Abstract
Pseudomonas aeruginosa, a gram-negative facultative pathogen, causes severe infections in immunocompromised and cystic fibrosis patients. However, the molecular details of the interaction between P. aeruginosa and mammalian cells are still largely unknown. Here we demonstrate that infection of human conjunctiva epithelial Chang cells with the well-characterized P. aeruginosa strain PAO-I results in rapid induction of apoptosis. Apoptosis was mediated by mitochondrial alterations, in particular mitochondrial depolarization, synthesis of reactive oxygen intermediates, and release of cytochrome c, as well as an activation of Jun N-terminal kinases (JNK). Stimulation of these events was dependent on upregulation of CD95 on infected cells, and a deficiency of CD95 or the CD95 ligand prevented mitochondrial changes, JNK activation, and apoptosis upon infection. Further, efficient apoptosis of Chang epithelial cells required infection with live P. aeruginosa, adhesion but not invasion of the bacteria, and expression of the type III secretion system in PAO-I. The data indicate a type III secretion system-dependent, sequential activation of several signaling pathways by P. aeruginosa PAO-I, resulting in apoptosis of the infected cell.
Apoptosis of mammalian host cells has been shown to be a hallmark of infection by some bacteria, viruses, and parasites (10, 28). A paradigm for bacterium-induced apoptosis is Shigella flexneri (40), which secretes the protein IpaB into the host cell via the type III secretion system (41). IpaB binds to and activates caspase 1, resulting in release of interleukin 1 and finally apoptosis of the infected cell (3, 18). Salmonella enterica serovar Typhimurium exploits a very similar system, secreting via the type III secretion system the protein SipB, which also induces apoptosis by binding to and activation of caspase 1 (17). In contrast, Yersinia enterocolitica appears to trigger apoptosis by the inhibition of several survival pathways active in the mammalian cell and by balancing proapoptotic pathways (17). Yersinia releases two proteins, YopJ and YopP, into the cytoplasm of the infected cell, which inhibit NF-κB and mitogen-activated protein kinases as well as dephosphorylate tyrosine residues (25, 27, 31). The exact mechanisms of how these events lead to apoptosis are unknown.
However, the molecular mechanisms of apoptosis induced by other bacteria, including enteropathogenic Escherichia coli (6), Listeria monocytogenes (30), and Staphylococcus aureus (24), are almost unknown.
Apoptosis has been shown to be mediated by a whole variety of signaling pathways. In particular, caspases, which cleave several intracellular proteins, have been demonstrated to play a pivotal role in almost any form of apoptosis (26, 33). Caspases are activated by proapoptotic receptors, e.g., CD95 or the tumor necrosis factor receptor, but also by stress stimuli, e.g., radiation, heat, or oxygen radicals (1, 23). Caspases are known to cleave several intracellular proteins, which finally results in DNA cleavage, disturbance of the mitochondrial function, and changes of the cell membrane (32). Mitochondria constitute a second system which has been shown to be highly important in many forms of apoptosis (22). They respond to many apoptotic stimuli with a release of cytochrome c and/or apoptosis-inducing factor, opening of the permeability transition pore, and depolarization of the mitochondrial membrane potential (22). Finally, the stimulation of stress kinases, Jun N-terminal kinase (JNK) and p38-K, has been invoked as being important for apoptosis, in particular upon application of stress stimuli (2).
Pseudomonas aeruginosa infections play an important and growing role in the clinic. In particular, patients with cystic fibrosis are highly susceptible to P. aeruginosa infections, very often developing chronic bronchiolar infection and eventually dying from the infection. Thus, the molecular mechanisms of host-P. aeruginosa interactions need further definition.
Here we identify molecular mechanisms involved in the induction of apoptosis by P. aeruginosa. We demonstrate that P. aeruginosa triggered an activation of JNK and of mitochondrial alterations typical for apoptosis. Induction of these signaling events seemed to be dependent on upregulation of endogenous CD95 on the surface of infected cells, and deficiency of either CD95 or CD95 ligand confined cellular resistance to P. aeruginosa-triggered apoptosis. In addition, our results indicate that adhesion of P. aeruginosa to mammalian cells and expression of a type III secretion system are required for CD95 upregulation, activation of JNK, induction of mitochondrial changes, and finally apoptosis.
MATERIALS AND METHODS
Chemicals, bacteria, animals, and cells.
Dihydroethidine (DHE), 5,5′, 6,6′-tetrachloro-1, 1′, 3, 3′-tetraethylbenzimidazolylcarbocyanineiodide (JC1), and 3, 3′-dihexyloxacarbocyanineiodide [DiOC6(3)] were from Molecular Probes (Mobitec, Goettingen, Germany). All other chemicals were from Sigma-Aldrich (Deisenhofen, Germany) if not otherwise indicated. C3He/N, lpr, and gld mice were obtained from Charles River (Sulzfeld, Germany). The human conjunctiva epithelial cell line Chang was purchased from American Type Culture Collection (Manassas, Va.). Chang cells were cultured in RPMI-1640 medium supplemented with 2 mM glutamine and 5% heat-inactivated fetal calf serum (FCS) (Life Technologies, Karlsruhe, Germany). Cells were grown as monolayers in tissue culture flasks in a humified atmosphere (5% CO2–95% air) at 37°C. To obtain ex vivo lung fibroblasts, mice were euthanized and lungs were surgically removed, dissected into 2- by 2-mm tissue fragments, and maintained in Dulbecco modified Eagle medium supplemented with 10% heat-inactivated FCS, 2 mM glutamine, 10 mM HEPES, 100 U of penicillin/ml, and 100 μg of streptomycin/ml (all from Life Technologies). Fibroblasts were harvested after 10 to 12 days and seeded in the same medium without penicillin-streptomycin for infection experiments.
The P. aeruginosa strains PAO-I and the type III secretion-negative mutant of P. aeruginosa PAO-I (PAK pcrD::Sm) were kindly provided by S. M. Fleiszig (University of California, Berkeley) and J. Heesemann (Max von Pettenkofer-Institut, Muenchen, Germany), respectively.
Infection experiments.
For infection experiments, bacteria were grown overnight at 37°C on tryptic soy agar plates, resuspended in tryptic soy broth (Difco Laboratories, Detroit, Mich.) to an optical density of 0.25, shaken at 130 rpm for 1 h at 37°C to reach the mid-logarithmic phase, pelleted by centrifugation, and resuspended in fresh tryptic soy broth. Chang cells were seeded 24 h prior to the infection in RPMI-1640 medium supplemented with 2 mM l-glutamine and 5% FCS at cell numbers yielding subconfluent cell layers for the infection experiments. Prior to the infection, cells were washed twice in RPMI-1640 with 2 mM l-glutamine and maintained in the same medium during the infection. Murine fibroblasts were infected in Dulbecco modified Eagle medium with 2 mM glutamine. For infection periods longer than 90 min, medium was supplemented with 1% heat-inactivated FCS. Infection was started by inoculation of cells at a bacteria/host cell ratio of 1,000:1. Additional experiments were performed using bacteria/host cell ratios of 100:1 and 10:1, also resulting in apoptosis of the host cells but with slower kinetics. Synchronous infection conditions and an enhanced bacteria-host cell interaction were achieved by a 2-min centrifugation (35 × g) step. The end of the centrifugation was defined as the start point in all experiments. After the infection time, cells were collected using cell dissociation solution (Sigma-Aldrich).
