Background.
Compared with calcineurin inhibitor–based immunosuppression, belatacept (BELA)-based treatment has been associated with better renal function but higher acute rejection rates. This phase 2 study (NCT02137239) compared the antirejection efficacy of BELA plus everolimus (EVL) with tacrolimus (TAC) plus mycophenolate mofetil (MMF), each following lymphocyte-depleting induction and rapid corticosteroid withdrawal.
Methods.
Patients who were de novo renal transplant recipients seropositive for Epstein-Barr virus were randomized to receive BELA+EVL or TAC+MMF maintenance therapy after rabbit antithymocyte globulin induction and up to 7 d of corticosteroids. The primary endpoint was the rate of biopsy-proven acute rejection at month 6.
Results.
Because of an unanticipated BELA supply constraint, enrollment was prematurely terminated at 68 patients, of whom 58 were randomized and transplanted (intention-to-treat [ITT] population: n = 26, BELA+EVL; n = 32, TAC+MMF). However, 25 patients received BELA+EVL‚ and 33 received TAC+MMF (modified ITT population). In the ITT population, the 6-mo biopsy-proven acute rejection rates were 7.7% versus 9.4% in the BELA+EVL versus TAC+MMF group. The corresponding 24-mo biopsy-proven acute rejection rates were 19.2% versus 12.5% in the ITT population and 16.0% versus 15.2% in the mITT population; all events were Banff severity grade ≤IIA and similar between groups. One patient in each group experienced graft loss unrelated to acute rejection. The 24-mo mean unadjusted estimated glomerular filtration rates were 71.8 versus 68.7 mL/min/1.73 m2 in the BELA+EVL versus TAC+MMF groups. Posttransplant lymphoproliferative disorder was reported for 1 patient in each group. No deaths or unexpected adverse events were observed.
Conclusions.
A steroid-free maintenance regimen of BELA+EVL may be associated with biopsy-proven acute rejection rates comparable to TAC+MMF.
Calcineurin inhibitor (CNI; tacrolimus [TAC] or cyclosporine)–based regimens are the standard of care for maintenance immunosuppression following kidney transplantation, usually in conjunction with an antimetabolite such as mycophenolate mofetil (MMF) or mycophenolate sodium, with or without corticosteroids.1,2 The use of CNI-based regimens has reduced acute rejection rates in the first year after transplant. However, CNIs are associated with nephrotoxicity that may limit long-term allograft survival,2,3 as well as increase the risk of cardiovascular disease (hypertension, dyslipidemia), posttransplant diabetes, and neurotoxicity.4 Additionally, long-term corticosteroid treatment is linked to posttransplant diabetes, cataracts, osteoporosis, and an increased risk of cardiovascular events.5,6 Consequently, CNI- and corticosteroid-sparing immunosuppressive strategies remain an area of continued interest in renal transplantation.
Belatacept (BELA) is a modified immunoglobulin (IgG1) cytotoxic T-lymphocyte–associated antigen 4 fusion protein that selectively blocks the CD28–CD80/86 costimulation pathway, resulting in inhibition of T-cell activation and proliferation.7 In 2011, BELA was approved in combination with basiliximab induction, MMF, and corticosteroids as maintenance therapy for the prophylaxis of acute rejection in Epstein-Barr virus (EBV)–seropositive patients receiving a renal transplant. In the phase 3 BENEFIT and BENEFIT-EXT trials,8-10 renal transplant recipients treated with BELA had improved cardiovascular and metabolic profiles, reduced incidence of chronic allograft nephropathy at 12 mo, and significantly higher levels of renal function that were sustained through 36 mo after transplant compared with those treated with cyclosporine.9-12 Patients treated with BELA also had a reduced incidence of de novo anti-HLA donor-specific antibody formation than patients treated with cyclosporine.13,14 Patient and graft survival rates were comparable between treatment arms through 36 mo after transplant.15,16 Among recipients of kidneys from living or standard-criteria deceased donors in the long-term follow-up of the BENEFIT study, BELA treatment resulted in a significantly higher rate of survival with a functioning graft at 60 and 84 mo after transplant than cyclosporine treatment.9-12,14 However, numerically higher biopsy-proven acute rejection rates and higher Banff severity grades of acute cellular rejection were observed among patients treated with BELA in both BENEFIT and BENEFIT-EXT, primarily during the first 6 mo after transplant. At 12 mo after transplant, acute rejection rates for BELA versus cyclosporine were 17% versus 7% in BENEFIT and 18% versus 14% in BENEFIT-EXT.9,10
These higher acute rejection rates have led to the exploration of alternative BELA-based regimens. Exploratory analyses and independent investigator-led studies revealed that highly differentiated CD57+/CD28− or CD38+/CD28− memory T cells refractory to costimulation blockade likely contribute to the higher early acute rejection rates observed with BELA.17,18 Mechanistic target of rapamycin inhibitors (mTORis) such as sirolimus (SRL) or everolimus (EVL), when combined with BELA, have been shown to inhibit the proliferation of CD8+/CD28−/CD38+ memory T cells refractory to costimulatory blockade, which may explain the lower acute rejection rates observed with these immunosuppressive combinations.18-20 Use of a T- or B-cell–depleting induction agent such as rabbit antithymocyte globulin (rATG; Thymoglobulin) has also been shown to be more effective in preventing rejection than basiliximab, a non–lymphocyte-depleting monoclonal antibody that blocks T-cell activation through CD25.21
In a phase 2 prospective multicenter study, renal transplant recipients received rATG induction followed by a rapid corticosteroid withdrawal and 1 of 3 regimens: BELA+SRL, BELA+MMF, or TAC+MMF.22 Acute rejection rates at 12 mo were lower with BELA+SRL (4%) and TAC+MMF (3%) than with BELA+MMF (15%).22 Additional analyses from this phase 2 trial and the phase 3 BENEFIT trial showed a higher proportion of circulating regulatory T cells (thought to contribute to greater immunologic tolerance) in patients treated with BELA+SRL compared with BELA+MMF or TAC+MMF.23 These data formed the rationale for further exploration of the antirejection efficacy of combination treatment with BELA and an mTORi in the present phase 2 clinical trial, which was designed to evaluate acute rejection rates in patients receiving rATG induction and rapid corticosteroid withdrawal followed by maintenance treatment with either BELA+EVL or TAC+MMF.
