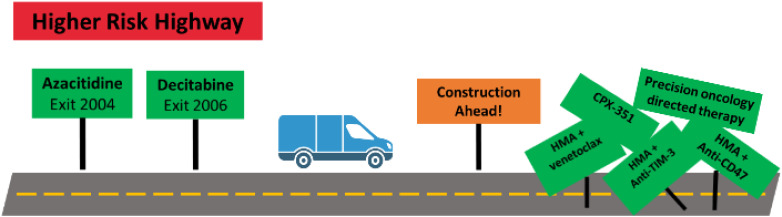
Visual Abstract
Abstract
Myelodysplastic syndromes (MDS) are typically a hematologic malignancy of older adults characterized by dysplastic hematopoiesis, cytopenia(s), and risk of acute myeloid leukemia transformation. The treatment approach to MDS depends largely on risk stratification of an individual's disease, most commonly using the Revised International Prognostic Scoring System, which takes into account peripheral blood cytopenias and bone marrow blast percentage and cytogenetics. The current standard of care for patients with higher-risk MDS (HR-MDS) includes hypomethylating agents (HMAs), decitabine and azacitidine, and allogenic stem cell transplant for patients able to undergo this therapy. However, leukemic transformation remains a significant challenge, and outcomes with these current therapies are still dismal. There are several novel therapies in development aiming to improve upon the outcomes of single-agent HMA therapy using combination strategies with HMAs. Here we discuss the current standard of care for HR-MDS treatment and explore some of the most promising combination therapies coming out of the pipeline for HR-MDS.
Learning Objectives
Learn about novel therapies for higher-risk myelodysplastic syndromes (MDS) that are currently in development
Understand the role of allogenic hematopoietic stem cell transplant for higher-risk MDS
Background
Myelodysplastic syndromes (MDS) are clonal myeloid malignancies of older adults in whom dysplastic hematopoiesis results in cytopenia(s) and carries the risk of developing acute myeloid leukemia (AML) (Figure 1).1,2 The treatment approach to MDS depends largely on risk stratification of an individual's disease, most commonly using the Revised International Prognostic Scoring System (IPSS-R), which takes into account peripheral blood cytopenias and bone marrow blast percentage and cytogenetics.2 The IPSS-R separates MDS into 5 risk categories (very low, low, intermediate, high, very high) with median survival and risk of developing AML worsening from very low-risk to very high-risk disease. The current standard of care for patients with lower-risk MDS (IPSS-R very low-risk, low-risk, and sometimes intermediate-risk groups) is typically supportive care and disease-modifying treatments to decrease transfusion burden, such as immunosuppression for MDS with hypoplastic features, lenalidomide for MDS with del(5q), and the recently approved luspatercept for MDS with ring sideroblasts.3 More aggressive therapy with hypomethylating agents (HMAs, decitabine and azacitidine) and allogenic stem cell transplant is standard of care for higher-risk MDS (HR-MDS), with the primary goal to prevent progression to AML.4 At times, it is appropriate to treat lower-risk MDS with HMAs as well, but this is beyond the scope of this discussion.
Figure 1.
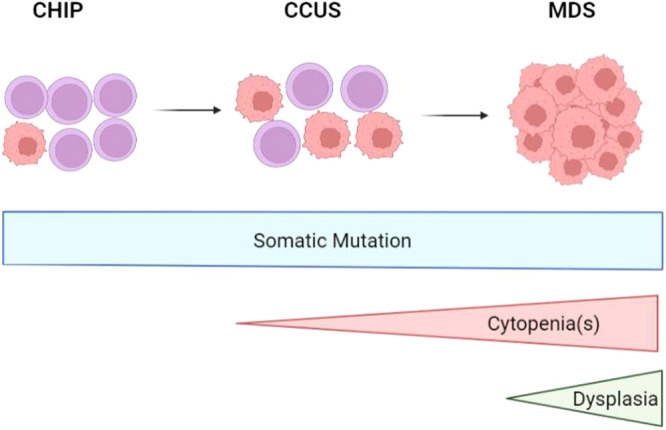
Disease progression from CHIP to CCUS to MDS as somatic mutations, cytopenias, and dysplasia develops. CCUS, clonal cytopenia of undetermined significance; CHIP, clonal hematopoiesis of indeterminate potential. Created in biorender.com.
Of importance, molecular data are not incorporated into the IPSS-R, and it is known that certain mutations portend better or worse prognoses than the IPSS-R would predict.5 Therefore, the Molecular International Prognostic Scoring System (IPSS-M) was developed to incorporate gene mutations into the MDS prognostic scoring system.6 The IPSS-M was created as a continuous index, defined as a weighted sum of prognostic variables consisting of (1) hemoglobin, platelets, and bone marrow blasts; (2) IPSS-R cytogenetic category; (3) 22 binary features derived from the presence of mutations in 21 predictive genes; and (4) 1 feature representing the number of mutations from a group of 17 additional genes.6 This approach grouped 2957 patients into the following risk categories: very low, low, moderate (moderate low and moderate high), high, and very high. When compared with the IPSS-R, the IPSS-M performed better at categorizing patients in terms of leukemia-free survival, leukemic transformation, and overall survival (OS). Finally, 46% (1246) of patients were restratified from the IPSS-R to a different risk category in the IPSS-M; 74% (926 patients) moved from a lower- to a higher-risk group and 26% (320 patients) moved from a higher- to a lower-risk group.
