Abstract
Cucumber (Cucumis sativus L.) is a crop plant being the third most-produced vegetable developed as a new model plant. Heavy metal pollution is a serious global problem that affects crop production. An industrial activity has led to high emissions of Cd into the environment. Plants realize adaptive strategies to diminish the toxic effects of Cd. They can remove excess toxic ions of heavy metals from the cytoplasm to the outside of cells using the metal/proton antiport. The proton gradient needed for the action of the antiporter is generated by the plasma membrane (PM) H+-ATPase (EC 3.6.3.14). We have shown that treatment of cucumber plants with Cd stimulated the diamine oxidase (DAO, EC 1.4.3.6) activity in roots. Under cadmium stress, the PM H+-ATPase activity also increased in cucumber seedlings. The stimulating effect of Cd on the PM H+-ATPase activity and expression of three genes encoding this enzyme (CsHA2, CsHA4, CsHA8) was reduced by aminoguanidine (AG, a DAO inhibitor). Moreover, we have observed that H2O2 produced by DAO promotes the formation of NO in the roots of seedlings. The results presented in this work showed that DAO may be an element of the signal transduction pathway, leading to enhanced PM H+-ATPase activity under cadmium stress.
Keywords: DAO, Cd, PM H+-ATPase, cucumber, heavy metals, hydrogen peroxide, nitric oxide
1. Introduction
Abiotic stresses are considered as major limiting factors that affect crop production. As estimated, around 90% of all arable lands are subjected to environmental stresses such as drought, low or high temperature, salinity, heavy metal exposure, and others [1]. In recent years, heavy metal pollution has become a more serious global problem. Among the heavy metals, cadmium is one of the most phytotoxic agents. Cd is easily taken up by plant roots and can accumulate for long periods of time inside a food chain (in plants and animals) [2]. This toxic metal has a negative effect on the growth and development of plants [3]. Cd reduces seed germination, early seedling growth, and plant bio-mass [4]. It was shown that the toxic effect of Cd ions on photosynthesis plays a significant role in the inhibition of plant growth [5]. Cd has been found to mediate oxidative stress, but unlike other heavy metals, it does not directly affect the production of reactive oxygen species (ROS) through the reaction of Fenton and/or Haber Weiss. Nevertheless, the production of ROS such as hydroxyl radical, hydrogen peroxide, singlet oxygen, and superoxide radicals after Cd exposure has been reported in plants [6].
Plants have developed several adaptive strategies to fight the toxic effects of Cd. An important element of detoxification is the exclusion of Cd outside the cell or its accumulation in metabolically less active parts of the plant [4]. Removing Cd from the cell is related to immobilizing it in the cell wall or chelation. Cd could be chelated in the apoplast and this limits its harmful effects in the cell [7]. Plants can remove excess toxic heavy metal ions from the cytoplasm to the outside of cells using the heavy metal/proton antiport systems. The cation diffusion facilitator (CDF) family proteins are membrane divalent cation transporters that transfer metal ions out of the cytoplasm into extracellular space or into vacuoles, and they act as metal2+/H+ antiporters [8]. The mechanism of Cd detoxification that relies on Cd2+/H+ antiport activity in plant plasma membrane has been reported [9]. Plant CDFs are called metal tolerance proteins (MTPs). The proton gradient needed for the action of the MTP antiporter located in the plasma membrane is generated by the plasma membrane proton pump (PM H+-ATPase).
PM H+-ATPase is a functional single polypeptide chain with a mass of about 100 kDa. The protein can oligomerize to form dimeric and hexameric complexes [10]. PM H+-ATPase belongs to the P-type ATPase superfamily. Among the P-type ATPases identified in plants, none exchange sodium and potassium, as does the animal Na+/K+-ATPase. Plants possess proton vector pumping ATPase (H+-ATPase), which combines ATP hydrolysis with proton transport from the cytoplasm out of the cell to the apoplast, thus creating an electrochemical gradient across the plasma membrane [11,12]. Generation of an electrochemical gradient across membrane results in a proton-motive force that is used by secondary transport for assimilation of various nutrients, and on the other hand, for releasing ions and toxic substances from cells [13]. Aside from the regulation of growth and development processes, the PM H+-ATPase also plays a role in the plastic adaptation of plants to changing conditions, especially stressogenic ones.
The plant PM H+-ATPases are a multigene family of proteins, and a total of 10 genes have been found in Cucumis sativus, seven of which (CsHA1, CsHA2, CsHA3, CsHA4, CsHA8, CsHA9, CsHA10) are expressed in the roots [14]. The PM H+-ATPases are differentially expressed according to environmental factors. Several studies have indicated that the H+-ATPase gene expression might be changed by environmental factors: salt [15], low temperature [16], heavy metals [17,18], dehydration [19], or mechanical stress [20]. Aside from the genetic regulation of a proton pump, its activity might be fast modulated posttranslationally at the protein level, mainly through reversible phosphorylation. PM H+-ATPase contains ten transmembrane domains. The catalytic domain is between transmembrane domains 4 and 5. The N- and C-termini of the protein are located on the cytoplasmic side [11]. The C-terminal region is involved in enzyme regulation. Phosphorylation of the penultimate residue, a Thr, and the subsequent binding of regulatory 14–3–3 proteinresult in enzyme activation.
It has been reported that PM H+-ATPase activity is changed due to Cd action. The effect of metal on plasma membrane H+-ATPase activity depends on the time of plant exposure as well as heavy metal concentration. In short time (up to a few hours) Cd-exposed plants, the inhibition of PM H+-ATPase activity was observed [17,21]. However, a longer time of plant treatment with Cd led to increased activity of the enzyme [9,18]. A plasma membrane is the first cellular structure to be exposed to the toxic effects of cadmium. This metal often causes membrane damage, which increases the permeability of ions and the loss of valuable substances [21]. An increase in PM H+-ATPase activity is necessary to generate a proton gradient across the plasma membrane in order to both replenish lost substances and get rid of excess toxic ions.
Plants also adapt to Cd toxicity by activating signaling pathways that allow them to function (grow and develop) under cadmium stress conditions. ROS-signaling is very important in this process. It was previously mentioned that the accumulation of cadmium caused the enhanced production of ROS, which can act as signaling molecules in the plants’ defense [22]. There are many potential sources of hydrogen peroxide production in plant cells. One of them is the degradation of polyamines (PAs), which contributes to an increase in extracellular H2O2 level. PAs play a crucial role in the responses of plants to abiotic stresses. They are oxidatively deaminated by amine oxidases including flavin adenine dinucleotide (FAD)-dependent polyamine oxidases (PAO, EC 1.5.3.3) and copper amine oxidases (CuAO, EC 1.4.3.6), also called diamine oxidases (DAO). DAO and PAO are responsible for the oxidation of PAs in plants, which occurs with production of H2O2 [23]. Amine oxidases require oxygen and water to deaminate PAs, and forming H2O2 and aldehyde as a result of cutting off the amino group [24].
DAO is the enzyme that transforms a primary amino group –NH2 with copper as a cofactor. Oxygen is the acceptor of the charge carried by this enzyme, which shows a preference for putrescine as a diamine [25]. DAO converts putrescine to 4-aminobutanal, which spontaneously changes into a cyclic compound Δ1-pyrroline. The other products are ammonia and hydrogen peroxide [24,26,27]. This oxidase tends to form homodimers with the weight of each subunit about 70–90 kDa. There are 33 conserved amino acid residues and the 2,4,5-trihydroxyphenylalanine quinone cofactor in each subunit near the catalytic site. Additionally, a coordination bond between the copper (II) ion and three histidine residues is formed in each subunit [24]. DAO is located in the cell wall and loosely associated with it [24,27]. PAO catabolize higher polyamines (e.g., spermmidine and spermine). It was reported that PAO is highly expressed in monocots whereas DAO is present at high levels in dicotyledons [25], as shown in the examples of species from the Fabaceae family (e.g., peas (Pisum), chickpeas (Cicer), lentils (Lens), soybeans (Glycine)) [25]. Diamine oxidase is also often found in rapidly growing tissues [24].
