Abstract
Peanut allergy is a lifelong, increasingly prevalent, and potentially life-threatening disease burdening families and communities. Dietary, particularly polyunsaturated fatty acids (PUFAs), intakes can exert positive effects on immune and inflammatory responses, and the red blood cell (RBC) membrane lipidome contains stabilized metabolic and nutritional information connected with such responses. The fatty-acid-based membrane lipidome profile has been exploratorily evaluated in a small cohort of patients (eight males and one female, age range 4.1–21.7 years old, body mass index BMI < 25) with angioedema and/or anaphylaxis after peanut ingestion. This analysis was performed according to an ISO 17025 certified robotic protocol, isolating mature RBCs, extracting membrane lipids, and transforming them to fatty acid methyl esters for gas chromatography recognition and quantification. Comparison with a group of age- and BMI-matched healthy individuals and with benchmark interval values of a healthy population evidenced significant differences, such as higher levels of ω-6 (arachidonic acid), lower values of ω-3 eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), together with an increased ω-6/ω-3 ratio in allergic patients. A significant inverse correlation was also found between specific immunoglobulin E (IgE) levels and ω-6 di-homo-gamma-linolenic acid (DGLA) and total PUFAs. Results of this preliminary study encourage screenings in larger cohorts, also in view of precision nutrition and nutraceuticals strategies, and stimulate interest to expand basic and applied research for unveiling molecular mechanisms that are still missing and individuating treatments in chronic allergic disorders.
Keywords: peanut allergy, allergic inflammation, red blood cell, membrane lipidome, ω-6 fatty acids, ω-3 fatty acids, polyunsaturated fatty acids
1. Introduction
Peanut allergy (PA) is one of the most common food allergies in the pediatric age, affecting approximately 1–3% of children in Western countries, and the prevalence has been increasing in the last decades worldwide [1,2]. Compared with other food allergies, PA is usually lifelong and associated with higher rates of accidental exposure, severe reactions, and potentially fatal anaphylaxis. This, in turn, has a significant impact on the management of the patient, also from a nutritional point of view. The immune system and impaired immune maturation are involved in tolerance breakdown and the development of immune-mediated diseases such as food allergies [3]. The involvement of lipids is progressively recognized both as basic constituents of cellular structures involved in immune regulation and components of diets [4].
1.1. Fatty Acids as Structural and Functional Constituents of Cell Membranes and Relationship with Diet
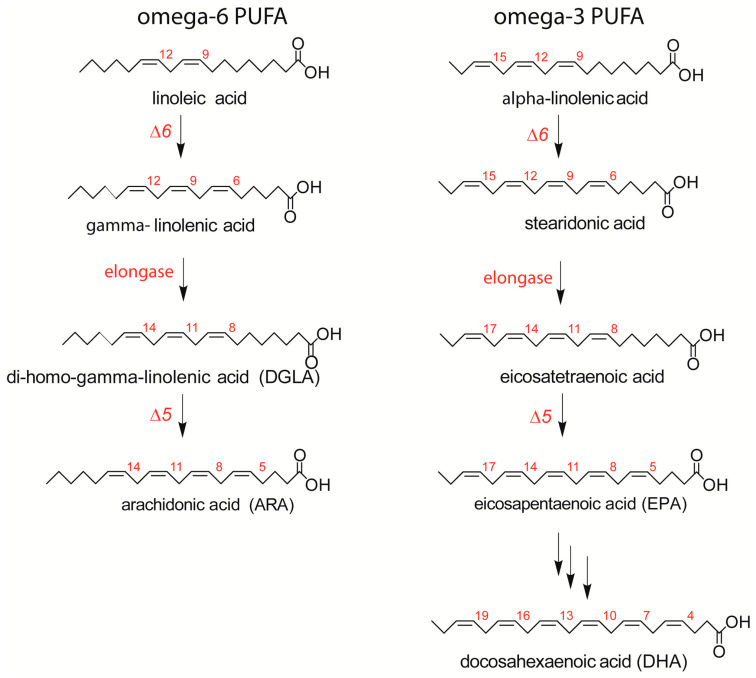
Fatty acids are distinguished by their structures into saturated, monounsaturated, and polyunsaturated families (SFA, MUFA, and PUFA), with an important dietary dependence on PUFAs, which are not directly produced by human metabolism. The omega-6 (ω-6) and omega-3 (ω-3) precursors, linoleic and alpha-linolenic acids, are processed after intake by desaturase and elongase enzymes to form long-chain PUFAs (LC-PUFAs), as shown in Figure 1 [5].
Figure 1.
The omega-6 (ω-6) and omega-3 (ω-3) pathways of main long-chain polyunsaturated fatty acid (LCPUFA) biosynthesis, with the interplay of desaturase and elongase enzymes starting from essential (dietary) fatty acid precursors.
LC-PUFAs incorporated into membrane phospholipids are crucial for their subsequent enzymatic release and, consequently, the formation of bioactive lipid mediators (prostaglandins, leukotrienes, etc.), playing important and well-known biological roles in the development and regulation of the immune system and inflammatory process [6,7]. It is worth recalling that allergic patients start to exclude food(s) from their diets, and this is an initial step for introducing fewer essential fatty acids (EFAs) or breaking the balance between ω-6 and ω-3 intakes, i.e., between pro- and anti-inflammatory components. Indeed, an optimal ω-6/ω-3 balance realizes the ordered sequence of ω-6 LCPUFAs derived mediators, which initiate the “reactivity” response, followed by mediators derived from ω-3 LCPUFAs called “specialized proresolving mediators” (SPMs), which bring resolution and tissue repair [8,9]. Only with the balanced production of these mediators can the overall cell response occur in a physiological manner. Dietary unbalances are recognized to be risk factors for atopy, asthma, and allergy [2,10], and fatty acid supplementations are proposed for the treatment of allergic conditions [11,12]. On the other hand, the association between ω-3 and ω-6 intakes and clinical improvements in food allergy patients [13] are under discussion and, more importantly, are not combined yet with the examination of the patient’s fatty acids status, per se or during supplementation, in particular utilizing erythrocyte membrane lipidome information, which is a reliable mirror of fatty acid intake and metabolism in the body tissues [14].
