Abstract
Stroke accounts for the second leading cause of death and a major cause of disability, with limited therapeutic strategy in both the acute and chronic phases. Blood-based biomarkers are intensively researched and widely recognized as useful tools to predict the prognoses of patients confronted with therapeutically limited diseases. We performed a systematic review of the circulating biomarkers in IS patients with prognostic value, with a focus on microRNAs and exosomes as predictive biomarkers of motor and cognitive recovery. We identified 63 studies, totalizing 72 circulating biomarkers with prognostic value in stroke recovery, as follows: 68 miRNAs and exosomal-miRNAs being identified as predictive for motor recovery after stroke, and seven biomarkers being predictive for cognitive recovery. Twelve meta-analyses were performed using effect sizes (random-effects and fixed-effects model). The most significant correlation findings obtained after pooling were with miR-21, miR-29b, miR-125b-5p, miR-126, and miR-335. We identified several miRNAs that were correlated with clinical outcomes of stroke severity and recovery after ischemic stroke, providing predictive information on motor and cognitive recovery. Based on the current state of research, we identified serum miR-9 and neutrophil miR-29b as the most promising biomarkers for in-depth follow-up studies, followed by serum miR-124 and plasma miR-125b.
Keywords: microRNAs, extracellular vesicles, disease biomarkers, ischemic stroke, prognosis, stroke recovery
1. Introduction
Stroke affects millions of people, accounting for the second leading cause of death and a major cause of disability [1]. Stroke is a heterogenous disease, and its two forms (ischemic and hemorrhagic) differ in terms of clinical features and disease progression; ischemic stroke (IS) is responsible for 80–85% of cases, while 10–15% are classified as hemorrhagic stroke, which includes subarachnoid hemorrhage and intraparenchymal hemorrhage [2,3].
Thrombolysis with rt-PA (intravenous recombinant tissue plasminogen activator) or thrombectomy are therapeutically restricted to a limited category of patients, i.e., those presenting to thrombolysis centers within 3–4.5 h of stroke onset [4].
Dysphasia, hemiparesis and incontinence, painful spasticity related to motor impairment, and post-stroke depression (PSD) are some of the most common reported long-term dysfunctions after stroke [5]. Moreover, unmodifiable factors, such as metabolic status of patients, i.e., hyperlipidemia, hyperglycemia, and comorbidities such as diabetes and hypertension have a high impact on post-stroke recovery [6,7].
About 50–60% of post-stroke patients experience motor dysfunction [8] and the majority of stroke survivors experience delayed recovery of motor and sensory function, or even worsening of neurological function [9]. Therefore, neurorehabilitation strategies have been developed to reduce neurological deficit and promote effective sensorimotor integration in motor and sensory-related impairments [10,11,12,13].
Novel therapeutic approaches were evidenced in experimental stroke models with the aim to improve functional recovery after stroke. However, despite promising pre-clinical results obtained using drugs, cell-based therapies, or mesenchymal stem cell-derived exosomes, the translation from bench to bedside has proven to be challenging [14].
Although many studies have focused on neuroprotective and recovery strategies in ischemic stroke, there is limited data on circulating biomarkers with prognostic and diagnostic value. Blood-based biomarkers are intensively researched and widely recognized as useful tools with which to predict the prognoses and survival rates of stroke patients [15]. Thus, the use of accessible biomarkers detected by quantitative polymerase chain reaction (qPCR)-based technics from biofluids could contribute to predicting with a high-accuracy long-term sensorimotor recovery [16].
MicroRNAs (miRNAs) emerged as post-transcriptional modulators and activators of signaling pathways in numerous diseases [17,18]. Thus, an altered profile of miRNA expression has been reported in blood samples of stroke patients, with multiple dysregulated miRNAs involved in cerebral ischemic-reperfusion processes, such as excitotoxicity, oxidative stress, neuroinflammation, and neuron cell death processes [19].
After ischemic injury, different miRNAs are responsible for communication between brain cells and may provide insights into neuronal repair, vasculogenesis, and neurogenesis [20]. Moreover, miRNAs produced by ischemic brain cells could cross the blood-brain barrier (BBB) packed in extracellular vesicles or exosomes, and exhibit remarkable stability in human biofluids, i.e., plasma or serum. There is hope that their expression profiling may directly reflect disease status and prognosis [21,22,23].
Therefore, by quantifying miRNA expression profiles and identifying those associated with unfavorable outcomes we could identify patients at risk for delayed and poor functional recovery and guide rehabilitation strategies. Indeed, compared to protein and metabolic biomarkers, stroke-specific miRNAs show high expression levels immediately after ischemic injury in serum and plasma samples [24]. Most importantly, there is an urgent need to find those blood miRNA biomarkers that correlate with long-term outcomes in the first days after stroke onset. In addition, in animal models of stroke, miRNAs and their carriers such as exosomes and other extracellular vehicles (EVs) play modulatory roles in brain remodeling and repair mechanisms of the neurovascular unit [25,26].
This systematic review aims to identify and summarize circulating miRNAs and EV-derived miRNAs that could serve as early-warning markers of long-term outcomes and predictors of physical recovery in stroke patients. To this end, we compared multiple studies reporting blood miRNA expression profiles within serum, plasma, and circulating cells in the early and late stages of stroke recovery. Then, we selected all miRNAs reported in electronic databases with prognostic value on the functional recovery of stroke patients. Moreover, whenever multiple comparable studies analyzed the same miRNA biomarker, we used meta-analytic techniques to obtain a pooled estimate of the correlation between miRNA expression and recovery assessment scores.
2. Methods
2.1. Search Strategy
We searched the following electronic databases: Medline PubMed, Scopus, Web of Science, Embase, and Crossref. We used different combinations of the following keyword search terms: (microRNA OR miRNA OR miR OR micro-RNA) AND (NIHSS OR NIH Stroke Scale OR NIH Stroke Score OR National Institutes of Health Stroke Scale) OR (modified Rankin Score OR mRS) OR (MMSE OR Mini-mental state examination) OR (MoCA OR Montreal Cognitive Assessment) AND (Stroke OR acute stroke OR ischemic stroke OR ischemic stroke OR cerebral ischemia OR cerebrovascular accident OR cerebral infarction).
2.2. Inclusion and Exclusion Criteria
For our search, we included the following studies: (1) cohort and case-control studies that evaluate the value of miRNAs in the prognosis of AIS (acute ischemic stroke); (2) CT/MRI-based diagnosis of stroke event in patients ≥ age 18 years; (3) studies retrieved between January 2011 and August 2022, with the primary source of quantitative research in a peer-reviewed journal or thesis published in English or German; (4) AIS diagnosis within 24 h after stroke onset; (5) blood collection time between 24 h after stroke up to 1 year after a stroke event, considering the period of 3–6 months after stroke as the most critical period of time for effective rehabilitation and fast recovery in stroke patients [13]; (6) measure(s) of physical and cognitive outcome using validated motor and cognitive assessment scales (e.g., NIHSS, mRS and/or mBI, MMSE, MoCA); and (7) studies which reported a measure for the prognostic value of said biomarker, e.g., sensitivity, specificity, area under the curve (for dichotomial outcomes), or correlation coefficients between miRNA expression and the results of a scale that assessed physical and cognitive outcome.
We excluded from the searched studies: (1) animal model studies; (2) studies evaluating non-stroke patients; (3) article not available in English; and (4) conference proceedings. Two Chinese-language studies were included because the English-language abstract provided sufficient information for the purpose of this review, with the exception of quality assessment.
2.3. Data Extraction and Quality Assessment
Two reviewers worked independently to extract the following data from eligible studies: demographic data of stroke cohorts (age/sex), inclusion/exclusion criteria, general patient information (comorbidities and stroke-related risk factors), choice of sample for miRNA quantification, miRNA collection timing, expression levels of circulating miRNAs, miRNA quantification method, disability scale used, disability score timing, and the diagnostic and prognostic performance of studied miRNAs.
2.4. Quality Assessment
We assessed the quality of included studies using the REMARK criteria [27]. This quality assessment tool comprised 8 items originally designed for studies of diagnostic and prognostic tumor-marker studies, evaluating the quality of study design and reporting, as well as the thoroughness of biomarker assessment. Similar systematic reviews aimed at identifying circulating biomarkers with prognostic value in stroke recovery used the REMARK criteria for checking the quality of studies included [7,28]. The items were evaluated as “yes” or “no”, and the percentage of “yes” responses was used as an overall quality score. The quality assessment scores of all the included papers in this review are available in Supplemental Table S4.
2.5. Statistical Analysis
Data analysis was performed using R 4.2 and package meta (correlation meta-analysis) [29,30]. To ensure meaningful pooling in the meta-analysis of correlation, we converted Pearson’s r and Kendall’s τ into Spearman’s ρ, the most commonly reported statistic in the included articles, based on the table provided by Gilpin for meta-analyses [31].
Pooling was performed using fixed-effects and random-effects (DerSimonian-Laird [32]) methods, and heterogeneity was evaluated using the I2 and Cochran’s Q (χ2) method [33,34]. Due to the small sample sizes in our paper and the low power of publication bias tests [35,36], we did not check for publication bias.
We did not pool across multiple outcome measures (e.g., correlations between miRNA expression and both NIHSS and mRS scores), following the conclusions of Puhan et al. and the recommendations of the Cochrane Scientific Committee [37,38]. Subsequently, all studies in the correlation meta-analysis employed the NIHSS score.
3. Results
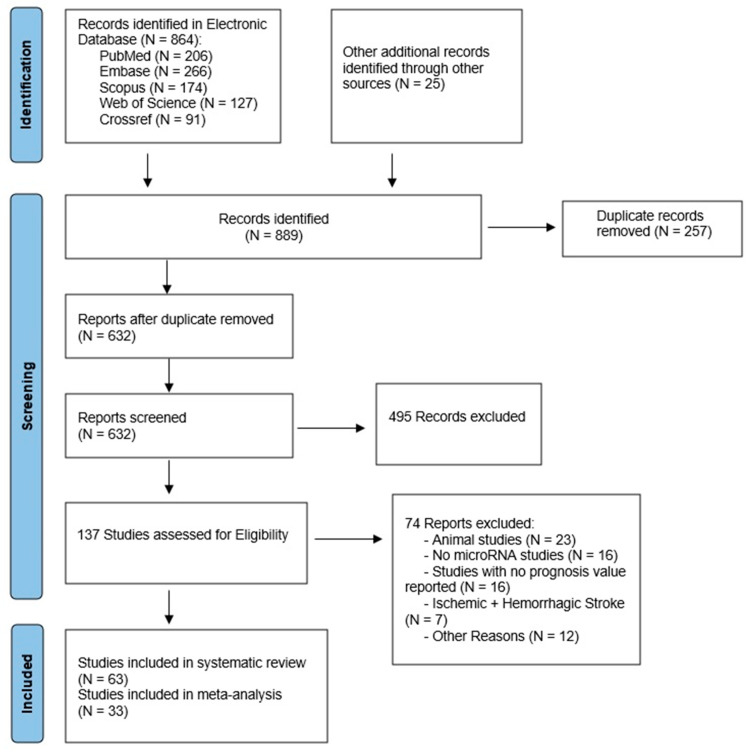
Based on the search keywords, we identified 889 records in the following electronic databases: Pubmed, Embase, Scopus, Web of Science, and Crossref. After removing all the duplicates, 632 studies were carried out for screening. Subsequently, we screened the retrieved articles based on the abstract and title, leaving 137 studies assessed for eligibility. After a complete full-text analysis of the remaining studies, 63 studies met our inclusion and exclusion criteria and were included in our review (Figure 1).
Figure 1.
Flowchart with selection criteria of research studies included in review.
