Abstract
Expanding knowledge of the molecular mechanisms at the basis of tumor development, especially the cross-talk between oncogenic pathways, will possibly lead to better tailoring of anticancer therapies. Nuclear factor erythroid 2-related factor 2 (NRF2) plays a central role in cancer progression, not only because of its antioxidant activity but also because it establishes cross-talk with several oncogenic pathways, including Heat Shock Factor1 (HSF1), mammalian target of rapamycin (mTOR), and mutant (mut) p53. Moreover, the involvement of NRF2 in gammaherpesvirus-driven carcinogenesis is particularly interesting. These viruses indeed hijack the NRF2 pathway to sustain the survival of tumor cells in which they establish a latent infection and to avoid a too-high increase of reactive oxygen species (ROS) when these cancer cells undergo treatments that induce viral replication. Interestingly, NRF2 activation may prevent gammaherpesvirus-driven oncogenic transformation, highlighting how manipulating the NRF2 pathway in the different phases of gammaherpesvirus-mediated carcinogenesis may lead to different outcomes. This review will highlight the mechanistic interplay between NRF2 and some oncogenic pathways and its involvement in gammaherpesviruses biology to recapitulate published evidence useful for potential application in cancer therapy.
Keywords: NRF2, p53, gammaherpesviruses, oxidative stress, reactive oxygen species (ROS), cancer therapy, chemoresistance, p62/SQSTM1, inflammation, KEAP1, p21, mTOR, NFkB, apoptosis, autophagy, STAT3
1. Introduction
Nuclear factor erythroid 2-related factor 2 (NRF2) is a transcription factor that protects cells from oxidative stress by regulating various phase II detoxifying/antioxidant enzymes, such as heme oxygenase 1 (HO-1), NAD(P)H quinone oxidoreductase 1 (NQO1), catalase, superoxide dismutase (SOD), and glutathione (GSH) [1]. NRF2 activation must be tightly regulated to sustain cell survival, particularly in cancer cells characterized by a high level of intracellular reactive oxygen species (ROS) responsible for DNA damage induction [2]. ROS increase in cancer cells may be due to the accumulation of dysfunctional mitochondria due to the dysregulation of autophagy, particularly in its selective form, mitophagy [3,4]. Of note, NRF2 plays a key role in regulating several mitochondrial activities, e.g., it increases the mitochondrial membrane potential (ΔΨ) and the availability of substrates for respiration and adenosine triphosphate (ATP) production [5,6]. NRF2 can also increase nicotinamide adenine dinucleotide phosphate (NADPH) by up-regulating gene encoding glucose-6-phosphate dehydrogenase (G6PD), enzymes of the pentose phosphate pathway (PPP), malic enzyme 1 (ME1), and isocitrate dehydrogenase 1 (IDH1) [6].
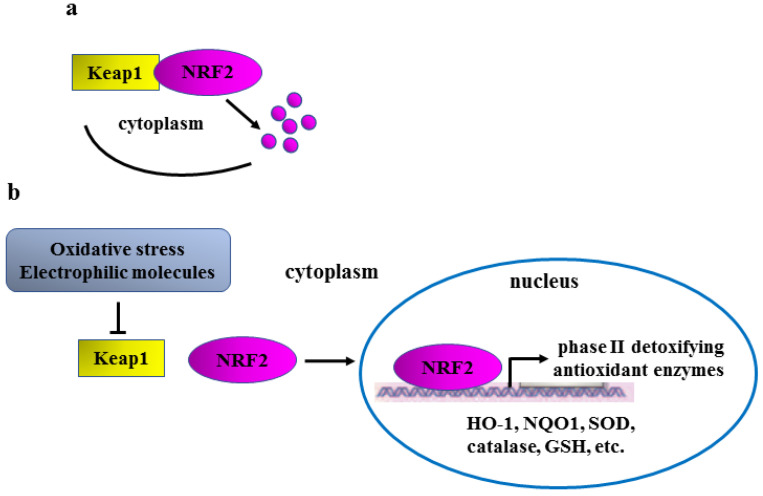
NRF2 activation is tightly regulated by Kelch-like ECH-associated protein 1 (Keap1), an ubiquitin ligase that, in unstressed conditions, interacts with NRF2 triggering its proteasomal degradation in the cytoplasm [7] (Figure 1a). Oxidative stress and/or electrophilic molecules, such as plant-derived phenolic compounds, modify cysteine residues 15, 16, and 17 within Keap1 protein, changing its conformation and thus preventing its binding to NRF2 for proteasomal degradation (Figure 1b, left part). Following this “canonical activation”, NRF2 is stabilized and can then translocate into the nucleus to induce the transcription of phase II detoxifying/antioxidant target genes, including HO-1, NQO1, catalase, SOD, and GSH (Figure 1b, right part), to restore cellular redox homeostasis [8].
Figure 1.
Schematic representation of Keap1/NRF2 regulation. (a) In unstressed conditions, Keap1 binds to NRF2, inducing its proteasomal degradation (purple dots). (b) Oxidative stress or electrophilic molecules change Keap1 conformation (inverted T sign), impairing Keap1/NRF2 binding (left part); NRF2 is therefore stabilized and can translocate into the nucleus to activate the transcription of phase II detoxifying/antioxidant genes (right part).
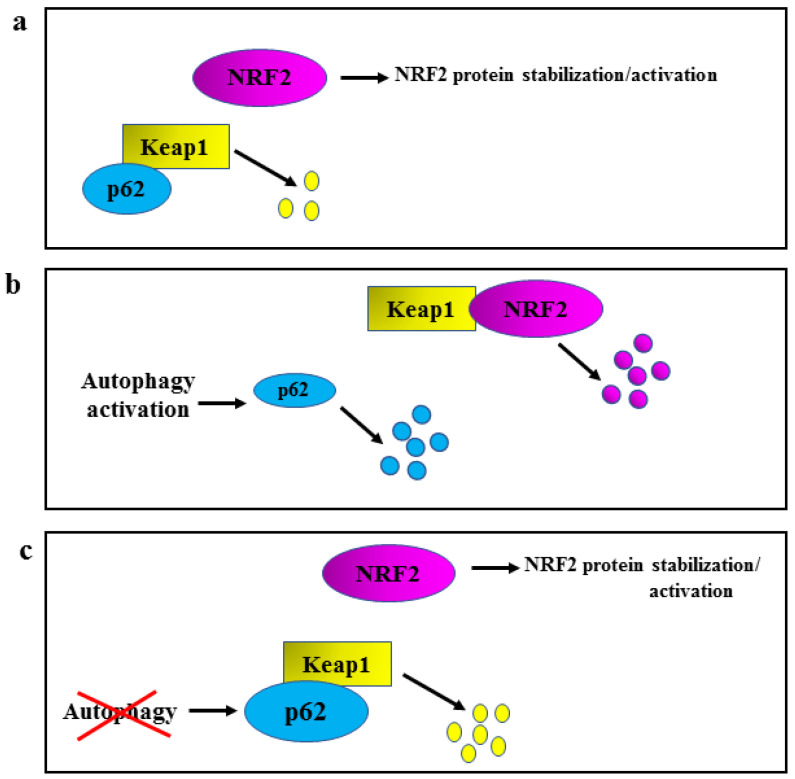
Another important molecule involved in NRF2 regulation is p62/sequestosome 1 (SQSTM1), which induces a “non-canonical stabilization/activation” of NRF2 by triggering Keap1 proteasomal degradation [9,10,11] (Figure 2a). Such regulation of NRF2 intervenes with autophagy, whose activation promotes p62/SQSTM1 degradation [9,10,11] (Figure 2b, left part). Indeed, the reduction of p62/SQSTM1 (degraded through autophagy) keeps Keap1 stabilized so it can bind NRF2 and induce NRF2 degradation (Figure 2b, right part); on the other hand, when autophagy is compromised by several means, p62/SQSTM1 accumulates and binds Keap1 triggering Keap1 proteasomal degradation (Figure 2c, lower part), and inducing the stabilization of NRF2 (Figure 2c, upper part) [9,10,11]. In this manner, the stabilization of NRF2 results as a compensatory mechanism to limit ROS increase due to autophagy dysregulation.