MATERIALS AND METHODS
Study Design
In this prospective, randomized, multicenter, parallel-group, phase 2 study (NCT02137239), patients were randomly assigned in a 1 to 1 ratio, stratified by the study site/center, to receive BELA+EVL or TAC+MMF as maintenance immunosuppression, following induction with rATG and corticosteroids (Figure S1, SDC, http://links.lww.com/TXD/A480). The planned study duration was 24 mo, with an 8-wk follow-up period for safety evaluation after the last dose of study treatment. Patients treated with BELA who discontinued treatment or completed the study and did not continue treatment with BELA after the study were seen 12 and 24 wks after the last BELA dose to test for the development of anti-BELA antibodies.
This study was conducted according to the ethical principles of the Declaration of Helsinki, the Declaration of Istanbul, and the Transplantation Society, and the trial protocol (IM103177) was approved by the institutional review board or independent ethics committee at each participating site (Table S1, SDC, http://links.lww.com/TXD/A480). All patients provided written informed consent before enrollment.
Patients and Treatment
Adult patients aged 18 to 75 y who were EBV seropositive and scheduled to receive a renal allograft from a living or deceased standard-criteria donor were enrolled. Patients at low-to-moderate immunologic risk were eligible, whereas those with ≥20% panel reactive antibodies, a positive T-cell lymphocytotoxic antibody crossmatch, or history of previous graft loss because of rejection were excluded. Patients who were cytomegalovirus negative and scheduled to receive a kidney from a cytomegalovirus-positive donor, recipients of a kidney from an extended-criteria donor or one donated following cardiac death, and patients who were EBV seronegative or whose EBV serostatus was unknown were also excluded.
All patients received rATG 1.5 mg/kg intravenously (IV) on the day of transplant, beginning intraoperatively before vascular reperfusion of the allograft, and then daily for 3 to 10 d (as tolerated) to reach a total cumulative dose of 3.0 to 5.5 mg/kg. All rATG doses were preceded by premedication with methylprednisolone IV. Patients in the BELA+EVL arm received the first infusion of BELA (10 mg/kg IV) on study day 1 between 12 and 24 h after completion of the initial rATG infusion. Thereafter, BELA was administered at 10 mg/kg IV on days 5, 14, 29, 43, 57, 71, and 84, followed by 5 mg/kg IV every 4 wks until trial completion. EVL was administered at an initial dose of 3.0 mg/d (1.5 mg twice daily) starting on day 3, with subsequent dosing adjusted to achieve whole blood trough concentrations of 6 to10 ng/mL for the first 3 mo after transplantation and 4 to 8 ng/mL thereafter. At the investigator’s discretion, initiation of EVL therapy could be deferred up to day 14. Patients in the TAC+MMF arm were given an initial dose of TAC, 0.1 mg/kg/d orally in 2 divided doses, after which the dose was adjusted to achieve whole blood trough concentrations of 4 to 11 ng/mL. MMF was administered, orally or IV, at a dose of 0.5 to 2.0 g/d (0.25–1.0 g twice daily) as tolerated.
All patients received methylprednisolone IV in tapering doses, beginning with 500 mg on day 1, 250 mg on day 2, and 125 mg on day 3, followed by oral corticosteroids equivalent to 60 mg prednisone on day 4. Oral corticosteroids were rapidly tapered and discontinued by day 7. However, patients still receiving rATG between days 8 and 10 continued to receive a single dose of methylprednisolone IV as prophylaxis before administration of each dose of rATG.
Endpoints
The primary endpoint was the rate of clinically suspected and biopsy-proven acute rejection at 6 mo after transplant. Secondary objectives included the severity of biopsy-proven acute rejection at 12 and 24 mo after transplant; rates of patient and graft survival at 6, 12, and 24 mo after transplant; renal function, as determined by estimated glomerular filtration rate (eGFR) per the 4-variable Modification of Diet in Renal Disease study equation24 and from the degree of proteinuria (calculated as the urinary protein-to-creatinine ratio from random-voided urine specimens collected at 3, 6, 12, and 24 mo); the rate of de novo donor-specific antibody formation at 12 and 24 mo; and safety and tolerability.
Diagnosis of acute rejection was based on clinical suspicion confirmed by biopsy findings consistent with cellular- or antibody-mediated acute rejection using the Banff classification of renal allograft pathology.25,26 Per protocol, clinical suspicion was based on the presence of ≥1 of the following: an unexplained increase in serum creatinine concentration ≥25% from baseline values, decreased urine output, fever or allograft tenderness, or clinically suspected acute rejection for any other reason.
Statistical Considerations
A total of 240 patients were targeted for enrollment, but enrollment was prematurely discontinued on December 31, 2016, because of an unanticipated BELA manufacturing–related supply constraint (owing to the transition to a novel manufacturing process). However, even at the originally targeted enrollment of 240 patients, this exploratory study was not designed to be sufficiently powered to demonstrate statistically significant treatment differences for any outcome measure.
The primary endpoint was assessed in the intention-to-treat (ITT) population, defined as all patients who were randomized, transplanted, and received ≥1 dose of rATG and ≥1 dose of study treatment (BELA or TAC), as well as by the subgroup (donor condition [living versus deceased] and recipient sex, race, and presence of diabetes). Safety outcomes were assessed in the modified ITT (mITT) population, whereby patients were grouped according to the treatment they actually received, regardless of randomization.
