Abstract
The subfamily-G ATP-binding cassette (ABCG) transporters play important roles in regulating cholesterol homeostasis. Recent progress in the structural data of ABCG1 and ABCG5/G8 disclose putative sterol binding sites that suggest the possible cholesterol translocation pathway. ABCG1 and ABCG5/G8 share high similarity in the overall molecular architecture, and both transporters appear to use several unique structural motifs to facilitate cholesterol transport along this pathway, including the phenylalanine highway and the hydrophobic valve. Interestingly, ABCG5/G8 is known to transport cholesterol and phytosterols, whereas ABCG1 seems to exclusively transport cholesterol. Ligand docking analysis indeed suggests a difference in recruiting sterol molecules to the known sterol-binding sites. Here, we further discuss how the different and shared structural features are relevant to their physiological functions, and finally provide our perspective on future studies in ABCG cholesterol transporters.
Keywords: ABCG1, ABCG5, ABCG8, structural motif, sterol trasportation, TICE, RCT
1. Introduction
ATP-binding cassette (ABC) transporters are ubiquitous in all living organisms, and play a crucial role in maintaining body homeostasis by functioning as pumps. They actively transport a variety of substrates across cell membranes utilizing ATP catalysis [1,2,3], the mechanism that is congruent with the ATPase function at their cytoplasmic nucleotide binding domain (NBD). The transmembrane domain (TMD) collaborates with the NBD to initiate the transport of substrates while anchoring the protein within the bilayer [3]. On the basis of similarities in the gene and amino acid sequences, as well as the structural topology of human ABC proteins, they are classified into seven subfamilies, ranging from ABCA to ABCG [4,5].
So far, mammalian ABCG proteins are uniquely known to efflux lipid-like compounds, such as cholesterol, and recent progress in ABCG structural data has underscored their importance in understanding the mechanism of ABC transporters [6]. Cholesterol is a key component of cell membranes and the precursor of bile acids, steroid hormones and vitamin D which are vital for body health [7]. Cholesterol can be obtained by de novo biosynthesis or via dietary uptakes in the guts. However, not all imported cholesterol is metabolized in the cells, and the excess amount of free cholesterol has to be cleared via two metabolic pathways, namely trans-intestinal cholesterol efflux (TICE) and reverse cholesterol transport (RCT) [8,9]. ABCG cholesterol transporters, i.e., ABCG1, ABCG4, ABCG5, and ABCG8 contribute to different stages of these removal processes [10]. Pathogenic mutations in these transporters have been known to be associated with various metabolic and cardiovascular diseases [11,12].
ABCG1 is a homodimer localized to the plasma membrane and late endosomes, functioning synergistically with ABCA1 to facilitate cholesterol efflux toward high-density lipoprotein (HDL) particles and thereby reducing total cholesterol in macrophages and peripheral tissues [13,14]. Alongside cholesterol, ABCG1 also transports phospholipids, though the specifics of the protein-ligand interactions are still poorly understood [15]. The malfunction of ABCG1 and ABCA1 results in Tangier disease, a severe form of familial HDL deficiency [16].
The ABCG5/G8 transporter is an obligatory heterodimer predominantly expressed on the apical surface of hepatocytes along the bile ducts and of enterocytes on the brush-boarder membranes [17]. ABCG5/G8 is the primary transporter in hepatobiliary and trans-intestinal cholesterol secretion. This protein was first described in the disease Sitosterolemia, a rare hereditary lipid storage disorder, characterized by the buildup of plant sterols (i.e., phytosterols) in plasma and tissues [18,19]. In addition to the phytosterols, sitosterolemic patients absorb a greater portion of dietary cholesterol, and they release less cholesterol into the bile. If lacking normal de novo cholesterol synthesis by HMG-CoA reductase activity, sitosterolemic patients can suffer much higher absorption of plant sterols through the intestine [11,20,21].
Due to their importance in regulating cholesterol homeostasis, ABCG sterol transporters have received a lot of attention in physiological studies and therapeutic development; yet, there remains a growing need to investigate their structure-function relationship to understand their molecular mechanism. Recent progress in the determination of ABCG1 and ABCG5/G8 structures has shed light on the biochemical and functional aspects of ABCG sterol proteins. In this article, we will highlight the available structural data of ABCG1 and ABCG5/G8 and the resolved cholesterol binding sites, and propose reasoning for the molecular mechanism applied by these transporters.
2. The General Structure of ABCG1 and ABCG5/G8
Site-directed mutagenesis studies and the crystal structure of ABCG5/G8, the first ABCG protein to have its structure identified, gave researchers a general notion of the structure and function of ABCG proteins [22,23,24]. The recent elucidation of other ABCG protein structures has provided more details regarding the organization of structural motifs and elements in these transporters [25,26]. The general structure of each ABCG half-transporter consists of a TMD, NBD, and an extracellular domain (ECD) [22]. Homo- or heterodimerization is required for these proteins to be expressed on the cell surface [27]. In a functional state, these proteins have six transmembrane helices (TMHs) in each half-transporter. TMH2 and 5 are central within the dimer interface, likely creating a pathway for substrate translocation. The distances between TMH1 and 2 as well as TMH3 and 4 form two loops, designated extracellular loop1 (ECL1) and ECL2, which are part of the ECD. Before coming to an end with TMH6, the C terminus of TMH5 forms a well-conserved re-entry helix which stretches to ECL3 [22,28]. The re-entry helices and ECLs contain conserved residues and form ECDs which may cooperate potentially in substrate release and direct binding of acceptor molecules [29,30]. The N-terminal portion of the TMH1 contains an orthogonal connecting helix (CnH), which is interfacial to the membrane bilayer and serves as the link between the TMD and the NBD. In this area, there is a coupling helix (CpH) formed by the loop between TMH2 and TMH3. Additionally, a helix with a conserved glutamate identifies the E-helix is present in close proximity to CnH. The CnH, CpH, and E-helix form a triple-helical bundle in this region, providing an immediate interface between NBD and TMD [22].
