Abstract
Caffeic acid belongs to the polyphenol compounds we consume daily, often in the form of coffee. Even though it is less explored than caffeic acid phenethyl ester, it still has many positive effects on human health. Caffeic acid can affect cancer, diabetes, atherosclerosis, Alzheimer’s disease, or bacterial and viral infections. This review focuses on the molecular mechanisms of how caffeic acid achieves its effects.
Keywords: caffeic acid, cancer, diabetes, obesity, atherosclerosis, Alzheimer’s disease
1. Introduction
When we drink coffee (even the one without caffeine) or red wine, we consume a molecule with highly diverse and interesting effects on our health: a natural polyphenolic compound called caffeic acid. A heavy coffee drinker can consume up to 500 mg of caffeic acid per day; people who do not drink coffee consume up to 25 mg of caffeic acid [1]. However, coffees (and red wine) are not the only sources of caffeic acid in our diet. Many other plant products contain caffeic acid, including apples, plums, lingonberries, black chokeberries, and many herbs of the mint family, e.g., sage, thyme, oregano, marjoram, oregano, or spearmint [1]. Black chokeberries seem to be the most potent source of caffeic acid (645 mg/100 g of dry weight). In comparison, the caffeic acid content in coffee ranges from 9 to 14 mg/100 g [2] or up to 87 mg/100 g, according to [1]. Other sources of caffeic acid are its naturally occurring esters: chlorogenic acid [3], rosmarinic acid [4], and caffeic acid phenethyl ester [5].
Unlike the information about caffeic acid content in various food, data about caffeic acid plasma levels in humans are scarce. It seems that both caffeic acid absorption and metabolism are fast [6], and 1 h after consuming 300 mL of red wine, the caffeic acid level reached a concentration of 28 nM [7].
The structure of caffeic acid (aromatic core, conjugated double bond, and hydroxyl groups) allows it to function as an antioxidant, but its effects are far from limited only to that. The published data show effects on various types of cancers, diabetes, obesity, and neurodegenerative diseases like Alzheimer’s or Parkinson’s. This review focuses on the mechanisms of those caffeic acid effects.
2. Caffeic Acid as an Antioxidant
The antioxidant effects of caffeic acid play an essential role in many beneficial effects on human health. Khan and coworkers summarized the antioxidant effects of caffeic acid against various types of free radicals supremely [8]. Therefore, we will mention the antioxidant (and prooxidant) effects of caffeic acid only briefly.
Caffeic acid consists of an aromatic core substituted in position 1 with an unsaturated three-carbon chain containing a carboxylic group and in positions 4 and 5 with two hydroxyl groups. It belongs to the so-called hydroxycinnamic acid group: aromatic acids with a C6–C3 skeleton. Caffeic acid’s structure represents an effective trap for radicals; the combination of an aromatic core with a conjugated side chain (Figure 1) allows for an easy delocalization of unpaired electrons. By giving hydrogen to quench the radicals, caffeic acid serves as a primary antioxidant [9]. The hydroxyl group in the paraposition towards the side chain stabilizes free electrons even better. Another way how caffeic acid works as an antioxidant is by chelating the metals with its two hydroxyl groups. Metal ions decompose peroxide into free radicals. By preventing them from doing it, caffeic acid functions as a secondary antioxidant [9].
Figure 1.
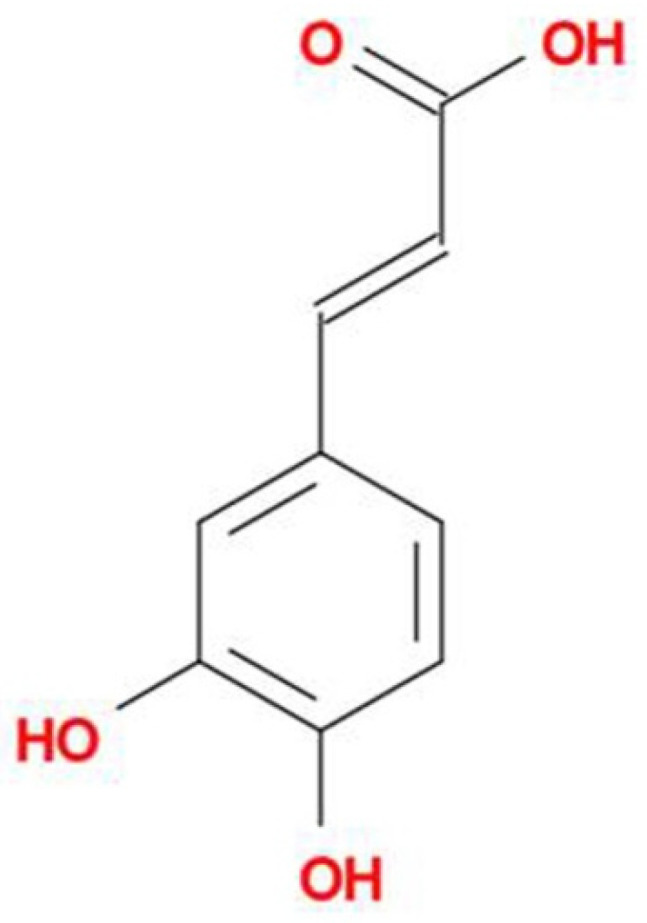
Chemical structure of caffeic acid.
Nevertheless, the chelating ability of caffeic acid is also responsible for its occasional pro-oxidant ability. After chelating Cu2+, the Cu2+ can be reduced to Cu+. That leads to a cascade of reactions, which produce, among others, superoxide radicals and hydroxyl radicals [10]. A large amount of endogenous copper in the human body occurs in, e.g., lymphocytes. Therefore, the combination of caffeic acid and endogenous copper ions can result in oxidative damage, e.g., DNA breaks [11].
Caffeic acid also prevents the formation of reactive oxygen species (ROS) by inhibiting 5-lipoxygenase. This enzyme turns arachidonic acid into leukotrienes and participates in forming ROS [12].
3. Caffeic Acid and Cancer
Multiple studies exist that describe the antiproliferative effect of caffeic acid against various types of cancer cells. Caffeic acid can affect cancer cells alone or in combination with anticancer drugs, which could decrease the anticancer drug dose or help prevent or overcome resistance against those drugs.
3.1. Cancer Prevention
Cooking meat, especially well-done meat, forms heterocyclic amines [13], compounds that act as mutagens and carcinogens [14]. Caffeic acid can inhibit the synthesis of some of them, e.g., PhIP (2-amino-1-methyl-6-phenyl-imidazo[4,5-b]pyridine), which occurs in heated protein-rich food [15]. Caffeic acid probably reacts with phenylacetaldehyde, an intermediate product in PhIP synthesis [16]. Caffeic acid also increased the efflux of PhIP into the intestine lumen by upregulation of ABC transporters p-glycoprotein and breast cancer resistance protein (BCRP) in the apical membrane of the intestine cells [17].
3.2. Liver Cancer
Liver cancer is the sixth most common cancer in the world [18], and hepatocellular carcinoma represents the most-diagnosed type among liver cancer cases [19]. Besides chronic hepatitis B and hepatitis C virus infection, its risk factors also include obesity, tobacco, and alcohol usage, and its incidence is generally increasing [19]. One reason for this cancer type’s relatively high morality is that hepatocellular carcinoma responds poorly to treatment due to its high vascularization [20]. The key molecule responsible for angiogenesis in hepatocellular carcinoma cells is a vascular endothelial growth factor (VEGF);both hypoxia-inducible factor 1α (HIF-1α) and pro-inflammatory NF-κB upregulate the expression of VEGF [21,22]. In hepatocellular carcinoma HepG2 and HCC97Hcells, caffeic acid (20 µM) reducedJNK-1-mediated stabilization of HIF-1α and, in this way, decreased the level of active HIF-1α available [21]. In HepG2 cells, caffeic acid (100 µM) inhibited the activity of NF-κB/IL-6/STAT3 signaling, which decreased the expression of VEGF [23]. It also inhibited another downstream product of NF-κB: matrix metalloproteinase 9 (MM-9), which promotes tumor invasiveness and metastases [20,24]. By reducing the expression of both VEGF and MM-9, caffeic acid acted as a potent anti-tumor agent against hepatocellular carcinoma cells. According to Yang and coworkers [25], caffeic acid (20 μM) also decreased the expression of mortalin(mitochondrial 70 kDa heat shock protein), which is an upstream inducer of PI3kB, NF-kB, and VEGF signaling. They observed those effects in three hepatocellular cell lines (HepG2 cells, Hep3Bcells, and sorafenib-resistant HuH7 cells). In hepatocellular carcinoma WCH-17A cells, a higher concentration of caffeic acid(1 mM) blocked proliferation and induced apoptosis by disrupting mitochondrial potential [26]. In rat hepatoma N1-S1 cells, caffeic acid (1 mM) inhibited lactate efflux and, in this way, decreased the effectiveness of anaerobic metabolism [27]. Concerning in vivo experiments, in rats with hepatocellular carcinoma induced by diethylnitrosamine, caffeic acid (100 mg/kg) reduced the histopathological changes and normalized levels of alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bile acid, total cholesterol, HDL and LDL [28]. To summarize, the primary way how caffeic acid affects hepatocellular carcinoma in vitro is inhibiting VEGF expression and upstream pathways (Table 1); in vivo it positively affects hepatic function and reduces histopathological changes.
Table 1.
Altered protein and mRNA expression in various types of cancer cells when exposed to caffeic acid.
| Caffeic Acid | ||
|---|---|---|
| hepatocellular carcinoma | ||
| HepG2, HCC97H | 20 μM | ↓HIF-1α |
| HepG2 | 100 μM | ↓NF-κB/IL-6/STAT3 |
| ↓VEGF, MM-9 | ||
| HepG2, Hep3B, sorafenib-resistant HuH7 | 20 μM | ↓mortalin |
| breast cancer | ||
| MCF7 | 50 μM | ↓ER, PKB/Akt |
| ↓IGF-1R | ||
| MCF7 | 171 μg/mL | ↑p21 mRNA |
| ↑MCL1 mRNA | ||
| skin cancer | ||
| human dermal fibroblasts and mouse skin | 40 μM | ↑XPC, XPA, PTEN |
| ↑TFIIH-p44, ERCC1 | ||
| squamous cell carcinoma | 15 mg/kg | ↓iNOS, VEGF |
| ↑p53 | ||
| A431, SK-MEL-5, SK-MEL-28 | 40 μM | ↓ERK1/2 |
| HaCaT | 100 μM | ↓NF-kB/Snail |
| lung cancer | ||
| H1299 cells and H1299-xenografts | 100 μM | ↑Bid, Bax |
| (with paclitaxel) | ↑ cas-3/7, cas-9 | |
| ↑p-JNK, p-ERK1/2 | ||
| A549 | 100 μM | ↑survivin, Bcl-2 |
| LA-795 | 60 μM | ↓p-MEK1/2, p-ERK1/2 |
| ↓cyclin D, vimentin | ||
| ↓beta-catenin | ||
| ↓TMEM16A | ||
| oral cancer | ||
| CAL-27 | 65 μg/mL | ↑p53 |
| ↑PRODH | ||
| cervical cancer | ||
| HTB-34 (ATCC-CRL1550) | 100 μM | ↑AMPK, GLUT1 |
| ↓ACLY, SCD1, ELOVL6 | ||
| SiHa | 100 μM | ↑AMPK |
| ↓ACC1, SREPB1c | ||
| ↑ACLY, ELOVL6 | ||
| C-4I | 100 μM | ↑E-cadherin |
| ↓vimentin | ||
| ↑TIMP-1 and -2 mRNA |
3.3. Breast Cancer
The effects of caffeic acid on breast cancer cells are less described than those of caffeic acid phenethyl ester, and the information about their mechanism is scarce. Breast cancer is the most commonly diagnosed cancer in women [29] and the most common cancer overall [30,31]. The presence or absence of estrogen receptors, progesterone receptors, and receptors for an endothelial growth factor (HER2) plays a significant role in breast cancer therapy and survival [32].
