Abstract
(1) Background: We aimed to evaluate the risk of hepatitis B virus (HBV) reactivation in lung cancer patients treated with tyrosine kinase inhibitor (TKI), particularly in those with resolved HBV infection. (2) Methods: In this retrospective hospital-based cohort study, we screened all lung cancer patients with positive hepatitis B core antibodies (anti-HBc) receiving systemic antineoplastic treatment during the period from January 2011 to December 2020. Cumulative incidences of HBV reactivation, and their hazard ratios (HRs), were evaluated after adjusting patient mortality as a competing risk. (3) Results: Among 1960 anti-HBc-positive patients receiving systemic therapy, 366 were HBsAg-positive and 1594 were HBsAg-negative. In HBsAg-positive patients without prophylactic NUC, 3-year cumulative incidences of HBV reactivation were similar between patients receiving chemotherapy and patients receiving TKI (15.0%, 95% confidence interval (CI): 0–31.2% vs. 21.2%, 95% CI: 10.8–31.7%; p = 0.680). Likewise, 3-year cumulative incidences of HBV-related hepatitis were similar between the two groups (chemotherapy vs. TKI: 15.0%, 95% CI: 0–31.2% vs. 9.3%, 95% CI: 2.8–15.7%; p = 0.441). In 521 HBsAg-negative TKI users, the 3-year cumulative incidence of HBV reactivation was only 0.6% (95% CI: 0.0–1.9%). From multivariable regression analysis, we found that the only independent risk factor for HBV reactivation in TKI users was HBsAg positivity (HR 53.8, 95% CI: 7.0–412.9; p < 0.001). (4) Conclusion: Due to high risks of HBV reactivation in HBsAg-positive TKI users, NUC prophylaxis can be considered. However, in patients with resolved HBV infection, such risks are lower, and therefore regular monitoring is recommended.
Keywords: hepatitis B virus reactivation, anti-HBc, lung cancer, tyrosine kinase inhibitor
1. Introduction
Chronic hepatitis B virus (HBV) infection is highly prevalent. It causes a major concern during systemic antineoplastic therapy for cancer patients [1,2]. HBV reactivation may not only delay antineoplastic treatments, but may also cause fulminant hepatitis flare, hepatic failure, and death [3,4]. Among HBV carriers without nucleos(t)ide analogue (NUC) prophylaxis, cytotoxic chemotherapy is a well-known risk factor for HBV reactivation, and the incidence of HBV reactivation can be as high as 20–50% [5,6]. In addition to chemotherapy, tyrosine kinase inhibitors (TKIs) have been reported to increase the risk of HBV reactivation. TKIs such as Bcr-Abl multikinase inhibitors (including imatinib, nilotinib, and dasatinib) are used for chronic myelogenous leukemia, gastrointestinal stromal tumor, and desmoid tumor [7,8]. In lung cancer patients, epidermal growth factor receptor (EGFR)-TKI treatment has been considered as a risk factor for HBV reactivation [9]. Growing evidence suggests the involvement of TKI in HBV reactivation [10].
With repeated reports on HBV reactivation, TKI has been categorized into a class of moderate risk of HBV reactivation in guidelines on clinical practice [11]. According to American Society of Clinical Oncology (ASCO), hepatitis B surface antigen (HBsAg)-positive patients are recommended to start antiviral prophylaxis before TKI therapy [12]. However, few studies have systematically investigated risks of HBV reactivation among TKI users, without estimated incidence rate on the HBV reactivation. Furthermore, HBV reactivation caused by TKI therapy is rarely reported among patients with resolved HBV infection, which is defined as negative HbsAg but positive hepatitis B core antibody (anti-HBc) present in blood [10]. Particularly, the incidence of HBV reactivation in patients with resolved HBV infection remains undetermined. Here, we aimed to systematically investigate HBV reactivation risks in lung cancer patients receiving TKI, including those being HBV carriers or with resolved HBV infection.
