Abstract
Tea (Camellia sinensis [L.] O. Kuntze) is an important global economic crop and is considered to enhance health. However, the functions of many genes in tea plants are unknown. Virus-induced gene silencing (VIGS) mediated by tobacco rattle virus (TRV) is an effective tool for the analysis of gene functions, although this method has rarely been reported in tea plants. In this study, we established an effective VIGS-mediated gene knockout technology to understand the functional identification of large-scale genomic sequences in tea plants. The results showed that the VIGS system was verified by detecting the virus and using a real-time quantitative reverse transcription PCR (qRT-PCR) analysis. The reporter gene CsPOR1 (protochlorophyllide oxidoreductase) was silenced using the vacuum infiltration method, and typical photobleaching and albino symptoms were observed in newly sprouted leaves at the whole plant level of tea after infection for 12 d and 25 d. After optimization, the VIGS system was successfully used to silence the tea plant CsTCS1 (caffeine synthase) gene. The results showed that the relative caffeine content was reduced 6.26-fold compared with the control, and the level of expression of CsPOR1 decreased by approximately 3.12-fold in plants in which CsPOR1 was silenced. These results demonstrate that VIGS can be quickly and efficiently used to analyze the function of genes in tea plants. The successful establishment of VIGS could eliminate the need for tissue culture by providing an effective method to study gene function in tea plants and accelerate the process of functional genome research in tea.
Keywords: tea plant, virus-induced gene silencing (VIGS), tobacco rattle virus (TRV), CsPOR1, CsTCS1
1. Introduction
VIGS is an RNA-silencing technology that is based on the mechanism of plant resistance to viral infections [1,2] and is specifically induced by viral replication and transcription. Viral vectors with specific exogenous genes that contain double-stranded RNA (dsRNA) play an important role in the process of VIGS. The dsRNAs are degraded into small interfering RNA (siRNAs) that vary in length from 21 to 23 nt and are processed by a Dicer-like nuclease. These siRNAs target the corresponding plant gene mRNAs, causing their degradation [3]. The mRNA of homologous gene sequences is degraded or modified by methylation, which causes changes in the plant phenotype or physiological indicators, thus, enabling the identification of functional genes [4,5]. VIGS technology does not require genetic transformation, and it can be easily used to acquire mutants. With its advantages of high speed, low cost, and high-throughput, VIGS technology currently serves as one of the most attractive technological tools in the field of functional genomics research.
Kumaga et al. [1] first applied VIGS technology to plants and inserted the tobacco PDS (phytoene desaturase) gene into tobacco mosaic virus (TMV). Inoculation with the altered TMV resulted in epistatic leaves that were chlorotic and whitened, which was accompanied by a significant decrease in the level of PDS mRNA. However, the TMV-induced virus had a short duration of silent phenotype, which limited its application. Tobacco rattle virus (TRV) is advantageous for its wide host range, high silencing efficiency, and long duration; thus, it has been widely used in a variety of plants [6,7], including strawberry (Fragaria × ananassa Duch.) [8], cotton (Gossypium spp.) [9], and potato (Solanum tuberosum L.) [10]. In addition, some economic trees have also been successfully established based on the system, including lychee (Litchi chinensis Sonn.) [11]. TRV has become the most widely used virus in the construction of VIGS systems.
The enzyme protochlorophyllide oxidoreductase (POR) catalyzes a light-dependent step in chlorophyll biosynthesis that is essential to photosynthesis, which catalyzes the reduction in the photosensitizer and substrate protochlorophyllide to form the pigment chlorophyllide [12,13,14,15]. Based on the function of the POR gene, it was used as a visual gene in the VIGS systems of Ananas bracteatus var. pineapple, Nicotiana benthamiana, and Arabidopsis thaliana, and a photo-bleaching phenomenon occurred after POR1 silencing [16,17].
Tea plants (Camellia sinensis [L.] O. Kuntze) are one of the most important economic trees in China and have been cultivated for thousands of years. Currently, with the development of tea genomics [18], an increasing number of perfect genomic libraries have been established (http://bigd.big.ac.cn/gsa/s/21CxCeKK, http://tpia.teaplant.org, accessed on 8 June 2022). However, there are relatively few methods to analyze the function of genes in tea plants, and the lack of studies on the stable genetic transformation of tea plants has caused a bottleneck in the research on tea biology. However, a TRV-VIGS system is highly efficient, takes less time, and is relatively simple to perform; therefore, it can compensate for the problems described above and enable the quick and accurate study of gene functions [19,20]. Caffeine is one of the most important components of tea plants, and it has a crucial impact on the quality of tea. CsTCS1 (caffeine synthase) catalyzes the last two steps of caffeine synthesis; the level of its expression affects the content of caffeine in the tea plants [21]. Yu et al. [22] transformed CsTCS1 into tobacco by heterologous transformation. The researchers detected the presence of caffeine synthase in tobacco, but the transformants did not produce caffeine. Mohanpuria et al. [23] used RNA interference (RNAi) technology to knock out CsTCS1 in tea plants, which reduced the contents of caffeine and theobromine in the gene knockdown tea plant. Deng et al. introduced fragments of CsTCS1 into E. coli to establish a prokaryotic expression system. The results showed that caffeine was present in its extracellular secretions. To our knowledge, there are currently no reports of the use of VIGS in tea plants. In this study, the TRV-mediated VIGS system of tea plants was established using the reporter gene CsPOR1, which encodes protochlorophyllide oxidoreductase. This system was used to silence CsTCS1. The results accurately determined the function of related endogenous genes in tea plants, which should improve the breeding efficiency of tea plants and create new varieties. Therefore, the establishment of the tea plant VIGS system can improve the efficiency of tea gene function research, avoid the problem of the incomplete or non-expression of tea plant endogenous genes in heterologous model organisms, introduce a new way to study the gene function of tea, and provide innovative tea plant germplasm. This study aimed to establish a TRV-mediated VIGS system for tea plants.
2. Results
2.1. TRV Can Infect Fuding Dabaicha
Three groups of plants were used to demonstrate that Fuding Dabaicha can be infected with tobacco rattle virus (TRV). The first group consisted of the newly sprouted leaves, including photobleached leaves, albino leaves, reddish leaves, and pale pink leaves of plants that were infected by Agrobacterium that harbored pTRV1 + pTRV2-CsPOR1, and Agrobacterium that harbored pTRV1 + pTRV2-CsTCS1. The second group consisted of the newly sprouted leaves that were not silenced and were treated with Agrobacterium that harbored the empty vectors pTRV1 and pTRV2. The final group consisted of untreated newly sprouted leaves that had not been treated with Agrobacterium. The leaves were randomly collected for RT-PCR detection. The results clearly showed that the transcripts of viral pTRV1 and pTRV2 were successfully detected in newly germinated tea leaves from plants that were infected with the virus (Figure 1). Simultaneously, we observed varying degrees of black spots in the newly reddish and albino leaves of the CsPOR1-silenced plants (Figure S1), which were attributed to the background TRV viral effect, whereas the black spot symptoms were not observed in the control group pTRV2. This also indicated that TRV had successfully infected the tea plants, and these phenomena are consistent with those described by Di Stilio et al. [24]. This shows that recombinant TRV can effectively replicate and spread in the Fuding Dabaicha plants.
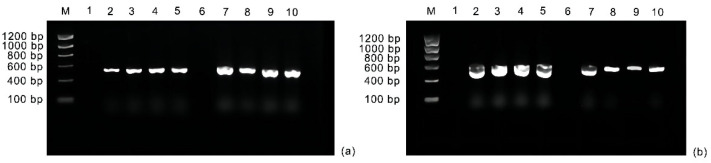
Figure 1.