In ER-positive breast cancer cell line MCF7, caffeic acid acted as an antiestrogen [33]; it downregulated the expression of estrogen receptor (ER), insulin-like growth factor 1 (IGF-1) receptor, and the level of activated PKB/Akt kinase, as well as suppressed the growth of cells. ER, IGF1 receptor and PKB/Akt participate in growth regulation pathways in estrogen-sensitive breast cancer cells [33]. In the ER-negative breast cancer cell line MDA-MB-231, the effect of caffeic acid was less prominent [33]. The same study also associated a moderate to high consumption of coffee with a lower breast cancer invasiveness in vivo [33]. In another study using ER-positive MCF7 cells, caffeic acid (171 μg/mL) stimulated the expression of the p21 gene (CDKN1A) [34]; the protein product of this gene arrests the cell cycle. Nevertheless, caffeic acid also stimulated the gene expression of a gene encoding anti-apoptotic protein MCL1 (myeloid leukemia cell differentiation protein) [34], which is not desirable when treating cancer cells. In a triple-negative MDA-MB-231 breast adenocarcinoma line, caffeic acid (50 μM)decreased the migration ability of cancer cells [35,36].
To summarize, caffeic acid inhibits estrogen receptor expression and PKB/Akt signaling in ER-positive cell lines (Table 1); in ER-negative cell lines, the antiproliferative effect is less prominent.
3.4. Skin Cancer
The major risk for skin cancer development is the skin’s exposure to UV light. Besides melanomas, skin cancers include non-melanoma skin cancers, e.g., basal cell carcinoma or squamous cell carcinoma.
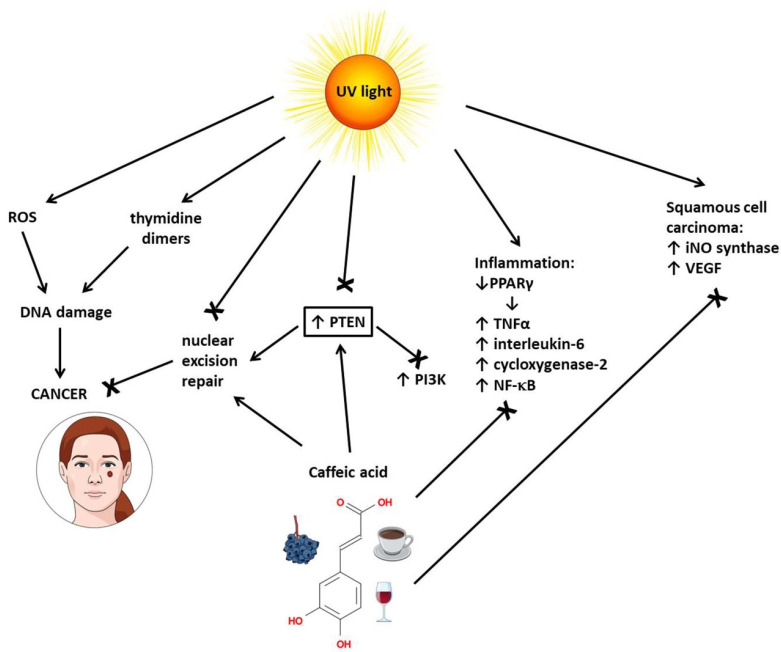
Caffeic acid protects the skin against cancer on multiple fronts (Figure 2). In the skin, UV light forms ROS that can break the sugar–phosphate spine of DNA [29]. By scavenging ROS, caffeic acid protects DNA against breakage [37]. UV light also forms thymidine dimers in the DNA strand; to repair thymidine dimers, the cell employs a repairing mechanism called nuclear excision repair [29]. In human dermal fibroblasts and mouse skin, caffeic acid (40 μM) prevented the UVB-induced the loss of proteins necessary for nuclear excision repair: xeroderma pigmentosum protein C (XPC), general transcription factor IIH subunit (TFIIH-p44), xeroderma pigmentosum protein A (XPA), and excision repair cross-complementation group 1 (ERCC1), as well as the loss of PTEN [37]. PTEN inhibits the PI3K/Akt signaling pathway, which is often constitutively active in skin cancer cells due to mutations. Additionally, PTEN is necessary for nuclear excision repair [38]. In Swiss albino mice, the pretreatment with caffeic acid (15 mg/kg) prevented UVB light-induced inflammation. Caffeic acid decreased tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), cyclooxygenase-2 (COX-2), and NF-κB levels in the exposed mice, possibly by inhibiting the expression of peroxisome proliferator-activated receptor gamma (PPARγ) [39].
Figure 2.
Protective effect of caffeic acid against skin cancer. ROS means reactive oxygen species, PTEN means phosphatase and tensin homolog, PI3K means phosphoinositide 3-kinase, PPARγ means peroxisome proliferator-activated receptor gamma, TNFα means tumor necrosis factor alpha, NF-κB means nuclear factor kappa B, iNO synthase means inducible nitric oxide synthase, and VEGF means vascular endothelial growth factor.
In squamous cell carcinoma induced in mice by chronic UVB irradiation, caffeic acid (15 mg/kg) downregulated the expression of inducible nitric oxide synthase (iNOS) and vascular endothelial growth factor (VEGF), upregulated p53expression, and reduced tumor growth [39]. In A431 skin cancer cells, SK-MEL-5 melanoma cells, and SK-MEL-28 melanoma cells, caffeic acid (40 μM directly inhibited ERK1/2 activity and, in this way, disrupted the MAP kinase signaling pathway that promotes tumor growth [40]. Caffeic acid significantly decreased the cell viability of cutaneous melanoma cell line SK-Mel-28 in the same doses that significantly increased the viability of the non-cancer cell line [41]. Caffeic acid also prevented the endothelial growth factor (EGF)-induced neoplastic transformation of human keratinocyte HaCat cells [40].
In transformed human keratinocyte HaCaT cells, caffeic acid decreased the activity of the NF-kB/Snail signaling pathway [42]. Snail inhibits E-cadherin; therefore, Snail inhibition promotes the migratory ability of cancer cells, i.e., metastases [43].
To summarize, caffeic acid can inhibit the PI3K/Akt, MAPK, and NF-kB signaling pathways in skin cancer cells (Table 1), decrease inflammation and oxidative stress and keep nuclear excision repair functional due to stimulation of PTEN expression.
3.5. Lung Cancer
Lung cancer is the most common cancer in men and the second-most common cancer in women [44]. Lung cancers include two main groups: non-small cell lung carcinoma and small cell lung carcinoma, which is more aggressive. The data concerning the effect of caffeic acid on lung cancer is controversial. Caffeic acid (600 μM) decreased the viability of human non-small-cell lung cancer H1229 cells but not control cells (human bronchial epithelium non-cancer cells) [45]. In H1299 cells, co-exposure to caffeic acid (100 μM) and cytostatic paclitaxel (10 μM) inhibited cell proliferation more than paclitaxel alone [45]. The co-exposure increased the expression of the pro-apoptotic proteins Bid and Bax, caspase-3/7 and 9 activity, and the expression of 6hosphor-JNK and 6hosphor-ERK1/2 in both H1299 cells and H1299-xenografts in nude mice [45]. Increased levels of phosphorylated p-JNK and p-ERK1/2 would typically represent bad news because the MAPK pathway canonically stimulates cell proliferation. Nevertheless, in some cancer types, activated JNK inhibits aerobic glycolysis and supports apoptosis [46]. According to Lin and coworkers [47], the co-treatment of H1299 cells with paclitaxel and 100 μM caffeic acid increased the viability of H1299 cells (paclitaxel concentration was not disclosed). The caffeic acid exposure also increased the expression of the anti-apoptotic proteins survivin and Bcl-2 in another non-small cell lung cancer cell line, A549 [47]. Nevertheless, in mouse lung adenocarcinoma LA-795 cells, caffeic acid (60 μM) decreased the cell viability to approximately 50%. It also decreased the protein expression of phospho-MEK1/2, phospho-ERK1/2 (members of MAPKinase pathway), cyclin D, beta-catenin (promoters of cell proliferation), and vimentin (a marker of epithelial-mesenchymal transition) [48]. The authors identified the inhibition of the calcium-activated chloride channel TMEM16A, a channel with multiple roles in cancer [49], as the primary mechanism behind those changes. In the mouse xenograft, caffeic acid (5.4 mg/kg) combined with doxorubicin (4.1 mg/kg) significantly decreased the size of tumors [48].
To summarize, most (but not all) data described the antiproliferative effect of caffeic acid against lung cancer; the mechanism often includes an alteration of the MAPK signaling pathway (Table 1).
3.6. Oral Cancer
Alcohol and tobacco consumption represents the major risk factors for this less prevalent type of cancer. The most common cancers of the oral cavity and pharynx are head and neck squamous cell carcinomas (HNSCC) [29,50].
Low concentrations of ethanol (2.5–10 mM) increased the growth and migration activity of oral squamous cell carcinoma cells [51]; caffeic acid (50 and 100 μM) reversed the effect. The same authors [52] described that caffeic acid (50 and 100 μM) decreased the viability of the human head and neck squamous carcinoma cells (HNSCC) line (Detroit 562) due to cell cycle arrest in G0/G1 phase. In human tongue squamous cell carcinoma cells (CAL-27), caffeic acid (65 μg/mL) decreased the cell viability while increasing the protein expression of p53, a protein able to promote cell cycle arrest and apoptosis [53]. It also increased the protein expression of proline dehydrogenase/proline oxidase (Table 1), a major enzyme that degrades proline in cells [53]. An increased proline level in cancer cells is connected with a poorer prognosis [54].
3.7. Cervical Cancer
Cervical cancer is the fourth most common cancer in the world for women, with the incidence higher in countries with lower incomes [55]. The primary cause of this type of cancer is infection with human papillomavirus (HPV) [55]. Several publications have described a positive effect of caffeic acid against this type of cancer cells.
