Abstract
One in three cancer deaths worldwide are caused by gastric and colorectal cancer malignancies. Although the incidence and fatality rates differ significantly from country to country, the rates of these cancers in East Asian nations such as South Korea and Japan have been increasing each year. Above all, the biggest danger of this disease is how challenging it is to recognize in its early stages. Moreover, most patients with these cancers do not present with any disease symptoms before receiving a definitive diagnosis. Currently, volatile organic compounds (VOCs) are being used for the early prediction of several other diseases, and research has been carried out on these applications. Exhaled VOCs from patients possess remarkable potential as novel biomarkers, and their analysis could be transformative in the prevention and early diagnosis of colon and stomach cancers. VOCs have been spotlighted in recent studies due to their ease of use. Diagnosis on the basis of patient VOC analysis takes less time than methods using gas chromatography, and results in the literature demonstrate that it is possible to determine whether a patient has certain diseases by using organic compounds in their breath as indicators. This study describes how VOCs can be used to precisely detect cancers; as more data are accumulated, the accuracy of this method will increase, and it can be applied in more fields.
Keywords: volatile organic compounds, early diagnosis, mass spectroscopy, gastric and colorectal cancer
1. Introduction
The incidence and mortality rate of gastric and colorectal cancer are increasing in East Asia [1,2]. By admission, most patients have already missed the appropriate time for early diagnosis [3,4]. Additionally, the design and establishment of medical systems in developing countries is challenging due to poor financial support from governments [5,6].
Although there are many methods for cancer diagnosis, existing invasive methods such as endoscopy and blood tests impose a heavy burden on patients [7,8]. For instance, patients with gastric and colorectal cancers need to regularly undergo endoscopic examination of the stomach and intestine, respectively [9,10]. To address this problem, a variety of new biomarkers have been designed [7,11].
In recent studies, researchers studying patients’ exhalations with the aim of effectively diagnosing disease have had some success [12,13]. To apply this technique in cancer diagnosis, it is necessary to compare the standardized exhalation parameters obtained from healthy individuals with those sampled from patients [14,15]. This method involves the chemical evaluation of exhaled air and the identification and quantification of compounds such as aldehydes and ketones [16]. It is possible to rapidly analyze the obtained organic matter using analytical chemical assays [17,18]. More studies are required in order to accumulate a large database, with the ultimate goal of significantly lowering mortality rates. This review highlights the importance of mass spectroscopy as a tool to analyze VOCs in the diagnosis of patients with gastric and colorectal cancer.
2. Gastric and Colorectal Cancer
This paper addresses in detail gastric and colorectal cancers, which belong to the class of gastrointestinal (GI) cancers that includes all cancers of the organs of the digestive tract [19,20]. GI cancer stem cells (CSCs) are resistant to conventional therapies such as chemotherapy and radiotherapy, and GI cancers are the most lethal and common types of cancer worldwide [21]. There are geographical factors influencing their incidence, and these cancers are especially prevalent in most East Asian countries [22,23]. Furthermore, over one million new cases of these cancers are diagnosed every year worldwide [24,25].
2.1. Gastric Cancer
Gastric cancer (GC) is third most common cause of cancer death worldwide [26]. Representing over 90% of GC cases, adenocarcinomas are growths of malignant cells within the lining of the stomach [27]. In the upper digestive system, including the esophagus and stomach, normal tissues can grow in a disordered fashion into carcinoid tumors [28]. The process happens slowly, and is more likely to occur with increasing age [29]. As this disease shows no symptoms in its early stages, it is often diagnosed after it metastasizes to other organs [30].
Depending on the section of the stomach in which the cancer first develops, symptoms tend to progress differently; stomach cancers can be divided into four major categories on this basis: adenocarcinomas, GI stromal tumors, neuroendocrine tumors, and lymphomas [31]. Adenocarcinomas are the most common among these, and there are two major types: intestinal and diffuse [32,33]. The former have a better patient prognosis, whereas the latter are normally discovered submucosally, tend to spread out, and are extremely difficult to detect [34,35,36]. There are also other GCs, but they are extremely infrequent [37].
2.2. Colorectal Cancer
Colorectal cancer (CRC) is also divided into colon or rectal cancer, depending on the area in which the cancer first develops [38]. As they have much in common, these cancers are frequently classified together [38,39]. Most colorectal tumors grow from small clumps called polyps on the inner lining of the colon or rectum [40,41]. Polyps have differing tendencies to transform into cancer depending on their type, and not all polyps result in cancer [42,43,44]. Additionally, it takes several years for polyps to develop into cancer [45].
Polyps can be classified into adenomatous, hyperplastic, inflammatory, and sessile serrated varieties [46,47]. Hyperplastic and inflammatory polyps are commonly found, and the adenomatous variety is precancerous [43,48]. Additionally, sessile serrated polyps are often considered adenomas, as they have a higher risk of developing into CRC [49,50]. If the discovered polyps include the following factors, they are at risk of developing into cancer: larger than 1 cm, greater than three in number, and dysplasia discovery after polyp removal [51,52,53]. As time goes by, precancerous polyps develop into cancer in the colon or rectum wall [54,55]. Given that most CRCs are adenocarcinomas (similar to gastric cancers), the cancers originate in cells inside the inner layer which produce mucus as lubrication to protect the colon and rectum [56]. Signet ring cell and mucinous cancers may be associated with a worse prognosis than other adenocarcinomas [57,58,59]. There are also other less common CRCs, such as carcinoid and GI tumors, lymphomas, and sarcomas [60,61].
Although there are differences between these cancer types, patients may not experience any symptoms before cancer diagnosis is made at the early stage or while developing to a later stage [62,63,64,65]. Therefore, early detection, diagnosis, and staging using biomarkers are critical to cancer treatment [66,67].
3. What Are Biomarkers?
Biomarkers are indicators that can be used to detect alterations in the body, for example, proteins, DNA, RNA as well as VOCs [68,69,70]. The biomarkers can be used to differentiate between normal and pathological conditions, predict treatment response, and enable objective measurement in the case of a specific disease or cancer [71]. The following conditions should be met, objectively measured, and evaluated in order for an indicator to be defined as a biomarker: normal biological process, disease progression, and drug responsiveness to treatment methods [72].
Biomarkers can be classified into two main groups—invasive or not (Table 1). For example, biomarkers requiring examination of patient body fluids such as blood and serum [73] are invasive, whereas noninvasive sources include breath, urine, and feces [74,75,76]. Additionally, invasive biomarkers can be substituted by analyzing a headspace gas and its medium from a cultured cell line in vitro [77,78]. Further studies on biomarkers for use in disease diagnosis, particularly cancers, are constantly being conducted and expanded to apply their scope in clinical practice [79].
Table 1.
Typical biomarkers and their use.
| Type of Biomarker | Characteristics | Cancer | Refs. | |
|---|---|---|---|---|
| Invasive | Blood | Shows chemicals and proteins originated by cancer cells | Prostate, ovarian, and testicular cancer | [80,81,82,83,84] |
| Endoscopy | Used to identify idiopathic symptoms and observe prognosis | Gastric and colorectal cancer | [85,86,87,88,89,90,91] | |
| Noninvasive | Nipple aspirate fluid | Indicates the degree of disease progression and enables early diagnosis | Breast cancer | [92,93] |
| Urine | Highly sensitive and economical source of biomarkers, allows surveillance of therapeutic result | Prostate, bladder, endometrial, and pancreatic cancer | [94,95,96,97,98,99] | |
| Breath | Accurate detection in a short time, can be used to predict cancers | Lung, breast, gastric, and colorectal cancer | [100,101,102,103,104,105,106,107] | |
| Sweat | Measurable in small quantities and is not limited by consultation space restrictions | Lung cancer | [108,109] | |
4. Breathomics
Breathomics has been a center of research attention since Linus Pauling revealed a complex mixture of an estimated 250 VOCs in human breath [110]. Typical examinations for cancer are based on imaging and blood analysis [111,112]. Computed tomography, for instance, physically and financially burdens patients because of radiation exposure and expense [113]. Thus, breathomics using VOCs obtained from exhaled breath samples is generating a great deal of interest [114].
In 2021, Tsou et al. demonstrated and generalized the concept of how VOCs obtained from patients with cancer could work as biomarkers compared with other noninvasive biomarkers [113]. Although most conventional detection methods have high sensitivity, there are several limitations to these analyses, such as the need for specialized facilities and the financial burden [115,116]. On the other hand, the method of using exhaled breath has numerous advantages, such as high sensitivity, simplicity, and low cost [117,118,119].
Most tests for gastric and colorectal cancers are similar, although there are slight differences [120]. Patients may be reluctant to agree to invasive medical tests such as gastroscopy [121,122]. Therefore, if they are proven to be feasible and valuable for clinical use, health technologies will continue to develop a variety of biomarkers using VOCs from patients’ exhaled breath to relieve this psychological burden [123].
5. Methods for VOC Measurement
The instruments used to analyze patients’ exhaled breath include gas chromatography–mass spectroscopy (GC–MS), collection tools such as the Tenax TA (pipe), and Tedlar bags for sample storage [124,125]. The pipes are especially useful for storage of low concentrations of exhaled gas because they contain a solid absorbent [126,127]. In brief, for the analysis process, a subject suspected of having a disease breathes into a Tedlar bag through a pipe [128]. Next, the collected sample is analyzed using GC–MS and the patient’s VOC profile is compared with VOC profiles obtained from healthy individuals [129,130,131]. It is critical that atmospheric VOCs are also collected in other tubes in order to know in advance the variables that may affect the experiment [113]. Various other analytical instruments have also been used, such as ion mobility spectrometry, selected ion flow tube–mass spectrometry (SIFT-MS), proton transfer reaction–mass spectrometry (PTR-MS), and comprehensive 2D gas chromatography [132].
SIFT-MS, which learns numerous data using extreme gradient boosting (XGBoost), is a point of convergence between specific and reliable quantification, and it is much sought after [133,134,135,136]. In other words, SIFT-MS combined with big data is useful for the qualitative analysis of VOCs in real time [136]. Before everything else, the tool classifies subjects based on their physical condition and the result of VOC analyses [137,138].
6. Cancer-Related VOCs in Exhaled Breath
Global Cancer Statistics reported that 46% of people worldwide experienced breast, lung, prostate, and gastric and colorectal cancers in 2020 [139] (Table 2). Aldehydes and ketones, which are primarily expressed in all cancers, are discussed in [140]. Although common chemicals such as alcohols and benzenes were also noted, they were considered to be from exogenous factors such as smoking (Table 3). The aim of this work was to clarify the biochemical pathways of aldehydes and ketones in order to determine their origins. The concentration changes in exhaled breath from GI cancer patients can be directly associated with biomarkers of cancer quantification because the metabolic processes of cancer cells produce or reduce abnormal organic compounds compared with normal cells [140,141]. An analysis of the Cancer Odor Database (COD) developed by Janfaza et al. indicates that some VOCs contribute to particular types of cancer and have potential as biomarkers [142,143].
Table 2.
The ranked cancers related to eight typical VOCs based on references.
| Rank | Cancer | Volatile Organic Compounds | Refs. |
|---|---|---|---|
| 1 | Breast | Alkanes, Aldehydes, Esters, Ketones | [119,144] |
| 2 | Lung | Alcohols, Aldehydes, Ethers | [145,146] |
| 3 | Colon | Alcohols, Aldehydes, Alkanes, Ketones | [131,147] |
| 4 | Prostate | Acetones, Alcohols, Aldehyde Ammonias | [148,149] |
| 5 | Stomach | Alcohols, Aldehydes, Ketones | [150,151] |
Table 3.
The altered VOCs in gastric and colorectal cancer patients.
| Cancer | Volatile Organic Compounds | Ref. | |
|---|---|---|---|
| Colon | Increased | Alcohols, Aldehydes (Benzaldehyde), Acetone (Ketones), Indole | [131,140,152,153,154] |
| Decreased | Benzene Ethyl | [140] | |
| Stomach | Increased | Alcohols (Phenol, 2-Butoxy-Ethanol), Aldehydes (Benzaldehyde, Propanal), Acetone (Ketones) | [140,150,155,156,157] |
| Decreased | Pentanoic acid, 1,3,5-Trimethylbenzene | [140] | |
6.1. Aldehydes
As indicated in Table 2, aldehydes are associated with all five of the specified types of cancer (breast, lung, colon, prostate, and stomach). Among the aldehydes, hexanal, nonanal, and heptanal aldehydes are commonly detected in patients’ exhaled breath, and in blood, saliva, and urine [143].
Since the composition of the membrane lipids in cancer cells is changed, some saturated and unsaturated lipids are observed at altered levels compared to the profiles associated with normal, healthy individuals [158,159]. Increased concentrations of unsaturated fatty acids might promote the production of some aldehydes through lipid peroxidation [160,161,162]. For this reason, the metabolism of aldehydes in cancer cells differs from that in normal cells [163,164].