1.2. Fatty-Acid-Based Membrane Lipidome Analysis
Fatty acid detection can be performed in plasma lipids or blood cell lipids, in particular red blood cells (RBCs), providing different types of information. Diet composition of the days before blood withdrawal strongly affects fatty acid plasma levels, in contrast to RBC membranes, where fatty-acid-based phospholipid composition reflects a balance between nutritional and metabolic factors (i.e., fatty acids transformation into phospholipids). Easiness of the sampling and work-up procedures leads to be more prone to choose plasma or whole blood specimens, but knowledge of the importance of membranes for cell signaling and precision medicine applications strongly indicates RBC membrane isolation and analysis to obtain information on the stabilized metabolic and nutritional status of patients [14]. Indeed, the fatty-acid-based membrane lipidome profile of mature RBCs (mean lifetime of 120 days) was developed and used as a nutritional, homeostatic, and metabolic biomarker in several human physiopathological states [15,16]. Certified laboratory protocol with fatty acid identification and quantitation following ISO 17025 international requirements ensures the reliability and repeatability of the results, as well as the quality of the data. With such a procedure, observational clinical studies were performed identifying membrane profiles in different health conditions [17,18,19,20,21,22]. This information opens the way to personalize the approach of membrane lipid therapy, a natural medicine tool effective in several health conditions [23,24].
2. Results
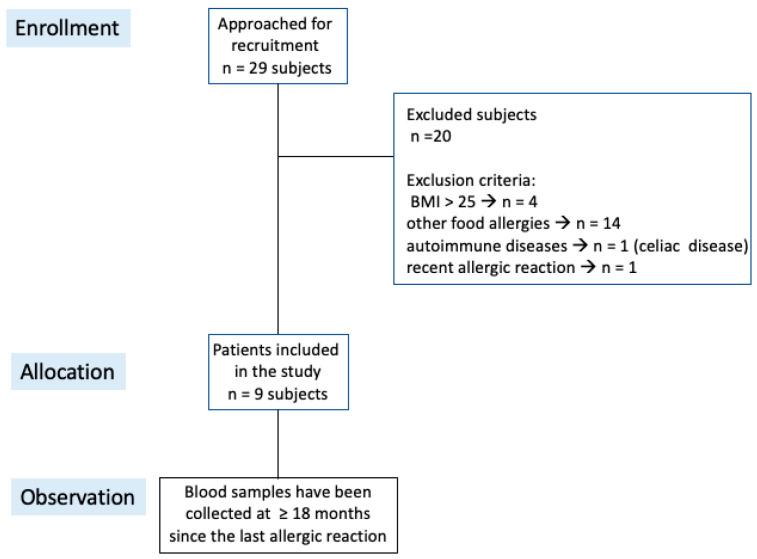
With the aim of exploring the relevance of the membrane lipidome profile in food allergy, a cohort of peanut-allergic patients was selected among subjects followed along years at the Pediatric Immunopathology and Allergology Unit of the university hospital. As shown in the diagram of Figure 2, we recruited n = 29 subjects with a clear history of angioedema and/or anaphylaxis, and exclusion criteria were applied (body mass index (BMI) > 25, other food allergies, autoimmune diseases, and allergic reactions in the last 18 months). The final cohort presented n = 9 patients (age 12 ± 5.6 years old). At the same time, 15 healthy individuals (8 females and 7 males) with BMI < 25 and age = 17 ± 4 years old were selected from the anonymous database available at the Lipidomic Laboratory, for which informed consent for research use was gathered at the moment of blood withdrawal, and respect of EU general data protection regulation 2016/679 (GDPR) guaranteed by the ISO 17025 certification. The controls did not have allergic problems. Mature RBC membrane fatty acid values compared with those of the patients were obtained by the same procedure. The observational study focused on the fatty acid composition of RBC membranes addressing the following key points: (a) the status of ω-6 vs. ω-3 fatty acid residues of the membrane phospholipids as the expression of proinflammatory tendency in allergic patients, and (b) the correlation of membrane lipidome profile features with specific immunoglobulin E (IgE) levels.
Figure 2.
Consort diagram of the patients in this study.
Clinical and laboratory data from peanut-allergic patients are described in Table 1. All patients had a history of angioedema and/or anaphylaxis after ingestion of peanuts; however, the recruited patients did not have any allergic reactions in the last 18 months.
Table 1.
Peanuts allergic patient characteristics.
| Sex | Age (Years) |
BMI (Centil) |
BMI (Kg/mq) |
Other Food Allergies |
Inhalant Allergies |
Reaction on Exposure to Peanuts |
Allergic Comorbidity * |
Totals IgE (UI/mL) | Peanut-Specific IgE (UI/mL) | Molecular Diagnosis (kU/L) |
|---|---|---|---|---|---|---|---|---|---|---|
| M | 4.6 | 80 | 18 | - | + | ANAPHYLAXIS | R | 965 | >100 | 100 (Arah2) |
| M | 11.7 | 80 | 22.7 | - | + | ANGIOEDEMA | A-R-AD | 965 | 0.50 | NA |
| M | 10.1 | 75 | 20.5 | - | - | ANGIOEDEMA | R-AD | 39.5 | 32.5 | NA |
| M | 21.7 | 96 | 28.7 | Walnut | + | ANAPHYLAXIS | R-C | 152 | 1 | 10.3 (Arah9) |
| M | 13.1 | 40 | 19.7 | - | + | ANAPHYLAXIS | A-R | 340 | 0.54 | 0.27 (Arah2) |
| M | 17.6 | 77 | 24.5 | Walnut | + | ANAPHYLAXIS | A-R-C | 587 | 5.29 | 6.97 (Arah9) |
| F | 12.7 | 80 | 23.9 | - | + | ANAPHYLAXIS | R-AD | 276 | >100 | NA |
| M | 12.4 | 10 | 16.5 | Hazelnut | - | ANGIOEDEMA | none | 34.5 | 2.96 | NA |
| M | 4.1 | 75 | 17.2 | Hazelnut | + | ANGIOEDEMA + INTESTINAL symptoms | none | 1833 | 48.8 | NA |
* A, asthma; R, rhinitis; C, conjunctivitis; AD, atopic dermatitis; NA, not available.