Our search strategy followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria [39]. The comprehensive search of the 63 included studies yielded a total of 72 circulating biomarkers with predictive values for stroke severity and recovery in IS patients. Specifically, these biomarkers were 60 non-exosomal circulating miRNA species isolated from blood components (plasma, serum, PBMCs, and other circulating cells) and 12 extracellular vesicle-derived miRNAs isolated from serum and plasma exosomes and endothelial microvesicles (Table 1).
Table 1.
Overview of identified circulating miRNAs in blood specimens which provide prognostic value in stroke patients.
| Study and Author | Country | N | Featured miRNAs | MiRNA Source | miRNA Sample Timing |
Clinical Outcome Measured |
|---|---|---|---|---|---|---|
| Ma et al., 2022 [40] | China | 72/56 | EMV-miR-125a-5p | Plasma | <24 h | NIHSS |
| Peng et al., 2015 [41] | China | 72/52 | miR-let-7e | Serum | <24 h | NIHSS |
| Wu et al., 2017 [42] | China | 131/50 | miR-23b-3p, -29b-3p, -181a-5p, -21-5p | Serum | <24 h | NIHSS, BI, mRS |
| Kijpaisalratana et al., 2020 [43] | Thailand | 23/35 | miR-125a-5p, -125b-5p, -433-5p | Serum | <72 h | NIHSS |
| Kautzky et al., 2022 [44] | Germany | 40/40 | miR-125a-5p, miR-125b-5p | Plasma | <24 h | NIHSS |
| Zhu et al., 2019 [45] | China | 170/170 | miR-143 | PBMC | < 24 h | NIHSS |
| Niu et al., 2021 [46] | China | 453/143 | Exosomal-miR-369-3p, -493-3p, -379-5p, -1296-5p | Plasma | <72 h | NIHSS |
| Li et al., 2022 [47] | China | 50/42 | miR-29b | Leukocytes | <6 h | NIHSS |
| Kotb et al., 2019 [48] | Lebanon | 44/22 | miR-146a | Serum | <24 h | GCS |
| Wu et al., 2020 [49] | China | 112/112 | miR-99b | Plasma | NR | GOS |
| Huang et al., 2016 [50] | China | 76/38 | miR-132 | Serum | Chronic | MoCA |
| Zhai et al., [51] | China | 108/76 | miR-195, -497 | Serum | <72 h | NIHSS, MoCA |
| Ma et al., 2019 [52] | China | 33/20 | miR-93 | Neutrophils and plasma | <6 h | NIHSS, BI, mRS |
| Lin et al., 2022 [53] | China | 96 | miR-411-5p | Serum | Before rt-PA, 24 h after rt-PA, and at 3 months | NIHSS |
| Xiang et al., 2017 [54] | China | 40/46 | let-7i | Plasma | 24 h after thrombolysis | NIHSS |
| Wang et al., 2020 [55] | China | 76 | let-7i | Serum | Chronic (within 1 year) | MoCA |
| Jickling et al., 2016 [56] | USA | 106/106 | let-7i | Circulating leukocytes | <72 h | NIHSS |
| Wang et al., 2019 [57] | China | 40/40 | miR-124 | Serum | <24 h | NIHSS |
| Yuan et al., 2022 [58] | China | 77 | miR-21, -132, -200b | Serum | 14 days | MMSE |
| Zhou and Qi, 2021 [59] | China | 108/108 | miR-124 | Serum | At 24, 48, and 72 h | GOS |
| Ji et al., 2016 [60] | China | 65/66 | Exosomal-miR-9, -124 | Serum | Mean 16.5 h | NIHSS |
| He et al., 2019 [61] | China | 94 | miR-124-3p, -125b-5p, -192-5p, -125b-5p | Plasma | 24 h after thrombolysis | NIHSS |
| He et al., 2019 [62] | China | 84 | miR-125b-5p, -206 | Plasma | 24 h after thrombolysis | NIHSS |
| Qi et al., 2021 [63] | China | 10/10 | Exosomal-miR-124-3p | Serum | At 2, 4, 6 h | NIHSS |
| Jin and Xing, 2017 [64] | China | 106/110 | miR-126, -130a, -185, -221, -222, -218, -378, -19a, -296, -101, -206 | Plasma | <24 h (after thrombolysis) | NIHSS |
| Jin and Xing, 2018 [65] | China | 148/148 | miR-126, -218, -130a, -185, -222 | Plasma | <24 h | NIHSS |
| Chen et al., 2020 [66] | China | 215/215 | miR-9, -195 | Plasma | <24 h | NIHSS |
| Yang et al., 2018 [67] | China | 96/45 | miR-195 | Plasma | <72 h | NIHSS |
| Liu et al., 2021 [68] | China | 170/100 | miR-21 | PBMCs + serum | <24 h | NIHSS |
| Zhou and Zhang, 2014 [69] | China | 68/21 | miR-21 | Plasma | <24 h | NIHSS |
| Wang et al., 2015 [70] | China | 58/59 | miR-29b | WBCs | <72 h | NIHSS |
| Ma et al., 2020 [71] | China | 60/40 | miR-29b | Neutrophils | <6 h | NIHSS |
| Abdelaleem et al., 2022 [72] | Egypt | 77/71 | miR-9, -106a | Serum | At diagnosis | NIHSS |
| Xue et al., 2018 [73] | China | 65/55 | miR-9 | Serum | At diagnosis | NIHSS |
| Xie et al., 2019 [74] | China | 40 IS + 40 Controls | miR-124 | Plasma | NR | NIHSS |
| Guo et al., 2020 [75] | China | 170/65 | miR-24, -29b | Serum | NR | NIHSS |
| Yuan et al., 2016 [76] | China | 152/136 | miR-335 | Plasma | <24 h | NIHSS |
| Yuan et al., 2016 [77] | China | 152/136 | miR-26b | Plasma | <24 h | NIHSS |
| Zhaou et al., 2016 [78] | China | 168/104 | miR-335 | Plasma | <24 h | NIHSS |
| Zhong et al., 2021 [79] | China | 89/39 | miR-497 | Serum | At admission (<24 h) and at discharge | NIHSS |
| Song et al., 2021 [80] | China | 80/30 | miR-409-3p | Serum | <9 h | NIHSS |
| Zhang et al., 2020 [81] | China | 93/70 | EMVs-miR-155 | Plasma | (<24 h) | NIHSS |
| Zeng et al., 2013 [82] | China | 105 | miR-210 | Leukocytes | <72 h | NIHSS, mRS |
| Yang et al., 2020 [83] | China | 79/60 | miR-135b | Serum | <24 h and 14 days after admission | NIHSS |
| Liu et al., 2019 [84] | China | 40/25 | miR-128 | Lymphocytes | <72 h | NIHSS, mRS |
| Zhao et al., 2020 [85] | China | 43/26 | miR-494 | Lymphocytes | <6 h | |
| Sheikhbahaei et al., 2019 [86] | Iran | 33/17 | miR-503 | Serum | <72 h + 3 months | NIHSS, mRS |
| Guo et al., 2022 [87] | China | 142/50 | miR-185, -424 | Serum | <24 h | NIHSS, mRS |
| Fu et al., 2019 [88] | China | 108/97 | miR-451 | Whole blood | <12 h | NIHSS |
| Ye et al., 2021 [89] | China | 43/43 | Exosomal-miR-27-3p | Serum | On admission | NIHSS |
| Otero-Ortega et al., 2021 [90] | Spain | 81/22 | miR-100-5p | Serum | <24h | NIHSS, mRS |
| Yang et al., 2016 [91] | China | 114/58 | miR-107, -128b, -153 | Plasma | <24 h | NIHSS |
| Zhou et al., 2018 [92] | China | 50/50 | Exosomal-miR-134 | Serum | 24-, 48- and 72 h | NIHSS |
| Chen et al., 2018 [93] | China | 128/102 | miR-146b | Serum | < 24 h | NIHSS |
| Chen et al., 2017 [94] | China | 50/33 | Exosomal-miR-223 | Serum | < 72 h | NIHSS |
| Liang et al., 2016 [95] | China | 102/97 | miR-34a-5p | Plasma | <12 h | NIHSS |
| Wang et al., 2014 [96] | China | 79/75 | miR-223 | Leucocytes | At 24, 48, 72 h | NIHSS |
| Wang et al., 2021 [97] | China | 88/88 | miR-9-5p, -128-3p | Serum | <6 h | mRS |
| Jia et al., 2015 [98] | China | 146/96 | miR-145 | Serum | <24 h | NIHSS |
| Liang et al., 2019 [99] | China | 62/62 | miR-140-5p | Plasma | <24 h | HAMD |
| Hu et al., 2020 [100] | China | 257 | miR-22 | Plasma | <7 days | HAMD |
| Cui et al., 2021 [101] | China | 136 | miR-221-3p | Serum | Chronic | NIHSS, HAMD |
| Zeng et al., 2011 [102] | China | 112/60 | miR-210 | Leucocytes | <3 days, 7 days, 14 days | NIHSS |
Legend: IS = ischemic stroke; N = number of IS patients/controls; Abbreviations: BI, Barthel Index; EMV, endothelial microvesicles; GCS, Glasgow Coma Scale; GOS, Glasgow Outcome Scale; HAMD, Hamilton Depression Rating Scale; MoCA, Montreal Cognitive Assessment; MMSE, Mini-Mental State Examination; mRS, Modified Rankin Score; NIHSS, National Institutes of Health Stroke Scale; PBMC, peripheral blood mononuclear cells.
3.1. Characteristics of Included Studies
The 63 included papers consisted of 55 case-control studies (IS patients vs. non-stroke controls) and eight cohort studies (which defined two subgroups according to long-term outcomes). These articles evaluated the relationship between circulating miRNA biomarkers and stroke severity and disability, summarizing a total of 5842 AIS, 81 TIA (transient ischemic attack), and 3852 control participants.
A total of 19 studies recruited fewer than 100 individuals, with two studies including fewer than 50 individuals. Of the 63 included studies, 12 included less than 50 IS patients. The main features of the included studies are provided in Table 1: author and year, country, number of patients recruited, featured miRNAs and miRNA sampling time, and clinical outcome studied. Demographic data of patients recruited in our review is provided in Supplemental Table S1.
A total of 22 studies (34.9%) included statistically significant differences in the prevalence of comorbidities between study groups; specifically, 21 studies (33.3%) registered a differing frequency of hypertension, 19 (30.1%) diabetes, and three (4.7%) cardiopathy. A single study (1.5%) reported the Charlson Comorbidity Index, which differed between IS patients and controls. 19 studies (30.1%) reported a differing metabolic profile between stroke patients and controls regarding carbohydrate and lipid metabolism and renal and systemic inflammatory markers.
A total of 23 studies (36.5%) used the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) classification to evaluate etiologic subtypes of ischemic stroke.
3.2. Methodological Assessment
Following Lai et al. [7], the percentages of “yes” among the eight questions of REMARK criteria [27] were used to evaluate the methodological quality of the included studies; the scores are shown in Supplemental Table S4. A prospective design was used in all of the included studies and also all authors used pre-defined clinical outcomes; however, only three studies (4.7%) described any form of blinding. The enrollment period for IS patients was satisfactorily specified in 44 studies (69.8%); almost all studies (98.4%) adequately described the measurement method of the biomarkers. Only two studies (3.1%) performed any form of sample size estimation. A total of 26 studies (41.2%) were deemed to correctly take into account candidate variables, either by including differing baseline characteristics in multivariate models, or by showing no significant differences at baseline.