2. Materials and Methods
2.1. Study Design
This was a retrospective cohort study conducted in Taichung Veteran General Hospital, a tertiary referral center in central Taiwan. We screened all patients with newly diagnosed lung cancer during the period from Jan 2011 to December 2020. Patients with a positive anti-HBc in blood were included in this study. We excluded those patients with hepatitis C virus or human immunodeficiency virus co-infection and also those without a complete HBV survey, i.e., anti-HBc in HbsAg-negative cases. Patients were followed up until October 2021. All methods of this study were approved by the Institutional Review Board of Taichung Veterans General Hospital (IRB CF20175B) and performed in accordance with the Declaration of Helsinki and the relevant guidelines and regulations.
2.2. Study Subgroups
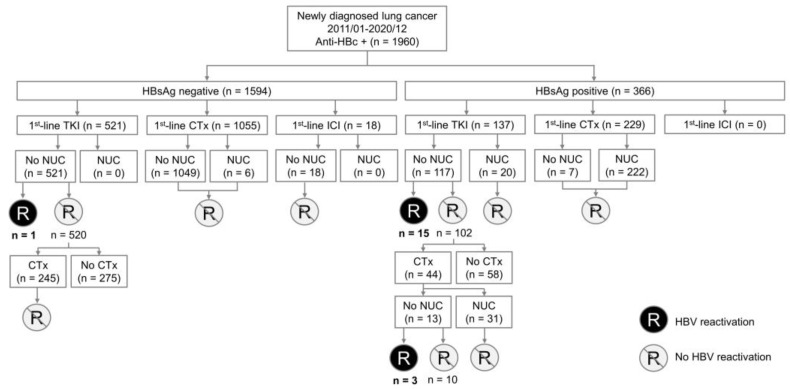
Subjects were categorized according to the status of HbsAg, antineoplastic treatments, and NUC prophylaxis (Figure 1). The HbsAg status was divided into HbsAg negative or positive groups. Three types of antineoplastic treatments were: TKI, chemotherapy, and immune checkpoint inhibitor (ICI). Before the start of antineoplastic treatments, patients either received or did not receive the prophylactic NUC. For those patients who received first-line TKI, followed by second-line chemotherapy without NUC prophylaxis, the risk of HBV reactivation was respectively evaluated during the TKI treatment or chemotherapy period.
Figure 1.
Algorithm for study participants enrollment and the risk of hepatitis B reactivation in various conditions. CTx, chemotherapy; HBV, hepatitis B virus; ICI, immune checkpoint inhibitor; NUC, nuceos(t)ide analogues; TKI, tyrosine kinase inhibitor.
2.3. HBV Reactivation and HBV-Related Hepatitis
In HBsAg-positive patients, HBV reactivation was defined as one of the following conditions: (1) a ≥ 100-fold increase in HBV DNA relative to the baseline level; (2) HBV DNA ≥ 1000 IU/mL in a patient with prior undetectable level; or (3) HBV DNA ≥ 10,000 IU/mL with an unknown baseline level.14 In patients with resolved HBV infection, i.e., positive anti-HBc and negative HBsAg, HBV reactivation was defined as one of the following conditions: (1) detectable HBV DNA; or (2) reappearance of HBsAg (seroconversion) [13]. HBV-related hepatitis after systemic antineoplastic treatment was defined as follows: serum alanine aminotransferase (ALT) of >2 times the baseline level, and >2 times the upper limit of normal levels [14]. Hepatitis induced by causes other than antineoplastic therapies was not considered to be HBV-related hepatitis.
2.4. Major Outcome
The main outcome was the occurrence of HBV reactivation or HBV-related hepatitis. The 3-year cumulative incidence was calculated. To assess the cumulative incidence of HBV reactivation, subjects were followed up from the date of systemic antineoplastic treatment until one of the following had occurred: main outcome, end of clinic follow-up, initiating NUC before another course of antineoplastic treatment, or patient mortality.
2.5. Statistics Analyses
To compare intergroup differences for categorical and continuous variables, Fisher’s exact test, Pearson’s chi-square test, and Mann–Whitney U test were used. The cumulative incidences of HBV reactivation/HBV-related hepatitis, and the overall survivals were estimated using the Kaplan–Meier method. Inter-group differences were assessed using the stratified log-rank test. Cumulative incidences were determined after adjusting patient mortality as a competing risk factor. Factors associated with HBV reactivation were assessed using the Cox proportional hazard model. The strength of association was presented as the Hazard ratio (HR) and 95% confidence interval (CI). In this study, we used the two-tailed statistical tests, and significance was set at p < 0.05. All analyses were performed on the IBM SPSS Statistics package, version 23 (IBM Corporation, Armonk, NY, USA).