CLINICAL CASE
A 67-year-old woman with osteoarthritis presented with fatigue and was found to have normocytic anemia (hemoglobin , 7.5 g/dL) and thrombocytopenia (platelets, 32 000/µL) with a normal white blood cell and absolute neutrophil count (4.46 × K/µL and 2.46 × K/µL, respectively). A bone marrow biopsy specimen showed a hypercellular (90%) marrow with 12% myeloid blasts, >15% ring sideroblasts, and >50% dysplasia in granulopoiesis and megakaryopoiesis, consistent with a diagnosis of MDS with excess blasts 2. Fluorescence in situ hybridization was positive for a del5q32 abnormality (unfortunately, karyotype was not done) and molecular testing revealed a deleterious TP53 mutation with a variant allele frequency of 81.5%. Her IPSS-R score at the time of MDS diagnosis was 6.5, putting her in the very high-risk category.
Current therapies for higher-risk MDS
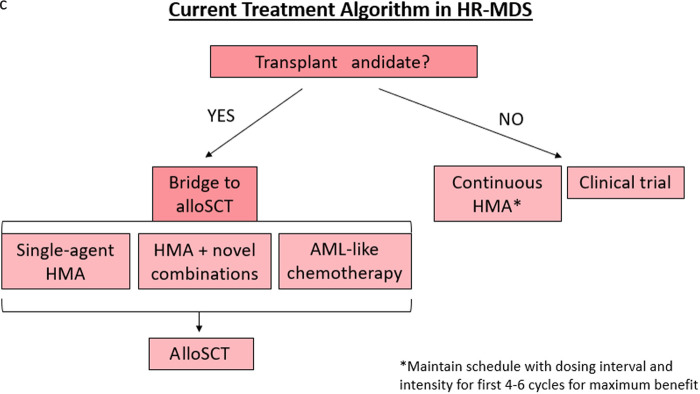
The current treatment algorithm for HR-MDS is depicted in Figure 2. The HMAs azacitidine and decitabine are the only therapies approved by the US Food and Drug Administration (FDA) for MDS (approved in 2004 and 2006, respectively), typically deployed when a patient is at least in the intermediate-risk IPSS-R category and/or has excess bone marrow blasts (>5%).7,8 While the mechanisms of action of HMAs are complicated and numerous, the most well-described mechanism is inhibition of the enzyme DNA methyltransferase, which methylates DNA, leading to transcriptional suppression.4,9 DNA hypermethylation, and thus aberrant gene silencing, plays a key role in MDS disease progression and cell lineage differentiation arrest, but when this methylation is inhibited by HMAs, these genes are reexpressed appropriately.4,9 Furthermore, there is evidence that HMAs can also paradoxically reactivate oncogenes, can cause differentiation, and be directly cytotoxic.10
Figure 2.
Treatment algorithm for HR-MDS based on current FDA-approved regimens.
Leading to azacitidine's approval was the phase 3, randomized controlled trial AZA-001 that compared outcomes of 358 patients with HR-MDS treated with azacitidine (75 mg/m2/d for 7 days every 28 days) or 1 of 3 conventional care regimens (best supportive care only [transfusions, antibiotics, granulocyte colony stimulating factor for neutropenic infections], low-dose cytarabine [20 mg/m2/d for 14 days every 28 days], or conventional induction/consolidation).11 Azacitidine demonstrated increased OS compared with conventional care regimen, 24.4 months vs 15 months, respectively (P = .0001). In addition, there was a 74% OS improvement with azacitidine (hazard ratio [HR], 0.58; 95% confidence interval [CI], 0.43-0.77).
Unsuccessful attempts have been made to improve upon single-agent azacitidine by using this in combination with various other agents; however, while these were negative trials, they have provided valuable information to the field. The randomized phase 2 SWOG S1117 trial compared standard-of-care azacitidine with azacitidine in combination with either lenalidomide or vorinostat in 277 patients with HR-MDS.12 This trial showed an overall response rate (ORR) of 38% in the azacitidine group with no significant improvement in ORR in the combination groups. The randomized phase 3 PANTHER trial investigated single-agent azacitidine vs azacitidine plus pevonedistat in 324 patients with HR-MDS, 103 patients with AML with blasts 20% to 30%, and 27 patients with chronic monomyelocytic leukemia (CMML).13 There was no statistically significant difference in median OS between the single-agent azacitidine arm (16.8 months) and the combination arm (20.3 months) (P = .181) in the intention-to-treat population; similarly, in the HR-MDS subset (21.6 months vs 17.5 months [HR, 0.785; P = .092], respectively), AML subset, and CMML subset, no differences in OS were seen. However, in both treatment arms, median OS improved once patients received at least 6 cycles of therapy, highlighting the importance of patients remaining on HMA therapy, whether alone or in combination, as long as possible, and indeed, the PANTHER trial was designed to ensure dose reductions and premature therapy discontinuation occurred as little as possible.13 Finally, it should be noted that the longer OS seen with single-agent azacitidine in AZA-001 of 24.4 months has yet to be repeated in these subsequent studies; median OS with single-agent azacitidine was only 15 months in this S1117 study and 17.5 months in the phase 3 PANTHER trial.12,13
Decitabine was approved following the phase 3, randomized control trial in 170 patients with MDS receiving decitabine (15 mg/m2 intravenously [IV] every 8 hours for 3 days every 6 weeks; of note, this strays from modern dosing) or best supportive care (consisting of transfusions and/or hematopoietic growth factors).9 Complete response rate was 9% and hematologic improvement (HI) rate was 13% in the decitabine group compared with the 0% ORR in the supportive care group. However, there was only a trend toward improvement in time to AML transformation or survival in the decitabine group vs supportive care group (12.1 months vs 7.8 months, respectively; P = .16), but a significant improvement was seen in higher-risk IPSS groups (12.0 months vs 6.8 months; P = .03). Recently, an oral formation of decitabine (decitabine coupled with cedazuridine to facilitate its oral bioavailability [Inqovi; Taiho Oncology]) was FDA approved in 2020 based on the comparable systemic drug concentrations, pharmacodynamics, and safety with oral cedazuridine/decitabine compared with IV decitabine with comparable clinical efficacy.14,15
