Abstract
Human macrophages are hosts for Mycobacterium tuberculosis, the causative agent of tuberculosis, which killed approximately 1.87 million people in 1997. Human alveolar macrophages do not express α- or β-defensins, broad-spectrum antimicrobial peptides which are expressed in macrophages from other species more resistant to infection with M. tuberculosis. It has been previously reported that M. tuberculosis is susceptible to killing by defensins, which may explain the difference in resistance. Defensin peptides have been suggested as a possible therapeutic strategy for a variety of infectious diseases, but development has been hampered by difficulties in their large-scale production. Here we report the cellular synthesis of human β-defensin 2 via highly efficient mRNA transfection of human macrophages. This enabled mycobactericidal and mycobacteristatic activity by the macrophages. Although human macrophages are difficult to transfect with plasmid vectors, these studies illustrate that primary macrophages are permissive for mRNA transfection, which enabled expression of a potentially therapeutic protein.
Tuberculosis continues to infect and kill approximately 2 million people each year world wide. It is estimated that one out of three humans is infected, leading to 8,000,000 new cases of active tuberculosis each year (10). The number of cases of tuberculosis is expected to double by the year 2020. Greater knowledge of the mechanisms of resistance to this pathogen, as well as new therapeutics, is needed. One of the first cell types to encounter Mycobacterium tuberculosis after inhalation is the macrophage. However, M. tuberculosis multiplies rapidly in cultured human macrophages even when they are stimulated with cytokines (9). Therefore, other elements of the immune system may assist macrophages in limiting the multiplication of tubercle bacilli in the approximately one-third of the earth's human population that is infected with M. tuberculosis but does not develop active disease (10).
One important element of our innate immune defenses against microorganisms are small antimicrobial peptides known as defensins (6). These small (30 to 50 amino acids) cationic peptides are found in a variety of mammalian myeloid and epithelial cells and are bactericidal or bacteristatic for a broad spectrum of microbes, including M. tuberculosis (21, 23). Defensins are primarily divided into two subclasses, α- and β-defensins, based on their structural characteristics and are found in a variety of tissues and cell types. They are among the most abundant components in phagocytic cells, where they participate in the oxygen-independent killing of ingested microorganisms. In epithelial cells, such as in the small-intestinal crypts (25), the female reproductive tract (27), and the trachea (8), they have been predicted to provide a first line of host defense by acting in the luminal contents as a component of the innate immune response. In the mammalian airway, β-defensins have been found in tracheal mucosa (8), nasal secretions (3) and bronchoalveolar lavage fluid (30) at concentrations which are antimicrobial in vitro, suggesting that they can perform this function in vivo.
While defensins are found in rabbit (26) and bovine (28) macrophages, they are absent from human macrophages (K.O.K. and G.D., unpublished data). Although defensins have been proposed for use as therapeutics (12), the chemical synthesis of these peptides is a challenge due to the complex pattern of disulfide bonds which stabilize their structure (18), and recombinant methods do not produce sufficient yields (14, 31). An alternative to using defensin proteins as antimicrobial agents, using DNA to encode the defensins for intracellular expression in a macrophage cell line, resulting in greater resistance to Histoplasma capsulatum, has been described elsewhere (4). To date, however, there are very few reports of primary human macrophage transfection with DNA plasmids. Studies which quantitate transfection efficiency report that only about 2% of the cells express the reporter gene (i.e., enhanced green fluorescent protein [eGFP]) (29, 32, 33).
We have previously observed that primary murine macrophages efficiently accumulate RNA delivered as a complex with cationic lipids both in vivo and in vitro and that the RNA is biologically active for at least 24 h following uptake (17). In addition, the RNA preferentially distributes in vivo to macrophage-rich tissues, including the lung, which is the primary site of infection in tuberculosis (17, 20). Therefore, we sought to determine whether human monocyte-derived macrophages (MDM) could be efficiently transfected with an mRNA encoding human β-defensin 2 (HBD-2) and whether the levels produced would be sufficient to inhibit the intracellular growth of a virulent strain of M. tuberculosis.
MATERIALS AND METHODS
Macrophage isolation and culture.
Monocytes were isolated from human whole blood by centrifugation through Ficoll-Hypaque. The mononuclear cell layer was washed in RPMI 1640 and saline, and the monocytes were counted. Approximately 106 monocytes were dispensed into the wells of 24-well plates (Falcon; Becton Dickinson, San Jose, Calif.) and allowed to adhere for 1 h. The monolayers were then washed three times to remove nonadherent cells. The resulting cell monolayers consisted of <95% monocytes as determined by hydrolysis of the nonspecific esterase substrate fluorescein diacetate and epifluorescence microscopy. The few remaining nonmonocytes appeared to be lymphocytes based on morphology. Monocyte monolayers were then cultured at 37°C for 8 days to allow for differentiation into macrophage-like cells prior to infection with M. tuberculosis Erdman. Alternatively, cells were placed at 100,000 cells/well into eight-well chambered coverslips (Nalge-Nunc International, Naperville, Ill.) and allowed to adhere for 2 h in RPMI 1640 including penicillin (0.05 U/ml), streptomycin (0.05 μg/ml), l-glutamine, and 10% autologous human serum. Nonadherent cells were then removed with three washes with warm phosphate-buffered saline (PBS), and the medium was replaced with antibiotic-free Macrophage-SFM (Gibco-BRL, Gaithersburg, Md.). The monocytes were then allowed to differentiate into macrophages for 6 to 7 days at 37°C in 5% CO2.