In Table 2, the results of the mature RBC fatty-acid-based membrane lipidome analysis are shown; as in previous work, the main fatty acids of the RBC membrane phospholipids are grouped to give a cohort of 10 SFA, MUFA, and PUFA components, the latter ones representing the ω-6 and ω-3 metabolic cascades shown in Figure 1 [15]. Each unsaturated fatty acid of the cohort is recognized by appropriate standards, ensuring that it is not superimposed to geometrical or positional isomers. Calibration with standard references allows to calculate the quantity of each fatty acid in the sample and express it as a relative quantitative amount (% rel. quant.) over the total of the 10 fatty acid quantities. From these values, the ω-6/ω-3 ratio, related to pro- and anti-inflammatory balance [25], and peroxidation index (PI), as a measure of the PUFA contribution to elevating membrane peroxidizability [26,27], were calculated. Each of the 10 fatty acids and related indexes were compared with the 15 age- and BMI-matched healthy controls and with the benchmark of interval values referred to a healthy population described in previous works [15,16,17]. In Table 2, significant changes observed between patients and controls are reported with their p-values.
Table 2.
Fatty acid profile of mature red blood cell membranes of peanut-allergic patients (n = 9) in comparison with the reference interval values of healthy population reported in refs. [15,16,17] and with age- and BMI-matched healthy controls (n = 15).
| Fatty Acids † | Reference
Interval Values |
Healthy Controls
(n = 15) ‡ (% rel. quant.) |
Patients (n = 9) ‡ (% rel. quant.) |
|---|---|---|---|
| 16:0 | 17–27 | 22.78 ± 1.02 | 22.03 ± 1.26 |
| 9-cis 16:1 | 0.2–05 | 0.26 ± 0.11 | 0.20 ± 0.06 |
| 18:0 | 13–20 | 18.04 ± 1.36 | 16.57 ± 1.07 * |
| 9-trans 18:1 | 0.1–0.3 | 0.11 ± 0.03 | 0.20 ± 0.03 *** |
| 9-cis 18:1 | 9–18 | 18.70 ± 1.39 | 18.83 ± 2.26 |
| 11-cis 18:1 | 0.7–1.3 | 1.03 ± 0.15 | 1.04 ± 0.18 |
| 18:2 ω-6 | 9–16 | 13.45 ± 1.14 | 12.96 ± 1.20 |
| 20:3 ω-6 (DGLA) | 1.9–2.4 | 2.06 ± 0.44 | 1.95 ± 0.35 |
| 20:4 ω-6 (ARA) | 13–17 | 16.19 ± 0.64 | 21.68 ± 1.9 *** |
| mono-trans ARA | 0.1–0.4 | 0.07 ± 0.05 | 0.14 ± 0.06 ** |
| 20:5 ω-3 (EPA) | 0.5–0.9 | 0.78 ± 0.12 | 0.47 ± 0.21 *** |
| 22:6 ω-3 (DHA) | 5–7 | 6.54 ± 1.03 | 3.92 ± 1.27 *** |
| SFA | 30–45 | 40.82 ± 1.34 | 38.60 ± 0.65 *** |
| MUFA | 13–23 | 19.99 ± 1.46 | 20.07 ± 2.35 |
| PUFA ω-6 | 24–34 | 31.69 ± 1.65 | 36.59 ± 1.93 *** |
| PUFA ω-3 | 5.7–9 | 7.32 ± 1.12 | 4.39 ± 1.43 *** |
| PUFA TOT | 28–39 | 39.01 ± 1.64 | 40.98 ± 2.30 * |
| SFA/MUFA | 1.7–2 | 2.05 ± 0.18 | 1.94 ± 0.21 |
| ω-6/ω-3 | 3.5–5.5 | 4.44 ± 0.84 | 9.31 ± 3.47 *** |
| Tot Trans | ≤0.4 | 0.18 ± 0.05 | 0.35 ± 0.07 *** |
| PI | 138–151 | 139.81 ± 8.15 | 129.95 ± 12.04 * |
† Fatty acids are reported from the gas chromatographic analysis (GC) after transformation of membrane phospholipids of mature RBCs into fatty acid methyl esters (FAMEs); ‡ values are expressed as relative percentages (mean ± SD) of the quantitative values of each fatty acid obtained by calibration curves of the standards and referred to the representative 10 fatty acids cohort. Statistical analyses comparing patients with healthy controls gave the following p-values: * ≤0.026; ** ≤0.0022; *** ≤0.0001.
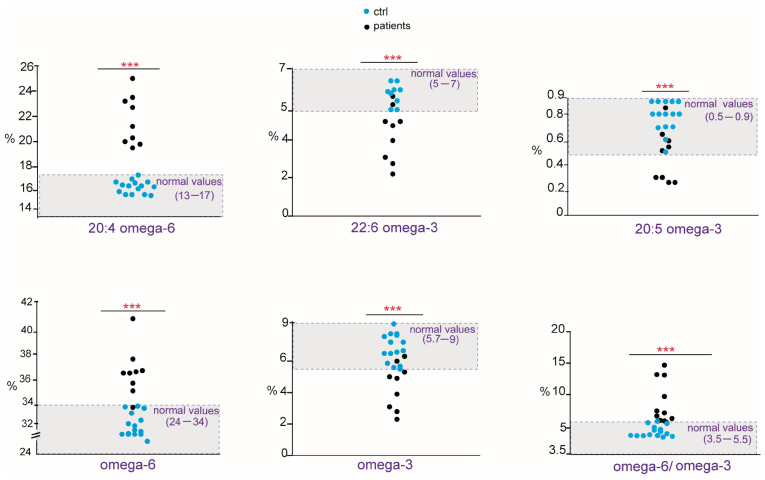
As shown in Table 2, in allergic patients, we found a marked unbalance mainly concerning the PUFA residues of cell membrane phospholipids. Compared with the cohort of healthy controls, the patients showed a significant increase in ω-6 20:4 (arachidonic acid, ARA), PUFA ω-6 and the ratio ω-6/ω-3, whereas ω-3 20:5 (eicosapentaenoic acid, EPA), 22:6 (docosahexaenoic acid, DHA), total PUFA ω-3, and the PI value were significantly diminished. In addition, total saturated fatty acids (SFAs) and stearic acid (18:0) were significantly lower than controls. The distribution of PUFA values and ratio as scattered dot plots showing each patient is presented in Figure 3 in comparison with those found in healthy controls, reporting also the benchmark of interval values in the healthy population. Although the cohort of patients is small, in Figure 3, it is possible to appreciate that their membrane fatty acid values are all positioned outside (lower or higher) both healthy controls and benchmark interval values. It is worth underlining that levels of trans fatty acids isomers of oleic and arachidonic acids were also evaluated since these compounds are available as standard references [17,18] and found to be significantly increased, although within the values reported for the benchmark (≤0.4).
