Abstract
Betulin, or naturally occurring triterpene, possesses promising antiproliferative activity. To further explore this potential, thirty-eight betulin acid ester derivatives modified at the C-28 position were tested for antitumor activities. Four human cancer cell lines, MV4-11 (leukemia), A549 (lung), PC-3 (prostate), MCF-7 (breast) as well as the normal BALB/3T3 (mouse fibroblasts) cell line were examined using MTT and SRB assays. A few derivatives exhibited strong antiproliferative activity with IC50 values between 2 and 5 µM. Subsequent mechanistic studies revealed that some derivatives induced apoptosis by inducing caspase-3/7 activity. A strong structure–activity correlation of tested compounds has been proposed along with experimental and in silico pharmacokinetic properties.
Keywords: betulin, anticancer activity, apoptosis, caspase 3/7, lipophilicity, ADME
1. Introduction
Cancer is one of the most serious threats to public health worldwide. After cardiovascular diseases, it is second-leading cause of mortality [1]. Chemotherapy agents still remain the key method to combat cancer, but developing resistance to currently used anticancer drugs forces the search for new agents with diverse mechanisms of action [2].
Chemical compounds isolated from plants are one of the richest sources of new drug candidates and are widely used in many different areas of pharmacotherapy [3,4]. Abundant in many plants, triterpene, also called betulin 1 is an example of such a compound. It possesses anticancer, antiviral, antibacterial and anti-inflammatory properties [5,6,7,8,9]. High natural availability and ease of obtaining as well as broad biological activity makes it a good candidate for further research as a potential drug.
It has been shown that betulin 1 selectively affects various cancer cells and is a low-toxic compound towards normal cells. It inhibits proliferation of more than 20 different cancer cell lines. Li et al. showed that betulin 1 induces apoptosis in human cancer cells such as HeLa (cervix carcinoma), HepG2 (hepatoma), A549 (lung adenocarcinoma), MCF-7 (breast cancer), SK-HEP-1 (hepatoma), PC-3 (prostate carcinoma), NCI-H460 (lung carcinoma) and K562 (erythroleukemia) [10]. These activities against a broad spectrum of different cell lines undergo many different mechanisms of action, which include apoptosis induction, inhibition of cell viability and metastasis, interference with angiogenesis and differentiation, change in signal transductions or DNA synthesis [11]. In addition, it was observed that depolarization of the mitochondrial membrane potential and mitochondrial release of cytochrome c followed the betulin 1 treatment. Moreover, betulin-induced apoptosis was mediated by sequential activation of caspase-9 and caspase-3/-7 and cleavage of poly(ADP-ribose) polymerase (PARP) [10].
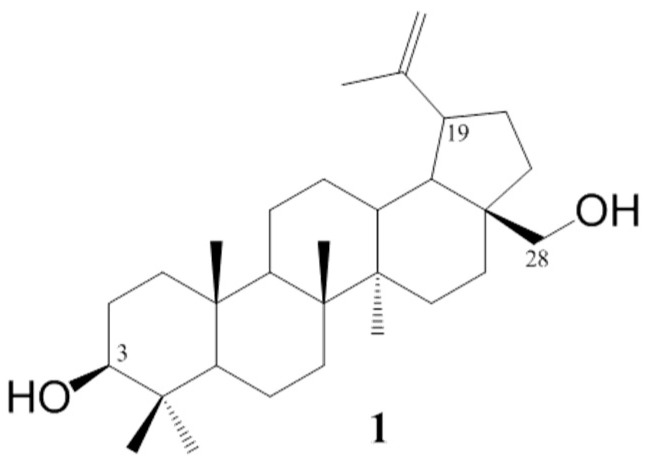
The betulin 1 chemical structure contains two hydroxyl groups at carbons C–3 and C–28 and an ispropenyl moiety attached to carbon C–19 (Figure 1). These moieties allow for chemically modifying the betulin 1 structure to enhance its biological activity. The use of standard methods of chemical synthesis and biocatalytic processes allowed for obtaining a number of active pentacyclic triterpene derivatives [12,13].
Figure 1.
Chemical structure of betulin.
A broad spectrum of derivatives such as esters [14], amides [15] and conjugates [16] exhibited a very good 1–5 µM range of anticancer activity potential against most clinically important cancers. To better understand their antitumor activity, a number of the molecular mechanisms of action studies have been conducted. A vast majority of them have shown that the induction of apoptotic cell death is an essential mechanism of anticancer agents’ activity [8,15,17].
Yang et al. obtained a group of six betulin acid ester derivatives and demonstrated their high antiproliferative activity with an apoptosis-induction mechanism [15]. In continuation of an ongoing program aiming at developing novel anticancer drugs, we decided to explore this small, but promising group of betulin 1 derivatives. Considering already described, potent pharmacophore groups, we have significantly widened the panel of derivatives by introducing new moieties [6]. Simultaneously with already published antiviral studies [6], and previously untested in the context of cytotoxic activity, compounds were tested for their antiproliferative activity against four cancer lines and one normal cell line. Considering the high cell line and mechanism specificity of active betulin derivatives and to verify if a previously described mechanism was retained in new compounds, we performed an induction of apoptosis analysis. Additionally, to widen and further explore the possible mechanistic pathway of tested derivatives, a caspase-3/7 and cell cycle arrest assays were conducted. Furthermore, to assess the potential of active compounds as potential drugs, an experimental and in silico analyses of lipophilicity and pharmacokinetic parameters were conducted. Finally, to facilitate the further search for new derivatives from the group of derivatives, the activity–structure relationship was proposed.
2. Results and Discussion
2.1. Preparation and Structure of Betulin Derivatives
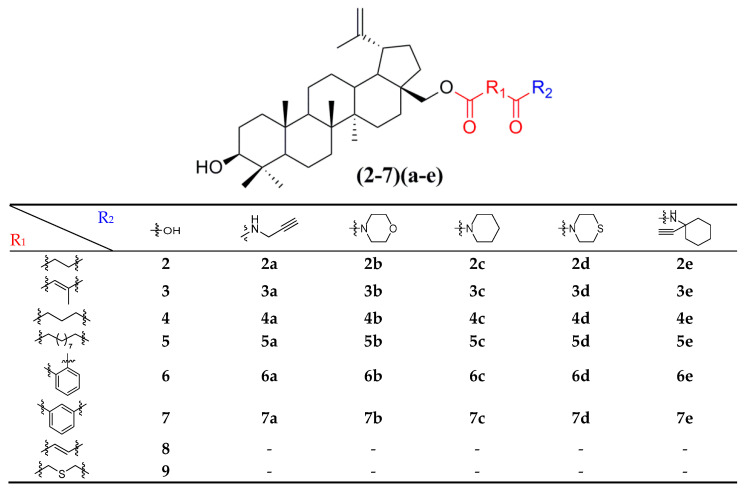
Thirty-eight betulin acid ester derivatives were synthetized, as published previously [6]. Briefly, in the first step, the 28-hydroxyl group of betulin was esterified with various dicarboxylic acids (R1) to produce esters 2–9. Next, derivatives 2–7 were acylated using five different amines (R2) to produce amides (2–7)(a–e). The wide variety of substituents, including those containing heteroatoms, aliphatic, aromatic, linear and cyclic systems, as well as peer-to-peer synthesis, allows for the precise correlation of biological activity with the diverse structures of derivatives. The structures of tested compounds have been presented in Scheme 1.
Scheme 1.
Structures of betulin derivatives used.
2.2. Antiproliferative Activity
2.2.1. Overview of Cell Lines and Compounds Tested
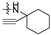
The in vitro antiproliferative activity tests have been conducted against four human cancer cell lines: MV4-11 biphenotypic B myelomonocytic leukemia cell line, A549 lung carcinoma cell line, MCF-7 breast carcinoma cell line, PC-3 prostate carcinoma cell line and one normal BALB/3T3 mouse fibroblast cell line. To preliminarily determine the active compounds, all derivatives were tested against the MV4-11 cell line. Based on these results, ten of the most active amide derivatives (half minimum inhibitory concentration, IC50 < 10 µM; compounds: 2(a–d), 3b, 3d, 4a, 4b, 4d, 5b), four dicarboxylic acid precursors (2, 3, 4, 5) as well as doxorubicin and betulin 1 as positive controls were chosen for further studies against remaining cell lines. All tested compounds were dissolved in DMSO and diluted with a culture medium to a desired concentration. Four piperidine derivatives (3c, 4c, 5c, 7c) were not soluble in DMSO; ergo, studies were not conducted on them. Cell viability, after 72 h incubation of the cells with tested compounds, was determined using tetrazolinum dye MTT (for MV4-11 cells) or sulforhodamine B (SRB test, for MCF-7, A549, PC-3, BALB/3T3 cells) colorimetric assays.
2.2.2. Initial Screening and Activity against MV4-11 and BALB/3T3 Cell Lines
Thirty-five compounds were tested in vitro against the MV4-11 leukemia cell line and compared with doxorubicin, cisplatin and betulin 1 as positive controls. Betulin’s half minimum inhibitory concentration (IC50 = 18.16 µM) was preconceived as a cutoff value for active compounds. The results, presented in the form of a heatmap, are shown in Table 1. The color scheme visualizes active compounds in green and inactive ones in red. Color saturation corresponds to their degree of activity as indicated on the scale.
