Abstract
High rates of cell proliferation and protein synthesis in pancreatic cancer are among many factors leading to endoplasmic reticulum (ER) stress. To restore cellular homeostasis, the unfolded protein response (UPR) activates as an adaptive mechanism through either the IRE1, PERK, or ATF6 pathways to reduce the translational load and process unfolded proteins, thus enabling tumor cells to proliferate. Under severe and prolonged ER stress, however, the UPR may promote adaptation, senescence, or apoptosis under these same pathways if homeostasis is not restored. In this review, we present evidence that high levels of ER stress and UPR activation are present in pancreatic cancer. We detail the mechanisms by which compounds activate one or many of the three arms of the UPR and effectuate downstream apoptosis and examine available data on the pre-clinical and clinical-phase ER stress inducers with the potential for anti-tumor efficacy in pancreatic cancer. Finally, we hypothesize a potential new approach to targeting pancreatic cancer by increasing levels of ER stress and UPR activation to incite apoptotic cell death.
Keywords: unfolded protein response (UPR), endoplasmic reticulum (ER) stress, pancreatic cancer, apoptosis
1. Introduction
Pancreatic cancer continues to be the third most common cancer-related death in the United States, accounting for 7% of all cancer deaths and 3% of all cancers [1]. Pancreatic ductal adenocarcinoma (PDAC) develops in the exocrine pancreas and is by far the most prevalent pancreatic cancer, accounting for more than 90% of all cases [2]. As of 2022, it is estimated that 62,210 people will be diagnosed with pancreatic cancer and 49,830 will die of the disease, with a current five-year survival rate of 11% [1]. Proportionally, men are at a slightly higher risk than women for pancreatic cancer.
Precision medicine with the incorporation of universal sequencing may improve the care of patients. However, only around one in six patients harbor a pathogenic germline variant, among which around half are in homologous recombination repair-related genes [3]. In patients with pathogenic BRCA1/2 or PALB2 mutations, the incorporation of platinum-based therapies and PARP inhibitors such as Olaparib brings modest improvements in outcomes [3,4,5]. More recently, two patients were treated with TCR gene therapy targeting the KRAS G12D driver mutation. One patient with metastatic refractory pancreatic cancer had a response after a single infusion of autologous T cells, which was continued at 6 months [6].
Poor prognosis in pancreatic cancer could be related to primary chemo-resistance [7]. Desmoplasia and the tumor microenvironment are two factors implicated in resistance to treatment, such as primary resistance to immunotherapy. Understanding and targeting cellular homeostasis could help to improve drug delivery and ultimately treatment outcomes [8]. Several experimental studies examined the unfolded protein response (UPR) and its correlation with cellular homeostasis and stress as a possible target in cancer [9]. As pancreatic cancer cells are severely hypoxic and bear cell stress, we hypothesize that increasing cell stress might induce apoptosis in pancreatic tumor cells [10]. In this article, we will review and examine the mechanisms involved in cell and ER stress, the role of the UPR in pancreatic cancer and its activation, and strategies to increase cell stress and tumor cell death through UPR activation.
2. Mechanisms of Cellular Homeostasis
In cellular metabolism, the mitochondria are involved in several essential functions such as energy generation, calcium signaling, stress response, cell differentiation, and apoptosis [11,12,13]. In the case of defective mitochondria, cellular homeostasis may be compromised, and quality control mechanisms are activated to preserve the balance between homeostasis and cell death [14]. Mitophagy can also be triggered through multiple signaling cascades in response to persistent defects [14].
An important part of cellular homeostasis with relevance to cancer metabolism is protein homeostasis. The endoplasmic reticulum (ER) is an interconnected single membrane-bound network that serves as a protein factory, where about one-third of all proteins, secretory or membrane-associated, are folded [15]. An ER quality control system exists to preserve the balance of the folded proteins [16]. This control system can detect correctly folded proteins exiting the ER to their destination, as well as misfolded or unfolded proteins [15]. This mechanism prevents misfolded or unfolded proteins from transiting the secretory pathway and assures that misfolded proteins are directed toward a degradative pathway [16,17]. ER-associated degradation (ERAD) is the process by which proteins that are terminally misfolded are transported from the ER to the cytosol and on to proteasome degradation [15].
To correctly recognize and promote the ubiquitination of misfolded proteins, the ERAD system is composed of multiple components that integrate protein complexes with the ER membrane [15]. In yeast, three membrane protein complexes that define different complexes (ERAD-L, ERAD-M, and ERAD-C) have been identified and proposed [15]. When proteins are misfolded, it is proposed that they are degraded by these complexes [15]. The progressive accumulation of unfolded proteins activates the ER stress receptors, which, together with molecular chaperones, activate the ERAD system and the unfolded protein response (UPR) to enhance the clearance of misfolded proteins [15].
The UPR is an adaptive response to the activation of the Integrated Stress Response (ISR) [17]. The ISR is an evolutionarily conserved intracellular signaling network that is activated when a cell experiences various intrinsic or extrinsic insults. Extrinsic activators of the ISR include amino acid depletion, glucose deprivation, and hypoxia, among others [18]. Conversely, there are intrinsic insults to the cell and, in particular, the ER can activate the ISR and specifically the UPR to induce changes in intraluminal calcium, altered glycosylation, nutrient deprivation, pathogen infection, expression of folding-defective proteins, and changes in redox status [17]. UPR activation may lead to resolved homeostasis, but under prolonged and unresolved stress, the signaling pathway will progress to apoptotic mechanisms [17].
3. Mechanisms of Unfolded Protein Response and Cancer
Beyond the multiple functions of the UPR in cellular homeostasis, cancer development, and maintenance, of particular interest are the endoplasmic reticulum (ER) membrane stress sensors [17]. These stress sensors include inositol-requiring enzyme 1 (IRE1), double-stranded RNA-activated protein kinase (PKR)–like ER kinase (PERK), and activating transcription factor 6 (ATF6) [17,18,19,20,21,22]. PERK is one of the four transmembrane initiators of intracellular signaling that comprise the ISR. Increasing evidence shows that UPR sensors are related to oncogenic programming and cancer stem cells (CSC), including the work by Pattabiraman and Weinberg, as well as the work of Ingrid Caras, which have propelled further research and clinical trials into CSC-targeted therapies [23,24].
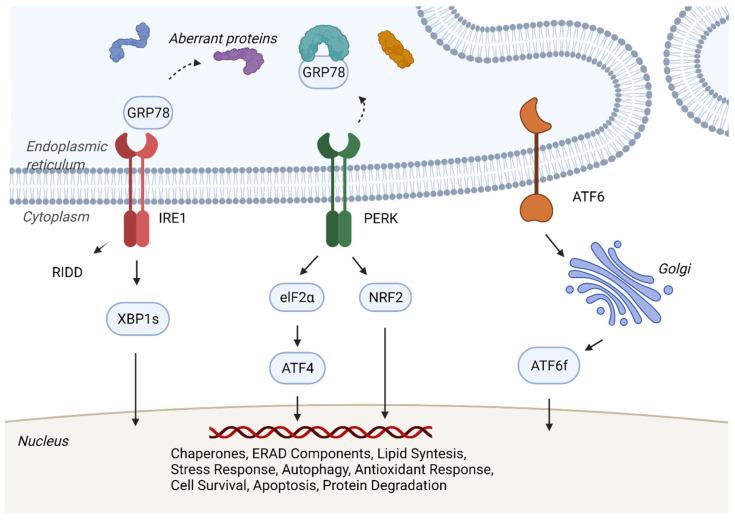
As the ER maintains the correct balance of secreted and transmembrane proteins, the UPR functions as an adaptive response to the accumulation of folded proteins and the imbalances of the capacity of ER protein folding and demand [17,25,26,27]. The ER transmembrane sensors IRE1, PERK, and ATF6 regulate proteostasis and homeostasis through separate and overlapping pathways [16] (Figure 1).