To further evaluate the effect of HMAs on outcomes of patients in various prognostic groups, Zeidan et al16 retrospectively evaluated 632 patients with HR-MDS to determine if various prognostic scoring systems could predict probability of response to HMA. This evaluation found that no matter the scoring system used to prognosticate MDS, it could be associated with OS, but no scoring system was able to predict response to HMA. Furthermore, patients with HR-MDS no matter the scoring system had a median OS of only 11 to 16 months.16
Finally, in higher-risk MDS, an allogenic stem cell transplant (alloSCT) is the only potential possibility to achieve a cure for this disease.17 Indeed, it is recommended that patients fit enough for alloSCT should pursue this treatment (determination of “fitness” is beyond the scope of this article).17 AlloSCT has been established as the standard of care for younger patients with MDS, but historically, this has not been the case for older adults with MDS.18 Therefore, 2 studies sought to evaluate the benefit of alloSCT in this population.18,19 BMT CTN 1102 assessed alloSCT with reduced intensity conditioning (RIC) in patients 50 to 75 years old with HR-MDS comparing patients with and without a suitable human leukocyte antigen (HLA)-matched donor.18 Of 384 total patients, 67.7% (260 patients) had a matched donor and 32.3% (124 patients) did not have a matched donor and instead received HMA and/or supportive care (indeed, a large percentage of these patients did not receive standard-of-care HMA). Patients with a suitable HLA-matched donor experienced improved outcomes, including increased OS (absolute improvement in 3-year OS was 21.3% [95% CI, 10.2-31.8; P = .0001]) and leukemia-free survival (absolute improvement in 3-year LFS was 15.2% [95% CI, 13.3-29.1; P = .003]). Interestingly, OS was significantly shorter in patients without a response to HMA prior to alloSCT (HR, 1.64; P = .0097).18 A second study, the VidazaAllo Study, compared continuous azacitidine in patients without a donor to 4 to 6 cycles of azacitidine followed by alloSCT with RIC in patients with a suitable HLA-matched donor.19 In total, 162 patients began azacitidine but only 108 patients (67%) proceeded to either alloSCT or continuous azacitidine, with most patients dropping out of the study due to disease progression or death mostly from infection. One-year transplant-related mortality was 19% (95% CI, 11%-28%; P = .0065). Patients in the alloSCT arm experienced improved relapse-free survival (13.6% in the alloSCT arm vs 0% in the azacitidine arm) and 3-year event-free survival (34% in the alloSCT arm [95% CI, 22%-47%] vs 0% in the azacitidine arm; P = .0001). However, there was no significant difference in 3-year OS between the 2 groups (50% in alloSCT arm [95% CI, 39%-61%] and 32% in the azacitidine arm [95% CI, 14%-52%]; P = .12). It should be noted that certain pathogenic mutations portend worse alloSCT outcomes in patients with MDS, importantly TP53 mutations and RAS mutations, in patients older than 40 years who received RIC.20
Currently, in our clinical practice, we advise fit patients with higher-risk MDS to pursue alloSCT and employ HMA therapy as a means to decrease bone marrow blast count to <5% (patients with <5% marrow blasts show better post-transplant outcomes than patients with higher blast percentages, even if they do not achieve a complete response [CR; <5% marrow blasts with normal maturation of all cell lines, persistent dysplasia will be noted, blood hemoglobin ≥11 g/dL, neutrophils ≥1.0x109/L, platelets ≥100x109/L, blasts 0%]) or improve other factors of the MDS disease state while the patient is being evaluated for alloSCT.21 It should be noted that optimal therapy to achieve blast count reduction in MDS prior to alloSCT is unknown, but HMAs have proven to have a tolerable safety profile. The advent of HMA and venetoclax combinations in MDS (while not yet FDA approved) begs the question of when to use HMA combinations in this setting, and while the best usage of this combination prior to transplant is still being investigated, we recommend to weigh risks and benefits and consider the side effect profile before adding venetoclax to an HMA regimen in this setting.
Clearly, these data demonstrate that there is significant room for improvement on the current therapies for MDS. Furthermore, at the time HMAs were approved, there was little understanding of how gene mutations affect the MDS disease course and outcomes of patients with MDS, making the current MDS landscape ripe for molecularly determined therapeutic advancements. Finally, continued investigation is needed to improve transplant optimization and outcomes in these patients.
Can we learn from the AML experience?
CLINICAL CASE (Continued)
Returning to our 67-year-old otherwise healthy woman with very high-risk MDS, given her HR-MDS and excellent performance status, we would aim to treat her with a more effective regimen than standard-of-care, single-agent HMA in order to decrease her disease burden to optimize her for alloSCT. In the following section, we summarize data from the AML experience as well as several promising combination therapies currently under investigation in late-stage clinical trials that would be options for this patient in the future.
