Abstract
A rodent respiratory experimental model has proved useful for investigating the immune mechanisms responsible for clearance of bacteria from the lungs. Immunohistochemical studies in immune and nonimmune rats have identified the cellular kinetics of response to bacterial pulmonary infection for CD8+, CD4+, and γδ+ T cells; B cells; and the expression of major histocompatibility complex class II (MHC-II). During the course of bacterial clearance, there was no apparent proliferation or extravasation of lymphocytes, nor was there increased expression of MHC-II in nonimmune animals despite an influx of polymorphonuclear leukocytes, whereas in immunized animals there was an early influx of CD8+ and γδ+ T cells, followed by enhanced expression of the MHC-II marker, cellular infiltration by polymorphonuclear leukocytes, and finally an increased number of CD4+ T cells. Depletion of CD8+ T cells confirmed their vital contribution in the preprimed immune response to pulmonary infection by significantly decreasing the animals' ability to clear bacteria following challenge.
In the area of pulmonary bacterial infection it is still unclear which cells mediate the rapid upregulation and control of the inflammatory response which results in the resolution of infection and the minimization of local tissue damage (7, 19, 47). Classical innate immune responses involving macrophages and polymorphonuclear leukocytes (PMNs) have been seen to assist both immune and nonimmune animals in clearing infections from the lung over time. However, the cellular influx in immune animals is far greater and more rapid than in nonimmune controls (18, 19). This rapid cellular response in the immune animals also corresponds to enhanced bacterial clearance and the more rapid resolution of inflammation, thus decreasing the opportunity for continued damage to local tissue.
Alveolar macrophages, PMNs, and γδ+ T cells have all demonstrated surveillance and phagocytic roles at mucosal surfaces (4, 40). Major histocompatibility complex class II (MHC-II)-restricted CD4+ T cells have been associated with clearance of bacterial infection (14), whereas MHC-I-restricted CD8+ T cells appear to be more involved with the clearance of infections, producing endogenous protein products such as in viral infections (11) or occasionally, for intracellular bacteria such as Listeria monocytogenes, via the MHC-I alternative pathway (37, 38).
The establishment of a mucosal immunization model looking at the kinetics of clearance of nontypeable Haemophilus influenzae (NTHI) in both immune and nonimmune rats and mice has enabled us to study the humoral and cellular interactions critical for enhanced clearance of bacteria from the lungs (18, 19). Comparison of the rat and mouse models demonstrated that the kinetics of cellular infiltration, inflammatory cytokine production, and bacterial clearance follow similar patterns. Whereas antibody and PMNs appeared to be important factors utilized by previously immunized animals in clearing NTHI from the lungs during the latter phases of infection, prepriming of the immune system to clear the bacteria from the lung is controlled by mechanisms set in place at a very early time point post-NTHI challenge.
To further investigate early control mechanisms in pulmonary bacterial clearance, the kinetics of recruitment of various lymphocyte subsets (including CD4+, CD8+, and γδ+ T cells) and the levels of MHC-II marker expression were investigated in immune and nonimmune rats following challenge with NTHI. The observation that CD8+ T cells were rapidly recruited to the lung in immunized animals following bacterial challenge led the investigators to deplete this cell type in mice. The mouse and rat models have been found to provide a similar response to NTHI following pulmonary challenge in both immunized and nonimmune animals. The combined data of rapid CD8+ T-cell recruitment and enhanced bacterial clearance in animals with their full complement of CD8+ T cells confirmed the role of these cells as a key cellular component in the upregulation of bacterial clearance from immunized animals.
MATERIALS AND METHODS
Antigen.
NTHI biotype II (NTHI 289) originally isolated from the sputum of an adult patient suffering from chronic bronchitis and detailed previously was used in the present study (19).
Immunization and antigen challenge.
Immunization and pulmonary challenge were performed as previously described (19). In summary, groups of four to five specific-pathogen-free (SPF) White Wistar male rats or BALB/c mice that were 8 to 10 weeks old were immunized with formalin-killed whole-cell NTHI 289 emulsified in a 1:1 ratio in incomplete Freund adjuvant (Sigma, St. Louis, Mo.) and injected subserosally to each Peyer's patch so that each animal received approximately 5 × 108 CFU (rats) or 1 × 107 CFU (mice) of bacteria. On day 14 the animals received an intratracheal (i.t.) boost of formalin-killed NTHI 289, with rats and mice receiving 50 or 20 μl of 1010 CFU/ml, respectively. Animals were allowed to recover, kept under SPF conditions, and challenged with live NTHI 289 21 days after the primary immunization. Challenge was done via a tracheal cannula with a total of 5 × 108 CFU in 50 μl for rats and 2 × 106 CFU in 20 μl for mice. This was dispersed into the lungs with small volumes of air from a syringe. Animals were killed by an overdose of pentobarbitone sodium administered 0.5, 1, 2, 4, 8, 12, and 24 h after lung inoculation. Phosphate-buffered saline (PBS) was substituted for NTHI 289 in control animals.
Bacterial clearance.