The involvement of amine oxidases in polyamine catabolism contributes to the adaptation of plants to adverse environmental conditions. Under aluminum stress, an increase in diamine oxidase activity was observed in pea root nodules. This increase in DAO activity was responsible for enhanced accumulation of hydrogen peroxide in nodules [28].
Aside from H2O2, PAs produce nitric oxide (NO) during their catabolism. Tun et al. [29] reported that PAs induced NO biosynthesis in Arabidopsis thaliana seedlings. NO is a key signaling molecule in plants, regulating a lot of physiological processes. Moreover, NO plays an important role in the regulation of plant responses to both abiotic and biotic stress conditions. DAO could participate in nitric oxide production in plants under environmental stresses. CuAO8 regulates arginine-dependent NO generation in Arabidopsis thaliana [30]. It was shown that the cuao8 mutant lines displayed a decreased NO production in seedlings after elicitor and salinity treatment. The review of Gill et al. [31] showed the importance of NO as a Cd stress modulator in crop plants.
In our earlier study, we showed that under cadmium stress, the PM H+-ATPase activity increased in the roots of cucumber seedlings [18]. It was found that signaling molecules NO and H2O2 are important in the modification of plasma membrane proton pump activity under abiotic stress conditions including salinity and low temperature [15]. Considering this, we decided to verify whether amine oxidases can contribute to the modification of the PM H+-ATPase activity in Cucumis sativus L. seedlings under cadmium stress. For this purpose, we performed experiments in which we determined the activities of DAO and PAO in cucumber roots; DAO activity in plants treated with Cd and/or AG (aminoguanidine, DAO inhibitor); activity of plasma membrane H+-ATPase and expression of genes encoding PM H+-ATPase in cucumber seedlings treated with Cd and/or AG; NO and H2O2 level in plants treated with Cd and/or AG. The obtained results suggest that DAO may be an important element of the signal transduction pathway, leading to an increase in PM H+-ATPase activity under cadmium stress.
2. Results
2.1. Activities of DAO and PAO in Cucumber Roots
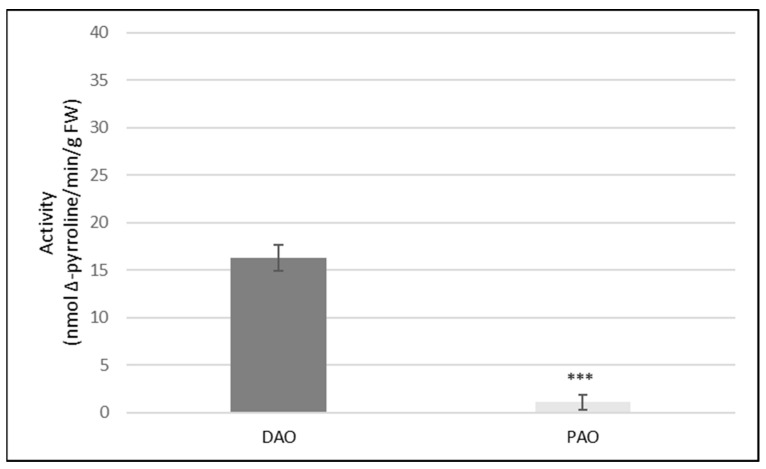
There are two main types of amine oxidases (AOs) in plants including copper-containing (DAO) and FAD-dependent (PAO). In our study, activities of both AOs, DAO and PAO, were measured in the roots of cucumber seedlings grown under the control (non-stress) conditions. It was shown that DAO activity was almost sixteen times greater than PAO activity (Figure 1).
Figure 1.
Activities of DAO and PAO in the roots of cucumber seedlings. Amine oxidase activities were measured in control cucumber plants, according to the method described in the Materials and Methods. Results are the means ± SD of five independent experiments with each experiment performed in six replicates. There was a significant difference between DAO and PAO activity (asterisks indicate the significant differences, where *** p < 0.001).
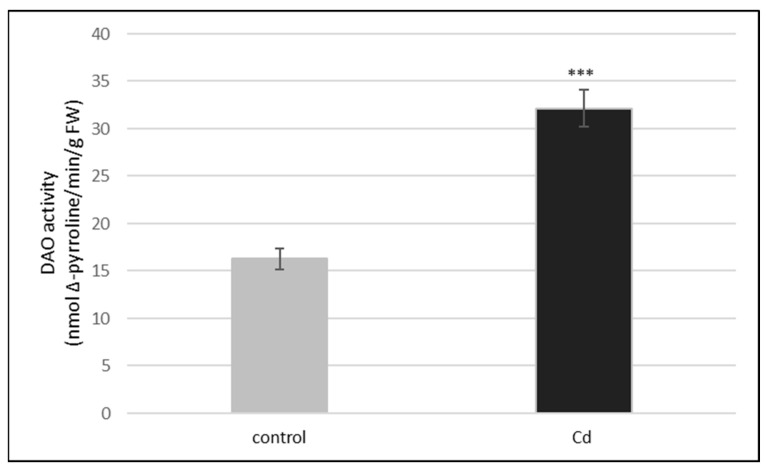
We observed that the treatment of plants with cadmium (10 µM Cd) changed the activity of DAO but had no effect on the activity of PAO in cucumber roots. PAO activity was always at a very low level, regardless of the plant treatment with cadmium (Repository 1). On the other hand, exposure of plants to cadmium stimulated twice DAO activity (Figure 2). To make sure that the determined activity, stimulated by Cd, is actually DAO activity, we used a DAO inhibitor (i.e., 0.1 mM aminoguanidine (AG)). The addition of AG greatly (about 90%) reduced DAO activity in both the control and cadmium-treated plants (Table 1).
Figure 2.
DAO activity in the roots of cucumber seedlings treated with 10 µM Cd. DAO activity was measured in the control plants and plants, which after 3 days of treatment with Cd were transferred to the control conditions for another 3 days (Cd). The data are presented as means ± SD from three independent experiments with each experiment performed in six replicates. There was a significant difference between DAO activity in the control and Cd treated plants (asterisks indicate the significant differences, where *** p < 0.001).
Table 1.