To discriminate the effects of P. aeruginosa adhesion to Chang cells from those of invasion, we incubated P. aeruginosa PAO-I for 15 min with a peptide corresponding to the amino acids 103 to 117 of the cystic fibrosis conductance regulator (CFTR) (1 μM; GRIIASYDPD NKEER; Birsner and Grob, Freiburg, Germany) previously shown to block P. aeruginosa internalization (29). This domain in CFTR binds to P. aeruginosa lipopolysaccharide (LPS), an event required for uptake of P. aeruginosa (29). Pretreated bacteria were washed once with phosphate-buffered saline (PBS) and immediately employed for infection of Chang epithelial cells. Possible toxic effects of the CFTR peptide on the viability of P. aeruginosa were excluded by plating untreated and CFTR-treated bacteria on tryptic soy agar plates and determining the number of CFU.
For the production of PAO-I supernatants, mid-logarithmic PAO-I cultures were pelleted by centrifugation and subsequently sterile filtered through a 0.2 μM filter (see Fig. 1F). In addition, PAO-I supernatants were produced by culturing 3 × 107, 3 × 108, or 3 × 109 PAO-I for 3 h at 37°C in 7 ml of RPMI medium (see Table 2 and Fig. 1B and 2B). After the bacteria were pelleted by centrifugation, supernatants were filtered through a 0.2 μM sterile filter unit and used for the treatment of Chang epithelial cells. These conditions correspond to those of infection experiments, with a bacteria-to-cell ratio of 10:1, 100:1, or 1,000:1. All preparations yielded equivalent results. P. aeruginosa PAO-I was heat inactivated by incubation for 20 min at 80°C. Supernatants and heat-inactivated samples were tested for the presence of growing bacteria by inoculation of 10 to 100 μl on tryptic soy broth. No viable bacteria could be detected. Equal volumes of heat-inactivated bacteria or bacterial suspensions representing equal numbers of bacteria were used for infection experiments.
FIG. 1.
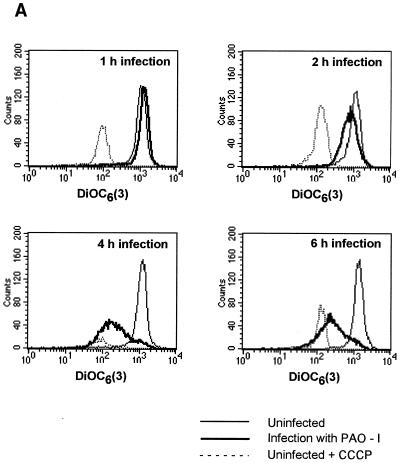
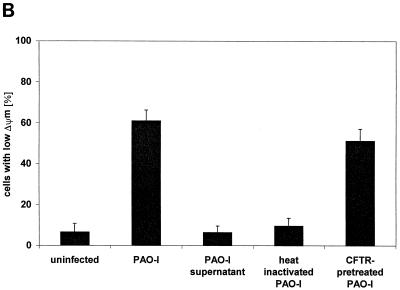
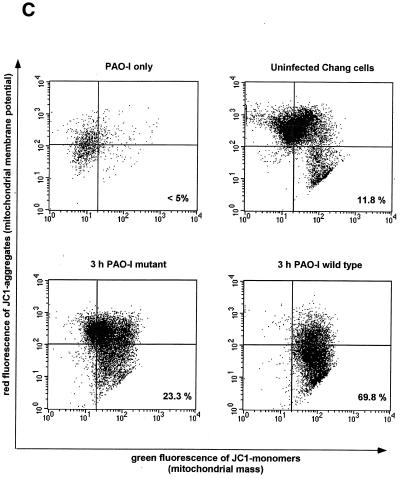
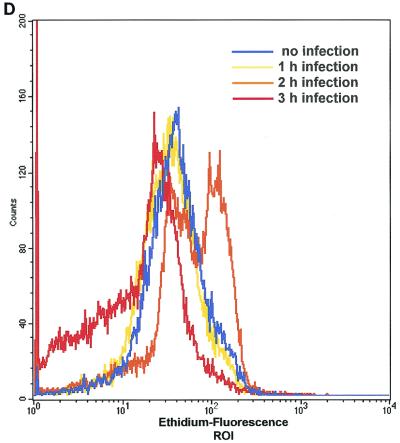
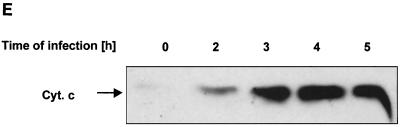
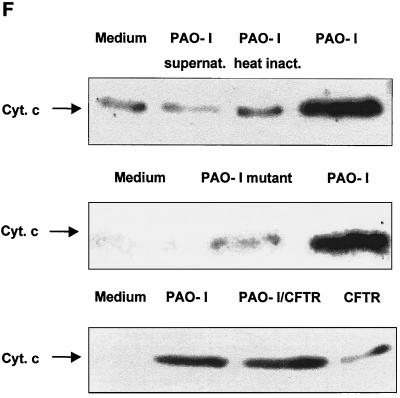
Infection of Chang epithelial cells with P. aeruginosa PAO-I induces alterations of mitochondrial functions. (A to C) PAO-I infection of Chang epithelial cells induces a progressive mitochondrial depolarization which is not observed after infection with heat-inactivated PAO-I, PAO-I supernatants, or a type III secretion system-deficient PAO-I mutant. Pretreatment with CFTR peptide does not alter mitochondrial depolarization. Likewise, infection induces a synthesis of ROS (D) and a release of cytochrome c into the cytosol of infected cells (E and F). Chang conjunctiva epithelial cells were infected with the indicated P. aeruginosa PAO-I preparations for the indicated time (A) or 3 h (B and C), stained with DiOC6(3) (A and B) or JC1 (C), and subjected to FACS analysis. Depolarization with carbonyl cyanide-m-chlorophenylhydrazone served as a positive control (A). In panel C, cells in the lower right quadrant represent cells with depolarized mitochondrial membrane potential. For determination of ROS synthesis (D), Chang cells were infected with P. aeruginosa PAO-I for the indicated time and stained with DHE, and ROS synthesis was analyzed by FACS. Cytochrome c (Cyt. c) release (E and F) was determined by immunoblotting cytosol preparations from uninfected Chang cells or Chang cells infected for the indicated times (E) or 3 h (F) with PAO-I as indicated. Data are representative of three (A, C, D, and E) or two (F) similar experiments. The bars in panel B show means ± SD.
TABLE 2.
