Abstract
Background: Human albumin (HA) infusion is potentially effective for the management of hyponatremia in liver cirrhosis, but the current evidence is very limited. Methods: In this retrospective study, 2414 cirrhotic patients who were consecutively admitted to our hospital between January 2010 and June 2014 were included in the Hospitalization outcome cohort, and 339 cirrhotic patients without malignancy who were consecutively admitted to our department between December 2014 and April 2021 were included in the Long-term outcome cohort. The development and improvement of hyponatremia were compared between patients who received HA infusion during hospitalizations and did not. Logistic and Cox regression analyses were performed to evaluate the association of development and improvement of hyponatremia during hospitalizations with the outcomes. Odds ratios (ORs) and hazard ratios (HRs) were calculated. Results: In the two cohorts, HA infusion significantly decreased the incidence of hyponatremia and increased the rate of improvement of hyponatremia in cirrhotic patients during hospitalizations. In the Hospitalization outcome cohort, the development of hyponatremia during hospitalizations was significantly associated with increased in-hospital mortality (OR = 2.493, p < 0.001), and the improvement of hyponatremia during hospitalizations was significantly associated with decreased in-hospital mortality (OR = 0.599, p = 0.014). In the Long-term outcome cohort, the development of hyponatremia during hospitalizations was significantly associated with decreased long-term survival (HR = 0.400, p < 0.001), and the improvement of hyponatremia during hospitalizations was not significantly associated with long-term survival (HR = 1.085, p = 0.813). Conclusions: HA infusion can effectively prevent the development of hyponatremia and improve hyponatremia in cirrhotic patients during hospitalizations, which may influence the patients’ outcomes.
Keywords: liver cirrhosis, hyponatremia, human albumin, prevention, treatment
1. Introduction
Hyponatremia is the most common electrolyte disorder in liver cirrhosis [1]. It has been reported that 49.40%, 21.60%, and 1.20% of patients with liver cirrhosis and ascites have a serum sodium level of <135, 130, and 120 mmol/L, respectively [2]. Hyponatremia is associated with increased morbidity and mortality [3,4]. Notably, serum sodium level has been incorporated in the model for end-stage liver disease (MELD) score to determine the priority of liver transplantation [5]. Correction of hyponatremia can improve the cognitive function and quality of life in patients with liver cirrhosis [6,7], but its benefits on the prognosis remain unclear.
Until now, the treatment of hyponatremia in liver cirrhosis remains a clinical challenge [1]. Fluid restriction is often ineffective [8], diuretics withdrawal can worsen the severity of ascites [1], hypertonic saline is reserved for patients with severe hyponatremia and its secondary potentially life-threatening complications [9,10], and vaptans have not been sufficiently approved in clinical practice [9,10]. Human albumin (HA), which has been recommended to manage hepatorenal syndrome, spontaneous bacterial peritonitis, and large volume paracentesis [11,12,13], seems to be effective for the management of hyponatremia. However, the recommendations are heterogeneous among the current guidelines [9,10,14,15], primarily due to the lack of relevant evidence. To the best of our knowledge, no study has specifically explored the role of HA infusion on the prevention of hyponatremia in patients with liver cirrhosis, and only four cohort studies [16,17,18,19] have evaluated the role of HA infusion on the treatment of hyponatremia in patients with liver cirrhosis. Notably, among these published studies, the study design, severity of liver cirrhosis, and outcome assessment are heterogeneous.
For these reasons, our current study has three-fold objectives: (1) to clarify whether HA infusion could prevent the development of hyponatremia in liver cirrhosis; (2) to evaluate whether HA infusion could improve the severity of hyponatremia in liver cirrhosis; and (3) to explore whether the development and improvement of hyponatremia could influence the short- and long-term outcomes of patients with liver cirrhosis.
2. Methods
2.1. Study Design
This retrospective observational study was approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command. The ethical approval number is Y2022-087. It includes two parts (i.e., Hospitalization outcome cohort and Long-term outcome cohort). In the Hospitalization outcome cohort, potentially eligible patients were screened from our retrospective database where all patients with a diagnosis of liver cirrhosis consecutively admitted to our hospital from January 2010 to June 2014 were enrolled and their outcomes during hospitalizations were observed [20]. In the Long-term outcome cohort, potentially eligible patients were screened from our prospective database where all patients with a diagnosis of liver cirrhosis and without malignancy consecutively admitted to the Department of Gastroenterology of our hospital from December 2014 to April 2021 were enrolled and their outcomes during follow-up were observed [21]. If serum sodium level was measured at least twice during hospitalizations, the patients would be considered in the current study.
Liver cirrhosis was diagnosed based on disease history, laboratory tests, endoscopic findings, ultrasonographic findings, and liver histology, if available. Hyponatremia was defined as a serum sodium level of <135 mmol/L, and the severity of hyponatremia was classified as mild (135–130 mmol/L), moderate (130–125 mmol/L), and severe (<125 mmol/L) [9,10]. Hyponatremia at admission was defined as the first serum sodium level measured at admission was <135 mmol/L. Hyponatremia during hospitalizations was defined as the first serum sodium level measured at admission was within the reference range (i.e., 135–145 mmol/L), but the serum sodium level rechecked during hospitalizations was <135 mmol/L. As mentioned in our previous study [20], HA was prescribed at the discretion of attending physicians, and its primary indications mainly included post-paracentesis, ascites, and hypoalbuminemia. Based on the current practice guideline, the treatments of hyponatremia mainly included water restriction, withdrawal of diuretics, hypertonic saline, and tolvaptan [22].
The data were collected regarding demographics (i.e., age and sex), etiology of liver cirrhosis (i.e., hepatitis B virus (HBV), hepatitis C virus (HCV), and alcohol), regular laboratory data (i.e., hemoglobin (Hb), white blood cell (WBC), platelet (PLT), total bilirubin (TBIL), albumin (ALB), alanine aminotransferase (ALT), alkaline phosphatase (AKP), serum creatinine (Scr), sodium (Na), potassium (K), prothrombin time (PT), and international normalized ratio (INR)). The patients’ conditions (i.e., hepatocellular carcinoma (HCC), hypokalemia, acute upper gastrointestinal bleeding (AUGIB), infection, ascites, and paracentesis) and drugs (i.e., desmopressin, terlipressin, furosemide, torasemide, spironolactone, hydrochlorothiazide, bumetanide, hypertonic saline, tolvaptan, and K supplement) that may affect serum sodium level were collected. The use of HA infusion and its dosage were also collected. All-cause death was recorded. Child–Pugh and MELD scores [23] were calculated.
2.2. Prevention of Hyponatremia
When the role of HA infusion for the prevention of hyponatremia was explored, the patients who underwent hemodialysis during hospitalizations or were diagnosed with hypernatremia or hyponatremia at admission were further excluded. Eligible patients assigned to the HA group should have received HA infusion before the development of hyponatremia or the last measurement of serum sodium level during hospitalizations. Otherwise, the remaining eligible patients were assigned to the control group. The development of hyponatremia was the outcome of interest as well as death. The development of hyponatremia was defined as hyponatremia was not observed at admission, but hyponatremia developed during hospitalizations.
2.3. Treatment of Hyponatremia
When the role of HA infusion for the treatment of hyponatremia was explored, the patients who underwent hemodialysis during hospitalizations, were diagnosed with hypernatremia at admission, or did not recheck serum sodium level after diagnosis of hyponatremia were further excluded. Eligible patients assigned to the HA group should have received HA infusion during the period from the diagnosis of hyponatremia to the last measurement of serum sodium level. Otherwise, the remaining eligible patients were assigned to the control group. The improvement of hyponatremia was the outcome of interest as well as death. The improvement of hyponatremia was defined as a reduction in the severity of hyponatremia.
2.4. Statistical Analyses
Continuous variables were reported as mean ± standard deviation and median (range) and compared by the non-parametric Mann–Whitney U test. Categorical variables were reported as frequency (percentage) and compared by the chi-square test. A 1:1 propensity score matching (PSM) analysis was performed. The matching factors included age, sex, Child–Pugh score, MELD score, hypokalemia, AUGIB, infection, ascites, paracentesis, desmopressin, terlipressin, furosemide, torasemide, spironolactone, hydrochlorothiazide, bumetanide, tolvaptan, hypertonic saline, and K supplement. Logistic regression analyses were conducted to explore the relationships of HA infusion with the development/improvement of hyponatremia and the effects of the development/improvement of hyponatremia on in-hospital death. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Cox regression analyses were also performed to explore the effects of the development/improvement of hyponatremia on long-term survival. Hazard ratios (HRs) and 95% CIs were calculated. Subgroup analyses were performed according to the presence of HCC and ascites and the use of paracentesis, if possible. In the Long-term outcome cohort, Kaplan–Meier curves were further drawn to demonstrate the cumulative survival and compared by the Log-rank test, and subgroup analyses were performed according to the use of HA infusion. A two-tailed p < 0.05 was considered statistically significant. All statistical analyses were performed with IBM SPSS 20.0 (IBM Crop, Armonk, NY, USA) software, Stata/SE 12.0 (Stata Corp, College Station, TX, USA) software, and GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) software.
3. Results
3.1. Hospitalization Outcome Cohort
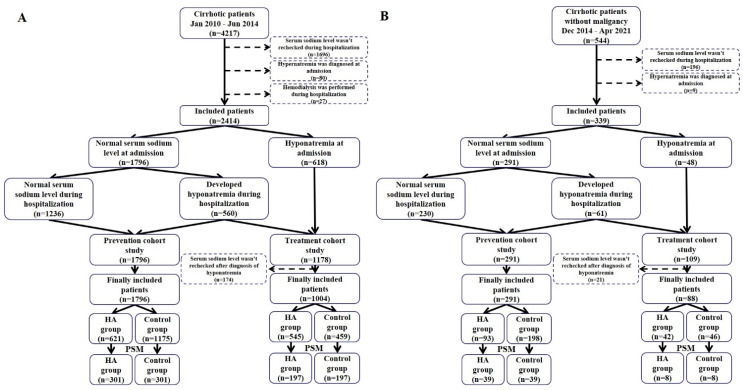
Patients. Overall, 4217 patients were screened, of whom 2414 were included in the Hospitalization outcome cohort (Figure 1A). Among them, 618 patients had hyponatremia at admission, 560 patients developed hyponatremia during hospitalizations, and 1236 patients had normal serum sodium level both at admission and during hospitalizations.