3.3. Collection and Profiling of miRNAs-Based Biomarkers
Blood samples were analyzed from 5842 AIS patients, 81 TIA patients, and 3852 controls, involving 72 circulating biomarkers. Plasma, serum, and various circulating cells were possible sources for miRNA extraction (Table 1). Some studies used microarray assays for miRNA selection, then the expression of which was quantified by using RT-qPCR assays.
Of the 72 miRNA-based biomarkers, 42 miRNAs were analyzed from serum (six from serum extracellular vesicles), 43 were collected from plasma (six from plasma extracellular vesicles), and 11 from circulating cells (four from leukocytes, two from lymphocytes, two from neutrophils, two from peripheral mononuclear cells (PBMCs), and one from white blood cells in general). Two studies employed miRNAs isolated from two different sources, such as PBMCs and serum, and neutrophils and plasma [52,68] (Table 1). miRNAs analysis was carried out at different time points from stroke onset: 59 miRNAs within 24 h; six miRNAs within 24 h after thrombolysis; 17 miRNAs within 72 h; three miRNAs at seven days, four miRNAs at 14 days; two miRNAs at 3 months; and three miRNAs within one year (Table 1).
3.4. Prognostic Tools and Prediction of Physical Recovery in IS Patients
All included studies used validated clinical scales to estimate the neurological recovery and functional outcome of stroke survivors, scales which have been proven to accurately discriminate between poor- and good-outcome patients [103]. For the purpose of the systematic review, we included studies that assessed stroke severity and long-term disability by using various instruments (see below), and we included studies that estimate cognitive and motor function at different time points.
To assess neurological deficit severity and short-term outcomes in IS patients, the National Institutes of Health Stroke Scale (NIHSS), Glasgow Outcome Scale (GOS), and Glasgow Coma Scale (GCS) were measured in early diagnosed IS patients. For long-term prognosis, studies evaluated motor recovery by using the Modified Rankin Score (mRS) and Barthel Index (BI). The Montreal Cognitive Assessment (MoCA), Hamilton Depression Rating Scale, and Mini-Mental State Examination (MMSE) addressed cognitive outcomes in IS patients.
Outcomes were measured after stroke at different time points, from hospital admission to a certain period after discharge, ranging from 1 to 3 months up to one year. In 58 studies, neurological deficit severity was evaluated using NIHSS: at 6, 24, 48, and 72 h, 7 days, and 3 months after stroke onset. NIHSS was used as a short-term prognostic scale within 24, 48, and 72 h after stroke onset in 43 studies, and four studies assessed the long-term prognosis of IS patients with the NIHSS score at 7 days [62,84] and 3 months [70,88]. GOS score was measured in two studies at 30 days [49,59], whereas in one study physical outcomes within 24 h were evaluated by GCS [48].
mRS and BI were used to evaluate long-term prognosis. mRS score was evaluated in 26 studies at different time points: six studies at hospital admission, five studies within 72 h [52,82,86,87,89], two studies at 7 days [52,84], one study at 12 days [42], two studies at 14 days [53,58], and 10 studies at 3 months [53,61,62,70,84,86,90,92,94,97]. BI was evaluated in two studies at patient discharge [42], on admission, and 7 days after stroke onset [52].
MMSE score was used to define cognitive recovery in one study at 14 days after stroke [58], whereas MoCA score assessed cognitive impairments in three studies in the chronic phase of stroke patients (within 1 year) [50,51,55]. To measure depression symptoms among post-stroke patients, three studies evaluated HAMD scores within 2–3 weeks and at 3 months after stroke onset [99,100,101].
Of the studies measuring mRS outcomes, eight studies defined a poor outcome as mRS > 2, and two studies as mRS ≥ 2, with the remainder not dichotomizing mRS results into poor or favorable prognoses. Finally, of the studies that used the NIHSS scale and defined dichotomial outcomes, an NIHSS score ≥ 7 was considered a poor prognosis.
3.5. Clinical Utility of miRNA-Based Biomarkers in Prediction of Neurological Recovery after Stroke
To further analyze the predictive value of included biomarkers as functional recovery tools, we grouped each study by miRNA and extracted miRNA profiling features, namely miRNA source, miRNA collection timing, disability scale used, disability score timing, and the correlation coefficient and/or sensitivity, specificity, and area under the curve (Supplemental Tables S2 and S3).
The 63 included studies analyzed 72 predictive biomarkers in stroke recovery, comparing miRNA levels between unfavorable/poor and favorable/good outcomes of stroke patients. Of the 72 biomarkers evaluated in the early and late phase of stroke recovery, 68 miRNAs and exosomal-miRNAs were analyzed with respect to motor recovery after stroke, i.e., 65 were related to NIHSS score, nine were related to RS score and three to BI score, and seven for cognitive recovery, i.e., three were related to HAMD score, four were related to MoCA score, and three were related to MMSE score.
Correlation coefficients (Spearman, Kendall, and Pearson) between miRNA expressions and neurological endpoints were analyzed for 68 of the 72 biomarkers, involving in total 5451 IS patients, 81 TIA patients, and 3700 controls. The expression of 10 miRNAs (miR-195, -497, -21, -132, -29b, -24, -409-3p, -135b, -185, and -424) were very strongly correlated with neurological outcome [50,51,58,69,75,80,83,87], whereas the levels of 25 miRNAs had a strong correlation with stroke recovery scores. No statistical significance was obtained for 17 miRNAs. The definitions of very strong (|r| > 0.75), strong (0.75 > |r| > 0.5), moderate (0.5 > |r| > 0.25), and weak (|r| < 0.25) correlation refer to the rule of thumb proposed by Cohen in 1988 [104]. The correlation coefficient between each miRNA and distinct clinical endpoints, appreciating motor and cognitive stroke recovery, is provided in Supplemental Table S3.
A total of 13 studies performed ROC analyses to analyze the prognosis of IS patients (neurological deficit severity and post-stroke disability) of the 15 circulating biomarkers. Of the 15 differentially expressed miRNAs, five miRNAs (miR-132, let-7i, miR-140-5p, miR-22, and miR-221-3p) [50,55,99,100,101] were upregulated in patients with cognitive impairments after stroke, and 12 miRNAs (miR-24, miR-29b, miR-210, miR-411-5p, miR-124-3p, miR-125b-5p, miR-192-5p, miR-210, miR-185, miR-424, miR-100-5p, and miR-210) [53,61,62,75,82,87,90,102] were downregulated in poor-prognosis patients with motor deficits.
The highest area under the curve (AUC) value for predicting post-stroke cognitive impairment in patients was reported for miR-132 (0.961 (95% CI: 0.931–0.991)) and miR-411-5p exhibited the highest AUC value (0.900) for detecting neurological motor deficit in stroke patients [53]. Moreover, the lowest AUC value (0.647) was reported for miR-192-5p [61] for assessing motor disability. Three articles each reported having AUC values of at least 0.90, with the remainder reporting less than 0.90 [50,53,101]. miR-132 also exhibited the highest sensitivity (94.9%) and specificity (86.7%) [50].
The duration from onset to blood collection varied from the acute phase (from 6, 24, and 72 h up to 14 days) to the chronic phase (i.e., within one year). A total of 63 miRNAs were associated with short-term prognosis of cognitive and motor function. A total of 5 miRNAs were associated with long-term prognosis of motor function, assessed by the GOS [49,59], mRS [84], and NIHSS scores [70,88] at 1–3 months. Moreover, cognitive recovery evaluated by the MMSA, MoCA, and HAMD within 1 year was related to four miRNAs [55,99,100,101]. Most of the studies isolated miRNAs at the same time as the patient’s clinical score assessment, specifically at the time of hospital admission of IS patients. However, in five studies, miRNA quantification was made in both the acute and chronic phases. The following miRNAs have been identified as stroke recovery biomarkers, with prognostic values evaluated at different time points: miR-210 was evaluated at 3 days, 7 days, and 14 days [102]; miR-411-5p was isolated from serum before rt-PA, 24 h after rt-PA, and 3 months (90 days) after stroke onset [53]; miR-497 was assessed from serum at admission and at discharge [79]; miR-135b was isolated from serum within 24 h and at day 14 after stroke onset [83]; and MiR-503 from serum was quantified within 72 h + 3 months [86].
In the acute phase of stroke, longitudinal analysis of blood miRNAs at different time points has been identified in four studies as follows: miR-124 at 24, 48, and 72 h [59]; miR-124-3p at 2, 4, and 6 h [63]; serum miR-134 at 24, 48 and 72 h [92]; and miR-223 from leucocytes at 24, 48, and 72 h [96]. Five studies evaluated miRNA expression profiling [53,54,61,62,64] after thrombolysis therapy, with one study assessing miRNA expression before and after thrombolysis therapy [53]. Nine studies evaluated the relation of 11 miRNAs isolated in the acute phase with a clinical score, evaluated in the chronic period (or at least a few days after miRNA collection). Specifically, the concentration of miR-23b-3p and miR-29b-3p as determined within 24 h was correlated with mRS and BI at discharge (within 12 days) [42]; miR-93 isolated within 6 h was associated with BI at 7 days [52]; miR-124 assessed within 72 h was correlated to GOS at 30 days [59]; miR-125b-5p and miR-206 assessed at 24 h after thrombolysis was correlated with NIHSS score on day 7 [62]. miR-29b isolated within 72 h was correlated with NIHSS at 3 months [70]; miR-128 assessed within 72 days correlated with NIHSS at 7 days and mRS at 3 months [84]; miR-451 assessed within 12 h correlated with NIHSS at 3 months [88]; miR-140-5p assessed within 24 h was associated with HAMD at 3 months [99]; and miR-22 assessed within 7 days was correlated with HAMD at 1 month [100].
3.6. Meta-Analysis of Correlations
Of the 63 studies of 72 miRNAs that were obtained through the literature search, the following were identified as being usable in the meta-analysis of correlations (i.e., at least two studies to allow for pooling, fitting the predefined inclusion and exclusion criteria, and having computed a correlation with the NIHSS score): let-7i (two studies), miR-9 (four studies), miR-21 (two studies), miR-29b (four studies), miR-124 (three studies), miR-124-3p (two studies), miR-125a-5p (two studies), miR-125b-5p (four studies), miR-126 (two studies), miR-185 (three studies), miR-195 (three studies), and miR-335 (two studies). In total, 29 studies were included, providing in total 33 data points (since a few studies analyzed more than one miRNA).
The median REMARK score for the 29 studies (percentage of “yes” responses in the quality assessment questionnaire) was 62.5% (i.e., five “yes” responses out of eight items), indicating moderate quality. Two studies only had English-language abstracts which provided sufficient information for pooling, but not for quality assessment.