Biopsy-proven acute rejection rates were summarized by treatment group at 6, 12, and 24 mo after transplant using point estimates (and 95% confidence intervals [CIs]) of the proportion of patients who experienced ≥1 episode of clinically suspected and biopsy-proven acute rejection. Two-sided 95% CIs were generated for the differences between treatment groups. A descriptive summary of eGFR was provided at months 3, 6, 12, and 24 after transplant. GFR was assumed to be 0 (or close to it) in patients with end-stage kidney disease receiving maintenance dialysis, as defined in the statistical analysis plan. The month-3 eGFR served as the baseline for adjustment of subsequent eGFR values for each patient at 6, 12, and 24 mo after transplant. To analyze changes from month-3 eGFR, adjusted renal function estimates were based on a linear mixed-effects model with treatment, month (categorical variable), month-3 eGFR, and the interaction of treatment-by-month as covariates. For any patient with ≥1 missing eGFR value because of graft loss or death, the missing values were imputed as 0.
RESULTS
Patient Disposition and Baseline Characteristics
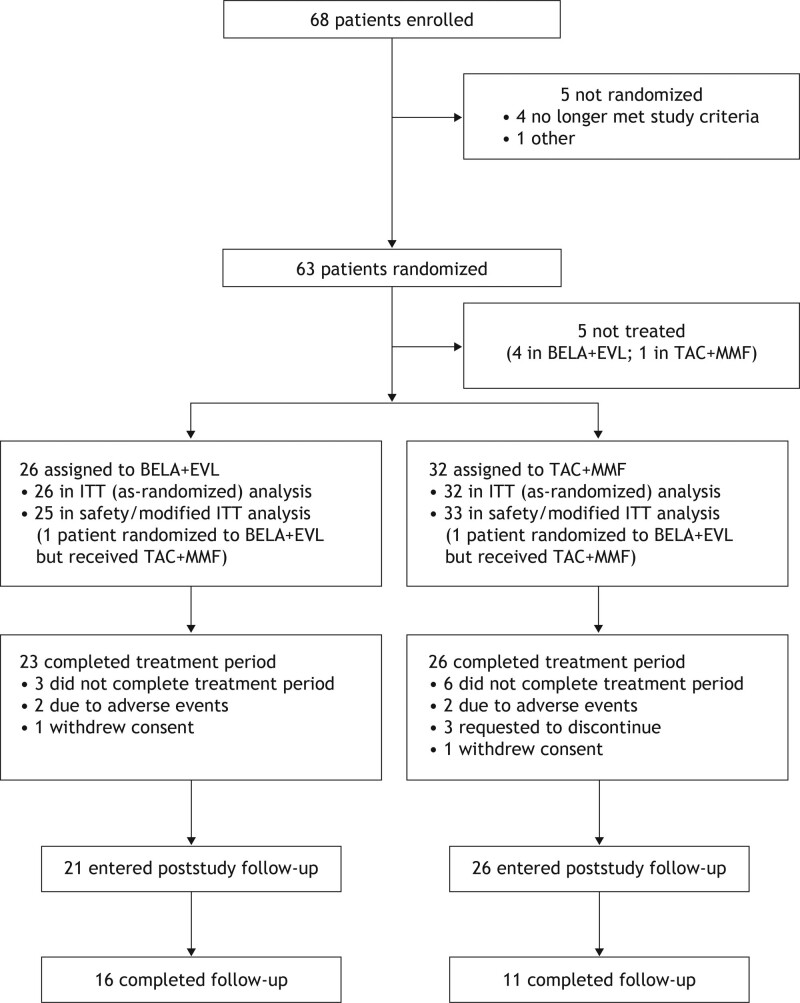
From December 24, 2015, to December 31, 2016, 68 patients were enrolled. Enrollment was then prematurely discontinued because of a BELA manufacturing–related supply constraint. The study completed on February 5, 2019 (Table 1; Figure 1). Sixty-three patients were randomized, of whom 58 underwent renal transplantation and were treated (ITT population: 26 in the BELA+EVL group and 32 in the TAC+MMF group). One patient who was randomized to BELA+EVL inadvertently received TAC+MMF throughout the 2-y study period; this was documented as an important protocol deviation (mITT population: 25 in the BELA+EVL group and 33 in the TAC+MMF group). In the BELA+EVL and TAC+MMF groups, 3 and 6 patients, respectively, discontinued assigned therapy during the treatment period and did not enter follow-up (Figure 1).
TABLE 1.
Demographic and baseline disease characteristics (ITT population)
| BELA+EVL (n = 26)a | TAC+MMF (n = 32)a | |
|---|---|---|
| Age, mean (SD), y | 51.7 (12.8) | 50.8 (10.9) |
| Age ≥65 y, n (%) | 4 (15.4) | 4 (12.5) |
| Male, n (%) | 21 (80.8) | 23 (71.9) |
| Race, n (%) | ||
| White | 23 (88.5) | 21 (65.6) |
| Black/African American | 3 (11.5) | 6 (18.8) |
| Other | 0 | 5 (15.6) |
| Kidney donor type | ||
| Living, n (%) | 23 (88.5) | 25 (78.1) |
| Deceased, n (%) | 3 (11.5) | 7 (21.9) |
| Renal transplant setting, n (%) | ||
| First | 26 (100.0) | 31 (96.9) |
| Second | 0 | 1 (3.1) |
| Panel reactive antibody | ||
| Mean (SD) | 2.4 (7.4) | 0.5 (1.5) |
| Median | 0 | 0 |
| Patients with panel reactive antibody >20%, n (%) | 1 (3.8) | 0 |
| Recipient/donor HLA mismatches, n (%) | ||
| 0–3 | 9 (34.6) | 15 (46.9) |
| 4–6 | 17 (65.4) | 17 (53.1) |
| Primary etiology of ESKD, n (%) | ||
| Glomerular disease | 10 (38.5) | 5 (15.6) |
| Diabetes | 5 (19.2) | 5 (15.6) |
| Hypertensive nephrosclerosis | 3 (11.5) | 7 (21.9) |
| Polycystic kidney disease | 3 (11.5) | 9 (28.1) |
| Renovascular disease | 2 (7.7) | 0 |
| Tubulointerstitial disease | 1 (3.8) | 0 |
| Other | 2 (7.7) | 6 (18.8) |
| Cytomegalovirus serostatus before randomization, n (%) | ||
| Positive | 18 (69.2) | 22 (68.8) |
| Negative/unknown | 8 (30.8) | 10 (31.2) |
aRefers to patients who were randomized and treated; 1 patient in the BELA+EVL arm received TAC+MMF for the 2-y study period.