Detection of TRV transcripts in VIGS leaves with phenotypic changes of tea plants: (a). 1–5 detected the strand of viral pTRV2, and 6–10 detected the strand of viral pTRV1 (1, uninfected seedlings; 6, uninfected cuttings; 2, seedlings infected with pTRV1 + pTRV2; 7, cuttings infected with pTRV1 + pTRV2; 3–5, seedlings infected with pTRV1 + pTRV2-CsPOR1; 8–10, cuttings infected with pTRV1 + pTRV2-CsPOR1); (b) 1–5 detected the strand of viral pTRV1, and 6–10 detected the strand of viral pTRV2 (1, uninfected seedlings; 6, uninfected cuttings; 2, seedlings infected with pTRV1 + pTRV2; 7, cuttings infected with pTRV1 + pTRV2; 3–5, seedlings infected with pTRV1 + pTRV2-CsTCS1; 8–10, cuttings infected with pTRV1 + pTRV2-CsTCS1). M: DL1200 marker.
As described by Kalbande and Patil [25], contamination with Agrobacterium was checked using the primers oriV-F-5′-CTACGGCCAGGCAATCTACC-3′ and oriV-R-5′-GAGCTGCATGTGTCAGAGGT-3′ (designed to amplify 130 bp of the oriV gene on the Ti plasmid). All eight putative transformants were tested using all the primers described above, which included the following: four-column-infected tea plants with pTRV1 + pTRV2, two tea plants infected with pTRV1 + pTRV2-CsPOR1, and two tea plants infected with pTRV1 + pTRV2-CsTCS1). The PCR with oriV primers confirmed the absence of Agrobacterium itself as a pathogen in eight putative transformants and wild-type plants (Figure S4).
2.2. TRV-Mediated VIGS System Was Successfully Used in the Tea plants
2.2.1. The TRV-Mediated VIGS System Was Successfully Used in the Tea Seedlings
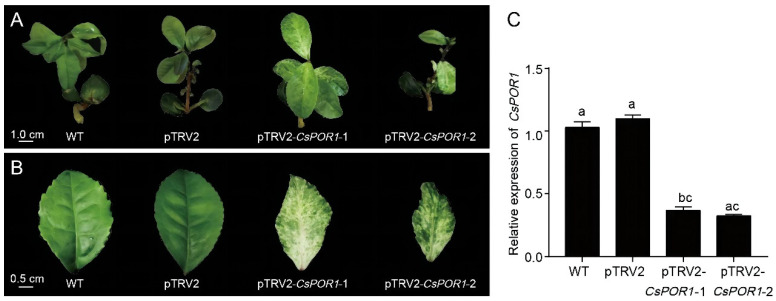
To test whether the TRV-based vector can effectively induce the silencing of endogenous genes in tea seedlings, CsPOR1 was selected as the visual marker. POR1 is one of the key genes in chlorophyll biosynthesis. Therefore, inhibition of this gene will lead to photodegradation of the chlorophyll or changes in the green color of plants. All the tea seedlings that were treated with the TRV virus survived, indicating that the vacuum-infiltration method can be used for VIGS experiments. All the newly developed leaves of the wild-type tea seedlings and those that were infected with pTRV1 + pTRV2 Agrobacterium were a normal shade of green after 25 d. Simultaneously, five of the tea seedlings infected with pTRV1 + pTRV2-CsPOR1 Agrobacterium exhibited a photo-bleached phenotype in the leaves that grew after 25 d (Figure 2A). Two of the tea seedlings with obvious photo-bleaching symptoms were selected as the materials for the qRT-PCR experiments, and they were then named pTRV2-CsPOR1-1 and pTRV2-CsPOR1-2. The symptoms of photo-bleaching in the leaves of the silenced plants became increasingly significant as the plants grew (Figure 2B). To further verify whether CsPOR1 was silent, the level of CsPOR1 mRNA in the leaves infected with pTRV2 + pTRV2-CsPOR1 was measured using a qRT-PCR. Compared with the wild-type tea seedlings and those infected with pTRV1 + pTRV2, the content of the CsPOR1 transcripts was significantly reduced in the leaves infected with pTRV1 + pTRV2-CsPOR1 (Figure 2C). These observations indicated that the TRV-mediated VIGS system could silence the visual marker gene (CsPOR1) in the tea seedling leaves. The results also showed that the TRV-VIGS system was successfully applied to tea seedlings and could significantly reduce the level of expression of endogenous target genes in the leaves, thereby achieving gene silencing.
Figure 2.
TRV-mediated VIGS of the CsPOR1 gene in tea seedling leaves. (A,B) WT, wild-type tea seedlings; pTRV2, infected seedlings with pTRV1 + pTRV2 Agrobacterium; pTRV2-CsPOR1-1 and pTRV2-CsPOR1-2, infected seedlings with pTRV1 + pTRV2-CsPOR1 Agrobacterium. (C) The expression of CsPOR1 was down-regulated in the photo-bleached leaves of tea seedlings. WT, wild-type tea seedlings; pTRV2, infected with pTRV1 + pTRV2 Agrobacterium; pTRV2-CsPOR1-1 and pTRV2-CsPOR1-2, infected with pTRV1 + pTRV2-CsPOR1 Agrobacterium. The error bars represent the mean ± SE of the three independent experiments. Different lowercase letters indicate a significant difference at p < 0.05 by Duncan’s multiple range test. SE, standard error; TRV, tobacco rattle virus; WT, wild type.
2.2.2. TRV-Mediated VIGS System Was Successfully Used in the Tea Cuttings
The vacuum infiltration method, which was developed by increasing the amount of sonication, was used to infect the tea cuttings. New sprouting leaves of uninfected tea cuttings and those infected with pTRV1 + pTRV2 Agrobacterium were still green after 20 d of infiltration (Figure 3B,C). However, the leaves of tea cuttings infected with pTRV1 + pTRV2-CsPOR1 Agrobacterium that had grown for 20 d were albino (Figure 3A). Among the 23 tea cuttings, the new leaves of eight cuttings were albino. The efficiency of silencing was 34.7%. One of the silenced plants was selected as experimental material for the qRT-PCR. The cuttings of the albino leaves were named pTRV2-CsPOR1-albino. The qRT-PCR results revealed that the expression of CsPOR1 in the leaves that were albino (Figure 3A) was successfully knocked down, as shown in Figure 3D. The results also indicated that the VIGS system can still be used successfully in tea cuttings.
Figure 3.
TRV-mediated VIGS of the CsPOR1 gene in tea-cutting leaves. (A) Untreated tea cuttings. (B) New leaves with normal symptoms 20 d after infection with pTRV1 + pTRV2. (C) New leaves with albino symptoms 20 d after infection with pTRV1 + pTRV2-CsPOR1. (D) Expression of CsPOR1 was downregulated in the leaves of tea cuttings that were albino or reddish. WT, uninfected cuttings; pTRV2, infection with pTRV1 + pTRV2 Agrobacterium; pTRV2-CsPOR1-albino and pTRV2-CsPOR1-reddish, infection with pTRV1 + pTRV2-CsPOR1 Agrobacterium. The error bars represent the mean ± SE of the three independent experiments. Different lowercase letters indicate significant differences at p < 0.05 by Duncan’s multiple range test. qRT-PCR, real-time quantitative reverse transcription PCR; SE, standard error; TRV, tobacco rattle virus; VIGS, virus-induced gene silencing.