Lungs lavages with PBS and homogenization have been previously described (19). Bacterial counts were determined from homogenate and lung lavage following overnight incubation of serial dilutions on chocolate blood agar (Oxoid, Hampshire, England) in 5% CO2 at 37°C.
Histology and immunocytochemistry.
Lungs from exsanguinated rats were infused and inflated with cold 0.25% (vol/vol) paraformaldehyde-lysine-periodate (PLP) fixative (0.2 M lysine phosphate buffer and 0.2 M l-lysine monohydrochloride [BDH Chemicals, Ltd., Poole, England] in 0.1 M sodium phosphate buffer [pH 7.4]) containing 0.02 M sodium–m-periodate (Sigma) and 0.25% (vol/vol) paraformaldehyde (Sigma, Poole, England). Following excision of the lung, the lobes were placed in fresh PLP fixative for 18 h at 4°C. Following 24 h of washing at 4°C in 0.05 M sodium phosphate buffer (pH 7.4) containing 7% (wt/vol) sucrose, the tissue was embedded in OCT (Tissue Tek; Miles, Inc., Elkhart, Ind.) and frozen in a mixture of acetone and dry ice. Blocks of frozen tissue were stored at −80°C.
Sections (6 μm) were stained with mouse anti-rat CD4 (clone W3/25) at 1/25, mouse anti-rat CD8-biotin (clone MRC OX-8) at 1/10; mouse anti-rat CD45R (clone HIS24) at 1/800, a pan-B-cell marker; mouse anti-rat gamma/delta T-cell receptor (clone V65) at 1/5; mouse anti-rat Ia (monomorphic)-biotin (clone MRC OX-6) at 1/15; or mouse anti-rat macrophages (clone ED2) at 1/200. All were obtained from Serotec (Kidlington, Oxford, United Kingdom). Goat anti-mouse immunoglobulin G (IgG biotinylated; Amersham, Little Chalfont, Buckinghamshire, United Kingdom) was used as a secondary antibody for gamma/delta and CD4+ T-cell staining and diluted 1/400 in a 1:10 dilution of normal rat serum in stock Tris buffer (STB; 49.5 mM Tris, 13.7 mM NaCl, 0.3 mM KCl, 1 mM phosphate buffer [Amresco, Solon, Ohio]; pH 7.6) to reduce nonspecific background staining.
Following washing of slides in Tris-buffered saline (TBS; 0.08% [vol/vol] NaCl, 10% [wt/vol] STB) to remove the OCT, the tissue was blocked with 10% normal goat serum (Sigma) for 5 min before applying the primary antibody for 1 h at 37°C. Washing of the slides in TBS for 3 min preceded dehydration through a series of graded alcohols and a 3-min immersion in 3% (vol/vol) H2O2 in methanol to remove endogenous peroxidase, before rehydration through the graded alcohols and washing for 3 min in TBS. A biotinylated secondary antibody was then applied (this step was not required if the primary antibody was already biotinylated) for 30 min at room temperature before washing the mixture for 3 min in TBS. Slides were incubated with Vectastain Elite ABC reagent (Vector, Burlingame, Calif.) for 30 min, washed in TBS for 5 min, and developed for 5 min in DAB-imidazole (0.05% 3,3′-diaminobenzadine tetrahydrochloride [Sigma] in dinitroaminoformamide–0.085 M Na2HPO4–0.085 M NaH2PO4–10 mM imidazole–0.03% [vol/vol] H2O2). The reaction was stopped by immersion in water for 5 min. The slides were counterstained in hematoxylin, dehydrated through graded alcohols and xylene, and mounted under glass using DPX mountant.
Semiquantitative analysis of positive cells was achieved by assessing 25 fields of four sections from two NTHI-challenged immune and nonimmune rats at each time point against the nonimmune PBS-challenged animals. Estimation of cell numbers was achieved by counting positively stained cells per field (magnified at ×40) in the infected areas of the tissue.
In vivo CD8+ T-cell depletion.
CD8+ T-cell depletion of mice was achieved according to well-established protocols of i.p. injection of 1 mg of anti-CD8 (clone 2.43.1, rat IgG2b; American Type Culture Collection) 24 h prior to challenge. The use of clone 2.43.1 to deplete mice of CD8+ T cells prior to challenge was previously assessed by flow cytometry analysis of splenic leukocytes. The treatment was found to be very effective, with <1% CD8+ T lymphocytes remaining following treatment (27). Control animals were treated with purified rat IgG (Calbiochem, La Jolla, Calif.).
Statistical analysis.
Clearance data have been expressed as the means ± the standard errors of the mean. Statistical significance has been tested for by one-way analysis of variance (ANOVA; Macintosh Systat).
RESULTS
Recruitment of CD8+ T cells is enhanced by mucosal immunization.