3. A Closer Look at the Structures of ABCG1 and ABCG5/G8
3.1. ECD: Hydrophobic Valve, ECLs
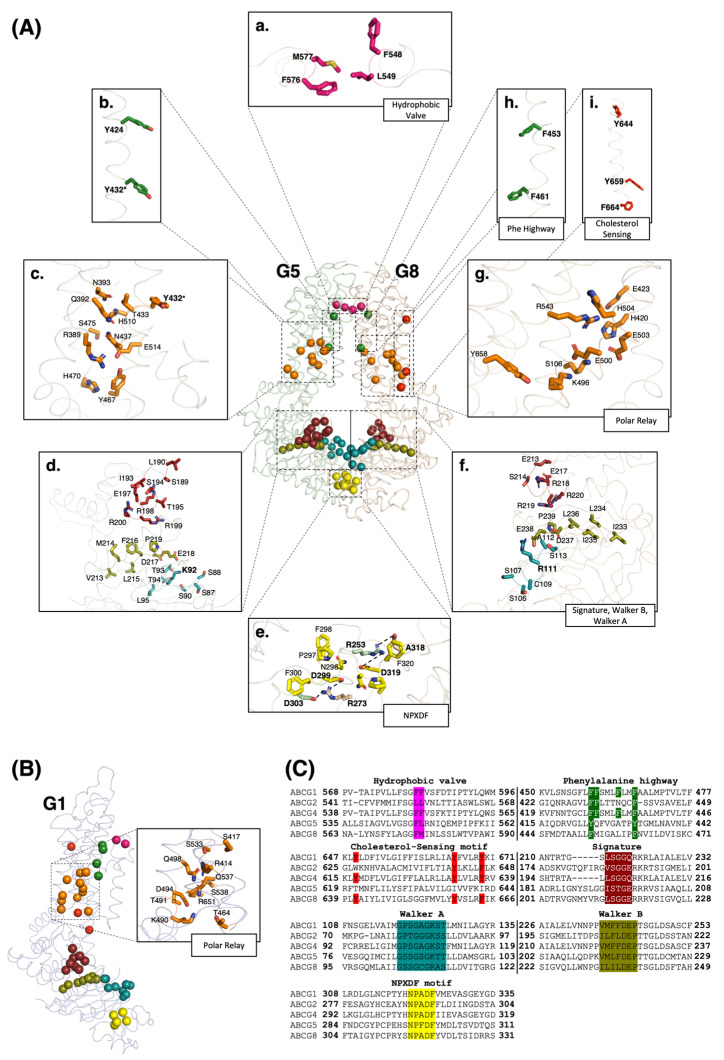
Just beneath the ECLs, TMH5 contains two conserved hydrophobic residues in ABCG members, primarily phenylalanines in the sterol transporters (Figure 1A,C). Studies on drug-efflux ABCG2 have suggested that these residues function as a hydrophobic valve to control entry to the upper cavity, receiving substrate before releasing it into the extracellular side [22,29,31,32]. This process is assumed to occur when the valves close during an inward relaxed conformation and open upon ATP hydrolysis during the change to an outward conformation. In addition to the hydrophobic valve, the ECLs also contain several conserved residues in ABCG proteins and some disease-causing mutations have been located in this area showing the importance of the ECDs in the substrate efflux to the extracellular matrix [33]. Moreover, in ECL3, ABCG5 and ABCG8 have a total of five cysteine residues, whereas the ABCG1 monomer has two cysteine residues which are believed to form intramolecular disulfide bonds [34]. Interestingly, ABCG1 has a shorter ECL3 than ABCG5/G8 and does not contain an N-glycosylation site [35].
Figure 1.
Motifs and elements in ABCG1 and ABCG5/G8. (A) Stick illustration of structural motifs shown on the crystal structure of ABCG5/G8 (PDB ID: 8CUB). (a–i) In the extracellular region, hydrophobic valve residues are shown in pink; in the TMDs, phenylalanine highway, cholesterol-sensing motif, and polar relay are represented in green, red, and orange, respectively; in the NBDs, signature, Walker A, Walker B, and NPXDF motifs are illustrated in brick red, deep teal, deep olive, and yellow, respectively. * The tyrosin residue labelled in the polar relay and phenylalanine highway is common in these two motifs. (B) The same conserved motifs with comparable colors in the structure of an ABCG1 half transporter. The zoomed-in picture shows the involved residues appear to form a polar relay network on the TMD of an ABCG1 monomer. These residues are highly conserved in mammals (see Supplementary Materials). (C) The sequence alignment illustrates conserved residues of structural motifs shown in panels A and B in all ABCG subfamily members (colors are picked in accordance with the color code in panels (A,B)).
3.2. TMD: Polar Relay, Cholesterol-Sensing Motif, Phenylalanine Highway
In close proximity to CnH and CpH in ABCG5/G8, a network of conserved polar residues, primarily Histidine, Asparagine, Glutamic acid, and Lysine form a polar relay generated by a network of hydrogen bonds and salt bridges (Figure 1A). This region is located immediately above the triple-helical bundle and is believed to act as a flexible hinge for subunit motions with a low energy barrier. The hydrogen bonds tying the TMD polar relay together may make this network more malleable than a buried hydrophobic core [22]. In ABCG1, this relay has yet to be defined. Here, using multiple sequence alignment analysis in the same region as that in ABCG5/G8, we identified several conserved polar residues that could potentially perform a comparable role as a polar relay in ABCG1 (Figure 1B).
The cholesterol recognition amino acid consensus (CRAC) motif, characterized by the sequence marker of L/V-X (1-5)-Y-X (1-5)-R/K, was initially described in enzymes that are involved in cholesterol production, such as HMG CoA Reductase and SCAP [36]. In ABCG members, this motif was first described in ABCG1 as one of the cholesterol sensing motifs. Former research revealed that tyrosine residues, Y649, Y667, and Y672 on TMH6 are components of this motif in ABCG1 [37]. This motif is critical for both protein function and stability and can be utilized to monitor the membrane cholesterol level by the protein. In previous research, the stability of the Y667L mutant protein in the presence and absence of cholesterol was studied. This revealed that unlike Y649L and Y672L, Y667L displays reduced cholesterol efflux, but can still be stabilized by the addition of cholesterol, suggesting that Y667 is essential for protein stability. A sequence analysis study showed that the tyrosine residues representing this motif are conserved in almost all ABCG proteins with the exception of ABCG5 [37,38,39] (Figure 1C). In ABCG8, this element is represented by three residues of Y644, Y659, and F664 (Figure 1A). The role of this motif in ABCG8 has not been determined yet, but based on the structural homology and sequence conservation, we hypothesize that it contributes to the sterol sensing function of ABCG5/G8 [40].
By analyzing the amino acid sequences of ABCG proteins, we recently found several well-conserved phenylalanine residues on TMH2 directly beneath the hydrophobic valve and above a cavity that is open to the cytoplasm (Figure 1A,C). The equivalent residues on ABCG5 are replaced by tyrosines, yet maintain the same spatial conservation towards the TMD dimer interface. These residues, particularly the first and the last phenylalanine, create an array of phenylalanine clusters in this region, namely the phenylalanine highway. We have proposed that this phenylalanine highway may play a role in ligand capture, through the molecular clamp, as well as the subsequent maintenance of substrate orientation and transfer to the hydrophobic valve [41].
3.3. NBD: Walker A, Walker B, Signature Motif, NPXDF Motif
The canonical Walker A, Walker B, and signature motifs are three highly conserved regions within the NBDs of ABCG transporters that are essential for nucleotide binding and subsequent hydrolysis [11,42] (Figure 1A,C). The interactions of one monomer’s Walker A and B motifs with the signature motif of the opposing monomer form NBDs of each transporter. ATP is believed to bind at the junction of two NBDs, where Walker A stabilizes this bond mostly through a conserved lysine or arginine residue and Walker B motif’s aspartate recruits a magnesium ion into the nucleotide-binding site [43]. The ATP catalysis depends on the conserved catalytic glutamate, located just downstream of the Walker B motif [44,45,46,47]. In ABCG sterol transporters, although no enzymatic study has been reported yet, it is hypothesized that the carboxylic group of glutamate coordinates a water molecule that promotes nucleophilic attack on the γ-phosphate of bound ATP to initiate its hydrolysis [43,47].