2.3. Optimized VIGS System in Tea
Based on the TRV-VIGS system of the tea seedlings, a TRV-VIGS system suitable for the tea cuttings was explored using the osmotic buffer that that was used for it. The amount of MgCl2 in the buffer was increased to 16 g·L−1, and the duration of the contact between the plant and the mixed bacterial solution was extended from 1 h to 2 h. An ultrasonic treatment step was added before the vacuum infiltration. The OD600 and vacuum pressure values suitable for the TRV-VIGS of the tea seedling system were explored (Table 1 and Table 2). The results indicated that the most effective conditions to infiltrate the tea seedlings included the following: an OD600 of 1.2, vacuum pressure of 0.7 kPa, and osmosis buffer of 4.74 g·L−1 of MS, 2 mol·L−1 of 6-BA, 2 mol·L−1 of As, and 100 umol·L−1 of NAA. The silencing efficiency of this system was 12.5%. Most of the tea cuttings were successfully infiltrated at an OD600 of 1.2, vacuum pressure of 0.8 kPa, and osmosis buffer of 4.74 g·L−1 of MS, 2 mol·−1 of 6-BA, 2 mol·L−1 of As, 100 umol·L−1 of NAA, and 16 g·L−1 of MgCl2, and the silencing efficiency of this system was 34.7%.
Table 1.
The efficiency of infection in different vacuum pressures and Agrobacterium densities (Tea seedlings).
| Combinations of Infiltration Buffer | OD600 | Vacuum Pressure | Number of Infected Seedlings |
Number of Successful Infected Seedlings |
Infection Efficiency (%) |
|---|---|---|---|---|---|
| 4.74 g·L−1 MS + 2 mol·L−1 6-benzylaminopurine + 2 mol·L−1 acetosyringone + 100 umol·L−1 1-naphthylacetic acid | 1.0 | 0.6 | 40 | 0 | 0 |
| 1.2 | 0.6 | 40 | 0 | 0 | |
| 1.5 | 0.6 | 40 | 0 | 0 | |
| 1.0 | 0.7 | 40 | 0 | 0 | |
| 1.2 | 0.7 | 40 | 5 | 12.5 | |
| 1.5 | 0.7 | 40 | 0 | 0 | |
| 1.0 | 0.8 | 40 | 0 | 0 | |
| 1.2 | 0.8 | 40 | 1 | 2.5 | |
| 1.5 | 0.8 | 40 | 0 | 0 |
Table 2.
The efficiency of infection following different vacuum pressures and Agrobacterium densities (Tea cuttings).
| Combinations of Infiltration Buffer | OD600 | Vacuum Pressure | Number of Infected Cuttings |
Number of Successful Infected Cuttings |
Infection Efficiency (%) |
|---|---|---|---|---|---|
| 4.74 g·L−1 MS + 2 mol·L−1 6-benzylaminopurine + 2 mol·L−1 acetosyringone + 100 umol·L−1 1-naphthylacetic acid + 16 g·L−1 MgCl2 | 1.0 | 0.6 | 25 | 0 | 0 |
| 1.2 | 0.6 | 24 | 0 | 0 | |
| 1.5 | 0.6 | 20 | 0 | 0 | |
| 1.0 | 0.7 | 25 | 0 | 0 | |
| 1.2 | 0.7 | 25 | 0 | 0 | |
| 1.5 | 0.7 | 26 | 0 | 0 | |
| 1.0 | 0.8 | 24 | 1 | 4.16 | |
| 1.2 | 0.8 | 23 | 8 | 34.7 | |
| 1.5 | 0.8 | 26 | 2 | 7.69 |
2.4. Prediction of Gene Silencing Times by the Phenotypic Changes of CsPOR1-Silenced Plants
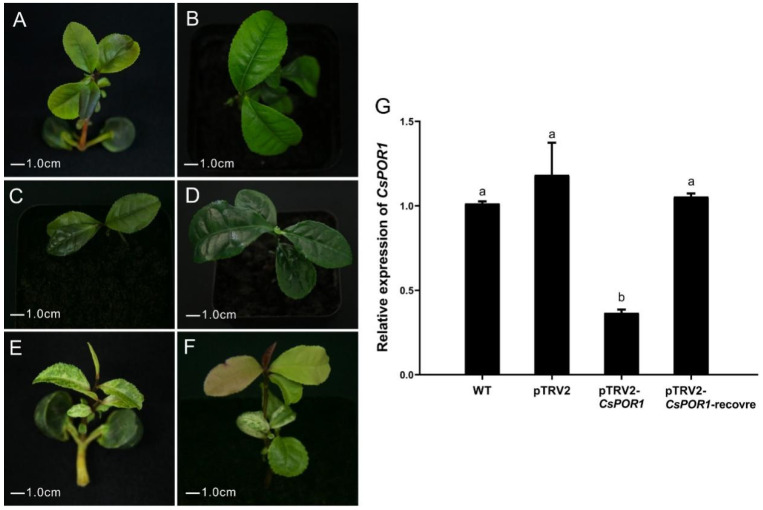
The tea seedlings were infected by pTRV1 + pTRV2-CsPOR1 Agrobacterium, and the nascent leaves that grew after 25 d in the infected part of the upper plant exhibited symptoms of photo bleaching (Figure 4E). Newly sprouted leaves of the wild-type tea seedlings and those infected with pTRV1 + pTRV2 Agrobacterium were still green at 25 d after infiltration (Figure 4A,C). After 65 d, the young leaves of those plants infected with pTRV1 + pTRV2-CsPOR1 Agrobacterium were restored to green (Figure 4F). However, after 65 d, the leaves on the wild-type plants and those infected with pTRV1 + pTRV2 Agrobacterium still remained green (Figure 4B,D). The photo-bleaching phenomenon was observed to last for more than 40 d.
Figure 4.
Leaf phenotypic and CsPOR1 expression changed after treatment with pTRV1 + pTRV2-CsPOR1. (A,B) Phenotype of tea seedling leaves at 25 d and 65 d after infection with Agrobacterium that harbored pTRV1 + pTRV2. (C,D) Phenotype of the leaves at 25 d after infection and 65 d of the control plants that had not been treated. (E,F) Phenotype of the leaves at 25 d and 65 d after infection with pTRV1 + pTRV2-CsPOR1 Agrobacterium. (G) The relative level of changes in the expression of CsPOR1 gene after the leaves had recovered phenotypically. WT, wild-type tea seedlings; pTRV2, tea seedlings infected with pTRV1 + pTRV2; pTRV2-CsPOR1 and pTRV2-CsPOR1-recovered, tea seedlings infected with pTRV1 + pTRV2-CsPOR1. The error bars represent the mean ± SE of three independent experiments. Different lowercase letters indicate significant differences at p < 0.05 by Duncan’s multiple range test. SE, standard error; TRV, tobacco rattle virus; WT, wild type.
Newly sprouted leaves after 65 d from the untreated plants (wild-type), non-silenced plants (vacuum-infiltrated with pTRV1 + pTRV2 Agrobacterium), those silenced for green leaves (vacuum-infiltrated with pTRV1 + pTRV2-CsPOR1 Agrobacterium), and the photo-bleached leaves after 25 d of silenced plants (vacuum-infiltrated with pTRV1 + pTRV2-CsPOR1 Agrobacterium) were collected for the qRT-PCR analysis. The silenced plants that exhibited photo-bleached leaves were named pTRV2-CsPOR1. The silenced plants with green leaves were named pTRV2-CsPOR1-recover. The qRT-PCR results confirmed that the expression of CsPOR1 was still knocked down in the photo-bleached leaves of the infected plants and recovered when the leaves turned green (Figure 4G).