Figure 3.
Scattered dot plots of fatty acids, families, and indexes significantly changed in peanut-allergic patients (black dots) compared with healthy controls (blue dots). Data are reported in Table 2. The benchmark of interval values in healthy population is highlighted in light gray in the graphs. p-values, *** ≤ 0.0001.
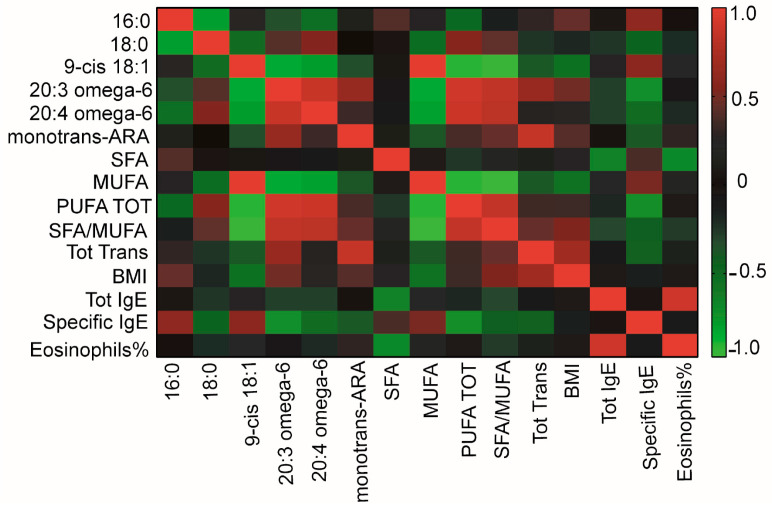
Patients’ clinical and laboratory data (Table 1) were then correlated to membrane FA values (Table 2), resulting in the heatmap shown in Figure 4.
Figure 4.
Heatmap showing correlations between patients’ clinical and laboratory data (Table 1) and fatty acid values (Table 2).
As detailed in Table 3, significant inverse correlations were found for peanut-specific IgE with ω-6 20:3 di-homo-gamma-linolenic acid DGLA and total PUFA (p-value = 0.033 and p-value = 0.038, respectively).
Table 3.
Correlation (expressed by the Spearman correlation coefficient, r) of the fatty acid values of Table 2 with the peanut-specific IgE shown in Table 1. See also heatmap in Figure 4.
| Correlation | r (+/−) | p-Value |
|---|---|---|
| 16:0—specific IgE | 0.6 | 0.086 |
| 18:0—specific IgE | −0.469 | 0.205 |
| 9-cis 18:1—specific IgE | 0.5 | 0.1 |
| 20:3 ω-6—specific IgE | −0.724 | 0.033 * |
| 20:4 ω-6—specific IgE | −0.517 | 0.162 |
| Monotrans-ARA—specific IgE | −0.3 | 0.391 |
| SFA—specific IgE | 0.377 | 0.30 |
| MUFA—specific IgE | 0.519 | 0.155 |
| Total PUFA—specific IgE | −0.711 | 0.038 * |
| Tot trans—specific IgE | −0.444 | 0.235 |
* Statistically significant correlations.
3. Discussion
This is the first study to explore the relevance of the membrane lipidome profile in patients with peanut allergy. We observed a clear unbalance between ω-6 and ω-3 fatty acids in this cohort of patients, mainly due to an increase in ω-6 20:4 (ARA) and a decrease in ω-3 (EPA and DHA). Considering the role of ARA as a precursor of proinflammatory eicosanoids [9], but also as a result of the metabolism of omega-6 linoleic acid taken from the diet (see Figure 1), our data support the synergy of metabolism and diet to build up a specific profile of the cell membranes of peanut-allergic patients [28]. The role of omega-3, both as essential components of the diet and as modulators of the immune and anti-inflammatory processes, is well known [28], and in our cohort, a significant reduction in ω-3 fatty acids involves EPA and DHA. Examining in detail the patients’ values in Figure 3, in nine patients, the DHA values resulted to be in the lower ranges compared with both healthy controls and the benchmark of the healthy population. As shown in Figure 1, EPA and DHA are the members of the ω-3 cascade containing the highest number of double bonds among all PUFAs (see Figure 1). Besides their transformation into bioactive lipid mediators with immune-stimulating and anti-inflammatory activities [12], DHA exerts a greater influence on membrane biophysical properties such as flexibility, fluidity, and thickness [29,30]. It is also clear that the quantity and quality of fat intake directly from seafood (fish, algae) can influence the levels of these fatty acids in cells; however, all patients apparently had regular fish consumption in their nut-free diet, not different from the healthy controls. Another cause of EPA and DHA diminutions can be the efficiency of the metabolic transformations in these patients, as shown in Figure 1. Indeed, humans have a limited capability to synthesize LC PUFA from the precursor alpha-linolenic acid [31], so that daily intakes of 250 mg EPA and DHA are indicated by the most relevant food safety agencies, such as EFSA (European Food Safety Authority) [32]. These preliminary data on ω-3 deficiency must be deepened in a study with larger cohorts.
We also consider it relevant that the ω-6 components came into the scenario of allergic patients, in particular with an increase in arachidonic acid, with known roles in the propagation of inflammatory responses and cellular reactivity. Moreover, ω-6 DGLA, which is a precursor of arachidonic acid and prostaglandins (PG series 1) [33], showed an inverse correlation with specific IgE levels. Literature data connect DGLA supplementation with an increase in PGD1 and improvement in atopic dermatitis, although in an animal model [34]. Moreover, prostaglandin E1 (PGE1) originates from DGLA, and a synthetic analog misoprostol has been reported to modulate histamine release from basophils [35]. It must be underlined that clinical trials on gamma-linolenic acid (GLA) supplementation as a precursor of DGLA (see Figure 1) gave very heterogeneous results, but no data are yet reported on the follow-up of the GLA-DGLA metabolic transformation and incorporation of DGLA in membrane phospholipids. Evidently, the possibility of monitoring DGLA levels in the RBC membrane offers the best strategy to follow up subjects during treatments. Moreover, total PUFA levels were negatively correlated with specific IgE, and both correlations underline the importance of deepening the PUFA status in patients in view of its importance for cell signaling. On the other hand, despite multiple evidence for PUFA relevance in different health conditions [6,7,9,13,33], the punctual follow-up of patients is still missing in clinical approaches.
