucleotide carrying an AP-1 binding site (Promega) served as an unspecific competitor in this experiment. The presence of p65 in lysates from toxin-treated cells was demonstrated by supershift induction with specific antibodies (anti-p65); supershifted bands are marked with a triangle. The NF-κB complexes are marked with a diamond. Data were derived from the same gel, but different scanning parameters were chosen. A variety of antibodies of irrelevant specificity and several other oligonucleotides encompassing binding sites for transcription factors other than NF-κB were also tested and were found to have no effect on the formation of the NF-κB complex.
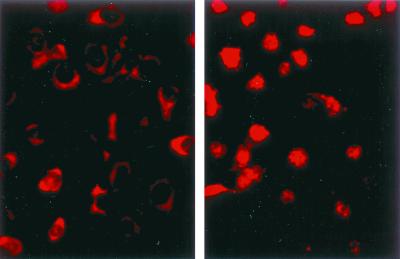
Induction of DNA-binding activity is usually accompanied by translocation of NF-κB to the nucleus. Whether activation of NF-κB in toxin-treated cells would proceed to this point was addressed in the next experiments. Antibodies to p65 were used to stain ECV304 cells. The staining remained cytoplasmatic in cells treated with the nonfunctional H35R mutant toxin. In contrast, nuclear translocation of p65 was observed in cells that had been treated with active alpha-toxin (Fig. 4).
FIG. 4.
Immunofluorescence detection of p65 translocation into the nuclei of toxin-treated cells. ECV304 cells were treated with a nonlytic alpha-toxin variant (left) or with the lytic wild-type toxin (right). After 1 h of treatment, the cells were fixed, permeabilized, and stained for p65.
Secretion of IL-8 by THP-1 and ECV304 cells after treatment with low doses of alpha-toxin.
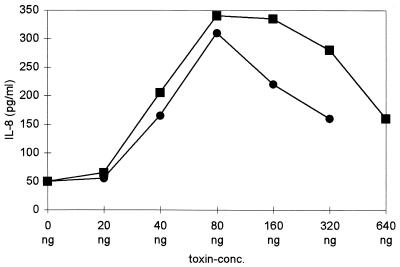
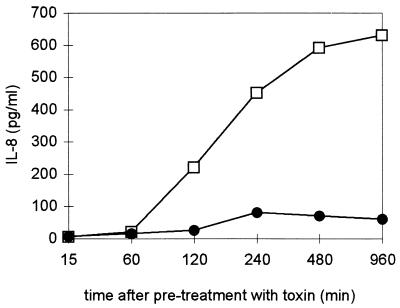
NF-κB activation in intact cells is a requirement for the transcription of many stress response genes. Since treatment with alpha-toxin caused a reduction of cellular ATP, it was not self-evident whether NF-κB activation would be followed by gene transcription and protein synthesis. Therefore, we examined supernatants of toxin-treated cells for several mediators of the inflammatory response. While TNF-α, RANTES, and IL-1β concentrations remained unchanged, alpha-toxin provoked a significant dose- and time-dependent increase in IL-8 levels (P < 0.005 for the comparison of maximal levels of dose responses of treated cultures of either cell type versus those of controls) (Fig. 5 and 6). Bell-shaped dose-response curves were observed with both cell types, with a maximum at 40 to 160 ng of alpha-toxin/ml. At concentrations below 80 ng/ml, cells remained impermeable to propidium iodide throughout the experiment, so the effect of toxin could not have been due to the release of preformed IL-8. IL-8 secretion commenced 2 h after toxin treatment, approximately 1 h after the maximum of NF-κB binding activity had been reached.
FIG. 5.
Dose-dependent response to alpha-toxin in ECV304 and THP-1 cells. IL-8 levels in supernatants were measured 24 h after toxin treatment (squares, THP-1; circles, ECV304). The bell-shaped dose-response curve and the maximum levels shown are representative (n = 7 for THP-1 cells, n = 5 for ECV304 cells). The lytic activity per nanogram of toxin varied between different toxin preparations; therefore, P values were calculated for the comparison of maxima in treated samples versus those of controls (see text).
