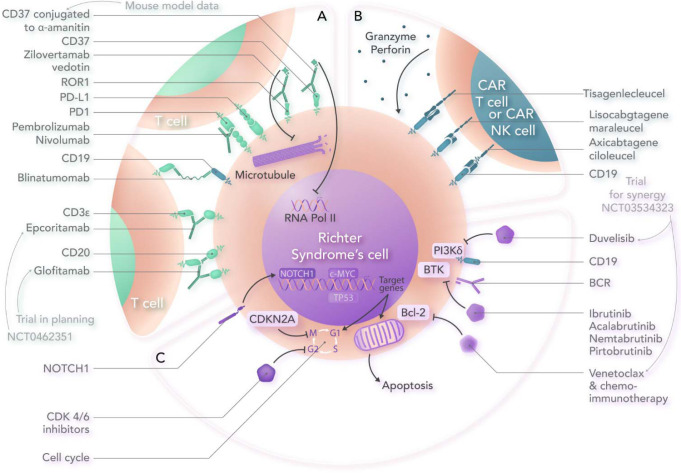
Visual Abstract
Abstract
Richter's syndrome (RS) is an aggressive histologic transformation of chronic lymphocytic leukemia (CLL), most commonly to diffuse large B-cell lymphoma (DLBCL). Outcomes are generally poor, with complete remission (CR) rates of only about 20% and less than 20% long-term survival with chemoimmunotherapy (CIT). RS is biologically heterogeneous, and in 80% of patients with CLL who develop DLBCL, the disease is clonally related to the CLL. Clonally unrelated cases are genetically and immunologically distinct from clonally related DLBCL-RS, have more favorable responses to CIT, and are best treated as de novo DLBCL. Relatively favorable outcomes with CIT are also seen in patients who have never previously received treatment for CLL and who lack TP53 mutation or deletion. For the remaining patients, treatment on a clinical trial is optimal. Fortunately, numerous agents are now in clinical development that show encouraging results. Here we review clinical data for some of the most promising approaches. DLBCL-RS tumor cells frequently express programmed cell death 1 protein (PD-1), and several studies have demonstrated activity for PD-1 inhibitors, especially in combination with ibrutinib. The BCL2 inhibitor venetoclax in combination with R-EPOCH CIT achieved CR in 50% of patients, and a study of venetoclax–R-CHOP is ongoing. The noncovalent Bruton's tyrosine kinase inhibitor pirtobrutinib has achieved responses in approximately two-thirds of heavily pretreated patients and, given its favorable toxicity profile, appears ideally suited to combining with other active agents. Finally, we review available data for bispecific antibodies, antibody-drug conjugates, and chimeric antigen receptor T-cell therapy, which, after revolutionizing the treatment of DLBCL, are now being evaluated in RS.
Learning Objectives
Understand the clinical features, diagnosis, and prognostic features of Richter's syndrome
Understand the novel therapeutic approaches and how to select the optimal approach
CLINICAL CASE
The patient, a fit 73-year-old woman with Richter's syndrome (RS), was treated for chronic lymphocytic leukemia (CLL) in 2011 with 6 cycles of fludarabine, cyclophosphamide, and rituximab (FCR) chemoimmunotherapy (CIT). She achieved complete remission (CR) but progressed with CLL in 2017. At progression, genomic evaluation revealed unmutated IGHV with IGHV4-39-IGHJ5 (subset no. 8) utilization. A chromosomal analysis revealed a diploid karyotype, with trisomy 12 identified in 34% of interphases by fluorescence in situ hybridization (FISH). Next generation sequencing showed a NOTCH1 mutation. In 2018 she was enrolled in a clinical trial with 2 years of fixed-duration ibrutinib and venetoclax, achieving CR with undetectable minimal residual disease to a level lower than 10−4 in the bone marrow. In 2021, just over a year after completion of ibrutinib and venetoclax, she progressed with rapid nodal enlargement, splenomegaly, and constitutional symptoms. Positron emission tomography/computed tomography (PET/CT) showed widespread nodal disease, with a maximum standardized uptake value (SUV) of 10.9. Biopsy confirmed diffuse large B-cell lymphoma (DLBCL). Genotyping of the biopsy specimen showed clonally related disease, with identical IGHV4-39-IGHJ5 utilization; no analyzable metaphases for karyotyping; trisomy 12 by FISH; NOTCH1 mutation; no TP53 mutation. Analysis of untransformed CLL cells at the time of progression showed trisomy 12 with a complex karyotype (46,XX,add (1(p36.1),-7,add(7)(q22),-10,+12,-14,+2mar).
Diagnosis
RS affects 2% to 15% of CLL patients, with an incidence of 0.5% to 1% per year.1,2 RS is defined as the development of a histologically aggressive lymphoma in a patient with s previous or concurrent diagnosis of CLL/small lymphocytic lymphoma, most commonly (~90% of cases) DLBCL, which in 80% of cases is clonally related to the underlying CLL. Transformation to classical Hodgkin lymphoma (CHL; ~10% of cases) or rare lymphoma subtypes occurs less frequently.3 The bulk of this review focuses on the DLBCL subtype (DLBCL-RS).