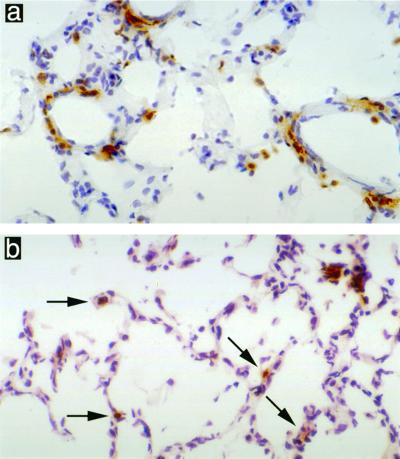
The number of CD8+ T cells found in normal rat lung was assessed by immunohistochemical staining of PBS-challenged rats. A proportion of the cells in lymphoid aggregates were found to be CD8+, and between one and two CD8+ T cells were found per alveolar sac throughout the lung (Fig. 1). Nonimmune rats challenged with NTHI neither increased nor decreased the number of CD8+ T cells in the lung postchallenge; however, animals previously immunized with NTHI showed marked extravasation of CD8+ T cells from blood vessels into the lung tissue. This extravasation was seen as early as 30 min postchallenge with NTHI and increased markedly over the following 24 h (Fig. 2, Table 1).
FIG. 1.
Lungs of PBS-challenged rats showing two to four CD4+ T cells per alveoli (a) and one to two CD8+ T cells per alveoli (b). Magnification, ×400.
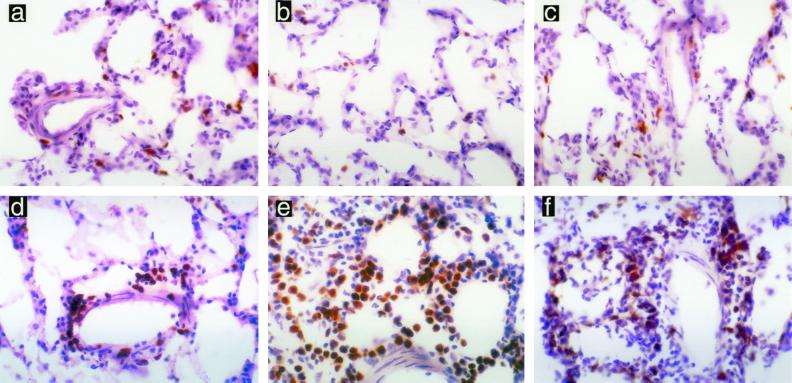
FIG. 2.
CD8+ T cells present in the rat lung following pulmonary challenge with NTHI. Nonimmune animals demonstrated no increase in CD8+ T cells at 1 h (a), 8 h (b), or 24 h (c). Immune animals showed marked increase in CD8+ T cells at 1 h (d), 8 h (e), and 24 h (f). Magnification, ×400.
TABLE 1.
Cell types and surface markers identified in pulmonary tissue at increasing time points postchallenge with NTHIa
| Animal group and time postchallenge (h) | Semiquantitative score for cell type:
|
||||
|---|---|---|---|---|---|
| γδ+ | CD4+ | CD8+ | MHC-II | B | |
| Immune | |||||
| 0.5 | + | + | ++ | + | + |
| 1 | ++ | + | +++ | + | + |
| 2 | ND | + | ++ | ++ | + |
| 4 | − | + | ++ | ++ | + |
| 8 | ND | ++ | +++++ | ++++ | ++ |
| 24 | − | +++ | +++++ | +++ | +++ |
| Nonimmune | |||||
| 0.5 | − | + | + | + | + |
| 1 | − | + | + | + | + |
| 2 | ND | + | + | + | + |
| 4 | − | + | + | + | + |
| 8 | ND | + | + | + | + |
| 24 | − | + | + | + | + |
Analysis of the cell type present was performed by immunoperoxidase staining using monoclonal antibodies. Values represent a semiquantitative score as expressed by the cell number per field magnified ×40 in infected tissues and are scored as follows: −, no cells seen on multiple sections; +, 1 to 5 cells present; ++, 6 to 10 cells present; +++, 11 to 20 cells present; ++++, 20 to 50 cells present; +++++, >50 cells present. ND, not done.
γδ+ T-cell recruitment is transiently enhanced following mucosal immunization.
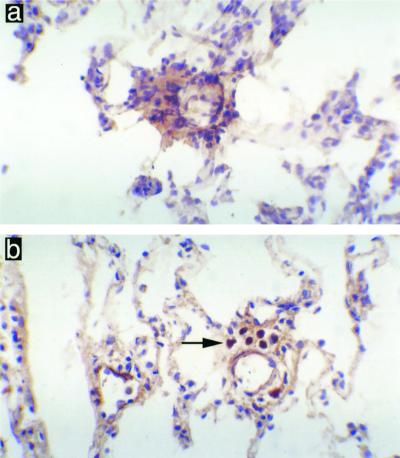
Very few γδ+ T cells were detected in the lung tissue of nonimmune animals challenged with NTHI. Extravasation of γδ+ T cells from blood vessels occurred at 30 min and 1 h following challenge with NTHI in immunized animals (Fig. 3, Table 1). This accumulation of γδ+ T cells was temporary, and by 24 h postchallenge very few positive cells were sporadically detected near the larger bronchi.
FIG. 3.
γδ+ T cells present in the rat lung 1 h following pulmonary challenge with NTHI. Nonimmune animals demonstrated no γδ+ T cells around the pulmonary blood vessels (a), while immune animals showed accumulations of γδ+ T cells around the pulmonary blood vessels (b). Magnification, ×400.