2.5. CsTCS1 Silencing Inhibited the Formation of Caffeine in the Leaves of Fuding Dabaicha
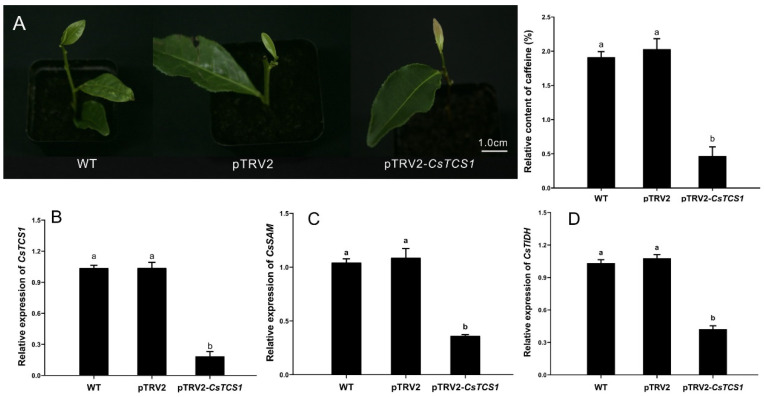
The level of expression of CsTCS1 in the tea plant leaves was downregulated to study the function of CsTCS1 using the TRV-VIGS system. In this study, tea cuttings were selected as experimental materials to measure the relative content of caffeine, in order to avoid interference in the experimental results caused by the individual differences of tea seedlings. The cuttings infected with pTRV1 + pTRV2-CsTCS1 grew new leaves that were pale pink after 12 d. Four of the 20 treated cuttings exhibited these symptoms, and the cutting with the most significant pink symptoms was selected for the experiment and named pTRV2-CsTCS1. The silencing efficiency of CsTCS1 was 20%. The newly developed leaves of the pTRV1 + pTRV2 cuttings treated with Agrobacterium and the wild-type cuttings were normal green. The content of caffeine in the new leaves of the cuttings was determined using HPLC (Figure S2). Compared with the green leaves of the wild-type cuttings and those infected with pTRV1 + pTRV2, the content of caffeine decreased by 6.26-fold in the pale pink leaves of cuttings infected with pTRV2-CsTCS1 (Figure 5A). This is consistent with the results described by Mohanpuria et al. [23]. To detect the relative level of expression of CsTCS1 and the changes in levels of expression of the key genes involved in caffeine synthesis after silencing, the levels of expression of CsTCS1, CsSAM (S-adenosylmethionine), and CsTIDH (hypoxanthine nucleotide dehydrogenase) were determined by a qRT-PCR. The results showed that the level of expression of CsTCS1 was significantly reduced (Figure 5B). Simultaneously, the levels of expression of CsSAM and CsTIDH were also significantly reduced (Figure 5C,D).
Figure 5.
Silencing of CsTCS1 in a leaf of tea plant using the VIGS system. (A) Relative content of caffeine. WT, untreated tea cuttings; pTRV2, tea cuttings infected by pTRV1 + pTRV2 Agrobacterium; pTRV2-CsTCS1, tea cuttings infected by pTRV1 + pTRV2-CsTCS1 Agrobacterium. (B–D) The relative level of expression of CsTCS1 (B), CsSAM (C), CsTIDH (D). WT, untreated tea cuttings; pTRV2, vacuum-infiltrated with pTRV1 + pTRV2; pTRV2-CsTCS1, vacuum-infiltrated with pTRV1 + pTRV2-CsTCS1. The error bars represent the mean ± SE of the three independent experiments. Different lowercase letters indicate significant differences at p < 0.05 by Duncan’s multiple range tests. SE, standard error; TRV, tobacco rattle virus; VIGS, virus-induced gene signaling.
3. Discussion
VIGS technology has been widely used in gene function analysis, particularly in plants that are difficult to transform genetically, such as PeHSF (Populus euphratica) [26], Chinese narcissus (Narcissus tarzetta L.) NtMYB3 [27], Jatropha curcas JcKASII [28], and Malus crabapple McMYB10 [29]. However, there have been no reports on the use of VIGS technology in tea plants. In this study, a VIGS transformation system for the tea plant was established, which lays the foundation for the analysis of gene functions in tea plants.
There are multiple reporter genes in plants, but they vary greatly given plant diversity. Whether the genes are anthocyanin-dominant or chlorophyll- or carotenoid-dominant is uncertain, and reporter genes should be selected based on the plant characteristics [29,30,31]. PDS, CHS (chalcone synthase), Ftsh (filamentation temperature sensitive H), ChlH (magnesium chelatase subunit H), and the POR1 genes are commonly used as reporter genes in VIGS systems. Kumagai et al. [1] used PDS as a marker gene in Nicotiana tabacum L.; Chen et al. [30] used CHS as a marker gene in Petunia hybrida; Saitoh and Terauchi [32] used Ftsh as a reporter gene in Nicotiana tabacum L.; Jia et al. [33] used ChlH as a reporter gene in Prunus persica; and Li and Ma [17] used POR1 as a marker gene in Ananas comosus var. bracteatus. This study used tea plant CsPOR1 as a marker gene. We chose the 308 bp coding sequence of CsPOR1 as a target gene fragment to insert into the TRV2 vector to construct pTRV2-CsPOR1, followed by successful silencing of the POR1 gene. This result is consistent with a previous report that fragments of 200–1300 bp can be used to induce gene silencing [34]. The silencing of CsPOR1 in the tea plant appeared to demonstrate a photo-bleaching phenomenon.
Previous studies have indicated that the vacuum infiltration methods are suitable for VIGS in younger seedlings and woody plants [35,36]; therefore, this study selected the vacuum infiltration technique as the best method of infection. The infiltration conditions, such as Agrobacterium solution and vacuum pressure, play important roles in the efficiency of VIGS [29]. In this study, we chose different combinations to explore the best OD600 and vacuum pressure. The results showed that an OD600 of 1.2 and an osmotic pressure of 0.7 kPa provided the best conditions for the infiltration of the tea plant sexual systems to utilize VIGS. The silencing efficiency was 12.5%. In addition, an OD600 of 1.2 and an osmotic pressure of 0.8 kPa provided the most effective infiltration conditions for the tea plant clones. In this case, the silencing efficiency was 34.7%. This system successfully transferred the TRV vector to tea plants. Some studies have suggested that cultivating plants at room temperature between 22 and 25 °C could help to increase the intensity of gene silencing by prolonging its duration [19,37]. Therefore, in this experiment, the temperature was maintained at 25 °C to obtain the best silencing efficiency, which is consistent with the previous data described above.
The plant material affects the efficiency of TRV-mediated VIGS [3]. VIGS experiments often utilize bulbs, leaves [33,38,39,40], seeds [31,41,42], stem segments [3], and cotyledon segments as plant materials. In this study, we chose cuttings from the current year’s growth and seedlings with cotyledons as plant materials. Among them, the advantage of cuttings compared with the plant materials described above is that it is convenient to utilize materials that will not require cultivation for a long time to perform the VIGS experiments. The advantage of seedlings is that they have large cotyledons, rapid growth, and produce a silent phenotype in a short period time.
It is difficult to determine if the gene is silenced in a TRV-VIGS system when the silenced target gene has no distinct phenotypic characteristics [3]. To solve this problem, this study analyzed CsPOR1 using a qRT-PCR analysis and observed silent plant leaf phenotypic changes to predict the timing of target gene silencing. The results showed that the gene silencing time is approximately 40 d. This study will greatly facilitate research on gene functions that have no phenotypic traits.
CsTCS1 (caffeine synthase) is the most critical enzyme in the synthesis of caffeine. This study used the TRV-mediated VIGS system to reduce the expression of CsTCS1 in tea leaves. The results of VIGS silencing showed that CsTCS1 controls the biosynthesis of caffeine. After CsTCS1 was inhibited, the levels of expression of the key genes CsSAM and CsTIDH involved in caffeine synthesis were also reduced. Based on the results, the new leaves of the cuttings grew pale pink when CsTCS1 was silenced, and we hypothesized that the silencing of CsTCS1 in the leaves could have inhibited the genes related to the control chlorophyll synthesis [43] and upregulated the expression of genes that control anthocyanin synthesis [44], resulting in the changes in leaf color in the CsTCS1-silenced plants. In addition, the accumulation of caffeine in tea leaves was reduced. These results indicate that the TRV-mediated VIGS system is suitable for the functional verification of tea tree genes and that this system is stable and reliable.