Bacterial inoculum.
To prepare mycobacterial suspensions, we collected the mycobacterial lawn from the surface of Middlebrook 7H11 agar plates when growth had reached mid-log phase. Mycobacteria were placed into 5 ml of Macrophage-SFM in 16-by-125-mm round-bottom borosilicate glass screw-cap culture tubes with glass beads (8 to 10 mm3; Fisher Scientific) and then vortexed in pulses six times. Clumps of mycobacteria were allowed to settle at unit gravity for 45 min. Supernatant containing a predominantly single cell suspension was then transferred to a new tube and allowed to settle for an additional 30 min. The supernatant was then transferred to 16-by-125-mm flat-bottom borosilicate glass screw-cap culture tubes (Fisher Scientific), and the numbers of bacterial cells were determined spectrophotometrically in a nephrometer (Becton Dickinson CrystalScan). Mycobacterial suspensions were diluted to an optical density of 1 McFarland unit/ml (108 cells/ml).
Infection of macrophages.
The growth kinetics of M. tuberculosis can be reproducibly measured in monolayers of human MDM when they are infected with a low inoculum in tissue culture. These procedures were performed under biosafety level 3 conditions in the Mycobacteriology Laboratory at National Jewish Medical and Research Center, Denver, Colo. This laboratory has developed and standardized an in vitro system for testing antimycobacterial drugs (22). This standardized procedure has been further developed to be used for the study of agents which may modulate macrophage activity (K.O.K. and M.H., unpublished data). Macrophage monolayers were infected by replacing the medium with Macrophage-SFM containing the appropriate numbers of M. tuberculosis bacilli. Infection was allowed to continue for 1 h, after which the monolayers were vigorously washed twice with RPMI 1640-saline and incubated further in Macrophage-SFM.
Production of mRNA.
A DNA fragment encoding eGFP was amplified from the retroviral plasmid pMXI-eGFP (provided by Gary Nolan, Cleveland Clinic) using PCR primers which incorporated the XbaI (5′) and SacI (3′) restriction sites. The PCR product was digested with these two enzymes to mature the ends and then cloned into the SacI to XbaI sites of pSP64-poly(A) (Promega, Madison, Wis.). After amplification and purification from Escherichia coli, the pSP64-eGFP-poly(A) plasmid was linearized at the end of the poly(A) addition tract using EcoRI. Capped mRNA encoding eGFP was made by in vitro transcription using the Message Machine kit (Ambion, Austin, Tex.) according to a protocol supplied by the manufacturer. The DNA template was removed by treatment of the reactions with DNase I for 30 min. The mRNA was purified by extraction with phenol-chloroform-isoamyl alcohol (Ambion, Austin, Tex.), followed by the removal of low-molecular-weight constituents by column chromatography over Sephadex G-50 spin columns (NICKspin columns; Pharmacia, Uppsala, Sweden). The resulting mRNA had an A260/A280 ratio of approximately 1.95. The mRNA was stored at −70°C until use. HBD-2 cDNA was produced by reverse transcription-PCR using human tracheal epithelial cell mRNA as a template and published primer sequences (13). The cDNA was cloned into the SmaI site of pBluescript. Templates for the in vitro transcription of HBD-2 mRNA were made via PCR from the HBD-2 cDNA using an upstream oligonucleotide bearing a promoter for bacteriophage T7 RNA polymerase and a downstream oligonucleotide bearing a 25-residue oligo(dT) extension for the templated addition of a poly(A) tail to the in vitro transcript. In vitro transcription was carried out as described for the eGFP template.
Transfection.
For the transfection of one well containing approximately 100,000 cells, 2 μg of eGFP mRNA was combined with 1 μg of Oligofectin G (Sequitur, Natik, Mass.) in 0.2 ml of serum-free, antibiotic-free RPMI 1640 in a 5-ml polystyrene culture tube (Falcon). The mixture was vortexed at high speed for 30 s and then allowed to stand at 25°C (room temperature) for 15 min. The macrophage monolayers were washed once with PBS, and the medium was replaced with the mRNA-Oligofectin G complex or the yeast tRNA-Oligofectin G complex (for controls) in RPMI 1640. The cultures were then returned to the incubator for 2 h, after which fetal bovine serum was added to 10%. The cells were incubated for an additional 4 h and then fixed with neutral buffered formalin for 30 min at 4°C. After fixation, the cells were washed extensively with 1 M glycine (pH 7.2) in order to inactivate the residual formaldehyde and to retard the development of autofluorescence. The fixed cells were then allowed to stand overnight at 4°C in the dark to allow full oxidation of the eGFP chromophore, which is essential for the development of fluorescent properties. The cells were examined and recorded using a Nikon Diaphot inverted microscope fitted with epifluorescence illumination and a charge-coupled device camera system (Nu200; Photometrics, Tucson, Ariz.). The Fluorescence intensity was recorded during 0.3-s exposures with a gain setting of 4 using IP Lab Spectrum software (Scanalytics, Vienna, Va.). The intensity was integrated within the region defined by the cell, and the average background of an area devoid of cells was subtracted.
Immunohistochemistry.
