Abstract
Rheumatoid arthritis (RA) is a common condition affecting approximately 1% of the general population. RA is a multisystem disorder that causes progressive articular destruction through synovial inflammation. One of the most common extraarticular manifestations of RA is pulmonary involvement, where all compartments of the pulmonary system can be impacted (e.g., pulmonary vasculature, pleura, parenchyma, and the airways). Although it has been known for decades that a portion of patients with RA develop interstitial lung disease, and recent advancements in understanding the genetic risk and treatment for RA-interstitial lung disease have drawn attention, more recent data have begun to highlight the significance of airway disease in patients with RA. Yet, little is known about the underlying pathogenesis, clinical impact, or optimal treatment strategies for airway disease in RA. This review will focus on airway disease involvement in patients with RA by highlighting areas of clinical inquiry for pulmonologists and rheumatologists and discuss areas for future research. Finally, we discuss a potential screening algorithm for providers when approaching patients with RA with respiratory complaints.
Keywords: rheumatoid arthritis, asthma, COPD, bronchiectasis, bronchiolitis
Rheumatoid Arthritis: Autoantibodies and Airway Disease Overview
Rheumatoid arthritis (RA) is the most common autoimmune arthritis, affecting 1% of the general population (1). Manifestations of RA lead to significant morbidity and mortality and are costly to the healthcare system and the patient suffering from the disease (2). End-organ damage is induced by antibody-mediated inflammation during the clinical phase of disease in RA. Antibodies to citrullinated protein antigens (ACPAs) have been shown to play a pathogenic role in synovial disease and are associated with worse RA parenchymal lung disease (3–6).
ACPAs are measured clinically through anticyclic citrullinated peptide tests (anti-CCP). In addition to their presence in individuals with established RA, ACPAs can also be detected in serum 3–5 years before the onset of joint disease in at-risk individuals who develop RA (7). Citrullinated peptides develop via the activity of peptidyl arginine deiminase, a calcium-dependent enzyme that catalyzes the citrullination of arginine to citrulline (3). Multiple citrullinated peptides have been associated with RA (e.g., citrullinated filaggrin, fibrinogen, vimentin, histones, α-enolase), and these citrullinated autoantigens are then taken up by the adaptive immune system through dendritic cells, followed by T and B cell interactions, which ultimately generate ACPAs. This process is often mediated by the “shared epitope,” which confers genetic RA risk via the HLA-DRB1 allele of the major histocompatibility complex, which increases the affinity of HLA for citrullinated peptides (8). ACPAs are suggested to exert their pathologic effects in the synovium through several indiscriminate mechanisms; for instance, ACPAs have been shown to bind to Fc receptors of myeloid lineage cells and activate the complement system in vitro (9). Also, ACPA has been shown to stimulate macrophages via Toll-like receptor 4 and Fcy receptor to induce TNF (tumor necrosis factor) production, which is also likely potentiated by rheumatoid factor (10, 11). ACPAs have also been shown to induce osteoclastogenesis via direct binding of osteoclasts (12).
Primary clinical manifestations of RA are musculoskeletal in nature, leading to a debilitating, erosive synovial arthropathy. RA is a multisystem, autoimmune, inflammatory condition that impacts multiple systems. Historically, RA pulmonary involvement has been synonymous with lung parenchymal disease; however, this review highlights another pulmonary compartment often overlooked in RA: the airways.
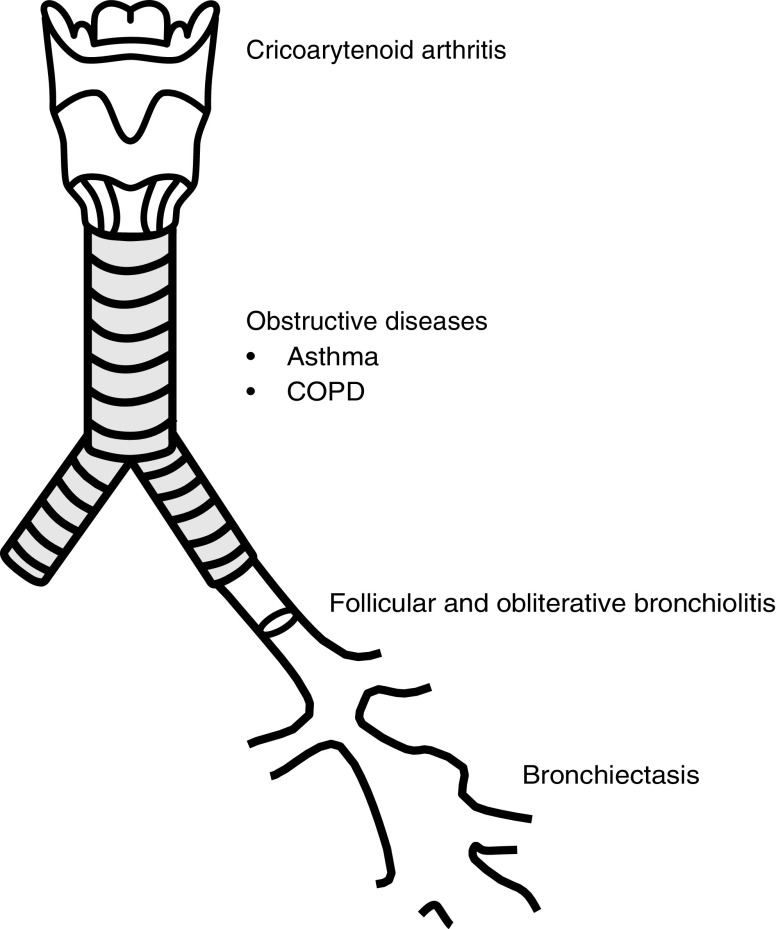
Airway disease is a heterogenous set of disorders that comprises asthma, chronic obstructive pulmonary disease (COPD), bronchiectasis, and inflammatory bronchiolitis syndromes (Figure 1). The underlying pathogenesis of these conditions varies; however, the nomenclature of airway disease applies to these disorders. This section describes what is known about each airway manifestation in RA, the epidemiology of these conditions in RA, the treatment impact for patients with RA, and the impact on clinical outcomes for patients with RA.
Figure 1.
Graphic representation of the manifestations of airway disease in patients with rheumatoid arthritis. COPD = chronic obstructive pulmonary disease.
One component of RA airway involvement is synovitis of the cricoarytenoid joint. Cricoarytenoid arthritis presents with laryngeal symptoms such as dysphonia (13), hoarseness, change in vocal quality, or a foreign body sensation (14); in rare cases it can progress to acute respiratory failure requiring emergent tracheostomy (15). Given that cricoarytenoid arthritis represents a synovial manifestation of RA, we chose not to further highlight the entity in this review, although it is a recognized airway manifestation seen in patients with RA.
Asthma
Asthma is an inflammatory airway disorder typically mediated by an inciting antigen that leads to a heterogenous, hyperinflammatory response. Recent advances in expanded biologic therapy directed at various upstream inflammatory mediators highlight the importance of emerging endotypes in asthma (16). These biologic treatment pathways can be divided roughly into two primary divisions: high and low T-helper cell type 2 (Th2) (termed type 1 and type 2 immune response as well), based on their expression of interleukin-4 (IL-4), IL-5, and IL-13, which are classically secreted by Th2 cells (cluster of differentiation 4+ [CD4+]) (16). Type 2 asthma, as triggered by these upstream cytokines (IL-4, IL-5, and IL-13), leads to high antibody titers and eosinophilia, which results in a cellular phenotype typified by eosinophils, mast cells, basophils, group 2 innate lymphoid cells, and immunoglobulin E (IgE)-producing B cells (16). Treatment of asthma typically consists of a regimen including antiinflammatory therapy directed at the specific inflammatory endotype of the patient and bronchodilator therapy aimed at reducing airway obstruction through bronchiolar smooth muscle relaxation.
Asthma is more common in RA cohorts than population controls (17, 18). The overall incidence of asthma in a Taiwanese RA cohort was 2.07-fold greater than their non-RA cohort controlling for age and sex (17). In addition, investigators using the Nurses’ Health Study data have shown that subjects with elevated RA autoantibodies who developed RA during observation were more likely to develop asthma as well (19). The Nurses’ Health Study data also show that the presence of baseline COPD or asthma at study enrollment increases the hazard ratio for incident RA development during the course of prospective evaluation, even when adjusting for important confounders (20). Although these recent large studies show a robust correlation between asthma and RA, previous observational data from smaller studies found no such link (21, 22).
