Abstract
The temporomandibular joint (TMJ) is a specialized synovial joint that is crucial for the movement and function of the jaw. TMJ osteoarthritis (TMJ OA) is the result of disc dislocation, trauma, functional overburden, and developmental anomalies. TMJ OA affects all joint structures, including the articular cartilage, synovium, subchondral bone, capsule, ligaments, periarticular muscles, and sensory nerves that innervate the tissues. The present review aimed to illustrate the main pathomechanisms involving cartilage and bone changes in TMJ OA and some therapeutic options that have shown potential restorative properties regarding these joint structures in vivo. Chondrocyte loss, extracellular matrix (ECM) degradation, and subchondral bone remodeling are important factors in TMJ OA. The subchondral bone actively participates in TMJ OA through an abnormal bone remodeling initially characterized by a loss of bone mass, followed by reparative mechanisms that lead to stiffness and thickening of the condylar osteochondral interface. In recent years, such therapies as intraarticular platelet-rich plasma (PRP), hyaluronic acid (HA), and mesenchymal stem cell-based treatment (MSCs) have shown promising results with respect to the regeneration of joint structures or the protection against further damage in TMJ OA. Nevertheless, PRP and MSCs are more frequently associated with cartilage and/or bone repair than HA. According to recent findings, the latter could enhance the restorative potential of other therapies (PRP, MSCs) when used in combination, rather than repair TMJ structures by itself. TMJ OA is a complex disease in which degenerative changes in the cartilage and bone develop through intricate mechanisms. The regenerative potential of such therapies as PRP, MSCs, and HA regarding the cartilage and subchondral bone (alone or in various combinations) in TMJ OA remains a matter of further research, with studies sometimes obtaining discrepant results.
Keywords: osteoarthritis, temporomandibular joint, bone remodeling, chondrocyte death, regenerative therapy
1. Introduction
The temporomandibular joint (TMJ) is a synovial joint that allows mandibular motion relative to the cranial base and distributes normal function (chewing and speaking) and parafunction stresses (clenching and bruxism). The ability of condylar cartilage to rebuild in response to changes in condylar repositioning, articular function, and particular mechanical loading is the most remarkable biological characteristic that distinguishes it from other types of cartilage [1,2,3,4,5,6,7,8].
Unlike hyaline articular cartilage, which solely contains type II collagen, TMJ mandibular condyle fibrocartilage contains both type I and type II collagen [9]. Because of this, TMJ demonstrated enhanced healing capability and interstitial cartilage development. Another distinguishing characteristic of TMJ is that, in contrast to the articular cartilage in other joints, which is a primary cartilage, the cartilage of the mandibular condyle is a secondary cartilage [10,11,12]. The most frequent type of arthritis affecting TMJ is temporomandibular joint osteoarthritis (TMJ OA), which has a significant clinical prevalence and adverse effects on the TMJ [7,13]. TMJ OA is a chronic degenerative disease that affects both the cartilage and the subchondral bone [14]. TMJ OA is primarily characterized by chondrocyte death, extracellular matrix (ECM) degradation, and subchondral bone remodeling [13].
In recent years, several therapeutic strategies have been tested beyond their ability to improve OA-related symptoms, with research focusing on their regenerative potential. In this respect, platelet-rich plasma (PRP), hyaluronic acid (HA), and mesenchymal stem cells (MSCs) (either alone, or in various combinations) have shown some promising results with regard to the restoration of the normal cartilage and bone aspect in OA (or a delayed progression of disease-related changes), as shown by objective assessment methods of their effects (such as imaging techniques or histological examination) [1,3]. Nevertheless, studies report sometimes discrepant findings.
The present review aimed to illustrate the main pathomechanisms involving cartilage and bone changes in TMJ OA and some therapeutic options that have shown potential restorative properties regarding these joint structures in vivo. We conducted a search of the literature regarding the pathogenesis of cartilage and bone degeneration in TMJ OA. In order to present the role of PRP, HA, and MSCs in the treatment of TMJ OA, we researched original articles published between 2019–present using the keywords “temporomandibular” and “osteoarthritis” and “platelet-rich plasma”/”hyaluronic acid”/”mesenchymal stem cells”. Of the resulting searches, we read and selected the articles that involved either experimental animals or human subjects with degenerative TMJ involvement and used objective measures to investigate cartilage and bone changes (imaging techniques, histological assessment).
2. Cartilage Involvement in Temporomandibular Joint Osteoarthritis
Mechanical factors that cause excessive or unbalanced joint loading influence the onset and progression of TMJ OA. Mechanical factors include injuries (which cause a change in the mechanical properties of the articular disc, cartilage degradation, and the production of inflammatory and pain mediators), parafunctions (which determine articular disc dislocation and degenerative changes within the condyle and articular eminence), increased friction within the TMJ, unstable occlusion, and functional overload [15]. In addition to the local causes, systemic risk factors for TMJ OA include aging, female sex, metabolic and autoimmune illnesses, dietary inadequacies, hormonal variables, and a predisposed genetic profile [16].
Degenerative changes result from a disruption in the remodeling of the TMJ. Remodeling is the essential biological response to TMJ loading. It maintains the balance of the joint, function, and occlusion. Excessive or prolonged TMJ overload and a reduction in TMJ adaptability may result in incorrect remodeling [17].
The degradation of collagen and proteoglycans in cartilage leads to fibrillation, erosion, and cracking in the superficial cartilage layer [18]. This process spreads to a deeper layer of cartilage and enlarges to generate erosions over time. The articular surface of the TMJ exhibits exceptional adaptability. The hyaline cartilage of the body’s weight-bearing joints is more robust to compressive loading, whereas the fibrocartilage of the TMJ is more resistant to shear force [19].
Early cartilage deterioration may be caused by metabolic or mechanical causes. It is a sequence of biomechanical changes in the joint’s hard and soft tissues, which in turn triggers the immunological response. Immune cells initiate an inflammatory response by releasing cytokines and chemokines, among other inflammatory mediators. The process is accompanied by the activation of the complement system and the production of cartilage-degrading molecules, including matrix metalloproteinases (MMPs) and prostaglandin E (PGE), further deteriorating the articular cartilage. This leads to the eventual degeneration and abrasion of joint cartilage and the remodeling of the subchondral bone through the onset of a local inflammatory response [17,20].
The defining characteristics of TMJ OA are both radiological and clinical [21]. The clinical manifestations include tenderness in the joint region, pain during mouth opening and lateral excursion, and grating or crepitus. Cortical bone degradation, flattening of joint compartments, and productive bone alterations such as sclerosis and osteophytes are radiographic manifestations of the disease [22].
As cartilage’s resident cells, chondrocytes are the essential mediators in maintaining the homeostasis of the cartilage matrix. Compromised chondrocyte activity and survival would disrupt cartilage homeostasis and hasten the progression of OA [9].
The quality of condylar bone is strongly related to the development of TMJ osteoarthritis. The gradual cartilage degeneration is caused by improper regulation of chondrocytes and an imbalance between tissue destruction and production [14]. Chondrocytes regulate the equilibrium between ECM synthesis and breakdown. However, this balance can be disrupted if catabolic and synthesis activity is not connected [23]. In addition, the number of apoptotic chondrocytes increases dramatically in OA, which is directly associated with the endoplasmic reticulum and death receptor pathways [24].
Hypertrophic chondrocytes induce the breakdown of the ECM and calcification of cartilage. This denatured cartilage adapts less mechanically to damaging stimuli, such as trauma [25]. Additionally, angiogenesis has been implicated in TMJ OA [14,26].
2.1. Chondrocyte Apoptosis
Multiple studies have shown that an increase in chondrocyte turnover initiates the deterioration of condylar cartilage [27]. Apoptosis, autophagy, and necroptosis are important in chondrocyte death. Apoptosis and necroptosis promote articular cartilage degeneration [14]. Chondrocyte apoptosis creates space for neovascularization, and it is believed that the apoptotic bodies created by this process constitute the origin of cartilage mineralization [25].
Calcium is essential for chondrocyte death, and mechanical strain can raise calcium concentration in chondrocytes [28]. Endoplasmic reticulum stress (ERS) induced by calcium influx can cause death in chondrocytes; this phenomenon is called ERS-mediated apoptosis [29]. A high concentration of intracellular calcium can activate inducible nitric oxide synthase (iNOS). By releasing cytochrome C (Cyt C) and caspase-9, nitric oxide (NO) generated by iNOS limits mitochondrial respiration and leads to chondrocyte death [30].
In addition, tumor necrosis factor (TNF) and fibroblast growth factor receptor 1 (FGFR1) can promote chondrocyte apoptosis by working on the death receptor pathway [31]. Necroptosis is an important phenomenon in OA and is caused by oxidative stress. The breakdown of the cartilage is accelerated by necroptosis, which is mediated by TNF and receptor-interacting proteins 1 and 3 (RIP1/RIP3). According to studies, inhibiting apoptosis promotes the necroptosis pathway [32].
Chondrocytes recycle or reuse large macromolecules through a process known as autophagy, which is believed to be a self-protective mechanism. In most cases, aberrant mortality of chondrocytes not only results in a decrease in the total number of chondrocytes but also initiates the degeneration of cartilage and the breakdown of subchondral bone [33].
Autophagy is a crucial survival strategy for chondrocytes in OA [33]. The primary step of autophagy is the production of autophagosomes, which sequester organelles or macromolecules that have been eliminated. Eventually, autophagosomes merge with lysosomes to generate autolysosomes, which destroy the stored materials and release tiny molecules that can be reused. In the early stage of TMJ OA, the autophagy markers beclin 1 and light chain 3 beta (LC3B) rise, but they decrease considerably in the late phase [24]. Early autophagy increases and protects chondrocytes against environmental alterations. Cartilage destruction is connected with the suppression of autophagy and cell death [24,34].
Furthermore, the endoplasmic reticulum-associated proteins ERN1, mTORC1, and EIF2AK3 trigger apoptosis and limit autophagy. The ERN1–MTORC1–EIF2AK3 signaling axis is the name given to this pathway [24]. As a result, controlling autophagy may be a valuable method for treating TMJ OA.
2.2. ECM Degeneration
The cartilage ECM is mainly made up of collagen fibers and big proteoglycans. It not only serves as a protective structure for cartilage against elastic and shear pressures, but it also governs chondrocyte behavior via matrix–cell interactions [35].
MMPs and a disintegrin and metalloproteinase production with thrombospondin motifs (ADAMTS) initiate ECM breakdown in TMJ OA. Through the bone morphogenetic protein (BMP) pathway, the degradation of type II collagen (Col2A1) promotes the hypertrophy of chondrocytes, hence accelerating the course of TMJ OA [36]. In addition, cartilage mineralization has been demonstrated to contribute to the development of TMJ OA [25].
Activation of 2A-adrenergic receptor signals through the extracellular regulated protein kinases 1 and 2 (ERK1/2) and protein kinase A (PKA) pathways increases the synthesis of matrix degradation-associated enzymes, such as MMP-3 and MMP-13. Osteopontin, an inflammatory agent, stimulates the production of MMPs through the NF-κB signaling pathway [37].
The HTRA1–DDR2–MMP-13 axis is essential for ECM breakdown. This process starts with the overexpression of high-temperature requirement A1 (HTRA1) and the breakdown of pericellular matrix components, including type VI collagen. Col2A1 can activate the transmembrane protein Discoidin Domain Receptor Tyrosine Kinase 2 (DDR2) in the absence of a pericellular matrix. DDR2 ultimately triggers MMP-13 and hastens TMJ OA [38].
2.3. Molecular Signaling in the Development of TMJ OA
2.3.1. Wnt/β-Catenin Signaling Pathway
The Wnt/β-catenin signaling pathway has been researched for decades in stem cell self-renewal, cell proliferation and differentiation throughout embryonic development, and adult tissue homeostasis. It is a conserved cellular communication system that influences the onset of OA and other forms of arthritis [9,39].
Intracellular β-catenin is stabilized and translocated to the nucleus thanks to a complex formed by Frizzled and low-density lipoprotein receptor-related proteins 5 or 6 (LRP5/6). Signaling through the Wnt/β-catenin pathway begins at the cell surface when the Wnt glycoprotein binds to specific receptors. After attaching to nuclear transcription factors, β-catenin protein allows for expressing Wnt-targeted proteins [9].
Progressive TMJ abnormalities, joint space narrowing, and OA-like TMJ defects have been documented in β-catenin conditional activation mice, β-catenin(ex3) Agc1ER. MMP-13, ADAMTS-4, and ADAMTS-5, which promote cartilage breakdown, were highly raised, whereas Col-X, a chondrocyte hypertrophy-related protein, was similarly upregulated in β-catenin(ex3) Agc1ER mice [9].
Furthermore, lower cell proliferation and higher cell death were found in these mice’s condylar cartilage. This evidence suggests that β-catenin plays a crucial role in TMJ pathophysiology and that Wnt/β-catenin signaling is a possible therapeutic target for treating TMJ OA [40].
2.3.2. TGF-β and BMP Signaling
Transforming growth factor β (TGF-β)/BMP signaling has been widely investigated concerning bone formation. It performs a variety of functions throughout life. The TGF-β superfamily consists of more than forty members, including TGF-βs, BMPs, and activin. They are embedded in the bone matrix and govern bone remodeling or influence the production of bone and cartilage [9,41].
The TGF-β signal pathway initiates intracellular signaling following the creation and activation of a heteromeric complex of types II and I serine/threonine kinase receptors, followed by the phosphorylation of particular Smad proteins, R-Smads. The phosphorylated R-Smads can heterodimerize with co-Smad, Smad4, ultimately translocating to the nucleus and activating the transcription of target genes [42].
In the TGF-β/Smad3 signaling pathway, sphingosine 1-phosphate (S1P), a bioactive lipid, is produced to function as an intracellular mediator or extracellular ligand for different receptors, resulting in inflammation, cell migration, and angiogenesis. The interaction between TGF-β/Smad3 and S1P/S1P3 and Smad3/S1P3 signaling in chondrocytes may play a role in the development of TMJ OA [43].
Additionally, it has been documented that overexpressing TGF-β1 causes aberrant subchondral bone remodeling that causes mice to develop TMJ OA and degrade mandibular condylar cartilage [9].