Figure 2.
Schematic representation of autophagy/p62/Keap1/NRF2 regulation. (a) p62/SQSTM1, herein shortened as p62, triggers a basal Keap1 proteasomal degradation (yellow dots) controlling NRF2 stabilization/activation. (b) Autophagy activation degrades p62 (left part, blue dots), stabilizing Keap1 that can bind NRF2 and induce NRF2 degradation (right part, purple dots). (c) When autophagy is compromised (red cross), p62 accumulates and can bind Keap1 and trigger Keap1 proteasomal degradation (yellow dots), inducing the stabilization of NRF2.
Similarly to p62, p21(Cip1/WAF1) (target of oncosuppressor p53) can bind to Keap1 and interrupt the Keap1/NRF2 interaction, activating NRF2 [12]. Moreover, a direct interaction between NRF2 and p21(Cip1/WAF1) has been also reported [12]. Post-translational modifications, such as phosphorylation, may also regulate NRF2 nuclear translocation. Intriguingly, phosphorylation may influence NRF2 activity both positively and negatively, depending on the residues that undergo phosphorylation and on the kinases that mediate this process. For example, protein kinase C (PKC) may positively influence NRF2 activity [13], while glycogen synthase kinase 3β (GSK-3β) inhibits it [14], underscoring the complex mechanisms involved in the regulation of NRF2 activity.
2. NRF2 and Oncogenic Pathways
Another fundamental function of NRF2 is to counteract inflammation that, along with ROS detoxification, plays a key role in preventing carcinogenesis, as evidenced by several studies performed in vitro and in vivo in animal models [15]. The role of NRF2 in inflammation and carcinogenesis mostly depends on its interaction with several molecular pathways. One of the most relevant pathways interacting with NRF2 is represented by heat shock factor 1 (HSF1), the master regulator of the heat shock response. HSF1 and NRF2 control overlapping target genes such as heat shock protein (HSP)70 and p62/SQSTM1 [16,17]. HSP70 plays a crucial role in the folding of proteins involved in key processes, such as the DNA damage response (DDR) of both DNA single and double-strand breaks [18]. HSP70 also sustains lysosomal membrane stability. In this regard, we have demonstrated that the pharmacological inhibition of HSP70 triggers necroptotic cell death in lymphoma cells due to the leakage of lysosomal proteases into the cytosol [19]. Of note, HSP70 collaborates with HSP90, another chaperone whose function is crucial for the folding of a variety of proteins, including oncogenes such as c-Myc and mutant p53 (mutp53) [20,21,22,23,24].
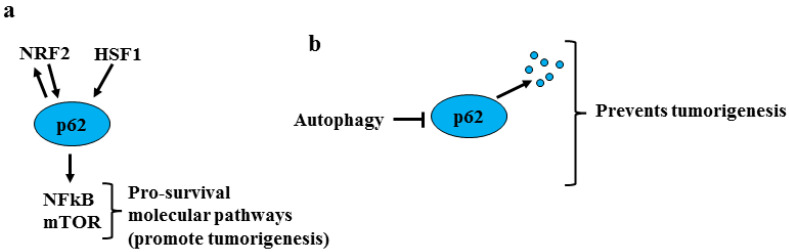
The other common target of NRF2 and HSF1 is p62/SQSTM1, known to play a pro-tumorigenic role not only because it promotes NRF2 stabilization [9] but also because it activates pro-survival molecular pathways such as nuclear factor kappa B (NFkB) and mammalian target of rapamycin (mTOR) [25] (Figure 3a). It has been suggested that autophagy suppresses tumorigenesis by eliminating p62/SQSTM1 [26] (Figure 3b). Interestingly, it has been shown that the reduction of p62/SQSTM1 and its effect on NRF2 may increase ROS levels and the release of inflammatory cytokines by stromal fibroblasts, promoting epithelial cell carcinogenesis [27]. Thus, it is known that oxidative stress induces DNA damage and cancer onset and that long-lasting chronic inflammation may favor all steps of carcinogenesis [28]. Hence, NRF2 transient activation is considered to be mainly cytoprotective during the first phases of carcinogenesis because it limits both DNA damage and inflammation.
Figure 3.
Role of p62/SQSTM1 in tumorigenesis. (a) P62/SQSTM1, herein shortened as p62, is a common target of NRF2 and HSF1 activates pro-survival molecular pathways such as NFkB and mTOR that promote tumorigenesis. (b) The degradation of p62 by autophagy activation counteracts the first phases of tumorigenesis.
Another important pathway that may directly interact with NRF2 is the phosphatidylinositol 3-kinase (PI3K)/AKT/mTOR, which is involved in regulating a variety of vital cellular processes [29]. This pathway has been reported to regulate NRF2 positively and negatively, depending on the cellular context [30]. Although further investigations will better clarify the relationship between NRF2 and mTOR, it has been reported that mutations in the Nrf2 gene increase the susceptibility of cancer cells to the cytotoxic effects of mTOR inhibitors [31]. Regarding the interplay between NRF2 and mTOR, we have recently shown that the mTOR/p-4EBP1 axis is hyper-phosphorylated following NRF2 activation by Dimethyl fumarate (DMF) [32], a molecule that induces the succination and inactivation of KEAP1 [33]. The activation of mTOR represents a mechanism of resistance of primary effusion lymphoma (PEL) cells undergoing DMF treatment, even if this molecule can still impair PEL survival. Indeed, DMF treatment induces de-phosphorylation and, therefore, inactivation of signal transducers and activators of transcription 3 (STAT3), a pro-survival transcription factor constitutively activated in PEL [32,34]. The cytotoxic effect of DMF against PEL correlates with ROS reduction; hence the traditional knowledge that ROS are only harmful by-products of respiration is being replaced by the finding that they are also important signaling molecules able to sustain oncogenic pathways [35]. In addition, we also found that DMF increases phosphorylation of extracellular signal-regulated kinase (ERK) 1/2, which represents another mechanism of resistance to the treatment due to the induction of pro-survival autophagy [32]. Furthermore, NRF2 inhibition by Brusatol, a quassinoid extracted from Brucea javanica able to interfere with NRF2-mediated defense mechanisms [36,37], can restore cancer cell chemosensitivity [38,39]. This indicates that a fine regulation of NRF2 activation is required to balance cancer cell survival/death outcomes [40].
As anticipated above, a key role in NRF2 regulation is played by its phosphorylation mediated by several kinases, whose activation may be influenced, in turn, by NRF2, in feedback loops. Among those kinases, there are the above-mentioned PI3K/AKT and ERK1/2, but also protein kinase C (PKC) [13], c-jun N-terminal kinase (JNK) [41], and p38 MAPK [42]. Interestingly, the activation of NRF2 can negatively influence nuclear factor kappa B (NFkB), the master regulator of cytokines transcription [43]. This may be one of the mechanisms through which NRF2 counteracts the production of pro-inflammatory molecules, including IL6, IL1β, TNFα, COX2, and iNOS. Even if NRF2 and STAT3 [44] can oppositely regulate the initial steps of tumorigenesis, they may synergize to sustain tumor progression, as reported in the case of breast cancer [45], for example. Interestingly, both STAT3 and NRF2 can be phosphorylated by PERK (protein kinase R-like ER kinase) either directly or indirectly through the phosphorylation of GSK3β during the activation of the unfolded protein response (UPR) [46]. UPR is a protective mechanism that helps cells cope with endoplasmic reticulum /ER stress [47]. However, while the direct phosphorylation of NRF2 by PERK results in NRF2 activation, the one mediated by GSK3β inhibits NRF2 [46]. Notably, although UPR is mainly a cell adaptive response to stress, a delicate balance of the activation of its three sensors, namely PERK, IRE1 (inositol-requiring enzyme 1) alpha, and ATF6 (cyclic AMP-dependent transcription factor-6), dictates the final cell death/survival outcome [48]. This decision is also influenced by the pathways activated/inhibited downstream of the UPR sensors, which include NRF2 [47].
