Abstract
Central nervous system (CNS) trauma, such as traumatic brain injury (TBI) and spinal cord injury (SCI), represents an increasingly important health burden in view of the preventability of most injuries and the complex and expensive medical care that they necessitate. These injuries are characterized by different signs of neurodegeneration, such as oxidative stress, mitochondrial dysfunction, and neuronal apoptosis. Cumulative evidence suggests that the transcriptional factor nuclear factor erythroid 2-related factor 2 (Nrf2) plays a crucial defensive role in regulating the antioxidant response. It has been demonstrated that several natural compounds are able to activate Nrf2, mediating its antioxidant response. Some of these compounds have been tested in experimental models of SCI and TBI, showing different neuroprotective properties. In this review, an overview of the preclinical studies that highlight the positive effects of natural bioactive compounds in SCI and TBI experimental models through the activation of the Nrf2 pathway has been provided. Interestingly, several natural compounds can activate Nrf2 through multiple pathways, inducing a strong antioxidant response against CNS trauma. Therefore, some of these compounds could represent promising therapeutic strategies for these pathological conditions.
Keywords: Nrf2, oxidative stress, spinal cord injury, traumatic brain injury
1. Introduction
Injuries to the central nervous system (CNS), such as traumatic brain injury (TBI) and spinal cord injury (SCI), represent a serious medical burden. The heterogeneity of the lesions by age and severity makes it difficult to find appropriate treatments [1,2,3].
The primary lesion triggers a dramatic inflammatory response that results in the activation of microglia, the invasion of peripheral immune cells, and the release of inflammatory mediators that damage tissue and lead to scar formation [4]. In this way, over the hours or months following the insult, the secondary lesion induces necrosis and apoptosis in both the damaged and healthy peripheral tissue [5]. The pathophysiological mechanisms underlying CNS lesions are complex and involve an inflammatory response, oxidative stress, mitochondrial dysfunction, blood-brain barrier (BBB) disruption, and so on. Among these, oxidative stress, one of the important mechanisms, occurs at the beginning of the damage and accompanies the entire traumatic event [6]. The reactive oxygen species (ROS) are small molecules derived from oxygen that are highly responsive, such as peroxides, superoxides, hydroxyl radicals, and singlet oxygen. ROS can affect both the severity and size, as well as the progression of TBI and SCI [7]. Under physiological conditions, a certain ROS level is important to maintain normal cellular function and adequately balance ROS levels [8]. However, an imbalance of these molecules results in their harmful interaction with nucleic acids, carbohydrates, lipids, and proteins [9,10]. Indeed, the interaction of these molecules with the lipid double layer determines the lipid peroxidation that induces damage and cell death [11]. Furthermore, it has been demonstrated that after TBI and SCI, oxidative stress can induce the oxidation of mitochondrial proteins, with a consequent reduction of mitochondrial activity and bioenergetic capacities [12,13]. However, low levels of these modifications, under physiological conditions, can serve the cell as beneficial responses to stress [14,15]. Contrarily, high levels of these modifications are responsible for enzymatic dysfunctions. In this regard, in recent decades, nuclear factor erythroid 2-related factor 2 (Nrf2) has received more attention due to its important role in cellular defense against different endogenous and exogenous stresses [16,17].
Nrf2 is a pleiotropic transcription factor that is responsible for the defense mechanism against oxidative stress and inflammatory damage, by regulating the expression of several genes [16]. Cumulative evidence suggests that Nrf2 plays a crucial role in orchestrating the antioxidant response in the brain [18], promoting the expression of several antioxidant enzymes, such as heme oxygenase-1 (HO-1), NADPH Quinone Dehydrogenase 1 (NQO1), glutathione (GSH) peroxidase, and other antioxidant proteins [19]. Indeed, Nrf2 demonstrates a protective role in many pathological processes, and its activation is involved in several CNS diseases [18,20], including TBI and SCI [21,22].
In this review, the pathophysiology of SCI and TBI will be illustrated, describing in detail the potential role of Nrf2 underlying oxidative stress as a trigger in the pathological process of these conditions. The biological role of Nrf2 and its involvement in the regulation of the intracellular redox balance will also be mentioned. There is growing interest in understanding the molecular mechanisms underlying Nrf2 activation or inhibition, for potential therapeutic purposes. In this regard, this article intends to analyze in depth the mechanism of Nrf2 as the main regulator of oxidative stress, also summarizing the natural compounds based on the regulation of the Nrf2 pathway for the future development of more effective treatments for TBI and SCI.
2. Methodology
In this review, the selected publications range from 2018 to 2022. In order to write the paragraphs, “5. Therapeutics interventions targeting the Nrf-2 in Spinal Cord Injury (SCI)” and “6. Therapeutics interventions targeting the Nrf-2 in Traumatic Brain Injury (TBI)”, the bibliography research in PubMed was performed using the following keywords: “spinal cord injury”, or “traumatic brain injury”, and “Nrf-2” and “oxidative stress”. We have specifically selected in vivo and in vitro studies that describe the progress of the investigations of natural compounds in the activation of Nrf2 signaling and their potential applications in TBI and SCI management (Figure 1).
Figure 1.
Prisma flow diagram details the methodology that was applied to choose the preclinical studies that were used for the writing of the review. Duplicate articles were excluded from the total number of studies that were recorded. Instead, we selected the articles that describe the role of natural compounds in the activation of Nuclear Factor Erythroid-2 Related Factor 2 (Nrf-2) signaling, which is important to promote injury repair and restore functional deficits (The PRISMA Statement is published in [23]).
3. The Biological Role of Nrf-2
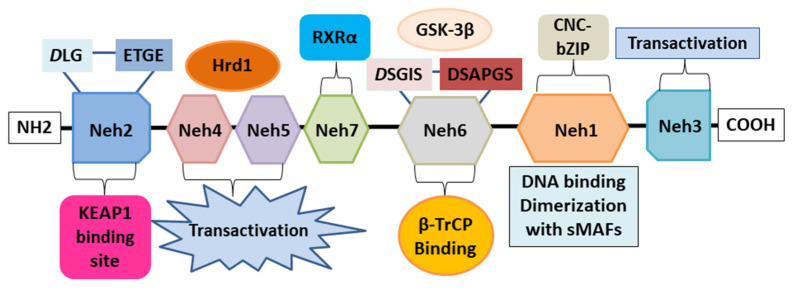
Nrf2, encoded in humans by the NFE2L2 gene, is a transcription factor that regulates the gene expression of several antioxidant and cytoprotective enzymes through a promoter sequence known as the antioxidant response element (ARE). ARE is a promoter element found in many cytoprotective genes, and therefore, Nrf2 plays an important role in the cellular defense systems against ARE-dependent environmental stresses. Nrf2 belongs to the Cap’n’Collar (CNC) subfamily of basic region leucine zipper (bZIP) transcription factors [24]. Nrf2 possesses seven conserved Nrf2-ECH homology (Neh) domains with different functions to control Nrf2 transcriptional activity (Figure 2). The bZip in the Neh1 domain interacts with AREs for gene transcription activation, while the Neh2 domain specifically recognizes the domain of Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1 (Keap1), to mediate the ubiquitination and degradation of Nrf2. The Neh3-5 domains bind various transcriptional components, acting as transcriptional activation domains, while the Neh6 domain binds the E3 ubiquitin ligase protein β-transducin repeat-containing protein (β-TrCP), involved in the degradation of Nrf2 in cells subjected to oxidative stress [25]. The Neh7 domain mediates interaction with the retinoic X receptor alpha (RXRα), a repressor of Nrf2 activity [26]. The presence of multiple domains allows Nrf2 to regulate the transcriptional activation of its target genes at different levels, thus regulating transcriptional, post-transcriptional, and post-translational regulation. Nrf2 activation is induced during oxidative stress, inflammation, and exposure to various stimuli such as growth factors, allowing cells to respond to different forms of stress. Indeed, Nrf2 is responsible for the regulation of more than 200 genes, all of which have the ARE promoter. Nrf2-regulated genes encode enzymes involved in xenobiotic and endobiotic metabolism, inflammatory responses, oxidative stress, and lipid, carbohydrate, and protein degradation metabolism [27].
Figure 2.
The structural architecture of Nrf2. The structure of Nrf2 contains seven domains, Neh domains, Neh1-Neh7. Neh1 contains a bZip motif, where the basic region is responsible for DNA binding and the zip dimerizes with other binding partners such as sMAFs. Neh2 contains ETGE and DLG motifs, involved in the interaction with Keap1 and subsequent Keap1-mediated proteasomal degradation. Neh3, Neh4, and Neh5 domains are transactivation domains of Nrf2. In particular, Neh4 and five domains interact with the Hrd1 responsible for Nrf2 degradation. The Neh6 domain contains two redox-independent degrons, DSGIS and DSAPGS, that bind to the E3 ubiquitin ligase β-TrCP involved in the Nrf2 degradation in oxidatively stressed cells. The neh7 domain mediates the interaction with RXRα, which represses Nrf2 activity. Nuclear factor erythroid 2-related factor 2: Nrf2; Nrf2-ECH homology: Neh; basic region leucine zipper: bZip; Cap’n’Collar: CNC; small muscleaponeurotic fibrosarcoma: sMAF; Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1: Keap1; β-transducing repeat-containing protein: β-TrCP; retinoic X receptor alpha: RXRα; Glycogen synthase kinase-3: GSK-3β; HMG-CoA reductase degradation 1: Hrd1.
Nrf2 is a transcription factor expressed in all cell types and is regulated, under different conditions, mainly by four ubiquitylation, by ubiquitin ligase E3, and by proteasomal degradation. Under physiological conditions, its basal protein levels are generally kept low due to proteasome degradation mediated by its negative regulator Keap1 [28]. Indeed in the cytoplasm, Keap1 creates a ubiquitin E3 ligase complex with Cullin3 which, by targeting Nrf2, induces its polyubiquitination and the rapid degradation of the proteasome [29,30]. During oxidative stress, electrophiles and ROS react with Keap1 sensor cysteines, protecting Nrf2 from Keap1-driven polyubiquitination and proteasome degradation [31,32]. This induces the translocation of the Nrf2 into the nucleus, where its accumulation allows the binding with the small muscleaponeurotic fibrosarcoma (sMAF) oncogene, with consequent activation of the expression of cytoprotective genes containing ARE [28,30,33]. Nrf2 activity is modulated at multiple levels, including regulation of the stability of the Nrf2 protein. Glycogen synthase kinase-3 β (GSK-3β) phosphorylates a motif present in the Neh6 domain, promoting the ubiquitination and degradation of Nrf2 [34,35]. GSK-3β phosphorylates Nrf2, creating a degradation domain that is recognized by β-TrCP and tagging for proteasomal degradation by the Keap1-Cullin 1 (CUL1) and RING-box protein 1 (RBX1) complex [36,37]. Likewise, CR6-interacting Factor 1, involved with proteins inducible by DNA damage, by binding the Neh2 domain and the C-terminal regions of Neh1 and Neh3 induce the degradation of Neh2 [38]. Nrf2 activity is also modulated by post-translational modifications that induce changes in its binding partners. Some proteins, such as c-jun N-terminal kinase (JNK) [39], protein kinase C (PKC) [40], extracellular signal-regulated protein kinases (ERK) [39], protein kinase R-like endoplasmic reticulum kinase [41], phosphoinositide 3-kinase (PI3K)/AKT, and casein kinase 2 [42], regulate Nrf2 phosphorylation by increasing its stability and transcriptional activity. Instead, proteins such as p38 and GSK-3β induce the phosphorylation of Nrf2 reducing its stability [35].
It is known that the Nrf2-Keap1 axis exerts a protective effect in various disorders that see oxidative stress and inflammation as the main pathological mechanisms [43]. Nrf2 is initially controlled at the transcriptional level by several transcription factors, such as the nuclear factor kappa-light chain-enhancer of activated B lymphocytes (NF-ĸB) [44,45]. The NFE2L2 promoter contains an NF-ĸB binding site which allows it to regulate Nrf2. Some signaling pathways, such as the PI3K-AKT pathway [46] and the Notch signaling pathway [47], seem to increase NFE2L2 transcription in order to allow Nrf2 to carry out the basal Nrf2 antioxidant program. It was discovered that at the post-transcriptional level it can also be regulated by microRNAs such as miR-144, which appears to be linked to a reduction in the transcriptional Nrf2 activity [48]. Other miRNAs, such as miR-28 [49], miR-34 [50], miR-93 [51], and miR-153 [52], appear to be involved in the regulation of Nrf2.
Nrf2/ARE signaling protects cells and tissues from oxidative stress [53] by negatively controlling the NF-kB signaling pathway that regulates inflammatory responses and cell damage [54]. Indeed, once translocated to the NF-κB nucleus, it induces the expression of proinflammatory cytokines (IL-1, IL-6, TNF-α), COX-2, and iNOS. Nrf2 inhibits the transcription of NF-κB by inhibiting its nuclear translocation [55]. Furthermore, high levels of Nrf2 enhance the levels of cellular HO-1, and consequently, increase the expression levels of phase II enzymes, blocking the degradation of IκB-α and the inhibitor of IκB-α [56]. Nrf2 can directly modulate ROS and RNS activating antioxidant enzymes, such as superoxide dismutase (SOD), GSH peroxidase, and members of peroxiredoxin [57,58]. Following oxidative stress, Nrf2 translocates into the nucleus where it induces the transcription of several antioxidant enzymes, such as GSH-dependent antioxidant enzymes (glutathione peroxidase 2, GPX2) and glutathione S-transferases (GST) [58]. Nrf2 also promotes the conversion of glutathione disulfide (GSSG) to GSH by glutathione reductase (GSR) [59], reducing oxidized thioredoxins by thioredoxin reductase (TrxR) [60] and Prx-SO2H by sulfiredoxin (Srx), as illustrated in Figure 3 [61].
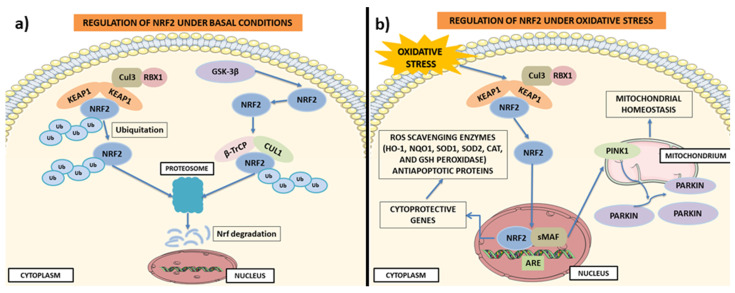
Figure 3.