Recruitment of CD4+ T cells is enhanced in late-stage clearance following mucosal immunization.
As with the normal distribution of CD8+ T cells in PBS-challenged rats, CD4+ T cells were scattered throughout the lung tissue, with between two and four cells per alveolar sac, and occasional aggregation occurred around the medium to large blood vessels (Fig. 1). There was no change in the number of CD4+ T cells present in the lung tissue in nonimmune rats following challenge with NTHI (Fig. 4, Table 1). Previously immunized animals showed no initial influx of CD4+ T cells, as was noted with the CD8+ T cells; however, by 8 h after challenge with NTHI a slight increase in CD4+ T cells was detected (Fig. 4, Table 1). This influx continued over time, and a moderate extravasation of CD4+ T cells from blood vessels into lung tissue was observed by 24 h post-bacterial challenge.
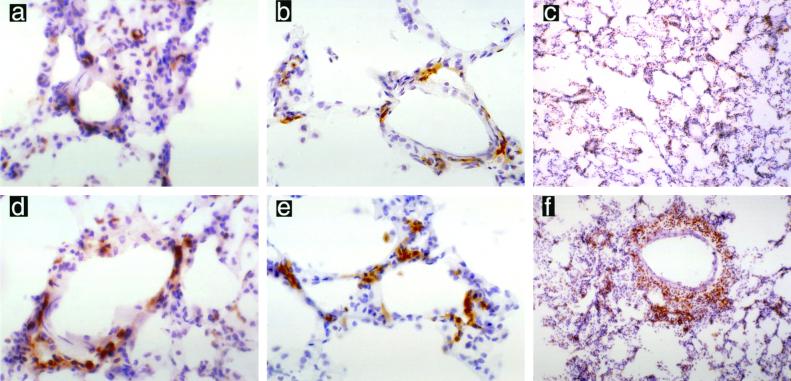
FIG. 4.
CD4+ T cells present in the rat lung following pulmonary challenge with NTHI. Nonimmune animals demonstrated no increase in CD4+ T cells at 1 h (a) (magnification, ×400), 8 h (b) (magnification, ×400), or 24 h (c) (magnification, ×200). Immune animals showed no increase in CD4+ T cells at 1 h (d) (magnification, ×400); however, a slight increase in CD4+ T cells was noted by 8 h (e) (magnification, ×400), and a marked infiltration of CD4+ T cells was seen surrounding blood vessels by 24 h (f) (magnification, ×100).
MHC-II expression increases in the mid to late stages in mucosally immunized animals in response to pulmonary bacterial challenge.
In order to investigate the possible upregulation of bacterial antigen presentation following exposure to NTHI, the kinetics of MHC-II expression were investigated. Normal levels of MHC-II were assessed in PBS-challenged animals, and MHC-II was found to be expressed on approximately two to four cells per alveolar sac. In nonimmune animals challenged with NTHI neither the level of expression nor the number of cells expressing the MHC-II marker increased nor decreased with time postchallenge.
Rats previously immunized with NTHI showed a level of MHC-II expression in the lung similar to that of the nonimmune animals at 30 min postchallenge with NTHI (Table 1); however, as time increased the number of cells expressing the MHC-II marker increased until 24 h postchallenge. The levels of MHC-II expression appeared to increase most near the blood vessels, with some extravasation of positive cells. The majority of cells staining positive for the MHC-II marker in the lung tissue also stained positive for the B-cell marker. Very few cells were dendriform in shape.
CD8+ T-cell depletion reduces pulmonary bacterial clearance in mucosally immunized animals.
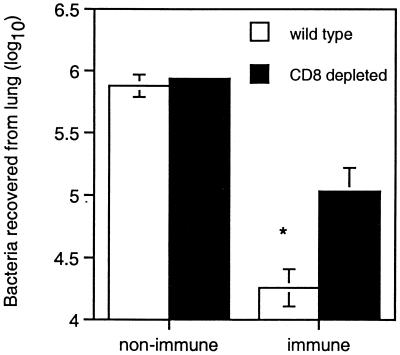
Previous experiments have demonstrated that mucosal immunization significantly enhances the ability of rats and mice to clear NTHI from the lungs compared with the nonimmune animals (18, 19). The immunohistochemical studies presented here indicate a rapid upregulation of CD8+ T cells in mucosally immunized animals challenged with NTHI (Fig. 2, Table 1). To further confirm the significance of this cell type in bacterial clearance following immunization, mice depleted of CD8+ were immunized and challenged with NTHI. Mice were used in preference to rats due to the well-established depletion protocols for CD8+ T-cell depletion in mice (27). It had previously been established that the rat and mouse models followed similar patterns in relation to bacterial clearance following pulmonary challenge with NTHI (data not included). While the depletion of CD8+ T cells from nonimmune animals did not affect their ability to clear bacteria from the lung, the depletion of CD8+ T cells from mucosally immunized mice significantly inhibited pulmonary clearance of bacteria compared to wild-type controls (Fig. 5, P <0.05).