Inhibition of DAO activity by AG. DAO activity was measured in the control plants (control, −AG) or in plants treated for 3 days with aminoguanidyne added to the nutrient solution before the collection (control, +AG) as well as in plants, which after 3 days of treatment with Cd were transferred to the control conditions without (Cd, −AG) or with aminoguanidyne (Cd, +AG) for another 3 days.
| DAO Activity (pmol Δ-Pyrroline/min/g FW) | ||
|---|---|---|
| −AG | +AG | |
| Control | 16.3 (±1.1) | 2.7 (±0.7) |
| Cd | 32.1 (±1.9) | 3.3 (±1.2) |
2.2. Activity of Plasma Membrane H+-ATPase in Roots of Cucumber Seedlings Treated with Cd and/or AG
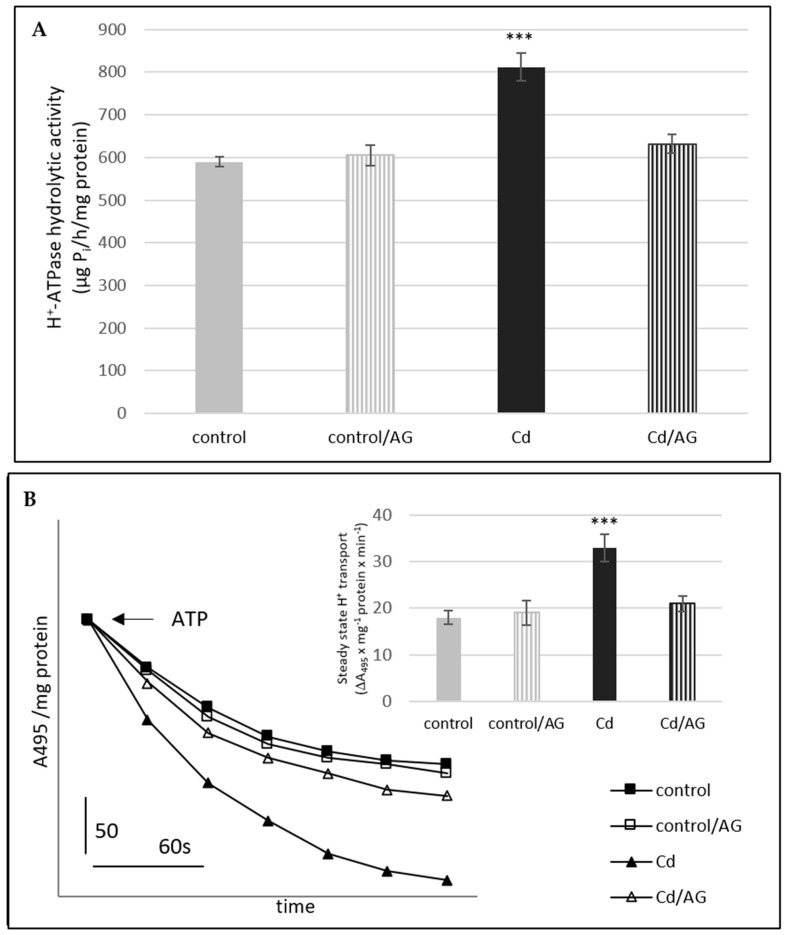
Treatment of the cucumber seedlings with cadmium increased the H+-ATPase activity in the plasma membrane fraction isolated from roots (Figure 3A,B). Cd activated the H+-pumping into vesicles to a much greater extent than the hydrolysis of ATP. The proton transport was stimulated by 89% whereas the hydrolytic activity by 37%. To verify whether the increased enzyme activity could be attributed to DAO action, the effect of aminoguanidine on the proton pump was studied in seedlings under the control and cadmium stress conditions. As we earlier indicated, treatment of the plants with cadmium and then transferring them to the control conditions increased the activity of the plasma membrane proton pump [18]. For this reason, AG was added to the medium after cadmium removal. It was found that the stimulating effect of cadmium on the plasma membrane H+-ATPase activity (both H+ transport and ATP hydrolysis) was totally abolished by AG (Cd/AG). In contrast, aminoguanidine had no effect on PM H+-ATPase activity in the control plants, not treated with Cd.
Figure 3.
Effect of cadmium and/or AG on the hydrolytic activity of H+-ATPase (A) and the ATP-dependent proton transport (B) measured in the plasma membrane vesicles. The plasma membranes (50 μg of protein) were isolated from control roots (control), roots of plants treated for 3 days before harvesting with aminoguanidyne added to control nutrient solution (control/AG), roots of plants, which after 3 days of treatment with 10 µM Cd were transferred to the control conditions for another 3 days (Cd), and the roots of plants, which after 3 days of treatment with 10 µM Cd were transferred to the control nutrient solutions with aminoguanidyne (Cd/AG). Hydrolytic activity of H+-ATPase was measured as described in the Materials and Methods. Results are the means ± SD of three independent experiments with each experiment performed in triplicate (A). After equilibration of membranes with the reaction medium (for at least 5 min), vesicle acidification was initiated by the addition of ATP to give a final concentration of 3 mM. The formation of a ΔpH gradient in the vesicles was monitored as the changes in acridine orange absorbance (A495). The values presented (B) are representative for the results obtained in three independent experiments with each experiment conducted in triplicate. The results in the inner diagrams (the steady state of H+ transport are the means ± SD from those three independent experiments (asterisks indicate the significant differences, where *** p < 0.001).
2.3. Effect of Cd and/or AG on the Expression Level of PM H+-ATPase Genes
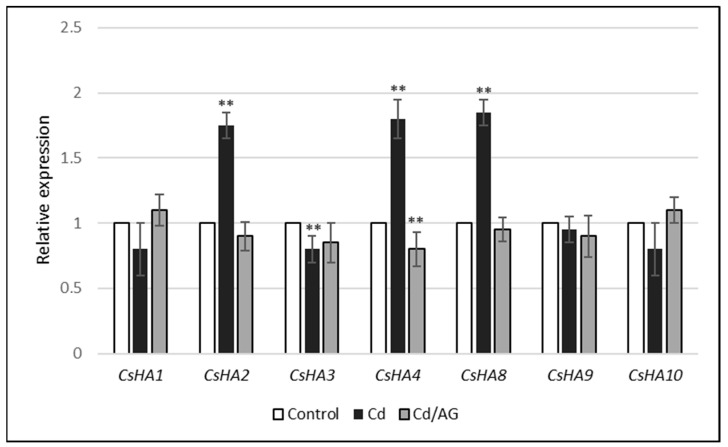
To evaluate the expression level of CsHA genes in the roots of seedlings treated with cadmium and/or AG, a real-time PCR assay was performed. We noticed that the relative expression of PM H+-ATPase genes in cucumber roots was affected by Cd. Increased CsHA2, CsHA4, and CsHA8 transcript levels were observed. On the other hand, the expression of other genes (CsHA1, CsHA3, CsHA9, and CsHA10) remained unchanged (Figure 4). Additionally, it was shown that the increase in the expression level of three isoforms (CsHA2, CsHA4, CsHA8) was completely diminished when the plants were treated with cadmium and then with AG (Figure 4). It is worth mentioning that the transcript level of genes encoding plasma membrane H+-ATPase in the control cucumber roots differed significantly (Table 2). The smallest gene expression was found in the case of the isoforms CsHA1 and CsHA10. In contrast, the greatest levels of gene expression were demonstrated for isoforms CsHA2, CsHA3, and CsHA8.
Figure 4.
Relative expression of PM H+-ATPase genes in cucumber roots exposed to Cd and/or AG. To determine the expression of PM H+-ATPase genes, real-time PCR analysis was performed as described in the Materials and Methods. The RNA was isolated from the control roots (control) and roots treated with 10 µM Cd (Cd) or 10 µM Cd and AG (Cd/AG). Values are the means of three replications. Error bars represent SD (asterisks indicate the significant differences, where ** p < 0.01).
Table 2.
