Abstract
The genus Anaplasma (Anaplasmataceae, Rickettsiales) includes tick-transmitted bacterial species of importance to both veterinary and human medicine. Apart from the traditionally recognized six Anaplasma species (A. phagocytophilum, A. platys, A. bovis, A. ovis, A. centrale, A. marginale), novel strains and candidate species, also of relevance to veterinary and human medicine, are emerging worldwide. Although species related to the zoonotic A. platys and A. phagocytophilum have been reported in several African and European Mediterranean countries, data on the presence of these species in sub-Saharan countries are still lacking. This manuscript reports the investigation of Anaplasma strains related to zoonotic species in ruminants in Senegal by combining different molecular tests and phylogenetic approaches. The results demonstrated a recent introduction of Candidatus (Ca) Anaplasma turritanum, a species related to the pathogenic A. platys, possibly originating by founder effect. Further, novel undetected strains related to Candidatus (Ca) Anaplasma cinensis were detected in cattle. Based on groEL and gltA molecular comparisons, we propose including these latter strains into the Candidatus (Ca) Anaplasma africanum species. Finally, we also report the emergence of Candidatus (Ca) A. boleense in Senegal. Collectively, results confirm that Anaplasma species diversity is greater than expected and should be further investigated, and that Anaplasma routine diagnostic procedures and epidemiological surveillance should take into account specificity issues raised by the presence of these novel strains, suggesting the use of a One Health approach for the management of Anaplasmataceae in sub-Saharan Africa.
Keywords: obligate intracellular bacteria, Anaplasma diversity, tick-borne diseases, zoonosis, one health
1. Introduction
Bacteria belonging to Anaplasmataceae (Alphaproteobacteria: Rickettsiales) are tick-transmitted, Gram-negative, obligate intracellular bacteria that replicate in both vertebrate and invertebrate host cells [1] and are significantly relevant both to veterinary and public health [2]. According to Dumler and coworkers [1], this family comprises four classified genera (Anaplasma, Ehrlichia, Neorickettsia, and Wolbachia), with the genus Anaplasma currently including six species with variable pathogenicity [1,2,3]. Additionally, novel strains and candidate species [2,4] have been recently recorded (Table 1).
Table 1.
Characteristics of Anaplasma species and unclassified genovariants 1.
| Species | Main Hosts | Pathogenicity | Cell; Tropism |
Primary Vectors | Distribution | Reference |
|---|---|---|---|---|---|---|
| A. phagocytophilum | Mammals | Vertebrates including human | Granulocytes | Ixodes spp. | The Americas, Eurasia, Africa | [1] |
| A. platys | Dog | Cyclic thrombocytopenia in dogs, human infection | Platelets | Rhipicephalus spp. | Worldwide | [1,5,6] |
| A. bovis | Cattle | Bovine anaplasmosis, human infection | Monocytes | Various tick species | South Europe, the Americas, Africa, Asia | [1,7] |
| A. ovis | Sheep | Ovine anaplasmosis, human infection | Erythrocytes | Dermacentor spp., Rhipicephalus spp. | Asia, Africa, Europe, N. America | [8,9] |
| A. marginale | Cattle | Bovine anaplasmosis | Erythrocytes | Various tick species | Worldwide | [1,9] |
| A. centrale | Cattle | Mild anaplasmosis | Erythrocytes | Rhipicephalus spp. | Worldwide | [1,9] |
| Ca A. turritanum | ruminants, cats | Not established | Platelets, granulocytes | Rhipicephalus spp., Haemaphysalis spp. | South Europe, Asia, Africa | [2,10,11] |
| Ca A. cinensis | Cattle | Not established | Not established | R. microplus | Asia, Africa | [2,12] |
| Ca A. odocoilei | Deer | Not established | Platelets | A. americanum, | USA, Mexico | [13] |
| A. capra | Humans, ruminants, dogs | Human infection | Erythrocytes | Various tick species | Europe, Asia | [14,15] |
| A. phagocytophilum like-1 (japan strains) | Sika deer, cattle, goat, sheep | Not established | Not established | Various tick species | Asia, Europe, Africa | [3,16,17] |
|
A. phagocytophilum like-2; (A. boleense) |
Cattle | Not established | Not established | Various tick species | Asia, Africa | [18,19,20] |
1 Genovariants based solely on 16S rRNA, 23S rRNA, and rpoB genes are not considered in this table.
A. phagocytophilum is the most relevant species in terms of animal and human tick-borne diseases within the genus, being the causative agent of ruminant tick-borne fever and granulocytic anaplasmosis of horses, dogs, and humans. Similarly, A. platys and A. marginale are of importance in veterinary medicine, as they cause cyclic thrombocytopenia in dogs and bovine anaplasmosis, respectively.
In addition to A. phagocytophilum, A. platys, A. bovis, A. capra, and A. ovis have been shown to be pathogenic to humans (Table 1).
In the last 10 years, novel Anaplasma strains related to but genetically distinct from the zoonotic A. phagocytophilum and A. platys have been identified worldwide.