Cells were grown on eight-chamber slides, fixed in formalin at 4°C, and washed in 1 M glycine. Immunohistochemistry was carried out as described earlier (34), using specific HBD-2 antibody (a gift of T. Ganz). This antiserum was raised in rabbits following conjugation to ovalbumin, using Freund incomplete adjuvant and Hunter's Titermax. Nonimmune serum was used as a specificity control and visualized using the Vector ABC kit (Vectorlabs). Acid-fast staining of mycobacteria was carried out using Difco TB auramine-O stain according to the protocol supplied by the manufacturer (Becton Dickinson Microbiology Systems, Sparks, Md.).
Enumeration of mycobacterial CFU.
Macrophage monolayers were infected with M. tuberculosis Erdman at a 10:1 ratio for 1 h. Infected cells were then transfected with increasing concentrations of mRNA encoding eGFP or HBD-2. Mycobacterial CFU were measured 4 days after infection. Infected macrophage monolayers were lysed at the end of the growth period with 1 ml of 0.25% sodium dodecyl sulfate (SDS) for 10 min. Wells were then scraped, and the lysate was transferred into plastic culture tubes. Lysate was diluted with 7H9 medium to neutralize the SDS/ spread onto Middlebrook 7H11 plates for colony growth for 21 days at 37°C, and then counted.
RESULTS
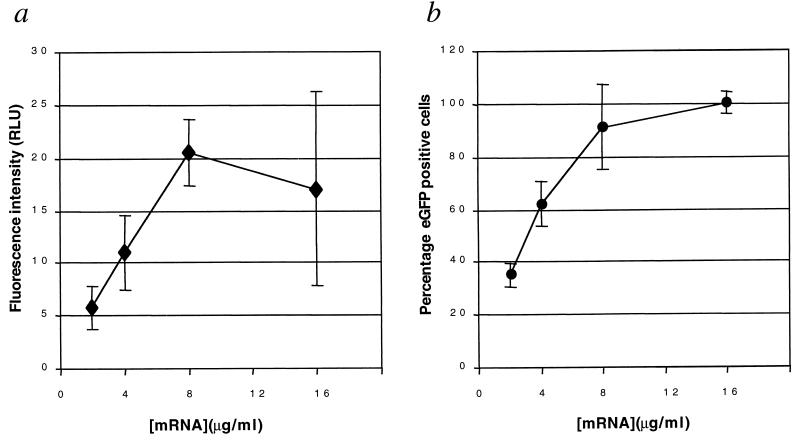
We first used mRNA encoding eGFP to determine the transfection efficiency and the optimal mRNA/lipid ratio and concentration. Several different ratios of RNA to cationic lipid were tested (data not shown). The ratio which provided the best GFP expression at 2 μg of RNA per ml was tested at higher concentrations of RNA as well. Figure 1 shows the results achieved with 8 μg of eGFP mRNA per ml (300 μg/ml of lipid), where >90% of macrophages exhibited fluoresence, indicating successful penetration of the mRNA into the cytoplasm of most of the macrophages. The average fluorescence intensity of the cells increased with the concentration of mRNA applied, up to 8 μg/ml. Increasing the mRNA concentration to 16 μg/ml did not result in a further increase (Fig. 2a). Figure 2b shows that the frequency of eGFP expression exceeded levels reported for the transfection of eGFP-encoding plasmids into macrophages by at least 40-fold (>90% positive) compared to results previously reported for plasmids (2% positive) (14, 18). Infection with M. tuberculosis did not reduce the transfection efficiency of eGFP mRNA into human MDM (data not shown).
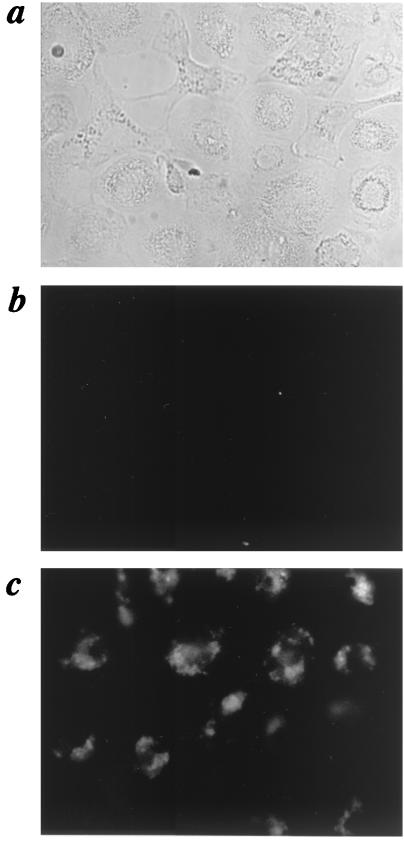
FIG. 1.
Fluorescence of human-monocyte-derived macrophages after mRNA transfection. (a) Phase-contrast image of MDM transfected with yeast tRNA as negative control for autofluorescence. Magnification, ×400. (b) Fluorescence image of panel a showing dim autofluorescence. (c) Fluorescence image of macrophages transfected with mRNA encoding eGFP/showing enhanced fluorescence for the majority of the macrophages. The images shown are representative of five experiments.
FIG. 2.
Intensity and frequency of eGFP expression following mRNA transfection. (a) Fluorescence intensity of human MDM following transfection with increasing amounts of eGFP mRNA-Oligofectin G complex. The fluorescence intensity is represented as relative light units (RLU), with the average background of the image subtracted. (b) Frequency of eGFP-positive cells as a function of increasing concentration of transfection complex. Each point represents the percentage of cells in three fields which are more than two standard deviations above the RNA control for that concentration.
Relative toxicities of mRNAs encoding GFP versus HBD-2.
