Visual Abstract
Learning Objectives
Understand the evidence base for the safety and efficacy of caplacizumab in iTTP, including through comparison of observational data
Assess the bleeding risk associated with caplacizumab use in iTTP
Analyze the cost-effectiveness of caplacizumab in the treatment of iTTP
CLINICAL CASE
A 21-year-old woman with a history of Hashimoto's thyroiditis presented with microangiopathic hemolytic anemia, headaches, and a petechial rash accompanied by new-onset menorrhagia. Her platelet count was 10 000/µl (normal, 150 000-400 000/µl), her lactate dehydrogenase (LDH) level was 1199 IU/L (normal, 94-250 IU/L), and her PLASMIC score was computed to be 7 (high pretest probability of severe ADAMTS13 deficiency). Therapeutic plasma exchange (TPE) and prednisone were initiated immediately. Plasma ADAMTS13 activity returned at less than 5% (normal, ≥67%), with an inhibitor titer of 1.4 Bethesda units (normal, <0.4 units), confirming the diagnosis of immune thrombotic thrombocytopenic purpura (iTTP). The patient's platelet count rose rapidly, reaching 247 000/µl after 6 daily TPE treatments. During the same period, her LDH declined to 145 IU/L, accompanied by resolution of her presenting symptoms. The patient received the first of 4 doses of rituximab and was subsequently discharged home on hospital day 8. In unselected patients with iTTP, does the routine addition of caplacizumab to TPE improve outcomes compared to TPE, corticosteroids, and rituximab alone?
Caplacizumab is a bivalent, single-domain antibody approved for use in unselected patients (all-comers) with iTTP and functions by inhibiting the interaction between platelets and the von Willebrand factor A1 domain. As the first novel therapy for iTTP in decades, caplacizumab could be a major step toward the long-sought goal of replacing TPE in the management of this disease. The administration of caplacizumab rapidly raises the platelet count in most iTTP patients and significantly shortens the time to platelet count normalization.1 In the randomized trial data, caplacizumab is associated with a reduction in TPE treatments and hospital length of stay as well as in the composite outcome of iTTP- related death, disease recurrence, or major thromboembolic events. In considering the potential benefits of caplacizumab, enthusiasm must be balanced against concerns about its efficacy, its safety, and its high cost (~$270 000- $450 000 per course). Here, we evaluate whether in unselected patients with iTTP the routine addition of caplacizumab improves outcomes compared to a TPE-based regimen alone.
Assessing efficacy and unmet need
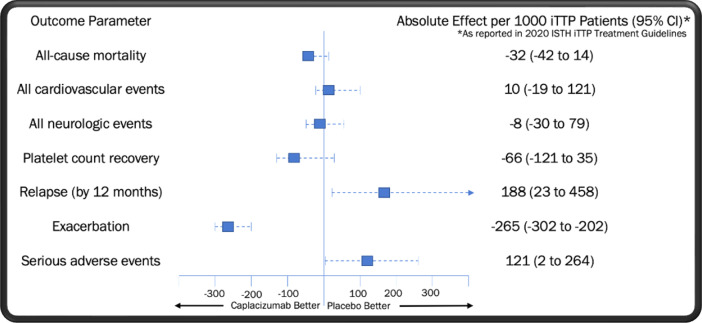
Two high-quality randomized controlled trials of caplacizumab in iTTP have been performed: TITAN (phase 2) and HERCULES (phase 3).1,2 Caplacizumab met its primary end point in these studies, reducing the median time to platelet count recovery by approximately 4.6 hours in HERCULES and 1.9 days in TITAN compared to TPE alone.1,2 In further examining the pooled trial results, we note the hierarchy of outcomes in iTTP from most to least clinically important as outlined by the expert panel on iTTP management convened by the International Society on Thrombosis and Haemostasis (ISTH)3: (1) all-cause mortality, (2) all cardiovascular events, (3) neurological events, (4) platelet count recovery, (5) relapse, (6) time to relapse, (7) acute renal injury or need for dialysis, (8) length of stay, (9) exacerbation of disease, and (10) normalization of ADAMTS13 level.