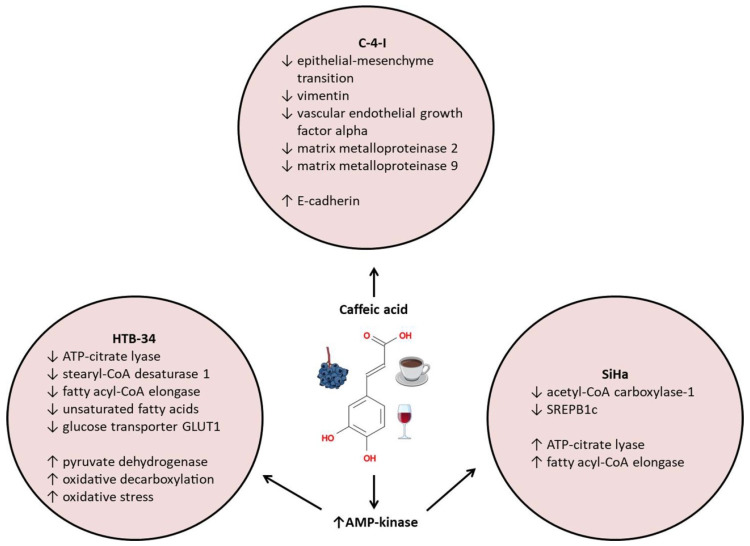
A combination of cisplatin (11 μM) and caffeic acid (300 μM) significantly increased apoptosis in cervical cancer cell lines HeLa (HPV-18-positive), SiHa, and CaSki (HPV-16-positive), and C33A (HPV-negative) when compared to cisplatin itself [56]. In the non-cancerous VERO cell line, neither cisplatin nor caffeic acid nor their combination significantly increased the number of apoptotic cells [56]. Tyszka-Czochara and coworkers published three articles describing the effects of caffeic acid (and a combination of caffeic acid and metformin) on cervical cancer cell lines. The first article [57] showed that, in the aggressive metastatic human cervical HTB-34 (ATCC-CRL1550) cancer cell line, the exposure to caffeic acid (100 μM) activated AMP-kinase (AMPK), a metabolic sensor with an anti-tumor effect [58,59]. Activated AMPK decreased protein expression of ATP citrate lyase (ACLY), stearoyl-CoA desaturase 1 (SCD1), and fatty acyl-CoA elongase-6 (ELOVL6), enzymes necessary for fatty acid synthesis. The combination of caffeic acid (100 μM) and metformin (10 mM) potentiated these effects [57]. The level of unsaturated fatty acids in HTB-34 cells dropped significantly after exposure. Cancer cells need fatty acids to form new membranes when cells grow. Therefore, fatty acid deprivation in cancer cells inhibits their proliferation. Caffeic acid (100 μM) exposure also decreased the expression of the glucose transporter GLUT1 and increased the activity of mitochondrial pyruvate dehydrogenase, oxidative decarboxylation, and oxidative stress in HTB-34 cells [57]. In the second article [60], caffeic acid (100 μM) decreased the cell viability of metastatic cervical cancer cells (SiHa) but not normal human fibroblasts (FB). Caffeic acid also increased oxidative stress in SiHa cells but not FB cells.
Caffeic acid activates AMPK, which then inhibits acetyl-CoA carboxylase-1 (ACC1) activity and the expression of SREPB1c [57]. Unlike in HTB-34 cells, the exposure increases the protein expression of ATP citrate lyase and fatty acyl-CoA elongase and fails to change the level of lipids in cells [57]. The third article [61] focused on the effect of caffeic acid on epithelial–mesenchyme transition. Losing markers of epithelial cells, e.g., E-cadherin, and gaining mesenchymal phenotype with markers such as vimentin, makes carcinoma cells more aggressive. A typical signaling molecule that promotes epithelial–mesenchyme transition is transforming growth factor beta (TGF-β). Caffeic acid (100 μM) increased the E-cadherin expression and decreased vimentin expression in the human cervical squamous cell line C-4I exposed to TGF-β, and, in this way, it effectively reversed the epithelial–mesenchyme transition. (TGF-β stimulates the epithelial–mesenchyme transition). Caffeic acid also increased mRNA levels of TIMP-1 and TIMP2 (tissue inhibitors of metalloproteinases 1 and 2), and decreased mRNA levels of VEGFA (vascular endothelial growth factor A), metalloproteinases MMP-2, and MMP-9 [57], essential for aggressive tumor growth and metastases [62].
To summarize, in cervical cancer cells, caffeic acid increases the expression of AMPK (Table 1), which then deregulates the expression of enzymes involved in the fatty acid synthesis (Figure 3). Caffeic acid also prevents the epithelial–mesenchyme transition by increasing the expression of E-cadherin and decreasing the expression of vimentin and metalloproteinases (Table 1) (Figure 3).
Figure 3.
Effects of caffeic acid on metastatic human cervical cancer cells. SREPB1c means sterol regulatory element-binding proteins, and CoA means coenzyme A.
4. Caffeic Acid and Diabetes, Obesity, and Metabolic Syndrome
The Mayo Clinic website describes metabolic syndrome as “a cluster of conditions that occur together, increasing your risk of heart disease, stroke, and type 2 diabetes. These conditions include increased blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol or triglyceride levels”(https://www.mayoclinic.org/diseases-conditions/metabolic-syndrome/symptoms-causes/syc-20351916 accessed on 8 August 2022). Published data show that caffeic acid has a wide range of effects against these conditions.
4.1. Diabetes
Castro and coworkers [63] showed that caffeic acid (50 mg/kg) reduced blood glucose levels in streptozocin-induced diabetic mice. They attributed this effect to the ability of caffeic acid to modulate purinergic signaling and, in this way, reduce oxidative stress and act in an anti-inflammatory way. In a similar diabetic model, caffeic acid (35 mg/kg) normalized blood insulin levels and antioxidant parameters: superoxide dismutase (SOD), CAD protein, and glutathione [64]. In alloxan-induced diabetic mice, caffeic acid (50 mg/kg) decreased blood glucose levels, increased hepatic glucokinase (GCK) levels, normalized body weight, and reduced LDL blood levels [65]. Caffeic acid also lowered serum levels of liver enzymes such as alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), and blood urea, and showed protective and regenerative effects on the kidney and liver. In streptozotocin-induced gestational diabetes in rats, caffeic acid (in a dose-dependent manner) normalized fetus weight, blood lipids, and antioxidant enzymes superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT), and glutathione negatively altered by diabetes [66]. In insulin-resistant neural cells of high fat diet-induced diabetic rats, caffeic acid increased the expression of the leptin receptor, phospho-JAK2, GLUT3, Akt, and PI3K, and in this way, sensitized cells to insulin signaling [67]. It also increased glucose intake in neural cells. These effects lead authors to suggest that caffeic acid can ameliorate memory function.
In human umbilical vein endothelial cells, HUVECs, caffeic acid (100 μM) inhibited the formation of advanced glycosylation end products, decreased the expression of inflammatory factors interleukin-1β (IL-1β), interleukin-18 (IL-18), and caspase-1, and decreased the production of reactive oxygen species [68]. In the same type of cells, a much lower concentration of caffeic acid (10 nM) improved intracellular redox status and decreased pro-inflammatory NF-κB signaling [69]. In the human stabilized endothelial cell line Ea.hy926, 10 nM caffeic acid showed a similar effect [70]. Additionally, 10 nM caffeic acid decreased apoptosis in Ea.hy926 cells exposed to high glucose. In the context of published data describing the various effects of caffeic acid, the biological activity of caffeic acid at a concentration of 10 nM is remarkable. A higher concentration of caffeic acid (10 μM) also decreased the expression of the receptor for advanced glycation end-products (RAGE) and inflammatory stress marker C-reactive protein (CRP), as well as vascular cell adhesion molecule-1 (VCAM-1), and monocyte chemoattractant protein-1 (MCP-1), in cultured human endothelial cells (HEC) [71].
In mice with chronic stress-induced insulin resistance, caffeic acid (5 and 10 mg/kg)decreased serum levels of glycosylated hemoglobin, tumor necrosis factor-α (TNF-α), and interleukin-1β (IL-1β) [72]. Caffeic acid (various concentrations) also improved oxidative stress in Fe2+-induced pancreatic injury: it normalized the level of glutathione, superoxide dismutase (SOD), and catalase (CAT) activity [73].
Approximately 75% of glucose in the blood is cleared by skeletal muscle. To make this possible, glucose transporter GLUT4 must reach the cell membrane. Both insulin signaling and exercise activate the GLUT4 transport while using different signaling pathways. An essential step in the exercise-activated pathway is activating AMPkinase (AMPK). Caffeic acid (100 μM and 1 mM) activated AMPK and its downstream target acetyl-CoA-carboxylase (ACC) in rat skeletal muscle [74]. In this way, caffeic acid helps decrease hyperglycemia if combined with physical exercise.
To summarize, in subjects with diabetes, caffeic acid decreases oxidative stress and inflammation, stimulates insulin sensitivity by inducing PI3K/Akt signaling, prevents damage caused by advanced glycation end-products, and increases the presence of GLUT4 in muscles by activating AMPK.
4.2. Obesity
Caffeic acid can also influence fat tissue. Two basic types of adipocytes exist in our bodies: white and brown. The brown adipocytes are more prone to start lipolysis (which leads to losing weight). The reason for this is a higher number of mitochondria in brown adipocytes [75]. Both β3-adrenergic stimulation and cold exposure can activate brown adipocytes and make them start lipolysis to gain energy, while white adipocytes serve more like a passive depot of energy storage. Nevertheless, it is possible to transform white adipocytes into brown ones [75].
Caffeic acid (5 μM, 10 μM, and 50 μM) decreased the expression of key genes of white adipogenic differentiation, including adiponectin, CAAT/enhancer-binding protein alpha (CEBPA), and fatty acid-binding protein 4 (FABP4), and increased the expression of brown adipocyte markers: cell death activator CIDE-A (CIDEA), and uncoupling protein 1 (UCP1) in human Simpson-Golabi-Behmel syndrome (SGB) adipocytes [76]. Caffeic acid also decreased protein expression of PPARγ and lipid accumulation and increased glycerol release [76]. Such results suggest a positive effect of caffeic acid on the “browning” of white adipocytes. Interestingly, a more robust effect was achieved by combining caffeic acid with its derivative chlorogenic acid [76]. In AML12 cells (mouse liver cells), caffeic acid (50 μM) decreased the lipid accumulation and the expression of endoplasmic reticulum stress markers induced by palmitate (250 μM) [77]. It also increased the expression of autophagy markers: microtubule-associated protein 1A/1B light chain 3B (LC3) and autophagy-related 7 (ATG7) [77]. In the differentiated pre-adipocyte cell line 3T3-L1, caffeic acid (31.25 μM and 62.5 μM) significantly reduced lipid content and inhibited intracytoplasmic reactive oxygen species [78]. Caffeic acid (50 μM) also significantly decreased PPARγ protein expression and lipid accumulation in primary-cultured rainbow trout adipocytes [79]. PPARγ represents a major regulator of adipogenesis, especially adipocyte differentiation and lipid accumulation [80]. When the adipocytes were co-exposed to obesitogen rosiglitazone, caffeic acid reversed its effect [79].