3. Results
3.1. Study Subjects
The algorithm and categories of the study participants we enrolled are illustrated in Figure 1. A total of 1960 patients with a positive anti-HBc were analyzed. Among them, 366 were HBsAg-positive and 1594 were HBsAg-negative. Their baseline characteristics are shown in Table 1. Most patients were middle aged or older, in their late cancer stages, and with histological adenocarcinoma. Half of them were non-smokers. Two thirds of patients received chemotherapy as first-line treatment, while the remaining one third received TKI.
Table 1.
Clinical characteristics of the included patients. N/A, not available; TKI, tyrosine kinase inhibitor.
| N = 1960 | |
|---|---|
| Age, years (range) | 63.0 (55.0–71.0) |
| Gender, n (%) | |
| Male | 1176 (60.0%) |
| Female | 784 (40.0%) |
| Smoking, n (%) | |
| No | 955 (48.7%) |
| Yes | 847 (43.2%) |
| N/A | 158 (8.1%) |
| Stage, n (%) | |
| I | 165 (8.4%) |
| II | 85 (4.3%) |
| III | 349 (17.8%) |
| IV | 1361 (69.4%) |
| Histology, n (%) | |
| Squamous cell carcinoma | 245 (12.5%) |
| Adenocarcinoma | 1423 (72.6%) |
| Small cell carcinoma | 133 (6.8%) |
| Others | 159 (8.1%) |
| First-line treatment, n (%) | |
| Chemotherapy | 1284 (65.5%) |
| Tyrosine kinase inhibitor (TKI) EGFR-TKI Gefitinib Erlotinib Afatinib Osimertinib ALK-inhibitor Crizotinib Ceritinib Alectinib Brigatinib |
658 (33.6%) 630 (32.1%) 182 (9.3%) 269 (13.7%) 145 (7.4%) 34 (1.7%) 28 (1.4%) 14 (0.7%) 3 (0.1%) 8 (0.4%) 3 (0.1%) |
| Immune checkpoint inhibitor | 18 (1.0%) |
| HBV serology, n (%) | |
| Positive HBsAg | 366 (18.7%) |
| Negative HBsAg and negative anti-HBs | 314 (16.0%) |
| Negative HBsAg and positive anti-HBs | 1280 (65.3%) |
3.2. HBV Reactivation under HBV Prophylaxis
Among HBsAg-positive patients, only a small proportion of TKI users (14.6%) received prophylactic NUC, while most patients (92.7%) received prophylactic NUC before chemotherapy. For patients with resolved HBV infection, the use of NUC prophylaxis was rare (0.4%). Entecavir (75.6%) was the most frequently prescribed NUC, followed by tenofovir (10.0%), telbivudine (9.7%), and lamivudine (4.7%). None of the patients under HBV prophylaxis developed HBV reactivation during the course of antineoplastic treatment.
3.3. HBV Reactivation without HBV Prophylaxis
Clinical data of patients developing HBV reactivation after systemic antineoplastic treatment are shown in Table 2. Of the 19 patients with HBV reactivation, the peak ALT level exceeded > 10 times of the upper limits in 5 patients. Two patients died of hepatic failure. Among HBsAg-positive patients receiving chemotherapy with HBV reactivation, all of them developed HBV-related hepatitis. Among HBsAg-positive TKI users, 15 developed HBV reactivation, while 8 met the criteria of HBV-related hepatitis. Only one HBsAg-negative TKI user had developed HBV reactivation. All TKI users with HBV reactivation had received EGFR-TKI. No TKI user had liver failure or died after HBV reactivation.
Table 2.