FIG. 5.
Bacteria recovered from lung in wild-type and CD8-depleted nonimmune and immune mice 4 h after challenge with NTHI (∗, P < 0.05). Values represent the mean ± standard error of the mean for four mice. Statistical analysis was done by using ANOVA (Macintosh Systat).
DISCUSSION
Clearance of bacteria from the lungs has been found to be a complex interaction of events. The experimental model used in this study focuses on the clearance of bacteria from the lung and compares immunized to nonimmune animals. The model has been well established for the study of immune mechanisms of bacterial clearance from the lung, and kinetic studies have demonstrated marked differences in cellular infiltration into the lung tissues of immunized and nonimmunized animals after challenge with NTHI (19, 20). During the course of bacterial clearance, there was no apparent proliferation nor extravasation of lymphocytes, nor was there increased expression of MHC-II in nonimmune animals, whereas in immunized animals there was an early influx of CD8+ and γδ+ T cells, followed by enhanced expression of the MHC-II marker, cellular infiltration by PMNs (19), and finally an increased number of CD4+ T cells. The depletion of CD8+ T cells confirmed their vital contribution in the preprimed immune response to pulmonary infection by decreasing the animals' ability to clear bacteria following challenge. The expression of MHC-I would be of interest to determine the upregulation and use of this pathway for antigen presentation.
This study of NTHI infection in the rat lung has demonstrated the importance of CD8+ T cells in protection from bacterial infection in immune animals. CD8+ T cells have classically been restricted by the MHC-I pathway and, as such, thought to be primarily involved with providing protection against organisms producing intracellular peptides, such as those produced from viral infections (12, 46). Nevertheless, recent work has pointed to the ability of CD8+ T cells to be activated through MHC-I type presentation of peptides processed from exogenous protein sources (31, 37, 43). Both macrophages (37, 43) and bacterially stimulated intestinal epithelial cells (1, 2) have been shown to act as antigen-presenting cells via the MHC-I pathway. The ability of NTHI to enter respiratory tract epithelial cells has been demonstrated both in adenoidal tissue in vivo (17) and in lung epithelial cells in vitro (44) and could conceivably result in MHC-I presentation to CD8+ T cells at both the primary immunization site in the gut and the effector site in the respiratory tract. This bacterial stimulation of the epithelial cells could also result in the release of chemokines such as interleukin-8, which results in the attraction of PMNs (15, 16), and macrophage inflammatory protein 1β (MIP-1β), which is selectively chemotactic for CD8+ T cells rather than CD4+ T cells (25).
The major roles attributed to CD8+ T cells are those of the suppressor role, as seen in the intestinal lining (5), and the cytolytic role, as seen when acting on macrophages infected by L. monocytogenes (23). CD8+ T-cell control is also apparent in the downregulation of the γδ+ T-cell response, as demonstrated in β2-microglobulin gene-deficient mice challenged with Mycobacterium bovis (35), which results in the overproliferation of γδ+ T cells. Chemokines, such as MIP-1β and lymphotactin, produced by γδ+ T cells and CD8+ T cells, have been found to attract CD8+ T cells to sites of infection (3, 9). This feedback mechanism of CD8+ T-cell attraction, due partially to the production of chemokines from γδ+ T cells (3), would further strengthen the evidence for the ability to preprime γδ+ T cells by immunization, since no CD8+ T cells accumulated in nonimmunized animals.
γδ+ T cells may act either to protect the host's pulmonary tissue from the pathogenic insult by targeting the inflammatory response to epithelial necrosis (29) or to help achieve the fine balance required between the induction of tolerance (28), regulation of allergic response (32, 41), and stimulation of an appropriate inflammatory response to infection (4). Stimulation and initial influx of the γδ+ T cells, as seen in immunized animals, may be due either to small, nonpeptidic, phosphorylated compounds (24) or to alkylamines derived from the bacteria (6).
The increased numbers of γδ+ T cells found in the immunized animals, combined with the demonstration of proliferation in response to specific allergens (42), provides evidence for the ability to prime γδ+ T cells to respond to specific antigens. This priming may be due to either a direct response, as seen with αβ+ T cells following immunization (30), or an indirect stimulation of γδ+ T cells via MHC-II-restricted CD4+ T cells, as seen with the expansion of γδ+ T cells in the spleen of mice infected with Leishmania major (39). Nevertheless, upon viewing the time frame of cellular response in the current model, we found that γδ+ T cells increased and decreased in number well before CD4+ T cells showed any signs of expansion. It is very plausible, however, that CD4+ T cells were actively secreting stimulatory cytokines well before proliferation. Antigen specificity and γδ+ T-cell depletion studies will help define their role in pulmonary bacterial clearance.
With the lack of gamma interferon found in the bronchoalveolar lavage fluid of rats (20) and the evidence that γδ+ T cells can contain intracellular stores of interleukin-4 (41), the early presence of γδ+ T cells at the site of infection may help mediate a Th2 type response postimmunization. This would be beneficial in optimizing the levels of IgA at the mucosal surface (34).