Regulation of the Nrf2 pathway. (a) Regulation of Nrf2 under basal conditions. Under basal conditions, Nrf2 is sequestered by Keap1 in the cytosol and ubiquitinated. The formation of the Keap1-Cul3-Rbx1 complex induces the degradation of the Nrf2 proteasome. In this way, Nrf2 expression is maintained in the cytosol at low levels. However, the degradation of Nrf2 can also be promoted by GSK-3β. GSK-3β by phosphorylating Nrf2 forms a recognition motif for the E3 ligase adapter β-TrCP. Formation of the GSK-3β/β-TrCP/Cul1 complex promotes Keap1-independent degradation of Nrf2. (b) Regulation of Nrf2 under oxidative stress. When cells are under severe oxidative stress, Nrf2 is released from Keap1 binding and is translocated from the cytosol to the nucleus. In the nucleus, it binds to ARE gene sequences and promotes the transcription of cytoprotective genes responsible for the translation of ROS scavenging enzymes (HO-1, NQO1, SOD, SOD2, CAT and GSH peroxidase) and antiapoptotic proteins. Furthermore, Nrf2 positively regulates PINK1 by promoting mitochondrial homeostasis through several mechanisms, such as the removal of damaged mitochondria. The image was created using the image bank of Servier Medical Art (Available online: http://smart.servier.com/, accessed on 15 November 2022), licensed under a Creative Commons Attribution 3.0 Unported License (Available online: https://creativecommons.org/licenses/by/3.0/, accessed on 15 November 2022). Nuclear factor E2-related factor 2: Nrf2; antioxidant response element: ARE; small muscleaponeurotic fibrosarcoma: sMAF; Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1: Keap1; β-transducing repeat-containing protein: β-TrCP; Cullin 3: CUL3; RING-box protein 1: RBX1; PTEN Induced Kinase 1: PINK1; Glycogen synthase kinase-3 β: GSK-3β; heme oxygenase-1: HO-1; NADPH Quinone Dehydrogenase 1: NQO1; reactive oxygen species: ROS; superoxide dismutase: SOD; glutathione: GSH; catalase: CAT.
Noteworthily, Nrf2 regulates mitochondrial function through the regulation of some key metabolic genes, for example, by improving glycolytic flux, amino acid metabolism, and glutaminolysis. Active Nrf2 is also involved in maintaining the integrity of mitochondrial DNA, an important regulator of cell death and inflammation [62]. Furthermore, Nrf2 is also a regulator of mitochondrial biogenesis via the activation of the mitochondrial transcription factor A (TFAM), the mitochondrial transcription factor B2 (TFBM2), and the nuclear respiratory factor-1 (Nrf-1) [63]. Additionally, regulating the expression of the transcriptional co-activator peroxisome proliferator activator gamma co-activator 1 alpha (PGC-1α), which is a major regulator of mitochondrial function and biogenesis, is another example of Nrf2 activity [64]. Additionally, when cells are subjected to oxidative stress, Nrf2 influences mitochondrial biogenesis also by inducing the process of mitophagy [65,66]. Nrf2 improves autophagy by promoting the expression of autophagic genes encoding SQSTM1/p62 [67], protein 2 containing the calcium-binding domain and spiral coil (CALCOCO2/NDP52), kinase 1 similar to unc-51 (ULK1), protein autophagic 5 (ATG5), and gamma-protein-like 1 associated with the aminobutyric acid receptor (GABARAPL1) [68].
For the purpose of improving the neuroprotective potential of Nrf2 signaling pathway activation, several natural flavanol compounds with antioxidant, anti-inflammatory, and iron-chelating properties have been tested. In this regard, it was demonstrated that intravenous or oral administration of epicatechin passes the BBB, improving vascular function and leading to the reduction both of oxidant species and free radicals through the activation of the Nrf2 signaling pathway [69]. Besides the activation of the Nrf2 pathway, it was highlighted that epicatechin can act also on activator protein-1 (AP-1) signaling pathways, thus protecting the astrocytes exposed to hemoglobin [70]. Additionally, it was demonstrated that epicatechin has a neuroprotective effect in the brain after TBI, by activating the Nrf2 pathway and inhibiting the expression of HO-1, and reducing iron deposition [71].
It is known that HO-1 overexpression could alter iron homeostasis and aggravate iron deposition in the brain. Excess iron is a pathological feature, promoting ROS generation and subsequent DNA damage; lipid peroxidation leads to BBB destruction, neuronal death, and neurological deficits [72,73]. Second, HO-1 overexpression and increased brain iron deposition could be linked to chronic inflammation. A previous study [74] demonstrated that exacerbated oxidative stress and iron accumulation could be promoted by inflammatory processes. Thus, natural compounds such as epicatechin, by activating Nrf2 and also inhibiting HO-1 expression and iron deposition, may show important neuroprotective effects. Nevertheless, several studies in Nrf2- and HO-1-deficient mice confirmed the neuroprotective function of Nrf2, and the deleterious effects of HO-1 in the pathologic process of intracerebral hemorrhage [20,75].
4. Pathophysiology of SCI
SCI is a pathological condition with a high mortality rate, and it reduces life expectancy depending on the severity of the insult and the age of the patient [76]. The available treatments for this condition are generally aimed at relieving the symptoms of patients by improving their quality of life [77]. Most spinal cord injuries are due to road accidents, falls, assaults, and sports injuries [78]. However, SCI can also be caused by other conditions, such as musculoskeletal diseases, congenital problems such as spinal bifida, infectious diseases, and cancers [79].
The initial phase immediately following the injury is known as the “primary injury”, it is an irreversible process that can be caused by bone fragmentation and ligament tear [80,81]. The primary lesion is followed by a cascade of events, including increased cell permeability, apoptotic signaling, excitotoxicity, and vascular and inflammatory damage, which will eventually lead to neuronal damage and death, known as the “secondary injury” [82,83,84]. The secondary lesion can be divided into three phases: acute (within a few days), subacute (from 2 days to 2 weeks), and chronic (over 2 weeks). During the acute phase, vascular damage can cause bleeding and disruption of the spinal cord barrier, which attracts the rapid infiltration of inflammatory cells such as neutrophils, resulting in the release of various pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin 1-alpha (IL-1α), interleukin 1-beta (IL-1β), and interleukin-6 (IL-6) [85,86]. During the subacute phase, arterial vessel damage impairs vascular supply and causes edema, leading to further neuronal and vascular damage, in particular causing the death of the oligodendrocytes responsible for the demyelination of the axons [87,88]. Furthermore, during this phase, the alteration of ionic homeostasis causes mitochondrial dysfunction [89,90], responsible for the excessive production of ROS and reactive nitrogen species (RNS) contributing to tissue damage [91]. During the chronic SCI phase, the damage expansion leads to the activation of astrocytes that produce excessive levels of the extracellular matrix. This alters vascular remodeling, the composition of the extracellular matrix, and the reorganization of neural circuits, inducing the formation of cystic cavities, axonal death, and the maturation of glial scars [92,93,94].
Since the complex pathophysiological changes make the secondary lesion more lethal than the primary one, the most promising approach in the treatment of SCI is to inhibit the secondary lesion and promote functional recovery [95]. In this regard, a large amount of evidence has shown that Nrf2 is involved in the pathogenesis of SCI and easily responds to traumatic injuries [96].
5. Therapeutics Interventions Targeting the Nrf-2 in SCI
Several evidences have shown that the Nrf2 improves spinal cord ischemia–reperfusion injury in neurons, and astrocytes, by activating antioxidant, antiapoptotic, and survival neuronal responses [97,98]. Therefore, strategies that can activate the Nrf2 pathway could be employed as a therapeutic tool to reduce oxidative stress and inhibit apoptotic processes that are activated in the damaged spinal cord. In a study performed on SCI rats, the effects of polyadine on oxidative stress, apoptosis, and the Nrf2 pathway underlying the SCI were evaluated. Polydatin administration (20 or 40 mg/kg, intraperitoneal) improved spinal cord impairment and locomotor function in SCI rats and also reduced injury-induced congestion, edema, and structural damage. Polydatin upregulated SOD and reduced malondialdehyde (MDA) levels, decreasing oxidative damage. Furthermore, it also decreased the levels of caspase-3 and split Bax and increased the levels of Bcl-2 in the spinal cord tissues, demonstrating its anti-apoptotic effect. Polydatin may have exerted its antioxidant and antiapoptotic action probably via the Nrf2 signaling pathway. Indeed, after treatment with the compound, the levels of nuclear Nrf2 and cytoplasmic HO-1 in spinal cord tissues were significantly elevated. Consistent with the findings observed in spinal cord tissues, including lipopolysaccharides (LPS)-stimulated BV2 microglia, polydatin treatment significantly reduced ROS and lactate dehydrogenase (LDH) production and inhibited BV2 cell apoptosis. These results, instead, were reversed with the Nrf2-knockdowns (KO) in LPS-induced BV2 cells [99].
Rosmarinic acid is a water-soluble polyphenolic phytochemical compound that has been shown to be protective against ischemic stroke [100], and against the neuronal cell damage induced by H2O2 [101]. These findings prompted researchers to investigate the effect of this compound on SCI and its underlying molecular mechanisms. After 7 days from the initial injury, the administration of rosmarinic acid (10, 20 or 40 mg/kg, intraperitoneal) reduced the areas of hemorrhage and swelling, the area of nerve cell destruction, reduced glial cell proliferation, and inflammatory cell infiltration. The treatment also increased the number of neurons in a dose-dependent manner, inducing functional recovery and a reduction in tissue damage, also after 28 days of treatment, highlighting the long-term effect of this compound. Like polydatin, rosmarinic acid also attenuated the damage-induced apoptosis by reducing the levels of Bax, cleaved caspase-9, and cleaved caspase-3, and increasing the level of Bcl-2. It promoted neuroprotection by increasing the expression of neurotrophic factors such as, neurofilament H (NF-H) and brain-derived neurotrophic factor (BDNF). Furthermore, the compound reduced oxidative stress by significantly increasing the activity of SOD, catalase (CAT), and GSH peroxidase, and reducing MDA. Subsequently, the proteomic analysis identified Nrf2 and NF-κB pathways as possible targets of rosmarinic acid, demonstrating that these beneficial effects could be mediated by this pathway. To confirm these data, the in vitro models of H2O2-induced oxidative injury and LPS-induced inflammatory injury in PC12 cells were used. PC12 cells is a cell line of rat, originated from the neural crest and widely used as model for neural differentiation as well as to evaluate the effects of differentiation. Additionally, in vitro, rosmarinic acid improved the antiapoptotic response and oxidative damage via Nrf2/HO-1 signaling [102].
Ginsenoside Rb1 is a natural compound that regulates the immune balance and acts as a scavenger of free radicals; therefore, the potential antioxidant effect has been evaluated in SCI. Treatment with ginsenoside Rb1 (10 mg/kg, intraperitoneal) improved hind limb motor function in rats that showed higher scores than those in the untreated SCI group. Histopathologically the compound reduced hemorrhage, neuronal degeneration/necrosis, and the infiltration of mononuclear cells and lymphocytes. After 7 days, the treatment significantly reduced serum MDA levels but increased SOD, CAT, and GSH levels. Instead, compared to the untreated SCI group, ginsenoside Rb1 significantly increased the expression of eNOS, HSP90, Nrf2, NQO1, and HO-1 proteins in the spinal cord. Ultimately, the SCI rats treated with ginsenoside Rb1 were injected with the eNOS inhibitor L-name to investigate the mechanism behind the protective effect. In this way, it was shown that ginsenoside Rb1 protected the spinal cord from damage-induced oxidative stress via the eNOS/Nrf2/ARE signaling pathway [103]. In another study, ginsenoside Rg1 decreased neuronal edema and bleeding in the damaged spinal cord and reduced inflammatory cell infiltration and cell necrosis. In addition, the treatment preserved the spinal cord structure from further injury and improved the motor function of the hind limbs in the SCI rat model. The treatment of SCI rats with ginsenoside Rb1 and Nrf2 inhibitor all-trans retinoic acid confirmed that the compound’s antioxidant and anti-inflammatory effects are regulated by the Nrf2/HO-1 signaling pathway [104]. The same results were obtained in rats administrated with notoginsenoside R1 (25 mg/kg/day, intraperitoneal) for 21 days, 2 h after SCI. These antioxidative, antiapoptotic, and anti-inflammatory effects of notoginsenoside R1 were reversed when SCI rats that had received notoginsenoside R1 were also treated with ML385, an inhibitor of the Nrf2/HO-1 signaling pathway. This confirmed that activation of the Nrf2/HO-1 signaling pathway after SCI may have an important implication in the management of SCI [105].
Luteolin, a flavonoid that possesses antioxidant [106], anti-inflammatory, and neuroprotective properties [107], has also been observed to be capable of activating Nrf2 gene expression. In in an ischemia–reperfusion SCI model, luteolin pretreatment (50 and 100 mg/kg, intraperitoneal) exhibited an improvement in neurological dysfunction of the lower hind limbs, demonstrating that this compound could protect animals from SCI. Pretreatment significantly reduced cellular apoptosis in the spinal cord of rats with an injury in a dose-dependent manner, and counteracted oxidative stress by lowering the oxidative activity of MDA and xantina oxidase. It also increased the antioxidant activity of SOD and GSH peroxidase and increased the levels of Nrf2 protein in the spinal cord 48 h after injury [108]. In this regard, in a subsequent study, it was explored whether pretreatment with this compound would improve recovery from SCI by activating Nrf2 and downstream target genes. Therefore, luteolin-pretreated animals were also treated with an Nrf2 inhibitor ML385 (30 mg/kg daily) for 14 consecutive days prior to the injury. Pretreatment with ML385 also reversed luteolin-induced pathological and functional improvements. Therefore, the results of the study showed that the anti-inflammatory, antioxidant, and neuroprotective activities of luteolin were closely related to the activation of Nrf2 [109].