Clinical data of patients developing HBV reactivation after systemic anticancer treatment. 3TC, lamivudine; ALT, alanine aminotransferase; ASP8273, an EGFR TKI; ETV, entecavir; LdT, telbivudine; TAF, tenofovir alafenamide; TKI, tyrosine kinase inhibitor. # The treatment duration in patients receiving first-line TKI only was counted since the initiation of TKI, while those receiving chemotherapy with previous TKI treatment was counted since the initiation of chemotherapy. $ HBV reactivation without HBV-related hepatitis. @ HBV prophylaxis before chemotherapy.
| Patient No. | Baseline Characteristics | Antineoplastic Treatment | HBV Reactivation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age/Sex | HBsAg | ALT (U/L) | Medication Courses | Duration # (Month) |
Peak ALT (U/L) |
HBV Viral Load (IU/mL) | Rescue NUC Therapy | Clinical Outcome | |
| 1 | 58/F | Positive | 48 | TKI then chemotherapy | 5.1 | 277 | >1.70 × 108 | ETV | liver failure |
| 2 | 67/F | Positive | 51 | TKI then chemotherapy | 0.9 | 169 | 1.05 × 105 | ETV | ALT decreased |
| 3 | 60/F | Positive | 15 | TKI then chemotherapy | 15.6 | 1074 | >1.70 × 108 | ETV | liver failure |
| 4 | 45/F | Positive | 63 | Erlotinib | 25.0 | 19 $ | 2.25 × 104 | ETV @ | |
| 5 | 62/F | Positive | 30 | Gefitinib | 1.9 | 140 | 7.03 × 103 | ETV | ALT decreased |
| 6 | 48/F | Positive | 20 | Gefitinib then osimertinib | 19.6 | 161 | 8.00 × 104 | ETV | ALT decreased |
| 7 | 50/M | Positive | 27 | Erlotinib then osimertinib | 24.4 | 16 $ | >1.70 × 108 | TAF @ | |
| 8 | 57/M | Positive | 20 | Gefitinib | 9.3 | 65 $ | 6.44 × 107 | ETV | ALT decreased |
| 9 | 70/M | Positive | 19 | Gefitinib | 1.6 | 125 | 2.82 × 105 | ETV | ALT decreased |
| 10 | 57/F | Positive | 30 | Gefitinib | 14.5 | 31 $ | 4.51 × 104 | ETV @ | |
| 11 | 70/M | Positive | 21 | Erlotinib | 4.0 | 355 | 1.03 × 105 | LdT | ALT decreased |
| 12 | 49/F | Positive | 12 | Erlotinib | 13.3 | 52 $ | 5.51 × 104 | nil | |
| 13 | 66/F | Positive | 20 | Afatinib | 0.5 | 1293 | >1.70 × 108 | ETV | ALT decreased |
| 14 | 67/F | Positive | 32 | ASP8273 then gefitinib | 5.1 | 1227 | 1.11 × 105 | ETV | ALT decreased |
| 15 | 67/M | Positive | 40 | Afatinib | 31.9 | 58 $ | 2.47 × 106 | nil | |
| 16 | 60/M | Positive | 104 | Erlotinib | 2.5 | 191 | 5.93 × 106 | ETV | ALT decreased |
| 17 | 59/F | Positive | 21 | Erlotinib then osimertinib | 13.1 | 552 | 7.93 × 106 | ETV | ALT decreased |
| 18 | 59/M | Positive | 44 | Erlotinib | 8.4 | 26 $ | 3.87 × 106 | ETV @ | |
| 19 | 67/M | Negative | 16 | Afatinib then osimertinib | 25.9 | 529 | 2.45 × 105 | ETV + 3TC | ALT decreased |
3.4. HBsAg-Positive Patients without HBV Prophylaxis
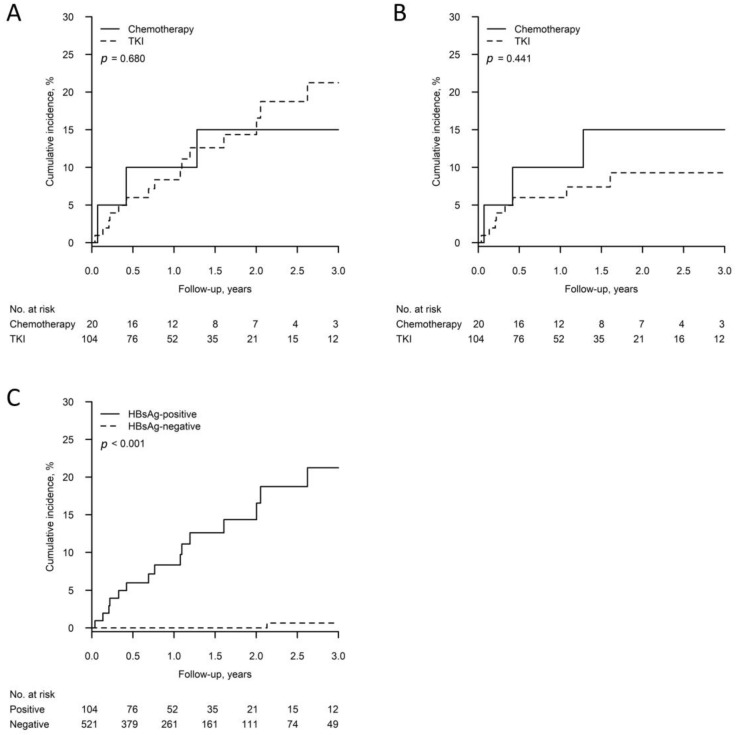