Table 1.
Growth inhibition (IC50, µM) efficiency of reference compounds (betulin 1, cisplatin and doxorubicin) and betulin derivatives on leukemia cell line; n/s-not studied due to insolubility in DMSO.
| R2 |
|
|
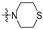
|
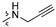
|
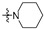
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | ||||||||||||||||
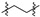
|
2 (23.18) | 2b (3.94) | 2d (3.16) | 2a (2.03) | 2c (5.78) | 2e (52.54) | ||||||||||
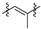
|
3 (19.95) | 3b (8.90) | 3d (4.23) | 3a (12.52) | 3c (n/s) | 3e (22.18) | ||||||||||

|
4 (24.01) | 4b (6.27) | 4d (7.04) | 4a (4.08) | 4c (n/s) | 4e (22.04) | ||||||||||
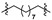
|
5 (5.01) | 5b (6.97) | 5d (43.56) | 5a (21.03) | 5c (n/s) | 5e (100.00) | ||||||||||
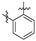
|
6 (34.38) | 6b (15.03) | 6d (14.31) | 6a (24.04) | 6c (100.00) | 6e (54.61) | ||||||||||
|
7 (23.54) | 7b (47.58) | 7d (72.24) | 7a (79.68) | 7c (n/s) | 7e (100.00) | ||||||||||
|
8 (15.25) | |||||||||||||||
|
9 (26.40) | |||||||||||||||
| Betulin 1 (18.16) | ||||||||||||||||
| Cisplatin (1.83) | ||||||||||||||||
| Doxorubicin (0.01) | ||||||||||||||||
| Scale: | 1 | 2 | 5 | 10 | 15 | 18.16 | 25 | 30 | 40 | 45 | 50 | |||||
The color scheme visualizes active compounds in green and inactive ones in red. Color saturation corresponds to their degree of activity as indicated on the scale.
The results show several strong structure–activity relationships. Briefly, almost all succinic (2 a–d), citraconic (3 a,b,d), glutaric (4 a,b,d) as well as two azelaic (5 a–b) acid amides show three to eleven-fold better activity than their parent ester compounds. In the case of succinic acid analogs, the 2a and 2d IC50 values were comparable to cisplatin (2.03, 3.16 and 1.83 µM, respectively). These findings correlate well with previous studies covering 28 substituted betulin derivatives containing a terminal triple bond as well as morpholine moieties [15,18]. Several other amides (2b, 2c, 3d, 5b, 4b, 4a, 4d) and azelaic acid ester 5 show IC50 between 3.94 and 7.04 µM. The presence of a terminal carboxylic group seems to be necessary for ester activity, as single carboxylic acid analogs of 5 do not exhibit activity at all [19].
A small, aliphatic linker such as succinic and glutaric tends to provide better activity with short, acyclic amines such as propargylamine. On the other hand, bigger or cyclic linkers such as phthalic, citraconic or azelaic tend to favor cyclic amines such as piperidine, morpholine or thiomorpholine. Interestingly, all m-phthalic and 1-ethynylcyclohexylamine derivatives were shown to be inactive compared to their precursors and reference compounds. In all cases, the activity drops dramatically from two to more than fifty-fold. These observations are consistent with previous studies [15]. The introduction of rigid aromatic or aliphatic rings with no heteroatoms may lead to reduced water solubility as well as cell membrane penetration, and thus degradation of activity [14,20], although, is it unclear why m-phthalic derivatives are less active than phthalic ones. Moreover, the activity of amides decreases with the elongation of a dicarboxylic acid linker and in the presence of aromatic rings in the following order: succinic > glutaric > citraconic > azelaic > phthalic > m-phthalic.
To acquire more in-depth insight into structure–activity relationships, it is crucial to compare these results with activity against normal, non-cancer cell lines such as BALB/3T3 mouse fibroblasts. The cytotoxicity data has been presented as a heatmap analogous to the MV4-11 results (Table 2). As expected, the antiproliferative activity of the tested compounds against normal cell lines was lower than that of the tumor one. The IC50 of positive controls betulin and doxorubicin was approximately 2 and 18 times lower than in the MV4-11 cell line, respectively. Preliminary analysis of the obtained data shows that structure–activity relationships are more dependent on the amines used rather than dicarboxylic linkers. The morpholine derivatives 2b, 3b and 5b with IC50 25.04, 24.69 and 20.36 µM were 3 to 6 times less active compared to MV4-11. Interestingly, propargylamine derivatives 2a and 4a almost retained their MV4-11 activity and were much less susceptible to cytotoxic agents than the BALB/3T3 cell line. Contrariwise, the thiomorpholine derivatives 2d, 3d and 4d lost their activity completely, beyond the maximum tested concentration used (50 µM).
Table 2.
Comparison of cytotoxicity (IC50) of the tested compounds against BALB/3T3 cell line depending on the dicarboxylic linker and amine present in their structure.
| R2 |
|
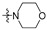
|
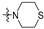
|
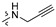
|
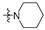
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | |||||||||||||||
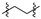
|
2 (53.37) | 2b (25.04) | 2d (50.00) | 2a (3.10) | 2c (32.71) | ||||||||||
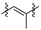
|
3 (37.17) | 3b (24.69) | 3d (50.00) | ||||||||||||

|
4 (37.90) | 4b (36.76) | 4d (50.00) | 4a (5.02) | |||||||||||
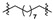
|
5 (23.44) | 5b (20.36) | |||||||||||||
| Betulin 1 (31.79) | |||||||||||||||
| Doxorubicin (0.18) | |||||||||||||||
| Scale: | 1 | 2 | 5 | 10 | 15 | 25 | 31.79 | 40 | 45 | 50 | |||||
The color scheme visualizes active compounds in green and inactive ones in red. Color saturation corresponds to their degree of activity as indicated on the scale.
2.2.3. Activity against A549, MCF-7 and PC-3 Cell Lines
After preliminary MV4-11 cell line antiproliferative screening, selected compounds (2, 2(a–d), 3, 3b, 3d, 4, 4a, 4b, 4d, 5, 5b) were tested on A549, MCF-7 and PC-3 cell lines to evaluate their influence (after 72 h of incubation) on cancer cells with diverse etiology (Table 3, Table 4 and Table 5). By comparing doxorubicin results, the susceptibility of cell lines increases in the following order PC-3 (IC50: 0.43 µM) < BALB/3T3 (IC50: 0.18 µM) < MCF-7 (IC50: 0.08 µM) < A549 (IC50: 0.04 µM) < MV4-11 (IC50: 0.01 µM). In the case of betulin 1, the sequence is: MCF-7 (IC50: 38.82 µM) < PC3 (IC50: 32.46 µM)~BALB/3T3 (IC50: 31.79 µM) < MV4-11 (IC50: 18.16 µM) < A549 (IC50: 15.51 µM). Betulin 1 shows a relatively small two-fold range from minimal to maximal IC50 values, whereas for doxorubicin, this parameter equals 43. Briefly, the A549 cell line shows similarly high susceptibility as leukemia MV4-11; on the other hand, MCF-7 and PC-3 cell lines are less leverageable and similar to the BALB/3T3 cell line. The structure–activity relationships of MCF-7 and PC-3 lines tend to promote morpholine moiety (b) with all tested dicarboxylic acid linkers (IC50 for MCF-7 12.27–21.61 µM and 17.86–24.84 µM for PC-3) over similar thiomorpholine ones (d, IC50 > 46.11 in all cases). This tendency also occurs in the case of the A549 cell line, but with lower IC50s (8.03–12.58 µM for morpholine derivatives (b) and 6.78–32.01 µM for thiomorpholine derivatives (d)).
Table 3.
Comparison of cytotoxicity (IC50, µM) of the tested compounds against A549cell line depending on the dicarboxylic linker and amine present in their structure.
| R2 |
|
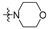
|
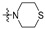
|
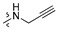
|
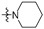
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | |||||||||||||||
|
2 (35.30) | 2b (8.03) | 2d (32.01) | 2a (19.15) | 2c (6.98) | ||||||||||
|
3 (28.70) | 3b (7.42) | 3d (6.78) | ||||||||||||

|
4 (32.48) | 4b (8.47) | 4d (40.20) | 4a (29.10) | |||||||||||
|
5 (4.91) | 5b (12.58) | |||||||||||||
| Betulin 1 (15.51) | |||||||||||||||
| Doxorubicin (0.04) | A549 | ||||||||||||||
| Scale: | 1 | 2 | 5 | 10 | 15.51 | 25 | 35 | 40 | 45 | 50 | |||||
The color scheme visualizes active compounds in green and inactive ones in red. Color saturation corresponds to their degree of activity as indicated on the scale.
Table 4.
