Abstract
Rod-like crystalline structures formed during eosinophilic Cryptococcus neoformans pneumonia in C57BL/6 mice. Crystals were found associated with yeast cells and free in host cell cytoplasm. The crystals apparently formed because of the interaction of a host protein with the cryptococcal polysaccharide. Crystal formation likely contributes to pathogenesis by causing cellular damage.
Cryptococcus neoformans causes life-threatening disease in 5 to 10% of patients with AIDS in the United States (32) and 30% of those in Africa (8). C. neoformans is unique among the pathogenic fungi in that it is encapsulated, yet it can be a facultative intracellular pathogen (9, 15). The cryptococcal polysaccharide capsule is required for virulence (17, 26) and interferes with multiple aspects of host immune responses (3). Polysaccharide accumulation is also associated with host cell destruction (15, 28).
Crystal formation during the inflammatory response to microbial agents is a relatively rare phenomenon. Crystalline inflammation has been associated with eosinophils, and pulmonary cryptococcal infection in some strains produces eosinophilic pneumonia (23, 24). In C57BL/6 mice, eosinophilic pneumonia is common 14 days after primary pulmonary infection (13). Crystalline pneumonia has been recently described for murine cryptococcal infection, with the crystals being assessed to be Charcot-Leyden crystals (22). During studies of pulmonary C. neoformans infection in C57BL/6 mice, we noted rod-like crystalline structures in close apposition to yeast cells. Crystal structures with similar appearance in macrophages of numerous species have been described (7, 25, 27, 29, 34, 35). In the murine lung, their presence has been termed acidophilic macrophage pneumonia, and this phenomenon occurs with varying frequency in healthy mice and in mice with various disease states (20, 36, 37). C57BL/6 mice are particularly prone to crystal formation (31). Recently, crystals with similar appearance have been shown to consist of Ym1 (T-lymphocyte-derived eosinophil chemotactic factor) (19).
Ultrastructure of crystals in murine infection.
Cultures of C. neoformans ATCC 24067, obtained from the American Type Culture Collection (Manassas, Va.) (16), were maintained, grown, and prepared as previously described (15). For animal studies, the guidelines for animal experimentation of the Albert Einstein College of Medicine were followed. Six- to 10-week-old C57BL/6 mice from the National Cancer Institute (Bethesda, Md.) and from Jackson Laboratories (Bar Harbor, Maine) were used in most experiments. Additional experiments were done with 129/SvJ (Jackson Laboratories), 129/SvEv (Taconic Farms, Germantown, N.Y.), and A/JCr (National Cancer Institute) mice. These strains were selected because we have used them for other studies of pulmonary cryptococcosis (12, 15). Mice were infected by intratracheal inoculation of 104 organisms of C. neoformans, as described previously (12). Mice were killed by cervical dislocation, their lungs were removed, and one lobe was fixed in 10% buffered formalin for light microscopy. The remainder of the lung was processed for electron microscopy, as described previously (13). C57BL/6 mice were studied 14 days after infection (three experiments) and 28 days after infection (two experiments). 129/SvEv, 129/SvJ, and A/JCr mice were each studied 14 and 28 days after infection in one experiment. Two mice were studied in each experimental group.