2.3.3. Indian Hedgehog Signaling
Indian hedgehog (Ihh), a signaling molecule, is essential for controlling the formation of the skeleton. During endochondral ossification, it is mainly expressed in pre-hypertrophic and hypertrophic chondrocytes. The production of parathyroid hormone-related protein (PTHrP) in periarticular tissue is one of the processes it controls during cartilage growth [44].
In TMJ osteoarthritic cartilage stimulated by unilateral anterior cross-bite (UAC), enhanced Ihh signaling promotes the terminal differentiation of deep zone chondrocytes. In contrast, the OA-like lesions and UAC-promoted chondrocyte terminal differentiation were rescued by the deletion of Smo in mice [9]. Another research found that Ihh facilitated OA development by controlling genes involved in cartilage deterioration and that Ihh inhibition mitigated the disease. Activation of Ihh, Smo, and Gli1 was detected in adjuvant-induced TMJ OA in mice [45], suggesting that Ihh signaling may exacerbate TMJ OA by encouraging chondrocyte hypertrophy.
2.3.4. FGF Signaling
Articular cartilage and the control of skeletal development are two of the essential functions of the FGF signaling system. There are 22 ligands in the FGF family, all of which bind to one of four different FGFRs to perform various tasks [46]. The binding of FGFs to the FGFRs’ extracellular domain is the usual starting point for FGF/FGFRs signaling. After the phosphorylation of the cytoplasmic tail of FGFRs and the recruitment of the corresponding target proteins, several different signaling processes are triggered.
Several signaling pathways are involved in the downstream signaling activities of FGF [47]. These include the phosphoinositide 3-kinase(PI3K)/Akt pathway, the phospholipase C (PLC) pathway, the mitogen-activated protein kinase (MAPK) pathway, and the signal transducers and activators of transcription (STAT) 1/p21 pathway. In addition, the FGF signaling pathway is connected with the development of OA via MEK/ERK, a critical downstream signaling molecule of FGFR1 [9].
FGF signaling may be increased in TMJ OA, as evidenced by the finding that ablation of FGRF1 in TMJ chondrocytes reduced TMJ OA development in particular OA models [48].
2.3.5. NF-κB Signaling
RelA, RelB, c-Rel, NF-κB1, and NF-κB2 are the five proteins that make up the NF-κB family. They interact with NF-κB inhibitors, form active complexes, move into the nucleus, bind to DNA, and control the expression of NF-κB-target genes [9]. The immune system, the inflammatory process, stress responses, cell proliferation, and cell death are all thought to be partially mediated by NF-κB.
The activation of the IKK-α/IKK-β/IKK-γ-NEMO complex is mediated by the TNF receptor (TNF-R), Toll-like receptor (TLR), or T-cell receptor (TCR) in the classical NF-κB signaling pathway. Inactive NF-κB dimers are linked to inhibitory NF-κB (I-κB) proteins in the cytoplasm. When mechanical and chemical cues stimulate cells, I-κBs are phosphorylated by I-κB kinases and degraded by the ubiquitin–proteasome system, allowing NF-κB heterodimers to translocate into the nucleus and promote the expression of target genes [9,49].
2.3.6. Notch Signaling
Necessary for cell differentiation and death, the Notch receptor is a single-pass transmembrane receptor at the cell surface. The highly conserved Notch signaling system consists of many components, including Notch ligands, Notch receptors, transcriptional effectors, and target genes [50]. Notch ligands bind to Notch receptors to commence the process, after which Notch receptors are cleaved, the intracellular domain of Notch receptors translocates to the nucleus, and target genes are activated [51].
The Notch signaling system controls the molecules involved in cartilage production and breakdown and hence plays a dual function in cartilage maintenance [52]. It is well known that Notch signaling is critical in the angiogenesis of condylar cartilage and disc, which is required to form TMJ OA [53]. Recent research has shown that changing Notch signaling may cause TMJ OA.
2.4. Angiogenesis in TMJ Osteoarthritis
Angiogenesis has been shown to enhance the development of OA [54]. Wang and colleagues discovered that mice with TMJ OA-like alterations increased the number of newly created blood vessels at the osteochondral junction [53]. In TMJ OA, these new blood vessels can carry inflammatory mediators and prolong inflammation [55]. When new blood arteries enter the cartilage, they stimulate chondrocyte enlargement and mineral deposition in the matrix. Through endochondral osteogenesis, osteophytes can integrate with newly created vessels on the joint’s surface to enhance complex tissue creation [14].
Vascular endothelial growth factor (VEGF) is important for angiogenesis and a key modulator of TMJ OA. Several transcription factors, including hypoxia-inducible factor-1 (HIF-1), promote VEGF expression [14,56]. The protein dickkopf-related protein-1 (DKK-1) was discovered in high concentrations in the synovial fluid of TMJ OA patients. It has been proposed that DKK-1 and high-mobility group box 1 (HMGB1) guide HIF-1 nuclear localization, enhancing VEGF production [56,57]. Interleukin-6 (IL-6) and IL-1 elevate VEGF levels via stimulating VEGF transcription in the nucleus. ERK1/2 stimulates the estrogen-related receptor γ (ERR γ). IL-1, on the other hand, directly stimulates NF-κB [26,58].
VEGF is generated by chondrocytes in articular cartilage and modulates autocrine levels of MMP-13 and tissue inhibitor of metalloproteinase-1 (TIMP-1). Reduced TIMP concentration and increased MMP expression disrupt the circulation of ECM components, collagen, and proteoglycans, which is shown by an increased breakdown. VEGF may increase articular cartilage deterioration by activating osteoclasts and allowing blood vessels to penetrate the cartilage [15,17].
Due to the breakdown of hyaluronic acid (HA) and the increased activity of free radicals, joint hydration is also reduced. When the pressure within a joint begins to surpass the capillary pressure, transient hypoxia and joint degeneration ensue. Reoxygenation is detected when the stress on the joint is reduced, and joint degeneration is halted.
During hypoxia and reperfusion cycles, free radicals are released. Free radicals hinder production and accelerate HA breakdown, lowering synovial fluid viscosity and increasing friction between joint surfaces [59]. Increased friction during TMJ movement causes permanent joint structure damage, internal articular disc derangement, and degenerative alterations [15,60].
3. Subchondral Bone Changes in Temporomandibular Joint Osteoarthritis
The main pathogenic mechanism highlighted at the level of the subchondral bone in TMJ OA is abnormal bone remodeling. This is due to complex mechanisms that include mechanical loading, inflammation, and degradation of the articular cartilage [9,13,61,62]. The onset of degenerative changes is characterized by a loss of bone mass which acts as a trigger for the development of TMJ OA and which participates in the degradation of articular cartilage [63]. Then follows slow bone repair mechanisms that cause an increase in bone density at the subchondral level, which leads to increased thickening and stiffness of the condylar osteochondral interface [64,65]. Erosions, osteophytes, the appearance of cyst-like lesions, and subchondral sclerosis represent the main radiographic changes evident in TMJ OA [20,61,66,67]. Clinically, patients present a decrease in mandibular mobility, pain during mastication and with the opening of the oral cavity, as well as joint sounds (cracking) during TMJ mobilization [68].
3.1. Bone Cells in Temporomandibular Osteoarthritis
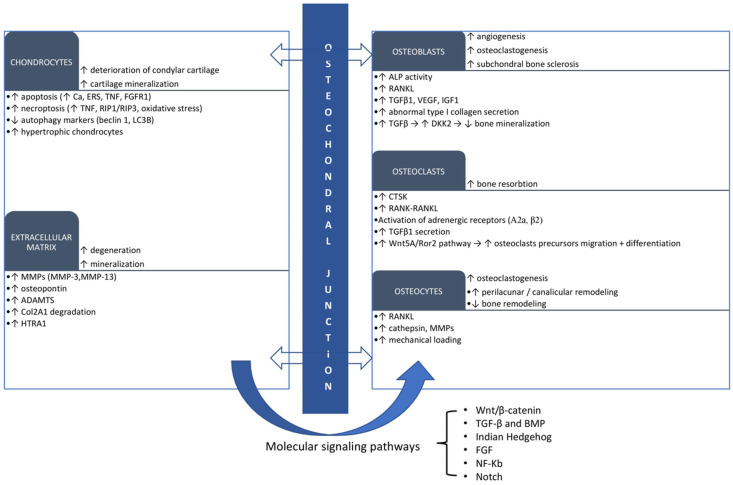
Bone remodeling in TMJ OA is associated with a decrease in the number and activity of osteoblasts, as well as an increase in osteoclast activity [63,64]. At the level of osteoblasts, bone-forming cells, a multitude of metabolic mechanisms with increased activity have been observed that favor angiogenesis, osteoclastogenesis, and subchondral bone sclerosis [69,70]. Thus, an increase in alkaline phosphatase (ALP) activity and an elevated expression of receptor activator of nuclear factor kappa-Β ligand (RANKL), transforming TGF-β1, VEGF, and insulin-like growth factor-1 (IGF-1) were highlighted in TMJ OA [71,72,73,74]. Moreover, osteoblasts participate directly in the formation of subchondral sclerosis characterized by an increase in bone density and volume, but associated with deficient mineralization [75,76]. This is due to the particular phenotype of osteoblasts that secrete abnormal type I collagen and that show increased expression of TGF-β which inhibits mineralization by stimulating the secretion of DKK2 [77,78].
Osteoclasts, the cells involved in bone resorption, present an increased number and intense activity in TMJ OA, the main activating mechanism of osteoclastogenesis being the binding of RANK with its ligand (RANKL) [79,80]. The most important degradative enzyme produced by osteoclasts that favors bone resorption is cathepsin K (CTSK), animal studies highlight its important role in the development of OA [81]. In addition, the migration and differentiation of osteoclast precursors are achieved through the WNT5A/receptor tyrosine kinase-like orphan receptor 2 (Ror2) pathway [82]. The activation of adrenergic receptors (β2 and α2A) via neurotransmitters determines, also through the RANKL pathway, the maturation of osteoclasts, and bone destruction [83]. Last but not least, osteoclast activity is stimulated by increased TGF-β1 secretion [84].
Although the data are limited, it seems that estrogen and progesterone hormones have a role in the occurrence of TMJ OA by direct action on bone cells [85,86]. Thus, at the onset of the disease, an increased level of estrogen has a protective effect by inhibiting the Wnt pathway and the activity of osteoclasts [85]. Moreover, the increased level of progesterone, by inhibiting NF-kB activity, has a beneficial effect on the subchondral bone, decreasing bone resorption [86].
Osteocytes, the most abundant bone cells, have an important role in osteoclastogenesis, being sensitive to joint mechanical loading and producing RANKL [87,88]. By secreting degradative enzymes such as CTS and MMPs, osteocytes react to different mechanical forces and resorb the bone matrix, leading to perilacunar/canalicular remodeling [89]. Studies have shown that a decrease in this bone remodeling determined by osteocytes can favor the onset of OA [90].
Figure 1 summarizes the role and the main metabolic changes occurring at the level of bone cells that have been highlighted in TMJ OA.
Figure 1.
The role and the main metabolic changes occurring at the level of articular cartilage, osteochondral junction, and subchondral bone highlighted in TMJ OA. ALP—alkaline phosphatase; RANKL—receptor activator of nuclear factor kappa-B ligand; RANK—receptor activator of nuclear factor kappa-B; TGFβ1—transforming growth factor β1; VEGF—vascular endothelial factor; IGF1—insulin-like growth factor 1; DKK2—dickkopf-2; CTSK—cathepsin K; Wnt5A/Ror2—Wnt5A/receptor tyrosine kinase-like orphan receptor 2; MMPs—matrix metalloproteinases, ERS—endoplasmic reticulum stress; TNF—tumor necrosis factor; FGFR1—fibroblast growth factor receptor 1; RIP1/RIP3—receptor-interacting proteins 1 and 3; LC3B—light chain 3 beta; ADAMTS—a disintegrin and metalloproteinase with thrombospondin motifs; Col2A1—type II collagen A1; HTRA1—high-temperature requirement A1.
3.2. Inflammation, Subchondral Bone and Temporomandibular Osteoarthritis
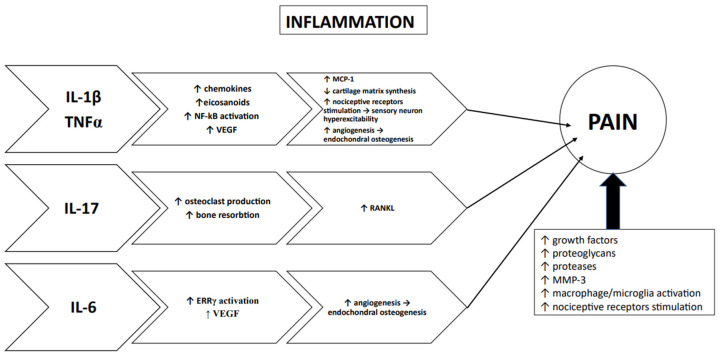
Although OA is not a systemic inflammatory condition, data support the role of various pro-inflammatory cytokines in TMJ OA both at the level of articular cartilage and of subchondral bone [91,92]. In the synovial fluid of these patients, an inflammatory environment characterized by an increased secretion of many molecules such as IL (IL-1β, -2, -12, -17, -18), TNF (TNFα and TNFβ) and interferon (IFN)-γ was highlighted. Among all these, the main pro-inflammatory cytokine secreted in TMJ OA is IL-12 [93].
IL-1β and TNFα actively participate in inflammation by increasing the expression of chemokines, eicosanoids, and various proteins [94,95,96]. Moreover, through the stimulation achieved by IL-1β, TMJ synoviocytes increase their production of monocyte chemoattractant protein-1 (MCP-1) [97]. Recently published data support that MCP-1 can be considered the trigger for the appearance, progression, and persistence of inflammation even in the absence of IL-1β [98]. In addition, the secretion of these cytokines correlates with the suppression of the synthesis of the articular cartilage matrix [99].
TNFα, IL-1β, and IL-17 directly modulate osteoclast production, as well as bone resorption, by increasing RANKL secretion at the level of osteoblasts and fibroblasts from the synovial membrane [100]. The results of a study on mice support the role of TLR4 in the occurrence of TMJ OA. Thus, through the MyD88/NF-kB activation pathway, TLR4 favors the appearance of OA changes, in particular, the degradation of both cartilage and subchondral bone [101].