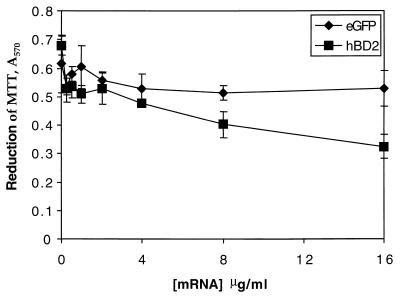
Cationic lipids are known to be toxic to mammalian cells at a high concentration (11), as are defensins (19). We therefore sought to determine the maximum dose of GFP mRNA-Oligofectin G complex that could be applied to the macrophages and whether HBD-2 mRNA had greater toxicity. Figure 3 shows the ability of human MDM to reduce MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] 24 h after treatment with increasing concentrations of either GFP mRNA or HBD-2 mRNA complexed with Oligofectin G. The reduction of MTT was not affected by the eGFP mRNA-cationic lipid complex until the concentration exceeded 32 μg/ml. In contrast, complexes of HBD-2 mRNA and Oligofectin G became toxic at 8 μg/ml, indicating that the proteins produced by translation of the mRNAs differed in toxicity as predicted.
FIG. 3.
Cell viability after transfection with mRNA lipid complexes. Cell viability was measured by incubation with 5 mg of MTT. After 24 h the absorbance of reduced MTT was measured at 585 nm for macrophages treated with Oligofectin G–HBD-2 mRNA complex (■) or Oligofectin G-eGFP mRNA complex (⧫). Cell viability was measured via reduction of MTT in at least three experiments, and representative results are presented here.
Production of HBD-2 and association with intracellular M. tuberculosis following mRNA transfection.
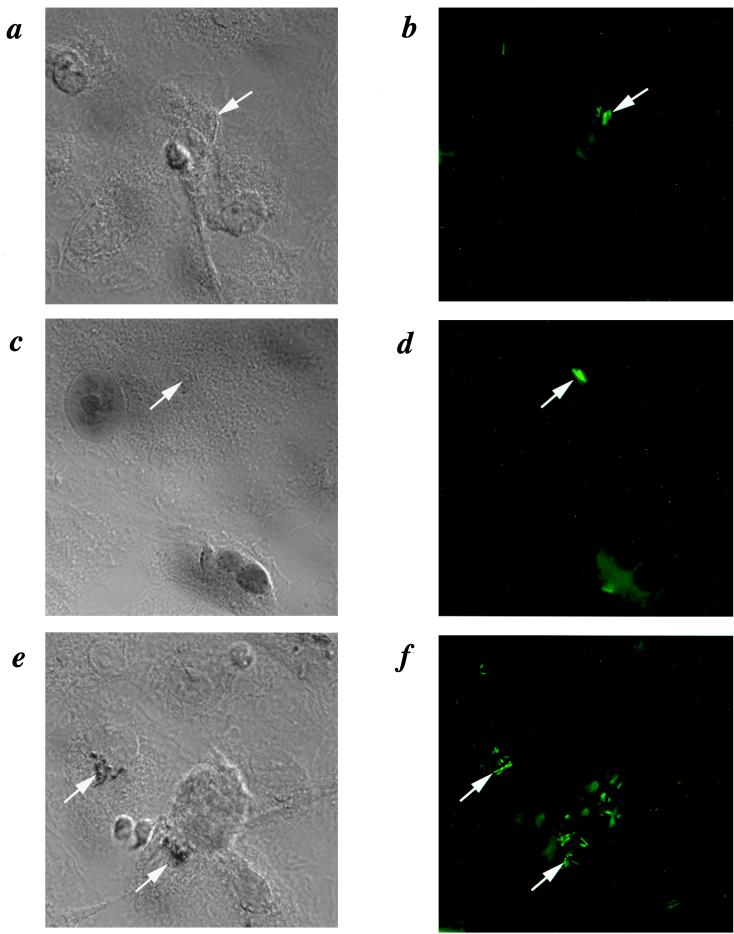
The ability of HBD-2 to affect the growth of M. tuberculosis within macrophages depends in part on the ability of the defensin protein to gain access to the mycobacteria. We therefore performed immunocytochemistry using a specific rabbit anti-human HBD-2 antiserum to determine if HBD-2 protein was produced following transfection with HBD-2 mRNA and to determine where in the macrophages the protein was localized. Figure 4a shows a lack of specific staining of M. tuberculosis-infected, HBD-2 mRNA-treated macrophages with preimmune rabbit serum. Figure 4b shows the presence of M. tuberculosis via auramine-O staining and epifluorescence microscopy. Infected macrophages transfected with mRNA encoding eGFP and stained with anti-HBD-2 antiserum showed a similar lack of staining for HBD-2 (Fig. 4c and d). However, when the specific anti-HBD-2 antiserum was used with infected macrophages which had been treated with HBD-2 mRNA 24 h earlier, specific staining was observed within the macrophages (Fig. 4e). The staining pattern is punctate and reminiscent of bacilli. A counterstain of the sample shown in Fig. 4e with auramine-O revealed that the cytoplasmic structures stained with anti-HBD-2 antiserum were acid-fast bacilli (Fig. 4f). These data indicate that HBD-2 was produced by the macrophages following transfection with HBD-2 mRNA and that the HBD-2 was able to gain access to the intracellular mycobacteria. However, not all of the acid-fast bacilli were positive for HBD-2 after treatment with mRNA.
FIG. 4.