Mulberrin is a natural compound of Ramulus mori which exhibits anti-inflammatory and antioxidant effects, therefore, it could be considered an effective therapeutic strategy to prevent the progression of SCI [110]. Mulberrin (15 or 30 mg/kg, by gavage), for 28 days, induced an improvement in motor function and attenuated the damage-induced apoptosis by the reduction of pro-apoptotic molecules (Bax, caspase-3, and PARP), and by the increase in the anti-apoptotic ones. Furthermore, the treatment resulted in a significant reduction of HO-1 and Nrf2 in the spinal cord tissue samples. These antioxidant effects of mulberrin were further confirmed in astrocytes treated with LPS in vitro. Therefore, the data of this study suggest that mulberrin could attenuate SCI by reducing miR-337 expressions which by regulating Nrf2 would reduce apoptosis, inflammation, and oxidative stress [111].
Another substance that appears to activate the Nrf2/HO-1 signaling pathway in the brain by attenuating oxidative stress and apoptosis-mediated cell death is maltol [112,113]. In the advanced phase of the damage (on the 7th and 14th days), the maltol (100 mg/kg, by gavage) improved locomotor function by decreasing the expression levels of the proapoptotic protein Bax and upregulating the antiapoptotic protein Bcl-2, thus attenuating neuronal apoptosis. Maltol could significantly inhibit H2O2-induced apoptosis in PC12 cells and could facilitate the expression of Nrf2 in the nucleus, thus suggesting that maltol could activate significantly the Nrf2 signaling pathway. This hypothesis was confirmed by the use of its ML385 inhibitor which reversed the inhibitory effect of maltol on ROS formation. Furthermore, maltol increased the level of mitophagy by activating the Nrf2/PINK1/Parkin signaling pathway in PC12 cells [114].
Perillaldehyde has been shown to be an activator of Nrf2 [115], therefore, it could be a potential therapeutic strategy for SCI. This hypothesis prompted a research group to explore these effects in an ischemia—reperfusion SCI rat model. The rats were pretreated for 7 days with perillaldehyde (36 and 72 mg/kg) administered intragastrically. Pretreatment-induced recovery of motor and neurological function also improved histological damage. In treated animals, functional and histological recovery was probably induced by the reduction of pro-inflammatory cytokines (IL-18 and IL-1β), by the inhibition of NLRP3 inflammasome activation, by the reduction of MDA, and by the increase in antioxidant enzymes (SOD, mitochondrial SOD, GSH peroxidase, and CAT). Furthermore, compared to the control group, the animals pretreated with the compound showed high Nrf2 and HO-1 expression. Moreover, pretreatment effectively inhibited the expression of phospho-NF-κB p65. The in vitro results also confirmed that perillaldehyde (0, 0.01, 0.1, 1, 10, 50, 100, 500 μM) counteracts the oxidative stress induced by the lesion probably by activating Nrf2/HO-1 [116]. Another natural compound that has been investigated for its possible role as an activator of Nrf2 is sinomenine [117,118]. After 14 days from the lesion, sinomenine (40 mg/kg, intraperitoneal) improved neurological deficits and reduced neuronal apoptosis, significantly decreasing inflammatory cytokines (IL-1β, IL-6, and TNF-α) and oxidative stress factors (MDA) in SCI rats. Contrarily, the treatment increased the activity of antioxidant enzymes such as SOD in the spinal cord. Furthermore, treatment with sinomenine induced an increase in the translocation of Nrf2 from the cytoplasm to the nucleus. To evaluate the role of sinomenineas as an activator of Nrf2, H2O2-stimulated and LPS-stimulated PC12 cells were treated with Nrf2 small RNA interference, demonstrating that Nrf2 silencing blocks the antioxidant and anti-inflammatory effects of sinomenine (10 µM) [119].
The data are summarized in Table 1, which shows that most natural compounds have a potential therapeutic efficacy for SCI management by inhibiting oxidative stress via Nrf2 activation (Figure 4). Indeed, once active, Nrf2 can downregulate inflammatory mediators (such as chemokines, and cytokines), and can downregulate NF-κB activity to achieve anti-inflammatory effects and regulate genes related to antioxidant mechanisms. Prevailing studies focus on the neuroprotective potential of these compounds by employing several mechanisms, including Nrf2/Keap1/ARE, Nrf2/PINK1/Parkin, NF-κB/TNF-α/ILs, and Bax/Bcl-2/caspases. Thus, the employment of these compounds that activate Nrf2 to effectively treat SCI could be a direction of future research.
Table 1.
Potential natural compounds Nrf2 activators for controlling SCI symptoms and improving functional recovery.
| Compound | Type of Compound |
SCI Time Frame | Experimental SCI Model | Targets | Potential Effects | Type of Study | Ref. |
|---|---|---|---|---|---|---|---|
| Polydatin | A stilbenoid glucoside | Acute SCI | Rats | Nuclear Nrf2 and cytoplasmic HO-1 | Polydatin is effective in ameliorating SCI, reducing oxidative stress and promoting antiapoptotic response via the Nrf2/HO-1 pathway. | In vivo | [99] |
| LPS-stimulated BV2 microglia | In vitro | ||||||
| Rosmarinic acid | A polyphenol | Sub-acute and chronic SCI | Rats | Nrf2/HO-1 and NF-κB | Rosmarinic acid exerts a neuroprotective effect on SCI and ameliorated the locomotor function by attenuating oxidative stress, apoptosis, and inflammation via modulating the Nrf2/HO-1 and NF-κB pathways. | In vivo | [102] |
| H2O2– and LPS-induced PC12 cells | In vitro | ||||||
| Ginsenoside Rb1 | A saponin | Sub-acute SCI | Rats | Endothelial NOS/Nrf2/ARE | Ginsenoside Rb1 improved the hind limb function score, protected the physiological function of spinal cord tissue, and exerted a protective effect against oxidative stress injury, enhancing the activity of the antioxidant enzyme and blocking lipid peroxidation, via the eNOS/Nrf2/HO-1 pathway. | In vivo | [103] |
| Ginsenoside Rb1 | A saponin | Acute SCI | Rats | Nrf2 and HO-1 | Ginsenoside Rg1 promoted a neuroprotective effect on SCI and ameliorated motor dysfunction after an injury, exerting antioxidative and anti-inflammatory effects via regulating the Nrf2/HO-1 signaling pathway. | In vivo | [104] |
| Notoginsenoside R1 | A saponin | Acute SCI | Rats | Nrf2 and HO-1 | Notoginsenoside R1 ameliorates the SCI condition by countering oxidative stress, neuronal apoptosis, and inflammation via activating the Nrf2/HO-1 signaling pathway. | In vivo | [105] |
| Luteolin | A flavonoid | Acute SCI | Ischemia–reperfusion SCI rats | Nrf2 | Luteolin exhibited a neuroprotective effect by alleviating oxidative stress, inhibiting inflammatory and neuronal apoptosis, probably through the signaling pathway Nrf2. | In vivo | [108] |
| Luteolin | A flavonoid | Acute SCI | Rats | Nrf2 | The neuroprotective efficacy of luteolin depends on the suppression of oxidative stress and neuronal apoptosis through signaling pathways involving Nrf2 activation and downstream gene expression. | In vivo | [109] |
| Mulberrin | An oxyresveratrol glycoside | Acute SCI | Rats | Nrf2 | Mulberrin could promote SCI recovery by reducing miR-337 expressions which, by regulating Nrf2, would reduce apoptosis, inflammation, and oxidative stress. | In vivo | [111] |
| LPS-stimulated Astrocytes |
In vitro | ||||||
| Maltol | An organic compound | SCI | Rats | Nrf2/PINK1/Parkin | Maltol could stimulate mitophagy and counteract the oxidative response and neuronal cell death induced by SCI by activating the Nrf2/PINK1/Parkin pathway. | In vivo | [114] |
| H2O2–-induced PC12 cells | In vitro | ||||||
| Perillaldehyde | An aldehyde | Acute SCI | Ischemia–reperfusion SCI rats | Nrf2/HO-1 | Perillaldehyde reduces oxidative stress and ameliorates ischemia–reperfusion SCI symptoms, probably activating the Nrf2/HO-1 signaling pathway. | In vivo | [116] |
| BV2 microglia OGD/R | In vitro | ||||||
| Sinomenine | An active alkaloid | Acute SCI | Rats | Nrf2 | Sinomenine has the potential therapeutic efficacy agent for SCI management by inhibiting inflammation and oxidative stress via Nrf2 activation. | In vivo | [119] |
| H2O2– and LPS-induced PC12 cells | In vitro |
Spinal cord injury: SCI; lipopolysaccharides: LPS; nuclear factor E2-related factor 2: Nrf2; heme oxygenase-1: HO-1; nuclear factor kappa-light chain-enhancer of activated B lymphocytes: NF-κB; nitric oxide synthase: NOS; catalase: CAT; antioxidant response element: ARE; BV2 microglia oxygen and glucose deprivation/reoxygenation: OGD/R.
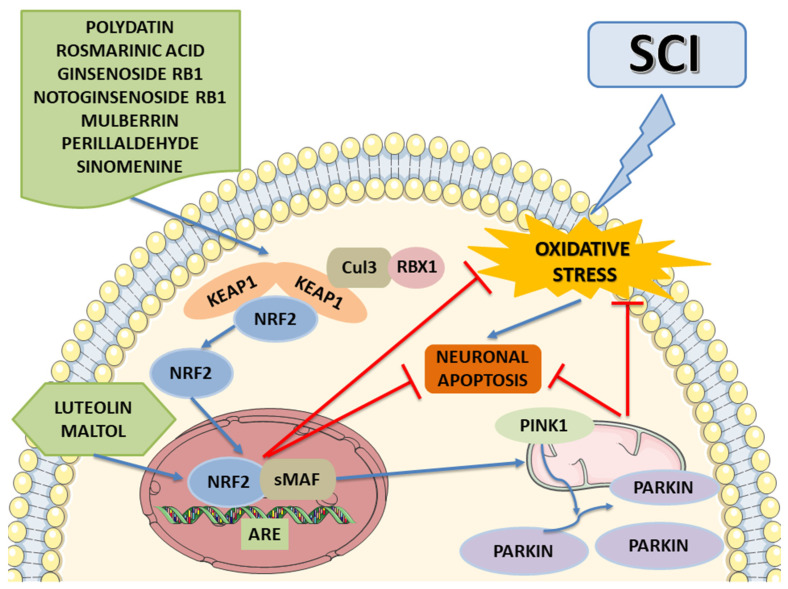
Figure 4.
The potential molecular mechanism of natural compounds on SCI. These natural compounds can activate the expression of Nrf2 and facilitate Nrf2′s translocation from the cytosol to the nuclear, thus inhibiting oxidative stress and protecting from neuronal apoptosis. Moreover, some of these promote mitophagy via enhancing the Nrf2/PINK1/Parkin pathway, suppressing neuronal apoptosis in SCI. The image was created using the image bank of Servier Medical Art (Available online: http://smart.servier.com/, accessed on 15 November 2022), licensed under a Creative Commons Attribution 3.0 Unported License (Available online: https://creativecommons.org/licenses/by/3.0/, accessed on 15 November 2022). Spinal cord injury: SCI; nuclear factor E2-related factor 2: Nrf2; antioxidant response element: ARE; small muscleaponeurotic fibrosarcoma: sMAF; Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1: Keap1; Cullin 3: CUL3; RING-box protein 1: RBX1; PTEN Induced Kinase 1: PINK1.
6. Pathophysiology of TBI
TBI is defined as acquired intracranial damage caused by a mechanical injury that occurs in the brain and compromises its function. TBI is caused by an external force such as a bump, a jolt to the head, a severe blow from an object, or a deep puncture of the skull through brain tissue [120]. When patients maintain good neurological function and the sequelae can be resolved completely without any treatment, TBI is referred to as mild. Conversely, a TBI is defined as moderate or severe when the injury causes a disability that makes the quality of life negligible [121].
The pathophysiology of TBI is divided into primary and secondary lesions. The primary damage occurs at the time of the exposure to external force and causes a mechanical breakdown of brain tissue [122]. The secondary injury has its onset hours after the traumatic event and it is due to the cascade of biochemical, cellular, and physiological events that are activated after the injury responsible for further damages [123,124]. Within 24 h of TBI, there is a disruption of normal blood flow, which generally causes ischemic events responsible for increases in ionic gradients and oxidative phosphorylation with consequent accumulation of lactate. High concentrations of lactate cause neuronal damage and disruption of the BBB and cerebral edema [125]. These mechanisms cause the depolarization of neurons and an increase in neurotransmitters, such as glutamate and aspartate, responsible for calcium (Ca2+), potassium, and sodium homeostasis [126]. The increase in intracellular Ca2+ promotes the activation of enzymes, such as caspases, responsible for cell death by apoptosis.
Oxidative stress also plays an important role in the development and pathogenesis of TBI. Indeed, it has been shown that ROS production induces lipid peroxidation of cell membranes, especially at the axonal level, increasing the neurodegeneration process [126,127]. Soon after, these events also cause the activation of an inflammatory response that stimulates macrophages, glial T cell lymphocytes, and neutrophils, to release pro-inflammatory cytokines, such as TNFα, IL-1β, and IL-6, and also contribute to the production of superoxide radicals [128,129]. It is worth noting that an increase in iron is also generated, which catalyzes the reaction of oxygen radicals and induces ferroptosis [130]. In this regard, it has been shown that Nrf2 plays a protective role in TBI by counteracting the oxidative and inflammatory response [44].
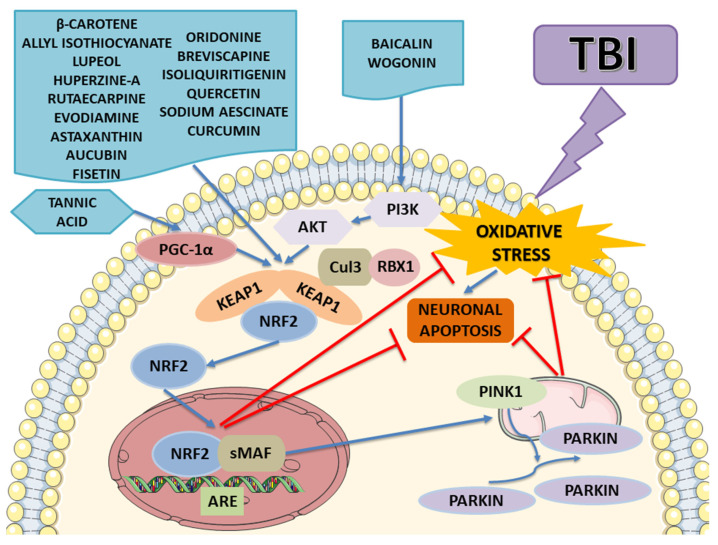
7. Therapeutics Interventions Targeting the Nrf-2 in TBI
To date, there is no efficacy treatment against TBI; therefore, it is important to find effective therapeutic strategies for TBI treatment [131]. Same as for SCI, recent evidence has demonstrated that the activation of Nrf2 mediated by different natural compounds and drugs could be a valid therapeutic strategy for TBI.