FIG. 6.
Time-dependent release of IL-8 into supernatants of alpha-toxin-treated THP-1 cells. Circles, nonlytic H35R toxin; squares, wild-type toxin.
Activation of an IL-8 promoter-reporter hybrid in toxin-treated cells.
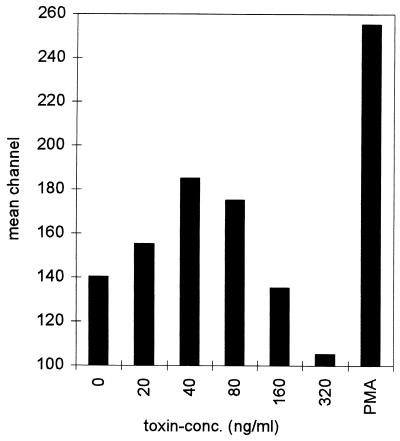
ECV304 cells were stably transfected with an IL-8–EGFP reporter hybrid, encompassing the proximal promoter of the IL-8 gene. This promoter region comprises an NF-κB site which is crucial for the transcriptional induction of IL-8 by various stimuli (16, 17). Reporter activity was assessed by flow cytometry. As shown in Fig. 7, alpha-toxin provoked increases in cellular fluorescence. A bell-shaped dose-response curve was again noted, with maximum increases in mean channel fluorescence occurring at 40 ng of alpha-toxin/ml. This corresponded to 40% of the maximum (PMA treatment).
FIG. 7.
Nonlytic doses of alpha-toxin productively activate the stably integrated proximal IL-8 promoter. ECV304 cells stably transfected with an IL-8–EGFP hybrid construct were treated with the depicted concentrations of alpha-toxin. FACScan analysis revealed toxin-dose-dependent increases in mean channel fluorescence, with maximum induction at 40 ng/ml. PMA (100 nM) served as a positive control. The experiment was repeated with virtually identical results.
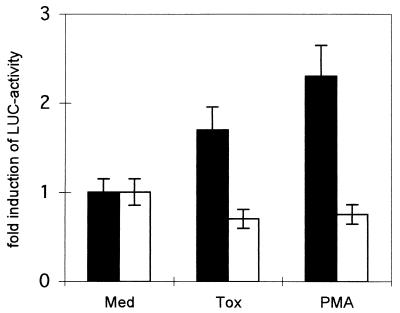
We then compared the activity of an IL-8 LUC reporter in wild-type 3T3 cells and 3T3 cells with a double knockout phenotype for NF-κB p50/p65. Induction by toxin was only seen in the wild-type cells (1.7-fold increase; P < 0.001), and reporter activity was reduced even by toxin in the double knockout cells (Fig. 8). Thus, NF-κB was indispensable for toxin-mediated transcriptional activation of IL-8.
FIG. 8.
No induction of pIL8LUC by alpha-toxin in p65/p50 knockout cells. 3T3 wild-type cells (black columns) or 3T3 cells carrying a p65/p50 double-knockout phenotype (white columns) were transiently transfected with pIL8LUC and were treated with media alone, alpha-toxin (40 μg/ml), or PMA. Luciferase activity in cell lysates was measured. The results are from three independent experiments; error bars indicate standard deviation.
NF-κB p50 and p65 are dispensable for alpha-toxin lesion repair.
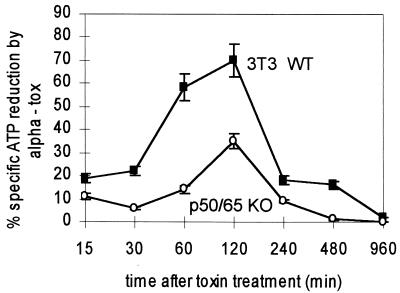
Apart from the regulation of soluble mediators, adhesion molecules, and other stress induced proteins, NF-κB has recently been assigned a role in the balancing of apoptotic death and survival. Fibroblasts exhibit a marked capacity to survive attack by alpha-toxin. To test whether NF-κB was involved in the repair process, wild-type 3T3 cells and NF-κB double knockout 3T3 cells were treated with alpha-toxin or H35R toxin and cellular ATP was measured. We observed that the depletion of cellular ATP in the NF-κB-deficient cells was only half as pronounced as that in wild-type cells (35% versus 70%; P < 0.01). In both cell lines, ATP levels returned to normal after 16 h. Thus, there was no requirement for NF-κB for the recovery process (Fig. 9).