RA and asthma both have a female preponderance (in adulthood). In adulthood, asthma is more common in women than men, increasingly nonatopic, and more likely to be classified as severe, when compared with age-matched men (23–25). Interestingly, in childhood asthma this paradigm is commonly reversed, and more young males are affected by asthma and have worse asthma severity (24). In RA, women are impacted in a 2:1 ratio compared with men, and RA-specific measures of disease severity are worse in women (26, 27). The role of female sex in RA and asthma in disease incidence severity implicates a potential role of sex hormones in disease pathogenesis in both conditions.
RA and Asthma: Clinical Implication of Codiagnosis
It is unknown if asthma in a patient with RA represents a unique inflammatory endotype mediated by autoantibody-associated inflammation, but there is some clue that patients with asthma with RA have worse outcomes (28). Luo and colleagues describe outcomes in the National Inpatient Sample and found that subjects with asthma with RA had worse in-hospital mortality and increased hospitalizations for asthma exacerbations when compared with subjects with asthma without RA (28). These data highlight the potential for RA to impact asthma-specific outcomes; however, to date, there are no published data that explore the impact of asthma on RA symptoms or RA disease control.
Little is known about the impact of asthma in patients with RA or the optimal approach to therapy in the individual patient with both diagnoses. Although there is a propensity for asthma within RA cohorts, there is no clinical guideline or society recommendation that guides screening for obstructive lung disease in patients with RA.
The role of biologic therapy is well explored in patients with RA, where adaptive and innate immune pathways have long been understood in the pathogenesis of synovial disease. Biologic therapy in RA consists of monoclonal antibody therapy directed at different components of these pathways, such as TNF-α, IL-1, IL-6, CD20+ B cells, and CD80/86 on antigen-presenting cells (29). Use of disease-modifying anti-rheumatic drugs (DMARDs) has been explored in populations with moderate to severe asthma following promising animal data; however, results have been largely negative or retracted in nearly all instances (30–32), with the exception of methotrexate. Methotrexate use in asthma has been shown to lead to modest reduction in daily steroid dose; however, there was no associated decrement in asthma control or forced expiratory volume in 1 second (FEV1) in these studies (33, 34).
As mentioned earlier, recent advances in biologic therapy in asthma have revolutionized the clinical approach to this condition. However, in contrast to RA, these biologic therapies in asthma are typically directed at Th2 inflammatory cascades. For instance, biologic therapies in asthma consist of antibodies directed at IgE, IL-4, IL-5, IL-13, and associated receptor antagonists (16).
Given the high coincidence of RA and asthma (17, 18, 20, 35), it is of paramount importance to the clinician to understand the impact that these diagnoses and discordant treatments have on pulmonary and joint-based outcomes. In addition, given the new frontier of biologic therapy in asthma endotypes and the potential implications of these therapies in patients with RA, there is reason to explore these important clinical considerations.
RA and Asthma: Possible Pathogenic Link
RA is caused by genetic–environmental interactions likely initiated at mucosal sites, such as within the lung airway. Although the exact etiology of RA remains unknown, it is known that the majority of patients with RA exhibit a systemic immune response to citrullinated peptides that leads to an autoantibody-mediated acute inflammatory response in the synovium (36). Clinically, ACPAs have high specificity for RA, and, when present in the absence of inflammatory arthritis, they strongly predict the future development of RA (3). This induction of abnormal immune responses to citrullinated peptides is hypothesized to occur in the lung in response to environmental insults, including smoking, where studies have reported the presence of ACPAs in sputum and bronchoalveolar lavage fluid from patients with RA and individuals at high risk of developing RA (37–39). Given the role that airway inflammation likely plays in the generation of ACPAs (35) and the role of ACPAs in RA pathogenesis, the association of asthma and RA leads to interesting scientific inquiry into a possible causal link.
Classically, RA inflammation is mediated through Th1 lymphocyte activity, and asthma is typically Th2 mediated. Conventional theory states that Th1 and Th2 diseases are inversely related, which has previously led to the assumption that RA and asthma are comorbidities and not biologically related disease states. However, multiple genetic and genomic studies have identified a Th1 phenotype within asthma populations (40–42). In addition, several studies have shown that Th1 and Th2 inflammation can occur simultaneously (43, 44), which indicates a potential role of systemic Th1 inflammatory states in subsets of subjects with asthma and thus a possible role for RA-related inflammation in asthma disease.
These interesting data regarding the role of the airway in production of ACPAs highlight the potential role of conditions like asthma as a risk factor for RA, which is supported by epidemiologic studies (20, 35, 45). However, it is important to point out that ACPAs and RA-mediated inflammation may also play a direct role in airway inflammation; therefore, it is also reasonable to consider the inverse: in some cases RA may lead to obstructive lung diseases such as asthma, which is also supported indirectly in epidemiologic studies (19, 46).
RA and COPD
COPD has a 10.1% prevalence worldwide (47). COPD is a clinical syndrome that encompasses a chronic respiratory disease typified by complete reversibility of airflow obstruction and alterations in pulmonary architecture. These structural alterations of COPD can manifest as emphysema, airway disease, or both. Although genetic conditions such as alpha-1 antitrypsin result in these same pathologic changes as COPD, most cases develop as a manifestation of inhalational smoke exposure (cigarettes or biomass).
Smoking is a shared risk factor for COPD and RA. In RA, smoking has been shown to be a modifiable risk factor in multiple studies (48–50). Interestingly, studies have also shown, in identical twin pairs with variable RA expression, that those twins who smoked were at higher risk for the development of RA, further supporting the role of environmental–gene interactions for RA and smoking (51, 52). Shared environmental risk factors certainly explain the high coincidence of many disorders with COPD, such as ischemic heart disease, atrial fibrillation, heart failure, osteoporosis, lung cancer, anxiety, and depression (53). In many cohorts of patients with RA, high rates of COPD have been found. Meta-analysis of these data shows the pooled prevalence of COPD in RA was 6.2% (95% confidence interval, 4.1–8.3%) (54). Although smoking is associated with RA and COPD, multiple analyses have found the risk for COPD in patients with RA remains significant after adjusting for smoking history (55, 56). Regardless, the presence of COPD in patients with RA has significant impact on morbidity and mortality (57).
Treatment of COPD and RA
There are clinical endotypes associated with COPD, although fewer than asthma. The two clinically important COPD endotypes are the aforementioned alpha-1 antitrypsin–deficient patients and those patients with COPD with elevated serum eosinophils. Elevated serum eosinophilia predicts response to corticosteroid therapy in COPD (53). Primary prevention is the most important intervention for COPD globally, and in the individual patient, where smoking cessation reduces risk of death and improves quality of life (53).
Despite the significant worldwide prevalence of COPD and poor clinical outcomes, there is very little clinical insight into COPD immunophenotypes, and as a result there are no effective immunomodulatory treatments aside from corticosteroids for patients with COPD. Given these limitations, it is unclear how treatment considerations for one disease should impact the other in coincident cases of RA and COPD (57). There has previously been concern regarding the use of biologic DMARD therapy in COPD, given potential risk for increased exacerbations; however, a large-scale, real-world trial design assessed this question and found no clear association between biologic DMARD use and poor COPD-specific outcomes (58). Like asthma, the use of conventional DMARDs has been explored in COPD populations, and methotrexate may exert a modest steroid-sparing effect in small studies. However, other conventional DMARD trials in COPD have been largely negative (59, 60). Clinicians should offer smoking cessation–directed interventions; however, it remains to be seen if more aggressive immune suppression for patients with RA with COPD impacts the airway disease manifestations or vice versa.
Shared Pathogenesis between COPD and RA
There are overlaps between RA and COPD in terms of autoimmune pathogenesis. For instance, higher levels of ACPAs have been detected in the serum of heavy smokers without RA at higher rates than control subjects (61). In addition, serum from patients with COPD has been shown to produce autoantibodies that react to antigens associated with known autoimmune conditions, including RA (62). Most interestingly, researchers identified a group of subjects with positive serum ACPAs before the development of RA who were also at increased risk for developing COPD when compared with a control population, which indicates a potential role for ACPAs in the pathogenesis of COPD in these patients (19).
RA and Bronchiolitis
Bronchioles are lower divisions of the airways that are typified by the absence of hyaline cartilage. These small airways are frequently involved in inflammatory disorders termed bronchiolitis.