Previous studies found that biliary cholesterol secretion was blocked by mutations of critical residues in the Walker A and Walker B motifs of ABCG5, but not by mutations at comparable sites in G8. When mutations were placed in the signature motif, the opposite outcome was seen. These findings collectively showed that the ABCG5 and ABCG8 NBDs are not functionally symmetric [47]. In ABCG1, mutating an essential glycine residue to alanine in the Walker A motif prevented its localization to the cell surface, abolished ABCG1′s ability to promote cholesterol esterification by oxidase and ACAT, and downregulated HDL-driven cholesterol efflux on the cultured mammalian cells. This indicated that the ATP binding domain in ABCG1 is essential for both lipid transport activity and protein trafficking [48].
NPXDF is a unique NBD motif in ABCG proteins and was first highlighted by a functional study on ABCG1. In this study, it has been unveiled that the cells expressing mutant ABCG1 for this motif show impaired cholesterol efflux activity compared with wild-type-expressing cells [49]. This motif was found to be closed upon dimerization between the two NBDs of half transporters, based on the ABCG5/G8 crystal structure and the ABCG2 cryo-EM structure [41,50]. It has been shown that key residues from the NPXDF motifs including two aspartate residues at the C-terminus of the short helical motif, NPXDFXXD, form two salt bridges with a positively charged arginine residue from the opposing half transporter. In ABCG5/G8, the first salt bridge is formed between the side chain of ABCG5Arg253 and the side chains of two aspartic acid residues, ABCG8Asp319, Asp323. The second pair is formed between the side chain of ABCG8Arg273 and the side chains of two aspartic acid residues, ABCG5Asp299, Asp303 (Figure 1A). Sequence alignment of the NPXDFXXD motif suggests that the residues forming the salt bridges are conserved in the ABCG family and across species [50] (Figure 1C).
4. Revelation of Cholesterol Binding Sites in ABCG Sterol Transporters and Logical Working Models
4.1. ABCG5/G8
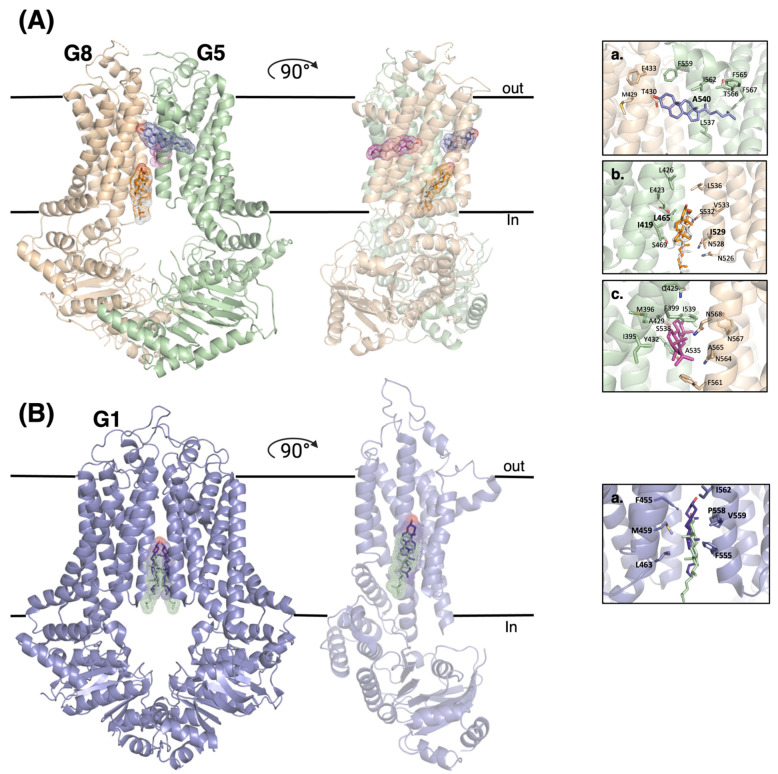
We recently determined a crystal structure of ABCG5/G8 in a cholesterol-complexed state. This structure provided evidence supporting the existence of a cholesterol molecule binding on the ABCG5-dominant side in the interface of two symmetrical proteins (site 3) [41] (Figure 2A(a)). In this peripheral binding site, cholesterol makes direct contact with the conserved ABCG5A540 residue. A mutant ABCG5A540F was previously shown to downregulate biliary cholesterol efflux in vivo and inhibited sterol-coupled ATPase activity in vitro [22]. The other binding site (site 1) within ABCG5/G8 has been determined to be located in the inner leaflet of the membrane (Figure 2A(b)). In this site, the isooctyl side chain of cholesterol facing the intracellular matrix of the membrane, close to Ile529 in TMH5 of ABCG5 and to Ile419 and Leu465 in TMH1 and TMH2 of ABCG8, respectively. The remaining bound cholesterol was found deeper within the central cavity of the TMD, situated halfway between the intracellular and extracellular leaflets and oriented parallel to the membrane on the ABCG8-dominant side (site 2) [25] (Figure 2A(c)).
Figure 2.
Cholesterol binding sites in the structures of ABCG1 and ABCG5/G8 proteins. (A) Comparison of cholesterol binding sites found on the determined structures of ABCG5/G8, as well as the involving residues. (a) This site (Site 3) was found in the crystal structure of ABCG5/G8 (PDB ID: 8CUB). (b,c). These binding sites (sites 1 and 2) were observed in the Cryo-EM structures of ABCG5/G8 (PDB ID: 7R8B and 7R8A, respectively). (B) Cartoon representation of ABCG1 in complex with two cholesterols forming a binding site. (a) Two cholesterol molecules colored in purple and green are from Cryo-EM structures of ABCG1, (PDB ID: 7FDV) and (PDB ID: 7R8D), respectively.
All three cholesterol binding sites on ABCG5/G8 take on uncommon “flipped” conformation in relation to the membrane’s general amphipathic nature (Figure 2A). Such flipped cholesterol conformations have been previously observed in membrane dynamics research as well as bound to other membrane proteins [51,52]. Spontaneous cholesterol flipping between leaflets is a common event in the plasma membrane, capable of undertaking intermediate horizontal orientation within the membrane’s core [51]. These orientations are similar to what is observed in ABCG5/G8′s binding sites 2 and 3. Site 1 contains a vertically oriented cholesterol molecule in a flipped conformation. The presence of hydrophobic residues as well as charged amino acids near the polar head likely helps bind and stabilize the sterol conformation [25]. The presence of this sterol also aids protein function as found through in vivo mouse biliary cholesterol transport [22]. Overhead near alanine 540 is site 3, which was found to be an important residue [22,40]. It is thus possible that ABCG5/G8 catalyzes cholesterol flipping from inner to outer leaflets, peripherally through its exterior surface, like P4-ATPase “credit card” models [53]. On the opposite dimer interface (site 2), a horizontal cholesterol molecule has been resolved and was able to bind cholesterol from a neutral orthogonal position within the membrane core, as seen through molecular dynamic simulations [25] (Figure 3A). However, the initial transition toward that horizontal state remains to be unknown, a future direction to further investigate the transporter’s mechanism.