Inflammation also plays an important role in the development of joint pain, a frequent symptom in these patients. Pro-inflammatory cytokines such as TNFα or IL-1β can directly stimulate nociceptive receptors and cause sensory neuron hyperexcitability [102,103]. Other molecules such as growth factors, proteoglycans, or proteases seem to be involved in the development of arthritic pain, MMP-3 being considered a hallmark for TMJ pain [104]. Moreover, promising results on murine studies were published, pointing to the important role of macrophage/microglia activation in the development of pain in these patients [105].
Cytokines such as IL-1β and IL-6 can facilitate the transcription of VEGF in the nucleus, thus leading to an increased expression of VEGF [58,106]. IL-1β has the ability to directly activate NF-kB, while IL-6, through ERK1/2, activates ERRγ [26,58,106,107].
VEGF is the main molecule responsible for angiogenesis, a pathogenic mechanism involved in the development of TMJ OA and characterized by the formation of new blood vessels at the osteochondral junction [53,54,108,109]. By invading the articular cartilage, it determines both the formation of mineral deposits at the level of the ECM as well as the hypertrophy of chondrocytes. In addition, it participates in endochondral osteogenesis by incorporating new blood vessels into the osteophytes [110]. In addition to angiogenesis, an important role is played by neurogenesis, both processes favoring the appearance of OA and being involved in the appearance of joint pain [111]. Angiogenesis and neurogenesis influence each other through vascular and nerve growth factors, leading to the emergence of neurovascular interaction involved in the TMJ OA progression [112,113].
Figure 2 shows the important role of inflammation in the changes at the level of the subchondral bone as well as its role in the occurrence of TMJ OA pain.
Figure 2.
The role of inflammation in the changes in the subchondral bone and the development of arthritic pain. IL-1β—interleukin 1β; TNFα—tumor necrosis factor α; IL-17—interleukin 17; IL-6—interleukin 6; VEGF—vascular endothelial growth factor; MCP-1—monocyte chemoattractant protein-1; RANKL—receptor activator of nuclear factor kappa-B ligand; ERRγ—estrogen-related receptor γ; NF-kB—nuclear factor kappa-light-chain-enhancer of activated B cells.
3.3. Mechanical Loading and Temporomandibular Osteoarthritis
The mechanical loading distributed on the surface of a joint is very important to maintain joint integrity and functionality, the bone microarchitecture being correlated with the direction and magnitude of the applied load [114]. Thus, the structure of the subchondral bone changes, highlighting changes such as: the increase in bone volume, the decrease in mineralization, the thickening of plates, and the decrease in the trabecular rod/plate ratio [115]. This is mainly due to the osteocytes that participate in the remodeling of the ECM through the increased secretion of degradative enzymes, as well as through the increase in their metabolic activity that leads to canalicular remodeling [116,117,118,119].
Although the TMJ is not a joint on which high mechanical load forces act, it seems that small alterations of the mechanical loading cause the appearance of degenerative changes [120]. The anatomical and positional changes in the fibrocartilaginous disc between the condyles and the joint fossa can be considered a cause of TMJ OA [121].
Another mechanism involved in the occurrence of TMJ OA is the decrease in the sensitivity of chondrocytes to mechanical loading, this being due to the knockdown of high mobility group protein B2 (HMGB2) [122]. Along with these, an abnormal subchondral bone remodeling characterized by a decrease in type I collagen production and increased bone resorption is involved in the development of TMJ OA [63]. Secondary to the increased resorptive activity, the data obtained from experimental models support the formation of new bone at the level of the condyles in the initial stages of degenerative changes [123].
4. Therapeutic Strategies Impacting Cartilage and/or Bone Changes in TMJ OA: Platelet-Rich Plasma, Hyaluronic Acid, and Stem Cell-Based Therapy
Presently, the most widely used treatment strategies in TMJ OA involve the diminishment of symptoms, with a focus on pain relief and improvement of mobility [124,125,126,127,128,129]. Nevertheless, recent studies support the regenerative or protective potential of certain therapies such as platelet-rich plasma (PRP), HA, and mesenchymal stem cells (MSCs) in OA [130,131,132]. The currently available data regarding the regenerative or protective potential of PRP, HA, and MSC-based therapy results from studies that used various treatment regimens and different assessment methods, which may partly explain the sometimes diverging findings.
4.1. Platelet-Rich Plasma (PRP)
In vitro research indicates that PRP (different formulations of autologous blood derivatives with a high concentration of platelets—cells involved in tissue healing) may bolster the proliferation of chondrocytes and MSCs with the concomitant deposition of type II collagen, thus potentially leading to cartilage repair [133]. Intraarticular PRP was proven effective in OA involving various joints, with studies showing that the treatment may significantly improve symptoms over various follow-up periods [134,135,136,137].
Diverse types of management options including PRP have shown promising results in in vitro and animal studies. Constructs of PRP embedded in different types of scaffolds and placed at the site of a cartilage defect where it exhibited positive effects on chondrocyte disposition, metabolic activity, glycosaminoglycan deposition, and collagen type II expression, as well as anti-apoptotic properties. Moreover, PRP was also used as a carrier for mesenchymal stem cells [138,139]. The molecular mechanisms through which PRP may contribute to tissue regeneration include its bioactivity. In this respect, growth factors such as TGFβ, IGF, VEGF, PDGF (platelet-derived growth factor), and bFGF (basic fibroblast growth factor) are known to be abundant in PRP and may encourage the proliferation of chondrogenic cells and the secretion of cartilaginous matrix components [140,141]. The development of PRP-based formulations for OA is based on several hypotheses including the potential anti-inflammatory and anti-catabolic properties of chemokines, and the anabolic effects of the growth factors found in PRP [142]. PRP inhibits serine/threonine kinase 1 (AKT1), phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), and NF-kB (PIK/AKT signaling) and hinders the release of pro-inflammatory cytokines such as TNFα and IL6 [143,144].
Recently published studies involving patients with TMJ OA suggest that PRP may induce condylar bone repair according to imaging findings (MRI—magnetic resonance imaging and/or CBCT—cone beam computed tomography) [145,146]. In animal models of TMJ OA, PRP therapy was associated with the improvement of the joint structure histological aspect (Table 1) [147,148,149]. Local injections of PRP have also shown beneficial effects on pain relief and improved maximal mouth opening in patients as well as animal models of TMJ OA [150,151,152,153].
Table 1.
Recent evidence from human and animal studies regarding the effects of PRP therapy on cartilage and/or bone changes in TMJ OA.
| Study | Study Description | Study Group(s) | Assessment | Main Findings: Changes Impacting the TMJ Cartilage and/or Bone |
|---|---|---|---|---|
| Liu et al., 2022 [145] | Comparison between PRP + physical therapy and PRP injections alone in patients with TMJ OA | Group 1: PRP injection Group 2: PRP injection + individualized comprehensive physical therapy |
CBCT and MRI at baseline followed by CBCT or MRI post-therapy | Condylar bone repair was seen in both groups (78.6% for PRP alone, and 81.5% for the combined treatment). There was no significant difference between the two groups with respect to imaging findings. |
| Arafat and Kamel, 2021 [147] | Analysis of the effects of PRP and/or HA injections in a rat model of TMJ OA | Group 1: Controls Group 2A: HA alone (2 injections at 7 and 21 days) Group 2B: PRP alone (2 injections at 7 and 21 days) Group 2C: HA + PRP (2 injections at 7 and 21 days) |
Histological assessment | The group treated with PRP alone exhibited a restoration of the fibrocartilage covering the entire surface of the condyle (yet with a decreased thickness) and a normal architecture of the subchondral bone. The combined treatment group showed better results, with a restored fibrocartilage layer and a normal aspect of the subchondral bone. |
| Coskun et al., 2019 [148] | Evaluation of PRP injections on cartilage and subchondral bone in a rodent model of TMJ OA (MIA-induced TMJ OA in rabbits) | Group 1: Single intraarticular injection of PRP Group 2: Three weekly injections of PRP Controls: Isotonic saline injections |
Histological assessment | An improvement was observed in the treated animals. However, there were no statistically significant differences between the treatment groups and the controls. |
| Li et al., 2021 [146] | Retrospective comparison between PRP and chitosan injections in patients with TMJ OA | Group 1: PRP injections Group 2: Chitosan injections |
CBCT or MRI | PRP treatment was associated with better results with respect to condylar bone repair, yet there was no statistically significant difference between the treatment groups. |
| AbuBakr et al., 2022 [149] | Comparative analysis of bone marrow MSC-derived microvesicles (BM-MSCs-MVs) and PRP in a murine model of TMJ OA (MIA-induced) | Group 1: No therapeutic intervention Group 2: BM-MSCs-MVs Group 3: PRP |
Histological assessment | The TMJ condyle structure was improved after treatment with PRP compared to the MIA-induced TMJ OA animals. Additionally, there was a diminishment in inflammatory cytokine values, as well as MMP3 and MMP13, and an increased type II collagen gene expression in both treatment groups. |
CBCT: cone beam computed tomography; MIA: monoiodoacetate; MMP: matrix metalloproteinase; MRI: magnetic resonance imaging.
Animal studies based on the histological assessment of the TMJ cartilage and bone showed that intraarticular PRP leads to a restoration of the fibrocartilage and an improved microarchitecture of the subchondral bone [147,148,149]. AbuBakr et al. also observed a decrease in MMP and pro-inflammatory cytokine values following treatment, with a concomitant upregulation of type II collagen gene expression in experimental animals [149]. Arafat and Kamel found that combined treatment with PRP + HA may be associated with superior results compared to PRP alone [147].
The imaging findings in patients with TMJ OA indicated that PRP could improve the aspect of the condylar bone [145,146]. Nevertheless, these studies did not use the same evaluation methods in all patients (either CBCT or MRI).
4.2. Hyaluronic Acid (HA)
Intraarticular HA injections have largely been used to reduce OA-related symptoms in larger joints such as the knees [154,155,156]. Apart from its viscoelastic behavior, HA has shown the ability to support cell growth and the chondrogenic differentiation of stem cells, as well as to provide binding sites for growth factors, favoring tissue healing. Certain HA formulations were associated with decreased pro-inflammatory cytokine levels and MMPs [157,158]. An important topic in cartilage tissue engineering has been designing HA hydrogel formulations to enhance regeneration. A sulforaphane-loaded hyaluronic acid hydrogel demonstrated protective effects against cartilage degradation by reducing the depletion of proteoglycans, increasing collagen type II, while also modulating NF-κB [159]. Nevertheless, a great number of recent studies focus largely on the biomaterials used as scaffolds or carriers, rather than on the properties of HA itself [160,161,162].
A significant improvement in pain levels and functionality was also seen in patients with TMJ OA following HA treatment [15,163,164]. HA therapy has also shown some beneficial effects regarding the regeneration of joint structures in TMJ OA in both patients and animal models (Table 2). However, some studies did not support the restorative effects of HA in TMJ OA [165,166,167,168,169,170].
Table 2.
Recent evidence from human and animal studies regarding the effects of HA injections on cartilage and/or bone changes in TMJ OA.
| Study | Study Description | Study Group(s) | Assessment | Main Findings: Changes Impacting the TMJ Cartilage and/or Bone |
|---|---|---|---|---|
| Arafat and Kamel, 2021 [147] | Analysis of the effects of HA and/or PRP injections in a rat model of TMJ OA | Group 1: Controls Group 2A: HA alone (2 injections at 7 and 21 days) Group 2B: PRP alone (2 injections at 7 and 21 days) Group 2C: HA + PRP (2 injections at 7 and 21 days) |
Histological assessment | The group treated with HA alone demonstrated an incomplete restoration of the fibrocartilage layer and the regeneration of the subchondral bone. The combined treatment group showed the best results, with a fully formed fibrocartilage and a normal aspect of the subchondral bone. |
| Iturriaga et al., 2021 [169] | Comparison between high and low molecular weight HA injections (HMW-HA versus LMW-HA) in a rabbit model of TMJ OA (MIA-induced) | Group 1: No therapeutic intervention (healthy TMJs, TMJ OA) Group 2: 30-day follow-up (TMJ OA, LMW-HA, HMW-HA) Group 3: 135-day follow-up (TMJ OA, LMW-HA, HMW-HA) |
Histological assessment | TMJ OA worsened without treatment. Both treatment groups exhibited cartilage and disc repair at 30 days, with better results in rabbit TMJs treated with LMW-HA. Nonetheless, a regression of tissue repair was seen in both treatment groups at 135 days. |
| Tolba et al., 2020 [170] | Evaluation of HMW-HA treatment in a rodent model of TMJ OA (CFA-induced) | Group 1: Controls (not injected) Group 2: Disease (CFA-induced arthritis) Group 3: Treated (CFA-induced arthritis + 3 weekly injections of HMW-HA) |
Histological assessment | The HMW-HA treatment group demonstrated restoration of the normal histological aspect of the TMJ (with respect to the condyle, the subchondral bone, and the articular disc). |
| Cen et al., 2022 [165] | Retrospective comparison between HA injections and oral glucosamine + diclofenac in TMJ OA patients | Group 1: Four biweekly injections of HA Group 2: Oral glucosamine + diclofenac |
CBCT | There was no statistically significant effect on bone changes in any of the treatment groups. |
| De Riu et al., 2019 [167] | A randomized clinical trial of HA compared to BMNC autograft treatment in patients exhibiting degenerative changes in the TMJ | Group 1: Arthrocentesis + BMNC Group 2: Arthrocentesis + HA |
MRI | There was no significant imaging evidence of regeneration in the HA group according to MRI scores. |
| Khairy et al., 2019 [166] | Comparative analysis of HA injections versus chondrogenic differentiated adipose-derived MSCs in patients with TMJ OA | Group 1: Arthrocentesis + HA injection Group 2: Arthrocentesis + chondrogenic differentiated adipose-derived MSCs |
CBCT | The HA-treated group showed no imaging evidence of restoration of the joint structures. |
| Köhnke et al., 2021 [168] | Analysis of treatment with HA and MSCs (separately and in combination HA + MSCs) in a rabbit model of TMJ OA (mechanical + collagenase-induced) | Group 1: AB serum Group 2: HA injection Group 3: MSCs Group 4: MSCs + HA |
Histological assessment Back-scattered electron imaging (BEI) |
Cartilage thickness was better in the HA treated group compared to the AB serum animals, with results approaching statistical significance. The HA + MSCs intervention showed the best results regarding cartilage thickness. According to BEI findings, there were no significant differences between the study groups with respect to subchondral mineralization. |
CBCT: cone beam computed tomography; CFA: complete Freund’s adjuvant; MIA: monoiodoacetate; MRI: magnetic resonance imaging.