CPX-351
CPX-351, liposomal cytarabine combined with daunorubicin, is currently FDA approved for adults with newly diagnosed AML with myelodysplasia-related changes or therapy-related AML, and given this, CPX-351 was investigated for use in high-risk MDS in 2 different prospective trials.22-24 First, a phase 2, multicenter Groupe Francophone des Myélodysplasies trial evaluated CPX-351 in 31 treatment-naive adult patients with HR-MDS >70 years old.23 All patients were initially considered for alloSCT with the goal of achieving blast clearance prior to transplant. CPX-351 induction (44 mg/m2 daunorubicin/100 mg/m2 days 1, 3, and 5) followed by at least 1 but up to 4 consolidation cycles aimed to achieve disease response prior to alloSCT. Twenty-three percent of patients achieved CR, 45% achieved marrow CR (mCR), 6% achieved HI of platelets plus neutrophils, and 89% of patients with bone marrow blasts >10% achieved <5% after induction. Finally, 22 patients went on to receive an alloSCT, with 5 alloSCTs still planned. Investigator choice, relapse, no available donor, and invasive fungal infection (each n = 1) were cited as reasons patients did not undergo transplantation. Five patients did not receive consolidation due to cytopenias, and 2 patients each did not undergo consolidation cycles due to cardiac toxicity and failure to achieve CR or partial response (PR; meets all CR criteria if normal before treatment except marrow blasts decreased by ≥50% over pretreatment but still >5%, cellularity/morphology not relevant). A second trial studied 2 different dose levels of CPX-351 in 19 HMA-naive adult patients with HR-MDS <70 years old; patients had to have >5% bone marrow blasts and be transplant eligible.22 At time of publication, dose escalation was complete and safety expansion was ongoing at dose level 2 (dose level 1: daunorubicin 29 mg/m2 and 65 mg/m2 cytarabine; dose level 2: daunorubicin 44 mg/m2 and 100 mg/m2 cytarabine). There have been no dose-limiting toxicities among these 19 patients. The overall response rate (CR + PR + HI), in 18 evaluable patients, was 38.9% with a CR in 4 patients, no PRs, and 3 patients with HI. Thirteen patients have been able to obtain an alloSCT thus far, and 4 are still candidates; for the 2 remaining patients, 1 patient had not yet completed induction at the time of publication and the other had progressed to AML. These studies have demonstrated feasibility of higher-intensity chemotherapy in high-risk, older patients with MDS as a potential option as a bridge to transplant if a suitable donor is identified.
Azacitidine in combination with venetoclax
Venetoclax was granted regular FDA approval in October 2020 in combination with HMA therapy (IV or subcutaneous only) or low-dose cytarabine for patients with newly diagnosed AML ≥75 years or unable to tolerate intensive chemotherapy. Venetoclax is an oral, potent, selective inhibitor of B-cell lymphoma 2, which is an antiapoptotic protein highly expressed in AML stem cells that rely on this protein for survival.25 Given the effectiveness of venetoclax combinations in AML, venetoclax in combination with azacitidine was investigated in HR-MDS, first in the phase 1b M15-522 trial in relapsed/refractory (r/r) disease and MDS-531 trial in previously untreated patients, as well as currently in the phase 3 VERONA trial in treatment-naive disease.26-29 Results from the phase 1b trials were encouraging; indeed, based on the results of the M15-531 trial, venetoclax with IV or subcutaneous azacitidine was granted Breakthrough Therapy Designation by the FDA.30 In 38 patients with r/r HR-MDS, the CR plus mCR (<5% bone marrow blasts but continued cytopenias), rate was 40% (3 and 12 patients, respectively), and 34% of patients achieved transfusion independence.26 Notably, past HMA therapy was allowed with study patients having received a median of 8 cycles of prior HMA. In the 78 patients with treatment-naive HR-MDS, results were even more impressive, as the CR and mCR rates were both 40%, and 46.5% of patients became transfusion independent.28,29 Fifty-one of these patients were treated at the recommended phase 2 dose of 14 days of 400 mg venetoclax. Interestingly, the median OS was 28 months in both groups of patients who did or did not achieve a CR.28 Furthermore, 23% of patients obtained an alloSCT following this treatment.
Given these data, this combination is currently being evaluated in the phase 3, randomized, double-blind VERONA study in adult patients with newly diagnosed HR-MDS with at least intermediate IPSS-R risk and without prior HMA exposure (standard azacitidine dosing with 14 days of 400 mg venetoclax, NCT04401748).27 These data demonstrate that IV or subcutaneous azacitidine in combination with venetoclax may be an effective therapy, especially for treatment-naive MDS. Indeed, given familiarity of use in AML, this combination has become a frequently used therapy for patients with HR-MDS eligible for an alloSCT but who are not eligible or willing to enroll in a clinical trial.