3. Interplay between NRF2 and p53
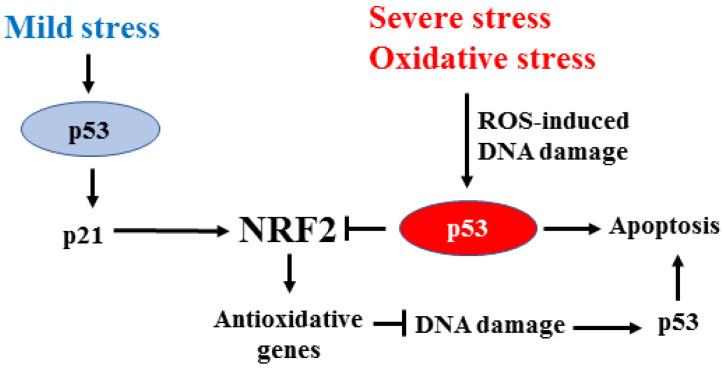
NRF2 may cross-talk with wild-type (wt) and mutant (mut)p53, inhibiting the wtp53 oncosuppressor functions and strengthening the mutp53 oncogenic functions. Both effects contribute to tumor progression and cancer cell resistance to the cytotoxic effects of anticancer therapies. The oncosuppressor p53 is the sensor of DNA damage that activates target genes involved in cell cycle arrest, senescence, or apoptosis, according to the extent of genotoxic damage [49,50]. In particular, high-intensity DNA damage mainly promotes wtp53 apoptotic function [51,52], and the impairment of apoptosis results in the loss of efficacy of cytotoxic therapies [53]. Interestingly, NRF2 and wtp53 share similarities in regulating the redox state, and they may also control each other [54]. Mild stress mainly activates wtp53 to induce p21, which may contribute to NRF2 stabilization [12], leading to cell protection from ROS-induced DNA damage [55]. On the other hand, high-intensity DNA damage, such as the oxidative stress, by inducing p53 post-translational modifications, specifically triggers wtp53 apoptotic activity [50,51,52] along with repression of NRF2 target genes, including x-CT, NQO1, and GST [56]. In this manner, p53/NRF2 interplay may balance the cell’s death/survival decision based on mild/severe stress (Figure 4).
Figure 4.
Interplay between p53 and NRF2. In response to mild stress, wtp53 is activated to mainly induce p21 (instead of apoptotic genes) that contributes to NRF2 stabilization. The antioxidant function of NRF2 protects cells from the DNA damage that usually triggers p53 apoptotic activation. Severe oxidative stress, with elevated ROS levels, activates wtp53 apoptotic function and represses the transcription of NRF2 target genes. The mild/severe stress balances the final NRF2/p53 dependent cell survival/death decision in a regulatory feedback loop.
We have shown that NRF2 activation, by, for instance, high glucose or natural compounds (e.g., sulforaphane or curcumin), may reduce p53 apoptotic function [24,38,57,58]. This outcome depends on the inhibition of homeodomain-interacting protein kinase 2 (HIPK2) [39,59] that specifically phosphorylates p53 at Ser46 for apoptotic activation [60]. NRF2, by counteracting oxidative stress, reduces the extent of DNA damage responsible for HIPK2 activation [61]. This outcome impairs the HIPK2/p53 pro-apoptotic activation in favor of the transcription of p21 that, in turn, sustains NRF2 activation [11]. The interplay between NRF2 and HIPK2 is quite intricate and still not completely unveiled. NRF2 may induce HIPK2 gene transcription [62]. The NRF2-induced HIPK2 protein undergoes post-transcriptional modifications by the redox state, leading to the transcription of several antioxidant genes in common with NRF2 (e.g., NQO1, HO-1), thus engaging a pro-survival cross-talk with NRF2 to the detriment of HIPK2 apoptotic activity [62]. NRF2 might also modulate HIPK2 indirectly, at the protein level, by favoring its proteasomal degradation [63,64]. Interestingly, HIPK2 protein regulation may influence its kinase and transcriptional activity and have a different impact on several biological processes [65,66,67,68,69]. In some conditions, HIPK2 may preferentially trigger the transcription of antioxidant genes and support the NRF2-mediated cytoprotective response instead of inducing p53 apoptotic activity, although this hypothesis still needs to be clarified. Therefore, a better understanding of the interplay between the NRF2 and HIPK2/wtp53 pathway could help to elucidate the pro-survival/apoptotic outcome in cancer, especially in the course of anticancer therapies. Since the NRF2 detoxifying activity is important in cancer prevention, cancer cells can hijack this protective mechanism to promote tumor progression [70]. In this regard, NRF2 inhibition could be a valuable strategy for efficiently reestablishing wtp53 apoptotic activity.
Besides being deregulated at the protein level, p53 is inactivated by gene mutations in almost 50% of all types of tumors; those missense mutations often diminish the p53 ability to bind specific DNA recognition sequences of wild-type target genes, losing their oncosupressor function [71]. Mutp53 proteins may sequester various tumor suppressors, including p53 itself (dominant-negative function) and the family members p63 and p73, inhibiting their oncosuppressor functions [72]. The characteristic of mutp53 proteins is their stabilization and increased expression that may depend on binding to cellular chaperones, including HSP/70 and HSP90 [73]. Although mutp53 proteins do not bind to canonical target gene promoters, some of them (i.e., R175H and R273H mutants) may still affect gene transcription by interacting with other transcription factors; this interaction enhances the oncogenic gain of function (GOF) of mutp53. Including increased cell proliferation, migration, and invasion, which contribute to various stages of tumor progression and cancer resistance to therapies [71,73,74,75].
Among the several oncogenic transcription factors that interact with mutp53 to promote cancer progression [76] is NRF2, although molecular interplay still needs to be fully elucidated. Mutp53 has been shown to induce a bi-directional NRF2 target regulation by repressing and activating the NRF2-dependent oxidative stress response [77,78]. Interestingly, in breast cancer cells, mutp53 interacts at the protein level with NRF2, tuning its activity to selectively transcribe genes that sustain cancer cell survival under oxidative stress, such as thioredoxin (TXN) and proteasome system (PSM), and repressing others such as heme oxygenase 1 (HMOX1) [79]. The interplay between mutp53 and NRF2 contributes to the increased survival of cancer cells under oxidative stress by, for instance, exploiting the thioredoxin system. TXN is associated with poor prognosis in breast cancer patients. Thus, a combination therapy that inhibits both TXN and mutp53 may synergistically reduce breast cancer cell growth [79]. The oncogenic interplay between NRF2 and mutp53 has been nicely demonstrated in vivo where, in a K-ras/p53 double mutant mouse model, Nrf2 depletion decreases pancreatic carcinogenesis and cancer invasion [80]. In another study, five mutp53 proteins have been shown to cooperate with NRF2 to induce the transcription of 26S proteasome and immunoproteasome genes in triple-negative breast cancer (TNBC) cell lines [81]. This cooperation enhances the degradation of tumor suppressor proteins and confers resistance to proteasome inhibitor therapy [81]. In addition, we have demonstrated that mutp53/HSP90 interaction activates a feedback loop between NRF2 and p62 that induces cancer chemoresistance in both pancreatic and breast cancer cells [22,24,39]. These findings suggest that a deeper understanding of the mutp53/NRF2 relationship may pave the way to new more efficient anticancer strategies.