Follicular Bronchiolitis
Follicular bronchiolitis (FB) develops in the setting of hyperplasia of bronchiole-associated lymphoid tissue. This bronchiole-associated lymphoid tissue hyperplasia is seen in many connective tissue disorders, including RA (63). Diagnosis of this condition is typically made on lung tissue examination after a surgical lung biopsy; however, there are imaging findings that are associated with FB, such as centrilobular nodules, hyperinflation, mosaicism, and air trapping, although these findings are nonspecific (64). Of interest, high-resolution computed tomography (HRCT) findings of bronchiolitis have several overlapping features with asthma, which can make the two diseases difficult to distinguish radiographically (64–66). Typically, FB requires surgical lung biopsy for diagnosis, given this nonspecific radiographic phenotype. On histopathology, FB is typified by lymphocytic proliferation and lymphoid follicles, with reactive germinal centers around the bronchioles accompanied by narrowing of the bronchiole lumen (67).
Given the rarity of RA-related FB, little is known about the expected clinical course; however, the primary clinical manifestations are a reduction in FEV1 and associated dyspnea. RA-FB is believed to be a progressive obstructive disease that responds briskly to immune suppression, given the cellular nature of its histopathology, although there are reports of a positive response to macrolide therapy as well (68, 69).
RA is also associated with obliterative bronchiolitis (OB) (also known as constrictive bronchiolitis and bronchiolitis obliterans). Like FB, OB occurs typically in secondary fashion and most commonly in the post–lung transplant and post–bone marrow transplant populations. However, RA is the most implicated connective tissue disease for OB (70). Imaging findings of OB are diffuse pulmonary infiltrates, bronchial wall thickening, and lobular areas of decreased attenuation with mosaicism indicating air trapping (70). The diagnosis of OB is clinical, based on the presence of an inciting event or condition (e.g., RA, lung transplantation, noxious chemical inhalation), marked airflow obstruction not believed to be related to COPD (for instance, the absence of smoking history), with or without biopsy evidence of bronchiolitis. Histopathologic examination of OB reveals concentric fibrosis of the bronchial wall with severe narrowing of the bronchiole lumen, occasionally associated with lymphocytic infiltrates within the walls of the bronchioles (70).
Outcomes in OB are generally worse when compared with FB, including cases associated with RA. In one case series of patients with RA who developed OB, all patients were on immunomodulatory therapy for RA at the time of OB onset. Of the 24 patients in the series, 52% had worsening symptoms despite directed therapy, 48% developed acute hypoxemic respiratory failure, 4 patients died of respiratory failure in the follow-up period, and 1 patient went for lung transplantation (70).
Treatment for OB in RA is generally a combination of inhaled bronchodilator therapy combined with aggressive immunomodulatory therapy with inhaled and oral corticosteroids, macrolides, cyclophosphamide, or etanercept.
Bronchiolitis conditions in RA are rare; however, they lead to significant morbidity and mortality and their diagnosis frequently requires surgical lung biopsy and significant immune modulatory therapy with early consideration of lung transplantation. However, as mentioned previously, there are no clear guidelines for screening patients with RA for obstructive lung diseases, which likely leads some patients to experience delayed referral to centers that specialize in diagnosis and management of inflammatory bronchiolitis and/or lung transplantation evaluation.
RA and Bronchiectasis
Bronchiectasis results from long-term damage to the airways causing them to widen and thicken, with increased production of mucous creating a nidus for recurrent infection. The hallmark condition of bronchiectasis, cystic fibrosis, results from inherited genetic abnormalities in transmembrane ion conductance channels in the airways leading to inappropriate mucous clearance and long-standing recurrent infections and bronchiectasis. Bronchiectasis can develop in many systemic conditions aside from cystic fibrosis, including RA.
Rates of bronchiectasis in patients with RA vary based on the method of screening used to detect airway abnormalities. In unselected patients with RA, the rate of bronchiectasis on HRCT is 17–30.5% (71), although the rate of symptomatic bronchiectasis in RA cohorts is much lower (between 2.4% and 3.1%) (72, 73).
Treatment Considerations of RA Bronchiectasis
Bronchiectasis in patients with RA leads to more irreversible obstruction than is seen in non-RA-related bronchiectasis (74, 75). Recurrent lower respiratory tract infections are a complex issue in patients with RA who commonly require immunomodulatory agents. Common RA regimens including corticosteroids, leflunomide, and biologic DMARDs have been shown to increase rates of lower respiratory tract infections (76, 77). The role of prophylactic antibiotics in RA-related bronchiectasis is unclear and left up to individual clinicians. It also unclear if immunomodulation has any impact on bronchiectasis severity or outcomes for patients with RA or if another approach should be used that targets possible underlying genetic or immunologic mechanisms directly (78).
Pathogenesis of RA Bronchiectasis
As referenced already, the pathogenesis of bronchiectasis in RA is unknown. There are multiple theories that outline possible mechanisms for airway manifestations of RA. Given the pathogenic role of ACPAs in RA, understanding the associations between bronchiectasis and ACPAs can offer insight into potential overlapping pathogenesis for RA and bronchiectasis. When comparing three groups—patients with early RA with ACPA positivity, ACPA-positive patients without RA, and normal ACPA-negative control subjects—there was significantly more airway thickening and bronchiectasis on HRCT in those groups who were ACPA-positive, even in the absence of RA (79). This highlights the role that the generation of ACPAs may play in the development of bronchiectasis seen on HRCT.
Another theory posits that bronchiectasis develops in RA related to an underlying genetic abnormality like the hallmark bronchiectasis condition: cystic fibrosis. In cystic fibrosis, the CFTR (cystic fibrosis transmembrane conductance regulator) gene is abnormal, leading to poor performance of the associated protein, which alters transmembrane conductance of sodium ions resulting in abnormal mucous layers. Researchers evaluated a population of subjects with RA with bronchiectasis for the common genetic mutations associated with cystic fibrosis and found that 15.4% of those patients were heterozygous for delta F508, the most common CFTR mutation in cystic fibrosis (80, 81). These findings, combined with the association of ACPAs and bronchiectasis, leads to a hypothesis that airway inflammation from bronchiectasis, which may develop due to genetic risk such as CFTR mutations, leads to autoantibody generation that can then play a role in RA pathogenesis and worsening airway disease.
Other theories of bronchiectasis in patients with RA include the possibility that recurrent infection related to chronic immune suppression predisposes patients with RA to bronchiectasis. For instance, patients with RA and bronchiectasis have frequent lower respiratory tract infections, and these infections were associated with preexisting sputum colonization and biologic DMARD use (77). The truth is likely some combination of all these factors: antibody-mediated inflammation, genetics, and a predisposition to recurrent lower respiratory tract infections all play a role in bronchiectasis seen in HRCT scans of patients with RA.
Screening Approaches to Patients with RA for Airway Disease
The most important screening tool for RA airway disease is the clinical history to elicit the presence of airway-related symptoms. Our recommendation is that clinicians caring for patients with RA evaluate and obtain a thorough history for the presence of symptoms that may be attributable to airway diseases, such as hoarseness, change in voice character/quality, foreign body sensation in the throat, dyspnea or shortness of breath, fatigue, wheezing, cough, recurrent lower respiratory tract infections, or chest tightness/pain. A combination of diagnostic tools guided by clinical history such as HRCT, pulmonary function testing with bronchodilator response, and/or bronchoprovocation testing should have adequate sensitivity and specificity in the symptomatic patient with RA to diagnose or largely exclude airway involvement. We have proposed a screening algorithm for clinicians when approaching the patient with RA with undifferentiated respiratory symptoms on screening (Figure 2)
Figure 2.