BELA, belatacept; ESKD, end-stage kidney disease; EVL, everolimus; ITT, intention-to-treat; MMF, mycophenolate mofetil; TAC, tacrolimus.
FIGURE 1.
Trial profile. aOne patient randomized to BELA+EVL received TAC+MMF for the 2-y study period. BELA, belatacept; EVL, everolimus; ITT, intention-to-treat; MMF, mycophenolate mofetil; TAC, tacrolimus.
Most patients were male and white, and all had negative T-cell and B-cell crossmatches at the time of transplantation. Demographic and baseline disease characteristics were generally well balanced between the treatment groups (Table 1). However, the BELA+EVL group had a numerically higher proportion of males and patients with 4 to 6 donor HLA mismatches and a numerically lower proportion of Black/African American individuals.
Treatment Exposure
In the mITT population, the median treatment duration was 757 d in the BELA+EVL group and 737 d in the TAC+MMF group (Table S2, SDC, http://links.lww.com/TXD/A480). In the BELA+EVL group, a median of 29 BELA infusions were administered. The average daily steroid doses at each timepoint were comparable in the 2 groups, as were the body weight–adjusted doses of rATG. TAC predose trough concentrations were maintained between 8 and 12 ng/mL for most of the 24-mo period (Table S3, SDC, http://links.lww.com/TXD/A480).
Clinically Suspected and Biopsy-proven Acute Rejection at 6 and 24 Mo
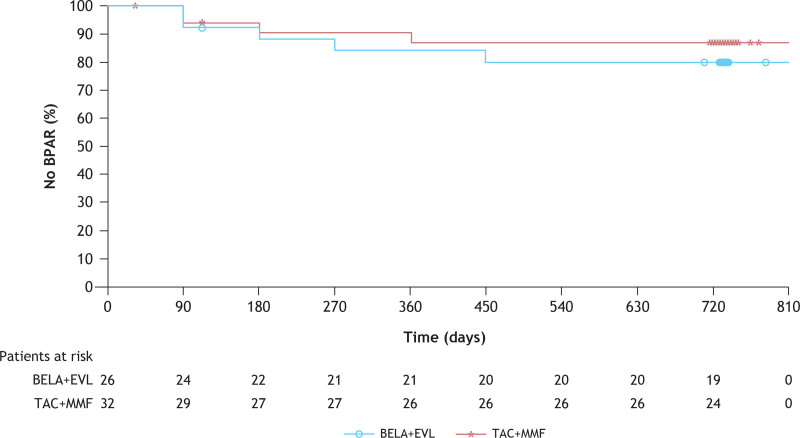
The episodes of clinically suspected and biopsy-proven acute rejection reported in this study were predominantly T-cell–mediated events, with or without an antibody-mediated (humoral) component; all were Banff grade I or IIA in severity (Table 2). No cases of pure antibody-mediated rejection were reported in either treatment group. In the ITT population, 7.7% (2/26) of patients in the BELA+EVL group and 9.4% (3/32) of patients in the TAC+MMF group had experienced ≥1 biopsy-proven acute rejection episode by month 6 (Table 2; Figure 2); 1 patient in the BELA+EVL group experienced 2 episodes. A humoral component was also identified in both patients treated with BELA+EVL who had biopsy-proven acute rejection; 1 patient had discontinued EVL 95 d before the onset of the biopsy-proven acute rejection episode because of the appearance of new-onset proteinuria, and the second patient, who started receiving EVL on day 6 after transplant, experienced biopsy-proven acute rejection on day 15 after transplant.
TABLE 2.
Rates and severity distributions of biopsy-proven acute rejection events at months 6 and 24a
| BELA+EVL (n = 26) | TAC+MMF (n = 32) | |
|---|---|---|
| Primary endpoint: BPAR at month 6 (ITT analysis)b | ||
| Patients with BPAR at month 6, n (%)a | 2 (7.7)e | 3 (9.4) |
| Difference from TAC+MMF (95% CI)c | −1.7 (−18.9 to 16.7) | |
| Banff grade events of acute cellular rejection (month 6), n (%)d | 3 (11.5) | 3 (9.4) |
| IA (mild acute) | 1 (3.8) | 0 |
| IB (mild acute) | 0 | 1 (3.1) |
| IIA (moderate acute) | 2 (7.7) | 2 (6.3) |
| IIB (moderate acute) | 0 | 0 |
| III (severe acute) | 0 | 0 |
| Humoral acute rejection events (month 6), n (%)d | 2 (7.7) | 0 |
| Humoral only | 0 | 0 |
| Humoral and cellular | 2 (7.7) | 0 |
| BPAR at month 24 (ITT analysis)a | ||
| Patients with BPAR at month 24, n (%)a | 5 (19.2) | 4 (12.5) |
| Difference from TAC+MMF (95% CI)c | 6.7 (−13.0 to 28.8) | |
| Banff grade events of acute cellular rejection (month 24), n (%)d | 5 (19.2) | 4 (12.5) |
| IA (mild acute) | 3 (11.5) | 1 (3.1) |
| IB (mild acute) | 0 | 1 (3.1) |
| IIA (moderate acute) | 2 (7.7) | 2 (6.3) |
| IIB moderate acute) | 0 | 0 |
| III (severe acute) | 0 | 0 |
| Humoral acute rejection events (month 6),n (%)d | 3 (11.5) | 0 |
| Humoral only | 0 | 0 |
| Humoral and cellular | 3 (11.5) | 0 |
| BELA + EVL (n = 25) | TAC + MMF (n = 33) | |
| BPAR at month 24 (mITT analysis)b | ||
| Patients with BPAR at month 24, n (%)a | 4 (16.0) | 5 (15.2) |
| Difference from TAC+MMF (95% CI)c | 0.8 (−18.8 to 23.6) |
aCellular (Banff IA or higher) or humoral BPAR.