A total of 104 HBsAg-positive patients received the first-line TKI without prophylactic NUC, with 15 developed HBV reactivation. For patients who received TKI followed by chemotherapy without NUC before chemotherapy (N = 13), we categorized them into the chemotherapy group. On the other hand, there were 20 HBsAg-positive patients (7 as first line and 13 with prior TKI use) receiving chemotherapy without prophylactic NUC, with 3 developing HBV reactivation (Figure 1). As shown in Figure 2A, the 3-year cumulative incidences of HBV reactivation in HBsAg-positive patients receiving chemotherapy and TKI were not significantly different (15.0%, 95% CI: 0–31.2% vs. 21.2%, 95% CI: 10.8–31.7%; p = 0.680). Moreover, as shown in Figure 2B, the 3-year cumulative incidences of HBV-related hepatitis were also not significantly different (chemotherapy vs. TKI: 15.0%, 95% CI: 0–31.2% vs. 9.3%, 95% CI: 2.8–15.7%; p = 0.441).
Figure 2.
(A) Cumulative incidence of HBV reactivation in HBsAg-positive patients without prophylactic NUC who received chemotherapy or tyrosine kinase inhibitor (TKI). (B) Comparison of cumulative HBV-related hepatitis incidence between HBsAg-positive patients without prophylactic NUC receiving chemotherapy and TKI. (C) Comparison of cumulative HBV reactivation incidence in patients receiving first-line TKI without prophylactic NUC between HBsAg-positive and HBsAg-negative groups. TKI, tyrosine kinase inhibitor.
3.5. HBsAg-Negative Patients without HBV Prophylaxis
Among 1594 patients with resolved HBV infection, 521 received TKI as first-line treatment, with only one developed HBV reactivation. That patient had negative anti-HBS, negative HBV viral load before EGFR-TKI treatment. The patient had HBV reactivation with reappearance of HBsAg, markedly elevated HBV viral load (245,000 IU/mL), as well as high ALT level of 529 U/l after 25.9 months of EGFR-TKI treatment (afatinib followed by osimertinib). No patient with resolved HBV infection developed HBV reactivation after chemotherapy or ICI treatment.
Among HBsAg-negative patients, 149 patients were tested for HBV viral load at baseline (136 patients with negative HBV viral load, 13 patients with detectable viral load). Among the 13 patients with detectable viral load, 6 patients received prophylactic NUC before systemic treatment. For the other 7 patients without prophylactic NUC, none of them developed HBV reactivation. The only one patient developing HBV reactivation among HBsAg-negative patients had undetectable HBV viral load at baseline.
3.6. TKI Users without HBV Prophylaxis
Of 349 patients who only received TKI, 16 developed HBV reactivation (15 HBsAg-positive, one HBsAg-negative patient). The group characteristics with or without HBV reactivation are shown in Supplementary Table S1. They showed no significant inter-group differences in age, gender, smoking status, histological types, and TKI types. In the reactivation group, all patients showed adenocarcinoma histology. In multivariable analysis, HBsAg positivity was the only independent risk factor for HBV reactivation for TKI users (HR 53.8, 95% CI: 7.0–412.9; p < 0.001) (Table 3). As shown in Figure 2C, the 3-year cumulative incidence of HBV reactivation in HBsAg-negative patients receiving first-line TKI without prophylactic NUC was merely 0.6% (95% CI: 0.0–1.9%), significantly different from HBsAg-positive patients (p < 0.001).