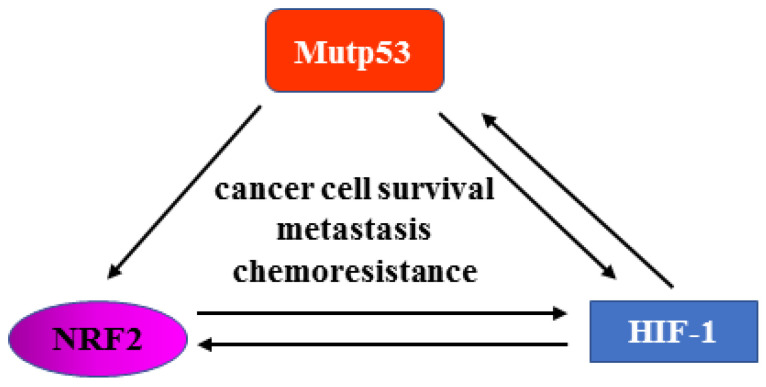
Hypoxia is a hallmark of solid tumors, and it is responsible for activating hypoxia-inducible factor-1 (HIF-1) oncogenic signaling to promote tumor progression, invasion, and chemoresistance [82]. There is an interplay between hypoxia and mutp53. Hypoxia sustains mutp53 activity, and mutp53 may induce the transcriptional activity of HIF-1 by stimulating the stability of the oxygen-dependent component of the HIF-1 transcription factor, that is, HIF-1alpha [83]. Besides HIF-1, tumor hypoxia activates NRF2 signaling to promote cancer survival, metastasis, and chemoresistance [64]. However, the two signaling pathways can also interact. Thus, NRF2 signaling can activate the HIF-1 response by, for instance, the activation of thioredoxin, though, on the other hand, HIF-1 signaling has been shown to increase NRF2 activation [64]. We can therefore picture a mechanistic interplay among mutp53, NRF2, and HIF-1 to sustain their oncogenic functions and promote tumor progression, invasion, and chemoresistance (Figure 5). Thus, it is conceivable that blocking one oncogenic pathway may influence other pathways and that synchronously blocking them may have greater success in anticancer therapy. For instance, mutp53 can be targeted at the protein level by inhibiting its binding to chaperone [73] or by inducing its autophagic degradation, as demonstrated by our study [84]. However, the interplay between mutp53 and autophagy is quite complex and context-dependent and needs further understanding to shape effective anticancer strategies [85].
Figure 5.
Reciprocal control between mutp53, NRF2, and HIF-1. Mutp53 can sustain NRF2 and HIF-1 activity. NRF2 and HIF-1 can control each other, as well as HIF-1 and mut-p53. The interplay among the oncogenic pathways contributes to tumor progression, metastasis, and chemoresistance.
Besides mutp53, other oncogenes have been reported to affect NRF2 activity, e.g., Myc has been shown to activate NRF2 and induce tumorigenesis in cells undergoing carcinogenic treatment [86]. Interestingly, c-Myc can establish an interplay with wtp53 as well as with mutp53, inhibiting the first [87] while sustaining the latter to promote pancreatic cancer cell survival [88].
4. NRF2 and Gammaherpesvirus-Driven Cancers
We have recently shown in vitro that the silencing of p62/SQSTM1 and NRF2 can counteract the Epstein-Barr virus (EBV)-driven B lymphocyte immortalization [89]. This effect correlates with the reduction of ROS, the main cause of DNA mutations, and the down-regulation of ATM. This kinase is essential to sense DNA damage and to trigger the DDR cascade in response to double-strand DNA breaks. This initiates DNA repair and helps to prevent the accumulation of DNA mutations. NRF2 or p62 /SQSTM1 knockdown also induces the downregulation of H2A histone family member X (H2AX) [89], another key player in DNA repair. Our findings agree with a previous study reporting that H2AX is degraded in oxidative stress conditions caused by a deficient antioxidant response [90]. NRF2 can also be regulated by the other human gammaherpesvirus [91], namely Kaposi’s sarcoma-associated herpesvirus (KSHV). This effect has been observed during de novo infection of human endothelial cells [92] or in naturally infected lymphoma cells [93]. In both cases, NRF2 activation positively regulates viral infection and cell survival/proliferation of virally infected cells.
Regarding gammaherpesvirus-driven carcinogenesis, it has been shown that, besides latent antigens, proteins expressed during the activation of the lytic cycle may contribute to cancer onset [94]. This is mainly because viral lytic proteins promote inflammation that, as said above, is strictly linked to carcinogenesis. NRF2 plays an important role in promoting viral replication, as indicated by the fact that the inhibition of NRF2 reduces the KSHV lytic cycle [93]. Interestingly, the NRF2 target HSP70 [15] is also indispensable for KSHV replication [95]. We have previously shown that the increase of reactive oxygen species (ROS), though it promotes the reactivation of KSHV from latency in lymphoma cells, must be moderate to allow viral replication [96]. Therefore, NRF2 activation is required to prevent too high an increase in ROS level that could promote cell death before viral particles are released [96]. This was observed in cells treated with phorbol diester 12-0-tetradecanoylphorbol-13-acetate (TPA), classic viral lytic cycle inducers that can exert a strong cytotoxic effect against gammaherpesvirus-harboring lymphoma cells [96]. It is also important to consider that EBV-induced NRF2 activation in monocytic cells contributes to the immune escape, which could indirectly facilitate cancer onset. We have found that EBV stabilizes NRF2 in these cells through autophagy inhibition and p62/SQSTM1 accumulation. The reduction of intracellular ROS, consequent to NRF2 activation, impairs the in vitro differentiation of monocytes into dendritic cells (DCs) [97]. Of note, DCs play a pivotal role in initiating the immune response toward new antigens, including viral or tumor antigens. Therefore, reducing their formation has a strong impact on immune response and viral immune escape.
5. Conclusions
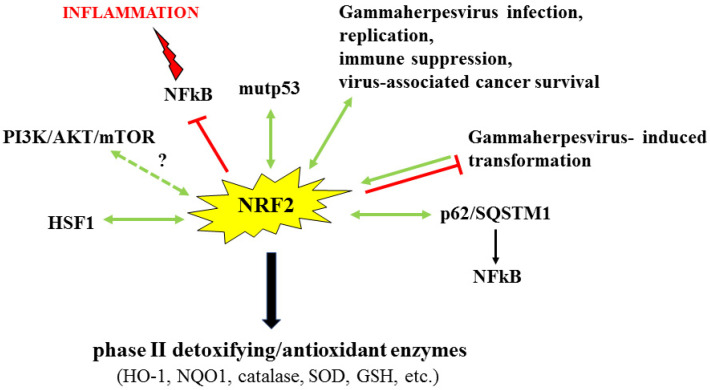
This update on the key role of NRF2 in cancer survival, progression, and chemoresistance highlights how several of its induced effects rely on the collaboration of NRF2 with oncogenic pathways, such as HSF1, mutp53, and mTOR, and with pro-tumorigenic molecules, such as p62/SQSTM1, sometimes in a feedback loop. Of note, we also highlight how NRF2 activation is exploited by gammaherpesvirus-driven carcinogenesis and immune suppression (Figure 6).