Comparison of cytotoxicity (IC50, µM) of the tested compounds against MCF-7 cell line depending on the dicarboxylic linker and amine present in their structure.
| R2 |
|
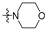
|
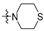
|
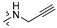
|
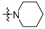
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | |||||||||||||||
|
2 (53.64) | 2b (19.27) | 2d (50.00) | 2a (29.79) | 2c (22.18) | ||||||||||
|
3 (38.22) | 3b (12.27) | 3d (34.05) | ||||||||||||

|
4 (43.93) | 4b (21.61) | 4d (50.00) | 4a (50.00) | |||||||||||
|
5 (12.27) | 5b (19.54) | |||||||||||||
| Betulin 1 (38.82) | |||||||||||||||
| Doxorubicin (0.08) | MCF-7 | ||||||||||||||
| Scale: | 1 | 2 | 5 | 10 | 15 | 25 | 35 | 38.82 | 45 | 50 | |||||
The color scheme visualizes active compounds in green and inactive ones in red. Color saturation corresponds to their degree of activity as indicated on the scale.
Table 5.
Comparison of cytotoxicity (IC50, µM) of the tested compounds against PC-3 cell line depending on the dicarboxylic linker and amine present in their structure.
| R2 |
|
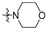
|
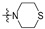
|
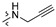
|
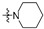
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R1 | |||||||||||||||
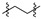
|
2 (68.55) | 2b (17.86) | 2d (50.00) | 2a (4.47) | 2c (30.88) | ||||||||||
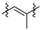
|
3 (34.42) | 3b (24.84) | 3d (46.11) | ||||||||||||
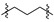
|
4 (36.19) | 4b (22.69) | 4d (50.00) | 3a (15.29) | |||||||||||
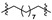
|
5 (19.77) | 5b (22.46) | |||||||||||||
| Betulin 1 (32.46) | |||||||||||||||
| Doxorubicin (0.43) | PC-3 | ||||||||||||||
| Scale: | 1 | 2 | 5 | 10 | 15 | 25 | 32.46 | 40 | 45 | 50 | |||||
The color scheme visualizes active compounds in green and inactive ones in red. Color saturation corresponds to their degree of activity as indicated on the scale.
2.2.4. Cross Cell Lines Summary of Antiproliferative Activity
The large variety of tested compounds and cell lines allows for a broad look at antiproliferative activity. The five cell lines studied can be divided into two groups because of their susceptibility to the tested compounds and structure–activity relationships. The first group contains A549 and MV4-11 cell lines and presented high susceptibility to the applied compounds. The second group, containing remaining lines MCF-7, PC-3 and BALB/3T3, exhibited very similar IC50 and structure–activity relationships.
Within all cancer cell lines, morpholine amides presented consistently high activity. Unfortunately, high activity against normal Balb/3T3 cell line results in a relatively low selectivity index (Table 6). Similarly, succinic-propargylamine derivate 2a has been found to be most active against MV4-11, PC-3 and BALB/3T3 cell lines. On the other hand, thiomorpholine amides of succinic (2d) and citraconic (3d) acid showed SI values of 15.8 and 11.8, respectively; therefore, in combination with one of the highest IC50 values (3.16 and 4.23 µM, respectively), this constitutes a solid basis that thiomorpholine derivatives are a promising group of anti-leukemia compounds and should be subject to further studies. Other derivatives exhibit selectivity indexes generally around or above 1, with an exception across all cell lines to propargylamine 2a and 4a compounds (ranges from 0.1 to 0.7). In the case of A549, all active derivatives (2b, 2c, 3b, 3d, 4b, 5) exhibited SI in a range from 3.1 to 7.4. For MCF-7 and PC-3 lines, SI fluctuates around 1.
Table 6.
The SI index = IC50 for normal cell line (BALB/3T3)/IC50 for respective cancerous cell line. A beneficial SI > 1.0 indicates a drug with efficacy against tumor cells greater than toxicity against normal cells.
| Compound | Cell Lines/Calculated Selectivity Index SI | |||
|---|---|---|---|---|
| MV4-11 | A549 | MCF-7 | PC-3 | |
| 2 | 2.3 | 1.5 | 1.0 | 0.8 |
| 2a | 1.5 | 0.2 | 0.1 | 0.7 |
| 2b | 6.4 | 3.1 | 1.3 | 1.4 |
| 2c | 5.7 | 4.7 | 1.5 | 1.1 |
| 2d | 15.8 | 1.6 | 1.0 | 1.0 |
| 3 | 1.9 | 1.3 | 1.0 | 1.1 |
| 3b | 2.8 | 3.3 | 2.0 | 1.0 |
| 3d | 11.8 | 7.4 | 1.5 | 1.1 |
| 4 | 1.6 | 1.2 | 0.9 | 1.0 |
| 4a | 1.2 | 0.2 | 0.1 | 0.3 |
| 4b | 5.9 | 4.3 | 1.7 | 1.6 |
| 4d | 7.1 | 1.2 | 1.0 | 1.0 |
| 5 | 4.7 | 4.8 | 1.9 | 1.2 |
| 5b | 2.9 | 1.6 | 1.0 | 0.9 |
| Betulin 1 | 1.8 | 2.0 | 0.8 | 1.0 |
| Doxorubicin | 18.0 | 4.5 | 2.3 | 0.4 |
In all cases, dicarboxylic esters were less active than betulin 1, except azelaic ester, which was more potent from 1.35 to 3.62-fold. Interestingly, the betulin 1 to 5 IC50 ratio was almost similar in the case of A549, MCF-7 and MV4-11 (3.16, 3.16 and 3.62, respectively) and PC-3 and BALB/3T3 (1.64 and 1.35, respectively). Furthermore, almost in all cell lines, azelaic ester 5 transformed into morpholine amide 5b, resulting in a decrease of activity, which is the opposite of previous morpholine-derivatives correlations.
2.3. Mechanistic Studies
2.3.1. Induction of Apoptosis
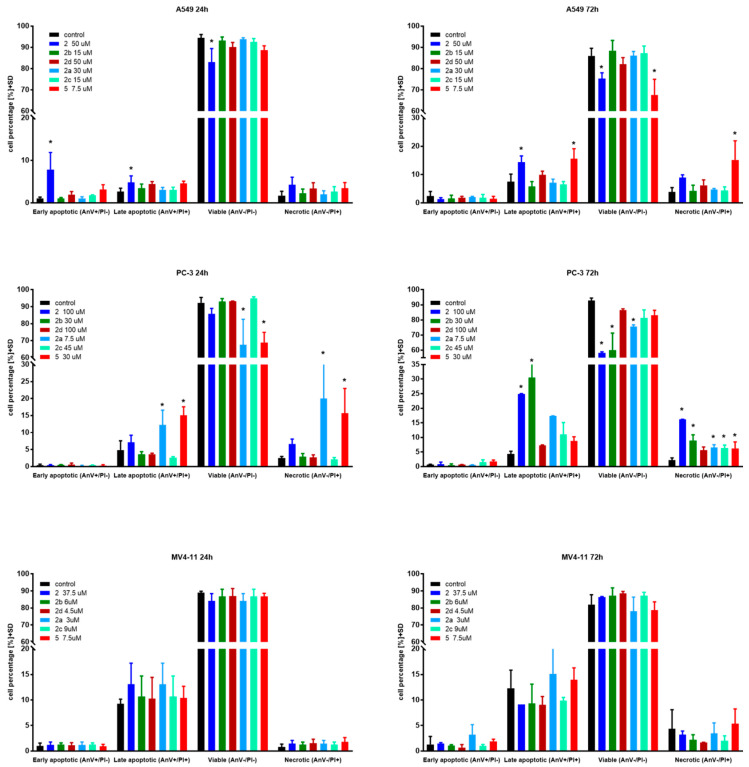
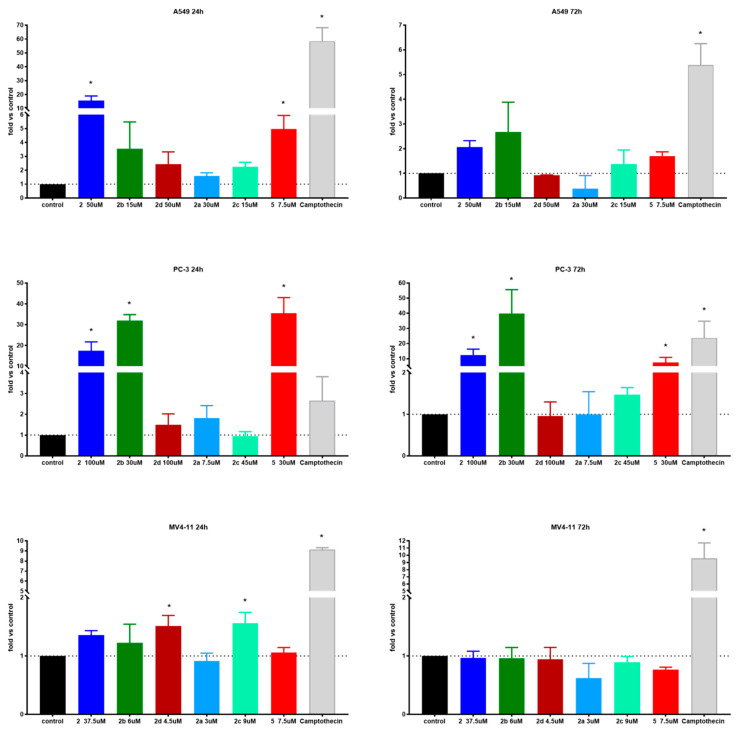
Apoptosis is one of a cell’s natural mechanisms for programmed cell death. It plays a crucial role in homeostasis by eliminating the unwanted or unnecessary cells. One of the most important functions of apoptosis is prevention of cancer. However, cancer cells often lose the ability to enter the path of apoptosis, which allows them to survive longer and grow in an uncontrolled manner. Regardless of that, apoptosis still remains the most important molecular mechanism to combat cancer cells; therefore, research is conducted to obtain agents with the ability to selectively induce apoptosis in cancer cell lines [21]. Previous research on betulin 1 and its derivatives revealed that apoptosis is a key mechanism of antitumor action of this group of compounds [10,15,22]. Based on that, to further evaluate the mechanism of action of obtained derivatives, we conducted apoptosis induction tests using the Annexin V (AnV)/Propidium iodide (PI) staining method after 24 and 72 h of incubation with tested compounds in about 1.5 × IC50 concentration. Results are presented in Figure 2.
