Abstract
Gallinacin-3 and gallopavin-1 (GPV-1) are newly characterized, epithelial β-defensins of the chicken (Gallus gallus) and turkey (Meleagris gallopavo), respectively. In normal chickens, the expression of gallinacin-3 was especially prominent in the tongue, bursa of Fabricius, and trachea. It also occurred in other organs, including the skin, esophagus, air sacs, large intestine, and kidney. Tracheal expression of gallinacin-3 increased significantly after experimental infection of chickens with Haemophilus paragallinarum, whereas its expression in the tongue, esophagus, and bursa of Fabricius was unaffected. The precursor of gallinacin-3 contained a long C-terminal extension not present in the prepropeptide. By comparing the cDNA sequences of gallinacin-3 and GPV-1, we concluded that a 2-nucleotide insertion into the gallinacin-3 gene had induced a frameshift that read through the original stop codon and allowed the chicken propeptide to lengthen. The striking structural resemblance of the precursors of β-defensins to those of crotamines (highly toxic peptides found in rattlesnake venom) supports their homology, even though defensins are specialized to kill microorganisms and crotamines are specialized to kill much larger prey.
Defensins are endogenous β-sheet peptides that contribute to the antimicrobial properties inherent in mammalian granulocytes, epithelial cells, and certain secretions. Three defensin subfamilies exist in vertebrates. Two of these, α- and β-defensins, occur in humans (11, 17) and the third, theta (θ)-defensins, has been identified to date only in leukocytes of the rhesus monkey (36). Compelling evidence indicates that α-, β-, and θ-defensins originated from a common ancestral defensin gene (22). Because only β-defensins have been found in birds, they may constitute the oldest of these three defensin subfamilies (15).
Human tissues express at least six α-defensins and three β-defensins. Four of the α-defensins (HNP-1, -2, -3, and -4) are stored within the primary (azurophil) granules of the neutrophil, and two others (HD-5 and -6) occur in cytoplasmic granules of small intestinal Paneth cells. HD-5 was also found in the vaginas and ectocervixes of healthy females and was expressed by inflamed fallopian tubes (26).
The β-defensins of humans (3, 14, 25, 32, 40, 43), mice (1, 24), and cattle (28, 31, 33, 35, 37, 42) have received considerable attention. Human β-defensin-1 (HBD-1) is expressed constitutively by epithelial cells throughout the body (43) and is especially prominent in genitourinary tract organs, including the vagina and kidneys (3, 40). HBD-2 is inducible and occurs in the skin, respiratory passages, and intestine (14, 25, 32). Impaired defensin function, which may or may not (18) be attributable to local airway hypersalinity, has been implicated in the pathogenesis of bronchopulmonary infections in cystic fibrosis patients (2, 12).
In cattle, β-defensin expression occurs in epithelial cells of the trachea, tongue, and intestine and in alveolar macrophages. In these sites, peptide expression is induced by lipopolysaccharide, injury, and/or cytokines (28, 31, 37). In contrast, bovine granulocytes express β-defensins in a constitutive manner (33, 35, 42).
α-Defensins are remarkably abundant in human, rabbit, and rat neutrophils (20), but only β-defensins (at least 13 different isoforms) appear in the neutrophils of cattle (33). β-Defensins also occur in the polymorphonucleated granulocytes (heterophils) of chickens and turkeys (4, 7, 15, 16). While defensins are often very prominent in granulocytes, they are not ubiquitous, since the neutrophils of mice (6), pigs (19), and horses (5) lack any defensins at all. Although avian β-defensins have been observed in bone marrow cells (4, 7, 15, 16), neither their induction during infection nor their expression in epithelial cells has been reported until now.
MATERIALS AND METHODS
cDNA cloning.
Total cellular RNA was purified from the tracheal tissues of chickens and turkeys using the Tri-Reagent RNA isolation procedure with reagents and procedures recommended by the manufacturer (Molecular Research Center, Cincinnati, Ohio). First-strand cDNA synthesis was done with an Advantage reverse transcription-PCR kit (Clontech, Palo Alto, Calif.) using primers designed according to the cDNA sequences of gallinacin-1 (Gal-1) and turkey heterophil peptide-1 (THP-1) (4). The sense primer P1 (5′-AAACCATGCGGATCGTGTACCTGC-3′) corresponded to the 5′ regions of both peptides. The antisense primer P2 (5′-GCAATGCCTAAACTGCACGACCAAAT-3′) was complementary to the 3′ cDNA preceding the poly(A) tails of both peptides. Chicken and turkey tracheal cDNAs were PCR amplified, inserted into TOPO-TA vectors (Invitrogen, Carlsbad, Calif.), and sequenced by the fluorescein-labeled dideoxynucleotide terminator method on an Applied Biosystems 373A DNA sequencer (Perkin-Elmer, Palo Alto, Calif.).
Tissue expression in the chicken.