To summarize, caffeic acid decreased lipid accumulation and promoted the white-to-brown transition of adipocytes.
4.3. Atherosclerosis
One of the major diseases connected with obesity is atherosclerosis. During atherosclerosis development, vascular inflammation plays a significant role. [81].
Caffeic acid (20 μM) showed a significant anti-atherosclerotic effect on human umbilical vein endothelial cells: it decreased interleukin-8 (IL-8) production, toll-like receptor 4 (TLR4) protein expression, and NF-κB signaling induced by the adipokine resistin [82]. In the same type of cells, caffeic acid (25 μM) also inhibited NF-κB-induced expression of adhesion molecules: intracellular adhesion molecule 1 (ICAM-1), vascular adhesion molecule 1 (VCAM-1), and E-selectin [83]. Once expressed on the cell surface, these adhesion molecules are responsible for interactions between blood components and vein endothelial cells [83]. Among others, they facilitate leukocyte adhesion to the endothelium, which represents one of the first steps in atherosclerosis development [84]. In male Wistar rats, caffeic acid (50 mg/kg, p.o.) improved the lipid profile and significantly reduced atherosclerotic lesions [85]. Oxidized LDL represents one of the major risk factors for atherosclerosis, as it causes endothelial dysfunction, an early event in the pathogenesis of cardiovascular diseases [84]. Caffeic acid (100 μM) decreased the activation of endothelial growth factor receptor (EGFR) stimulated by oxidized LDL in ECV-304 endothelial cells and GM-08133A smooth muscle cells [86].
To summarize, caffeic acid decreased pro-inflammatory NF-κB signaling and the expression of adhesive molecules ICAM-1, VCAM-1, and E-selectin in vascular endothelial cells.
5. Effects of Caffeic Acid on Brain-Related Diseases
Another pool of published data about caffeic acid describes its effect on brain-related diseases, with most data focusing on counteracting the symptoms of Alzheimer’s disease; a few others describe the effect of caffeic acid on depression or Parkinson’s disease.
5.1. Alzheimer’s Disease
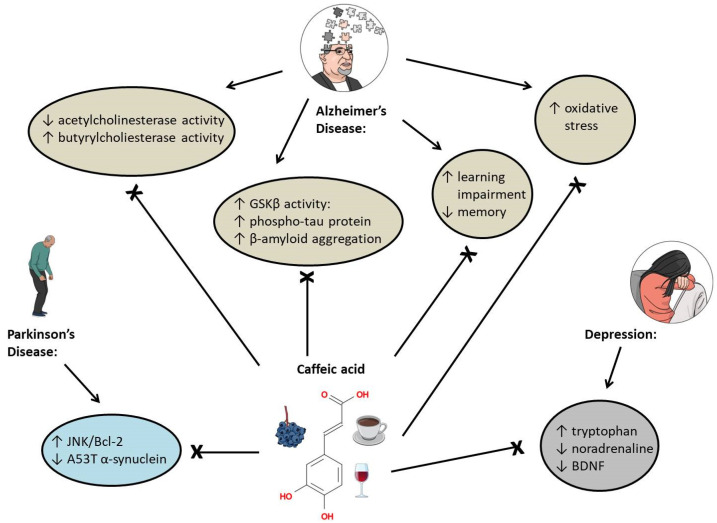
The main components of plaques found in the brains of patients with Alzheimer’s disease consist of β-amyloid peptides and tau proteins. The essential step for tau protein aggregation is tau phosphorylation which may also play a role in initiating β-amyloid toxicity. One of the kinases that phosphorylate tau protein is glycogen synthase kinase-3 beta (GSK3β); insulin signaling inhibits the activity of this kinase. Therefore, a hypothesis suggests that GSK3β deregulation in neurons may be a key point in developing Alzheimer’s disease [87].
Feeding hyperinsulinemic rats with caffeic acid (30 mg/kg b.w./day) for 30 weeks significantly improved their memory and learning impairments caused by a high-fat diet [88]. In the brain of hyperinsulinemic rats, caffeic acid normalized superoxide dismutase (SOD) activity and glutathione levels, inhibited glycogen synthase kinase 3β (GSK3β) activity, and decreased the level of β-amyloid and phosphorylated tau protein [88]. Sul and coworkers [89] found similar effects in vitro: the pretreatment with caffeic acid (10 μg/mL) decreased the level of phosphorylated tau protein and GSK3β stimulated by the exposure to 10 μM amyloid-β25-35 in rat pheochromocytoma cells PC12. In vitro, caffeic acid (800 μM) prevented the β-amyloid1-42 aggregation [90]. It also promoted the disaggregating of mature fibrils in an aqueous solution in the presence of liposomes, which simulated the presence of cell membranes [90]. In the rat model of Alzheimer’s disease established by injection of amyloid-β1-40 into the rats, caffeic acid (100 mg/kg for two weeks) significantly improved learning deficits and increased cognitive function (demonstrated by the Morris water maze task). Caffeic acid (100 mg/kg for two weeks) also suppressed oxidative stress, inflammation, NF-κB-p65 protein expression, and caspase-3 activity [91]. In a rat model of Alzheimer’s disease established by intracerebroventricularly administered streptozotocin, caffeic acid (40 mg/kg/day p.o.) showed a similar effect [92]. In an aluminum chloride-induced dementia in rats, caffeic acid (100 mg/kg, p.o.) improved cognitive ability and normalized acetylcholine esterase activity, nitrite and glutathione levels, as well as the protein expression of catalase (CAT) and glutathione-S-transferase (GST) in the brain [93]. In an amyloid-β25-35-injected Alzheimer’s disease mouse model, caffeic acid (50 mg/kg/day) improved cognitive functions and inhibited lipid peroxidation and nitric oxide formation in the brain [94]. The majority of people with Alzheimer’s disease suffer from decreased acetylcholine esterase activity and increased butyrylcholine esterase activity [95], and acetylcholinesterase and butyrylesterase inhibitors represent an effective treatment for the disease [96,97]. Caffeic acid (12 μg/mL) inhibited acetylcholinesterase and butyrylcholinesterase activity in the brain of untreated rats in vitro [98]. In acrolein-induced oxidative stress, a situation connected with Alzheimer’s disease [99], caffeic acid (25 μM) protected HT22 mouse hippocampal cells against ROS and glutathione depletion [100]. It also counteracted the disruptive effects of acrolein on p-ERK1/2, p-p38, and p-JNK1 expression [100].
To summarize, in subjects with Alzheimer’s disease, caffeic acid decreases oxidative stress and improves cognitive functions, probably by inhibiting NF-κB and GSK3β signaling and acetylcholinesterase and butyrylcholinesterase activity (Figure 4). Additionally, even though the authors failed to mention it in their papers, we consider the inhibitory effect of caffeic acid on 5-lipoxygenase as another factor in protecting the brain against damage [101,102,103,104].
Figure 4.
The effects of caffeic acid on brain-related diseases. GSKβ means glycogen synthase kinase 3β, A53T is a type of mutation, and BDNF means brain-derived neurotrophic factor.
5.2. Depression
In depressed rats, caffeic acid (10 and 30 mg/kg) normalized noradrenalin and tryptophan levels in a dose-dependent manner [105]. Caffeic acid also increased the expression of brain-derived neurotrophic factor (BDNF) in stressed mice; the effect was mediated by 5-lipoxygenase inhibition [106]. BDNF, a neurotrophin that modulates neuroplasticity in the brain, is regularly decreased in depressed patients [107].
5.3. Parkinson’s Disease
Protein α-synuclein controls vesicle trafficking in neurons [108]. Its A53T mutated form plays a significant role in developing Parkinson’s disease as its aggregates damage synaptic vesicles, mitochondria, and other cell structures [109]. In A53T α-synuclein transgenic mice, caffeic acid (10 mg/kg) activated the JNK/Bcl-2-mediated autophagy pathway and, in this way, reduced the level of A53T α-synuclein in the substantia nigra of the brain [110].
6. Antibacterial and Antiviral Activity of Caffeic Acid
6.1. Antibacterial Activity
The antibacterial activity of caffeic acid was tested mostly using Staphylococcus aureus, a Gram-positive pathogen able to form biofilms [111]. It is often resistant to antibiotics and disinfectants and, therefore, more difficult to treat [112].
Kwon and coworkers described that caffeic acid (1.0 mg/mL) inhibited the growth of Staphylococcus aureus [113]. They hypothesized that caffeic acid inhibited proline dehydrogenase (PRODH), an enzyme necessary for providing energy and managing the redox potential in cells [114]. Caffeic acid (10 mg/mL) also inhibited the secretion of α-hemolysin [115]. Staphylococcus aureus secretes α-hemolysin to promote the hemolysis of erythrocytes. α-hemolysin represents one of the major virulence factors of Staphylococcus aureus [115]. In the RN-4220 and –1199B resistant strains of Staphylococcus aureus, caffeic acid (1024 μg/mL) inhibited the MrsA and NorA efflux pumps responsible for the resistance [112]. Caffeic acid also showed promising inhibitory activity against tetR and tetM efflux pumps in silico, which could help fight tet efflux-based tetracycline-resistant bacteria [116]. Caffeic acid (1 mg/mL) inhibited the growth of four clinically significant bacteria: Escherichia coli, Pseudomonas aeruginosa, Listeria monocytogenes, and Staphylococcus aureus [117]. Caffeic acid inhibited their replication alone and when combined with Gentamycin, Ciprofloxacin, and Streptomycin [117]. Pinho and coworkers [118] confirmed the effectiveness of caffeic acid (5 mg/mL) against Staphylococcus aureus, Staphylococcus epidermidis, and a bit less against Klebsiella pneumoniae.
6.2. Antiviral Activity
Performing experiments withinfluenza virus A (IFV-A),poliovirus type 1 virus (PV1), and herpes simplex virus 1 (HSV1), Utsunomiya and coworkers [119] showed that caffeic acid (6 mM) inhibited the growth of both DNA and RNA viruses, with RNA viruses being possibly more sensitive. Additionally, the inhibitive effect depended on receiving caffeic acid up to three hours after infection; after that, the effect decreased [119]. Caffeic acid (400 μM) notably inhibited hepatitis C virus (HCV) replication, increased heme oxygenase-1 (HO-1) expression (HO-1 can trigger interferon α antiviral response), and erythroid 2-related factor 2 (Nrf2) expression [120]. In HepG2.2.15 cells, caffeic acid (40 μM) inhibited herpes B virus (HBV) DNA replication; in duck HBV-infected ducklings, caffeic acid (100 mg/kg/day) significantly decreased the level of HBV DNA in serum [121]. In HEp-2 and Vero cells, caffeic acid (8 mM) inhibited the multiplication of HSV1, but only if added early after infection; the addition of caffeic acid six hours after infection showed no effect [122]. Those results suggest that caffeic acid can inhibitHSV-1 multiplication only at the beginning of the process. Langland and coworkers [123] tested the effect of chelates consisting of caffeic acid and metal and non-metal ions against herpes simplex virus 1 (HSV1), herpes simplex virus 2 (HSV2), vaccinia virus (VACV), and a VSV-Ebola pseudo-typed virus. The antiviral activity of caffeic acid increased 100-fold with the addition of Fe3+, molybdate and phosphate [123]. Caffeic acid (1mM) also inhibited the growth of severe fever with thrombocytopenia syndrome virus (SFTSV); specifically, it inhibited the binding of the virus to the host cells [124]. In their later work, Ogawa and coworkers showed that the effect against SFTSV depends on the o-dihydroxybenzene backbone of caffeic acid [125].