Oridonine is the main constituent of Rabdosia rubescens, which acquired great interest for its role as a powerful activator of Nrf2 [132]. These properties prompted a research group to evaluate the potential effect of oridonine as an activator of Nrf2 in TBI management. Already 7 days after the injury, oridonine (20 mg/kg, intraperitoneal) improved the motor and cognitive function, reducing the volume of the cortical lesion, cerebral edema, the accumulation of macrophages reactivated after the lesion, and neuronal apoptosis. These outcomes are directly linked to the protective effect of oridonine against oxidative stress. Indeed, in the injured cortex a decrease in ROS, MDA, mitochondrial membrane, and adenosine triphosphate content was detected, which on the contrary were elevated after TBI. The same antioxidant effect of oridonine (0.5, 1, 2, and 4 μM) was also confirmed in H2O2-exposed mouse neuroblastoma (N2a) cells [133].
Breviscapine is an aglycone flavonoid extracted from the Erigeron plant that appears to be involved in the activation of ATPase and SOD after trauma [134]. Breviscapine (50 mg/kg, intraperitoneal) promoted the recovery of neurobehavioral functions and reduced lesion-induced apoptosis of neuronal cells. Indeed, in the brain tissues of treated TBI rats, the expression of Bax was inhibited, while that of Bcl-2 was increased. Furthermore, the treatment promoted the increased expression of Nrf2 and its related downstream proteins (HO-1 and NQO-1), consistent with mRNA levels, thus, highlighting its neuroprotective role via modulating Nrf2 [135].
Isoliquiritigenins are chalcone compounds, already known as a regulator of the Nrf2/ARE signaling pathway in animal models [136]. In line with these findings, isoliquiritigenin (20 mg/kg; intraperitoneally) one hour after injury improved neurological deficits, histopathologic lesion air, brain content, and vascular permeability. In agreement with the reduced water content, an increased expression of aquaporins was detected. Conversely, the expression level of the cleaved-caspase-3 was reduced, demonstrating the antiapoptotic effects exerted by the compound. The treatment counteracts TBI-induced oxidative stress by reducing the levels of MDA and increasing those of SOD and GSH peroxidase in the brain cortex tissue via Nrf2 activation. Indeed, in Nrf2-KO mice, isoliquiritigenin treatment failed to protect against damage-induced oxidative stress. These results obtained in vivo were also replicated in vitro in the OGD/R model using SH-SY5Y cells, where treatment with isoliquiritigenin (2, 5, 10, 20, and 40 µM) highlighted its role in activating the Nrf2 pathway, promoting the transfer of the Nrf2 protein from the cytoplasm to the nucleus [137].
7-D-Glucuronic acid-5,6-dihydroxyflavone (baicalin) is a flavonoid that crosses the BBB exerting neuroprotective effects in SCI [138]. These data have encouraged the study of the effects of this compound in a TBI mouse model. Baicalin (50, 100, and 150 mg/kg, intraperitoneal) improved neurological damage, reduced edema, and neuronal apoptosis, as demonstrated by an increased Bcl-2 protein expression and a decreased Bax and cleaved caspase-3. Furthermore, this compound exerted antioxidant effects by reducing MDA and increasing GSH peroxidase and SOD in the injured cerebral cortex. These effects are mediated by Nrf2 pathway activation, as demonstrated by the increased nuclear translocation of Nrf2 and its downstream antioxidant enzymes (NQO-1 and HO-1) after treatment with baicalin. Furthermore, the use of the PI3K/Akt inhibitor demonstrated the involvement of Akt in promoting the translocation of Nrf2 from the cytoplasm to the nucleus [139]. Another flavonoid that exerts antioxidant effects by activating Nrf2/HO-1 signaling in a PI3K/Akt-dependent manner is wogonin. Wogonin (40 mg/kg intraperitoneal), reduced cerebral edema, and improved behavioral deficits. This compound protected neuronal cells against apoptosis by increasing Bcl-2 protein expression in the CA1 region of the hippocampus of TBI rats and decreasing the apoptotic proteins caspase-3 and Bax. Furthermore, the compound exerted antioxidant actions, increasing the levels of antioxidant enzymes (GSH, CAT, and SOD) and decreasing ROS, MDA, and NOX2. Wogonin promoted the expression of phosphorylated Akt, Nrf2, and HO-1 in the hippocampus of TBI rats, demonstrating the antioxidant effects as mediated by the activation of Nrf2/HO-1 pathway in a PI3K/Akt-dependent manner [140]. One more flavonoid known for its antioxidant and neuroprotective effects is quercetin. In a model of TBI induced by a modified weight-drop device, treatment with intraperitoneal quercetin (5, 20, or 50 mg/kg) administered 0.5, 12, and 24 h after the injury, reduced cerebral edema and microgliosis 3 days after the injury. Furthermore, the compound counteracted oxidative stress by reducing MDA levels and increasing SOD, CAT, and GSH peroxidase in the cortex through Nrf2/HO-1 pathway activation [141]. The same result was also obtained following treatment with fisetin, another flavonoid extracted from vegetables and fruits [142], known for its anti-inflammatory and antioxidant properties [143]. Furthermore, after 3 days from the injury, treatment with fisetin (25, 50, and 75 mg/kg) protected against neuronal apoptosis, increasing Bcl-2 expression and reducing caspase-3 and Bax expression, and promoting the translocation of Nrf2 from the cytoplasm to the nucleus, thus activating the Nfr2/ARE pathway. Data was also confirmed by the use of Nrf2-KO mice, where the fisetin failed to counteract oxidative stress [144].
Another natural compound that exerts neuroprotective effects after TBI through possible involvement of the Nrf2-ARE pathway is curcumin, extracted from Curcuma longa L. Curcumin (50 and 100 mg/kg; intraperitoneal), especially at the higher dose, reduced injury-induced secondary brain damage, attenuated oxidative stress, and improved neurological function. Furthermore, the compound exerted anti-apoptotic effects by increasing the expression of Bcl-2 and reducing that of the cleaved caspase-3 in brain tissues. Furthermore, curcumin enhanced the translocation of Nrf2 from the cytoplasm to the nucleus, and by increasing the expression of downstream factors such as HO-1 and NQO1, demonstrated the role of this compound in counteracting the oxidative stress induced by the lesion [145]. In another study, at 24 h after the damage, curcumin reduced the levels of Nrf2 and related proteins. In Nrf2-KO mice, the neuroprotective effects of curcumin treatment were significantly reduced, highlighting that the therapeutic effect of this compound in TBI is related to the activation of the Nrf2 pathway [146].
Sodium aescinate, extracted from chestnut seeds, showed anti-inflammatory properties and exerted antioxidant effects in an SCI model [147]. In this regard, a research group led by Zhang L. et al. explored the effects of this saponin in the TBI mouse model. Sodium aescinate (0.5, 1, and 2 mg/kg; intraperitoneal) reduced TBI-induced neurological deficits, brain injury, and counteracted oxidative stress and neuronal apoptosis. Treatment promoted Nrf2 nuclear translocation and enhanced Nrf2-ARE binding. The same data were also obtained in vitro, suggesting that this compound provided neuroprotective effects, especially at 10 μM. Treatment with sodium aescinate in Nrf2-KO mice failed to counteract the oxidative stress induced by TBI, suggesting that also this compound exerts an activating effect on the Nrf2-ARE pathway [148].
Likewise, β-carotene exerts neuroprotective effects after TBI, through the possible involvement of the Nrf2-ARE pathway. In a TBI mouse model, administration of β-carotene (10, 20, 30, and 50 mg/kg; by gavage) improved both the cognitive and neural function. The β-carotene has increased the permeability to the BBB and protected the brain tissue from the oxidative stress TBI-induced. Indeed, especially at the dose of 30 mg/kg, the compound increased the levels of SOD and decreased those of MDA. The β-carotene after 7 days of treatment protected the neuronal cortex from TBI-induced apoptosis. Furthermore, it was shown that the treatment increased the translocation of Nrf2 into the nucleus, decreased the expression of Keap1, and increased the expression of the Nrf2′s downstream effectors (NQO-1 and HO-1), both at the protein level and mRNA [149].
Another carotenoid pigment that exhibits antioxidant, anti-inflammatory, immunomodulatory, and neuroprotective properties, is astaxanthin, which has also shown promise in counteracting oxidative stress in TBI [150,151]. In this regard, the mechanisms underlying the effects of the compound were studied in a mouse model of TBI. Already one day after the injury, astaxanthin (100 mg/kg; intraperitoneal) improved the neuronal function and significantly increased the expression levels of the Nrf2 protein and mRNA, consequently also increasing that of its downstream expression (HO-1 and NQO1). Furthermore, 24 h after injury, the treatment induced an increase in Nrf2 nuclear translocation, suggesting that the neuroprotective effects of Nrf2 against TBI-induced oxidative stress may be mediated by activation of the Nrf2/HO-1 pathway [152]. In another study, astaxanthin (25, 75, and 150 mg/kg) counteracted oxidative stress 1, 3, and 7 days after injury. Moreover, 3 days after the damage, this compound induced an improvement of the neurological functions compromised by the lesion, increasing the expression of peroxiredoxin 2 (Prx2), Nrf2, and sirtuin 1 (SIRT1). Conversely, it reduced the phosphorylation of kinase 1 for the regulation of the apoptosis signal (ASK1) and p38. Thus, it was demonstrated that astaxanthin could inhibit oxidative insults and neuronal apoptosis via activation of SIRT1/Nrf2/Prx2/ASK1/p38 signaling. Furthermore, 21 days after the damage induction, the treatment reduced neuronal apoptosis and lesion volume, suggesting that astaxanthin could improve TBI-induced neurological deficits even long term. Effects of astaxanthin on SIRT1/Nrf2/Prx2/ASK1/p38 signaling were also confirmed in vitro in H2O2-induced primary cortical neurons [153].
Tannic acid is a natural compound, recently known for reducing neurodegeneration and behavioral deficits in an ischemic stroke model [154]. For this reason, its neuromodulatory properties were investigated in a rat model of TBI. Treatment with tannic acid (50 mg/kg) was performed intraperitoneally 30 min before and 6 and 18 h after the injury. A total of 24 h after the injury, the treatment reduced neuronal damage, decreased brain edema, and improved behavioral changes. This polyphenol induced these effects by restoring GSH levels in penumbra tissue and increasing levels of antioxidant enzymes (GST, GSH peroxidase, CAT, and SOD). Additionally, the treatment also significantly increased the protein expression of PGC-1α and Nrf2, mitochondrial transcription factor A, and HO-1 after TBI. Tannic acid improves behavioral deficits, oxidative damage, and mitochondrial damage by activating the PGC-1α/Nrf2/HO-1 signaling pathway [155].
Furthermore, some isothiocyanates such as allyl isothiocyanate, extracted from radish, mustard, and wasabi, are already known for their ability to activate Nrf2 in cultured fibroblasts [156]. Treatment with allyl isothiocyanate given after an injury reduced the brain edema and BBB permeability by decreasing glial fibrillary acid protein (GFAP) and NF-κB levels, and increasing the levels of Nrf2, protein growth-associated 43 (GAP43), and neural cell adhesion molecules. This demonstrates how allyl isothiocyanate enhanced the expression of neuronal plasticity markers and endogenous antioxidant mechanisms through Nrf2 upregulation [157]. Similarly, treatment with lupeol (50 mg/kg), 7 days after the injury inhibited TBI-induced apoptotic cell death in mouse brains by reducing mitochondrial apoptotic signaling (caspase-3, Bax, cytochrome-C and Bcl2), and reduced the activation of glial cells in the cortex and hippocampus of the brain. Furthermore, lupeol promoted Nrf2/HO-1 expression and thereby reduced oxidative stress in the TBI mouse brain [158]. Furthermore, Huperzine-A, a natural sesquiterpene alkaloid, has been shown to have efficacy in improving behavioral alterations in a model of TBI [159]. In this regard, Huperzine-A (0.5 mg/kg) was administered intraperitoneally 30 min after the first injury, and once daily for one month. The treatment reduced brain edema, restored behavioral changes, and improved cognition by increasing synaptic proteins. The Huperzine-A treatment also inhibited the inflammatory response and enhanced the activity of antioxidant enzymes. Finally, this compound exerted its antioxidant effects by activating the Nrf2 pathway and promoting the translocation of Nrf2 from the cytoplasm to the nucleus [160].
Rutaecarpine derived from Euodia rutaecarpa can also activate the Nrf2 pathway and inhibit oxidative damage, as demonstrated in a model of craniofacial injury [161]. In a model of TBI, rutaecarpine (5, 10, 20 mg/kg) 24 h and 30 min before the lesion, and 2, 24, 48 and 72 h after TBI, improved cognitive impairment, inhibited neuronal apoptosis, and counteracted lesion-induced oxidative stress. The same result was also obtained in H2O2-induced PC12 cells. Furthermore, rutaecarpine promotes PGK1 and Nrf2 expressions, suggesting that it may exert its neuroprotective effects against TBI by activating the PGK1/Keap1/Nrf2 pathway [162]. The same result was also obtained by treatment with evodiamine (5, 10, 20 mg/kg), a quinazoline alkaloidal extracted from the fruit of Evodia rutaecarpa [163]. Similarly, aubucin, an iridoid glycoside, has been shown to be a promising compound due to its neuroprotective, antioxidant, and anti-inflammatory properties [164]. Its properties were tested in an in vitro model of H2O2-induced oxidative stress in primary cortical neurons. Cells were treated for 12 h with aubucin (50, 100 or 200 μg/mL). Treatment promoted the translocation of Nrf2 from the cytoplasm to the nucleus, activating the expression of antioxidant enzymes and thereby reducing oxidative stress and reducing apoptosis. Furthermore, in vivo treatment with aubucin (20 or 40 mg/kg) administered intraperitoneally at 30 min, 12, 24, and 48 h after the injury reduced brain edema, ameliorated histological damage, cognitive deficit, and short and long-term neurologic functions. In Nrf-KO mice, aubucin treatment did not reverse oxidative damage, demonstrating the implication of Nrf2 underpinning the neuroprotective and antioxidant effects of this compound [165].