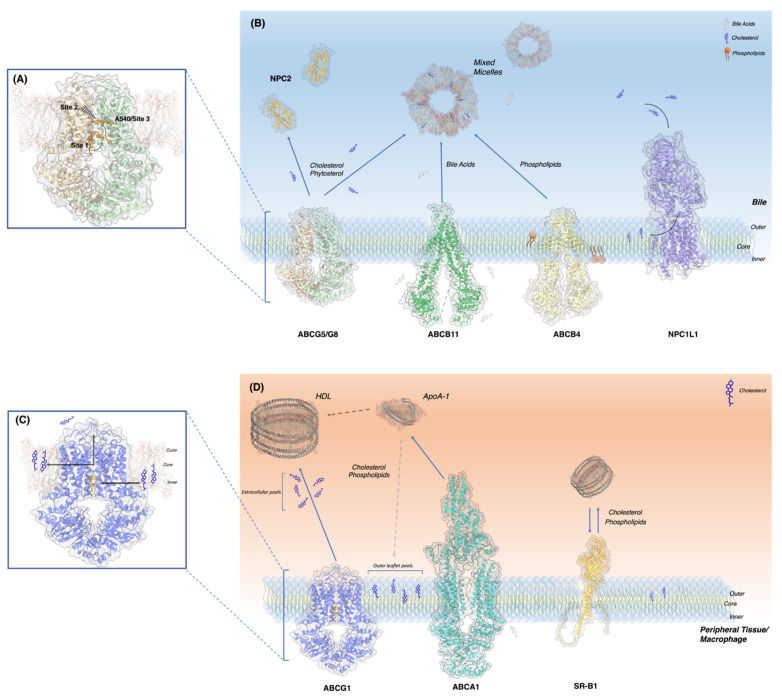
Figure 3.
Molecular and cellular view of ABCG5/G8 and ABCG1 in their microenvironments. (A) Binding sites 1–3 of ABCG5/G8. Site 2 is on the back side of the protein. (B) Lipid and bile salt transporters of the canalicular membrane including their substrates and acceptor molecules, ABCG5/G8, ABCB11, ABCB4, NPC1L1 and NPC2 shown in cartoon. (PDB ID: 8CUB, 6LR0, 7NIV, 7DF8, 1NEP). (C) ABCG1 binding site and cholesterol trajectory towards protein and post efflux. (D) ABCG1 and lipid transporters expressed on macrophage: ABCA1, ABCG1 and SR-B1 (PDB ID: 7R8D, 5XYJ) (Alphafold).
ABCG5/G8 is presented on the canalicular membranes’ outer leaflet, enriched with cholesterol and sphingomyelin (SM), two lipids that can generate liquid-ordered phases of membranes, causing asymmetrical phases between the membrane [54,55,56]. Liquid-ordered phases, equally referred to as lipid rafts or lipid microdomains, represent dense and rigid structures within the fluid plasma membrane, exhibiting detergent resistance [39,57,58]. This type of resistance is crucial when localized so closely to the bile, containing waste product, cholesterol and bile salts. The canalicular membrane experiences stringent interactions with bile acids’ detergent properties, leading some to speculate about the self-preserving functions of high-density lipid packing in the outer leaflets of the plasma membranes on the apical cell surface [59,60]. As discovered by an earlier study, membrane cholesterol within the canalicular membrane prevented the cytotoxic effects of external bile acids. Therefore, the resident lipid transporting proteins must function together, converging on the goal to maintain homeostasis [59].
The cohort of proteins embedded in the canalicular bilayer performs varying tasks, contributing to the maintenance of lipid levels within the cell, membrane, and bile. Functioning as an endogenous sterol and phytosterol transporter, ABCG5/G8 is involved in a perpetual push and pull with its sterol importing counterpart, Niemann-Pick C1-Like 1 (NPC1L1). As substrates bind, NPC1L1′s conformation changes, resulting in the formation of the cholesterol delivering tunnel, in turn, allowing the substrates to navigate towards the outer leaflet of the membrane [61]. Multidrug resistant protein 3 (MDR3) also known as ABCB4, is a phospholipid translocase embedded in the canalicular membrane [62]. Although it actively flips inner leaflet phospholipids and sterols to the outer leaflet, there is evidence supporting its subsequent role in substrate secretion towards the bile [63,64,65]. Furthermore, MDR3 is found to be essential for the proper function of ABCG5/G8, which is strongly believed to be caused by its involvement in the formation of mixed micelles through phospholipid secretion in tandem with ABCB11′s bile salt secretion [66,67]. Mixed micelles are very charged, small aggregates of phospholipids, cholesterol and bile salts, and these micelles form the basis of currently known ABCG5/G8 acceptor particles [68,69]. Acceptor particles will then intake the exported lipids from ABCG5/G8; however, it is unknown if direct protein-particle interactions are necessary. Besides bile micelles, recent studies detected the presence of Niemann Pick C2 (NPC2), a lysosomal cholesterol transporting protein, in the bile [70]. Lack of NPC2 was shown to downregulate biliary cholesterol secretion in vivo, whereas using an NPC2-overexpressing mouse model, the ABCG5/G8 expression was positively regulated and the cholesterol secretion was restored. This suggests that although mixed micelles are considered the “primary” acceptor particle of sterols from ABCG5/G8, biliary NPC2 could speculatively play a “secondary” acceptor role by expediting the sterol transport from ABCG5/G8 to mixed micelles with its quick load and unload rates. Further studies remain needed to confirm direct interactions between the two proteins and between the NPC2 and mixed micelles. Anatomically, the bile feeds through the common bile duct which links to the upper small intestine known as the duodenum and trickles to the medially located jujenum. As ABCG5/G8 is highly expressed on the duodenum and jujenum brush border enterocytes [11], the presence of lipid rafts [71] and bile may provide a similar plasma membrane microenvironment to that on the bile ducts for ABCG5/G8 functions (Figure 3B).
4.2. ABCG1
The cholesterol-bound structures of ABCG1 show an inward-facing conformation. Two cholesterol molecules have been observed to be located in symmetrical alignment in the cholesterol binding site (Figure 2B). Using the numbering of a recent cryo-EM structure (PDB ID: 7R8D), the hydrophobic interactions between cholesterol and the binding site are formed through the residues of Phe455, Met459, and Leu463 from TM2 of one monomer and with Phe555, Pro558, Val 559 and Ile562 from TM5 of the other monomer (Figure 2B—right panel). Site-directed mutagenesis supports the essential roles of these residues in cholesterol transport [72]. In both of these cholesterol-bound structures of ABCG1, cholesterol shows similar binding interactions, although in one (PDB ID: 7FDV), cholesterol is located slightly higher [35,72].