Figure 2.
Induction of apoptosis by tested compounds after 24 and 72 h. * statistically significant versus control cells, p < 0.05. Compounds were used in concentrations of about 1.5 × IC50.
In the case of the A549 lung carcinoma cell line after 24 h incubation time, one compound 2 showed significantly increased levels of early-apoptotic and apoptotic cells at the expense of the living cell population. As expected, longer treatments shifted the ratio towards apoptotic and necrotic cells. Additionally, after 72 h incubation time, compound 5 exhibited significantly upraised levels of apoptotic and necrotic cells compared to the control. A similar effect was observed on the PC-3 cell line after 24 h incubation with derivatives 2, 2a and 5. After 72 h incubation time, almost all compounds caused a significant uprising in the number of necrotic cells. Compounds 2 and 2b at PC-3 cells exhibited a similar profile as 5 at A549 after 72 h incubation time by shifting the ratio of the living cell population towards apoptotic and necrotic ones. The tested compounds did not influence apoptosis on the MV4-11 leukemia cell line, which may suggest a different mechanism of action.
Overall, apoptosis induction activity was exposed by succinic acid ester 2, its propargylamine 2a and morpholine 2b amides as well as azelaic acid ester 5.
2.3.2. Cell Cycle Arrest
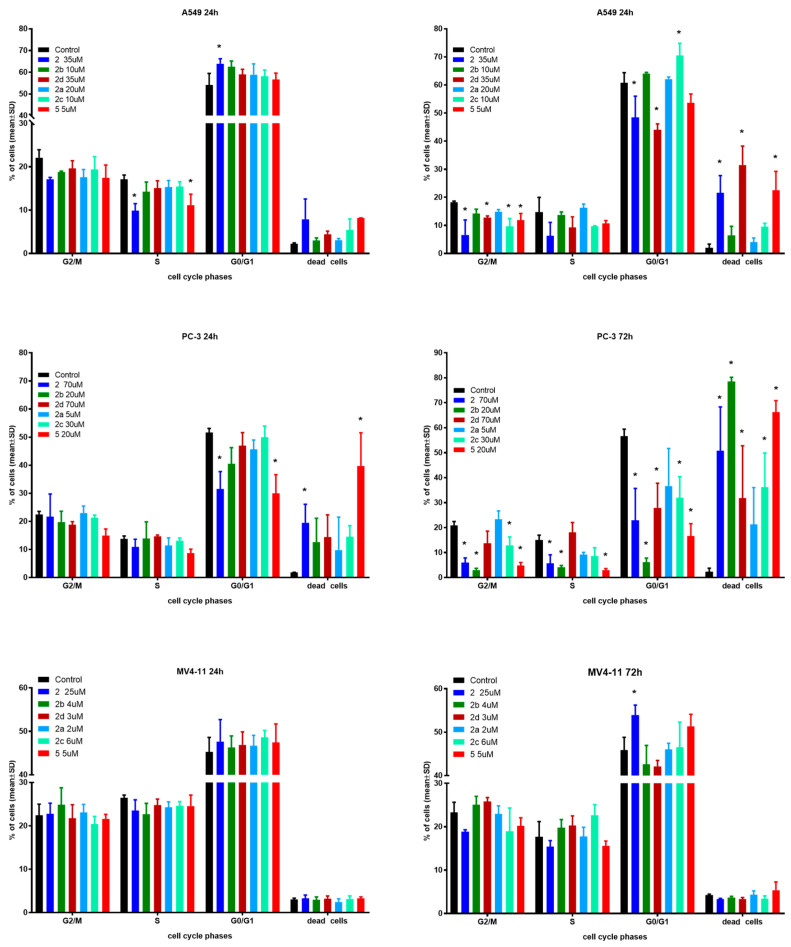
In the next step of mechanistic evaluation, the cell cycles of MV4-11, PC-3 and A549 cells were analyzed after 24 and 72 h of exposure to tested compounds in their IC50 concentrations (Figure 3).
Figure 3.
Influence of tested compounds on cell cycle after 24 and 72 h incubation. * statistically significant versus control cells, p < 0.05. Compounds were tested in concentrations of about 1 × IC50.
The effects of the selected derivatives on the cell cycle were analyzed by flow cytometry using PI staining.
In the case of A549 cells after 24 h incubation time, only derivative 2 showed significantly induced cell cycle arrest in the G0/G1 phase compared to the control. Compounds 2 and 5 reduced the number of cells in the S phase with an increased number of dead cells. After 72 h incubation time, compounds 2, 2d and 5 caused a greater percentage of dead cells with a decrease in G2/M and G0/G1 phases. Furthermore, compound 2c induced cycle arrest in the G0/G1 phase with a decrease in the G2/M phase.
The PC-3 cell line showed a similar tendency to A549 cells. A longer incubation time results in a higher percentage of dead cells, but this effect is much stronger compared to A549 cells. Compound 5 resulted in 80% dead cells versus 15% after the same 24 h incubation time used in the studies.
The MV4-11 cell cycle was generally not affected by the tested compounds in both incubation times. Only compound 2 exhibited a statistically significant increase in the number of cells in the G0/G1 phase compared to the reference, which resulted in a lower number of cells in the G2/M and S phases. There were no differences between the two incubation times used in the studies.
Regardless of the type of cell line, the obtained results do not indicate any influence on nucleic acid synthesis (S) and the G2/mitosis steps of cell cycle. As expected, longer incubation with more cytotoxic compounds resulted in a higher percentage of dead cells, which suggests time-dependent antiproliferative activity. When compared to control cells, only PC-3 cells after 72 h incubation time exhibited a high shift towards dead cells. The cell cycle arrest in the G0/G1 phase for some compounds mentioned earlier is in line with previous reports on the effect of betulin derivatives on tumor cell cycles [11,17,22].
2.3.3. Caspase-3/7 Activity
Caspase-3 and caspase-7 are cysteine-aspartic acid proteases that can directly execute apoptosis followed by sequential activation of caspase-8 or caspase-9. Activation of these enzymes leads to apoptotic cell death; thus, measuring their activity is a good method to determine the pro-apoptotic potential of tested compounds. One of the mechanisms of betulin’s antiproliferative activity is activation of these enzymes; therefore, to further examine the mechanism of action of tested derivatives, we decided to determine their levels [10]. In the current study, the selected derivatives have been tested after 24 and 72 h of incubation time with 1 × IC50 (data not showed) and 1.5 × IC50 concentrations. Results are presented in Figure 4.
Figure 4.
Caspase-3/7 activity of tested compounds after 24 and 72 h. * statistically significant versus control, p < 0.05. Compounds were tested in concentrations of about 1.5 × IC50.
The A549 lung carcinoma cell line presented hugely elevated caspase 3/7 activity after treatment with succinic acid ester 2 and its two amides 2c, 2b and azelaic ester 5 as well. As expected, extending of the incubation time from 24 to 72 h led to a several-fold decrease in activity, but was still statistically up-leveled. Caspases-3/7 are executioner enzymes, so as the percentage of dead cells increases, their activity also declines. In almost all cases, the 1.5 × IC50 compound concentration was needed to induce statistically important level increases. Interestingly, in the case of ester 5 and amide 2b and 2c derivatives, the caspase-3/7 elevated activity correlates well with a high degree of antiproliferative activity. On the other hand, succinic acid ester 2 shows highest caspase 3/7 activity and one of the lowest antiproliferative activities (Table 7). This might suggest a different mechanism of action for these derivatives.
Table 7.
Comparison of antiproliferative and caspase-3/7 activities of tested compounds on A549 and PC-3 cell lines.
| A549 | PC-3 | |||||
|---|---|---|---|---|---|---|
| Induction of Apoptosis | Caspase 3/7 Activity * | Antiproliferative Activity ** | Induction of Apoptosis | Caspase 3/7 Activity * | Antiproliferative Activity ** | |
| 2 | + | + | − | + | + | − |
| 2a | − | − | − | + | − | + |
| 2b | − | + | + | + | + | + |
| 2c | − | + | + | − | − | − |
| 2d | − | − | − | − | − | − |
| 5 | + | + | + | + | + | + |
* plus mark represents statistically important elevated activity of caspase 3/7 compared to control; ** plus mark represents antiproliferative IC50 lower than reference compound used (betulin 1).