Clinical features
Suggestive clinical features of DLBCL-RS are high-grade fevers, rapidly enlarging lymph nodes, unexplained weight loss, hypercalcemia, markedly elevated lactate dehydrogenase (LDH), and the development of extranodal disease. These features are nonspecific, and biopsy is always required for diagnostic confirmation and to obtain tissue for genomic evaluation, which is prognostically relevant and may direct therapeutic decisions.1
PET/CT and biopsy
We perform PET/CT in any patient with clinical suspicion for RS. In 1 study done prior to the targeted-agent era, the negative predictive value of a PET/CT maximum SUV (SUVmax) lower than 5 was 92%, with a positive predictive value of 38%.4 The positive predictive value of an SUVmax of 5 was lower in patients progressing on Bruton's tyrosine kinase inhibitors (BTKis) or PI3K-delta inhibitors, but an SUV lower than 5 retains excellent negative predictive value.5
We generally obtain a tissue diagnosis in patients with an SUV greater than or equal to 5 on PET/CT. However, there is considerable nuance to this decision, and clinical judgment can be exercised regarding features that increase or decrease the likelihood of RS being present. As an example, a patient with widespread lymph node progression clinically may be less likely to have RS than one with dominant and rapid progression at 1 or few sites. Similarly, significant differences in the SUVmax from 1 tumor site to another may suggest transformation at the site with a high SUV, while a patient with uniform SUVs of just above 5 throughout all lymph node groups may be more likely to have CLL progression. Overall, if there is suspicion, a biopsy should be considered. Excisional lymph node biopsy is ideal.1 If the lesion is inaccessible for surgical biopsy, then an image-guided core needle biopsy (not fine needle aspiration) should be performed. Where possible, the lesion with the highest SUV should be biopsied.
Risk factors, molecular pathogenesis, and prognosis
High-risk genomic characteristics of CLL increase the risk of transformation to RS—notably, unmutated IGHV status, IGHV stereotyped subset number 8 (IGHV4-39-IGHJ5), activating NOTCH1 mutations, TP53 deletion and/or mutation, and del11q.1,3 Near tetraploidy has been associated with a high risk of RS in patients receiving ibrutinib.6
The clonal relationship between the CLL cells and the RS cells is determined by sequencing the IGH gene in the transformed cells and comparing it to a concurrent or historical IGH sequence from the patient's CLL cells. Discordant light-chain expression between the CLL and the DLBCL-RS cells is, unfortunately, not a reliable surrogate for the lack of a clonal relationship, with cases reported in which light-chain discordance between CLL and RS cells exists despite identical IGH rearrangements.7 Given the distinct genomic characteristics and clinical outcomes of clonally related vs clonally unrelated cases, these are best thought of as distinct entities.
Clonally related DLBCL-RS is genomically distinct from clonally unrelated DLBCL-RS. The most common genomic alterations in clonally related DLBCL-RS are TP53 mutation (60%-80%),8 CDKN2A deletion (30%), MYC overexpression (40%), and activating NOTCH1 mutation (~30%).9 NOTCH1 mutations cluster among patients with trisomy 12 and are largely mutually exclusive with TP53 mutations and CDKN2A deletions.9 Notably, clonally unrelated DLBCL-RS has a lower rate of TP53 disruption (~20%), akin to de novo DLBCL. Additionally, stereotyped immunoglobulin genes (particularly IGHV4-39/IGHD6-13/IGHJ5) are found in 50% of clonally related DLBCL-RS but almost never in clonally unrelated DLBCL-RS.8
Finally, DLBCL-RS appears immunologically distinct from both CLL and from de novo DLBCL. Notably, the malignant B cells in DLBCL-RS express programmed cell death 1 protein (PD-1) in up to 80% of cases, while PD-1 expression is rare in de novo DLBCL, which may have therapeutic relevance.10
The risk of RS most likely relates to underlying disease biology, rather than treatment received. Among numerous randomized trials in the front-line and relapsed/refractory settings reviewed here,3 none showed a significant difference in RS incidence between treatment arms, except a lower rate for FCR vs FC in the CLL8 study.11 Notably, there was no difference in RS risk between treatment arms in the E1912 study of ibrutinib-rituximab vs FCR or the CLL14 study of chlorambucil-obinutuzumab vs venetoclax-obinutuzumab.12,13 The risk for RS, however, increases in studies in relapsed/refractory CLL compared to studies conducted in front-line patients, likely due to higher proportions of patients in relapsed/refractory studies with high-risk disease biology and clonal evolution during therapy.