PAO-I-induced apoptosis requires adhesion of live bacteria expressing a functional type III secretion systema
| Preparations used for infection | % Annexin-positive cells |
|---|---|
| No bacteria | 11.5 ± 2.9 |
| PAO-I wild type | 33.2 ± 4.9 |
| PAO-I supernatant | 11.4 ± 3.2 |
| PAO-I wild type, heat inactivated | 13.5 ± 4.7 |
| CFTR peptide-pretreated PAO-I wild type | 26.7 ± 2.9 |
| Type III secretion system-deficient PAO-I | 17.2 ± 5.6 |
Chang cells were left uninfected or infected for 3 h with PAO-I, PAO-I supernatants (from 3 × 109 PAO-I; 3 h), heat-inactivated PAO-I (20 min; 80°C), PAO-I pretreated with CFTR peptide (15 min; 1 μM), or a type III secretion system-defective PAO-I mutant, respectively. Apoptotic cell death was then determined by FACS analysis of FITC-annexin-stained cells. Cell debris and bacteria were excluded from the analysis by means of size gates (values are means ± SD; n = 3).
FIG. 2.
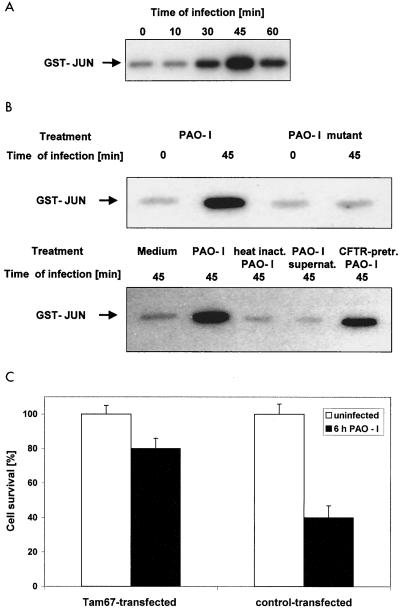
P. aeruginosa PAO-I induces activation of JNK. (A) P. aeruginosa PAO-I infection of Chang epithelial cells induces rapid activation of JNK. Cells were infected with PAO-I for the indicated time and lysed, JNK was immunoprecipitated, and kinase activity was determined by phosphorylation of GST-c-JUN followed by separation by SDS-PAGE, blotting, and autoradiography. Shown is a blot representative of three similar experiments. (B) PAO-I supernatants, heat-inactivated PAO-I, and type III secretion system-deficient P. aeruginosa PAO-I fail to activate JNK, whereas CFTR-pretreated PAO-I induced JNK activation. Cells were left uninfected or infected for 45 min as indicated. JNK activity was determined as described above. Experiments were repeated twice with similar results. (C) Transient transfection of Chang cells with Tam67 blocking JNK reduces apoptosis induced by P. aeruginosa infection. Shown is the percent survival of the cells as determined by DNA fragmentation after 6 h of infection (mean ± SD of three experiments).
Quantification of apoptosis.
Cells were washed twice in 10 mM HEPES (pH 7.4), 140 mM NaCl, and 5 mM CaCl2, resuspended at a concentration of 2 × 105 cells/200 μl in the same buffer supplemented with fluorescein isothiocyanate (FITC)-annexin (dilution, 1:100; Roche Biochemicals, Mannheim, Germany), incubated for 15 min, and subjected to fluorescence-activated cell sorter (FACS) analysis employing a FACS-calibur flow cytometer using the CELLQuest software (Becton Dickinson, Mountain View, Calif.). Morphological changes typical for apoptosis were identified by trypan blue staining of the cells. Those changes included chromatin condensation, nuclear fragmentation, and blebbing.
Alternatively, apoptosis was quantified by determination of DNA fragmentation. To this end, cells were in vivo labeled with 10 μCi of [3H]thymidine (80 Ci/mmol; NEN-DuPont)/ml for 24 h prior to the infection. Cells were harvested and disrupted by one cycle of freezing at −20°C and thawing, and unfragmented genomic DNA was collected by filtration through glass fiber filters (Pharmacia, Freiburg, Germany) and counted by liquid scintillation. Results are expressed as percent DNA fragmentation ± the standard deviation (SD) compared to those for control samples. Experiments were done in triplicate and repeated two times.
Cytochrome c release.
Cells were incubated for 30 min on ice in a solution containing 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES)-KOH (pH 7.4), 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM dithiothreitol, 10 μM cytochalasin B, and 10 μM (each) aprotinin and leupeptin. After Dounce homogenization, samples were centrifuged at 15,000 × g for 15 min. The supernatants were added to 5× reducing sodium dodecyl sulfate (SDS) sample buffer (60 mM Tris-HCl (pH 6.8), 2.3% SDS, 10% glycerol, and 5% β-mercaptoethanol) and boiled for 5 min at 95°C, and aliquots of the cytosolic fraction corresponding to 20 μg of total protein were subjected to SDS–15% polyacrylamide gel electrophoresis (PAGE). Proteins were transferred onto nitrocellulose membranes (Hybond; Amersham Pharmacia Biotech), the membranes were blocked for 1 h with 4% bovine serum albumin, and cytochrome c was detected by incubation with a monoclonal mouse anti-cytochrome c antibody (clone 7H8.2C12; Pharmingen) followed by alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) (Santa Cruz Inc., Santa Cruz, Calif.) and development with the Tropix enhanced chemiluminescence detection system (PE Applied Biosystems, Bedford, Mass.). Equal protein loading was controlled by Ponceau Red staining of the blot membranes. To further control equal amounts of total protein in each sample, the blots were stripped with 62.5 mM Tris-Cl (pH 6.8), 2% SDS, and 100 mM β-mercaptoethanol for 30 min at 60°C and reprobed with a mouse monoclonal anti-actin antibody (Roche Molecular Biochemicals, Mannheim, Germany).
Assessment of mitochondrial function.
For determination of mitochondrial membrane potential, cells were washed twice with PBS and then loaded with the cationic lipophilic fluorochrome DiOC6(3) (200 pM) by incubation for 30 min at 37°C. Cells were washed in PBS and submitted to FACS analysis. As a positive control, cells were subsequently treated for 15 min with a 1 μM concentration of the uncoupling agent carbonyl cyanide-m-chlorophenylhydrazone. Alternatively, cells were resupended in complete RPMI medium and loaded with the cationic lipophilic fluorochrome JC1 (2.5 μg/ml) for 10 min at 37°C. Cells were washed twice with PBS and submitted to FACS analysis. The red fluorescence of JC1 aggregates corresponded to the mitochondrial membrane potential, whereas the green fluorescence of JC1 monomers was indicative of the mitochondrial mass.
The production of reactive oxygen species was analyzed by staining of the cells with DHE. To this end, PBS-washed cells were incubated with DHE (5 μM) for 30 min at room temperature, washed, resuspended at a concentration of 106 cells/ml in PBS, and submitted to FACS analysis. Forward and side scatter were used to establish size gates and to exclude bacteria and cellular debris from the analysis.
CD95 translocation.