The results are summarized in Table 2, which shows that most natural compounds activate the Nrf2 pathway as illustrated in Figure 5. The experimental studies have shown that the Nrf2 activation signaling pathway mediated by these compounds protects against oxidative damage mitochondrial dysfunction, apoptosis, and inflammation. The studies above-mentioned highlight that the neuroprotective potential of these compounds is linked to different mechanisms, including Nrf2/Keap1/ARE, Nrf2/PINK1/Parkin, NF-κB/TNF-α/ILs, Bax/Bcl-2/caspases, PGK1/Keap1/Nrf2, and SIRT1/Nrf2/Prx2/ASK1/p38. Thus, targeting Nrf2 signaling pathway activation is a promising therapeutic strategy for TBI.
Table 2.
Potential natural compound Nrf2 activators for controlling TBI symptoms and improving functional recovery.
| Compund | Type of Compound | TBI Time Frame | Experimental TBI Models | Targets | Potential Effects | Type of Study | Ref. |
|---|---|---|---|---|---|---|---|
| Oridonine | An organic compound | Acute TBI | Mice | Nrf2/HO-1 pathway | Oridonine ameliorated functional damage and neuropathological changes in animals with TBI, enhancing mitochondrial function and reducing oxidative stress-induced neuroinflammation through activating the Nrf2/HO-1 pathway. | In vivo | [133] |
| H2O2-induced oxidant damage in N2a cells | In vitro | ||||||
| Breviscapine | An aglycone flavonoid | Acute TBI | Rats | Nrf2/HO-1 pathway | Breviscapin treatment ameliorated TBI-induced neuron cell apoptosis and improved neurobehavioral functions through the activation of the Nrf2 pathway and its related downstream proteins (HO-1 and NQO-1). | In vivo | [135] |
| Isoliquiritigenin | A flavonoid | Acute TBI | Mouse TBI and Nrf2-KO mice | Nrf2 pathway | Isoliquiritigenin treatment attenuated lesion-induced damage by counteracting oxidative stress via Nrf2 activation, highlighting its important therapeutic potential in TBI treatment. | In vivo | [137] |
| SH-SY5Y OGD/R | In vitro | ||||||
| Baicalin | A major bioactive flavone | Acute TBI | Mice | Akt/Nrf2 pathway | Baicalin induces neuroprotection and prevents TBI-induced oxidative stress by activating the Akt/Nrf2 pathway. | In vivo | [139] |
| Wogonin | A flavonoid | Acute TBI | Mice | PI3K/Akt/Nrf2/HO-1 pathway |
Wogonin protected the hippocampal damage TBI-induced by counteracting oxidative stress and neuronal death by activating the Nrf2/HO-1 pathway in a PI3K/Akt-dependent manner. | In vivo | [140] |
| Quercetin | A flavonoid | Acute TBI | Rats | Nrf2/HO-1 pathway | Quercetin activated the Nrf2/HO-1 pathway, thus protecting the animals from TBI-induced oxidative stress. | In vivo | [141] |
| Fisetin | A flavonoid | Acute TBI | Mice | Nrf2-ARE pathway | Fisetin treatment activated the Nrf2/HO-1 pathway, thus protecting the animals from TBI-induced oxidative stress and neuronal apoptosis. | In vivo | [144] |
| Curcumin | A diferuloylmethane | Acute TBI | Mice | Nrf2-ARE pathway | Curcumin attenuated the injury-induced oxidative stress and prevented neurological damage, possibly by activating the Nrf2-ARE pathway. | In vivo | [145] |
| Curcumin | A diferuloylmethane | Acute TBI | Mouse TBI and Nrf2-KO mice | Nrf2/HO-1 pathway | Curcumin has shown a neuroprotective role associated with the activation of the Nrf2 pathway, proving to be a potential therapeutic intervention in TBI management. | In vivo | [146] |
| Sodium aescinate | A triterpene saponin | Mouse TBI and Nrf2-KO mice | Nrf2-ARE pathway | Sodium aescinate, by activating the Nrf2-ARE pathway, exerts neuroprotective effects against oxidative stress and neuronal apoptosis TBI-induced, thus highlighting its promising therapeutic effects in the management of this pathology. | In vivo | [148] | |
| Neuron model of TBI | In vitro | ||||||
| β-carotene | A carotenoid | Acute TBI | Mice | Nrf2/HO-1 pathway | β-carotene ameliorated brain injury after TBI by regulating the Nrf2/Keap1-mediated antioxidant pathway. | In vivo | [149] |
| Astaxanthin | A carotenoid pigment | Acute TBI | Mice | Nrf2/HO-1 pathway | Astaxanthin treatment promoted neuroprotective effects in the TBI mouse model probably activating the Nrf2/HO-1 signaling pathway. | In vivo | [152] |
| Astaxanthin | A carotenoid pigment | Acute and chronic TBI | Mouse TBI and Nrf2-KO mice | SIRT1/Nrf2/Prx2/ASK1/p38 signaling | Astaxanthin decreased oxidative stress and neuronal death regulating the SIRT1/Nrf2/Prx2/ASK1/p38 signaling pathway, highlighting its promising therapeutic potential in TBI even in the long term. | In vivo | [153] |
| H2O2-induced oxidant damage in Primary Cortical Neurons | In vitro | ||||||
| Tannic acid | A natural polyphenol | Acute TBI | Rats | PGC-1α/Nrf2/HO-1 signaling pathway | Pretreatment with tannin acid 30 min before, and 6 and 18 h after injury improved behavioral deficits, counteracting TBI-induced oxidative stress and mitochondrial damage probably by activating PGC-1α/Nrf-2/HO-1 signaling pathway. | In vivo | [155] |
| Allyl isothiocyanate | A organosulfur compound | Acute TBI | Mice | Nrf2 pathway | Allyl isothiocyanate treatment ameliorated TBI damage and neurological deficit, enhancing the expression of neuronal plasticity markers and reducing oxidative stress through Nrf2 upregulation. | In vivo | [157] |
| Lupeol | A triterpenoid | Acute TBI | Mice | Nrf2 | Lupeol exerted neuroprotective effects and ameliorated memory and behavioral deficits, TBI-induced reducing glial cell activation, oxidative stress, and apoptosis likely through increasing Nrf2 levels in the brain. | In vivo | [158] |
| Huperzine-A | A sesquiterpene alkaloid | Acute and chronic TBI | Mice | Nrf2 | Huperzine-A induces neuroprotective effects in a TBI mouse model, reducing the oxidative stress response via the Nrf2 pathway. | In vivo | [160] |
| Rutaecarpine | An alkaloid | Acute TBI | Mice | PGK1/Keap1/Nrf2 pathway | Rutaecarpine protected against neuronal apoptosis and oxidative stress induced by TBI, by activating the PGK1/Keap1/Nrf2 pathway. | In vivo | [162] |
| H2O2-induced oxidant damage PC12 | In vitro | ||||||
| Evodiamine | A quinazoline alkaloidal | Acute TBI | Mice | PGK1/Keap1/Nrf2 pathway | Evodiamine protected against neuronal apoptosis and oxidative stress induced by TBI, by activating the PGK1/Keap1/Nrf2 pathway. | In vivo | [163] |
| H2O2-induced PC12 | In vitro | ||||||
| Aucubin | An iridoid glycoside | Acute TBI | H2O2-induced oxidant damage in primary cortical neurons | Nrf2-ARE signaling pathway | Aubucin, by activating the Nrf2 pathway, attenuated TBI-induced oxidative stress and neuronal apoptosis, improving neurological outcomes, and behavioral and cognitive deficits. | In vitro | [165] |
| Mouse TBI and Nrf2-KO mice | In vivo |
Traumatic brain injury: TBI; nuclear factor E2-related factor 2: Nrf2; heme oxygenase-1: HO-1; NADPH Quinone Dehydrogenase 1: NQO1; Nrf2-knockout: Nrf2-KO; antioxidant response elements: ARE; peroxisome proliferator–activated receptor gamma co-activator 1 alpha: PGC-1α; peroxiredoxin 2: Prx2; sirtuin 1: SIRT1; Phosphoinositide 3-kinases: PI3Ks; apoptosis signal-regulating kinase 1: ASK1; Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1: Keap1; oxygen and glucose deprivation/reoxygenation: OGD/R.
Figure 5.
The potential molecular mechanism of natural compounds on TBI. These natural compounds can activate the expression of Nrf2 and facilitate Nrf2’s translocation from the cytosol to the nuclear, thus, inhibiting oxidative stress and protecting from neuronal apoptosis. Tannic acid positively regulates the protein expression of PGC-1α, thereby activating the Nrf2/ARE pathway. Instead, aicalin and wogonin increase the level of phosphorylated AKT and PI3K and activates Nrf2, which translocates into the nucleus to increase its downstream effectors’ production, which are responsible for anti-apoptosis and anti-oxidative effects, thus realizing the repair of TBI. The image was created using the image bank of Servier Medical Art (Available online: http://smart.servier.com/, accessed on 15 November 2022), licensed under a Creative Commons Attribution 3.0 Unported License (Available online: https://creativecommons.org/licenses/by/3.0/, accessed on 15 November 2022). Traumatic brain injury: TBI; nuclear factor E2-related factor 2: Nrf2; antioxidant response element: ARE; small muscleaponeurotic fibrosarcoma: sMAF; Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1: Keap1; β-transducing repeat-containing protein: β-TrCP; Cullin 3: CUL3; RING-box protein 1: RBX1; PTEN Induced Kinase 1: PINK1; peroxisome proliferator–activated receptor gamma co-activator 1 alpha: PGC-1α; Phosphoinositide 3-kinases: PI3Ks.
8. Conclusions
The activation of Nrf2 mediated by several natural compounds promotes its translocation within the nucleus and induces the transcription of enzymes involved in counteracting oxidative stress, the mitochondrial response, and neuronal apoptosis. Nrf2 activation strategies are likely effective in preventing disease and slowing its progression. However, the current understanding of the mechanisms underlying the pathogenesis of these pathological conditions is still limited, and the different mechanisms following the activation of Nrf2/ARE remain to be further explored. Furthermore, clinical trials in patients with SCI and TBI that evaluate the therapeutic implications of these compounds are needed.
Acknowledgments
The authors would like to thank the Ministry of Health, Italy. We also thank Giovanni Schepici for his contribution during the revision of the manuscript.