bOne patient randomized to BELA+EVL was treated with TAC+MMF for the entire 2-y study period and was analyzed “as-randomized” (ie, the BELA+EVL group) in the ITT analysis and “as-treated” (ie, in the TAC+MMF group) in the mITT analysis.
cThe CI for the difference in BPAR was calculated using the normal approximation if X ≥ 5 and (N–X) ≥ 5 in both treatment groups. Otherwise, the exact method was used.
dA patient could have been counted in >1 category of acute rejection if >1 episode was experienced or findings consistent with both cellular and humoral rejection were identified on biopsy.
eOne patient in the BELA+EVL group experienced 2 episodes of BPAR.
BELA, belatacept; BPAR, biopsy-proven acute rejection; CI, confidence interval; EVL, everolimus; ITT, intention-to-treat; mITT, modified intention-to-treat; MMF, mycophenolate mofetil; TAC, tacrolimus.
FIGURE 2.
Kaplan-Meier analysis of the rates of BPARa >24 mo in the ITTb population. aCellular (Banff IA or higher) or humoral BPAR. bOne patient randomized to BELA+EVL was treated with TAC+MMF for the entire 2-y study period and was analyzed “as-randomized” (ie, in the BELA+EVL group) in the ITT analysis. BELA, belatacept; BPAR, biopsy-proven acute rejection; EVL, everolimus; ITT, intention-to-treat; MMF, mycophenolate mofetil; TAC, tacrolimus.
In the ITT population, 19.2% (5/26) of patients in the BELA+EVL group and 12.5% (4/32) of patients in the TAC+MMF group had experienced ≥1 episode of biopsy-proven acute rejection by 24 mo after transplant; in the mITT population, the rates of biopsy-proven acute rejection at 24 mo were similar at 16.0% (4/25) in the BELA+EVL group and 15.2% (5/32) in the TAC+MMF group (Table 2). Biopsy-proven acute rejection was treated with increased doses of corticosteroids in all 9 patients; 3 patients (1 in the BELA+EVL group; 2 in the TAC+MMF group) also received lymphocyte-depleting therapy.
Subgroup analyses of biopsy-proven acute rejection rates at 6 mo after transplant were uninformative because of the small number of study participants available for analysis (≤3 patients in each subgroup).
Patient and Graft Survival
No deaths were reported during the 2-y study period. During the study, graft loss was reported for 2 patients, both because of allograft thrombosis—1 in the BELA+EVL group following percutaneous angioplasty for renal artery stenosis (day 107) and 1 in the TAC+MMF group following transplant surgery involving a complicated vascular anastomosis (day 2). Neither of these patients experienced biopsy-proven acute rejection before graft loss.
Renal Function
At month 24, the unadjusted mean eGFR was higher in the BELA+EVL group than in the TAC+MMF group (71.8 versus 68.7 mL/min/1.73 m2); however, the baseline-adjusted mean eGFR was lower in the BELA+EVL group than in the TAC+MMF group (68.2 versus 71.4 mL/min/1.73 m2) (Table 3). This was attributed to the time at which graft loss occurred in each group. The month-3 value for eGFR was imputed to 0 for the patient treated with TAC+MMF who experienced graft loss on day 2, which meant that the impact of this imputation was reflected in (and lowered) the month-3 “baseline” value against which all subsequent adjusted mean eGFR values for the TAC+MMF group were calculated, whereas the graft loss in the BELA+EVL group did not occur until day 107 (after the month-3 study visit).
TABLE 3.
| BELA+EVL (n = 26) | TAC+MMF (n = 32) | |
|---|---|---|
| Unadjusted mean eGFR, mL/min/1.73 m2 (SD) [no. of patients] | ||
| Month 3 | 69.2 (22.0) [n = 25] | 62.2 (18.9) [n = 31] |
| Month 6 | 66.0 (24.7) [n = 25] | 63.9 (20.2) [n = 29] |
| Month 12 | 66.2 (22.4) [n = 25] | 62.0 (22.2) [n = 28] |
| Month 18 | 70.8 (23.0) [n = 23] | 67.5 (20.4) [n = 27] |
| Month 24 | 71.8 (21.9) [n = 24] | 68.7 (23.1) [n = 25] |
| Adjusted mean eGFR, mL/min/1.73 m2 (95% CI)d | ||
| Month 12 | 62.8 (55.8–69.8) | 65.2 (58.7–71.8) |
| Month 18 | 67.5 (61.0–74.0) | 69.4 (63.4–75.4) |
| Month 24 | 68.2 (60.9–75.5) | 71.4 (64.5–78.3) |
aRenal function was estimated using the Modification of Diet in Renal Disease equation (4-variable).
bOne patient randomized to BELA+EVL was treated with TAC+MMF for the entire 2-y study period and was analyzed “as-randomized” (ie, in the BELA+EVL group) in the ITT analysis.
ceGFR was imputed to 0 for the 2 patients who experienced graft loss (1 in each treatment group), but the timing of the imputation differed between treatment groups. The graft loss in the TAC+MMF group occurred on day 2, so imputation to an eGFR of 0 negatively affected the baseline (month 3) and all results after month 3; the graft loss in the BELA+EVL group occurred on day 107 and negatively affected all values after the month-3 baseline.
dAdjusted renal function estimates were based on a linear mixed-effects model with treatment, month (categorical variable), month-3 eGFR, and interaction of treatment-by-month as covariates. The model includes all post–month-3 data during the 24-month period. Missing eGFR values because of death or graft loss were imputed to 0.
BELA, belatacept; CI, confidence interval; eGFR, estimated glomerular filtration rate; EVL, everolimus; ITT, intention-to-treat; MMF, mycophenolate mofetil; TAC, tacrolimus.