The exclusion of subjects with a BMI > 25 from our cohort, as well as from the healthy controls, takes into account that overweight and obesity are known to increase arachidonic acid levels in RBC membranes [22,36], and we wanted to exclude interferences from known proinflammatory conditions. In addition, the distance of 18 months from the last allergic episode in patients and the exclusion of allergic conditions in the healthy cohort were carefully checked to eliminate interferences from immune reactivity. It is interesting to observe that in obesity, the membrane fatty acid asset had some common features with that of allergic patients (such as low levels of DHA and increased levels of ARA and omega-6/omega-3 ratio) [22,36], whereas an increase in the SFA/MUFA ratio was not found, which is known to influence membrane properties through an increased rigidity of RBC membranes [37].
The overall oxidative reactivity estimated with the peroxidizability of RBC membranes (peroxidation index, PI; cfr., Table 2) in allergic patients was lower than in controls. However, it must be underlined that we did not perform direct measurements of peroxidation processes through a measure of oxidation metabolites, and this is a limitation of the present study. In our analysis, we also measured trans fatty acid (TFA) isomers of oleic and arachidonic acids, which are known markers of endogenous cis-trans isomerization of the corresponding cis MUFA and PUFA caused by increased free radical production [38,39]. Previously, we reported TFA to increase in the blood cell membranes of children affected by atopic eczema/dermatitis syndrome [40]. In our allergic cohort, we found TFA significantly increased compared with healthy controls (0.35 ± 0.07 vs. 0.18 ± 0.05, p-value ≤ 0.0001) [15]; however, the threshold value of the benchmark (0.4%) was not overcome.
The detection of fatty acids and isomers is one of the various aspects of the analytical protocol that starts from the choice of mature RBC membranes for sampling, as detailed in the experimental part and discussed in previous research papers and reviews [16,20,21,22]. Precision medicine must rely on analytical data not only obtained by protocols unified among laboratories but also certified for the results and reliability by a competent auditing process, usually provided by national bodies of accreditation through compliance with the ISO 17025 regulation. The use of gas chromatographic methodology, with cis and trans fatty acid references and calibration procedures, allows to examine fatty acid levels with the highest molecular identification and sensitive quantification, and this is needed when membrane lipidome profiles are developed to identify disease onset [14,16,18,20]. Moreover, the inclusion of a robotic platform in the certified protocol reduces errors due to manual operations. Our small cohort certainly took advantage of such precise measures to evidence significant results, but we are aware that the sample size must be increased to achieve greater statistical power. Here, we preliminarily showed results of membrane-based diagnostics in peanut allergy patients, supporting the importance of this tool that has reached maturity and technological advancement to serve larger screenings.
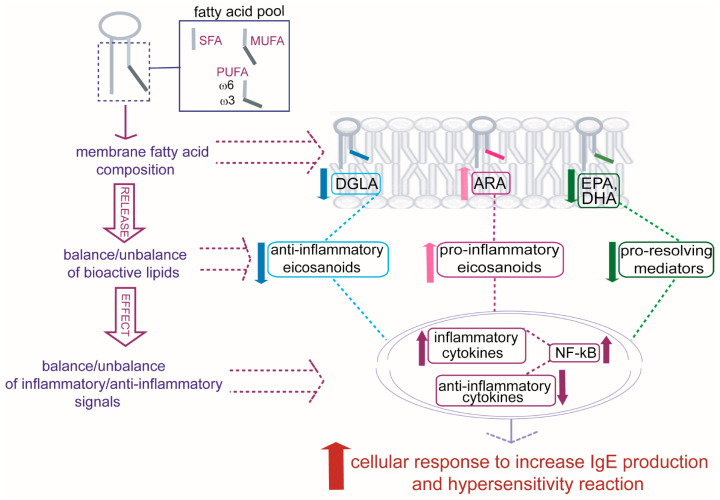
Further studies will be able to address an integrated vision in allergic patients in which specific alterations found in RBC membrane lipidome profiles mirror crucial changes in molecular components and related signaling to develop allergic reactivity [41]. Some of these changes are indicated in Figure 5, starting from the formation of the fatty acid pool, influenced by nutritional and metabolic contributions specific to each individual, and the consequences on the composition of membrane phospholipids. Once membranes are formed, they present different qualities and quantities of PUFAs that, in turn, can bring a balance/unbalance of bioactive lipids created in cells after the release of PUFA residues from phospholipids. An increase in ARA and a diminution in DGLA, EPA, and DHA in allergic patients can contribute to an unbalance of signaling with an influence on inflammatory and anti-inflammatory cytokine productions, as well as on a diminution in the protection from antigen-induced activation given by ω-3 proresolving mediators (epoxins, resolvins, protectins) [41], resulting in a general cellular response toward hypersensitivity and augmented IgE production [42].
Figure 5.
The individual fatty acid pool, derived from diet and metabolism, influences the formation of phospholipids, and an unbalance occurs in allergic patients among dihomo-gamma-linolenic acid (DGLA), arachidonic acid (ARA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA) residues present in cell membranes. The fatty acid composition has impact on formation of bioactive lipids and balance/unbalance of transcription factors (NF-kB, among others) for the production of inflammatory/anti-inflammatory cytokines influencing the cellular IgE production and the final hypersensitivity reaction.
Alteration of the PUFA composition in membranes is basic information to acquire and translate into precision medicine and nutrition strategies. In fact, the type and dosage of fatty acids able to rebalance the molecular status of patients can be personalized for each distinct profile and regularly monitored. This is particularly important in children, in which PUFAs are used to sustain the exponential growth of cells for all tissues, and their exact levels must be determined [43] in order to promptly individuate deficiency or excess with impact on normal tissue functioning [44,45]. Our exploratory study highlights cell membranes for effecting PUFA detection exactly in the active site of their immunomodulatory effects, evidencing molecular mechanisms that are still missing in the evaluation of PUFA treatments in chronic allergic disorders.