Figure 1.
Flow charts of patient selection in the Hospitalization outcome (panel A) and Long-term outcome (panel B) cohorts. Abbreviations: PSM: propensity score matching; HA: human albumin.
Prevention of hyponatremia. Overall, 1796 patients had normal serum sodium level at admission. Among them, 621 and 1175 patients were assigned to the HA and control groups, respectively. After PSM, 602 patients were included. Median total dosage of HA was 30 g (range: 10–530) in the HA group. The HA group had a significantly lower incidence of hyponatremia than the control group (16.30% versus 41.90%, p < 0.001) (Table 1). Similarly, logistic regression analysis also showed that HA infusion was significantly associated with decreased risk of developing hyponatremia during hospitalizations (OR = 0.270, 95% CI = 0.184–0.396, p < 0.001) (Figure 2A).
Table 1.
Hospitalization outcome cohort—Characteristics of patients in the prevention study after PSM.
| Variables | No. Pts | Overall | No. Pts | HA Group | No. Pts | Control Group |
p Value |
|---|---|---|---|---|---|---|---|
| Age (years) | 602 | 58.26 (21.14–87.82) 59.15 ± 11.85 |
301 | 58.46 (21.14–87.82) 59.12 ± 12.02 |
301 | 57.69 (29.66–86.00) 59.17 ± 11.69 |
0.916 |
| Sex (male) (%) | 602 | 389 (64.60%) | 301 | 192 (63.80%) | 301 | 197 (65.40%) | 0.670 |
| Etiology of liver cirrhosis | |||||||
| HBV (%) | 602 | 265 (44.00%) | 301 | 145 (48.20%) | 301 | 120 (39.90%) | 0.040 |
| HCV (%) | 602 | 57 (9.50%) | 301 | 33 (11.00%) | 301 | 24 (8.00%) | 0.210 |
| Alcohol (%) | 602 | 175 (29.10%) | 301 | 88 (29.20%) | 301 | 87 (28.90%) | 0.928 |
| HCC (%) | 602 | 167 (27.70%) | 301 | 80 (26.60%) | 301 | 87 (28.90%) | 0.524 |
| Hypokalemia (%) | 602 | 73 (12.10%) | 301 | 36 (12.00%) | 301 | 37 (12.30%) | 0.901 |
| AUGIB (%) | 602 | 177 (29.40%) | 301 | 88 (29.20%) | 301 | 89 (29.60%) | 0.929 |
| Infection (%) | 602 | 197 (32.70%) | 301 | 95 (31.60%) | 301 | 102 (33.90%) | 0.543 |
| Ascites (%) | 602 | 349 (58.00%) | 301 | 176 (58.50%) | 301 | 173 (57.50%) | 0.804 |
| Paracentesis * (%) | 602 | 43 (7.10%) | 301 | 18 (6.00%) | 301 | 25 (8.30%) | 0.268 |
| Laboratory tests | |||||||
| Hb (g/L) | 602 | 92.00 (27.00–169.00) 93.84 ± 28.00 |
301 | 91.00 (29.00–164.00) 91.83 ± 26.89 |
301 | 93.00 (27.00–169.00) 95.84 ± 28.97 |
0.132 |
| WBC (109/L) | 602 | 4.10 (0.50–33.50) 5.18 ± 3.94 |
301 | 4.00 (0.90–30.20) 4.96 ± 3.61 |
301 | 4.20 (0.50–33.50) 5.41 ± 4.24 |
0.342 |
| PLT (109/L) | 602 | 76.50 (9.00–775.00) 97.62 ± 81.23 |
301 | 74.00 (16.00–394.00) 89.86 ± 57.31 |
301 | 78.00 (9.00–775.00) 105.37 ± 99.06 |
0.327 |
| TBIL (μmol/L) | 602 | 24.65 (3.40–576.40) 40.77 ± 59.28 |
301 | 23.40 (3.40–423.50) 34.62 ± 46.53 |
301 | 26.70 (5.10–576.40) 46.92 ± 69.27 |
0.048 |
| ALB (g/L) | 602 | 30.60 (10.00–53.90) 31.11 ± 6.56 |
301 | 29.30 (13.50–48.50) 29.75 ± 6.42 |
301 | 32.30 (10.00–53.90) 32.47 ± 6.41 |
<0.001 |
| ALT (U/L) | 602 | 27.50 (4.00–1460.00) 51.84 ± 117.83 |
301 | 27.00 (4.00–730.00) 43.86 ± 58.71 |
301 | 28.00 (6.00–1460.00) 59.82 ± 155.69 |
0.882 |
| AKP (U/L) | 602 | 92.00 (1.30–782.00) 119.85 ± 93.81 |
301 | 91.80 (7.05–782.00) 122.82 ± 107.90 |
301 | 92.20 (1.30–511.00) 116.89 ± 77.27 |
0.437 |
| Scr (μmol/L) | 602 | 61.80 (2.60–742.00) 76.22 ± 66.70 |
301 | 62.00 (24.00–742.00) 75.20 ± 58.27 |
301 | 61.00 (2.60–715.00) 77.23 ± 74.26 |
0.688 |
| K (mmol/L) | 602 | 4.05 (2.05–6.14) 4.04 ± 0.52 |
301 | 4.03 (2.65–5.57) 4.04 ± 0.49 |
301 | 4.07 (2.05–6.14) 4.04 ± 0.55 |
0.849 |
| Na (mmol/L) | 602 | 139.30 (135.00–145.00) 139.39 ± 2.57 |
301 | 139.50 (135.00–145.00) 139.50 ± 2.52 |
301 | 139.10 (135.00–145.00) 139.29 ± 2.61 |
0.294 |
| PT (seconds) | 602 | 15.80 (11.00–51.00) 16.60 ± 4.16 |
301 | 16.00 (11.00–33.70) 16.48 ± 3.29 |
301 | 15.50 (11.30–51.00) 16.71 ± 4.89 |
0.349 |
| INR | 602 | 1.27 (0.79–11.70) 1.39 ± 0.65 |
301 | 1.28 (0.79–3.28) 1.35 ± 0.36 |
301 | 1.24 (0.82–11.70) 1.43 ± 0.85 |
0.634 |
| Child–Pugh score | 602 | 8 (5–14) 7.92 ± 1.91 |
301 | 8 (5–14) 7.89 ± 1.78 |
301 | 8 (5–13) 7.96 ± 2.03 |
0.929 |
| MELD score | 602 | 6.92 (−21.42–44.70) 8.03 ± 6.76 |
301 | 6.77 (−5.62–26.86) 7.69 ± 5.90 |
301 | 7.24 (−21.42–44.70) 8.37 ± 7.51 |
0.561 |
| Treatments | |||||||
| Desmopressin (%) | 602 | 46 (7.60%) | 301 | 18 (6.00%) | 301 | 28 (9.30%) | 0.125 |
| Terlipressin (%) | 602 | 0 | 301 | 0 | 301 | 0 | \ |
| Furosemide (%) | 602 | 390 (64.80%) | 301 | 196 (65.10%) | 301 | 194 (64.50%) | 0.864 |
| Torasemide (%) | 602 | 249 (41.40%) | 301 | 133 (44.20%) | 301 | 116 (38.50%) | 0.159 |
| Spironolactone (%) | 602 | 302 (50.20%) | 301 | 156 (51.80%) | 301 | 146 (48.50%) | 0.415 |
| Hydrochlorothiazide (%) | 602 | 4 (0.70%) | 301 | 3 (1.00%) | 301 | 1 (0.30%) | 0.316 |
| Bumetanide (%) | 602 | 8 (1.30%) | 301 | 4 (1.30%) | 301 | 4 (1.30%) | 1.000 |
| Tolvaptan (%) | 602 | 2 (0.30%) | 301 | 1 (0.30%) | 301 | 1 (0.30%) | 1.000 |
| Hypertonic saline # (%) | 602 | 10 (1.70%) | 301 | 4 (1.30%) | 301 | 6 (2.00%) | 0.524 |
| K supplement (%) | 602 | 434 (72.10%) | 301 | 219 (72.80%) | 301 | 215 (71.40%) | 0.716 |
| HA dosage (g) | 301 | 30 (10–530) 46.84 ± 46.42 |
301 | 30 (10–530) 46.84 ± 46.42 |
NA | NA | \ |
| Incidence of hyponatremia (%) | 602 | 175 (29.10%) | 301 | 49 (16.30%) | 301 | 126 (41.90%) | <0.001 |
Notes: * Data regarding use of paracentesis were extracted before the development of hyponatremia. # Data regarding use of hypertonic saline were extracted before the development of hyponatremia. Abbreviations: PSM: propensity score matching; Pts: patients; HA: human albumin; HBV: hepatitis B virus; HCV: hepatitis C virus; HCC: hepatocellular carcinoma; AUGIB: acute upper gastrointestinal bleeding, Hb: hemoglobin; WBC: white blood cell; PLT: platelet; TBIL: total bilirubin; ALB: albumin; ALT: alanine aminotransferase; AKP: alkaline phosphatase; Scr: serum creatinine; K: potassium; Na: sodium; PT: prothrombin time; INR: international normalized ratio; MELD: model for end-stage liver disease; NA: not applicable.