Cells were harvested using cell dissociation solution supplemented with 10 mM 1,10 phenanthroline (Sigma-Aldrich). Cells were washed twice with PBS containing 10 mM 1,10 phenanthroline and resuspended in a solution containing 132 mM NaCl, 1 mM CaCl2, 0.7 mM MgCl2, 20 mM HEPES (pH 7.3), 5.4 mM KCl, 0.8 mM MgSO4, 0.2% sodium azide, 2% FCS, and 10 mM 1,10 phenanthroline. Cells were then incubated for 45 min at 4°C with mouse anti-human CD95 (clone CH11; Biozol, Eching, Germany) or hamster anti-mouse CD95 (clone JO2; Becton Dickinson, Heidelberg, Germany), washed, and incubated for an additional 45 min with FITC-coupled goat anti-mouse or goat anti-hamster IgG (Becton Dickinson). Finally, cells were washed and subjected to FACS analysis.
JNK and p38-K activity.
To measure the activity of JNK and p38-K, cells were infected for the time indicated and lysed in a solution containing 25 mM HEPES (pH 7.4), 0.2% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, 125 mM NaCl, 10 mM (each) sodium fluoride, Na3VO4, and sodium pyrophosphate, and 10 μg (each) of aprotinin and leupeptin/ml. Lysates were centrifuged at 25,000 × g for 20 min, and JNK or p38-K was immunoprecipitated from the supernatant at 4°C for 1 h using polyclonal rabbit anti-human JNK or p38-K antisera (Santa Cruz Inc.). Immunocomplexes were immobilized on protein A/G (Santa Cruz Inc.), incubated for an additional 45 min at 4°C, washed twice in lysis buffer, twice in a buffer containing 132 mM NaCl, 20 mM HEPES, 5 mM KCl, 1 mM CaCl2, 0.7 mM MgCl2, 0.8 mM MgSO4, 1% NP-40 and 2 mM Na3 VO4, once in 100 mM Tris (pH 7.5)–0.5 M LiCl, and finally twice in kinase buffer consisting of 12.5 mM morpholinepropanesulfonic acid (pH 7.5), 12.5 mM β-glycerophosphate, 0.5 mM EGTA, 7.5 mM MgCl2, 0.5 mM NaF, 0.5 mM Na3VO4. Immunoprecipitates were resuspended in kinase buffer supplemented with 10 μCi of [γ-32P]ATP (6,000 Ci/mmol; NEN-DuPont)/sample, 10 μM ATP, and 1 μg of glutathione-S-transferase (GST)-c-JUN (amino acids 1 to 79) or GST-ATF-2 (amino acids 1 to 96)/ml. The samples were incubated at 30°C for 15 min, and incubation was stopped by addition of 5 μl of boiling 5× reducing SDS sample buffer. Samples were separated by SDS–10% PAGE and analyzed by autoradiography. The substrates GST-c-JUN and GST-ATF-2 were expressed in DH5α bacteria by incubation with isopropyl-β-d-thiogalactopyranoside (200 μM) for 4 h. Bacteria were lysed in a solution containing 25 mM HEPES (pH 7.4), 0.2% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, 125 mM NaCl, and 10 μg (each) of aprotinin and leupeptin/ml; GST fusion proteins were purified by binding to glutathione agarose and eluted in kinase buffer supplemented with 20 mM glutathione. The purity of the preparations was tested by SDS-PAGE and Coomassie staining.
In order to normalize all samples for equal amounts of protein, the cells were in vivo labeled with [3H]choline chloride (75 Ci/mmol; NEN-DuPont) for 24 h, and an aliquot of the lysates was scintillation counted. The immunoprecipitates were then adjusted for equal amounts of cell equivalents.
Inhibition of JNK by transfection of tam67.
Cotransfection of Chang cells with transdominant inhibitory pCEV/tam67 (50 μg/5 × 106 cells) or vector control (pCEV) with an expression vector for CD20 (pRc/CMV-cd20) (10 μg/5 × 106 cells) was performed with lipofectamine. After 12 h, cells were washed and labeled with [3H]thymidine for 24 h. Cells were then infected for 30 min and collected using cell dissociation solution, and CD20+ cells were sorted by incubation with 50 μg of a mouse anti-CD20 antibody (Dianova, Hamburg, Germany)/ml (60 min; 4°C). Cells were washed three times and further incubated (60 min; 4°C) with magnetic beads coated with a sheep anti-mouse Ig (Dynal, Hamburg, Germany) (14). Since the 5:1 ratio of pCEV/Tam67 to pRc/CMV-cd20 drives expression of Tam67 in any CD20+ cell, the selection of CD20+ cells permits effective sorting for Tam67-expressing cells. Apoptosis was determined by DNA fragmentation as described above. Trypan blue staining, detecting morphological changes indicative for apoptosis, served as a control. These changes included cell rounding, membrane blebbing, and chromatin condensation.
FACS experiments to determine CD95 expression of Tam67/CD20-transfected cells were performed as described above and were repeated twice.
Statistical evaluation.
All experiments were performed at least twice. Results are presented as means ± standard deviation (SD) if not otherwise indicated. Significance of the results was analyzed by the Student's t test.
RESULTS
PAO-I-induced apoptosis of Chang epithelial cells requires bacterial adhesion and expression of the type III secretion system.
To gain insight into the interactions between P. aeruginosa and mammalian cells, we investigated whether the well-defined laboratory P. aeruginosa strain PAO-I induces apoptosis of human Chang epithelial cells. The results reveal morphologic alterations of the cells indicative for apoptosis, i.e., cell rounding, membrane blebbing, and breakdown of phosphatidylserine asymmetry, as early as 3 h after infection with PAO-I. Apoptosis increased in the following hours, and complete cell death was observed within 9 h after initiation of the infection (Table 1).
TABLE 1.
Infection of Chang conjunctiva epithelial cells with wild-type P. aeruginosa PAO-I results in a time-dependent breakdown of phosphatidylserine asymmetry in the cytoplasm membranea
| Period of expt (h) | % Annexin-positive cells
|
|
|---|---|---|
| No infection | Infection with PAO-I | |
| 0 | 8.9 ± 1.5 | 9.1 ± 2.7 |
| 3 | 12.3 ± 1.1 | 28.0 ± 4.8 |
| 6 | 14.6 ± 2.5 | 53.7 ± 4.9 |
| 9 | 24.0 ± 5.0 | 81.1 ± 4.3 |
Chang conjunctiva epithelial cells were infected with P. aeruginosa PAO-I for the indicated time, stained with FITC-annexin, and subjected to FACS analysis. Data are the means ± SD of results from three experiments.