In Japan, bacteria phylogenetically clustering in a monophyletic clade distinct from but closely related to A. phagocytophilum have been identified in Haemaphysalis and Ixodes ticks infesting domestic and wild ruminants [17,21], and in China, Anaplasma strains designated as Candidatus (Ca) Anaplasma boleense were detected in Hyalomma asiaticum ticks infesting domestic ruminants [20]. Both the Japanese strains and Ca A. boleense have been identified in the Mediterranean area (Tunisia, Italy), where they were respectively described as A. phagocytophilum-like 2 and A. phagocytophilum-like 1, based on their 16S rRNA and groEL gene sequences [3,22,23]
Based on 16S rRNA, groEL, and gltA, Anaplasma strains related to the canine A. platys have been identified in Sardinian ruminants, cats, and Rhipicephalus ticks. Based on genetic comparisons, these strains were assigned to A. platys-like [2,10,11,24]. The same organism was detected in Tunisian ruminants [25] and in ticks from Costa Rica [26].
In China, novel Anaplasma strains genetically related to A. platys were identified in Rhipicephalus microplus by Guo and colleagues [12]. Recently, upon groEl and gltA comparisons, Mediterranean and Chinese A. platys-like strains were respectively assigned to the two novel, distinct species Candidatus (Ca) Anaplasma turritanum and Ca Anaplasma cinensis [2].
Reports on A. phagocytophilum, A. platys, and related strains are scarce in sub-Saharan Africa. Notably, Dahmani and colleagues [27] recorded the emergence of a potentially new species commonly infecting ruminants in Senegal, and provisionally named it Anaplasma cf. platys by comparison of concatenated 23S rRNA, 16S rRNA, and rpoB genes.
This paper investigates the presence of Anaplasma strains related to the zoonotic A. phagocytophilum and A. platys in Senegal ruminants by combining molecular tools targeting the Anaplasma 16S rRNA, groEL, and gltA genes. Sequencing, molecular typing, and phylogenetic analyses allowed us to demonstrate the emergence of Candidatus A. turritanum in Senegal and to postulate its recent introduction in the Mediterranean area by the founder effect. Moreover, the presence of A. phagocytophilum-like 2 and of novel bovine Anaplasma strains related to Ca A. cinensis is demonstrated for the first time in Senegal. Implications of the emergence of these Anaplasma species in sub-Saharan Africa on diagnostics, diversity, transmission, and public health are also discussed.
2. Results
The use of three distinct PCR tests for the detection of Anaplasma strains related to A. platys, combined with sequencing and Blast analysis (Table 2 and Table 3), allowed us to establish the presence of Ca A. turritanum in 92/176 ruminants (52%). Of these, 83/176 were sheep (47%), and 9/176 (0.05%) were goats, whereas Ca A. turritanum was not detected in bovines. The number of animals testing positive by groEL and gltA PCR were comparable. Sequencing of groEL amplicons obtained from 37 sheep and six goats revealed the presence of a unique sequence type with 98–100% similarity/homology to Ca A. turritanum strains isolated from cats and ruminants in Tunisia [25] and in Italy [10,11]. Similarly, gltA sequencing from 34 sheep and seven goats revealed the presence of a unique sequence type that was 100% homologous to the same ruminant strains.
Table 2.
Positivity of PCR tests specific for Anaplasma strains genetically related to A. phagocytophilum and A. platys.
| Host Species | N 1 | Ca A. turritanum | Cf Ca A. cinensis | A. phagocytophilum Group | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| gltA | groEL | Both gltA; and groEL |
At Least 1 Test | gltA | groEL | 16S rRNA | groEL; A. Phago |
groEL; A. Phago Like 1 |
groEL; A. Phago Like 2 |
||
| Sheep | 134 | 81 | 83 | 81 | 83 | 0 | 0 | 23 | 1 | - | 18 |
| Goat | 28 | 9 | 7 | 6 | 9 | 0 | 0 | - | - | - | - |
| Cattle | 14 | 0 | 0 | 0 | 0 | 3 | 3 | 5 | - | - | 5 |
| TOT | 176 | 90 | 90 | 87 | 92 | 3 | 3 | 28 | 1 | - | 23 |
1 total number of sampled animals.
Table 3.
Assignment of sequence types obtained in this study and similarity to sequences deposited in the GenBank.