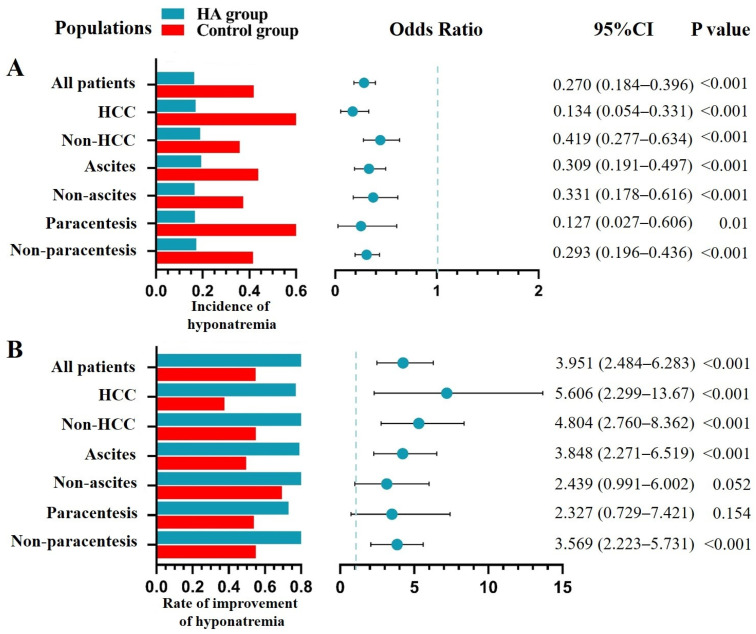
Figure 2.
HA infusion for the prevention and treatment of hyponatremia during hospitalizations. (Panel A): Bar plots displayed the incidence of hyponatremia in the HA and control groups, and forest plots showed the ORs with 95% CIs for the role of HA infusion for the prevention of hyponatremia. (Panel B): Bar plots displayed the rate of improvement of hyponatremia in the HA and control groups, and forest plots showed the ORs with 95% CIs for the role of HA infusion for the improvement of hyponatremia. Abbreviations: HA: human albumin; CI: confidence intervals; HCC: hepatocellular carcinoma.
Regardless of HCC, ascites, and paracentesis, the HA group had a significantly lower incidence of hyponatremia than the control group; and logistic regression analyses also showed that HA infusion was significantly associated with decreased risk of developing hyponatremia during hospitalizations (Figure 2A).
Five hundred and sixty patients developed hyponatremia during hospitalizations and 1236 did not. Among them, 77 patients died during hospitalizations. Causes of death were related (n = 65) and unrelated (n = 12) to liver diseases. Patients who developed hyponatremia during hospitalizations had a significantly higher in-hospital mortality than those who did not (7.10% versus 3.00%, p < 0.001). Results remained in both HA (10.40% versus 4.30%, p = 0.004) and control (5.60% versus 2.30%, p = 0.003) groups. Similarly, logistic regression analysis also showed that the development of hyponatremia during hospitalizations was significantly associated with increased in-hospital mortality (OR = 2.493, 95% CI = 1.576–3.944, p < 0.001). Results remained in both HA (OR = 2.555, 95% CI = 1.319–4.948, p = 0.005) and control (OR = 2.556, 95% CI = 1.345–4.857, p = 0.004) groups.
Treatment of hyponatremia. Overall, 1178 patients were diagnosed with hyponatremia at admission/during hospitalizations. Among them, 174 patients who did not recheck serum sodium level after the diagnosis of hyponatremia were excluded. Finally, 1004 patients were included. Among them, 545 and 459 patients were assigned to the HA and control groups, respectively. After PSM, 394 patients were included. Median total dosage of HA was 40 g (range: 10–380) in the HA group. The HA group had a significantly higher rate of improvement of hyponatremia than the control group (82.70% versus 54.80%, p < 0.001) (Table 2). Similarly, logistic regression analysis showed that HA infusion was significantly associated with increased rate of improvement of hyponatremia during hospitalizations (OR = 3.951, 95% CI = 2.484–6.283, p < 0.001) (Figure 2B).
Table 2.
Hospitalization outcome cohort—Characteristics of patients in the treatment study after PSM.
| Variables | No. Pts | Overall | No. Pts | HA Group | No. Pts | Control Group |
p Value |
|---|---|---|---|---|---|---|---|
| Age (years) | 394 | 58.10 (29.94–89.19) 59.48 ± 11.52 |
197 | 58.20 (37.88–85.92) 59.76 ± 11.91 |
197 | 57.97 (29.94–89.19) 59.19 ± 11.14 |
0.777 |
| Sex (male) (%) | 394 | 280 (71.10%) | 197 | 142 (72.10%) | 197 | 138 (70.10%) | 0.657 |
| Etiology of liver cirrhosis | |||||||
| HBV (%) | 394 | 153 (38.80%) | 197 | 82 (41.60%) | 197 | 71 (36.00%) | 0.256 |
| HCV (%) | 394 | 48 (12.20%) | 197 | 28 (14.20%) | 197 | 20 (10.20%) | 0.218 |
| Alcohol (%) | 394 | 137 (34.80%) | 197 | 66 (33.50%) | 197 | 71 (36.00%) | 0.597 |
| HCC (%) | 394 | 90 (22.80%) | 197 | 51 (25.90%) | 197 | 39 (19.80%) | 0.150 |
| Hypokalemia (%) | 394 | 63 (16.00%) | 197 | 33 (16.80%) | 197 | 30 (15.20%) | 0.680 |
| AUGIB (%) | 394 | 81 (20.60%) | 197 | 38 (19.30%) | 197 | 43 (21.80%) | 0.533 |
| Infection (%) | 394 | 161 (40.90%) | 197 | 81 (41.10%) | 197 | 80 (40.60%) | 0.918 |
| Ascites (%) | 394 | 270 (68.50%) | 197 | 136 (69.00%) | 197 | 134 (68.00%) | 0.828 |
| Paracentesis * (%) | 394 | 51 (12.90%) | 197 | 23 (11.70%) | 197 | 28 (14.20%) | 0.453 |
| Severity of hyponatremia | |||||||
| Mild (%)/Moderate (%)/Severe (%) | 394 | 313 (79.40%)/58 (14.70%)/23 (5.80%) | 197 | 155 (78.70%)/29 (14.70%)/13 (6.60%) | 197 | 158 (80.20%)/29 (14.70%)/10 (5.10%) | 0.811 |
| Laboratory tests | |||||||
| Hb (g/L) | 394 | 94.00 (35.00–180.00) 93.93 ± 28.35 |
197 | 93.00 (36.00–180.00) 94.04 ± 29.00 |
197 | 96.00 (35.00–157.00) 93.81 ± 27.75 |
0.812 |
| WBC (109/L) | 394 | 5.50 (0.50–31.10) 6.61 ± 4.64 |
197 | 5.70 (0.90–31.10) 6.88 ± 5.06 |
197 | 5.50 (0.50–30.70) 6.33 ± 4.17 |
0.636 |
| PLT (109/L) | 394 | 84.00 (5.00–464.00) 100.93 ± 71.42 |
197 | 81.00 (13.00–365.00) 99.57 ± 66.64 |
197 | 84.00 (5.00–464.00) 102.28 ± 76.04 |
0.907 |
| TBIL (μmol/L) | 394 | 33.30 (2.70–809.80) 72.40 ± 107.12 |
197 | 31.10 (2.70–454.70) 62.18 ± 82.29 |
197 | 36.40 (4.20–809.80) 82.63 ± 126.71 |
0.155 |
| ALB (g/L) | 394 | 29.00 (12.40–52.80) 29.58 ± 6.58 |
197 | 27.90 (12.40–50.00) 28.89 ± 6.60 |
197 | 29.60 (13.70–52.80) 30.27 ± 6.48 |
0.017 |
| ALT (U/L) | 394 | 31.00 (7.00–3471.00) 63.59 ± 197.28 |
197 | 30.00 (7.00–3471.00) 70.23 ± 256.54 |
197 | 33.00 (8.00–1335.00) 56.94 ± 110.16 |
0.508 |
| AKP (U/L) | 394 | 104.00 (35.00–1075.00) 143.88 ± 126.34 |
197 | 100.00 (39.00–586.00) 128.25 ± 91.63 |
197 | 109.00 (35.00–1075.00) 159.51 ± 152.04 |
0.122 |
| Scr (μmol/L) | 394 | 64.00 (24.00–761.00) 88.89 ± 85.07 |
197 | 65.00 (30.00–636.00) 88.10 ± 74.03 |
197 | 63.00 (24.00–761.00) 89.69 ± 95.02 |
0.242 |
| K (mmol/L) | 394 | 4.04 (2.09–6.95) 4.07 ± 0.68 |
197 | 4.08 (2.09–6.95) 4.09 ± 0.68 |
197 | 4.02 (2.17–6.37) 4.05 ± 0.69 |
0.600 |
| Na (mmol/L) | 394 | 132.55 (102.90–134.90) 131.54 ± 3.59 |
197 | 132.60 (115.80–134.90) 131.73 ± 3.20 |
197 | 132.40 (102.90–134.90) 131.35 ± 3.94 |
0.381 |
| PT (seconds) | 394 | 16.25 (11.00–63.30) 17.58 ± 5.21 |
197 | 16.50 (11.00–63.30) 17.66 ± 5.53 |
197 | 16.00 (11.20–40.90) 17.49 ± 4.87 |
0.427 |
| INR | 394 | 1.31 (0.81–11.70) 1.51 ± 0.81 |
197 | 1.32 (0.84–8.05) 1.49 ± 0.70 |
197 | 1.30 (0.81–11.70) 1.53 ± 0.92 |
0.600 |
| Child–Pugh score | 394 | 9 (5–15) 8.82 ± 2.16 |
197 | 9 (5–14) 8.70 ± 2.03 |
197 | 9 (5–15) 8.95 ± 2.28 |
0.695 |
| MELD score | 394 | 9.58 (−5.22–43.97) 11.12 ± 8.75 |
197 | 9.70 (−5.22–43.97) 10.95 ± 8.23 |
197 | 9.39 (−4.79–40.95) 11.28 ± 9.26 |
0.986 |
| Treatments | |||||||
| Desmopressin (%) | 394 | 11 (2.80%) | 197 | 7 (3.60%) | 197 | 4 (2.00%) | 0.359 |
| Terlipressin (%) | 394 | 0 | 197 | 0 | 197 | 0 | \ |
| Furosemide (%) | 394 | 294 (74.60%) | 197 | 146 (74.10%) | 197 | 148 (75.10%) | 0.817 |
| Torasemide (%) | 394 | 191 (48.50%) | 197 | 99 (50.30%) | 197 | 92 (46.70%) | 0.480 |
| Spironolactone (%) | 394 | 227 (57.60%) | 197 | 114 (57.90%) | 197 | 113 (57.40%) | 0.919 |
| Hydrochlorothiazide (%) | 394 | 4 (1.00%) | 197 | 2 (1.00%) | 197 | 2 (1.00%) | 1.000 |
| Bumetanide (%) | 394 | 9 (2.30%) | 197 | 5 (2.50%) | 197 | 4 (2.00%) | 0.736 |
| Tolvaptan (%) | 394 | 0 | 197 | 0 | 197 | 0 | \ |
| Hypertonic saline # (%) | 394 | 34 (8.60%) | 197 | 17 (8.60%) | 197 | 17 (8.60%) | 1.000 |
| K supplement (%) | 394 | 291 (73.90%) | 197 | 147 (74.60%) | 197 | 144 (73.10%) | 0.731 |
| HA dosage (g) | 197 | 40.00 (10.00–380.00) 53.20 ± 47.48 |
197 | 40.00 (10.00–380.00) 53.20 ± 47.48 |
NA | NA | \ |
| Improvement of hyponatremia (%) | 394 | 271 (68.80%) | 197 | 163 (82.70%) | 197 | 108 (54.80%) | <0.001 |
Notes: * Data regarding use of paracentesis were extracted after the development of hyponatremia. # Data regarding use of hypertonic saline were extracted after the development of hyponatremia. Abbreviations: PSM: propensity score matching; Pts: patients; HA: human albumin; HBV: hepatitis B virus; HCV: hepatitis C virus; HCC: hepatocellular carcinoma; AUGIB: acute upper gastrointestinal bleeding, Hb: hemoglobin; WBC: white blood cell; PLT: platelet; TBIL: total bilirubin; ALB: albumin; ALT: alanine aminotransferase; AKP: alkaline phosphatase; Scr: serum creatinine; K: potassium; Na: sodium; PT: prothrombin time; INR: international normalized ratio; MELD: model for end-stage liver disease; NA: not applicable.