The succinic acid derivatives had a similar influence on prostate carcinoma cell line PC-3 as of A549. After 24 h, the incubation time levels of caspase-3/7 were 2 to 35 times higher than the control. Contrary to MV4-11 cell line results, caspase levels were 3 to 15 times higher than positive control camptothecin. Activity was retained at a longer incubation time, but compared to camptothecin, it dropped below 1 one. The morpholine ester of 2–2b exhibited a very high level of caspase-3/7 after 24 and 72 h time at 1.5 × IC50 concentration. The activity of 5 was very high at shorter incubation times and dropped significantly later. The concentration-dependent increase of caspase-3/7 activity in the PC-3 cell line as well as the key role of these enzymes in apoptosis has been described before [23,24].
In the case of the MV4-11 leukemia cell line, only compounds 2c and 2d after 24 h exhibited increased levels of caspase-3/7. After 72 h, no effect on caspases was observed at any concentration used. The MFC-7 breast carcinoma cells do not express caspase-3/7 and, therefore, studies were not conducted on them [25].
2.4. Lipophilicity and ADME Properties
Lipophilicity is an important physicochemical parameter that determines the possibility of transporting substances through biological membranes. This descriptor is related to processes such as absorption and distribution, as well as the excretion of xenobiotics in the body. Lipophilicity also affects the ability of a compound to bind to plasma proteins and form a compound–receptor complex. The pharmacodynamic and pharmacokinetic properties of medicinal substances often correlate with their lipophilicity [26].
Among the different methods used to determine the lipophilicity parameter, in this work, we used the experimental reverse phase thin layer chromatography (RP-TLC) method as well as in silico calculation methods. The retention mechanism in the RP-TLC method is based on the partition of substances between two phases. Silica gel (RP-18 F254S plates) was used as the hydrophobic stationary phase. In contrast, the hydrophilic mobile phase was a binary mixture of acetone and an aqueous solution of TRIS buffer. The chromatographic analysis by RP-TLC is presented in the Supplementary Materials. The chromatographic parameters (RM0, log PTLC) determined for the carboxyacyl 2–9 and amide (2–7)(a–e) betulin derivatives are presented in Table 8. The literature lipophilicity (logPlit) and experimental (RM0) values of reference compounds are presented in Table S1.
Table 8.
The experimental values of lipophilicity for tested compounds.
| Compound | RM0 | −b | r | log PTLC |
|---|---|---|---|---|
| 2 | 5.910 | 0.071 | 0.982 | 8.191 |
| 2a | 4.863 | 0.059 | 0.997 | 6.796 |
| 2b | 5.024 | 0.059 | 0.990 | 7.011 |
| 2c | 5.728 | 0.065 | 0.994 | 7.949 |
| 2d | 5.898 | 0.068 | 0.987 | 8.176 |
| 2e | 6.027 | 0.070 | 0.993 | 8.347 |
| 3 | 5.410 | 0.065 | 0.995 | 7.525 |
| 3a | 5.326 | 0.063 | 0.996 | 7.413 |
| 3b | 5.086 | 0.060 | 0.991 | 7.093 |
| 3c | 5.650 | 0.064 | 0.993 | 7.845 |
| 3d | 5.711 | 0.066 | 0.993 | 7.926 |
| 3e | 6.098 | 0.070 | 0.997 | 8.441 |
| 4 | 5.250 | 0.062 | 0.996 | 7.312 |
| 4a | 5.158 | 0.061 | 0.993 | 7.189 |
| 4b | 4.961 | 0.057 | 0.995 | 6.926 |
| 4c | 5.012 | 0.055 | 0.989 | 6.994 |
| 4d | 5.008 | 0.057 | 0.998 | 6.989 |
| 4e | 5.920 | 0.068 | 0.995 | 8.205 |
| 5 | 5.949 | 0.068 | 0.998 | 8.247 |
| 5a | 5.998 | 0.069 | 0.995 | 8.308 |
| 5b | 5.572 | 0.062 | 0.995 | 7.740 |
| 5c | 5.768 | 0.062 | 0.988 | 8.002 |
| 5d | 5.044 | 0.055 | 0.983 | 7.037 |
| 5e | 6.093 | 0.068 | 0.991 | 8.435 |
| 6 | 5.979 | 0.071 | 0.988 | 8.283 |
| 6a | 6.016 | 0.072 | 0.986 | 8.332 |
| 6b | 5.979 | 0.070 | 0.983 | 8.284 |
| 6c | 5.875 | 0.063 | 0.986 | 8.145 |
| 6d | 6.255 | 0.072 | 0.981 | 8.652 |
| 6e | 6.412 | 0.074 | 0.985 | 8.861 |
| 7 | 5.817 | 0.066 | 0.996 | 8.067 |
| 7a | 5.839 | 0.067 | 0.990 | 8.096 |
| 7b | 5.153 | 0.058 | 0.959 | 7.182 |
| 7c | 5.773 | 0.063 | 0.985 | 8.008 |
| 7d | 5.980 | 0.066 | 0.986 | 8.284 |
| 7e | 5.746 | 0.061 | 0.987 | 7.972 |
| 8 | 5.129 | 0.063 | 0.995 | 7.150 |
| 9 | 6.087 | 0.073 | 0.988 | 8.427 |
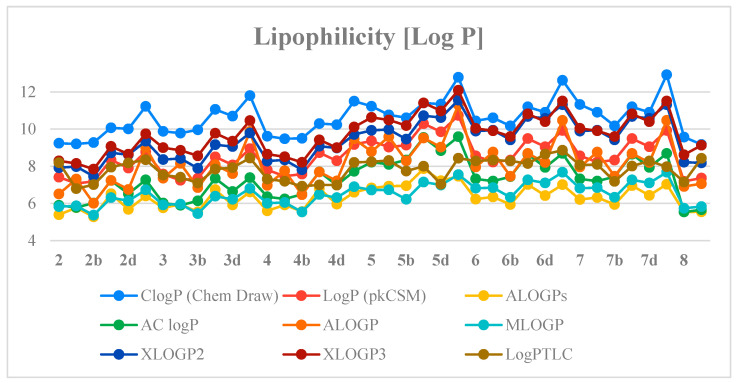
The values of the log PTLC of the compounds studied were in the range of 6.796–8.861. The propargylamine-containing derivative 2a was characterized by the lowest value of experimental lipophilicity (log PTLC = 6.796) and the highest activity against MV4-11, Balb/3T3 and PC-3 cells (IC50: 2.03, 3.10 and 4.47 µM, respectively) among all tested derivatives. The results indicated that introducing morpholinyl moiety derivatives (2–5)b led to the reduction of lipophilicity. The morpholine-derivatives 6b and 7b showed higher values of log PTLC than compounds (2–5)b, which is due to the presence of an aromatic ring in their chemical structure. The amides containing phthalic and isophthalic moieties 7(a, c–e) were inactive against cancer cell lines tested and had high experimental lipophilicity values (log PTLC = 7.972–8.284). It was also shown that compounds (2–6)e containing the cyclic 1-ethynylcyclohexylamine substituent had high values of the experimental lipophilicity parameter ranging from 8.205–8.861. This group also includes compound 6e with the highest log PTLC value among all of the studied betulin dicarboxylic acid ester derivatives.
Next, the previously determined experimental lipophilicity of the tested derivatives was compared with the theoretical values. The theoretical values of the partition coefficients (log P) were derived from eight calculation programs (C log P (Chem Draw Ultra 14.0, http://www.perkinelmer.co.uk/category/chemdraw), Log P (pkCSM), ALOGPs, AC log P, ALOGP, MLOGP, XLOGP2 and XLOGP3) [27,28]. The obtained results (Table S2, Supplementary Materials) show that compound 2a has the lowest value of the experimental and theoretical lipophilicity parameters. Determined theoretical values of log P for the tested compounds are in the range of 5.28–12.93. Analysis of the data obtained shows that the highest log P values are from C log P (Chem Draw) and the lowest from ALOGPs. Table S3 (Supplementary Materials) presents the correlation matrix for the theoretical parameters of lipophilicity of the compounds. Obtained correlation coefficients are in the range of 0.791–0.981. The highest correlation value was recorded for Log P (pkCSM) and AC log P (0.981). Figure 5 shows a comparison of the experimental lipophilicity values (log PTLC) for all derivatives with the corresponding values obtained from theoretical calculations.
Figure 5.
The profile of change for calculated and experimental lipophilicity of the studied compounds.
The study of biological activity of the synthesized compounds is complemented by evaluation of pharmacokinetic parameters such as drug similarity, ADME profile (Absorption, Distribution, Metabolism, Excretion) and toxicity. To assess the potential of the chemical compound to be active via oral administration, the criteria formulated by Lipinski and referred to as the Rule of Five are most often taken into account. Lipinski’s rule is based on four physicochemical parameters such as molecular weight (MW ≤ 500), lipophilicity (LogP ≤ 5), the number of hydrogen bond donors (nHBD ≤ 5) and acceptors (nHBA ≤ 10) [29]. These parameters are related to intestinal permeability and water solubility [30]. The calculated pharmacokinetic properties of the tested compounds are shown in Table 9.
Table 9.