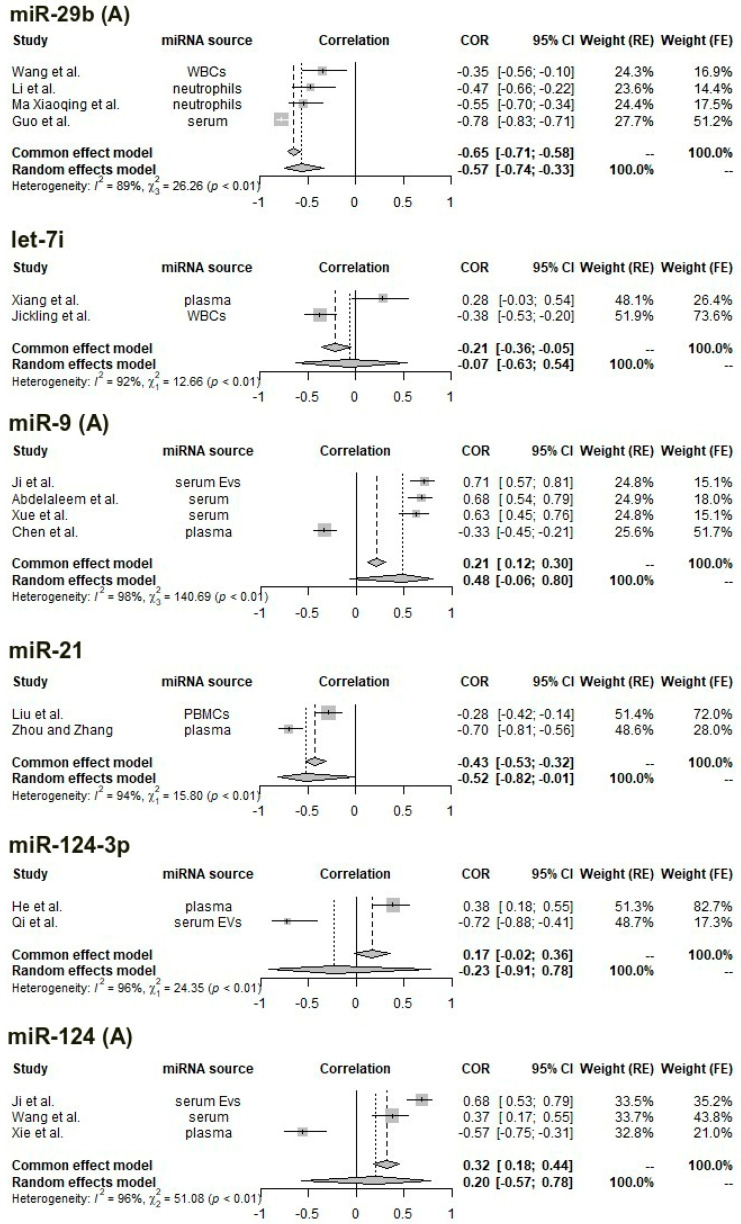
One analyzable study [57] performed the correlation in a sample consisting of both patients (n = 40) and controls (n = 40). Of the 29 included studies, 14 used plasma miRNAs (one from plasma extracellular vesicles), nine extracted miRNA from serum (two from serum extracellular vesicles), and five from circulating cells (two from neutrophils, one from peripheral mononuclear cells, and two from white blood cells in general). Subsequently, meta-analyses with pooling were computed for each miRNA. All suffer from very high heterogeneity (I2 > 90% and significant Cochran’s Q test results), with the exception of the four studies identified for miR-125b-5p with moderate heterogeneity (I2 = 50% and non-significant Cochran’s Q) and the two studies identified for miR-126 (I2 = 0%); however, the studies were performed by the same team, at the same center and during overlapping periods, so we cannot exclude patient overlap.
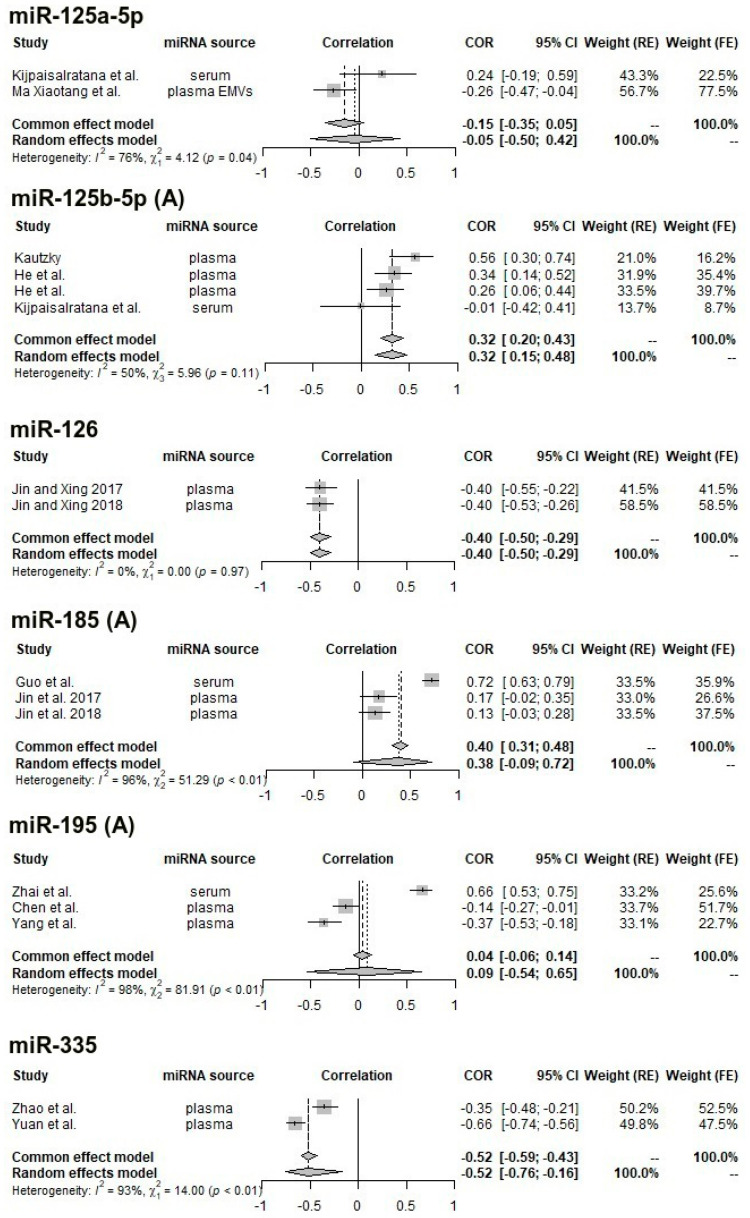
The miRNAs for which a significant correlation was obtained after pooling (reporting random-effects results for studies with high heterogeneity and fixed-effects estimates for low-heterogeneity studies) are miR-21 (pooled ρ = −0.52, 95% CI: −0.82–−0.01, random-effects model), miR-29b (pooled ρ = −0.57, 95% CI: −0.74–−0.33), miR-125b-5p (fixed-effects: pooled ρ = 0.32, 95% CI: 0.20–0.43, random-effects: pooled ρ = 0.32, 95% CI: 0.15–0.48), miR-126 (pooled ρ = −0.62, 95% CI: −0.86–−0.14, fixed-effects), and miR-335 (pooled ρ = −0.52, 95% CI: −0.76–−0.16, random-effects).
Starting from the observation that correlation coefficients differ significantly according to the miRNA source, we repeated the meta-analyses after grouping by miRNA source. In total, we were left with eight miRNAs with at least two studies using a similar sample: miR-9 (serum, three studies), miR-29b (circulating cells, three studies), miR-124 (serum, two studies), miR-125b-5p (plasma, three studies), miR-126 (plasma, two studies), miR-185 (plasma, two studies), miR-195 (plasma, two studies), and miR-335 (plasma, two studies).
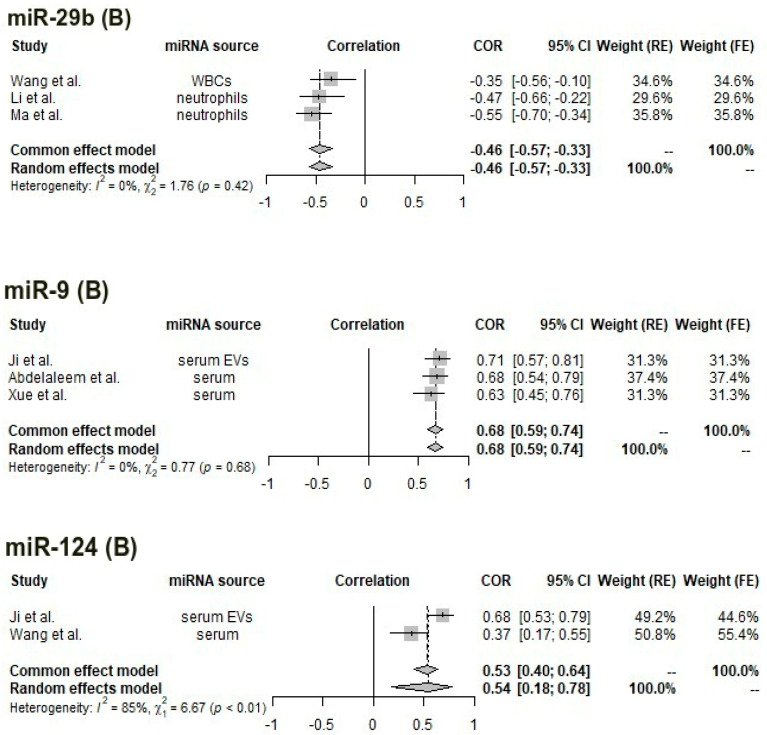
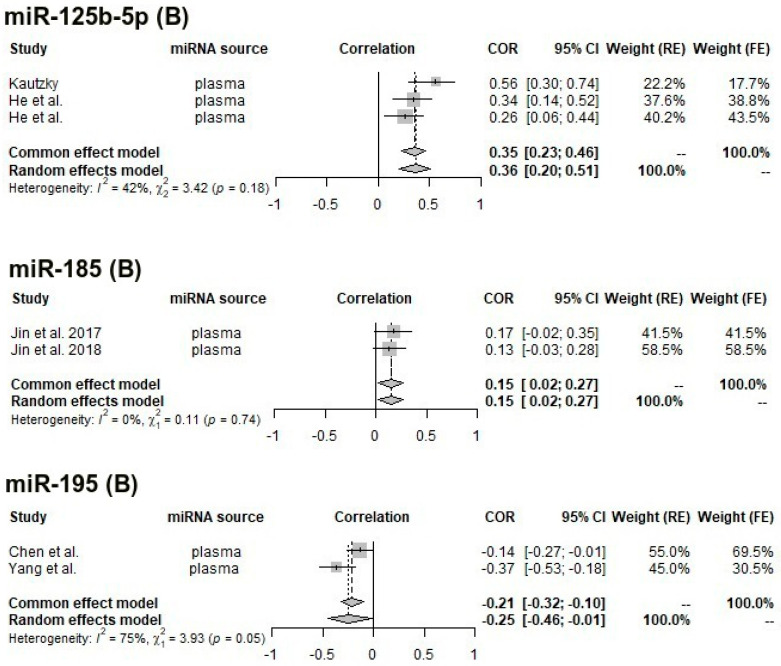
After subsetting for sample type, the following significant pooled effects were obtained: serum miR-9 (pooled ρ = 0.68, 95% CI: 0.59–0.74, fixed-effects model), white blood cell miR-29b (pooled ρ = −0.46, 95% CI: −0.57–−0.33, fixed-effects), serum miR-124 (pooled ρ = 0.54, 95% CI: 0.18–0.78, random-effects), plasma miR-125b-5p (pooled ρ = 0.35, 95% CI: 0.23–0.46, fixed-effects), plasma miR-185 (pooled ρ = 0.15, 95% CI: 0.02–0.27, fixed-effects), and plasma miR-195 (pooled ρ = −0.25, 95% CI: −0.46–−0.01).
Heterogeneity is noticeably lower for miR-9 (from an I2 value of 98% to 0%), miR-29b (from 89% to 0%), and miR-185 (from 96% to 0%) and improved slightly for miR-124 (from 96% to 85%), miR-125b-5p (from 50% to 42%), and miR-195 (from 98% to 75%).
The resulting forest plots are presented in Figure 2 and Figure 3 comparatively, with both fixed (common)-effects and random-effects estimates and heterogeneity measures.
Figure 2.
Forest plot for the correlation between the concentration of let-7i, miR-9, miR-21, miR-29b, miR-124, miR-124-3p, and NIHSS score; in certain cases, we address separately all reported studies (A) and (B) only studies with reported same miRNA source. References: miR-29b: [47,70,71,75]; let-7i: [54,56]; miR-9: [60,66,72,73]; miR-21: [68,69]; miR-124-3p: [61,63]; miR-124: [57,60,74].