Regardless of HCC and ascites, the HA group had a significantly higher rate of improvement of hyponatremia than the control group. This difference remained significant in patients who did not undergo paracentesis, but not in those who underwent paracentesis. Logistic regression analyses showed that HA infusion was significantly associated with increased rate of improvement of hyponatremia during hospitalizations in patients with HCC, non-HCC, ascites, and who did not undergo paracentesis, but not those without ascites or who underwent paracentesis (Figure 2B).
Six hundred and forty-two patients had improvement of hyponatremia during hospitalizations and 362 did not. Among them, 104 patients died during hospitalizations. Causes of death were related (n = 88) and unrelated (n = 16) to liver diseases. Patients who had improvement of hyponatremia during hospitalizations had a significantly lower in-hospital mortality than those who did not (8.60% versus 13.50%, p = 0.013). Results remained in control group (6.70% versus 14.20%, p = 0.008), but not in HA group (10.00% versus 12.90%, p = 0.309). Similarly, logistic regression analysis also showed that the improvement of hyponatremia during hospitalizations was significantly associated with decreased in-hospital mortality (OR = 0.599, 95% CI = 0.398–0.901, p = 0.014). Results remained in control group (OR = 0.435, 95% CI = 0.232–0.815, p = 0.009), but not in HA group (OR = 0.752, 95% CI = 0.434–1.304, p = 0.310).
3.2. Long-Term Outcome Cohort
Patients. Overall, 544 patients were screened, of whom 339 were included in the Long-term outcome cohort (Figure 1B). Among them, 48 patients had hyponatremia at admission, 61 patients developed hyponatremia during hospitalizations, and 230 patients had normal serum sodium level both at admission and during hospitalizations.
Prevention of hyponatremia. Overall, 291 patients had normal serum sodium level at admission. Among them, 93 and 198 patients were assigned to the HA and control groups, respectively. After PSM, 78 patients were included. Median total dosage of HA was 30 g (range: 10–150) in the HA group. The HA group had a significantly lower incidence of hyponatremia than the control group (7.70% versus 30.80%, p = 0.010) (Table 3). Similarly, logistic regression analysis also showed that HA infusion was significantly associated with decreased risk of developing hyponatremia during hospitalizations (OR = 0.188, 95% CI = 0.048–0.731, p = 0.016).
Table 3.
Long-term outcome cohort—Characteristics of patients in the prevention study after PSM.
| Variables | No. Pts | Overall | No. Pts | HA Group | No. Pts | Control Group | p Value |
|---|---|---|---|---|---|---|---|
| Age (years) | 78 | 57.93 (30.21–78.36) 57.49 ± 10.71 |
39 | 58.10 (30.21–78.36) 56.59 ± 11.40 |
39 | 58.10 (33.61–77.30) 58.39 ± 10.04 |
0.371 |
| Sex (male) (%) | 78 | 51 (65.40%) | 39 | 26 (66.70%) | 39 | 25 (64.10%) | 0.812 |
| Etiology of liver cirrhosis | |||||||
| HBV (%) | 78 | 32 (41.00%) | 39 | 18 (46.20%) | 39 | 14 (35.90%) | 0.357 |
| HCV (%) | 78 | 4 (5.10%) | 39 | 3 (7.70%) | 39 | 1 (2.60%) | 0.305 |
| Alcohol (%) | 78 | 30 (38.50%) | 39 | 14 (35.90%) | 39 | 16 (41.00%) | 0.642 |
| Hypokalemia (%) | 78 | 16 (20.50%) | 39 | 6 (15.40%) | 39 | 10 (25.60%) | 0.262 |
| AUGIB (%) | 78 | 27 (34.60%) | 39 | 12 (30.80%) | 39 | 15 (38.50%) | 0.475 |
| Infection (%) | 78 | 8 (10.30%) | 39 | 5 (12.80%) | 39 | 3 (7.70%) | 0.455 |
| Ascites (%) | 78 | 65 (83.30%) | 39 | 32 (82.10%) | 39 | 33 (84.60%) | 0.761 |
| Paracentesis * (%) | 78 | 0 | 39 | 0 | 39 | 0 | \ |
| Laboratory tests | |||||||
| Hb (g/L) | 78 | 85.00 (37.00–150.00) 89.95 ± 26.53 |
39 | 95.00 (43.00–150.00) 93.13 ± 24.82 |
39 | 80.00 (37.00–136.00) 86.77 ± 28.10 |
0.259 |
| WBC (109/L) | 78 | 3.70 (0.80–10.60) 4.15 ± 2.03 |
39 | 4.30 (1.00–10.60) 4.40 ± 1.96 |
39 | 3.50 (0.80–9.30) 3.90 ± 2.10 |
0.106 |
| PLT (109/L) | 78 | 77.50 (19.00–470.00) 100.92 ± 71.19 |
39 | 79.00 (19.00–470.00) 105.15 ± 80.12 |
39 | 77.00 (34.00–302.00) 96.69 ± 61.74 |
0.649 |
| TBIL (μmol/L) | 78 | 27.05 (8.00–281.10) 38.08 ± 40.23 |
39 | 27.50 (8.80–100.40) 33.92 ± 24.29 |
39 | 24.70 (8.00–281.10) 42.23 ± 51.52 |
0.901 |
| ALB (g/L) | 78 | 28.70 (19.00–38.00) 28.58 ± 4.40 |
39 | 26.80 (19.00–38.00) 27.25 ± 4.62 |
39 | 30.50 (21.50–37.00) 29.91 ± 3.78 |
0.009 |
| ALT (U/L) | 78 | 26.55 (8.17–613.24) 48.72 ± 75.91 |
39 | 27.30 (12.18–241.21) 47.07 ± 43.79 |
39 | 24.95 (8.17–613.24) 50.37 ± 98.76 |
0.169 |
| AKP (U/L) | 78 | 103.97 (31.00–2525.27) 171.11 ± 299.77 |
39 | 103.66 (31.00–983.93) 143.44 ± 152.69 |
39 | 104.62 (33.66–2525.27) 198.78 ± 396.49 |
0.964 |
| Scr (μmol/L) | 78 | 60.81 (40.21–178.55) 67.55 ± 22.28 |
39 | 72.04 (40.70–178.55) 74.86 ± 26.14 |
39 | 54.45 (40.21–99.20) 60.24 ± 14.58 |
0.004 |
| K (mmol/L) | 78 | 3.84 (2.42–5.19) 3.81 ± 0.49 |
39 | 3.86 (2.42–5.19) 3.85 ± 0.53 |
39 | 3.79 (2.84–4.70) 3.77 ± 0.44 |
0.433 |
| Na (mmol/L) | 78 | 138.10 (135.50–144.30) 138.62 ± 2.18 |
39 | 137.60 (135.50–144.30) 138.33 ± 2.09 |
39 | 138.40 (135.50–144.20) 138.92 ± 2.26 |
0.243 |
| PT (seconds) | 78 | 15.95 (12.50–27.40) 16.61 ± 2.73 |
39 | 16.00 (12.60–23.90) 16.66 ± 2.35 |
39 | 15.60 (12.50–27.40) 16.56 ± 3.10 |
0.330 |
| INR | 78 | 1.27 (0.94–2.55) 1.36 ± 0.28 |
39 | 1.30 (1.00–2.08) 1.37 ± 0.24 |
39 | 1.26 (0.94–2.55) 1.36 ± 0.33 |
0.298 |
| Child–Pugh score | 78 | 8 (5–12) 8.33 ± 1.30 |
39 | 8 (6–12) 8.41 ± 1.29 |
39 | 8 (5–11) 8.26 ± 1.31 |
0.905 |
| MELD score | 78 | 8.05 (−2.35–22.73) 8.22 ± 4.90 |
39 | 8.50 (−2.35–19.43) 9.18 ± 4.68 |
39 | 7.08 (−0.56–22.73) 7.26 ± 4.99 |
0.055 |
| Treatments | |||||||
| Desmopressin (%) | 78 | 0 | 39 | 0 | 39 | 0 | \ |
| Terlipressin (%) | 78 | 9 (11.50%) | 39 | 5 (12.80%) | 39 | 4 (10.30%) | 0.723 |
| Furosemide (%) | 78 | 28 (35.90%) | 39 | 13 (33.33%) | 39 | 15 (38.50%) | 0.637 |
| Torasemide (%) | 78 | 38 (48.70%) | 39 | 20 (51.30%) | 39 | 18 (46.20%) | 0.651 |
| Spironolactone (%) | 78 | 33 (42.30%) | 39 | 15 (38.50%) | 39 | 18 (46.20%) | 0.492 |
| Hydrochlorothiazide (%) | 78 | 0 | 39 | 0 | 39 | 0 | \ |
| Bumetanide (%) | 78 | 0 | 39 | 0 | 39 | 0 | \ |
| Tolvaptan (%) | 78 | 0 | 39 | 0 | 39 | 0 | \ |
| Hypertonic saline # (%) | 78 | 0 | 39 | 0 | 39 | 0 | \ |
| K supplement (%) | 78 | 62 (79.50%) | 39 | 30 (76.90%) | 39 | 32 (82.10%) | 0.575 |
| HA dosage (g) | 39 | 30.00 (10.00–150.00) 42.56 ± 32.18 |
39 | 30.00 (10.00–150.00) 42.56 ± 32.18 |
NA | NA | \ |
| Incidence of hyponatremia (%) |
78 | 15 (19.20%) | 39 | 3 (7.70%) | 39 | 12 (30.80%) | 0.010 |
Notes: * Data regarding use of paracentesis were extracted before the development of hyponatremia. # Data regarding use of hypertonic saline were extracted before the development of hyponatremia. Abbreviations: PSM: propensity score matching; Pts: patients; HA: human albumin; HBV: hepatitis B virus; HCV: hepatitis C virus; AUGIB: acute upper gastrointestinal bleeding, Hb: hemoglobin; WBC: white blood cell; PLT: platelet; TBIL: total bilirubin; ALB: albumin; ALT: alanine aminotransferase; AKP: alkaline phosphatase; Scr: serum creatinine; K: potassium; Na: sodium; PT: prothrombin time; INR: international normalized ratio; MELD: model for end-stage liver disease; NA: not applicable.