7. Summary
Caffeic acid has shown a wide range of effects beneficial to human health. Its inhibitive effects on cancer cell growth are mediated mainly by inhibiting the PI3K/Akt pathway, MAPK pathway and NF-kB signaling with the consequent inhibition of VEGF. In diabetic rodents, caffeic acid also decreased NF-kB signaling, decreased glucose blood levels, normalized hepatic enzyme levels, improved redox status, and decreased advanced glycation end-products signaling. In adipose tissue, caffeic acid promoted the shift from white adipocytes into brown adipocytes by affecting their differentiation markers. In vein endothelial cells, caffeic acid decreased NF-kB signaling and the expression of adhesive molecules that participates in forming of atherosclerotic plaques. In rodents with Alzheimer’s disease, caffeic acid improved cognitive skills and redox status and decreased the formation of beta-amyloid plaques; the mechanism of these changes correlated with decreased GSK3β levels. In rodents with induced depression, caffeic acid normalized tryptophan and noradrenalin levels; in rodents with Parkinson’s disease, caffeic acid decreased levels of mutated α-synuclein by inducing autophagy. Caffeic also demonstrated antibacterial and antiviral effects: it successfully inhibited the growth of resistant Staphyloccocus aureus strains, mostly by inhibiting their efflux pumps. It also inhibited DNA and RNA viruses’ growth as long as it was added at the beginning of the infection.
All these beneficial effects will undoubtedly please coffee lovers. Nevertheless, the question remains whether daily consumption of various beverages suffices to build up caffeic acid blood levels high enough to affect cells.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This work was supported by the project National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, ID Project No. LX22NPO5104)—Funded by the European Union—Next Generation EU and by Charles University Cooperatio METD.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.El-Seedi H.R., El-Said A.M., Khalifa S.A., Goransson U., Bohlin L., Borg-Karlson A.K., Verpoorte R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012;60:10877–10895. doi: 10.1021/jf301807g. [DOI] [PubMed] [Google Scholar]
- 2.Trandafir I., Nour V., Ionica M.E. Antioxidant capacity, phenolic acids and caffeine contents of some commercial coffees available on the Romanian market. Arch. Latinoam. De Nutr. 2013;63:87–94. [PubMed] [Google Scholar]
- 3.Lafay S., Morand C., Manach C., Besson C., Scalbert A. Absorption and metabolism of caffeic acid and chlorogenic acid in the small intestine of rats. Br. J. Nutr. 2006;96:39–46. doi: 10.1079/BJN20061714. [DOI] [PubMed] [Google Scholar]
- 4.Baba S., Osakabe N., Natsume M., Terao J. Orally administered rosmarinic acid is present as the conjugated and/or methylated forms in plasma, and is degraded and metabolized to conjugated forms of caffeic acid, ferulic acid and m-coumaric acid. Life Sci. 2004;75:165–178. doi: 10.1016/j.lfs.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Celli N., Dragani L.K., Murzilli S., Pagliani T., Poggi A. In vitro and in vivo stability of caffeic acid phenethyl ester, a bioactive compound of propolis. J. Agric. Food Chem. 2007;55:3398–3407. doi: 10.1021/jf063477o. [DOI] [PubMed] [Google Scholar]
- 6.Mirzaei S., Gholami M.H., Zabolian A., Saleki H., Farahani M.V., Hamzehlou S., Far F.B., Sharifzadeh S.O., Samarghandian S., Khan H., et al. Caffeic acid and its derivatives as potential modulators of oncogenic molecular pathways: New hope in the fight against cancer. Pharmacol. Res. 2021;171:105759. doi: 10.1016/j.phrs.2021.105759. [DOI] [PubMed] [Google Scholar]
- 7.Simonetti P., Gardana C., Pietta P. Plasma levels of caffeic acid and antioxidant status after red wine intake. J. Agric. Food Chem. 2001;49:5964–5968. doi: 10.1021/jf010546k. [DOI] [PubMed] [Google Scholar]
- 8.Khan F.A., Maalik A., Murtaza G. Inhibitory mechanism against oxidative stress of caffeic acid. J. Food. Drug. Anal. 2016;24:695–702. doi: 10.1016/j.jfda.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Damasceno S.S., Dantas B.B., Ribeiro-Filho J., Antonio M.A.D., Galberto M.d.C.J. Chemical Properties of Caffeic and Ferulic Acids in Biological System: Implications in Cancer Therapy. A Review. Curr. Pharm. Des. 2017;23:3015–3023. doi: 10.2174/1381612822666161208145508. [DOI] [PubMed] [Google Scholar]
- 10.Zheng L.F., Dai F., Zhou B., Yang L., Liu Z.L. Prooxidant activity of hydroxycinnamic acids on DNA damage in the presence of Cu(II) ions: Mechanism and structure-activity relationship. Food Chem. Toxicol. 2008;46:149–156. doi: 10.1016/j.fct.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Bhat S.H., Azmi A.S., Hadi S.M. Prooxidant DNA breakage induced by caffeic acid in human peripheral lymphocytes: Involvement of endogenous copper and a putative mechanism for anticancer properties. Toxicol. Appl. Pharmacol. 2007;218:249–255. doi: 10.1016/j.taap.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Cai H., Huang X.J., Xu S.T., Shen H., Zhang P.F., Huang Y., Jiang J.Y., Sun Y.J., Jiang B., Wu X.M., et al. Discovery of novel hybrids of diaryl-1,2,4-triazoles and caffeic acid as dual inhibitors of cyclooxygenase-2 and 5-lipoxygenase for cancer therapy. Eur. J. Med. Chem. 2016;108:89–103. doi: 10.1016/j.ejmech.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 13.Adeyeye S.A.O. Heterocyclic Amines and Polycyclic Aromatic Hydrocarbons in Cooked Meat Products: A Review. Polycycl. Aromat. Compd. 2018;40:1557–1567. doi: 10.1080/10406638.2018.1559208. [DOI] [Google Scholar]
- 14.Felton J.S., Knize M.G., Wu R.W., Colvin M.E., Hatch F.T., Malfatti M.A. Mutagenic potency of food-derived heterocyclic amines. Mutat. Res. 2007;616:90–94. doi: 10.1016/j.mrfmmm.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Zhang N., Chen Y.F., Zhao Y.L., Fan D.M., Li L.J., Yan B.W., Tao G., Zhao J.X., Zhang H., Wang M.F. Caffeic acid assists microwave heating to inhibit the formation of mutagenic and carcinogenic PhIP. Food Chem. 2020;317:8. doi: 10.1016/j.foodchem.2020.126447. [DOI] [PubMed] [Google Scholar]
- 16.Cheng K.W., Wong C.C., Chao J., Lo C., Chen F., Chu I.K., Che C.-M., Ho C.-T., Wang M. Inhibition of mutagenic PhIP formation byepigallocatechin gallateviascavenging ofphenylacetaldehyde. Mol. Nutr. Food Res. 2009;53:716–725. doi: 10.1002/mnfr.200800206. [DOI] [PubMed] [Google Scholar]
- 17.Hong Y.J., Yang S.Y., Nam M.H., Koo Y.C., Lee K.W. Caffeic Acid Inhibits the Uptake of 2-Amino-1-methyl-6-phenylimidazo 4,5-b pyridine (PhIP) by Inducing the Efflux Transporters Expression in Caco-2 Cells. Biol. Pharm. Bull. 2015;38:201–207. doi: 10.1248/bpb.b14-00495. [DOI] [PubMed] [Google Scholar]
- 18.web3. [(accessed on 8 August 2022)]. Available online: https://www.wcrf.org/cancer-trends/liver-cancer-statistics/
- 19.McGlynn K.A., Petrick J.L., El-Serag H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73((Suppl. S1)):4–13. doi: 10.1002/hep.31288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Espindola K.M.M., Ferreira R.G., Narvaez L.E.M., Rosario A., da Silva A.H.M., Silva A.G.B., Vieira A.P.O., Monteiro M.C. Chemical and Pharmacological Aspects of Caffeic Acid and Its Activity in Hepatocarcinoma. Front. Oncol. 2019;9:541. doi: 10.3389/fonc.2019.00541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu W.T., Yang Y., Zhang C., Zhang Y.J., Chen L.J., Shen J., Li G.Y., Li Z., Li L., Li Y., et al. Caffeic acid attenuates the angiogenic function of hepatocellular carcinoma cells via reduction in JNK-1-mediated HIF-1 alpha stabilization in hypoxia. RSC Adv. 2016;6:82774–82782. doi: 10.1039/C6RA07703J. [DOI] [Google Scholar]
- 22.Jiang F., Wang X.X., Liu Q.Q., Shen J., Li Z., Li Y., Zhang J.P. Inhibition of TGF-beta/SMAD3/NF-kappa B signaling by microRNA-491 is involved in arsenic trioxide-induced anti-angiogenesis in hepatocellular carcinoma cells. Toxicol. Lett. 2014;231:55–61. doi: 10.1016/j.toxlet.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 23.Wang L.L., Lu M., Yi M., Chen L.J., Shen J., Li Z., Li L., Yang Y., Zhang J.P., Li Y. Caffeic acid attenuates the autocrine IL-6 in hepatocellular carcinoma via the epigenetic silencing of the NF-kappa B-IL-6-STAT-3 feedback loop. RSC Adv. 2015;5:52952–52957. doi: 10.1039/C5RA05878C. [DOI] [Google Scholar]
- 24.Chung T.W., Moon S.K., Chang Y.C., Ko J.H., Lee Y.C., Cho G., Kim S.H., Kim J.G., Kim C.H. Novel and therapeutic effect of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma cells: Complete regression of hepatoma growth and metastasis by dual mechanism. FASEB J. 2004;18:1670–1681. doi: 10.1096/fj.04-2126com. [DOI] [PubMed] [Google Scholar]
- 25.Yang Y., Jin M., Dai Y., Shan W.Q., Chen S., Cai R., Yang H.J., Tang L.M., Li L. Involvement and Targeted Intervention of Mortalin-Regulated Proteome Phosphorylated-Modification in Hepatocellular Carcinoma. Front. Oncol. 2021;11:12. doi: 10.3389/fonc.2021.687871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brautigan D.L., Gielata M., Heo J., Kubicka E., Wilkins L.R. Selective toxicity of caffeic acid in hepatocellular carcinoma cells. Biochem. Biophys. Res. Commun. 2018;505:612–617. doi: 10.1016/j.bbrc.2018.09.155. [DOI] [PubMed] [Google Scholar]