Table 3.
Univariate and multivariable analysis for HBV reactivation in lung cancer patients receiving tyrosine kinase inhibitor treatment only.
| Variable | Total | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|
| n = 349 | Hazard Ratio (95% CI) | p Value | Hazard Ratio (95% CI) | p Value | |
| Age | 0.96 (0.92–1.00) | 0.067 | 0.97 (0.93–1.01) | 0.164 | |
| Gender | |||||
| Female | 204 | Reference | Reference | ||
| Male | 145 | 1.63 (0.61–4.35) | 0.327 | 1.18 (0.35–3.97) | 0.792 |
| Smoking habit | |||||
| Never smoker | 244 | Reference | Reference | ||
| Ever smoker | 75 | 2.04 (0.74–5.62) | 0.167 | 2.73 (0.72–10.32) | 0.140 |
| Tyrosine kinase inhibitor | |||||
| Gefitinib | 85 | Reference | Reference | ||
| Erlotinib | 141 | 0.52 (0.17–1.62) | 0.262 | 0.32 (0.10–1.04) | 0.059 |
| Afatinib | 77 | 0.43 (0.11–1.74) | 0.239 | 0.33 (0.08–1.42) | 0.135 |
| Osimertinib | 23 | 0.56 (0.07–4.70) | 0.596 | 0.35 (0.04–3.42) | 0.365 |
| HBV serology | |||||
| Negative HBsAg | 276 | Reference | Reference | ||
| Positive HBsAg | 73 | 62.20 (8.21–471.08) | <0.001 | 53.77 (7.00–412.90) | <0.001 |
3.7. Overall Survival
To evaluate the overall survival difference between TKI treatment patients with and without HBV reactivation, we focused on HBsAg-positive patients. Those receiving chemotherapy after TKI treatment without prophylactic NUC before chemotherapy were not included for analysis. There was no significant difference of overall survival between patients with and without HBV reactivation. Their median survival was 21.9 months (95% CI, 4.5–39.4) for the reactivation group, and 22.8 months (95% CI, 16.0–29.5) for the non-reactivation group (p = 0.582) (Supplementary Figure S1).
4. Discussion
Though TKI is a known risk factor for HBV reactivation, the risk of HBV reactivation has not yet been systematically determined in lung cancer patients. In this study, we found that the incidence of HBV reactivation in HBsAg-positive patients receiving TKI treatment was not low, and therefore prophylactic NUC should be considered. Furthermore, we first explored the incidence of HBV reactivation in lung cancer patients with resolved HBV infection, and the incidence of HBV reactivation was extremely low during systemic antineoplastic treatment. Although prophylactic NUC may not be required, regular monitoring of liver function and HBV viral load during the treatment course is suggested.
Our results clearly showed that the 3-year cumulative incidences of HBV reactivation or HBV-related hepatitis in TKI users were not significantly lower than that of patients receiving chemotherapy. In a previous study, the HBV reactivation rate among HBsAg-positive TKI uses was 9.36% (16/171) [9]. However, the cumulative incidence adjusted by competing mortality risk was not precisely calculated in the absence of control patients only receiving chemotherapy. Our present findings provided strong evidence for HBV prophylaxis in this population. However, we found that the rate of NUC prophylaxis in HBsAg-positive TKI users was quite low, when compared with patients receiving chemotherapy (14.6% vs. 92.7%). The low rate of NUC prophylaxis among TKI users was similar to the previous report [15]. The phenomenon may be contributed to the regulations of National Health Insurance reimbursement in Taiwan. Accordingly, the concept of NUC prophylaxis for HBsAg-positive TKI users needs to be promoted in real-world practice.
Our study first revealed that HBsAg was the most important risk factor for HBV reactivation in TKI users, and the 3-year cumulative incidence of HBV reactivation among TKI users with resolved HBV infection was very low. With a very low risk of HBV reactivation, NUC prophylaxis may not be cost-effective. However, we found that once reactivating HBV, patients might suffer from severe hepatitis flare, with a risk of hepatic failure. Therefore, a regular follow-up on HBV reactivation certainly helps to early detect a possibility of severe hepatitis flare. According to the recommendation from ASCO guidelines, patients with resolved HBV infection should be followed with frequent laboratories every 4 weeks including HBsAg, HBV DNA, and serum ALT. For patients who developed HBV reactivation (defined as appearance of HBV DNA at any level in the guideline), the follow-up frequency increased to every 2 weeks. Once the patient experiences active HBV disease (defined as reverse HBsAg seroconversion from HBsAg-negative to HBsAg-positive or an increase in ALT > 2 times the upper limit of normal levels), antiviral therapy should be given [12].