Figure 3.
Forest plot for the correlation between the concentration of miR-125a-5p, miR-125b-5p, miR-126, miR-185, miR-195, miR-335, and NIHSS score; in certain cases, we address separately all reported studies (A) and (B) only studies with reported same miRNA source. References: miR-125a-5p: [40,43]; miR-125b-5p: [43,44,61,62]; miR-126: [64,65]; miR-185: [64,65,87]; miR-195: [51,66,67]; miR-335: [76,78].
4. Discussion
Currently, the use of miRNAs as prognostic and diagnostic markers has been gaining recognition in stroke research due to their satisfactory stability in peripheral biofluids, and ease of quantification and validation [21,22,23]. Nonetheless, the field of circulating miRNAs as biomarkers in ischemic stroke is still in its early stages, with few studies being performed on the same miRNA and in similar conditions [15].
By conducting a systematic literature search, we identified all eligible studies which address miRNA expression profiles in blood samples of IS patients related to stroke severity and recovery. Our analysis yielded 72 circulating miRNA-based biomarkers, including 60 non-exosomal miRNAs and 12 exosome-derived miRNAs with predictive values for neurological deficit severity, disability, and stroke recovery (Table 1).
To our knowledge, only one systematic review analyzed the diagnostic and prognostic performance of miRNAs in stroke patients [24]. The authors have a different approach to the topic compared to ours, analyzing dichotomous outcomes of dysregulated miRNAs (i.e., sensitivity and specificity in predicting good or poor outcomes) within the acute phase (<24 h) of IS and ICH [24].
Our systematic review addressed for the first time the prognostic performance of circulating miRNA related to IS recovery, comparing correlation coefficients and ROC analysis between differential-expressed miRNAs and neurological assessment scores. For the meta-analysis of correlation coefficients, we included 29 studies, providing in total 33 data points, including only miRNAs retrieved in at least two studies, which were correlated with NIHSS score.
The prognostic value of miRNAs was analyzed in different blood specimens and at different time points for both miRNA quantification and stroke outcomes, offering a comprehensive picture of miRNAs in IS recovery. Of the 72 predictive biomarkers analyzed from ischemic stroke samples, 68 miRNAs exhibited prognostic value for motor recovery, and seven biomarkers indicate prognostic value for cognitive recovery.
The utility of these circulating biomarkers in predicting long-term outcomes of stroke patients comes with some concerns, which mainly stem from the heterogeneity of miRNA expression. The circulating biomarkers identified by our systematic review encounter a large variability in miRNA isolation profile: multiple different time points of blood sample collection; different miRNA sources evaluated; different vascular and metabolic factors harbored by some stroke patients; and different stroke subtypes between stroke patients.
The heterogeneity of miRNA expression is explained in part by the different blood components used for miRNA analysis [105], which is discussed in detail below. In our case, the literature review identified 42 miRNAs analyzed from serum, 43 miRNAs collected from plasma, and 11 miRNAs isolated from circulating cells. A systematic review analyzing predictive biomarkers for physical recovery highlighted the importance of a short window (24 ± 6 h) for blood sampling to provide prognostic information in the early phase of IS [7]. However, miRNA isolated in the acute stroke phase might provide ischemic injury changes rather than stroke recovery changes, according to Edwardson et al. [26]. We included a large window of blood collection timing for miRNA quantification, ranging from immediately after hospital admission (<6, 24, and 72 h) to the chronic phase of stroke (within one year), capturing all miRNAs which exhibited short-term and long-term prognostic value in IS patients.
Nonetheless, our results highlight the importance of more comprehensive blood sampling, ideally through longitudinal, repeated-measures approaches, which would capture the prognostic signature of miRNAs related to stroke recovery, a point also made by Edwardson et al. [26]. However, we identified only four studies that performed a longitudinal analysis of blood miRNAs at different time points: miR-124 [59]; miR-124-3p [63]; miR-134 [92]; and miR-223 [96]. Of particular note, we identified nine studies that evaluated the relationship between miRNAs assessed in the acute phase of stroke (in total 11 miRNAs) with long-term clinical outcomes (i.e., assessed in the chronic phase or at least a few days after miRNA collection) [15].
Another contributor to the heterogeneity of both miRNA expression and stroke outcome is the presence of comorbid conditions: although several studies analyze differential miRNA expression according to stroke subtype or comorbidities [94,106,107], we also identified 22 studies that have statistically significant differences in the prevalence of comorbidities between stroke patients and control groups, including hypertension, diabetes, cardiopathy, metabolic profiles of carbohydrate, lipid metabolism, and renal and systemic inflammatory markers, but do not attempt to investigate whether they act as confounders.
Only 23 (36.5%) studies distinguish between the etiologic subtypes of IS (as defined by the TOAST classification). Of these studies, it is only Tan et al. who investigated differences between miRNA profiles according to TOAST subtype: small-artery (SA), large-artery (LA), and cardioembolic; more specifically, differential miRNA expression between good and poor outcomes is only apparent for SA or LA stroke [108].
Yet another source of variability is heterogeneity in treatment, more specifically between patients undergoing or not undergoing thrombolysis: we identified five studies that evaluated miRNA expression profiling after thrombolysis [53,54,61,62,64]. Nonetheless, Lin et al., evaluated miR-411-5p before and after thrombolysis therapy [53], suggesting that it might have a smaller contribution to heterogeneity. So far, there were no large studies that demonstrated that tissue plasminogen activator might modify miRNA expression profiles, which further impacts the assessment of prognostic biomarkers [54].
Our analysis highlights 10 miRNAs (miR-195, -497, -21, -132, -29b, -24, -409-3p, -135b, -185, and -424) to be very strongly correlated with neurological outcome [50,51,58,69,75,80,83,87]. Of the studies with binary outcomes, miR-132 and miR-411-5p exhibited the highest AUC value (0.961 and 0.900, respectively) for detecting neurological motor deficit in stroke patients [53], with miR-132 exhibiting the highest sensitivity (94.9%) and specificity (86.7%) [50].
The only meta-analysis performed on the topic of circulating miRNAs in stroke patients is that of Deng et al., who investigated the utility of miRNAs in diagnosing ischemic stroke. The authors pooled results from multiple miRNAs [109]. While this approach is shared by a number of other miRNA-focused meta-analyses, we do not consider the pooled coefficients to have an obvious clinical significance. By grouping results by miRNA and then by miRNA source, we intended to obtain more meaningful results, with a higher clinical utility.
The meta-analysis focused on the connection between miRNAs and a quantitative estimate of stroke severity (in our case, NIHSS scores, typically at or soon after diagnosis). A vast majority of the included studies have approached the former topic, with most articles reporting correlation coefficients between miRNA expression and the NIHSS scores, an outcome measure that can easily be pooled.
One of the first observations regarding the meta-analysis of correlations is that the results depend significantly on the choice of miRNA source: serum, plasma, and specific blood cells. The existence of important differences between the miRNAome of plasma and serum in healthy individuals has been known since 2012: a striking observation is that the coagulation process releases cellular miRNAs into serum, which would imply that the miRNome of serum is more similar to that of whole blood than to that of plasma. Additionally, the authors showed that different qPCR platforms are in low agreement. These results are supported by evidence of variable changes in the miRNA profiles of whole blood, serum, and plasma induced by physical disturbance, long-term cold storage, and freeze-thaw cycles [110,111].
A study on patients with non-ST-elevation myocardial infarction has likewise identified several inconsistencies between serum and plasma: the levels of miR-1 and miR-208 are more variable in plasma, the expression of miR-133a and miR-26a are significantly different from controls only in serum, and miR-21 is increased in serum and decreased in plasma compared to controls [105].
Our meta-analysis cannot prove that the choice of serum, plasma, or cells is responsible for the identified heterogeneity, especially since other factors vary between studies (qPCR protocols, timing, patient selection, etc.) and few of the included articles rank well on the REMARK bias assessment. Nonetheless, these results highlight the importance of a comparative primary study of the serum, plasma, and circulating cell miRNA landscapes of stroke patients, in order to identify the most promising biomarkers.
Our meta-analysis lends support to the potential clinical utility and highlights the need for follow-up studies for the following biomarkers: serum miR-9, neutrophil/WBC miR-29b, plasma miR-125b-5p, and plasma miR-195. More specifically, we have found that serum miR-9 shows high performance as an indicator of stroke severity: the pooled Spearman’s correlation coefficient is relatively high (0.68), and there is a low heterogeneity between the three included studies, despite differing techniques (one study used exosomal miR-9), miRNA collection timing, comorbidities (one study included only diabetics with stroke), moderately low-quality assessment scores, and ethnicity (two Chinese studies and one Egyptian study). A slightly weaker association was found for white blood cell miR-29b (ρ = −0.46), but, like serum miR-9, it shows very low heterogeneity across the three included studies.
Plasma miR-125b-5p also shows potential, with a moderate pooled coefficient (0.43) and moderate heterogeneity, and to a lesser extent plasma miR-195 (with a relatively low pooled ρ = −0.25 and moderately high heterogeneity).
The main limitations of our study are represented by all aforementioned sources of heterogeneity in the differential expression of miRNAs, from methods, normalization, and timing of miRNA isolation, and the blood component used for miRNA analysis, to the intrinsic characteristics of the evaluated population. Indeed, different normalization strategies may lead to different outputs [112]. Most of the studies included in our review originate from China, with several studies from Thailand, Germany, Lebanon, the USA, Egypt, and Iran.
Thus, the detection of differential expression of miRNA profiles might vary among the study population. The limitations of our meta-analytic methods stem from the very limited sample sizes: no miRNA has been investigated by more than four studies, a number that drops to two to three after restricting the analysis according to biofluid.
For this reason, we did not test for publication bias, in order to avoid misleading false-negative results due to their low statistical power [35,36]. Nonetheless, very few included studies reported non-significant results, so the pooling results are likely to be skewed due to publication bias.
5. Conclusions
In this review, we identified 72 circulating predictive biomarkers of stroke severity and stroke recovery with respect to the motor and cognitive functions of IS patients.
Based on the current state of research, we identified serum miR-9 and neutrophil miR-29b as the most promising biomarkers for in-depth follow-up studies, followed by serum miR-124 and plasma miR-125b. Although circulating miRNAs could contribute to facilitating stroke diagnosis and predicting long-term outcomes, we identified multiple shortcomings which need to be addressed before the inclusion of miRNA-based panels in clinical practice: the lack of studies investigating long-term outcomes, the uninvestigated discrepancy between plasma and serum miRNA expression, and the high risk of bias and low sample size.
Acknowledgments
We would like to thank Roxana Surugiu for her generous assistance with this manuscript.
Supplementary Materials
The supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms24010251/s1.