A healthy 3-month-old chicken was sacrificed, and 21 tissue samples were obtained at necropsy. These were rinsed in cold, sterile saline, frozen immediately, and stored at −80°C until used. Total RNA purification and cDNA synthesis were done as described above. PCR primers were designed according to the cDNA sequences of Gal-1, Gal-2, and Gal-3. P3 (5′-CCCTTACCTCACTCTCATC-3′), corresponding to bp 222 to 240 of the Gal-1 cDNA sequence, and P2 (described above) were used to amplify Gal-1 and Gal-1α. P4 (5′-GTTCTGTAAAGGAGGGTCCTGCCAC-3′), corresponding to bp 114 to 138 of the Gal-2 cDNA sequence, and P5 (5′-ACTCTATAACACAAAACATATTGC-3′), complementary to bp 327 to 350 of the Gal-2 cDNA sequence, were used to amplify Gal-2. P2 and P6 (5′-CTGCCGCTTCCCACACATAG-3′), corresponding to bp 113 to 132 of the Gal-3 cDNA sequence, were used to amplify Gal-3. Two β-actin primers, P7 (5′-GAGCACCCTGTGCTGCTCACAGAGG-3′) and P8 (5′-CATTGCCAATGGTGATGACCTGACC-3′), corresponded to the sequence of chicken β-actin cDNA and were used to assess the quality and quantity of the chicken mRNA samples.
Thirty-five PCR cycles were performed with an automated thermal cycler, as follows: 94°C for 20 s, 55°C for 20 s, and 72°C for 40 s. We used a master reagent mixture to ensure tube-to-tube consistency in cDNA synthesis and PCR amplification. Reaction products were visualized after electrophoresis in 1.4% agarose gels.
Experimental infections.
Using a protocol that had been approved by the National Animal Disease Center (Ames, Iowa) Animal Care and Use Committee, mature female chickens were challenged via intranasal inoculation with 0.1 ml of an egg yolk inoculum that contained approximately 5 × 106 organisms of the Modesto strain of Haemophilus paragallinarum. Age-matched, noninfected control birds were housed under similar environmental conditions. Chickens were euthanized on the fifth day after infection, following the onset of clinical signs. Tissue samples were collected, snap frozen in liquid nitrogen, and stored at −80°C.
In situ hybridization.
Paraffin-embedded chicken tongue biopsy specimens were sectioned to a thickness of about 5 μm. The sections were baked at 60°C for 1 h, deparaffinized in two changes of xylene for 5 min, immersed in two changes of absolute ethanol for 1 min, and air dried for 10 min. Sections were treated at 37°C for 10 min with 40 μg of proteinase K per ml in phosphate-buffered saline (PBS), rinsed in PBS, and fixed at room temperature for 1 min with fresh 4% paraformaldehyde in PBS. After being rinsed with PBS, the sections were dehydrated through a graded ethanol series and air dried.
The specific antisense probe (5′-CACAGAATTCAGGGCATCAACCTCATATGCTCTTCCACAGCAGG-3′) was complementary to bp 160 to 203 of Gal-3. A sense probe of the same sequence was used as the control. Both probes were labeled using the BioPrime DNA labeling system (Life Technologies, Rockville, Md.). Briefly, 500 ng of oligonucleotides was mixed with 20 μl of 2.5× random primers solution, denatured by boiling for 5 min, and ice cooled for 5 min. To this was added 5 μl of 10× deoxynucleoside triphosphates, 1.3 μl of Klenow fragment, and distilled water to bring the total volume up to 50 μl. Reaction mixtures were incubated at 37°C for 60 min, ethanol precipitated twice with 5 μl of 3 M sodium acetate and 100 μl of cold 95% ethanol, frozen at −80°C for 2 h, and centrifuged at 15,000 × g for 10 min.
Hybridization and detection were carried out using an in situ hybridization and detection system (Life Technologies). DNA probes were dissolved at 0.5 μl/ml in hybridization buffer and 20% dextran sulfate solution. Probes were denatured by boiling for 10 min and chilled on ice for 5 min. Ten microliters of probe was used per slide, and slides were covered with 22- by 22-mm coverslips (Fisher Scientific, Hanover Park, Ill.) and hybridized in a humid chamber at 42°C overnight. After hybridization, the coverslips were removed, and the slides were immersed in three changes of 0.2× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate) at room temperature for 15 min.
Next, the slides were incubated with 200 μl of blocking solution at room temperature for 15 min in a humidified chamber. The blocking solution was then removed, and the slides were next incubated with 10 μl of streptavidin-alkaline phosphatase conjugate plus 90 μl of buffer for another 15 min. The slides were washed twice in Tris-buffered saline (100 mM Tris base, 150 mM sodium chloride, pH 7.5) for 15 min and once in alkaline-substrate buffer (100 mM Tris base, 150 mM sodium chloride, 50 mM MgCl2 · 6H2O, pH 9.5) for 5 min at room temperature. Color development was carried out in nitroblue tetrazolium–5-bromo-4-chloro-3-indolylphosphate solution at 37°C and terminated by rinsing the slides several times in deionized water. Slides were counterstained with safranin, dehydrated through a graded ethanol series (50, 70, 90, and 100%) for 1 min in each concentration, air dried, and permanently mounted with Gel/Mount (Biomeda Corp., Foster City, Calif.). Photographs were taken at a magnification of ×100 with a Nikon Optiphot microscope.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences described in this paper are as follows: Gal-1α, AF181951; Gal-3, AF181952; and GPV-1, AF18195.