Figure 6.
Interplay between NRF2 and oncogenic, pro-survival/inflammatory pathways. Not applicable Schematic representation of the interaction between NRF2 and several pathways that can control each other in a feedback loop. The antioxidant activity of NRF2 and its interaction with those pathways can have a key role in tumor progression.
Interestingly, the NRF2 detoxifying activity, hijacked by cancer cells as a protective mechanism, particularly in the course of anti-cancer treatments, is also important in cancer prevention. Therefore, NRF2 manipulation could be a valuable strategy both to prevent cancer and to inhibit its progression, e.g., through the restoration of wtp53 apoptotic activity. In addition, given the key role of NRF2 in gammaherpesvirus-driven carcinogenesis, targeting NRF2 could represent a promising strategy to counteract cancers associated with them. Indeed, NRF2 manipulation may help to reduce the capability of these viruses to infect target cells, to prevent the transformation of cells from which their associated cancers arise, to counteract viral lytic antigen expression and the inflammation that they promote, and, last but not least, to interfere with their-induced immunosuppression. Of note, in cancers associated with gammaherpesviruses, both NRF2 inhibition and NRF2 activation may represent a promising therapeutic approach, as ROS, unless they are too high, can sustain pro-survival, oncogenic pathways, such as STAT3 [32,98,99,100,101]. In light of these findings, future studies should be directed to a better understanding of NRF2 biology, its interaction with oncogenic pathways, and the role of this intricate cross-talk in the different steps of carcinogenesis to target them properly.
Acknowledgments
The authors thank the lab people for their technical support and helpful discussion.
Author Contributions
Conceptualization and writing, M.C. and G.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Italian Association for Cancer Research (AIRC) to G.D. (AIRC Id.16742) and M.C (AIRC Id.23040).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Li L., Dong H., Song E., Xu X., Liu L., Song Y. Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem. Biol. Interact. 2014;209:56–67. doi: 10.1016/j.cbi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Tripathi D., Nam A., Oldenburg D.J., Bendich A.J. Reactive Oxygen Species, Antioxidant Agents, and DNA Damage in Developing Maize Mitochondria and Plastids. Front. Plant Sci. 2020;11:596. doi: 10.3389/fpls.2020.00596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Gaetano A., Gibellini L., Zanini G., Nasi M., Cossarizza A., Pinti M. Mitophagy and Oxidative Stress: The Role of Aging. Antioxidants. 2021;10:794. doi: 10.3390/antiox10050794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klionsky D.J., Abdel-Aziz A.K., Abdelfatah S., Abdellatif M., Abdoli A., Abel S., Abeliovich H., Abildgaard M.H., Abudu Y.P., Acevedo-Arozena A., et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition) Autophagy. 2021;17:1–382. doi: 10.1080/15548627.2020.1797280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinkova-Kostova A.T., Abramov A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmstrom K.M., Kostov R.V., Dinkova-Kostova A.T. The multifaceted role of Nrf2 in mitochondrial function. Curr. Opin. Toxicol. 2016;1:80–91. doi: 10.1016/j.cotox.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kansanen E., Kuosmanen S.M., Leinone H., Levonen A.L. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox. Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rojo de la Vega M., Chapman E., Zhang D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell. 2018;34:21–43. doi: 10.1016/j.ccell.2018.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komatsu M., Kurokawa H., Waguri S., Taguchi K., Kobayashi A., Ichimura Y., Sou Y.S., Ueno I., Sakamoto A., Tong K.I., et al. The selective autophagy substrate p62 activates the stress responsive transcription factor Nrf2 through inactivation of Keap1. Nat. Cell Biol. 2010;12:213–223. doi: 10.1038/ncb2021. [DOI] [PubMed] [Google Scholar]
- 10.Jiang T., Harder B., Rojo de la Vega M., Wong P.K., Chapman E., Zhang D.D. p62 links autophagy and Nrf2 signaling. Free Radic. Biol. Med. 2015;88:199–204. doi: 10.1016/j.freeradbiomed.2015.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katsuragi Y., Ichimura Y., Komatsu M. Regulation of the Keap1–Nrf2 pathway by p62/SQSTM1. Curr. Opin. Toxicol. 2016;1:54–61. doi: 10.1016/j.cotox.2016.09.005. [DOI] [Google Scholar]
- 12.Chen W., Sun Z., Wang X.J., Jiang T., Huang Z., Fang D., Zhang D.D. Direct interaction between Nrf2 and p21(Cip1/WAF1) upregulates the Nrf2-mediated antioxidant response. Mol. Cell. 2009;34:663–673. doi: 10.1016/j.molcel.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang H.C., Nguyen T., Pickett C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002;277:42769–42774. doi: 10.1074/jbc.M206911200. [DOI] [PubMed] [Google Scholar]
- 14.Liu T., Lv Y.F., Zhao J.L., You Q.D., Jiang Z.Y. Regulation of Nrf2 by phosphorylation: Consequences for biological function and therapeutic implications. Free Radic. Biol. Med. 2021;168:129–141. doi: 10.1016/j.freeradbiomed.2021.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Saha S., Buttari B., Panieri E., Profumo E., Saso L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules. 2020;25:5474. doi: 10.3390/molecules25225474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dayalan Naidu S., Kostov R.V., Dinkova-Kostova A.T. Transcription factors Hsf1 and Nrf2 engage in crosstalk for cytoprotection. Trends Pharmacol. Sci. 2015;36:6–14. doi: 10.1016/j.tips.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Home T., Jensen R.A., Rao R. Heat shock factor 1 in protein homeostasis and oncogenic signal integration. Cancer Res. 2015;75:907–912. doi: 10.1158/0008-5472.CAN-14-2905. [DOI] [PubMed] [Google Scholar]
- 18.Sottile M.L., Nadin S.B. Heat shock proteins and DNA repair mechanisms: An updated overview. Cell Stress Chaperones. 2018;23:303–315. doi: 10.1007/s12192-017-0843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Granato M., Lacconi V., Peddis M., Lotti L.V., Di Renzo L., Gonnella R., Santarelli R., Trivedi P., Frati L., D’Orazi G., et al. HSP70 inhibition by 2-phenylethynesulfonamide induces lysosomal cathepsin D release and immunogenic cell death in primary effusion lymphoma. Cell Death Dis. 2013;4:e730. doi: 10.1038/cddis.2013.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Doyle S.M., Hoskins J.R., Kravats A.N., Heffner A.L., Garikapati S., Wickner S. Intermolecular Interactions between Hsp90 and Hsp70. J. Mol. Biol. 2019;431:2729–2746. doi: 10.1016/j.jmb.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J., Zhang L.L., Wu W., Guo H., Li Y., Sukhanova M., Venkataraman G., Huang S., Zhang H., Alikhan M., et al. Activation of MYC, a bona fide client of HSP90, contributes to intrinsic ibrutinib resistance in mantle cell lymphoma. Blood Adv. 2018;2:2039–2051. doi: 10.1182/bloodadvances.2018016048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilardini Montani M.S., Cecere N., Granato M., Romeo M.A., Falcinelli L., Ciciarelli U., D’Orazi G., Faggioni A., Cirone M. Mutant p53, Stabilized by Its Interplay with HSP90, Activates a Positive Feed-Back Loop Between NRF2 and p62 that Induces Chemo-Resistance to Apigenin in Pancreatic Cancer Cells. Cancers. 