The pharmacokinetic parameters of tested compounds (pkCSM-Biosig Lab—University of Melbourne http://biosig.unimelb.edu.au; accessed on 15 September 2022).
| Compound | MW | nHBA | nHBD | nROT | TPSA (Å2) | Caco-2 Perm. (log Papp, cm/s) |
|---|---|---|---|---|---|---|
| 2 | 542.80 | 4 | 2 | 6 | 83.83 | 0.633 |
| 2a | 579.87 | 4 | 2 | 7 | 75.63 | 0.703 |
| 2b | 611.91 | 5 | 1 | 6 | 76.07 | 0.713 |
| 2c | 609.94 | 4 | 1 | 6 | 66.84 | 0.611 |
| 2d | 627.97 | 5 | 1 | 6 | 66.84 | 0.627 |
| 2e | 647.99 | 4 | 2 | 7 | 75.63 | 0.654 |
| 3 | 554.81 | 4 | 2 | 5 | 83.83 | 0.653 |
| 3a | 591.88 | 4 | 2 | 6 | 75.63 | 0.723 |
| 3b | 623.92 | 5 | 1 | 5 | 76.07 | 0.733 |
| 3c | 621.95 | 4 | 1 | 5 | 66.84 | 0.631 |
| 3d | 639.98 | 5 | 1 | 5 | 66.84 | 0.647 |
| 3e | 660.00 | 4 | 2 | 6 | 75.63 | 0.674 |
| 4 | 556.83 | 4 | 2 | 7 | 83.83 | 0.609 |
| 4a | 593.89 | 4 | 2 | 8 | 75.63 | 0.708 |
| 4b | 625.94 | 5 | 1 | 7 | 76.07 | 0.719 |
| 4c | 623.96 | 4 | 1 | 7 | 66.84 | 0.617 |
| 4d | 642.00 | 5 | 1 | 7 | 66.84 | 0.633 |
| 4e | 662.01 | 4 | 2 | 8 | 75.63 | 0.659 |
| 5 | 612.94 | 4 | 2 | 11 | 83.83 | 0.461 |
| 5a | 650.00 | 4 | 2 | 12 | 75.63 | 0.569 |
| 5b | 682.04 | 5 | 1 | 11 | 76.07 | 0.580 |
| 5c | 680.07 | 4 | 1 | 11 | 66.84 | 0.478 |
| 5d | 698.11 | 5 | 1 | 11 | 66.84 | 0.494 |
| 5e | 718.12 | 4 | 2 | 12 | 75.63 | 0.521 |
| 6 | 590.85 | 4 | 2 | 5 | 83.83 | 0.683 |
| 6a | 627.91 | 4 | 2 | 6 | 75.63 | 0.668 |
| 6b | 659.95 | 5 | 1 | 5 | 76.07 | 0.699 |
| 6c | 657.98 | 4 | 1 | 5 | 66.84 | 0.614 |
| 6d | 676.02 | 5 | 1 | 5 | 66.84 | 0.630 |
| 6e | 696.03 | 4 | 2 | 6 | 75.63 | 0.620 |
| 7 | 590.85 | 4 | 2 | 5 | 83.83 | 0.714 |
| 7a | 627.91 | 4 | 2 | 6 | 75.63 | 0.724 |
| 7b | 659.95 | 5 | 1 | 5 | 76.07 | 0.736 |
| 7c | 657.98 | 4 | 1 | 5 | 66.84 | 0.652 |
| 7d | 676.02 | 5 | 1 | 5 | 66.84 | 0.667 |
| 7e | 696.03 | 4 | 2 | 6 | 75.63 | 0.675 |
| 8 | 540.79 | 4 | 2 | 5 | 83.83 | 0.661 |
| 9 | 574.87 | 5 | 2 | 7 | 83.83 | 0.684 |
The molecular weights of the tested compounds are in the range of 540.79–718.12 and are higher than the value adopted by Lipinski (MW ≤ 500), determining a chemical compound with good oral bioavailability. Furthermore, for the most active compound 2a, both the molecular weight (MW = 579.87) and the experimentally determined value of the lipophilicity parameter (Table 8, logPTLC = 6.796) violate this rule. The high molecular weight and higher lipophilicity of the compound result in poor water solubility and, therefore, low oral bioavailability. Oral drug delivery is the most common mode of administration and thus approximately 60% of the small-molecule drugs known on the market are used. Nevertheless, there is an increasing number of potential synthetic drug candidates that exhibit poor water solubility [31,32]. Various methods of orally formulating drugs with poor water solubility have been developed to overcome this limitation. These include crystal engineering methods (formation of metastable polymorphs or co-crystals), chemical modifications (conversion into prodrugs or salts), particle size reduction (micronization and nanoparticles) or amorphization (formation of solid dispersions). Another way is to use drug carrier systems such as micelles, nanoparticles, cyclodextrins and lipid-based preparations (e.g., liposomes) [31].
Taking into account another criterion for assessing drug-likeness, known as the Weber rule, a molecule of an orally administered drug should contain less than 10 rotating bonds (nROT ≤ 10) and its polar topological surface should be less than 140 Å2 (TPSA ≤ 140 Å2) [33]. Only 5–5e series compounds have a higher number of rotational bonds, and all other compounds satisfy this rule (Table 9).
A method for predicting intestinal permeability involving both passive and active absorption mechanisms of a new potential drug candidate is to use the Caco-2 model [34]. For the tested compounds, the Caco-2 perm values calculated by the pkCSM server are in the range of 0.461–0.736, which indicates their poor intestinal absorption [35]. The correlation between the molecular descriptors, anticancer activity and the RM0 values for betulin derivatives are presented in the Tables S4 and S5.
In general, the results suggest a strong inversely proportional correlation between compound lipophilicity and anticancer activity. The rule of five turned out to be unrelated to the activity of the tested compounds, as all of them do not satisfy the criteria, but still exhibit activity.
3. Conclusions
The search for new anticancer drugs is currently one of the main objectives of medicinal chemistry. After that, understanding of the mechanism of action of potential anti-cancer drugs is one of the most important steps in the advancement of chemotherapy of these diseases. In this paper, we have significantly widened and explored a new panel of betulin acid ester derivatives against existing and previously undescribed human cancer and normal cell lines. Furthermore, we have validated previous findings relating to their proapoptotic mechanisms of action. Considering the vast panel of possible mechanisms of actions of betulin derivatives, additional cell cycle arrest and caspase-3/7 activity assessments have been done.
Overall, derivatives with succinic acid linker 2(a–d) as well as morpholine amine (2–4)d exhibited very good cytotoxic activity. Thiomorpholine derivatives (2–4)d are good candidates for further studies, as they showed very good selectivity indexes and level of activity as well. On the other hand, all m-phthalic and 1-ethynylcyclohexyl derivatives were shown to be inactive. Subsequently, apoptosis detection and cell cycle analysis indicated induction of apoptosis as the main mechanisms of action of active compounds. With the exception of the leukemia MV4-11 cell line, proapoptotic activity correlates well with an increased level of apoptosis executioner enzymes–caspases-3/7. To complete the characterization of compounds, lipophilicity analysis linked logPTLC values with degree of antiproliferative activity.
The obtained results confirm the dependence of the activity and mechanisms of action of particular betulin derivatives on the cell line used, and at the same time, the need to study them for individual compounds. Additionally, the study presented strong findings that were consistent with the literature and the structure–activity relationships, which provide a solid basis for further studies on betulin derivatives as potent anticancer agents.
4. Materials and Methods
4.1. Compounds
Compounds were obtained from previous research (already published, [6]) and used simultaneously to carry out this study. Spectra data as well as melting points are available as supplementary materials in a previous paper [6].
4.2. Biological Evaluation
4.2.1. Cell Lines
Human biphenotypic B myelomonocytic leukemia MV4-11, murine normal fibroblast BALB/3T3 cells were obtained from the American Type Culture Collection (USA); and human lung carcinoma A549, human breast cancer MCF-7 and human prostate adenocarcinoma PC-3 were obtained from the European Collection of Authenticated Cell Cultures (UK). All the cell lines were maintained at the Hirszfeld Institute of Immunology and Experimental Therapy, PAS, Wroclaw, Poland.
MV4-11 and PC-3 cells were cultured in RPMI 1640 medium (HIIET PAS, Poland) with 1.0 mM sodium pyruvate (only MV4-11) and 10% fetal bovine serum (FBS) (all from Merck, Darmstadt, Germany). A549 cells were cultured in RPMI 1640 + Opti-MEM (1:1) ((HIIET PAS, Poland and Gibco, UK) supplemented with 5% fetal bovine serum (Merck, Darmstadt, Germany). The MCF-7 cells were cultured in Eagle medium (HIIET PAS, Poland) supplemented with 8 mg/mL of insulin and 1% of MEM NON-Essential amino acid (all Merck, Darmstadt, Germany). Normal fibroblasts BALB/3T3 cells were cultured in DMEM medium (Gibco, UK) supplemented with 10% fetal bovine serum and 1.0 mM sodium pyruvate (all from Merck, Darmstadt, Germany). All culture media were supplemented with 2 mM L-glutamine (Merck, Darmstadt, Germany), 100 units/ml penicillin, (Polfa Tarchomin S.A., Poland) and 100 µg/mL streptomycin (Merck, Darmstadt, Germany). All cell lines were grown at 37 °C in a 5% CO2 humidified atmosphere.