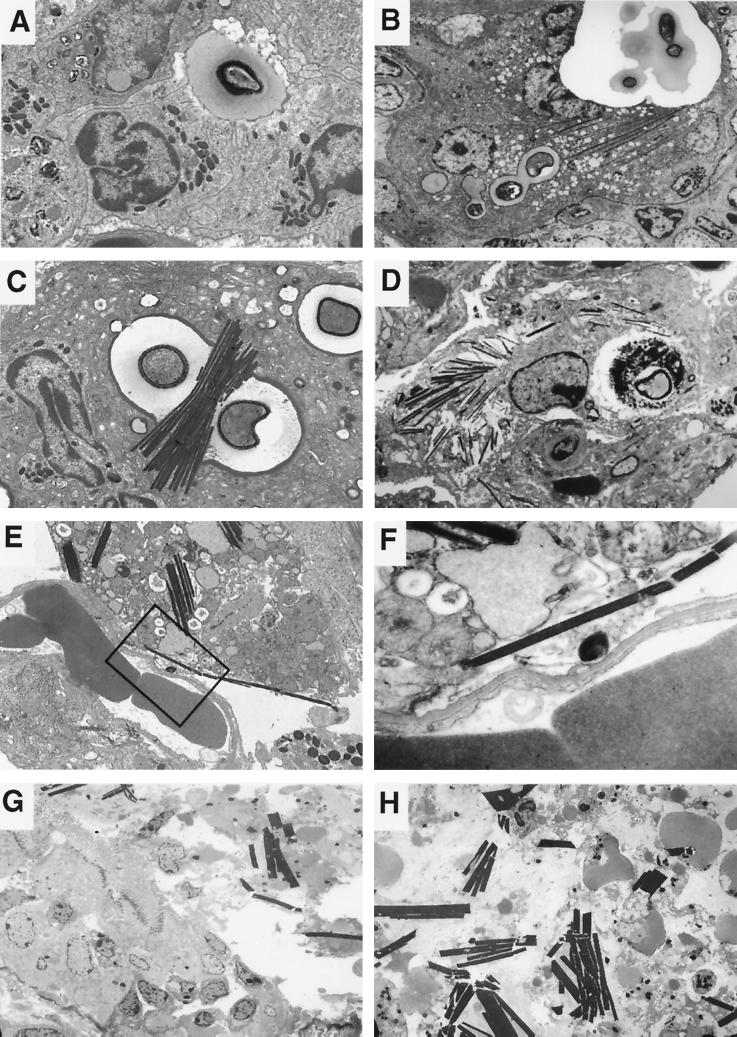
Crystalline structures were seen in the lungs of all C57BL/6 mice infected with C. neoformans in five of five independent experiments but were rarely seen in A/JCr, 129/SvEv, or 129/SvJ mice. In C57BL/6 mice, crystals were commonly seen 14 days after infection by transmission electron microscopy (Fig. 1). They were rarely present in lung tissue studied at earlier times after infection (data not shown). Deposits with the structural appearance of membranes were seen on the outer surfaces of the crystals and at times appeared to form a surrounding membrane (data not shown). The crystals were present in increased numbers 28 days after infection, at which time they often formed parallel stacks. The thickness of the crystalline structures appeared to be a multiple of the initial crystal thickness, suggesting side-to-side stacking. An internal structure was visible in some, with ultradense bands oriented along either the long or the short axis of the crystal. The distance between ultradense bands was determined by measuring the number of bands in 5 to 10 mm inside 10 crystals magnified 150,000 times. The mean distance between ultradense bands in either orientation was 0.42 nm (standard deviation, ±0.04 nm). An electron-dense ring frequently formed around the outer aspect of the cryptococcal polysaccharide capsule, with electron density and thickness similar to those of the crystals. The crystals were most often located in large multinucleated cells of macrophage origin based upon staining with Griffonia simplicifolia agglutinin B4 isolectin (14) but were occasionally inside eosinophils. Crystals were seen in cells containing C. neoformans and were absent from uninfected areas. Cells containing large numbers of crystals appeared to be dying, as they had rounded nuclei, clumped chromatin, and cytoplasmic disruption. Crystals protruded through the membrane of some cells, suggesting that crystal formation can lead to membrane disruption. By 28 days after infection, crystals were found extracellularly and in association with bronchial epithelial disruption.
FIG. 1.
(A) Eosinophils recruited to the site of infection discharge electron-dense granular contents at the surface of an extracellular Cryptococcus, organism, allowing contact of cryptococcal polysaccharide with granule proteins (day 14; magnification, ×3,000). (B) The location of the yeast cell and crystals within a large multinucleated cell at a magnification lower than that for panel A. An electron-dense rim forms around the yeast cell, and crystals subsequently polymerize in contact with yeast and in the cytoplasm (day 14; magnification, ×2,000). (C) Budding intracellular C. neoformans in murine lung tissue 14 days after infection with electron-dense rim surrounding the polysaccharide capsule. Crystals formed in association with the yeast cell (magnification, ×4,000). (D) On day 28 after infection, large numbers of crystals were seen inside multinucleated cells, some of which were dying (magnification, ×2,000). (E) Crystals disrupted host cell membranes and became extracellular (day 28 after infection; magnification, ×3,000). (F) Higher magnification of the area enclosed in the boxed area in panel E demonstrates membrane disruption (magnification, ×20,000). (G and H) On day 28 after infection, crystals disrupted the bronchial epithelium, with loss of cilia. Epithelial cell debris and larger extracellular crystals were seen inside bronchi (magnification, ×1,000).