Prognostic features
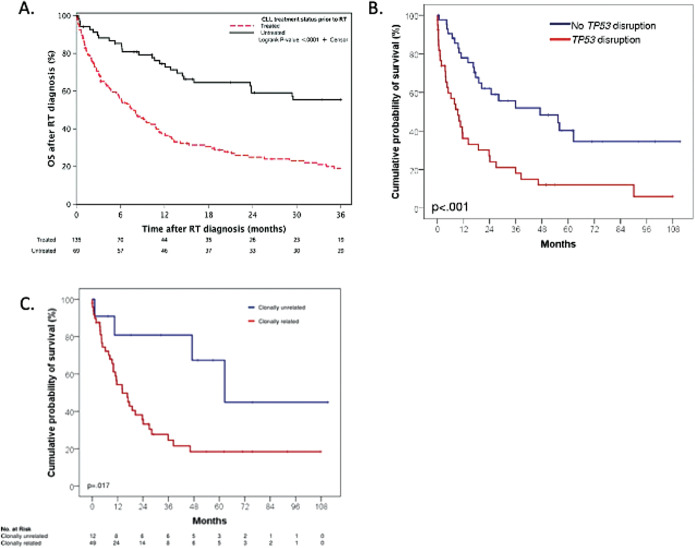
Patients untreated for CLL at the time of DLBCL-RS have relatively favorable progression-free survival (PFS; median 46.3 vs 7.8 months) (Figure 1A).14 Beyond this, the most important prognostic factor in DLBCL-RS is the clonal relationship of DLBCL-RS to the underlying CLL. In 1 study, patients with clonally unrelated disease had a median PFS of 62.5 months vs 14.2 months for clonally related disease (Figure 1B).8 Beyond clonal relationship to the underlying CLL (which is not available in most case series reported) and number of prior therapies for CLL, there is some discordance between different case series regarding the prognostic significance of certain markers. In the 2011 case series by Rossi et al, patients with TP53 disruption had a median PFS of 9.4 months vs 47.1 months without TP53 disruption (Figure 1B). In the Mayo Clinic series, however, TP53 disruption was not independently associated with inferior outcomes, with the most important negative prognostic markers being elevated LDH and prior treatment for CLL. In another study, a complex karyotype in the CLL cells and an increasing number of prior therapies for CLL were associated with inferior PFS/OS, while LDH and TP53 deletion by FISH were not.
Figure 1.
(A) PFS in Mayo Clinic patients with DLBCL-RS by prior CLL treatment status. Used with the permission of Wang et al.14 (B) PFS in patients in a case series according to the presence or absence of T53 mutation/deletion. (C) PFS of patients in a case series by clonal relationship to the underlying CLL. (B) and (C) used with the permission of Rossie et al.8 RT, Richter’s transformation.
Variants
The CHL subtype of RS (CHL-RS) is rare (~1/10th as frequent as DLBCL-RS) but is the second most common histologic transformation. A retrospective analysis of 94 patients demonstrated relatively favorable outcomes, especially for those patients who received chemotherapy with doxorubicin hydrochloride, bleomycin sulfate, vinblastine sulfate, and dacarbazine (ABVD; median OS, 13.2 years).15 Similar to data for DLBCL-RS, patients with no prior therapy for CLL had superior outcomes to previously treated patients, and those never treated with purine analogue chemotherapy had superior outcomes to those previously treated with purine analogues. Few patients underwent allogeneic stem cell transplant (alloSCT) in first remission, but within the limitations of the small numbers of patients analyzed, alloSCT did not appear to have an impact on survival outcomes.
CIT in the treatment of DLBCL-RS
Initial treatment with CIT, analogous to the treatment of de novo DLBCL, is generally given, but outcomes for patients with DLBCL-RS are poor. CIT studies have generally resulted in CR rates of 20% or lower, with a median overall survival (OS) of 6 to 12 months (Table 1).
Table 1.
Outcomes in DLBCL-RS with standard CIT
| Study and years of patient recruitment | Regimen | n | Median age (years) | Results | ||
|---|---|---|---|---|---|---|
| ORR | CRR | Median OS | ||||
| Anthracycline-containing regimens | ||||||
| Langerbeins et al16 (2003-2008) | R-CHOP | 15 | 69 (N/A) | 67% | 7% | 21 months |
| Dabaja et al17 (published 2000) | HyperCVXD | 29 | 61 (36-75) | 41% | 38% | 10 months |
| Tsimberidou et al18 (1999-2001) | Rituximab and GM-CSF with alternating hyperCVAD and MTX/cytarabine | 30 | 59 (27-79) | 43% | 18% | 8.5 months |
| Rogers et al19 (2006-2014) | R-EPOCH | 46 | 67 (38-83) | 39% | N/A | 5.9 months |
| Platinum-containing regimens | ||||||
| Tsimberidou et al20 (2004-2006) | OFAR1 | 20 | 59 (34-77) | 50% | 20% | 8 months |
| Tsimberidou et al21 (2007-2010) | OFAR2 | 35 | 63 (40-81) | 43% | 8.6% | 6.6 months |
| Fludarabine-containing regimens | ||||||
| Giles et al22 (1992-1996) | PFA or CFA | 12 | 59 (49-74) | 45% | N/A | 17 months |
| Tsimberidou et al23 (1997-2001) | FACPGM | 15 | 62 (42-74) | 5% | 5% | 2.2 months |
CFA, cyclophosphamide-fludarabine-arabinosyl cytosine; FACPGM, fludarabine–cytarabine–cyclophosphamide–cisplatin–GM-CSF; GM-CSF, granulocyte-macrophage colony-stimulating factor; HyperCVAD, fractionated cyclophosphamide-vincristine-liposomal daunorubicin- dexamethasone; MTX, methotrexate; N/A, not available; OFAR, oxaliplatin-fludarabine-cytarabine-rituximab; PFA, cisplatin, fludarabine, cytarabine.
As outlined above, attempts to improve outcomes through intensification of chemotherapy have been unsuccessful. As a result, there is no standard of care CIT regimen for DLBCL-RS, and there is an unmet need for effective treatments in this disease. Currently, treatment choice is based on age, comorbidities, prior therapies, and the experience of the treating center. Given poor outcomes with standard therapy, all patients should be treated in clinical trials where possible. Combinations with novel targeted therapies, checkpoint inhibitors, cellular therapy, and trials of several nonchemotherapy regimens are ongoing, as described below.