| Sequence Type | Gene (Primers Used); Reference | Animal Sources | BlastN | GenBank Accession Number(s) |
|---|---|---|---|---|
| Ovicaprine1 | groEL (EphplgroEL(569)F, EphplgroEL(1193)R, EplgroEL(1084)R); [30] |
37 sheep 6 goats |
98–100% Ca A. turritanum |
OP573342-384 |
| Bovine1 | groEL (EphplgroEL(569)F, EphplgroEL(1193)R, EplgroEL(1084)R); | 1 cattle | 96% Anaplasma sp. 88% Ca A. cinensis |
OP573278 |
| Bovine2 | groEL (EphplgroEL(569)F, EphplgroEL(1193)R, EplgroEL(1084)R); [30] |
1 cattle | 96% Anaplasma sp. 88% Ca A. cinensis |
OP573279 |
| Bovine3 | groEL (EphplgroEL(569)F, EphplgroEL(1193)R; EplgroEL(1084)R); [30] |
1 cattle | 96% Anaplasma sp. 88% Ca A. cinensis |
OP573280 |
| Ovine2 | groEL (EphplgroEL(569)F, EphplgroEL(1193)R; EphgroEL(1142)R); [30] | 1 sheep | 88% A. bovis | OP573277 |
| Ovibovine1 | groEL (APHAGOVAR2GROEL_F, APHAGOVAR2GROEL_R1, APHAGOVAR2GROEL_R2); [3] | 3 Cattle 3 Sheep |
99% Anaplasma sp. 91% A. boleense |
OP573323, OP573327-28, OP573332-33, OP573338 |
| Ovine1 | groEL (APHAGOVAR2GROEL_F, APHAGOVAR2GROEL_R1, APHAGOVAR2GROEL_R2); [3] | 13 Sheep | 99% Anaplasma sp. 91% A. boleense |
OP573324-26, OP573329-31, OP573334-37, OP573339-41 |
| Bovine4 | groEL (APHAGOVAR2GROEL_F, APHAGOVAR2GROEL_R1, APHAGOVAR2GROEL_R2); [3] | 1 Cattle | 99% Anaplasma sp. 91% A. boleense |
OP573322 |
| Ovicaprine2 | gltA (AplaLikeGLTAF1, AplaLikeGLTAR, AplaLikeGLTAF2); [2] | 7 goats | 99–100% Ca A. turritanum | OP573281-321 |
| Bovine5 | gltA (Pglt-F, Pglt-R1, Pglt-R2); [12] | 3 Cattle | 79% Anaplasma spp. 78,56% Ca A. cinensis |
OP654651-53 |
| Ovibovine2 | 16S rRNA (EE1, EE2, SSAP2f, SSAP2r); [22,23] | 4 cattle 15 Sheep |
100% Anaplasma sp. 99.83% A. boleense |
OP546293-94, OP546304-05, OP546296-98, OP546301-03, OP546306, OP546309-11, OP546313-16, OP546318 |
| Ovine3 | 16S rRNA (EE1, EE2, SSAP2f, SSAP2r); [22,23] | 2 sheep | 99.83% Anaplasma sp. 99.66% A. boleense |
OP546312, OP546317 |
| Ovine4 | 16S rRNA (EE1, EE2, SSAP2f, SSAP2r); [22,23] | 2 sheep | 100% A. phagocytophilum like2 |
OP546307, OP546299 |
| Ovine5 | 16S rRNA (EE1, EE2, SSAP2f, SSAP2r); [22,23] | 3 sheep | 100% A. phagocytophilum like2 |
OP546300, OP546295, OP546308 |
Novel Anaplasma strains (Table 2 and Table 3) were also detected in three varieties of cattle by both groEL and gltA PCRs. GroEL sequences were assigned to three distinct sequence types, whereas gltA sequencing generated an invariable sequence for the three animals and therefore a unique sequence type. GroEL sequence types showed a higher level of similarity (Table 3) with Anaplasma spp. isolated in Tunisian cattle [25] and droedaries [28] and with Ca A. cinensis strains isolated in China [12]. The invariable gltA sequence detected in the three animals was consistently more similar (Table 3) to Anaplasma spp. strains isolated in dromedaries in Egypt [29]) and to Ca A. cinensis isolated in China [12].
One ovine sample was unexpectedly positive for groEL semi-nested PCR specific for A. phagocytophilum (Table 3) and resulted in 88% similarity to various A. bovis strains.
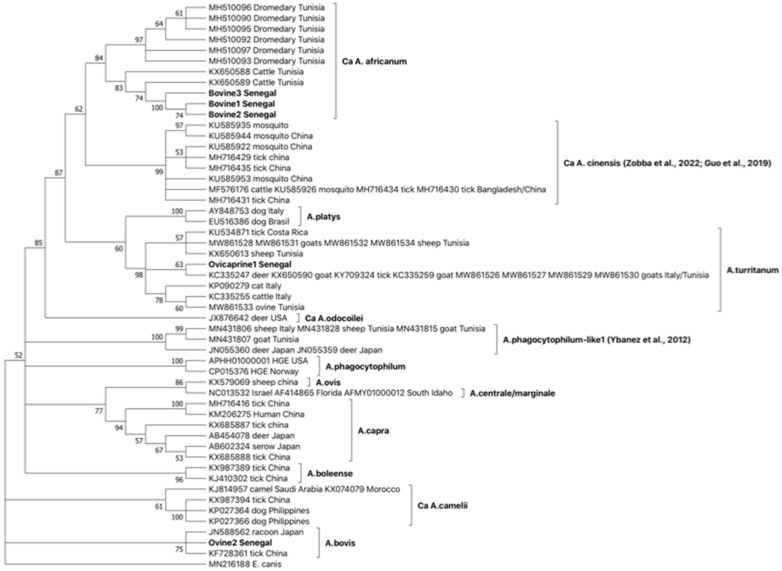
In phylogenetic trees (Figure 1), consistentl with BLASTN comparisons, the groEL invariable sequence type Ovicaprine1 Senegal is included in a statistically supported monophyletic clade together with Ca A. turritanum groEl sequences isolated in ticks and ruminants in Tunisia, Italy, and Costa Rica. This clade is closely related to A. platys and is basal to Ca A. cinensis. Interestingly, the three groEL sequence types Bovine1, Bovine2, and Bovine3 were grouped together in a statistically supported clade, together with groEL sequences isolated in dromedaries and cattle from Tunisia; this clade formed a Ca A. cinensis sister group.
Figure 1.
Phylogeny of the 4 groEL sequence types identified in Senegal and genetically related to A. platys with OTUs selected as representative of the different Anaplasma species and Ehrlichia canis chosen as an outgroup. The bootstrap consensus tree inferred from 1000 replicates is taken to represent the evolutionary history of the taxa analyzed. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together are shown next to the branches. This analysis involved 53 nucleotide sequence types, and there were a total of 405 positions in the final dataset. References in clades are: Ca A. cinensis [2,12], A. phagocytophilum-like1 [17].