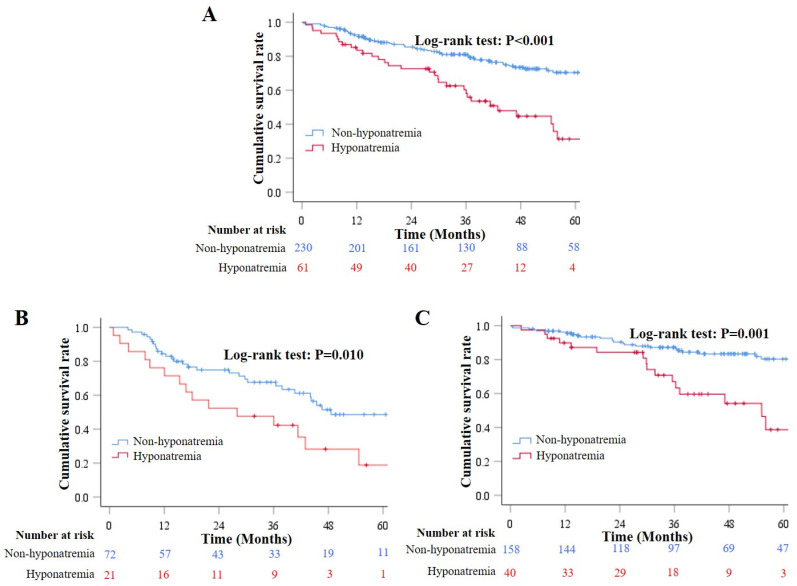
Sixty-one patients developed hyponatremia during hospitalizations and 230 did not. During a median follow-up period of 37.12 months (range: 0.30–82.55), 97 patients died. Causes of death were related (n = 70) and unrelated (n = 27) to liver diseases. Cox regression analysis demonstrated that the development of hyponatremia during hospitalizations was significantly associated with decreased long-term survival (HR = 0.400, 95% CI = 0.260–0.616, p < 0.001). Results remained in both HA (HR = 0.460, 95% CI = 0.250–0.845, p = 0.012) and control (HR = 0.380, 95% CI = 0.208–0.697, p = 0.002) groups. Kaplan–Meier curve analysis also showed that patients who developed hyponatremia during hospitalizations had a significantly lower cumulative survival rate than those who did not (Log-rank test: p < 0.001) (Figure 3A). Results remained in both HA (Log-rank test: p = 0.010) (Figure 3B) and control (Log-rank test: p = 0.001) (Figure 3C) groups.
Figure 3.
Long-term survival according to the development of hyponatremia during hospitalizations. (Panel A): Kaplan–Meier curves showed the cumulative survival rates in overall patients who developed and did not develop hyponatremia during hospitalizations. (Panel B): Kaplan–Meier curves showed the cumulative survival rates in overall patients who developed and did not develop hyponatremia during hospitalizations in the HA group. (Panel C): Kaplan–Meier curves showed the cumulative survival rates in overall patients who developed and did not develop hyponatremia during hospitalizations in the control group.
Treatment of hyponatremia. Overall, 109 patients were diagnosed with hyponatremia at admission/during hospitalizations. Among them, 21 patients who did not recheck serum sodium level after the diagnosis of hyponatremia were excluded. Finally, 88 patients were included. Among them, 42 and 46 patients were assigned to the HA and control groups, respectively. After PSM, 16 patients were included. Median total dosage of HA was 40 g (range: 20–180) in the HA group. The HA group had a significantly higher rate of improvement of hyponatremia than the control group (87.50% versus 37.50%, p = 0.039) (Table 4). Logistic regression analysis showed that HA infusion was not significantly associated with increased improvement of hyponatremia during hospitalizations (OR = 11.667, 95% CI = 0.922–147.563, p = 0.058).
Table 4.
Long-term outcome cohort—Characteristics of patients in the treatment study after PSM.
| Variables | No. Pts | Overall | No. Pts | HA Group | No. Pts | Control Group | p Value |
|---|---|---|---|---|---|---|---|
| Age (years) | 16 | 55.90 (32.78–80.79) 56.49 ± 13.32 |
8 | 56.69 (44.18–80.79) 58.80 ± 13.07 |
8 | 53.84 (32.78–70.79) 54.18 ± 14.04 |
0.529 |
| Sex (male) (%) | 16 | 10 (62.50%) | 8 | 4 (50.00%) | 8 | 6 (75.00%) | 0.320 |
| Etiology of liver cirrhosis | |||||||
| HBV (%) | 16 | 4 (25.00%) | 8 | 3 (37.50%) | 8 | 1 (12.50%) | 0.248 |
| HCV (%) | 16 | 3 (18.80%) | 8 | 1 (12.50%) | 8 | 2 (25.00%) | 0.522 |
| Alcohol (%) | 16 | 6 (37.50%) | 8 | 3 (37.50%) | 8 | 3 (37.50%) | 1.000 |
| Hypokalemia (%) | 16 | 3 (18.80%) | 8 | 3 (37.50%) | 8 | 0 | 0.055 |
| AUGIB (%) | 16 | 10 (62.50%) | 8 | 5 (62.50%) | 8 | 5 (62.50%) | 1.000 |
| Infection (%) | 16 | 3 (18.80%) | 8 | 2 (25.00%) | 8 | 1 (12.50%) | 0.522 |
| Ascites (%) | 16 | 16 (100.00%) | 8 | 8 (100.00%) | 8 | 8 (100.00%) | \ |
| Paracentesis * (%) | 16 | 0 | 8 | 0 | 8 | 0 | \ |
| Laboratory tests | |||||||
| Hb (g/L) | 16 | 75.00 (59.00–135.00) 81.94 ± 22.29 |
8 | 74.5 (59.00–100.00) 77.13 ± 16.39 |
8 | 76.00 (60.00–135.00) 86.75 ± 27.26 |
0.563 |
| WBC (109/L) | 16 | 6.45 (1.70–20.30) 7.16 ± 4.38 |
8 | 6.70 (2.30–20.30) 8.30 ± 5.70 |
8 | 6.25 (1.70–9.20) 6.03 ± 2.39 |
0.563 |
| PLT (109/L) | 16 | 96.50 (22.00–215.00) 100.13 ± 59.72 |
8 | 82.50 (22.00–215.00) 83.50 ± 60.95 |
8 | 123.00 (30.00–203.00) 103.00 ± 67.30 |
0.128 |
| TBIL (μmol/L) | 16 | 34.50 (13.10–281.10) 59.68 ± 71.23 |
8 | 31.10 (13.10–177.90) 53.69 ± 54.44 |
8 | 36.45 (14.70–281.10) 65.68 ± 88.46 |
0.834 |
| ALB (g/L) | 16 | 28.05 (19.00–34.00) 27.04 ± 4.32 |
8 | 25.95 (19.00–28.50) 25.03 ± 3.37 |
8 | 30.00 (19.30–34.00) 29.05 ± 4.40 |
0.015 |
| ALT (U/L) | 16 | 24.04 (10.26–613.24) 68.07 ± 146.75 |
8 | 23.16 (10.26–68.00) 27.53 ± 19.09 |
8 | 40.66 (10.92–613.24) 108.61 ± 205.00 |
0.294 |
| AKP (U/L) | 16 | 85.12 (43.51–351.14) 114.80 ± 78.75 |
8 | 90.87 (43.51–187.00) 97.97 ± 47.90 |
8 | 85.12 (62.00–351.14) 131.64 ± 101.72 |
0.529 |
| Scr (μmol/L) | 16 | 66.99 (37.66–99.20) 67.65 ± 15.69 |
8 | 68.25 (37.66–90.10) 67.61 ± 17.79 |
8 | 66.99 (51.90–99.20) 67.69 ± 14.52 |
0.916 |
| K (mmol/L) | 16 | 3.94 (2.72–4.51) 3.77 ± 0.49 |
8 | 3.68 (2.72–4.12) 3.51 ± 0.53 |
8 | 4.01 (3.64–4.51) 4.04 ± 0.27 |
0.040 |
| Na (mmol/L) | 16 | 133.95 (127.00–134.90) 133.27 ± 2.14 |
8 | 133.20 (127.00–134.70) 132.31 ± 2.68 |
8 | 134.6 (132.80–134.90) 134.23 ± 0.73 |
0.073 |
| PT (seconds) | 16 | 16.75 (13.80–23.90) 17.78 ± 3.62 |
8 | 18.35 (13.80–23.90) 18.68 ± 4.27 |
8 | 16.05 (14.00–22.20) 16.89 ± 2.83 |
0.529 |
| INR | 16 | 1.38 (1.06–2.08) 1.49 ± 0.37 |
8 | 1.54 (1.09–2.08) 1.58 ± 0.42 |
8 | 1.33 (1.06–2.04) 1.40 ± 0.32 |
0.344 |
| Child–Pugh score | 16 | 9 (7–12) 9.44 ± 1.36 |
8 | 9 (8–12) 9.75 ± 1.49 |
8 | 9 (7–11) 9.13 ± 1.25 |
0.451 |
| MELD score | 16 | 9.65 (5.37–18.77) 10.65 ± 4.48 |
8 | 9.65 (5.37–18.77) 11.07 ± 4.95 |
8 | 9.75 (5.42–16.07) 10.23 ± 4.25 |
0.834 |
| Treatments | |||||||
| Desmopressin (%) | 16 | 0 | 8 | 0 | 8 | 0 | \ |