Hematopoietic progenitor cell transplantation in DLBCL-RS
Evaluation of the impact of alloSCT and autologous stem cell transplant (autoSCT) is limited by the lack of prospective randomized studies, introducing selection bias into the comparison of survival between transplanted and nontransplanted patients. With these caveats, there appears to be curative potential of alloSCT, especially for patients who achieve a CR prior to transplant. In a European Society for Blood and Marrow Transplantation analysis of 25 patients (9/25 with progressive disease prior to alloSCT), there was a plateau on the long-term relapse-free survival (RFS) curve. However, overall results remained poor, with 3-year RFS of only 27% post alloSCT and with 47% relapse (10 with DLBCL-RS, 2 with CLL) and 26% nonrelapse mortality at 3 years. Chemosensitive disease and the use of reduced-intensity conditioning were associated with superior RFS (largely due to lower nonrelapse mortality), stressing the importance of more effective therapy prior to alloSCT. Thirty-four patients in the same cohort who had chemosensitive disease underwent autoSCT. No plateau was seen on the RFS curve after autoSCT, with 3-year RFS of 45% (11 relapses with DLBCL-RS and 6 with CLL).24
Novel approaches to DLBCL-RS treatment
Here, we cover reported clinical trial data on novel approaches in DLBCL-RS (Table 2). Unfortunately, the numbers of patients treated with most novel approaches are small, but several show encouraging results. In addition, the visual abstract also indicates some approaches with promising preclinical data and/or active clinical investigation, without publicly reported data available.
Table 2.
Novel approaches to DLBCL-RS treatment with published results
| Treatment | Number of patients | Median number prior Rx (CLL + RT) | ORR/CRR (%) | Median PFS/DOR(mo) | Median OS (mo) |
|---|---|---|---|---|---|
| Small-molecule targeted agents | |||||
| Venetoclax monotherapy38 | 7 | NR | 43/0 | NR/NR | NR |
| Acalabrutinib monotherapy25 | 25 | 1 for RT | 40/8 | 3.2/6.2 | NR |
| DTRM-555 (novel BTKi DTRMWXHS- 12– everolimus–pomalidomide)39 | 24 | 5 | 45/9 | NR/NR | NR |
| Pirtobrutinib26 | 9 | 6 (including 100% treated with covalent BTKi) | 67/NR | NR/NR | NR |
| CIT + targeted agents | |||||
| R-EPOCH− venetoclax28 | 26 | 1 for CLL, 0 for RT | 62/50 | 10.1/NR | 19.6 |
| Checkpoint inhibitors | |||||
| Pembrolizumab30 | 9 | 5 | 44/0 | NR/NR | 10.1 |
| Pembrolizumab31 | 23 (2 with CHL) | 3 for RT, NR for CLL | 5/0 (excluding 2 responders with CHL) | 1.6/NR | 3.8 |
| Ibrutinib-nivolumab40 | 24 | 3 | 43/35 | NR/10. | 13.8 |
| Ibrutinib- nivolumab41 | 20 | 2 | 65/10 | 5.0/6.9 | 10.3 |
| Venetoclax-obinutuzumab- atezolizumab33 | 7 | NR | 100/71 | Not reached/not reached | NR |
| Bispecific antibodies | |||||
| Blinatumomab monotherapy (Leukemia, in press) | 9 | 4 for CLL +2 for DLBCL-RS | 22/11 | 1.9/NR | 10.3 |
| Blinatumomab after R-CHOP34 | 31 | 2 for CLL | 54/39 | NR/NR | NR |
| Antibody-drug conjugates | |||||
| Zilovertamab vedotin36 | 6 | NR | 67/17 | NR/NR | NR |
| CAR T | |||||
| CD19 CAR T42 | 6 (DLBCL only) | 5 | 67/67 | NR/NR | NR |
| Axicabtagene ciloleucel43 | 8 | 4 | 100/63 | NR/NR | NR |
| Lisocabtagene maraleucel (European Breyanzi label) | 4 | NR | 50/25 | NR/2 | NR |
NR, not reported; RT, Richter’s transformation.
Small-molecule targeted agents
BTKis have dramatically improved outcomes for patients with CLL. However, results in DLBCL-RS have been more modest. Acalabrutinib monotherapy achieved responses, mostly partial responses, in 40% of patients, with a median duration of response (DOR) of 6 months.25 Pirtobrutinib, a noncovalent BTKi with a prolonged half-life, achieved at least partial responses in 6 of 9 very heavily pretreated patients with DLBCL-RS, all of whom had previously received a covalent BTKi, with a highly favorable toxicity profile.26 Data from a much larger cohort treated with pirtobrutinib are eagerly awaited.