Author Contributions
Conceptualization, S.S. and E.M.; writing—original draft preparation, S.S.; writing—review and editing, S.S. and E.M.; supervision, E.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by Current Research Funds 2022, Ministry of Health, Italy.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Faul M., Wald M.M., Xu L., Coronado V.G. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths, 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control; Atlanta, GA, USA: 2010. [Google Scholar]
- 2.Finkelstein E., Corso P.S., Miller T.R. The Incidence and Economic Burden of Injuries in the United States. Oxford University Press; New York, NY, USA: 2006. [Google Scholar]
- 3.Control C.f.D. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Volume 45 Centers for Disease Control and Prevention; Atlanta, GA, USA: 2003. [Google Scholar]
- 4.Trivedi A., Olivas A.D., Noble-Haeusslein L.J. Inflammation and spinal cord injury: Infiltrating leukocytes as determinants of injury and repair processes. Clin. Neurosci. Res. 2006;6:283–292. doi: 10.1016/j.cnr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borgens R.B., Liu-Snyder P. Understanding secondary injury. Q. Rev. Biol. 2012;87:89–127. doi: 10.1086/665457. [DOI] [PubMed] [Google Scholar]
- 6.Rajeev V., Fann D.Y., Dinh Q.N., Kim H.A., De Silva T.M., Lai M.K.P., Chen C.L., Drummond G.R., Sobey C.G., Arumugam T.V. Pathophysiology of blood brain barrier dysfunction during chronic cerebral hypoperfusion in vascular cognitive impairment. Theranostics. 2022;12:1639–1658. doi: 10.7150/thno.68304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Von Leden R.E., Yauger Y.J., Khayrullina G., Byrnes K.R. Central Nervous System Injury and Nicotinamide Adenine Dinucleotide Phosphate Oxidase: Oxidative Stress and Therapeutic Targets. J. Neurotrauma. 2017;34:755–764. doi: 10.1089/neu.2016.4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dröge W. Free radicals in the physiological control of cell function. Physiol. Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- 9.Finkel T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans M.D., Dizdaroglu M., Cooke M.S. Oxidative DNA damage and disease: Induction, repair and significance. Mutat. Res. /Rev. Mutat. Res. 2004;567:1–61. doi: 10.1016/j.mrrev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Harfoot C. Lipid metabolism in the rumen. Lipid Metab. Rumin. Anim. 1981:21–55. doi: 10.1016/b978-0-08-023789-3.50006-4. [DOI] [PubMed] [Google Scholar]
- 12.Jia Z.-Q., Li G., Zhang Z.-Y., Li H.-T., Wang J.-Q., Fan Z.-K., Lv G. Time representation of mitochondrial morphology and function after acute spinal cord injury. Neural Regen. Res. 2016;11:137. doi: 10.4103/1673-5374.175061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greco T., Glenn T.C., Hovda D.A., Prins M.L. Ketogenic diet decreases oxidative stress and improves mitochondrial respiratory complex activity. J. Cereb. Blood Flow Metab. 2016;36:1603–1613. doi: 10.1177/0271678X15610584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutierrez J., Ballinger S.W., Darley-Usmar V.M., Landar A. Free radicals, mitochondria, and oxidized lipids: The emerging role in signal transduction in vascular cells. Circ. Res. 2006;99:924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- 15.Cai Z., Yan L.-J. Protein oxidative modifications: Beneficial roles in disease and health. J. Biochem. Pharmacol. Res. 2013;1:15. [PMC free article] [PubMed] [Google Scholar]
- 16.He F., Ru X., Wen T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020;21:4777. doi: 10.3390/ijms21134777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson D.A., Johnson J.A. Nrf2—A therapeutic target for the treatment of neurodegenerative diseases. Free Radic. Biol. Med. 2015;88:253–267. doi: 10.1016/j.freeradbiomed.2015.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen G., Fang Q., Zhang J., Zhou D., Wang Z. Role of the Nrf2-ARE pathway in early brain injury after experimental subarachnoid hemorrhage. J. Neurosci. Res. 2011;89:515–523. doi: 10.1002/jnr.22577. [DOI] [PubMed] [Google Scholar]
- 19.Dinkova-Kostova A.T., Abramov A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J., Fields J., Zhao C., Langer J., Thimmulappa R.K., Kensler T.W., Yamamoto M., Biswal S., Doré S. Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic. Biol. Med. 2007;43:408–414. doi: 10.1016/j.freeradbiomed.2007.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yan W., Wang H.-D., Hu Z.-G., Wang Q.-F., Yin H.-X. Activation of Nrf2–ARE pathway in brain after traumatic brain injury. Neurosci. Lett. 2008;431:150–154. doi: 10.1016/j.neulet.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 22.Jiang T. Recent advances in the role of Nrf2 in spinal cord injury: Regulatory mechanisms and therapeutic Options. Front. Aging Neurosci. 2022;14:851257. doi: 10.3389/fnagi.2022.851257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moher D., Liberati A., Tetzlaff J., Altman D.G. RESEARCH METHODS & REPORTING-Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement-David Moher and colleagues introduce PRISMA, an update of the QUOROM guidelines for reporting systematic reviews and meta-analyses. BMJ. 2009;338:332. [Google Scholar]
- 24.Jaramillo M.C., Zhang D.D. The emerging role of the Nrf2–Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMahon M., Thomas N., Itoh K., Yamamoto M., Hayes J.D. Redox-regulated turnover of Nrf2 is determined by at least two separate protein domains, the redox-sensitive Neh2 degron and the redox-insensitive Neh6 degron. J. Biol. Chem. 2004;279:31556–31567. doi: 10.1074/jbc.M403061200. [DOI] [PubMed] [Google Scholar]
- 26.Wang H., Liu K., Geng M., Gao P., Wu X., Hai Y., Li Y., Li Y., Luo L., Hayes J.D. RXRα inhibits the NRF2-ARE signaling pathway through a direct interaction with the Neh7 domain of NRF2. Cancer Res. 2013;73:3097–3108. doi: 10.1158/0008-5472.CAN-12-3386. [DOI] [PubMed] [Google Scholar]
- 27.Hayes J.D., Dinkova-Kostova A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Canning P., Sorrell F.J., Bullock A.N. Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. 2015;88:101–107. doi: 10.1016/j.freeradbiomed.2015.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kobayashi A., Kang M.-I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto M., Kensler T.W., Motohashi H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018;98:1169–1203. doi: 10.1152/physrev.00023.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMahon M., Lamont D.J., Beattie K.A., Hayes J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. USA. 2010;107:18838–18843. doi: 10.1073/pnas.1007387107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baird L., Llères D., Swift S., Dinkova-Kostova A.T. Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proc. Natl. Acad. Sci. USA. 2013;110:15259–15264. doi: 10.1073/pnas.1305687110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Itoh K., Wakabayashi N., Katoh Y., Ishii T., O'Connor T., Yamamoto M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells. 2003;8:379–391. doi: 10.1046/j.1365-2443.2003.00640.x. [DOI] [PubMed] [Google Scholar]
- 34.Chowdhry S., Zhang Y., McMahon M., Sutherland C., Cuadrado A., Hayes J.D. Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene. 2013;32:3765–3781. doi: 10.1038/onc.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayes J.D., Chowdhry S., Dinkova-Kostova A.T., Sutherland C. Dual regulation of transcription factor Nrf2 by Keap1 and by the combined actions of β-TrCP and GSK-3. Biochem. Soc. Trans. 2015;43:611–620. doi: 10.1042/BST20150011. [DOI] [PubMed] [Google Scholar]
- 36.Salazar M., Rojo A.I., Velasco D., de Sagarra R.M., Cuadrado A. Glycogen synthase kinase-3β inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J. Biol. Chem. 2006;281:14841–14851. doi: 10.1074/jbc.M513737200. [DOI] [PubMed] [Google Scholar]
- 37.Rada P., Rojo A.I., Chowdhry S., McMahon M., Hayes J.D., Cuadrado A. SCF/β-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol. Cell. Biol. 2011;31:1121–1133. doi: 10.1128/MCB.01204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kang H.J., Hong Y.B., Kim H.J., Bae I. CR6-interacting factor 1 (CRIF1) regulates NF-E2-related factor 2 (NRF2) protein stability by proteasome-mediated degradation. J. Biol. Chem. 2010;285:21258–21268. doi: 10.1074/jbc.M109.084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan X., Xu C., Pan Z., Keum Y.S., Kim J.H., Shen G., Yu S., Oo K.T., Ma J., Kong A.N.T. Butylated hydroxyanisole regulates ARE-mediated gene expression via Nrf2 coupled with ERK and JNK signaling pathway in HepG2 cells. Mol. Carcinog. Publ. Coop. Univ. Tex. MD Cancer Cent. 2006;45:841–850. doi: 10.1002/mc.20234. [DOI] [PubMed] [Google Scholar]
- 40.Huang H.-C., Nguyen T., Pickett C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 41.Cullinan S.B., Zhang D., Hannink M., Arvisais E., Kaufman R.J., Diehl J.A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell. Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Apopa P.L., He X., Ma Q. Phosphorylation of Nrf2 in the transcription activation domain by casein kinase 2 (CK2) is critical for the nuclear translocation and transcription activation function of Nrf2 in IMR-32 neuroblastoma cells. J. Biochem. Mol. Toxicol. 2008;22:63–76. doi: 10.1002/jbt.20212. [DOI] [PubMed] [Google Scholar]
- 43.Cuadrado A., Rojo A.I., Wells G., Hayes J.D., Cousin S.P., Rumsey W.L., Attucks O.C., Franklin S., Levonen A.-L., Kensler T.W. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019;18:295–317. doi: 10.1038/s41573-018-0008-x. [DOI] [PubMed] [Google Scholar]
- 44.Sivandzade F., Prasad S., Bhalerao A., Cucullo L. NRF2 and NF-κB interplay in cerebrovascular and neurodegenerative disorders: Molecular mechanisms and possible therapeutic approaches. Redox Biol. 2019;21:101059. doi: 10.1016/j.redox.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wardyn J.D., Ponsford A.H., Sanderson C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015;43:621–626. doi: 10.1042/BST20150014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitsuishi Y., Taguchi K., Kawatani Y., Shibata T., Nukiwa T., Aburatani H., Yamamoto M., Motohashi H. Nrf2 redirects glucose and glutamine into anabolic pathways in metabolic reprogramming. Cancer Cell. 2012;22:66–79. doi: 10.1016/j.ccr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 47.Wakabayashi N., Skoko J.J., Chartoumpekis D.V., Kimura S., Slocum S.L., Noda K., Palliyaguru D.L., Fujimuro M., Boley P.A., Tanaka Y. Notch-Nrf2 axis: Regulation of Nrf2 gene expression and cytoprotection by notch signaling. Mol. Cell. Biol. 2014;34:653–663. doi: 10.1128/MCB.01408-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sangokoya C., Telen M.J., Chi J.-T. microRNA miR-144 modulates oxidative stress tolerance and associates with anemia severity in sickle cell disease. Blood J. Am. Soc. Hematol. 2010;116:4338–4348. doi: 10.1182/blood-2009-04-214817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang M., Yao Y., Eades G., Zhang Y., Zhou Q. MiR-28 regulates Nrf2 expression through a Keap1-independent mechanism. Breast Cancer Res. Treat. 2011;129:983–991. doi: 10.1007/s10549-011-1604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li N., Muthusamy S., Liang R., Sarojini H., Wang E. Increased expression of miR-34a and miR-93 in rat liver during aging, and their impact on the expression of Mgst1 and Sirt1. Mech. Ageing Dev. 2011;132:75–85. doi: 10.1016/j.mad.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Singh B., Ronghe A.M., Chatterjee A., Bhat N.K., Bhat H.K. MicroRNA-93 regulates NRF2 expression and is associated with breast carcinogenesis. Carcinogenesis. 2013;34:1165–1172. doi: 10.1093/carcin/bgt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang B., Teng Y., Liu Q. MicroRNA-153 Regulates NRF2 Expression and is Associated with Breast Carcinogenesis. Clin. Lab. 2016;62:39–47. doi: 10.7754/Clin.Lab.2015.150518. [DOI] [PubMed] [Google Scholar]
- 53.Kansanen E., Kuosmanen S.M., Leinonen H., Levonen A.-L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karin M., Yamamoto Y., Wang Q. The IKK NF-κB system: A treasure trove for drug development. Nat. Rev. Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 55.Yerra V.G., Negi G., Sharma S.S., Kumar A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013;1:394–397. doi: 10.1016/j.redox.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen L.-G., Zhang Y.-Q., Wu Z.-Z., Hsieh C.-W., Chu C.-S., Wung B.-S. Peanut arachidin-1 enhances Nrf2-mediated protective mechanisms against TNF-α-induced ICAM-1 expression and NF-κB activation in endothelial cells. Int. J. Mol. Med. 2018;41:541–547. doi: 10.3892/ijmm.2017.3238. [DOI] [PubMed] [Google Scholar]
- 57.Rangasamy T., Cho C.Y., Thimmulappa R.K., Zhen L., Srisuma S.S., Kensler T.W., Yamamoto M., Petrache I., Tuder R.M., Biswal S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke–induced emphysema in mice. J. Clin. Investig. 2004;114:1248–1259. doi: 10.1172/JCI200421146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 59.Harvey C., Thimmulappa R., Singh A., Blake D., Ling G., Wakabayashi N., Fujii J., Myers A., Biswal S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic. Biol. Med. 2009;46:443–453. doi: 10.1016/j.freeradbiomed.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rundlöf A.-K., Carlsten M., Arnér E.S. The core promoter of human thioredoxin reductase 1: Cloning, transcriptional activity, and Oct-1, Sp1, and Sp3 binding reveal a housekeeping-type promoter for the AU-rich element-regulated gene. J. Biol. Chem. 2001;276:30542–30551. doi: 10.1074/jbc.M101452200. [DOI] [PubMed] [Google Scholar]
- 61.Bae S.H., Sung S.H., Cho E.J., Lee S.K., Lee H.E., Woo H.A., Yu D.Y., Kil I.S., Rhee S.G. Concerted action of sulfiredoxin and peroxiredoxin I protects against alcohol-induced oxidative injury in mouse liver. Hepatology. 2011;53:945–953. doi: 10.1002/hep.24104. [DOI] [PubMed] [Google Scholar]