To identify further constituents of P. aeruginosa PAO-I-induced apoptosis of mammalian cells, we compared induction of apoptosis by live and heat-inactivated bacteria. In addition, we tested whether soluble proteins released by P. aeruginosa PAO-I were sufficient to trigger apoptotic cell death. To this end, we performed infection experiments with supernatants of PAO-I cultures. Our results show that neither heat-inactivated bacteria nor supernatants produced from 3 × 109 P. aeruginosa PAO-I during 3 h of culture killed Chang epithelial cells (Table 2). Upon adhesion, P. aeruginosa is internalized into epithelial cells (11, 19), which seems to be mediated by binding of bacterial LPS to CFTR, since invasion is blocked by preincubation of bacteria with a CFTR peptide representing the binding domain of LPS to CFTR (29). To investigate whether PAO-I-induced apoptosis was dependent on invasion, we performed infection experiments with CFTR peptide-pretreated PAO-I. The results demonstrate that pretreatment with the CFTR peptide did not decrease the cytotoxicity of PAO-I (Table 2). Control experiments demonstrated that pretreatment with the CFTR peptide almost completely blocked internalization of P. aeruginosa PAO-I into Chang cells, whereas adhesion was even increased (not shown). These data suggest that invasion of PAO-I is not required for its cytotoxic action and that the adhesion of live bacteria seems to be sufficient to induce apoptosis.
To further characterize bacterial constituents involved in cellular apoptosis, we tested the role of the bacterial type III secretion system, which is required for the delivery of several toxins to host cells (7). To this end, we performed infection experiments with wild-type PAO-I and with a type III secretion system-negative PAO-I mutant (20). The results reveal that the PAO-I type III secretion mutant almost completely failed to induce apoptosis of Chang cells (Table 2).
PAO-I infection induces alterations of mitochondrial functions.
To identify molecular mechanisms mediating P. aeruginosa-induced apoptosis of mammalian cells, we tested for an alteration of mitochondria, which have been shown to play a pivotal role in many forms of apoptotic cell death (13, 22). To examine whether infection of Chang epithelial cells with PAO-I induces a reduction of the mitochondrial membrane potential (Δψm), cells were harvested at various time points after infection and Δψm was analyzed after loading the cells with the cationic fluorescent dye DiOC6(3). The results demonstrate a time-dependent increase in the number of cells with a reduced Δψm (Fig. 1A). A moderate reduction in the Δψm of Chang cells was observed as early as 2 h after addition of the bacteria to the cells. The amount of cells with low Δψm and the extent of depolarization increased during prolonged infection periods, yielding about 60% living cells with low Δψm after 3 h of infection (Fig. 1B). All cells showed Δψm depolarization 4 to 6 h after infection (Fig. 1A). Supernatants of PAO-I cultures or heat-inactivated bacteria completely failed to induce a significant depolarization of Δψm (Fig. 1B). In contrast, inhibition of bacterial internalization by pretreatment with the CFTR peptide did not influence mitochondrial changes induced by P. aeruginosa (Fig. 1B), indicating that invasion is not required for mitochondrial changes during apoptosis. Deficiency of the type III secretion system in P. aeruginosa almost completely abolished the bacterial effect on Δψm (Fig. 1C).
To test whether mitochondrial membrane potential depolarization upon infection is linked to hyperproduction of reactive oxygen species, we performed FACS analyses of DHE-stained Chang cells. The ethidium derivative DHE penetrates into the cytosol of living cells, where it is oxidized in the presence of reactive oxygen intermediates (ROS) and peroxides to fluorescent products (39). The results confirm the notion of induction of mitochondrial changes by PAO-I and show an increase of ROS in PAO-I-infected Chang cells peaking 2 h after the start of the infection (Fig. 1D).
Next we examined whether treatment of Chang cells with PAO-I induces release of cytochrome c, a mitochondrial protein localized in the mitochondrial intermembrane space, into the cytosol. Our results reveal that cytochrome c is released from the mitochondria into the cytosol 2 to 3 h after P. aeruginosa infection (Fig. 1E), reaching a maximum at 4 h of infection. The time course of cytochrome c release was very similar to that observed for Δψm or induction of phosphatidylserine asymmetry (Fig. 1A and Table 1). Supernatants of PAO-I cultures, heat-inactivated bacteria, or type III secretion-deficient PAO-I mutants failed to induce significant cytochrome c release from mitochondria, whereas inhibition of bacterial internalization by pretreatment with the CFTR peptide did not change cytochrome c release (Fig. 1F).
PAO-I induces JNK activation.
To identify further pathways involved in P. aeruginosa-initiated apoptosis of mammalian cells, we tested the activation of Jun N-terminal kinases, which have been previously implicated in apoptosis signaling, in particular upon application of stress stimuli (36). To this end, Chang epithelial cells were infected with PAO-I, JNK, or p38-K and were immunoprecipitated, and the activity of the kinases was determined in an in vitro kinase assay. The results reveal a marked and rapid activation of JNK upon infection with PAO-I (Fig. 2A). In contrast, no significant activation of p38 kinases could be detected (data not shown). Deletion of the type III system in P. aeruginosa almost completely abrogated activation of JNK upon infection (Fig. 2B). In addition, PAO-I supernatants and heat-inactivated PAO-I failed to activate JNK, whereas CFTR-pretreated PAO-I activated JNK to the same extent as PAO-I (Fig. 2B). To determine the significance of JNK activation for P. aeruginosa-induced apoptosis, we transiently transfected cells with Tam67, whose product functions as a pseudosubstrate for JNK and prevents phosphorylation of endogenous substrates of JNK, thus functionally blocking JNK. Expression of Tam67 reduced apoptosis by 65%, suggesting that the activation of JNK by PAO-I plays a pivotal role in the induction of apoptosis by P. aeruginosa (Fig. 2C).
PAO-I triggers apoptosis via cellular CD95.