| Terlipressin (%) | 16 | 1 (6.30%) | 8 | 0 | 8 | 1 (12.50%) | 0.302 |
| Furosemide (%) | 16 | 9 (56.30%) | 8 | 5 (62.50%) | 8 | 4 (50.00%) | 0.614 |
| Torasemide (%) | 16 | 11 (68.80%) | 8 | 5 (62.50%) | 8 | 6 (75.00%) | 0.590 |
| Spironolactone (%) | 16 | 8 (50.00%) | 8 | 3 (37.50%) | 8 | 5 (62.50%) | 0.317 |
| Hydrochlorothiazide (%) | 16 | 0 | 8 | 0 | 8 | 0 | \ |
| Bumetanide (%) | 16 | 0 | 8 | 0 | 8 | 0 | \ |
| Tolvaptan (%) | 16 | 0 | 8 | 0 | 8 | 0 | \ |
| Hypertonic saline # (%) | 16 | 1 (12.50%) | 8 | 1 (12.50%) | 8 | 0 | 0.302 |
| K supplement (%) | 16 | 14 (87.50%) | 8 | 7 (87.50%) | 8 | 7 (87.50%) | 1.000 |
| HA dosage (g) | 8 | 40.00 (20.00–180.00) 60.00 ± 53.72 |
8 | 40.00 (20.00–180.00) 60.00 ± 53.72 |
NA | NA | \ |
| Improvement of hyponatremia (%) | 16 | 10 (62.50%) | 8 | 7 (87.50%) | 8 | 3 (37.50%) | 0.039 |
Notes: * Data regarding use of paracentesis were extracted after the development of hyponatremia. # Data regarding use of hypertonic saline were extracted after the development of hyponatremia. Abbreviations: PSM: propensity score matching; Pts: patients; HA: human albumin; HBV: hepatitis B virus; HCV: hepatitis C virus; AUGIB: acute upper gastrointestinal bleeding, Hb: hemoglobin; WBC: white blood cell; PLT: platelet; TBIL: total bilirubin; ALB: albumin; ALT: alanine aminotransferase; AKP: alkaline phosphatase; Scr: serum creatinine; K: potassium; Na: sodium; PT: prothrombin time; INR: international normalized ratio; MELD: model for end-stage liver disease; NA: not applicable.
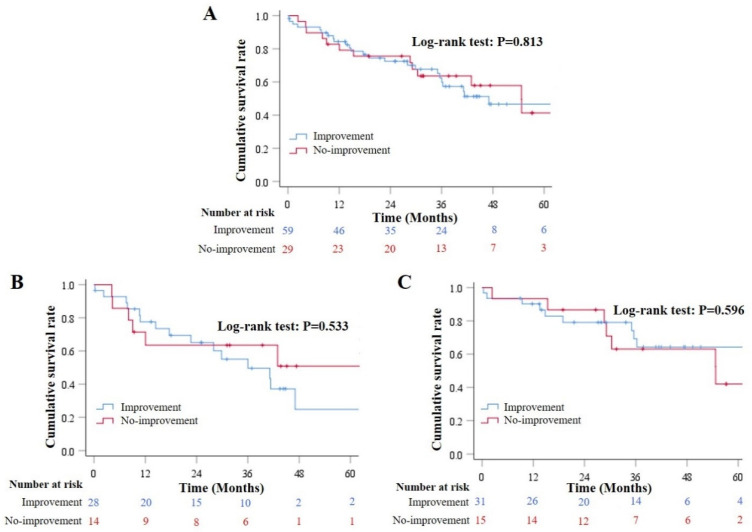
Fifty-nine patients had improvement of hyponatremia during hospitalizations and 29 did not. During a median follow-up period of 30.72 months (range: 0.21–76.18), 41 patients died. Causes of death were related (n = 37) and unrelated (n = 4) to liver diseases. Cox regression analysis demonstrated that the improvement of hyponatremia during hospitalizations was not significantly associated with increased long-term survival (HR = 1.085, 95% CI = 0.553–2.127, p = 0.813). Results remained in both HA (HR = 1.352, 95% CI = 0.523–3.495, p = 0.534) and control (HR = 0.864, 95% CI = 0.325–2.297, p = 0.769) groups. Kaplan–Meier curve analysis also demonstrated that the cumulative survival was not significantly different between patients who had improvement of hyponatremia during hospitalizations and those who did not (Log-rank test: p = 0.813) (Figure 4A). Results remained in both HA (Log-rank test: p = 0.533) (Figure 4B) and control (Log-rank test: p = 0.596) (Figure 4C) groups.
Figure 4.
Long-term survival according to the improvement of hyponatremia during hospitalizations. (Panel A): Kaplan–Meier curves showed the cumulative survival rates in overall patients who had and did not have improvement of hyponatremia during hospitalizations. (Panel B): Kaplan–Meier curves showed the cumulative survival rates in overall patients who had and did not have improvement of hyponatremia during hospitalizations in the HA group. (Panel C): Kaplan–Meier curves showed the cumulative survival rates in overall patients who had and did not have improvement of hyponatremia during hospitalizations in the control group.
4. Discussion
Our study has two major findings: (1) HA infusion can effectively reduce the incidence of hyponatremia during hospitalizations in patients with liver cirrhosis and normal serum sodium level at admission, and the development of hyponatremia during hospitalizations can worsen both in-hospital and long-term outcomes; and (2) HA infusion can effectively improve serum sodium level during hospitalizations in patients with liver cirrhosis and hyponatremia, and the improvement of hyponatremia should be beneficial for in-hospital outcome, but not for long-term outcome.
Hypervolemic hyponatremia is the most common type of hyponatremia in patients with liver cirrhosis, accounting for more than 90% [24]. It is mainly related to water retention secondary to increased secretion of antidiuretic hormone [25], which is caused by splanchnic vasodilation associated with portal hypertension, systemic inflammation, and hyperdynamic circulation in advanced liver cirrhosis [26,27]. HA can bind to endogenous and exogenous compounds, thereby exerting antioxidant activity, modulating inflammation and immune responses, improving cardiac function, and restoring endothelial integrity [12,28,29]. Therefore, HA infusion may be theoretically appropriate for the management of hyponatremia in patients with liver cirrhosis.
To the best of our knowledge, only four studies have explored the role of HA infusion for the treatment of hyponatremia [16,17,18,19]. In 2017, Shen et al.’s cohort study, which included 146 patients with hyponatremia, showed that a change of serum sodium level was similar between HA and crystalloid groups, and that HA infusion was associated with reduced 6-month mortality [16]. In 2018, Bajaj et al.’s cohort study, which included 1126 patients with hyponatremia, showed that HA group had a significantly higher rate of hyponatremia resolution, but a higher 30-day mortality than control group [17]. In 2021, China et al.’s post hoc analysis of ATTIRE trial, which included 206 patients with hyponatremia, showed that HA group had a significantly higher serum sodium level than control group [18]. In 2022, Zaccherini et al.’s post hoc analysis of ANSWER study, which included 431 patients with hyponatremia, showed that HA infusion can improve hyponatremia and reduce episodes of at least moderate hyponatremia in outpatients with cirrhosis and ascites [19].