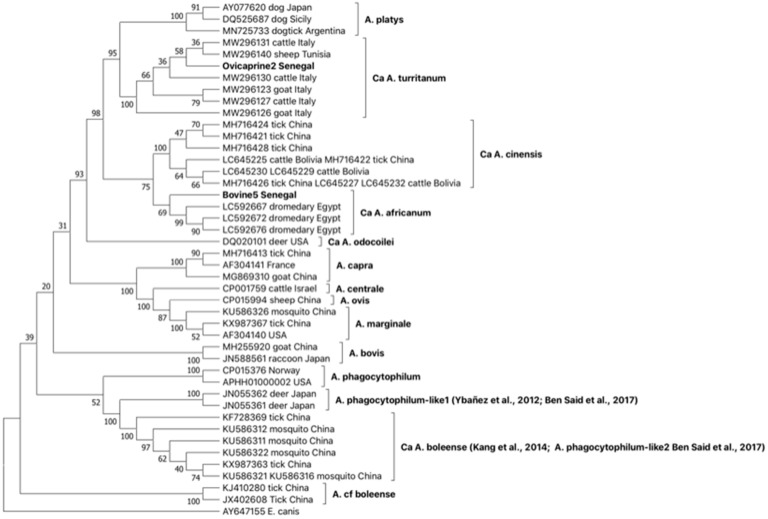
GltA phylogeny confirms that which was observed by groEL (Figure 2). The sequence type Ovicaprine2 Senegal groups with Ca A. turritanum sequences obtained from ruminants in Tunisia and Italy confirmed the circulation of this Anaplasma species in Senegal. Further, the sequence type Bovine5 Senegal, similar to that observed with groEL sequence types obtained from the same animals, is included together with gltA sequences rescued from dromedaries in Egypt in a statistically supported group distinct from but related to Ca A. cinensis.
Figure 2.
Phylogeny of the 2 gltA sequence types identified in Senegal and genetically related to A. platys with OTUs selected as representative of the different Anaplasma species and Ehrlichia canis chosen as an outgroup. The bootstrap consensus tree inferred from 1000 replicates is taken to represent the evolutionary history of the taxa analyzed. The percentage of replicate trees in which the associated taxa clustered together is shown next to the branches. This analysis involved 44 nucleotide sequence types, and there were a total of 483 positions in the final dataset. References in clades are: A. phagocytophilum-like1 [17,23], Ca A. boleense [20,23].
Investigation of strains related to A. phagocytophilum by nested 16S rRNA PCR resulted in 28/176 positive samples (23 sheep and five cattle, Table 2). Sequencing of 16S rRNA PCR products allowed us to assign sequences to four sequence types (Ovibovine2 Senegal, Ovine3 Senegal, Ovine4 Senegal, and Ovine5 Senegal) mostly similar to Ca A. boleense (A. phagocytophilum-like2. Table 2). Consistent with 16S rRNA results, out of the three PCR tests targeting the groEL gene, only the semi-nested PCR targeting the A. phagocytophilum-like2 (Ca A. boleense) groEL gene showed positivity (Table 2). Out of 28 16S rRNA PCR-positive animals, 23 were also positive for groEL (18 sheep and five cattle). Sequencing revealed the circulation of three sequence types; a sequence type rescued exclusively from cattle (Bovine4 Senegal), a sequence type circulating only in sheep (Ovine1 Senegal), and finally a sequence type (Ovibovine1 Senegal) rescued from both cattle and sheep.
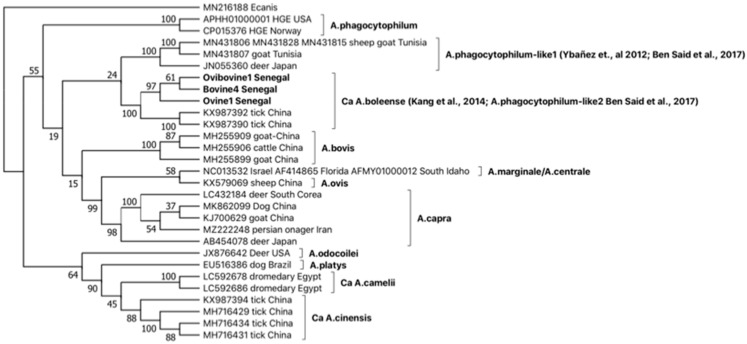
GroEL phylogeny of the three sequence types (Figure 3), together with sequences representative of the different Anaplasma strains, placed Ovine1 Senegal, Bovine4 Senegal, and Ovibovine Senegal in a monophyletic clade, including Ca A. boleense sequences isolated in Chinese ticks, consistent with 16S rRNA and groEL BLASTN results.
Figure 3.
Phylogeny of groEL sequence types identified in Senegal genetically related to A. phagocytophilum with OTUs selected as representative of the different Anaplasma species and Ehrlichia canis chosen as an outgroup. The bootstrap consensus tree inferred from 1000 replicates is taken to represent the evolutionary history of the taxa analyzed. The percentage of replicate trees in which the associated taxa clustered together is shown next to the branches. This analysis involved 29 nucleotide sequence types, and there were a total of 667 positions in the final dataset. References in clades are: A. phagocytophilum-like1 [17,23], Ca A. boleense [20,23].