Author Contributions
C.-C.B., A.-V.B. and D.C. wrote the main manuscript text and V.-F.C. and A.-O.M. prepared the Graphical Abstract. D.M.H., B.C. and A.P.-W. reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by grants from UEFISCDI, iBioStroke, project number 136/2020 under the umbrella of the ERA-NET EuroNanoMed project number 192/2020 (GA #723770 of the EU Horizon 2020 Research and Innovation Programme) to A.P.-W. and grant number 26/525/1/25.05.2022 “Computerized diagnosis and monitoring of patients with colorectal cancer” to D.C. and project no. 26/408/3, “Treatment of vascular ischemia using neuromodulatory mesenchymal cells-derived extracellular vesicles”, to B.C.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., et al. Global and Regional Mortality from 235 Causes of Death for 20 Age Groups in 1990 and 2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elkind M.S., Sacco R.L. Stroke Risk Factors and Stroke Prevention. Semin. Neurol. 1998;18:429–440. doi: 10.1055/s-2008-1040896. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization . Noncommunicable Diseases and Mental Health Cluster WHO STEPS Stroke Manual: The WHO STEPwise Approach to Stroke Surveillance. World Health Organization; Geneva, Switzerland: 2005. [Google Scholar]
- 4.Fonarow G.C., Zhao X., Smith E.E., Saver J.L., Reeves M.J., Bhatt D.L., Xian Y., Hernandez A.F., Peterson E.D., Schwamm L.H. Door-to-Needle Times for Tissue Plasminogen Activator Administration and Clinical Outcomes in Acute Ischemic Stroke before and after a Quality Improvement Initiative. JAMA. 2014;311:1632–1640. doi: 10.1001/jama.2014.3203. [DOI] [PubMed] [Google Scholar]
- 5.Creutzfeldt C.J., Holloway R.G., Walker M. Symptomatic and Palliative Care for Stroke Survivors. J. Gen. Intern. Med. 2012;27:853–860. doi: 10.1007/s11606-011-1966-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hermann D.M., Chopp M. Promoting Neurological Recovery in the Post-Acute Stroke Phase: Benefits and Challenges. Eur. Neurol. 2014;72:317–325. doi: 10.1159/000365171. [DOI] [PubMed] [Google Scholar]
- 7.Lai Y.-J., Hanneman S.K., Casarez R.L., Wang J., McCullough L.D. Blood Biomarkers for Physical Recovery in Ischemic Stroke: A Systematic Review. Am. J. Transl. Res. 2019;11:4603–4613. [PMC free article] [PubMed] [Google Scholar]
- 8.Schaechter J.D. Motor Rehabilitation and Brain Plasticity after Hemiparetic Stroke. Prog. Neurobiol. 2004;73:61–72. doi: 10.1016/j.pneurobio.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Alawieh A., Zhao J., Feng W. Factors Affecting Post-Stroke Motor Recovery: Implications on Neurotherapy after Brain Injury. Behav. Brain Res. 2018;340:94–101. doi: 10.1016/j.bbr.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murie-Fernández M., Irimia P., Martínez-Vila E., John Meyer M., Teasell R. Neuro-Rehabilitation after Stroke. Neurología. 2010;25:189–196. doi: 10.1016/S0213-4853(10)70008-6. [DOI] [PubMed] [Google Scholar]
- 11.Mureșanu D.F., Livinț Popa L., Chira D., Dăbală V., Hapca E., Vlad I., Văcăraș V., Popescu B.O., Cherecheș R., Strilciuc Ș., et al. Role and Impact of Cerebrolysin for Ischemic Stroke Care. J. Clin. Med. 2022;11:1273. doi: 10.3390/jcm11051273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolognini N., Russo C., Edwards D.J. The Sensory Side of Post-Stroke Motor Rehabilitation. Restor. Neurol. Neurosci. 2016;34:571–586. doi: 10.3233/RNN-150606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee K.B., Lim S.H., Kim K.H., Kim K.J., Kim Y.R., Chang W.N., Yeom J.W., Kim Y.D., Hwang B.Y. Six-Month Functional Recovery of Stroke Patients: A Multi-Time-Point Study. Int. J. Rehabil. Res. 2015;38:173–180. doi: 10.1097/MRR.0000000000000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo Y., Tang H., Li H., Zhao R., Huang Q., Liu J. Recent Advances in the Development of Neuroprotective Agents and Therapeutic Targets in the Treatment of Cerebral Ischemia. Eur. J. Med. Chem. 2019;162:132–146. doi: 10.1016/j.ejmech.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 15.Steliga A., Kowiański P., Czuba E., Waśkow M., Moryś J., Lietzau G. Neurovascular Unit as a Source of Ischemic Stroke Biomarkers—Limitations of Experimental Studies and Perspectives for Clinical Application. Transl. Stroke Res. 2020;11:553–579. doi: 10.1007/s12975-019-00744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfeiffer S., Sánchez-Lechuga B., Donovan P., Halang L., Prehn J.H.M., Campos-Caro A., Byrne M.M., López-Tinoco C. Circulating MiR-330-3p in Late Pregnancy Is Associated with Pregnancy Outcomes Among Lean Women with GDM. Sci. Rep. 2020;10:908. doi: 10.1038/s41598-020-57838-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larrea E., Sole C., Manterola L., Goicoechea I., Armesto M., Arestin M., Caffarel M.M., Araujo A.M., Araiz M., Fernandez-Mercado M., et al. New Concepts in Cancer Biomarkers: Circulating MiRNAs in Liquid Biopsies. Int. J. Mol. Sci. 2016;17:627. doi: 10.3390/ijms17050627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartel D.P. MicroRNAs: Target Recognition and Regulatory Functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoshnam S.E., Winlow W., Farzaneh M. The Interplay of MicroRNAs in the Inflammatory Mechanisms Following Ischemic Stroke. J. Neuropathol. Exp. Neurol. 2017;76:548–561. doi: 10.1093/jnen/nlx036. [DOI] [PubMed] [Google Scholar]
- 20.Khoshnam S.E., Winlow W., Farbood Y., Moghaddam H.F., Farzaneh M. Emerging Roles of MicroRNAs in Ischemic Stroke: As Possible Therapeutic Agents. J. Stroke. 2017;19:166–187. doi: 10.5853/jos.2016.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salido-Guadarrama I., Romero-Cordoba S., Peralta-Zaragoza O., Hidalgo-Miranda A., Rodríguez-Dorantes M. MicroRNAs Transported by Exosomes in Body Fluids as Mediators of Intercellular Communication in Cancer. OncoTargets Ther. 2014;7:1327–1338. doi: 10.2147/OTT.S61562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raoof R., Jimenez-Mateos E.M., Bauer S., Tackenberg B., Rosenow F., Lang J., Onugoren M.D., Hamer H., Huchtemann T., Körtvélyessy P., et al. Cerebrospinal Fluid MicroRNAs Are Potential Biomarkers of Temporal Lobe Epilepsy and Status Epilepticus. Sci. Rep. 2017;7:3328. doi: 10.1038/s41598-017-02969-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J., Li S., Li L., Li M., Guo C., Yao J., Mi S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinform. 2015;13:17–24. doi: 10.1016/j.gpb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bejleri J., Jirström E., Donovan P., Williams D.J., Pfeiffer S. Diagnostic and Prognostic Circulating MicroRNA in Acute Stroke: A Systematic and Bioinformatic Analysis of Current Evidence. J. Stroke. 2021;23:162–182. doi: 10.5853/jos.2020.05085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Forró T., Bajkó Z., Bălașa A., Bălașa R. Dysfunction of the Neurovascular Unit in Ischemic Stroke: Highlights on MicroRNAs and Exosomes as Potential Biomarkers and Therapy. Int. J. Mol. Sci. 2021;22:5621. doi: 10.3390/ijms22115621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edwardson M.A., Zhong X., Fiandaca M.S., Federoff H.J., Cheema A.K., Dromerick A.W. Plasma MicroRNA Markers of Upper Limb Recovery Following Human Stroke. Sci. Rep. 2018;8:12558. doi: 10.1038/s41598-018-31020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altman D.G., McShane L.M., Sauerbrei W., Taube S.E. Reporting Recommendations for Tumor Marker Prognostic Studies (REMARK): Explanation and Elaboration. PLoS Med. 2012;9:e1001216. doi: 10.1371/journal.pmed.1001216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasan N., McColgan P., Bentley P., Edwards R.J., Sharma P. Towards the Identification of Blood Biomarkers for Acute Stroke in Humans: A Comprehensive Systematic Review. Br. J. Clin. Pharm. 2012;74:230–240. doi: 10.1111/j.1365-2125.2012.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.R Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2022. [Google Scholar]
- 30.Balduzzi S., Rücker G., Schwarzer G. How to Perform a Meta-Analysis with R: A Practical Tutorial. Evid. Based Ment. Health. 2019;22:153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilpin A.R. Table for Conversion of Kendall’S Tau to Spearman’S Rho Within the Context of Measures of Magnitude of Effect for Meta-Analysis. Educ. Psychol. Meas. 1993;53:87–92. doi: 10.1177/0013164493053001007. [DOI] [Google Scholar]
- 32.DerSimonian R., Laird N. Meta-Analysis in Clinical Trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 33.Cochran W.G. Some Methods for Strengthening the Common Χ2 Tests. Biometrics. 1954;10:417–451. doi: 10.2307/3001616. [DOI] [Google Scholar]
- 34.Higgins J., Thompson S., Deeks J., Altman D. Statistical Heterogeneity in Systematic Reviews of Clinical Trials: A Critical Appraisal of Guidelines and Practice. J. Health Serv. Res. Policy. 2002;7:51–61. doi: 10.1258/1355819021927674. [DOI] [PubMed] [Google Scholar]
- 35.Lin L., Chu H., Murad M.H., Hong C., Qu Z., Cole S.R., Chen Y. Empirical Comparison of Publication Bias Tests in Meta-Analysis. J. Gen. Intern. Med. 2018;33:1260–1267. doi: 10.1007/s11606-018-4425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higgins J., Thomas J. In: Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Higgins J., Thomas J., Chandler J., Cumpston M., Li T., Page M., Welch V., editors. John Wiley & Sons; Hoboken, NJ, USA: 2019. [Google Scholar]
- 37.Puhan M.A., Soesilo I., Guyatt G.H., Schünemann H.J. Combining Scores from Different Patient Reported Outcome Measures in Meta-Analyses: When Is It Justified? Health Qual. Life Outcomes. 2006;4:94. doi: 10.1186/1477-7525-4-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mind Those Outcome Measures! A Guide to Enhanced Critical Appraisal of Outcomes|Cochrane Methods. [(accessed on 27 September 2022)]. Available online: https://methods.cochrane.org/news/mind-those-outcome-measures-guide-enhanced-critical-appraisal-outcomes.