Transcript level of genes encoding plasma membrane H+-ATPase in the roots of Cucumis sativus L. For the normalization of expression of each CsHA gene, a gene encoding TIP41-like protein was used as the internal standard.
| Gene | Transcript Level (Fluorescence Units) |
|---|---|
| CsHA1 | 0.01 |
| CsHA2 | 14.23 |
| CsHA3 | 13.25 |
| CsHA4 | 0.83 |
| CsHA8 | 7.33 |
| CsHA9 | 2.17 |
| CsHA10 | 0.18 |
2.4. NO Level in Roots of Cucumber Seedlings Treated with Cd and/or AG
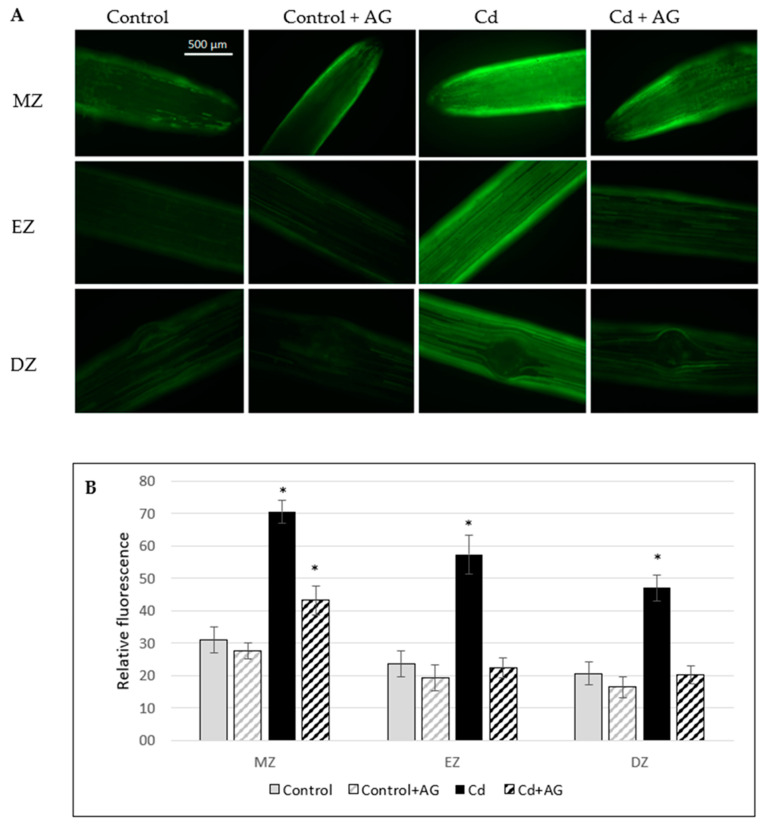
Polyamine catabolism may produce nitric oxide. It was found that the endogenous level of NO increased about 130% in the roots of plants exposed to 10 µM Cd for three days in comparison to the control (Figure 5). However, when the cadmium-stressed plants were transferred to the medium containing the DAO inhibitor (AG), the NO level significantly decreased in the roots, almost to the value observed in the control roots. This result suggests that the observed increase in the NO level in the tissues treated with Cd was dependent on DAO activity.
Figure 5.
Bio-imaging of NO level in the roots of cucumber seedlings treated with Cd in the presence or absence of aminoguanidyne (AG) (A) and the mean related fluorescence density ± SD (B). After 3 days of treatment with Cd, plants were transferred to the control conditions for another 3 days (Cd) or to the control nutrient solutions with aminoguanidyne (Cd + AG). At the same time, control plants were also treated with aminoguanidyne (control + AG). NO production was monitored by labeling with the NO-specific fluorescent dye DAF-2D and imaged using fluorescent microscopy. The images are representative for at least three roots for each treatment from three independent replications of the experiment. Asterisks on the figure indicate significant differences (* p < 0.05). The intensity of green fluorescence in the images was analyzed and expressed as the average number of pixels in green channel on a scale ranging from 0 to 255. MZ—meristematic zone, EZ—elongation zone, DZ—differentiation zone.
2.5. DAO-Induced H2O2 Level under Cadmium Stress
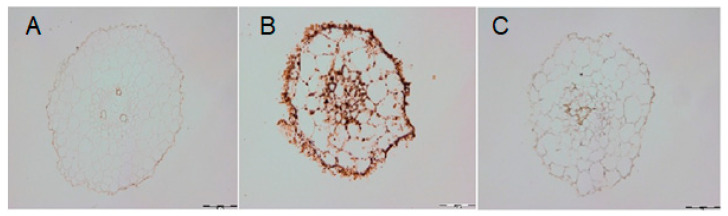
DAO can oxidize Put, leading to H2O2 accumulation. To explain the role of DAO in the Cd-induced increase of endogenous H2O2 content, the level of hydrogen peroxide was determined in the roots in the presence of aminoguanidine. Cucumber plants were grown with the addition of 10 µM CdCl2 and after 3 days, returned to the control medium without or with AG for another 3 days. The stimulating effect of Cd on H2O2 accumulation was significantly reduced (Figure 6).
Figure 6.
DAB staining and visualization of hydrogen peroxide in the roots of cucumber seedlings: control plants (A), plants treated with Cd (the plants after 3 days of treatment with Cd were transferred to the control conditions for another 3 days) (B) and plants, in which the heavy metal was withdrawn after 3 days, and then aminoguanidyne was added for the next 3 days (C).
2.6. H2O2-Dependent NO Generation in the Roots of Cucumber Seedlings
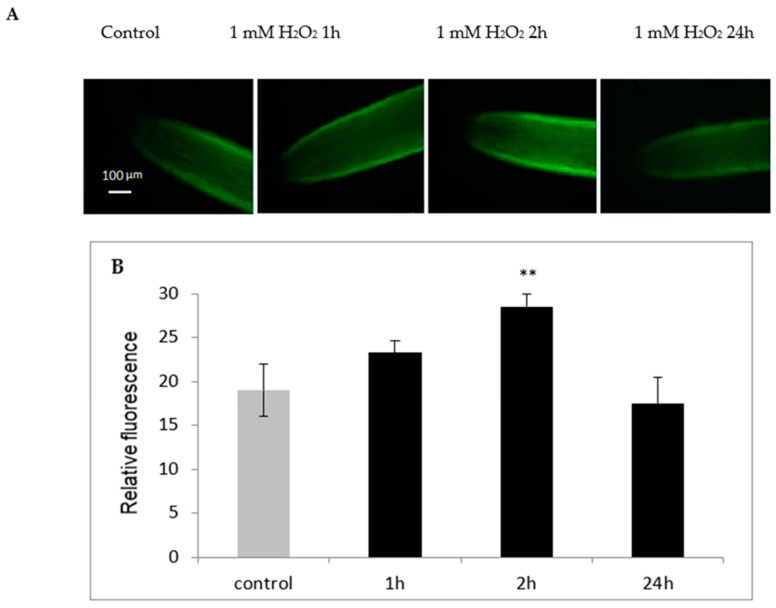
To verify the possible role of hydrogen peroxide in the production of NO in cucumber root tissue, plants were treated with 1 mM H2O2 for 1, 2, and 24 h and the level of NO was analyzed. It was indicated that hydrogen peroxide contributed to the increase in NO levels in plant roots treated with it for 2 h (Figure 7). However, in the roots of plants exposed to H2O2 for a longer time (24 h), the level of NO was similar as in the control plants not treated with H2O2.
Figure 7.
Bio-imaging of NO level in the roots of cucumber seedlings treated with H2O2 for 1, 2, and 24 h (A) and the mean related fluorescence density ± SD (B). Control plants were not treated with H2O2. NO production was monitored by labeling with the NO-specific fluorescent dye DAF-2D and imaged using fluorescent microscopy. The images are representative for at least three roots for each treatment from four independent replications of the experiment. Asterisks on the figure indicate significant differences (** p < 0.01).