2019;11:703. doi: 10.3390/cancers11050703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Romeo M.A., Gilardini Montani M.S., Benedetti R., Arena A., D’Orazi G., Cirone M. VPA and TSA Interrupt the Interplay between mutp53 and HSP70, Leading to CHK1 and RAD51 Down-Regulation and Sensitizing Pancreatic Cancer Cells to AZD2461 PARP Inhibitor. Int. J. Mol. Sci. 2022;23:2268. doi: 10.3390/ijms23042268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garufi A., Baldari S., Pettinari R., Gilardini Montani M.S., D’Orazi V., Pistritto G., Crispini A., Giorno E., Toietta G., Marchetti F., et al. A ruthenium(II)-curcumin compound modulates NRF2 expression balancing the cancer cell death/survival outcome according to p53 status. J. Exp. Clin. Cancer Res. 2020;39:122. doi: 10.1186/s13046-020-01628-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moscat J., Karin M., Diaz-Meco M.T. p62 in Cancer: Signaling Adaptor Beyond Autophagy. Cell. 2016;167:606–609. doi: 10.1016/j.cell.2016.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathew R., Karp C.M., Beaudoin B., Vuong N., Chen G., Chen H.Y., Bray K., Reddy A., Bhanot G., Gelinas C., et al. Autophagy suppresses tumorigenesis through elimination of p62. Cell. 2009;137:1062–1075. doi: 10.1016/j.cell.2009.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valencia T., Kim J.Y., Abu-Baker S., Moscat-Pardos J., Ahn C.S., Reina-Campos M., Duran A., Castilla E.A., Metallo C.M., Diaz-Meco M.T., et al. Metabolic reprogramming of stromal fibroblasts through p62-mTORC1 signaling promotes inflammation and tumorigenesis. Cancer Cell. 2014;26:121–135. doi: 10.1016/j.ccr.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greten F.R., Grivennikov S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity. 2019;51:27–41. doi: 10.1016/j.immuni.2019.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cirone M. Cancer cells dysregulate PI3K/AKT/mTOR pathway activation to ensure their survival and proliferation: Mimicking them is a smart strategy of gammaherpesviruses. Crit. Rev. Biochem. Mol. Biol. 2021;56:500–509. doi: 10.1080/10409238.2021.1934811. [DOI] [PubMed] [Google Scholar]
- 30.Aramburu J., Ortells M.C., Tejedor S., Buxade M., Lopez-Rodriguez C. Transcriptional regulation of the stress response by mTOR. Sci. Signal. 2014;7:re2. doi: 10.1126/scisignal.2005326. [DOI] [PubMed] [Google Scholar]
- 31.Shibata T., Saito S., Kokubu A., Suzuki T., Yamamoto M., Hirohashi S. Global downstream pathway analysis reveals a dependence of oncogenic NF-E2-related factor 2 mutation on the mTOR growth signaling pathway. Cancer Res. 2010;70:9095–9105. doi: 10.1158/0008-5472.CAN-10-0384. [DOI] [PubMed] [Google Scholar]
- 32.Gonnella R., Zarrella R., Santarelli R., Germano C.A., Gilardini Montani M.S., Cirone M. Mechanisms of Sensitivity and Resistance of Primary Effusion Lymphoma to Dimethyl Fumarate (DMF) Int. J. Mol. Sci. 2022;23:6773. doi: 10.3390/ijms23126773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saidu N.E.B., Kavian N., Leroy K., Jacob C., Nicco C., Batteux F., Alexandre J. Dimethyl fumarate, a two-edged drug: Current status and future directions. Med. Res. Rev. 2019;39:1923–1952. doi: 10.1002/med.21567. [DOI] [PubMed] [Google Scholar]
- 34.Granato M., Chiozzi B., Filardi M.R., Lotti L.V., Di Renzo L., Faggioni A., Cirone M. Tyrosine kinase inhibitor tyrphostin AG490 triggers both apoptosis and autophagy by reducing HSF1 and Mcl-1 in PEL cells. Cancer Lett. 2015;366:191–197. doi: 10.1016/j.canlet.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Reczek C.R., Chandel N.S. The Two Faces of Reactive Oxygen Species in Cancer. Annu. Rev. Cancer Biol. 2017;1:79–98. doi: 10.1146/annurev-cancerbio-041916-065808. [DOI] [Google Scholar]
- 36.Ren D., Villeneuve N.F., Jiang T., Wu T., Lau A., Toppin H.A., Zhang D.D. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Natl. Acad. Sci. USA. 2011;108:1433–1438. doi: 10.1073/pnas.1014275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olayanju A., Copple I.M., Bryan H.K., Edge G.T., Sison R.L., Wong M.W., Lai Z.Q., Lin Z.X., Dunn K., Sanderson C.M., et al. Brusatol provokes a rapid and transient inhibition of Nrf2 signaling and sensitizes mammalian cells to chemical toxicity-implications for therapeutic targeting of Nrf2. Free Radic. Biol. Med. 2015;78:202–212. doi: 10.1016/j.freeradbiomed.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garufi A., Traversi G., Gilardini Montani M.S., D’Orazi V., Pistritto G., Cirone M., D’Orazi G. Reduced chemotherapeutic sensitivity in high glucose condition: Implication of antioxidant response. Oncotarget. 2019;10:4691–4702. doi: 10.18632/oncotarget.27087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garufi A., Giorno E., Gilardini Montani M.S., Pistritto G., Crispini A., Cirone M., D’Orazi G. P62/SQSTM1/Keap1/NRF2 Axis Reduces Cancer Cells Death-Sensitivity in Response to Zn(II)-Curcumin Complex. Biomolecules. 2021;11:348. doi: 10.3390/biom11030348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu S., Lu H., Bail Y. Nrf2 in cancer: A double-edged sword. Cancer Med. 2019;8:2252–2267. doi: 10.1002/cam4.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J.K., Jang H.D. Nrf2-mediated HO-1 induction coupled with the ERK signaling pathway contributes to indirect antioxidant capacity of caffeic acid phenethyl ester in HepG2 cells. Int. J. Mol. Sci. 2014;15:12149–12165. doi: 10.3390/ijms150712149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma L., Liu J., Zhang X., Qi J., Yu W., Gu Y. p38 MAPK-dependent Nrf2 induction enhances the resistance of glioma cells against TMZ. Med. Oncol. 2015;32:69. doi: 10.1007/s12032-015-0517-y. [DOI] [PubMed] [Google Scholar]
- 43.Li W., Khor T.O., Xu C., Shen G., Jeong W.S., Yu S., Kong A. Activation of Nrf2-antioxidant signaling attenuates NF-kB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008;76:1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu H., Pardoll D., Jove R. STATs in cancer inflammation and immunity: A leading role for STAT3. Nat. Rev. Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim S.J., Saeidi S., Cho N.C., Kim S.H., Lee H.B., Han W., Noh D.Y., Surh Y.J. Interaction of Nrf2 with dimeric STAT3 induces IL-23 expression: Implications for breast cancer progression. Cancer Lett. 2021;500:147–160. doi: 10.1016/j.canlet.2020.11.047. [DOI] [PubMed] [Google Scholar]
- 46.Cullinan S.B., Zhang D., Hannink M., Arvisais E., Kaufman R.J., Diehl J.A. Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol. Cell Biol. 2003;23:7198–7209. doi: 10.1128/MCB.23.20.7198-7209.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Orazi G., Cirone M. Interconnected Adaptive Responses: A Way Out for Cancer Cells to Avoid Cellular Demise. Cancers. 2022;14:2780. doi: 10.3390/cancers14112780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madden E., Logue S.E., Healy S.J., Manie S., Samali A. The role of the unfolded protein response in cancer progression: From oncogenesis to chemoresistance. Biol. Cell. 2019;111:1–17. doi: 10.1111/boc.201800050. [DOI] [PubMed] [Google Scholar]