Association of HBD-2 with intracellular M. tuberculosis following transfection with mRNA encoding HBD-2. (a) Nomarski image (magnification, ×1,000) of cells transfected with mRNA encoding HBD-2 immunostained with preimmune serum and counterstained with auramine-O to show M. tuberculosis (arrows). (b) Fluorescence image of panel a showing locations of macrophages (dim green) and M. tuberculosis (bright green). (c) Cells transfected with control mRNA and stained with anti-HBD-2 monoclonal antibody, showing a lack of specific staining for HBD-2 corresponding to the M. tuberculosis shown in panel d. (d) Fluorescence image of panel c showing locations of M. tuberculosis. (e) Nomarski image of cells transfected with mRNA encoding HBD-2 immunostained with specific anti-HBD-2 serum and counterstained with auramine-O. The brown precipitate of the diaminobenzidine appears black under Nomarski optics and partially quenches the fluorescence from auramine-O seen in panel f. (f) Fluorescence image of panel c, showing the locations of M. tuberculosis.
Dose response of inhibition of M. tuberculosis growth.
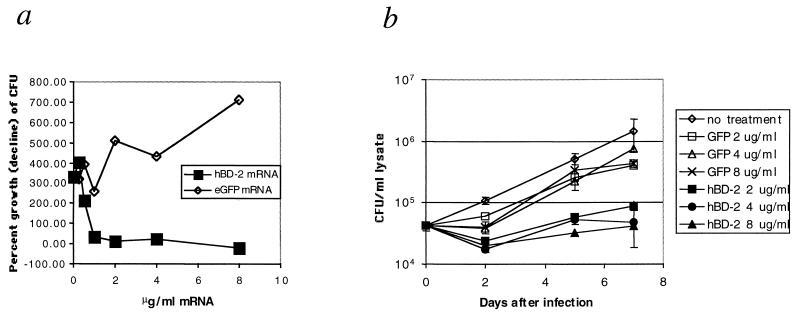
Since the HBD-2 produced by the macrophages was determined to bind to the intracellular mycobacteria, we next sought to determine if sufficient HBD-2 could be produced by the macrophages following mRNA transfection to inhibit the growth of M. tuberculosis. For this experiment, macrophage monolayers were infected with M. tuberculosis Erdman at a 10:1 ratio of bacilli to macrophages. We found this ratio to result in the infection of approximately 30% of the macrophages. At 1 h after infection, the monolayers were treated with increasing concentrations of HBD-2 mRNA or eGFP mRNA complexed with Oligofectin G ranging from 0.5 to 8 μg/ml. The monolayers were then incubated at 37°C and 5% CO2 for 4 days, after which the monolayers were lysed and the lysates were spread on 7H11 Middlebrook plates to determine the number of mycobacterial CFU remaining. The results are shown in Fig. 5a. The growth of M. tuberculosis was inhibited in cell monolayers treated with 0.5 μg of HBD-2 mRNA per ml but not in cells treated with eGFP mRNA. The growth of M. tuberculosis in the monolayers was prevented by treatment with 2 μg or more of HBD-2 mRNA per ml but was enhanced by treatment with the same concentrations of eGFP mRNA. Therefore, HBD-2 mRNA treatment resulted in concentration-dependent inhibition of mycobacterial growth, with an MIC of approximately 2 μg/ml (20 nM).
FIG. 5.
Inhibition of mycobacterial growth in MDM monolayers. (a) Growth of M. tuberculosis following transfection with mRNA encoding HBD-2 or eGFP. The percent growth or decline of the mycobacteria was calculated by dividing the average number of colonies counted on four replicate plates on day 4 by the average number from the time zero lysates, with the result multiplied by 100, e.g., (day 4/day 0) × 100. The results are representative of three experiments. (b) Duration of growth inhibition following mRNA transfection. Macrophage monolayers were infected with M. tuberculosis Erdman at a 10:1 ratio for 1 h. Infected cells were then transfected with either eGFP mRNA or HBD-2 mRNA at the indicated concentrations. Cultures were lysed at the days indicated, and mycobacterial CFU were measured as in panel a. Error bars indicate the standard deviation of four samples. These results are representative of four experiments.
Extended duration of M. tuberculosis growth inhibition following single administration.
Following the determination that macrophages transfected with HBD-2 mRNA could inhibit the growth of M. tuberculosis, we tested the duration of the growth inhibition. HBD-2 mRNA was administered as described above at concentrations of 2, 4, and 8 μg/ml and complexed with Oligofectin G. Monolayers were lysed, and the numbers of mycobacteral CFU were determined by growth on 7H11 plates 0, 2, 5, and 7 days after infection. The results are shown in Fig. 5b. Treatment with HBD-2 mRNA resulted in a reduction of CFU between days 0 and 2, whereas mycobacterial numbers remained constant in monolayers treated with eGFP mRNA and increased in untreated monolayers. Mycobacterium counts increased by two- to threefold between days 2 and 7 in cells treated with HBD-2 mRNA but increased approximately 10-fold in cells treated with eGFP mRNA. Mycobacteria in untreated monolayers increased 50-fold overall between days 0 and 7, while numbers of mycobacteria in the HBD-2 mRNA-treated cultures did not exceed those present at the beginning of the experiment. Therefore, the mycobacterial growth inhibition mediated by macrophages treated with a single addition of HBD-2 mRNA lasted for at least 7 days. Upon microscopic inspection of the monolayers, cells treated with HBD-2 mRNA appeared much healthier, with few signs of infection at the end of 7 days, whereas untreated cells or those which received mRNA encoding eGFP showed extensive cytopathology, with many dead cells by day 7 (data not shown).