Figure 1.
Unfolded Protein Response. A simplified version of unfolded protein response (UPR). Under stress, cells activate the adaptative UPR to establish cellular homeostasis and proteostasis. The sensors IRE1, PERK, and ATF6 are activated after the dissociation of GRP78, which is recruited due to the accumulation of misfolded and unfolded proteins. IRE1 oligomerization and auto-phosphorylation induce kinase and RNase activity leading to mRNA degradation (RIDD) and altered splicing of the XBP1 mRNA and production of the transcription factor XBP1s. PERK undergoes oligomerization and auto-phosphorylation, subsequently phosphorylating the eukaryotic translation initiation factor eIF2α, resulting in the translation of transcription factor ATF4. NRF2 is also phosphorylated by PERK and activates antioxidant responses. ATF6 activation occurs after transport to the Golgi apparatus where it undergoes S1P/S2P protease cleavage, yielding transcriptionally active ATF6f. The transcriptional events mediated by XBP1s, ATF4, and ATF6f promote the expression of chaperones and components of ER-associated protein degradation (ERAD), reducing protein translation and increasing ER capacity in order to restore homeostasis. Additionally, UPR-mediated gene expression also directly impacts autophagy, cytokine production, and apoptosis. Figure 1 was created with BioRender.com.
In cancer, the UPR plays a role in cell survival properties, carcinogenesis, and even mechanisms related to treatment resistance [17,28,29]. As a result of aberrant protein production and intrinsic stress in cancer, the UPR is highly active [17]. The tumor microenvironment is normally subjected to stress by intrinsic and extrinsic conditions (Table 1), including nutrient shortage, hypoxia, aberrant protein synthesis, and chemotherapy exposure [30,31,32]. These conditions can also upregulate UPR activation, with cellular homeostatic effects and consequently tumor survival. Furthermore, it is shown that the UPR is related to multiple other factors in cancer, including genomic instability, inflammation, cell survival, angiogenesis, and metastatic properties [30].
Table 1.
Factors that increase stress.
| Intrinsic Factors | Extrinsic |
|---|---|
| Oncogenic activation | Hypoxia |
| Altered Ploidy | Acidosis |
| Exacerbated Secretors | Nutrient depletion |
| Genomic Instability | VEGF |
| Redox imbalance | |
| Inward mutation |
Recent efforts have sought to better characterize the role of inflammation in tumorigenesis, specifically regarding the implication of chronic pancreatitis (CP) in pancreatic cancer development. Lowenfels et al. [33] and several meta-analyses have confirmed that CP patients face an increased risk of developing PDAC at the 10- and 20-year mark [34,35,36]. Although many factors may contribute to the development of PDAC from CP, one leading hypothesis is that chronic inflammation presents an optimal environment for oncogenic mutations, which could catalyze carcinogenesis for patients with a genetic predisposition or at environmental risk (tobacco, nicotine, or alcohol abuse) for pancreatic cancer [37]. Additionally, with the advancement of genetically engineered mouse models, the identification of acinar cells as the main cellular origin of PDAC and the transdifferentiation of acinar cells into pancreatic intraepithelial neoplasia (PanIN) adds context to the link between PDAC and CP [38].
Acinar-to-ductal metaplasia (ADM) is the process of pancreatic acinar cells differentiating into ductal-like cells [38]. Because acinar cells are synthetically very active and prone to ER stress, their plasticity represents an intrinsic defense mechanism to protect acinar cells from genetic and environmental pressures [38]. However, under sustained cell stress, such as inflammation during chronic pancreatitis and mutant KRAS or aberrant growth factor signaling, acinar cells differentiate through ADM into PanIN, a precancerous lesion and the main pathological basis of PDAC development [38]. Hingorani et al. determined the origin of PDAC using KRASG12D mice and found that PanIN formation was preceded by acinar-to-ductal metaplasia [39]. Furthermore, Carrière et al. subjected genetically engineered mice with a Lox-Stop-Lox (LSL) sequence followed by a K-Ras G12D point mutation (LSL-KRASG12D mice) to a series of caerulein injections (previously demonstrated to induce pancreatitis) and found that pancreatitis-induced acinar cell regeneration increased the propensity for pancreatic malignant transformation, confirming the role of inflammation in the development of PanINs and subsequent PDAC [40].
4. Evidence for UPR Being Important in Pancreatic Cancer
The UPR could be a pathway with therapeutic potential in PDAC [32]. In a study evaluating the cytotoxic effect of nemorosone, a polycyclic polyprenylated acylphloroglucinol derived from an ethanolic flower extract of Clusia Rosea on pancreatic cell lines, UPR response was induced after ER stress [32]. Pancreatic cell lines Capan-1, AsPC-1, and MIA-PaCa-2 were exposed to nemorosone in increasing concentrations. At 10 mM, a significant reduction in pancreatic cancer cell growth of at least 80% was observed. Among multiple effects observed, an evaluation of the expressed genes on the tested lines revealed them to be significantly associated with the response to cellular stress, as well as the regulation of apoptosis and cell cycle. Furthermore, most induced genes shared by all cell lines were found to be directly involved in the signaling network of ER stress and the UPR [32]. Many of the genes are induced by ATF4 and resemble pro-survival genes related to the UPR [32].
There is growing evidence that ER stress and UPR activation are abundant in pancreatic cancer [7,41]. In 2015, Kong et al. examined KRAS/Mek-mTOR signaling by implanting pancreatic tissue from PDAC patients who had pancreatic resections into wild-type mice, thus creating transgenic p48Cre/+, LSL-KRASG12D/+, and Tsc1flax/+ murine models [41]. By establishing these cell lines and implanting them orthotopically into wild-type mice, researchers found extensive necrotic components and confirmed high levels of hypoxia-induced ER stress in the tumor microenvironment [41]. A particular UPR component of interest is the ER chaperone protein glucose-related protein 78 (GRP78) [42,43]. GRP78, also called BiP (immunoglobulin heavy chain-binding protein) or HSPA5 (heat-shock protein A5), binds misfolded and unfolded proteins to correct the folding process. Additionally, it is upregulated in the context of ER stress, along with PERK and the other complexes that reduce protein synthesis [43,44]. GRP78 blocks the activation of IRE1, PERK, and ATF6 by binding to their ER luminal domains. Under ER stress, GRP78 dissociates, resulting in the activation of IRE1, PERK, and ATF6 and their downstream signaling pathways (Figure 1).