6.1.1. Metabolic Pathway of Aldehydes in Cancer and Normal Cells
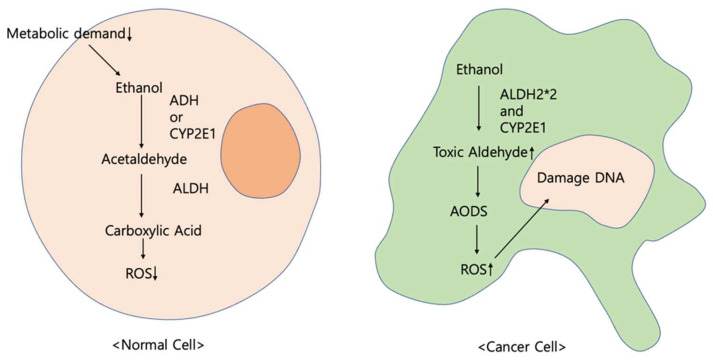
Ethanol, an alcohol, is oxidated in diverse metabolic mechanisms by enzymes such as aldehyde dehydrogenase (ALDH), alcohol dehydrogenase (ADH), and cytochrome P450 (CYP450) with hydrogen peroxide [165,166,167]. Generally, ethanol is oxidated to acetaldehyde by ADH [168,169]. Then, the aldehyde is degraded to carboxylic acid using ALDH [170,171].
Since metabolic demands are rarely lowered in normal cells, ALDH is not overexpressed to detoxify and lower reactive oxygen species (ROS) production [172]. In contrast, toxic aldehydes and ROS accumulate in cancer tissues [173].
The primary alcohol is typically metabolized in two steps in the liver [174]. First, ethanol is oxidated to acetaldehyde through enzymes such as ADH and cytochrome P2E1 (CYP2E1) [175]. With ALDH, acetaldehyde is additionally metabolized to acetate as a further step [176,177]. Above all, the results of these reactions depend on the enzymes; even acetaldehyde, which is toxic [178,179,180] and carcinogenic, has the potential to accumulate [176]. This accumulation has grave implications for DNA, suppressing DNA repair and damaging the antioxidative defense system (AODS) [181,182].
In the first step of oxidation, various acetaldehydes are generated through ADH1B and ADH1C, which belong to the same subfamily [183,184,185]. However, toxic acetaldehydes, which are the result of oxidation by CYP2E1 [167,186], produce ROS [174,187] and damage AODS [188]. As a result, insufficiently detoxified ROS cause the formation of DNA adducts [189,190].
In the second step, acetaldehyde is degraded by ALDH [166,191]. The enzyme has polymorphisms, such as ALDH2*1 and ALDH2*2 [188]. Among these, ALDH2*2 contributes to the accumulation of acetaldehyde [192,193,194] (Figure 1).
Figure 1.
Complete oxidation mechanism.
6.1.2. Cytochrome P450 and Reactive Oxygen Species
Cytochrome P450 (CYP450)
As a coenzyme containing heme, CYP450 is a multigenic family of proteins [195,196]. Most of these enzymes are responsible for different enzymatic reactions and are well known as electron transport oxidases [197,198]. Above all, CPY450 plays a key role in diverse metabolism and detoxification processes [199]. Moreover, the enzyme is involved in miscellaneous enzymatic reactions such as fatty acid metabolism [200,201]. CYP450 is primarily found within the endoplasmic reticulum, and in mitochondria in the liver [202]. CYP450 is classified based on electron transport proteins, for instance, microsomal and mitochondrial [203].
ROS production is closely related to CYP450 [204]. CYP450 enzymes, which can control carcinogenic activity, are involved in cancer initiation and promotion [205,206]. Furthermore, when CYP450s are overexpressed in a tumor cell, ROS are manufactured by the coenzymes [207,208]. Among the subfamily of CYP450 enzymes, CYP2E1 is mainly correlated with ROS production [41]. Specifically, the overexpression of CYP2E1 results in a high level of inflammatory cytokines compared to normal cells [209,210].
Reactive Oxygen Species
Although ROS are signaling molecules for normal cells, ROS generation can cause harm to autophagy, unfolded protein response, and several cellular organelles, with the potential to lead to disorder in normal cell viability [211,212]. For that reason, unnecessary ROS should be eradicated in order to maintain redox homeostasis [171].
Normal cells have enough adaptive ability to protect themselves from the adverse influences of ROS [213]. In contrast, where there is anomalous ROS production, redox imbalance can provoke advancement to the initiation and development of several cancer types. Additionally, the metabolism of cancer tumors generates high ROS concentrations [214].
At low ROS levels, biological processes of cancer cells such as development and survival are limited because cells have the capability of antioxidant activity to repair damage [215,216,217]. At high ROS concentrations, cellular organelles are damaged, and the DNA repair pathway is disrupted [218,219,220]. Additionally, increased oxidative stress results in a high rate of aldehyde production [143].
6.2. Ketones
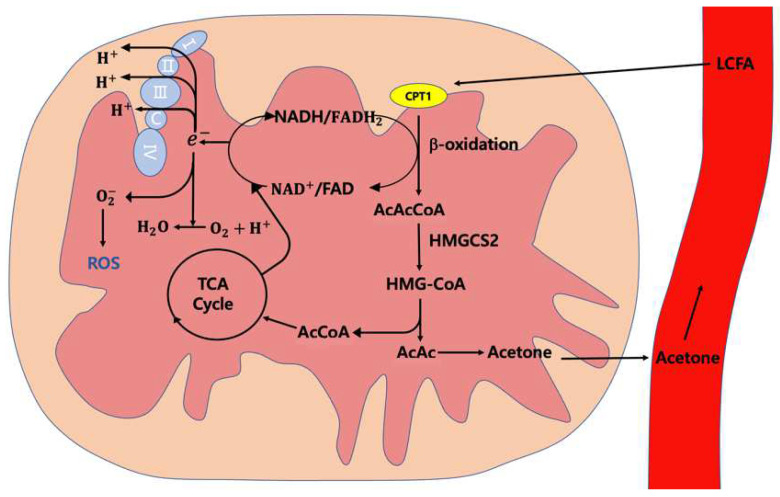
Similarly to aldehyde, ketones are derived from and affected by external factors such as diet [221]. Nevertheless, in many cancers, the production of ketones begins from a typical mechanism of increasing long-chain fatty acid (LCFA) oxidation to increase the ketone body production in the mitochondria of the liver [222,223]. As the first step in the catabolism of fatty acids, β-oxidation breaks down fatty acids using electron transport chain factors such as NADH and FADH2, and produces acetoacetyl-CoA (acac-CoA) [224,225].
Normal and tumor tissues regulate ketone bodies differently [226,227]. In normal tissue, ketone bodies regulate cellular energy supply from glucose to fatty acids and ketones to regulate blood glucose, since glucose provision is restricted by 3-hydroxy-3-methlglutaryl-CoA synthase 2 (HMGCS2) and solute carrier family 16 (SLC16A6) [228,229]. Additionally, ketone bodies can be degraded into acetyl-CoA to enter the tricarboxylic acid (TCA) cycle, which produces energy and enhances cell viability [230,231,232].
The mitochondrial structure of cancer cells is different to that of normal cells; ketone bodies may increase their oxidative stress via the TCA cycle [228]. Moreover, electrons are overproduced by NADH and in the TCA cycle and β-oxidation, and are moved into the mitochondria of cancer cells [232]. Additionally, the antioxidant system pathway is inhibited because of increased ROS and causes oxidative stress damage in low-carbohydrate conditions [215,233].
Acetoacetyl (AcAc) directly results in the formation of ketone bodies, which are released into the plasma [28,234,235]. As these ketones are weakly soluble, they are transported through blood vessels to the lungs and are then exhaled [236] (Figure 2).
Figure 2.
Cancer cell ketogenesis.
Acetone is the smallest ketone, and it is continuously produced during acetoacetate decarboxylation [237,238] even after being degraded into acetol by CYP2E1 [239]. There are different mechanisms involved in ketone production. For example, 2-nonanone is generated via nonane metabolism by CYP450 [240,241].
There are four ketones that are considered cancer biomarkers: 2-nonanone, 3-heptanone, 4-heptanone, and cyclohexanone [242,243]. Although there are limitations to their use in the detection and investigation of cancers, among these ketones, cyclohexanone is extensively observed in patients with chronic pulmonary disease and not in healthy individuals [244,245].
7. Summary and Future Perspectives
7.1. Summary
VOCs contain invaluable information about the biochemical metabolization of cancer cells [246]. According to some articles, some compounds are related to specific cancers and can be used to distinguish between patients and healthy people [100]. Aldehyde and ketone can be identified in the breath just minutes after being released from tissues because they are slightly soluble in blood [247].
As reported, 10 VOCs are associated with gastric and colorectal cancers, in addition to aromatics and hydrocarbons from exogenous factors. Although these organic compounds can all be deemed important biomarkers, hexanal and 3-heptanone are especially reported to be closely related to gastric and colorectal cancers according to studies using various methods, although exhaled breath has not been studied in this regard [248,249,250].
According to the other reports, many short-chain fatty acids (SCFAs), such as acetate, have been found at high concentrations in the exhaled breath of patients with colorectal [153] and gastric cancer [156] in comparison to healthy subjects. This result shows that SCFAs in the breath of GC patients might be generated by the metabolism of stomach cancer cells.
7.2. Future Perspectives
The studies described herein found significant cancer-related aspects of VOC profiles. In the medical field, biomarkers are a cornerstone of a paradigm shift towards a personalized medical system centered on prevention, with treatment based on experience and statistics beyond the existing collective diagnostic tests [251,252,253]. The global biomarker market is growing steadily [254]. Biomarker research on many diseases is growing alongside the development of the medical industry [71,255]. The development of more advanced biomarkers is in progress, and this is expected to bring more progressive biomarker use [140,256,257,258]. The fatality rates of some cancers are still high, in view of the fact that it is difficult to be aware of symptoms before the disease has developed to a fatal level, despite the use of advanced medical technologies [259]. To make matters worse, high costs make it difficult for patients to access medical tests without insurance [260,261,262].
The metabolization of aldehydes and ketones for gastric and colorectal cancer has been comprehensively reviewed in this article. Moreover, we have demonstrated that VOCs contain invaluable information about the biochemical metabolization of cancer cells. Therefore, the comprehensive analysis of discernible VOCs in patients’ exhaled breath may reduce the burden of invasive medical tests for patients, and may enable the early detection of cancer and the efficient prediction of prognosis following surgery with a small outlay.
Regarding instrumentation, SIFT-MS can be used to analyze considerable quantities of quantitative data with the XGBoost model and to predict cancers based on VOC factors [113,263]. Based on machine learning and deep learning algorithms, this instrument can accurately determine cancer using VOCs from patients’ exhaled breath and reduce the interference of environmental factors, resulting in accurate prediction models [113,264]. As science has advanced, big data associated with research on how VOCs are related to cancers has been accumulating for over fifty years, and thus, it can now be processed [265,266]. If large amounts of data continue to accumulate as additional research continues, further research will be still easier.
In addition to SIFT-MS, bioelectronic and olfactory-receptor-based sensors have shown remarkable sensitivity upon their merging into a primary transducer [267,268,269]. This has many advantages—it is simple to use and sufficiently inexpensive that it can be made available to everyone [132,267,268]. Thus, these are promising alternatives to conventional diagnostic instruments [270].
8. Conclusions
The study of VOCs from exhaled breath is an area of significant innovation [271]. It has a great deal of potential to yield biomarkers for GI cancer, although further studies are required because sufficient data have not yet been collected. Above all, the origin of VOCs can include exogenous factors, especially physical activities and smoking, which change the pattern of VOCs [272,273].
For instance, acetone, with an abnormal fruity odor, might be considered an adequate cancer biomarker [239]. However, the chemical cannot itself represent an appropriate biomarker because the acetone concentration in breath changes during activities such as exercising or fasting [274]. Furthermore, there are limitations in that the origins of most VOCs (e.g., 4-heptanone) are unclear [248], and thus, they are not recommended for use as biomarkers [275].
Similarly, there are still limitations to research on the origins of most VOCs [248]. However, analyzing big data with advanced instruments might be useful and helpful in solving the problem of VOCs related to gastric and colorectal cancer. Consequently, there is a possibility that, in the future, we will be able to easily prevent and treat cancer using these revolutionary biomarkers.