As compared to these previous studies, our current study has some strengths in terms of study design. First, the severity of liver cirrhosis may affect the efficacy of HA infusion in liver cirrhosis. In our study, PSM analyses were employed to balance the severity of liver cirrhosis between patients who received and did not receive HA. By comparison, in Shen et al.’s study [16], the HA group had significantly higher proportions of ascites, refractory ascites, and diuretic use and higher MELD score than the control group. In Bajaj et al.’s study [17], the HA group had significantly higher proportions of infection, spontaneous bacterial peritonitis, renal dysfunction, large volume paracentesis, and organ failure and higher Child–Pugh and MELD scores than the control group. Baseline characteristics of patients with hyponatremia were not clearly reported in China et al.’s [18] and Zaccherini et al.’s [19] studies. Second, HA was selectively infused in the control group in Shen et al.’s [16], China et al.’s [18], and Zaccherini et al.’s [19] studies, which may cause a bias in assessing the outcomes. By comparison, none received HA infusion in the control group in our study. Third, HA was infused at a median dosage of 225 g in Bajaj et al.’s [17] study during hospitalizations and a mean dosage of 239.4 g in China et al.’s [18] study during a 14-day period. It is more likely that high-dose HA infusion can cause serious adverse events, such as pulmonary edema [30]. By comparison, only a relatively low dosage of HA infusion (median: 40 g) during hospitalizations was employed in our study, which is similar to that in the ANSWER study [31]. Fourth, ascites and HCC are common predisposing factors of hyponatremia in patients with liver cirrhosis [32,33,34], and hyponatremia also significantly worsens the outcomes of cirrhotic patients with ascites [4,35] and HCC [36,37]. Thus, our study conducted subgroup analyses to evaluate the efficacy of HA infusion for correction of hyponatremia according to the presence of ascites and HCC, which have not been performed in previous studies yet.
No previous study has specifically explored the role of HA infusion for the prevention of hyponatremia in patients with liver cirrhosis. However, some studies, which primarily evaluated the efficacy of HA infusion for the prevention of post-paracentesis circulatory dysfunction in cirrhotic patients with ascites undergoing large volume paracentesis, reported that HA infusion could significantly decrease the incidence of hyponatremia after large volume paracentesis [38,39]. By comparison, our study has for the first time demonstrated that HA infusion may prevent from hyponatremia in general patients with liver cirrhosis during hospitalizations.
As known, hyponatremia is an important prognostic factor of patients with liver cirrhosis [3,40,41], because it can predispose to more severe complications [42,43,44]. Our study further confirmed that the development of hyponatremia significantly increased the risk of in-hospital and long-term death. By comparison, few studies explored the impact of the improvement of hyponatremia on the prognosis of liver cirrhosis. Until now, only a previous cohort study demonstrated the benefits of the improvement of hyponatremia by tolvaptan on the short-term survival of patients with liver cirrhosis [45]. Similarly, our study found that the improvement of hyponatremia was significantly associated with a lower risk of in-hospital death, but could not support its benefit in reducing the long-term mortality. This is probably because long-term outcome may be influenced by multiple factors in patients with liver cirrhosis, especially Child–Pugh [46] and MELD [47] scores. Additionally, despite the improvement of hyponatremia during hospitalizations, the progression of liver cirrhosis and recurrence of hyponatremia during follow-up had not been evaluated in our study.
Our study has several limitations. First, the type of hyponatremia (i.e., hypervolemic, hypovolemic, or euvolemic) was not clearly identified due to a lack of data regarding patients’ volume status. However, we performed subgroup analyses according to the presence of ascites. Second, the etiology of hyponatremia could not be clarified, but some potential risk factors, including hypokalemia, AUGIB, infection, ascites, paracentesis, desmopressin, terlipressin, furosemide, torasemide, spironolactone, hydrochlorothiazide, and bumetanide, were adjusted. Third, the development and improvement of hyponatremia during follow-up were not available. Fourth, the information regarding HA infusion after discharge were not available in the Long-term outcome cohort, thus the role of long-term HA infusion could not be explored. Fifth, the first or recurring episodes of hyponatremia could not be clearly identified. Finally, the patient selection bias was often unavoidable due to the retrospective nature of this study.
5. Conclusions
HA infusion may be effective for preventing and treating hyponatremia in patients with liver cirrhosis during hospitalizations, which may be beneficial for the patients’ outcomes. Randomized controlled trials should be warranted to clarify the role of HA infusion for the management of hyponatremia in patients with liver cirrhosis.
Acknowledgments
We are indebted to our study team for establishing and updating our retrospective and prospective databases, including Junna Dai, Cuihong Zhu, Yun Li, Ying Peng, Zheng Ning, Feifei Hou, Jiancheng Zhao, Han Deng, Ran Wang, Jing Li, Xintong Zhang, Dan Han, Tingxue Song, Zhong Peng, Wenchun Bao, Yingying Li, Kexin Zheng, Qianqian Li, Xiangbo Xu, Yang An, Le Wang, Fangfang Yi, Yanyan Wu, Li Luo, Yue Yin, Shixue Xu, Menghua Zhu, Mengyuan Peng, Yiyan Zhang, Weiwei Wang, Min Ding, Wentao Xu, Lu Chai, Xiaojie Zheng, and Xueying Wang, of whom all had worked for our study group.
Author Contributions
Conceptualization: X.Q.; methodology: Z.B. and X.Q.; formal analysis: Z.B., W.X. and X.Q.; data curation: Z.B., W.X., L.C., X.Z. and X.Q.; writing–original draft: Z.B. and X.Q.; writing–review and editing: Z.B., W.X., N.M.-S., C.A.P., G.C. and X.Q.; supervision: G.C. and X.Q. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Medical Ethical Committee of the General Hospital of Northern Theater Command. The ethical approval number is Y2022-087.
Informed Consent Statement
Patients’ written informed consents have been waived due to the retrospective nature of this study.
Data Availability Statement
The datasets generated or analyzed during this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was partially supported by the Young and Middle-aged Scientific and Technological Innovation Talents Support Plan Project of Shenyang (RC210011).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Alukal J.J., John S., Thuluvath P.J. Hyponatremia in Cirrhosis: An Update. Am. J. Gastroenterol. 2020;115:1775–1785. doi: 10.14309/ajg.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 2.Angeli P., Wong F., Watson H., Ginès P., Investigators C. Hyponatremia in cirrhosis: Results of a patient population survey. Hepatology. 2006;44:1535–1542. doi: 10.1002/hep.21412. [DOI] [PubMed] [Google Scholar]
- 3.Kim W.R., Biggins S.W., Kremers W.K., Wiesner R.H., Kamath P.S., Benson J.T., Edwards E., Therneau T.M. Hyponatremia and mortality among patients on the liver-transplant waiting list. N. Engl. J. Med. 2008;359:1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sersté T., Gustot T., Rautou P.E., Francoz C., Njimi H., Durand F., Valla D., Lebrec D., Moreau R. Severe hyponatremia is a better predictor of mortality than MELDNa in patients with cirrhosis and refractory ascites. J. Hepatol. 2012;57:274–280. doi: 10.1016/j.jhep.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Bernardi M., Gitto S., Biselli M. The MELD score in patients awaiting liver transplant: Strengths and weaknesses. J. Hepatol. 2011;54:1297–1306. doi: 10.1016/j.jhep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Ahluwalia V., Wade J.B., Thacker L., Kraft K.A., Sterling R.K., Stravitz R.T., Fuchs M., Bouneva I., Puri P., Luketic V., et al. Differential impact of hyponatremia and hepatic encephalopathy on health-related quality of life and brain metabolite abnormalities in cirrhosis. J. Hepatol. 2013;59:467–473. doi: 10.1016/j.jhep.2013.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahluwalia V., Heuman D.M., Feldman G., Wade J.B., Thacker L.R., Gavis E., Gilles H., Unser A., White M.B., Bajaj J.S. Correction of hyponatraemia improves cognition, quality of life, and brain oedema in cirrhosis. J. Hepatol. 2015;62:75–82. doi: 10.1016/j.jhep.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sigal S.H., Amin A., Chiodo J.A., Sanyal A. Management Strategies and Outcomes for Hyponatremia in Cirrhosis in the Hyponatremia Registry. Can. J. Gastroenterol. Hepatol. 2018;2018:1579508. doi: 10.1155/2018/1579508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Association for the Study of the Liver EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018;69:406–460. doi: 10.1016/j.jhep.2018.03.024. [DOI] [PubMed] [Google Scholar]
- 10.Aithal G.P., Palaniyappan N., China L., Härmälä S., Macken L., Ryan J.M., Wilkes E.A., Moore K., Leithead J.A., Hayes P.C., et al. Guidelines on the management of ascites in cirrhosis. Gut. 2021;70:9–29. doi: 10.1136/gutjnl-2020-321790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arroyo V., García-Martinez R., Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J. Hepatol. 2014;61:396–407. doi: 10.1016/j.jhep.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 12.Bernardi M., Angeli P., Claria J., Moreau R., Gines P., Jalan R., Caraceni P., Fernandez J., Gerbes A.L., O’Brien A.J., et al. Albumin in decompensated cirrhosis: New concepts and perspectives. Gut. 2020;69:1127–1138. doi: 10.1136/gutjnl-2019-318843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagdish R.K., Maras J.S., Sarin S.K. Albumin in Advanced Liver Diseases: The Good and Bad of a Drug! Hepatology. 2021;74:2848–2862. doi: 10.1002/hep.31836. [DOI] [PubMed] [Google Scholar]