Elevated GRP78 is related to poor prognosis in pancreatic cancer [45]. In one study, GRP78 was detected using tissue microarray-based immunohistochemistry in tissues from 180 pancreatic cancer patients [44]. Interestingly, not only was worse overall survival and a higher T-stage observed among patients with higher expression of GRP78 but the expression of GRP78 was also higher in tumor tissues than in the adjacent nontumor tissues [44]. In vitro, the regulation of GRP78 in PDAC cell lines affected the proliferation, migration, and invasion capacities [45]. Transcriptomic analysis of the PDAC cell line S2-VP10 evaluating the shRNA-mediated knockdown of GRP78 suggests that GRP78 interferes in multiple signaling pathways including cell-cycle, apoptosis, and actin-cytoskeleton regulation [45]. In Pdx1-Cre, KrasG12D/+, and p53f/+ murine models of PDAC, high GRP78 was also related to tumor development; however, in the same genetic background, mice pancreatic tissue bearing an additional GRP78f/+ allele reduced pancreatic tumorigenesis [46]. Resistance to chemotherapy can also be related to GRP78. Silencing GRP78 reduced efflux by ATP-binding cassette transporters, and in combination with chemotherapy, cells treated with silencing GRP78 exhibited significantly more cell death [47].
The relationship between IRE1 and the proliferative effects in pancreatic cancer cell lines has been reported [48]. Proliferation assays using fourteen pancreatic cancer cell lines showed a dose- and time-dependent growth inhibition by IRE1α-specific inhibitors, and subsequent cell cycle analysis showed that these IRE1α inhibitors caused growth arrest at either the G1 or G2/M phases (SU8686, MiaPaCa2) and induced apoptosis (Panc0327, Panc0403) [48]. In another study, suppression of IRE1 with IRE inhibitor STF-083010 alone reduced the viability of pancreatic cancer cell lines [49]. In the same study, sunitinib-, gemcitabine-, and chloroquine-treated mice showed a significant reduction in GRP78 expression, reduced cell proliferation, and increased apoptosis [48]. Studies in pancreatic cancer cell lines also showed that PERK activation can contribute to tumorigenesis [48]. GSK2656157, an ATP-competitive inhibitor, showed inhibition of stress-induced PERK autophosphorylation, eIF2α substrate phosphorylation, and decreased ATF4 and CAAT/enhancer binding protein–homologous protein in multiple cell lines [49]. Further oral administration of GSK2656157 resulted in dose-dependent inhibition of multiple human tumor xenograft growth in mice [49]. HSP70, similar to GRP78, is another chaperone that can trigger UPR-mediated cell death [50]. Modulating HSP70 is also another strategy to induce the UPR and stress, and agonists and antagonists are being developed as therapeutic strategies to modulate the UPR [50].
5. Molecular Induction of ER Stress and Apoptosis
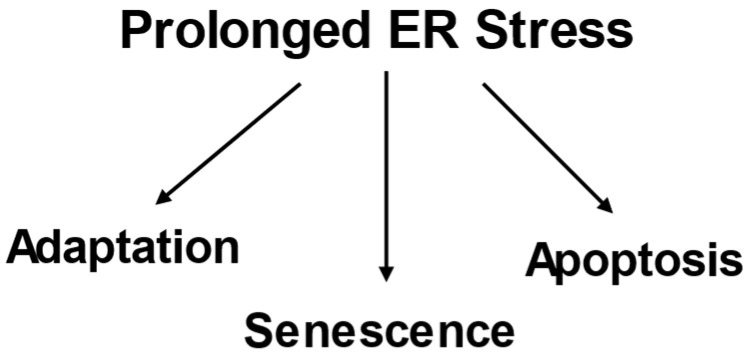
During tumorigenesis, rapid cell proliferation and high rates of protein synthesis deplete the tumor microenvironment (TME) of oxygen, nutrients, and glucose, leading to ER stress [7]. In addition, the heavy demand and limited capacity of ER protein folding during cell proliferation lead to an accumulation of improperly folded proteins. Under general ER stress such as abnormal TME, IRE1, PERK, and ATF6 have unique mechanisms to promote cell survival and regulate cellular homeostasis. However, failure to restore cellular homeostasis under prolonged ER stress may lead to adaptation, senescence, or cell death through apoptosis, as depicted in Figure 2.
Figure 2.
Cellular Responses to Prolonged Levels of ER Stress.
Mitigating ER stress and the effects of the UPR have been proposed for several cancers and diseases, particularly hematologic malignancies, where HSP90 inhibitors reduce ER stress by inhibiting the post-translational modification of proteins, thus reducing protein levels vital for cancer pathology and disrupting signaling cascades. Though many theories propose reducing ER stress, we propose a radically different approach of mechanistically increasing ER stress to levels the cell cannot overcome to induce apoptosis through the UPR, thus enhancing the effects of chemotherapy. A summary of agents and treatment modalities under recent investigation for their ability to induce ER stress and apoptotic cell death are listed below in Table 2.
Table 2.
Potential Agents to Activate UPR-Induced Apoptosis in Pancreatic Cancer.
| Agent | Mechanism of Action | Stage of Development | Clinical Trials | Comments |
|---|---|---|---|---|
| Gambogenic Acid (GNA) | ROS-dependent activation of IRE1 leads to prolonged ER stress; PERK activation → eIF2 phosphorylation inhibits protein synthesis [51,52,53] | Preclinical | No first-in-human trials | |
| Tigatuzumab | Death Receptor (DR)5 monoclonal antibody agonist; Induces TRAIL to bind DR5 initiating downstream caspase activation in tumors [54,55,56,57,58] | Phase 2 |
NCT01307891 NCT01220999 |
Combination with gemcitabine, sorafenib, nab-PAC |
| Minnelide | Water-soluble analog of triptolide; Inhibits GRP78; Upregulates IRE1 and PERK pathways to increase ER stress [59,60] | Phase 1/2 |
NCT03129139 NCT04896073 |
Single-agent and combination trials with Paclitaxel |
| BOLD-100 | Ruthenium (III) anticancer agent; inhibits GRP78 and increases ROS production; leads to ER stress [61,62,63] | Phase 1B | NCT04421820 | Combination with FOLFOX |
| Disulfram (Antabuse) | Binds Copper to form DSF-Cu complex; Induces ROS production → Increased levels of oxidized proteins → ER stress and UPR activation [64] | Phase 2 Completed, Phase 1 (recruiting) |
NCT03714555 NCT02671890 |
Combination with nab-PAC-gemcitabine, FOLFIRINOX, Gemcitabine |
| Radiation therapy | Radiation → ROS/RNS production → ER stress induction and UPR activation [65,66,67,68,69,70] | ER stress inducers sensitize cancer cells to radiation treatment | ||
| KRN5500 (Spicamycin analog) | Anti-Golgi drug; Inhibits protein synthesis and glycoprotein processing via altered Golgi (dilated cisternae) → Accumulation of unfolded proteins in ER lumen → apoptosis via intrinsic pathway [71,72] | Phase 1 completed |
NCT00017238 NCT00002923 |
No tumor response, three disease stabilizations observed in in-human trials. |
| Atovaquone | Ubiquinone analogue: Inhibits Complex III of ETC and oxidative phosphorylation → leads to oxidative and ER stress [73,74] | Phase 1 | NCT04648033 | Investigated in NSCLC, ovarian cancer |
| Auranofin | Gold (I) complex; inhibits thioredoxin reductases (TrxRs) → increased ROS species leading to ER stress; also targets PI3K/AKT/mTOR pathway [75,76] | Phase 1 |
NCT01747798 NCT03456700 |
Investigated in NSCLC, ovarian cancer |
Footnote: nab-PAC: nab-paclitaxel, CRC: Colorectal cancer NSCLC: Non-Small Cell Lung Cancer, FOLFIRINOX: fluorouracil, folinic acid, irinotecan, oxaliplatin, FOLFOX: fluorouracil, oxaliplatin + folinic acid.