3. Materials and Methods
3.1. Plant Material and Chemical Treatments
Cucumber seeds (Cucumis sativus L. var. Wisconsin), germinated for 48 h at 25 °C, were transferred to a nutrient medium for 6 days. The plants were grown in the nutrient solution contained: 1 mM MgSO4, 5 mM Ca(NO3)2, 5 mM KNO3, 1 mM KH2PO4, and microelements: 75 μM ferric citrate, 10 μM MnSO4, 5 μM H3BO4, 1 μM CuSO4, 0.01 μM ZnSO4, and 0.05 μM Na2MoO4. The plants were cultivated hydroponically without (control) or with 10 µM CdCl2 added to the nutrient solutions (pH 5.5) with a 16-h photoperiod (180 μmol m−2 s−1) at 25 °C during the day and 22 °C during the night. After 3 days, both the control and Cd-treated seedlings (Cd) were transferred to the fresh control nutrient medium (pH 6.5) for the next 3 days. Additionally, some of the control and cadmium treated plants were supplemented with 0.1 mM aminoguanidyne (AG), a DAO inhibitor, which was introduced to the medium after 3 days of cultivation when plants returned to the control medium. Such treatment conditions are compatible with our previous work [18].
3.2. Assay of DAO and PAO
Cucumber roots were used as a source of enzymes. DAO and PAO activities were estimated spectrophotometrically by a method based on the colorimetric assay of Δ-pyrroline using putrescine for DAO and spermidine for PAO as substrates [32]. One gram of fresh roots was homogenized at 4 °C in 100 mM K-phosphate (pH 7.0), containing 10 mM dithiothreitol, 10 µM pyridoxal, 0.1% Triton X-100. Homogenate was held 20 min on ice and centrifuged at 15,000× g for 20 min at 4 °C. The supernatant was used to determine the activity of diamine oxidase and polyamine oxidase.
The principle of the method is that Δ-pyrroline formed by the enzymatic oxidation of putrescine or spermidine can react with 2-aminobenzaldehyde to produce a yellowish-colored dihydroquinazolinium derivative. A spectrophotometric assay for the determination of Δ-pyrroline was performed according to Holmsted et al. [32], as modified by Federico and Angelini [33]. The 1 mL reaction mixture contained: supernatant, 50 mM K-phosphate, pH 7.5 or 6.5 (for DAO and PAO, respectively), 10 mM putrescine or spermidine (for DAO and PAO, respectively), 50 U catalase, and 0.1% 2-aminobenzaldehyde. The samples were incubated for two hours at 37 °C. After this time, the reaction was stopped by the addition of 125 μL of 10% TCA and centrifuged for 10 min at 10,000× g. The absorbance was measured at 430 nm (ε = 1.86 × 10−3 mol × cm−1).
3.3. Plasma Membrane Isolation and PM H+-ATPase Activity Determination
To obtain preparations containing highly purified plasma membrane (PM) vesicles, the method described by Kłobus [34] was used. PM was isolated from cucumber roots by an aqueous two-phase system. The PM obtained by this procedure was mainly composed of right-side-out vesicles and was used to determine hydrolytic ATPase activity. Some of the vesicles were turned to the inside-out oriented form using Brij58 and used for the measurements of ATP-dependent H+ transport across the PM.
The hydrolytic activity of the PM H+-ATPase was determined by measuring the phosphate released from ATP according to the procedure of Gallagher and Leonard [35]. Proton transport activity was determined by measuring the absorbance change of acridine orange at 495 nm (A495), according to Kłobus and Buczek [36]. After the equilibration of membranes with reaction medium (at least for 5 min), vesicle acidification was initiated by the addition of 3 mM Mg-ATP. For each combination, passive proton movement across the PM was measured without ATP in the reaction medium.
3.4. Determination of Endogenous NO
Nitric oxide concentration in roots was detected by fluorescent microscopy using the fluorescent NO indicator dye DAF-2DA (5,6-diaminofluorescein diacetate). Roots were briefly excised from the plants and incubated for 10 min in the dark in 20 mM HEPES-KOH, pH 7.4, containing 10 µM DAF-2DA. To remove excess fluorophore from the surface, roots were washed for 15 min in fresh HEPES-KOH buffer. NO-associated fluorescence was detected with a Zeiss Axio Image M2 fluorescent microscope using unchanged parameters for every measurement. For fluorescence observation, a Tag-YFP filter with an emission of 524 nm was used. The intensity of green fluorescence in the images was analyzed using Adobe Photoshop CC software and was expressed as the average number of pixels in a green channel on a scale ranging from 0 to 255.
3.5. Histochemical Detection of H2O2 in Cucumber Roots
H2O2 was identified by immersing whole six-day-old cucumber plants in a 1 mg/mL solution of DAB, in 0.33 mM MES-NaOH, pH 5.5, for 8 h in dark tubes according to the procedure of Thordal–Christensen et al. [37].
3.6. Protein Determination
Protein content was measured by the method of Bradford [38] in the presence of 0.02% Triton X-100, using BSA as the standard.
3.7. RNA Isolation and Analysis of Transcript Levels
To assess the expression of the genes encoding PM-H+-ATPase, CsHA1 (GenBank accession no. JK693835), CsHA2 (GenBank accession no. EU735752), CsHA3 (GenBank accession no. EF375892), CsHA4 (GenBank accession no. HO054960), CsHA8 (GenBank accession no. HO054964), CsHA9 (GenBank accession no. HO054965), and CsHA10 (GenBank accession no. HO054966), a real-time polymerase chain reaction (PCR) was performed using the LightCycler (2.0) system from Roche Diagnostics. For the standardization of expression of each CsHA gene, a gene encoding TIP41-like protein (GenBank accession no. GW881871) was used as the control. Total RNA was isolated from the roots of cucumber seedlings. A total of 50 mg of tissue was ground in a porcelain mortar under liquid nitrogen with Tri Reagent, following the manufacturer’s instructions (Sigma, St. Louis, MO, USA) and then reverse-transcribed into first-strand cDNA with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Waltham, MA, USA). The cDNA was then used as the template for PCR amplification with the Real-Time 2 × PCR Master Mix SYBR (A&A Biotechnology, Gdańsk, Poland) kit. The following amplification conditions were applied: 30 s at 95 °C, 45 cycles of 10 s at 95 °C, 10 s at 58 °C, and 12 s at 72 °C, with a final melting for 15 s at 65 °C.
3.8. Statistical Analysis
The quantitative PCR data was analyzed by the ΔΔCT method using Light Cycler 4.1 software (Roche). At least three independent experiments concerning protein and RNA extractions, the measurements of enzyme activity and gene expression were made, run in triplicate, and the means ± standard deviation (SD) of these results are presented in the figures. Results were statistically analyzed by the Student’s t-test. Asterisks in the figures indicate significant differences, (p < 0.05 one asterisk, p < 0.01 two asterisks, p < 0.001 three asterisks), and a statistical tool in R package (R Core Team 2021) was used.
The jamovi project (2021). jamovi. (Version 2.0) [Computer Software]. Retrieved from https://www.jamovi.org.
R Core Team (2021). R: A Language and environment for statistical computing (Version 4.0) [Computer software]. Retrieved from https://cran.r-project.org (R packages retrieved from MRAN snapshot 1 April 2021).
4. Discussion
In the presented research, we decided to analyze the responses of plants to stress factors, in which PM H+-ATPase plays a key role, using cucumber seedlings as research material. Cucumber (Cucumis sativus L.) is a crop plant and an economically important vegetable. According to Statista, in 2020, global production of cucumber increased to over 91 million metric tons, making cucumber the third most-produced vegetable. The nutritional value of the cucumber is low, but its delicate flavor makes it popular for salads and relishes. Because of the small number of genes, rich diversity of sex expression, suitability for vascular biology studies, and short lifecycle cucumber is being developed as a new model plant [39].