Three of 7 patients with DLBCL-RS responded to single-agent venetoclax in the phase 1 study.27 This led to a multicenter phase 2 study of venetoclax in addition to R-EPOCH (rituximab, etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin), which demonstrated an encouraging 50% CR rate.28 Standard dosing of dose-adjusted R-EPOCH was utilized. Cycle 1 was given without venetoclax. Prior to cycle 2, rapid venetoclax ramp-up over 5 days, with inpatient tumor lysis syndrome monitoring, was performed, with no tumor lysis syndrome seen. Subsequently, venetoclax at 400 mg/d for 10 days was given concurrently with cycle 2 through 6 of dose- adjusted R-EPOCH. Of note, TP53 alterations did not negatively affect the CR rate. Hematologic toxicity was substantial, and consequently, the median number of cycles of R-EPOCH given was 4. There were 2 therapy-related deaths, both occurring during cycle 1 (prior to the initiation of venetoclax). An additional cohort is being evaluated utilizing R-CHOP rather than R-EPOCH in the hope of mitigating hematologic toxicity and improving deliverability without compromising outcomes (NCT03054896). A retrospective analysis of 10 patients treated off protocol showed an encouraging 50% CR rate with venetoclax–R-CHOP.29
Antibody-based therapy
Immune checkpoint blockade has shown some promising results. A study of the PD-1 inhibitor pembrolizumab, as monotherapy, showed that 4 of 9 patients achieved PR,30 but a larger, multicenter follow-up study was disappointing.31 Somewhat superior results were seen with ibrutinib-nivolumab: 35% CR was seen in data from MD Anderson, with more favorable responses in ibrutinib-naive patients.32 A follow-up multicenter study showed a 65% overall response rate (ORR; 10% complete response rate [CRR]) but with a median DOR of only 6.9 months.32 More recently, atezolizumab-venetoclax-obinutuzumab achieved CR in 5 of 7 patients.33
Responses have been seen with blinatumomab either alone (Thompson et al, Leukemia 2022, accepted) or as consolidation after R-CHOP.34 Numerous bispecific antibodies are being studied in DLBCL, targeting CD19, CD20, CD22, CD37, and ROR1, reviewed elsewhere.35
Finally, the ROR1-targeting antibody-drug conjugate zilovertamab vedotin achieved an ORR of 50% in patients heavily pretreated for CLL and/or RS.36
Chimeric antigen receptor T-cell and chimeric antigen receptor natural killer cell therapy
In a retrospective evaluation of patients treated with axicabtagene ciloleucel for DLBCL-RS, 9 patients received the drug, 7 in combination with a BTKi.36 The ORR was 100% in 8 evaluable patients, with 5 of 8 CRs. Similarly, in preliminary results of an ongoing clinical trial in Israel, 8 patients with DLBCL-RS received anti-CD19 chimeric antigen receptor (CAR) T cells, and 5 patients achieved CR. In the TRANSCEND-NHL001 trial, 4 patients with DLBCL-RS were treated with lisocabtagene ciloleucel (liso-cel), and 2 of 4 patients responded, with 1 of 4 achieving CR (European Breyanzi label). A single patient with RS in a phase 1 study of CAR natural killer (NK) therapy achieved CR.37 Several studies with novel CAR NK products are ongoing.
Trials of interest
The ongoing PLATFORM trial (NCT03310619) is studying a combination of liso-cel plus targeted or immunotherapies in different combination cohorts. One cohort combined the checkpoint inhibitor durvalumab. One patient with relapsed/refractory DLBCL-RS achieved a CR for 2 years with this combination, but she unfortunately died of therapy-related myelodysplastic syndrome. On the ibrutinib as well as nivolumab combination cohorts in this trial, some other DLBCL-RS patients are experiencing durable remissions (data not published). Taken together, these results support further exploration of anti-CD19 CAR T for DLBCL-RS, potentially in combination with a BTKi as well as a checkpoint inhibitor. An investigator-initiated study of liso- cel–ibrutinib–nivolumab is planned at City of Hope National Medical Center and MD Anderson Cancer Center.
Bispecific antibodies targeting CD20 have achieved impressive rates of durable CRs in de novo DLBCL.44,45 Epcoritamab, a CD3x20 bispecific antibody, is being evaluated in DLBCL-RS (NCT04623541), and a multicenter investigator- initiated study is planned with glofitamab in RS (Davids M, personal communication).
Pirtobrutinib is active and very well tolerated in DLBCL-RS. Given its favorable toxicity profile, it appears an ideal agent to explore in combination with other active agents. A phase 2 study of pirtobrutinib-venetoclax-obinutuzumab in CLL and DLBCL-RS is planned at MD Anderson.
How we treat
We treat patients with CHL-RS similarly to patients with de novo CHL, usually with ABVD or similar as initial therapy. Anecdotally, we have seen excellent responses to PD-1 inhibition in patients with relapsed CHL-RS.
Among patients with DLBCL-RS, optimal risk stratification is essential. This requires consideration of patient fitness, extent of prior therapy, determination of clonal relationship to the underlying CLL, and TP53 mutation/deletion status. We recognize that IGHV sequencing of tumor tissue to determine the clonal relationship of the DLBCL-RS to the underlying CLL is not universally available.
The above evaluation allows us to identify the small groups of patients who have relatively favorable outcomes with CIT: (1) those with clonally unrelated disease (who should be treated as de novo DLBCL) and (2) those who have untreated CLL and may lack TP53 alteration. These patients can receive R-CHOP CIT alone, without alloSCT. For all other patients, the goal is to achieve remission and then proceed with alloSCT for eligible patients, which is potentially curative for RS and CLL.