On the horizon for higher-risk MDS
Anti-CD47 antibodies
CD47, aka integrin-associated protein, is a cell surface protein and important immune checkpoint for macrophages, as CD47 expression prevents phagocytosis by macrophages.31,32 Notably, CD47 is upregulated in MDS and on leukemic stem cells, and this expression may be augmented by azacitidine.32 Therefore, CD47 inhibitors are currently in development for the treatment of MDS, the furthest along clinically being magrolimab (Hu5F9-G4), which is a monoclonal antibody targeting CD47 and inducing tumor phagocytosis.33
Magrolimab in combination with azacitidine was initially evaluated in a phase 1b clinical trial of 95 untreated patients with HR-MDS who had at least an intermediate IPSS-R score.34 Azacitidine was administered as described previously, and magrolimab was given in a priming/intrapatient dose escalation regimen (1-30 mg/kg weekly).34 ORR (CR + PR + HI) was 46.3%, with a 33% (31 patients) CR rate and 11% (10 patients) HI rate. Interestingly, this combination appears effective in TP53-mutated patients as 40% (10 of 15 patients) of TP53-mutated MDS patients achieved a CR and 8% (2 patients) achieved HI.34 Of note, 83% (5/6 patients) of patients with TP53-mutated AML treated with magrolimab + azacitidine achieved a CR or CR with incomplete count recovery.35 As TP53 portends a poor prognosis in myeloid malignancies, this observation highlights the importance of incorporating molecular data into the prognostic evaluation of patients with MDS. Side effects from this combination were comparable to azacitidine monotherapy; only 1 patient discontinued therapy due to an adverse event (AE), and on-target anemia was the most common AE at 37%.33 The AE profile did prompt the FDA to place magrolimab on a brief clinical hold from February to April 2022 likely due to the on-target effect of hemolytic anemia, as aging red blood cells express lower levels of CD47 but more prophagocytic signals.36 However, clinical trials have since resumed enrollment.37
Currently enrolling is the phase 3, randomized ENHANCE trial in adults with untreated HR-MDS, intermediate to very high risk per IPSS-R score, comparing magrolimab plus azacitidine to placebo plus azacitidine (NCT04313881). In ENHANCE, azacitidine is administered per local prescribing practices, and magrolimab/placebo is given IV with a 1-mg/kg priming dose, then intrapatient dose escalation up to 30 mg/kg through cycle 1, then 30 mg/kg weekly dosing during cycle 2, followed by 30 mg/kg every 2 weeks dosing cycle 3 onward.33 Results from this trial are eagerly anticipated.
Sabatolimab
Further investigation into immunotherapeutic approaches in myeloid malignancies have led to the development of sabatolimab (MBG453) in MDS.38,39 Sabatolimab inhibits tim-3, which plays a role in regulating immune responses in malignancy.40 Importantly, tim-3 is expressed only on immune cells and leukemic myeloid cells, but normal hematopoietic stem cells lack expression.39,40
A phase 1b study of sabatolimab (1-2 infusions/month) in combination with HMA (standard dosing) in 51 HMA-naive, high-risk, and very high-risk patients with MDS, per IPSS-R score, showed promising results (NCT03066648).39 ORR (CR + PR + HI) was 33.3% (17 patients), with a 19.6% CR rate (10 patients), a 3.9% PR rate (2 patients), and a 9.8% HI rate (5 patients). This combination showed a median duration of response of 16.1 months (95% CI, 6.7 to not estimable) and an estimated 12-month progression-free survival rate of 51.9%. Sabatolimab + HMA also appeared efficacious in the 14 patients with MDS with TP53 mutations who experienced a 71.4% (10 patients) ORR (note this value includes CR + PR + HI as well as marrow CR) with a median duration of response of 21.5 months (95% CI, 6.7 to not estimable). Furthermore, 24.5% of patients achieved disease improvement in order to undergo alloSCT, and these patients did not experience graft-vs-host disease more than expected with HMA therapy alone. No patients discontinued therapy due to an AE, and the toxicity profile was similar to HMA alone.
Given these promising results, the STIMULUS clinical trial program was developed to further evaluate the effectiveness of sabatolimab in MDS, CMML, and AML.41 The phase 2 randomized, double-blind, placebo-controlled STIMULUS-MDS1 study in patients with HR-MDS evaluating sabatolimab 400 mg or placebo every 2 weeks in combination with either decitabine (20 mg/m2 for 5 days) or azacitidine (75 mg/m2 for 7 days) has completed accrual, and results are eagerly anticipated (NCT03946670). Furthermore, the phase 3 randomized, double- blind, placebo-controlled STIMULUS-MDS2 trial of sabatolimab 800 mg or placebo every 4 weeks in combination with azacitidine for patients with HR-MDS or CMML type 2 has begun accrual (NCT04266301).