- 62.Hu Q., Ren J., Li G., Wu J., Wu X., Wang G., Gu G., Ren H., Hong Z., Li J. The mitochondrially targeted antioxidant MitoQ protects the intestinal barrier by ameliorating mitochondrial DNA damage via the Nrf2/ARE signaling pathway. Cell Death Dis. 2018;9:403. doi: 10.1038/s41419-018-0436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Piantadosi C.A., Carraway M.S., Babiker A., Suliman H.B. Heme oxygenase-1 regulates cardiac mitochondrial biogenesis via Nrf2-mediated transcriptional control of nuclear respiratory factor-1. Circ. Res. 2008;103:1232–1240. doi: 10.1161/01.RES.0000338597.71702.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gureev A.P., Shaforostova E.A., Popov V.N. Regulation of mitochondrial biogenesis as a way for active longevity: Interaction between the Nrf2 and PGC-1α signaling pathways. Front. Genet. 2019;10:435. doi: 10.3389/fgene.2019.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kovac S., Angelova P.R., Holmström K.M., Zhang Y., Dinkova-Kostova A.T., Abramov A.Y. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2015;1850:794–801. doi: 10.1016/j.bbagen.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holmström K.M., Baird L., Zhang Y., Hargreaves I., Chalasani A., Land J.M., Stanyer L., Yamamoto M., Dinkova-Kostova A.T., Abramov A.Y. Nrf2 impacts cellular bioenergetics by controlling substrate availability for mitochondrial respiration. Biol. Open. 2013;2:761–770. doi: 10.1242/bio.20134853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y.-S., Ueno I., Sakamoto A., Tong K.I. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 68.Lippai M., Lőw P. The role of the selective adaptor p62 and ubiquitin-like proteins in autophagy. BioMed Res. Int. 2014;2014:832704. doi: 10.1155/2014/832704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chang C.F., Cho S., Wang J. (−)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann. Clin. Transl. Neurol. 2014;1:258–271. doi: 10.1002/acn3.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lan X., Han X., Li Q., Wang J. (−)-Epicatechin, a Natural Flavonoid Compound, Protects Astrocytes Against Hemoglobin Toxicity via Nrf2 and AP-1 Signaling Pathways. Mol. Neurobiol. 2017;54:7898–7907. doi: 10.1007/s12035-016-0271-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng T., Wang W., Li Q., Han X., Xing J., Qi C., Lan X., Wan J., Potts A., Guan F., et al. Cerebroprotection of flavanol (−)-epicatechin after traumatic brain injury via Nrf2-dependent and -independent pathways. Free Radic. Biol. Med. 2016;92:15–28. doi: 10.1016/j.freeradbiomed.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Guo X., Ma L., Li H., Qi X., Wei Y., Duan Z., Xu J., Wang C., You C., Tian M. Brainstem iron overload and injury in a rat model of brainstem hemorrhage. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2020;29:104956. doi: 10.1016/j.jstrokecerebrovasdis.2020.104956. [DOI] [PubMed] [Google Scholar]
- 73.Xu J., Xiao C., Song W., Cui X., Pan M., Wang Q., Feng Y., Xu Y. Elevated Heme Oxygenase-1 Correlates with Increased Brain Iron Deposition Measured by Quantitative Susceptibility Mapping and Decreased Hemoglobin in Patients with Parkinson’s Disease. Front. Aging Neurosci. 2021;13:656626. doi: 10.3389/fnagi.2021.656626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Urrutia P.J., Mena N.P., Núñez M.T. The interplay between iron accumulation, mitochondrial dysfunction, and inflammation during the execution step of neurodegenerative disorders. Front. Pharmacol. 2014;5:38. doi: 10.3389/fphar.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang J., Doré S. Heme oxygenase-1 exacerbates early brain injury after intracerebral haemorrhage. Brain. 2007;130:1643–1652. doi: 10.1093/brain/awm095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krause J., Saunders L. Risk of mortality and life expectancy after spinal cord injury: The role of health behaviors and participation. Top. Spinal Cord Inj. Rehabil. 2010;16:53–60. doi: 10.1310/sci1602-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khorasanizadeh M., Yousefifard M., Eskian M., Lu Y., Chalangari M., Harrop J.S., Jazayeri S.B., Seyedpour S., Khodaei B., Hosseini M. Neurological recovery following traumatic spinal cord injury: A systematic review and meta-analysis. J. Neurosurg. Spine. 2019;30:683–699. doi: 10.3171/2018.10.SPINE18802. [DOI] [PubMed] [Google Scholar]
- 78.National SCI Statistical Center . Facts and Figures at a Glance. Volume 10 University of Alabama at Birmingham; Birmingham, AL, USA: 2016. [Google Scholar]
- 79.McKinley W.O., Seel R.T., Hardman J.T. Nontraumatic spinal cord injury: Incidence, epidemiology, and functional outcome. Arch. Phys. Med. Rehabil. 1999;80:619–623. doi: 10.1016/S0003-9993(99)90162-4. [DOI] [PubMed] [Google Scholar]
- 80.Liu X., Liang F., Song W., Diao X., Zhu W., Yang J. Effect of Nrf2 signaling pathway on the improvement of intestinal epithelial barrier dysfunction by hyperbaric oxygen treatment after spinal cord injury. Cell Stress Chaperones. 2021;26:433–441. doi: 10.1007/s12192-020-01190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Katoh H., Yokota K., Fehlings M.G. Regeneration of spinal cord connectivity through stem cell transplantation and biomaterial scaffolds. Front. Cell. Neurosci. 2019;13:248. doi: 10.3389/fncel.2019.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Anjum A., Yazid M.D.i., Fauzi Daud M., Idris J., Ng A.M.H., Selvi Naicker A., Ismail O.H.R., Athi Kumar R.K., Lokanathan Y. Spinal cord injury: Pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int. J. Mol. Sci. 2020;21:7533. doi: 10.3390/ijms21207533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yip P.K., Malaspina A. Spinal cord trauma and the molecular point of no return. Mol. Neurodegener. 2012;7:6. doi: 10.1186/1750-1326-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rowland J.W., Hawryluk G.W., Kwon B., Fehlings M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- 85.Nakamura M., Houghtling R.A., MacArthur L., Bayer B.M., Bregman B.S. Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord. Exp. Neurol. 2003;184:313–325. doi: 10.1016/S0014-4886(03)00361-3. [DOI] [PubMed] [Google Scholar]
- 86.Ahuja C.S., Nori S., Tetreault L., Wilson J., Kwon B., Harrop J., Choi D., Fehlings M.G. Traumatic spinal cord injury—Repair and regeneration. Neurosurgery. 2017;80:S9–S22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 87.Beattie M.S., Farooqui A.A., BRESNAHAN J.C. Review of current evidence for apoptosis after spinal cord injury. J. Neurotrauma. 2000;17:915–925. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- 88.Dong H., Fazzaro A., Xiang C., Korsmeyer S.J., Jacquin M.F., McDonald J.W. Enhanced oligodendrocyte survival after spinal cord injury in Bax-deficient mice and mice with delayed Wallerian degeneration. J. Neurosci. 2003;23:8682–8691. doi: 10.1523/JNEUROSCI.23-25-08682.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhivotovsky B., Orrenius S. Calcium and cell death mechanisms: A perspective from the cell death community. Cell Calcium. 2011;50:211–221. doi: 10.1016/j.ceca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 90.Park E., Velumian A.A., Fehlings M.G. The role of excitotoxicity in secondary mechanisms of spinal cord injury: A review with an emphasis on the implications for white matter degeneration. J. Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- 91.Sugawara T., Chan P.H. Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid. Redox Signal. 2003;5:597–607. doi: 10.1089/152308603770310266. [DOI] [PubMed] [Google Scholar]
- 92.Croce C.M. Causes and consequences of microRNA dysregulation in cancer. Nat. Rev. Genet. 2009;10:704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Donnelly D.J., Popovich P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tran A.P., Warren P.M., Silver J. The biology of regeneration failure and success after spinal cord injury. Physiol. Rev. 2018;98:881–917. doi: 10.1152/physrev.00017.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Samarghandian S., Pourbagher-Shahri A.M., Ashrafizadeh M., Khan H., Forouzanfar F., Aramjoo H., Farkhondeh T. A pivotal role of the nrf2 signaling pathway in spinal cord injury: A prospective therapeutics study. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2020;19:207–219. doi: 10.2174/1871527319666200604175118. [DOI] [PubMed] [Google Scholar]
- 96.Kanninen K.M., Pomeshchik Y., Leinonen H., Malm T., Koistinaho J., Levonen A.-L. Applications of the Keap1–Nrf2 system for gene and cell therapy. Free Radic. Biol. Med. 2015;88:350–361. doi: 10.1016/j.freeradbiomed.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 97.Wang L., Yao Y., He R., Meng Y., Li N., Zhang D., Xu J., Chen O., Cui J., Bian J. Methane ameliorates spinal cord ischemia-reperfusion injury in rats: Antioxidant, anti-inflammatory and anti-apoptotic activity mediated by Nrf2 activation. Free Radic. Biol. Med. 2017;103:69–86. doi: 10.1016/j.freeradbiomed.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 98.Xu J., Huang G., Zhang K., Sun J., Xu T., Li R., Tao H., Xu W. Nrf2 activation in astrocytes contributes to spinal cord ischemic tolerance induced by hyperbaric oxygen preconditioning. J. Neurotrauma. 2014;31:1343–1353. doi: 10.1089/neu.2013.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lv R., Du L., Zhang L., Zhang Z. Polydatin attenuates spinal cord injury in rats by inhibiting oxidative stress and microglia apoptosis via Nrf2/HO-1 pathway. Life Sci. 2019;217:119–127. doi: 10.1016/j.lfs.2018.11.053. [DOI] [PubMed] [Google Scholar]
- 100.Cui H.-Y., Zhang X.-J., Yang Y., Zhang C., Zhu C.-H., Miao J.-Y., Chen R. Rosmarinic acid elicits neuroprotection in ischemic stroke via Nrf2 and heme oxygenase 1 signaling. Neural Regen. Res. 2018;13:2119. doi: 10.4103/1673-5374.241463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ghaffari H., Venkataramana M., Ghassam B.J., Nayaka S.C., Nataraju A., Geetha N., Prakash H. Rosmarinic acid mediated neuroprotective effects against H2O2-induced neuronal cell damage in N2A cells. Life Sci. 2014;113:7–13. doi: 10.1016/j.lfs.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 102.Ma Z., Lu Y., Yang F., Li S., He X., Gao Y., Zhang G., Ren E., Wang Y., Kang X. Rosmarinic acid exerts a neuroprotective effect on spinal cord injury by suppressing oxidative stress and inflammation via modulating the Nrf2/HO-1 and TLR4/NF-κB pathways. Toxicol. Appl. Pharmacol. 2020;397:115014. doi: 10.1016/j.taap.2020.115014. [DOI] [PubMed] [Google Scholar]
- 103.Dong X., Zheng L., Lu S., Yang Y. Neuroprotective effects of pretreatment of ginsenoside R b1 on severe cerebral ischemia-induced injuries in aged mice: Involvement of anti-oxidant signaling. Geriatr. Gerontol. Int. 2017;17:338–345. doi: 10.1111/ggi.12699. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Z., Yang K., Mao R., Zhong D., Xu Z., Xu J., Xiong M. Ginsenoside Rg1 inhibits oxidative stress and inflammation in rats with spinal cord injury via Nrf2/HO-1 signaling pathway. Neuroreport. 2021;33:81–89. doi: 10.1097/WNR.0000000000001757. [DOI] [PubMed] [Google Scholar]
- 105.Luo H., Bao Z., Zhou M., Chen Y., Huang Z. Notoginsenoside R1 alleviates spinal cord injury by inhibiting oxidative stress, neuronal apoptosis, and inflammation via activating the nuclear factor erythroid 2 related factor 2/heme oxygenase-1 signaling pathway. Neuroreport. 2022;33:451–462. doi: 10.1097/WNR.0000000000001803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhen J.-L., Chang Y.-N., Qu Z.-Z., Fu T., Liu J.-Q., Wang W.-P. Luteolin rescues pentylenetetrazole-induced cognitive impairment in epileptic rats by reducing oxidative stress and activating PKA/CREB/BDNF signaling. Epilepsy Behav. 2016;57:177–184. doi: 10.1016/j.yebeh.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 107.Nabavi S.F., Braidy N., Gortzi O., Sobarzo-Sanchez E., Daglia M., Skalicka-Woźniak K., Nabavi S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015;119:1–11. doi: 10.1016/j.brainresbull.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 108.Fu J., Sun H., Zhang Y., Xu W., Wang C., Fang Y., Zhao J. Neuroprotective effects of luteolin against spinal cord ischemia–reperfusion injury by attenuation of oxidative stress, inflammation, and apoptosis. J. Med. Food. 2018;21:13–20. doi: 10.1089/jmf.2017.4021. [DOI] [PubMed] [Google Scholar]
- 109.Fu J., Xu W., Zhang Y., Sun H., Zhao J. Luteolin Modulates the NF-E2-Related Factor 2/Glutamate–Cysteine Ligase Pathway in Rats with Spinal Cord Injury. J. Med. Food. 2021;24:218–225. doi: 10.1089/jmf.2020.4764. [DOI] [PubMed] [Google Scholar]
- 110.Zhang Z., Shi L. Anti-inflammatory and analgesic properties of cis-mulberroside A from Ramulus mori. Fitoterapia. 2010;81:214–218. doi: 10.1016/j.fitote.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 111.Xia P., Gao X., Duan L., Zhang W., Sun Y.F. Mulberrin (Mul) reduces spinal cord injury (SCI)-induced apoptosis, inflammation and oxidative stress in rats via miroRNA-337 by targeting Nrf-2. Biomed. Pharmacother. 2018;107:1480–1487. doi: 10.1016/j.biopha.2018.07.082. [DOI] [PubMed] [Google Scholar]
- 112.Guo N., Li C., Liu Q., Liu S., Huan Y., Wang X., Bai G., Yang M., Sun S., Xu C. Maltol, a food flavor enhancer, attenuates diabetic peripheral neuropathy in streptozotocin-induced diabetic rats. Food Funct. 2018;9:6287–6297. doi: 10.1039/C8FO01964A. [DOI] [PubMed] [Google Scholar]
- 113.Sha J.-Y., Zhou Y.-D., Yang J.-Y., Leng J., Li J.-H., Hu J.-N., Liu W., Jiang S., Wang Y.-P., Chen C. Maltol (3-hydroxy-2-methyl-4-pyrone) slows d-galactose-induced brain aging process by damping the Nrf2/HO-1-mediated oxidative stress in mice. J. Agric. Food Chem. 2019;67:10342–10351. doi: 10.1021/acs.jafc.9b04614. [DOI] [PubMed] [Google Scholar]
- 114.Mao Y., Du J., Chen X., Al Mamun A., Cao L., Yang Y., Mubwandarikwa J., Zaeem M., Zhang W., Chen Y., et al. Maltol Promotes Mitophagy and Inhibits Oxidative Stress via the Nrf2/PINK1/Parkin Pathway after Spinal Cord Injury. Oxidative Med. Cell. Longev. 2022;2022:1337630. doi: 10.1155/2022/1337630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fuyuno Y., Uchi H., Yasumatsu M., Morino-Koga S., Tanaka Y., Mitoma C., Furue M. Perillaldehyde inhibits AHR signaling and activates NRF2 antioxidant pathway in human keratinocytes. Oxidative Med. Cell. Longev. 2018;2018:9524657. doi: 10.1155/2018/9524657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zheng W., Liu B., Shi E. Perillaldehyde Alleviates Spinal Cord Ischemia-Reperfusion Injury Via Activating the Nrf2 Pathway. J. Surg. Res. 2021;268:308–317. doi: 10.1016/j.jss.2021.06.055. [DOI] [PubMed] [Google Scholar]