RESULTS
Gallinacins.
We sequenced 12 clones from the chicken trachea PCR product. One showed 99% identity with the Gal-1 cDNA sequence (Fig. 1), and its deduced mature amino acid sequence was identical to that of the Gal-1α peptide we previously purified from chicken leukocytes (15). The carboxyl-terminal glycine shown in the cDNA sequence was absent from the mature Gal-1α peptide, indicating that the native peptide undergoes C-terminal processing. The Gal-1α prepropeptide comprised 65 residues, including a signal sequence of 20 residues, a propiece with 5 residues, a mature peptide with 39 residues, and the C-terminal glycine discussed above. Three single-nucleotide substitutions distinguished Gal-1α from Gal-1, and each caused an amino acid change. In Gal-1α and Gal-1, the amino acids and codons were as follows, respectively: Ser9 and Asn9 (AGT and AAT), Ser20 and Tyr20 (TCC and TAC), and His32 and Tyr32 (TAC and CAC). We had originally purified Gal-1α from chicken leukocytes (15, 16), and while the present results suggest that it may also be expressed by chicken tracheal cells, its origin from tissue leukocytes cannot be excluded, as some macrophages can express β-defensins (29).
FIG. 1.
cDNA and peptide sequences of Gal-1α. The deduced chicken prepropeptide contains 65 residues with a mass of 7,286 Da and a pI of 10.21. The stop codon (TGA) is double underlined.
All of the other 11 cDNA clones obtained from the chicken tracheal PCR product encoded a novel β-defensin, Gal-3, whose sequence is shown in Fig. 2. The deduced Gal-3 prepropeptide contained 80 amino acids and had a mass of 8,723.3 Da and a pI of 9.42. The propeptide included a 20-residue signal sequence, followed by a short propiece and a typical cationic β-defensin domain. The latter contained 38 residues, and its mass and pI were 4,234 Da and 9.49, respectively. The defensin domain was followed by a distinctly unusual 22- to 24-residue anionic extension, (AY)EVD … NPH.
FIG. 2.
cDNA and peptide sequences of Gal-3. The deduced chicken prepropeptide contains 78 residues with a mass of 8,746 Da and a pI of 9.42. The stop codon (TGA) is double underlined, and the peptide's C-terminal extension is in boldface.
GPV-1.
We identified a homologous epithelial β-defensin in turkey tracheal tissue. Named GPV-1, its cDNA and deduced amino acid sequences are shown in Fig. 3. Unlike Gal-3, GPV-1 did not contain a C-terminal extension. Instead, its 240-bp reading frame encoded a 59-amino-acid residue and a cationic prepropeptide, with a calculated mass of 6,598 Da (oxidized cysteines) and pI of 9.49. The signal sequence and 3′ untranslated portion of GPV-1 cDNA were each 83% identical to THP-1 cDNA at the nucleotide level, but the sequences encoding the mature peptides were considerably more divergent.
FIG. 3.
cDNA and peptide sequences of Gallopavin 1. The deduced turkey prepropeptide contains 59 residues with a mass of 6,598 Da and a pI of 9.49. The stop codon (TGA) is double underlined.
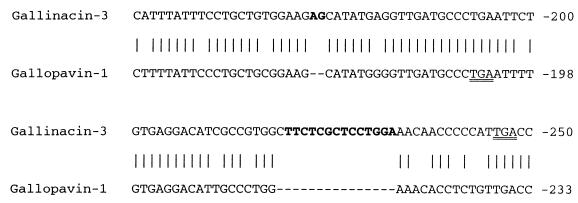
The Gal-3 and Gal-1 and -1α cDNA sequences showed ∼75% overall identity, which was most marked in their signal sequences and 3′ untranslated regions. The corresponding cDNA sequences of chicken Gal-3 and turkey GPV-1 were 91% identical. The C-terminal extension of Gal-3 evidently arose from the insertion of two bases just before its original TGA stop codon (retained in GPV-1), introducing a frameshift and read through of the old stop codon (Fig. 4). These changes, plus insertion of a 15-bp fragment not found in GPV-1, generated the “postpiece” of Gal-3 (Fig. 2). To confirm that the insert, frameshift, and extension did not result from a PCR artifact, we used another PCR primer set to prepare and amplify cDNA from the chicken tongue and bursa of Fabricius. Cloning and sequencing of both PCR products confirmed the above-described findings (data not shown).
FIG. 4.
Partial cDNA sequences of Gal-3 and Gallopavin 1. The inserted nucleotide bases of Gal-3 (i.e., those without counterparts in Gallopavin 1) are shown in boldface. Both in-frame TGA stop codons are double underlined.
Expression in healthy tissues.