4. Materials and Methods
4.1. Study Subjects
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and obtained ethical clearance from the ethics committee at Policlinico Tor Vergata, University of Rome Tor Vergata (n.82.21). Informed consent was obtained from each patient or patient’s legal guardians and each of the healthy controls. As reported in Figure 2, a total of 29 Italian native patients with peanut allergy (18 M and 11 F, age range 4.1–21.7 years) were identified at the Pediatric Immunopathology and Allergology Unit, Policlinico Tor Vergata, University of Rome Tor Vergata. Peanut allergy was defined by the following criteria: (a) history of significant peanut-related clinical symptoms, (b) positive skin prick test to peanut allergen (wheal ≥ 3 mm larger than the saline control), and (c) positive in vitro serum peanut IgE (CAP-FEIA) > 0.1 Ku/L. Exclusion criteria were as follows: (a) BMI > 25 Kg/mq, (b) other concomitant food allergies, and (c) autoimmune diseases. A final number of 9 peanut-allergic patients (8 males), age range 4.1–21.7 years, were included in the study. These criteria allowed appropriateness of patient enrollment in the study. Further, blood samples for the lipidome profile of red blood cell membranes were obtained ≥18 months since last allergic reaction to standardize time of testing and reduce the risk of interfering factors. Component-resolved diagnostics (CRDs) for Arah2 and Arah9 were available in 4 patients. As healthy controls, 15 healthy individuals (8 females and 7 males) with BMI < 25 and age 17 ± 4 years old were selected from an anonymous database available in the Lipidomic Laboratory, for which informed consent was gathered at the moment of blood withdrawal, and respect of EU general data protection regulation 2016/679 (GDPR) was ensured by the ISO 17025 certification. All controls did not have any history of allergic reactivity. The benchmark of reference interval values for mature RBC membranes served for observing controls and patients using the data reported for populations [15,16,17].
4.2. Isolation of Fatty Acids from RBC Membrane Phospholipids and Gas Chromatographic Analysis
Fatty-acid-based membrane lipidome analyses were performed by the Lipidomic Laboratory of Lipinutragen (Bologna, Italy). Blood samples (0.5 mL) collected in vacutainer tubes with ethylenediaminetetraacetic acid (EDTA) were treated according to the ISO17025 certified procedure (accredited Lab. #1836L) by robotic equipment and processed as described in previous studies [21,22,36,46]. Briefly, the mature cell fraction was isolated based on the higher density of the aged cells with control of diameter controlled by cell counter (Scepter 2.0 with Scepter Software Pro, EMD Millipore, Darmstadt, Germany) [47]. After phospholipid extraction, derivatization to fatty acid methyl esters (FAME) was performed, transforming membrane glycerophospholipids (mainly phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, phosphatidyl inositol, and plasmalogens) to examine up to 80% of the RBC membrane lipidome [48]. Fatty acids analysis was performed by gas chromatography (GC), and percentages are given as % relative quantitative (% rel. quant.), as previously described [21,22,36,46], comparing with the benchmark of the interval values of each fatty acid and index [15].
4.3. Statistical Analysis
Statistics were performed using GraphPad Prism 8.0 software (GraphPad Software, Inc., San Diego, CA, USA), applying unpaired t-test and Spearman correlation with a 95% confidence interval.
Acknowledgments
The study was carried out in the frame of the activities of M.S. for the PhD program in Immunology, Molecular Medicine, and Applied Biotechnology, University of Rome Tor Vergata, Rome, Italy.
Author Contributions
Conceptualization, C.F.; methodology, C.F., A.S., V.M. and L.C.; software, A.S., E.D.D. and F.D.N.; validation, A.S., E.D.D., C.F. and V.M.; formal analysis, F.D.N., E.D.D. and M.S.; investigation, A.S., F.D.N., E.D.D. and M.S.; resources, C.F., L.C. and V.M.; data curation, A.S. and E.D.D.; writing—original draft preparation, C.F., L.C. and V.M.; writing—review and editing, C.F., V.M. and L.C.; supervision, C.F., L.C. and V.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Policlinico Tor Vergata, University of Rome Tor Vergata (protocol code n.82.21; date of approval, 21 April 2021).
Informed Consent Statement
Informed consent was obtained from all subjects or from their parents in case of <18-year-old patients involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
C.F. is a cofounder of Lipinutragen srl, a spin-off of the CNR dedicated to membrane lipidomic analysis in human health. F.D.N. is an employee of Lipinutragen srl. This company did not have any role in the design and conduction of the present study.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lieberman J.A., Gupta R.S., Knibb R.C., Haselkorn T., Tilles S., Mack D.P., Pouessel G. The global burden of illness of peanut allergy: A comprehensive literature review. Allergy. 2021;76:1367–1384. doi: 10.1111/all.14666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xing Y.H., Wong G.W.K. Environmental Influences and Allergic Diseases in the Asia-Pacific Region: What Will Happen in Next 30 Years? Allergy Asthma Immunol. Res. 2022;14:21–39. doi: 10.4168/aair.2022.14.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Satitsuksanoa P., Jansen K., Globinska A., van de Veen W., Akdis M. Regulatory Immune Mechanisms in Tolerance to Food Allergy. Front. Immunol. 2018;9:2939. doi: 10.3389/fimmu.2018.02939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yaqoob P. Fatty acids as gatekeepers of immune cell regulation. Trends Immunol. 2003;24:639–645. doi: 10.1016/j.it.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Brown H.A., Marnett L.J. Introduction to Lipid Biochemistry, Metabolism, and Signaling. Chem. Rev. 2011;111:5817–5820. doi: 10.1021/cr200363s. [DOI] [PubMed] [Google Scholar]