FIG. 9.
Recovery of fibroblasts from sublethal attack is independent of p65/p50. The effect of alpha-toxin on cellular ATP levels over time was compared between 3T3 cells and 3T3 p65/p50 double-knockout cells. The data are from three independent experiments; error bars indicate standard deviation.
DISCUSSION
NF-κB activation has previously been described for cells undergoing attack by sublytic doses of the pore-forming C5b-9 complement complex (14), but analogous events provoked by a pore-forming bacterial toxin have not been reported. Here it is shown that the activation of NF-κB is indeed provoked by low concentrations of staphylococcal alpha-toxin, leading to the generation of IL-8, an important inflammatory mediator. Results obtained in experiments with transfected IL-8 reporter constructs clearly linked NF-κB activation to IL-8 induction.
The mechanisms underlying induction of NF-κB by alpha-toxin are not clear. However, the lack of activity of the H35R alpha-toxin mutant that retains the capacity of membrane binding and pre-pore formation (13) indicated that membrane permeabilization is crucial for activation of the transcription factor. Both THP-1 and ECV304 cells secreted IL-8 despite ATP depletion. The cholesterol-binding Alv toxin from Bacillus alvei has recently been shown to provoke IL-8 mRNA and protein production in neutrophils (15). It is probable that NF-κB activation was also involved in that case.
NFκ-B regulates numerous genes (20), e.g., those for RANTES (19), ICAM, and IL-8 (16, 17, 20). We did not observe an increase of RANTES upon alpha-toxin treatment of THP-1 cells or ECV304 cells, which might have been due to the lack of additional triggers required for induction in these cells. The NF-κB site in the proximal promoter of the IL-8 gene preferentially binds homodimers of p65 or c-Rel (17). While our supershift experiments indicated that p65 is present in the EMSA complexes, it remains to be investigated whether alpha-toxin preferentially induces certain NF-κB subtypes. Ongoing experiments are also being conducted to screen for other targets of NF-κB activation in toxin-treated cells.
A role for NF-κB as an important regulator of cell death is evolving (1, 8). A protective effect has been found in the majority of cases, but cell death has also occasionally been shown to depend on this transcription factor. ATP levels in 3T3 cells treated with alpha-toxin followed essentially the pattern observed earlier with human foreskin fibroblasts (26). The ATP decrease after toxin treatment was less pronounced in p65/p50 knockout cells. A possible explanation for this phenomenon would be that the inhibition of NF-κB p50/p65-mediated stress responses conserves ATP. Replenishment of intracellular ATP pools was complete after 16 h in both wild-type and p65/p50 knockout 3T3 cells, so NF-κB appeared to play no essential role in lesion repair. It is not excluded that other members of the Rel family of transcription factors might be involved in the recovery process.