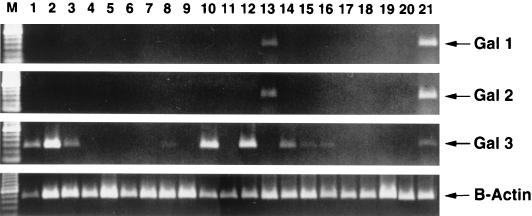
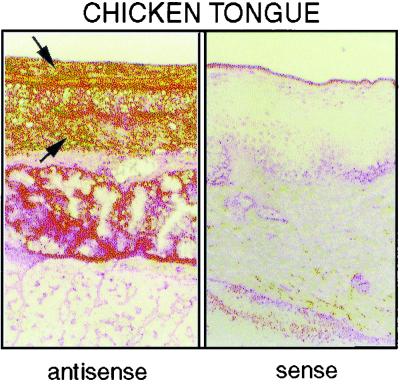
We examined the expression of Gal-1, -2, and -3 in 21 different healthy chicken tissues, including (i) skin, (ii) the gastrointestinal tract (tongue, esophagus, proventriculus, gizzard, liver, small intestine, large intestine, cloaca, bursa of Fabricius, and gall bladder), (iii) the respiratory tract (trachea, lung, and air sacs), (iv) the genitourinary tract (kidney, ovary, oviduct, and egg yolk sacs), and (v) miscellaneous tissues (spleen, pancreas, and bone marrow). Figure 5 shows that Gal-1 and -1α (our primers did not distinguish between them) and Gal-2 were expressed strongly only in healthy bone marrow and, to a lesser extent, in lung. In contrast, Gal-3 was weakly expressed in the bone marrow and was strongly expressed in the tongue, bursa of Fabricius, and trachea. Moderate Gal-3 expression was noted in the skin, esophagus and air sacs. Weaker expression of Gal-3 was seen in the large intestine, kidney, and ovary. Thus, whereas expression of Gal-1 (and -1α) and -2 was restricted to bone marrow cells, Gal-3 showed widespread expression in nonmyeloid cells. The expression of Gal-3 by epithelial cells of the tongue was confirmed by in situ hybridization (Fig. 6).
FIG. 5.
Expression of β-defensins in the tissues of normal chickens. Lanes: 1, skin; 2, tongue; 3, esophagus and crop; 4, proventriculus; 5, gizzard; 6, liver; 7, small intestine; 8, large intestine; 9, cloaca (coprodaeum, urodaeum, and proctodaeum); 10, bursa of Fabricius; 11, liver; 12, trachea; 13, lung; 14, air sacs (interclavicular, cervical, anterior thoracic, posterior thoracic, and abdominal); 15, kidney (cranial, middle, and caudal); 16, ovary; 17, oviduct (pooled ostium, magnum, isthmus, uterine shell gland, and vagina); 18, egg yolk sacs; 19, spleen; 20, pancreas; 21, bone marrow; M, ladder standards. Each tissue sample was obtained from a single individual chicken.
FIG. 6.
Gal-3 expression in the chicken tongue (in situ hybridization). (Left panel) Section of tongue that was probed with antisense message. Sites of Gal-3 expression (arrows) are stained brown. (Right panel) Control that was processed with the corresponding sense probe.
Inducibility.
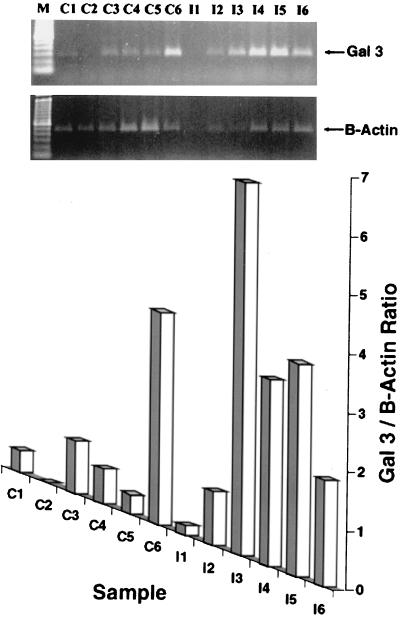
To determine if tracheal Gal-3 production was constitutive or increased in response to infection, we challenged six chickens with H. paragallinarum, reserving six noninfected, age-matched animals of the same lines as controls (Fig. 7). The results were analyzed quantitatively by phosphorimaging the PCR products and normalizing Gal-3 expression to that of β-actin in the same sample. One sample from an infected chicken (sample I-1) lacked both Gal-3 and β-actin and was therefore not analyzed further. The other 11 tracheal samples (6 control and 5 infected) were analyzed by a Mann-Whitney rank sum test. The median values were 1.140 (control) and 6.800 (infected). These differences were significant (T = 43.000; P [exact] = 0.017), indicating that tracheal expression of Gal-3 rose in response to H. paragallinarum infection. In contrast, the expression of Gal-3 in the skin, tongue, esophagus, and bursa of Fabricius did not differ significantly in tissues from control and infected chickens, suggesting that it was constitutive in nature (data not shown).
FIG. 7.
Induction of Gal-3 in the chicken trachea after infection. Tracheal tissues were obtained from six control chickens (lanes C) and six infected chickens (lanes I) on the fifth day after the experimental group had been infected with H. paragallinarum. Reverse transcription-PCR was performed as described in the text, using primers specific for Gal-3 and chicken β-actin. The bar graph shows the Gal-3/β-actin ratio.