While some animal research suggested that HA could exhibit certain restorative or protective properties, in patients with TMJ OA, imaging findings (CBCT or MRI) indicated that HA alone does not improve the aspect of the cartilage or condylar bone [165,166,167]. However, the methodology and the treatment regimens varied greatly between studies.
Notably, the combination treatment of HA and PRP in a murine model of TMJ OA exhibited better results with respect to the regeneration of joint structures compared to HA alone [147]. Moreover, the addition of HA to MSCs may improve cartilage repair according to the results obtained by Köhnke et al. in a rabbit model of TMJ OA [168]. These findings suggest that HA could enhance the regenerative potential of other therapies (PRP, MSCs) rather than restore normal TMJ structures by itself.
4.3. Mesenchymal Stem Cells (MSCs)
MSCs may derive from a wide array of tissues such as the synovium, the umbilical cord, adipose tissue, dental pulp, and bone marrow. In recent years, the promising findings with respect to the important regenerative potential of MSC-based therapies in OA have been gathering interest [171,172,173].
Several mechanisms through which transplanted MSCs could restore the normal aspect of the arthritic joint have been proposed. In vitro studies indicated that, under controlled conditions, MSCs may differentiate into cartilage, bone, ligament, and tendon structures. Moreover, the exosomes (extracellular vesicles) released by these cells may carry various bioactive molecules. It has been shown that MSCs may exhibit immunomodulatory and anti-inflammatory effects and may promote angiogenesis [174,175,176]. MSCs could hinder the degradation of the cartilage extracellular matrix. Additionally, under the influence of MSCs, the macrophage-like synoviocytes may switch to the M2 phenotype, rather than the predominantly pro-inflammatory M1 by secreting prostaglandin-E2 and indoleamine 2,3-dioxygenase [177].
In TMJ OA, their use in patients and experimental animals was tied to numerous favorable effects including cartilage restoration and delayed degradation, chondrogenic and osteogenic differentiation, as well as the improvement in subchondral bone volume and structure. Moreover, MSCs have exhibited anti-inflammatory and trophic effects in TMJ OA in addition to potential pain reduction and improved functionality [178,179,180,181,182]. Research conducted in the last years describes histological changes and imaging findings suggesting joint structure regeneration following treatment (Table 3). However, studies differ in terms of the types of MSCs used, the treatment regimens and follow-up periods, as well as assessment methods [179,180,181,182,183,184,185,186,187,188].
Table 3.
Recent evidence from human and animal studies regarding the effects of MSCs on cartilage and/or bone changes in TMJ OA.
| Study | Study Description | Study Group(s) | Assessment | Main Findings: Changes Impacting the TMJ Cartilage and/or Bone |
|---|---|---|---|---|
| Gomez et al., 2021 [185] | Analysis of cartilage regeneration after MSCs + PRP treatment in mice with surgically induced TMJ condyle cartilage damage (CCD, extended until the subchondral bone) in a murine model | Group 1: Human MSCs embedded into preclotted PRP Group 2: Untreated animals with TMJ condylar cartilage damage Group 3: Sham group |
Histological analysis | At 6 weeks, the macroscopic aspect of the treated lesions displayed cartilage-like tissue occupying the damaged sections. The authors noted that a collagen and GAG matrix with chondrocytes filled the treated areas. |
| Khairy et al., 2019 [166] | Analysis of HA injections versus chondrogenic differentiated adipose-derived MSCs in patients with TMJ OA | Group 1: Arthrocentesis + HA injection Group 2: Arthrocentesis + chondrogenic differentiated adipose-derived MSCs |
CBCT | Compared to the HA group, the patients treated with chondrogenic differentiated adipose-derived MSCs demonstrated CT evidence of remodeling. |
| Kim et al., 2019 [186] | Evaluation of the effects of different concentrations of human umbilical cord-derived MSCs in a rabbit model of TMJ OA (MIA-induced) | Group 1: Controls Group 2: Untreated TMJ OA Group 3: Dexamethasone Group 4–6: Low, Median and High concentrations of MSCs (chondrogenic differentiation) at 4 weeks post-induction of TMJ OA |
Histological assessment Micro-CT |
The authors described the regenerative effects (including potential chondrogenesis) in all MSC concentrations. The median dose of MSCs exhibited the best results regarding cartilage protection and restoration. Moreover, MSC treatment demonstrated potent anti-inflammatory effects. |
| Köhnke et al., 2021 [168] | Analysis of treatment with MSCs and HA (separately and in combination) in a rabbit model of TMJ OA (mechanical + collagenase-induced) | Group 1: AB serum Group 2: HA injection Group 3: MSCs Group 4: MSCs + HA |
Histological assessment Back-scattered electron imaging (BEI) |
Cartilage thickness was significantly better in the MSCs only treated group compared to the AB serum animals and the combination MSCs + HA group was significantly, superior to both AB serum and the HA only group. However, there were no significant differences between the study groups with respect to subchondral mineralization according to BEI. |
| Zhang et al., 2019 [187] | Examination of human embryonic stem cell-derived MSC exosomes in a mouse model of TMJ OA (MIA-induced) | Group 1: Phosphate-buffered saline Group 2: MSC exosomes Group 3: Sham group |
Micro-CT Histological assessment |
MSC exosome treatment demonstrated reparative and regenerative effects in mice with TMJ OA according to the histological analysis, with more prominent changes being described at 8 weeks. The imaging findings indicated that MSC exosome therapy had a beneficial effect on bone volume and structure. |
| Ogasawara et al., 2020 [188] | Analysis of intravenous stem cells from human exfoliated deciduous teeth (SHED-CM) effect in a mouse model of TMJ OA (mechanical stress-induced—forced maximal mouth opening 3 h/day for 5 or 10 days) | Group 1: Mechanical stress for 5 days (pre-treatment group) Group 2: Mechanical stress for 10 days + intravenous SHED-CM (days 6–10) Group 3: Mechanical stress for 10 days + intravenous DMEM (days 6–10) |
Micro-CT (high-resolution) Histological assessment |
SHED-CM therapy appeared to stimulate tissue repair and regeneration. The high-resolution micro-CT findings in the TMJ condyles of experimental animals showed that the SHED-CM group had a smoother surface of the cartilage and a reduced subchondral bone resorption. |
| De Riu et al., 2019 [167] | A randomized clinical trial of BMNC autograft treatment compared to HA injection in patients with degenerative changes in the TMJ | Group 1: Arthrocentesis + BMNC Group 2: Arthrocentesis + HA |
MRI | There was no imaging evidence of regeneration related to BMNC treatment according to the MRI scoring system. |
| AbuBakr et al., 2022 [149] | Comparative analysis of bone marrow MSC-derived microvesicles (BM-MSCs-MVs) and PRP in a murine model of TMJ OA (MIA-induced) | Group 1: No intervention Group 2: BM-MSCs-MVs Group 3: PRP |
Histological assessment | The condylar structure was improved after treatment with BM-MSCs-MVs. Moreover, there was a decrease in inflammatory cytokine levels, as well as MMP3 and MMP13, and an increased type II collagen gene expression. BM-MSCs-MVs were found to be superior to PRP in some respects. |
| Carboni et al., 2019 [184] | Evaluation of abdominal adipose tissue-derived MSCs in patients with internal derangement of the TMJ | Group 1: Adipose-derived MSCs Group 2: Saline injection |
MRI | The MRI findings showed restoration of the joint structures in the treatment group. |
CBCT: cone beam computed tomography; GAG: glycosaminoglycan; DMEM: Dulbecco’s Modified Eagle Medium; MIA: monoiodoacetate; MMP: matrix metalloproteinase; Micro-CT: micro-computed tomography; MRI: magnetic resonance imaging.
Notable histological signs of cartilage regeneration were seen in animal models of TMJ OA (induced chemically, surgically, or mechanically or by a combination of chemical factors and mechanical stress). Moreover, some studies indicated that MSCs-based therapy could be superior to other treatment strategies for TMJ OA such as intra-articular injections of PRP and HA for the restoration of joint structures [149,168].
In patients with TMJ OA, imaging findings (CBCT, MRI) showed regenerative joint changes related to MSCs-based therapy [183]. In patients treated with MSCs, Carboni et al. described a better MRI aspect of the TMJ structures compared to controls receiving saline injections [184]. Based on CBCT evidence, Khairy et al. found that adipose-derived MSCs had better effects with respect to joint remodeling compared to HA injections [166].
However, in the study conducted by De Riu et al., there was no significant evidence of joint reparation following treatment with BMNC (bone marrow nucleated cell concentrate) according to the MRI scoring system [167].
5. Conclusions
Several potential mechanisms of TMJ OA have been hypothesized in the literature. The majority of these purported processes are predicated on the assumption that joint overloading, excessive pressures, and trauma all have a role in disturbing the structural integrity of the TMJ structure. Understanding the chain of biological processes that culminate in TMJ cartilage and bone degeneration is the first step in developing a complete model of the pathophysiology of TMJ OA.
Certain treatment strategies are linked to positive outcomes regarding the regeneration of joint structures over time in TMJ OA. While studies used diverse treatment regimens and evaluation methods, more prominent findings were described in MSCs-based treatment and PRP compared to HA. According to recent findings, the latter could enhance the restorative potential of other therapies (PRP, MSCs) when used in combination, rather than repair TMJ structures by itself.
TMJ OA is a complex disease in which degenerative changes in the cartilage and bone develop through intricate mechanisms. The regenerative potential of such therapies as PRP, MSCs, and HA regarding the cartilage and subchondral bone (alone or in various combinations) in TMJ OA remains a matter of further research, with studies sometimes obtaining discrepant results.