- 39.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma X., Liao X., Liu J., Wang Y., Wang X., Chen Y., Yin X., Pan Q. Circulating Endothelial Microvesicles and Their Carried MiR-125a-5p: Potential Biomarkers for Ischaemic Stroke. Stroke Vasc. Neurol. 2022 doi: 10.1136/svn-2021-001476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng G., Yuan Y., Wu S., He F., Hu Y., Luo B. MicroRNA Let-7e Is a Potential Circulating Biomarker of Acute Stage Ischemic Stroke. Transl. Stroke Res. 2015;6:437–445. doi: 10.1007/s12975-015-0422-x. [DOI] [PubMed] [Google Scholar]
- 42.Wu J., Fan C.-L., Ma L.-J., Liu T., Wang C., Song J.-X., Lv Q.-S., Pan H., Zhang C.-N., Wang J.-J. Distinctive Expression Signatures of Serum MicroRNAs in Ischaemic Stroke and Transient Ischaemic Attack Patients. Thromb. Haemost. 2017;117:992–1001. doi: 10.1160/TH16-08-0606. [DOI] [PubMed] [Google Scholar]
- 43.Kijpaisalratana N., Nimsamer P., Khamwut A., Payungporn S., Pisitkun T., Chutinet A., Utoomprurkporn N., Kerr S.J., Vongvasinkul P., Suwanwela N.C. Serum MiRNA125a-5p, MiR-125b-5p, and MiR-433-5p as Biomarkers to Differentiate between Posterior Circulation Stroke and Peripheral Vertigo. BMC Neurol. 2020;20:372. doi: 10.1186/s12883-020-01946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kautzky V. Ph.D. Thesis. Ludwig-Maximilians-Universität München; München, Germany: 2022. Identifizierung von Einflussfaktoren auf die Konzentrationen der Zirkulierenden microRNAs miR-125a-5p, miR-125b-5p und miR-143-3p Nach Ischämischem Schlaganfall. Text. [Google Scholar]
- 45.Zhu X., Ding J., Wang B., Wang J., Xu M. Circular RNA DLGAP4 Is Down-Regulated and Negatively Correlates with Severity, Inflammatory Cytokine Expression and pro-Inflammatory Gene MiR-143 Expression in Acute Ischemic Stroke Patients. Int. J. Clin. Exp. Pathol. 2019;12:941–948. [PMC free article] [PubMed] [Google Scholar]
- 46.Niu M., Li H., Li X., Yan X., Ma A., Pan X., Zhu X. Circulating Exosomal MiRNAs as Novel Biomarkers Perform Superior Diagnostic Efficiency Compared with Plasma MiRNAs for Large-Artery Atherosclerosis Stroke. Front. Pharm. 2021;12:791644. doi: 10.3389/fphar.2021.791644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li G., Ma X., Zhao H., Fan J., Liu T., Luo Y., Guo Y. Long Non-Coding RNA H19 Promotes Leukocyte Inflammation in Ischemic Stroke by Targeting the MiR-29b/C1QTNF6 Axis. CNS Neurosci. Ther. 2022;28:953–963. doi: 10.1111/cns.13829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kotb H.G., Ibrahim A.H., Mohamed E.F., Ali O.M., Hassanein N., Badawy D., Abdelatty Aly E. The Expression of MicroRNA 146a in Patients with Ischemic Stroke: An Observational Study. Int. J. Gen. Med. 2019;12:273–278. doi: 10.2147/IJGM.S213535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu X., Zhang X., Li D., Zhu Z. Plasma Level of MiR-99b May Serve as Potential Diagnostic and Short-term Prognostic Markers in Patients with Acute Cerebral Infarction. J. Clin. Lab. Anal. 2020;34:e23093. doi: 10.1002/jcla.23093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huang S., Zhao J., Huang D., Zhuo L., Liao S., Jiang Z. Serum MiR-132 Is a Risk Marker of Post-Stroke Cognitive Impairment. Neurosci. Lett. 2016;615:102–106. doi: 10.1016/j.neulet.2016.01.028. [DOI] [PubMed] [Google Scholar]
- 51.Zhai Y., Zhu Z., Li H., Zhao C., Huang Y., Wang P. MiR-195 and MiR-497 in Acute Stroke and Their Correlations with Post-Stroke Cognitive Impairment. Int. J. Clin. Exp. Pathol. 2020;13:3092–3099. [PMC free article] [PubMed] [Google Scholar]
- 52.Ma Q., Li G., Tao Z., Wang J., Wang R., Liu P., Luo Y., Zhao H. Blood MicroRNA-93 as an Indicator for Diagnosis and Prediction of Functional Recovery of Acute Stroke Patients. J. Clin. Neurosci. 2019;62:121–127. doi: 10.1016/j.jocn.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 53.Lin X., Wang W., Tao T., Zhang D., Mao L., He X. Synthetic Role of MiR-411-5p and CT Perfusion Information in Predicting Clinical Outcomes after Thrombolysis in Acute Cerebral Infarction. Acta Neurol. Belg. 2022 doi: 10.1007/s13760-022-02041-9. [DOI] [PubMed] [Google Scholar]
- 54.Xiang W., Tian C., Lin J., Wu X., Pang G., Zhou L., Pan S., Deng Z. Plasma Let-7i and MiR-15a Expression Are Associated with the Effect of Recombinant Tissue Plasminogen Activator Treatment in Acute Ischemic Stroke Patients. Thromb. Res. 2017;158:121–125. doi: 10.1016/j.thromres.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 55.Wang Z.-Q., Li K., Huang J., Huo T.-T., Lv P.-Y. MicroRNA Let-7i Is a Promising Serum Biomarker for Post-Stroke Cognitive Impairment and Alleviated OGD-Induced Cell Damage in Vitro by Regulating Bcl-2. Front. Neurosci. 2020;14:215. doi: 10.3389/fnins.2020.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jickling G.C., Ander B.P., Shroff N., Orantia M., Stamova B., Dykstra-Aiello C., Hull H., Zhan X., Liu D., Sharp F.R. Leukocyte Response Is Regulated by MicroRNA Let7i in Patients with Acute Ischemic Stroke. Neurology. 2016;87:2198–2205. doi: 10.1212/WNL.0000000000003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J., Huang Q., Ding J., Wang X. Elevated Serum Levels of Brain-Derived Neurotrophic Factor and MiR-124 in Acute Ischemic Stroke Patients and the Molecular Mechanism. 3 Biotech. 2019;9:386. doi: 10.1007/s13205-019-1914-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yuan M., Guo Y.-S., Zhang X.-X., Gao Z.-K., Shen X.-Y., Han Y., Bi X. Diagnostic Performance of MiR-21, MiR-124, MiR-132, and MiR-200b Serums in Post-Stroke Cognitive Impairment Patients. Folia Neuropathol. 2022;60:228–236. doi: 10.5114/fn.2022.118187. [DOI] [PubMed] [Google Scholar]
- 59.Zhou X., Qi L. MiR-124 Is Downregulated in Serum of Acute Cerebral Infarct Patients and Shows Diagnostic and Prognostic Value. Clin. Appl. Thromb. Hemost. 2021;27:10760296211035446. doi: 10.1177/10760296211035446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji Q., Ji Y., Peng J., Zhou X., Chen X., Zhao H., Xu T., Chen L., Xu Y. Increased Brain-Specific MiR-9 and MiR-124 in the Serum Exosomes of Acute Ischemic Stroke Patients. PLoS ONE. 2016;11:e0163645. doi: 10.1371/journal.pone.0163645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.He X.-W., Shi Y.-H., Liu Y.-S., Li G.-F., Zhao R., Hu Y., Lin C.-C., Zhuang M.-T., Su J.-J., Liu J.-R. Increased Plasma Levels of MiR-124-3p, MiR-125b-5p and MiR-192-5p Are Associated with Outcomes in Acute Ischaemic Stroke Patients Receiving Thrombolysis. Atherosclerosis. 2019;289:36–43. doi: 10.1016/j.atherosclerosis.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 62.He X.-W., Shi Y.-H., Zhao R., Liu Y.-S., Li G.-F., Hu Y., Chen W., Cui G.-H., Su J.-J., Liu J.-R. Plasma Levels of MiR-125b-5p and MiR-206 in Acute Ischemic Stroke Patients After Recanalization Treatment: A Prospective Observational Study. J. Stroke Cereb. Dis. 2019;28:1654–1661. doi: 10.1016/j.jstrokecerebrovasdis.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 63.Qi Z., Zhao Y., Su Y., Cao B., Yang J.-J., Xing Q. Serum Extracellular Vesicle–Derived MiR-124-3p as a Diagnostic and Predictive Marker for Early-Stage Acute Ischemic Stroke. Front. Mol. Biosci. 2021;8:685088. doi: 10.3389/fmolb.2021.685088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jin F., Xing J. Circulating Pro-Angiogenic and Anti-Angiogenic MicroRNA Expressions in Patients with Acute Ischemic Stroke and Their Association with Disease Severity. Neurol. Sci. 2017;38:2015–2023. doi: 10.1007/s10072-017-3071-x. [DOI] [PubMed] [Google Scholar]
- 65.Jin F., Xing J. Circulating MiR-126 and MiR-130a Levels Correlate with Lower Disease Risk, Disease Severity, and Reduced Inflammatory Cytokine Levels in Acute Ischemic Stroke Patients. Neurol. Sci. 2018;39:1757–1765. doi: 10.1007/s10072-018-3499-7. [DOI] [PubMed] [Google Scholar]
- 66.Chen X., Zhang X., Su C., Huang S. Long Noncoding RNA HULC in Acute Ischemic Stroke: Association with Disease Risk, Severity, and Recurrence-free Survival and Relation with IL-6, ICAM1, MiR-9, and MiR-195. J. Clin. Lab. Anal. 2020;34:e23500. doi: 10.1002/jcla.23500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang G., Liu Z., Wang L., Chen X., Wang X., Dong Q., Zhang D., Yang Z., Zhou Q., Sun J., et al. MicroRNA-195 Protection against Focal Cerebral Ischemia by Targeting CX3CR1. J. Neurosurg. 2018;131:1445–1454. doi: 10.3171/2018.5.JNS173061. [DOI] [PubMed] [Google Scholar]
- 68.Liu C., Huang H., Li Y., Zhao H. The Relationship of Long Non-Coding RNA Maternally Expressed Gene 3 with MicroRNA-21 and Their Correlation with Acute Ischemic Stroke Risk, Disease Severity and Recurrence Risk. Clin. Neurol. Neurosurg. 2021;210:106940. doi: 10.1016/j.clineuro.2021.106940. [DOI] [PubMed] [Google Scholar]
- 69.Zhou J., Zhang J. Identification of MiRNA-21 and MiRNA-24 in Plasma as Potential Early Stage Markers of Acute Cerebral Infarction. Mol. Med. Rep. 2014;10:971–976. doi: 10.3892/mmr.2014.2245. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y., Huang J., Ma Y., Tang G., Liu Y., Chen X., Zhang Z., Zeng L., Wang Y., Ouyang Y.-B., et al. MicroRNA-29b Is a Therapeutic Target in Cerebral Ischemia Associated with Aquaporin 4. J. Cereb. Blood Flow Metab. 2015;35:1977–1984. doi: 10.1038/jcbfm.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma X., Yun H.J., Elkin K., Guo Y., Ding Y., Li G. MicroRNA-29b Suppresses Inflammation and Protects Blood-Brain Barrier Integrity in Ischemic Stroke. Mediat. Inflamm. 2022;2022:e1755416. doi: 10.1155/2022/1755416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abdelaleem O.O., Shaker O.G., Mohamed M.M., Ahmed T.I., Elkhateeb A.F., Abdelghaffar N.K., Ahmed N.A., Khalefa A.A., Hemeda N.F., Mahmoud R.H. Differential Expression of Serum TUG1, LINC00657, MiR-9, and MiR-106a in Diabetic Patients with and Without Ischemic Stroke. Front. Mol. Biosci. 2022;8:758742. doi: 10.3389/fmolb.2021.758742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xue Y., Li M., Liu D., Zhu Q., Chen H. Expression of MiR-9 in the Serum of Patients with Acute Ischemic Stroke and Its Effect on Neuronal Damage. Int. J. Clin. Exp. Pathol. 2018;11:5885–5892. [PMC free article] [PubMed] [Google Scholar]
- 74.Xie Z., Liu B., Zhou M., Chen Y. Expression and clinical significance of plasma miR-124 in acute ischemic stroke. J. Pract. Med. 2019;24:343–345. [Google Scholar]