In Table 1, we display the pooled odds ratios (OR) for each of these outcomes as determined by the ISTH expert committee via the fixed-effect method.3 No significant improvement was seen in all-cause mortality or platelet count recovery at 28 to 30 days with the addition of caplacizumab. Nevertheless, it is important to evaluate the trend toward mortality benefit found in the group receiving caplacizumab (OR = 0.27; 95% CI, 0.05-1.34), as even a small improvement in survival may be of clinical relevance. Three pieces of evidence should be considered when assessing whether this observation is the result of underpowering, meaning that the true OR favors treatment with caplacizumab. First, no similar signal for benefit was seen in the occurrence of cardiovascular events, an expected driver of mortality in iTTP, with patients who received caplacizumab displaying a modest trend toward an increased risk of adverse cardiovascular outcomes. Second, 3 observational studies utilizing historical controls did not identify a statistically significant improvement in all-cause mortality with the use of caplacizumab.4-6 Third, caplacizumab was associated with a significantly increased risk of iTTP relapse in the US Food and Drug Administration registration trials, with the rate of fatal relapses not reported in any study to date. Unmeasured deaths from relapses linked to caplacizumab could cause the true mortality rate in patients receiving the drug to be underestimated.
Table 1.
Pooled outcomes from randomized trials of caplacizumaba
| Clinical importance rankingb | Outcome | Caplacizumab | Placebo | Relative effect: odds ratio (95% CI) | Absolute effect: per 1000 iTTP patients (95% CI) |
|---|---|---|---|---|---|
| 1 | All-cause mortality | 1/108 (0.9%) | 5/112 (4.5%) | 0.27 (0.05-1.34) | −32 (−42 to 14) |
| 2 | All cardiovascular events | 4/106 (3.8%) | 3/110 (2.7%) | 1.39 (0.31-6.23) | 10 (−19 to 121) |
| 3 | Neurologic events | 3/106 (2.8%) | 4/110 (3.6%) | 0.77 (0.17-3.47) | −8 (−30 to 79) |
| 4 | Platelet count recovery | 96/108 (88.9%) | 92/112 (82.1%) | 1.71 (0.8-3.63) | −66 (−121 to 35) |
| 5 | Relapse (at 1 mo) | 14/108 (13.0%) | 0/112 (0.0%) | 9.08 (3.06-26.89) | Not estimable (0 events in placebo) |
| 5 | Relapse (at 12 mo)c | 11/36 (30.6%) | 3/37 (8.1%) | 4.17 (1.31-13.27) | 188 (23 to 458) |
| 6 | Time to relapse | Not reported | Not reported | N/A | N/A |
| 7 | Acute renal injury/dialysis | Not reported | Not reported | N/A | N/A |
| 8 | Days in hospitald | Mean (95% CI) difference: 4.5 (7.32-1.68) days lower with caplacizumab | N/A | N/A | |
| 8 | Days of TPE | Mean (95% CI) difference: 3.69 (5.35-2.02) days lower with caplacizumab | N/A | N/A | |
| 9 | Exacerbation | 6/108 (5.6%) | 39/112 (34.8%) | 0.17 (0.09-0.32) | −265 (−302 to −202) |
| 10 | Normal ADAMTS13 level after TPE | Not reported | Not reported | N/A | N/A |
| N/A | Serious adverse events | 36/106 (34.0%) | 24/110 (21.8%) | 1.84 (1.01-3.34) | 121 (2 to 264) |
As reported in the 2020 ISTH iTTP Treatment Guidelines supplement.
As defined by the 2020 ISTH iTTP Treatment Guidelines.
Data only available for TITAN trial.
Data only available for HERCULES trial.
A closer look at the HERCULES trial data reveals that improvement in the composite outcome seen with caplacizumab was driven by a reduction in iTTP exacerbations,1 a metric ranked ninth out of 10 in clinical importance by the ISTH committee. Exacerbations consist of a decrease in the platelet count to less than 150 000/µl together with an increase in LDH within 30 days of discontinuing TPE.7 These episodes, which occur in approximately 30% to 40% of patients and are rarely symptomatic, invariably require the resumption of TPE.1 While cumbersome to manage and distressing for patients, practically all exacerbations resolve uneventfully with continued TPE, consistent with the low clinical priority assigned to these events by the ISTH panel.