6. Gambogenic Acid (GNA)
Gambogenic acid (GNA), a compound isolated from the traditional Chinese medicine gamboge, has shown promise in inducing apoptosis by increasing ER stress through several different mechanisms. In colorectal cancer (CRC) xenografts and murine models, GNA induced ER stress-mediated apoptosis in a reactive oxygen species (ROS)-dependent manner, activating the IRE1 pathway to induce prolonged ER stress [51]. More recently, GNA was shown to inhibit CRC proliferation in vitro by inducing apoptosis [52]. Upon further examination, while GNA upregulated the expression of IRE1, PERK, and downstream eIF2 and ATF4, GNA-induced ER stress was deduced to occur from inhibition of protein synthesis resulting from eIF2 phosphorylation [52]. When GNA’s effect was examined in vivo using an azoxymethane (AOM)/dextran sulfate sodium (DSS) mouse model of colitis-associated cancer (CAC) established in BALB/c mice, GNA clearly suppressed colorectal tumors while also increasing the expression of CHOP and GRP78, indicating GNA suppressed tumor growth by triggering ER stress [52]. In nasopharyngeal carcinoma, however, in addition to increasing GRP78 and CHOP expression, treatment with GNA resulted in a dramatic efflux of intracellular chloride ions and an opening of the chloride ion channel [53]. As apoptotic volume decrease (AVD) through chloride channels is an early hallmark of apoptotic events, this suggests a possible pleiotropic mechanism in which GNA induces apoptosis through both ER stress and chloride channel opening.
7. Tigatuzumab
Aside from increasing ER stress, immunotherapies known to induce apoptosis through caspase activation by targeting proteins associated with mitochondrial are also of interest. Tigatuzumab, a human monoclonal antibody that acts as a death receptor 5 agonist, has shown preclinical promise in inducing tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) activity in tumor cells [54]. TRAIL, a member of the TNF superfamily of cytokines, is expressed commonly in colon, gastric, pancreatic, and other types of human cancers, with little to no expression in normal tissue and has a marked ability to bind death receptor 5 (DR5) and trigger apoptosis in tumor cells via downstream caspase activation [55]. As TRAIL targets multiple receptors, agonistic monoclonal antibodies such as tigatuzumab that specifically bind DR5 may be advantageous anticancer agents due to a more targeted and stronger apoptosis-inducing activity [54]. Preclinical studies of TRA-8, a murine DR5 agonist, in pancreatic cancer cell lines showed single-agent anti-tumor efficacy and increased efficacy in combination with doxorubicin, paclitaxel, and gemcitabine [56]. Increased sensitivity to treatment was observed in tissue with higher DR5 expression levels [56]. The clinical efficacy of Tigatuzumab in combination with FDA-approved chemotherapeutic agents is described in Table 3.
Table 3.
Phase 2 clinical efficacy of Tigatuzumab (TIG) in combination with gemcitabine, sorafenib, and nab-PAC/Abraxane.
| Trial Phase | Combination Agents | Type of Cancer | Progression-Free Survival (PFS) | Overall Survival (OS) | Conclusions |
|---|---|---|---|---|---|
| Phase II | Tigatuzumab + Gemcitabine | Unresectable or metastatic pancreatic cancer | 52.5% PFS at 16 weeks; not significant from historical data at 44% seen with gemcitabine alone [57] | 8.2 months; comparable to 3.6–6.8 months (gemcitabine alone), 3.8–11.1 months (gemcitabine + other agents), 11.1 months (FOLFIRINOX trial) [57] | Marginal increase in overall survival with TIG compared to gemcitabine alone suggests possible contribution of TIG to anti-tumor effects of gemcitabine; TIG may be clinically active [57] |
| Phase II | Tigatuzumab + Sorafenib | Advanced hepatocellular carcinoma | Time to progression (TTP): 3.9 months in 6/6 mg/kg TIG + SOR; 2.8 months in SOR alone (small sample size p = 0.988) [54] | 12.2 months in TIG + SOR; 8.2 months in SOR alone (small sample size p = 0.737) [54] | TIG + SOR failed to meet primary efficacy endpoint of TTP. However, combination was well tolerated and suggests possible increase in OS for TIG [54] |
| Phase II | Tigatuzumab + nab-PAC/Abraxane | Metastatic triple-negative breast cancer (TNBC) | 2.8 months overall in TIG + nab-PAC, 3.8 months in patients with objective response; 3.7 months in nab-PAC arm [58] | Overall response rate (ORR): 28% (CI 14.9–45.0% in TIG + nab-PAC; 38% (CI 18.0–61.1%) in nab-PAC arm [58] | 3 complete responses (CR) + 1 near CR in TIG + nab-PAC arm; no CR in nab-PAC arm; does not support further research of TIG + nab-PAC; however, notable increase in complete responses suggests further investigation of anti-DR5 agents [58] |
8. Minnelide
Preclinical studies demonstrated that triptolide, a diterpenoid triepoxide, had high efficacy in inhibiting pancreatic cancer cells in vitro and blocking tumor growth and metastasis in vivo [59]. Mujumdar et al. unveiled how triptolide induces cell death by increasing ER stress and activating UPR-mediated apoptosis [59]. Mechanistically, triptolide initially increased GRP78 expression 4 h after treatment, reflecting an initial cellular survival mechanism, but decreased GRP78 expression by the 24 h timepoint, activating the ER stress pathway to initiate apoptotic death [59]. Moreover, treatment with triptolide resulted in sustained increases of IRE1 and eIF2 levels, induced the expression of CHOP mRNA in multiple cell lines, and led to increased levels of ER stress, indicating that treatment with triptolide induces the UPR in pancreatic cancer cells through the PERK-eIF2 and IRE1 pathways [59].
Given triptolides’ poor solubility limiting its clinical use, Minnelide was developed as a highly water-soluble analog that exhibited a greater preclinical efficacy, showing great potential for its clinical application. In a Phase 1 dose escalation and pharmacokinetic study of Minnelide in pancreatic and gastric tumors, 36% of patients saw a partial metabolic response following cycle 1, and stable metabolic disease was observed in 52% of patients (n = 19) [60]. Using RECIST criteria (n = 10) after cycle 2, a partial response was observed in one patient (gastric) and stable disease was seen in six patients (5 pancreas, 1 rectal). Although neutropenia was observed as a common dose-limiting toxicity, this was rapidly reversible, and a revised treatment schedule has been developed to allow for more sustained treatment [60]. Currently, a Phase 1 clinical trial is underway, evaluating the treatment-related adverse events and anti-tumor activity of Minnelide alone and in combination with paclitaxel in patients with advanced solid tumors, mainly pancreatic and gastric (NCT03129139).