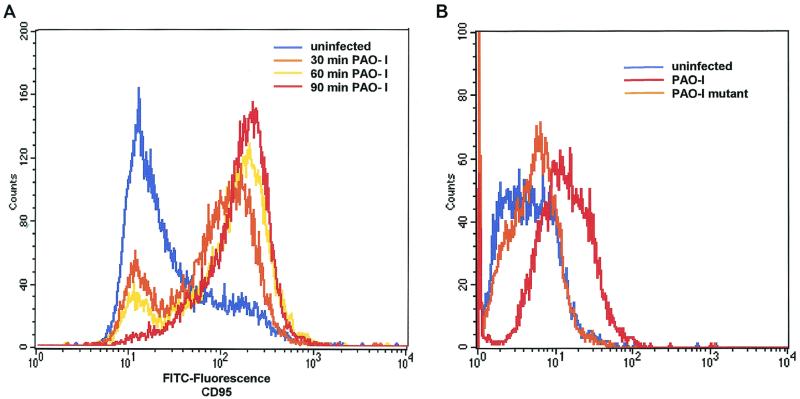
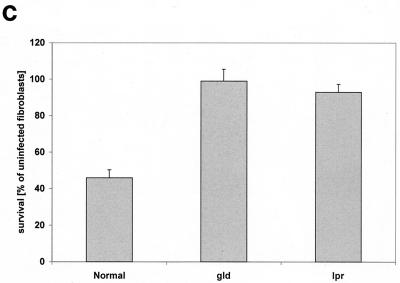
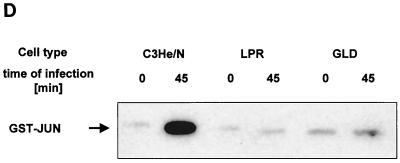
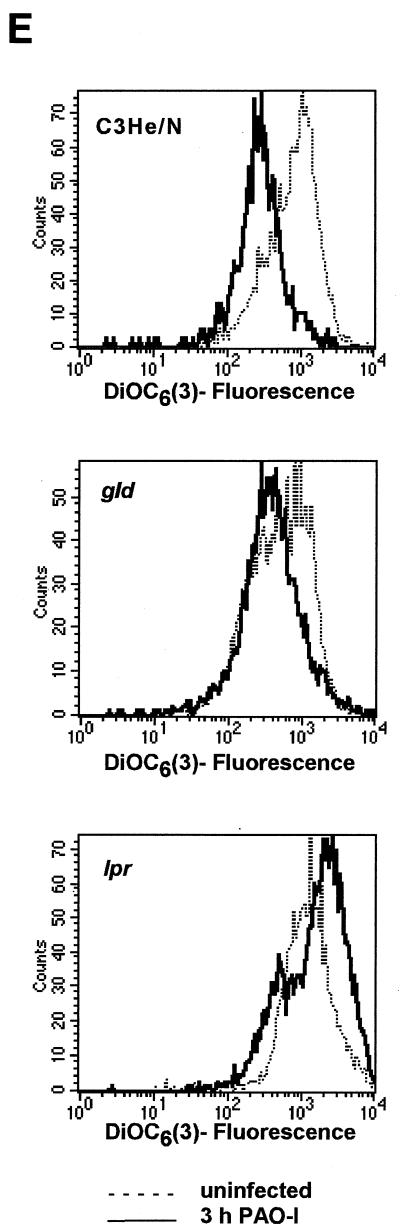
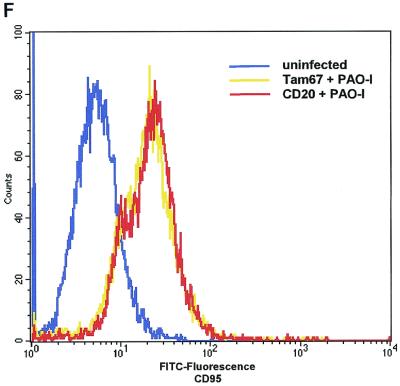
An upregulation of cell surface CD95 and CD95 ligand resulting in the activation of this death receptor has been recently shown to be pivotal for the induction of apoptosis by several P. aeruginosa strains (12). We therefore investigated whether PAO-I infection also induces an increased cell surface expression of CD95 and whether the observed alterations of mitchondria and JNK activity depend on the cellular CD95 and CD95 ligand. Our results show that PAO-I infection triggers a time-dependent upregulation of CD95 expression on the surface of Chang epithelial cells (Fig. 3A), whereas infection with type III secretion system-deficient mutant P. aeruginosa almost completely failed to upregulate CD95 (Fig. 3B). The significance of CD95 upregulation upon infection with PAO-I is indicated by the finding that fibroblasts from lpr or gld mice, which lack a functional CD95 or CD95 ligand, failed to undergo significant apoptosis (Fig. 3C), to activate JNK (Fig. 3D), or to induce mitochondrial depolarization (Fig. 3E) upon infection with PAO-I. Control fibroblasts from C3He/N mice expressing functional CD95 and CD95 ligand readily responded to the infection (Fig. 3C to E). Vice versa, inhibition of JNK by Tam 67 expression did not inhibit CD95 upregulation, indicating that CD95 acts upstream of JNK in the induction of apoptosis (Fig. 3F). These data suggest an important role of CD95 in PAO-I induced apoptotic signaling.
FIG. 3.
P. aeruginosa PAO-I triggers apoptosis via cellular CD95. Infection with P. aeruginosa PAO-I triggers a time-dependent upregulation of CD95 on the surface of Chang epithelial cells (A), which is dependent on a functional type III secretion system (B). Mouse fibroblasts lacking functional CD95 (lpr) or a CD95 receptor (gld) fail to undergo apoptosis (C), JNK activation (D), or complete mitochondrial depolarization (E) in response to infection with PAO-I. PAO-I-induced CD95 upregulation is not prevented by functional JNK inhibition (F). (A and B) Human Chang epithelial cells were left uninfected or infected as indicated or for 90 min (B) with P. aeruginosa PAO-I. CD95 exposure on the cell surface was determined by FACS analysis of immunostained cells. (C) Primary lung fibroblasts from C3He/N (control), lpr, and gld mice were labeled for 24 h with [3H]thymidine and infected for 6 h with PAO-I. Apoptosis was determined by DNA fragmentation. Shown is the percentage of DNA fragmentation ± SD of three independent experiments. (D) Primary lung fibroblasts from C3He/N (control), lpr, and gld mice were infected for 45 min with PAO-I. Cells were lysed, JNK was immunoprecipitated, and JNK activity was determined by phosphorylation of GST-c-JUN followed by separation by SDS-PAGE, blotting, and autoradiography. (E) Primary lung fibroblasts from C3He/N (control), lpr, and gld mice were infected for 3 h with PAO-I. The Δψm was then determined by FACS analysis after loading of the cells with DiOC6(3). (F) Control or Tam67-transfected Chang cells were infected with PAO-I for 45 min, and CD95 expression was determined by FACS analysis after immunostaining for CD95. Shown are representative results. Experiments were repeated twice.
DISCUSSION
In the present study we demonstrate the induction of apoptosis in mammalian epithelial cells upon infection with P. aeruginosa PAO-I and provide insight into the molecular details triggering apoptosis. We show that P. aeruginosa employs its type III secretion system to induce an upregulation of CD95 on infected cells, resulting in stimulation of JNK and mitochondrial alterations, which finally triggers apoptosis. The study further reveals that adhesion, but not internalization, of P. aeruginosa PAO-I is required for induction of apoptosis in epithelial cells, which is in accordance with earlier studies showing a dissociation of cytotoxicity and bacterial internalization (8).
Induction of apoptosis of mammalian host cells has been shown for several bacteria, including Y. enterocolitica (25), E. coli (6), S. aureus (24), Salmonella serovar Typhimurium (17), S. flexneri (40), and L. monocytogenes (30). However, the signaling machinery in the host cell that triggers cell death is still largely unknown. Our results indicate the activation of two distinct proapoptotic signaling pathways by P. aeruginosa. First, P. aeruginosa infection induces several changes of mitochondria typical for apoptosis. Those changes include cytochrome c release, synthesis of reactive oxygen intermediates, and Δψm depolarization, hallmarks of signaling in apoptosis. Second, P. aeruginosa infection triggers activation of JNK, which has been previously shown to be activated by several proapoptotic stimuli (2, 36). The significance of JNK activation is suggested by the finding that inhibition of JNK by Tam67 transfection at least partially prevents P. aeruginosa-triggered apoptosis. A recent study revealed a translocation of the stress-activated protein kinases (SAPK)/JNK to the mitochondrium and association of the kinase with Bcl-xL upon irradiation of the cells (21). Translocated SAPK/JNK phosphorylated and thus inactivated Bcl-xL, suggesting a link between the mitochondrial and the SAPK/JNK pathways in apoptosis, which might be also functional upon infection of epithelial cells with P. aeruginosa. The suggestion of a mechanistic link between these two death-inducing pathways is further supported by results demonstrating a requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway (34).