In modeling the benefit of caplacizumab as part of their submission to the National Institute for Health and Care Excellence,8,9 Sanofi anticipated an absolute mortality rate of 3.8% per iTTP episode in patients receiving caplacizumab at specialized iTTP “centers of excellence.” This outcome is similar to what is routinely being achieved without caplacizumab in many large academic medical centers throughout the United States.10-12 To further evaluate this question, we compared data from 4 recent observational studies of caplacizumab against results from the pooled placebo arms of the TITAN and HERCULES trials as well as the experience without caplacizumab in the Harvard TMA Research Collaborative (Table 2).1,2 With the caveat of cross-study comparisons, no clear mortality benefit was seen in patients who received caplacizumab, and hospital length of stay appeared similar between the 2 groups.
Table 2.
Comparison of key outcome measures in patients treated with caplacizumab vs standard of care
| Parameter | Caplacizumab observational experience A14 | Caplacizumab observational experience B4 | Caplacizumab observational experience C5 | Caplacizumab observational experience D6 | Pooled RCT placebo arms1,2 | Harvard collaborative without caplacizumab11,17,18 |
|---|---|---|---|---|---|---|
| Number of subjects | 60 | 90 | 85 | 44 | 112 | 124 |
| Mortality per episode (%) | 1.7 | 1.1 | 6.0 | 4.5 | 4.5 | 3.7 |
| Number of TPE sessions | 9 (2-41)a | 5 (4-7) | 7 (5-14) | 8.5 (6-12.5) | 10.2b | 15 (8-23) |
| Hospital LOS (days) | 18 (5-79)a | 13 (9-19) | 12 (8-24) | 12 (9-15) | 12 (4-53)c | 12 (8-20) |
All values reported as median (interquartile range) unless otherwise noted.
Reported as median (range).
Weighted mean from both studies.
Reported in HERCULES trial only.
LOS, length of stay; NR, not reported.
Evaluating safety
Within hours of administration, caplacizumab induces a severe acquired type 2M von Willebrand disease.13 Accordingly, in the randomized trial data caplacizumab is associated with higher rates of adverse bleeding-related events,1,2 the type and severity of which are generally not observed in iTTP patients managed primarily with TPE.11 Examination of the HERCULES results reveals several hemorrhagic complications that were unique to the treatment arm, including upper and lower gastrointestinal hemorrhage (9 events), abdominal wall hematoma (1 event), injection site hemorrhage (3 events), hemorrhagic stroke (1 event), eye hemorrhage (1 event), hemorrhagic ovarian cyst (1 event), subarachnoid hemorrhage (1 event), and hemoptysis (2 events).1 Bleeding-related complications unique to the placebo arm included vessel puncture site bruising (2 events), mouth hemorrhage (1 event), hemorrhagic transformation of stroke (1 event), and postprocedural hematoma (1 event). Consistent with these outcomes, bleeding events drove the ISTH panel's finding that caplacizumab significantly increases serious adverse events compared to TPE (OR, 1.84; 95% CI, 1.01-3.34).3
All large observational studies of caplacizumab to date confirm the high rate of hemorrhagic complications in patients receiving the drug, though nonstandard or incomplete reporting limits the utility of these experiences in assessing risk.4-6,14 Concerns around bleeding were further highlighted by 2 recent case series. In 20 patients treated initially with caplacizumab instead of TPE, 1 individual experienced a subdural hematoma requiring neurosurgical intervention and rescue therapy with von Willebrand factor concentrate.15 Another series reported that 1 of 77 patients treated with caplacizumab experienced fatal intracranial hemorrhage soon after receiving the drug.6 By contrast, no episodes of intracranial hemorrhage were observed in 219 consecutive episodes of iTTP treated with TPE during 13 years of data collected by the Harvard registry.11
In the TITAN and HERCULES studies, caplacizumab use was associated with fewer TPE treatments and a more than 4-fold increase in iTTP relapse compared to placebo.1,2 Because caplacizumab increases platelet count rapidly but does not influence the production of autoantibody, it is possible that patients receiving the drug may be undertreated with TPE. However, a higher proportion of the placebo arms in both studies received rituximab, which may have contributed to the marked disparity in relapse rates. Further research is required to determine if this observation was due to undertreatment with TPE, inadequate immunosuppression in the caplacizumab arms, or another cause.