- 49.Vousden K.H., Prives C. Blinded by the Light: The Growing Complexity of p53. Cell. 2009;137:413–431. doi: 10.1016/j.cell.2009.04.037. [DOI] [PubMed] [Google Scholar]
- 50.Murray-Zmijewski F., Slee E.A., Lu X. A complex barcode underlies the heterogeneous response of p53 to stress. Nat. Rev. Mol. Cell Biol. 2008;9:702–712. doi: 10.1038/nrm2451. [DOI] [PubMed] [Google Scholar]
- 51.Puca R., Nardinocchi L., Sacchi A., Rechavi G., Givol D., D’Orazi G. HIPK2 modulates p53 activity towards pro-apoptotic transcription. Mol. Cancer. 2009;8:85. doi: 10.1186/1476-4598-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aubrey B.J., Kelly G.L., Janic A., Herold M.J., Strasser A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018;25:104–113. doi: 10.1038/cdd.2017.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pistritto G., Trisciuoglio D., Ceci C., Garufi A., D’Orazi G. Apoptosis as anticancer mechanism: Function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY) 2016;8:603–619. doi: 10.18632/aging.100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rotblat B., Melino G., Knight R.A. NRF2 and p53: Januses in cancer? Oncotarget. 2012;3:1272–1283. doi: 10.18632/oncotarget.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sablina A.A., Budanov A.V., Ilyinskaya G.V., Agapova L.S., Kravchenko J.E., Chumakov P.M. The antioxidant function of the p53 tumor suppressor. Nat. Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Faraonio R., Vergara P., Di Marzo D., Pierantoni M.G., Napolitano M., Russo T., Cimino F. p53 suppresses the Nrf2-dependent transcription of antioxidant response genes. J. Biol. Chem. 2006;281:39776–39784. doi: 10.1074/jbc.M605707200. [DOI] [PubMed] [Google Scholar]
- 57.Garufi A., D’Orazi G. High glucose dephosphorylates serine 46 and inhibits p53 apoptotic activity. J. Exp. Clin. Cancer Res. 2014;33:79. doi: 10.1186/s13046-014-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garufi A., Pistritto G., Baldari S., Toietta G., Cirone M., D’Orazi G. p53-Dependent PUMA to DRAM antagonistic interplay as a key molecular switch in cell-fate decision in normal/high glucose conditions. J. Exp. Clin. Cancer Res. 2017;36:126. doi: 10.1186/s13046-017-0596-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.D’Orazi G., Garufi A., Cirone M. Nuclear factor erythroid 2 (NF-E2) p45-related factor 2 interferes with homeodomain-interacting protein kinase 2/p53 activity to impair solid tumors chemosensitivity. IUBMB Life. 2020;72:1634–1639. doi: 10.1002/iub.2334. [DOI] [PubMed] [Google Scholar]
- 60.D’Orazi G., Cecchinelli B., Bruno T., Manni I., Higashimoto Y., Saito S., Gostissa M., Coen S., Marchetti A., Del Sal G., et al. Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat. Cell Biol. 2002;4:11–19. doi: 10.1038/ncb714. [DOI] [PubMed] [Google Scholar]
- 61.Hofmann T.G., Glas C., Bitomsky N. HIPK2: A tumour suppressor that controls DNA damage-induced cell fate and cytokinesis. Bioessays. 2013;35:55–64. doi: 10.1002/bies.201200060. [DOI] [PubMed] [Google Scholar]
- 62.Torrente L., Sanchez C., Moreno R., Chowdhry S., Cabello P., Isono K., Koseki H., Honda T., Hayes J.D., Dinkova-Kostova A.T., et al. Crosstalk between NRF2 and HIPK2 shapes cytoprotective responses. Oncogene. 2017;36:6204–6212. doi: 10.1038/onc.2017.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nardinocchi L., Puca R., D’Orazi G. HIF-1alpha antagonizes p53-mediated apoptosis by triggering HIPK2 degradation. Aging (Albany NY) 2011;3:33–43. doi: 10.18632/aging.100254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Toth R.K., Warfel N.A. Strange Bedfellows: Nuclear Factor, Erythroid 2-Like 2 (Nrf2) and Hypoxia-Inducible Factor 1 (HIF-1) in Tumor Hypoxia. Antioxidants. 2017;6:27. doi: 10.3390/antiox6020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feng Y., Zhou L., Sun X., Li Q. Homeodomain-interacting protein kinase 2 (HIPK2): A promising target for anti-cancer therapies. Oncotarget. 2017;8:20452–20461. doi: 10.18632/oncotarget.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garufi A., Traversi G., Cirone M., D’Orazi G. HIPK2 role in the tumor-host interaction: Impact on fibroblasts transdifferentiation CAF-like. IUBMB Life. 2019;71:2055–2061. doi: 10.1002/iub.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Conte A., Valente V., Paladino S., Pierantoni G.M. HIPK2 in cancer biology and therapy: Recent findings and future perspectives. Cell Signal. 2022;101:110491. doi: 10.1016/j.cellsig.2022.110491. [DOI] [PubMed] [Google Scholar]
- 68.De la Vega L., Grishina I., Moreno R., Kruger M., Braun T., Schmitz M.L. A redox-regulated SUMO/acetylation switch of HIPK2 controls the survival threshold to oxidative stress. Mol. Cell. 2012;46:472–483. doi: 10.1016/j.molcel.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 69.Wook Choi D., Yong Choi C. HIPK2 modification code for cell death and survival. Mol. Cell Oncol. 2014;1:e955999. doi: 10.1080/23723548.2014.955999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DeNicola G.M., Karreth F.A., Humpton T.J., Gopinathan A., Wei C., Frese K., Mangal D., Yu K.H., Yeo C.J., Calhoun E.S., et al. Oncogene-induced NRF2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freed-Pastor W.A., Prives C. Mutant p53: One name, many proteins. Genes Dev. 2012;26:1268–1296. doi: 10.1101/gad.190678.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xu J., Reumers J., Couceiro J.R., De Smet F., Gallardo R., Rudyak S., Cornelis A., Rozenski J., Zwolinska A., Marine J.C., et al. Gain of function of mutant p53 by coaggregation with multiple tumor suppressors. Nat. Chem. Biol. 2011;7:285–295. doi: 10.1038/nchembio.546. [DOI] [PubMed] [Google Scholar]
- 73.Schulz-Heddergott R., Moll U.M. Gain-of-Function (GOF) Mutant p53 as Actionable Therapeutic Target. Cancers. 2018;10:188. doi: 10.3390/cancers10060188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muller P.A., Vousden K.H. Mutant p53 in cancer: New functions and therapeutic opportunities. Cancer Cell. 2014;25:304–317. doi: 10.1016/j.ccr.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mantovani F., Collavin L., Del Sal G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019;26:199–212. doi: 10.1038/s41418-018-0246-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.D’Orazi G., Cirone M. Mutant p53 and Cellular Stress Pathways: A Criminal Alliance That Promotes Cancer Progression. Cancers. 2019;11:614. doi: 10.3390/cancers11050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kalo E., Kogan-Sakin I., Solomon H., Bar-Nathan E., Shay M., Shetzer Y., Dekel E., Goldfinger N., Buganim Y., Stambolsky P., et al. Mutant p53R273H attenuates the expression of phase 2 detoxifying enzymes and promotes the survival of cells with high levels of reactive oxygen species. J. Cell Sci. 2012;125:5578–5586. doi: 10.1242/jcs.106815. [DOI] [PubMed] [Google Scholar]