Both mitochondrial changes and JNK activation seem to be initiated by CD95-CD95 ligand interactions, which are promoted by cell surface upregulation of the receptor-ligand pair upon infection. Our data showing that JNK inhibition by Tam67 expression does not prevent CD95 upregulation exclude a significant function of JNK in this process. However, upregulation of CD95 by P. aeruginosa infection critically, but not completely, depends on expression of a type III secretion system in P. aeruginosa. This situation is reminiscent of induction of apoptosis by S. flexneri and Salmonella serovar Typhimurium, which introduce several proapoptotic proteins, including IpaB and SipB, into the infected cells (7, 17). However, these factors directly activate caspases and may not require the upregulation of endogenous death receptors as observed for P. aeruginosa (3, 12). The upregulation of endogenous death receptors also differs from the mechanisms of apoptosis induction employed by Y. enterocolitica, which induces cell death by inhibition of survival pathways rather than by direct triggering of cell death (25, 27, 31). Thus, the present study suggests a novel mechanism of type III secretion system-regulated apoptosis. The P. aeruginosa type III secretion system has been shown to be involved in the release of several toxins, including ExoS, ExoT, ExoU, and ExoY (20, 35). Expression of ExoU has been shown to correlate with the cytotoxicity of P. aeruginosa; however, cellular proteins targeted by ExoU are unknown (9). Since P. aeruginosa mutants lacking ExoU do not induce epithelial cell death, ExoU might be a good candidate to be involved in the upregulation of CD95 and CD95 ligand, finally mediating epithelial cell apoptosis. However, a recent study suggested that ExoU predominantly induced necrosis in macrophages (16), which was not observed in our experiments on human epithelial cells and murine primary lung fibroblasts. This study suggested that another yet-unidentified type III-dependent factor triggers apoptosis by P. aeruginosa (16). ExoS, which exhibits ADP-ribosyltransferase activity, and ExoT seem to be involved predominantly in the regulation of bacterial uptake, and it has been shown that they block internalization of P. aeruginosa into mammalian cells (5). The biological function of ExoY, which seems to be an adenylate cyclase, in bacterial toxicity and/or invasion is still unknown (37). Even if these considerations suggest ExoU as the primary candidate to induce CD95-CD95 ligand upregulation and apoptotic signaling, further studies—beyond the focus of the present study—employing different mutants have to show the role of these toxins in the induction of mammalian cell apoptosis by P. aeruginosa.
Our data show that deficiency of the type III system does not completely abrogate apoptosis upon infection with P. aeruginosa PAO-I. In particular, some limited mitochondrial changes triggered by P. aeruginosa infection are still detected after infection with the type III mutant. The notion of a type III system-independent apoptosis is consistent with earlier findings indicating that P. aeruginosa pili are also able to initiate apoptosis (4). Further, P. aeruginosa LPS is already sufficient to induce an upregulation of the Trail-APO 2 ligand in monocytes and macrophages (15); an effect of LPS on the CD95-CD95 ligand has yet to be tested. Thus, it is possible that multiple factors, including type III-dependent toxins, pili, and LPS, upregulate CD95 upon infection with P. aeruginosa.
Induction of host cell apoptosis upon infection with P. aeruginosa might be beneficial for the bacterium, since the apoptosis of epithelial cells may break the epithelial cell barrier and thus permit the bacterium to access the submucosa. On the other hand, the induction of apoptosis might be also beneficial for the host: the activation of JNK during apoptosis might result in AP-I-dependent gene transcription and finally the synthesis and release of cytokines and/or defensins, killing the bacteria and/or protecting other cells from the infection. Second, apoptotic bodies are rapidly internalized by neighboring cells, and the uptake of P. aeruginosa packed in those apoptotic bodies may enable the cells to fuse the endosome with lysosomes and, thus, to digest the pathogen. Finally, the presentation of bacterial antigens by dendritic cells is most effectively activated by a combination of apoptotic and necrotic cells (38). Therefore, the induction of apoptosis might be an initial step in the specific immune response to P. aeruginosa.
In summary, we provide evidence for the activation of several signaling pathways by P. aeruginosa PAO-I to trigger apoptosis of mammalian host cells. In particular, the activation of the JNK signaling pathway and the alterations of mitochondria seem to play a pivotal role in the induction of mammalian cell apoptosis by P. aeruginosa.
ACKNOWLEDGMENTS
This work was supported by a grant of the Deutsche Forschungsgemeinschaft Gu 335-10/2 and ALSAC to E.G.
REFERENCES
- 1.Ashkenazi A, Dixit V M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 2.Basu S, Kolesnick R N. Stress signals for apoptosis: ceramide and c-Jun kinase. Oncogene. 1998;17:3277–3285. doi: 10.1038/sj.onc.1202570. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Smith M R, Thirumalai K, Zychlinski A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 4.Comolli J C, Waite L L, Mostov K E, Engel J N. Pili binding to asialo-GM1 on epithelial cells can mediate cytotoxicity or bacterial internalization by Pseudomonas aeruginosa. Infect Immun. 1999;67:3207–3214. doi: 10.1128/iai.67.7.3207-3214.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowell B A, Chen D Y, Frank D W, Vallis A J, Fleiszig S M J. ExoT of cytotoxic Pseudomonas aeruginosa prevents uptake by corneal epithelial cells. Infect Immun. 2000;68:403–406. doi: 10.1128/iai.68.1.403-406.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crane J K, Majumdar S, Pickhardt D F. Host cell death due to enteropathogenic Escherichia coli has features of apoptosis. Infect Immun. 1999;67:2575–2584. doi: 10.1128/iai.67.5.2575-2584.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donnenberg M S. Pathogenic strategies of bacteria. Nature. 2000;406:768–774. doi: 10.1038/35021212. [DOI] [PubMed] [Google Scholar]
- 8.Evans D J, Frank D W, Finck-Barbançon V, Wu C, Fleiszig S M. Pseudomonas aeruginosa invasion and cytotoxicity are independent events, both of which involve protein tyrosine kinase activity. Infect Immun. 1998;66:1453–1459. doi: 10.1128/iai.66.4.1453-1459.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finck-Barbançon V, Goranson J, Zhu L, Sawa T, Wiener-Kronish J P, Fleiszig S M, Wu C, Mende-Mueller L, Frank D W. ExoU expression by Pseudomonas aeruginosa correlates with acute cytotoxicity and epithelial injury. Mol Microbiol. 1997;25:547–557. doi: 10.1046/j.1365-2958.1997.4891851.x. [DOI] [PubMed] [Google Scholar]