- 27.Wilkins L.R., Brautigan D.L., Wu H.P., Yarmohammadi H., Kubicka E., Serbulea V., Leitinger N., Liu W., Haaga J.R. Cinnamic Acid Derivatives Enhance the Efficacy of Transarterial Embolization in a Rat Model of Hepatocellular Carcinoma. Cardiovasc. Interv. Radiol. 2017;40:430–437. doi: 10.1007/s00270-016-1515-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z., Wang D., Qiao S.L., Wu X.Y., Cao S.Y., Wang L., Su X.J., Li L. Metabolic and microbial signatures in rat hepatocellular carcinoma treated with caffeic acid and chlorogenic acid. Sci. Rep. 2017;7:10. doi: 10.1038/s41598-017-04888-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bunz F. Principles of Cancer Genetics. 2nd ed. Springer Science+Business Media; Dordrecht, The Netherland: 2016. [Google Scholar]
- 30.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 31.web2. [(accessed on 15 August 2022)]. Available online: https://www.wcrf.org/cancer-trends/breast-cancer-statistics/
- 32.Vici P., Pizzuti L., Natoli C., Gamucci T., Di Lauro L., Barba M., Sergi D., Botti C., Michelotti A., Moscetti L., et al. Triple positive breast cancer: A distinct subtype? Cancer Treat. Rev. 2015;41:69–76. doi: 10.1016/j.ctrv.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Rosendahl A.H., Perks C.M., Zeng L., Markkula A., Simonsson M., Rose C., Ingvar C., Holly J.M.P., Jernstrom H. Caffeine and Caffeic Acid Inhibit Growth and Modify Estrogen Receptor and Insulin-like Growth Factor I Receptor Levels in Human Breast Cancer. Clin. Cancer Res. 2015;21:1877–1887. doi: 10.1158/1078-0432.CCR-14-1748. [DOI] [PubMed] [Google Scholar]
- 34.Rezaei-Seresht H., Cheshomi H., Falanji F., Movahedi-Motlagh F., Hashemian M., Mireskandari E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: An in silico and in vitro study. Avicenna J. Phytomedicine. 2019;9:574–586. doi: 10.22038/AJP.2019.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabala-Dzik A., Rzepecka-Stojko A., Kubina R., Jastrzebska-Stojko Z., Stojko R., Wojtyczka R.D., Stojko J. Comparison of Two Components of Propolis: Caffeic Acid (CA) and Caffeic Acid Phenethyl Ester (CAPE) Induce Apoptosis and Cell Cycle Arrest of Breast Cancer Cells MDA-MB-231. Molecules. 2017;22:1554. doi: 10.3390/molecules22091554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kabala-Dzik A., Rzepecka-Stojko A., Kubina R., Wojtyczka R.D., Buszman E., Stojko J. Caffeic Acid Versus Caffeic Acid Phenethyl Ester in the Treatment of Breast Cancer MCF-7 Cells: Migration Rate Inhibition. Integr. Cancer Ther. 2018;17:1247–1259. doi: 10.1177/1534735418801521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balupillai A., Nagarajan R.P., Ramasamy K., Govindasamy K., Muthusamy G. Caffeic acid prevents UVB radiation induced photocarcinogenesis through regulation of PTEN signaling in human dermal fibroblasts and mouse skin. Toxicol. Appl. Pharmacol. 2018;352:87–96. doi: 10.1016/j.taap.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 38.Ming M., He Y.Y. PTEN in DNA damage repair. Cancer Lett. 2012;319:125–129. doi: 10.1016/j.canlet.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Balupillai A., Prasad R.N., Ramasamy K., Muthusamy G., Shanmugham M., Govindasamy K., Gunaseelan S. Caffeic Acid Inhibits UVB-induced Inflammation and Photocarcinogenesis Through Activation of Peroxisome Proliferator-activated Receptor- in Mouse Skin. Photochem. Photobiol. 2015;91:1458–1468. doi: 10.1111/php.12522. [DOI] [PubMed] [Google Scholar]
- 40.Yang G., Fu Y., Malakhova M., Kurinov I., Zhu F., Yao K., Li H.T., Chen H.Y., Li W., Lim D.Y., et al. Caffeic Acid Directly Targets ERK1/2 to Attenuate Solar UV-Induced Skin Carcinogenesis. Cancer Prev. Res. 2014;7:1056–1066. doi: 10.1158/1940-6207.CAPR-14-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pelinson L.P., Assmann C.E., Palma T.V., da Cruz I.B.M., Pillat M.M., Manica A., Stefanello N., Weis G.C.C., Alves A.D., de Andrade C.M., et al. Antiproliferative and apoptotic effects of caffeic acid on SK-Mel-28 human melanoma cancer cells. Mol. Biol. Rep. 2019;46:2085–2092. doi: 10.1007/s11033-019-04658-1. [DOI] [PubMed] [Google Scholar]
- 42.Yang Y., Li Y., Wang K.B., Wang Y., Yin W.Q., Li L. P38/NF-kappa B/Snail Pathway Is Involved in Caffeic Acid-Induced Inhibition of Cancer Stem Cells-Like Properties and Migratory Capacity in Malignant Human Keratinocyte. PLoS ONE. 2013;8:e58915. doi: 10.1371/journal.pone.0058915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y., Zhou B.P. TNF-alpha/NF-kappaB/Snail pathway in cancer cell migration and invasion. Br. J. Cancer. 2010;102:639–644. doi: 10.1038/sj.bjc.6605530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.web1. [(accessed on 8 August 2022)]. Available online: https://www.wcrf.org/cancer-trends/lung-cancer-statistics/
- 45.Min J., Shen H., Xi W., Wang Q., Yin L., Zhang Y.F., Yu Y., Yang Q., Wang Z.N. Synergistic Anticancer Activity of Combined Use of Caffeic Acid with Paclitaxel Enhances Apoptosis of Non-Small-Cell Lung Cancer H1299 Cells in Vivo and in Vitro. Cell. Physiol. Biochem. 2018;48:1433–1442. doi: 10.1159/000492253. [DOI] [PubMed] [Google Scholar]
- 46.Papa S., Choy P.M., Bubici C. The ERK and JNK pathways in the regulation of metabolic reprogramming. Oncogene. 2019;38:2223–2240. doi: 10.1038/s41388-018-0582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lin C.L., Chen R.F., Chen J.Y.F., Chu Y.C., Wang H.M., Chou H.L., Chang W.C., Fong Y., Chang W.T., Wu C.Y., et al. Protective Effect of Caffeic Acid on Paclitaxel Induced Anti-Proliferation and Apoptosis of Lung Cancer Cells Involves NF-kappa B Pathway. Int. J. Mol. Sci. 2012;13:6236–6245. doi: 10.3390/ijms13056236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bai X., Li S.T., Liu X.Y., An H.L., Kang X.J., Guo S. Caffeic Acid, an Active Ingredient in Coffee, Combines with DOX for Multitarget Combination Therapy of Lung Cancer. J. Agric. Food Chem. 2022;70:8326–8337. doi: 10.1021/acs.jafc.2c03009. [DOI] [PubMed] [Google Scholar]
- 49.Crottes D., Jan L.Y. The multifaceted role of TMEM16A in cancer. Cell Calcium. 2019;82:102050. doi: 10.1016/j.ceca.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gupta B., Johnson N.W., Kumar N. Global Epidemiology of Head and Neck Cancers: A Continuing Challenge. Oncology. 2016;91:13–23. doi: 10.1159/000446117. [DOI] [PubMed] [Google Scholar]
- 51.Dziedzic A., Kubina R., Kabala-Dzik A., Wojtyczka R.D., Morawiec T., Buldak R.J. Caffeic Acid Reduces the Viability and Migration Rate of Oral Carcinoma Cells (SCC-25) Exposed to Low Concentrations of Ethanol. Int. J. Mol. Sci. 2014;15:18725–18741. doi: 10.3390/ijms151018725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dziedzic A., Kubina R., Kabala-Dzik A., Tanasiewicz M. Induction of Cell Cycle Arrest and Apoptotic Response of Head and Neck Squamous Carcinoma Cells (Detroit 562) by Caffeic Acid and Caffeic Acid Phenethyl Ester Derivative. Evid. Based Complement. Altern. Med. 2017;2017:6793456. doi: 10.1155/2017/6793456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Celinska-Janowicz K., Zareba I., Lazarek U., Teul J., Tomczyk M., Palka J., Miltyk W. Constituents of Propolis: Chrysin, Caffeic Acid, p-Coumaric Acid, and Ferulic Acid Induce PRODH/POX-Dependent Apoptosis in Human Tongue Squamous Cell Carcinoma Cell (CAL-27) Front. Pharmacol. 2018;9:336. doi: 10.3389/fphar.2018.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Phang J.M., Liu W., Hancock C., Christian K.J. The proline regulatory axis and cancer. Front. Oncol. 2012;2:60. doi: 10.3389/fonc.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vu M., Yu J., Awolude O.A., Chuang L. Cervical cancer worldwide. Curr. Probl. Cancer. 2018;42:457–465. doi: 10.1016/j.currproblcancer.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Koraneekit A., Limpaiboon T., Sangka A., Boonsiri P., Daduang S., Daduang J. Synergistic effects of cisplatin-caffeic acid induces apoptosis in human cervical cancer cells via the mitochondrial pathways. Oncol. Lett. 2018;15:7397–7402. doi: 10.3892/ol.2018.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tyszka-Czochara M., Konieczny P., Majka M. Caffeic Acid Expands Anti-Tumor Effect of Metformin in Human Metastatic Cervical Carcinoma HTB-34 Cells: Implications of AMPK Activation and Impairment of Fatty Acids De Novo Biosynthesis. Int. J. Mol. Sci. 2017;18:462. doi: 10.3390/ijms18020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zannella V.E., Cojocari D., Hilgendorf S., Vellanki R.N., Chung S., Wouters B.G., Koritzinsky M. AMPK regulates metabolism and survival in response to ionizing radiation. Radiother. Oncol. 2011;99:293–299. doi: 10.1016/j.radonc.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 59.Chomanicova N., Gazova A., Adamickova A., Valaskova S., Kyselovic J. The role of AMPK/mTOR signaling pathway in anticancer activity of metformin. Physiol. Res. 2021;70:501–508. doi: 10.33549/physiolres.934618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tyszka-Czochara M., Bukowska-Strakova K., Majka M. Metformin and caffeic acid regulate metabolic reprogramming in human cervical carcinoma SiHa/HTB-35 cells and augment anticancer activity of Cisplatin via cell cycle regulation. Food Chem. Toxicol. 2017;106:260–272. doi: 10.1016/j.fct.2017.05.065. [DOI] [PubMed] [Google Scholar]