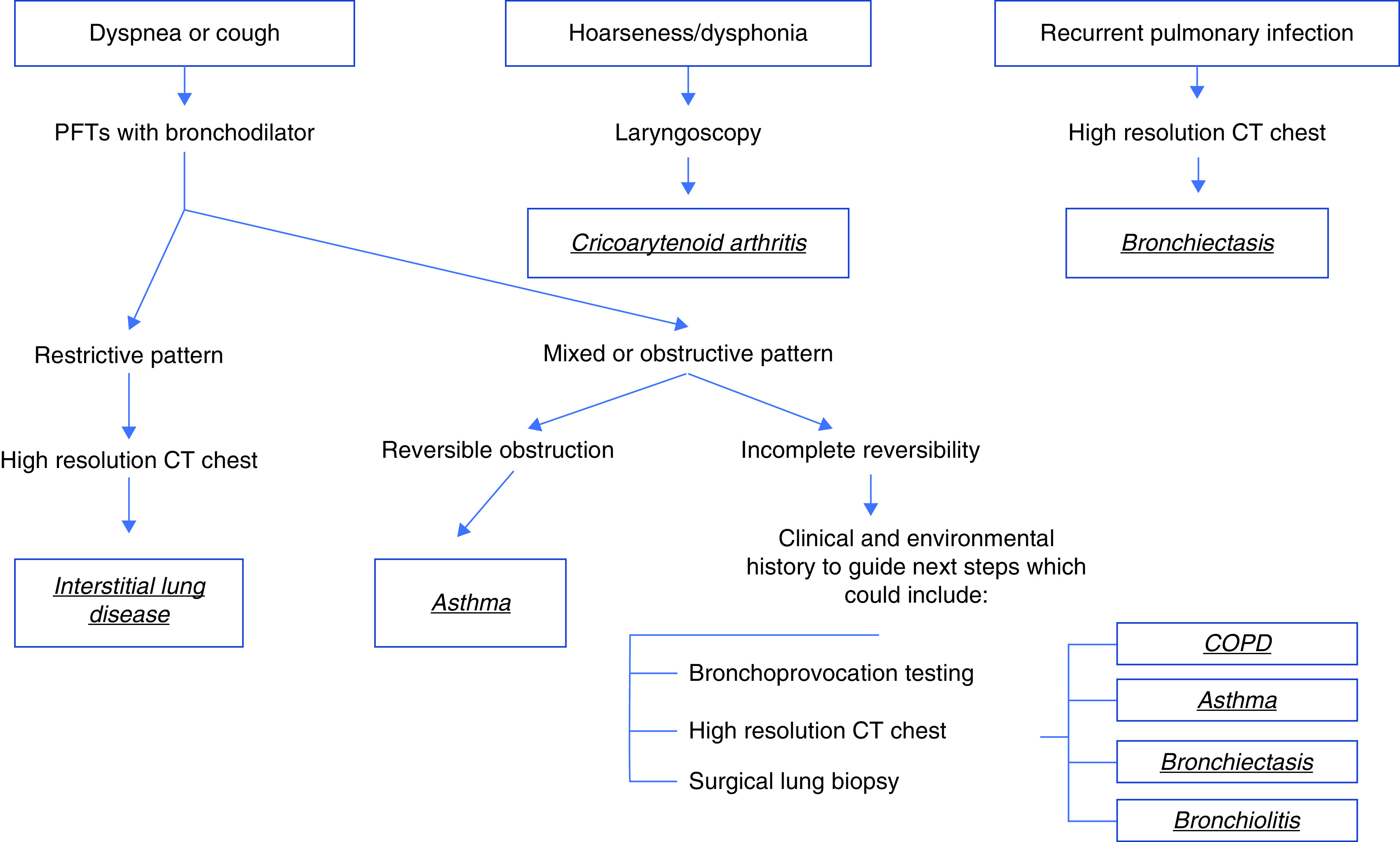
Proposed algorithm for evaluation of patients with rheumatoid arthritis (RA) based on positive screen during clinical evaluation for dyspnea, recurrent lower respiratory tract infections, or voice changes/dysphonia. COPD = chronic obstructive pulmonary disease; CT = computed tomography; PFTs = pulmonary function tests.
The diagnosis of any of these airway diseases in RA will have treatment implications in the symptomatic patient with RA and will typically require a multidisciplinary approach for treatment combining rheumatologists; pulmonologists who specialize in asthma, COPD, interstitial lung disease (ILD), or bronchiectasis; and/or otolaryngologists for laryngoscopy. In addition, consideration between pulmonologists and rheumatologists should focus on the role of DMARD therapy in the individual patients with comorbid RA and airway disease (for instance, consideration of nonbiologic therapy in patients with recurrent infections in the setting of bronchiectasis).
Future Directions in RA Airway Disease Research
Data have been presented so far in this review that highlight two exciting hypotheses: model 1, inflammation in the lung at the airway mucosal surface leads to the development of ACPAs and subsequent RA (Figure 3A); and model 2, systemic RA inflammation impacts airway mucosa leading to a unique autoantibody-mediated airway disease endotype (Figure 3B).
Figure 3.
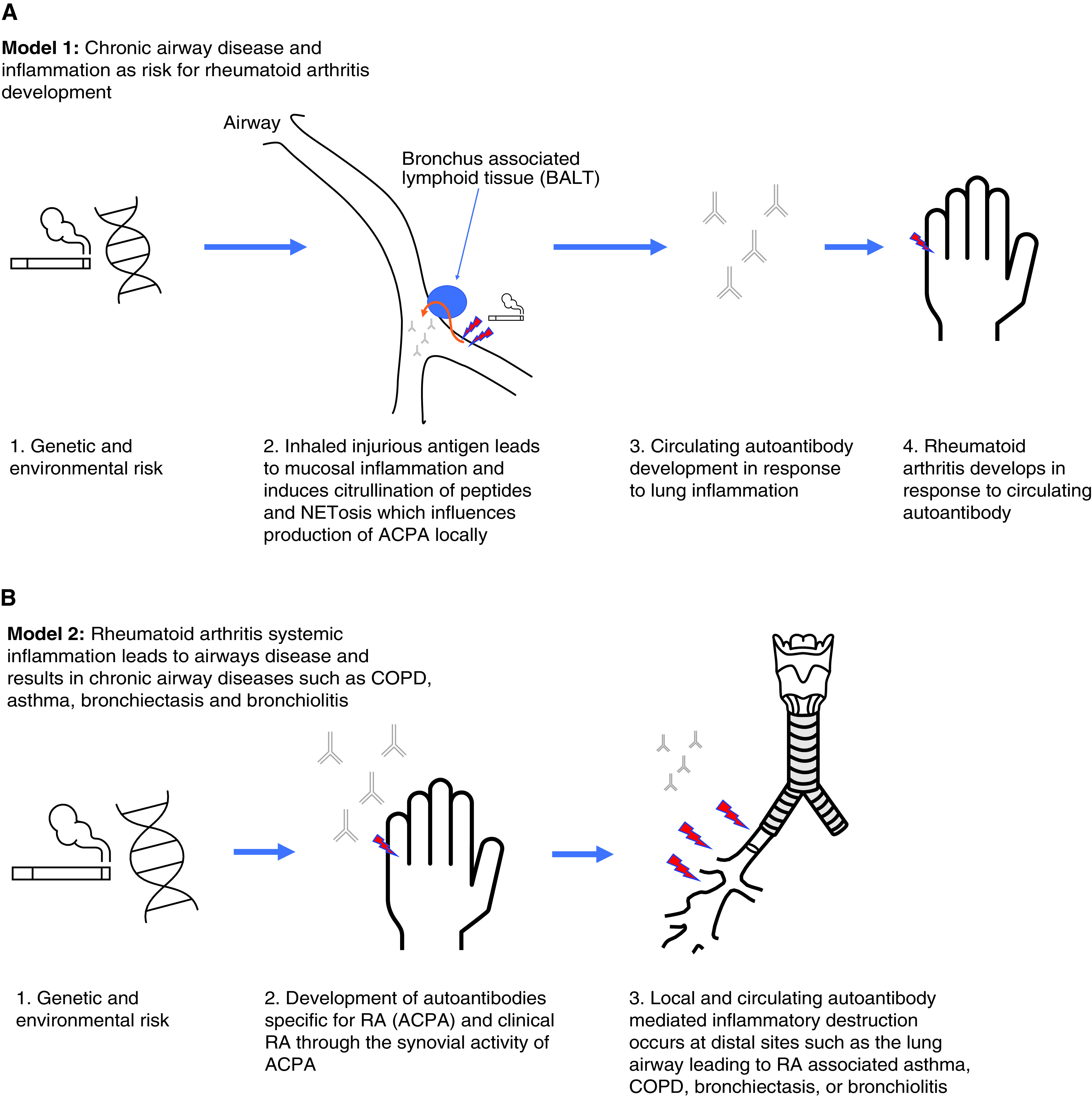
(A) Model 1: Chronic airway disease and inflammation as risk for rheumatoid arthritis (RA) development. Data that support model 1 of development of antibodies to citrullinated protein antigens (ACPAs) in chronic lung disease are found in References 7, 19, 20, 35, 38, 39, 45, 79, 80, 83, 85, and 92. (B) Model 2: Airway diseases such as asthma, chronic obstructive pulmonary disease (COPD), bronchiectasis, and bronchiolitis result as a direct manifestation of RA autoantibody-mediated inflammation and represent a unique autoimmune endotype of airway disease. Data that support model 2 of RA-mediated lung inflammation causing airway phenotypes are found in References 28, 46, 56, 68, and 93–95. NETosis = neutrophil extracellular trap formation.
In the experimental view of airway–RA synergy represented by model 1, the airway involvement in these cases does not reflect a manifestation of inflammation from RA; instead, the underlying airway involvement predates clinical RA and leads to the generation of ACPAs. In this scenario, early identification and intervention of airway disease and immunomodulation for those patients at risk could be an important clinical step to disrupting RA pathogenesis. In addition, it is paramount that clinical investigators understand the pathobiology of disease initiation from airway inflammation to interrupt or alter the typical disease course.