3. Discussion
Apart from the six widely recognized species included in the genus Anaplasma, novel strains and candidate species have been reported in the last 10 years worldwide. The emergence of these novel strains related to A. phagocytophilum and A. platys, together with the acknowledgment of A. platys and A. capra as zoonotic agents, has raised concerns in both veterinary and public health.
Among species related to A. platys, Ca A. turritanum was recently identified and described in several European and African Mediterranean Countries [2]. Reports of these strains in African sub-Saharan countries are still lacking.
This study reports the emergence of Ca A. turritanum in a sub-Saharan African country (Senegal). By considering cumulative positivity to both groEL and gltA PCR, Ca A. turritanum was detected in some 50% of the tested ruminants from Senegal, with a prevalence consistent with values previously reported in the Mediterranean area [10]. Ca A. turritanum prevalence seems higher in sheep (83/134, 62%) with respect to goats (9/28, 32%), although it should be taken into consideration that the number of sampled animals was much lower in goats than in sheep. Further, Ca A. turritanum was not detected in bovines. Cohen’s kappa coefficient (κ) was 0.97, indicating almost perfect agreement between Ca A. turritanum groEL and gltA pcr tests and validating their use in the diagnostics routine.
Considering groEL and gltA results obtained in this study, Anaplasma cf. platys strains previously identified in Senegal by Dahamani and coworkers [27] by comparing concatenated 23S rRNA, 16S rRNA, and the rpoB genes in the same animals should be assigned to the Ca A. turritanum species.
In spite of a high prevalence of Ca A. turritanum in Senegal, ruminants of both gltA and groEL sequencing resulted in a single sequence type circulating in sheep and goats from Senegal; taking into account the high genetic variability of Ca A. turritanum in Italian and Tunisian ruminants [2], we postulate that Ca A. turritanum originated in the Mediterranean area, and that its expansion to Sub-Saharan Countries was hampered by the Sahara Desert, which worked as a natural barrier to hosts’ and vectors’ diffusion and contact. Under this scenario, the absence of genetic variability of Ca A. turritanum in Senegal could be explained by its recent introduction and by the founder effect possibly resulting from the ingress of a single Ca A. turritanum strain in this country, for instance, through zootechnical practices or carried by ticks transported by migratory birds.
Furthermore, based on gltA and groEL comparisons (Figure 1 and Figure 2), the emergence of novel Anaplasma strains forming a monophyletic clade closely related to the recently described Ca A. cinensis [2,12] is also reported in Senegalese cattle. Considering groEL and gltA philogenies, we propose assigning these latter novel strains to the novel species Ca A. africanum. The Ca A. africanum clade includes strains previously described in dromedary camels from Egypt and reported as Anaplasma sp. [29], in dromedaries from Tunisia, deposited in the GenBank under A. platys-like designation [28], and in cattle from Tunisia [25]. Finally, the emergence of strains belonging to Ca A. boleeense is demonstrated in Senegal by the identification of three groEL sequence types in ruminants (Table 3), included in a monophyletic cluster together with strains rescued from ticks in China (Figure 3).
The identification of new Anaplasma strains related to A. platys and A. phagocytophilum (pathogenic to both animals and humans) raises concerns in the management of these infections in sub-Saharan Africa and points to the importance of a One Health approach in taking actions in order to establish geographical distribution, host and vector tropism, and pathogenicity of these novel strains. Further, for some of them, the acknowledgment of their zoonotic potential (e.g., A. capra) reinforces the hypothesis that Anaplasma diversity and the number of potential zoonotic species included in this genus could be greater than that suspected in the past, and it could justify more effort in investigating the presence of possible emerging novel strains worldwide.
In conclusion, we recommend that these novel strains should be included in the diagnostic routine and epidemiological surveillance of tick-transmitted pathogens. Finally, serological and molecular diagnostic tools and past data should be reconsidered in light of the possibility of coinfection with traditional Anaplasma species, routinely diagnosed in the past, and these novel genetically related strains.
4. Materials and Methods
4.1. Ethics Approval and Consent to Participate
Animal blood samples were collected by veterinarians according to good practice and following Senegalese regulations with the agreement of owners.
4.2. Samples Collection and DNA Extractions
A total of 176 EDTA blood samples were used in this study. Blood was collected from 176 clinically healthy ruminants (Table 2) in June 2014 in the Keur Momar Sarr Senegal region (15°55′0.0012″ N, 15°58′0.0012″ W). Samples were stored at −20 °C until use. After thawing (before DNA extraction), blood samples were split into 100µL aliquots. DNA was extracted from 100 µL blood aliquots with the DNeasy Blood and Tissue Kit (Qiagen, Milano, Italy) according to vendor instructions.
4.3. PCR Strategies
The presence of species related to A. phagocytophilum and A. platys in samples was investigated using different PCR tests targeting the 16S rRNA and groEL and gltA genes (Table 2 and Table 3). To investigate strains related to A. platys, 3 different PCR tests were used: (1) a semi-nested PCR targeting 515 bp of the Ca A. turritanum groeEL gene [30,31]; (2) a semi-nested PCR targeting 947 bp of Ca A. turritanum gltA gene [2]; (3) a semi-nested PCR targeting 660 bp of the gltA gene of species related A. platys [12]. To investigate strains related to A. phagocytophilum, samples were initially screened with a nested PCR test [22,23] specific to the 16S rRNA gene of the A. phagocytophilum group (A. phagocytophilum, A. phagocytophilum-like1 (Japanese strains)), A. phagocytophilum-like2 (Ca A. boleense). Samples positive for 16S rRNA PCR were screened with 3 additional PCR tests (Table 3): (1) a semi-nested PCR targeting 573 bp of the A. phagocytophilum groEL gene [30,31]; (2) a nested PCR targeting 1446 bp of the A. phagocytophilum-like1 (Japanese strains) groEL gene [17]; (3) a semi-nested PCR targeting 792 bp of the A. phagocytophilum-like2 (Ca A. boleense) groEL gene [3].