In addition to cholesterol, other known lipid substrates like SM are also seen superficially on the cavity’s exterior. How the SM molecule enters the protein and gets transported remains elusive, although it may equally facilitate the recruitment and entry of cholesterol, possibly through an SM binding site in the inner leaflet cavity [73,74]. ABCG1 then promotes lipid translocation from the inner to outer leaflet and helps prevent the cytotoxic effects of excess cholesterol and sphingomyelin packing within the membrane, which would impact membrane fluidity and dynamics and cause membrane protein misfolding. ABCG1 thus appears to regulate its own function by dissipating the high cholesterol environment in which it is localized to [39], an environment shared by ABCG1, ABCA1, and Scavenger receptor- B1 (SR-B1), all participating in RCT (Figure 3D). ABCG1 has been long theorized to transport sterols to HDL molecules. Although, this topic remains contentious between two theories. The first argues that ABCG1 redistributes free cholesterol from the inner to outer leaflet of the membrane. HDL particles would then gather sterols by binding the membrane [48,75]. The other theory suggests ABCG1 effluxes ligands into extracellular pools, where the lipoproteins would intake them [76,77,78] (Figure 3C). HDL can bind membranes without the presence of protein intermediates [79], and at high enough concentrations, will remove outer leaflet cholesterol. Yet, at low lipoprotein concentrations, only extracellular cholesterol can be picked up [77]. These extracellular pools are exclusively caused by ABCG1 as ABCA1 is unable to perform this task, likely due to the temporary storage of ligands within its ECD [80] (Figure 3D). ABCA1 exports ligands to apoA-1, through direct and indirect ways. Around 10% of apoA-1 binds ABCA1 directly [81], while the remaining removed outer leaflet free cholesterol pools, whose formation could be the result of both ABCG1 and ABCA1, albeit this remains a debated topic [48,82,83,84] (Figure 3D).
5. Outlook
5.1. Challenge to Reveal Conformations of the Catalytic Cycle during the Sterol Transport
Structural analyses of ABCG1 and ABCG5/G8 agree on a number of enzymatic and genetic data and provide a wealth of molecular platforms to further investigate the structure-function relationship of ABCG sterol transporters by additional biochemical and biophysical approaches. Currently, several models exist in the inward-facing conformation, whether in the presence or absence of antibodies or nucleotide ligands [35,41,72]; yet there is only one nucleotide-bound outward-facing structure [25]. At the modest resolution of 3–4 Å, essentially all inward-facing models can be superimposed to the first ABCG5/G8 crystal structure. This suggests that, whether by X-ray crystallography or cryo-EM, there is still a huge gap and urgent need to capture intermediate conformations during the catalytic cycle. This will remain a major challenge in the coming decades, but technological advancement in either crystallography or electron microscopy will hold great promise. In addition, with the sophistication of molecular dynamics simulation, rigorous biophysical studies and computation characterizations, will address the dynamic aspects of sterol transporter mechanism.
5.2. Challenge to Determine Substrate Specificity and Selectivity on ABCG Sterol Transporters
Recent structures of ABCG1 or ABCG5/G8 revealed several bona fide sterol molecules. As cholesterol is part of lipid bilayers of cellular membranes, it may be tricky to differentiate substrate sterols and membrane structural sterols. Using computer-based molecular docking analysis, we observed differential cholesterol binding profiles on either transporters, suggesting sterol selectivity on ABCG1 and ABCG5/G8. In addition, ABCG1 appears to bind preferably cholesterol, suggesting substrate specificity among ABCG sterol transporters [41]. To support this hypothesis, it is necessary to exploit functional reconstitution of native and purified ABCG sterol transporters in their native membrane lipid environments and in the presence of a variety of sterol lipids and nucleotides, which is yet understudied. With the advancement of molecular dynamics simulation of large macromolecules, the existing structures and models of ABCG5/G8 and ABCG1 should promote interdisciplinary biochemical, biophysical and computational approaches to elucidate substrate specificity and sterol selectivity among ABCG sterol transporters.
Acknowledgments
We thank the critical feedback from all reviewers. This work was supported by a National New Investigator Award from the Heart and Stroke Foundation of Canada and a Canadian Institutes of Health Research Project Grant (PJT-180640) to JYL. SS is a recipient of the Undergraduate Research Opportunities Program (UROP) scholarship at the University of Ottawa.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24010484/s1.
Author Contributions
Conceptualization, F.R., D.F., G.G. and J.-Y.L.; formal analysis, F.R., D.F., G.G. and S.S.; visualization, F.R., D.F. and G.G.; writing–original draft, F.R., D.F. and G.G.; writing–review & editing, F.R., D.F., G.G., S.S. and J.-Y.L.; funding acquisition, J.-Y.L.; project administration, J.-Y.L.; resources, J.-Y.L.; supervision, J.-Y.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Canadian Institutes of Health Research Project Grant (PJT-180640) and Heart and Stroke Foundation of Canada National New Investigator Award.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kuchler K. The ABC of ABCs: Multidrug Resistance and Genetic Diseases. Volume 278. Wiley Online Library; Hoboken, NJ, USA: 2011. p. 3189. [DOI] [PubMed] [Google Scholar]
- 2.Locher K.P. Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat. Struct. Mol. Biol. 2016;23:487–493. doi: 10.1038/nsmb.3216. [DOI] [PubMed] [Google Scholar]
- 3.Rees D.C., Johnson E., Lewinson O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009;10:218–227. doi: 10.1038/nrm2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linton K.J., Higgins C.F. Structure and function of ABC transporters: The ATP switch provides flexible control. Pflügers Arch.-Eur. J. Physiol. 2007;453:555–567. doi: 10.1007/s00424-006-0126-x. [DOI] [PubMed] [Google Scholar]
- 5.Liu X. ABC family transporters. Drug Transp. Drug Dispos. Eff. Toxic. 2019;1141:13–100. doi: 10.1007/978-981-13-7647-4_2. [DOI] [PubMed] [Google Scholar]