Author Contributions
Conceptualization, A.C., L.A.M. and A.M.B.; methodology, E.R.; software, I.B. and I.I.R.; validation, E.R. and B.-I.T.; formal analysis, L.A.M., A.C. and A.M.B.; investigation, I.R.M.; resources, B.-I.T., E.R. and P.R.; data curation, E.R. and L.A.M.; writing—original draft preparation, A.C., L.A.M. and A.M.B.; writing—review and editing, E.R. and B.-I.T.; visualization, I.B. and P.R.; supervision, E.R.; project administration, B.-I.T. and E.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Van Bellinghen X., Idoux-Gillet Y., Pugliano M., Strub M., Bornert F., Clauss F., Schwinté P., Keller L., Benkirane-Jessel N., Kuchler-Bopp S., et al. Temporomandibular Joint Regenerative Medicine. Int. J. Mol. Sci. 2018;19:446. doi: 10.3390/ijms19020446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gomes L.R., Gomes M.R., Jung B., Paniagua B., Ruellas A.C., Gonçalves J.R., Styner M.A., Wolford L.M., Cevidanes L. Diagnostic Index of Three-Dimensional Osteoarthritic Changes in Temporomandibular Joint Condylar Morphology. J. Med. Imaging. 2015;2:1. doi: 10.1117/1.JMI.2.3.034501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haigler M.C., Abdulrehman E., Siddappa S., Kishore R., Padilla M., Enciso R. Use of platelet-rich plasma, platelet-rich growth factor with arthrocentesis or arthroscopy to treat temporomandibular joint osteoarthritis: Systematic review with meta-analyses. J. Am. Dent. Assoc. 2018;149:940–952. doi: 10.1016/j.adaj.2018.07.025. [DOI] [PubMed] [Google Scholar]
- 4.Hunter D.J., Bierma-Zeinstra S. Osteoarthritis. Lancet. 2019;393:1745–1759. doi: 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 5.Aryaei A., Vapniarsky N., Hu J.C., Athanasiou K.A. Recent Tissue Engineering Advances for the Treatment of Temporomandibular Joint Disorders. Curr. Osteoporos. Rep. 2016;14:269–279. doi: 10.1007/s11914-016-0327-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Monasterio G., Castillo F., Betancur D., Hernández A., Flores G., Díaz W., Hernández M., Vernal R. Temporomandibular Joint Pathology-Current Approaches and Understanding. IntechOpen; London, UK: 2018. Osteoarthritis of the Temporomandibular Joint: Clinical and Imagenological Diagnosis, Pathogenic Role of the Immuno- Inflammatory Response, and Immunotherapeutic Strategies Based on T Regulatory Lymphocytes. [Google Scholar]
- 7.Zhao Y., An Y., Zhou L., Wu F., Wu G., Wang J., Chen L. Animal Models of Temporomandibular Joint Osteoarthritis: Classification and Selection. Front. Physiol. 2022;13:859517. doi: 10.3389/fphys.2022.859517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakano H., Watahiki J., Kubota M., Maki K., Shibasaki Y., Hatcher D., Miller A.J. Micro X-Ray Computed Tomography Analysis for the Evaluation of Asymmetrical Condylar Growth in the Rat. Orthod. Craniofac. Res. 2003;6:168–172. doi: 10.1034/j.1600-0544.2003.252.x. [DOI] [PubMed] [Google Scholar]
- 9.Lu K., Ma F., Yi D., Yu H., Tong L., Chen D. Molecular Signaling in Temporomandibular Joint Osteoarthritis. J. Orthop. Translat. 2022;32:21–27. doi: 10.1016/j.jot.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizoguchi I., Nakamura M., Takahashi I., Kagayama M., Mitani H. A Comparison of the Immunohistochemical Localization of Type I and Type II Collagens in Craniofacial Cartilages of the Rat. Cells Tissues Organs. 1992;144:59–64. doi: 10.1159/000147286. [DOI] [PubMed] [Google Scholar]
- 11.Kumar D., Schooler J., Zuo J., McCulloch C.E., Nardo L., Link T.M., Li X., Majumdar S. Trabecular Bone Structure and Spatial Differences in Articular Cartilage MR Relaxation Times in Individuals with Posterior Horn Medial Meniscal Tears. Osteoarthr. Cartil. 2013;21:86–93. doi: 10.1016/j.joca.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sellam J., Berenbaum F. The Role of Synovitis in Pathophysiology and Clinical Symptoms of Osteoarthritis. Nat. Rev. Rheumatol. 2010;6:625–635. doi: 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 13.Wang X.D., Zhang J.N., Gan Y.H., Zhou Y.H. Current Understanding of Pathogenesis and Treatment of TMJ Osteoarthritis. J. Dent. Res. 2015;94:666–673. doi: 10.1177/0022034515574770. [DOI] [PubMed] [Google Scholar]
- 14.Li B., Guan G., Mei L., Jiao K., Li H. Pathological Mechanism of Chondrocytes and the Surrounding Environment during Osteoarthritis of Temporomandibular Joint. J. Cell. Mol. Med. 2021;25:4902–4911. doi: 10.1111/jcmm.16514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Derwich M., Mitus-Kenig M., Pawlowska E. Interdisciplinary Approach to the Temporomandibular Joint Osteoarthritis—Review of the Literature. Medicina. 2020;56:225. doi: 10.3390/medicina56050225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cevidanes L.H., Walker D., Schilling J., Sugai J., Giannobile W., Paniagua B., Benavides E., Zhu H., Marron J.S., Jung B.T., et al. 3D osteoarthritic changes in TMJ condylar morphology correlates with specific systemic and local biomarkers of disease. Osteoarthr. Cartil. 2014;22:1657–1667. doi: 10.1016/j.joca.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka E., Detamore M.S., Mercuri L.G. Degenerative Disorders of the Temporomandibular Joint: Etiology, Diagnosis, and Treatment. J. Dent. Res. 2008;87:296–307. doi: 10.1177/154405910808700406. [DOI] [PubMed] [Google Scholar]
- 18.Breedveld F.C. Osteoarthritis—The Impact of a Serious Disease. Rheumatology. 2004;43:i4–i8. doi: 10.1093/rheumatology/keh102. [DOI] [PubMed] [Google Scholar]
- 19.Milam S.B. Pathogenesis of Degenerative Temporomandibular Joint Arthritides. Odontology. 2005;93:7–15. doi: 10.1007/s10266-005-0056-7. [DOI] [PubMed] [Google Scholar]
- 20.Kalladka M., Quek S., Heir G., Eliav E., Mupparapu M., Viswanath A. Temporomandibular Joint Osteoarthritis: Diagnosis and Long-Term Conservative Management: A Topic Review. J. Indian Prosthodont. Soc. 2013;14:6–15. doi: 10.1007/s13191-013-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Massilla Mani F., Sivasubramanian S.S. A Study of Temporomandibular Joint Osteoarthritis Using Computed Tomographic Imaging. Biomed. J. 2016;39:201–206. doi: 10.1016/j.bj.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Emshoff R., Rudisch A. Validity of Clinical Diagnostic Criteria for Temporomandibular Disorders. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2001;91:50–55. doi: 10.1067/moe.2001.111129. [DOI] [PubMed] [Google Scholar]
- 23.Utreja A., Dyment N.A., Yadav S., Villa M.M., Li Y., Jiang X., Nanda R., Rowe D.W. Cell and Matrix Response of Temporomandibular Cartilage to Mechanical Loading. Osteoarthr. Cartil. 2016;24:335–344. doi: 10.1016/j.joca.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang H., Wen Y., Zhang M., Liu Q., Zhang H., Zhang J., Lu L., Ye T., Bai X., Xiao G., et al. MTORC1 Coordinates the Autophagy and Apoptosis Signaling in Articular Chondrocytes in Osteoarthritic Temporomandibular Joint. Autophagy. 2019;16:271–288. doi: 10.1080/15548627.2019.1606647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan J., Qin W., Xiao B., Wan Q., Tay F.R., Niu L., Jiao K. Pathological Calcification in Osteoarthritis: An Outcome or a Disease Initiator? Biol. Rev. 2020;95:960–985. doi: 10.1111/brv.12595. [DOI] [PubMed] [Google Scholar]
- 26.Zhao H., Liu S., Ma C., Ma S., Chen G., Yuan L., Chen L., Zhao H. Estrogen-Related Receptor γ Induces Angiogenesis and Extracellular Matrix Degradation of Temporomandibular Joint Osteoarthritis in Rats. Front. Pharmacol. 2019;10:1290. doi: 10.3389/fphar.2019.01290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlier E., Relic B., Deroyer C., Malaise O., Neuville S., Collée J., Malaise M., De Seny D. Insights on Molecular Mechanisms of Chondrocytes Death in Osteoarthritis. Int. J. Mol. Sci. 2016;17:2146. doi: 10.3390/ijms17122146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H., Zhang X.Y., Wu T.J., Cheng W., Liu X., Jiang T.T., Wen J., Li J., Ma Q.L., Hua Z.C. Endoplasmic reticulum stress regulates rat mandibular cartilage thinning under compressive mechanical stress. J. Biol. Chem. 2013;288:18172–18183. doi: 10.1074/jbc.M112.407296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Z., Zhou M., Wang Q., Zhu M., Chen S., Li H. Mechanical and Hypoxia Stress Can Cause Chondrocytes Apoptosis through over-Activation of Endoplasmic Reticulum Stress. Arch. Oral Biol. 2017;84:125–132. doi: 10.1016/j.archoralbio.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 30.Ren H., Yang H., Xie M., Wen Y., Liu Q., Li X., Liu J., Xu H., Tang W., Wang M. Chondrocyte Apoptosis in Rat Mandibular Condyles Induced by Dental Occlusion Due to Mitochondrial Damage Caused by Nitric Oxide. Arch. Oral Biol. 2019;101:108–121. doi: 10.1016/j.archoralbio.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 31.Dobsak T., Heimel P., Tangl S., Schwarze U.Y., Schett G., Gruber R. Impaired Periodontium and Temporomandibular Joints in Tumour Necrosis Factor-α Transgenic Mice. J. Clin. Periodontol. 2017;44:1226–1235. doi: 10.1111/jcpe.12799. [DOI] [PubMed] [Google Scholar]
- 32.Zhang C., Lin S., Li T., Jiang Y., Huang Z., Wen J., Cheng W., Li H. Mechanical Force-Mediated Pathological Cartilage Thinning Is Regulated by Necroptosis and Apoptosis. Osteoarthr. Cartil. 2017;25:1324–1334. doi: 10.1016/j.joca.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Cheng N.-T., Guo A., Meng H. The Protective Role of Autophagy in Experimental Osteoarthritis, and the Therapeutic Effects of Torin 1 on Osteoarthritis by Activating Autophagy. BMC Musculoskelet. Disord. 2016;17:1–8. doi: 10.1186/s12891-016-0995-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu L., Chen Y., Tooze S.A. Autophagy Pathway: Cellular and Molecular Mechanisms. Autophagy. 2017;14:207–215. doi: 10.1080/15548627.2017.1378838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi Y., Hu X., Cheng J., Zhang X., Zhao F., Shi W., Ren B., Yu H., Yang P., Li Z., et al. A Small Molecule Promotes Cartilage Extracellular Matrix Generation and Inhibits Osteoarthritis Development. Nat. Commun. 2019;10:1914. doi: 10.1038/s41467-019-09839-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lian C., Wang X., Qiu X., Wu Z., Gao B., Liu L., Liang G., Zhou H., Yang X., Peng Y., et al. Collagen Type II Suppresses Articular Chondrocyte Hypertrophy and Osteoarthritis Progression by Promoting Integrin β1−smad1 Interaction. Bone Res. 2019;7:8. doi: 10.1038/s41413-019-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiao K., Zeng G., Niu L.-N., Yang H.-X., Ren G.-T., Xu X.-Y., Li F.-F., Tay F.R., Wang M.-Q. Activation of α2a-Adrenergic Signal Transduction in Chondrocytes Promotes Degenerative Remodelling of Temporomandibular Joint. Sci. Rep. 2016;6:30085. doi: 10.1038/srep30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ge C., Mohamed F., Binrayes A., Kapila S., Franceschi R.T. Selective Role of Discoidin Domain Receptor 2 in Murine Temporomandibular Joint Development and Aging. J. Dent. Res. 2017;97:321–328. doi: 10.1177/0022034517738190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu M., Tang D., Wu Q., Hao S., Chen M., Xie C., Rosier R.N., O’Keefe R.J., Zuscik M., Chen D. Activation of β-Catenin Signaling in Articular Chondrocytes Leads to Osteoarthritis-like Phenotype in Adult β-Catenin Conditional Activation Mice. J. Bone Miner. Res. 2009;24:12–21. doi: 10.1359/jbmr.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Y., Shu B., Xie R., Huang J., Zheng L., Zhou X., Xiao G., Zhao L., Chen D. Deletion of axin1 in Condylar Chondrocytes Leads to Osteoarthritis-like Phenotype in Temporomandibular Joint via Activation of β-Catenin and FGF Signaling. J. Cell. Physiol. 2018;234:1720–1729. doi: 10.1002/jcp.27043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang Y., Wu X., Lei W., Pang L., Wan C., Shi Z., Zhao L., Nagy T.R., Peng X., Hu J., et al. TGF-β1–Induced Migration of Bone Mesenchymal Stem Cells Couples Bone Resorption with Formation. Nat. Med. 2009;15:757–765. doi: 10.1038/nm.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yi J.J., Barnes A.P., Hand R., Polleux F., Ehlers M.D. TGF-β Signaling Specifies Axons during Brain Development. Cell. 2010;142:144–157. doi: 10.1016/j.cell.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li W., Zhao S., Yang H., Zhang C., Kang Q., Deng J., Xu Y., Ding Y., Li S. Potential Novel Prediction of TMJ-Oa: Mir-140-5p Regulates Inflammation through SMAD/TGF-β Signaling. Front. Pharmacol. 2019;10:15. doi: 10.3389/fphar.2019.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alman B.A. The Role of Hedgehog Signalling in Skeletal Health and Disease. Nat. Rev. Rheumatol. 2015;11:552–560. doi: 10.1038/nrrheum.2015.84. [DOI] [PubMed] [Google Scholar]
- 45.Yang H., Zhang M., Liu Q., Zhang H., Zhang J., Lu L., Xie M., Chen D., Wang M. Inhibition of IHH Reverses Temporomandibular Joint Osteoarthritis via a PTH1R Signaling Dependent Mechanism. Int. J. Mol. Sci. 2019;20:3797. doi: 10.3390/ijms20153797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ornitz D.M., Itoh N. The Fibroblast Growth Factor Signaling Pathway. Wiley Interdiscip. Rev. Dev. Biol. 2015;4:215–266. doi: 10.1002/wdev.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Su N. FGF Signaling: Its Role in Bone Development and Human Skeleton Diseases. Front. Biosci. 2008;13:2842. doi: 10.2741/2890. [DOI] [PubMed] [Google Scholar]