DISCUSSION
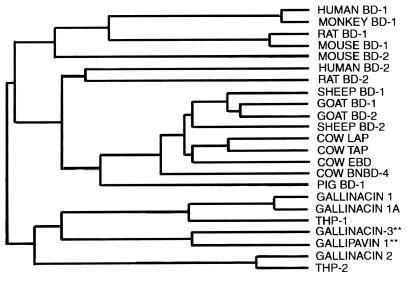
A database search (BLAST) performed on the nucleotide sequences of Gal-3 and GPV-1 identified only chicken Gal-1 and turkey THP-1 as their close relatives. When this search was performed on the peptide sequences of Gal-3 and GPV-1, many additional β-defensin homologues were recognized. The relationship of Gal-3 and GPV-1 to other β-defensins is shown in a dendrogram, based on amino acid sequences (Fig. 8).
FIG. 8.
Dendrogram. Avian and mammalian β-defensins are shown. The relationship is based on amino acid sequence homology. TAP, LAP, and EBD, β-defensins from epithelial cells of the bovine trachea, tongue, and intestine, respectively.
An unusual structural feature of the Gal-3 precursor was the 22- to 24-residue peptide domain that extended beyond its expected C terminus. The mechanism that established this was discussed above. The functional significance (if any) of the extension is unknown. Since we did not purify mature Gal-3 peptide, we cannot exclude its posttranslational removal by limited proteolysis. Very similar peptide architecture has been noted in certain defensin-like peptides from invertebrates. MGD-1, a 39-residue, defensin-like antibacterial peptide from hemocytes of the Mediterranean mussel, Mytilus galloprovincialis, provides the best example (23). It has a signal peptide of 21 residues (versus 20 residues in Gal-3), an active peptide of 39 amino acids (versus 38 residues in Gal-3), and an acidic, 21-residue carboxyl-terminal extension (versus 22 to 24 residues in Gal-3). The insect defensins of bees (Apis mellifera and Bombus pascuorum) have C-terminal extensions that make them about 12 residues longer than other insect defensins (10, 27). What, if anything, the extensions contribute to function remains to be determined. “Big defensin,” an antimicrobial peptide of Limulus, the horseshoe crab, has a 35-residue N-terminal hydrophobic domain and a 37-residue, C-terminal β-defensin-like domain (30). Here, both domains contribute significantly to the peptide's antimicrobial properties (30).
The extensive expression of Gal-3 in the chicken tongue, evident in Fig. 5 and 6, is reminiscent of findings reported for cattle (31) and pigs (34). Immunohistochemical studies showed that β-defensin was concentrated in a 0.1-mm-thick layer at the cornified tips of filiform papillae on the dorsal tongue of the pig and in superficial squamous cell layers of its buccal mucosa, presumably constituting an antimicrobial barrier against organisms that enter the mouth (34).
The prominent Gal-3 expression in the bursa of Fabricius (Fig. 5) was fascinating, given recent reports that describe interactions between defensins and B or T lymphocytes. For example, human β-defensins are chemotactic for immature dendritic cells and memory T cells and bind CCR6, a chemokine receptor preferentially expressed by these cells (41). There is also evidence that defensins can enhance systemic immunoglobulin G antibody responses in vitro and in vivo, acting through help provided by CD4+ Th1- and Th2-type cytokines (21). Finally, α-defensins and prodefensins have been recovered as HLA-DR-associated ligands from the peripheral blood mononuclear cells of two patients with plasmacytoma (13).
Figure 9 compares the primary sequences of Gal-3 and GPV-1 to each other and to those of the myeloid β-defensins of these fowl. Except for its C-terminal extension, Gal-3 resembles GPV-1 more closely than it resembles myeloid gallinacins. Although other factors may have contributed to maintaining the stability of epithelial β-defensins, a need to retain structural features involved in receptor-ligand interactions (e.g., with lymphocytes or cytokine receptors) may have constrained their ability to diverge.
FIG. 9.
Primary sequences of chicken and turkey β-defensins. Heterophils are equivalent to polymorphonuclear neutrophils in humans. Identical residues in each set are connected by vertical lines. Boldface indicates conserved residues.
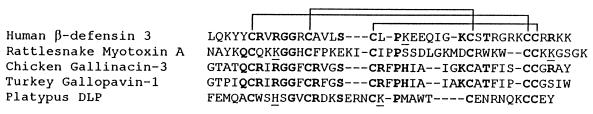
It is noteworthy that β-defensins show striking similarities to certain toxins. Figure 10 aligns chicken Gal-3 and turkey GPV-1 with three other peptides: (i) crotamine, a myotoxic peptide found in rattlesnake (Crotalus viridis viridis) venom; (ii) a β-defensin-like peptide from the venom of the male duck-billed platypus (Ornithorhynchus anatiformis) (8, 38); and (iii) HBD-3 (the HBD-3 sequence is entry gi:8163794 on the National Center for Biotechnology server of the National Institutes of Health [http://www.ncbi.nlm.nih.gov/]).
FIG. 10.
A little further from the tree? The amino acid sequences of Gal-3, Gal-1, HBD-3, platypus defensin-like peptide (DLP), and a rattlesnake crotamine are shown, with gaps (–) placed to maximize alignment. Cysteine connectivity, shown at the top, is identical for all of these peptides. Boldface indicates residues identical to those found in both Gal-3 and GPV-1. Underlining shows conservative substitutions of residues found in both Gal-3 and GPV-1.