Polyamines are molecules, which not only participate in plant growth and development, but are also involved in the adaptation of plants to environmental stresses. Polyamine catabolism seems to be particularly important in the process of plant adaptation to unfavorable environmental conditions. Two kinds of enzymes are involved in PA catabolism, copper-dependent diamine oxidase, and flavin adenine dinucleotide (FAD)-dependent polyamine oxidase. Both DAO and PAO are responsible for the oxidation of PAs, which occurs together with the production of H2O2 [23]. Analyses of the activity of both enzymes in cucumber seedlings indicated that PAO activity was very low whereas DAO activity was almost sixteen times greater (Figure 1). Our results are in line with the data provided by Moschou et al. [25]. In plants, DAO occurs at high level in dicots, particularly pea, chickpea, lentil, and soybean seedlings, loosely associated with cell wall. In contrast, PAO is highly expressed in monocots. Diamine oxidase is also often found in rapidly growing tissues [24]. We have also shown that the treatment of cucumber plants with cadmium changed the activity of the DAO but had no effect on the activity of PAO in roots. Under all of the tested conditions, PAO activity maintained at a very low level, regardless of the treatment (Personal communication, M. Janicka, available in repository). Considering the available data [25] and the results obtained as well as working with dicot and very young plants (Cucumis sativus seedlings), showing the very low level of PAO activity, in further experiments, only the activity of DAO was analyzed. Treatment of plants with cadmium stimulated DAO activity twice (Figure 2). AG (a DAO inhibitor) significantly reduced enzyme activity in both the control and cadmium-treated plants (Table 1). The involvement of amine oxidases in polyamine catabolism is related to their role in plant defense responses. Under aluminum stress, an increase of diamine oxidase activity in pea root nodules was observed. This increase in DAO activity was responsible for the enhanced accumulation of hydrogen peroxide in nodules [28].
In our experiments, treatment of the cucumber seedlings with cadmium increased the H+-ATPase activity in the plasma membrane fraction (Figure 3A,B). This enzyme functions as a proton pump, which couples ATP hydrolysis to proton transport and creates an electrochemical H+ gradient across plasma membrane. This gradient is used by secondary transporters [40]. Aside from the regulation of physiological processes, the plasma membrane proton pump participates in the adaptation of plants to stress factors [13]. Heavy metals often lead to disturbances in plants including membrane damage and ion imbalance. In such conditions, maintaining ionic homeostasis and replacing the loss of substances in reparative processes is a significant matter. Because the active transport of ions across the plasma membrane requires increased generation of a proton gradient by PM H+-ATPase, regulation of its activity is an important cellular mechanism for stress tolerance. To investigate whether the increased enzyme activity observed in plants treated with Cd could be attributed to DAO action, the effect of AG, the inhibitor of DAO, on the membrane proton pump was studied in cucumber seedlings (Figure 3A,B). The stimulating effect of Cd on enzyme activity was totally abolished by AG, suggesting that DAO may be involved in the modification of PM proton pump functioning under cadmium stress. Since the AG does not change the activity of the plasma membrane proton pump under control conditions (Figure 3), it seems that the participation of DAO in the activation of the enzyme (PM H+-ATPase) is important only when the plants are exposed to cadmium stress.
Polyamine catabolism may be involved in the regulation of gene expression of proteins associated with other processes. In the Arabidopsis mutant defective in AtPAO4 (polyamine oxidase gene), altered expression of genes related to abiotic stress responses and the metabolism of flavonoids and lignins was observed [24]. Hydrogen peroxide formed in the apoplast during the catabolism of PAs triggers a cascade of reactions, leading to increased expression of specific genes encoding superoxide dismutase, ascorbate peroxidase, pathogenesis-related proteins, kinases, transcription factors, and other stress response proteins. The application of spermine to tobacco leaves, mimicking apoplastic accumulation of polyamines as a result of incompatible plant–pathogen interactions, increased the expression of marker genes for hypersensitivity reactions. On the other hand, the observed stimulation of gene expression was decreased by inhibitors of diamine and polyamine oxidase. This indicates the participation of H2O2, resulting from the polyamine decomposition, in the regulation of the expression of these genes [24]. In our experiments, cadmium-induced changes in PM H+-ATPase activity were observed and these changes were suggested to be due to the action of DAO (Figure 3A,B). The modification of the activity of plasma membrane proton pump can take place at the level of gene transcription. For this reason, the expression level of CsHA genes (encoding isoforms of plasma membrane H+-ATPase in cucumber) was analyzed in seedling roots treated with cadmium and/or AG. We noticed that relative expression of PM H+-ATPase genes in cucumber roots was affected by Cd. An increase in the CsHA2, CsHA4, and CsHA8 transcript levels was observed (Figure 4). Moreover, this increase was completely diminished by the presence of AG. It can therefore be suggested that the hydrogen peroxide generated during the breakdown of polyamines may be responsible for both changes in the expression of genes encoding PM H+-ATPase and an increase in its activity at the protein level. Earlier, we observed that treatment of cucumber seedlings with H2O2 contributes to increased expression of PM H+-ATPase genes in roots [41].
We have shown that DAO activity generates H2O2 in cucumber roots under the cadmium stress condition (Figure 6). DAO can oxidize Put to induce H2O2 accumulation [23]. Our previous studies have found that in cucumber plants treated with cadmium, accumulation of hydrogen peroxide in roots was observed [42]. To examine the role of DAO in the Cd-induced increasing of H2O2 content, aminoguanidine was used. It was confirmed that Cd enhances H2O2 accumulation via the activity of DAO (Figure 6). Previous studies have shown that the plasma membrane proton pump plays an essential role in the adaptation of cucumber seedlings to cadmium stress [17,18,42]. New data suggest that diamine oxidase, through the production of hydrogen peroxide, may be an important element of the signal transduction pathway, leading to the modification of PM H+-ATPase activity under cadmium stress conditions.
Nitric oxide appears to be another player in plant reactions to cadmium. We have observed that short time (2 h) treatment of plants with H2O2 promotes the formation of NO in the roots of cucumber seedlings (Figure 7). Both nitric oxide and hydrogen peroxide are involved in plant responses to biotic as well as abiotic stresses [15,43,44]. The review of Gill et al. [31] clearly indicated the importance of nitric oxide in cadmium stress tolerance in crop plants. We have demonstrated earlier that pretreatment of cucumber plants with the NO donor (sodium nitroprusside) or with H2O2 increased the hydrolytic and transporting activities of PM H+-ATPase [15]. In this study, it was observed that hydrogen peroxide could contribute to an increase in endogenous NO levels, suggesting that the stimulation of H+-ATPase in the plasma membrane under Cd stress could be caused by the action of DAO, which, through the production of H2O2, could lead to an increase in NO level. In plants, the signal transduction pathways are still elucidated. In a number of abiotic and biotic responses, H2O2 generation occurs in parallel with NO, and these molecules can act both synergistically and independently [45,46]. Tun et al. [29] reported that PAs induced NO biosynthesis in Arabidopsis thaliana seedlings. Additionally, in Arabidopsis, copper amine oxidase 1 (CuAO1) contributes to ABA and PA-induced NO biosynthesis [47]. Moreover, copper amine oxidase 8 regulates arginine-dependent NO production in Arabidopsis [30]. NO biosynthesis as a result of PA catabolism, catalyzed by DAO, may explain many functions of PA mediated stress responses. In our study, the endogenous levels of NO increased in plants treated with Cd (Figure 5). However, when the cadmium-stressed plants were additionally treated with the DAO inhibitor, the NO level decreased. This result suggests that the increase in NO content in the tissues treated with Cd is dependent on DAO activity.