- 48.Wang Z., Huang J., Zhou S., Luo F., Tan Q., Sun X., Ni Z., Chen H., Du X., Xie Y., et al. Loss of FGFR1 in Chondrocytes Inhibits Osteoarthritis by Promoting Autophagic Activity in Temporomandibular Joint. J. Biol. Chem. 2018;293:8761–8774. doi: 10.1074/jbc.RA118.002293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Q., Lenardo M.J., Baltimore D. 30 Years of NF-ΚB: A Blossoming of Relevance to Human Pathobiology. Cell. 2017;168:37–57. doi: 10.1016/j.cell.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.D’Souza B., Meloty-Kapella L., Weinmaster G. Canonical and Non-Canonical Notch Ligands. Curr. Top. Dev. Biol. 2010;92:73–129. doi: 10.1016/S0070-2153(10)92003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kopan R., Ilagan M.X. The Canonical Notch Signaling Pathway: Unfolding the Activation Mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Saito T., Tanaka S. Molecular Mechanisms Underlying Osteoarthritis Development: Notch and NF-ΚB. Arthritis Res. Ther. 2017;19:94. doi: 10.1186/s13075-017-1296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Q.-Y., Dai J., Kuang B., Zhang J., Yu S.-B., Duan Y.-Z., Wang M.-Q. Osteochondral Angiogenesis in Rat Mandibular Condyles with Osteoarthritis-like Changes. Arch. Oral Biol. 2012;57:620–629. doi: 10.1016/j.archoralbio.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Ashraf S., Walsh D.A. Angiogenesis in Osteoarthritis. Curr. Opin. Rheumatol. 2008;20:573–580. doi: 10.1097/BOR.0b013e3283103d12. [DOI] [PubMed] [Google Scholar]
- 55.Pesesse L., Sanchez C., Henrotin Y. Osteochondral Plate Angiogenesis: A New Treatment Target in Osteoarthritis. Joint Bone Spine. 2011;78:144–149. doi: 10.1016/j.jbspin.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Feng Y., Ke J., Cao P., Deng M., Li J., Cai H., Meng Q., Li Y., Long X. HMGB1-Induced Angiogenesis in Perforated Disc Cells of Human Temporomandibular Joint. J. Cell. Mol. Med. 2017;22:1283–1291. doi: 10.1111/jcmm.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang S.-J., Li W., Li Y.-J., Fang W., Long X. Dickkopf-Related Protein 1 Induces Angiogenesis by Upregulating Vascular Endothelial Growth Factor in the Synovial Fibroblasts of Patients with Temporomandibular Joint Disorders. Mol. Med. Rep. 2015;12:4959–4966. doi: 10.3892/mmr.2015.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J., Cai H., Meng Q., Li Y., Chen G., Fang W., Long X. IL-1β-Regulating Angiogenic Factors Expression in Perforated Temporomandibular Disk Cells via NF-ΚB Pathway. J. Oral Pathol. Med. 2016;45:605–612. doi: 10.1111/jop.12420. [DOI] [PubMed] [Google Scholar]
- 59.Landes C.A., Goral W., Mack M.G., Sader R. 3-D Sonography for Diagnosis of Osteoarthrosis and Disk Degeneration of the Temporomandibular Joint, Compared with MRI. Ultrasound. Med. Biol. 2006;32:627–632. doi: 10.1016/j.ultrasmedbio.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 60.Bonjardim L.R., Gavião M.B., Pereira L.J., Castelo P.M., Garcia R.C. Signs and Symptoms of Temporomandibular Disorders in Adolescents. Braz. Oral Res. 2005;19:93–98. doi: 10.1590/S1806-83242005000200004. [DOI] [PubMed] [Google Scholar]
- 61.Wu F., An Y., Zhou L., Zhao Y., Chen L., Wang J., Wu G. Whole-Transcriptome Sequencing and Cerna Interaction Network of Temporomandibular Joint Osteoarthritis. Front. Genet. 2022;13:962574. doi: 10.3389/fgene.2022.962574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Abrahamsson A.K., Kristensen M., Arvidsson L.Z., Kvien T.K., Larheim T.A., Haugen I.K. Frequency of Temporomandibular Joint Osteoarthritis and Related Symptoms in a Hand Osteoarthritis Cohort. Osteoarthr. Cartil. 2017;25:654–657. doi: 10.1016/j.joca.2016.12.028. [DOI] [PubMed] [Google Scholar]
- 63.Embree M., Ono M., Kilts T., Walker D., Langguth J., Mao J., Bi Y., Barth J.L., Young M. Role of subchondral bone during early-stage experimental TMJ osteoarthritis. J. Dent. Res. 2011;90:1331–1338. doi: 10.1177/0022034511421930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burr D.B., Gallant M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012;8:665–673. doi: 10.1038/nrrheum.2012.130. [DOI] [PubMed] [Google Scholar]
- 65.Zhang J., Liao L., Zhu J., Wan X., Xie M., Zhang H., Zhang M., Lu L., Yang H., Jing D., et al. Osteochondral Interface Stiffening in Mandibular Condylar Osteoarthritis. J. Dent. Res. 2018;97:563–570. doi: 10.1177/0022034517748562. [DOI] [PubMed] [Google Scholar]
- 66.Gharavi S.M., Qiao Y., Faghihimehr A., Vossen J. Imaging of the Temporomandibular Joint. Diagnostics. 2022;12:1006. doi: 10.3390/diagnostics12041006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhao Y.-P., Zhang Z.-Y., Wu Y.-T., Zhang W.-L., Ma X.-C. Investigation of the Clinical and Radiographic Features of Osteoarthrosis of the Temporomandibular Joints in Adolescents and Young Adults. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2011;111:e27–e34. doi: 10.1016/j.tripleo.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 68.AlZarea B.K. Temporomandibular Disorders (TMD) in Edentulous Patients: A Review and Proposed Classification (Dr. Bader’s Classification) J. Clin. Diagn. Res. 2015;9:ZE06. doi: 10.7860/JCDR/2015/13535.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katsimbri P. The Biology of Normal Bone Remodelling. Eur. J. Cancer Care. 2017;26:e12740. doi: 10.1111/ecc.12740. [DOI] [PubMed] [Google Scholar]
- 70.Wang T., Wen C.-Y., Yan C.-H., Lu W.-W., Chiu K.-Y. Spatial and Temporal Changes of Subchondral Bone Proceed to Microscopic Articular Cartilage Degeneration in Guinea Pigs with Spontaneous Osteoarthritis. Osteoarthr. Cartil. 2013;21:574–581. doi: 10.1016/j.joca.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Tat S.K., Pelletier J.-P., Lajeunesse D., Fahmi H., Duval N., Martel-Pelletier J. Differential Modulation of RANKL Isoforms by Human Osteoarthritic Subchondral Bone Osteoblasts: Influence of Osteotropic Factors. Bone. 2008;43:284–291. doi: 10.1016/j.bone.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abed É., Delalandre A., Lajeunesse D. Beneficial Effect of Resveratrol on Phenotypic Features and Activity of Osteoarthritic Osteoblasts. Arthritis Res. Ther. 2017;19:151. doi: 10.1186/s13075-017-1365-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Corrado A., Neve A., Cantatore F.P. Expression of Vascular Endothelial Growth Factor in Normal, Osteoarthritic and Osteoporotic Osteoblasts. Clin. Exp. Med. 2011;13:81–84. doi: 10.1007/s10238-011-0170-5. [DOI] [PubMed] [Google Scholar]
- 74.Hilal G., Martel-Pelletier J., Pelletier J.-P., Ranger P., Lajeunesse D. Osteoblast-like Cells from Human Subchondral Osteoarthritic Bone Demonstrate an Altered Phenotype in Vitro: Possible Role in Subchondral Bone Sclerosis. Arthritis Rheumatol. 1998;41:891–899. doi: 10.1002/1529-0131(199805)41:5<891::AID-ART17>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 75.Bianco D., Todorov A., Čengić T., Pagenstert G., Schären S., Netzer C., Hügle T., Geurts J. Alterations of Subchondral Bone Progenitor Cells in Human Knee and Hip Osteoarthritis Lead to a Bone Sclerosis Phenotype. Int. J. Mol. Sci. 2018;19:475. doi: 10.3390/ijms19020475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li G., Yin J., Gao J., Cheng T.S., Pavlos N.J., Zhang C., Zheng M.H. Subchondral Bone in Osteoarthritis: Insight into Risk Factors and Microstructural Changes. Arthritis Res. Ther. 2013;15:223. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chan T.F., Couchourel D., Abed É., Delalandre A., Duval N., Lajeunesse D. Elevated Dickkopf-2 Levels Contribute to the Abnormal Phenotype of Human Osteoarthritic Osteoblasts. J. Bone Miner. Res. 2011;26:1399–1410. doi: 10.1002/jbmr.358. [DOI] [PubMed] [Google Scholar]
- 78.Sanchez C., Mazzucchelli G., Lambert C., Comblain F., DePauw E., Henrotin Y. Comparison of Secretome from Osteoblasts Derived from Sclerotic versus Non-Sclerotic Subchondral Bone in Oa: A Pilot Study. PLoS ONE. 2018;13:e0194591. doi: 10.1371/journal.pone.0194591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Borciani G., Montalbano G., Baldini N., Cerqueni G., Vitale-Brovarone C., Ciapetti G. Co–Culture Systems of Osteoblasts and Osteoclasts: Simulating in Vitro Bone Remodeling in Regenerative Approaches. Acta Biomater. 2020;108:22–45. doi: 10.1016/j.actbio.2020.03.043. [DOI] [PubMed] [Google Scholar]
- 80.Jaiprakash A., Prasadam I., Feng J.Q., Liu Y., Crawford R., Xiao Y. Phenotypic Characterization of Osteoarthritic Osteocytes from the Sclerotic Zones: A Possible Pathological Role in Subchondral Bone Sclerosis. Int. J. Mol. Sci. 2012;8:406–417. doi: 10.7150/ijbs.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Soki F.N., Yoshida R., Paglia D.N., Duong L.T., Hansen M.F., Drissi H. Articular Cartilage Protection in CTSK -/- Mice Is Associated with Cellular and Molecular Changes in Subchondral Bone and Cartilage Matrix. J. Cell. Physiol. 2018;233:8666–8676. doi: 10.1002/jcp.26745. [DOI] [PubMed] [Google Scholar]
- 82.Yang T., Zhang J., Cao Y., Zhang M., Jing L., Jiao K., Yu S., Chang W., Chen D., Wang M. WNT5A/ror2 Mediates Temporomandibular Joint Subchondral Bone Remodeling. J. Dent. Res. 2015;94:803–812. doi: 10.1177/0022034515576051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiao K., Niu L., Xu X., Liu Y., Li X., Tay F.R., Wang M. Norepinephrine Regulates Condylar Bone Loss via Comorbid Factors. J. Dent. Res. 2015;94:813–820. doi: 10.1177/0022034515577677. [DOI] [PubMed] [Google Scholar]
- 84.Zheng L., Pi C., Zhang J., Fan Y., Cui C., Zhou Y., Sun J., Yuan Q., Xu X., Ye L., et al. Aberrant Activation of Latent Transforming Growth Factor-β Initiates the Onset of Temporomandibular Joint Osteoarthritis. Bone Res. 2018;6:26. doi: 10.1038/s41413-018-0027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ye T., Sun D., Mu T., Chu Y., Miao H., Zhang M., Yang H., Liu Q., Lu L., Xing X., et al. Differential Effects of High-Physiological Oestrogen on the Degeneration of Mandibular Condylar Cartilage and Subchondral Bone. Bone. 2018;111:9–22. doi: 10.1016/j.bone.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 86.Xue X.-T., Kou X.-X., Li C.-S., Bi R.-Y., Meng Z., Wang X.-D., Zhou Y.-H., Gan Y.-H. Progesterone Attenuates Temporomandibular Joint Inflammation through Inhibition of NF-ΚB Pathway in Ovariectomized Rats. Sci. Rep. 2017;7:15334. doi: 10.1038/s41598-017-15285-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Robling A.G., Bonewald L.F. The Osteocyte: New Insights. Annu. Rev. Physiol. 2020;82:485–506. doi: 10.1146/annurev-physiol-021119-034332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiong J., Onal M., Jilka R.L., Weinstein R.S., Manolagas S.C., O’Brien C.A. Matrix-Embedded Cells Control Osteoclast Formation. Nat. Med. 2011;17:1235–1241. doi: 10.1038/nm.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dole N.S., Mazur C.M., Acevedo C., Lopez J.P., Monteiro D.A., Fowler T.W., Gludovatz B., Walsh F., Regan J.N., Messina S., et al. Osteocyte-Intrinsic TGF-β Signaling Regulates Bone Quality through Perilacunar/Canalicular Remodeling. Cell Rep. 2017;21:2585–2596. doi: 10.1016/j.celrep.2017.10.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mazur C.M., Woo J.J., Yee C.S., Fields A.J., Acevedo C., Bailey K.N., Kaya S., Fowler T.W., Lotz J.C., Dang A., et al. Osteocyte Dysfunction Promotes Osteoarthritis through MMP13-Dependent Suppression of Subchondral Bone Homeostasis. Bone Res. 2019;7:34. doi: 10.1038/s41413-019-0070-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goldring M.B., Otero M. Inflammation in Osteoarthritis. Curr. Opin. Rheumatol. 2011;23:471–478. doi: 10.1097/BOR.0b013e328349c2b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Larheim T.A., Abrahamsson A.-K., Kristensen M., Arvidsson L.Z. Temporomandibular Joint Diagnostics Using CBCT. Dentomaxillofac. Radiol. 2015;44:20140235. doi: 10.1259/dmfr.20140235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vernal R., Velásquez E., Gamonal J., Garcia-Sanz J.A., Silva A., Sanz M. Expression of Proinflammatory Cytokines in Osteoarthritis of the Temporomandibular Joint. Dentomaxillofac. Radiol. 2008;53:910–915. doi: 10.1016/j.archoralbio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 94.Ogura N., Tobe M., Sakamaki H., Nagura H., Abiko Y., Kondoh T. Tumor Necrosis Factor-Alpha Increases Chemokine Gene Expression and Production in Synovial Fibroblasts from Human Temporomandibular Joint. J. Oral Pathol. Med. 2005;34:357–363. doi: 10.1111/j.1600-0714.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 95.Ogura N., Akutsu M., Tobe M., Sakamaki H., Abiko Y., Kondoh T. Microarray Analysis of Il-1β-Stimulated Chemokine Genes in Synovial Fibroblasts from Human TMJ. J. Oral Pathol. Med. 2007;36:223–228. doi: 10.1111/j.1600-0714.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 96.Tanimoto K., Iwabuchi Y., Tanne Y., Kamiya T., Inubushi T., Kunimatsu R., Mitsuyoshi T., Tanne K. Interleukin-1 Beta Affects Cyclooxygenase-2 Expression and Cartilage Metabolism in Mandibular Condyle. Arch. Oral Biol. 2011;56:1412–1418. doi: 10.1016/j.archoralbio.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 97.Ogura N., Satoh K., Akutsu M., Tobe M., Kuyama K., Kuboyama N., Sakamaki H., Kujiraoka H., Kondoh T. MCP-1 Production in Temporomandibular Joint Inflammation. J. Dent. Res. 2010;89:1117–1122. doi: 10.1177/0022034510376041. [DOI] [PubMed] [Google Scholar]