For all PCR tests, cycling conditions and mixing were as described in the original papers.
4.4. Sequencing, Sequence Types Assignment, and Phylogenetic Analyses
Amplicons were purified by using the DNA Clean and Concentrator kit (Zymo Research, Milano, Italy), according to the manufacturer’s instruction. DNA samples’ quality and quantity were assessed using the Nano Drop (Eppendorf, Milano, Italy). PCR products were automatically sequenced (BMR Genomics, Padova, Italy) on both strands. Chromatograms were edited with Chromas 2.2 (Technelysium, Helensvale, Australia), and sequences were aligned with Clustal X version 2.0 [32]. All sequences obtained in this study were deposited in the GenBank database (accession numbers are shown in Table 3).
Sequences were assigned to unique sequence types named after the host and identified by progressive numbers. Sequence types were challenged against the GenBank database with standard nucleotide BLAST (BlastN; https://blast.ncbi.nlm.nih.gov, accessed on 1 September 2022). Sequence types were also used as operational taxonomic units (OTUs) in phylogenetic analyses.
In particular, groEL sequence types obtained in this study by 2 semi-nested PCRs (Table 3) were aligned with 48 unique groEL sequences representative of Anaplasma species identified in ticks and vertebrate hosts worldwide. Alignment was inputted in MEGA11 [33] to reconstruct OTUs phylogeny. Phylogenetic analysis was inferred using the maximum likelihood method based on the Tamura 3-parameter model [34], identified as the best model by MEGA11. The robustness of trees was evaluated by bootstrapping over 1000 reiterations [35]. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Tamura 3-parameter model and then selecting the topology with a superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 1.3501)). This analysis involved 53 nucleotide sequences. There were 405 positions in the final dataset.
Furthermore, gltA sequence types obtained in this study by 2 semi-nested PCRs (Table 3) were aligned with 42 unique gltA sequences representative of Anaplasma species identified in ticks and vertebrate hosts worldwide. Alignment was inputted in MEGA11. Phylogeny was reconstructed using the maximum likelihood method based on the Tamura 3-parameter model and evaluated by bootstrapping over 1000 replicates. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the Tamura 3-parameter model and then selecting the topology with a superior log likelihood value. A discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 2.3476)). This analysis involved 44 nucleotide sequences. There were 483 positions in the final dataset.
Finally, groEL sequence types obtained in this study by semi-nested PCR (Table 3) were aligned to 26 unique groEL sequences representative of Anaplasma species identified in ticks and vertebrate hosts worldwide. As above, trees were reconstructed using the maximum likelihood method based on the Tamura 3-parameter model and evaluated by bootstrapping. In this case, a discrete Gamma distribution was used to model evolutionary rate differences among sites (5 categories (+G, parameter = 0.5603)). The analysis involved 29 nucleotide sequences, and there were 667 positions in the final dataset.
Author Contributions
Conceptualization, A.A.; Methodology, A.A. and R.Z.; Validation, A.A., R.Z., and B.D.; Formal Analysis, R.Z. and A.A; Investigation, R.Z., A.A., C.M., E.S., R.P., M.D., O.M., and B.D.; Data Curation, A.A, B.D., A.S., and R.Z.; Writing—Original Draft Preparation, A.A.; Writing—Review and Editing, R.Z., C.M., M.D., O.M., B.D., R.P., E.S., A.S., M.P., and A.A.; Supervision, A.A.; Funding Acquisition, A.A. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the University of Sassari, grant entitled “Fondo Ateneo per la Ricerca” (FAR), anno 2020.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dumler J.S., Barbet A.F., Bekker C.P.J., Dasch G.A., Palmer G.H., Ray S.C., Rikihisa Y., Rurangirwa F.R. Reorganization of Genera in the Families Rickettsiaceae and Anaplasmataceae in the Order Rickettsiales: Unification of Some Species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, Descriptions of Six New Species Combi. Int. J. Syst. Evol. Microbiol. 2001;51:2145–2165. doi: 10.1099/00207713-51-6-2145. [DOI] [PubMed] [Google Scholar]
- 2.Zobba R., Schianchi E., Ben Said M., Belkahia H., Messadi L., Piredda R., Pittau M., Alberti A. GltA Typing of Anaplasma Strains Related to A. platys: Taxonomical and One Health Implications. Ticks Tick. Borne. Dis. 2022;13:101850. doi: 10.1016/j.ttbdis.2021.101850. [DOI] [PubMed] [Google Scholar]