New-onset diabetes mellitus was reported for 4 patients in each group. No clinically significant changes in mean systolic or diastolic blood pressure were noted during the study. Lipid-lowering and antihypertensive medication use was comparable between groups, both at baseline and month 24 (Table S4, SDC, http://links.lww.com/TXD/A480).
Donor-specific Antibodies
No patient in the BELA+EVL group developed de novo donor-specific antibodies. De novo donor-specific antibodies (class I) were detected in 1 patient in the TAC+MMF group at month 24.
Safety
In the mITT analysis at month 24, the safety profiles of the 2 treatment regimens were generally similar, with no new or unexpected adverse events (AEs) observed. Although similar proportions of patients in each treatment group experienced AEs of any cause, a higher proportion of patients treated with BELA+EVL experienced treatment-related AEs compared with patients treated with TAC+MMF (72.0% [18/25] versus 42.4% [14/33]) (Table 4). However, serious AEs were less frequent with BELA+EVL than TAC+MMF (52.0% [13/25] versus 60.6% [20/33]) (Table 4). Similar proportions of patients in the BELA+EVL and TAC+MMF groups experienced serious infections (16.0% [4/25] and 15.2% [5/33], respectively). Urinary tract infection was the most common serious infection, reported for 12% (3/25) of patients in the BELA+EVL group and for 0 of 33 (0%) in the TAC+MMF group (Table 4).
TABLE 4.
Safety summary at 24 mo (mITT analysis)a
| BELA+EVL (n = 25) | TAC+MMF (n = 33) | |
|---|---|---|
| Patients with any AE of any cause, n (%)b | 25 (100.0) | 32 (97.0) |
| Mouth ulceration | 10 (40.0) | 0 |
| Hypophosphatemia | 9 (36.0) | 8 (24.2) |
| Leukopenia | 8 (32.0) | 7 (21.2) |
| Constipation | 5 (20.0) | 10 (30.3) |
| Diarrhea | 5 (20.0) | 10 (30.3) |
| Nausea | 5 (20.0) | 13 (39.4) |
| Hyperkalemia | 1 (4.0) | 11 (33.3) |
| Patients with any serious AE of any cause, n (%)c | 13 (52.0) | 20 (60.6) |
| Urinary tract infection | 3 (12.0) | 0 |
| Acute cholecystitis | 2 (8.0) | 0 |
| Blood creatinine increased | 1 (4.0) | 3 (9.1) |
| Acute kidney injury | 0 | 2 (6.1) |
| Febrile neutropenia | 0 | 2 (6.1) |
| Neutropenia | 0 | 4 (12.1) |
| AEs of special interest, n (%) | ||
| Any central nervous system infection | 0 | 0 |
| Drug-induced liver injury | 0 | 0 |
| New-onset diabetes after transplant | 4 (16.0) | 4 (12.1) |
| Progressive multifocal leukoencephalopathy | 0 | 0 |
| Posttransplant lymphoproliferative disorder | 1 (4.0) | 1 (3.0) |
| Tuberculosis (any site) | 0 | 0 |
| Patients with any treatment-related AE, n (%) | 18 (72.0) | 14 (42.4) |
| AEs of any cause leading to study drug discontinuation, n (%) | 2 (8.0) | 2 (6.1) |
aOne patient randomized to BELA+EVL was treated with TAC+MMF for the entire 2-y study period and was analyzed “as-treated” (ie, in TAC+MMF group) in the mITT analysis.
bPreferred terms reported for ≥30% of patients in either treatment group are presented.
cPreferred terms reported for ≥2 patients in either treatment group are presented.
AE, adverse event; BELA, belatacept; EVL, everolimus; mITT, modified intention-to-treat; MMF, mycophenolate mofetil; TAC, tacrolimus.
Two cases of posttransplant lymphoproliferative disorder (PTLD) were reported, 1 in each treatment group. One patient treated with BELA+EVL who reported abnormal-appearing bowel movements was found to have PTLD localized to the colon with no evidence of systemic or central nervous system involvement. BELA was discontinued for this patient, who subsequently completed a course of chemotherapy for PTLD. This patient was alive with a functioning graft at the last follow-up visit at week 104 but died poststudy (783 d after randomization) because of complications from PTLD. Central nervous system PTLD was reported for 1 patient treated with TAC+MMF. MMF was discontinued, and the patient completed a course of chemotherapy for PTLD. This patient also completed the week 104 study visit alive and with a functioning graft. In each group, 1 case of malignancy other than PTLD was reported (basal cell carcinoma in the BELA+EVL group; multiple myeloma in the TAC+MMF group). No cases of progressive multifocal leukoencephalopathy were reported during the study.
AEs led to treatment discontinuation in 2 patients in the BELA+EVL group (PTLD and worsening vascular skin lesions) and 2 patients in the TAC+MMF group (plasma cell myeloma and renal transplant failure). The renal transplant failure event represented an episode of acute kidney injury from which the patient subsequently recovered.
Laboratory values between baseline and month 24 were generally consistent with those expected in a population of renal allograft recipients during the perioperative period and subsequent follow-up. Other than low lymphocyte counts—reported for 84% (21/25) and 70% (23/33) of patients in the BELA+EVL and TAC+MMF groups, respectively—there were few marked laboratory abnormalities.
DISCUSSION
In this prospective, randomized phase 2 clinical trial that incorporated rATG induction and rapid corticosteroid withdrawal in combination with BELA plus an mTORi (BELA+EVL) or standard-of-care (TAC+MMF) maintenance immunosuppression, biopsy-proven acute rejection rates at 6 mo after kidney transplant were similar. In addition, the severity distribution of acute rejection events was similar in both groups, which is in sharp contrast with observations in the phase 3 BENEFIT and BENEFIT-EXT trials, wherein patients treated with BELA experienced biopsy-proven acute rejection events that were greater in severity than patients treated with cyclosporine.9,10 At 24 mo after transplant, cumulative biopsy-proven acute rejection rates were similar across groups in the mITT population analysis. There were no deaths in either treatment group and no graft losses following biopsy-proven acute rejection episodes. The isolated cases of graft loss in each treatment group occurred because of technical complications associated with transplant surgery or a posttransplant diagnostic and therapeutic intervention. Clinically meaningful improvements in unadjusted eGFR were observed in both groups. Only 1 patient in the TAC+MMF group developed de novo donor-specific antibodies. No clinically meaningful treatment differences in mean systolic or diastolic blood pressures were observed.