Abbreviations
| VOCs | Volatile Organic Compounds |
| GI | Gastrointestinal |
| CSCs | Cancer Stem Cells |
| GC | Gastric Cancer |
| CRC | Colorectal Cancer |
| GC-MS | Gas Chromatography–Mass Spectroscopy |
| SIFT-MS | Selected Ion Flow Tube–Mass Spectrometry |
| PTR-MS | Proton Transfer Reaction–Mass Spectrometry |
| ALDH | Aldehyde Dehydrogenase |
| ADH | Alcohol Dehydrogenase |
| CYP450 | Cytochrome P450 |
| ROS | Reactive Oxygen Species |
| CYP2E1 | Cytochrome P2E1 |
| AODS | Antioxidative Defense System |
| LCFA | Long-Chain Fatty Acid |
| acac-COA | Acetoacetyl-CoA |
| HMGCS2 | 3-Hydroxy-3-Methlglutaryl-CoA Synthase 2 |
| SLC16A6 | Solute Carrier Family 16 |
| TCA | Tricarboxylic Acid |
| AcAc | Acetoacetyl |
| SCFAs | Short-Chain Fatty Acids |
Author Contributions
Conceptualization, J.C.; writing—original draft preparation, J.C., S.A., S.H. and Y.S.; writing—review and editing, J.C., S.A., S.H., Y.S., T.G.C. and I.K.; supervision, T.G.C. and S.S.K.; project administration, I.K. and S.S.K.; funding acquisition, T.G.C. and S.S.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST), NRF-2018R1A6A1A03025124 to S.S.K. and NRF-2020R1I1A1A01069013 to T.G.C.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pourhoseingholi M.A., Vahedi M., Baghestani A.R. Burden of gastrointestinal cancer in Asia; An overview. Gastroenterol. Hepatol. Bed Bench. 2015;8:19–27. [PMC free article] [PubMed] [Google Scholar]
- 2.Huang J., Ngai C.H., Deng Y., Tin M.S., Lok V., Zhang L., Yuan J., Xu W., Zheng Z.-J., Wong M.C.S. Cancer Incidence and Mortality in Asian Countries: A Trend Analysis. Cancer Control. 2022;29 doi: 10.1177/10732748221095955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford A., Kunik M.E., Schulz P., Williams S.P., Singh H. Missed and delayed diagnosis of dementia in primary care: Prevalence and contributing factors. Alzheimer Dis. Assoc. Disord. 2009;23:306–314. doi: 10.1097/WAD.0b013e3181a6bebc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crosby D., Bhatia S., Brindle K.M., Coussens L.M., Dive C., Emberton M., Esener S., Fitzgerald R.C., Gambhir S.S., Kuhn P., et al. Early detection of cancer. Science. 2022;375:eaay9040. doi: 10.1126/science.aay9040. [DOI] [PubMed] [Google Scholar]
- 5.Han W. Health Care System Reforms in Developing Countries. J. Public Health Res. 2012;1:e31–e207. doi: 10.4081/jphr.2012.e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rahman M.M., Ghoshal U.C., Ragunath K., Jenkins G., Edwards C., Hasan M., Taylor-Robinson S.D. Biomedical research in developing countries: Opportunities, methods, and challenges. Indian J. Gastroenterol. 2020;39:292–302. doi: 10.1007/s12664-020-01056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent J.L., Bogossian E., Menozzi M. The Future of Biomarkers. Crit. Care Clin. 2020;36:177–187. doi: 10.1016/j.ccc.2019.08.014. [DOI] [PubMed] [Google Scholar]
- 8.Beer T.M. Novel blood-based early cancer detection: Diagnostics in development. Am. J. Manag. Care. 2020;26:S292–S299. doi: 10.37765/ajmc.2020.88533. [DOI] [PubMed] [Google Scholar]
- 9.Niknam N., Obanor S., Lee L.A. Endoscopic methods for the detection and treatment of gastric cancer. Curr. Opin. Gastroenterol. 2022;38:436–442. doi: 10.1097/MOG.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 10.Ortega-Morán J.F., Azpeitia A., Sánchez-Peralta L.F., Bote-Curiel L., Pagador B., Cabezón V., Saratxaga C.L., Sánchez-Margallo F.M. Medical needs related to the endoscopic technology and colonoscopy for colorectal cancer diagnosis. BMC Cancer. 2021;21:467. doi: 10.1186/s12885-021-08190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y., Hu Y., Di Wang D., Yu K., Wang L., Zou Y., Zhao C., Zhang X., Wang P., Ying K. The analysis of volatile organic compounds biomarkers for lung cancer in exhaled breath, tissues and cell lines. Cancer Biomark. 2012;11:129–137. doi: 10.3233/CBM-2012-00270. [DOI] [PubMed] [Google Scholar]
- 12.Grob N.M., Aytekin M., Dweik R.A. Biomarkers in exhaled breath condensate: A review of collection, processing and analysis. J. Breath Res. 2008;2:037004. doi: 10.1088/1752-7155/2/3/037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaloumenou M., Skotadis E., Lagopati N., Efstathopoulos E., Tsoukalas D. Breath Analysis: A Promising Tool for Disease Diagnosis—The Role of Sensors. Sensors. 2022;22:1238. doi: 10.3390/s22031238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibrahim W., Carr L., Cordell R., Wilde M.J., Salman D., Monks P.S., Thomas P., Brightling E.C., Siddiqui S., Greening N.J. Breathomics for the clinician: The use of volatile organic compounds in respiratory diseases. Thorax. 2021;76:514–521. doi: 10.1136/thoraxjnl-2020-215667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peters A.L., Gerritsen M.G., Brinkman P., Zwinderman K.A., Vlaar A.P., Bos L.D. Volatile organic compounds in exhaled breath are independent of systemic inflammatory syndrome caused by in-travenous lipopolysaccharide infusion in humans: Results from an experiment in healthy volunteers. J. Breath Res. 2017;11:026003. doi: 10.1088/1752-7163/aa6545. [DOI] [PubMed] [Google Scholar]
- 16.Gashimova E., Osipova A., Temerdashev A., Porkhanov V., Polyakov I., Perunov D., Dmitrieva E. Exhaled breath analysis using GC-MS and an electronic nose for lung cancer diagnostics. Anal. Methods. 2021;13:4793–4804. doi: 10.1039/D1AY01163D. [DOI] [PubMed] [Google Scholar]
- 17.Hashoul D., Haick H. Sensors for detecting pulmonary diseases from exhaled breath. Eur. Respir. Rev. 2019;28:190011. doi: 10.1183/16000617.0011-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Subali A.D., Wiyono L., Yusuf M., Zaky M.F.A. The potential of volatile organic compounds-based breath analysis for COVID-19 screening: A systematic review & meta-analysis. Diagn. Microbiol. Infect. Dis. 2021;102:115589. doi: 10.1016/j.diagmicrobio.2021.115589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taniguchi H., Moriya C., Igarashi H., Saitoh A., Yamamoto H., Adachi Y., Imai K. Cancer stem cells in human gastrointestinal cancer. Cancer Sci. 2016;107:1556–1562. doi: 10.1111/cas.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Y., Shi L., He X., Luo Y. Gastrointestinal cancers in China, the USA, and Europe. Gastroenterol. Rep. 2021;9:91–104. doi: 10.1093/gastro/goab010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdi E., Latifi-Navid S., Latifi-Navid H., Safaralizadeh R. LncRNA polymorphisms and upper gastrointestinal cancer risk. Pathol. Res. Pract. 2020;218:153324. doi: 10.1016/j.prp.2020.153324. [DOI] [PubMed] [Google Scholar]
- 22.Sekiguchi M., Oda I., Matsuda T., Saito Y. Epidemiological Trends and Future Perspectives of Gastric Cancer in Eastern Asia. Digestion. 2021;103:22–28. doi: 10.1159/000518483. [DOI] [PubMed] [Google Scholar]
- 23.Wong M.C., Ding H., Wang J., Chan P.S., Huang J. Prevalence and risk factors of colorectal cancer in Asia. Intest. Res. 2019;17:317–329. doi: 10.5217/ir.2019.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xi Y., Xu P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021;14:101174. doi: 10.1016/j.tranon.2021.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rawla P., Barsouk A. Epidemiology of gastric cancer: Global trends, risk factors and prevention. Gastroenterol. Rev. 2019;14:26–38. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thrift A.P., El-Serag H.B. Burden of Gastric Cancer. Clin. Gastroenterol. Hepatol. 2020;18:534–542. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakr R., Massoud M., Aftimos G., Chahine G. Gastric Adenocarcinoma Secondary to Primary Gastric Diffuse Large B-cell Lymphoma. J. Gastric Cancer. 2017;17:180–185. doi: 10.5230/jgc.2017.17.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaudhry S.R., Liman M.N.P., Peterson D.C. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Anatomy, Abdomen and Pelvis, Stomach. [PubMed] [Google Scholar]
- 29.Basendowah M.H., Ashour M.A., Hassan A.Y., Alshaynawi S., Alyazidi L.K. Multiple Small Intestinal Neuroendocrine Tumors with Findings of Intestinal Obstruction. Cureus. 2021;13:e17629. doi: 10.7759/cureus.17629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zakaria A., Alnimer L., Byrd G., Piper M., Raphael M., Warren B., Piper M. Asymptomatic Ileal Neuroendocrine “Carcinoid” Tumor Incidentally Diagnosed on Colorectal Cancer Screening Colonoscopy: Does Routine TI Intubation Matter? Case Rep. Gastrointest. Med. 2021;2021:6620036. doi: 10.1155/2021/6620036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richman D.M., Tirumani S.H., Hornick J., Fuchs C.S., Howard S., Krajewski K., Ramaiya N., Rosenthal M. Beyond gastric adenocarcinoma: Multimodality assessment of common and uncommon gastric neoplasms. Abdom. Radiol. 2016;42:124–140. doi: 10.1007/s00261-016-0901-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu B., El Hajj N., Sittler S., Lammert N., Barnes R., Meloni-Ehrig A. Gastric cancer: Classification, histology and application of molecular pathology. J. Gastrointest. Oncol. 2012;3:251–261. doi: 10.3978/j.issn.2078-6891.2012.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van der Kaaij R.T., Koemans W.J., van Putten M., Snaebjornsson P., Luijten J.C., van Dieren J.M., Cats A., Lemmens V.E.P.P., Verhoeven R.H.A., van Sandick J.W., et al. A population-based study on intestinal and diffuse type adenocarcinoma of the oesophagus and stomach in the Netherlands between 1989 and 2015. Eur. J. Cancer. 2020;130:23–31. doi: 10.1016/j.ejca.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 34.Song M., An J.Y., Noh S.H., Shin S.K., Lee Y.C., Kim H., Hyunki K. Abstract 783: High microsatellite instability predicts good prognosis in intestinal type gastric cancers. Cancer Res. 2010;70:783. doi: 10.1158/1538-7445.AM10-783. [DOI] [PubMed] [Google Scholar]
- 35.Onitilo A.A., Aryal G., Engel J.M. Hereditary Diffuse Gastric Cancer: A Family Diagnosis and Treatment. Clin. Med. Res. 2012;11:36–41. doi: 10.3121/cmr.2012.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petrelli F., Berenato R., Turati L., Mennitto A., Steccanella F., Caporale M., Dallera P., de Braud F., Pezzica E., di Bartolomeo M., et al. Prognostic value of diffuse versus intestinal histotype in patients with gastric cancer: A systematic review and meta-analysis. J. Gastrointest. Oncol. 2017;8:148–163. doi: 10.21037/jgo.2017.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Recio-Boiles A., Babiker H.M. Gastric Cancer. StatPearls; Treasure Island, FL, USA: 2022. [Google Scholar]
- 38.Paschke S., Jafarov S., Staib L., Kreuser E.-D., Maulbecker-Armstrong C., Roitman M., Holm T., Harris C.C., Link K.-H., Kornmann M. Are Colon and Rectal Cancer Two Different Tumor Entities? A Proposal to Abandon the Term Colorectal Cancer. Int. J. Mol. Sci. 2018;19:2577. doi: 10.3390/ijms19092577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tamas K., Walenkamp A., de Vries E., van Vugt M., Beets-Tan R., van Etten B., de Groot D., Hospers G. Rectal and colon cancer: Not just a different anatomic site. Cancer Treat. Rev. 2015;41:671–679. doi: 10.1016/j.ctrv.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Marley A.R., Nan H. Epidemiology of colorectal cancer. Int. J. Mol. Epidemiol. Genet. 2016;7:105–114. [PMC free article] [PubMed] [Google Scholar]
- 41.Sharzehan M.A.K., Sito H., Abdullah N., Alexiou A., Papadakis M., Jamal R., Tan S.C. Association between CYP2E1 polymorphisms and colorectal cancer risk: A systematic review and meta-analysis. Sci. Rep. 2022;12:20149. doi: 10.1038/s41598-022-24398-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bujanda L., Cosme A., Gil I., Arenas-Mirave J.I. Malignant colorectal polyps. World J. Gastroenterol. 2010;16:3103–3111. doi: 10.3748/wjg.v16.i25.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shussman N., Wexner S.D. Colorectal polyps and polyposis syndromes. Gastroenterol. Rep. 2014;2:1–15. doi: 10.1093/gastro/got041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith R.A., Fedewa S., Siegel R. Early colorectal cancer detection-Current and evolving challenges in evidence, guidelines, policy, and practices. Adv. Cancer Res. 2021;151:69–107. doi: 10.1016/bs.acr.2021.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Waldum H., Fossmark R. Gastritis, Gastric Polyps and Gastric Cancer. Int. J. Mol. Sci. 2021;22:6548. doi: 10.3390/ijms22126548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ko H.M., Harpaz N., McBride R.B., Cui M., Ye F., Zhang D., Ullman A.T., Polydorides A.D. Serrated colorectal polyps in inflammatory bowel disease. Mod. Pathol. 2015;28:1584–1593. doi: 10.1038/modpathol.2015.111. [DOI] [PubMed] [Google Scholar]