- 3.Zobba R., Ben Said M., Belkahia H., Pittau M., Cacciotto C., Pinna Parpaglia M.L., Messadi L., Alberti A. Molecular Epidemiology of Anaplasma Spp. Related to A. phagocytophilum in Mediterranean Small Ruminants. Acta Trop. 2020;202:105286. doi: 10.1016/j.actatropica.2019.105286. [DOI] [PubMed] [Google Scholar]
- 4.Rar V., Golovljova I. Anaplasma, Ehrlichia, and “ Candidatus Neoehrlichia” Bacteria: Pathogenicity, Biodiversity, and Molecular Genetic Characteristics, a Review. Infect. Genet. Evol. 2011;11:1842–1861. doi: 10.1016/j.meegid.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 5.Maggi R.G., Mascarelli P.E., Havenga L.N., Naidoo V., Breitschwerdt E.B. Co-Infection with Anaplasma platys, Bartonella henselae and Candidatus Mycoplasma haematoparvum in a Veterinarian. Parasit. Vectors. 2013;6:103. doi: 10.1186/1756-3305-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arraga-Alvarado C.M., Qurollo B.A., Parra O.C., Berrueta M.A., Hegarty B.C., Breitschwerdt E.B. Case Report: Molecular Evidence of Anaplasma platys Infection in Two Women from Venezuela. Am. J. Trop. Med. Hyg. 2014;91:1161–1165. doi: 10.4269/ajtmh.14-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu M., Chen Q., Qin X., Lyu Y., Teng Z., Li K., Yu L., Jin X., Chang H., Wang W., et al. Anaplasma bovis Infection in Fever and Thrombocytopenia Patients—Anhui Province, China, 2021. China CDC Wkly. 2022;4:249–253. doi: 10.46234/ccdcw2022.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chochlakis D., Ioannou I., Tselentis Y., Psaroulaki A. Human Anaplasmosis and Anaplasma ovis Variant. Emerg. Infect. Dis. 2010;16:1031–1032. doi: 10.3201/eid1606.090175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rar V., Tkachev S., Tikunova N. Genetic Diversity of Anaplasma Bacteria: Twenty Years Later. Infect. Genet. Evol. 2021;91:104833. doi: 10.1016/j.meegid.2021.104833. [DOI] [PubMed] [Google Scholar]
- 10.Zobba R., Anfossi A.G., Parpaglia M.L.P., Dore G.M., Chessa B., Spezzigu A., Rocca S., Visco S., Pittau M., Alberti A. Molecular Investigation and Phylogeny of Anaplasma spp. in Mediterranean Ruminants Reveal the Presence of Neutrophil-Tropic Strains Closely Related to A. platys. Appl. Environ. Microbiol. 2014;80:271–280. doi: 10.1128/AEM.03129-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zobba R., Anfossi A.G., Visco S., Sotgiu F., Dedola C., Pinna Parpaglia M.L., Battilani M., Pittau M., Alberti A. Cell Tropism and Molecular Epidemiology of Anaplasma platys-like Strains in Cats. Ticks Tick. Borne. Dis. 2015;6:272–280. doi: 10.1016/j.ttbdis.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Guo W.P., Zhang B., Wang Y.H., Xu G., Wang X., Ni X., Zhou E.M. Molecular Identification and Characterization of Anaplasma capra and Anaplasma platys- like in Rhipicephalus microplus in Ankang, Northwest China. BMC Infect. Dis. 2019;19:434. doi: 10.1186/s12879-019-4075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tate C.M., Howerth E.W., Mead D.G., Dugan V.G., Luttrell M.P., Sahora A.I., Munderloh U.G., Davidson W.R., Yabsley M.J. Anaplasma odocoilei Sp. Nov. (Family Anaplasmataceae) from White-Tailed Deer (Odocoileus Virginianus) Ticks Tick Borne Dis. 2013;4:110–119. doi: 10.1016/j.ttbdis.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H., Zheng Y.C., Ma L., Jia N., Jiang B.G., Jiang R.R., Huo Q.B., Wang Y.W., Liu H.B., Chu Y.L., et al. Human Infection with a Novel Tick-Borne Anaplasma species in China: A Surveillance Study. Lancet Infect. Dis. 2015;15:663–670. doi: 10.1016/S1473-3099(15)70051-4. [DOI] [PubMed] [Google Scholar]
- 15.Jouglin M., Blanc B., de la Cotte N., Bastian S., Ortiz K., Malandrin L. First Detection and Molecular Identification of the Zoonotic Anaplasma capra in Deer in France. PLoS ONE. 2019;14:e0219184. doi: 10.1371/journal.pone.0219184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seo M.G., Ouh I.O., Kwon O.D., Kwak D. Molecular Detection of Anaplasma phagocytophilum-like Anaplasma spp. and Pathogenic A. phagocytophilum in Cattle from South Korea. Mol. Phylogenet. Evol. 2018;126:23–30. doi: 10.1016/j.ympev.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Ybañez A.P., Matsumoto K., Kishimoto T., Inokuma H. Molecular Analyses of a Potentially Novel Anaplasma species Closely Related to Anaplasma phagocytophilum Detected in Sika Deer (Cervus Nippon Yesoensis) in Japan. Vet. Microbiol. 2012;157:232–236. doi: 10.1016/j.vetmic.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 18.de Jesus Fernandes S., Matos C.A., Freschi C.R., de Souza Ramos I.A., Machado R.Z., André M.R. Diversity of Anaplasma species in Cattle in Mozambique. Ticks Tick Borne Dis. 2019;10:651–664. doi: 10.1016/j.ttbdis.