The safety profile of the immunosuppressive drug combinations observed in this study was consistent with those of the individual agents (BELA,9,10 TAC,27 MMF,28 and EVL29). No unexpected AEs or other safety concerns were noted. New-onset diabetes was reported in 4 patients in each group. Because of an unexpected BELA supply constraint (owing to the transition to a novel manufacturing process), enrollment in this study was prematurely discontinued, resulting in the recruitment of only 68 of the 240 patients that were planned. Of these 68 patients, 58 were randomized, transplanted, and analyzed. This reduced sample size affected data interpretation by amplifying the impact of an isolated protocol deviation in which 1 patient was randomly assigned to BELA+EVL but instead received TAC+MMF for the entire study duration. This patient subsequently developed biopsy-proven acute rejection, resulting in differences in the rates of biopsy-proven acute rejection between the ITT and the mITT analyses. The small sample size and important differences in the timing of the single graft loss in each treatment group relative to the month-3 renal function “baseline” also resulted in discordant adjusted eGFR outcomes between the ITT and mITT analyses.
The findings reported here are consistent with those of earlier trials in which lymphocyte-depleting induction therapy, coupled with a short course of corticosteroids by maintenance immunosuppression with BELA plus an mTORi (EVL), were associated with rates and severity distributions of biopsy-proven acute rejection events that were similar to those observed with standard-of-care treatment with TAC+MMF.22,30 In the phase 2 corticosteroid avoidance study of Ferguson et al,22 the rates and severity distributions of biopsy-proven acute rejection events were similar in the BELA+SRL and TAC+MMF treatment groups, and all such events responded to corticosteroids or lymphocyte-depleting therapy, with 12-mo rates of survival with a functioning graft of 91% and 100%, respectively. In another single-center, prospective study30 in which renal transplant recipients (N = 40) received alemtuzumab and corticosteroid induction followed by SRL and BELA, no clinical rejection occurred during the first 12 mo. Four patients experienced biopsy-proven acute rejection during the first year after transplant, all of whom were successfully treated with methylprednisolone IV.30 At 5 y after transplant, patient and graft survival rates were 100% and 95%, respectively.30
In the current study, the initial rATG infusion was separated from the BELA infusion by 12 to 24 h, and no patient experienced graft loss due to perioperative renovascular thrombosis. This was also true for patients who received alemtuzumab induction followed by BELA+SRL in the study by Kirk et al,31 wherein an intervening period between BELA and alemtuzumab infusions was introduced to avoid AEs caused by coinfusion of these biologic agents. In contrast, Ferguson et al22 reported 4 graft losses, of which 2 were attributed to allograft thrombosis (BELA+SRL and BELA+MMF); and in the Clinical Trials in Organ Transplantation-10 study,32 treatment with lymphocyte induction therapy (either alemtuzumab or rATG) followed by BELA was associated with perioperative allograft thrombosis in 2 patients and graft loss in 3 patients. Together, these findings support the avoidance of coadministering agents from these 2 groups of biologic therapies during the perioperative period.
In conclusion, although conducted in a limited patient population, the results from this study are consistent with previous reports22,30 and suggest that renal allograft recipients receiving lymphocyte-depleting induction therapy and rapid corticosteroid withdrawal, followed by maintenance immunosuppression with BELA plus an mTORi (EVL), have acute rejection rates and severity distributions comparable to those observed in patients treated with a CNI-based regimen (TAC+MMF).
ACKNOWLEDGMENTS
The authors thank the patients and investigators who participated in this trial. They would like to acknowledge Deborah Pocetti, RPh, for her contribution as the clinical trial manager for this study. Professional medical writing and editorial assistance were provided by Vasupradha Vethantham, PhD, and Kelsey Hogan, MS, of Ashfield MedComms, an Inizio company, and were funded by Bristol Myers Squibb.
Supplementary Material
Footnotes
This work was funded by Bristol Myers Squibb, Princeton, NJ.
V.R.P. has received consulting fees from CareDx and Transplant Genomics. J.O. has received consulting fees from Sigilon Therapeutics, has participated in the data safety and monitoring board for Sigilon Therapeutics and Avengebio, has a leadership/ fiduciary role in CellTrans Inc, and has stock/stock options in Sigilon Therapeutics and CellTrans Inc. L.A. is an employee of Bristol Myers Squibb. M.P. is an employee of and owns stock in Bristol Myers Squibb. R.N.F. has received consulting fees from Genentech, Novartis, Veloxis Pharmaceuticals, and Mallinckrodt Pharmaceuticals and has a leadership/fiduciary role at the American Society of Transplantation, OPTN/UNOS. The other authors declare no conflicts of interest.