Upregulation of MHC-II was observed by 2 h postchallenge with NTHI in immunized animals. The levels of MHC-II expression continued to rise until live bacteria had been eliminated from the lung. The precise role for the upregulation of MHC-II expression in this model is ambiguous. Previous work on respiratory tract infection with Moraxella catarrhalis had demonstrated the importance of dendritic cell infiltration along the length of the trachea following bacterial challenge (33). However, the majority of cells showing MHC-II expression in the bronchi and alveolar sacs of immunized animals following pulmonary challenge with NTHI also stained positively for B-cell markers. While there is evidence that dendritic cells are extremely important in their surveillance role in the respiratory tract (22) and while in vitro they are more efficient at antigen presentation to T cells than macrophages, their ability to uptake and present antigen without macrophage help is minimal (21, 36). The relationship between dendritic cells and macrophages is complex, with small numbers of lung interstitial macrophages enhancing the ability of dendritic cells to act as antigen-presenting cells, whereas large numbers of macrophages have an inhibitory effect on the process (21). For this reason, the MHC-II recruitment observed in this model may be a demonstration that it is more effective for the respiratory tract to recruit different groups of antigen-presenting cells to separate but interconnecting locations in order to maximize their antigen-processing potential.
The kinetics of change in the CD4+ T-cell numbers were very different, with no proliferation of CD4+ T cells at the early time points postchallenge with NTHI. It is thought that these T cells play an important role in enhancing the clearance of bacteria in immune compared to nonimmune rats through both innate (8) and acquired (13) mechanisms. In addition to the traditional antigen-presenting pathways for CD4+ T-cell activation, bacterial entry into intestinal epithelial cells can result in the presentation of antigen to CD4+ T cells via MHC-II (26). Furthermore, it has been shown that the transfer of purified preprimed CD4+ T cells or purified preprimed T cells from mucosally immunized rats to naive recipients, followed by either challenge with homologous strains of Pseudomonas aeruginosa (14) or NTHI (45) resulted in enhanced bacterial clearance compared to control animals receiving T cells from nonimmunized animals. Interestingly, T-cell transfer experiments from immunized to naive mice have also been shown to enhance innate mechanisms such as the recruitment and activation of macrophages and polymorphs to the site of challenge in experiments involving L. monocytogenes (10). CD4+ T-cell depletion studies will help us to clarify their role in respiratory immunity to NTHI.
Kinetic studies on the influx of cells to the lungs of animals challenged with NTHI have shown an enhancement of cell numbers in immune compared with nonimmune animals over time. The accumulation of γδ+ T cells observed in immune but not in nonimmune rats demonstrates the possibility that mucosal immunization has the ability to preprime these cells to respond to specific antigen. Additionally, the massive and rapid extravasation of CD8+ T cells to the lung post-bacterial challenge in immunized animals, combined with inhibition of bacterial clearance following CD8+ T-cell depletion, demonstrates the importance of this cell type in the clearance of NTHI.
ACKNOWLEDGMENTS
We thank Rosario Rosaria and Capital Pathology for advice and help in the immunohistological techniques.
This work was supported by National Health and Medical Research Grant 951089 and a collaborative Research Scholarship from the John Curtin School of Medical Research, Australian National University, Canberra, Australia.
REFERENCES
- 1.Blumberg R S. Current concepts in mucosal immunity II. One size fits all: nonclassical MHC molecules fulfill multiple roles in epithelial cell function. Am J Physiol. 1998;2(Pt. 1):G227–G231. doi: 10.1152/ajpgi.1998.274.2.G227. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg R S, Simister N, Christ A D, Israel E J, Colgan S P, Balk S P. MHC-like molecules on mucosal epithelial cells. In: Kagnoff M F, Kiyono H, editors. Essentials of mucosal immunology. San Diego, Calif: Academic Press, Inc.; 1996. pp. 85–99. [Google Scholar]
- 3.Boismenu R, Feng L, Xia Y Y, Chang J C C, Havran W L. Chemokine expression by intraepithelial γδ T cells: implications for the recruitment of inflammatory cells to damaged epithelia. J Immunol. 1996;157:985–992. [PubMed] [Google Scholar]
- 4.Boismenu R, Havran W L. γδ T cells in host defense and epithelial cell biology. Clin Immunol Immunopathol. 1998;86:121–133. doi: 10.1006/clin.1997.4468. [DOI] [PubMed] [Google Scholar]