5. Conclusions
In summary, this work characterizes for the first time the role of DAO in the adaptation of plants to cadmium stress via regulation of PM H+-ATPase activity. Treatment of cucumber plants with Cd stimulated both DAO and PM H+-ATPase activities in cucumber seedling roots. The stimulating effect of Cd on the PM H+-ATPase was reduced by AG. Moreover, it was observed that H2O2 produced by DAO promotes the formation of NO in the roots of plants. It seems that DAO may be an element of the signal transduction pathway, leading to enhanced PM H+-ATPase activity under cadmium stress. Our results provide new insights into the PA mediated signaling, in which proton pump may function as a potential target, and both H2O2 and NO act as mediators.
Acknowledgments
We thank Beata Kuligowska for her excellent technical assistance.
Author Contributions
Conceptualization, M.J.; Formal analysis, M.J., M.R. and K.K.; Investigation, M.J., N.N., A.M., D.J. and K.K.; Methodology, M.J., M.R., N.N., A.M. and K.K.; Writing—original draft, M.J.; Writing—review & editing, M.J., M.R. and K.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented are available in this manuscript and in the repository at: https://www.repozytorium.uni.wroc.pl/dlibra/publication/139358/edition/128721#, https://www.repozytorium.uni.wroc.pl/dlibra/publication/139336/edition/128680 (accessed on 10 September 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Fancy N.N., Bahlmann A.K., Gary J., Loake G.J. Nitric oxide function in plant abiotic stress. Plant Cell Environ. 2017;40:462–472. doi: 10.1111/pce.12707. [DOI] [PubMed] [Google Scholar]
- 2.Genchi G., Sinicropi M.S., Lauria G., Carocci A., Catalano A. The effects of cadmium. Int. J. Environ. Res. Public Health. 2020;17:3782. doi: 10.3390/ijerph17113782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen H., Li Y., Ma X., Guo L.H.Y., Ren Z., Kuang Z., Zhang X., Zhang Z. Analysis of potential strategies for cadmium stress tolerance revealed by transcriptome analysis of upland cotton. Sci. Rep. 2019;9:86. doi: 10.1038/s41598-018-36228-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rasafi T., Oukarroum A., Haddioui A., Song H., Kwon E.E., Bolan N., Tack F.M.G., Sebastian A., Prasad M.N.V., Rinklebe J. Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2022;52:675–726. doi: 10.1080/10643389.2020.1835435. [DOI] [Google Scholar]
- 5.Sebastian A., Prasad M.N.V. Iron-and manganese-assisted cadmium tolerance in Oryza sativa L.: Lowering of rhizotoxicity next to functional photosynthesis. Planta. 2015;241:1519–1528. doi: 10.1007/s00425-015-2276-6. [DOI] [PubMed] [Google Scholar]
- 6.Singh I., Shah K. Evidences for suppression of cadmium induced oxidative stress in presence of sulphosalicylic acid in rice seedlings. Plant Growth Regul. 2015;76:99–110. doi: 10.1007/s10725-015-0023-4. [DOI] [Google Scholar]
- 7.van Belleghem F., Cuypers A., Semane B., Smeets K., Vangronsveld J., d’Haen J., Valcke R. Subcellular localization of cadmium in roots and leaves of Arabidopsis thaliana. New Phytol. 2007;173:495–508. doi: 10.1111/j.1469-8137.2006.01940.x. [DOI] [PubMed] [Google Scholar]
- 8.Ram H., Kaur A., Gandass N., Singh S., Desmukh R., Sonah H., Sharma T.R. Molecular characterization and expression dynamics of MTP genes under various spatiotemporal stages and metal stress conditions in rice. PLoS ONE. 2019;14:e0217360. doi: 10.1371/journal.pone.0217360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burzyński M., Kolano E. In vivo and in vitro effects of copper and cadmium on the plasma membrane H+-ATPase from cucumber (Cucumis sativus L.) and maize (Zea mays L.) roots. Acta Physiol. Plant. 2003;25:39–45. doi: 10.1007/s11738-003-0034-z. [DOI] [Google Scholar]
- 10.Kanczewska J., Marco S., Vandermeeren C., Maudoux O., Rigaud J.L., Boutry M. Activation of the plant plasma membrane H+-ATPase by phosphorylation and binding of 14-3-3 proteins converts a dimmer into a hexamer. Proc. Natl. Acad. Sci. USA. 2005;102:11675–11680. doi: 10.1073/pnas.0504498102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duby G., Boutry M. The plant plasma membrane proton pump ATPase: A highly regulated P-type ATPase with multiple physiological roles. Pflug. Arch.—Eur. J. Physiol. 2009;457:645–655. doi: 10.1007/s00424-008-0457-x. [DOI] [PubMed] [Google Scholar]
- 12.Ding M., Zhang M., Zheng H., Hayashi Y., Zhu Y., Kinoshita T. Molecular basis of plasma membrane H+-ATPase function and potential application in the agricultural production. Plant Physiol. Biochem. 2021;168:10–16. doi: 10.1016/j.plaphy.2021.09.036. [DOI] [PubMed] [Google Scholar]
- 13.Janicka-Russak M. Plant plasma membrane H+-ATPase in adaptation of plants to abiotic stresses. In: Shanker A., Venkateswarlu B., editors. Abiotic Stress Response in Plants—Physiological, Biochemical and Genetic Perspectives. InTech; Rijeka, Croatia: 2011. pp. 197–218. [Google Scholar]
- 14.Wdowikowska A., Klobus G. The plasma membrane proton pump gene family in cucumber. Acta Physiol. Plant. 2016;38:135. doi: 10.1007/s11738-016-2152-4. [DOI] [Google Scholar]
- 15.Janicka M., Reda M., Czyżewska K., Kabała K. Involvement of signalling molecules NO, H2O2 and H2S in modification of plasma membrane proton pump in cucumber roots subjected to salt or low temperature stress. Funct. Plant Biol. 2018;45:428–439. doi: 10.1071/FP17095. [DOI] [PubMed] [Google Scholar]
- 16.Ahn S., Im Y., Chung G., Seong K., Cho B. Sensitivity of plasma membrane H+-ATPase of cucumber root system in response to low root temperature. Plant Cell Rep. 2000;19:831–835. doi: 10.1007/s002999900190. [DOI] [PubMed] [Google Scholar]
- 17.Janicka-Russak M., Kabała K., Burzyński M., Kłobus G. Response of plasma membrane H+-ATPase to heavy metal stress in Cucumis sativus roots. J. Exp. Bot. 2008;59:3721–3728. doi: 10.1093/jxb/ern219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janicka-Russak M., Kabała K., Burzyński M. Different effect of cadmium and copper on H+-ATPase activity in plasma membrane vesicles from Cucumis sativus roots. J. Exp. Bot. 2012;63:4133–4142. doi: 10.1093/jxb/ers097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Surowy T., Boyer J. Low water potentials affect expression of genes encoding vegetative storage proteins and plasma membrane proton ATPase in soybean. Plant Mol. Biol. 1991;16:252–262. doi: 10.1007/BF00020556. [DOI] [PubMed] [Google Scholar]
- 20.Ouffatole M., Arango M., Boutry M. Identification and expression of tree new Nicotianaplumbaginifolia genes which encode isoforms of a plasma-membrane H+-ATPase, and one of which is induced by mechanical stress. Planta. 2000;210:715–722. doi: 10.1007/s004250050672. [DOI] [PubMed] [Google Scholar]