Precision oncology/biomarker-based therapies
Ivosidenib and enasidenib are isocitrate dehydrogenase 1 and 2 (IDH) inhibitors, respectively, FDA approved as a single agent for the treatment of AML.42,43 Given their efficacy in AML, these inhibitors are currently being studied for the treatment of MDS. The phase 2 Idiome study investigated single-agent ivosidenib in 32 patients with IDH1-mutated MDS (3 cohorts: high-risk disease r/r to HMA, first-line high-risk disease, low-risk after erythropoietin-stimulating agent failure) and found, in 26 evaluable patients, an ORR of 69% (18 patients), with 12 patients achieving CR (46%), 1 PR (3.8%), and 5 HI (19.2%).44 Median OS was 14 months. This study is still ongoing (NCT03503409). Single-agent enasidenib was studied in the phase 2 Ideal trial in 45 patients with IDH2-muated MDS (same cohorts as the Idiome trial above).45 In the 26 evaluable patients, ORR was 42% (11 patients), with 6 patients (55%) achieving CR, 2 PR (18%), and 3 HI (27.3%). Median OS was 17.3 months. Differentiation syndrome occurred in 7 patients across both trials; 1 patient died, and the rest recovered without sequalae.44,45
Another promising target in MDS is the retinoic acid receptor alpha (RARA).46 This novel target is currently being investigated in the phase 3 SELECT-MDS-1 trial of RARA agonist tamibarotene in combination with azacitidine, compared with placebo plus azacitidine, in patients with HR-MDS who have RARA overexpression (NCT04797780). Tamibarotene has shown efficacy with an expected safety profile in an earlier phase trial in newly diagnosed patients with AML with RARA overexpression.47
Summary
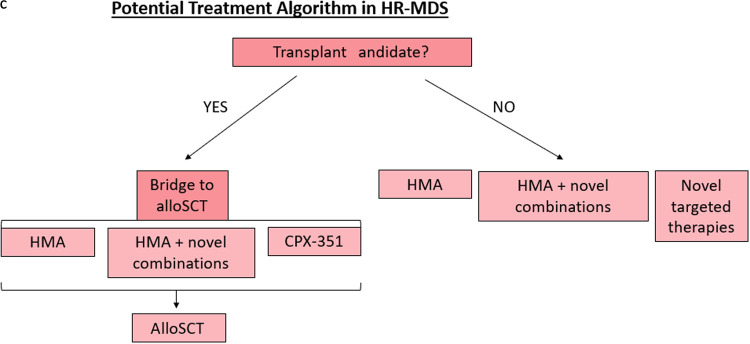
HR-MDS is a devastating malignancy that currently has a paucity of treatment options. However, there is now a growing armamentarium for MDS therapy with multiple promising combinations in late stages of development (Figure 3). Still, these investigational therapies, including CPX-351 for MDS, require further investigation with longer study follow-up, future randomized trials with larger sample sizes, survival correlation, and determination of response signals among disease subsets. As our understanding of the molecular pathogenesis of MDS improves and our treatment options expand, we anticipate providing effective therapies for our patients with HR-MDS that improve both quality of life and survival.
Figure 3.
Treatment algorithm for HR-MDS based on therapies under development.
Contributor Information
Kristin L. Koenig, The Ohio State University, Columbus, OH
Uma Borate, The Ohio State University, Columbus, OH.
Conflict-of-interest disclosure
Kristin L. Koenig has no competing financial interests to declare.
Uma Borate has provided consulting for Novartis, Abbvie, and Genentech and has research funding from Novartis, Abbvie, and Jazz.
Off-label drug use
Kristin L. Koenig: magrolimab, sabatolimab, ivosidenib, enasidenib, and tamibarotene are discussed.
Uma Borate: magrolimab, sabatolimab, ivosidenib, enasidenib, and tamibarotene are discussed.
References
- 1.Gu S, Xia J, Tian Y, Zi J, Ge Z. A novel scoring system integrating molecular abnormalities with IPSS-R can improve the risk stratification in patients with MDS. BMC Cancer. 2021;21(1):134. doi: 10.1186/s12885-021-07864-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg PL, Tuechler H, Schanz J, et al.. Revised International Prognostic Scoring System for myelodysplastic syndromes. Blood. 2012;120(12):2454-2465. doi: 10.1182/blood-2012-03-420489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carraway HE, Saygin C. Therapy for lower-risk MDS. Hematology Am Soc Hematol Educ Program. 2020;2020(1):426-433. doi: 10.1182/hematology.2020000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stomper J, Rotondo JC, Greve G, Lübbert M. Hypomethylating agents (HMA) for the treatment of acute myeloid leukemia and myelodysplastic syndromes: mechanisms of resistance and novel HMA-based therapies. Leukemia. 2021;35(7):1873-1889. doi: 10.1038/s41375-021-01218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bejar R, Elli P, Torsten H, et al.. Somatic mutations in MDS patients are associated with clinical features and predict prognosis independent of the IPSS-R: analysis of combined datasets from the International Working Group for Prognosis in MDS-Molecular Committee. Blood. 2015;126(23):907. doi: 10.1182/blood.V126.23.907.907 [DOI] [Google Scholar]
- 6.Bernard E, Tuechler H, Greenberg PL, et al.. Molecular international prognosis scoring system for myelodysplastic syndromes. NEJM Evid. 2022;1(7). [DOI] [PubMed] [Google Scholar]
- 7.Kaminskas E, Farrell AT, Wang Y-C, Sridhara R, Pazdur R. FDA drug approval summary: azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist. 2005;10(3):176-182. doi: 10.1634/theoncologist.10-3-176. [DOI] [PubMed] [Google Scholar]
- 8.Drug approval package: Dacogen (decitabine) NDA #021790. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2006/021790s000_dacogentoc.cfm. Accessed 18 May 2022.
- 9.Kantarjian H, Issa J-P, Rosenfeld C-S, et al.. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106(8):1794-1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y-C, Kwon J, Fabiani E, et al.. Demethylation and up-regulation of an oncogene after hypomethylating therapy. N Engl J Med. 2022;386(21):1998-2010. doi: 10.1056/NEJMoa2119771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenaux P, Mufti GJ, Santini V, et al.. Azacitidine (AZA) treatment prolongs overall survival (OS) in higher-risk MDS patients compared with conventional care regimens (CCR): results of the AZA-001 phase III study. Blood. 2007;110(11):817. doi: 10.1182/blood.V110.11.817.817. [DOI] [Google Scholar]
- 12.Sekeres MA, Othus M, List AF, et al.. Randomized phase II study of azacitidine alone or in combination with lenalidomide or with vorinostat in higher- risk myelodysplastic syndromes and chronic myelomonocytic leukemia: North American Intergroup Study SWOG S1117. J Clin Oncol. 2017;35(24): 2745-2753. doi: 10.1200/JCO.2015.66.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.No significant benefit for pevonedistat plus azacitidine in higher-risk myelodysplastic syndrome. The ASCO Post. https://ascopost.com/issues/february-25-2022/no-significant-benefit-for-pevonedistat-plus-azacitidine-in-higher-risk-myelodysplastic-syndrome/. Accessed 30 June 2022.