Even though homology at the nucleotide level is no longer evident, HBD-3 and chicken Gal-3 are clearly the descendants of common ancestral genes. The platypus peptide has not been cloned, but its solution structure and cysteine disulfide pairing are identical to those of the bovine β-defensin BNBD-12 (39). The crotalid myotoxins, several of which have been cloned, share the identical disulfide pairing motif (9). Because the signal peptide of defensins typically shows greater conservation than does the defensin domain, we performed a BLAST search on the amino acid sequence of HBD-3 and obtained 62 hits from among the 509,459 sequences analyzed. Remarkably, 19 of the first 20 hits were peptides that contained six cysteines with an identical disulfide pairing pattern. Of these, 11 were mammalian β-defensins (including BNBD-1), 2 were avian β-defensins (gallinacin and THP), and 6 were rattlesnake peptides of the crotamine type (Table 1). Since the signal sequence search did not use any sequence or conformational information derived from the cysteine-rich β-defensin or toxin domains, it seems highly improbable that rattlesnake crotamines and β-defensins are not homologous. While this dual nature of peptides derived from a common gene is new, perhaps Lucretius (who also anticipated atomic structure) had something similar in mind when he wrote in De Rerum Natura, “Quod ali cibus est aliis fuat acre venenum.” (Lucretius [98 to 55 BCE] in De Rerum Natura. Translation: “What is food to one may be a fierce poison to others.”)
TABLE 1.
Signal sequence conservationa
| Species | Accession no. | Peptide | No. of residues/total (%)
|
|
|---|---|---|---|---|
| Identical | Conserved | |||
| Human | AF217245 | HBD-3 | 22/22 (100) | 22/22 (100) |
| Chimpanzee | AF209855 | β-Defensin-2 | 13/20 (65) | 18/20 (90) |
| Human | AF040153 | HBD-2 | 13/20 (65) | 18/20 (90) |
| Mouse | AF092929 | β-Defensin-3 | 12/17 (70) | 13/17 (75) |
| Pig | AF031666 | β-Defensin-1 | 11/20 (55) | 14/20 (70) |
| Turkey | AF033338 | THP-2 | 14/20 (70) | 15/20 (75) |
| Chicken | AF033336 | Gallinacin | 14/20 (70) | 15/20 (75) |
| Rat | AF068861 | β-Defensin-2 | 11/18 (61) | 14/18 (77) |
| Mouse | AF068861 | β-Defensin-4 | 11/18 (61) | 13/18 (72) |
| Tropical rattlesnake | AF044674 | Crotamine | 12/21 (57) | 15/21 (71) |
| Prairie rattlesnake | JC5324 | Myotoxin A | 12/21 (57) | 15/21 (71) |
| Bovine | AAD43032.1 | BNBD-12 | 12/20 (60) | 14/20 (70) |
| Bovine | S76279 | LAP (tongue) | 11/15 (73) | 13/15 (86) |
| Bovine | M63023 | TAP (trachea) | 11/15 (73) | 13/15 (86) |
| Bovine | AF000362 | EAP (enteric) | 10/15 (66) | 12/15 (79) |
REFERENCES
- 1.Bals R, Goldman M J, Wilson J M. Mouse beta-defensin 1 is a salt-sensitive antimicrobial peptide present in epithelia of the lung and urogenital tract. Infect Immun. 1998;66:1225–1232. doi: 10.1128/iai.66.3.1225-1232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bals R, Wang X, Wu Z, Freeman T, Bafna V, Zasloff M, Wilson J M. Human beta-defensin 2 is a salt-sensitive peptide antibiotic expressed in human lung. J Clin Investig. 1998;102:874–880. doi: 10.1172/JCI2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bensch K W, Raida M, Magert H J, Schulz-Knappe P, Forssmann W G. hBD-1: a novel beta-defensin from human plasma. FEBS Lett. 1995;368:331–335. doi: 10.1016/0014-5793(95)00687-5. [DOI] [PubMed] [Google Scholar]
- 4.Brockus C W, Jackwood M W, Harmon B G. Characterization of beta-defensin prepropeptide mRNA from chicken and turkey bone marrow. Anim Genet. 1998;29:283–289. doi: 10.1046/j.1365-2052.1998.00338.x. [DOI] [PubMed] [Google Scholar]
- 5.Couto M A, Harwig S S, Cullor J S, Hughes J P, Lehrer R I. Identification of eNAP-1, an antimicrobial peptide from equine neutrophils. Infect Immun. 1992;60:3065–3071. doi: 10.1128/iai.60.8.3065-3071.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisenhauer P B, Lehrer R I. Mouse neutrophils lack defensins. Infect Immun. 1992;60:3446–3447. doi: 10.1128/iai.60.8.3446-3447.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans E W, Beach G G, Wunderlich J, Harmon B G. Isolation of antimicrobial peptides from avian heterophils. J Leukoc Biol. 1994;56:661–665. doi: 10.1002/jlb.56.5.661. [DOI] [PubMed] [Google Scholar]
- 8.Fenner P J, Williamson J A, Myers D. Platypus envenomation—a painful learning experience. Med J Aust. 1992;157:829–832. doi: 10.5694/j.1326-5377.1992.tb141302.x. [DOI] [PubMed] [Google Scholar]