4.2.2. Determination of Antiproliferative Activity
The solutions of the tested compounds were prepared by dissolving the substances in DMSO to the desired concentration (25 mM). Then, the tested compounds were diluted in a culture medium to reach the final concentrations. Before addition of the tested compounds (24 h prior), the cells were plated in 96-well plates (Sarstedt, Germany) at a density of 1 × 104, 0.75 × 104 (MCF-7) or 0.5 × 104 (A549) cells per well. The assay was performed after 72 h of exposure to 100, 10, 1 and 0.1 µM concentration of the tested agents. The in vitro cytotoxic effect of all agents was examined using the MTT (MV4-11) or SRB assay, as described previously [36]. Briefly, the MTT method showed the cells viability, and the number of cells that converted the water soluble MTT reagent (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to an insoluble formazan product. The SRB method determined the amount of cellular proteins. The concentration of the colorimetric probe (formazan in MTT or sulforhodamine B in SRB method) was determined by an optical density measurement using a standard microplate reader for 96-well plates. After optical density measurement, using the data of the mean absorbances of control cells (AbCC), the absorbances of cells treated by the tested compound (AbTr) (for each concentration separately) and the absorbance of the cultured medium (AbCM) the % of cell proliferation inhibition was calculated according to the following formula:
From the obtained proliferation inhibition values, effect-concentration curves of the compound used were plotted. The results were calculated as an IC50 (inhibitory concentration 50%) of the concentration of the tested agent, which is cytotoxic for 50% of the cancer cells. IC50 values were calculated for each experiment separately using the Prolab-3 system based on Cheburator 0.4 software [36] and mean values (±SD) are presented. Each compound in each concentration was tested in triplicate in a single experiment, which was repeated 3–5 times.
4.2.3. Cell Cycle Analysis
The MV4-11, A549 and PC-3 cells were seeded on 24-well plates (Sarstedt, Germany) for a final volume of 1.5 mL. The cells were exposed to the tested compounds 2, 2a–d and 5 at concentrations of about IC50 for 24 and 72 h. After incubation, the cells were collected and 1 × 106 of cells were washed twice in cold PBS and fixed for 24 h in 70% ethanol at −20 °C, the cells were washed twice in PBS and incubated with RNAse (8 μg/mL, Fermentas, Germany) at 37 °C for 1 h. The cells were stained for 30 min. with propidium iodide (50 μg/mL, Sigma Aldrich, Germany) at 4 °C and the cellular DNA content was analyzed by flow cytometry using BD LSRFortessa cytometer (BD Bioscience, San Jose, CA, USA). Compounds at each concentration were tested at least three times independently. The obtained results were analyzed using Flowing software 2.5.1 (Cell Imaging Core, Turku Centre for Biotechnology, University of Turku and Åbo Akademi University, Turku, Finland).
4.2.4. Apoptosis Determination by Annexin V Staining
The MV4-11, A549 and PC-3 cells were seeded on 24-well plates (Sarstedt, Germany) for a final volume of 1.5 mL. The cells were exposed to the tested compounds 2, 2a–d and 5 at concentrations of about 1.5×IC50 for 24 and 72 h. After incubation, the cells were collected and 2 × 105 of cells were washed twice with PBS. APC-Annexin V (BD Pharmingen) was dissolved in a concentration of 1 mg/mL in a binding buffer (Hepes buffer: 10 mM HEPES/NaOH, pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, (HIIET, Poland) and the cells were suspended in 200 µL of this 1 mg/mL solution (freshly prepared each time). After 15 min of incubation in the dark at room temperature, the propidium iodide (PI) solution (1 mg/mL) was added prior to the analysis to give a final concentration of 0.1 mg/mL. Data acquisition was performed by flow cytometry using a BD LSRFortessa cytometer. The compounds were tested at least three times independently. Results were analyzed using Flowing software 2.5.1. The data were displayed as a two-color dot plot with an APC-Annexin V vs. PI. Double-negative cells were live cells, PI+/Annexin V+ were late apoptotic, PI-/Annexin V+ were early apoptotic cells and PI+/Annexin V- were necrotic cells.
4.2.5. Caspase-3/7 Activity Determination
The MV4-11, A549 and PC-3 cells were seeded on 48-well plates for a final volume of 1 mL. The cells were exposed to the tested compounds at concentrations of about 1.5 × IC50 or campthothecin (0.05–0.1 µg/mL) as a positive control, for 24 and 72 h. After incubation, the cells were collected and centrifuged (5 min., 4 °C, 250× g). Cells were suspended in 50 µL of ice-cold lysis buffer (50 mM HEPES, 10% (w/v) sucrose, 150 mM NaCl, 2 mM EDTA and 1% (v/v) Triton X-100, pH 7.3, HIIET, Poland) and incubated 30 min. at 40 °C. After the incubation, 40 µL of each sample was transferred to a white, 96-well plate (Corning, CA, USA) containing 160 µL of the reaction buffer (20 mM HEPES, 10 % sucrose, 100 mM NaCl, 1 mM EDTA, 10 mM DTT, 0.02 % Trition X-100, pH 7.3) (HIIET, Wroclaw, Poland) with 9 µM AC-DEVD-AMC (Cayman Chemical) fluorogenic substrate (λex = 360 nm, λem = 460 nm). The fluorescence increase correlated with the caspase-3/7 level was continuously recorded at 37 °C for 120 min using a Biotek Synergy H4 (Biokom, Warsaw, Poland). Compounds were tested in duplicates in a single experiment and each experiment was repeated at least three times independently. Results were normalized to the number of cells in each well and are reported as mean relative caspase-3/7 activity compared to the untreated control sample ± SD.
4.3. Lipophilicity
The RP-TLC chromatographic procedure used to determine the experimental logP value is included in the Supplementary Materials. To calculate the theoretical values of logP partition coefficients, the Chem Draw software (Perkin Elmer Informatics) was used to determine the Clog P values as well as transform the structures of the studied molecules into a simplified molecular-input line-entry system (SMILES). Based on SMILES codes, and using freely available web tools, such as pkCSM (Biosig Lab—University of Melbourne) and ALOGPS 2.1 (Virtual Computational Chemistry Laboratory), further theoretical logP values were calculated [28].
4.4. In Silico Analysis
Prediction of the pharmacokinetic profile of the tested compounds, including the calculation of molecular and physicochemical descriptors, as well as the assessment of similarity to drugs, was performed using the pkCSM-Biosig Lab—University of Melbourne (http://biosig.unimelb.edu.au) server.
4.5. Statistical Analysis
All datasets were analyzed using t-test. P-values lower than 0.05 were considered as statistically significant. Statistical analysis was performed using Statsoft Statistica 10 software.
Acknowledgments
The authors are grateful to Stanisław Boryczka for his valuable contribution to the creation of the presented study.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24010196/s1. Table S1. The literature lipophilicity (logPlit) and experimental (RM0) values of reference compounds; Table S2. The theoretical values of lipophilicity for tested compounds; Table S3. The correlation matrix for theoretically obtained lipophilicity parameters of tested compounds; Table S4. The correlation between the molecular descriptors and the RM0 values for betulin derivatives; Table S5. The correlation between the anticancer activity (IC50) and the RM0 values for betulin derivatives. Reference [37] is cited in the supplementary materials.