Cost-effectiveness considerations
At current pricing, the cost of a single course of caplacizumab can approach US $500 000. Given that a highly effective standard of care already exists for iTTP, we sought to understand whether the cost of caplacizumab is justified. Using decision tree analysis and Markov modeling to evaluate cost-effectiveness, we found that (1) the cost of managing a case of TTP rises almost 4-fold with the use of caplacizumab, (2) the price of caplacizumab would have to fall by approximately 80% for the drug to be minimally cost-effective, and (3) the average hospital stay in patients receiving TPE alone would have to more than triple for any decrease in inpatient time associated with caplacizumab to generate savings.16 At an incremental cost-effectiveness ratio of US $1.48 million per quality-adjusted life-year, caplacizumab is not cost-effective from a US health systems perspective. For comparison, a conservative (higher-end) willingness-to-pay threshold of 3 times per capita gross domestic product was US $195 300 per quality-adjusted life-year in 2019. Our modeling utilized a best-case scenario that is highly favorable to caplacizumab, such as assuming the drug carries a mortality benefit, muting the impact of caplacizumab-associated relapse, using disease and remission utilities preset to minimize the incremental cost-effectiveness ratio, and entirely ignoring drug-induced bleeding events. Given these factors, it is likely that the actual costs associated with the use of caplacizumab are significantly higher than estimated by our analysis.
Conclusion and recommendations
We believe that the available data do not support the routine use of caplacizumab in unselected patients with iTTP (grade IB). Major outcomes of clinical concern such as mortality and cardiovascular and neurological events are not significantly reduced by caplacizumab. The possible up-front reduction in TPE requirement and hospital stay associated with caplacizumab must be balanced against the significant safety signals seen for relapse and hemorrhage, the full impacts of which are not captured in the current literature. We also note that the adjunctive use of caplacizumab is cost-ineffective at its current US price point, raising questions of equity, fair access, and health systems impact. Further data may demonstrate that caplacizumab has value in the management of certain iTTP subpopulations, such as critically ill or refractory patients, those with severe allergic reactions to plasma, or those with the high pretest probability of severe ADAMTS13 deficiency being seen in settings without ready access to TPE. We await future studies to clarify the role of this novel therapy in the management of iTTP.
Conflict-of-interest disclosure
George Goshua: no competing financial interests to declare.
Pavan K. Bendapudi: no competing financial interests to declare.
Off-label drug use
George Goshua: off-label use of rituximab in iTTP is discussed.
Pavan K. Bendapudi: off-label use of rituximab in iTTP is discussed.