9. BOLD-100/KP1339
BOLD-100/KP1339, a ruthenium (III)-based anticancer agent, has shown promising anticancer activity in preclinical studies on solid tumors for its ability to increase ER stress [61]. Compared to platinum-based anticancer agents that target DNA, this ruthenium-based metallic agent acts on proteins and has been shown to increase the production of ROS while downregulating GRP78 and upregulating PERK and downstream CHOP, increasing ER stress and promoting ER-dependent apoptosis [61,62]. In a Phase I clinical trial, stable disease was observed for patients with non-small-cell lung cancer, sarcoma, and colorectal cancer tumors, and a partial response or disease stabilization was observed in patients with gastrointestinal neuroendocrine tumors [63]. Given the documented safety with manageable adverse effects observed in the Phase I clinical trial, a Phase 1B trial is currently underway investigating the combination of BOLD-100/KP1339 and FOLFOX for patients with advanced solid tumors (NCT04421820).
10. Radiation Therapy to Activate UPR
Ionizing radiation is known to generate ROS and reactive nitrogen species causing oxidative damage to macromolecules in the mitochondria, leading to ER stress and activation of the UPR. With a multitude of clinical studies suggesting only around a 30% response rate for pancreatic cancer to radiation therapy [65,66,67,68,69,70], a combination with ER stress inducers may offer clinical benefits for overcoming radioresistance [70]. In glioblastoma multiforme (GBM), when exposing patient-derived GBM stem cells (GSCs) to the ER stress-inducing agent 2-deoxy-D-glucose (2-DG), dose-dependent decreases in tumor viability were observed, along with increased apoptotic markers, indicating that increasing ER stress pharmacologically sensitized GBM cells to radiotherapy [70]. Although the pharmacological induction of ER stress has yet to be investigated in combination with radiation therapy in pancreatic cancer, activating the CHOP-mediated apoptosis pathway through ER stress-inducing agents may potentially sensitize pancreatic cancer tumors to radiation and improve response rates.
11. Conclusions
Given the heterogeneity of pancreatic cancer in terms of mutations, stressors, and tumor microenvironment by the time of detection, identifying a common context of vulnerability expressed across a wide array of cases could be an attractive approach to broadly targeting this aggressive malignancy. Several preclinical and clinical investigations over the past decade have presented evidence of both intrinsic and extrinsic stressors in the tumor biology of pancreatic cancer, contributing to increased ER stress and the activation of the unfolded protein response. Although UPR activation canonically serves to enable tumors to proliferate under mild to moderate ER stress, we hypothesize that increasing the load and prolonging ER stress may be an Achilles’ heel in this adaptive mechanism to stress cells to induce apoptotic cell death. Here, we present several therapeutics with the potential to increase ER stress and critically enhance the effects of currently used chemotherapies. Further preclinical models and clinical phase investigations are warranted to better characterize the safety and efficacy of these therapeutics and offer insight into the potential anti-tumor effects of ER stress inducers. In particular, the downstream effectors of apoptosis need to be well understood, along with the stress conditions that potentiate their activation. This information will lead to more refined therapeutics. Further clinical-stage research will also examine the toxicities in patients associated with modulating ER stress, which will be critical information for developing therapeutics that exploit the UPR pathway.
Acknowledgments
The authors would like to acknowledge the Purple Pansies foundation for their inspiration and support toward novel laboratory discoveries and the enduring fight against pancreas cancer.
Author Contributions
Conceptualization: G.B., R.M.M., P.L.S.U.J., G.K., H.H. and D.D.V.H.; Methodology: G.B., R.M.M., P.L.S.U.J., G.K., H.H. and D.D.V.H.; Software: G.B., R.M.M., P.L.S.U.J., G.K., H.H. and D.D.V.H.; Formal analysis: G.B., R.M.M., P.L.S.U.J., G.K., H.H. and D.D.V.H.; Investigation: G.B., R.M.M., P.L.S.U.J., G.K., H.H. and D.D.V.H.; Resources: G.B., R.M.M., P.L.S.U.J., G.K., H.H. and D.D.V.H.; Data curation: G.B., R.M.M., P.L.S.U.J., G.K., H.H. and D.D.V.H.; Writing—Original Draft Preparation: G.B., R.M.M., P.L.S.U.J., G.K., H.H. and D.D.V.H.; Writing—Review and Editing: G.B., R.M.M., P.L.S.U.J., G.K., H.H. and D.D.V.H.; Funding acquisition: G.B., R.M.M., P.L.S.U.J., G.K., H.H. and D.D.V.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.American Cancer Society Facts & Figures 2022. American Cancer Society. Atlanta, Ga. 2022. [(accessed on 10 October 2022)]. Available online: https://www.cancer.org/cancer/pancreatic-cancer/about/key-statistics.html.
- 2.Sarantis P., Koustas E., Papadimitropoulou A., Papavassiliou A.G., Karamouzis M.V. Pancreatic ductal adenocarcinoma: Treatment hurdles, tumor microenvironment and immunotherapy. World J. Gastrointest. Oncol. 2020;12:173–181. doi: 10.4251/wjgo.v12.i2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uson P.L.S., Jr., Samadder N.J., Riegert-Johnson D., Boardman L., Borad M.J., Ahn D., Sonbol M.B., Faigel D.O., Fukami N., Pannala R., et al. Clinical Impact of Pathogenic Germline Variants in Pancreatic Cancer: Results from a Multicenter, Prospective, Universal Genetic Testing Study. Clin. Transl. Gastroenterol. 2021;12:e00414. doi: 10.14309/ctg.0000000000000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Golan T., Hammel P., Reni M., Van Cutsem E., Macarulla T., Hall M.J., Park J.-O., Hochhauser D., Arnold D., Oh D.-Y., et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N. Engl. J. Med. 2019;381:317–327. doi: 10.1056/NEJMoa1903387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Reilly E.M., Lee J.W., Zalupski M., Capanu M., Park J., Golan T., Tahover E., Lowery M.A., Chou J.F., Sahai V., et al. Randomized, Multicenter, Phase II Trial of Gemcitabine and Cisplatin with or Without Veliparib in Patients With Pancreas Adenocarcinoma and a Germline BRCA/PALB2 Mutation. J. Clin. Oncol. 2020;38:1378–1388. doi: 10.1200/JCO.19.02931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leidner R., Silva N.S., Huang H., Sprott D., Zheng C., Shih Y.-P., Leung A., Payne R., Sutcliffe K., Cramer J., et al. Neoantigen T-Cell Receptor Gene Therapy in Pancreatic Cancer. N. Engl. J. Med. 2022;386:2112–2119. doi: 10.1056/NEJMoa2119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson C.M., Talty A., Logue S.E., Mnich K., Gorman A.M., Samali A. An Emerging Role for the Unfolded Protein Response in Pancreatic Cancer. Cancers. 2021;13:261. doi: 10.3390/cancers13020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold F., Gout J., Wiese H., Weissinger S.E., Roger E., Perkhofer L., Walter K., Scheible J., Bozzo C.P., Lechel A., et al. RINT1 Regulates SUMOylation and the DNA Damage Response to Preserve Cellular Homeostasis in Pancreatic Cancer. Cancer Res. 2021;81:1758–1774. doi: 10.1158/0008-5472.CAN-20-2633. [DOI] [PubMed] [Google Scholar]