The dose dependency of NF-κB activation deserves emphasis. When nucleated cells are permeabilized by toxin doses that provoke only partial cellular ATP depletion, lesion repair may follow. In fibroblasts, evidence is available that this repair is due to closure of the toxin channels (24, 26). In the absence of an alternative explanation, we assume that this also underlies the capacity of THP-1 and ECV304 cells to recuperate from toxin attack. Productive transcriptional activation will occur only within the narrow window of subcytocidal toxin concentrations; when they are exceeded, the repair mechanism is overrun and cell death ensues. While it is possible that cell death could elicit a response of bystander cells, it should be noted that in the present study, IL-8 secretion and reporter activity could be observed at toxin concentrations that did not significantly affect ATP levels. With THP-1 cells, we have also found that these toxin concentrations do not affect the response to a subsequent challenge with lipopolysaccharide (unpublished observation). The bell-shaped dose-response curves of NF-κB activation and IL-8 production underscore the contention that subcytocidal toxin concentrations can elicit a cellular response. When toxin-producing bacteria invade and multiply in tissues, it is likely that at any given time, certain cells will be exposed to the critical toxin concentrations that provoke such active cellular responses. Production of inflammatory cytokines is, of course, but one of countless possible reactions that will follow as a consequence of transcriptional activation, which may turn out to be a common event occurring in the wake of membrane permeabilization by pore-forming toxins. The ensuing cellular responses may contribute to both short- and long-range pathophysiological processes that are encountered during bacterial infections.
ACKNOWLEDGMENTS
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 490) and the Verband der Chemischen Industrie.
REFERENCES
- 1.Barkett M, Gilmore T D. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene. 1999;18:6910–6924. doi: 10.1038/sj.onc.1203238. [DOI] [PubMed] [Google Scholar]
- 2.Beg A A, Sha W C, Bronson R T, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 3.Bhakdi S, Bayley H, Valeva A, Walev I, Walker B, Kehoe M, Palmer M. Staphylococcal alpha-toxin, streptolysin-O, and Escherichia coli hemolysin: prototypes of pore-forming bacterial cytolysins. Arch Microbiol. 1996;165:73–79. doi: 10.1007/s002030050300. [DOI] [PubMed] [Google Scholar]
- 4.Bhakdi S, Muhly M, Korom S, Hugo F. Release of interleukin-1 beta associated with potent cytocidal action of staphylococcal alpha-toxin on human monocytes. Infect Immun. 1989;57:3512–3519. doi: 10.1128/iai.57.11.3512-3519.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhakdi S, Muhly M, Korom S, Schmidt G. Effects of Escherichia coli hemolysin on human monocytes. Cytocidal action and stimulation of interleukin 1 release. J Clin Investig. 1990;85:1746–1753. doi: 10.1172/JCI114631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabiaux V, Wolff C, Ruysschaert J M. Interaction with a lipid membrane: a key step in bacterial toxins virulence. Int J Biol Macromol. 1997;21:285–298. doi: 10.1016/s0141-8130(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 8.Foo S Y, Nolan G P. NF-κB to the rescue: RELs, apoptosis and cellular transformation. Trends Genet. 1999;15:229–235. doi: 10.1016/s0168-9525(99)01719-9. [DOI] [PubMed] [Google Scholar]
- 9.Ghosh S. Regulation of inducible gene expression by the transcription factor NF-κB. Immunol Res. 1999;19:183–189. doi: 10.1007/BF02786486. [DOI] [PubMed] [Google Scholar]
- 10.Gilmore T D. The Rel/NF-κB signal transduction pathway: introduction. Oncogene. 1999;18:6842–6844. doi: 10.1038/sj.onc.1203237. [DOI] [PubMed] [Google Scholar]
- 11.Grimminger F, Rose F, Sibelius U, Meinhardt M, Potzsch B, Spriestersbach R, Bhakdi S, Suttorp N, Seeger W. Human endothelial cell activation and mediator release in response to the bacterial exotoxins Escherichia coli hemolysin and staphylococcal alpha-toxin. J Immunol. 1997;159:1909–1916. [PubMed] [Google Scholar]