This airway–RA link is intriguing, given the potential role of the lung and airway mucosa in RA disease pathogenesis via production of ACPAs, as noted previously in this review. In this model, lung injury, mediated by smoking or microbiota pressures such as mycobacterial disease, induce ACPAs potentially via the effect of neutrophil-mediated inflammation and neutrophil extracellular traps (NETs) (8, 39, 79, 82–84). NETs externalize digested peptides for antigen-presenting cells and serve as antigenic targets for ACPA (82).
In RA subjects, NET formation is increased in sputum neutrophils, and elevated levels of sputum ACPAs are associated with citrullinated protein containing NETs (83, 85, 86). In addition, NET peptides can be internalized by antigen-presenting cells and presented in a major histocompatibility complex class II–dependent manner to antigen-specific T cells in association with production of ACPAs (87). Together, these data support that NET peptides can be an initial antigenic trigger of ACPAs and that this process likely occurs in the lungs, but further studies are necessary to understand if there is a causal link between NETs in the lung, ACPAs, and RA (Figure 4).
Figure 4.
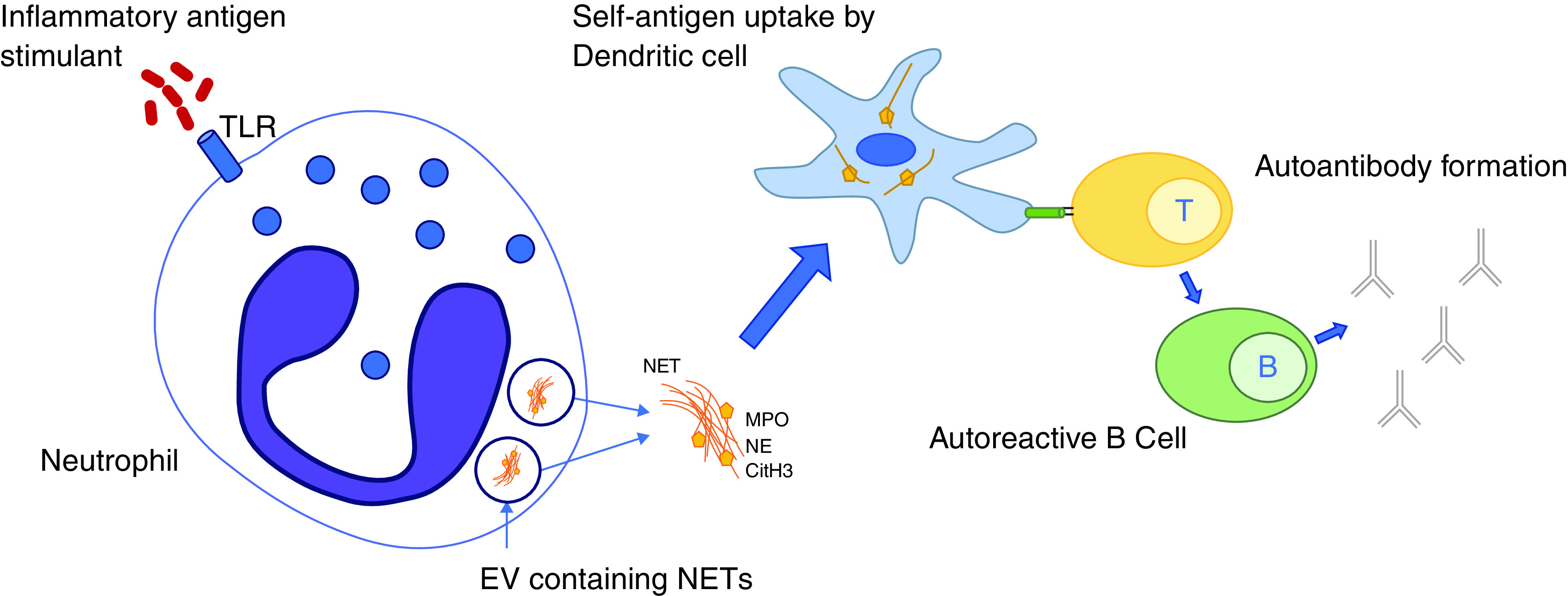
Neutrophil extracellular trap formation (NETosis) occurs when an antigenic stimulus, such as bacterial infection, stimulates the release of decondensed nucleolar material from the neutrophil, which, in addition to innate immune function capabilities, contains self-antigens. These self-antigens are then taken up by dendritic cells and through T and B cell interactions lead to the production of autoantibodies such as antibodies to citrullinated peptide antigens (ACPAs). CitH3 = citrullinated histone 3; EV = extracellular vesicle; MPO = myeloperoxidase; NE = neutrophil elastase; NET = neutrophil extracellular trap; TLR = Toll-like receptor.
Genome-wide association studies have unlocked the framework of underlying genetic risks for multiple diseases, including RA-ILD (88). RA-ILD is an interesting comparison to the other diseases discussed herein. Currently, management of RA lung disease varies significantly; RA-ILD is treated with aggressive immune suppression targeted at pulmonary manifestations of RA inflammation, whereas asthma or COPD in a patient with RA is thought of exclusively as a comorbidity. Meanwhile, the recent genetic insights into RA-ILD found shared genetic risks with a similar ILD, idiopathic pulmonary fibrosis (IPF). Might it be that RA-ILD is a more similar condition to IPF, and something about the ongoing lung injury predisposes to the development of RA in some individuals? This field remains an active area of discussion and research but has very important implications when considering asthma in a patient with RA or RA-bronchiectasis, given the genetic associations with cystic fibrosis already discussed. Genetic and epigenetic studies are necessary to understand how the interaction of environmental factors influences the coincidence of these diseases.
These RA-ILD and RA-bronchiectasis genetic insights also raise the specter of the usefulness of DMARD therapy in RA-related lung diseases. Perhaps the efficacy of DMARD therapy in lung disease for patients with RA is blunted because of the underlying genetic risks for lung manifestations that are not primarily inflammation driven, (i.e., such as IPF, in which outcomes are worse with an immune-suppressive approach [89]). Much work is still required to understand how treatment for lung disease in RA is best approached.
When considering model 2, presented here in Figure 2B, it is important to point out that airway abnormalities in RA (based on HRCT analysis) are high (30%) but not universal (71). Therefore, many patients with RA must develop RA in the absence of underlying airway involvement. It is also important to point out that in our current understanding of the role of ACPAs in lung disease, there is evidence of worsened outcomes for RA parenchymal disease but no data to date that describe the role of ACPAs in RA airway disease (90, 91). As such, it is important to understand if these patients with RA airway disease have distinct clinical phenotypes that lead to alterations in treatment response or other differential outcomes.
The next vital step for pulmonary physicians caring for patients with RA is to understand if airway disease in RA represents an RA manifestation (Figure 2B). Current approaches to management of these conditions (asthma, COPD, bronchiolitis, and bronchiectasis) in patients with RA largely ignores the potential that inflammation from RA contributes to the condition; however, given our limited understanding in these cases, this distinction may have important treatment considerations. Prospective, longitudinal work is required to understand this link between the airway and RA, which should include multiple specialties and research disciplines.
Another current limitation to understanding the impact of these disease states on one another is the nature of clinical and translational research study design, which typically studies a single disease in isolation. For instance, most studies of RA treatment outcomes would likely exclude someone with significant asthma, COPD, or bronchiectasis. We believe that many questions raised in this review will remain unanswered unless trials specifically designed with these two models in mind are undertaken. For instance, it is paramount to include subjects with RA with lung disease in treatment study designs to understand the impact of DMARD therapy for RA on airway diseases in coexistent states and vice versa. In addition, it is vital to undertake prospective evaluation of subjects with chronic lung disease, such as asthma, COPD, and bronchiectasis, who are serially monitored for the development of autoantibodies and subsequent RA to determine if there is validity to model 1 (Figure 2A).
Nearly all the data presented in this review are associative in nature. Given this, there are limitations to the conclusions that can be drawn. We caution clinicians and investigators when approaching this issue that more mechanistic studies are needed to better address the questions that are raised herein.
This review highlights the next steps in understanding this association between RA and the airways; however, it remains clear that the current standard of care of these patients should enlist in-depth consultation with diverse specialties, and decisions about treatment risks and benefits should be made in concert with pulmonary and rheumatology providers.