We stress that, where possible, all patients, especially those with poor-risk disease, should be enrolled in clinical trials. Outside clinical trials, patients predicted to have poor response to CIT (patients with TP53 alterations and patients with clonally related disease who have previously received treatment for CLL) could be considered for an alternative approach, utilizing off-label therapy. The 2 approaches we generally utilize for such patients are R-CHOP or R-EPOCH combined with venetoclax or ibrutinib at 420 mg/d plus nivolumab at 3 mg/kg intravenously every 2 weeks. In general, we favor R-CHOP–venetoclax in patients who are CIT-naive, given the approximate 50% CR rate with CIT plus venetoclax and the lesser degree of toxicity compared to R-EPOCH-venetoclax seen in our experience and in the de novo DLBCL literature. For patients previously treated with CIT for CLL or DLBCL-RS, we favor ibrutinib- nivolumab, especially in patients who are BTKi-naive, given that response rates to this regimen are substantially higher in BTKi- naive (64%) vs BTKi pretreated patients (23%).40 When utilizing PD-1/programmed cell death 1 ligand 1 inhibitors as a bridge to alloSCT, it is important to remember the potential for increased severe acute graft-versus-host disease risk post alloSCT. This risk may be ameliorated by the use of posttransplant cyclophosphamide.46
CLINICAL CASE (Continued)
The patient was enrolled in a clinical trial with R-CHOP-venetoclax (NCT03984448). Cycle 1 (R-CHOP alone, standard doses) was poorly tolerated, with grade 4 ileus and febrile neutropenia. Subsequent cycles, with the addition of venetoclax per protocol, were given without vincristine and with a dose reduction to 400 mg/m2 of cyclophosphamide and 25 mg/m2 of doxorubicin and were well tolerated. She achieved CR on PET/CT after cycle 3 and undetectable minimal residual disease to a level lower than 10−6 in the bone marrow by next generation sequencing following cycle 6, at which point she proceeded with alloSCT from a matched unrelated donor. She remains well thus far and in CR 3 months post alloSCT.
Conflict-of-interest disclosure
Philip A. Thompson: research funding: Adaptive Biotechnologies, Genentech, AbbVie, Pharmacyclics, Amgen, Lilly; advisory board: Adaptive Biotechnologies, Janssen, Pharmacyclics, AstraZeneca, Beigene, AbbVie, Genentech, Lilly; lecturer: Janssen Australia.
Tanya Siddiqi: speaker: Astra Zeneca, Bristol Myers Squibb, BeiGene; advisory board: Astra Zeneca, BeiGene, Bristol Myers Squibb, Celgene, AbbVie, Pharmacyclics, Gilead; honoraria: Dava Oncology, ResearchToPractice.
Off-label drug use
Ibrutinib and nivolumab for Richter's syndrome; venetoclax combined with chemoimmunotherapy for Richter's syndrome.
References
- 1.Parikh SA, Kay NE, Shanafelt TD. How we treat Richter syndrome. Blood. 2014;123(11):1647-1657. doi: 10.1182/blood-2013-11-516229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts AW, Davids MS, Pagel JM, et al.. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374(4):311-322. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossi D, Spina V, Gaidano G. Biology and treatment of Richter syndrome. Blood. 2018;131(25):2761-2772. doi: 10.1182/blood-2018-01-791376. [DOI] [PubMed] [Google Scholar]
- 4.Falchi L, Keating MJ, Marom EM, et al.. Correlation between FDG/PET, histology, characteristics, and survival in 332 patients with chronic lymphoid leukemia. Blood. 2014;123(18):2783-2790. doi: 10.1182/blood-2013-11-536169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Rabe KG, Bold MS, et al.. The role of 18F-FDG-PET in detecting Richter's transformation of chronic lymphocytic leukemia in patients receiving therapy with a B-cell receptor inhibitor. Haematologica. 2020;105(11):2675-2678. doi: 10.3324/haematol.2019.240564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller CR, Ruppert AS, Heerema NA, et al.. Near-tetraploidy is associated with Richter transformation in chronic lymphocytic leukemia patients receiving ibrutinib. Blood Adv. 2017;1(19):1584-1588. doi: 10.1182/bloodadvances.2017007302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyamura K, Osada H, Yamauchi T, et al.. Single clonal origin of neoplastic B-cells with different immunoglobulin light chains in a patient with Richter's syndrome. Cancer. 1990;66(1):140-144. doi:. [DOI] [PubMed] [Google Scholar]
- 8.Rossi D, Spina V, Deambrogi C, et al.. The genetics of Richter syndrome reveals disease heterogeneity and predicts survival after transformation. Blood. 2011;117(12):3391-3401. doi: 10.1182/blood-2010-09-302174. [DOI] [PubMed] [Google Scholar]
- 9.Chigrinova E, Rinaldi A, Kwee I, et al.. Two main genetic pathways lead to the transformation of chronic lymphocytic leukemia to Richter syndrome. Blood. 2013;122(15):2673-2682. doi: 10.1182/blood-2013-03-489518. [DOI] [PubMed] [Google Scholar]
- 10.He R, Ding W, Viswanatha DS, et al.. PD-1 expression in chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and large B-cell Richter transformation (DLBCL-RT): a characteristic feature of DLBCL-RT and potential surrogate marker for clonal relatedness. Am J Surg Pathol. 2018;42(7):843-854. doi: 10.1097/PAS.0000000000001077. [DOI] [PubMed] [Google Scholar]