The mechanism of HBV reactivation among TKI users remains unclear. Tyrosine kinase receptor-mediated signaling pathways are crucial for immune activation and the proliferation of lymphocytes. TKIs block these pathways which lead to impaired lymphocyte function and concomitant HBV reactivation. However, further studies are mandated for determining the mechanisms [16,17].
Several limitations of this study are acknowledged. First, because most TKI users did not receive HBV prophylaxis during the study period, the baseline serum HBV viral load was usually not checked before TKI treatments. Therefore, changes of HBV viral loads from the baseline were not fully evaluated. On the other hand, serum HBV viral loads were typically checked when suspecting HBV-related hepatitis, the risk of HBV-related hepatitis, i.e., one of the major outcomes in this study, was likely precisely evaluated. Second, among HBsAg-positive TKI users, the serum HBV viral load was checked only when hepatitis had occurred, or in preparation for a second-line chemotherapy. The incidence of HBV reactivation would likely be underestimated. However, these factors would not change the conclusion of this study in that the HBV reactivation risk in TKI users was not lower than that of patients receiving chemotherapy. Third, some patients received chemotherapy immediately after TKI discontinuation without prophylactic NUC. They could have complicated the interpretation of the cause of HBV reactivation. Further studies to clarify the role of prior TKI treatment in HBV reactivation during the second-line chemotherapy may be interesting; however, HBV prophylaxis before chemotherapy should not be omitted.
5. Conclusions
In lung cancer patients receiving TKI treatment, since the risk of HBV reactivation is not low, NUC prophylaxis should be considered. Given such a low HBV reactivation risk in lung cancer patients with resolved HBV infection, regular monitoring is suggested.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12010231/s1, Supplementary Table S1. Characteristics of lung cancer patients receiving tyrosine kinase inhibitor treatment only with or without HBV reactivation. Supplementary Figure S1. Overall survival of HBsAg-positive lung cancer patients receiving first-line tyrosine kinase inhibitor treatment with and without HBV reactivation.
Author Contributions
Conceptualization: P.-H.L., T.-Y.L. and Y.-H.H.; methodology: P.-H.L., T.-Y.L. and J.-S.T.; software: P.-H.L., T.-Y.L. and J.-S.T.; validation: P.-H.L. and J.-S.T.; formal analysis: P.-H.L.; investigation: P.-H.L.; resources: Y.-H.H., K.-H.H. and K.-C.C.; data curation: Y.-H.H. and Y.-W.H.; writing—original draft preparation: P.-H.L.; writing—review and editing: J.-S.T. and T.-Y.L.; supervision: H.L., G.-C.C. and T.-Y.Y.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Taichung Veterans General Hospital (IRB No. CF20175B).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Razavi-Shearer D., Gamkrelidze I., Nguyen M.H., Chen D.-S., Van Damme P., Abbas Z., Abdulla M., Abou Rached A., Adda D., Aho I., et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol. Hepatol. 2018;3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 2.Ramsey S.D., Unger J.M., Baker L.H., Little R.F., Loomba R., Hwang J.P., Chugh R., Konerman M.A., Arnold K., Menter A.R., et al. Prevalence of hepatitis B virus, hepatitis C virus, and HIV infection among patients with newly diagnosed cancer from academic and community oncology practices. JAMA Oncol. 2019;5:497–505. doi: 10.1001/jamaoncol.2018.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loomba R., Rowley A., Wesley R., Liang T.J., Hoofnagle J.H., Pucino F., Csako G. Systematic review: The effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann. Intern. Med. 2008;148:519–528. doi: 10.7326/0003-4819-148-7-200804010-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandalà M., Fagiuoli S., Francisci D., Bruno R., Merelli B., Pasulo L., Tondini C., Labianaca R., Roila F. Hepatitis B in immunosuppressed cancer patients: Pathogenesis, incidence and prophylaxis. Crit. Rev. Oncol. Hematol. 2013;87:12–27. doi: 10.1016/j.critrevonc.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Lok A., McMahon B.J. Chronic hepatitis B. Hepatology. 2007;45:507–539. doi: 10.1002/hep.21513. [DOI] [PubMed] [Google Scholar]