- 5.Brandtzaeg P, Halstensen T S, Kett K, Krajci P, Kvale D, Rognum T O, Scott H, Sollid L M. Immunobiology and immunopathology of human gut mucosa: humoral immunity and intraepithelial lymphocytes. Gastroenterology. 1989;97:1562–1584. doi: 10.1016/0016-5085(89)90406-x. [DOI] [PubMed] [Google Scholar]
- 6.Bukowski J F, Morita C T, Brenner M B. Human gamma delta T cells recognize alkylamines derived from microbes, edible plants, and tea: implications for innate immunity. Immunity. 1999;11:57–65. doi: 10.1016/s1074-7613(00)80081-3. [DOI] [PubMed] [Google Scholar]
- 7.Buret A, Dunkley M L, Pang G, Clancy R L, Cripps A W. Pulmonary immunity to Pseudomonas aeruginosa in intestinally immunized rats: roles of alveolar macrophages, tumor necrosis factor alpha, and interleukin-1α. Infect Immun. 1994;62:5335–5343. doi: 10.1128/iai.62.12.5335-5343.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell P A. The neutrophil, a professional killer of bacteria, may be controlled by T cells. Clin Exp Immunol. 1990;79:141–143. doi: 10.1111/j.1365-2249.1990.tb05169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of rantes, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 10.Czuprynski C J, Henson P M, Campbell P A. Enhanced accumulation of inflammatory neutrophils and macrophages mediated by transfer of T cells from mice immunized with Listeria monocytogenes. J Immunol. 1985;134:3449–3454. [PubMed] [Google Scholar]
- 11.Doherty P C. Cell mediated immunity in virus infections. Scand J Immunol. 1997;46:528–540. doi: 10.1046/j.1365-3083.1997.d01-170.x. [DOI] [PubMed] [Google Scholar]
- 12.Doherty P C, Topham D J, Tripp R A, Cardin R D, Brooks J W, Stevenson P G. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol Rev. 1997;159:105–117. doi: 10.1111/j.1600-065x.1997.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 13.Dunkley M, Pabst R, Cripps A. An important role for intestinally-derived T cells in respiratory defence. Immunol Today. 1995;16:231–236. doi: 10.1016/0167-5699(95)80165-0. [DOI] [PubMed] [Google Scholar]
- 14.Dunkley M L, Clancy R L, Cripps A W. A role for CD4+ T cells from orally immunized rats in enhanced clearance of Pseudomonas aeruginosa from the lung. Immunology. 1994;83:362–369. [PMC free article] [PubMed] [Google Scholar]
- 15.Eckmann L, Jung H C, Schürer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff M F. Differential cytokine expression by human intestinal epithelial cell: regulation expression of interleukin 8. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 16.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forsgren J, Samuelson A, Ahlin A, Jonasson J, Rynnel-Dagoo B, Lindberg A. Haemophilus influenzae resides and multiplies intracellularly in human adenoid tissue as demonstrated by in situ hybridization and bacterial viability assay. Infect Immun. 1994;62:673–679. doi: 10.1128/iai.62.2.673-679.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foxwell A R. Mechanisms of immunity to nontypeable Haemophilus influenzae in the lung. Ph.D. thesis. Canberra, Australia: University of Canberra; 1998. [Google Scholar]
- 19.Foxwell A R, Kyd J M, Cripps A W. Characteristics of the immunological response in the clearance of nontypeable Haemophilus influenzae from the lung. Immunol Cell Biol. 1998;76:323–331. doi: 10.1046/j.1440-1711.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 20.Foxwell A R, Kyd J M, Cripps A W. Kinetics of inflammatory cytokines in the clearance of nontypeable Haemophilus influenzae from the lung. Immunol Cell Biol. 1998;76:556–559. doi: 10.1046/j.1440-1711.1998.00771.x. [DOI] [PubMed] [Google Scholar]
- 21.Gong J L, McCarthy K M, Rogers R A, Schneeberger E E. Interstitial lung macrophages interact with dendritic cells to present antigenic peptides derived from particulate antigens to T cells. Immunology. 1994;81:343–351. [PMC free article] [PubMed] [Google Scholar]
- 22.Holt P G, Haining S, Nelson D J, Sedgwick J D. Origin and steady-state turnover of class II MHC-bearing dendritic cells in the epithelium of the conducting airway. J Immunol. 1994;153:153–161. [PubMed] [Google Scholar]
- 23.Jiang X, Gregory S H, Wing E J. Immune CD8+ T lymphocytes lyse Listeria monocytogenes-infected hepatocytes by a classical MHC class I-restricted mechanism. J Immunol. 1997;158:287–293. [PubMed] [Google Scholar]
- 24.Julia M R, Serra P, Matamoros N, Raga S, Matinez P. Small cytoplasmic antigens from Pseudomonas aeruginosa stimulate gamma/delta T lymphocytes. Scand J Immunol. 1998;48:672–678. doi: 10.1046/j.1365-3083.1998.00445.x. [DOI] [PubMed] [Google Scholar]
- 25.Jung H C, Eckmann L, Yang S-K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Investig. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaiserlian D, Vidal K, Revillard J-P. Murine enterocytes can present soluble antigens to specific class II-restricted CD4+ T cells. Eur J Immunol. 1989;19:1513–1516. doi: 10.1002/eji.1830190827. [DOI] [PubMed] [Google Scholar]