- 21.Fodor E., Szabo-Nagy A., Erdei L. The effects of cadmium on the fluidity and H+-ATPase activity of plasma membrane from sunflower and wheat roots. J. Plant Physiol. 1995;147:87–92. doi: 10.1016/S0176-1617(11)81418-5. [DOI] [Google Scholar]
- 22.Liu J., Hasanuzzaman M., Wen H., Zhang J., Peng T., Sun H., Zhao Q. High temperature and drought stress cause abscisic acid and reactive oxygen species accumulation and suppress seed germination growth in rice. Protoplasma. 2019;256:1217–1227. doi: 10.1007/s00709-019-01354-6. [DOI] [PubMed] [Google Scholar]
- 23.Cona A., Rea G., Angelini R., Federico R., Tavladoraki P. Functions of amine oxidases in plant development and defense. Trends Plant Sci. 2006;11:80–88. doi: 10.1016/j.tplants.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 24.Tavladoraki P., Cona A., Federico R., Tempera G., Viceconte N., Saccoccio S., Battaglia V., Toninello A., Agostinelli E. Polyamine catabolism: Target for antiproliferative therapies in animals and stress tolerance strategies in plants. Amino Acids. 2012;42:411–426. doi: 10.1007/s00726-011-1012-1. [DOI] [PubMed] [Google Scholar]
- 25.Moschou P., Paschalidis K., Roubelakis-Angelakis K. Plant polyamine catabolism. Plant Signals Behav. 2008;3:1061–1066. doi: 10.4161/psb.3.12.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alcázar R., Altabell T., Marco F., Bortolotti C., Reymond M., Koncz C., Carrasco P., Tiburcio A. Polyamines: Molecules with regulatory functions in plant abiotic stress tolerance. Planta. 2010;231:1237–1249. doi: 10.1007/s00425-010-1130-0. [DOI] [PubMed] [Google Scholar]
- 27.Gill S.S., Tuteja N. Polyamines and abiotic stress tolerance in plants. Plant Signal. Behav. 2010;5:26–33. doi: 10.4161/psb.5.1.10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sujkowska-Rybkowska M., Borucki W. Localization of hydrogen peroxide accumulation and diamine oxidase activity in pea root nodules under aluminum stress. Micron. 2014;57:13–22. doi: 10.1016/j.micron.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 29.Tun N.N., Santa-Catarina C., Begum T., Silveria V., Handro W., Floh E.I.S., Schere G.F.E. Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 2006;47:346–354. doi: 10.1093/pcp/pci252. [DOI] [PubMed] [Google Scholar]
- 30.Groβ F., Rudolf E.E., Thiele B., Durner J., Astier J. Copper amine oxidase 8 regulates arginine-dependent nitric oxide production in Arab. Thaliana. J. Exp. Bot. 2017;68:2149–2162. doi: 10.1093/jxb/erx105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gill S.S., Hasanuzzaman M., Nahar K., Macovei A., Tuteja N. Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol. Biochem. 2013;63:254–261. doi: 10.1016/j.plaphy.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Holmsted B., Larsson L., Tham R. Further studies on spectrometric method for the determination of amine oxidase activity. Biochim. Biophys. Acta. 1961;48:182–186. doi: 10.1016/0006-3002(61)90530-3. [DOI] [PubMed] [Google Scholar]
- 33.Federico R., Angelini R. Distribution of polyamines and their related catabolic enzymes in etiolated and light-grown Leguminosae seedlings. Planta. 1988;173:317–321. doi: 10.1007/BF00401018. [DOI] [PubMed] [Google Scholar]
- 34.Kłobus G. The role of plasma membrane-bound activities in nitrate transport into sealed plasma membrane vesicles from Cucumis sativus L. roots. In: Baluška F., Čiamporová M., Gašparíková O., Barlow P.W., editors. Structure and Function of Roots. Kulwer Academic Publisher; Dordrecht, The Netherlands: 1995. pp. 133–140. Developments in Plant and Soil Science. [Google Scholar]
- 35.Gallahger S.R., Leonard R.T. Effect of vanadate, molybdate and azide on membrane associated ATPase and soluble phosphatase activities of corn roots. Plant Physiol. 1982;70:1335–1340. doi: 10.1104/pp.70.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kłobus G., Buczek J. The role of plasma membrane oxidoreductase activity in proton transport. J. Plant Physiol. 1995;146:103–107. doi: 10.1016/S0176-1617(11)81974-7. [DOI] [Google Scholar]
- 37.Thordal-Christensen H.Z., Wei Z., Collinge Y. Subcellular localization of H2O2 in plants, H2O2 accumulation in papillae and hypersensitive response during barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
- 38.Bradford M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principles of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 39.Lv J., Qi J., Shi Q., Shen D., Zhang S., Shao G., Li H., Sun Z., Weng Y., Shang Y., et al. Genetic diversity and population structure of cucumber (Cucumis sativus L.) PLoS ONE. 2012;7:e46919. doi: 10.1371/journal.pone.0046919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Serrano R. Structure and function of plasma membrane ATPase. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989;40:61–94. doi: 10.1146/annurev.pp.40.060189.000425. [DOI] [Google Scholar]
- 41.Janicka-Russak M., Kabała K., Wdowikowska A., Kłobus G. Response of plasma membrane H+-ATPase to low temperature in cucumber roots. J. Plant Res. 2012;125:291–300. doi: 10.1007/s10265-011-0438-6. [DOI] [PubMed] [Google Scholar]
- 42.Jakubowska D., Janicka M. The role of brassinosteroids in the regulation of the plasma membrane H+-ATPase and NADPH oxidase under cadmium stress. Plant Sci. 2017;264:37–47. doi: 10.1016/j.plantsci.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Neill S.J., Desikan D., Clarke A., Hancock J.T. Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol. 2002;128:13–16. doi: 10.1104/pp.010707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yan J., Tsuichihara N., Etoh T., Iwai S. Reactive oxygen species and nitric oxide are involved in ABA inhibition of stomatal opening. Plant Cell Environ. 2007;30:1320–1325. doi: 10.1111/j.1365-3040.2007.01711.x. [DOI] [PubMed] [Google Scholar]
- 45.Qiao W., Li C., Fan L.M. Cross-talk between nitric oxide and hydrogen peroxide in plant responses to abiotic stresses. Environ. Exp. Bot. 2014;100:84–93. doi: 10.1016/j.envexpbot.2013.12.014. [DOI] [Google Scholar]
- 46.Zhang F., Wang Y., Yang Y., Wu H., Wang D., Liu J. Involvement of hydrogen peroxide and nitric oxide in salt resistance in the calluses from Populus euphratica. Plant Cell Environ. 2007;30:775–785. doi: 10.1111/j.1365-3040.2007.01667.x. [DOI] [PubMed] [Google Scholar]
- 47.Wimalasekera R., Villa C., Begum T., Schere G.F.E. Copper Amine Oxidase1 (CuAO1) of Arabidopsis thaliana contributes to abscisic acid- and polyamine-induced nitric oxide biosynthesis and abscisic acid signal transduction. Mol. Plant. 2011;4:663–678. doi: 10.1093/mp/ssr023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented are available in this manuscript and in the repository at: https://www.repozytorium.uni.wroc.pl/dlibra/publication/139358/edition/128721#, https://www.repozytorium.uni.wroc.pl/dlibra/publication/139336/edition/128680 (accessed on 10 September 2021).