- 6.Kerr I.D., Hutchison E., Gerard L., Aleidi S.M., Gelissen I.C. Mammalian ABCG-transporters, sterols and lipids: To bind perchance to transport? Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2021;1866:158860. doi: 10.1016/j.bbalip.2020.158860. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y., Yutuc E., Griffiths W.J. Cholesterol metabolism pathways–are the intermediates more important than the products? FEBS J. 2021;288:3727–3745. doi: 10.1111/febs.15727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouimet M., Barrett T.J., Fisher E.A. HDL and reverse cholesterol transport: Basic mechanisms and their roles in vascular health and disease. Circ. Res. 2019;124:1505–1518. doi: 10.1161/CIRCRESAHA.119.312617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vrins C.L., Ottenhoff R., van den Oever K., de Waart D.R., Kruyt J.K., Zhao Y., van Berkel T.J., Havekes L.M., Aerts J.M., van Eck M. Trans-intestinal cholesterol efflux is not mediated through high density lipoprotein. J. Lipid Res. 2012;53:2017–2023. doi: 10.1194/jlr.M022194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerr I.D., Haider A.J., Gelissen I.C. The ABCG family of membrane-associated transporters: You don’t have to be big to be mighty. Br. J. Pharmacol. 2011;164:1767–1779. doi: 10.1111/j.1476-5381.2010.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berge K.E., Tian H., Graf G.A., Yu L., Grishin N.V., Schultz J., Kwiterovich P., Shan B., Barnes R., Hobbs H.H. Accumulation of dietary cholesterol in sitosterolemia caused by mutations in adjacent ABC transporters. Science. 2000;290:1771–1775. doi: 10.1126/science.290.5497.1771. [DOI] [PubMed] [Google Scholar]
- 12.Meurs I., Lammers B., Zhao Y., Out R., Hildebrand R.B., Hoekstra M., Van Berkel T.J., Van Eck M. The effect of ABCG1 deficiency on atherosclerotic lesion development in LDL receptor knockout mice depends on the stage of atherogenesis. Atherosclerosis. 2012;221:41–47. doi: 10.1016/j.atherosclerosis.2011.11.024. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy M.A., Barrera G.C., Nakamura K., Baldán Á., Tarr P., Fishbein M.C., Frank J., Francone O.L., Edwards P.A. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 2005;1:121–131. doi: 10.1016/j.cmet.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Wang S., Smith J.D. ABCA1 and nascent HDL biogenesis. Biofactors. 2014;40:547–554. doi: 10.1002/biof.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sano O., Kobayashi A., Nagao K., Kumagai K., Kioka N., Hanada K., Ueda K., Matsuo M. Sphingomyelin-dependence of cholesterol efflux mediated by ABCG1. J. Lipid Res. 2007;48:2377–2384. doi: 10.1194/jlr.M700139-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Cavelier C., Lorenzi I., Rohrer L., von Eckardstein A. Lipid efflux by the ATP-binding cassette transporters ABCA1 and ABCG1. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2006;1761:655–666. doi: 10.1016/j.bbalip.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Brown J.M., Yu L. Protein mediators of sterol transport across intestinal brush border membrane. Cholest. Bind. Cholest. Transp. Proteins. 2010;51:337–380. doi: 10.1007/978-90-481-8622-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salen G., Horak I., Rothkopf M., Cohen J., Speck J., Tint G., Shore V., Dayal B., Chen T., Shefer S. Lethal atherosclerosis associated with abnormal plasma and tissue sterol composition in sitosterolemia with xanthomatosis. J. Lipid Res. 1985;26:1126–1133. doi: 10.1016/S0022-2275(20)34286-3. [DOI] [PubMed] [Google Scholar]
- 19.Miettinen T.A. Phytosterolaemia, xanthomatosis and premature atherosclerotic arterial disease: A case with high plant sterol absorption, impaired sterol elimination and low cholesterol synthesis. Eur. J. Clin. Investig. 1980;10:27–35. doi: 10.1111/j.1365-2362.1980.tb00006.x. [DOI] [PubMed] [Google Scholar]
- 20.Lee M.-H., Lu K., Hazard S., Yu H., Shulenin S., Hidaka H., Kojima H., Allikmets R., Sakuma N., Pegoraro R. Identification of a gene, ABCG5, important in the regulation of dietary cholesterol absorption. Nat. Genet. 2001;27:79–83. doi: 10.1038/83799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salen G., Shefer S., Nguyen L., Ness G.C., Tint G., Shore V. Sitosterolemia. J. Lipid Res. 1992;33:945–955. doi: 10.1016/S0022-2275(20)41411-7. [DOI] [PubMed] [Google Scholar]
- 22.Lee J.-Y., Kinch L.N., Borek D.M., Wang J., Wang J., Urbatsch I.L., Xie X.-S., Grishin N.V., Cohen J.C., Otwinowski Z. Crystal structure of the human sterol transporter ABCG5/ABCG8. Nature. 2016;533:561–564. doi: 10.1038/nature17666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakulj L., Vissers M.N., Tanck M.W., Hutten B.A., Stellaard F., Kastelein J.J., Dallinga-Thie G.M. ABCG5/G8 polymorphisms and markers of cholesterol metabolism: Systematic review and meta-analysis [S] J. Lipid Res. 2010;51:3016–3023. doi: 10.1194/jlr.M008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusuhara H., Sugiyama Y. ATP-binding cassette, subfamily G (ABCG family) Pflügers Arch.-Eur. J. Physiol. 2007;453:735–744. doi: 10.1007/s00424-006-0134-x. [DOI] [PubMed] [Google Scholar]
- 25.Sun Y., Wang J., Long T., Qi X., Donnelly L., Elghobashi-Meinhardt N., Esparza L., Cohen J.C., Xie X.-S., Hobbs H.H. Molecular basis of cholesterol efflux via ABCG subfamily transporters. Proc. Natl. Acad. Sci. USA. 2021;118:e2110483118. doi: 10.1073/pnas.2110483118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris A., Wagner M., Du D., Raschka S., Nentwig L.-M., Gohlke H., Smits S.H., Luisi B.F., Schmitt L. Structure and efflux mechanism of the yeast pleiotropic drug resistance transporter Pdr5. Nat. Commun. 2021;12:5254. doi: 10.1038/s41467-021-25574-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okiyoneda T., Kono T., Niibori A., Harada K., Kusuhara H., Takada T., Shuto T., Suico M.A., Sugiyama Y., Kai H. Calreticulin facilitates the cell surface expression of ABCG5/G8. Biochem. Biophys. Res. Commun. 2006;347:67–75. doi: 10.1016/j.bbrc.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Biemans-Oldehinkel E., Doeven M.K., Poolman B. ABC transporter architecture and regulatory roles of accessory domains. FEBS Lett. 2006;580:1023–1035. doi: 10.1016/j.febslet.2005.11.079. [DOI] [PubMed] [Google Scholar]
- 29.Khunweeraphong N., Szöllősi D., Stockner T., Kuchler K. The ABCG2 multidrug transporter is a pump gated by a valve and an extracellular lid. Nat. Commun. 2019;10:5433. doi: 10.1038/s41467-019-13302-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khunweeraphong N., Mitchell-White J., Szöllősi D., Hussein T., Kuchler K., Kerr I.D., Stockner T., Lee J.Y. Picky ABCG5/G8 and promiscuous ABCG2-a tale of fatty diets and drug toxicity. FEBS Lett. 2020;594:4035–4058. doi: 10.1002/1873-3468.13938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutter C.A., Timachi M.H., Hürlimann L.M., Zimmermann I., Egloff P., Göddeke H., Kucher S., Štefanić S., Karttunen M., Schäfer L.V. The extracellular gate shapes the energy profile of an ABC exporter. Nat. Commun. 2019;10:1–13. doi: 10.1038/s41467-019-09892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kodan A., Yamaguchi T., Nakatsu T., Matsuoka K., Kimura Y., Ueda K., Kato H. Inward-and outward-facing X-ray crystal structures of homodimeric P-glycoprotein CmABCB1. Nat. Commun. 2019;10:2260. doi: 10.1038/s41467-018-08007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Banerjee A., Moreno A., Pata J., Falson P., Prasad R. ABCG: A new fold of ABC exporters and a whole new bag of riddles! Adv. Protein Chem. Struct. Biol. 2021;123:163–191. doi: 10.1016/bs.apcsb.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Wakabayashi K., Nakagawa H., Adachi T., Kii I., Kobatake E., Kudo A., Ishikawa T. Identification of cysteine residues critically involved in homodimer formation and protein expression of human ATP-binding cassette transporter ABCG2: A new approach using the flp recombinase system. J. Exp. Oncol. 2006;5:205–222. [PubMed] [Google Scholar]