- 47.Perea García J., Arribas J., Cañete Á., García J.L., Álvaro E., Tapial S., Narváez C., Vivas Lopez A., Brandáriz L., Hernández-Villafranca S., et al. Association of Polyps with Early-Onset Colorectal Cancer and Throughout Surveillance: Novel Clinical and Molecular Implications. Cancers. 2019;11:1900. doi: 10.3390/cancers11121900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim M., Vogtmann E., Ahlquist D.A., Devens M.E., Kisiel J.B., Taylor W.R., White B.A., Hale V.L., Sung J., Chia N., et al. Fecal Metabolomic Signatures in Colorectal Adenoma Patients Are Associated with Gut Microbiota and Early Events of Colorectal Cancer Pathogenesis. mBio. 2020;11:e03186-19. doi: 10.1128/mBio.03186-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Obuch J.C., Pigott C.M., Ahnen D.J. Sessile Serrated Polyps: Detection, Eradication, and Prevention of the Evil Twin. Curr. Treat. Options Gastroenterol. 2015;13:156–170. doi: 10.1007/s11938-015-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murakami T., Sakamoto N., Nagahara A. Endoscopic diagnosis of sessile serrated adenoma/polyp with and without dyspla-sia/carcinoma. World J. Gastroenterol. 2018;24:3250–3259. doi: 10.3748/wjg.v24.i29.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eichenseer P.J., Dhanekula R., Jakate S., Mobarhan S., Melson J.E. Endoscopic Mis-sizing of Polyps Changes Colorectal Cancer Surveillance Recommendations. Dis. Colon Rectum. 2013;56:315–321. doi: 10.1097/DCR.0b013e31826dd138. [DOI] [PubMed] [Google Scholar]
- 52.Alecu M., Simion L., Straja N., Brătucu E. Multiple polyps and colorectal cancer. Chirurgia. 2014;109:342–346. [PubMed] [Google Scholar]
- 53.Ateş Ö., Sivri B., Kiliçkap S. Evaluation of risk factors for the recurrence of colorectal polyps and colorectal cancer. Turk. J. Med. Sci. 2017;47:1370–1376. doi: 10.3906/sag-1601-63. [DOI] [PubMed] [Google Scholar]
- 54.Balchen V., Simon K. Colorectal cancer development and advances in screening. Clin. Interv. Aging. 2016;11:967–976. doi: 10.2147/CIA.S109285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xia L., Chen J., Zhang Y., Yu P., Wang P., Jiao M., Liu Y., Xu K., Liu X., Yang H. Analysis of the therapeutic effect of transanal endoscopic microsurgery on large rectal adenoma. J. Minimal Access Surg. 2022;18:571. doi: 10.4103/jmas.jmas_273_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coleman O., Haller D. Microbe–Mucus Interface in the Pathogenesis of Colorectal Cancer. Cancers. 2021;13:616. doi: 10.3390/cancers13040616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fanali C., Lucchetti D., Farina M., Corbi M., Cufino V., Cittadini A., Sgambato A. Cancer stem cells in colorectal cancer from pathogenesis to therapy: Controversies and perspectives. World J. Gastroenterol. 2014;20:923–942. doi: 10.3748/wjg.v20.i4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steel M.J., Bukhari H., Gentile L., Telford J., Schaeffer D.F. Colorectal adenocarcinomas diagnosed following a negative faecal immunochemical test show high-risk pathological features in a colon screening programme. Histopathology. 2020;78:710–716. doi: 10.1111/his.14278. [DOI] [PubMed] [Google Scholar]
- 59.Zhang J., Xie X., Wu Z., Hu H., Cai Y., Li J., Ling J., Ding M., Li W., Deng Y. Mucinous Adenocarcinoma Predicts Poor Response and Prognosis in Patients with Locally Advanced Rectal Cancer: A Pooled Analysis of Individual Participant Data From 3 Prospective Studies. Clin. Color. Cancer. 2021;20:e240–e248. doi: 10.1016/j.clcc.2021.06.004. [DOI] [PubMed] [Google Scholar]
- 60.Nitsche U., Zimmermann A., Späth C., Müller T., Maak M., Schuster T., Slotta-Huspenina J., Käser S.A., Michalski C.W., Janssen K.-P., et al. Mucinous and Signet-Ring Cell Colorectal Cancers Differ from Classical Adenocarcinomas in Tumor Biology and Prognosis. Ann. Surg. 2013;258:775–783. doi: 10.1097/SLA.0b013e3182a69f7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu X., Li Y.-Q., Li Q.-G., Ma Y.-L., Peng J.-J., Cai S. Mucinous Adenocarcinomas Histotype Can Also be a High-Risk Factor for Stage II Colorectal Cancer Patients. Cell. Physiol. Biochem. 2018;47:630–640. doi: 10.1159/000490018. [DOI] [PubMed] [Google Scholar]
- 62.Chow J.S., Chen C.C., Ahsan H., Neugut I.A. A Population-Based Study of the Incidence of Malignant Small Bowel Tumours: SEER, 1973–1990. Int. J. Epidemiol. 1996;25:722–728. doi: 10.1093/ije/25.4.722. [DOI] [PubMed] [Google Scholar]
- 63.Pan S.Y., Morrison H. Epidemiology of cancer of the small intestine. World J. Gastrointest. Oncol. 2011;3:33–42. doi: 10.4251/wjgo.v3.i3.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park E.J., Baek J.-H., Choi G.-S., Park W.C., Yu C.S., Kang S.-B., Min B.S., Kim J.H., Kim H.R., Lee B.H., et al. The Role of Primary Tumor Resection in Colorectal Cancer Patients with Asymptomatic, Synchronous, Unresectable Metastasis: A Multicenter Randomized Controlled Trial. Cancers. 2020;12:2306. doi: 10.3390/cancers12082306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Desmond B.J., Dennett E.R., Danielson K.M. Circulating Extracellular Vesicle MicroRNA as Diagnostic Biomarkers in Early Colorectal Cancer—A Review. Cancers. 2019;12:52. doi: 10.3390/cancers12010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Majumdar S.R., Fletcher R.H., Evans A.T. How does colorectal cancer present? Symptoms, duration, and clues to location. Am. J. Gastroenterol. 1999;94:3039–3045. doi: 10.1111/j.1572-0241.1999.01454.x. [DOI] [PubMed] [Google Scholar]
- 67.Ogunwobi O.O., Mahmood F., Akingboye A. Biomarkers in Colorectal Cancer: Current Research and Future Prospects. Int. J. Mol. Sci. 2020;21:5311. doi: 10.3390/ijms21155311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Landegren U., Hammond M. Cancer diagnostics based on plasma protein biomarkers: Hard times but great expectations. Mol. Oncol. 2020;15:1715–1726. doi: 10.1002/1878-0261.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kerr K.M., Bibeau F., Thunnissen E., Botling J., Ryška A., Wolf J., Öhrling K., Burdon P., Malapelle U., Büttner R. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer. 2021;154:161–175. doi: 10.1016/j.lungcan.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 70.Janssens E., van Meerbeeck J.P., Lamote K. Volatile organic compounds in human matrices as lung cancer biomarkers: A sys-tematic review. Crit. Rev. Oncol. Hematol. 2020;153:103037. doi: 10.1016/j.critrevonc.2020.103037. [DOI] [PubMed] [Google Scholar]
- 71.Califf R.M. Biomarker definitions and their applications. Exp. Biol. Med. 2018;243:213–221. doi: 10.1177/1535370217750088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Puntmann O.V. How-to guide on biomarkers: Biomarker definitions, validation and applications with examples from cardiovascular disease. Postgrad. Med. J. 2009;85:538–545. doi: 10.1136/pgmj.2008.073759. [DOI] [PubMed] [Google Scholar]
- 73.Chu G.C.W., Lazare K., Sullivan F. Serum and blood based biomarkers for lung cancer screening: A systematic review. BMC Cancer. 2018;18:181. doi: 10.1186/s12885-018-4024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sun Y.-J., Li A.-W., Zhang Y.-C., Dai J.-H., Zhu X. Evaluation of Urine NDRG1 as Noninvasive Biomarker for Bladder Cancer Diagnosis. Clin. Lab. 2021;67 doi: 10.7754/Clin.Lab.2020.191020. [DOI] [PubMed] [Google Scholar]
- 75.Ministro P., Martins D. Fecal biomarkers in inflammatory bowel disease: How, when and why? Expert Rev. Gastroenterol. Hepatol. 2017;11:317–328. doi: 10.1080/17474124.2017.1292128. [DOI] [PubMed] [Google Scholar]
- 76.Pham Y.L., Beauchamp J. Breath Biomarkers in Diagnostic Applications. Molecules. 2021;26:5514. doi: 10.3390/molecules26185514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Roslund K., Lehto M., Pussinen P., Hartonen K., Groop P.-H., Halonen L., Metsälä M. Identifying volatile in vitro biomarkers for oral bacteria with proton-transfer-reaction mass spectrometry and gas chromatography–mass spectrometry. Sci. Rep. 2021;11:16897. doi: 10.1038/s41598-021-96287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mapar M., Rydzak T., Groves R.A., Lewis I.A. Biomarker enrichment medium: A defined medium for metabolomic analysis of microbial pathogens. Front. Microbiol. 2022;13:957158. doi: 10.3389/fmicb.2022.957158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.DeLouize A.M., Eick G., Karam S.D., Snodgrass J.J. Current and future applications of biomarkers in samples collected through minimally invasive methods for cancer medicine and population-based research. Am. J. Hum. Biol. 2021;34:e23665. doi: 10.1002/ajhb.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alford A.V., Brito J.M., Yadav K.K., Yadav S.S., Tewari A.K., Renzulli J. The Use of Biomarkers in Prostate Cancer Screening and Treatment. Rev. Urol. 2017;19:221–234. doi: 10.3909/riu0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muinao T., Boruah H.P.D., Pal M. Multi-biomarker panel signature as the key to diagnosis of ovarian cancer. Heliyon. 2019;5:e02826. doi: 10.1016/j.heliyon.2019.e02826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Arda E., Arikan G., Akdere H., Akgul M., Yuksel I. Predictive and prognostic impact of preoperative complete blood count based systemic inflammatory markers in testicular cancer. Int. Braz. J. Urol. 2020;46:216–223. doi: 10.1590/s1677-5538.ibju.2018.0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kalinich M., Haber D.A. Cancer detection: Seeking signals in blood. Science. 2018;359:866–867. doi: 10.1126/science.aas9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Paproski R.J., Jovel J., Wong G.K.-S., Lewis J.D., Zemp R.J. Enhanced Detection of Cancer Biomarkers in Blood-Borne Extracellular Vesicles Using Nanodroplets and Focused Ultrasound. Cancer Res. 2017;77:3–13. doi: 10.1158/0008-5472.CAN-15-3231. [DOI] [PubMed] [Google Scholar]
- 85.Ladigan-Badura S., Vangala D.B., Engel C., Bucksch K., Hueneburg R., Perne C., Nattermann J., Steinke-Lange V., Rahner N., Schackert H.K., et al. Value of upper gastrointestinal endoscopy for gastric cancer surveillance in patients with Lynch syndrome. Int. J. Cancer. 2020;148:106–114. doi: 10.1002/ijc.33294. [DOI] [PubMed] [Google Scholar]
- 86.Ma W., Wang K., Nguyen L.H., Joshi A., Cao Y., Nishihara R., Wu K., Ogino S., Giovannucci E.L., Song M., et al. Association of Screening Lower Endoscopy with Colorectal Cancer Incidence and Mortality in Adults Older Than 75 Years. JAMA Oncol. 2021;7:985. doi: 10.1001/jamaoncol.2021.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wellington J., Scott B., Kundu S., Stuart P., Koch K.L. Effect of endoscopic pyloric therapies for patients with nausea and vomiting and functional obstructive gastroparesis. Auton. Neurosci. 2016;202:56–61. doi: 10.1016/j.autneu.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 88.Afonso J., Saraiva M.M., Ferreira J.P.S., Cardoso H., Ribeiro T., Andrade P., Parente M., Jorge R.N., Macedo G. Automated detection of ulcers and erosions in capsule endoscopy images using a convolutional neural network. Med. Biol. Eng. Comput. 2022;60:719–725. doi: 10.1007/s11517-021-02486-9. [DOI] [PubMed] [Google Scholar]