- 9.Fox J W, Elzinga M, Tu A T. Amino acid sequence and disulfide bond assignment of myotoxin a isolated from the venom of Prairie rattlesnake (Crotalus viridis viridis) Biochemistry. 1979;18:678–684. doi: 10.1021/bi00571a020. [DOI] [PubMed] [Google Scholar]
- 10.Fujiwara S, Imai J, Fujiwara M, Yaeshima T, Kawashima T, Kobayashi K. A potent antibacterial protein in royal jelly. Purification and determination of the primary structure of royalisin. J Biol Chem. 1990;265:11333–11337. [PubMed] [Google Scholar]
- 11.Ganz T, Weiss J. Antimicrobial peptides of phagocytes and epithelia. Semin Hematol. 1997;34:343–354. [PubMed] [Google Scholar]
- 12.Goldman M J, Anderson G M, Stolzenberg E D, Kari U P, Zasloff M, Wilson J M. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–560. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 13.Halder T M, Bluggel M, Heinzel S, Pawelec G, Meyer H E, Kalbacher H. Defensins are dominant HLA-DR-associated self-peptides from CD34(−) peripheral blood mononuclear cells of different tumor patients (plasmacytoma, chronic myeloid leukemia) Blood. 2000;95:2890–2896. [PubMed] [Google Scholar]
- 14.Harder J, Meyer-Hoffert U, Teran L M, Schwichtenberg L, Bartels J, Maune S, Schroder J M. Mucoid Pseudomonas aeruginosa, TNF-α, and IL-1beta, but not IL-6, induce human beta-defensin-2 in respiratory epithelia. Am J Respir Cell Mol Biol. 2000;22:714–721. doi: 10.1165/ajrcmb.22.6.4023. [DOI] [PubMed] [Google Scholar]
- 15.Harwig S S, Swiderek K M, Kokryakov V N, Tan L, Lee T D, Panyutich E A, Aleshina G M, Shamova O V, Lehrer R I. Gallinacins: cysteine-rich antimicrobial peptides of chicken leukocytes. FEBS Lett. 1994;342:281–285. doi: 10.1016/0014-5793(94)80517-2. [DOI] [PubMed] [Google Scholar]
- 16.Harwig S S, Swiderek K M, Kokryakov V, Lee T D, Lehrer R I. Primary structure of Gallinacin-1, an antimicrobial β-defensin from chicken leukocytes. In: Crabbs J, editor. Techniques in protein chemistry. IV. San Diego, Calif: Academic Press; 1994. pp. 81–88. [Google Scholar]
- 17.Huttner K M, Bevins C L. Antimicrobial peptides as mediators of epithelial host defense. Pediatr Res. 1999;45:785–794. doi: 10.1203/00006450-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Knowles M R, Robinson J M, Wood R E, Pue C A, Mentz W M, Wager G C, Gatzy J T, Boucher R C. Ion composition of airway surface liquid of patients with cystic fibrosis as compared with normal and disease-control subjects. J Clin Investig. 1997;100:2588–2595. doi: 10.1172/JCI119802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokryakov V N, Harwig S S, Panyutich E A, Shevchenko A A, Aleshina G M, Shamova O V, Korneva H A, Lehrer R I. Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 1993;327:231–236. doi: 10.1016/0014-5793(93)80175-t. [DOI] [PubMed] [Google Scholar]
- 20.Lehrer R I, Lichtenstein A K, Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- 21.Lillard J W, Jr, Boyaka P N, Chertov O, Oppenheim J J, McGhee J R. Mechanisms for induction of acquired host immunity by neutrophil peptide defensins. Proc Natl Acad Sci USA. 1999;96:651–656. doi: 10.1073/pnas.96.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L, Zhao C, Heng H H, Ganz T. The human beta-defensin-1 and alpha-defensins are encoded by adjacent genes: two peptide families with differing disulfide topology share a common ancestry. Genomics. 1997;43:316–320. doi: 10.1006/geno.1997.4801. [DOI] [PubMed] [Google Scholar]
- 23.Mitta G, Vandenbulcke F, Hubert F, Roch P. Mussel defensins are synthesised and processed in granulocytes then released into the plasma after bacterial challenge J. Cell Sci. 1999;112:4233–4242. doi: 10.1242/jcs.112.23.4233. [DOI] [PubMed] [Google Scholar]
- 24.Morrison G M, Davidson D J, Dorin J R. A novel mouse beta defensin, Defb2, which is upregulated in the airways by lipopolysaccharide. FEBS Lett. 1999;442:112–116. doi: 10.1016/s0014-5793(98)01630-5. [DOI] [PubMed] [Google Scholar]
- 25.O'Neil D A, Porter E M, Elewaut D, Anderson G M, Eckmann L, Ganz T, Kagnoff M F. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–6724. [PubMed] [Google Scholar]
- 26.Quayle A J, Porter E M, Nussbaum A A, Wang Y M, Brabec C, Yip K P, Mok S C. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152:1247–1258. [PMC free article] [PubMed] [Google Scholar]