- 14.Garcia-Manero G, Griffiths EA, Steensma DP, et al.. Oral cedazuridine/decitabine for MDS and CMML: a phase 2 pharmacokinetic/pharmacodynamic randomized crossover study. Blood. 2020;136(6):674-683. doi: 10.1182/blood.2019004143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim N, Norsworthy KJ, Subramaniam S, et al.. FDA approval summary: decitabine and cedazuridine tablets for myelodysplastic syndromes. Clin Cancer Res. 2022;28(16):3411-3416. doi: 10.1158/1078-0432.CCR-21-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zeidan AM, Sekeres MA, Garcia-Manero G, et al; MDS Clinical Research Consortium. Comparison of risk stratification tools in predicting outcomes of patients with higher-risk myelodysplastic syndromes treated with azanucleosides. Leukemia. 2016;30(3):649-657. doi: 10.1038/leu.2015.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Witte T, Bowen D, Robin M, et al.. Allogeneic hematopoietic stem cell transplantation for MDS and CMML: recommendations from an international expert panel. Blood. 2017;129(13):1753-1762. doi: 10.1182/blood-2016-06-724500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakamura R, Saber W, Martens MJ, et al.. Biologic assignment trial of reduced-intensity hematopoietic cell transplantation based on donor availability in patients 50-75 years of age with advanced myelodysplastic syndrome. J Clin Oncol. 2021;39(30):3328-3339. doi: 10.1200/JCO.20.03380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kröger N, Sockel K, Wolschke C, et al.. Comparison between 5-azacytidine treatment and allogeneic stem-cell transplantation in elderly patients with advanced MDS according to donor availability (VidazaAllo Study). J Clin Oncol. 2021;39(30):3318-3327. doi: 10.1200/JCO.20.02724. [DOI] [PubMed] [Google Scholar]
- 20.Lindsley RC, Saber W, Mar BG, et al.. Prognostic mutations in myelodysplastic syndrome after stem-cell transplantation. N Engl J Med. 2017;376(6):536-547. doi: 10.1056/NEJMoa1611604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayer HG, Kröger M, Beyer J, et al; Cooperative German Transplant Study Group. Reduced intensity conditioning for allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia: disease status by marrow blasts is the strongest prognostic factor. Bone Marrow Transplant. 2003;31(12):1089-1095. doi: 10.1038/sj.bmt.1704062. [DOI] [PubMed] [Google Scholar]
- 22.Jacoby MA, Sallman DA, Scott BL, et al.. A pilot study of CPX-351 (Vyxeos ©) for transplant eligible, higher risk patients with myelodysplastic syndrome. Blood. 2021;138(suppl 1):540. doi: 10.1182/blood-2021-151137 [DOI] [Google Scholar]
- 23.Peterlin P, Turlure P, Chevallier P, et al.. CPX 351 as first line treatment in higher risk MDS: a phase II trial by the GFM. Blood. 2021;138(suppl 1): 243. [Google Scholar]
- 24.Food and Drug Administration. FDA approves first treatment for certain types of poor-prognosis acute myeloid leukemia. 2020. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-certain-types-poor-prognosis-acute-myeloid-leukemia. Accessed 24 May 2022.
- 25.DiNardo CD, Jonas BA, Pullarkat V, et al.. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383(7):617-629. doi: 10.1056/NEJMoa2012971. [DOI] [PubMed] [Google Scholar]
- 26.Zeidan AM, Pollyea DA, Garcia JS, et al.. A phase 1b study evaluating the safety and efficacy of venetoclax as monotherapy or in combination with azacitidine for the treatment of relapsed/refractory myelodysplastic syndrome. Blood. 2019; 134(suppl 1):565. [Google Scholar]
- 27.Zeidan AM, Garcia JS, Fenaux P, et al.. Phase 3 VERONA study of venetoclax with azacitidine to assess change in complete remission and overall survival in treatment-naïve higher-risk myelodysplastic syndromes. J Clin Oncol. 2021;39:TPS7054. doi: 10.1200/JCO.2021.39.15_suppl.TPS7054 [DOI] [Google Scholar]
- 28.Garcia-Manero G, Wei AH, Porkka K, et al.. Poster: mds-420: sabatolimab plus hypomethylating agents (HMAs) in patients with high-/very high-risk myelodysplastic syndrome (HR/vHR-MDS) and newly diagnosed acute myeloid leukemia (ND-AML): subgroup analysis of a phase 1 study. Clin Lymphoma Myeloma Leuk. 2021;21:s230. doi: 10.1016/s2152-2650(21)01447-6. [DOI] [Google Scholar]
- 29.Garcia JS, Wei AH, Jacoby MA, et al.. Molecular responses are observed across mutational spectrum in treatment-naïve higher-risk myelodysplastic syndrome patients treated with venetoclax plus azacitidine. Blood. 2021;138:241. [Google Scholar]
- 30.AbbVie News Center. Venetoclax (VENCLEXTA®) granted US FDA Breakthrough Therapy Designation (BTD) in higher risk myelodysplastic syndrome (MDS). https://news.abbvie.com/news/press-releases/venetoclax-venclexta-granted-us-fda-breakthrough-therapy-designation-btd-in-higher-risk-myelodysplastic-syndrome-mds.htm. Accessed 11 May 2022.