- 89.Gockel I., Hoffmeister A. Endoscopic or Surgical Resection for Gastro-Esophageal. Cancer. 2018;115:513–519. doi: 10.3238/arztebl.2018.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Owaki T., Matsumoto M., Okumura H., Uchicado Y., Kita Y., Setoyama T., Sasaki K., Sakurai T., Omoto I., Shimada M., et al. Endoscopic ultrasonography is useful for monitoring the tumor response of neoadjuvant chemoradiation therapy in esophageal squamous cell carcinoma. Am. J. Surg. 2012;203:191–197. doi: 10.1016/j.amjsurg.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 91.Yamada Y., Arao T., Gotoda T., Taniguchi H., Oda I., Shirao K., Shimada Y., Hamaguchi T., Kato K., Hamano T., et al. Identification of prognostic biomarkers in gastric cancer using endoscopic biopsy samples. Cancer Sci. 2008;99:2193–2199. doi: 10.1111/j.1349-7006.2008.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Shaheed S.-U., Tait C., Kyriacou K., Linforth R., Salhab M., Sutton C. Evaluation of nipple aspirate fluid as a diagnostic tool for early detection of breast cancer. Clin. Proteom. 2018;15:3. doi: 10.1186/s12014-017-9179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sauter E.R., Winn J.N., Dale P.S., Wagner-Mann C. Nipple aspirate fluid color is associated with breast cancer. Cancer Detect. Prev. 2006;30:322–328. doi: 10.1016/j.cdp.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 94.Berenguer C.V., Pereira F., Pereira J.A.M., Câmara J.S. Volatilomics: An Emerging and Promising Avenue for the Detection of Potential Prostate Cancer Biomarkers. Cancers. 2022;14:3982. doi: 10.3390/cancers14163982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ng K., Stenzl A., Sharma A., Vasdev N. Urinary biomarkers in bladder cancer: A review of the current landscape and future directions. Urinary biomarkers in bladder cancer: A review of the current landscape and future directions. Urol. Oncol. 2021;39:41–51. doi: 10.1016/j.urolonc.2020.08.016. [DOI] [PubMed] [Google Scholar]
- 96.Debernardi S., Blyuss O., Rycyk D., Srivastava K., Jeon C.Y., Cai H., Cai Q., Shu X., Crnogorac-Jurcevic T. Urine biomarkers enable pancreatic cancer detection up to 2 years before diagnosis. Int. J. Cancer. 2022 doi: 10.1002/ijc.34287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Njoku K., Chiasserini D., Jones E.R., Barr C.E., O’Flynn H., Whetton A.D., Crosbie E.J. Urinary Biomarkers and Their Potential for the Non-Invasive Detection of Endometrial Cancer. Front. Oncol. 2020;10:559016. doi: 10.3389/fonc.2020.559016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bhat A., Ritch C.R. Urinary biomarkers in bladder cancer: Where do we stand? Curr. Opin. Urol. 2019;29:203–209. doi: 10.1097/MOU.0000000000000605. [DOI] [PubMed] [Google Scholar]
- 99.Batista R., Vinagre N., Meireles S., Vinagre J., Prazeres H., Leão R., Máximo V., Soares P. Biomarkers for Bladder Cancer Diagnosis and Surveillance: A Comprehensive Review. Diagnostics. 2020;10:39. doi: 10.3390/diagnostics10010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiang L., Wu S., Hua Q., Bao C., Liu H. Volatile Organic Compounds in Human Exhaled Breath to Diagnose Gastrointestinal Cancer: A Meta-Analysis. Front. Oncol. 2021;11:606915. doi: 10.3389/fonc.2021.606915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Jia Z., Zhang H., Ong C.N., Patra A., Lu Y., Lim C.T., Venkatesan T. Detection of Lung Cancer: Concomitant Volatile Organic Compounds and Metabolomic Profiling of Six Cancer Cell Lines of Different Histological Origins. ACS Omega. 2018;3:5131–5140. doi: 10.1021/acsomega.7b02035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang J., Tian Y., Luo Z., Qian C., Li W., Duan Y. Breath volatile organic compound analysis: An emerging method for gastric cancer detection. J. Breath Res. 2021;15:044002. doi: 10.1088/1752-7163/ac2cde. [DOI] [PubMed] [Google Scholar]
- 103.Markar S.R., Chin S., Romano A., Wiggins T., Antonowicz S., Paraskeva P., Ziprin P., Darzi A., Hanna G.B. Breath Volatile Organic Compound Profiling of Colorectal Cancer Using Selected Ion Flow-tube Mass Spectrometry. Ann. Surg. 2019;269:903–910. doi: 10.1097/SLA.0000000000002539. [DOI] [PubMed] [Google Scholar]
- 104.Phillips M., Altorki N., Austin J.H., Cameron R.B., Cataneo R.N., Greenberg J., Kloss R., Maxfield R.A., Munawar M.I., Pass H.I., et al. Prediction of lung cancer using volatile biomarkers in breath1. Cancer Biomark. 2007;3:95–109. doi: 10.3233/CBM-2007-3204. [DOI] [PubMed] [Google Scholar]
- 105.Dent A.G., Sutedja T.G., Zimmerman P.V. Exhaled breath analysis for lung cancer. J. Thorac. Dis. 2013;5:S540–S550. doi: 10.3978/j.issn.2072-1439.2013.08.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Phillips M., Cataneo R.N., Lebauer C., Mundada M., Saunders C. Breath mass ion biomarkers of breast cancer. J. Breath Res. 2017;11:016004. doi: 10.1088/1752-7163/aa549b. [DOI] [PubMed] [Google Scholar]
- 107.Krilaviciute A., Heiss J.A., Leja M., Kupcinskas J., Haick H., Brenner H. Detection of cancer through exhaled breath: A systematic review. Oncotarget. 2015;6:38643–38657. doi: 10.18632/oncotarget.5938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Brunmair J., Gotsmy M., Niederstaetter L., Neuditschko B., Bileck A., Slany A., Feuerstein M.L., Langbauer C., Janker L., Zanghellini J., et al. Finger sweat analysis enables short interval metabolic biomonitoring in humans. Nat. Commun. 2021;12:5993. doi: 10.1038/s41467-021-26245-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Brasier N., Eckstein J. Sweat as a Source of Next-Generation Digital Biomarkers. Digit. Biomark. 2019;3:155–165. doi: 10.1159/000504387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hintzen K.F.H., Grote J., Wintjens E.A.G.W., Lubbers T., Eussen M.M.M., van Schooten F.J., Bouvy N.D., Peeters A. Breath analysis for the detection of digestive tract malignancies: Systematic review. BJS Open. 2021;5:zrab013. doi: 10.1093/bjsopen/zrab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Woo S., Atun R., Ward Z., Scott A.M., Hricak H., Vargas H.A. Diagnostic performance of conventional and advanced imaging modalities for assessing newly diagnosed cervical cancer: Systematic review and meta-analysis. Eur. Radiol. 2020;30:5560–5577. doi: 10.1007/s00330-020-06909-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Loke S.Y., Lee A.S.G. The future of blood-based biomarkers for the early detection of breast cancer. Eur. J. Cancer. 2018;92:54–68. doi: 10.1016/j.ejca.2017.12.025. [DOI] [PubMed] [Google Scholar]
- 113.Tsou P.-H., Lin Z.-L., Pan Y.-C., Yang H.-C., Chang C.-J., Liang S.-K., Wen Y.-F., Chang C.-H., Chang L.-Y., Yu K.-L., et al. Exploring Volatile Organic Compounds in Breath for High-Accuracy Prediction of Lung Cancer. Cancers. 2021;13:1431. doi: 10.3390/cancers13061431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kuo T.-C., Tan C.-E., Wang S.-Y., Lin A.O., Su B.-H., Hsu M.-T., Lin J., Cheng Y.-Y., Chen C.-S., Yang Y.-C., et al. Human Breathomics Database. Database. 2020;2020:baz139. doi: 10.1093/database/baz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kim H., Yang J.M., Jin Y., Jheon S., Kim K., Lee C.T., Chung J.-H., Paik J.H. MicroRNA expression profiles and clinicopathological implications in lung adenocarcinoma according to EGFR, KRAS, and ALK status. Oncotarget. 2016;8:8484–8498. doi: 10.18632/oncotarget.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lu S., Kong H., Hou Y., Ge D., Huang W., Ou J., Yang D., Zhang L., Wu G., Song Y., et al. Two plasma microRNA panels for diagnosis and subtype discrimination of lung cancer. Lung Cancer. 2018;123:44–51. doi: 10.1016/j.lungcan.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 117.Marzorati D., Mainardi L., Sedda G., Gasparri R., Spaggiari L., Cerveri P. A review of exhaled breath: A key role in lung cancer diagnosis. J. Breath Res. 2019;13:034001. doi: 10.1088/1752-7163/ab0684. [DOI] [PubMed] [Google Scholar]
- 118.Piqueret B., Bourachot B., Leroy C., Devienne P., Mechta-Grigoriou F., D’Ettorre P., Sandoz J.-C. Ants detect cancer cells through volatile organic compounds. iScience. 2022;25:103959. doi: 10.1016/j.isci.2022.103959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leemans M., Bauër P., Cuzuel V., Audureau E., Fromantin I. Volatile Organic Compounds Analysis as a Potential Novel Screening Tool for Breast Cancer: A Systematic Review. Biomark. Insights. 2022;17:1–19. doi: 10.1177/11772719221100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Naito Y., Uchiyama K., Kinoshita Y., Fukudo S., Joh T., Suzuki H., Takahashi S., Ueno F., Fujiwara Y., Arakawa T., et al. A Questionnaire-Based Survey on Screening for Gastric and Colorectal Cancer by Physicians in East Asian Countries in 2010. Digestion. 2012;86:94–106. doi: 10.1159/000339342. [DOI] [PubMed] [Google Scholar]
- 121.Krist A.H., Davidson K.W., Mangione C.M., Barry M.J., Cabana M., Caughey A.B., Davis E.M., Donahue K.E., Doubeni C.A., Kubik M.T., et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:962–970. doi: 10.1001/jama.2021.1117. [DOI] [PubMed] [Google Scholar]
- 122.Yu B., Hazlewood P.J., Yin X., Li S., Yue H., Xu K., Xu S., Mi Y. Effect of electroacupuncture on discomfort during gastroscopy: A study protocol for a randomized controlled trial. Trials. 2022;23:364. doi: 10.1186/s13063-022-06165-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou J., Huang Z.-A., Kumar U., Chen D.D. Review of recent developments in determining volatile organic compounds in exhaled breath as biomarkers for lung cancer diagnosis. Anal. Chim. Acta. 2017;996:1–9. doi: 10.1016/j.aca.2017.09.021. [DOI] [PubMed] [Google Scholar]
- 124.Brown V.M., Crump D.R., Plant N.T., Pengelly I. Evaluation of the stability of a mixture of volatile organic compounds on sorbents for the determination of emissions from indoor materials and products using thermal desorption/gas chromatography/mass spectrometry. J. Chromatogr. A. 2014;1350:1–9. doi: 10.1016/j.chroma.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 125.Martínez R.A.S., Hernández J.M.P., Terol G.L., Gallego-Jara J., García-Marcos L., Díaz M.C., Puente T.D.D. Data preprocessing workflow for exhaled breath analysis by GC/MS using open sources. Sci. Rep. 2020;10:22008. doi: 10.1038/s41598-020-79014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee J.H., Batterman S.A., Jia C., Chernyak S. Ozone Artifacts and Carbonyl Measurements Using Tenax GR, Tenax TA, Carbopack B, and Carbopack X Adsorbents. J. Air Waste Manag. Assoc. 2006;56:1503–1517. doi: 10.1080/10473289.2006.10464560. [DOI] [PubMed] [Google Scholar]
- 127.Yu Q., Chen J., Fu W., Muhammad K.G., Li Y., Liu W., Xu L., Dong H., Wang D., Liu J., et al. Smartphone-Based Platforms for Clinical Detections in Lung-Cancer-Related Exhaled Breath Biomarkers: A Review. Biosensors. 2022;12:223. doi: 10.3390/bios12040223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lawal O., Ahmed W.M., Nijsen T.M., Goodacre R., Fowler S.J. Exhaled breath analysis: A review of ‘breath-taking’ methods for off-line analysis. Metabolomics. 2017;13:110. doi: 10.1007/s11306-017-1241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Daughtrey E.H., Jr., Oliver K.D., Adams J.R., Kronmiller K.G., Lonneman W.A., McClenny W.A. A comparison of sampling and analysis methods for low-ppbC levels of volatile organic compounds in ambient air. J. Environ. Monit. 2001;3:166–174. doi: 10.1039/b007158g. [DOI] [PubMed] [Google Scholar]
- 130.Altomare D.F., di Lena M., Porcelli F., Trizio L., Travaglio E., Tutino M., Dragonieri S., Memeo V., de Gennaro G. Exhaled volatile organic compounds identify patients with colorectal cancer. Br. J. Surg. 2013;100:144–150. doi: 10.1002/bjs.8942. [DOI] [PubMed] [Google Scholar]
- 131.De Vietro N., Aresta A., Rotelli M.T., Zambonin C., Lippolis C., Picciariello A., Altomare D.F. Relationship between cancer tissue derived and exhaled volatile organic compound from colorectal cancer patients. Preliminary results. J. Pharm. Biomed. Anal. 2019;180:113055. doi: 10.1016/j.jpba.2019.113055. [DOI] [PubMed] [Google Scholar]
- 132.Sun X., Shao K., Wang T. Detection of volatile organic compounds (VOCs) from exhaled breath as noninvasive methods for cancer diagnosis. Anal. Bioanal. Chem. 2015;408:2759–2780. doi: 10.1007/s00216-015-9200-6. [DOI] [PubMed] [Google Scholar]
- 133.Noh B., Youm C., Goh E., Lee M., Park H., Jeon H., Kim O.Y. XGBoost based machine learning approach to predict the risk of fall in older adults using gait outcomes. Sci. Rep. 2021;11:12183. doi: 10.1038/s41598-021-91797-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Paleczek A., Grochala D., Rydosz A. Artificial Breath Classification Using XGBoost Algorithm for Diabetes Detection. Sensors. 2021;21:4187. doi: 10.3390/s21124187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.La Nasa J., Modugno F., Colombini M.P., Degano I. Validation Study of Selected Ion Flow Tube-Mass Spectrometry (SIFT-MS) in Heritage Science: Characterization of Natural and Synthetic Paint Varnishes by Portable Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2019;30:2250–2258. doi: 10.1007/s13361-019-02305-4. [DOI] [PubMed] [Google Scholar]