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Guo W.P., Tian J.H., Lin X.D., Ni X.B., Chen X.P., Liao Y., Yang S.Y., Dumler J.S., Holmes E.C., Zhang Y.Z. Extensive Genetic Diversity of Rickettsiales Bacteria in Multiple Mosquito Species. Sci. Rep. 2016;6:38770. doi: 10.1038/srep38770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang Y.J., Diao X.N., Zhao G.Y., Chen M.H., Xiong Y., Shi M., Fu W.M., Guo Y.J., Pan B., Chen X.P., et al. Extensive Diversity of Rickettsiales Bacteria in Two Species of Ticks from China and the Evolution of the Rickettsiales. BMC Evol. Biol. 2014;14:167. doi: 10.1186/s12862-014-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yoshimoto K., Matsuyama Y., Matsuda H., Sakamoto L., Matsumoto K., Yokoyama N., Inokuma H. Detection of Anaplasma bovis and Anaplasma phagocytophilum DNA from Haemaphysalis Megaspinosa in Hokkaido, Japan. Vet. Parasitol. 2010;168:170–172. doi: 10.1016/j.vetpar.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Ben Said M., Belkahia H., Alberti A., Zobba R., Bousrih M., Yahiaoui M., Daaloul-Jedidi M., Mamlouk A., Gharbi M., Messadi L. Molecular Survey of Anaplasm a species in Small Ruminants Reveals the Presence of Novel Strains Closely Related to A. phagocytophilum in Tunisia. Vector-Borne Zoonotic Dis. 2015;15:580–590. doi: 10.1089/vbz.2015.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben Said M., Belkahia H., El Mabrouk N., Saidani M., Ben Hassen M., Alberti A., Zobba R., Bouattour S., Bouattour A., Messadi L. Molecular Typing and Diagnosis of Anaplasma spp. Closely Related to Anaplasma phagocytophilum in Ruminants from Tunisia. Ticks Tick Borne Dis. 2017;8:412–422. doi: 10.1016/j.ttbdis.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Chisu V., Zobba R., Lecis R., Sotgiu F., Masala G., Foxi C., Pisu D., Alberti A. GroEL Typing and Phylogeny of Anaplasma species in Ticks from Domestic and Wild Vertebrates. Ticks Tick Borne Dis. 2018;9:31–36. doi: 10.1016/j.ttbdis.2017.10.012. [DOI] [PubMed] [Google Scholar]
- 25.Ben Said M., Belkahia H., El Mabrouk N., Saidani M., Alberti A., Zobba R., Cherif A., Mahjoub T., Bouattour A., Messadi L. Anaplasma platys-like Strains in Ruminants from Tunisia. Infect. Genet. Evol. 2017;49:226–233. doi: 10.1016/j.meegid.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Campos-Calderón L., Ábrego-Sánchez L., Solórzano-Morales A., Alberti A., Tore G., Zobba R., Jiménez-Rocha A.E., Dolz G. Molecular Detection and Identification of Rickettsiales Pathogens in Dog Ticks from Costa Rica. Ticks Tick. Borne. Dis. 2016;7:1198–1202. doi: 10.1016/j.ttbdis.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Dahmani M., Davoust B., Sambou M., Bassene H., Scandola P., Ameur T., Raoult D., Fenollar F., Mediannikov O. Molecular Investigation and Phylogeny of Species of the Anaplasmataceae Infecting Animals and Ticks in Senegal. Parasit. Vectors. 2019;12:495. doi: 10.1186/s13071-019-3742-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selmi R., Ben Said M., Dhibi M., Ben Yahia H., Messadi L. Improving Specific Detection and Updating Phylogenetic Data Related to Anaplasma platys-like Strains Infecting Camels (Camelus Dromedarius) and Their Ticks. Ticks Tick Borne Dis. 2019;10:1–13. doi: 10.1016/j.ttbdis.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Mohamed W.M.A., Ali A.O., Mahmoud H.Y.A.H., Omar M.A., Chatanga E., Salim B., Naguib D., Anders J.L., Nonaka N., Moustafa M.A.M., et al. Exploring Prokaryotic and Eukaryotic Microbiomes Helps in Detecting Tick-Borne Infectious Agents in the Blood of Camels. Pathogens. 2021;10:351. doi: 10.3390/pathogens10030351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alberti A., Zobba R., Chessa B., Addis M.F., Sparagano O., Parpaglia M.L.P., Cubeddu T., Pintori G., Pittau M. Equine and Canine Anaplasma phagocytophilum Strains Isolated on the Island of Sardinia (Italy) Are Phylogenetically Related to Pathogenic Strains from the United States. Appl. Environ. Microbiol. 2005;71:6418–6422. doi: 10.1128/AEM.71.10.6418-6422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alberti A., Addis M.F., Sparagano O., Zobba R., Chessa B., Cubeddu T., Parpaglia M.L.P., Ardu M., Pittau M. Anaplasma phagocytophilum, Sardinia, Italy. Emerg. Infect. Dis. 2005;11:1322–1324. doi: 10.3201/eid1108.050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larkin M.A., Blackshields G., Brown N.P., Chenna R., Mcgettigan P.A., Mcwilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X Version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 33.Tamura K., Stecher G., Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamura K. Estimation of the Number of Nucleotide Substitutions When There Are Strong Transition-Transversion and G+C-Content Biases. Mol. Biol. Evol. 1992;9:678–687. doi: 10.1093/oxfordjournals.molbev.a040752. [DOI] [PubMed] [Google Scholar]
- 35.Felsenstein J. Confidence Limits on Phylogenies: An Approach Using the Bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.