DISCUSSION
Since the discovery of inducible defensin genes in mammalian cells, it has been hypothesized that their expression could be exogenously modulated to enhance the host defense against pathogenic microorganisms (7). In this study we demonstrate that cultured primary human macrophages can be efficiently transfected with mRNA encoding these potentially therapeutic proteins, resulting in enhanced microbicidal activity. This strongly supports the hypothesis that defensins act as host defense molecules both in vivo and in vitro.
The efficiency of transfection observed following delivery of an eGFP mRNA-Oligofectin G complex (>90%) was approximately 40-fold greater than was previously been reported for cultured human macrophages using electroporation or lipoplex-mediated delivery of DNA reporter vectors (29, 32, 33). We have previously observed highly efficient uptake of RNA-cationic lipid complex by murine macrophages both in vitro and in vivo (17, 20). However, DNA-cationic lipid complexes are taken up by primary murine macrophages with similar efficiency (K.O.K., unpublished data), which indicates that the uptake of nucleic acid by macrophages is not the limiting step in expression. If DNA and RNA complexed with cationic lipids penetrate the cytoplasm at similar levels, then the inefficiency of DNA expression relative to RNA may be related to the transport of the DNA to the nucleus or related to the promoter activity, neither of which are required for translation of mRNA.
Several cationic lipid formulations were examined in these studies, many of which showed efficacy in delivering exogenous mRNA to the cytoplasm. However, Oligofectin G was effective at a lower concentration and was less toxic to the macrophages relative to Lipofectamine, DOTAP, Lipofectin, or DMRIE-cholesterol (data not shown). The toxicity of the HBD-2 mRNA-cationic lipid complexes was greater than for the GFP mRNA-cationic lipid complex. This may be due to the reported toxicity of HBD-2 for mammalian cells (19) rather than the lipid portion of the complex. The toxicity of the GFP-cationic lipid complex observed at concentrations of >32 μg of RNA per ml is most likely due to the complex rather than to the mRNA or the lipid, since the components of the complex were either not toxic in the concentration range tested (the GFP mRNA) or were only toxic at much greater concentrations (Oligofectin G). Such toxicity has been reported for other nucleic acid-cationic lipid complexes (11).
Immunostaining for HBD-2 after transfection of M. tuberculosis-infected macrophages was mainly observed associated with intracellular M. tuberculosis rather than in the cytoplasm of the macrophages. Mycobacteria have been reported to reside in phagosomes which do not normally mature to lysosomes (5). The localization of HBD-2 to this intracellular compartment is somewhat surprising, as it is not obvious how the HBD-2 gained access to the bacilli. In the epithelial cells where HBD-2 is normally synthesized, it is directly secreted via the trans-Golgi and is not stored intracellularly (6). In contrast, the α-defensins are stored in cytoplasmic granules of the polymorphonuclear leukocytes or Paneth cells (24). It is possible that the HBD-2 synthesized from the transfected mRNA was secreted from the macrophages soon after synthesis. After secretion, the newly synthesized HBD-2 would have to gain access to the intracellular bacilli via the endocytic process or via direct penetration of the macrophage plasma membrane, and then the membrane of the mycobacterium-containing phagosome. The mycobacterium-containing phagosome has also been reported to exchange material with the extracellular medium via the recycling endosome compartment (2). It is therefore possible that HBD-2 secreted by the macrophages reentered the cells by endocytosis and was then transported into the mycobacterium-containing phagosome. However, since defensins have been shown to bind to and penetrate the plasma membranes of mammalian cells, direct diffusion of the newly synthesized HBD-2 from the trans-Golgi or extracellular medium directly into the phagosomes cannot be ruled out. Elucidation of the route by which HBD-2 gains access to intracellular mycobacteria will require further studies. However, following exposure to the mycobacterium the high affinity of defensins for bacterial cell membranes would tend to cause the accumulation of defensin on the surface of the bacilli. This result was observed, with immunostaining of HBD-2 mainly localized to the bacilli. We have also observed high accumulation of fluorescently labeled human neutrophil peptide 1 on M. avium in human macrophages within 5 min of addition to the medium, while similarly labeled bovine serum albumin was excluded from the cells (data not shown).
Exposure of intracellular mycobacteria to defensins in the extracellular medium helps to explain how alveolar macrophages, which do not normally synthesize defensins, might utilize defensins synthesized by nearby cells, including epithelia and neutrophils, to limit the multiplication of M. tuberculosis following inhalation and phagocytosis.
The growth of intracellular mycobacteria was inhibited as a result of transfecting mRNA encoding HBD-2 but not GFP in a dose-dependent manner. The 50% inhibitory concentration (IC50) for HBD-2 mRNA was 2 μg/ml (∼20 nM), which was approximately fourfold less than the dose which was toxic to the macrophages. It is unclear whether the IC50 represents transfection of 50% of the macrophages with sufficient mRNA to completely inhibit growth of the bacilli or whether all of the macrophages were transfected with a similar amount of HBD-2 mRNA, which was sufficient to mediate 50% inhibition of growth. The inhibition of growth was robust and remained evident for at least 7 days of culture with a single addition of HBD-2 mRNA.