- 78.Tung M.C., Lin P.L., Wang Y.C., He T.Y., Lee M.C., Yeh S.D., Chen C.Y., Lee H. Mutant p53 confers chemoresistance in non-small cell lung cancer by upregulating Nrf2. Oncotarget. 2015;6:41692–41705. doi: 10.18632/oncotarget.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lisek K., Campaner E., Ciani Y., Walerych D., Del Sal G. Mutant p53 tunes the NRF2-dependent antioxidant response to support survival of cancer cells. Oncotarget. 2018;9:20508–20523. doi: 10.18632/oncotarget.24974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hamada S., Taguchi K., Masamune A., Yamamoto M., Shimosegawa T. Nrf2 promotes mutant K-ras/p53-driven pancreatic carcinogenesis. Carcinogenesis. 2017;38:661–670. doi: 10.1093/carcin/bgx043. [DOI] [PubMed] [Google Scholar]
- 81.Walerych D., Lisek K., Sommaggio R., Piazza S., Ciani Y., Dalla E., Rajkowska K., Gaweda-Walerych K., Ingallina E., Tonelli C., et al. Proteasome machinery is instrumental in a common gain-of-function program of the p53 missense mutants in cancer. Nat. Cell Biol. 2016;18:897–909. doi: 10.1038/ncb3380. [DOI] [PubMed] [Google Scholar]
- 82.Muz B., de la Puente P., Azab F., Azab A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia. 2015;3:83–92. doi: 10.2147/HP.S93413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Amelio I., Mancini M., Petrova V., Cairns R.A., Vikhreva P., Nicolai S., Marini A., Antonov A.A., Le Quesne J., Baena Acevedo J.D., et al. p53 mutants cooperate with HIF-1 in transcriptional regulation of extracellular matrix components to promote tumor progression. Proc. Natl. Acad. Sci. USA. 2018;115:E10869–E10878. doi: 10.1073/pnas.1808314115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Garufi A., Pucci D., D’Orazi V., Cirone M., Bossi G., Avantaggiati M.L., D’Orazi G. Degradation of mutant p53H175 protein by Zn(II) through autophagy. Cell Death Dis. 2014;5:e1271. doi: 10.1038/cddis.2014.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shi Y., Vakifahmetoglu-Norberg H. Mutant p53 as a regulator and target of autophagy. Front. Oncol. 2021;10:607149. doi: 10.3389/fonc.2020.607149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang Y.C., Hsiao J.R., Jiang S.S., Chang J.Y., Chu P.Y., Liu K.J., Fang H.L., Lin L.M., Chen H.H., Huang Y.W., et al. c-MYC-directed NRF2 drives malignant progression of head and neck cancer via glucose-6-phosphate dehydrogenase and transketolase activation. Theranostics. 2021;11:5232–5247. doi: 10.7150/thno.53417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Arena A., Gilardini Montani M.S., Romeo M.A., Benedetti R., Gaeta A., Cirone M. DNA damage triggers an interplay between wtp53 and c-Myc affecting lymphoma cell proliferation and Kaposi sarcoma herpesvirus replication. Biochim. Biophys. Acta Mol. Cell Res. 2022;1869:119168. doi: 10.1016/j.bbamcr.2021.119168. [DOI] [PubMed] [Google Scholar]
- 88.Romeo M.A., Gilardini Montani M.S., Arena A., Benedetti R., D’Orazi G., Cirone M. c-Myc Sustains Pancreatic Cancer Cell Survival and mutp53 Stability through the Mevalonate Pathway. Biomedicines. 2022;10:2489. doi: 10.3390/biomedicines10102489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gilardini Montani M.S., Tarquini G., Santarelli R., Gonnella R., Romeo M.A., Benedetti R., Arena A., Faggioni A., Cirone M. p62/SQSTM1 promotes mitophagy and activates the NRF2-mediated antioxidant and anti-inflammatory response restraining EBV-driven B lymphocyte proliferation. Carcinogenesis. 2022;43:277–287. doi: 10.1093/carcin/bgab116. [DOI] [PubMed] [Google Scholar]
- 90.Gruosso T., Mieulet V., Cardon M., Bourachot B., Kieffer Y., Devun F., Dubois T., Dutreix M., Vincent-Salomon A., Miller K.M., et al. Chronic oxidative stress promotes H2AX protein degradation and enhances chemosensitivity in breast cancer patients. EMBO Mol. Med. 2016;8:527–549. doi: 10.15252/emmm.201505891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cirone M. EBV and KSHV Infection Dysregulates Autophagy to Optimize Viral Replication, Prevent Immune Recognition and Promote Tumorigenesis. Viruses. 2018;10:599. doi: 10.3390/v10110599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gjyshi O., Bottero V., Veettil M.V., Dutta S., Singh V.V., Chikoti L., Chandran B. Kaposi’s sarcoma-associated herpesvirus induces Nrf2 during de novo infection of endothelial cells to create a microenvironment conducive to infection. PLoS Pathog. 2014;10:e1004460. doi: 10.1371/journal.ppat.1004460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gjyshi O., Flaherty S., Veettil M.V., Johnson K.E., Chandran B., Bottero V. Kaposi’s sarcoma-associated herpesvirus induces Nrf2 activation in latently infected endothelial cells through SQSTM1 phosphorylation and interaction with polyubiquitinated Keap1. J. Virol. 2015;89:2268–2286. doi: 10.1128/JVI.02742-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rosemarie Q., Sugden B. Epstein-Barr Virus: How Its Lytic Phase Contributes to Oncogenesis. Microorganisms. 2020;8:1824. doi: 10.3390/microorganisms8111824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baquero-Perez B., Whitehouse A. Hsp70 Isoforms Are Essential for the Formation of Kaposi’s Sarcoma-Associated Herpesvirus Replication and Transcription Compartments. PLoS Pathog. 2015;11:e1005274. doi: 10.1371/journal.ppat.1005274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Granato M., Gilardini Montani M.S., Angiolillo C., D’Orazi G., Faggioni A., Cirone M. Cytotoxic Drugs Activate KSHV Lytic Cycle in Latently Infected PEL Cells by Inducing a Moderate ROS Increase Controlled by HSF1, NRF2 and p62/SQSTM1. Viruses. 2018;11:8. doi: 10.3390/v11010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gilardini Montani M.S., Santarelli R., Granato M., Gonnella R., Torrisi M.R., Faggioni A., Cirone M. EBV reduces autophagy, intracellular ROS and mitochondria to impair monocyte survival and differentiation. Autophagy. 2019;15:652–667. doi: 10.1080/15548627.2018.1536530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Granato M., Rizzello C., Romeo M.A., Yadav S., Santarelli R., D’Orazi G., Faggioni A., Cirone M. Concomitant reduction of c-Myc expression and PI3K/AKT/mTOR signaling by quercetin induces a strong cytotoxic effect against Burkitt’s lymphoma. Int. J. Biochem. Cell Biol. 2016;79:393–400. doi: 10.1016/j.biocel.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 99.Granato M., Rizzello C., Gilardini Montani M.S., Cuomo L., Vitillo M., Santarelli R., Gonnella R., D’Orazi G., Faggioni A., Cirone M. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J. Nutr. Biochem. 2017;41:124–136. doi: 10.1016/j.jnutbio.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 100.Granato M., Gilardini Montani M.S., Santarelli R., D’Orazi G., Faggioni A., Cirone M. Apigenin, by activating p53 and inhibiting STAT3, modulates the balance between pro-apoptotic and pro-survival pathways to induce PEL cell death. J. Exp. Clin. Cancer Res. 2017;36:167. doi: 10.1186/s13046-017-0632-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Granato M., Gilardini Montani M.S., Filardi M., Faggioni A., Cirone M. Capsaicin triggers immunogenic PEL cell death, stimulates DCs and reverts PEL-induced immune suppression. Oncotarget. 2015;6:29543–29554. doi: 10.18632/oncotarget.4911. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.