Immunoelectron microscopy.
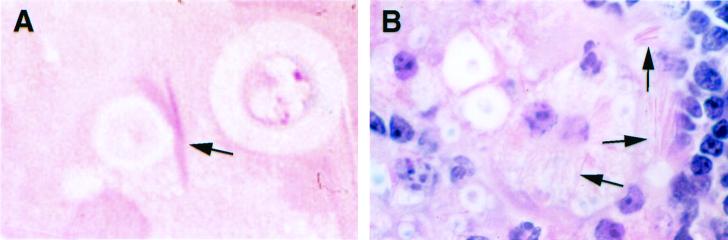
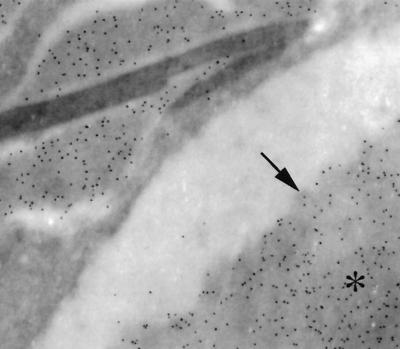
Because the appearance of the crystals was similar to that of eosinophil-specific granule cores (data not shown) (31), we initially hypothesized that they were composed of eosinophilic major basic protein (MBP). Staining of the crystals with eosin demonstrated their acidophilic nature (Fig. 2). Polyclonal rabbit antiserum to murine MBP was generously donated by James J. Lee and Nancy A. Lee (Mayo Clinic, Scottsdale, Ariz.). Unicryl-embedded (BB International, Cardiff, England), ultrathin lung tissue sections were blocked and then incubated in rabbit antiserum to murine MBP diluted 1:100 or in prebled rabbit serum for 1 h at room temperature. Grids were then incubated in goat anti-rabbit immunoglobulin G (IgG) conjugated to 10-nm-diameter gold particles (Goldmark Biologicals, Phillipsburg, N.J.) diluted 1:30 for 2 h at room temperature and fixed in 2% glutaraldehyde. Immunoelectron microscopy showed binding of immune antiserum to murine MBP to the eosinophil-specific granule core and matrix but not to the crystals. No labeling of eosinophils was seen with normal rabbit serum. The electron density of the crystals was similar to that of the cryptococcal cell wall, which contains polymerized melanin in vivo. To investigate whether the crystals were melanin, we performed immunoelectron microscopy with two melanin-binding monoclonal antibodies (MAbs), 11B1 or 6D2 (both IgM), or murine IgM (Southern Biotechnology Associates, Birmingham, Ala.) as a control, as described previously (33). The crystals remained unlabeled, although gold particles were observed in the cryptococcal cell wall, which is consistent with cell wall melanization (33). Next, we evaluated whether the crystals were composed of polysaccharide, which is abundant in infected tissue (18). In 1-μm-thick deplasticized sections, the crystals did not stain with periodic acid Schiff's reagents, which stain polysaccharide. Localization of C. neoformans capsular polysaccharide (CNPS) by immunoelectron microscopy was done on ultrathin sections of Epon-embedded tissue by using MAb 2H1, an IgG1 that binds to glucuronoxylomannan, the major component of capsular polysaccharide, as described previously (4, 5). Murine IgG (Sigma) was used as a control. CNPS was identified in vacuoles containing the crystals and surrounding the outer membranes of the crystals, but the crystals were not labeled (Fig. 3). On the basis of these results, we concluded that the crystals were not composed of MBP, melanin, or polysaccharide.
FIG. 2.