- 27.Rees J A, Moniatte M, Bulet P. Novel antibacterial peptides isolated from a European bumblebee, Bombus pascuorum (Hymenoptera, Apoidea) Insect Biochem Mol Biol. 1997;27:413–422. doi: 10.1016/s0965-1748(97)00013-1. [DOI] [PubMed] [Google Scholar]
- 28.Russell J P, Diamond G, Tarver A P, Scanlin T F, Bevins C L. Coordinate induction of two antibiotic genes in tracheal epithelial cells exposed to the inflammatory mediators lipopolysaccharide and tumor necrosis factor alpha. Infect Immun. 1996;64:1565–1568. doi: 10.1128/iai.64.5.1565-1568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryan L K, Rhodes J, Bhat M, Diamond G. Expression of beta-defensin genes in bovine alveolar macrophages. Infect Immun. 1998;66:878–881. doi: 10.1128/iai.66.2.878-881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saito T, Kawabata S, Shigenaga T, Takayenoki Y, Cho J, Nakajima H, Hirata M, Iwanaga S. A novel big defensin identified in horseshoe crab hemocytes: isolation, amino acid sequence, and antibacterial activity. J Biochem (Tokyo) 1995;117:1131–1137. doi: 10.1093/oxfordjournals.jbchem.a124818. [DOI] [PubMed] [Google Scholar]
- 31.Schonwetter B S, Stolzenberg E D, Zasloff M A. Epithelial antibiotics induced at sites of inflammation. Science. 1995;267:1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- 32.Schroder J M, Harder J. Human beta-defensin-2. Int J Biochem Cell Biol. 1999;31:645–651. doi: 10.1016/s1357-2725(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 33.Selsted M E, Tang Y Q, Morris W L, McGuire P A, Novotny M J, Smith W, Henschen A H, Cullor J S. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J Biol Chem. 1993;268:6641–6648. [PubMed] [Google Scholar]
- 34.Shi J, Zhang G, Wu H, Ross C, Blecha F, Ganz T. Porcine epithelial beta-defensin 1 is expressed in the dorsal tongue at antimicrobial concentrations. Infect Immun. 1999;67:3121–3127. doi: 10.1128/iai.67.6.3121-3127.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang Y Q, Selsted M E. Characterization of the disulfide motif in BNBD-12, an antimicrobial beta-defensin peptide from bovine neutrophils. J Biol Chem. 1993;268:6649–6653. [PubMed] [Google Scholar]
- 36.Tang Y Q, Yuan J, Osapay G, Osapay K, Tran D, Miller C J, Ouellette A J, Selsted M E. A cyclic antimicrobial peptide produced in primate leukocytes by the ligation of two truncated alpha-defensins. Science. 1999;286:498–502. doi: 10.1126/science.286.5439.498. [DOI] [PubMed] [Google Scholar]
- 37.Tarver A P, Clark D P, Diamond G, Russell J P, Erdjument-Bromage H, Tempst P, Cohen K S, Jones D E, Sweeney R W, Wines M, Hwang S, Bevins C L. Enteric beta-defensin: molecular cloning and characterization of a gene with inducible intestinal epithelial cell expression associated with Cryptosporidium parvuminfection. Infect Immun. 1998;66:1045–1056. doi: 10.1128/iai.66.3.1045-1056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tonkin M A, Negrine J. Wild platypus attack in the antipodes. A case report. J Hand Surg. 1994;19:162–164. doi: 10.1016/0266-7681(94)90155-4. [DOI] [PubMed] [Google Scholar]
- 39.Torres A M, de Plater G M, Doverskog M, Birinyi-Strachan L C, Nicholson G M, Gallagher C H, Kuchel P W. Defensin-like peptide-2 from platypus venom: member of a class of peptides with a distinct structural fold. Biochem J. 2000;348:649–656. [PMC free article] [PubMed] [Google Scholar]
- 40.Valore E V, Park C H, Quayle A J, Wiles K R, McCray P B, Jr, Ganz T. Human beta-defensin-1: an antimicrobial peptide of urogenital tissues. J Clin Investig. 1998;101:1633–1642. doi: 10.1172/JCI1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang D, Chertov O, Bykovskaia S N, Chen Q, Buffo M J, Shogan J, Anderson M, Schroder J M, Wang J M, Howard O M, Oppenheim J J. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286:525–528. doi: 10.1126/science.286.5439.525. [DOI] [PubMed] [Google Scholar]
- 42.Yount N Y, Yuan J, Tarver A, Castro T, Diamond G, Tran P A, Levy J N, McCullough C, Cullor J S, Bevins C L, Selsted M E. Cloning and expression of bovine neutrophil beta-defensins. Biosynthetic profile during neutrophilic maturation and localization of mature peptide to novel cytoplasmic dense granules. J Biol Chem. 1999;274:26249–26258. doi: 10.1074/jbc.274.37.26249. [DOI] [PubMed] [Google Scholar]
- 43.Zhao C, Wang I, Lehrer R I. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996;396:319–322. doi: 10.1016/0014-5793(96)01123-4. [DOI] [PubMed] [Google Scholar]