The number of viable mycobacteria in the macrophages declined by approximately 50% in the first 24 h after infection when the macrophages were treated with HBD-2 mRNA (Fig. 5). These data imply that a true bactericidal effect could potentially be achieved by administering the mRNA to the cultures at 2-day intervals. This is consistent with other data we have gathered (not shown) using luciferase mRNA as the reporter for murine macrophages. The dosing schedule may lend itself to further optimization for maximum antimycobacterial activity of HBD-2 mRNA, as may the structure and chemistry of the mRNA itself. The native mRNA encoding HBD-2 contains relatively long 5′- and 3′-untranslated regions (UTRs) predicted to have extensive secondary structure of unknown function but which maintain extensive homology with other β-defensins (6). Stability and translational efficiency may be improved by replacement of the native UTRs with those from β-globin, which is a very stable and efficiently translated mRNA in most cell types (17). The mRNA may also be further stabilized by alteration of the 2′OH groups (15) and by replacing some of the bridging phosphate groups with phosphorothioate groups (16) without abolishing translational activity (1).
ACKNOWLEDGMENTS
We thank Tom Ganz for the generous gift of anti-HBD-2 antiserum and Lisa Ryan, Nancy Connell, David Riches, and Peter Henson for critical reading of the manuscript. Oligofectin-G was generously provided by Tod Woolf, Sequiteur, Natik, Mass.
G.D. was supported by grants from the NIH (HL53400) and the Cystic Fibrosis Foundation.
REFERENCES
- 1.Aurup H, Siebert A, Benseler F, Williams D, Eckstein F. Translation of 2′-modified mRNA in vitro and in vivo. Nucleic Acids Res. 1994;22:4963–4968. doi: 10.1093/nar/22.23.4963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemens D L, Horwitz M A. The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J Exp Med. 1996;184:1349–1355. doi: 10.1084/jem.184.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cole A M, Dewan P, Ganz T. Innate antimicrobial activity of nasal secretions. Infect Immun. 1999;67:3267–3275. doi: 10.1128/iai.67.7.3267-3275.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Couto M A, Liu L, Lehrer R I, Ganz T. Inhibition of intracellular Histoplasma capsulatum replication by murine macrophages that produce human defensin. Infect Immun. 1994;62:2375–2378. doi: 10.1128/iai.62.6.2375-2378.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deretic V, Fratti R A. Mycobacterium tuberculosis phagosome. Mol Microbiol. 1999;31:1603–1609. doi: 10.1046/j.1365-2958.1999.01279.x. [DOI] [PubMed] [Google Scholar]
- 6.Diamond G, Bevins C L. Beta-defensins: endogenous antibiotics of the innate host defense response. Clin Immunol Immunopathol. 1998;88:221–225. doi: 10.1006/clin.1998.4587. [DOI] [PubMed] [Google Scholar]
- 7.Diamond G, Russell J P, Bevins C L. Inducible expression of an antibiotic peptide gene in lipopolysaccharide-challenged tracheal epithelial cells. Proc Natl Acad Sci USA. 1996;93:5156–5160. doi: 10.1073/pnas.93.10.5156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond G, Zasloff M, Eck H, Brasseur M, Maloy W L, Bevins C L. Tracheal antimicrobial peptide, a novel cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci USA. 1991;88:3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Douvas G S, Looker D L, Vatter A E, Crowle A J. Gamma interferon activates human macrophages to become tumoricidal and leishmanicidal but enhances replication of macrophage-associated mycobacteria. Infect Immun. 1985;50:1–8. doi: 10.1128/iai.50.1.1-8.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dye C, Scheele S, Dolin P, Pathania V, Raviglione M C. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 11.Freedland S J, Malone R W, Borchers H M, Zadourian Z, Malone J G, Bennett M J, Nantz M H, Li J H, Gumerlock P H, Erickson K L. Toxicity of cationic lipid-ribozyme complexes in human prostate tumor cells can mimic ribozyme activity. Biochem Mol Med. 1996;59:144–153. doi: 10.1006/bmme.1996.0080. [DOI] [PubMed] [Google Scholar]
- 12.Ganz, T., and R. I. Lehrer. 1995. Defensins: pharmacology and therapeutics. 66:191–205. [DOI] [PubMed]
- 13.Harder J, Bartels J, Christophers E, Schroder J-M. A peptide antibiotic from human skin. Nature. 1997;387:861. doi: 10.1038/43088. [DOI] [PubMed] [Google Scholar]
- 14.Harwig S L, Ganz T, Lehrer R I. Neutrophil defensins: purification, characterization and antimicrobial testing. Methods Enzymol. 1994;236:160–170. doi: 10.1016/0076-6879(94)36015-4. [DOI] [PubMed] [Google Scholar]
- 15.Heidenreich O, Benseler F, Fahrenholz A, Eckstein F. High activity and stability of hammerhead ribozymes containing 2′-modified pyrimidine nucleosides and phosphorothioates. J Biol Chem. 1994;269:2131–2138. [PubMed] [Google Scholar]