- 117.Wu W.N., Wu P.F., Chen X.L., Zhang Z., Gu J., Yang Y.J., Xiong Q.J., Ni L., Wang F., Chen J.G. Sinomenine protects against ischaemic brain injury: Involvement of co-inhibition of acid-sensing ion channel 1a and L-type calcium channels. Br. J. Pharmacol. 2011;164:1445–1459. doi: 10.1111/j.1476-5381.2011.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang Z., Liu Y., Yuan F., Li Z., Huang S., Shen H., Yuan B. Sinomenine inhibits microglia activation and attenuates brain injury in intracerebral hemorrhage. Mol. Immunol. 2014;60:109–114. doi: 10.1016/j.molimm.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 119.Zhang L., Zhang W., Zheng B., Tian N. Sinomenine attenuates traumatic spinal cord injury by suppressing oxidative stress and inflammation via Nrf2 pathway. Neurochem. Res. 2019;44:763–775. doi: 10.1007/s11064-018-02706-z. [DOI] [PubMed] [Google Scholar]
- 120.Huynh L.M., Burns M.P., Taub D.D., Blackman M.R., Zhou J. Chronic neurobehavioral impairments and decreased hippocampal expression of genes important for brain glucose utilization in a mouse model of mild TBI. Front. Endocrinol. 2020;11:556380. doi: 10.3389/fendo.2020.556380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Summers C.R., Ivins B., Schwab K.A. Traumatic brain injury in the United States: An epidemiologic overview. Mt. Sinai J. Med. J. Transl. Pers. Med. 2009;76:105–110. doi: 10.1002/msj.20100. [DOI] [PubMed] [Google Scholar]
- 122.Lorente L., Martín M.M., Almeida T., Abreu-González P., Ramos L., Argueso M., Riaño-Ruiz M., Solé-Violán J., Jiménez A. Total antioxidant capacity is associated with mortality of patients with severe traumatic brain injury. BMC Neurol. 2015;15:115. doi: 10.1186/s12883-015-0378-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lozano D., Gonzales-Portillo G.S., Acosta S., de la Pena I., Tajiri N., Kaneko Y., Borlongan C.V. Neuroinflammatory responses to traumatic brain injury: Etiology, clinical consequences, and therapeutic opportunities. Neuropsychiatr. Dis. Treat. 2015;11:97. doi: 10.2147/NDT.S65815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McKee C.A., Lukens J.R. Emerging roles for the immune system in traumatic brain injury. Front. Immunol. 2016;7:556. doi: 10.3389/fimmu.2016.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jalloh I., Carpenter K.L., Helmy A., Carpenter T.A., Menon D.K., Hutchinson P.J. Glucose metabolism following human traumatic brain injury: Methods of assessment and pathophysiological findings. Metab. Brain Dis. 2015;30:615–632. doi: 10.1007/s11011-014-9628-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hinzman J.M., Thomas T.C., Quintero J.E., Gerhardt G.A., Lifshitz J. Disruptions in the regulation of extracellular glutamate by neurons and glia in the rat striatum two days after diffuse brain injury. J. Neurotrauma. 2012;29:1197–1208. doi: 10.1089/neu.2011.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Farkas O., Povlishock J.T. Cellular and subcellular change evoked by diffuse traumatic brain injury: A complex web of change extending far beyond focal damage. Prog. Brain Res. 2007;161:43–59. doi: 10.1016/S0079-6123(06)61004-2. [DOI] [PubMed] [Google Scholar]
- 128.Jarrahi A., Braun M., Ahluwalia M., Gupta R.V., Wilson M., Munie S., Ahluwalia P., Vender J.R., Vale F.L., Dhandapani K.M. Revisiting traumatic brain injury: From molecular mechanisms to therapeutic interventions. Biomedicines. 2020;8:389. doi: 10.3390/biomedicines8100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abbasi-Kangevari M., Ghamari S.-H., Safaeinejad F., Bahrami S., Niknejad H. Potential therapeutic features of human amniotic mesenchymal stem cells in multiple sclerosis: Immunomodulation, inflammation suppression, angiogenesis promotion, oxidative stress inhibition, neurogenesis induction, MMPs regulation, and remyelination stimulation. Front. Immunol. 2019;10:238. doi: 10.3389/fimmu.2019.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tang S., Gao P., Chen H., Zhou X., Ou Y., He Y. The role of iron, its metabolism and ferroptosis in traumatic brain injury. Front. Cell. Neurosci. 2020;14:590789. doi: 10.3389/fncel.2020.590789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Loane D.J., Stoica B.A., Faden A.I. Neuroprotection for traumatic brain injury. Handb. Clin. Neurol. 2015;127:343–366. doi: 10.1016/B978-0-444-52892-6.00022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zheng X., Wu Q., Song Z., Zhang H., Zhang J., Zhang L., Zhang T., Wang C., Wang T. Effects of Oridonin on growth performance and oxidative stress in broilers challenged with lipopolysaccharide. Poult. Sci. 2016;95:2281–2289. doi: 10.3382/ps/pew161. [DOI] [PubMed] [Google Scholar]
- 133.Zhao X.J., Zhu H.Y., Wang X.L., Lu X.W., Pan C.L., Xu L., Liu X., Xu N., Zhang Z.Y. Oridonin Ameliorates Traumatic Brain Injury-Induced Neurological Damage by Improving Mitochondrial Function and Antioxidant Capacity and Suppressing Neuroinflammation through the Nrf2 Pathway. J. Neurotrauma. 2022;39:530–543. doi: 10.1089/neu.2021.0466. [DOI] [PubMed] [Google Scholar]
- 134.Jiang L., Hu Y., He X., Lv Q., Wang T.-h., Xia Q.-j. Breviscapine reduces neuronal injury caused by traumatic brain injury insult: Partly associated with suppression of interleukin-6 expression. Neural Regen. Res. 2017;12:90. doi: 10.4103/1673-5374.198990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Li F., Wang X., Zhang Z., Gao P., Zhang X. Breviscapine provides a neuroprotective effect after traumatic brain injury by modulating the Nrf2 signaling pathway. J. Cell Biochem. 2019;120:14899–14907. doi: 10.1002/jcb.28751. [DOI] [PubMed] [Google Scholar]
- 136.Hou Z., Chen L., Fang P., Cai H., Tang H., Peng Y., Deng Y., Cao L., Li H., Zhang B. Mechanisms of triptolide-induced hepatotoxicity and protective effect of combined use of isoliquiritigenin: Possible roles of Nrf2 and hepatic transporters. Front. Pharmacol. 2018;9:226. doi: 10.3389/fphar.2018.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang M., Huang L.-L., Teng C.-H., Wu F.-F., Ge L.-y., Shi Y.-J., He Z.-L., Liu L., Jiang C.-J., Hou R.-N. Isoliquiritigenin provides protection and attenuates oxidative stress-induced injuries via the Nrf2-ARE signaling pathway after traumatic brain injury. Neurochem. Res. 2018;43:2435–2445. doi: 10.1007/s11064-018-2671-z. [DOI] [PubMed] [Google Scholar]
- 138.Cao Y., Li G., Wang Y.-f., Fan Z.-k., Yu D.-s., Bi Y.-l. Neuroprotective effect of baicalin on compression spinal cord injury in rats. Brain Res. 2010;1357:115–123. doi: 10.1016/j.brainres.2010.07.108. [DOI] [PubMed] [Google Scholar]
- 139.Fang J., Wang H., Zhou J., Dai W., Zhu Y., Zhou Y., Wang X., Zhou M. Baicalin provides neuroprotection in traumatic brain injury mice model through Akt/Nrf2 pathway. Drug Des. Dev. Ther. 2018;12:2497–2508. doi: 10.2147/DDDT.S163951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Feng Y., Ju Y., Yan Z., Ji M., Yang M., Wu Q., Wang L., Sun G. Protective role of wogonin following traumatic brain injury by reducing oxidative stress and apoptosis via the PI3K/Nrf2/HO-1 pathway. Int. J. Mol. Med. 2022;49:53. doi: 10.3892/ijmm.2022.5109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Song J., Du G., Wu H., Gao X., Yang Z., Liu B., Cui S. Protective effects of quercetin on traumatic brain injury induced inflammation and oxidative stress in cortex through activating Nrf2/HO-1 pathway. Restor. Neurol. Neurosci. 2021;39:73–84. doi: 10.3233/RNN-201119. [DOI] [PubMed] [Google Scholar]
- 142.Arai Y., Watanabe S., Kimira M., Shimoi K., Mochizuki R., Kinae N. Dietary Intakes of Flavonols, Flavones and Isoflavones by Japanese Women and the Inverse Correlation between Quercetin Intake and Plasma LDL Cholesterol Concentration. J. Nutr. 2000;130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 143.Zhou C.-H., Wang C.-X., Xie G.-B., Wu L.-Y., Wei Y.-X., Wang Q., Zhang H.-S., Hang C.-H., Zhou M.-L., Shi J.-X. Fisetin alleviates early brain injury following experimental subarachnoid hemorrhage in rats possibly by suppressing TLR 4/NF-κB signaling pathway. Brain Res. 2015;1629:250–259. doi: 10.1016/j.brainres.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 144.Zhang L., Wang H., Zhou Y., Zhu Y., Fei M. Fisetin alleviates oxidative stress after traumatic brain injury via the Nrf2-ARE pathway. Neurochem Int. 2018;118:304–313. doi: 10.1016/j.neuint.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 145.Dai W., Wang H., Fang J., Zhu Y., Zhou J., Wang X., Zhou Y., Zhou M. Curcumin provides neuroprotection in model of traumatic brain injury via the Nrf2-ARE signaling pathway. Brain Res. Bull. 2018;140:65–71. doi: 10.1016/j.brainresbull.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 146.Dong W., Yang B., Wang L., Li B., Guo X., Zhang M., Jiang Z., Fu J., Pi J., Guan D., et al. Curcumin plays neuroprotective roles against traumatic brain injury partly via Nrf2 signaling. Toxicol. Appl. Pharm. 2018;346:28–36. doi: 10.1016/j.taap.2018.03.020. [DOI] [PubMed] [Google Scholar]
- 147.Cheng P., Kuang F., Ju G. Aescin reduces oxidative stress and provides neuroprotection in experimental traumatic spinal cord injury. Free Radic. Biol. Med. 2016;99:405–417. doi: 10.1016/j.freeradbiomed.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 148.Zhang L., Fei M., Wang H., Zhu Y. Sodium aescinate provides neuroprotection in experimental traumatic brain injury via the Nrf2-ARE pathway. Brain Res. Bull. 2020;157:26–36. doi: 10.1016/j.brainresbull.2020.01.019. [DOI] [PubMed] [Google Scholar]
- 149.Chen P., Li L., Gao Y., Xie Z., Zhang Y., Pan Z., Tu Y., Wang H., Han Q., Hu X., et al. β-carotene provides neuroprotection after experimental traumatic brain injury via the Nrf2-ARE pathway. JIN. 2019;18:153–161. doi: 10.31083/j.jin.2019.02.120. [DOI] [PubMed] [Google Scholar]
- 150.Yuan J.P., Peng J., Yin K., Wang J.H. Potential health-promoting effects of astaxanthin: A high-value carotenoid mostly from microalgae. Mol. Nutr. Food Res. 2011;55:150–165. doi: 10.1002/mnfr.201000414. [DOI] [PubMed] [Google Scholar]
- 151.Zhang M., Cui Z., Cui H., Cao Y., Wang Y., Zhong C. Astaxanthin alleviates cerebral edema by modulating NKCC1 and AQP4 expression after traumatic brain injury in mice. BMC Neurosci. 2016;17:60. doi: 10.1186/s12868-016-0295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gao F., Wu X., Mao X., Niu F., Zhang B., Dong J., Liu B. Astaxanthin provides neuroprotection in an experimental model of traumatic brain injury via the Nrf2/HO-1 pathway. Am. J. Transl. Res. 2021;13:1483–1493. [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang X.S., Lu Y., Li W., Tao T., Peng L., Wang W.H., Gao S., Liu C., Zhuang Z., Xia D.Y., et al. Astaxanthin ameliorates oxidative stress and neuronal apoptosis via SIRT1/NRF2/Prx2/ASK1/p38 after traumatic brain injury in mice. Br. J. Pharm. 2021;178:1114–1132. doi: 10.1111/bph.15346. [DOI] [PubMed] [Google Scholar]
- 154.Ashafaq M., Tabassum H., Parvez S. Modulation of behavioral deficits and neurodegeneration by tannic acid in experimental stroke challenged Wistar rats. Mol. Neurobiol. 2017;54:5941–5951. doi: 10.1007/s12035-016-0096-8. [DOI] [PubMed] [Google Scholar]
- 155.Salman M., Tabassum H., Parvez S. Tannic Acid Provides Neuroprotective Effects Against Traumatic Brain Injury Through the PGC-1α/Nrf2/HO-1 Pathway. Mol. Neurobiol. 2020;57:2870–2885. doi: 10.1007/s12035-020-01924-3. [DOI] [PubMed] [Google Scholar]
- 156.Ernst I.M., Wagner A.E., Schuemann C., Storm N., Höppner W., Döring F., Stocker A., Rimbach G. Allyl-, butyl-and phenylethyl-isothiocyanate activate Nrf2 in cultured fibroblasts. Pharmacol. Res. 2011;63:233–240. doi: 10.1016/j.phrs.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 157.Caglayan B., Kilic E., Dalay A., Altunay S., Tuzcu M., Erten F., Orhan C., Gunal M.Y., Yulug B., Juturu V. Allyl isothiocyanate attenuates oxidative stress and inflammation by modulating Nrf2/HO-1 and NF-κB pathways in traumatic brain injury in mice. Mol. Biol. Rep. 2019;46:241–250. doi: 10.1007/s11033-018-4465-4. [DOI] [PubMed] [Google Scholar]
- 158.Ahmad R., Khan A., Rehman I.U., Lee H.J., Khan I., Kim M.O. Lupeol Treatment Attenuates Activation of Glial Cells and Oxidative-Stress-Mediated Neuropathology in Mouse Model of Traumatic Brain Injury. Int. J. Mol. Sci. 2022;23:6086. doi: 10.3390/ijms23116086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mei Z., Zheng P., Tan X., Wang Y., Situ B. Huperzine A alleviates neuroinflammation, oxidative stress and improves cognitive function after repetitive traumatic brain injury. Metab. Brain Dis. 2017;32:1861–1869. doi: 10.1007/s11011-017-0075-4. [DOI] [PubMed] [Google Scholar]
- 160.Mei Z., Hong Y., Yang H., Sheng Q., Situ B. Huperzine A protects against traumatic brain injury through anti-oxidative effects via the Nrf2-ARE pathway. Iran. J. Basic Med. Sci. 2021;24:1455–1461. doi: 10.22038/IJBMS.2021.58169.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Han M., Hu L., Chen Y. Rutaecarpine may improve neuronal injury, inhibits apoptosis, inflammation and oxidative stress by regulating the expression of ERK1/2 and Nrf2/HO-1 pathway in rats with cerebral ischemia-reperfusion injury. Drug Des. Dev. Ther. 2019;13:2923. doi: 10.2147/DDDT.S216156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xu M., Li L., Liu H., Lu W., Ling X., Gong M. Rutaecarpine Attenuates Oxidative Stress-Induced Traumatic Brain Injury and Reduces Secondary Injury via the PGK1/KEAP1/NRF2 Signaling Pathway. Front. Pharm. 2022;13:807125. doi: 10.3389/fphar.2022.807125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu M., Wang W., Lu W., Ling X., Rui Q., Ni H. Evodiamine prevents traumatic brain injury through inhibiting oxidative stress via PGK1/NRF2 pathway. Biomed. Pharmacother. 2022;153:113435. doi: 10.1016/j.biopha.2022.113435. [DOI] [PubMed] [Google Scholar]
- 164.Zeng X., Guo F., Ouyang D. A review of the pharmacology and toxicology of aucubin. Fitoterapia. 2020;140:104443. doi: 10.1016/j.fitote.2019.104443. [DOI] [PubMed] [Google Scholar]
- 165.Wang H., Zhou X.M., Wu L.Y., Liu G.J., Xu W.D., Zhang X.S., Gao Y.Y., Tao T., Zhou Y., Lu Y., et al. Aucubin alleviates oxidative stress and inflammation via Nrf2-mediated signaling activity in experimental traumatic brain injury. J. Neuroinflammation. 2020;17:188. doi: 10.1186/s12974-020-01863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.