- 12.Jonas D, Walev I, Berger T, Liebetrau M, Palmer M, Bhakdi S. Novel path to apoptosis: small transmembrane pores created by staphylococcal alpha-toxin in T lymphocytes evoke internucleosomal DNA degradation. Infect Immun. 1994;62:1304–1312. doi: 10.1128/iai.62.4.1304-1312.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jursch R, Hildebrand A, Hobom G, Tranum-Jensen J, Ward R, Kehoe M, Bhakdi S. Histidine residues near the N terminus of staphylococcal alpha-toxin as reporters of regions that are critical for oligomerization and pore formation. Infect Immun. 1994;62:2249–2256. doi: 10.1128/iai.62.6.2249-2256.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilgore K S, Schmid E, Shanley T P, Flory C M, Maheswari V, Tramontini N L, Cohen H, Ward P A, Friedl H P, Warren J S. Sublytic concentrations of the membrane attack complex of complement induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-κB activation. Am J Pathol. 1997;150:2019–2031. [PMC free article] [PubMed] [Google Scholar]
- 15.König B, Koller M, Prevost G, Piemont Y, Alouf J E, Schreiner A, König W. Activation of human effector cells by different bacterial toxins (leukocidin, alveolysin, and erythrogenic toxin A): generation of interleukin-8. Infect Immun. 1994;62:4831–4837. doi: 10.1128/iai.62.11.4831-4837.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunsch C, Lang R K, Rosen C A, Shannon M F. Synergistic transcriptional activation of the IL-8 gene by NF-κB p65 (RelA) and NF-IL-6. J Immunol. 1994;153:153–164. [PubMed] [Google Scholar]
- 17.Kunsch C, Rosen C A. NF-κB subunit-specific regulation of the interleukin-8 promoter. Mol Cell Biol. 1993;13:6137–6146. doi: 10.1128/mcb.13.10.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin K I, Lee S H, Narayanan R, Baraban J M, Hardwick J M, Ratan R R. Thiol agents and Bcl-2 identify an alphavirus-induced apoptotic pathway that requires activation of the transcription factor NF-κB. J Cell Biol. 1995;131:1149–1161. doi: 10.1083/jcb.131.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson P J, Kim H T, Manning W C, Goralski T J, Krensky A M. Genomic organization and transcriptional regulation of the RANTES chemokine gene. J Immunol. 1993;151:2601–2612. [PubMed] [Google Scholar]
- 20.Pahl H L. Activators and target genes of Rel/NF-κB transcription factors. Oncogene. 1999;18:6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 21.Palmer M, Jursch R, Weller U, Valeva A, Hilgert K, Kehoe M, Bhakdi S. Staphylococcus aureus alpha-toxin. Production of functionally intact, site-specifically modifiable protein by introduction of cysteine at positions 69, 130, and 186. J Biol Chem. 1993;268:11959–11962. [PubMed] [Google Scholar]
- 22.Roebuck K A. Oxidant stress regulation of IL-8 and ICAM-1 gene expression: differential activation and binding of the transcription factors AP-1 and NF-κB. Int J Mol Med. 1999;4:223–230. doi: 10.3892/ijmm.4.3.223. [DOI] [PubMed] [Google Scholar]
- 23.Sha W C, Liou H C, Tuomanen E I, Baltimore D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 24.Valeva A, Walev I, Gerber A, Klein J, Palmer M, Bhakdi S. Staphylococcal alpha-toxin: repair of a calcium-impermeable pore in the target cell membrane. Mol Microbiol. 2000;36:467–476. doi: 10.1046/j.1365-2958.2000.01865.x. [DOI] [PubMed] [Google Scholar]
- 25.Walev I, Martin E, Jonas D, Mohamadzadeh M, Müller-Klieser W, Kunz L, Bhakdi S. Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect Immun. 1993;61:4972–4979. doi: 10.1128/iai.61.12.4972-4979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walev I, Palmer M, Martin E, Jonas D, Weller U, Höhn-Bentz H, Husmann M, Bhakdi S. Recovery of human fibroblasts from attack by the pore-forming alpha-toxin of Staphylococcus aureus. Microb Pathog. 1994;17:187–201. doi: 10.1006/mpat.1994.1065. [DOI] [PubMed] [Google Scholar]
- 27.Walev I, Reske K, Palmer M, Valeva A, Bhakdi S. Potassium-inhibited processing of IL-1 beta in human monocytes. EMBO J. 1995;14:1607–1614. doi: 10.1002/j.1460-2075.1995.tb07149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]