- 9.Dong L., Khoonkari M., Avril T., Chevet E., Kruyt F.A.E. The unfolded protein response as regulator of cancer stemness and differentiation: Mechanisms and implications for cancer therapy. Biochem. Pharmacol. 2021;192:114737. doi: 10.1016/j.bcp.2021.114737. [DOI] [PubMed] [Google Scholar]
- 10.Shah V.M., Sheppard B.C., Sears R.C., Alani A.W.G. Hypoxia: Friend or Foe for drug delivery in Pancreatic Cancer. Cancer Lett. 2020;492:63–70. doi: 10.1016/j.canlet.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keener J., Sneyd J. Cellular Homeostasis. In: Keener J., Sneyd J., editors. Mathematical Physiology. Interdisciplinary Applied Mathematics. Volume 8/1. Springer; New York, NY, USA: 2009. [DOI] [Google Scholar]
- 12.Liput M., Magliaro C., Kuczynska Z., Zayat V., Ahluwalia A., Buzanska L. Tools and approaches for analyzing the role of mitochondria in health, development and disease using human cerebral organoids. Dev. Neurobiol. 2021;81:591–607. doi: 10.1002/dneu.22818. [DOI] [PubMed] [Google Scholar]
- 13.Osellame L.D., Blacker T.S., Duchen M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012;26:711–723. doi: 10.1016/j.beem.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konstantinos P., Lionaki E., Tavernarakis N. Mechanisms of mitophagy in cellular homeostasis, physiology and pathology. Nat. Cell Biol. 2018;20:1013–1022. doi: 10.1038/s41556-018-0176-2. [DOI] [PubMed] [Google Scholar]
- 15.Hoseki J., Ushioda R., Nagata K. Mechanism and components of endoplasmic reticulum-associated degradation. J. Biochem. 2010;147:19–25. doi: 10.1093/jb/mvp194. [DOI] [PubMed] [Google Scholar]
- 16.Ellgaard L., Helenius A. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 2003;4:181191. doi: 10.1038/nrm1052. [DOI] [PubMed] [Google Scholar]
- 17.Malhotra J.D., Kaufman R.J. Seminars in Cell & Developmental Biology. Volume 18. Academic Press; Cambridge, MA, USA: 2007. The endoplasmic reticulum and the unfolded protein response. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian X., Zhang S., Zhou L., Seyhan A.A., Borrero L.H., Zhang Y., El-Deiry W.S. Targeting the integrated stress response in cancer therapy. Front. Pharmacol. 2021;12:747837. doi: 10.3389/fphar.2021.747837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bertolotti A., Zhang Y., Hendershot L.M., Harding H.P., Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 20.Shen J., Chen X., Hendershot L., Prywes R. ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev. Cell. 2002;3:99–111. doi: 10.1016/S1534-5807(02)00203-4. [DOI] [PubMed] [Google Scholar]
- 21.Walter P., Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 22.Han J., Kaufman R.J. Physiological/pathological ramifications of transcription factors in the unfolded protein response. Genes. 2017;31:1417–1438. doi: 10.1101/gad.297374.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pattabiraman D.R., Weinberg R.A. Tackling the cancer stem cells—What challenges do they pose? Nat. Rev. Drug Discov. 2014;13:497–512. doi: 10.1038/nrd4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caras I.W. Two cancer stem cell-targeted therapies in clinical trials as viewed from the standpoint of the cancer stem cell model. Stem Cells Transl. Med. 2020;9:821–826. doi: 10.1002/sctm.19-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saito A., Imaizumi K. Unfolded Protein Response-Dependent Communication and Contact among Endoplasmic Reticulum, Mitochondria, and Plasma Membrane. Int. J. Mol. Sci. 2018;19:3215. doi: 10.3390/ijms19103215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frakes A.E., Dillin A. The UPR(ER): Sensor and Coordinator of Organismal Homeostasis. Mol. Cell. 2017;66:761–771. doi: 10.1016/j.molcel.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 27.Wang M., Kaufman R.J. Protein misfolding in the endoplasmic reticulum as a conduit to human disease. Nature. 2016;529:326–335. doi: 10.1038/nature17041. [DOI] [PubMed] [Google Scholar]
- 28.Ma Y., Hendershot L.M. The role of the unfolded protein response in tumour development: Friend or foe? Nat. Rev. Cancer. 2004;4:966–977. doi: 10.1038/nrc1505. [DOI] [PubMed] [Google Scholar]
- 29.Chevet E., Hetz C., Samali A. Endoplasmic reticulum stress-activated cell reprogramming in oncogenesis. Cancer Discov. 2015;5:586–597. doi: 10.1158/2159-8290.CD-14-1490. [DOI] [PubMed] [Google Scholar]
- 30.Siwecka N., Rozpędek W., Pytel D., Wawrzynkiewicz A., Dziki A., Diehl J.A., Majsterek I. Dual role of endoplasmic reticulum stress-mediated unfolded protein response signaling pathway in carcinogenesis. Int. J. Mol. Sci. 2019;20:4354. doi: 10.3390/ijms20184354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urra H., Dufey E., Avril T., Chevet E., Hetz C. Endoplasmic reticulum stress and the hallmarks of cancer. Trends Cancer. 2016;2:252–262. doi: 10.1016/j.trecan.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 32.Holtrup F., Bauer A., Fellenberg K., Hilger R.A., Wink M., Hoheisel J.D. Microarray analysis of nemorosone-induced cytotoxic effects on pancreatic cancer cells reveals activation of the unfolded protein response (UPR) Br. J. Pharmacol. 2011;162:1045–1059. doi: 10.1111/j.1476-5381.2010.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowenfels A.B., Maisonneuve P., Cavallini G., Ammann R.W., Lankisch P.G., Andersen J.R., DiMagno E.P., Andren-Sandberg A., Domellof L. Pancreatitis and the Risk of Pancreatic Cancer. N. Engl. J. Med. 1993;328:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 34.Kirkegård J., Mortensen F.V., Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am. J. Gastroenterol. 2017;112:1366–1372. doi: 10.1038/ajg.2017.218. [DOI] [PubMed] [Google Scholar]