4. Materials and Methods
4.1. Plant Material and Growing Conditions
The first type of explant material was the F1 hybrid seeds of ‘Fuding Dabaicha’ (♀) × Longjing 43’ (♂) [45]. Seeds with the episperm removed were placed in a 4 cm tall tray that contained the nutrient solution for cultivation. The composition of the nutrients included 100 uL·L−1 of 6-benzylaminopurine (6-BA), 600 uL·L−1 of gibberellic acid (GA3), and 5 mL·L−1 of chloramphenicol. The seedlings were grown for 20 d at 25 °C in a greenhouse at Guizhou University (26°44′ N, 106°65′ E), Guiyang, China, with a relative humidity of 60–70% and 16 h/8 h light/dark. The seedlings were used for vacuum infiltration experiments when they had grown to 2–3 cm. The second type of explant material utilized was cuttings of Fuding Dabaicha grown in the current year [46], which were obtained from the Guizhou Academy of Agricultural Sciences (26°12′ N, 106°08′ E), Guiyang, China. Cuttings of the same growth status were selected for the vacuum infiltration experiments.
4.2. Total RNA Extraction
The total RNA was extracted using a TRIzol Up Plus RNA Kit (TianGen Biochemical Technology Co., Ltd., Beijing, China), following the manufacturer’s instructions. The RNA quality was checked by electrophoresis on a 1.0% (w/v) agarose gel. The cDNA was synthesized by reverse transcription using a Prime ScriptTM II 1st strand cDNA Synthesis Kit (Beijing Solarbio Technology Co., Ltd., Beijing, China).
4.3. Cloning of a Partial Sequence of CsPOR1
Parts of the sequences of CsPOR1 (GenBank MH925309.1) and CsTCS1 (GenBank AB031280.1) were amplified using PrimeSTAR® Max (Junotec Biotechnology Co., Ltd., Wuhan, China) high-fidelity DNA polymerase amplification of the target fragment. The PCR reaction system was 25 µL of PrimeSTAR Max Premix positive. A volume of 2.5 µL of the forward primer and reverse primer and 3.0 µL of cDNA were brought to a volume of 50 µL with sterile distilled water. The PCR reaction conditions were as follows: 95 °C for 5 min; 34 cycles of 95 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min; 72 °C for 10 min, and storage at 4 °C. The PCR product was ligated into the cloning vector pMD18-T (Junuod Biotechnology Co., Ltd., Wuhan, China) and transformed into E. coli DH5α competent cells (Kewen Biotechnology Co., Ltd., Changsha, China) by heat shock. The cells were then plated overnight at 37 °C and cultured. Resistance to 50 mg/L of kanamycin and the universal primer M13-47/48 were used to amplify the bacterial liquid by PCR, and the positive recombinants that were screened were sent to the company for sequencing (Figure S3). The sequences were compared with those obtained from GenBank for confirmation (Table 3).
Table 3.
List of primers used in this study.
| Usage | Primer Name | Primer Sequence |
|---|---|---|
| Partial gene cloning |
CsPOR1 | F: CCAAGGCTCTAGCTGAAACA R: GAAAGGAGGAAGTGTCCAAGAT |
| Partial gene cloning |
CsTCS1 | F: CTGAATTGGTTTCACAGGGATTG R: TGTGGTCTTCGGTAGCTTTG |
| TRV virus testing |
pTRV1 | F: AAAGTGACTGGTGTGCCTAAA R: CTTGCAGAGCAGGAACTCTATC |
| TRV virus testing |
pTRV2 | F: CTACTACTACCAAGGCGAACAC R: GTCGTCAAGCCACTTCCTAA |
| Vector construction |
pTRV2-CsPOR1 | F: GAGGGTGGGTGATACCATATTC R: GCTGCTGTTTGTGCTCTTATAG |
| Vector construction |
pTRV2-CsTCS1 | F: GACACCTTCAATATACCCAGCTA R: ACTAGGATGATACTTGTGGTCTTC |
| qRT -PCR | qRT -PCR CsPOR1 | F: CCAAGGCTCTAGCTGAAACA R: GTCAAGTGAGGCAAGGTCTAA |
| qRT -PCR | qRT -PCR CsTCS1 | F: ACAAGCCCTCCTGTTGTAAG R: CCTCTTGGGATCTAGCATTGAG |
| qRT-PCR | qRT -PCR CsTIDH | F: GCGCCATCCGACTATGATTT R: CCACTACTTGTCCTCCATCTTTC |
| qRT-PCR | qRT -PCR CsSAM | F: CGATGAGACCGTGACAAATGA R: GAAGGGTTGAGGTGGAAGATG |
| qRT-PCR | Actin | F: CAGACCGTATGAGCAAGGAAAT R: GTGCTTAGGGATGCAAGGATAG |
4.4. Vector Construction
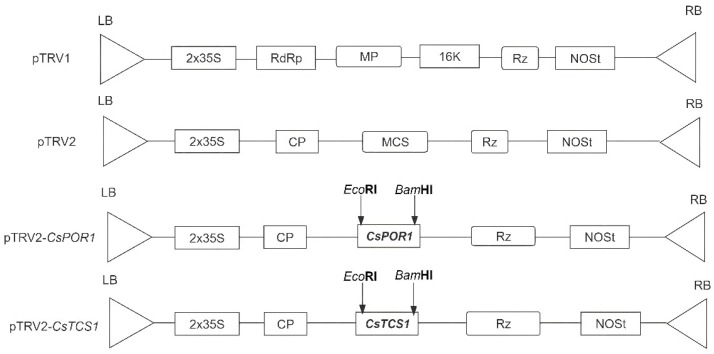
The fragments of CsPOR1 and CsTCS1 used for VIGS were assembled into the pTRV2 virus vector (Figure 6).
Figure 6.
Map of the pTRV1, pTRV2, pTRV2-CsPOR1, and pTRV2-CsTCS1 vectors used in this study. LB, left border; RB, right border; RdRp, RNA-dependent RNA polymerase; MP, movement protein; 16k, 16 kD protein; Rz, self-cleaving ribozyme; NOSt, NOS terminator; CP, coat protein; MCS, multiple cloning site.
Construction of the pTRV2-CsPOR1 and pTRV2-CsTCS1 vector. The pTRV2-CsPOR1 vector was constructed using a 308 bp fragment of CsPOR1 with EcoRI and BamHI restriction sites. The PCR product and pTRV2 vector, which was digested by EcoRI and BamHI restriction enzymes and recovered, were ligated using T4 DNA ligase (Covent Biotechnology Co., Ltd., Changsha, China) overnight at 16 °C, and then transformed into E. coli DH5α. The specific primers for pTRV2-CsPOR1-F and pTRV2-CsPOR1-R were used to verify the insertion of the 308 bp product by PCR with a bacterial solution (Table 3). Moreover, the 332 bp CsTCS1 fragment was selected and cloned into the pTRV2 vector with EcoRI and BamHI restriction sites using T4 DNA ligase (Covent Biotechnology Co., Ltd., Changsha, China).