- 61.Tyszka-Czochara M., Lasota M., Majka M. Caffeic Acid and Metformin Inhibit Invasive Phenotype Induced by TGF-beta1 in C-4I and HTB-35/SiHa Human Cervical Squamous Carcinoma Cells by Acting on Different Molecular Targets. Int. J. Mol. Sci. 2018;19:266. doi: 10.3390/ijms19010266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng H., Takahashi H., Murai Y., Cui Z., Nomoto K., Niwa H., Tsuneyama K., Takano Y. Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth, invasion, metastasis and angiogenesis of gastric carcinoma. Anticancer Res. 2006;26:3579–3583. [PubMed] [Google Scholar]
- 63.Castro M.F.V., Stefanello N., Assmann C.E., Baldissarelli J., Bagatini M.D., da Silva A.D., da Costa P., Borba L., da Cruz I.B.M., Morsch V.M., et al. Modulatory effects of caffeic acid on purinergic and cholinergic systems and oxi-inflammatory parameters of streptozotocin-induced diabetic rats. Life Sci. 2021;277:12. doi: 10.1016/j.lfs.2021.119421. [DOI] [PubMed] [Google Scholar]
- 64.Xu W.G., Luo Q., Wen X.Y., Xiao M., Mei Q.J. Antioxidant and anti-diabetic effects of caffeic acid in a rat model of diabetes. Trop. J. Pharm. Res. 2020;19:1227–1232. doi: 10.4314/tjpr.v19i6.17. [DOI] [Google Scholar]
- 65.Orsolic N., Sirovina D., Odeh D., Gajski G., Balta V., Sver L., Jembrek M.J. Efficacy of Caffeic Acid on Diabetes and Its Complications in the Mouse. Molecules. 2021;26:3262. doi: 10.3390/molecules26113262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu Y., Liu S.K., Wang H., Su W.H. Protective Effect of Caffeic Acid on Streptozotocin Induced Gestational Diabetes Mellitus in Rats: Possible Mechanism. Pak. J. Zool. 2021;53:1045–1052. doi: 10.17582/journal.pjz/20200106060120. [DOI] [Google Scholar]
- 67.Chang W.C., Kuo P.L., Chen C.W., Wu J.S.B., Shen S.C. Caffeic acid improves memory impairment and brain glucose metabolism via ameliorating cerebral insulin and leptin signaling pathways in high-fat diet-induced hyperinsulinemic rats. Food Res. Int. 2015;77:24–33. doi: 10.1016/j.foodres.2015.04.010. [DOI] [Google Scholar]
- 68.Cao X.Y., Xia Y., Zeng M., Wang W.Y., He Y., Liu J.L. Caffeic Acid Inhibits the Formation of Advanced Glycation End Products (AGEs) and Mitigates the AGEs-Induced Oxidative Stress and Inflammation Reaction in Human Umbilical Vein Endothelial Cells (HUVECs) Chem. Biodivers. 2019;16:9. doi: 10.1002/cbdv.201900174. [DOI] [PubMed] [Google Scholar]
- 69.Fratantonio D., Speciale A., Canali R., Natarelli L., Ferrari D., Saija A., Virgili F., Cimino F. Low nanomolar caffeic acid attenuates high glucose-induced endothelial dysfunction in primary human umbilical-vein endothelial cells by affecting NF-B and Nrf2 pathways. Biofactors. 2017;43:54–62. doi: 10.1002/biof.1312. [DOI] [PubMed] [Google Scholar]
- 70.Natarelli L., Ranaldi G., Leoni G., Roselli M., Guantario B., Comitato R., Ambra R., Cimino F., Speciale A., Virgili F., et al. Nanomolar Caffeic Acid Decreases Glucose Uptake and the Effects of High Glucose in Endothelial Cells. PLoS ONE. 2015;10:19. doi: 10.1371/journal.pone.0142421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toma L., Sanda G.M., Niculescu L.S., Deleanu M., Stancu C.S., Sima A.V. Caffeic acid attenuates the inflammatory stress induced by glycated LDL in human endothelial cells by mechanisms involving inhibition of AGE-receptor, oxidative, and endoplasmic reticulum stress. Biofactors. 2017;43:685–697. doi: 10.1002/biof.1373. [DOI] [PubMed] [Google Scholar]
- 72.Choudhary S., Mourya A., Ahuja S., Sah S.P., Kumar A. Plausible anti-inflammatory mechanism of resveratrol and caffeic acid against chronic stress-induced insulin resistance in mice. Inflammopharmacology. 2016;24:347–361. doi: 10.1007/s10787-016-0287-y. [DOI] [PubMed] [Google Scholar]
- 73.Salau V.F., Erukainure O.L., Ibeji C.U., Koorbanally N.A., Islam M.S. Ferric-Induced Pancreatic Injury Involves Exacerbation of Cholinergic and Proteolytic Activities, and Dysregulation of Metabolic Pathways: Protective Effect of Caffeic Acid. Biol. Trace Elem. Res. 2020;196:517–527. doi: 10.1007/s12011-019-01937-7. [DOI] [PubMed] [Google Scholar]
- 74.Tsuda S., Egawa T., Ma X., Oshima R., Kurogi E., Hayashi T. Coffee polyphenol caffeic acid but not chlorogenic acid increases 5’ AMP-activated protein kinase and insulin-independent glucose transport in rat skeletal muscle. J. Nutr. Biochem. 2012;23:1403–1409. doi: 10.1016/j.jnutbio.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 75.Virtanen K.A., Nuutila P. Brown adipose tissue in humans. Curr. Opin. Lipidol. 2011;22:49–54. doi: 10.1097/MOL.0b013e3283425243. [DOI] [PubMed] [Google Scholar]
- 76.Vasileva L.V., Savova M.S., Amirova K.M., Balcheva-Sivenova Z., Ferrante C., Orlando G., Wabitsch M., Georgiev M.I. Caffeic and Chlorogenic Acids Synergistically Activate Browning Program in Human Adipocytes: Implications of AMPK- and PPAR-Mediated Pathways. Int. J. Mol. Sci. 2020;21:9740. doi: 10.3390/ijms21249740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim H.M., Kim Y., Lee E.S., Huh J.H., Chung C.H. Caffeic acid ameliorates hepatic steatosis and reduces ER stress in high fat diet-induced obese mice by regulating autophagy. Nutrition. 2018;55–56:63–70. doi: 10.1016/j.nut.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 78.Mariana B.D., Tiago L.S., Ramon R.P.P.B.d.M., Jamile M.F., Tiago S.M., Richard R.C.M., Hector G.R., Dânya B.L., Alice M.C.M., Maria G.R.d.Q. Caffeic acid reduces lipid accumulation and reactive oxygen species production in adipocytes. Afr. J. Pharm. Pharmacol. 2018;12:263–268. doi: 10.5897/AJPP2018.4937. [DOI] [Google Scholar]
- 79.Lutfi E., Babin P.J., Gutierrez J., Capilla E., Navarro I. Caffeic acid and hydroxytyrosol have anti-obesogenic properties in zebrafish and rainbow trout models. PLoS ONE. 2017;12:21. doi: 10.1371/journal.pone.0178833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee J.E., Ge K. Transcriptional and epigenetic regulation of PPARgamma expression during adipogenesis. Cell Biosci. 2014;4:29. doi: 10.1186/2045-3701-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pamukcu B., Lip G.Y., Devitt A., Griffiths H., Shantsila E. The role of monocytes in atherosclerotic coronary artery disease. Ann. Med. 2010;42:394–403. doi: 10.3109/07853890.2010.497767. [DOI] [PubMed] [Google Scholar]
- 82.Lee E.S., Park S.H., Kim M.S., Han S.Y., Kim H.S., Kang Y.H. Caffeic Acid Disturbs Monocyte Adhesion onto Cultured Endothelial Cells Stimulated by Adipokine Resistin. J. Agric. Food Chem. 2012;60:2730–2739. doi: 10.1021/jf203774y. [DOI] [PubMed] [Google Scholar]
- 83.Moon M.K., Lee Y.J., Kim J.S., Kang D.G., Lee H.S. Effect of Caffeic Acid on Tumor Necrosis Factor-Alpha-Induced Vascular Inflammation in Human Umbilical Vein Endothelial Cells. Biol. Pharm. Bull. 2009;32:1371–1377. doi: 10.1248/bpb.32.1371. [DOI] [PubMed] [Google Scholar]
- 84.Mudau M., Genis A., Lochner A., Strijdom H. Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovasc. J. Afr. 2012;23:222–231. doi: 10.5830/CVJA-2011-068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang Y., Kaur G., Kumar M., Kushwah A.S., Kabra A., Kainth R. Caffeic Acid Prevents Vascular Oxidative Stress and Atherosclerosis against Atherosclerogenic Diet in Rats. Evid. Based Complement. Altern. Med. 2022;2022:8. doi: 10.1155/2022/8913926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vacaresse N., Vieira O., Robbesyn F., Jurgens G., Salvayre R., Negre-Salvayre A. Phenolic antioxidants trolox and caffeic acid modulate the oxidized LDL-induced EGF-receptor activation. Br. J. Pharmacol. 2001;132:1777–1788. doi: 10.1038/sj.bjp.0703981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hernandez F., Lucas J.J., Avila J. GSK3 and tau: Two convergence points in Alzheimer’s disease. J. Alzheimer Dis. 2013;33((Suppl. S1)):S141–S144. doi: 10.3233/JAD-2012-129025. [DOI] [PubMed] [Google Scholar]
- 88.Chang W.C., Huang D.W., Lo Y.M., Tee Q.Q., Kuo P., Wu J.S., Huang W.C., Shen Z.C. Protective Effect of Caffeic Acid against Alzheimer’s Disease Pathogenesis via Modulating Cerebral Insulin Signaling, beta-Amyloid Accumulation, and Synaptic Plasticity in Hyperinsulinemic Rats. J. Agric. Food Chem. 2019;67:7684–7693. doi: 10.1021/acs.jafc.9b02078. [DOI] [PubMed] [Google Scholar]
- 89.Sul D., Kim H.S., Lee D., Joo S.S., Hwang K.W., Park S.Y. Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci. 2009;84:257–262. doi: 10.1016/j.lfs.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 90.Andrade S., Loureiro J.A., Pereira M.C. Caffeic acid for the prevention and treatment of Alzheimer’s disease: The effect of lipid membranes on the inhibition of aggregation and disruption of A beta fibrils. Int. J. Biol. Macromol. 2021;190:853–861. doi: 10.1016/j.ijbiomac.2021.08.198. [DOI] [PubMed] [Google Scholar]
- 91.Wang Y.L., Wang Y.T., Li J.F., Hua L.L., Han B., Zhang Y.Z., Yang X.P., Zeng Z.L., Bai H.Y., Yin H.L., et al. Effects of caffeic acid on learning deficits in a model of Alzheimer’s disease. Int. J. Mol. Med. 2016;38:869–875. doi: 10.3892/ijmm.2016.2683. [DOI] [PubMed] [Google Scholar]