- 136.Španěl P., Smith D. Progress in SIFT-MS: Breath analysis and other applications. Mass Spectrom. Rev. 2010;30:236–267. doi: 10.1002/mas.20303. [DOI] [PubMed] [Google Scholar]
- 137.Longo V., Forleo A., Ferramosca A., Notari T., Pappalardo S., Siciliano P., Capone S., Montano L. Blood, urine and semen Volatile Organic Compound (VOC) pattern analysis for assessing health environmental impact in highly polluted areas in Italy. Environ. Pollut. 2021;286:117410. doi: 10.1016/j.envpol.2021.117410. [DOI] [PubMed] [Google Scholar]
- 138.Capone S., Tufariello M., Forleo A., Longo V., Giampetruzzi L., Radogna A.V., Casino F., Siciliano P. Chromatographic analysis of VOC patterns in exhaled breath from smokers and nonsmokers. Biomed. Chromatogr. 2017;32:e4132. doi: 10.1002/bmc.4132. [DOI] [PubMed] [Google Scholar]
- 139.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 140.Dima A.C., Balaban D.V., Dima A. Diagnostic Application of Volatile Organic Compounds as Potential Biomarkers for Detecting Digestive Neoplasia: A Systematic Review. Diagnostics. 2021;11:2317. doi: 10.3390/diagnostics11122317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hanna G.B., Boshier P.R., Markar S.R., Romano A. Accuracy and Methodologic Challenges of Volatile Organic Compound-Based Exhaled Breath Tests for Cancer Diagnosis: A Systematic Review and Meta-analysis. JAMA Oncol. 2019;5:e182815. doi: 10.1001/jamaoncol.2018.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Janfaza S., Nojavani M.B., Khorsand B., Nikkhah M., Zahiri J. Cancer Odor Database (COD): A critical databank for cancer diagnosis research. Database. 2017;2017:bax055. doi: 10.1093/database/bax055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Janfaza S., Khorsand B., Nikkhah M., Zahiri J. Digging deeper into volatile organic compounds associated with cancer. Biol. Methods Protoc. 2019;4:bpz014. doi: 10.1093/biomethods/bpz014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mangler M., Freitag C., Lanowska M., Staeck O., Schneider A., Speiser D. Volatile organic compounds (VOCs) in exhaled breath of patients with breast cancer in a clinical setting. Ginekol. Polska. 2012;83:730–736. [PubMed] [Google Scholar]
- 145.Ligor M., Ligor T., Bajtarevic A., Ager C., Pienz M., Klieber M., Denz H., Fiegl M., Hilbe W., Weiss W., et al. Determination of volatile organic compounds in exhaled breath of patients with lung cancer using solid phase microextraction and gas chromatography mass spectrometry. Clin. Chem. Lab. Med. 2009;47:550–560. doi: 10.1515/CCLM.2009.133. [DOI] [PubMed] [Google Scholar]
- 146.Wang P., Huang Q., Meng S., Mu T., Liu Z., He M., Li Q., Zhao S., Wang S., Qiu M. Identification of lung cancer breath biomarkers based on perioperative breathomics testing: A prospective observational study. eClinicalMedicine. 2022;47:101384. doi: 10.1016/j.eclinm.2022.101384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Liu D., Ji L., Li M., Li D., Guo L., Nie M., Wang D., Lv Y., Bai Y., Liu M., et al. Analysis of volatile organic compounds released from SW480 colorectal cancer cells and the blood of tumor-bearing mice. Transl. Cancer Res. 2019;8:2736–2751. doi: 10.21037/tcr.2019.10.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Peng G., Hakim M., Broza Y.Y., Billan S., Abdah-Bortnyak R., Kuten A., Tisch U., Haick H. Detection of lung, breast, colorectal, and prostate cancers from exhaled breath using a single array of nanosensors. Br. J. Cancer. 2010;103:542–551. doi: 10.1038/sj.bjc.6605810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maiti K.S., Fill E., Strittmatter F., Volz Y., Sroka R., Apolonski A. Towards reliable diagnostics of prostate cancer via breath. Sci. Rep. 2021;11:18381. doi: 10.1038/s41598-021-96845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Durán-Acevedo C.M., Jaimes-Mogollón A.L., Gualdrón-Guerrero O.E., Welearegay T.G., Martinez-Marín J.D., Caceres-Tarazona J.M., Acevedo Z.C.S., Beleño-Saenz K.D.J., Cindemir U., Österlund L., et al. Exhaled breath analysis for gastric cancer diagnosis in Colombian patients. Oncotarget. 2018;9:28805–28817. doi: 10.18632/oncotarget.25331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ślefarska-Wolak D., Heinzle C., Leiherer A., Ager C., Muendlein A., Mezmale L., Leja M., Corvalan A., Drexel H., Królicka A., et al. Volatilomic Signatures of AGS and SNU-1 Gastric Cancer Cell Lines. Molecules. 2022;27:4012. doi: 10.3390/molecules27134012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vernia F., Valvano M., Fabiani S., Stefanelli G., Longo S., Viscido A., Latella G. Are Volatile Organic Compounds Accurate Markers in the Assessment of Colorectal Cancer and Inflammatory Bowel Diseases? A Review. Cancers. 2021;13:2361. doi: 10.3390/cancers13102361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Altomare D.F., Picciariello A., Rotelli M.T., De Fazio M., Aresta A., Zambonin C.G., Vincenti L., Trerotoli P., De Vietro N. Chemical signature of colorectal cancer: Case–control study for profiling the breath print. BJS Open. 2020;4:1189–1199. doi: 10.1002/bjs5.50354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ferrari A., Neefs I., Hoeck S., Peeters M., Van Hal G. Towards Novel Non-Invasive Colorectal Cancer Screening Methods: A Comprehensive Review. Cancers. 2021;13:1820. doi: 10.3390/cancers13081820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jung Y.J., Seo H.S., Kim J.H., Song K.Y., Park C.H., Lee H.H. Advanced Diagnostic Technology of Volatile Organic Compounds Real Time analysis Analysis from Exhaled Breath of Gastric Cancer Patients Using Proton-Transfer-Reaction Time-of-Flight Mass Spectrometry. Front. Oncol. 2021;11:560591. doi: 10.3389/fonc.2021.560591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hong Y., Che X., Su H., Mai Z., Huang Z., Huang W., Chen W., Liu S., Gao W., Zhou Z., et al. Exhaled breath analysis using on-line preconcentration mass spectrometry for gastric cancer diagnosis. Biol. Mass Spectrom. 2020;56:e4588. doi: 10.1002/jms.4588. [DOI] [PubMed] [Google Scholar]
- 157.Chin S., Romano A., Doran S.L.F., Hanna G.B. Cross-platform mass spectrometry annotation in breathomics of oesophageal-gastric cancer. Sci. Rep. 2018;8:5139. doi: 10.1038/s41598-018-22890-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Baronzio G., Freitas I., Griffini P., Bertone V., Pacini F., Mascaro G., Razzini E., Gramaglia A. Omega-3 fatty acids can improve radioresponse modifying tumor interstitial pressure, blood rheology and membrane peroxidability. Anticancer Res. 1994;14:1145–1154. [PubMed] [Google Scholar]
- 159.Mika A., Pakiet A., Czumaj A., Kaczynski Z., Liakh I., Kobiela J., Perdyan A., Adrych K., Makarewicz W., Sledzinski T. Decreased Triacylglycerol Content and Elevated Contents of Cell Membrane Lipids in Colorectal Cancer Tissue: A Lipidomic Study. J. Clin. Med. 2020;9:1095. doi: 10.3390/jcm9041095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ayala A., Muñoz M.F., Argüelles S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014;2014:360438. doi: 10.1155/2014/360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Guillen M.D., Goicoechea E. Toxic oxygenated alpha, beta-unsaturated aldehydes and their study in foods: A review. Crit. Rev. Food Sci. Nutr. 2008;48:119–136. doi: 10.1080/10408390601177613. [DOI] [PubMed] [Google Scholar]
- 162.Sutaria S.R., Gori S.S., Morris J.D., Xie Z., Fu X.-A., Nantz M.H. Lipid Peroxidation Produces a Diverse Mixture of Saturated and Unsaturated Aldehydes in Exhaled Breath That Can Serve as Biomarkers of Lung Cancer—A Review. Metabolites. 2022;12:561. doi: 10.3390/metabo12060561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Jelski W., Chrostek L., Szmitkowski M. The Activity of Class I, III, and IV of Alcohol Dehydrogenase Isoenzymes and Aldehyde Dehydrogenase in Gastric Cancer. Am. J. Dig. Dis. 2007;52:531–535. doi: 10.1007/s10620-006-9454-0. [DOI] [PubMed] [Google Scholar]
- 164.Lee N., Spears M.E., Carlisle A.E., Kim D. Endogenous toxic metabolites and implications in cancer therapy. Oncogene. 2020;39:5709–5720. doi: 10.1038/s41388-020-01395-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zakhari S. Overview: How is alcohol metabolized by the body? Alcohol Res. Health. 2006;29:245–254. [PMC free article] [PubMed] [Google Scholar]
- 166.Jelski W., Szmitkowski M. Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) in the cancer diseases. Clin. Chim. Acta. 2008;395:1–5. doi: 10.1016/j.cca.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 167.Jiang Y., Zhang T., Kusumanchi P., Han S., Yang Z., Liangpunsakul S. Alcohol Metabolizing Enzymes, Microsomal Ethanol Oxidizing System, Cytochrome P450 2E1, Catalase, and Aldehyde Dehydrogenase in Alcohol-Associated Liver Disease. Biomedicines. 2020;8:50. doi: 10.3390/biomedicines8030050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mello T., Ceni E., Surrenti C., Galli A. Alcohol induced hepatic fibrosis: Role of acetaldehyde. Mol. Asp. Med. 2008;29:17–21. doi: 10.1016/j.mam.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 169.Park B., Kim J.-H., Lee E.-G., Jung S.-Y., Lee S.Y., Kang H.-S., Han J.H. Role of aldehyde dehydrogenases, alcohol dehydrogenase 1B genotype, alcohol consumption, and their combination in breast cancer in East-Asian women. Sci. Rep. 2020;10:6564. doi: 10.1038/s41598-020-62361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Vasiliou V., Pappa A., Petersen D.R. Role of aldehyde dehydrogenases in endogenous and xenobiotic metabolism. Chem. Interact. 2000;129:1–19. doi: 10.1016/S0009-2797(00)00211-8. [DOI] [PubMed] [Google Scholar]
- 171.Chung J., Huda N., Shin Y., Han S., Akter S., Kang I., Ha J., Choe W., Choi T.G., Kim S.S. Correlation between Oxidative Stress and Transforming Growth Factor-Beta in Cancers. Int. J. Mol. Sci. 2021;22:13181. doi: 10.3390/ijms222413181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mackus M., Van De Loo A.J., Garssen J., Kraneveld A.D., Scholey A., Verster J.C. The Role of Alcohol Metabolism in the Pathology of Alcohol Hangover. J. Clin. Med. 2020;9:3421. doi: 10.3390/jcm9113421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dinavahi S.S., Bazewicz C.G., Gowda R., Robertson G.P. Aldehyde Dehydrogenase Inhibitors for Cancer Therapeutics. Trends Pharmacol. Sci. 2019;40:774–789. doi: 10.1016/j.tips.2019.08.002. [DOI] [PubMed] [Google Scholar]
- 174.Doody E.E., Groebner J.L., Walker J.R., Frizol B.M., Tuma D.J., Fernandez D.J., Tuma P.L. Ethanol metabolism by alcohol dehydrogenase or cytochrome P4502E1 differentially impairs hepatic protein trafficking and growth hormone signaling. Am. J. Physiol. Liver Physiol. 2017;313:G558–G569. doi: 10.1152/ajpgi.00027.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Orywal K., Szmitkowski M. Alcohol dehydrogenase and aldehyde dehydrogenase in malignant neoplasms. Clin. Exp. Med. 2016;17:131–139. doi: 10.1007/s10238-016-0408-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Salaspuro M. Acetaldehyde as a common denominator and cumulative carcinogen in digestive tract cancers. Scand. J. Gastroenterol. 2009;44:912–925. doi: 10.1080/00365520902912563. [DOI] [PubMed] [Google Scholar]
- 177.Xu D., Han H., He Y., Lee H., Wu D., Liu F., Liu X., Liu Y., Lu Y., Ji C. A Hepatocyte-Mimicking Antidote for Alcohol Intoxication. Adv. Mater. 2018;30:e1707443. doi: 10.1002/adma.201707443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lieber C.S. Metabolic effects of acetaldehyde. Biochem. Soc. Trans. 1988;16:241–247. doi: 10.1042/bst0160241. [DOI] [PubMed] [Google Scholar]
- 179.Kazimírová V., Rebroš M. Production of Aldehydes by Biocatalysis. Int. J. Mol. Sci. 2021;22:4949. doi: 10.3390/ijms22094949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Waris S., Patel A., Ali A., Mahmood R. Acetaldehyde-induced oxidative modifications and morphological changes in isolated human erythrocytes: An in vitro study. Environ. Sci. Pollut. Res. 2020;27:16268–16281. doi: 10.1007/s11356-020-08044-4. [DOI] [PubMed] [Google Scholar]