- 35.Skarda L., Kowal J., Locher K.P. Structure of the human cholesterol transporter ABCG1. J. Mol. Biol. 2021;433:167218. doi: 10.1016/j.jmb.2021.167218. [DOI] [PubMed] [Google Scholar]
- 36.Epand R.M. Cholesterol and the interaction of proteins with membrane domains. Prog. Lipid Res. 2006;45:279–294. doi: 10.1016/j.plipres.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 37.Sharpe L.J., Rao G., Jones P.M., Glancey E., Aleidi S.M., George A.M., Brown A.J., Gelissen I.C. Cholesterol sensing by the ABCG1 lipid transporter: Requirement of a CRAC motif in the final transmembrane domain. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids. 2015;1851:956–964. doi: 10.1016/j.bbalip.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Kenny D.J., Plichta D.R., Shungin D., Koppel N., Hall A.B., Fu B., Vasan R.S., Shaw S.Y., Vlamakis H., Balskus E.P. Cholesterol metabolism by uncultured human gut bacteria influences host cholesterol level. Cell Host Microbe. 2020;28:245–257.e6. doi: 10.1016/j.chom.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ristovski M., Farhat D., Bancud S.E.M., Lee J.-Y. Lipid Transporters Beam Signals from Cell Membranes. Membranes. 2021;11:562. doi: 10.3390/membranes11080562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xavier B.M., Jennings W.J., Zein A.A., Wang J., Lee J.-Y. Structural snapshot of the cholesterol-transport ATP-binding cassette proteins. Biochem. Cell Biol. 2019;97:224–233. doi: 10.1139/bcb-2018-0151. [DOI] [PubMed] [Google Scholar]
- 41.Farhat D., Rezaei F., Ristovski M., Yang Y., Stancescu A., Dzimkova L., Samnani S., Couture J.-F., Lee J.-Y. Structural analysis of cholesterol binding and sterol selectivity by ABCG5/G8. J. Mol. Biol. 2022;434:167795. doi: 10.1016/j.jmb.2022.167795. [DOI] [PubMed] [Google Scholar]
- 42.Higgins C.F. ABC transporters: From microorganisms to man. Annu. Rev. Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 43.Hung L.-W., Wang I.X., Nikaido K., Liu P.-Q., Ames G.F.-L., Kim S.-H. Crystal structure of the ATP-binding subunit of an ABC transporter. Nature. 1998;396:703–707. doi: 10.1038/25393. [DOI] [PubMed] [Google Scholar]
- 44.Payen L.F., Gao M., Westlake C.J., Cole S.P., Deeley R.G. Role of carboxylate residues adjacent to the conserved core Walker B motifs in the catalytic cycle of multidrug resistance protein 1 (ABCC1) J. Biol. Chem. 2003;278:38537–38547. doi: 10.1074/jbc.M305786200. [DOI] [PubMed] [Google Scholar]
- 45.Sauna Z.E., Müller M., Peng X.-H., Ambudkar S.V. Importance of the conserved Walker B glutamate residues, 556 and 1201, for the completion of the catalytic cycle of ATP hydrolysis by human P-glycoprotein (ABCB1) Biochemistry. 2002;41:13989–14000. doi: 10.1021/bi026626e. [DOI] [PubMed] [Google Scholar]
- 46.Tombline G., Bartholomew L.A., Tyndall G.A., Gimi K., Urbatsch I.L., Senior A.E. Properties of P-glycoprotein with mutations in the “catalytic carboxylate” glutamate residues. J. Biol. Chem. 2004;279:46518–46526. doi: 10.1074/jbc.M408052200. [DOI] [PubMed] [Google Scholar]
- 47.Zhang D.-W., Graf G.A., Gerard R.D., Cohen J.C., Hobbs H.H. Functional asymmetry of nucleotide-binding domains in ABCG5 and ABCG8. J. Biol. Chem. 2006;281:4507–4516. doi: 10.1074/jbc.M512277200. [DOI] [PubMed] [Google Scholar]
- 48.Vaughan A.M., Oram J.F. ABCG1 redistributes cell cholesterol to domains removable by high density lipoprotein but not by lipid-depleted apolipoproteins. J. Biol. Chem. 2005;280:30150–30157. doi: 10.1074/jbc.M505368200. [DOI] [PubMed] [Google Scholar]
- 49.Wang F., Li G., Gu H.-m., Zhang D.-w. Characterization of the role of a highly conserved sequence in ATP binding cassette transporter G (ABCG) family in ABCG1 stability, oligomerization, and trafficking. Biochemistry. 2013;52:9497–9509. doi: 10.1021/bi401285j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H., Huang C.-S., Yu X., Lee J., Vaish A., Chen Q., Zhou M., Wang Z., Min X. Cryo-EM structure of ABCG5/G8 in complex with modulating antibodies. Commun. Biol. 2021;4:526. doi: 10.1038/s42003-021-02039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu R.-X., Baoukina S., Tieleman D.P. Cholesterol flip-flop in heterogeneous membranes. J. Chem. Theory Comput. 2019;15:2064–2070. doi: 10.1021/acs.jctc.8b00933. [DOI] [PubMed] [Google Scholar]
- 52.Wu H., Cao R., Wei S., Pathan-Chhatbar S., Wen M., Wu B., Schamel W.W., Wang S., OuYang B. Cholesterol Binds in a Reversed Orientation to TCRβ-TM in Which Its OH Group is Localized to the Center of the Lipid Bilayer. J. Mol. Biol. 2021;433:167328. doi: 10.1016/j.jmb.2021.167328. [DOI] [PubMed] [Google Scholar]
- 53.Andersen J.P., Vestergaard A.L., Mikkelsen S.A., Mogensen L.S., Chalat M., Molday R.S. P4-ATPases as phospholipid flippases—Structure, function, and enigmas. Front. Physiol. 2016;7:275. doi: 10.3389/fphys.2016.00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Quinn P.J., Wolf C. The liquid-ordered phase in membranes. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2009;1788:33–46. doi: 10.1016/j.bbamem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Eckhardt E.R., Moschetta A., Renooij W., Goerdayal S.S., van Berge-Henegouwen G.P., Van Erpecum K.J. Asymmetric distribution of phosphatidylcholine and sphingomyelin between micellar and vesicular phases: Potential implications for canalicular bile formation1. J. Lipid Res. 1999;40:2022–2033. doi: 10.1016/S0022-2275(20)32426-3. [DOI] [PubMed] [Google Scholar]
- 56.Amigo L., Mendoza H., Zanlungo S., Miquel J.F., Rigotti A., González S., Nervi F. Enrichment of canalicular membrane with cholesterol and sphingomyelin prevents bile salt-induced hepatic damage. J. Lipid Res. 1999;40:533–542. doi: 10.1016/S0022-2275(20)32458-5. [DOI] [PubMed] [Google Scholar]