4.5. Impact and Optimization of the Conversion Systems
4.5.1. Tea Plant Infection
pTRV1, pTRV2, pTRV2-CsPOR1, and pTRV2-CsTCS1 were introduced into the Agrobacterium GV3101 competent state using the freeze–thaw method. Each strain of Agrobacterium was inoculated into solid YEP media, which contained 100 mg of L−1 kanamycin and 50 mg·L−1 of rifampin, and the 28 °C activated culture was grown for 48 h. Single colonies were cultured in the corresponding liquid YEP media until the cells reached an OD600 of 1.0–1.5. The Agrobacterium cells were centrifuged at 6000 rpm for 6 min. The bacterial fluid was collected and suspended in 4.74 g·L−1 of MS, 2 mol·L−1 of 6-BA, 2 mol·L−1 of acetosyringone (AS), 100 umol·L−1 of 1-napthalene acetic acid (NAA), and 16 g·L−1 of MgCl2, pH 5.6. The OD600 was adjusted to 1.2. pTRV1 was mixed with pTRV2, pTRV2-CsPOR1, and pTRV2-CsTCS1 bacterial liquid 1:1, and the two bacterial liquids were fully mixed at room temperature [47,48]. The two types of tea plant materials were vacuum infiltrated. This process was conducted on the tea seedlings using a blade to introduce 3–4 wounds around the cotyledons. All the leaves were removed, and the tea seedlings were placed in a Buchner flask that contained mixed bacterial liquid and vacuum infiltrated. The inoculated seedlings were grown in a greenhouse at 25 °C at 16 h/8 h light/dark. Shears were used to cut the tea cuttings to 15–20 cm. A slice of a mature leaf was retained, and the tea cuttings were then placed in a Buchner flask that contained mixed bacterial liquid for vacuum infiltration. They were kept in the dark for three days and then grown at 25 °C in a greenhouse with a 16 h/8 h light/dark cycle.
4.5.2. Optimization of the Transformation System
Two-factor multi-level experiments identified the most suitable vacuum pressure and OD600 of Agrobacterium for the TRV-mediated VIGS system of tea. The same conditions were used for the vacuum infiltration time, osmosis buffer components, and soaking time. Agrobacterium strains that contained pTRV1, pTRV2, and pTRV-CsPOR1 were resuspended in the two different infiltration buffers, and the final OD600 values of 1, 1.2, and 1.5 were obtained, respectively. The vacuum pressure (0.6 kPa, 0.7 kPa, and 0.8 kPa) and OD600 (1, 1.2, and 1.5) were combined in pairs and divided into nine groups to explore the best conditions. Agrobacterium pTRV1 and pTRV2, and pTRV1 and pTRV-CsPOR1, were mixed at a 1:1 ratio and incubated for 1 or 2 h at room temperature. Multiple tea seedlings and cuttings were randomly selected and infiltrated with different vacuum pressures and optical densities of Agrobacterium.
4.6. Silencing and HPLC Analysis of CsTCS1 for Caffeine Content
The CsTCS1 involved in caffeine biosynthesis was silenced in the tea plant using the optimal system that resulted from Section 2.5, and a non-silenced plant was used as the control. The non-silenced plants were infected with pTRV1 + pTRV2 Agrobacterium. When the CsTCS1 gene was silenced, the caffeine in the leaves of the CsTCS1-silenced plants, wild-type plants, and non-silenced plants was determined by high-performance liquid chromatography (HPLC) (Hitachi Limited Co., Ltd., Tokyo, Japan). A total of 1.0 g of ground tea sample was weighed on a scale that was accurate to 0.0001 g and placed in a 500 mL flask. A total of 4.5 g of magnesium oxide and 300 mL of ultrapure water were brought to the boil in a boiling water bath and extracted for 20 min. The flask was shaken every 5 min. After extraction, the liquid was immediately filtered under heat and reduced pressure. The filtrate was transferred to a 500 mL volumetric flask, and after it had cooled, the volume was brought up to scale with ultrapure water and mixed well. A portion of the test solution was passed through a 45 PM membrane filter [49]. The conditions for the HPLC included a detection wavelength of 240 nm; a mobile phase of methanol: ultrapure water (3:7, v/v); a flow rate of 1 mL/min; a column temperature of 40 °C; and an injection volume of 10 ul, followed by ISO 17027:1995 (2013) (ISO). The relative content of caffeine was calculated using Equation (1).
| (1) |
4.7. Analysis of Expression by qRT-PCR
The total RNA was extracted using a TRIzol-A+ kit (TianGen Biochemical Technology Co., Ltd., Beijing, China). The cDNA was synthesized by reverse transcription using a PrimeScriptTM II first strand cDNA Synthesis Kit (Solarbio Technology Co., Ltd., Beijing, China) and diluted 10-fold for real-time PCR detection. To determine the levels of expression of the CsPOR1 and CsTCS1 genes, Primer Premier 5.0 was used to design specific primers for CsPOR1 and CsTCS1. The actin gene from tea was used as an internal reference (Table 1). A real-time quantitative reverse transcription PCR (qRT-PCR) assay was performed on a Bio-Rad CFX ConnectTM real-time quantitative PCR instrument (Bio-Rad, Hercules, CA, USA). The qRT-PCR used was a general-purpose high-sensitivity dye-based quantitative PCR detection kit from Nanjing Novozan Biotechnology Co., Ltd. (Nanjing, China). The qRT-PCR reaction system was utilized according to the manufacturer’s instructions. The conditions and system of the qRT-PCR were as follows: one cycle of denaturation (94 °C, 5 min), followed by 40 cycles of amplification (94 °C, 30 s; 50 °C, 45 s) and signal acquisition (72 °C, 113 s); 50 µL of the qRT-PCR selection system, which included 20 µL of nuclease-free water, 25 µL of Pfu PCR Mix (Biorun Biotechnology Co., Ltd., Wuhan, China), 1 µL of template, 2 µL of primer(+) (100 μM), and 2 µL of primer(-) (100 μM). The relative levels of expression of the genes were analyzed using 2−ΔCt.
4.8. Data Analysis
The data were subjected to an analysis of variance (ANOVA) using SPSS 18.0 (IBM, Inc., Armonk, NY, USA); error bars represent the mean ± standard deviation of data from the three independent experiments. Different letters indicate significant differences at p < 0.05 and extremely significant differences at p < 0.01. The qRT-PCR data were analyzed using SPSS for the significant difference analysis (p < 0.05). The data were analyzed using Microsoft Excel 2007 (Redmond, WA, USA), and a sequence analysis was performed using DNAMAN 5.0 and the database from a public website (https://www.ncbi.nlm.nih.gov/, accessed on 8 June 2022).
5. Conclusions
This study attempted to ameliorate the lack of a complete genetic transformation system for tea plants and other problems. Tea was genetically transformed without the need for tissue culture for the first time by utilizing a TRV-VIGS system. This method provides an effective method to study the function of genes in tea plants and accelerate the process of functional genome research in tea.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24010392/s1.