Light microscopic staining of intracellular crystals (arrows). (A) Crystals from a 1-μm-thick section of mouse lung 14 days after infection stained with eosin. (B) Staining of a 5-μm-thick section of mouse lung 28 days after infection with hematoxylin and eosin showing a macrophage containing a large number of intracellular crystals. Magnification, ×1,000 (panels A and B).
FIG. 3.
Immunoelctron microscopy shows the presence of gold label for cryptococcal polysaccharide surrounding the outer membrane of the crystals but not over the crystals. The asterisk is located on a cryptococcal capsule. The arrow points to artifactual contraction of the capsule from the edge of a phagosome. magnification, ×15,000).
In vitro crystal formation.
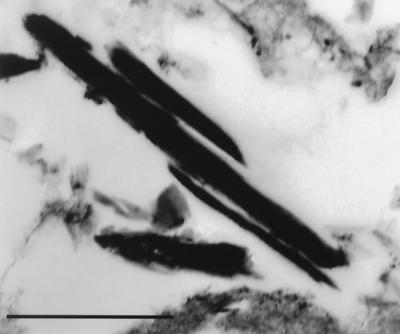
Peritoneal inflammatory cells from male Sprague Dawley rats from the National Cancer Institute and Charles River Laboratories (Raleigh, N.C.) were obtained as described previously (13). Rats were used for this experiment because they are a plentiful source of peritoneal inflammatory cells that contain a high percentage of eosinophils. Nonadherent cells were plated in 24-well plates at a final concentration of 1.5 × 106 cells in 1 ml of RPMI containing 10% heat-inactivated fetal calf serum with 1% penicillin or streptomycin (incubation medium). C. neoformans strains ATCC 24067, 3501, or Cap 67 (an acapsular strain isogenic with strain 3501) were added at a final concentration of 1.5 × 105 yeast cells/ml. To induce phagocytosis of C. neoformans and eosinophil degranulation, 200 μg of MAb 2H1/ml was added, and the plates were incubated at 37°C with 8% CO2. Every 24 h, the medium was aspirated and replaced with 1 ml of medium with 200 μg of MAb 2H1/ml. At various times after infection, the contents of the wells were aspirated, centrifuged at 330 × g for 5 min, and resuspended in Trump's fixative. Cell blocks were embedded in araldite-Epon and processed for electron microscopy as described above. For each specimen, all areas of five noncontiguous grids were examined. In a preliminary experiment, crystal formation was maximal after incubation for 3 days and was less evident at 24 h. After incubation for 5 days, wells were overwhelmed with C. neoformans and most cells had degenerated. As a result, incubation for 3 days was used for subsequent experiments. Crystals formed in large numbers when either of two encapsulated strains of C. neoformans and MAb to the polysaccharide capsule were added to rat peritoneal inflammatory cells (Fig. 4). In contrast, crystals rarely formed when an acapsular strain was used.
FIG. 4.
Crystals formed after incubation of C. neoformans strain 24067 with rat peritoneal inflammatory cells in vitro for 3 days. Bar, 1 μm.
CNPS from ATCC 3501 was isolated as described previously (6). Rat peritoneal inflammatory cells were prepared as described above, and 1.5 × 106 cells in incubation medium were incubated with 1, 10, or 100 μg of CNPS/ml and 200 μg of MAb 2H1/ml for 3 days at 37°C. The incubation medium was changed daily. For the sample containing 1 μg of CNPS/ml, additional CNPS was added on days 1 and 2. For the 10-μg/ml sample, additional CNPS was added on day 2. In all wells containing CNPS, MAb 2H1 was added to the replacement medium on days 1 and 2 at a final concentration of 200 μg/ml. To demonstrate a requirement for capsular polysaccharide for crystal formation, 1.5 × 106 sheep erythrocytes (RBCs) conjugated to MAb 2H1 were incubated with eosinophils under the same conditions. Sheep RBCs were conjugated to MAb 2H1 by incubation of a 5% cell suspension in 0.25% glutaraldehyde for 1 h at room temperature with continuous stirring (2, 30). Attachment of MAb 2H1 to the RBC surface was assessed by demonstration of agglutination following the addition of CNPS. Crystals formed in assays containing 1 or 10 μg of CNPS/ml and MAb 2H1. When 100 μg of CNPS/ml was used, inflammatory cells degenerated. The addition of complexes of CNPS and MAb induced frequent crystal formation, whereas very rare crystals formed when antigen-antibody complexes consisting of sheep RBCs conjugated to anticapsular MAb were added to inflammatory cells, despite internalization of these complexes. These results demonstrate that capsular polysaccharide contributes to crystal formation. Although we cannot definitively state that these crystals are the same as those observed in murine pulmonary infection because homology of murine Ym1 with a rat eosinophil chemotactic factor is unknown, similar crystals in rats have been described previously (34).