- 6.Miles E.A., Childs C.E., Calder P.C. Long-Chain Polyunsaturated Fatty Acids (LCPUFAs) and the Developing Immune System: A Narrative Review. Nutrients. 2021;13:247. doi: 10.3390/nu13010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar N.G., Contaifer D., Madurantakam P., Carbone S., Price E.T., Van Tassell B., Brophy D.F., Wijesinghe D.S. Dietary Bioactive Fatty Acids as Modulators of Immune Function: Implications on Human Health. Nutrients. 2019;11:2974. doi: 10.3390/nu11122974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Waehler R. Fatty acids: Facts vs. fiction. Int. J. Vitam. Nutr. Res. 2021;27:1–21. doi: 10.1024/0300-9831/a000713. [DOI] [PubMed] [Google Scholar]
- 9.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devereux G., Seaton A. Diet as a risk factor for atopy and asthma. J. Allergy Clin. Immunol. 2005;115:1109–1117. doi: 10.1016/j.jaci.2004.12.1139. [DOI] [PubMed] [Google Scholar]
- 11.Hoppenbrouwers T., Hogervorst J.H.C., Garssen J., Wichers H.J., Willemsen L.E.M. Long Chain Polyunsaturated Fatty Acids (LCPUFAs) in the Prevention of Food Allergy. Front. Immunol. 2019;10:1118. doi: 10.3389/fimmu.2019.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sartorio M.U.A., Pendezza E., Coppola S., Paparo L., D’Auria E., Zuccotti G.V., Canani R.B. Potential Role of Omega-3 Polyunsaturated Fatty Acids in Pediatric Food Allergy. Nutrients. 2022;14:152. doi: 10.3390/nu14010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brigham E.P., Woo H., McCormack M., Rice J., Koehler K., Vulcain T., Wu T.S., Koch A., Sharma S., Kolandooz F., et al. Omega-3 and Omega-6 Intake Modifies Asthma Severity and Response to Indoor Air Pollution in Children. Am. J. Respir. Crit. Care Med. 2019;199:1478–1486. doi: 10.1164/rccm.201808-1474OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenna J.T., Plourde M., Stark K.D., Jones P.J., Lin Y.H. Best practices for the design, laboratory analysis, and reporting of trials involving fatty acids. Am. J. Clin. Nutr. 2018;108:211–227. doi: 10.1093/ajcn/nqy089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferreri C., Chatgilialoglu C. Role of fatty acid-based functional lipidomics in the development of molecular diagnostic tools. Expert Rev. Mol. Diagn. 2012;12:767–780. doi: 10.1586/erm.12.73. [DOI] [PubMed] [Google Scholar]
- 16.Ferreri C., Masi A., Sansone A., Giacometti G., Larocca A.V., Menounou G., Scanferlato R., Tortorella S., Rota D., Conti M., et al. Fatty Acids in Membranes as Homeostatic, Metabolic and Nutritional Biomarkers: Recent Advancements in Analytics and Diagnostics. Diagnostics. 2017;7:1. doi: 10.3390/diagnostics7010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreri C., Chatgilialoglu C. Membrane Lipidomics for Personalized Health. John Wiley & Sons Inc.; Hoboken, NJ, USA: 2015. pp. 1–184. [Google Scholar]
- 18.Sansone A., Tolika E., Louka M., Sunda V., Deplano S., Melchiorre M., Anagnostopoulos D., Chatgilialoglu C., Formisano C., Di Micco R., et al. Hexadecenoic Fatty Acid Isomers in Human Blood Lipids and Their Relevance for the Interpretation of Lipidomic Profiles. PLoS ONE. 2016;11:e0152378. doi: 10.1371/journal.pone.0152378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giacometti G., Ferreri C., Sansone A., Chatgilialoglu C., Marzetti C., Spyratou E., Georgakilas A.G., Marini M., Abruzzo P.M., Bolotta A., et al. High predictive values of RBC membrane-based diagnostics by biophotonics in an integrated approach for Autism Spectrum Disorders. Sci. Rep. 2017;7:9854. doi: 10.1038/s41598-017-10361-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amezaga J., Arranz S., Urruticoechea A., Ugartemendia G., Larraioz A., Louka M., Uriarte M., Ferreri C., Tueros I. Altered Red Blood Cell Membrane Fatty Acid Profile in Cancer Patients. Nutrients. 2018;10:1853. doi: 10.3390/nu10121853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amezaga J., Ugartemendia G., Larraioz A., Bretana N., Iruretagoyena A., Camba J., Urruticoechea A., Ferreri C., Tueros I. Altered Levels of Desaturation and omega-6 Fatty Acids in Breast Cancer Patients’ Red Blood Cell Membranes. Metabolites. 2020;10:469. doi: 10.3390/metabo10110469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jauregibeitia I., Portune K., Rica I., Tueros I., Velasco O., Grau G., Castano L., Di Nolfo F., Ferreri C., Arranz S. Potential of Erythrocyte Membrane Lipid Profile as a Novel Inflammatory Biomarker to Distinguish Metabolically Healthy Obesity in Children. J. Pers. Med. 2021;11:337. doi: 10.3390/jpm11050337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicolson G.L., Ash M.E. Lipid Replacement Therapy: A natural medicine approach to replacing damaged lipids in cellular membranes and organelles and restoring function. Biochim. Biophys. Acta-Biomembr. 2014;1838:1657–1679. doi: 10.1016/j.bbamem.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Torres M., Parets S., Fernandez-Diaz J., Beteta-Gobel R., Rodriguez-Lorca R., Roman R., Llado V., Rossello C.A., Fernandez-Garcia P., Escriba P.V. Lipids in Pathophysiology and Development of the Membrane Lipid Therapy: New Bioactive Lipids. Membranes. 2021;11:919. doi: 10.3390/membranes11120919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris W.S., Tintle N.L., Imamura F., Qian F., Korat A.V.A., Marklund M., Djousse L., Bassett J.K., Carmichael P.H., Chen Y.Y., et al. Blood n-3 fatty acid levels and total and cause-specific mortality from 17 prospective studies. Nat. Commun. 2021;12:2329. doi: 10.1038/s41467-021-22370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jove M., Mota-Martorell N., Pradas I., Galo-Licona J.D., Martin-Gari M., Obis E., Sol J., Pamplona R. The Lipidome Fingerprint of Longevity. Molecules. 2020;25:4343. doi: 10.3390/molecules25184343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puca A.A., Andrew P., Novelli V., Anselmi C.V., Somalvico F., Cirillo N.A., Chatgilialoglu C., Ferreri C. Fatty acid profile of erythrocyte membranes as possible biomarker of longevity. Rejuvenation Res. 2008;11:63–72. doi: 10.1089/rej.2007.0566. [DOI] [PubMed] [Google Scholar]