- 11.Fischer K, Bahlo J, Fink AM, et al.. Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood. 2016;127(2):208-215. doi: 10.1182/blood-2015-06-651125. [DOI] [PubMed] [Google Scholar]
- 12.Shanafelt TD, Wang XV, Hanson CA, et al.. Long-term outcomes for ibrutinib-rituximab and chemoimmunotherapy in CLL: updated results of the E1912 trial. Blood. 2022;140(2):112-120. doi: 10.1182/blood.2021014960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Sawaf O, Zhang C, Lu T, et al.. Minimal residual disease dynamics after venetoclax-obinutuzumab treatment: extended off-treatment follow-up from the randomized CLL14 study. J Clin Oncol. 2021;39(36):4049-4060. doi: 10.1200/JCO.21.01181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Tschautscher MA, Rabe KG, et al.. Clinical characteristics and outcomes of Richter transformation: experience of 204 patients from a single center. Haematologica. 2020;105(3):765-773. doi: 10.3324/haematol.2019.224121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stephens DM, Boucher K, Kander E, et al.. Hodgkin lymphoma arising in patients with chronic lymphocytic leukemia: outcomes from a large multi-center collaboration. Haematologica. 2021;106(11):2845-2852. doi: 10.3324/haematol.2020.256388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langerbeins P, Busch R, Anheier N, et al.. Poor efficacy and tolerability of R-CHOP in relapsed/refractory chronic lymphocytic leukemia and Richter transformation. Am J Hematol. 2014;89(12):E239-E243. doi: 10.1002/ajh.23841. [DOI] [PubMed] [Google Scholar]
- 17.Dabaja BS, O'Brien SM, Kantarjian HM, et al.. Fractionated cyclophosphamide, vincristine, liposomal daunorubicin (daunoXome), and dexamethasone (hyperCVXD) regimen in Richter's syndrome. Leuk Lymphoma. 2001;42(3):329-337. doi: 10.3109/10428190109064589. [DOI] [PubMed] [Google Scholar]
- 18.Tsimberidou AM, Kantarjian HM, Cortes J, et al.. Fractionated cyclophosphamide, vincristine, liposomal daunorubicin, and dexamethasone plus rituximab and granulocyte-macrophage-colony stimulating factor (GM-CSF) alternating with methotrexate and cytarabine plus rituximab and GM-CSF in patients with Richter syndrome or fludarabine-refractory chronic lymphocytic leukemia. Cancer. 2003;97(7):1711-1720. doi: 10.1002/cncr.11238. [DOI] [PubMed] [Google Scholar]
- 19.Rogers KA, Huang Y, Ruppert AS, et al.. A single-institution retrospective cohort study of first-line R-EPOCH chemoimmunotherapy for Richter syndrome demonstrating complex chronic lymphocytic leukaemia karyotype as an adverse prognostic factor. Br J Haematol. 2018;180(2):259-266. doi: 10.1111/bjh.15035. [DOI] [PubMed] [Google Scholar]
- 20.Tsimberidou AM, Wierda WG, Plunkett W, et al.. Phase I-II study of oxaliplatin, fludarabine, cytarabine, and rituximab combination therapy in patients with Richter's syndrome or fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2008;26(2):196-203. doi: 10.1200/JCO.2007.11.8513. [DOI] [PubMed] [Google Scholar]
- 21.Tsimberidou AM, Wierda WG, Wen S, et al; Chronic Lymphocytic Leukemia Research Consortium. Phase I-II clinical trial of oxaliplatin, fludarabine, cytarabine, and rituximab therapy in aggressive relapsed/refractory chronic lymphocytic leukemia or Richter syndrome. Clin Lymphoma Myeloma Leuk. 2013;13(5):568-574. doi: 10.1016/j.clml.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giles FJ, Obrien S, Kantarjian HM. Sequential cis-platinum, fludarabine, and arabinosyl cytosine (PFA) or cyclophosphamide, fludarabine and arabinosyl cytosine (CFA) in patients with Richter's syndrome. Blood. 1996; 88(suppl 1):93a. doi: 10.3109/10428199909145949. [DOI] [Google Scholar]
- 23.Tsimberidou AM, O'Brien SM, Cortes JE, et al.. Phase II study of fludarabine, cytarabine (Ara-C), cyclophosphamide, cisplatin and GM-CSF (FACPGM) in patients with Richter's syndrome or refractory lymphoproliferative disorders. Leuk Lymphoma. 2002;43(4):767-772. doi: 10.1080/10428190290016872. [DOI] [PubMed] [Google Scholar]
- 24.Cwynarski K, van Biezen A, de Wreede L, et al.. Autologous and allogeneic stem-cell transplantation for transformed chronic lymphocytic leukemia (Richter's syndrome): a retrospective analysis from the Chronic Lymphocytic Leukemia Subcommittee of the Chronic Leukemia Working Party and Lymphoma Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2012;30(18):2211- 2217. doi: 10.1200/JCO.2011.37.4108. [DOI] [PubMed] [Google Scholar]
- 25.Eyre TA, Schuh A, Wierda WG, et al.. Acalabrutinib monotherapy for treatment of chronic lymphocytic leukaemia (ACE-CL-001): analysis of the Richter transformation cohort of an open-label, single-arm, phase 1-2 study. Lancet Haematol. 2021;8(12):e912-e921. doi: 10.1016/S2352-3026(21)00305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mato AR, Shah NN, Jurczak W, et al.. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397(10277):892-901. doi: 10.1016/S0140-6736(21)00224-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davids MS, Pagel JM, Kahl BS, et al.. Bcl-2 inhibitor ABT-199 (GDC-0199) monotherapy shows anti-tumor activity including complete remissions in high-risk relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL). Blood. 2013;122(21):872. doi: 10.1182/blood.V122.21.872.872.23803709 [DOI] [Google Scholar]