Author Contributions
Conceptualization, G.L.; methodology, G.L., X.Y. and Y.L.; software, G.L. and X.Y.; validation, G.L. and Y.L.; formal analysis, G.L., Y.L. and X.Y.; investigation, G.L. and Y.L.; resources, Y.L. and L.L.; data curation, G.L. and X.Y.; writing—original draft preparation, G.L.; writing—review and editing, G.L., X.Y. and L.L.; visualization, G.L., Y.L. and L.L.; supervision, Y.L. and L.L.; project administration, L.L.; funding acquisition, X.Y. and L.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Natural Science Foundation of China (3226180451); Guizhou Province Outstanding Young Scientific and Technological Talent Cultivation Project (Qiankehe Platform Talent [2019]5651); and Guizhou Province Science and Technology Planning Project (Qiankehe Support [2021] General 111).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kumagai M.H., Donson J., Della-Cioppa G., Harvey D., Hanley K., Grill L.K. Cytoplasmic inhibition of carotenoid biosynthesis with virus-derived RNA. Proc. Natl. Acad. Sci. USA. 1995;92:1679–1683. doi: 10.1073/pnas.92.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kjemtrup S., Sampson K.S., Peele C.G., Nguyen L.V., Conkling M.A., Thompson W.F., Robertson D. Gene silencing from plant DNA carried by a Geminivirus. Plant J. Cell Mol. Biol. 1998;14:91–100. doi: 10.1046/j.1365-313X.1998.00101.x. [DOI] [PubMed] [Google Scholar]
- 3.Shen J., Si W., Wu Y., Xu Y., Wang J., Cheng T., Zhang Q., Pan H. Establishment and Verification of An Efficient Virus-induced Gene Silencing System in Forsythia. Hortic. Plant J. 2021;7:81–88. doi: 10.1016/j.hpj.2020.09.001. [DOI] [Google Scholar]
- 4.Mandar R.G., Arunima P., Indranil D., Prakash P.K. RETRACTED ARTICLE: Virus-induced gene silencing for functional analysis of selected genes. Plant Cell Rep. 2008;27:209–219. doi: 10.1007/s00299-007-0460-2. [DOI] [PubMed] [Google Scholar]
- 5.Senthil-Kumar M., Mysore K.S. New dimensions for VIGS in plant functional genomics. Trends Plant Sci. 2011;16:656–665. doi: 10.1016/j.tplants.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Huang C., Qian Y., Li Z., Zhou X. Virus-induced gene silencing and its application in plant functional genomics. Sci. China Life Sci. 2012;55:99–108. doi: 10.1007/s11427-012-4280-4. [DOI] [PubMed] [Google Scholar]
- 7.Zhou X., Huang C. Virus-induced gene silencing using begomovirus satellite molecules. Methods Mol. Biol. 2012;894:57–67. doi: 10.1007/978-1-61779-882-5_4. [DOI] [PubMed] [Google Scholar]
- 8.Ji T., Li C., Zhen-yun H., Yun-cong Y. Tobacco rattle virus mediated gene silencing in strawberry plants. Plant Cell Tissue Organ Cult. PCTOC. 2015;120:1131–1138. [Google Scholar]
- 9.Wang J.T.Y.Y. Cotton Leaf Curl Multan Virus-Derived Viral Small RNAs Can Target Cotton Genes to Promote Viral Infection. Front. Plant Sci. 2016;7:1162. doi: 10.3389/fpls.2016.01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faivre-Rampant O., Gilroy E.M., Hrubikova K., Hein I., Millam S., Loake G.J., Birch P., Taylor M., Lacomme C. Potato virus X-induced gene silencing in leaves and tubers of potato. Plant Physiol. 2004;134:1308–1316. doi: 10.1104/pp.103.037507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X., Zhang J., Wu Z., Lai B., Huang X., Qin Y., Wang H., Hu G. Functional characterization of a glucosyltransferase gene, LcUFGT1, involved in the formation of cyanidin glucoside in the pericarp of Litchi chinensis. Physiol. Plant. 2015;156:139–149. doi: 10.1111/ppl.12391. [DOI] [PubMed] [Google Scholar]
- 12.Gabruk M., Mysliwa-Kurdziel B. Light-Dependent Protochlorophyllide Oxidoreductase: Phylogeny, Regulation, and Catalytic Properties. Biochemistry. 2015;54:5255–5262. doi: 10.1021/acs.biochem.5b00704. [DOI] [PubMed] [Google Scholar]
- 13.Scrutton N.S., Groot M.L., Heyes D.J. Excited state dynamics and catalytic mechanism of the light-driven enzyme protochlorophyllide oxidoreductase. Phys. Chem. Chem. Phys. 2012;14:8818–8824. doi: 10.1039/c2cp23789j. [DOI] [PubMed] [Google Scholar]
- 14.Damien S., Bertrand L., Stéphan C., Stéphanie B., Solène M., Emmanuelle B., Pierre R., Sabine B., Yohann C., Didier N., et al. An algal photoenzyme converts fatty acids to hydrocarbons. Science. 2017;357:903–907. doi: 10.1126/science.aan6349. [DOI] [PubMed] [Google Scholar]
- 15.Zhang S., Heyes D.J., Feng L., Sun W., Johannissen L.O., Liu H., Levy C.W., Li X., Yang J., Yu X., et al. Structural basis for enzymatic photocatalysis in chlorophyll biosynthesis. Nature. 2019;574:722–725. doi: 10.1038/s41586-019-1685-2. [DOI] [PubMed] [Google Scholar]
- 16.Lee J., Lee H., Song J., Jung Y.J., Reinbothe S., Park Y., Lee S.Y., Pai H. Cell Growth Defect Factor1/CHAPERONE-LIKE PROTEIN OF POR1 Plays a Role in Stabilization of Light-Dependent Protochlorophyllide Oxidoreductase in Nicotiana benthamiana and Arabidopsis. Plant Cell. 2013;25:3944–3960. doi: 10.1105/tpc.113.111096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li R.X., Ma J. Screening, Cloning and Functional Identification of POR Gene in the Albino of Ananas Comosus var Bracteatus Leaves Using VIGS Technique. Sichuan Agricultural University/Landscape Architecture; Chengdu, China: 2018. [Google Scholar]
- 18.Xia E., Tong W., Hou Y., An Y., Chen L., Wu Q., Liu Y., Yu J., Li F., Li R., et al. The Reference Genome of Tea Plant and Resequencing of 81 Diverse Accessions Provide Insights into Its Genome Evolution and Adaptation. Mol. Plant. 2020;13:1013–1026. doi: 10.1016/j.molp.2020.04.010. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y., Schiff M., Dinesh-Kumar S.P. Virus-induced gene silencing in tomato. Plant J. Cell Mol. Biol. 2002;31:777–786. doi: 10.1046/j.1365-313X.2002.01394.x. [DOI] [PubMed] [Google Scholar]
- 20.Romero I., Tikunov Y., Bovy A. Virus-induced gene silencing in detached tomatoes and biochemical effects of phytoene desaturase gene silencing. J. Plant Physiol. 2011;168:1129–1135. doi: 10.1016/j.jplph.2010.12.020. [DOI] [PubMed] [Google Scholar]
- 21.Deng W., Jin Y., Li M., Ma L., Zhang Z. Prokaryotic Expression of Caffeine Synthase Gene (TCS1), Its Polyclonal Antibody Preparation and Identification. Bull. Bot. Res. 2015;35:333–339. [Google Scholar]
- 22.Yu Y., Jiang C., Wan X., Li X. Expression of tea caffeine synthase cDNA in tobacco. J. Northwest A F Univ. 2007;35:181–186. [Google Scholar]
- 23.Mohanpuria P., Kumar V., Ahuja P.S., Yadav S.K. Producing low-caffeine tea through post-transcriptional silencing of caffeine synthase mRNA. Plant Mol.Biol. 2011;76:523–534. doi: 10.1007/s11103-011-9785-x. [DOI] [PubMed] [Google Scholar]