- 57.Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 58.McGuinn K.P., Mahoney M.G. Epidermal Cells. Springer; Berlin/Heidelberg, Germany: 2014. Lipid rafts and detergent-resistant membranes in epithelial keratinocytes; pp. 133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou Y., Doyen R., Lichtenberger L.M. The role of membrane cholesterol in determining bile acid cytotoxicity and cytoprotection of ursodeoxycholic acid. Biochim. et Biophys. Acta (BBA) Biomembr. 2009;1788:507–513. doi: 10.1016/j.bbamem.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Temel R.E., Tang W., Ma Y., Rudel L.L., Willingham M.C., Ioannou Y.A., Davies J.P., Nilsson L.-M., Yu L. Hepatic Niemann-Pick C1–like 1 regulates biliary cholesterol concentration and is a target of ezetimibe. J. Clin. Investig. 2007;117:1968–1978. doi: 10.1172/JCI30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang C.-S., Yu X., Fordstrom P., Choi K., Chung B.C., Roh S.-H., Chiu W., Zhou M., Min X., Wang Z. Cryo-EM structures of NPC1L1 reveal mechanisms of cholesterol transport and ezetimibe inhibition. Sci. Adv. 2020;6:eabb1989. doi: 10.1126/sciadv.abb1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daleke D.L. Regulation of transbilayer plasma membrane phospholipid asymmetry. J. Lipid Res. 2003;44:233–242. doi: 10.1194/jlr.R200019-JLR200. [DOI] [PubMed] [Google Scholar]
- 63.Morita S.-y., Terada T. Molecular mechanisms for biliary phospholipid and drug efflux mediated by ABCB4 and bile salts. BioMed Res. Int. 2014;2014:954781. doi: 10.1155/2014/954781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Elferink R.O., Ottenhoff R., van Wijland M., Smit J., Schinkel A., Groen A.K. Regulation of biliary lipid secretion by mdr2 P-glycoprotein in the mouse. J. Clin. Investig. 1995;95:31–38. doi: 10.1172/JCI117658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elferink R.O., Ottenhoff R., van Wijland M., Frijters C., Van Nieuwkerk C., Groen A. Uncoupling of biliary phospholipid and cholesterol secretion in mice with reduced expression of mdr2 P-glycoprotein. J. Lipid Res. 1996;37:1065–1075. doi: 10.1016/S0022-2275(20)42016-4. [DOI] [PubMed] [Google Scholar]
- 66.Langheim S., Yu L., Von Bergmann K., Lütjohann D., Xu F., Hobbs H.H., Cohen J.C. ABCG5 and ABCG8 require MDR2 for secretion of cholesterol into bile. J. Lipid Res. 2005;46:1732–1738. doi: 10.1194/jlr.M500115-JLR200. [DOI] [PubMed] [Google Scholar]
- 67.Hirano H., Kurata A., Onishi Y., Sakurai A., Saito H., Nakagawa H., Nagakura M., Tarui S., Kanamori Y., Kitajima M. High-speed screening and QSAR analysis of human ATP-binding cassette transporter ABCB11 (bile salt export pump) to predict drug-induced intrahepatic cholestasis. Mol. Pharm. 2006;3:252–265. doi: 10.1021/mp060004w. [DOI] [PubMed] [Google Scholar]
- 68.Maeda H. A thermodynamic analysis of charged mixed micelles in water. J. Phys. Chem. B. 2005;109:15933–15940. doi: 10.1021/jp052082p. [DOI] [PubMed] [Google Scholar]
- 69.Kosters A., Kunne C., Looije N., Patel S.B., Elferink R.P.O., Groen A.K. The mechanism of ABCG5/ABCG8 in biliary cholesterol secretion in mice1. J. Lipid Res. 2006;47:1959–1966. doi: 10.1194/jlr.M500511-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yamanashi Y., Takada T., Yoshikado T., Shoda J.I., Suzuki H. NPC2 regulates biliary cholesterol secretion via stimulation of ABCG5/G8-mediated cholesterol transport. Gastroenterology. 2011;140:1664–1674. doi: 10.1053/j.gastro.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 71.Danielsen E.M., Hansen G.H. Lipid rafts in epithelial brush borders: Atypical membrane microdomains with specialized functions. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2003;1617:1–9. doi: 10.1016/j.bbamem.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Xu D., Li Y., Yang F., Sun C.-R., Pan J., Wang L., Chen Z.-P., Fang S.-C., Yao X., Hou W.-T. Structure and transport mechanism of the human cholesterol transporter ABCG1. Cell Rep. 2022;38:110298. doi: 10.1016/j.celrep.2022.110298. [DOI] [PubMed] [Google Scholar]
- 73.Hirayama H., Kimura Y., Kioka N., Matsuo M., Ueda K. ATPase activity of human ABCG1 is stimulated by cholesterol and sphingomyelin [S] J. Lipid Res. 2013;54:496–502. doi: 10.1194/jlr.M033209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fridkis-Hareli M., Sharp A., Abel L., Globerson A. Thymocyte development in an in vitro constructed chimera of irradiated fetal thymus and lymphohemopoietic cells. Thymus. 1991;18:225–235. [PubMed] [Google Scholar]
- 75.Pagler T.A., Wang M., Mondal M., Murphy A.J., Westerterp M., Moore K.J., Maxfield F.R., Tall A.R. Deletion of ABCA1 and ABCG1 impairs macrophage migration because of increased Rac1 signaling. Circ. Res. 2011;108:194–200. doi: 10.1161/CIRCRESAHA.110.228619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ong D.S., Anzinger J.J., Leyva F.J., Rubin N., Addadi L., Kruth H.S. Extracellular cholesterol-rich microdomains generated by human macrophages and their potential function in reverse cholesterol transport. J. Lipid Res. 2010;51:2303–2313. doi: 10.1194/jlr.M005660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Freeman S.R., Jin X., Anzinger J.J., Xu Q., Purushothaman S., Fessler M.B., Addadi L., Kruth H.S. ABCG1-mediated generation of extracellular cholesterol microdomains. J. Lipid Res. 2014;55:115–127. doi: 10.1194/jlr.M044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jin X., Sviridov D., Liu Y., Vaisman B., Addadi L., Remaley A.T., Kruth H.S. ABCA1 mediates ApoA-I and ApoA-I Mimetic Peptide Mobilization of Extracellular Cholesterol Microdomains Deposited by Macrophages. Arterioscler. Thromb. Vasc. Biol. 2016;36:2283. doi: 10.1161/ATVBAHA.116.308334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Axmann M., Plochberger B., Mikula M., Weber F., Strobl W.M., Stangl H. Plasma Membrane Lipids: An Important Binding Site for All Lipoprotein Classes. Membranes. 2021;11:882. doi: 10.3390/membranes11110882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ishigami M., Ogasawara F., Nagao K., Hashimoto H., Kimura Y., Kioka N., Ueda K. Temporary sequestration of cholesterol and phosphatidylcholine within extracellular domains of ABCA1 during nascent HDL generation. Sci. Rep. 2018;8:6170. doi: 10.1038/s41598-018-24428-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Phillips M.C. Is ABCA1 a lipid transfer protein? J. Lipid Res. 2018;59:749–763. doi: 10.1194/jlr.R082313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vedhachalam C., Ghering A.B., Davidson W.S., Lund-Katz S., Rothblat G.H., Phillips M.C. ABCA1-induced cell surface binding sites for ApoA-I. Arterioscler. Thromb. Vasc. Biol. 2007;27:1603–1609. doi: 10.1161/ATVBAHA.107.145789. [DOI] [PubMed] [Google Scholar]
- 83.Gelissen I.C., Harris M., Rye K.-A., Quinn C., Brown A.J., Kockx M., Cartland S., Packianathan M., Kritharides L., Jessup W. ABCA1 and ABCG1 synergize to mediate cholesterol export to apoA-I. Arterioscler. Thromb. Vasc. Biol. 2006;26:534–540. doi: 10.1161/01.ATV.0000200082.58536.e1. [DOI] [PubMed] [Google Scholar]
- 84.Karten B., Campenot R.B., Vance D.E., Vance J.E. Expression of ABCG1, but not ABCA1, correlates with cholesterol release by cerebellar astroglia. J. Biol. Chem. 2006;281:4049–4057. doi: 10.1074/jbc.M508915200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.