Crystals with an appearance similar to those in the present study have been associated with eosinophilic infiltrates (1, 11, 21, 31, 37). Consequently, many investigators have hypothesized that they represent a crystallized eosinophil protein, such as lysophospholipase (Charcot-Leyden crystals) (22, 37). However, Charcot-Leyden crystals from nonprimate species have never been definitively described (10, 11). Rather, the recent report identifying very similar crystals as composed of an eosinophil chemotactic factor, Ym1, provides the likely explanation for the coincidence of crystals and eosinophil infiltrates, as eosinophils are recruited in response to this protein (19). That Ym1 has chitinase activity and is present in large amounts in association with C. neoformans, which, like other fungi, contains cell wall chitin, supports the view of Guo et al. (19) that Ym1 may be part of the host response to microorganisms that contain chitin. Further, these investigators suggest that Ym1 may be involved in binding to extracellular polysaccharide. Here, we found that crystals formed at the edge of the polysaccharide capsule and were closely associated with cytoplasmic CNPS deposits. CNPS is a polysaccharide with a high molecular weight, which may promote protein polymerization through excluded volume effects in the phagosome.
Crystal formation appears to be a cytotoxic phenomenon. The formation of larger collections of these structures was associated with disruption and death of macrophages and bronchial epithelial cell destruction. Since macrophages are the principal effector cells against C. neoformans, crystal formation may interfere with host defense mechanisms and promote persistence of infection. Furthermore, polymerization of a host cell protein by exposure to CNPS during cryptococcal infection may eliminate a potentially microbicidal protein from solution. The reason for the strikingly frequent appearance of these polymers in murine cryptococcal pneumonia may be related to two unusual qualities of this fungal infection: chronicity and abundant polysaccharide production in tissue. The conversion of a host cell protein from an effector molecule into a polymer that damages host cells may represent a novel mechanism of virulence for CNPS and may contribute to the chronicity and persistence associated with cryptococcal infection. While the overall impact of crystal formation in cryptococcal infection is unknown, the association of crystals with disrupted cells suggests that the net effect of crystal formation is harmful to the host.
Acknowledgments
This work was supported by NIH grant AI01341 (M.F.), by NIH grants AI33774, AI33142, and HL59842 (A.C.), and by a Burroughs Wellcome Developmental Therapeutics Award (A.C.). This support is gratefully acknowledged.
We thank James J. Lee and Nancy A. Lee for generously providing antiserum to murine MBP, Gerald Gleich, Liise-Anne Pirofski, and Matthew Scharff for critical reading of the manuscript, Ann Dvorak for helpful discussion, Jorge Bermudez for processing of tissue for light microscopy, and Clemen Cayetano and Valentin Storovoytov for technical assistance with electron microscopy.