- 181.Inoue K., Fukunaga M., Kiriyama T., Komura S. Accumulation of Acetaldehyde in Alcohol-Sensitive Japanese: Relation to Ethanol and Acetaldehyde Oxidizing Capacity. Alcohol. Clin. Exp. Res. 1984;8:319–322. doi: 10.1111/j.1530-0277.1984.tb05519.x. [DOI] [PubMed] [Google Scholar]
- 182.Weng M.-W., Lee H.-W., Park S.-H., Hu Y., Wang H.-T., Chen L.C., Rom W., Huang W., Lepor H., Wu X.-R., et al. Aldehydes are the predominant forces inducing DNA damage and inhibiting DNA repair in tobacco smoke carcinogenesis. Proc. Natl. Acad. Sci. USA. 2018;115:E6152–E6161. doi: 10.1073/pnas.1804869115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Bosron W.F., Li T.-K. Genetic polymorphism of human liver alcohol and aldehyde dehydrogenases, and their relationship to alcohol metabolism and alcoholism. Hepatology. 1986;6:502–510. doi: 10.1002/hep.1840060330. [DOI] [PubMed] [Google Scholar]
- 184.Chang J.S., Straif K., Guha N. The role of alcohol dehydrogenase genes in head and neck cancers: A systematic review and meta-analysis of ADH1B and ADH1C. Mutagenesis. 2011;27:275–286. doi: 10.1093/mutage/ger073. [DOI] [PubMed] [Google Scholar]
- 185.Edenberg H.J., McClintick J.N. Alcohol Dehydrogenases, Aldehyde Dehydrogenases, and Alcohol Use Disorders: A Critical Review. Alcohol. Clin. Exp. Res. 2018;42:2281–2297. doi: 10.1111/acer.13904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jeong K.-S., Soh Y., Jeng J., Felder M.R., Hardwick J.P., Song B.J. Cytochrome P450 2E1 (CYP2E1)-Dependent Production of a 37-kDa Acetaldehyde–Protein Adduct in the Rat Liver. Arch. Biochem. Biophys. 2000;384:81–87. doi: 10.1006/abbi.2000.2119. [DOI] [PubMed] [Google Scholar]
- 187.Tamura M., Ito H., Matsui H., Hyodo I. Acetaldehyde is an oxidative stressor for gastric epithelial cells. J. Clin. Biochem. Nutr. 2014;55:26–31. doi: 10.3164/jcbn.14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Seitz H.K., Stickel F. Acetaldehyde as an underestimated risk factor for cancer development: Role of genetics in ethanol metabolism. Genes Nutr. 2009;5:121–128. doi: 10.1007/s12263-009-0154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Veith A., Moorthy B. Role of cytochrome P450s in the generation and metabolism of reactive oxygen species. Curr. Opin. Toxicol. 2017;7:44–51. doi: 10.1016/j.cotox.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fang J.L., Vaca C.E. Detection of DNA adducts of acetaldehyde in peripheral white blood cells of alcohol abusers. Carcinogenesis. 1997;18:627–632. doi: 10.1093/carcin/18.4.627. [DOI] [PubMed] [Google Scholar]
- 191.Jung S.-J., Hwang J.-H., Park E.-O., Lee S.-O., Chung Y.-J., Chung M.-J., Lim S., Lim T.-J., Ha Y., Park B.-H., et al. Regulation of Alcohol and Acetaldehyde Metabolism by a Mixture of Lactobacillus and Bifidobacterium Species in Human. Nutrients. 2021;13:1875. doi: 10.3390/nu13061875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bosron W.F., Ehrig T., Li T.-K. Genetic Factors in Alcohol Metabolism and Alcoholism. Semin. Liver Dis. 1993;13:126–135. doi: 10.1055/s-2007-1007344. [DOI] [PubMed] [Google Scholar]
- 193.Seitz H.K., Matsuzaki S., Yokoyama A., Homann N., Väkeväinen S., Wang X.D. Alcohol and cancer. Alcohol Clin. Exp. Res. 2001;25:137S–143S. doi: 10.1111/j.1530-0277.2001.tb02388.x. [DOI] [PubMed] [Google Scholar]
- 194.Yang S.-S., Chen Y.-H., Hu J.-T., Chiu C.-F., Hung S.-W., Chang Y.-C., Chiu C.-C., Chuang H.-L. Aldehyde Dehydrogenase Mutation Exacerbated High-Fat-Diet-Induced Nonalcoholic Fatty Liver Disease with Gut Microbiota Remodeling in Male Mice. Biology. 2021;10:737. doi: 10.3390/biology10080737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vasav A.P., Barvkar V.T. Phylogenomic analysis of cytochrome P450 multigene family and their differential expression analysis in Solanum lycopersicum L. suggested tissue specific promoters. BMC Genom. 2019;20:116. doi: 10.1186/s12864-019-5483-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ghosh A., Sumi M.P., Tupta B., Okamoto T., Aulak K., Tsutsui M., Shimokawa H., Erzurum S.C., Stuehr D.J. Low levels of nitric oxide promotes heme maturation into several hemeproteins and is also therapeutic. Redox Biol. 2022;56:102478. doi: 10.1016/j.redox.2022.102478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Guengerich F.P. Common and Uncommon Cytochrome P450 Reactions Related to Metabolism and Chemical Toxicity. Chem. Res. Toxicol. 2001;14:611–650. doi: 10.1021/tx0002583. [DOI] [PubMed] [Google Scholar]
- 198.Guengerich F.P. Mechanisms of Cytochrome P450-Catalyzed Oxidations. ACS Catal. 2018;8:10964–10976. doi: 10.1021/acscatal.8b03401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Esteves F., Rueff J., Kranendonk M. The Central Role of Cytochrome P450 in Xenobiotic Metabolism—A Brief Review on a Fascinating Enzyme Family. J. Xenobiotics. 2021;11:7. doi: 10.3390/jox11030007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hardwick J.P. Cytochrome P450 omega hydroxylase (CYP4) function in fatty acid metabolism and metabolic diseases. Biochem. Pharmacol. 2008;75:2263–2275. doi: 10.1016/j.bcp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 201.Sarparast M., Dattmore D., Alan J., Lee K.S.S. Cytochrome P450 Metabolism of Polyunsaturated Fatty Acids and Neurodegeneration. Nutrients. 2020;12:3523. doi: 10.3390/nu12113523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Manikandan P., Nagini S. Cytochrome P450 Structure, Function and Clinical Significance: A Review. Curr. Drug Targets. 2018;19:38–54. doi: 10.2174/1389450118666170125144557. [DOI] [PubMed] [Google Scholar]
- 203.Omura T., Gotoh O. Evolutionary origin of mitochondrial cytochrome P450. J. Biochem. 2017;161:399–407. doi: 10.1093/jb/mvx011. [DOI] [PubMed] [Google Scholar]
- 204.Wang T.-S., Lin C.-P., Chen Y.-P., Chao M.-R., Li C.-C., Liu K.-L. CYP450-mediated mitochondrial ROS production involved in arecoline N -oxide-induced oxidative damage in liver cell lines. Environ. Toxicol. 2018;33:1029–1038. doi: 10.1002/tox.22588. [DOI] [PubMed] [Google Scholar]
- 205.Modugno F., Knoll C., Kanbour-Shakir A., Romkes M. A Potential Role for the Estrogen-metabolizing Cytochrome P450 Enzymes in Human Breast Carcinogenesis. Breast Cancer Res. Treat. 2003;82:191–197. doi: 10.1023/B:BREA.0000004376.21491.44. [DOI] [PubMed] [Google Scholar]
- 206.Guengerich F.P., Avadhani N.G. Roles of Cytochrome P450 in Metabolism of Ethanol and Carcinogens. Adv. Exp. Med. Biol. 2018;1032:15–35. doi: 10.1007/978-3-319-98788-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pillai V.C., Snyder O.R., Gumaste U., Thekkumkara T.J., Mehvar R. Effects of transient overexpression or knockdown of cytochrome P450 reductase on reactive oxygen species generation and hypoxia reoxygenation injury in liver cells. Clin. Exp. Pharmacol. Physiol. 2011;38:846–853. doi: 10.1111/j.1440-1681.2011.05622.x. [DOI] [PubMed] [Google Scholar]
- 208.Qi Y., Toyooka T., Kashiwagi H., Yanagiba Y., Koda S., Ohta H., Wang R.-S. 2,4-Dimethylaniline generates phosphorylated histone H2AX in human urothelial and hepatic cells through reactive oxygen species produced by cytochrome P450 2E1. Arch. Toxicol. 2018;92:3093–3101. doi: 10.1007/s00204-018-2289-6. [DOI] [PubMed] [Google Scholar]
- 209.Gonzalez F.J. Role of cytochromes P450 in chemical toxicity and oxidative stress: Studies with CYP2E1. Mutat. Res. Mol. Mech. Mutagen. 2005;569:101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 210.Wang C., Gao N., Yang L., Guo Y., Fang Y., Wang T., Xu C., Li G.f., Zhou J., Zhang Y., et al. Stat4 rs7574865 polymorphism promotes the occurrence and progression of hepatocellular carcinoma via the Stat4/CYP2E1/FGL2 pathway. Cell Death Dis. 2022;13:130. doi: 10.1038/s41419-022-04584-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ali I., Shah S.Z.A., Jin Y., Li Z.-S., Ullah O., Fang N.-Z. Reactive oxygen species-mediated unfolded protein response pathways in preimplantation embryos. J. Vet. Sci. 2017;18:1–9. doi: 10.4142/jvs.2017.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chang K.-C., Liu P.-F., Chang C.-H., Lin Y.-C., Chen Y.-J., Shu C.-W. The interplay of autophagy and oxidative stress in the pathogenesis and therapy of retinal degenerative diseases. Cell Biosci. 2022;12:1. doi: 10.1186/s13578-021-00736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Matic I. The major contribution of the DNA damage-triggered reactive oxygen species production to cell death: Implications for antimicrobial and cancer therapy. Curr. Genet. 2017;64:567–569. doi: 10.1007/s00294-017-0787-3. [DOI] [PubMed] [Google Scholar]
- 214.Kumari S., Badana A.K., Murali M.G., Shailender G., Malla R. Reactive Oxygen Species: A Key Constituent in Cancer Survival. Biomark. Insights. 2018;13:1177271918755391. doi: 10.1177/1177271918755391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Snezhkina A.V., Kudryavtseva A.V., Kardymon O.L., Savvateeva M.V., Melnikova N.V., Krasnov G.S., Dmitriev A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Med. Cell. Longev. 2019;2019:6175804. doi: 10.1155/2019/6175804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Filomeni G., de Zio D., Cecconi F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015;22:377–388. doi: 10.1038/cdd.2014.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., Castoria G., Migliaccio A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Aguiar P.H.N., Furtado C., Repolês B.M., Ribeiro G.A., Mendes I.C., Peloso E.F., Gadelha F.R., Macedo A.M., Franco G.R., Pena S.D.J., et al. Oxidative Stress and DNA Lesions: The Role of 8-Oxoguanine Lesions in Trypanosoma cruzi Cell Viability. PLoS Negl. Trop. Dis. 2013;7:e2279. doi: 10.1371/journal.pntd.0002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Srinivas U.S., Tan B.W.Q., Vellayappan B.A., Jeyasekharan A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019;25:101084. doi: 10.1016/j.redox.2018.101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nakamura H., Takada K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021;112:3945–3952. doi: 10.1111/cas.15068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stubbs B.J., Cox P.J., Evans R.D., Santer P., Miller J.J., Faull O.K., Magor-Elliott S., Hiyama S., Stirling M., Clarke K. On the Metabolism of Exogenous Ketones in Humans. Front. Physiol. 2017;8:848. doi: 10.3389/fphys.2017.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Broza Y.Y., Kremer R., Tisch U., Gevorkyan A., Shiban A., Best L.A., Haick H. A nanomaterial-based breath test for short-term follow-up after lung tumor resection. Nanomed. Nanotechnol. Biol. Med. 2013;9:15–21. doi: 10.1016/j.nano.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 223.Carley A.N., Maurya S.K., Fasano M., Wang Y., Selzman C.H., Drakos S.G., Lewandowski E.D. Short-Chain Fatty Acids Outpace Ketone Oxidation in the Failing Heart. Circulation. 2021;143:1797–1808. doi: 10.1161/CIRCULATIONAHA.120.052671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Adeva-Andany M.M., Carneiro-Freire N., Seco-Filgueira M., Fernández-Fernández C., Mouriño-Bayolo D. Mitochondrial beta-oxidation of saturated fatty acids in humans. Mitochondrion. 2019;46:73–90. doi: 10.1016/j.mito.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 225.Van der Sluis R., Erasmus E. Xenobiotic/medium chain fatty acid: CoA ligase—A critical review on its role in fatty acid metabolism and the detoxification of benzoic acid and aspirin. Expert Opin. Drug Metab. Toxicol. 2016;12:1169–1179. doi: 10.1080/17425255.2016.1206888. [DOI] [PubMed] [Google Scholar]
- 226.Grabacka M., Pierzchalska M., Dean M., Reiss K. Regulation of Ketone Body Metabolism and the Role of PPARalpha. Int. J. Mol. Sci. 2016;17:2093. doi: 10.3390/ijms17122093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Longo R., Peri C., Cricrì D., Coppi L., Caruso D., Mitro N., de Fabiani E., Crestani M. Ketogenic Diet: A New Light Shining on Old but Gold Biochemistry. Nutrients. 2019;11:2497. doi: 10.3390/nu11102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Feng S., Wang H., Liu J., Aa J., Zhou F., Wang G. Multi-dimensional roles of ketone bodies in cancer biology: Opportunities for cancer therapy. Pharmacol. Res. 2019;150:104500. doi: 10.1016/j.phrs.2019.104500. [DOI] [PubMed] [Google Scholar]