M.P. contributed substantially to the conception and design of the work. V.R.P., B.M., L.G., J.O., R.G., T.P., H.Y., and R.N.F. contributed substantially to data collection. V.R.P., R.N.F., L.A., and M.P. contributed substantially to data analysis and interpretation. V.R.P., R.N.F., M.P., and L.A. contributed substantially to writing the article. All authors contributed substantially to critical review and final approval of the article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Wagner M, Earley AK, Webster AC, et al. Mycophenolic acid versus azathioprine as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst Rev. 2015;(12):CD007746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azzi JR, Sayegh MH, Mallat SG. Calcineurin inhibitors: 40 years later, can’t live without. J Immunol. 2013;191:5785–5791. [DOI] [PubMed] [Google Scholar]
- 3.Ekberg H. Calcineurin inhibitor sparing in renal transplantation. Transplantation. 2008;86:761–767. [DOI] [PubMed] [Google Scholar]
- 4.Farouk SS, Rein JL. The many faces of calcineurin inhibitor toxicity-what the FK? Adv Chronic Kidney Dis. 2020;27:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veenstra DL, Best JH, Hornberger J, et al. Incidence and long-term cost of steroid-related side effects after renal transplantation. Am J Kidney Dis. 1999;33:829–839. [DOI] [PubMed] [Google Scholar]
- 6.Roland M, Gatault P, Doute C, et al. Immunosuppressive medications, clinical and metabolic parameters in new-onset diabetes mellitus after kidney transplantation. Transpl Int. 2008;21:523–530. [DOI] [PubMed] [Google Scholar]
- 7.Larsen CP, Pearson TC, Adams AB, et al. Rational development of LEA29Y (belatacept), a high-affinity variant of CTLA4-Ig with potent immunosuppressive properties. Am J Transplant. 2005;5:443–453. [DOI] [PubMed] [Google Scholar]
- 8.Nulojix. Prescribing Information. Bristol-Myers Squibb; 2018. [Google Scholar]
- 9.Durrbach A, Pestana JM, Pearson T, et al. A phase III study of belatacept versus cyclosporine in kidney transplants from extended criteria donors (BENEFIT-EXT study). Am J Transplant. 2010;10:547–557. [DOI] [PubMed] [Google Scholar]
- 10.Vincenti F, Charpentier B, Vanrenterghem Y, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant. 2010;10:535–546. [DOI] [PubMed] [Google Scholar]
- 11.Durrbach A, Pestana JM, Florman S, et al. Long-term outcomes in belatacept- versus cyclosporine-treated recipients of extended criteria donor kidneys: final results from BENEFIT-EXT, a phase III randomized study. Am J Transplant. 2016;16:3192–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vincenti F, Rostaing L, Grinyo J, et al. Belatacept and long-term outcomes in kidney transplantation. N Engl J Med. 2016;374:333–343. [DOI] [PubMed] [Google Scholar]
- 13.Bray RA, Gebel HM, Townsend R, et al. Posttransplant reduction in preexisting donor-specific antibody levels after belatacept- versus cyclosporine-based immunosuppression: post hoc analyses of BENEFIT and BENEFIT-EXT. Am J Transplant. 2018;18:1774–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bray RA, Gebel HM, Townsend R, et al. De novo donor-specific antibodies in belatacept-treated vs cyclosporine-treated kidney-transplant recipients: post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Am J Transplant. 2018;18:1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincenti F, Larsen CP, Alberu J, et al. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant. 2012;12:210–217. [DOI] [PubMed] [Google Scholar]
- 16.Pestana JO, Grinyo JM, Vanrenterghem Y, et al. Three-year outcomes from BENEFIT-EXT: a phase III study of belatacept versus cyclosporine in recipients of extended criteria donor kidneys. Am J Transplant. 2012;12:630–639. [DOI] [PubMed] [Google Scholar]
- 17.Espinosa J, Herr F, Tharp G, et al. CD57(+) CD4 T cells underlie belatacept-resistant allograft rejection. Am J Transplant. 2016;16:1102–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro-Rojas CM, Godarova A, Shi T, et al. mTOR inhibitor therapy diminishes circulating CD8+ CD28- effector memory T cells and improves allograft inflammation in belatacept-refractory renal allograft rejection. Transplantation. 2020;104:1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoji J, Leung JC, Tang Q, et al. The use of mTOR inhibitors prevents acute cellular rejection in kidney transplantation on belatacept therapy [abstract]. Am J Transplant. 2019;19(Suppl 3):104. [Google Scholar]
- 20.Diekmann F. Immunosuppressive minimization with mTOR inhibitors and belatacept. Transpl Int. 2015;28:921–927. [DOI] [PubMed] [Google Scholar]
- 21.Koyawala N, Silber JH, Rosenbaum PR, et al. Comparing outcomes between antibody induction therapies in kidney transplantation. J Am Soc Nephrol. 2017;28:2188–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferguson R, Grinyó J, Vincenti F, et al. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant. 2011;11:66–76. [DOI] [PubMed] [Google Scholar]
- 23.Bestard O, Cassis L, Cruzado JM, et al. Costimulatory blockade with mTor inhibition abrogates effector T-cell responses allowing regulatory T-cell survival in renal transplantation. Transpl Int. 2011;24:451–460. [DOI] [PubMed] [Google Scholar]
- 24.Levey AS, Coresh J, Balk E, et al. ; National Kidney Foundation. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137–147. [DOI] [PubMed] [Google Scholar]
- 25.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. [DOI] [PubMed] [Google Scholar]
- 26.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55:713–723. [DOI] [PubMed] [Google Scholar]
- 27.Jouve T, Noble J, Rostaing L, et al. An update on the safety of tacrolimus in kidney transplant recipients, with a focus on tacrolimus minimization. Expert Opin Drug Saf. 2019;18:285–294. [DOI] [PubMed] [Google Scholar]
- 28.Knight SR, Russell NK, Barcena L, et al. Mycophenolate mofetil decreases acute rejection and may improve graft survival in renal transplant recipients when compared with azathioprine: a systematic review. Transplantation. 2009;87:785–794. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Liu D, Li J, et al. Efficacy and safety of everolimus for maintenance immunosuppression of kidney transplantation: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0170246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitz R, Fitch ZW, Xu H, et al. Kidney transplantation using alemtuzumab, belatacept, and sirolimus: five-year follow-up. Am J Transplant. 2020;20:3609–3619. [DOI] [PubMed] [Google Scholar]
- 31.Kirk AD, Guasch A, Xu H, et al. Renal transplantation using belatacept without maintenance steroids or calcineurin inhibitors. Am J Transplant. 2014;14:1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newell KA, Mehta AK, Larsen CP, et al. Lessons learned: early termination of a randomized trial of calcineurin inhibitor and corticosteroid avoidance using belatacept. Am J Transplant. 2017;17:2712–2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.