- 14.Italian Association for the Study of the Liver. Italian Society of Transfusion Medicine Immunohaematology AISF-SIMTI Position Paper: The appropriate use of albumin in patients with liver cirrhosis. Dig. Liver Dis. 2016;48:4–15. doi: 10.1016/j.dld.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Runyon B.A. Management of adult patients with ascites due to cirrhosis: An update. Hepatology. 2009;49:2087–2107. doi: 10.1002/hep.22853. [DOI] [PubMed] [Google Scholar]
- 16.Shen N.T., Barraza L.H., Anam A.K., Patel P., Jesudian A. Benefit of Albumin Infusion in Hospitalized Patients With Cirrhosis and Hyponatremia: A Retrospective Cohort Study. J. Gastroenterol. Hepatol. Res. 2017;6:2441–2445. doi: 10.17554/j.issn.2224-3992.2017.06.724. [DOI] [Google Scholar]
- 17.Bajaj J.S., Tandon P., OʼLeary J.G., Biggins S.W., Wong F., Kamath P.S., Garcia-Tsao G., Maliakkal B., Lai J.C., Fallon M., et al. The Impact of Albumin Use on Resolution of Hyponatremia in Hospitalized Patients with Cirrhosis. Am. J. Gastroenterol. 2018;113:1339. doi: 10.1038/s41395-018-0119-3. [DOI] [PubMed] [Google Scholar]
- 18.China L., Freemantle N., Forrest E., Kallis Y., Ryder S.D., Wright G., O’Brien A. Targeted Albumin Therapy Does Not Improve Short-Term Outcome in Hyponatremic Patients Hospitalized with Complications of Cirrhosis: Data From the ATTIRE Trial. Am. J. Gastroenterol. 2021;116:2292–2295. doi: 10.14309/ajg.0000000000001488. [DOI] [PubMed] [Google Scholar]
- 19.Zaccherini G., Baldassarre M., Tufoni M., Nardelli S., Piano S., Alessandria C., Neri S., Foschi F.G., Levantesi F., Bedogni G., et al. Correction and Prevention of Hyponatremia in Patients with Cirrhosis and Ascites: Post Hoc Analysis of the ANSWER Study Database. Am. J. Gastroenterol. 2022 doi: 10.14309/ajg.0000000000001995. [DOI] [PubMed] [Google Scholar]
- 20.Bai Z., Bernardi M., Yoshida E.M., Li H., Guo X., Méndez-Sánchez N., Li Y., Wang R., Deng J., Qi X. Albumin infusion may decrease the incidence and severity of overt hepatic encephalopathy in liver cirrhosis. Aging. 2019;11:8502–8525. doi: 10.18632/aging.102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin Y., Li Y., Shao L., Yuan S., Liu B., Lin S., Yang Y., Tang S., Meng F., Wu Y., et al. Effect of Body Mass Index on the Prognosis of Liver Cirrhosis. Front. Nutr. 2021;8:700132. doi: 10.3389/fnut.2021.700132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.European Association for the Study of the Liver EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J. Hepatol. 2010;53:397–417. doi: 10.1016/j.jhep.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Peng Y., Qi X., Guo X. Child-Pugh Versus MELD Score for the Assessment of Prognosis in Liver Cirrhosis: A Systematic Review and Meta-Analysis of Observational Studies. Medicine. 2016;95:e2877. doi: 10.1097/MD.0000000000002877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Attar B. Approach to Hyponatremia in Cirrhosis. Clin. Liver Dis. 2019;13:98–101. doi: 10.1002/cld.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rondon-Berrios H., Velez J.C.Q. Hyponatremia in Cirrhosis. Clin. Liver Dis. 2022;26:149–164. doi: 10.1016/j.cld.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John S., Thuluvath P.J. Hyponatremia in cirrhosis: Pathophysiology and management. World J. Gastroenterol. 2015;21:3197–3205. doi: 10.3748/wjg.v21.i11.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernardi M., Moreau R., Angeli P., Schnabl B., Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: From peripheral arterial vasodilation to systemic inflammation hypothesis. J. Hepatol. 2015;63:1272–1284. doi: 10.1016/j.jhep.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Caraceni P., Abraldes J.G., Ginès P., Newsome P.N., Sarin S.K. The search for disease-modifying agents in decompensated cirrhosis: From drug repurposing to drug discovery. J. Hepatol. 2021;75((Suppl. S1)):S118–S134. doi: 10.1016/j.jhep.2021.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Martinez R., Caraceni P., Bernardi M., Gines P., Arroyo V., Jalan R. Albumin: Pathophysiologic basis of its role in the treatment of cirrhosis and its complications. Hepatology. 2013;58:1836–1846. doi: 10.1002/hep.26338. [DOI] [PubMed] [Google Scholar]
- 30.China L., Freemantle N., Forrest E., Kallis Y., Ryder S.D., Wright G., Portal A.J., Becares Salles N., Gilroy D.W., O’Brien A., et al. A Randomized Trial of Albumin Infusions in Hospitalized Patients with Cirrhosis. N. Engl. J. Med. 2021;384:808–817. doi: 10.1056/NEJMoa2022166. [DOI] [PubMed] [Google Scholar]
- 31.Caraceni P., Riggio O., Angeli P., Alessandria C., Neri S., Foschi F.G., Levantesi F., Airoldi A., Boccia S., Svegliati-Baroni G., et al. Long-term albumin administration in decompensated cirrhosis (ANSWER): An open-label randomised trial. Lancet. 2018;391:2417–2429. doi: 10.1016/S0140-6736(18)30840-7. [DOI] [PubMed] [Google Scholar]
- 32.Porcel A., Díaz F., Rendón P., Macías M., Martín-Herrera L., Girón-González J.A. Dilutional hyponatremia in patients with cirrhosis and ascites. Arch. Intern. Med. 2002;162:323–328. doi: 10.1001/archinte.162.3.323. [DOI] [PubMed] [Google Scholar]
- 33.Fortune B., Cardenas A. Ascites, refractory ascites and hyponatremia in cirrhosis. Gastroenterol. Rep. 2017;5:104–112. doi: 10.1093/gastro/gox010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ginès P., Cárdenas A. The management of ascites and hyponatremia in cirrhosis. Semin. Liver Dis. 2008;28:43–58. doi: 10.1055/s-2008-1040320. [DOI] [PubMed] [Google Scholar]
- 35.Thuluvath P.J., Alukal J.J., Zhang T. Impact of Hyponatremia on Morbidity, Mortality, and Resource Utilization in Portal Hypertensive Ascites: A Nationwide Analysis. J. Clin. Exp. Hepatol. 2022;12:871–875. doi: 10.1016/j.jceh.2021.10.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kegasawa T., Sakamori R., Maesaka K., Yamada R., Tahata Y., Urabe A., Kodama T., Hikita H., Imanaka K., Ohkawa K., et al. Lower Serum Sodium Levels Are Associated with the Therapeutic Effect of Sorafenib on Hepatocellular Carcinoma. Dig. Dis. Sci. 2021;66:1720–1729. doi: 10.1007/s10620-020-06380-6. [DOI] [PubMed] [Google Scholar]
- 37.Biolato M., Miele L., Vero V., Racco S., Di Stasi C., Iezzi R., Zanché A., Pompili M., Rapaccini G.L., La Torre G., et al. Hepatocellular carcinoma treated by conventional transarterial chemoembolization in field-practice: Serum sodium predicts survival. World J. Gastroenterol. 2014;20:8158–8165. doi: 10.3748/wjg.v20.i25.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bernardi M., Caraceni P., Navickis R.J., Wilkes M.M. Albumin infusion in patients undergoing large-volume paracentesis: A meta-analysis of randomized trials. Hepatology. 2012;55:1172–1181. doi: 10.1002/hep.24786. [DOI] [PubMed] [Google Scholar]
- 39.Kütting F., Schubert J., Franklin J., Bowe A., Hoffmann V., Demir M., Pelc A., Nierhoff D., Töx U., Steffen H.M. Insufficient evidence of benefit regarding mortality due to albumin substitution in HCC-free cirrhotic patients undergoing large volume paracentesis. J. Gastroenterol. Hepatol. 2017;32:327–338. doi: 10.1111/jgh.13421. [DOI] [PubMed] [Google Scholar]
- 40.Lopes-Secundo T.M., Sevá-Pereira T., Correa B.R., Silva N.C.M., Imbrizi M.R., Cunha-Silva M., Soares E.C., Almeida J.R.S. Serum sodium, model for end-stage liver disease, and a recent invasive procedure are risk factors for severe acute-on-chronic liver failure and death in cirrhotic patients hospitalized with bacterial infection. Eur. J. Gastroenterol. Hepatol. 2018;30:1055–1059. doi: 10.1097/MEG.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 41.Ennaifer R., Cheikh M., Romdhane H., El Elj R., Ben Nejma H., Bougassas W., Bel Hadj N. Hyponatremia in cirrhosis: Risk factors and prognostic value. Tunis Med. 2016;94:401–405. [PubMed] [Google Scholar]
- 42.Kim J.H., Lee J.S., Lee S.H., Bae W.K., Kim N.H., Kim K.A., Moon Y.S. The association between the serum sodium level and the severity of complications in liver cirrhosis. Korean J. Intern. Med. 2009;24:106–112. doi: 10.3904/kjim.2009.24.2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barakat A.A., Metwaly A.A., Nasr F.M., El-Ghannam M., El-Talkawy M.D., Taleb H.A. Impact of hyponatremia on frequency of complications in patients with decompensated liver cirrhosis. Electron. Phys. 2015;7:1349–1358. doi: 10.14661/1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenq C.C., Tsai M.H., Tian Y.C., Chang M.Y., Lin C.Y., Lien J.M., Chen Y.C., Fang J.T., Chen P.C., Yang C.W. Serum sodium predicts prognosis in critically ill cirrhotic patients. J. Clin. Gastroenterol. 2010;44:220–226. doi: 10.1097/MCG.0b013e3181aabbcd. [DOI] [PubMed] [Google Scholar]
- 45.Jia J., Xie W., Ding H., Mao H., Guo H., Li Y., Wang X., Wang J., Lu W., Li C., et al. Utility and safety of tolvaptan in cirrhotic patients with hyponatremia: A prospective cohort study. Ann. Hepatol. 2017;16:123–132. doi: 10.5604/16652681.1226823. [DOI] [PubMed] [Google Scholar]
- 46.Pugh R.N., Murray-Lyon I.M., Dawson J.L., Pietroni M.C., Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 47.Malinchoc M., Kamath P.S., Gordon F.D., Peine C.J., Rank J., ter Borg P.C. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864–871. doi: 10.1053/he.2000.5852. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated or analyzed during this study are available from the corresponding author on reasonable request.