Footnotes
CME will be available for this article at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Gabriel SE. The epidemiology of rheumatoid arthritis. Rheum Dis Clin North Am . 2001;27:269–281. doi: 10.1016/s0889-857x(05)70201-5. [DOI] [PubMed] [Google Scholar]
- 2. Minaur NJ, Jacoby RK, Cosh JA, Taylor G, Rasker JJ. Outcome after 40 years with rheumatoid arthritis: a prospective study of function, disease activity, and mortality. J Rheumatol Suppl . 2004;69:3–8. [PubMed] [Google Scholar]
- 3. Luban S, Li ZG. Citrullinated peptide and its relevance to rheumatoid arthritis: an update. Int J Rheum Dis . 2010;13:284–287. doi: 10.1111/j.1756-185X.2010.01553.x. [DOI] [PubMed] [Google Scholar]
- 4. Restrepo JF, del Rincón I, Battafarano DF, Haas RW, Doria M, Escalante A. Clinical and laboratory factors associated with interstitial lung disease in rheumatoid arthritis. Clin Rheumatol . 2015;34:1529–1536. doi: 10.1007/s10067-015-3025-8. [DOI] [PubMed] [Google Scholar]
- 5. Rocha-Muñoz AD, Ponce-Guarneros M, Gamez-Nava JI, Olivas-Flores EM, Mejía M, Juárez-Contreras P, et al. Anti-cyclic citrullinated peptide antibodies and severity of interstitial lung disease in women with rheumatoid arthritis. J Immunol Res . 2015;2015:151626. doi: 10.1155/2015/151626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ytterberg AJ, Joshua V, Reynisdottir G, Tarasova NK, Rutishauser D, Ossipova E, et al. Shared immunological targets in the lungs and joints of patients with rheumatoid arthritis: identification and validation. Ann Rheum Dis . 2015;74:1772–1777. doi: 10.1136/annrheumdis-2013-204912. [DOI] [PubMed] [Google Scholar]
- 7. Holers VM, Demoruelle MK, Kuhn KA, Buckner JH, Robinson WH, Okamoto Y, et al. Rheumatoid arthritis and the mucosal origins hypothesis: protection turns to destruction. Nat Rev Rheumatol . 2018;14:542–557. doi: 10.1038/s41584-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bang SY, Lee KH, Cho SK, Lee HS, Lee KW, Bae SC. Smoking increases rheumatoid arthritis susceptibility in individuals carrying the HLA-DRB1 shared epitope, regardless of rheumatoid factor or anti-cyclic citrullinated peptide antibody status. Arthritis Rheum . 2010;62:369–377. doi: 10.1002/art.27272. [DOI] [PubMed] [Google Scholar]
- 9. Trouw LA, Haisma EM, Levarht EW, van der Woude D, Ioan-Facsinay A, Daha MR, et al. Anti-cyclic citrullinated peptide antibodies from rheumatoid arthritis patients activate complement via both the classical and alternative pathways. Arthritis Rheum . 2009;60:1923–1931. doi: 10.1002/art.24622. [DOI] [PubMed] [Google Scholar]
- 10. Sokolove J, Zhao X, Chandra PE, Robinson WH. Immune complexes containing citrullinated fibrinogen costimulate macrophages via Toll-like receptor 4 and Fcγ receptor. Arthritis Rheum . 2011;63:53–62. doi: 10.1002/art.30081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sokolove J, Johnson DS, Lahey LJ, Wagner CA, Cheng D, Thiele GM, et al. Rheumatoid factor as a potentiator of anti-citrullinated protein antibody-mediated inflammation in rheumatoid arthritis. Arthritis Rheumatol . 2014;66:813–821. doi: 10.1002/art.38307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, et al. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. J Clin Invest . 2012;122:1791–1802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamdan AL, Khalifee E, Berjawi G. Unilateral cricoarytenoid joint ankylosis in rheumatoid arthritis. Ear Nose Throat J . 2020;99:11–12. doi: 10.1177/0145561319825734. [DOI] [PubMed] [Google Scholar]
- 14. Hamdan AL, Sarieddine D. Laryngeal manifestations of rheumatoid arthritis. Autoimmune Dis . 2013;2013:103081. doi: 10.1155/2013/103081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pradhan P, Bhardwaj A, Venkatachalam VP. Bilateral cricoarytenoid arthritis: a cause of recurrent upper airway obstruction in rheumatoid arthritis. Malays J Med Sci . 2016;23:89–91. [PMC free article] [PubMed] [Google Scholar]
- 16. McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med . 2019;199:433–445. doi: 10.1164/rccm.201810-1944CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shen TC, Lin CL, Wei CC, Tu CY, Li YF. The risk of asthma in rheumatoid arthritis: a population-based cohort study. QJM . 2014;107:435–442. doi: 10.1093/qjmed/hcu008. [DOI] [PubMed] [Google Scholar]
- 18. Kim SY, Min C, Oh DJ, Choi HG. Increased risk of asthma in patients with rheumatoid arthritis: A longitudinal follow-up study using a national sample cohort. Sci Rep . 2019;9:6957. doi: 10.1038/s41598-019-43481-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zaccardelli A, Liu X, Ford JA, Cui J, Lu B, Chu SH, et al. Elevated anti-citrullinated protein antibodies prior to rheumatoid arthritis diagnosis and risks for chronic obstructive pulmonary disease or asthma. Arthritis Care Res (Hoboken) . 2021;73:498–509. doi: 10.1002/acr.24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ford JA, Liu X, Chu SH, Lu B, Cho MH, Silverman EK, et al. Asthma, chronic obstructive pulmonary disease, and subsequent risk for incident rheumatoid arthritis among women: a prospective cohort study. Arthritis Rheumatol . 2020;72:704–713. doi: 10.1002/art.41194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Olsson AR, Wingren G, Skogh T, Svernell O, Ernerudh J. Allergic manifestations in patients with rheumatoid arthritis. APMIS . 2003;111:940–944. doi: 10.1034/j.1600-0463.2003.1111004.x. [DOI] [PubMed] [Google Scholar]
- 22. Kaptanoglu E, Akkurt I, Sahin O, Hocaoglu S, Nacitarhan V, Elden H, et al. Prevalence of atopy in rheumatoid arthritis in Sivas, Turkey: a prospective clinical study. Rheumatol Int . 2004;24:267–271. doi: 10.1007/s00296-003-0369-1. [DOI] [PubMed] [Google Scholar]
- 23. Zein JG, Erzurum SC. Asthma is different in women. Curr Allergy Asthma Rep . 2015;15:28. doi: 10.1007/s11882-015-0528-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zein JG, Denson JL, Wechsler ME. Asthma over the adult life course: gender and hormonal influences. Clin Chest Med . 2019;40:149–161. doi: 10.1016/j.ccm.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 25. Fuseini H, Newcomb DC. Mechanisms driving gender differences in asthma. Curr Allergy Asthma Rep . 2017;17:19. doi: 10.1007/s11882-017-0686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sokka T, Toloza S, Cutolo M, Kautiainen H, Makinen H, Gogus F, et al. QUEST-RA Group Women, men, and rheumatoid arthritis: analyses of disease activity, disease characteristics, and treatments in the QUEST-RA study. Arthritis Res Ther . 2009;11:R7. doi: 10.1186/ar2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tengstrand B, Ahlmén M, Hafström I. The influence of sex on rheumatoid arthritis: a prospective study of onset and outcome after 2 years. J Rheumatol . 2004;31:214–222. [PubMed] [Google Scholar]
- 28. Luo Y, Fan X, Jiang C, Arevalo Molina AB, Salgado M, Xu J. Rheumatoid arthritis is associated with increased in-hospital mortality in asthma exacerbations: a nationwide study. Clin Rheumatol . 2018;37:1971–1976. doi: 10.1007/s10067-018-4114-2. [DOI] [PubMed] [Google Scholar]
- 29. Woodrick RS, Ruderman EM. Safety of biologic therapy in rheumatoid arthritis. Nat Rev Rheumatol . 2011;7:639–652. doi: 10.1038/nrrheum.2011.145. [DOI] [PubMed] [Google Scholar]
- 30. Dasgupta A, Radford K, Arnold DM, Thabane L, Nair P. The effects of rituximab on serum IgE and BAFF. Allergy Asthma Clin Immunol . 2013;9:39. doi: 10.1186/1710-1492-9-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holgate ST, Noonan M, Chanez P, Busse W, Dupont L, Pavord I, et al. Efficacy and safety of etanercept in moderate-to-severe asthma: a randomised, controlled trial. Eur Respir J. 2011;37:1352–1359. doi: 10.1183/09031936.00063510. [DOI] [PubMed] [Google Scholar]
- 32. Erin EM, Leaker BR, Nicholson GC, Tan AJ, Green LM, Neighbour H, Zacharasiewicz AS, Turner J, Barnathan ES, Kon OM, Barnes PJ, Hansel TT. The effects of a monoclonal antibody directed against tumor necrosis factor-alpha in asthma. Am J Respir Crit Care Med . 2006;174:753–762. doi: 10.1164/rccm.200601-072OC. [DOI] [PubMed] [Google Scholar]