- 98.Ibi M., Horie S., Kyakumoto S., Chosa N., Yoshida M., Kamo M., Ohtsuka M., Ishisaki A. Cell–Cell Interactions between Monocytes/Macrophages and Synoviocyte-like Cells Promote Inflammatory Cell Infiltration Mediated by Augmentation of MCP-1 Production in Temporomandibular Joint. Biosci. Rep. 2018;38:BSR20171217. doi: 10.1042/BSR20171217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang X.D., Cui S.J., Liu Y., Luo Q., Du R.J., Kou X.X., Zhang J.N., Zhou Y.H., Gan Y.H. Deterioration of Mechanical Properties of Discs in Chronically Inflamed TMJ. J. Dent. Res. 2014;93:1170–1176. doi: 10.1177/0022034514552825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wehmeyer C., Pap T., Buckley C.D., Naylor A.J. The Role of Stromal Cells in Inflammatory Bone Loss. Clin. Exp. Immunol. 2017;189:1–11. doi: 10.1111/cei.12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu X., Cai H., Cao P., Feng Y., Jiang H., Liu L., Ke J., Long X. TLR4 Contributes to the Damage of Cartilage and Subchondral Bone in Discectomy-Induced Tmjoa Mice. J. Cell. Mol. Med. 2020;24:11489–11499. doi: 10.1111/jcmm.15763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schaible H.-G. Nociceptive Neurons Detect Cytokines in Arthritis. Arthritis Res. Ther. 2014;16:470. doi: 10.1186/s13075-014-0470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaneyama K., Segami N., Nishimura M., Suzuki T., Sato J. Importance of Proinflammatory Cytokines in Synovial Fluid from 121 Joints with Temporomandibular Disorders. Br. J. Oral Maxillofac. Surg. 2002;40:418–423. doi: 10.1016/S0266-4356(02)00215-2. [DOI] [PubMed] [Google Scholar]
- 104.Ishimaru J.-I., Oguma Y., Goss A.N. Matrix Metalloproteinase and Tissue Inhibitor of Metalloproteinase in Serum and Lavage Synovial Fluid of Patients with Temporomandibular Joint Disorders. Br. J. Oral Maxillofac. Surg. 2000;38:354–359. doi: 10.1054/bjom.2000.0306. [DOI] [PubMed] [Google Scholar]
- 105.Wang G.Y., Shi X.Q., Wu W., Gueorguieva M., Yang M., Zhang J. Sustained and Repeated Mouth Opening Leads to Development of Painful Temporomandibular Disorders Involving Macrophage/Microglia Activation in Mice. Pain. 2018;159:1277–1288. doi: 10.1097/j.pain.0000000000001206. [DOI] [PubMed] [Google Scholar]
- 106.Xiao M., Hu Z., Jiang H., Li C., Guo H., Fang W., Long X. The Expression of Netrin-1 in the MIA-Induced Osteoarthritic Temporomandibular Joint in Mice. Sci. Rep. 2021;11:15695. doi: 10.1038/s41598-021-95251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang J., Liu T., Wen X., Zhou Z., Yan J., Gao J., Zuo J. Estrogen-Related Receptors: Novel Potential Regulators of Osteoarthritis Pathogenesis. Mol. Med. 2021;27:5. doi: 10.1186/s10020-021-00270-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qin W., Zhang Z., Yan J., Han X., Niu L.-N., Jiao K. Interaction of Neurovascular Signals in the Degraded Condylar Cartilage. Front. Bioeng. Biotechnol. 2022;10:901749. doi: 10.3389/fbioe.2022.901749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bednarczyk E. Chondrocytes in Vitro Systems Allowing Study of OA. Int. J. Mol. Sci. 2022;23:10308. doi: 10.3390/ijms231810308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bonnet C.S. Osteoarthritis, Angiogenesis and Inflammation. Rheumatology. 2005;44:7–16. doi: 10.1093/rheumatology/keh344. [DOI] [PubMed] [Google Scholar]
- 111.Ashraf S., Wibberley H., Mapp P.I., Hill R., Wilson D., Walsh D.A. Increased Vascular Penetration and Nerve Growth in the Meniscus: A Potential Source of Pain in Osteoarthritis. Ann. Rheum. Dis. 2010;70:523–529. doi: 10.1136/ard.2010.137844. [DOI] [PubMed] [Google Scholar]
- 112.Mapp P.I., Walsh D.A. Mechanisms and Targets of Angiogenesis and Nerve Growth in Osteoarthritis. Nat. Rev. Rheumatol. 2012;8:390–398. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 113.Yu X., Qi Y., Zhao T., Fang J., Liu X., Xu T., Yang Q., Dai X. NGF Increases FGF2 Expression and Promotes Endothelial Cell Migration and Tube Formation through PI3K/Akt and ERK/MAPK Pathways in Human Chondrocytes. Osteoarthr. Cartil. 2019;27:526–534. doi: 10.1016/j.joca.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 114.Frost H.M. From Wolff’s Law to the Utah Paradigm: Insights about Bone Physiology and Its Clinical Applications. Anat. Rec. 2001;262:398–419. doi: 10.1002/ar.1049. [DOI] [PubMed] [Google Scholar]
- 115.Shiraishi K., Chiba K., Okazaki N., Yokota K., Nakazoe Y., Kidera K., Yonekura A., Tomita M., Osaki M. In Vivo Analysis of Subchondral Trabecular Bone in Patients with Osteoarthritis of the Knee Using Second-Generation High-Resolution Peripheral Quantitative Computed Tomography (HR-Pqct) Bone. 2020;132:115155. doi: 10.1016/j.bone.2019.115155. [DOI] [PubMed] [Google Scholar]
- 116.Bonewald L.F. The Amazing Osteocyte. J. Bone Miner. Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tang S.Y., Herber R.-P., Ho S.P., Alliston T. Matrix Metalloproteinase-13 Is Required for Osteocytic Perilacunar Remodeling and Maintains Bone Fracture Resistance. J. Bone Miner. Res. 2012;27:1936–1950. doi: 10.1002/jbmr.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Qing H., Ardeshirpour L., Divieti Pajevic P., Dusevich V., Jähn K., Kato S., Wysolmerski J., Bonewald L.F. Demonstration of Osteocytic Perilacunar/Canalicular Remodeling in Mice during Lactation. J. Bone Miner. Res. 2012;27:1018–1029. doi: 10.1002/jbmr.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kogawa M., Wijenayaka A.R., Ormsby R.T., Thomas G.P., Anderson P.H., Bonewald L.F., Findlay D.M., Atkins G.J. Sclerostin Regulates Release of Bone Mineral by Osteocytes by Induction of Carbonic Anhydrase 2. J. Bone Miner. Res. 2013;28:2436–2448. doi: 10.1002/jbmr.2003. [DOI] [PubMed] [Google Scholar]
- 120.Fujisawa T., Kuboki T., Kasai T., Sonoyama W., Kojima S., Uehara J., Komori C., Yatani H., Hattori T., Takigawa M. A Repetitive, Steady Mouth Opening Induced an Osteoarthritis-like Lesion in the Rabbit Temporomandibular Joint. J. Dent. Res. 2003;82:731–735. doi: 10.1177/154405910308200914. [DOI] [PubMed] [Google Scholar]
- 121.Das S.K. TMJ Osteoarthritis and Early Diagnosis. J. Oral Biol. Craniofac. Res. 2013;3:109–110. doi: 10.1016/j.jobcr.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou Y., Lu H., Deng L., Lin C.-H., Pennington Klein K., Wu M. HMGB2 Is Associated with Pressure Loading in Chondrocytes of Temporomandibular Joint: In Vitro and in Vivo Study. Cytokine. 2020;126:154875. doi: 10.1016/j.cyto.2019.154875. [DOI] [PubMed] [Google Scholar]
- 123.Zhang J., Jiao K., Zhang M., Zhou T., Liu X.-D., Yu S.-B., Lu L., Jing L., Yang T., Zhang Y., et al. Occlusal Effects on Longitudinal Bone Alterations of the Temporomandibular Joint. J. Dent. Res. 2013;92:253–259. doi: 10.1177/0022034512473482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ren H., Liu J.Y., Liu Y.C., Bao G., Kang H. Comparative effectiveness of low-level laser therapy with different wavelengths and transcutaneous electric nerve stimulation in the treatment of pain caused by temporomandibular disorders: A systematic review and network meta-analysis. J. Oral Rehabil. 2022;49:138–149. doi: 10.1111/joor.13230. [DOI] [PubMed] [Google Scholar]
- 125.Derwich M., Mitus-Kenig M., Pawlowska E. Orally Administered NSAIDs—General Characteristics and Usage in the Treatment of Temporomandibular Joint Osteoarthritis—A Narrative Review. Pharmaceuticals. 2021;14:219. doi: 10.3390/ph14030219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sabbagh H., Sabbagh A., Heppner A., Auer C., Wichelhaus A., Hoffmann L. Patients’ perceptions on temporomandibular disorder treatment with hydrostatic oral splints-a pilot study. BDJ Open. 2022;8:1–6. doi: 10.1038/s41405-022-00096-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li D.T.S., Leung Y.Y. Temporomandibular Disorders: Current Concepts and Controversies in Diagnosis and Management. Diagnostics. 2021;11:459. doi: 10.3390/diagnostics11030459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Marotta N., Ferrillo M., Demeco A., Drago Ferrante V., Inzitari M.T., Pellegrino R., Pino I., Russo I., de Sire A., Ammendolia A. Effects of Radial Extracorporeal Shock Wave Therapy in Reducing Pain in Patients with Temporomandibular Disorders: A Pilot Randomized Controlled Trial. Appl. Sci. 2022;12:3821. doi: 10.3390/app12083821. [DOI] [Google Scholar]
- 129.Minervini G., Fiorillo L., Russo D., Lanza A., D’Amico C., Cervino G., Meto A., Di Francesco F. Prosthodontic Treatment in Patients with Temporomandibular Disorders and Orofacial Pain and/or Bruxism: A Review of the Literature. Prosthesis. 2022;4:253–262. doi: 10.3390/prosthesis4020025. [DOI] [Google Scholar]
- 130.Bartolotti I., Roseti L., Petretta M., Grigolo B., Desando G. A Roadmap of In Vitro Models in Osteoarthritis: A Focus on Their Biological Relevance in Regenerative Medicine. J. Clin. Med. 2021;10:1920. doi: 10.3390/jcm10091920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Main B.J., Maffulli N., Valk J.A., Rodriguez H.C., Gupta M., El-Amin S.F., III, Gupta A. Umbilical Cord-Derived Wharton’s Jelly for Regenerative Medicine Applications: A Systematic Review. Pharmaceuticals. 2021;14:1090. doi: 10.3390/ph14111090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Matsuzaka Y., Yashiro R. Therapeutic Strategy of Mesenchymal-Stem-Cell-Derived Extracellular Vesicles as Regenerative Medicine. Int. J. Mol. Sci. 2022;23:6480. doi: 10.3390/ijms23126480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chung P.Y., Lin M.T., Chang H.P. Effectiveness of platelet-rich plasma injection in patients with temporomandibular joint osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019;127:106–116. doi: 10.1016/j.oooo.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 134.Saita Y., Kobayashi Y., Nishio H., Wakayama T., Fukusato S., Uchino S., Momoi Y., Ikeda H., Kaneko K. Predictors of Effectiveness of Platelet-Rich Plasma Therapy for Knee Osteoarthritis: A Retrospective Cohort Study. J. Clin. Med. 2021;10:4514. doi: 10.3390/jcm10194514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kikuchi N., Yoshioka T., Arai N., Sugaya H., Hyodo K., Taniguchi Y., Okuno K., Kanamori A., Yamazaki M. A Retrospective Analysis of Clinical Outcome and Predictive Factors for Responders with Knee Osteoarthritis to a Single Injection of Leukocyte-Poor Platelet-Rich Plasma. J. Clin. Med. 2021;10:5121. doi: 10.3390/jcm10215121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Heidari N., Slevin M., Zeinolabediny Y., Meloni D., Olgiati S., Wilson A., Noorani A., Azamfirei L. Comparison of the Effect of MFAT and MFAT + PRP on Treatment of Hip Osteoarthritis: An Observational, Intention-to-Treat Study at One Year. J. Clin. Med. 2022;11:1056. doi: 10.3390/jcm11041056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Smedslund G., Kjeken I., Musial F., Sexton J., Østerås N. Interventions for osteoarthritis pain: A systematic review with network meta-analysis of existing Cochrane reviews. Osteoarthr. Cartil. 2022;4:100242. doi: 10.1016/j.ocarto.2022.100242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Patel S., Mishra N.P., Chouhan D.K., Nahar U., Dhillon M.S. Chondroprotective effects of multiple PRP injections in osteoarthritis by apoptosis regulation and increased aggrecan synthesis-Immunohistochemistry based Guinea pig study. J. Clin. Orthop. Trauma. 2022;25:101762. doi: 10.1016/j.jcot.2022.101762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tatsis D., Vasalou V., Kotidis E., Anestiadou E., Grivas I., Cheva A., Koliakos G., Venetis G., Pramateftakis M.-G., Ouzounidis N., et al. The Combined Use of Platelet-Rich Plasma and Adipose-Derived Mesenchymal Stem Cells Promotes Healing. A Review of Experimental Models and Future Perspectives. Biomolecules. 2021;11:1403. doi: 10.3390/biom11101403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pötter N., Westbrock F., Grad S., Alini M., Stoddart M.J., Schmal H., Kubosch D., Salzmann G., Kubosch E.J. Evaluation of the influence of platelet-rich plasma (PRP), platelet lysate (PL) and mechanical loading on chondrogenesis in vitro. Sci. Rep. 2021;11:1–11. doi: 10.1038/s41598-021-99614-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cecerska-Heryć E., Goszka M., Serwin N., Roszak M., Grygorcewicz B., Heryć R., Dołęgowska B. Applications of the regenerative capacity of platelets in modern medicine. Cytokine Growth Factor Rev. 2021;64:84–94. doi: 10.1016/j.cytogfr.2021.11.003. [DOI] [PubMed] [Google Scholar]
- 142.Del Amo C., Perez-Valle A., Atilano L., Andia I. Unraveling the Signaling Secretome of Platelet-Rich Plasma: Towards a Better Understanding of Its Therapeutic Potential in Knee Osteoarthritis. J. Clin. Med. 2022;11:473. doi: 10.3390/jcm11030473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Andia I., Atilano L., Maffulli N. Moving toward targeting the right phenotype with the right platelet-rich plasma (PRP) formulation for knee osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2021;13:1759720X211004336. doi: 10.1177/1759720X211004336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Tucker J.D., Goetz L.L., Duncan M.B., Gilman J.B., Elmore L.W., Sell S.A., McClure M.J., Quagliano P.V., Martin C.C. Randomized, Placebo-Controlled Analysis of the Knee Synovial Environment Following Platelet-Rich Plasma Treatment for Knee Osteoarthritis. PM&R. 2021;13:707–719. doi: 10.1002/pmrj.12561. [DOI] [PubMed] [Google Scholar]
- 145.Liu S.S., Xu L.L., Fan S., Lu S.J., Jin L., Liu L.K., Yao Y., Cai B. Effect of platelet-rich plasma injection combined with individualised comprehensive physical therapy on temporomandibular joint osteoarthritis: A prospective cohort study. J. Oral Rehabil. 2022;49:150–159. doi: 10.1111/joor.13261. [DOI] [PubMed] [Google Scholar]