- 6.Paul S., Saxena A., Terrin N., Viveiros K., Balk E.M., Wong J.B. Hepatitis B virus reactivation and prophylaxis during solid tumor chemotherapy: A systematic review and meta-analysis. Ann. Intern. Med. 2016;164:30–40. doi: 10.7326/M15-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voican C., Mir O., Loulergue P., Dhooge M., Brezault C., Dréanic J., Chaussade S., Pol S., Coriat R. Hepatitis B virus reactivation in patients with solid tumors receiving systemic anticancer treatment. Ann. Oncol. 2016;27:2172–2183. doi: 10.1093/annonc/mdw414. [DOI] [PubMed] [Google Scholar]
- 8.Wang L.-Y., Chu S.-C., Lo Y., Yang Y.-Y., Chan K.A. Association of Bcr-Abl Tyrosine Kinase Inhibitors With Hepatitis B Virus Reactivation Requiring Antiviral Treatment in Taiwan. JAMA Netw Open. 2021;4:e214132. doi: 10.1001/jamanetworkopen.2021.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yao Z.-H., Liao W.-Y., Ho C.-C., Chen K.-Y., Shih J.-Y., Chen J.-S., Lin Z.Z., Lin C.C., Yang J.C., Yu C.J. Incidence of hepatitis B reactivation during epidermal growth factor receptor tyrosine kinase inhibitor treatment in non–small-cell lung cancer patients. Eur. J. Cancer. 2019;117:107–115. doi: 10.1016/j.ejca.2019.05.032. [DOI] [PubMed] [Google Scholar]
- 10.Chang C.-S., Tsai C.-Y., Yan S.-L. Hepatitis B reactivation in patients receiving targeted therapies. Hematology. 2017;22:592–598. doi: 10.1080/10245332.2017.1321882. [DOI] [PubMed] [Google Scholar]
- 11.Loomba R., Liang T.J. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: Current concepts, management strategies, and future directions. Gastroenterology. 2017;152:1297–1309. doi: 10.1053/j.gastro.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hwang J.P., Feld J.J., Hammond S.P., Wang S.H., Alston-Johnson D.E., Cryer D.R., Hershman D.L., Loehrer A.P., Sabichi A.L., Symington B.E., et al. Hepatitis B virus screening and management for patients with cancer prior to therapy: ASCO provisional clinical opinion update. J. Clin. Oncol. 2020;38:3698–3715. doi: 10.1200/JCO.20.01757. [DOI] [PubMed] [Google Scholar]
- 13.Terrault N.A., Lok A.S., McMahon B.J., Chang K.M., Hwang J.P., Jonas M.M., Brown R.S., Jr., Bzowej N.H., Wong J.B. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liao Y.-J., Li Y.-C., Lee S.-W., Wu C.-Y., Yang S.-S., Yeh H.-Z., Chang C.S., Lee T.Y. Lamivudine versus entecavir in the rescue of chemotherapy-induced hepatitis B flare-up. J. Chin. Med. Assoc. 2017;80:758–765. doi: 10.1016/j.jcma.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y.-H., Liang J.-D., Sheng W.-H., Tien F.-M., Chen C.-Y., Tien H.-F. Hepatitis B reactivation during treatment of tyrosine kinase inhibitors-Experience in 142 adult patients with chronic myeloid leukemia. Leuk. Res. 2019;81:95–97. doi: 10.1016/j.leukres.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Wang B., Mufti G., Agarwal K. Reactivation of hepatitis B virus infection in patients with hematologic disorders. Haematologica. 2019;104:435–443. doi: 10.3324/haematol.2018.210252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee P.-H., Lee T.-Y., Chang G.-C. Hepatitis B flare during osimertinib targeted therapy in a lung cancer patient with a resolved hepatitis B virus infection. Eur. J. Cancer. 2020;130:272–274. doi: 10.1016/j.ejca.2020.02.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.