- 229.Hegardt F.G. Mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase: A control enzyme in ketogenesis. Biochem. J. 1999;338:569–582. doi: 10.1042/bj3380569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cotter D.G., Schugar R.C., Crawford P.A. Ketone body metabolism and cardiovascular disease. Am. J. Physiol. Circ. Physiol. 2013;304:H1060–H1076. doi: 10.1152/ajpheart.00646.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Noebels J.L., Avoli M., Rogawski M.A., Olsen R.W., Delgado-Escueta A.V. Jasper’s Basic Mechanisms of the Epilepsies. National Center for Biotechnology Information; Bethesda, MD, USA: 2012. [PubMed] [Google Scholar]
- 232.Puchalska P., Crawford P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017;25:262–284. doi: 10.1016/j.cmet.2016.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Olson K.R., Gao Y. Effects of inhibiting antioxidant pathways on cellular hydrogen sulfide and polysulfide metabolism. Free Radic. Biol. Med. 2019;135:1–14. doi: 10.1016/j.freeradbiomed.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 234.Halestrap A.P. The monocarboxylate transporter family-Structure and functional characterization. IUBMB Life. 2011;64:1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 235.Halestrap A.P., Wilson M.C. The monocarboxylate transporter family-Role and regulation. IUBMB Life. 2011;64:109–119. doi: 10.1002/iub.572. [DOI] [PubMed] [Google Scholar]
- 236.Mazzone P.J. Analysis of Volatile Organic Compounds in the Exhaled Breath for the Diagnosis of Lung Cancer. J. Thorac. Oncol. 2008;3:774–780. doi: 10.1097/JTO.0b013e31817c7439. [DOI] [PubMed] [Google Scholar]
- 237.Bondoc F.Y., Bao Z., Hu W.-Y., Gonzalez F.J., Wang Y., Yang C.S., Hong J.-Y. Acetone catabolism by cytochrome P450 2E1: Studies with CYP2E1-null mice. Biochem. Pharmacol. 1999;58:461–463. doi: 10.1016/S0006-2952(99)00111-2. [DOI] [PubMed] [Google Scholar]
- 238.You I., Kim E.B. Genome-based species-specific primers for rapid identification of six species of Lactobacillus acidophilus group using multiplex PCR. PLoS ONE. 2020;15:e0230550. doi: 10.1371/journal.pone.0230550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ruzsányi V., Kalapos M.P. Breath acetone as a potential marker in clinical practice. J. Breath Res. 2017;11:024002. doi: 10.1088/1752-7163/aa66d3. [DOI] [PubMed] [Google Scholar]
- 240.Edwards J.E., Rose R.L., Hodgson E. The metabolism of nonane, a JP-8 jet fuel component, by human liver microsomes, P450 isoforms and alcohol dehydrogenase and inhibition of human P450 isoforms by JP-8. Chem. Interactions. 2005;151:203–211. doi: 10.1016/j.cbi.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 241.Leung T., Rajendran R., Singh S., Garva R., Krstic-Demonacos M., Demonacos C. Cytochrome P450 2E1 (CYP2E1) regulates the response to oxidative stress and migration of breast cancer cells. Breast Cancer Res. 2013;15:R107. doi: 10.1186/bcr3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Thomas R.D., Green M.R., Wilson C., Weckle A.L., Duanmu Z., Kocarek T.A., Runge-Morris M. Cytochrome P450 expression and metabolic activation of cooked food mutagen 2-amino-1-methyl-6-phenylimidazo [4,5-b]pyridine (PhIP) in MCF10A breast epithelial cells. Chem. Biol. Interact. 2006;160:204–216. doi: 10.1016/j.cbi.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 243.Wang C., Sun B., Guo L., Wang X., Ke C., Liu S., Zhao W., Luo S., Guo Z., Zhang Y., et al. Volatile Organic Metabolites Identify Patients with Breast Cancer, Cyclomastopathy and Mammary Gland Fibroma. Sci. Rep. 2014;4:5383. doi: 10.1038/srep05383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Mochalski P., Sponring A., King J., Unterkofler K., Troppmair J., Amann A. Release and uptake of volatile organic compounds by human hepatocellular carcinoma cells (HepG2) in vitro. Cancer Cell Int. 2013;13:72–79. doi: 10.1186/1475-2867-13-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ratiu I.A., Ligor T., Bocos-Bintintan V., Mayhew A.C., Buszewski B. Volatile Organic Compounds in Exhaled Breath as Fingerprints of Lung Cancer, Asthma and COPD. J. Clin. Med. 2020;10:32. doi: 10.3390/jcm10010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Van der Schee M., Pinheiro H., Gaude E. Breath biopsy for early detection and precision medicine in cancer. Ecancermedicalscience. 2018;12:ed84. doi: 10.3332/ecancer.2018.ed84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Haick H., Broza Y.Y., Mochalski P., Ruzsanyi V., Amann A. Assessment, origin, and implementation of breath volatile cancer markers. Chem. Soc. Rev. 2013;43:1423–1449. doi: 10.1039/C3CS60329F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Silva C.L., Passos M., Câmara J.S. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br. J. Cancer. 2011;105:1894–1904. doi: 10.1038/bjc.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Arasaradnam R.P., McFarlane M.J., Ryan-Fisher C., Westenbrink E., Hodges P., Thomas M.G., Chambers S., O’Connell N., Bailey C., Harmston C., et al. Detection of Colorectal Cancer (CRC) by Urinary Volatile Organic Compound Analysis. PLoS ONE. 2014;9:e108750. doi: 10.1371/journal.pone.0108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Mochalski P., Leja M., Gasenko E., Skapars R., Santare D., Sivins A., Aronsson D.E., Ager C., Jaeschke C., Shani G., et al. Ex vivo emission of volatile organic compounds from gastric cancer and non-cancerous tissue. J. Breath Res. 2018;12:046005. doi: 10.1088/1752-7163/aacbfb. [DOI] [PubMed] [Google Scholar]
- 251.Gosho M., Nagashima K., Sato Y. Study Designs and Statistical Analyses for Biomarker Research. Sensors. 2012;12:8966–8986. doi: 10.3390/s120708966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Van Keulen K.E., Jansen M.E., Schrauwen R.W., Kolkman J.J., Siersema P.D. Volatile organic compounds in breath can serve as a non-invasive diagnostic biomarker for the detection of advanced adenomas and colorectal cancer. Aliment. Pharmacol. Ther. 2020;51:334–346. doi: 10.1111/apt.15622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Xiang W., Wang R., Bai D., Yu T.-H., Chen X.-Z., on behalf of the SIGES Research Groups Helicobacter Pylori Related Gastric Cancer Screening and Cost-Effectiveness Analysis: A Hospital-Based Cross-Sectional Study (SIGES) Nutr. Cancer. 2022;74:2769–2778. doi: 10.1080/01635581.2021.2022168. [DOI] [PubMed] [Google Scholar]
- 254.Selleck M.J., Senthil M., Wall N.R. Making Meaningful Clinical Use of Biomarkers. Biomark. Insights. 2017;12:1–7. doi: 10.1177/1177271917715236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Stenman U.H. Biomarker development, from bench to bedside. Crit. Rev. Clin. Lab. Sci. 2016;53:69–86. doi: 10.3109/10408363.2015.1075468. [DOI] [PubMed] [Google Scholar]
- 256.Hampel H., Lista S., Khachaturian Z.S. Development of biomarkers to chart all Alzheimer’s disease stages: The royal road to cutting the therapeutic Gordian Knot. Alzheimer’s Dement. 2012;8:312–336. doi: 10.1016/j.jalz.2012.05.2116. [DOI] [PubMed] [Google Scholar]
- 257.Pitkänen A., Löscher W., Vezzani A., Becker A.J., Simonato M., Lukasiuk K., Gröhn O., Bankstahl J.P., Friedman A., Aronica E., et al. Advances in the development of biomarkers for epilepsy. Lancet Neurol. 2016;15:843–856. doi: 10.1016/S1474-4422(16)00112-5. [DOI] [PubMed] [Google Scholar]
- 258.Schapira A.H. Recent developments in biomarkers in Parkinson disease. Curr. Opin. Neurol. 2013;26:395–400. doi: 10.1097/WCO.0b013e3283633741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lewis D.R., Siembida E.J., Seibel N.L., Smith A.W., Mariotto A.B. Survival outcomes for cancer types with the highest death rates for adolescents and young adults, 1975–2016. Cancer. 2021;127:4277–4286. doi: 10.1002/cncr.33793. [DOI] [PubMed] [Google Scholar]
- 260.Huang H.-Y., Shi J.-F., Guo L.-W., Bai Y.-N., Liao X.-Z., Liu G.-X., Mao A.-Y., Ren J.-S., Sun X.-J., Zhu X.-Y., et al. Expenditure and financial burden for the diagnosis and treatment of colorectal cancer in China: A hospital-based, multicenter, cross-sectional survey. Chin. J. Cancer. 2017;36:41. doi: 10.1186/s40880-017-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Henderson R.H., French D., Maughan T., Adams R., Allemani C., Minicozzi P., Coleman M.P., McFerran E., Sullivan R., Lawler M. The economic burden of colorectal cancer across Europe: A population-based cost-of-illness study. Lancet Gastroenterol. Hepatol. 2021;6:709–722. doi: 10.1016/S2468-1253(21)00147-3. [DOI] [PubMed] [Google Scholar]
- 262.Rice D.R., Farooq A., Hyer J.M., Paredes A.Z., Bae J., Tsilimigras D.I., Pawlik T.M. Health expenditures and financial burden among patients with major gastrointestinal cancers relative to other common cancers in the United States. Surgery. 2020;167:985–990. doi: 10.1016/j.surg.2020.02.029. [DOI] [PubMed] [Google Scholar]
- 263.Subramoniam V.A.B.M., Mathew L. Detection of COPD and Lung Cancer with electronic nose using ensemble learning methods. Clin. Chim. Acta. 2021;523:231–238. doi: 10.1016/j.cca.2021.10.005. [DOI] [PubMed] [Google Scholar]
- 264.Španěl P., Smith D. Quantification of volatile metabolites in exhaled breath by selected ion flow tube mass spectrometry, SIFT-MS. Clin. Mass Spectrom. 2020;16:18–24. doi: 10.1016/j.clinms.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Pauling L., Robinson A.B., Teranishi R., Cary P. Quantitative Analysis of Urine Vapor and Breath by Gas-Liquid Partition Chromatography. Proc. Natl. Acad. Sci. USA. 1971;68:2374–2376. doi: 10.1073/pnas.68.10.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Ferraris V.A., Information P.E.K.F.C. What do dogs, ancient Romans, Linus Pauling, and mass spectrometry have in common? Early lung cancer and exhaled breath. J. Thorac. Cardiovasc. Surg. 2015;151:313–314. doi: 10.1016/j.jtcvs.2015.09.120. [DOI] [PubMed] [Google Scholar]
- 267.Brooks S.W., Moore D.R., Marzouk E.B., Glenn F.R., Hallock R. Canine Olfaction and Electronic Nose Detection of Volatile Organic Compounds in the Detection of Cancer: A Review. Cancer Investig. 2015;33:411–419. doi: 10.3109/07357907.2015.1047510. [DOI] [PubMed] [Google Scholar]
- 268.Janfaza S., Nojavani M.B., Nikkhah M., Alizadeh T., Esfandiar A., Ganjali M.R. A selective chemiresistive sensor for the cancer-related volatile organic compound hexanal by using molecularly imprinted polymers and multiwalled carbon nanotubes. Mikrochim. Acta. 2019;186:137. doi: 10.1007/s00604-019-3241-z. [DOI] [PubMed] [Google Scholar]
- 269.Dung T.T., Oh Y., Choi S.-J., Kim I.-D., Oh M.-K., Kim M. Applications and Advances in Bioelectronic Noses for Odour Sensing. Sensors. 2018;18:103. doi: 10.3390/s18010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Misra R., Acharya S., Sushmitha N. Nanobiosensor-based diagnostic tools in viral infections: Special emphasis on COVID-19. Rev. Med. Virol. 2021;32:e2267. doi: 10.1002/rmv.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Finamore P., Pedone C., Lelli D., Costanzo L., Bartoli I.R., De Vincentis A., Grasso S., Parente F.R., Pennazza G., Santonico M., et al. Analysis of volatile organic compounds: An innovative approach to heart failure characterization in older patients. J. Breath Res. 2018;12:026007. doi: 10.1088/1752-7163/aa8cd4. [DOI] [PubMed] [Google Scholar]
- 272.Heaney L.M., Kang S., Turner M.A., Lindley M.R., Thomas C.L.P. The Impact of a Graded Maximal Exercise Protocol on Exhaled Volatile Organic Compounds: A Pilot Study. Molecules. 2022;27:370. doi: 10.3390/molecules27020370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Blanchet L., Smolinska A., Baranska A., Tigchelaar E., Swertz M., Zhernakova A., Dallinga J.W., Wijmenga C., van Schooten F.J. Factors that influence the volatile organic compound content in human breath. J. Breath Res. 2017;11:016013. doi: 10.1088/1752-7163/aa5cc5. [DOI] [PubMed] [Google Scholar]
- 274.Nagamine K., Mineta D., Ishida K., Katayama K., Kondo T. Mixed effects of moderate exercise and subsequent various food ingestion on breath acetone. J. Breath Res. 2023;17:016004. doi: 10.1088/1752-7163/ac9ed4. [DOI] [PubMed] [Google Scholar]
- 275.Smith D., Wang T., Spanĕl P. On-line, simultaneous quantification of ethanol, some metabolites and water vapour in breath following the ingestion of alcohol. Physiol. Meas. 2002;23:477–489. doi: 10.1088/0967-3334/23/3/301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.