- 31.Eladl E, Tremblay-LeMay R, Rastgoo N, et al.. Role of CD47 in hematological malignancies. J Hematol Oncol. 2020;13(1):96. doi: 10.1186/s13045-020-00930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marple AH, DeZern AE. Please “eat me”: can magrolimab put higher-risk MDS back on the immunologic menu? Hematologist. 2021;18(4):15. doi: 10.1182/hem.V18.4.2021410. [DOI] [Google Scholar]
- 33.Garcia-Manero G, Daver NG, Xu J, et al.. Magrolimab + azacitidine versus azacitidine + placebo in untreated higher risk (HR) myelodysplastic syndrome (MDS): the phase 3, randomized, ENHANCE study. J Clin Oncol. 2021;39(suppl 1):TPS7055. doi: 10.1200/JCO.2021.39.15_suppl.TPS7055. [DOI] [Google Scholar]
- 34.Sallman DA, Al Malki MM, Steven Asch A, et al.. Magrolimab in combination with azacitidine for untreated higher-risk myelodysplastic syndromes (HR-MDS): 5F9005 phase 1b study results. J Clin Oncol. 2022;40(16, suppl): 7017-7017. doi: 10.1200/JCO.2022.40.16_suppl.7017 [DOI] [Google Scholar]
- 35.Sallman DA, Asch AS, Al Malki MM, et al.. The first-in-class anti-CD47 antibody magrolimab (5F9) in combination with azacitidine is effective in MDS AM and L patients: ongoing phase 1b results. Blood. 2019;134(suppl 1):569. [Google Scholar]
- 36.Safety concerns prompt pause of magrolimab trials. Cancer Discov. 2022;12(4):877-878. doi: 10.1158/2159-8290.CD-NB2022-0012. [DOI] [PubMed] [Google Scholar]
- 37.FDA lifts partial clinical hold on MDS AM. https://www.gilead.com/news-and-press/press-room/press-releases/2022/4/fda-lifts-partial-clinical-hold-on-mds-and-aml-magrolimab-studies. Accessed 11 May 2022.
- 38.Wei AH. Sabatolimab plus hypomethylating agents (HMAs) in patients (pts) with high-/very high-risk myelodysplastic syndrome (HR/VHR-MDS) and acute myeloid leukemia (AML): subgroup analysis of a phase 1 study. https://library.ehaweb.org/eha/2021/eha2021-virtual-congress/324576/andrew.h.wei.sabatolimab.plus.hypomethylating.agents.28hmas29.in.patients.28pts29.html?f=listing%3D3%2Abrowseby%3D8%2Asortby%3D1%2Amedia%3D1. Accessed 18 May 2022.
- 39.Brunner AM, Esteve J, Porkka K, et al.. Efficacy and safety of sabatolimab (MBG453) in combination with hypomethylating agents (HMAs) in patients (pts) with very high/high-risk myelodysplastic syndrome (vHR/HR-MDS) and acute myeloid leukemia (AML): final analysis from a phase Ib study. Blood. 2021;138(1):244. doi: 10.1182/blood-2021-146039 [DOI] [Google Scholar]
- 40.Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol. 2020;20(3):173-185. doi: 10.1038/s41577-019-0224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeidan AM, Esteve J, Giagounidis A, et al.. The STIMULUS program: clinical trials evaluating sabatolimab (MBG453) combination therapy in patients (pts) with higher-risk myelodysplastic syndromes (HR-MDS) or acute myeloid leukemia (AML). Blood. 2020;136(suppl 1)45-46. [Google Scholar]
- 42.Food and Drug Administration. FDA approves ivosidenib as first-line treatment for AML with IDH1 mutation. 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ivosidenib-first-line-treatment-aml-idh1-mutation. Accessed 20 December 2019.
- 43.Food and Drug Administration. FDA granted regular approval to enasidenib for the treatment of relapsed or refractory AML. 2019. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-granted-regular-approval-enasidenib-treatment-relapsed-or-refractory-aml. Accessed 26 May 2022.
- 44.Ades L, Dimicoli-Salazar S, Sebert M, et al.. Enasidenib (ENA) is effective in patients with IDH2 mutated myelodysplastic syndrome (MDS): the ideal phase 2 study by the GFM Group. Blood. 2021;138(suppl 1):63. doi: 10.1182/blood-2021-147898. [DOI] [Google Scholar]
- 45.Ades L, Dimicoli-Salazar S, Sebert M, et al.. Enasidenib (ENA) is effective in patients with IDH2 mutated myelodysplastic syndrome (MDS): the ideal phase 2 study by the GFM Group. Blood. 2021;138(suppl1):63. [Google Scholar]
- 46.McKeown MR, Johannessen L, Lee E, Fiore C, di Tomaso E. Antitumor synergy with SY-1425, a selective RARα agonist, and hypomethylating agents in retinoic acid receptor pathway activated models of acute myeloid leukemia. Haematologica. 2019;104(4):e138-e142. doi: 10.3324/haematol.2018.192807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Syros Pharmaceuticals, Inc. Tamibarotene for higher-risk MDS and AML: Syros Pharmaceuticals, Inc. (SYRS). https://www.syros.com/programs/tamibarotene. Accessed 26 May 2022.