- 16.Heidenreich O, Xu X, Swiderski P, Rossi J J, Nerenberg M. Correlation of activity with stability of chemically modified ribozymes in nuclei suspension. Antisense Nucleic Acid Drug Dev. 1996;6:111–118. doi: 10.1089/oli.1.1996.6.111. [DOI] [PubMed] [Google Scholar]
- 17.Kisich K O, Malone R W, Feldstein P A, Erickson K L. Specific inhibition of macrophage TNF-alpha expression by in vivo ribozyme treatment. J Immunol. 1999;163:2008–2016. [PubMed] [Google Scholar]
- 18.Lauth X, Nesin A, Briand J P, Roussel J P, Hetru C. Isolation, characterization and chemical synthesis of a new insect defensin from Chironomus plumosus (Diptera) Insect Biochem Mol Biol. 1998;28:1059–1066. doi: 10.1016/s0965-1748(98)00101-5. [DOI] [PubMed] [Google Scholar]
- 19.Lichtenstein A K, Ganz T, Sestedt M E, Lehrer R I. In vitro tumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood. 1986;68:1407–1410. [PubMed] [Google Scholar]
- 20.Malone R W, Felgner P L, Verma I. Cationic liposome mediated RNA transfection. Proc Natl Acad Sci USA. 1989;86:6077–6081. doi: 10.1073/pnas.86.16.6077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyakawa Y, Ratnakar P, Gururuaj Rao A, Costello M L, Mathieu-Costello O, Lehrer R I, Catanzaro A. In vitro activity of the antimicrobial peptides human and rabbit defensins and porcine leukocyte protegrin against Mycobacterium tuberculosis. Infect Immun. 1996;64:926–932. doi: 10.1128/iai.64.3.926-932.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mor N, Simon B, Heifets L. Bacteriostatic and bactericidal activities of benzoxazinorifamycin KRM-1648 against Mycobacterium tuberculosis and Mycobacterium avium in human macrophages. Antimicrob Agents Chemother. 1996;40:1482–1485. doi: 10.1128/aac.40.6.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogata K B, Linzer A, Zuberi R I, Ganz T, Lehrer R I, Catanzaro A. Activity of defensins from human neutrophilic granulocytes against Mycobacterium avium-Mycobacterium intracellulare. Infect Immun. 1992;60:4720–4725. doi: 10.1128/iai.60.11.4720-4725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouellette A J. IV. Paneth cell antimicrobial peptides and the biology of the mucosal barrier. Am J Physiol. 1999;277:G257–G261. doi: 10.1152/ajpgi.1999.277.2.G257. [DOI] [PubMed] [Google Scholar]
- 25.Ouellette A J, Selsted M E. Paneth cell defensins: endogenous peptide components of intestinal host defense. FASEB J. 1996;10:1280–1289. doi: 10.1096/fasebj.10.11.8836041. [DOI] [PubMed] [Google Scholar]
- 26.Patterson-Delafield J, Szklarek D, Martinez R J, Lehrer R I. Microbicidal cationic proteins of rabbit alveolar macrophages: amino acid composition and functional attributes. Infect Immun. 1981;31:723–731. doi: 10.1128/iai.31.2.723-731.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Quayle A J, Martin Porter E, Nussbaum A A, Wang Y-M, Brabec C, Mok S C. Gene expression, immunolocalization and secretion of human defensin-5 in human female genital tract. Am J Pathol. 1998;152:1247–1258. [PMC free article] [PubMed] [Google Scholar]
- 28.Ryan L K, Rhodes J, Bhat M, Diamond G. Expression of beta-defensin genes in bovine alveolar macrophages. Infect Immun. 1998;66:878–881. doi: 10.1128/iai.66.2.878-881.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simoes S, Slepushkin V, Pretzer E, Dazin P, Gaspar R, Pedroso de Lima M C, Duzgunes N. Transfection of human macrophages by lipoplexes via the combined use of transferrin and pH-sensitive peptides. J Leukoc Biol. 1999;65:270–279. doi: 10.1002/jlb.65.2.270. [DOI] [PubMed] [Google Scholar]
- 30.Travis S M, Conway B A, Zabner J, Smith J J, Anderson N N, Singh P K, Greenberg E P, Welsh M J. Activity of abundant antimicrobials of the human airway. Am J Respir Cell Mol Biol. 1999;20:872–879. doi: 10.1165/ajrcmb.20.5.3572. [DOI] [PubMed] [Google Scholar]
- 31.Valore E V, Ganz T. Laboratory production of antimicrobial peptides in native conformation. Methods Mol Biol. 1997;78:115–131. doi: 10.1385/0-89603-408-9:115. [DOI] [PubMed] [Google Scholar]
- 32.Van Tendeloo V F, Snoeck H W, Lardon F, Vanham G L, Nijs G, Lenjou M, Hendriks L, Van Broeckhoven C, Moulijn A, Rodrigus I, Verdonk P, Van Bockstaele D R, Berneman Z N. Nonviral transfection of distinct types of human dendritic cells: high-efficiency gene transfer by electroporation into hematopoietic progenitor- but not monocyte-derived dendritic cells. Gene Ther. 1998;5:700–707. doi: 10.1038/sj.gt.3300626. [DOI] [PubMed] [Google Scholar]
- 33.Weir J P, Meltzer M S. Transfection of human immunodeficiency virus type 1 proviral DNA into primary human monocytes. Cell Immunol. 1993;148:157–165. doi: 10.1006/cimm.1993.1098. [DOI] [PubMed] [Google Scholar]
- 34.Yount N Y, Yuan J, Tarver A, Castro T, Diamond G, Tran P A, Levy J N, McCullough C, Cullor J S, Bevins C L, Selsted M E. Cloning and expression of bovine neutrophil beta-defensins. Biosynthetic profile during neutrophilic maturation and localization of mature peptide to novel cytoplasmic dense granules. J Biol Chem. 1999;274:26249–26258. doi: 10.1074/jbc.274.37.26249. [DOI] [PubMed] [Google Scholar]