REFERENCES
- 1.Ali B A, Shortland J R, Hudson G. Origin of crystalloid inclusions in macrophages. II. Evidence for derivation from eosinophil granulocyte breakdown. Br J Exp Pathol. 1981;62:662–668. [PMC free article] [PubMed] [Google Scholar]
- 2.Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969;6:43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- 3.Buchanan K L, Murphy J W. What makes Cryptococcus neoformans a pathogen? Emerg Infect Dis. 1998;4:71–83. doi: 10.3201/eid0401.980109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman D L, Kozel T R, Lendvai N, Mukherjee J, Pirofski L-A, Rivera J, Rosas A L, Scharff M D, Valadon P, Westin K, Zhong Z. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998;42:1437–1446. doi: 10.1128/aac.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadevall A, Mukherjee J, Devi S J N, Schneerson R, Robbins J B, Scharff M D. Antibodies elicited by a Cryptococcus neoformans-tetanus toxoid conjugate vaccine have the same specificity as those elicited in infection. J Infect Dis. 1992;165:1086–1093. doi: 10.1093/infdis/165.6.1086. [DOI] [PubMed] [Google Scholar]
- 6.Cherniak R, Reiss E, Turner S H. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr Res. 1982;103:239–250. [Google Scholar]
- 7.Collet A J. Fine structure of the alveolar macrophage of the cat and modifications of its cytoplasmic components during phagocytosis. Anat Rec. 1970;167:277–290. doi: 10.1002/ar.1091670303. [DOI] [PubMed] [Google Scholar]
- 8.Desmet P, Kayembe K D, De Vroey C. The value of cryptococcal serum antigen screening among HIV-positive/AIDS patients in Kinshasa, Zaire. AIDS. 1989;3:77–78. doi: 10.1097/00002030-198902000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Diamond R D, Bennett J E. Growth of Cryptococcus neoformans within human macrophages in vitro. Infect Immun. 1973;7:231–236. doi: 10.1128/iai.7.2.231-236.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dvorak A M, Weller P F, Monahan-Earley R A, Letourneau L, Ackerman S J. Ultrastructural localization of Charcot-Leyden crystal protein (lysophospholipase) and peroxidase in macrophages, eosinophils, and extracellular matrix of the skin in the hypereosinophilic syndrome. Lab Investig. 1990;62:590–607. [PubMed] [Google Scholar]
- 11.El-Hashimi W. Charcot-Leyden crystals. Am J Pathol. 1971;65:311–324. [PMC free article] [PubMed] [Google Scholar]
- 12.Feldmesser M, Casadevall A. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J Immunol. 1997;158:790–799. [PubMed] [Google Scholar]
- 13.Feldmesser M, Casadevall A, Kress Y, Spira G, Orlofsky A. Eosinophil-Cryptococcus neoformans interactions in vivo and in vitro. Infect Immun. 1997;65:1899–1907. doi: 10.1128/iai.65.5.1899-1907.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldmesser M, Kress Y, Casadevall A. Effect of antibody to capsular polysaccharide on eosinophilic pneumonia in murine infection with Cryptococcus neoformans. J Infect Dis. 1998;177:1639–1646. doi: 10.1086/515314. [DOI] [PubMed] [Google Scholar]
- 15.Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun. 2000;68:4225–4237. doi: 10.1128/iai.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franzot S P, Mukherjee J, Cherniak R, Chen L-C, Hamdan J S, Casadevall A. Microevolution of a standard strain of Cryptococcus neoformans resulting in differences in virulence and other phenotypes. Infect Immun. 1998;66:89–97. doi: 10.1128/iai.66.1.89-97.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fromtling R A, Shadomy H J, Jacobson E S. Decreased virulence in stable, acapsular mutants of Cryptococcus neoformans. Mycopathologia. 1982;79:23–29. doi: 10.1007/BF00636177. [DOI] [PubMed] [Google Scholar]
- 18.Goldman D L, Lee S C, Casadevall A. Tissue localization of Cryptococcus neoformans glucuronoxylomannan in the presence and absence of specific antibody. Infect Immun. 1995;63:3448–3453. doi: 10.1128/iai.63.9.3448-3453.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo L, Johnson R S, Schuh J C. Biochemical characterization of endogenously formed eosinophilic crystals in the lungs of mice. J Biol Chem. 2000;275:8032–8037. doi: 10.1074/jbc.275.11.8032. [DOI] [PubMed] [Google Scholar]