References
- 1.Scully M, Cataland SR, Peyvandi F, et al; HERCULES Investigators. Caplacizumab treatment for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2019;380(4):335-346. doi: 10.1056/NEJMoa1806311. [DOI] [PubMed] [Google Scholar]
- 2.Peyvandi F, Scully M, Kremer Hovinga JA, et al; TITAN Investigators. Caplacizumab for acquired thrombotic thrombocytopenic purpura. N Engl J Med. 2016;374(6):511-522. doi: 10.1056/NEJMoa1505533. [DOI] [PubMed] [Google Scholar]
- 3.Zheng XL, Vesely SK, Cataland SR, et al.. ISTH guidelines for treatment of thrombotic thrombocytopenic purpura. J Thromb Haemost. 2020;18(10):2496-2502. doi: 10.1111/jth.15010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coppo P, Bubenheim M, Azoulay E, et al.. A regimen with caplacizumab, immunosuppression, and plasma exchange prevents unfavorable outcomes in immune-mediated TTP. Blood. 2021;137(6):733-742. doi: 10.1182/blood.2020008021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutt T, Shaw RJ, Stubbs M, et al.. Real-world experience with caplacizumab in the management of acute TTP. Blood. 2021;137(13):1731-1740. doi: 10.1182/blood.2020007599. [DOI] [PubMed] [Google Scholar]
- 6.Pascual Izquierdo MC, Mingot-Castellano ME, Kerguelen Fuentes AE, et al.. Real-world effectiveness of caplacizumab vs standard of care in immune thrombotic thrombocytopenic purpura [published online 5 August 2022]. Blood Adv. doi: 10.1182/bloodadvances.2022008028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scully M, Cataland S, Coppo P, et al; International Working Group for Thrombotic Thrombocytopenic Purpura. Consensus on the standardization of terminology in thrombotic thrombocytopenic purpura and related thrombotic microangiopathies. J Thromb Haemost. 2017;15(2):312-322. doi: 10.1111/jth.13571. [DOI] [PubMed] [Google Scholar]
- 8.Hughes M, Prescott C, Elliott N, Adler AI. NICE guidance on caplacizumab for treating acute acquired thrombotic thrombocytopenia purpura. Lancet Haematol. 2021;8(1):e14-e15. doi: 10.1016/S2352-3026(20)30406-3. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Health and Care Excellence. Final appraisal document: caplacizumab with plasma exchange and immunosuppression for treating acute acquired thrombotic thrombocytopenic purpura. https://www.nice.org.uk/guidance/ta667/documents/final-appraisal-determination-document. Accessed 18 October 2022.
- 10.Goshua G, Prasad V, Lee AI, Bendapudi PK. Accurate accounting of caplacizumab cost effectiveness. Lancet Haematol. 2021;8(5):e315. doi: 10.1016/S2352-3026(21)00052-1. [DOI] [PubMed] [Google Scholar]
- 11.Colling M, Sun L, Upadhyay V, et al.. Deaths and complications associated with the management of acute immune thrombotic thrombocytopenic purpura. Transfusion. 2020;60(4):841-846. doi: 10.1111/trf.15721. [DOI] [PubMed] [Google Scholar]
- 12.Chaturvedi S, Antun AG, Farland A, et al.. Race, rituximab, and relapse in TTP. Blood. 2022;140(12):1335-1344. doi: 10.1182/blood.2022016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Völker LA, Kaufeld J, Miesbach W, et al.. ADAMTS13 and VWF activities guide individualized caplacizumab treatment in patients with aTTP. Blood Adv. 2020;4(13):3093-3101. doi: 10.1182/bloodadvances.2020001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Völker LA, Kaufeld J, Miesbach W, et al.. Real-world data confirm the effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura. Blood Adv. 2020;4(13):3085-3092. doi: 10.1182/bloodadvances.2020001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knöbl P EK, Buxhofer-Ausch V, Thaler J, Gleixner K, Sperr W. Management of thrombotic thrombocytopenic purpura (TTP) without plasma exchange: an update on the Austrian experience. Paper presented at: ISTH Congress 2022; London, UK; 2022. [Google Scholar]
- 16.Goshua G, Sinha P, Hendrickson JE, Tormey C, Bendapudi PK, Lee AI. Cost effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura. Blood. 2021;137(7):969-976. doi: 10.1182/blood.2020006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bendapudi PK, Li A, Hamdan A, et al.. Impact of severe ADAMTS13 deficiency on clinical presentation and outcomes in patients with thrombotic microangiopathies: the experience of the Harvard TMA Research Collaborative. Br J Haematol. 2015;171(5):836-844. doi: 10.1111/bjh.13658. [DOI] [PubMed] [Google Scholar]
- 18.Sun L, Mack J, Li A, et al.. Predictors of relapse and efficacy of rituximab in immune thrombotic thrombocytopenic purpura. Blood Adv. 2019;3(9):1512-1518. doi: 10.1182/bloodadvances.2019031039. [DOI] [PMC free article] [PubMed] [Google Scholar]