- 24.Di Stilio V.S., Kumar R.A., Oddone A.M., Tolkin T.R., Salles P., McCarty K. Virus-induced gene silencing as a tool for comparative functional studies in Thalictrum. PLoS ONE. 2010;5:e12064. doi: 10.1371/journal.pone.0012064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalbande B.B., Patil A.S. Plant tissue culture independent Agrobacterium tumefaciens mediated In-planta transformation strategy for upland cotton (Gossypium hirsutum) J. Genet. Eng. Biotechnol. 2016;14:9–18. doi: 10.1016/j.jgeb.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen Z., Yao J., Sun J., Chang L., Wang S., Ding M., Qian Z., Zhang H., Zhao N., Sa G., et al. Populus euphratica HSF binds the promoter of WRKY1 to enhance salt tolerance. Plant Sci. 2015;235:89–100. doi: 10.1016/j.plantsci.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 27.Zhou P., Peng J., Zeng M., Wu L., Fan Y., Zeng L. Virus-induced gene silencing (VIGS) in Chinese narcissus and its use in functional analysis of NtMYB3. Hortic. Plant J. 2021;7:565–572. doi: 10.1016/j.hpj.2021.04.009. [DOI] [Google Scholar]
- 28.Ye J., Qu J., Bui H.T., Chua N.H. Rapid analysis of Jatropha curcas gene functions by virus-induced gene silencing. Plant Biotechnol. J. 2009;7:964–976. doi: 10.1111/j.1467-7652.2009.00457.x. [DOI] [PubMed] [Google Scholar]
- 29.Jie Z., Ji T., De-qiang T., Ke-ting L., Yong-jun Z., Yun-cong Y. An optimized TRV-based virus-induced gene silencing protocol for Malus crabapple. Plant Cell Tissue Organ Cult. PCTOC. 2016;126:499–509. [Google Scholar]
- 30.Chen J.C., Jiang C.Z., Gookin T.E., Hunter D.A., Clark D.G., Reid M.S. Chalcone synthase as a reporter in virus-induced gene silencing studies of flower senescence. Plant Mol. Biol. 2004;55:521–530. doi: 10.1007/s11103-004-0590-7. [DOI] [PubMed] [Google Scholar]
- 31.Xiao Z., Xing M., Liu X., Fang Z., Yang L., Zhang Y., Wang Y., Zhuang M., Lv H. An efficient virus-induced gene silencing (VIGS) system for functional genomics in Brassicas using a cabbage leaf curl virus (CaLCuV)-based vector. Planta. 2020;252:42. doi: 10.1007/s00425-020-03454-7. [DOI] [PubMed] [Google Scholar]
- 32.Saitoh H., Terauchi R. Virus-induced silencing of FtsH gene in Nicotiana benthmiana causes a striking bleached leaf phenotype. Genes Genet. Syst. 2002;77:335–340. doi: 10.1266/ggs.77.335. [DOI] [PubMed] [Google Scholar]
- 33.Jia H., Guo J., Qin L., Shen Y. Virus-induced PpCHL Hgene silencing in peach leaves (Prunus persica) Hortic. Sci. Biotechnol. 2010;85:528–532. doi: 10.1080/14620316.2010.11512709. [DOI] [Google Scholar]
- 34.Liu E., Page J.E. Optimized cDNA libraries for virus-induced gene silencing (VIGS) using tobacco rattle virus. Plant Methods. 2008;4:5. doi: 10.1186/1746-4811-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuanzhong J., Shenglong Y., Lijun W., Yanjiao D., Wanxiang L., Hong L., Di Fan, Faqi Z., Keming L. Heterologous gene silencing induced by tobacco rattle virus (TRV) is efficient for pursuing functional genomics studies in woody plants. Plant Cell Tissue Organ Cult. PCTOC. 2014;116:163–174. [Google Scholar]
- 36.Yan H.X., Fu D.Q., Zhu B.Z., Liu H.P., Shen X.Y., Luo Y.B. Sprout vacuum-infiltration: A simple and efficient agroinoculation method for virus-induced gene silencing in diverse solanaceous species. Plant Cell Rep. 2012;31:1713–1722. doi: 10.1007/s00299-012-1285-1. [DOI] [PubMed] [Google Scholar]
- 37.Fu D.Q., Zhu B.Z., Zhu H.L., Zhang H.X., Xie Y.H., Jiang W.B., Zhao X.D., Luo K.B. Enhancement of virus-induced gene silencing in tomato by low temperature and low humidity. Mol. Cells. 2006;21:153–160. [PubMed] [Google Scholar]
- 38.Ali Z., Abul-faraj A., Li L., Ghosh N., Piatek M., Mahjoub A., Aouida M., Piatek A., Baltes N.J., Voytas D.F., et al. Efficient Virus-Mediated Genome Editing in Plants Using the CRISPR/Cas9 System. Mol. Plant. 2015;8:1288–1291. doi: 10.1016/j.molp.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 39.Chen X., Duan X., Wang S., Wu W., Zhang X. Virus-induced gene silencing (VIGS) for functional analysis of MYB80 gene involved in Solanum lycopersicum cold tolerance. Protoplasma. 2019;256:409–418. doi: 10.1007/s00709-018-1302-5. [DOI] [PubMed] [Google Scholar]
- 40.Li H., Zhang D., Xie K., Wang Y., Liao Q., Hong Y., Liu Y. Efficient and high-throughput pseudorecombinant-chimeric Cucumber mosaic virus-based VIGS in maize. Plant Physiol. 2021;187:2865–2876. doi: 10.1093/plphys/kiab443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jing J.L., Cheng H.W., Qiao J.X., Yue P.L., Xian F.L., Hong Y.Q. Overexpression and VIGS system for functional gene validation in oriental melon (Cucumis melo var. makuwa Makino) Plant Cell Tissue Organ Cult. PCTOC. 2019;137:275–284. [Google Scholar]
- 42.Zhang J., Wang F., Zhang C., Zhang J., Chen Y., Liu G., Zhao Y., Hao F., Zhang J. A novel VIGS method by agroinoculation of cotton seeds and application for elucidating functions of GhBI-1 in salt-stress response. Plant Cell Rep. 2018;37:1091–1100. doi: 10.1007/s00299-018-2294-5. [DOI] [PubMed] [Google Scholar]
- 43.Li N.N., Lu J.L., Li Q.S., Zheng X.Q., Wang X.C., Wang L., Wang Y.C., Ding C.Q., Liang Y.R., Yang Y.J. Dissection of Chemical Composition and Associated Gene Expression in the Pigment-Deficient Tea Cultivar ‘Xiaoxueya’ Reveals an Albino Phenotype and Metabolite Formation. Front. Plant Sci. 2019;10:1543. doi: 10.3389/fpls.2019.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li P., Ye Z., Fu J., Xu Y., Shen Y., Zhang Y., Tang D., Li P., Zuo H., Tong W., et al. CsMYB184 regulates caffeine biosynthesis in tea plants. Plant Biotechnol. J. 2022;20:1012–1014. doi: 10.1111/pbi.13814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang P., Zhang J., Su J., Wang P., Liu J., Liu B., Feng D., Wang J., Wang H. The chloroplast min system functions differentially in two specific nongreen plastids in Arabidopsis thaliana. PLoS ONE. 2013;8:e71190. doi: 10.1371/journal.pone.0071190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Q., Xu K., Yi L., Hou Y., Li D., Hu H., Zhou F., Song P., Yu Y., Wei Q., et al. A rapid, simple, and highly efficient method for VIGS and in vitro-inoculation of plant virus by INABS applied to crops that develop axillary buds and can survive from cuttings. BMC Plant Biol. 2021;21:545. doi: 10.1186/s12870-021-03331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu H., Fu D., Zhu B., Yan H., Shen X., Zuo J., Zhu Y., Luo Y. Virus-induced gene silencing in eggplant (Solanum melongena) J. Integr. Plant Biol. 2012;54:422–429. doi: 10.1111/j.1744-7909.2012.01102.x. [DOI] [PubMed] [Google Scholar]
- 48.Hua X., Leifeng X., Panpan Y., Yuwei C., Yuchao T., Guoren H., Suxia Y., Jingyi L., Jun M. Virus-induced Phytoene Desaturase (PDS) Gene Silencing Using Tobacco Rattle Virus in Lilium × formolongi. Hortic. Plant J. 2019;5:31–38. [Google Scholar]
- 49.Bispo M.S., Veloso M.C.C., Pinheiro H.L.C., De Oliveira R.F.S., Reis J.O.N., De Andrade J.B. Simultaneous determination of caffeine, theobromine, and theophylline by high-performance liquid chromatography. J. Chromatogr. Sci. 2002;40:45–48. doi: 10.1093/chromsci/40.1.45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.