- 20.Horn H A, Congdon C C, Eschenbrenner A B, Andervont H B, Stewart H L. Pulmonary adenomatosis in mice. J Natl Cancer Inst. 1952;12:1297–1315. [PubMed] [Google Scholar]
- 21.Hudson G. Crystalloid material in macrophages of mouse bone marrow. Acta Anat. 1968;71:100–107. doi: 10.1159/000143176. [DOI] [PubMed] [Google Scholar]
- 22.Huffnagle G B, Boyd M B, Street N E, Lipscomb M F. IL-5 is required for eosinophil recruitment, crystal deposition, and mononuclear cell recruitment during a pulmonary Cryptococcus neoformans infection in genetically susceptible mice (C57BL/6) J Immunol. 1998;160:2393–2400. [PubMed] [Google Scholar]
- 23.Huffnagle G B, Chen G-H, Curtis J L, McDonald R A, Strieter R M, Toews G B. Down-regulation of the afferent phase of T cell-mediated pulmonary inflammation and immunity by a high melanin-producing strain of Cryptococcus neoformans. J Immunol. 1995;155:3507–3516. [PubMed] [Google Scholar]
- 24.Huffnagle G B, Lipscomb M F, Lovchik J A, Hoag K A, Street N E. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. J Leukoc Biol. 1994;55:35–42. doi: 10.1002/jlb.55.1.35. [DOI] [PubMed] [Google Scholar]
- 25.Komiyama A, Spicer S S. Microendocytosis in eosinophilic leukocytes. J Cell Biol. 1975;64:622–635. doi: 10.1083/jcb.64.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozel T R, Cazin J., Jr Nonencapsulated variant of Cryptococcus neoformans. I. Virulence studies and characterization of soluble polysaccharide. Infect Immun. 1971;3:287–294. doi: 10.1128/iai.3.2.287-294.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leake E S, Wright M J. The fine structure of lung macrophages from rhesus and squirrel monkeys, with special reference to the large numbers of mitochondria. Am Rev Respir Dis. 1976;114:581–592. doi: 10.1164/arrd.1976.114.3.581. [DOI] [PubMed] [Google Scholar]
- 28.Lee S C, Kress Y, Zhao M-L, Dickson D W, Casadevall A. Cryptococcus neoformans survive and replicate in human microglia. Lab Investig. 1995;73:871–879. [PubMed] [Google Scholar]
- 29.Marshall G E, Shortland J R, Hudson G. Crystalloid material in cells of the murine mononuclear phagocyte system. J Anat. 1988;157:217–228. [PMC free article] [PubMed] [Google Scholar]
- 30.Mota G, Galatiue C, Sjoquist J, Ghetie V. A non-dissociable IgG-protein A complex. Ann Immunol. 1983;134C:331–340. doi: 10.1016/s0769-2625(83)80127-5. [DOI] [PubMed] [Google Scholar]
- 31.Murray A B, Luz A. Acidophilic macrophage pneumonia in laboratory mice. Vet Pathol. 1990;27:274–281. doi: 10.1177/030098589002700409. [DOI] [PubMed] [Google Scholar]
- 32.Powderly W G. Cryptococcal meningitis and AIDS. Clin Infect Dis. 1993;17:837–842. doi: 10.1093/clinids/17.5.837. [DOI] [PubMed] [Google Scholar]
- 33.Rosas A L, Nosanchuk J D, Feldmesser M, Cox G M, McDade H C, Casadevall A. Synthesis of polymerized melanin by Cryptococcus neoformans in infected rodents. Infect Immun. 2000;68:2845–2853. doi: 10.1128/iai.68.5.2845-2853.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simon Gerard T. Splenic macrophages. In: Carr I, Daems W T, editors. The reticuloendothelial system: a comprehensive treatise. I. Morphology. London, United Kingdom: Plenum Press; 1980. pp. 469–497. [Google Scholar]
- 35.Simon G T, Burke J S. Electron microscopy of the spleen. Am J Pathol. 1970;58:451–469. [PMC free article] [PubMed] [Google Scholar]
- 36.Ward J M. Pulmonary pathology of the motheaten mouse. Vet Pathol. 1978;15:170–178. doi: 10.1177/030098587801500203. [DOI] [PubMed] [Google Scholar]
- 37.Zyngier F R, Brockbank A. Electron microscopy of the lung in experimental Toxocara canis infection. Ann Trop Med Parasitol. 1974;68:229–233. doi: 10.1080/00034983.1974.11686940. [DOI] [PubMed] [Google Scholar]