- 92.Deshmukh R., Kaundal M., Bansal V., Samardeep Caffeic acid attenuates oxidative stress, learning and memory deficit in intra-cerebroventricular streptozotocin induced experimental dementia in rats. Biomed. Pharmacother. 2016;81:56–62. doi: 10.1016/j.biopha.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 93.Khan K.A., Kumar N., Nayak P.G., Nampoothiri M., Shenoy R.R., Krishnadas N., Rao C.M., Mudgal J. Impact of caffeic acid on aluminium chloride-induced dementia in rats. J. Pharm. Pharmacol. 2013;65:1745–1752. doi: 10.1111/jphp.12126. [DOI] [PubMed] [Google Scholar]
- 94.Kim J.H., Wang Q., Choi J.M., Lee S., Cho E.J. Protective role of caffeic acid in an A beta(25-35)-induced Alzheimer’s disease model. Nutr. Res. Pract. 2015;9:480–488. doi: 10.4162/nrp.2015.9.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brimijoin S. Molecular forms of acetylcholinesterase in brain, nerve and muscle: Nature, localization and dynamics. Prog. Neurobiol. 1983;21:291–322. doi: 10.1016/0301-0082(83)90015-1. [DOI] [PubMed] [Google Scholar]
- 96.Pohanka M. Inhibitors of acetylcholinesterase and butyrylcholinesterase meet immunity. Int. J. Mol. Sci. 2014;15:9809–9825. doi: 10.3390/ijms15069809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Knez D., Coquelle N., Pislar A., Zakelj S., Jukic M., Sova M., Mravljak J., Nachon F., Brazzolotto X., Kos J., et al. Multi-target-directed ligands for treating Alzheimer’s disease: Butyrylcholinesterase inhibitors displaying antioxidant and neuroprotective activities. Eur. J. Med. Chem. 2018;156:598–617. doi: 10.1016/j.ejmech.2018.07.033. [DOI] [PubMed] [Google Scholar]
- 98.Oboh G., Agunloye O.M., Akinyemi A.J., Ademiluyi A.O., Adefegha S.A. Comparative Study on the Inhibitory Effect of Caffeic and Chlorogenic Acids on Key Enzymes Linked to Alzheimer’s Disease and Some Pro-oxidant Induced Oxidative Stress in Rats’ Brain-In Vitro. Neurochem. Res. 2013;38:413–419. doi: 10.1007/s11064-012-0935-6. [DOI] [PubMed] [Google Scholar]
- 99.Bradley M.A., Markesbery W.R., Lovell M.A. Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radic. Biol. Med. 2010;48:1570–1576. doi: 10.1016/j.freeradbiomed.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang Y.J., Jin M.H., Pi R.B., Zhang J.J., Chen M.H., Ouyang Y., Liu A.M., Chao X.J., Liu P.Q., Liu J., et al. Protective effects of caffeic acid and caffeic acid phenethyl ester against acrolein-induced neurotoxicity in HT22 mouse hippocampal cells. Neurosci. Lett. 2013;535:146–151. doi: 10.1016/j.neulet.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 101.Liang G.J., Shi B., Luo W.N., Yang J.Q. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015;11:10. doi: 10.1186/s12993-015-0064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pan Y.Q., Zhang P., Yang J.Q., Su Q.A. 5-lipoxygenase expression in a brain damage model induced by chronic oral administration of aluminum. Neural Regen. Res. 2010;5:1634–1638. [Google Scholar]
- 103.Song Y., Wei E.Q., Zhang W.P., Zhang L., Liu J.R., Chen Z. Minocycline protects PC12 cells from ischemic-like injury and inhibits 5-lipoxygenase activation. Neuroreport. 2004;15:2181–2184. doi: 10.1097/00001756-200410050-00007. [DOI] [PubMed] [Google Scholar]
- 104.Yang J.Q., Zhou Q.X., Liu B.Z., He B.C. Protection of mouse brain from aluminum-induced damage by caffeic acid. CnsNeurosci. Ther. 2008;14:10–16. doi: 10.1111/j.1755-5949.2007.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang D., Zhang L., Yang J.Q., Luo Y., Cui T., Du T.T., Jiang X.H. Evaluation on monoamine neurotransmitters changes in depression rats given with sertraline, meloxicam or/and caffeic acid. Genes Dis. 2019;6:167–175. doi: 10.1016/j.gendis.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dzitoyeva S., Imbesi M., Uz T., Dimitrijevic N., Manev H., Manev R. Caffeic acid attenuates the decrease of cortical BDNF transcript IV mRNA induced by swim stress in wild-type but not in 5-lipoxygenase-deficient mice. J. Neural Transm. 2008;115:823–827. doi: 10.1007/s00702-008-0034-7. [DOI] [PubMed] [Google Scholar]
- 107.Lin C.C., Huang T.L. Brain-derived neurotrophic factor and mental disorders. Biomed. J. 2020;43:134–142. doi: 10.1016/j.bj.2020.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maroteaux L., Campanelli J.T., Scheller R.H. Synuclein: A neuron-specific protein localized to the nucleus and presynaptic nerve terminal. J. Neurosci. 1988;8:2804–2815. doi: 10.1523/JNEUROSCI.08-08-02804.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ozansoy M., Basak A.N. The central theme of Parkinson’s disease: Alpha-synuclein. Mol. Neurobiol. 2013;47:460–465. doi: 10.1007/s12035-012-8369-3. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Y., Wu Q.M., Zhang L., Wang Q., Yang Z.X., Liu J., Feng L.Y. Caffeic acid reduces A53T alpha-synuclein by activating JNK/Bcl-2-mediated autophagy in vitro and improves behaviour and protects dopaminergic neurons in a mouse model of Parkinson’s disease. Pharmacol. Res. 2019;150:14. doi: 10.1016/j.phrs.2019.104538. [DOI] [PubMed] [Google Scholar]
- 111.Khan F., Bamunuarachchi N.I., Tabassum N., Kim Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food. Chem. 2021;69:2979–3004. doi: 10.1021/acs.jafc.0c07579. [DOI] [PubMed] [Google Scholar]
- 112.Dos Santos J.F.S., Tintino S.R., de Freitas T.S., Campina F.F., Irwin R.D.A., Siqueira-Junior J.P., Coutinho H.D.M., Cunha F.A.B. In vitro e in silico evaluation of the inhibition of Staphylococcus aureus efflux pumps by caffeic and gallic acid. Comp. Immunol. Microbiol. Infect. Dis. 2018;57:22–28. doi: 10.1016/j.cimid.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 113.Kwon Y.I., Apostolidis E., Labbe R.G., Shetty K. Inhibition of Staphylococcus aureusby Phenolic Phytochemicals of Selected Clonal Herbs Species ofLamiaceaeFamily and Likely Mode of Action through Proline Oxidation. Food Biotechnol. 2007;21:71–89. doi: 10.1080/08905430701191205. [DOI] [Google Scholar]
- 114.Servet C., Ghelis T., Richard L., Zilberstein A., Savoure A. Proline dehydrogenase: A key enzyme in controlling cellular homeostasis. Front. Biosci. (Landmark Ed.) 2012;17:607–620. doi: 10.2741/3947. [DOI] [PubMed] [Google Scholar]
- 115.Luis A., Silva F., Sousa S., Duarte A.P., Domingues F. Antistaphylococcal and biofilm inhibitory activities of gallic, caffeic, and chlorogenic acids. Biofouling. 2014;30:69–79. doi: 10.1080/08927014.2013.845878. [DOI] [PubMed] [Google Scholar]
- 116.Sivakumar S., Girija A.S.S., Priyadharsini J.V. Evaluation of the inhibitory effect of caffeic acid and gallic acid on tetR and tetM efflux pumps mediating tetracycline resistance in Streptococcus sp. using computational approach. J. King Saud Univ. Sci. 2020;32:904–909. doi: 10.1016/j.jksus.2019.05.003. [DOI] [Google Scholar]
- 117.Saavedra M.J., Borges A., Dias C., Aires A., Bennett R.N., Rosa E.S., Simoes M. Antimicrobial activity of phenolics and glucosinolate hydrolysis products and their synergy with streptomycin against pathogenic bacteria. Med. Chem. 2010;6:174–183. doi: 10.2174/1573406411006030174. [DOI] [PubMed] [Google Scholar]
- 118.Pinho E., Ferreira I.C., Barros L., Carvalho A.M., Soares G., Henriques M. Antibacterial potential of northeastern Portugal wild plant extracts and respective phenolic compounds. Biomed. Res. Int. 2014;2014:814590. doi: 10.1155/2014/814590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Utsunomiya H., Ichinosei M., Ikeda K., Uozaki M., Morishita J., Kuwahara T., Koyama A.H., Yamasaki H. Inhibition by caffeic acid of the influenza A virus multiplication in vitro. Int. J. Mol. Med. 2014;34:1020–1024. doi: 10.3892/ijmm.2014.1859. [DOI] [PubMed] [Google Scholar]
- 120.Shen J., Wang G.F., Zuo J.P. Caffeic acid inhibits HCV replication via induction of IFN alpha antiviral response through p62-mediated Keap1/Nrf2 signaling pathway. Antivir. Res. 2018;154:166–173. doi: 10.1016/j.antiviral.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 121.Wang G.F., Shi L.P., Ren Y.D., Liu Q.F., Liu H.F., Zhang R.J., Li Z., Zhu F.H., He P.L., Tang W., et al. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir. Res. 2009;83:186–190. doi: 10.1016/j.antiviral.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 122.Ikeda K., Tsujimoto K., Uozaki M., Nishide M., Suzuki Y., Koyama A.H., Yamasaki H. Inhibition of multiplication of herpes simplex virus by caffeic acid. Int. J. Mol. Med. 2011;28:595–598. doi: 10.3892/ijmm.2011.739. [DOI] [PubMed] [Google Scholar]
- 123.Langland J., Jacobs B., Wagner C.E., Ruiz G., Cahill T.M. Antiviral activity of metal chelates of caffeic acid and similar compounds towards herpes simplex, VSV-Ebola pseudotyped and vaccinia viruses. Antivir. Res. 2018;160:143–150. doi: 10.1016/j.antiviral.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 124.Ogawa M., Shirasago Y., Ando S., Shimojima M., Saijo M., Fukasawa M. Caffeic acid, a coffee-related organic acid, inhibits infection by severe fever with thrombocytopenia syndrome virus in vitro. J. Infect. Chemother. 2018;24:597–601. doi: 10.1016/j.jiac.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 125.Ogawa M., Shirasago Y., Tanida I., Kakuta S., Uchiyama Y., Shimojima M., Hanada K., Saijo M., Fukasawa M. Structural basis of antiviral activity of caffeic acid against severe fever with thrombocytopenia syndrome virus. J. Infect. Chemother. 2021;27:397–400. doi: 10.1016/j.jiac.2020.10.015. [DOI] [PubMed] [Google Scholar]