- 33. Knarborg M, Hilberg O, Hoffmann HJ, Dahl R. Methotrexate as an oral corticosteroid-sparing agent in severe asthma: the emergence of a responder asthma endotype. Eur Clin Respir J . 2014;1:10.3402/ecrj.v1.25037. doi: 10.3402/ecrj.v1.25037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Davies H, Olson L, Gibson P. Methotrexate as a steroid sparing agent for asthma in adults. Cochrane Database Syst Rev . 2000;1998:CD000391. doi: 10.1002/14651858.CD000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zaccardelli A, Liu X, Ford JA, Cui J, Lu B, Chu SH, et al. Asthma and elevation of anti-citrullinated protein antibodies prior to the onset of rheumatoid arthritis. Arthritis Res Ther . 2019;21:246. doi: 10.1186/s13075-019-2035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet . 2016;388:2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 37. Deane KD, Norris JM, Holers VM. Preclinical rheumatoid arthritis: identification, evaluation, and future directions for investigation. Rheum Dis Clin North Am . 2010;36:213–241. doi: 10.1016/j.rdc.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kelmenson LB, Demoruelle MK, Deane KD. The complex role of the lung in the pathogenesis and clinical outcomes of rheumatoid arthritis. Curr Rheumatol Rep . 2016;18:69. doi: 10.1007/s11926-016-0618-4. [DOI] [PubMed] [Google Scholar]
- 39. Willis VC, Demoruelle MK, Derber LA, Chartier-Logan CJ, Parish MC, Pedraza IF, et al. Sputum autoantibodies in patients with established rheumatoid arthritis and subjects at risk of future clinically apparent disease. Arthritis Rheum . 2013;65:2545–2554. doi: 10.1002/art.38066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Modena BD, Tedrow JR, Milosevic J, Bleecker ER, Meyers DA, Wu W, et al. Gene expression in relation to exhaled nitric oxide identifies novel asthma phenotypes with unique biomolecular pathways. Am J Respir Crit Care Med . 2014;190:1363–1372. doi: 10.1164/rccm.201406-1099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li X, Hawkins GA, Ampleford EJ, Moore WC, Li H, Hastie AT, et al. Genome-wide association study identifies TH1 pathway genes associated with lung function in asthmatic patients. J Allergy Clin Immunol . 2013;132:313–20.e15. doi: 10.1016/j.jaci.2013.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Raundhal M, Morse C, Khare A, Oriss TB, Milosevic J, Trudeau J, et al. High IFN-γ and low SLPI mark severe asthma in mice and humans. J Clin Invest . 2015;125:3037–3050. doi: 10.1172/JCI80911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol . 1996;97:1288–1296. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 44. Voraphani N, Gladwin MT, Contreras AU, Kaminski N, Tedrow JR, Milosevic J, et al. An airway epithelial iNOS-DUOX2-thyroid peroxidase metabolome drives Th1/Th2 nitrative stress in human severe asthma. Mucosal Immunol . 2014;7:1175–1185. doi: 10.1038/mi.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Friedlander HM, Ford JA, Zaccardelli A, Terrio AV, Cho MH, Sparks JA. Obstructive lung diseases and risk of rheumatoid arthritis. Expert Rev Clin Immunol . 2020;16:37–50. doi: 10.1080/1744666X.2019.1698293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Prisco L, Moll M, Wang J, Hobbs BD, Huang W, Martin LW, et al. Relationship between rheumatoid arthritis and pulmonary function measures on spirometry in the UK Biobank. Arthritis Rheumatol . 2021;73:1994–2002. doi: 10.1002/art.41791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet . 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hoovestol RA, Mikuls TR. Environmental exposures and rheumatoid arthritis risk. Curr Rheumatol Rep . 2011;13:431–439. doi: 10.1007/s11926-011-0203-9. [DOI] [PubMed] [Google Scholar]
- 49. Heliövaara M, Aho K, Aromaa A, Knekt P, Reunanen A. Smoking and risk of rheumatoid arthritis. J Rheumatol . 1993;20:1830–1835. [PubMed] [Google Scholar]
- 50. Saag KG, Cerhan JR, Kolluri S, Ohashi K, Hunninghake GW, Schwartz DA. Cigarette smoking and rheumatoid arthritis severity. Ann Rheum Dis . 1997;56:463–469. doi: 10.1136/ard.56.8.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Silman AJ, Newman J, MacGregor AJ. Cigarette smoking increases the risk of rheumatoid arthritis: results from a nationwide study of disease-discordant twins. Arthritis Rheum . 1996;39:732–735. doi: 10.1002/art.1780390504. [DOI] [PubMed] [Google Scholar]
- 52. Klareskog L, Stolt P, Lundberg K, Källberg H, Bengtsson C, Grunewald J, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum . 2006;54:38–46. doi: 10.1002/art.21575. [DOI] [PubMed] [Google Scholar]
- 53. Celli BR, Wedzicha JA. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med . 2019;381:1257–1266. doi: 10.1056/NEJMra1900500. [DOI] [PubMed] [Google Scholar]
- 54. Ma Y, Tong H, Zhang X, Wang M, Yang J, Wu M, et al. Chronic obstructive pulmonary disease in rheumatoid arthritis: a systematic review and meta-analysis. Respir Res . 2019;20:144. doi: 10.1186/s12931-019-1123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, Vassallo R, et al. Incidence and mortality of obstructive lung disease in rheumatoid arthritis: a population-based study. Arthritis Care Res (Hoboken) . 2013;65:1243–1250. doi: 10.1002/acr.21986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sparks JA, Lin TC, Camargo CA, Jr, Barbhaiya M, Tedeschi SK, Costenbader KH, et al. Rheumatoid arthritis and risk of chronic obstructive pulmonary disease or asthma among women: A marginal structural model analysis in the Nurses’ Health Study. Semin Arthritis Rheum . 2018;47:639–648. doi: 10.1016/j.semarthrit.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hyldgaard C, Ellingsen T, Bendstrup E. COPD: an overlooked cause of excess mortality in patients with rheumatoid arthritis. Lancet Respir Med . 2018;6:326–327. doi: 10.1016/S2213-2600(18)30056-0. [DOI] [PubMed] [Google Scholar]
- 58. Hudson M, Dell’Aniello S, Shen S, Simon TA, Ernst P, Suissa S. Comparative safety of biologic versus conventional synthetic DMARDs in rheumatoid arthritis with COPD: a real-world population study. Rheumatology (Oxford) . 2020;59:820–827. doi: 10.1093/rheumatology/kez359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bergsøe CM, Sivapalan P, Saeed MI, Eklöf J, Saghir Z, Sørensen R, et al. Risk of chronic obstructive pulmonary disease exacerbation in patients who use methotrexate: a nationwide study of 58,580 outpatients. Biomedicines . 2021;9:604. doi: 10.3390/biomedicines9060604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Loza MJ, Watt R, Baribaud F, Barnathan ES, Rennard SI. Systemic inflammatory profile and response to anti-tumor necrosis factor therapy in chronic obstructive pulmonary disease. Respir Res . 2012;13:12. doi: 10.1186/1465-9921-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ruiz-Esquide V, Gómara MJ, Peinado VI, Gómez Puerta JA, Barberá JA, Cañete JdeD, et al. Anti-citrullinated peptide antibodies in the serum of heavy smokers without rheumatoid arthritis: a differential effect of chronic obstructive pulmonary disease? Clin Rheumatol . 2012;31:1047–1050. doi: 10.1007/s10067-012-1971-y. [DOI] [PubMed] [Google Scholar]
- 62. Packard TA, Li QZ, Cosgrove GP, Bowler RP, Cambier JC. COPD is associated with production of autoantibodies to a broad spectrum of self-antigens, correlative with disease phenotype. Immunol Res . 2013;55:48–57. doi: 10.1007/s12026-012-8347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shaw M, Collins BF, Ho LA, Raghu G. Rheumatoid arthritis-associated lung disease. Eur Respir Rev . 2015;24:1–16. doi: 10.1183/09059180.00008014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pipavath SJ, Lynch DA, Cool C, Brown KK, Newell JD. Radiologic and pathologic features of bronchiolitis. AJR Am J Roentgenol . 2005;185:354–363. doi: 10.2214/ajr.185.2.01850354. [DOI] [PubMed] [Google Scholar]