- 75.Guo C., Zhong C., Li Q., Gao Y., Li W., Ou Y. Expressions and neural function prognostic evaluation of serum microRNA-24 and microRNA-29b in elderly patients with acute ischemic stroke. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32:78–82. doi: 10.3760/cma.j.cn121430-20190715-00014. [DOI] [PubMed] [Google Scholar]
- 76.Yuan M., Yuan H., Zhou C., Liu F., Lin C., Tang Y. The Significance of Low Plasma MiR-335 Level in Patients with Acute Cerebral Infarction May Be Associated with the Loss of Control of CALM1 Expression. Int. J. Clin. Exp. Med. 2016;9:19595–19601. [Google Scholar]
- 77.Yuan M., Tang Y., Zhou C., Liu F., Chen L., Yuan H. Elevated Plasma CaM Expression in Patients with Acute Cerebral Infarction Predicts Poor Outcomes and Is Inversely Associated with MiR-26b Expression. Int. J. Neurosci. 2016;126:408–414. doi: 10.3109/00207454.2015.1020537. [DOI] [PubMed] [Google Scholar]
- 78.Zhao B., Zhu Z., Hao J., Wan Z., Guo X. Decreased Plasma MiR-335 Expression in Patients with Acute Ischemic Stroke and Its Association with Calmodulin Expression. J. Int. Med. Res. 2016;44:1331–1338. doi: 10.1177/0300060516665707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhong C., Yin C., Niu G., Ning L., Pan J. MicroRNA MiR-497 Is Closely Associated with Poor Prognosis in Patients with Cerebral Ischemic Stroke. Bioengineered. 2021;12:2851–2862. doi: 10.1080/21655979.2021.1940073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Song X.-D., Li S.-X., Zhu M. Plasma MiR-409-3p Promotes Acute Cerebral Infarction via Suppressing CTRP3. Kaohsiung J. Med. Sci. 2021;37:324–333. doi: 10.1002/kjm2.12327. [DOI] [PubMed] [Google Scholar]
- 81.Zhang H., Chen G., Qiu W., Pan Q., Chen Y., Chen Y., Ma X. Plasma Endothelial Microvesicles and Their Carrying MiRNA-155 Serve as Biomarkers for Ischemic Stroke. J. Neurosci. Res. 2020;98:2290–2301. doi: 10.1002/jnr.24696. [DOI] [PubMed] [Google Scholar]
- 82.Zeng L., Liu J., Wang Y., Wang L., Weng S., Chen S., Yang G.-Y. Cocktail Blood Biomarkers: Prediction of Clinical Outcomes in Patients with Acute Ischemic Stroke. Eur. Neurol. 2013;69:68–75. doi: 10.1159/000342896. [DOI] [PubMed] [Google Scholar]
- 83.Yang S., Zhan X., He M., Wang J., Qiu X. MiR-135b Levels in the Peripheral Blood Serve as a Marker Associated with Acute Ischemic Stroke. Exp. Ther. Med. 2020;19:3551–3558. doi: 10.3892/etm.2020.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu P., Han Z., Ma Q., Liu T., Wang R., Tao Z., Li G., Li F., Zhang S., Li L., et al. Upregulation of MicroRNA-128 in the Peripheral Blood of Acute Ischemic Stroke Patients Is Correlated with Stroke Severity Partially through Inhibition of Neuronal Cell Cycle Reentry. Cell Transpl. 2019;28:839–850. doi: 10.1177/0963689719846848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao H., Li G., Wang R., Tao Z., Ma Q., Zhang S., Han Z., Yan F., Li F., Liu P., et al. Silencing of MicroRNA-494 Inhibits the Neurotoxic Th1 Shift via Regulating HDAC2-STAT4 Cascade in Ischaemic Stroke. Br. J. Pharmacol. 2020;177:128–144. doi: 10.1111/bph.14852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sheikhbahaei S., Manizheh D., Mohammad S., Hasan T.M., Saman N., Laleh R., Mahsa M., Sanaz A.K., Shaghayegh H.J. Can MiR-503 Be Used as a Marker in Diabetic Patients with Ischemic Stroke? BMC Endocr. Disord. 2019;19:42. doi: 10.1186/s12902-019-0371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guo C., Yao Y., Li Q., Gao Y., Cao H. Expression and Clinical Value of MiR-185 and MiR-424 in Patients with Acute Ischemic Stroke. Int. J. Gen. Med. 2022;15:71–78. doi: 10.2147/IJGM.S340586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fu C., Chen S., Cai N., Liu Z., Wang P., Zhao J. Potential Neuroprotective Effect of MiR-451 Against Cerebral Ischemia/Reperfusion Injury in Stroke Patients and a Mouse Model. World Neurosurg. 2019;130:e54–e61. doi: 10.1016/j.wneu.2019.05.194. [DOI] [PubMed] [Google Scholar]
- 89.Ye Z., Hu J., Xu H., Sun B., Jin Y., Zhang Y., Zhang J. Serum Exosomal MicroRNA-27-3p Aggravates Cerebral Injury and Inflammation in Patients with Acute Cerebral Infarction by Targeting PPARγ. Inflammation. 2021;44:1035–1048. doi: 10.1007/s10753-020-01399-3. [DOI] [PubMed] [Google Scholar]
- 90.Otero-Ortega L., Alonso-López E., Pérez-Mato M., Laso-García F., Gómez-de Frutos M.C., Diekhorst L., García-Bermejo M.L., Conde-Moreno E., Fuentes B., de Leciñana M.A., et al. Circulating Extracellular Vesicle Proteins and MicroRNA Profiles in Subcortical and Cortical-Subcortical Ischaemic Stroke. Biomedicines. 2021;9:786. doi: 10.3390/biomedicines9070786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang Z.-B., Li T.-B., Zhang Z., Ren K.-D., Zheng Z.-F., Peng J., Luo X.-J. The Diagnostic Value of Circulating Brain-Specific MicroRNAs for Ischemic Stroke. Intern. Med. 2016;55:1279–1286. doi: 10.2169/internalmedicine.55.5925. [DOI] [PubMed] [Google Scholar]
- 92.Zhou J., Chen L., Chen B., Huang S., Zeng C., Wu H., Chen C., Long F. Increased Serum Exosomal MiR-134 Expression in the Acute Ischemic Stroke Patients. BMC Neurol. 2018;18:198. doi: 10.1186/s12883-018-1196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chen Z., Wang K., Huang J., Zheng G., Lv Y., Luo N., Liang M., Huang L. Upregulated Serum MiR-146b Serves as a Biomarker for Acute Ischemic Stroke. Cell Physiol. Biochem. 2018;45:397–405. doi: 10.1159/000486916. [DOI] [PubMed] [Google Scholar]
- 94.Chen Y., Song Y., Huang J., Qu M., Zhang Y., Geng J., Zhang Z., Liu J., Yang G.-Y. Increased Circulating Exosomal MiRNA-223 Is Associated with Acute Ischemic Stroke. Front. Neurol. 2017;8:57. doi: 10.3389/fneur.2017.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liang T., Lou J. Increased Expression of Mir-34a-5p and Clinical Association in Acute Ischemic Stroke Patients and in a Rat Model. Med. Sci. Monit. 2016;22:2950–2955. doi: 10.12659/MSM.900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang Y., Zhang Y., Huang J., Chen X., Gu X., Wang Y., Zeng L., Yang G.-Y. Increase of Circulating MiR-223 and Insulin-like Growth Factor-1 Is Associated with the Pathogenesis of Acute Ischemic Stroke in Patients. BMC Neurol. 2014;14:77. doi: 10.1186/1471-2377-14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang Q., Wang F., Fu F., Liu J., Sun W., Chen Y. Diagnostic and Prognostic Value of Serum MiR-9-5p and MiR-128-3p Levels in Early-Stage Acute Ischemic Stroke. Clinics. 2021;76:e2958. doi: 10.6061/clinics/2021/e2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jia L., Hao F., Wang W., Qu Y. Circulating MiR-145 Is Associated with Plasma High-Sensitivity C-Reactive Protein in Acute Ischemic Stroke Patients. Cell Biochem. Funct. 2015;33:314–319. doi: 10.1002/cbf.3116. [DOI] [PubMed] [Google Scholar]
- 99.Liang H.-B., He J.-R., Tu X.-Q., Ding K.-Q., Yang G.-Y., Zhang Y., Zeng L.-L. MicroRNA-140-5p: A Novel Circulating Biomarker for Early Warning of Late-Onset Post-Stroke Depression. J. Psychiatr. Res. 2019;115:129–141. doi: 10.1016/j.jpsychires.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 100.Hu J., Zhou W., Zhou Z., Yang Q., Xu J., Dong W. MiR-22 and Cerebral Microbleeds in Brainstem and Deep Area Are Associated with Depression One Month after Ischemic Stroke. Braz. J. Med. Biol. Res. 2020;53:e9162. doi: 10.1590/1414-431x20209162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cui Y., Ma G., Kong F., Song L. Diagnostic Values of MiR-221-3p in Serum and Cerebrospinal Fluid for Post-Stroke Depression and Analysis of Risk Factors. Iran. J. Public Health. 2021;50:1241. doi: 10.18502/ijph.v50i6.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zeng L., Liu J., Wang Y., Wang L., Weng S., Tang Y., Zheng C., Cheng Q., Chen S., Yang G.-Y. MicroRNA-210 as a Novel Blood Biomarker in Acute Cerebral Ischemia. Front. Biosci. 2011;3:1265–1272. doi: 10.2741/330. [DOI] [PubMed] [Google Scholar]
- 103.Bang O.Y., Park H.Y., Yoon J.H., Yeo S.H., Kim J.W., Lee M.A., Park M.H., Lee P.H., Joo I.S., Huh K. Predicting the Long-Term Outcome after Subacute Stroke within the Middle Cerebral Artery Territory. J. Clin. Neurol. 2005;1:148–158. doi: 10.3988/jcn.2005.1.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Routledge; New York, NY, USA: 1988. [Google Scholar]
- 105.Mompeón A., Ortega-Paz L., Vidal-Gómez X., Costa T.J., Pérez-Cremades D., Garcia-Blas S., Brugaletta S., Sanchis J., Sabate M., Novella S., et al. Disparate MiRNA Expression in Serum and Plasma of Patients with Acute Myocardial Infarction: A Systematic and Paired Comparative Analysis. Sci. Rep. 2020;10:5373. doi: 10.1038/s41598-020-61507-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhou B., Li B., Feng P., Wang X., Gao H., Xu L., Wang T., Guo X. Identification of a MiRNA Biomarker for the Large Artery Atherosclerosis Subtype of Acute Ischemic Stroke. Folia Neuropathol. 2022;60:210–220. doi: 10.5114/fn.2022.117248. [DOI] [PubMed] [Google Scholar]
- 107.Modak J.M., Roy-O’Reilly M., Zhu L., Staff I., McCullough L.D. Differential MicroRibonucleic Acid Expression in Cardioembolic Stroke. J. Stroke Cereb. Dis. 2019;28:121–124. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tan K.S., Armugam A., Sepramaniam S., Lim K.Y., Setyowati K.D., Wang C.W., Jeyaseelan K. Expression Profile of MicroRNAs in Young Stroke Patients. PLoS ONE. 2009;4:e7689. doi: 10.1371/journal.pone.0007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deng Y., Huang P., Zhang F., Chen T. Association of MicroRNAs with Risk of Stroke: A Meta-Analysis. Front. Neurol. 2022;13:865265. doi: 10.3389/fneur.2022.865265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang K., Yuan Y., Cho J.-H., McClarty S., Baxter D., Galas D.J. Comparing the MicroRNA Spectrum between Serum and Plasma. PLoS ONE. 2012;7:e41561. doi: 10.1371/journal.pone.0041561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Glinge C., Clauss S., Boddum K., Jabbari R., Jabbari J., Risgaard B., Tomsits P., Hildebrand B., Kääb S., Wakili R., et al. Stability of Circulating Blood-Based MicroRNAs—Pre-Analytic Methodological Considerations. PLoS ONE. 2017;12:e0167969. doi: 10.1371/journal.pone.0167969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Faraldi M., Gomarasca M., Sansoni V., Perego S., Banfi G., Lombardi G. Normalization Strategies Differently Affect Circulating MiRNA Profile Associated with the Training Status. Sci. Rep. 2019;9:1584. doi: 10.1038/s41598-019-38505-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Upon reasonable request from the corresponding author.