- 28.Davids MS, Rogers KA, Tyekucheva S, et al.. Venetoclax plus dose-adjusted R-EPOCH for Richter syndrome. Blood. 2022;139(5):686-689. doi: 10.1182/blood.2021011386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hampel P, Parikh S, Wierda W, et al.. Venetoclax-based treatment of patients with Richter syndrome: outcomes from a multicenter retrospective study. HemaSphere. 2022;6(June):549-550. doi: 10.1097/01.HS9.0000845488.18043.aa. [DOI] [Google Scholar]
- 30.Ding W, LaPlant BR, Call TG, et al.. Pembrolizumab in patients with CLL and Richter transformation or with relapsed CLL. Blood. 2017;129(26):3419-3427. doi: 10.1182/blood-2017-02-765685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armand P, Murawski N, Molin D, et al.. Pembrolizumab in relapsed or refractory Richter syndrome. Br J Haematol. 2020;190(2):e117-e120. doi: 10.1111/bjh.16762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Younes A, Sehn LH, Johnson P, et al; PHOENIX Investigators. Randomized phase III trial of ibrutinib and rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in non-germinal center B-cell diffuse large B-cell lymphoma. J Clin Oncol. 2019;37(15):1285-1295. doi: 10.1200/JCO.18.02403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jain N, Ferrajoli A, Thompson PA, et al.. Venetoclax, obinutuzumab and atezolizumab (PD-L1 checkpoint inhibitor) for treatment for patients with Richter transformation. Blood. 2021;138(suppl 1):1550. doi: 10.1182/blood-2021-154279. [DOI] [Google Scholar]
- 34.Guieze R, Ysebaert L, Roos-Weil D, et al.. Blinatumomab for patients with Richter's syndrome: a multicenter phase 2 trial from the Filo Group. Blood. 2021;138(suppl 1):3570. doi: 10.1182/blood-2021-147467. [DOI] [Google Scholar]
- 35.Tavarozzi R, Manzato E. The role of bispecific antibodies in non-Hodgkin's lymphoma: from structure to prospective clinical use. Antibodies (Basel). 2022;11(1):16. doi: 10.3390/antib11010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M, Mei M, Barr PM, et al.. Zilovertamab vedotin (MK-2140) for the treatment of non-Hodgkin lymphoma: the phase 1 dose escalation and cohort expansion WAVELINE-001 study of an anti-ROR1 antibody-drug conjugate. HemaSphere. 2022;6(June):1116-1117. doi: 10.1097/01.HS9.0000847788.96420.63. [DOI] [Google Scholar]
- 37.Liu E, Marin D, Banerjee P, et al.. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N Engl J Med. 2020;382(6):545-553. doi: 10.1056/NEJMoa1910607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davids MS, Roberts AW, Seymour JF, et al.. Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35(8):826-833. doi: 10.1200/JCO.2016.70.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mato AR, Schuster SJ, Foss FM, et al.. A once daily, oral, triple combination of BTK inhibitor, mTOR inhibitor and IMiD for treatment of relapsed/refractory Richter's transformation and de novo diffuse large B-cell lymphoma. Blood. 2020;136(suppl 1):21-22. doi: 10.1182/blood-2020-138896. [DOI] [Google Scholar]
- 40.Jain N, Ferrajoli A, Basu S, et al.. A phase II trial of nivolumab combined with ibrutinib for patients with Richter transformation. Blood. 2018;132(suppl 1):296. doi: 10.1182/blood-2018-99-120355. [DOI] [Google Scholar]
- 41.Younes A, Brody J, Carpio C, et al.. Safety and activity of ibrutinib in combination with nivolumab in patients with relapsed non-Hodgkin lymphoma or chronic lymphocytic leukaemia: a phase 1/2a study. Lancet Haematol. 2019;6(2):e67-e78. doi: 10.1016/S2352-3026(18)30217-5. [DOI] [PubMed] [Google Scholar]
- 42.Benjamini O, Shimoni A, Besser M, et al.. Safety and efficacy of CD19-CAR T cells in Richter's transformation after targeted therapy for chronic lymphocytic leukemia. Blood. 2020;136(suppl 1):40. doi: 10.1182/blood-2020-138904. [DOI] [Google Scholar]
- 43.Kittai AS, Bond DA, William B, et al.. Clinical activity of axicabtagene ciloleucel in adult patients with Richter syndrome. Blood Adv. 2020;4(19):4648-4652. doi: 10.1182/bloodadvances.2020002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hutchings M, Mous R, Clausen MR, et al.. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet. 2021;398(10306):1157-1169. doi: 10.1016/S0140-6736(21)00889-8. [DOI] [PubMed] [Google Scholar]
- 45.Hutchings M, Morschhauser F, Iacoboni G, et al.. Glofitamab, a novel, bivalent CD20-targeting T-cell–engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase I trial. J Clin Oncol. 2021;39(18):1959-1970. doi: 10.1200/JCO.20.03175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saberian C, Abdel-Wahab N, Abudayyeh A, et al.. Post-transplantation cyclophosphamide reduces the incidence of acute graft-versus-host disease in patients with acute myeloid leukemia/myelodysplastic syndromes who receive immune checkpoint inhibitors after allogeneic hematopoietic stem cell transplantation. J Immunother Cancer. 2021;9(2):e001818. doi: 10.1136/jitc-2020-001818. [DOI] [PMC free article] [PubMed] [Google Scholar]