- 65. Jensen SP, Lynch DA, Brown KK, Wenzel SE, Newell JD. High-resolution CT features of severe asthma and bronchiolitis obliterans. Clin Radiol . 2002;57:1078–1085. doi: 10.1053/crad.2002.1104. [DOI] [PubMed] [Google Scholar]
- 66. Copley SJ, Wells AU, Müller NL, Rubens MB, Hollings NP, Cleverley JR, et al. Thin-section CT in obstructive pulmonary disease: discriminatory value. Radiology . 2002;223:812–819. doi: 10.1148/radiol.2233010760. [DOI] [PubMed] [Google Scholar]
- 67. Lu J, Ma M, Zhao Q, Meng F, Wang D, Cai H, et al. The clinical characteristics and outcomes of follicular bronchiolitis in Chinese adult patients. Sci Rep . 2018;8:7300. doi: 10.1038/s41598-018-25670-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Aerni MR, Vassallo R, Ryu JH. Successful treatment of follicular bronchiolitis with macrolide. Chest . 2005;128:428S. [Google Scholar]
- 69. Howling SJ, Hansell DM, Wells AU, Nicholson AG, Flint JD, Müller NL. Follicular bronchiolitis: thin-section CT and histologic findings. Radiology . 1999;212:637–642. doi: 10.1148/radiology.212.3.r99se04637. [DOI] [PubMed] [Google Scholar]
- 70. Duarte AC, Cordeiro A, Soares J, Gonçalves P. Follicular bronchiolitis, a frequently misdiagnosed condition. Pulmonology . 2019;25:62–64. doi: 10.1016/j.pulmoe.2019.02.002. [DOI] [PubMed] [Google Scholar]
- 71. Wilczynska MM, Condliffe AM, McKeon DJ. Coexistence of bronchiectasis and rheumatoid arthritis: revisited. Respir Care . 2013;58:694–701. doi: 10.4187/respcare.01857. [DOI] [PubMed] [Google Scholar]
- 72. Aronoff A, Bywaters EG, Fearnley GR. Lung lesions in rheumatoid arthritis. BMJ . 1955;2:228–232. doi: 10.1136/bmj.2.4933.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Allain J, Saraux A, Guedes C, Valls I, Devauchelle V, Le Goff P. Prevalence of symptomatic bronchiectasis in patients with rheumatoid arthritis. Rev Rhum Engl Ed . 1997;64:531–537. [PubMed] [Google Scholar]
- 74. McMahon MJ, Swinson DR, Shettar S, Wolstenholme R, Chattopadhyay C, Smith P, et al. Bronchiectasis and rheumatoid arthritis: a clinical study. Ann Rheum Dis . 1993;52:776–779. doi: 10.1136/ard.52.11.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bilgici A, Ulusoy H, Kuru O, Celenk C, Unsal M, Danaci M. Pulmonary involvement in rheumatoid arthritis. Rheumatol Int . 2005;25:429–435. doi: 10.1007/s00296-004-0472-y. [DOI] [PubMed] [Google Scholar]
- 76. Jenks KA, Stamp LK, O’Donnell JL, Savage RL, Chapman PT. Leflunomide-associated infections in rheumatoid arthritis. J Rheumatol . 2007;34:2201–2203. [PubMed] [Google Scholar]
- 77. Geri G, Dadoun S, Bui T, Del Castillo Pinol N, Paternotte S, Dougados M, et al. Risk of infections in bronchiectasis during disease-modifying treatment and biologics for rheumatic diseases. BMC Infect Dis . 2011;11:304. doi: 10.1186/1471-2334-11-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Flume PA, Chalmers JD, Olivier KN. Rheumatoid arthritis-associated bronchiectasis: authors’ reply. Lancet . 2019;393:2036. doi: 10.1016/S0140-6736(19)30012-1. [DOI] [PubMed] [Google Scholar]
- 79. Demoruelle MK, Weisman MH, Simonian PL, Lynch DA, Sachs PB, Pedraza IF, et al. Brief report: airways abnormalities and rheumatoid arthritis-related autoantibodies in subjects without arthritis: early injury or initiating site of autoimmunity? Arthritis Rheum . 2012;64:1756–1761. doi: 10.1002/art.34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Puéchal X, Fajac I, Bienvenu T, Desmazes-Dufeu N, Hubert D, Kaplan JC, et al. Increased frequency of cystic fibrosis deltaF508 mutation in bronchiectasis associated with rheumatoid arthritis. Eur Respir J . 1999;13:1281–1287. doi: 10.1183/09031936.99.13612889. [DOI] [PubMed] [Google Scholar]
- 81. Puéchal X, Bienvenu T, Génin E, Berthelot JM, Sibilia J, Gaudin P, et al. Mutations of the cystic fibrosis gene in patients with bronchiectasis associated with rheumatoid arthritis. Ann Rheum Dis . 2011;70:653–659. doi: 10.1136/ard.2010.142760. [DOI] [PubMed] [Google Scholar]
- 82. Elkayam O, Segal R, Bendayan D, van Uitert R, Onnekink C, Pruijn GJ. The anti-cyclic citrullinated peptide response in tuberculosis patients is not citrulline-dependent and sensitive to treatment. Arthritis Res Ther . 2010;12:R12. doi: 10.1186/ar2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Demoruelle MK, Harrall KK, Ho L, Purmalek MM, Seto NL, Rothfuss HM, et al. Anti-citrullinated protein antibodies are associated with neutrophil extracellular traps in the sputum in relatives of rheumatoid arthritis patients. Arthritis Rheumatol . 2017;69:1165–1175. doi: 10.1002/art.40066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, et al. NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med . 2013;5:178ra40. doi: 10.1126/scitranslmed.3005580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Demoruelle MK, Bowers E, Lahey LJ, Sokolove J, Purmalek M, Seto NL, et al. Antibody responses to citrullinated and noncitrullinated antigens in the sputum of subjects with rheumatoid arthritis and subjects at risk for development of rheumatoid arthritis. Arthritis Rheumatol . 2018;70:516–527. doi: 10.1002/art.40401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Okamoto Y, et al. Sputum neutrophil extracellular trap subsets associate with IgA anti-citrullinated protein antibodies in subjects at-risk for rheumatoid arthritis Arthritis Rheumatol 2021 10.1002/art.41948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Carmona-Rivera C, Carlucci PM, Moore E, Lingampalli N, Uchtenhagen H, James E, et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci Immunol . 2017;2:eaag3358. doi: 10.1126/sciimmunol.aag3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Juge P-A, Lee JS, Ebstein E, Furukawa H, Dobrinskikh E, Gazal S, et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med . 2018;379:2209–2219. doi: 10.1056/NEJMoa1801562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Raghu G, Anstrom KJ, King TE, Jr, Lasky JA, Martinez FJ, Idiopathic Pulmonary Fibrosis Clinical Research Network Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med . 2012;366:1968–1977. doi: 10.1056/NEJMoa1113354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Giles JT, Danoff SK, Sokolove J, Wagner CA, Winchester R, Pappas DA, et al. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis . 2014;73:1487–1494. doi: 10.1136/annrheumdis-2012-203160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Zhu J, Zhou Y, Chen X, Li J. A metaanalysis of the increased risk of rheumatoid arthritis-related pulmonary disease as a result of serum anticitrullinated protein antibody positivity. J Rheumatol . 2014;41:1282–1289. doi: 10.3899/jrheum.131341. [DOI] [PubMed] [Google Scholar]
- 92. Quirke AM, Perry E, Cartwright A, Kelly C, De Soyza A, Eggleton P, et al. Bronchiectasis is a model for chronic bacterial infection inducing autoimmunity in rheumatoid arthritis. Arthritis Rheumatol . 2015;67:2335–2342. doi: 10.1002/art.39226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Devouassoux G, Cottin V, Lioté H, Marchand E, Frachon I, Schuller A, et al. Groupe d’Etudes et de Recherche sur les Maladies “Orphelines” Pulmonaires (GERM“O”P) Characterisation of severe obliterative bronchiolitis in rheumatoid arthritis. Eur Respir J . 2009;33:1053–1061. doi: 10.1183/09031936.00091608. [DOI] [PubMed] [Google Scholar]
- 94. Fernández Pérez ER, Krishnamoorthy M, Brown KK, Huie TJ, Fischer A, Solomon JJ, et al. FEV1 over time in patients with connective tissue disease-related bronchiolitis. Respir Med . 2013;107:883–889. doi: 10.1016/j.rmed.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kinoshita M, Higashi T, Tanaka C, Tokunaga N, Ichikawa Y, Oizumi K. Follicular bronchiolitis associated with rheumatoid arthritis. Intern Med . 1992;31:674–677. doi: 10.2169/internalmedicine.31.674. [DOI] [PubMed] [Google Scholar]