- 35.Raimondi S., Lowenfels A.B., Morselli-Labate A.M., Maisonneuve P., Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract. Res. Clin. Gastroenterol. 2010;24:349–358. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Tong G.X., Geng Q.Q., Chai J., Cheng J., Chen P.L., Liang H., Shen X.R., Wang D.B. Association between pancreatitis and subsequent risk of pancreatic cancer: A systematic review of epidemiological studies. Asian Pac. J. Cancer Prev. 2014;15:5029–5034. doi: 10.7314/APJCP.2014.15.12.5029. [DOI] [PubMed] [Google Scholar]
- 37.Umans D.S., Hoogenboom S.A., Sissingh N.J., Lekkerkerker S.J., Verdonk R.C., van Hooft J.E. Pancreatitis and pancreatic cancer: A case of the chicken or the egg. World J. Gastroenterol. 2021;27:3148–3157. doi: 10.3748/wjg.v27.i23.3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L., Xie D., Wei D. Pancreatic Acinar-to-Ductal Metaplasia and Pancreatic Cancer. Methods Mol. Biol. 2019;1882:299–308. doi: 10.1007/978-1-4939-8879-2_26. [DOI] [PubMed] [Google Scholar]
- 39.Hingorani S.R., Petricoin E.F., Maitra A., Rajapakse V., King C., Jacobetz M.A., Ross S., Conrads T.P., Veenstra T.D., Hitt B.A., et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/S1535-6108(03)00309-X. [DOI] [PubMed] [Google Scholar]
- 40.Carrière C., Young A.L., Gunn J.R., Longnecker D.S., Korc M. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem. Biophys. Res. Commun. 2009;382:561–565. doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kong B., Cheg T., Wu W., Regel I., Raulefs R., Friess H., Erkan M., Esposito I., Kleeff J., Michalski C.W. Hypoxia-induced endoplasmic reticulum stress characterizes a necrotic phenotype of pancreatic cancer. Oncotarget. 2015;6:32154. doi: 10.18632/oncotarget.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu G., Luo H., Zhu X. Targeting the GRP78 pathway for cancer therapy. Front. Med. 2020;7:351. doi: 10.3389/fmed.2020.00351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niu Z., Wang M., Zhou L., Yao L., Liao Q., Zhao Y. Elevated GRP78 expression is associated with poor prognosis in patients with pancreatic cancer. Sci. Rep. 2015;5:16067. doi: 10.1038/srep16067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dauer P., Sharma N.S., Gupta V.K., Durden B., Hadad R., Banerjee S., Dudeja V., Saluja A., Banerjee S. ER stress sensor, glucose regulatory protein 78 (GRP78) regulates redox status in pancreatic cancer thereby maintaining “stemness”. Cell Death Dis. 2019;10:132. doi: 10.1038/s41419-019-1408-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen J., Ha D.P., Zhu G., Rangel D.F., Kobielak A., Gill P.S., Groshen S., Dubeau L., Lee A.S. GRP78 haploinsufficiency suppresses acinar-to-ductal metaplasia, signaling, and mutant Kras-driven pancreatic tumorigenesis in mice. Proc. Natl. Acad. Sci. USA. 2017;114:E4020–E4029. doi: 10.1073/pnas.1616060114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gifford J.B., Huang W., Zeleniak A.E., Hindoyan A., Wu H., Donahue T.R., Hill R. Expression of GRP78, Master Regulator of the Unfolded Protein Response, Increases Chemoresistance in Pan-creatic Ductal Adenocarcinoma. Mol. Cancer Ther. 2016;15:1043–1052. doi: 10.1158/1535-7163.MCT-15-0774. [DOI] [PubMed] [Google Scholar]
- 47.Chien W., Ding L.-W., Sun Q.-Y., Torres-Fernandez L.A., Tan S.Z., Xiao J., Lim S.L., Garg M., Lee K.L., Kitajima S., et al. Selective inhibition of unfolded protein response induces apoptosis in pancreatic cancer cells. Oncotarget. 2014;5:4881. doi: 10.18632/oncotarget.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thakur P.C., Miller-Ocuin J.L., Kguyen K., Matsuda R., Singhi Ad Zeh H.J., Bahary N. Inhibition of endoplasmic-reticulum-stress-mediated autophagy enhances the effectiveness of chemotherapeutics on pancreatic cancer. J. Transl. Med. 2018;16:190. doi: 10.1186/s12967-018-1562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Atkins C., Liu Q., Minthorn E., Zhang S.-Y., Figueroa D.J., Moss K., Stanley T.B., Sanders B., Goetz A., Gaul N., et al. Characterization of a novel PERK kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2013;73:1993–2002. doi: 10.1158/0008-5472.CAN-12-3109. [DOI] [PubMed] [Google Scholar]
- 50.Terrab L., Wipf P. Hsp70 and the unfolded protein response as a challenging drug target and an inspiration for probe molecule development. ACS Med. Chem. Lett. 2020;11:232–236. doi: 10.1021/acsmedchemlett.9b00583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhao Q., Zhong J., Bi Y., Liu Y., Liu Y., Guo J., Pan L., Tan Y., Yu X. Gambogenic acid induces Noxa-mediated apoptosis in colorectal cancer through ROS-dependent activation of IRE1α/JNK. Phytomedicine. 2020;78:153306. doi: 10.1016/j.phymed.2020.153306. [DOI] [PubMed] [Google Scholar]
- 52.Liu C., Xu J., Guo C., Chen X., Qian C., Zhang X., Zhou P., Yang Y. Gambogenic Acid Induces Endoplasmic Reticulum Stress in Colorectal Cancer via the Aurora A Pathway. Front. Cell Dev. Biol. 2021;9:736350. doi: 10.3389/fcell.2021.736350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su J., Xu T., Jiang G., Hou M., Liang M., Cheng H., Li Q. Gambogenic acid triggers apoptosis in human nasopharyngeal carcinoma CNE-2Z cells by activating volume-sensitive outwardly rectifying chloride channel. Fitoterapia. 2019;133:150–158. doi: 10.1016/j.fitote.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Cheng A.-L., Kang Y.-K., He A.R., Lim H.Y., Ryoo B.-Y., Hung C.-H., Sheen I.-S., Izumi N., Austin T., Wang Q., et al. Safety and efficacy of tigatuzumab plus sorafenib as first-line therapy in subjects with advanced hepatocellular carcinoma: A phase 2 randomized study. J. Hepatol. 2015;63:896–904. doi: 10.1016/j.jhep.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 55.Forero-Torres A., Shah J., Wood T., Posey J., Carlisle R., Copigneaux C., Luo F., Wojtowicz-Praga S., Percent I., Saleh M. Phase I trial of weekly tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5) Cancer Biother. Radiopharm. 2010;25:13–19. doi: 10.1089/cbr.2009.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeRosier L.C., Vickers S.M., Zinn K.R., Huang Z., Wang W., Grizzle W.E., Sellers J., Stockard C.R., Zhou T., Oliver P.G., et al. TRA-8 anti-DR5 monoclonal antibody and gemcitabine induce apoptosis and inhibit radiologically validated orthotopic pancreatic tumor growth. Mol. Cancer Ther. 2007;6:3198–3207. doi: 10.1158/1535-7163.MCT-07-0299. [DOI] [PubMed] [Google Scholar]