Author Contributions
P.P.: conceptualization, methodology, validation, writing—original draft preparation, project administration; M.Ś.: methodology, validation, writing—review and editing E.C., G.B. and E.B.: methodology, writing—review and editing, supervision, funding acquisition; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the Medical University of Silesia grant number [PCN-1-042/K/2/F and PCN-1-044/K/2/F].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 2.Ward R.A., Fawell S., Floc’h N., Flemington V., McKerrecher D., Smith P.D. Challenges and Opportunities in Cancer Drug Resistance. Chem. Rev. 2021;121:3297–3351. doi: 10.1021/acs.chemrev.0c00383. [DOI] [PubMed] [Google Scholar]
- 3.Potterat O., Hamburger M. Drug discovery and development with plant-derived compounds. In: Petersen F., Amstutz R., editors. Progress in Drug Research. 2nd ed. Volume 65. Birkhäuser Verlag; Basel, Switzerland: 2008. pp. 45–118. [DOI] [PubMed] [Google Scholar]
- 4.Kabała-Dzik A., Rzepecka-Stojko A., Kubina R., Iriti M., Wojtyczka R.D., Buszman E., Stojko J. Flavonoids, bioactive components of propolis, exhibit cytotoxic activity and induce cell cycle arrest and apoptosis in human breast cancer cells MDA-MB-231 and MCF-7—A comparative study. Cell. Mol. Biol. 2018;64:1–10. doi: 10.14715/cmb/2018.64.8.1. [DOI] [PubMed] [Google Scholar]
- 5.Grymel M., Lalik A., Kazek-Kęsik A., Szewczyk M., Grabiec P., Erfurt K. Design, Synthesis and Preliminary Evaluation of the Cytotoxicity and Antibacterial Activity of Novel Triphenylphosphonium Derivatives of Betulin. Molecules. 2022;27:5156. doi: 10.3390/molecules27165156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pęcak P., Orzechowska B., Chrobak E., Boryczka S. Novel betulin dicarboxylic acid ester derivatives as potent antiviral agents: Design, synthesis, biological evaluation, structure-activity relationship and in-silico study. Eur. J. Med. Chem. 2021;225:113738. doi: 10.1016/j.ejmech.2021.113738. [DOI] [PubMed] [Google Scholar]
- 7.Ouyang T., Yin H., Yang J., Liu Y., Ma S. Tissue regeneration effect of betulin via inhibition of ROS/MAPKs/NF-ĸB axis using zebrafish model. Biomed. Pharmacother. 2022;153:113420. doi: 10.1016/j.biopha.2022.113420. [DOI] [PubMed] [Google Scholar]
- 8.Guo W.B., Zhang H., Yan W.Q., Liu Y.M., Zhou F., Cai D.S., Zhang W.X., Huang X.M., Jia X.H., Chen H.S., et al. Design, synthesis, and biological evaluation of ligustrazine–betulin amino-acid/dipeptide derivatives as anti-tumor agents. Eur. J. Med. Chem. 2020;185:111839. doi: 10.1016/j.ejmech.2019.111839. [DOI] [PubMed] [Google Scholar]
- 9.Gonçalves S., Silva G., Pitta I., Rêgo M., Gnoato C., Rocha Pitta M. Novel betulin derivatives inhibit IFN-γ and modulates COX-2 expression. Nat. Prod. Res. 2018;12:1702–1711. doi: 10.1080/14786419.2018.1528581. [DOI] [PubMed] [Google Scholar]
- 10.Li Y., He K., Huang Y., Zheng D., Gao C., Cui L., Jin Y. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog. 2010;49:630–640. doi: 10.1002/mc.20638. [DOI] [PubMed] [Google Scholar]
- 11.Zhao J., Li R., Pawlak A., Henklewska M., Sysak A., Wen L., Yi J.E., Obmińska-Mrukowicz B. Antitumor activity of betulinic acid and betulin in canine cancer cell lines. In Vivo. 2018;32:1081–1088. doi: 10.21873/invivo.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachořík J., Urban M. Biocatalysis in the Chemistry of Lupane Triterpenoids. Molecules. 2021;26:2271. doi: 10.3390/molecules26082271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nurul Atikah Binti A.Y., Mat Hadzir N., Efliza Ashari S. Identification and Optimisation of Lipase-Catalysed Synthesis of Betulinic Acid Amide in a Solvent System. J. Appl. Chem. 2016;2016:149326. doi: 10.1155/2016/5149326. [DOI] [Google Scholar]
- 14.Drag-Zalesinska M., Kulbacka J., Saczko J., Wysocka T., Zabel M., Surowiak P., Drag M. Esters of betulin and betulinic acid with amino acids have improved water solubility and are selectively cytotoxic toward cancer cells. Bioorg. Med. Chem. Lett. 2009;19:4814–4817. doi: 10.1016/j.bmcl.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 15.Yang S.J., Liu M.C., Xiang H.M., Zhao Q., Xue W., Yang S. Synthesis and in vitro antitumor evaluation of betulin acid ester derivatives as novel apoptosis inducers. Eur. J. Med. Chem. 2015;102:249–255. doi: 10.1016/j.ejmech.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Suman P., Patel A., Solano L., Jampana G., Gardner Z.S., Holt C.M., Jonnalagadda S.C. Synthesis and cytotoxicity of Baylis-Hillman template derived betulinic acid-triazole conjugates. Tetrahedron. 2017;73:4214–4226. doi: 10.1016/j.tet.2016.11.056. [DOI] [Google Scholar]
- 17.Yan X., Yang L., Feng G., Yu Z., Xiao M., Cai W., Xing Y., Bai S., Guo J., Wang Z., et al. Lup-20(29)-en-3β,28-di-yl-nitrooxy acetate affects MCF-7 proliferation through the crosstalk between apoptosis and autophagy in mitochondria. Cell Death Dis. 2018;9:241. doi: 10.1038/s41419-017-0255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boryczka S., Bębenek E., Wietrzyk J., Kempińska K., Jastrzębska M., Kusz J., Nowak M. Synthesis, Structure and Cytotoxic Activity of New Acetylenic Derivatives of Betulin. Molecules. 2013;18:4526–4543. doi: 10.3390/molecules18044526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kommera H., Kaluperović G.N.K., Kalbitz J., Paschke R. Synthesis and Anticancer Activity of Novel Betulinic acid and Betulin Derivatives. Arch. Pharm. 2010;8:449–457. doi: 10.1002/ardp.201000011. [DOI] [PubMed] [Google Scholar]
- 20.Kvasnica M., Sarek J., Klinotova E., Dzuba P., Hajduch M. Synthesis of phthalates of betulinic acid and betulin with cytotoxic activity. Bioorg. Med. Chem. 2005;13:3447–3454. doi: 10.1016/j.bmc.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 21.Pfeffer C.M., Singh A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018;19:448. doi: 10.3390/ijms19020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han Y.-H., Mun J.-G., Jeon H.D., Kee J.-Y., Hong S.-H. Betulin Inhibits Lung Metastasis by Inducing Cell Cycle Arrest, Autophagy, and Apoptosis of Metastatic Colorectal Cancer Cells. Nutrients. 2020;12:66. doi: 10.3390/nu12010066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamalidehghan B., Ghafouri-Fard S., Motevaseli E., Ahmadipour F. Inhibition of human prostate cancer (PC-3) cells and targeting of PC-3-derived prostate cancer stem cells with koenimbin, a natural dietary compound from Murraya koenigii (L) Spreng. Drug Des. Devel. Ther. 2018;12:1119–1133. doi: 10.2147/DDDT.S156826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kong E.H., Kim Y.J., Kim Y.J., Cho H.J., Yu S.N., Kim K.Y., Chang J.H., Ahn S.C. Piplartine induces caspase-mediated apoptosis in PC-3 human prostate cancer cells. Oncol. Rep. 2008;20:785–792. doi: 10.3892/or_00000075. [DOI] [PubMed] [Google Scholar]
- 25.Janicke R.U. MCF-7 breast carcinoma cells do not express caspase-3. Breast Cancer Res. Treat. 2009;117:219–221. doi: 10.1007/s10549-008-0217-9. [DOI] [PubMed] [Google Scholar]
- 26.Ciura K., Dziomba S., Nowakowska J., Markuszewski M. Thin layer chromatography in drug discovery process. J. Chromatogr. A. 2017;1520:9–22. doi: 10.1016/j.chroma.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 27. [(accessed on 15 September 2022)]. Available online: http://biosig.unimelb.edu.au.
- 28. [(accessed on 24 September 2022)]. Available online: http://www.vcclab.org.
- 29.Lipinski C., Lombardo F., Dominy B., Feeney P. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997;23:3–25. doi: 10.1016/S0169-409X(96)00423-1. [DOI] [PubMed] [Google Scholar]
- 30.Attique S.A., Hassan M., Usman M., Atif R.M., Mahboob S., Al-Ghanim K.A., Bilal M., Nawaz M.Z. A Molecular Docking Approach to Evaluate the Pharmacological Properties of Natural and Synthetic Treatment Candidates for Use Against Hypertension. Int. J. Environ. Res. Public Health. 2019;16:923. doi: 10.3390/ijerph16060923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alqahtani M., Kazi M., Alsenaidy M., Ahmad M. Advances in Oral Drug Delivery. Front. Pharmacol. 2021;12:618411. doi: 10.3389/fphar.2021.618411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prasad V., De Jesús K., Mailankody S. The high price of anticancer drugs: Origins, implications, barriers, solutions. Nat. Rev. Clin. Oncol. 2017;14:381–390. doi: 10.1038/nrclinonc.2017.31. [DOI] [PubMed] [Google Scholar]
- 33.Veber D., Johnson S., Cheng H.Y., Smith B.R., Ward K.W., Kopple K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002;45:2615–2623. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 34.Falcón-Cano G., Molina C., Cabrera-Pérez M. Reliable Prediction of Caco-2 Permeability by Supervised Recursive Machine Learning Approaches. Pharmaceutics. 2022;14:1998. doi: 10.3390/pharmaceutics14101998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel R.D., Kumar S.P., Patel C.N., Shankar S.S., Pandya H.A., Solanki H.A. Parallel screening of drug-like natural compounds using Caco-2 cell permeability QSAR model with applicability domain, lipophilic ligand efficiency index and shape property: A case study of HIV-1 reverse transcriptase inhibitors. J. Mol. Struct. 2017;1146:80–95. doi: 10.1016/j.molstruc.2017.05.019. [DOI] [Google Scholar]
- 36.Czarnecka M., Świtalska M., Wietrzyk J., Maciejewska G., Gliszczyńska A. Synthesis, Characterization, and In Vitro Cancer Cell Growth Inhibition Evaluation of Novel Phosphatidylcholines with Anisic and Veratric Acids. Molecules. 2018;23:2022. doi: 10.3390/molecules23082022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kadela-Tomanek M., Jastrzębska M., Marciniec K., Chrobak E., Bębenek E., Boryczka S. Lipophilicity, pharmacokinetic properties, and molecular docking study on SARS-CoV-2 target for betulin triazole derivatives with attached 1,4-quinone. Pharmaceutics. 2021;13:781. doi: 10.3390/pharmaceutics13060781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.