- 28.Miles E.A., Calder P.C. Can early omega-3 fatty acid exposure reduce risk of childhood allergic disease? Nutrients. 2017;9:784. doi: 10.3390/nu9070784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calder P.C. Docosahexaenoic Acid. Ann. Nutr. Met. 2016;69:8–21. doi: 10.1159/000448262. [DOI] [PubMed] [Google Scholar]
- 30.Hishikawa D., Valentine W.J., Iizuka-Hishikawa Y., Shindou H., Shimizu T. Metabolism and functions of docosahexaenoic acid-containing membrane glycerophospholipids. FEBS Lett. 2017;591:2730–2744. doi: 10.1002/1873-3468.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ghasemi Fard S., Wang F.L., Sinclair A.J., Elliott G., Turchini G.M. How does high DHA fish oil affect health? A systematic review of evidence. Crit. Rev. Food Sci. Nutr. 2019;59:1684–1727. doi: 10.1080/10408398.2018.1425978. [DOI] [PubMed] [Google Scholar]
- 32.EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA), Scientific Opinion on principles for deriving and applying Dietary Reference Values. EFSA J. 2010;8:1458. [Google Scholar]
- 33.Sergeant S., Rahbar E., Chilton F.H. Gamma-linolenic acid, Dihomo-gamma linolenic, Eicosanoids and Inflammatory Processes. Eur. J. Pharmacol. 2016;785:77–86. doi: 10.1016/j.ejphar.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amagai Y., Oida K., Matsuda A., Jung K., Kakutani S., Tanaka T., Matsuda K., Jang H., Ahn G., Xia Y., et al. Dihomo-gamma-linolenic acid prevents the development of atopic dermatitis through prostaglandin D-1 production in NC/Tnd mice. J. Dermatol. Sci. 2015;79:30–37. doi: 10.1016/j.jdermsci.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Babakhin A.A., Nolte H., DuBuske L.M. Effect of misoprostol on the secretion of histamine from basophils of whole blood. Ann. Allergy Asthma Immunol. 2000;84:361–365. doi: 10.1016/S1081-1206(10)62787-1. [DOI] [PubMed] [Google Scholar]
- 36.Jauregibeitia I., Portune K., Rica I., Tueros I., Velasco O., Grau G., Trebolazabala N., Castano L., Larocca A.V., Ferreri C., et al. Fatty Acid Profile of Mature Red Blood Cell Membranes and Dietary Intake as a New Approach to Characterize Children with Overweight and Obesity. Nutrients. 2020;12:3446. doi: 10.3390/nu12113446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sot J., García-Arribas A.B., Abad B., Arranz S., Portune K., Andrade F., Martín-Nieto A., Velasco O., Arana E., Tueros I., et al. Erythrocyte Membrane Nanomechanical Rigidity Is Decreased in Obese Patients. Int. J. Mol. Sci. 2022;23:1920. doi: 10.3390/ijms23031920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung W.-L., Hwang L.S., Shahidi F., Pan M.-H., Wang Y., Ho C.-T. Endogenous formation of trans fatty acids: Health implications and potential dietary intervention. J. Funct. Foods. 2016;25:14–24. doi: 10.1016/j.jff.2016.05.006. [DOI] [Google Scholar]
- 39.Chatgilialoglu C., Ferreri C., Melchiorre M., Sansone A., Torreggiani A. Lipid geometrical isomerism: From chemistry to biology and diagnostics. Chem. Rev. 2014;114:255–284. doi: 10.1021/cr4002287. [DOI] [PubMed] [Google Scholar]
- 40.Ferreri C., Angelini F., Chatgilialoglu C., Dellonte S., Moschese V., Rossi P., Chini L. Trans fatty acids and atopic eczema/dermatitis syndrome: The relationship with a free radical cis-trans isomerization of membrane lipids. Lipids. 2005;4:661–667. doi: 10.1007/s11745-005-1428-7. [DOI] [PubMed] [Google Scholar]
- 41.Hogenkamp A., Ehlers A., Garssen J., Willemsen L.E.M. Allergy modulation by N-3 long chain polyunsaturated fatty acids and fat soluble nutrients and the Mediterranean diet. Front. Pharmacol. 2020;11:1244;. doi: 10.3389/fphar.2020.01244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Djuricic I., Calder P.C. Beneficial outcomes of omega-6 and omega-3 polyunsaturated fatty acids in human health: An update for 2021. Nutrients. 2021;13:2421. doi: 10.3390/nu13072421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stratakis N., Gielen M., Margetaki K., de Groot R.H.M., Apostolaki M., Chalkiadaki G., Vafeiadi M., Leventakou V., Godschalk R.W., Kogevinas M., et al. PUFA status at birth and allergy-related phenotypes in childhood: A pooled analysis of the Maastricht Essential Fatty Acid Birth (MEFAB) and RHEA birth cohorts. Br. J. Nutr. 2018;119:202–210. doi: 10.1017/S0007114517003348. [DOI] [PubMed] [Google Scholar]
- 44.Simon D., Eng P.A., Borelli S., Kagi R., Zimmermann C., Zahner C., Drewe J., Hess L., Ferrari G., Lautenschlager S., et al. Gamma-Linolenic Acid Levels Correlate with Clinical Efficacy of Evening Primrose Oil in Patients with Atopic Dermatitis. Adv. Ther. 2014;31:180–188. doi: 10.1007/s12325-014-0093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shek L.P., Chong M.F.F., Lim J.Y., Soh S.E., Chong Y.S. Role of Dietary Long-Chain Polyunsaturated Fatty Acids in Infant Allergies and Respiratory Diseases. Clin. Dev. Immunol. 2012;2012:730568. doi: 10.1155/2012/730568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolotta A., Pini A., Abruzzo P.M., Ghezzo A., Modesti A., Gamberi T., Ferreri C., Bugamelli F., Fortuna F., Vertuani S., et al. Effects of tocotrienol supplementation in Friedreich’s ataxia: A model of oxidative stress pathology. Exp. Biol. Med. 2020;245:201–212. doi: 10.1177/1535370219890873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vandervegt S.G.L., Ruben A.M.T., Werre J.M., Palsma D.M.H., Verhoef C.W., Degier J., Staal G.E.J. Counterflow centrifugation of red cell populations-a cell age-related separation technique. Br. J. Haematol. 1985;61:393–403. doi: 10.1111/j.1365-2141.1985.tb02843.x. [DOI] [PubMed] [Google Scholar]
- 48.Hodson L., Skeaff C.M., Fielding B.A. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog. Lipid Res. 2008;47:348–380. doi: 10.1016/j.plipres.2008.03.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.