- 146.Li F.L., Wu C.B., Sun H.J., Zhou Q. Comparison of autologous platelet-rich plasma and chitosan in the treatment of temporomandibular joint osteoarthritis: A retrospective cohort study. J. Oral Maxillofac. Surg. 2021;79:324–332. doi: 10.1016/j.joms.2020.09.016. [DOI] [PubMed] [Google Scholar]
- 147.Arafat S., Kamel S. Biochemical and Histological Evaluation of different intra-articular injections as therapy for Temporomandibular Joint Osteoarthritis in Rats. Egypt. Dent. J. 2021;67:1165–1175. doi: 10.21608/edj.2021.65528.1532. [DOI] [Google Scholar]
- 148.Coskun U., Candirli C., Kerimoglu G., Taskesen F. Effect of platelet-rich plasma on temporomandibular joint cartilage wound healing: Experimental study in rabbits. J. Craniomaxillofac. Surg. 2019;47:357–364. doi: 10.1016/j.jcms.2018.12.004. [DOI] [PubMed] [Google Scholar]
- 149.AbuBakr N., Fares A.E., Mostafa A., Farag D.B. Mesenchymal stem cells-derived microvesicles versus platelet-rich plasma in the treatment of monoiodoacetate-induced temporomandibular joint osteoarthritis in Albino rats. Heliyon. 2022;8:e10857. doi: 10.1016/j.heliyon.2022.e10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Liapaki A., Thamm J.R., Ha S., Monteiro J.L., McCain J.P., Troulis M.J., Guastaldi F.P. Is there a difference in treatment effect of different intra-articular drugs for temporomandibular joint osteoarthritis? A systematic review of randomized controlled trials. Int. J. Oral Maxillofac. Surg. 2021;50:1233–1243. doi: 10.1016/j.ijom.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 151.Al-Moraissi E.A., Wolford L.M., Ellis E., Neff A. The hierarchy of different treatments for arthrogenous temporomandibular disorders: A network meta-analysis of randomized clinical trials. J. Craniomaxillofac. Surg. 2020;48:9–23. doi: 10.1016/j.jcms.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 152.Xie Y., Zhao K., Ye G., Yao X., Yu M., Ouyang H. Effectiveness of Intra-Articular Injections of Sodium Hyaluronate, Corticosteroids, Platelet-Rich Plasma on Temporomandibular Joint Osteoarthritis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Evid. Based Dent. Pract. 2022;22:101720. doi: 10.1016/j.jebdp.2022.101720. [DOI] [PubMed] [Google Scholar]
- 153.Gutiérrez I.Q., Sábado-Bundó H., Gay-Escoda C. Intraarticular injections of platelet rich plasma and plasma rich in growth factors with arthrocenthesis or arthroscopy in the treatment of temporomandibular joint disorders: A systematic review. J. Stomatol. Oral Maxillofac. Surg. 2021;123:e327–e335. doi: 10.1016/j.jormas.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 154.Smith C., Patel R., Vannabouathong C., Sales B., Rabinovich A., McCormack R., Belzile E.L., Bhandari M. Combined intra-articular injection of corticosteroid and hyaluronic acid reduces pain compared to hyaluronic acid alone in the treatment of knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2019;27:1974–1983. doi: 10.1007/s00167-018-5071-7. [DOI] [PubMed] [Google Scholar]
- 155.Han Y., Huang H., Pan J., Lin J., Zeng L., Liang G., Yang W., Liu J. Meta-analysis comparing platelet-rich plasma vs hyaluronic acid injection in patients with knee osteoarthritis. Pain Med. 2019;20:1418–1429. doi: 10.1093/pm/pnz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu Z., He Z., Shu L., Li X., Ma M., Ye C. Intra-articular platelet-rich plasma combined with hyaluronic acid injection for knee osteoarthritis is superior to platelet-rich plasma or hyaluronic acid alone in inhibiting inflammation and improving pain and function. Arthrosc. J. Arthrosc. Relat. Surg. 2021;37:903–915. doi: 10.1016/j.arthro.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 157.Diaz-Rodriguez P., Marino C., Vázquez J.A., Caeiro-Rey J.R., Landin M. Targeting joint inflammation for osteoarthritis management through stimulus-sensitive hyaluronic acid based intra-articular hydrogels. Mater. Sci. Eng. C. 2021;128:112254. doi: 10.1016/j.msec.2021.112254. [DOI] [PubMed] [Google Scholar]
- 158.Kim Y.S., Guilak F. Engineering Hyaluronic Acid for the Development of New Treatment Strategies for Osteoarthritis. Int. J. Mol. Sci. 2022;23:8662. doi: 10.3390/ijms23158662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.do Nascimento M.H.M., Ambrosio F.N., Ferraraz D.C., Windisch-Neto H., Querobino S.M., Nascimento-Sales M., Alberto-Silva C., Christoffolete M.A., Franco M.K., Kent B., et al. Sulforaphane-loaded hyaluronic acid-poloxamer hybrid hydrogel enhances cartilage protection in osteoarthritis models. Mater. Sci. Eng. C. 2021;128:112345. doi: 10.1016/j.msec.2021.112345. [DOI] [PubMed] [Google Scholar]
- 160.Zhou T., Ran J., Xu P., Shen L., He Y., Ye J., Wu L., Gao C. A hyaluronic acid/platelet-rich plasma hydrogel containing MnO2 nanozymes efficiently alleviates osteoarthritis in vivo. Carbohydr. Polym. 2022;292:119667. doi: 10.1016/j.carbpol.2022.119667. [DOI] [PubMed] [Google Scholar]
- 161.Liu W., Ma M., Lei Z., Xiong Z., Tao T., Lei P., Hu Y., Jiang X., Xiao J. Intra-articular injectable hydroxypropyl chitin/hyaluronic acid hydrogel as bio-lubricant to attenuate osteoarthritis progression. Mater. Des. 2022;217:110579. doi: 10.1016/j.matdes.2022.110579. [DOI] [Google Scholar]
- 162.Yang R., Chen M., Yang X., Sun W., Lu C., Hui Q., Shi C., Li X., Wang X. Modified poloxamer 407 and hyaluronic acid thermosensitive hydrogel-encapsulated keratinocyte growth factor 2 improves knee osteoarthritis in rats. Mater. Des. 2021;210:110086. doi: 10.1016/j.matdes.2021.110086. [DOI] [Google Scholar]
- 163.Chęciński M., Chęcińska K., Turosz N., Kamińska M., Nowak Z., Sikora M., Chlubek D. The Administration of Hyaluronic Acid into the Temporomandibular Joints’ Cavities Increases the Mandible’s Mobility: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022;11:1901. doi: 10.3390/jcm11071901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ferreira N., Masterson D., de Lima R.L., de Souza Moura B., Oliveira A.T., da Silva Fidalgo T.K., Carvalho A.C., DosSantos M.F., Grossmann E. Efficacy of viscosupplementation with hyaluronic acid in temporomandibular disorders: A systematic review. J. Craniomaxillofac. Surg. 2018;46:1943–1952. doi: 10.1016/j.jcms.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 165.Cen X., Pan X., Zhang B., Liu C., Huang X., Zhao Z. Hyaluronan injection versus oral glucosamine and diclofenac in the treatment of temporomandibular joint osteoarthritis. Clin. Oral Investig. 2022;26:2703–2710. doi: 10.1007/s00784-021-04241-8. [DOI] [PubMed] [Google Scholar]
- 166.Khairy M., Abulmagd I., Sabry D. Evaluation of the therapeutic potentials of adipose Derived stem cells in comparison to hyaluronic acid in temporomandibular joint osteoarthritis A multi assessment study. Egypt. J. Oral Maxillofac. Surg. 2019;10:102–117. doi: 10.21608/omx.2020.25885.1054. [DOI] [Google Scholar]
- 167.De Riu G., Vaira L.A., Carta E., Meloni S.M., Sembronio S., Robiony M. Bone marrow nucleated cell concentrate autograft in temporomandibular joint degenerative disorders: 1-year results of a randomized clinical trial. J. Cranio-Maxillofac. Surg. 2019;47:1728–1738. doi: 10.1016/j.jcms.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 168.Köhnke R., Ahlers M.O., Birkelbach M.A., Ewald F., Krueger M., Fiedler I., Busse B., Heiland M., Vollkommer T., Gosau M., et al. Temporomandibular Joint Osteoarthritis: Regenerative Treatment by a Stem Cell Containing Advanced Therapy Medicinal Product (ATMP)—An In Vivo Animal Trial. Int. J. Mol. Sci. 2021;22:443. doi: 10.3390/ijms22010443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Iturriaga V., Vásquez B., Bornhardt T., Del Sol M. Effects of low and high molecular weight hyaluronic acid on the osteoarthritic temporomandibular joint in rabbit. Clin. Oral Investig. 2021;25:4507–4518. doi: 10.1007/s00784-020-03763-x. [DOI] [PubMed] [Google Scholar]
- 170.Tolba Y.M., Omar S.S., Nagui D.A., Nawwar M.A. Effect of high molecular weight hyaluronic acid in treatment of osteoarthritic temporomandibular joints of rats. Arch. Oral Biol. 2020;110:104618. doi: 10.1016/j.archoralbio.2019.104618. [DOI] [PubMed] [Google Scholar]
- 171.Ragni E., Perucca Orfei C., Sinigaglia F., de Girolamo L. Joint Tissue Protective and Immune-Modulating miRNA Landscape of Mesenchymal Stromal Cell-Derived Extracellular Vesicles under Different Osteoarthritis-Mimicking Conditions. Pharmaceutics. 2022;14:1400. doi: 10.3390/pharmaceutics14071400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ong W.K., Chakraborty S., Sugii S. Adipose Tissue: Understanding the Heterogeneity of Stem Cells for Regenerative Medicine. Biomolecules. 2021;11:918. doi: 10.3390/biom11070918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ragni E., Colombini A., Viganò M., Libonati F., Perucca Orfei C., Zagra L., de Girolamo L. Cartilage Protective and Immunomodulatory Features of Osteoarthritis Synovial Fluid-Treated Adipose-Derived Mesenchymal Stem Cells Secreted Factors and Extracellular Vesicles-Embedded miRNAs. Cells. 2021;10:1072. doi: 10.3390/cells10051072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yu H., Huang Y., Yang L. Research progress in the use of mesenchymal stem cells and their derived exosomes in the treatment of osteoarthritis. Ageing Res. Rev. 2022;80:101684. doi: 10.1016/j.arr.2022.101684. [DOI] [PubMed] [Google Scholar]
- 175.Gadelkarim M., Hafez A., Awad A.K., Shehata M.A., AbouEl-Enein A., Alsadek M.E., Deeb M.A., Afifi A.M. Safety and Efficacy of Adipose-Derived Mesenchymal Stem Cells for Knee Osteoarthritis: A Systematic Review and Meta-Analysis. Joint Bone Spine. 2022;89:105404. doi: 10.1016/j.jbspin.2022.105404. [DOI] [PubMed] [Google Scholar]
- 176.Song Y., Zhang J., Xu H., Lin Z., Chang H., Liu W., Kong L. Mesenchymal stem cells in knee osteoarthritis treatment: A systematic review and meta-analysis. J. Orthop. Translat. 2020;24:121–130. doi: 10.1016/j.jot.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ragni E., Perucca Orfei C., De Luca P., Colombini A., Viganò M., de Girolamo L. Secreted Factors and EV-miRNAs Orchestrate the Healing Capacity of Adipose Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis. Int. J. Mol. Sci. 2020;21:1582. doi: 10.3390/ijms21051582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhao Y., Xie L. An update on mesenchymal stem cell-centered therapies in temporomandibular joint osteoarthritis. Stem Cells Int. 2021;2021:6619527. doi: 10.1155/2021/6619527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Matheus H.R., Özdemir Ş.D., Guastaldi F.P. Stem cell-based therapies for temporomandibular joint osteoarthritis and regeneration of cartilage/osteochondral defects: A systematic review of preclinical experiments. Osteoarthr. Cartil. 2022;30:1174–1185. doi: 10.1016/j.joca.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 180.Luo P., Jiang C., Ji P., Wang M., Xu J. Exosomes of stem cells from human exfoliated deciduous teeth as an anti-inflammatory agent in temporomandibular joint chondrocytes via miR-100-5p/mTOR. Stem Cell Res. Ther. 2019;10:1–2. doi: 10.1186/s13287-019-1341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Zhang B., Tian X., Qu Z., Liu J., Yang L., Zhang W. Efficacy of extracellular vesicles from mesenchymal stem cells on osteoarthritis in animal models: A systematic review and meta-analysis. Nanomedicine. 2021;16:1297–1310. doi: 10.2217/nnm-2021-0047. [DOI] [PubMed] [Google Scholar]
- 182.Sembronio S., Tel A., Tremolada C., Lazzarotto A., Isola M., Robiony M. Temporomandibular Joint Arthrocentesis and Microfragmented Adipose Tissue Injection for the Treatment of Internal Derangement and Osteoarthritis: A Randomized Clinical Trial. J. Oral Maxillofac. Surg. 2021;79:1447–1456. doi: 10.1016/j.joms.2021.01.038. [DOI] [PubMed] [Google Scholar]
- 183.Chęciński M., Chęcińska K., Turosz N., Kamińska M., Nowak Z., Sikora M., Chlubek D. Autologous Stem Cells Transplants in the Treatment of Temporomandibular Joints Disorders: A Systematic Review and Meta-Analysis of Clinical Trials. Cells. 2022;11:2709. doi: 10.3390/cells11172709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Carboni A., Amodeo G., Perugini M., Arangio P., Orsini R., Scopelliti D. Temporomandibular Disorders Clinical and Anatomical Outcomes After Fat-Derived Stem Cells Injection. J. Craniofac. Surg. 2019;30:793–797. doi: 10.1097/SCS.0000000000004884. [DOI] [PubMed] [Google Scholar]
- 185.Gomez M., Wittig O., Diaz-Solano D., Cardier J.E. Mesenchymal stromal cell transplantation induces regeneration of large and full-thickness cartilage defect of the temporomandibular joint. Cartilage. 2021;13:1814S–1821S. doi: 10.1177/1947603520926711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kim H., Yang G., Park J., Choi J., Kang E., Lee B.K. Therapeutic effect of mesenchymal stem cells derived from human umbilical cord in rabbit temporomandibular joint model of osteoarthritis. Sci. Rep. 2019;9:1–4. doi: 10.1038/s41598-019-50435-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhang S., Teo K.Y., Chuah S.J., Lai R.C., Lim S.K., Toh W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. doi: 10.1016/j.biomaterials.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 188.Ogasawara N., Kano F., Hashimoto N., Mori H., Liu Y., Xia L., Sakamaki T., Hibi H., Iwamoto T., Tanaka E., et al. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental temporomandibular joint osteoarthritis. Osteoarthr. Cartil. 2020;28:831–841. doi: 10.1016/j.joca.2020.03.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.