- 10.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 11.Fleiszig S M, Zaidi T S, Fletcher E L, Preston M J, Pier G B. Pseudomonas aeruginosa invades corneal epithelial cells during experimental infection. Infect Immun. 1994;62:3485–3493. doi: 10.1128/iai.62.8.3485-3493.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grassmé H, Kirschnek S, Riethmueller J, Riehle A, von Kürthy G, Lang F, Weller M, Gulbins E. Host defense to Pseudomonas aeruginosa requires CD95/CD95 ligand interactions on epithelial cells. Science. 2000;290:527–530. doi: 10.1126/science.290.5491.527. [DOI] [PubMed] [Google Scholar]
- 13.Green D R, Reed J C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 14.Gulbins E, Bissonnette R, Mahboubi A, Martin S, Nishioka W, Brunner T, Baier G, Baier-Bitterlich G, Byrd C, Lang F, Kolesnick R N, Altman A, Green D. FAS-induced apoptosis is mediated via a ceramide-initiated RAS signaling pathway. Immunity. 1995;2:341–351. doi: 10.1016/1074-7613(95)90142-6. [DOI] [PubMed] [Google Scholar]
- 15.Halaas O, Vik R, Ashkenazi A, Espevik T. Lipopolysaccharide induces expression of APO2 ligand/TRAIL in human monocytes and macrophages. Scand J Immunol. 2000;51:244–250. doi: 10.1046/j.1365-3083.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- 16.Hauser A R, Engel J N. Pseudomonas aeruginosa induces type-III-secretion-mediated apoptosis of macrophages and epithelial cells. Infect Immun. 1999;67:5530–5537. doi: 10.1128/iai.67.10.5530-5537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hersh D, Monack D M, Smith M R, Ghori N, Falkow S, Zychlinski A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hilbi H, Moss J E, Hersh D, Chen Y, Arondel J, Banerjee S, Flavell R A, Yuan J, Sansonetti P J, Zychlinsky A. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem. 1998;273:32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 19.Hirakata Y, Izumikawa K, Yamaguchi T, Igimi S, Furuya N, Maesaki S, Tomono K, Yamada Y, Kohno S, Yamaguchi K, Kamihira S. Adherence to and penetration of human intestinal Caco-2 epithelial cell monolayers by Pseudomonas aeruginosa. Infect Immun. 1998;66:1748–1751. doi: 10.1128/iai.66.4.1748-1751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornef M W, Roggenkamp A, Geiger A M, Hogardt M, Jacobi C A, Heesemann J. Triggering the ExoS regulon of Pseudomonas aeruginosa: a GFP-reporter analysis of exoenzyme (Exo) S, ExoT and ExoU synthesis. Microb Pathog. 2000;29:329–343. doi: 10.1006/mpat.2000.0398. [DOI] [PubMed] [Google Scholar]
- 21.Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, Cheng K, Chen Y N, Campbell A, Sudha T, Yuan Z M, Narula J, Weichselbaum R, Narlin C, Kufe D. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-xL in response to DNA-damage. J Biol Chem. 2000;275:322–327. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- 22.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Annu Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 23.McIlroy D, Sakahira H, Talanian R V, Nagata S. Involvement of caspase 3-activated DNase in internucleosomal DNA cleavage induced by diverse apoptotic stimuli. Oncogene. 1999;18:4401–4408. doi: 10.1038/sj.onc.1202868. [DOI] [PubMed] [Google Scholar]
- 24.Menzies B E, Kourteva I. Internalization of Staphylococcus aureus by endothelial cells induces apoptosis. Infect Immun. 1998;66:5994–5998. doi: 10.1128/iai.66.12.5994-5998.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunez G, Benedict M A, Hu Y, Inohara N. Caspases: the proteases of the apoptotic pathway. Oncogene. 1998;17:3237–3245. doi: 10.1038/sj.onc.1202581. [DOI] [PubMed] [Google Scholar]
- 27.Palmer L E, Hobbie S, Galán J E, Bliska J B. YopJ of Yersinia pseudo-tuberculosis is required for the inhibition of macrophage TNF-production and downregulation of the MAP kinase p38 and JNK. Mol Microbiol. 1998;27:953–965. doi: 10.1046/j.1365-2958.1998.00740.x. [DOI] [PubMed] [Google Scholar]
- 28.Payne C M, Bernstein C, Bernstein H. Apoptosis overview emphasizing the role of oxidative stress, DNA damage and signal-transduction pathways. Leuk Lymphoma. 1995;19:43–93. doi: 10.3109/10428199509059662. [DOI] [PubMed] [Google Scholar]
- 29.Pier G B, Grout M, Zaidi T S. Cystic fibrosis transmembrane conductance regulator is an epithelial cell receptor for clearance of Pseudomonas aeruginosa from the lung. Proc Natl Acad Sci USA. 1997;94:12088–12093. doi: 10.1073/pnas.94.22.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers H W, Callery M P, Deck B, Unanue E R. Listeria monocytogenes induces apoptosis of infected hepatocytes. J Immunol. 1996;156:679–684. [PubMed] [Google Scholar]
- 31.Schesser K, Spiik A K, Dukuzumuremyi J M, Neurath M F, Petterson S, Wolf-Watz H. The yopJ locus is required for Yersinia-mediated inhibition of NFkappa B activation and cytokine expression: YopJ contains a eukaryotic SH-2 like domain that is essential for its repressive activity. Mol Microbiol. 1998;28:1067–1079. doi: 10.1046/j.1365-2958.1998.00851.x. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi A. Caspase: executioner and undertaker of apoptosis. Int J Hematol. 1999;70:226–232. [PubMed] [Google Scholar]
- 33.Thornberry N A, Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 34.Tournier C, Hess P, Yang D D, Xu J, Turner T K, Nimnual A, Bar-Sagi D, Jones S N, Flavell R A, Davis R J. Requirement of JNK for stress-induced activation of the cytochrome c mediated pathway. Science. 2000;288:870–874. doi: 10.1126/science.288.5467.870. [DOI] [PubMed] [Google Scholar]
- 35.Vallis A J, Finck-Barbançon V, Yahr T L, Frank D W. Biological effects of Pseudomonas aeruginosa type III secreted proteins on CHO cells. Infect Immun. 1999;67:2040–2044. doi: 10.1128/iai.67.4.2040-2044.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, Haimowitz-Friedman A, Fuks Z, Kolesnick R N. Requirement for ceramide-initiated SAPK/JNK signalling in stress-induced apoptosis. Nature. 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 37.Yahr T L, Vallis A J, Hancock M K, Barbieri J T, Frank D W. Exo Y, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci USA. 1999;95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yrlid U, Wick M. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J Exp Med. 2000;191:613–624. doi: 10.1084/jem.191.4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zamzami N, Marchetti P, Castedo I M, Decaudin D, Macho A, Hirsch T, Susin S A, Petit P X, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182:367–377. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 41.Zychlinsky A, Kenny B, Menard R, Prevost M C, Holland I B, Sansonetti P J. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994;11:619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]