- 27.Karupiah G, Buller R M L, van Rooijen N, Duarte C J, Chen J. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J Virol. 1996;70:8301–8309. doi: 10.1128/jvi.70.12.8301-8309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ke Y, Lake J P, Ziegler H K, Kapp J A. γδ T lymphocytes regulate the induction and maintenance of oral tolerance. J Immunol. 1997;158:3610–3618. [PubMed] [Google Scholar]
- 29.King D P, Hyde D M, Jackson K A, Novosad D M, Ellis T N, Putney L, Stovall M Y, Van Winkle L S, Beaman B L, Ferrick D A. Cutting edge: protective response to pulmoary injury requires gamma delta T lymphocytes. J Immunol. 1999;162:5033–5036. [PubMed] [Google Scholar]
- 30.Kyd J M, Dunkley M L, Cripps A W. Enhanced respiratory clearance of nontypeable Haemophilus influenzae following mucosal immunization with P6 in a rat model. Infect Immun. 1995;63:2931–2940. doi: 10.1128/iai.63.8.2931-2940.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matinez-Kinader B, Lipford G B, Wagner H, Heeg K. Sensitization of MHC class I-restricted T cells to exogenous proteins: evidence for an alternative class I-restricted antigen presentation pathway. Immunology. 1995;86:287–295. [PMC free article] [PubMed] [Google Scholar]
- 32.McMenamin C, Pimm C, McKersey M, Holt P G. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γδ T cells. Science. 1994;265:1869–1871. doi: 10.1126/science.7916481. [DOI] [PubMed] [Google Scholar]
- 33.McWilliam A S, Nelson D, Thomas J A, Holt P G. Rapid dendritic cell recruitment is a hallmark of the acute inflammatory response at mucosal surfaces. J Exp Med. 1994;179:1331–1336. doi: 10.1084/jem.179.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mosmann T R, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 35.Muller D, Pakpreo P, Filla J, Pederson K, Cigel F, Malkovska V. Increased gamma-delta T-lymphocyte response to Mycobacterium bovis BCG in major histocompatibility complex class I-deficient mice. Infect Immun. 1995;63:2361–2366. doi: 10.1128/iai.63.6.2361-2366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nair S, Buiting A M J, Rouse R J D, Van Rooijen N, Huang L, Rouse B T. Role of macrophages and dendritic cells in primary cytotoxic T lymphocyte responses. Int Immunol. 1995;7:679–688. doi: 10.1093/intimm/7.4.679. [DOI] [PubMed] [Google Scholar]
- 37.Pfeifer J D, Wick M J, Roberts R L, Findlay K, Normark S J, Harding C V. Phagocytic processing of bacterial antigens for class I MHC presentation to T cells. Science. 1993;361:359–362. doi: 10.1038/361359a0. [DOI] [PubMed] [Google Scholar]
- 38.Rock K L. A new foreign policy: MHC class I molecules monitor the outside world. Immunol Today. 1996;17:131–137. doi: 10.1016/0167-5699(96)80605-0. [DOI] [PubMed] [Google Scholar]
- 39.Rosat J-P, Conceiçao-Silva F, Waanders G A, Beermann F, Wilson A, Owen M J, Hayday A C, Huang S, Aguet M, MacDonald H R, Louis J A. Expansion of γδ+ T cells in BALB/c mice infected with Leishmania major is dependent upon Th-2 type CD4+ T cells. Infect Immun. 1995;63:3000–3004. doi: 10.1128/iai.63.8.3000-3004.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sibille Y, Reynolds H Y. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990;141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- 41.Spinozzi F, Agea E, Bistoni O, Forenza N, Bertotto A. γδ T cells, allergen recognition and airway inflammation. Immunol Today. 1998;19:22–26. doi: 10.1016/s0167-5699(97)01182-1. [DOI] [PubMed] [Google Scholar]
- 42.Spinozzi F, Agea E, Bistoni O, Forenza N, Monaco A, Riccardi C, Grignani F, Bertotto A. Increased allergen-specific, steroid-sensitive γδ T cells in bronchoalveolar lavage fluid from patients with asthma. Ann Intern Med. 1996;124:223–227. doi: 10.7326/0003-4819-124-2-199601150-00005. [DOI] [PubMed] [Google Scholar]
- 43.Suto R, Srivastava P K. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 44.van Schilfgaarde M, van Alphen L, Eijk P, Everts V, Dankert J. Paracytosis of Haemophilus influenzae through cell layers of NCI-H292 lung epithelial cells. Infect Immun. 1995;63:4729–4737. doi: 10.1128/iai.63.12.4729-4737.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wallace F J, Cripps A W, Clancy R L, Husband A J, Witt C S. A role for intestinal T lymphocytes in bronchus mucosal immunity. Immunology. 1991;74:68–73. [PMC free article] [PubMed] [Google Scholar]
- 46.Welsh R M, Lin M-Y, Lohman B L, Varga S M, Zarozinski C C, Selin L K. αβ and γδ T-cell networks and their roles in natural resistance to viral infections. Immunol Rev. 1997;159:79–93. doi: 10.1111/j.1600-065x.1997.tb01008.x. [DOI] [PubMed] [Google Scholar]
- 47.Wilson N R, Dunkley M L, Buret A, Young B, Cripps A W. Histopathology of the lung following intratracheal challenge with live Pseudomonas aeruginosa in intestinally immunized rats. Immunol Cell Biol. 1995;73:440–445. doi: 10.1038/icb.1995.68. [DOI] [PubMed] [Google Scholar]