- 57.Forero-Torres A., Infante J.R., Waterhouse D., Wong L., Vickers S., Arrowsmith E., He A.R., Hart L., Trent D., Wade J., et al. Phase 2, multicenter, open-label study of tigatuzumab (CS-1008), a humanized monoclonal antibody targeting death receptor 5, in combination with gemcitabine in chemotherapy-naive patients with unresectable or metastatic pancreatic cancer. Cancer Med. 2013;2:925–932. doi: 10.1002/cam4.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forero-Torres A., Varley K.E., Abramson V.G., Li Y., Vaklavas C., Lin N.U., Liu M.C., Rugo H.S., Nanda R., Storniolo A.M., et al. Translational Breast Cancer Research Consortium (TBCRC). TBCRC 019: A Phase II Trial of Nanoparticle Albumin-Bound Paclitaxel with or without the Anti-Death Receptor 5 Monoclonal Antibody Tigatuzumab in Patients with Triple-Negative Breast Cancer. Clin. Cancer Res. 2015;21:2722–2729. doi: 10.1158/1078-0432.CCR-14-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mujumdar N., Banerjee S., Chen Z., Sangwan V., Chugh R., Dudeja V., Yamamoto M., Vickers S.M., Saluja A.K. Triptolide activates unfolded protein response leading to chronic ER stress in pancreatic cancer cells. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;306:G1011–G1020. doi: 10.1152/ajpgi.00466.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greeno E., Borazanci E., Gockerman J., Korn R., Saluja A., Von Hoff D. Phase I dose escalation and pharmokinetic study of 14-O phosphonooxymethyltriptolide. In Proceedings of the 106th Annual Meeting of the American Association for Cancer Research, 18–22 April 2015; Philadelphia, P.A., Ed.; AACR. Cancer Res. 2015;75((Suppl. S15)):nr CT207. doi: 10.1158/1538-7445.AM2015-CT207. [DOI] [Google Scholar]
- 61.Neuditschko B., Legin A.A., Baier D., Schintlmeinster A., Reipert S., Wagner M., Keppler K.K., Berger W., Meier-Menches S.M., Gerner C. Interaction with Ribosomal Proteins Accompanies Stress Induction of the Anticancer Metallodrug BOLD-100/KP1339 in the Endoplasmic Reticulum. Angew. Chem. Int. Ed. Engl. 2021;60:5063–5068. doi: 10.1002/anie.202015962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Flocke L.S., Trondl R., Jakupec M.A., Keppler B.K. Molecular mode of action of NKP-1339—A clinically investigated ruthenium-based drug—Involves ER- and ROS-related effects in colon carcinoma cell lines. Investig. New Drugs. 2016;34:261–268. doi: 10.1007/s10637-016-0337-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burris H.A., Bakewell S., Bendell J.C., Infante J., Jones S.F., Spigel D.R., Weiss G.J., Ramanathan R.K., Ogden A., Von Hoff D. Safety and activity of IT-139, a ruthenium-based compound, in patients with advanced solid tumours: A first-in-human, open-label, dose-escalation phase I study with expansion cohort. ESMO Open. 2017;1:e000154. doi: 10.1136/esmoopen-2016-000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ekinci E., Rohondia S., Khan R., Dou Q.P. Repurposing Disulfiram as An Anti-Cancer Agent: Updated Review on Literature and Patents. Recent Pat. Anticancer. Drug Discov. 2019;14:113–132. doi: 10.2174/1574892814666190514104035. [DOI] [PubMed] [Google Scholar]
- 65.Cardenes H.R., Moore A.M., Johnson C.S., Yu M., Helft P., Chiorean E.G., Vinson J., Howard T.J., Stephens A.W., Tai D.F., et al. A phase II study of gemcitabine in combination with radiation therapy in patients with localized, unresectable, pancreatic cancer: A Hoosier Oncology Group study. Am. J. Clin. Oncol. 2011;34:460–465. doi: 10.1097/COC.0b013e3181e9c103. [DOI] [PubMed] [Google Scholar]
- 66.McGinn C.J., Zalupski M.M., Shureiqi I., Robertson J.M., Eckhauser F.E., Smith D., Brown D., Hejna G., Strawderman M., Normolle D., et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J. Clin. Oncol. 2001;19:4202–4208. doi: 10.1200/JCO.2001.19.22.4202. [DOI] [PubMed] [Google Scholar]
- 67.Murphy J.D., Adusumilli S., Griffith K.A., Ray M.E., Zalupski M.M., Lawrence T.S., Ben-Josef E. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:801–808. doi: 10.1016/j.ijrobp.2006.12.053. [DOI] [PubMed] [Google Scholar]
- 68.Wolff R.A., Evans D.B., Gravel D.M., Lenzi R., Pisters P.W., ELee J., Janjan N.A., Charnsangavej C., Abbruzzese J.L. Phase I trial of gemcitabine combined with radiation for the treatment of locally advanced pancreatic adenocarcinoma. Clin. Cancer Res. 2001;7:2246–2253. [PubMed] [Google Scholar]
- 69.Nguyen L., Dobiasch S., Schneider G., Schmid R.M., Azimzadeh O., Kanev K., Buschmann D., Pfaffl M.W., Bartzsch S., Schmid T.E., et al. Impact of DNA repair and reactive oxygen species levels on radioresistance in pancreatic cancer. Radiother. Oncol. 2021;159:265–276. doi: 10.1016/j.radonc.2021.03.038. [DOI] [PubMed] [Google Scholar]
- 70.Shah S.S., Rodriguez G.A., Musick A., Walters W.M., Cordoba N., Barbarite E., Marlow M., Marples B., Prince J., Komotar R., et al. Targeting Glioblastoma Stem Cells with 2-Deoxy-D-Glucose (2-DG) Potentiates Radiation-Induced Unfolded Protein Response (UPR) Cancers. 2019;11:159. doi: 10.3390/cancers11020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shah S.S., Rodriguez G.A., Musick A., Walters W.M., de Cordoba N., Barbarite E., Marlow M.M., Marples B., Prince J.S., Komotar R.J., et al. KRN5500, a spicamycin derivative, exerts anti-myeloma effects through impairing both myeloma cells and osteoclasts. Br. J. Haematol. 2011;155:328–339. doi: 10.1111/j.1365-2141.2011.08844.x. [DOI] [PubMed] [Google Scholar]
- 72.Supko J.G., Eder JPJr Ryan D.P., Seiden M.V., Lynch T.J., Amrein P.C., Kufe D.W., Clark J.W. Phase I clinical trial and pharmacokinetic study of the spicamycin analog KRN5500 administered as a 1-hour intravenous infusion for five consecutive days to patients with refractory solid tumors. Clin. Cancer Res. 2003;9:5178–5186. [PubMed] [Google Scholar]
- 73.Ashton T.M., McKenna W.G., Kunz-Schughart L.A., Higgins G.S. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin. Cancer Res. 2018;24:2482–2490. doi: 10.1158/1078-0432.CCR-17-3070. [DOI] [PubMed] [Google Scholar]
- 74.Guo Y., Hu B., Fu B., Zhu H. Atovaquone at clinically relevant concentration overcomes chemoresistance in ovarian cancer via inhibiting mitochondrial respiration. Pathol. Res. Pract. 2021;224:153529. doi: 10.1016/j.prp.2021.153529. [DOI] [PubMed] [Google Scholar]
- 75.Abdalbari F.H., Telleria C.M. The gold complex auranofin: New perspectives for cancer therapy. Discov. Oncol. 2021;12:42. doi: 10.1007/s12672-021-00439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li H., Hu J., Wu S., Wang L., Cao X., Zhang X., Dai B., Cao M., Shao R., Zhang R., et al. Auranofin-mediated inhibition of PI3K/AKT/mTOR axis and anticancer activity in non-small cell lung cancer cells. Oncotarget. 2016;7:3548–3558. doi: 10.18632/oncotarget.6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.