Abstract
A defined allelic-replacement mutant of the sly gene, encoding a thiol-activated cytolysin, from a European isolate of Streptococcus suis serotype 2 was generated and characterized. Unlike the parental strain, it is nonhemolytic, noncytotoxic for cultured macrophage-like cells, avirulent in a mouse infection model, yet only slightly attenuated in a porcine model of systemic infection.
Streptococcus suis has been described as the etiological agent for a number of infectious disease syndromes in pigs, including arthritis, septicemia, meningitis, and pneumonia. S. suis produces a secreted hemolysin (suilysin) which has been suggested as playing a role in virulence (2, 4–7, 10). In order to investigate the role played by suilysin in the pathogenesis of a European serotype 2 isolate of S. suis, we generated a defined allelic-replacement mutant of the sly gene and compared the wild-type organism with the sly mutant in a number of assays.
Bacterial strains and media.
S. suis type 2 strain P1/7 was grown on Columbia agar (Oxoid) containing 10% defibrinated horse blood or in liquid cultures of Todd-Hewitt broth (Oxoid) supplemented with 7% fetal calf serum (FCS) (Gibco). Allelic-replacement mutants of S. suis were maintained on 1 μg of erythromycin (Sigma) per ml.
Mutagenesis of the suilysin gene from S. suis type 2.
An erythromycin resistance gene cassette was introduced into an EcoRV site within a 1,278-bp fragment of the sly gene, contained in the vector pT7-Blue (Stratagene), amplified by PCR using primers suis1 (5′-AGCTTGACTTACGAGCCACAAGAG-3′) and suis2 (5′-CCACCATTCCCAAGCTAATCCTGT-3′) with chromosomal DNA from P1/7 as a template. The resulting plasmid, pSUI-erm, contains the erm gene in the same orientation as the sly gene. Plasmid pSUI-erm was introduced into P1/7 by electroporation (22.5 V/cm, 25 μF, and 1,000 Ω). A transformant which had undergone a double-crossover event, confirmed by Southern hybridization and PCR, with concomitant insertion mutation of the sly gene, was isolated and named S7c.
Phenotypic analysis of S7c.
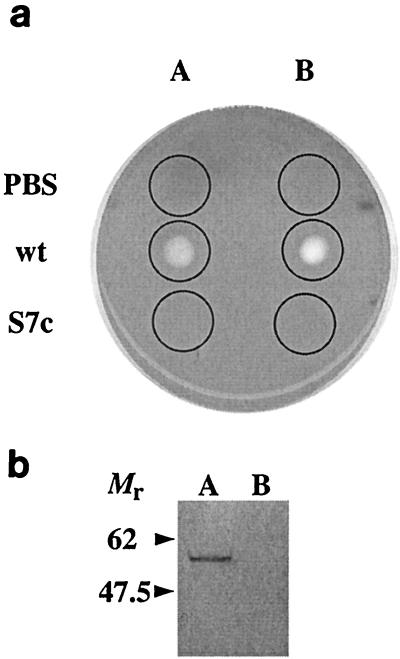
Overnight growth of suilysin mutant S7c on Columbia blood agar plates revealed no β-hemolysis. Secreted proteins from anaerobically grown overnight cultures of P1/7 and S7c were concentrated 100-fold by ammonium sulfate (50%, wt/vol) precipitation; then 10 μl each of these preparations was spotted onto a Columbia horse blood agar plate and incubated at 37°C for 30 min. The proteins from P1/7 show clear zones of hemolysis, whereas there is a complete absence of hemolytic activity in the proteins obtained from S7c (Fig. 1a). The hemolytic activity from the wild-type parental bacteria was enhanced by the addition of β-mercaptoethanol, as previously reported (7) (Fig. 1a).
FIG. 1.
Characterization of P1/7 and S7c culture supernatants. (a) Overnight culture supernatants from wild type P1/7 (wt) and S7c (S7c) were concentrated 100-fold by ammonium sulfate precipitation, and 10-μl samples, indicated by circles, were overlaid onto a 7% (vol/vol) horse blood agar plate, followed by incubation at 37°C for 60 min. Samples in track B were treated with β-mercaptoethanol to a final concentration of 1 mM. PBS, phosphate-buffered saline. (b) Western blot of culture supernatants from P1/7 and S7c using antisuilysin monoclonal antibody. Proteins secreted from overnight culture supernatants from P1/7 (track A) and S7c (track B) were concentrated 25 times, separated on a polyacrylamide gel, and Western blotted onto nitrocellulose, followed by development with a monoclonal antibody specific for suilysin. The band corresponding to suilysin in track A is completely absent from track B. Molecular weights are in thousands.
The lack of expression of suilysin was confirmed by Western blotting. Supernatants from aerobically grown cultures of both P1/7 and S7C (sterilized using a 0.22-μm-pore-size filter and concentrated 25-fold using Amicon filters) were probed with a monoclonal antibody (INT-STS-28-02; A. C. Jacobs, Intervet) raised against purified suilysin. An immunoreactive protein with the expected molecular weight (54,000) was clearly present in the supernatant from P1/7 (Fig. 1b, track A), whereas there was no immunoreactive material present in that from S7c (Fig. 1b, track B). These results show that an allelic-replacement insertion mutant lacking functional sly was generated in S. suis and that this mutant was not hemolytic.
Cell culture and cytotoxicity assay.
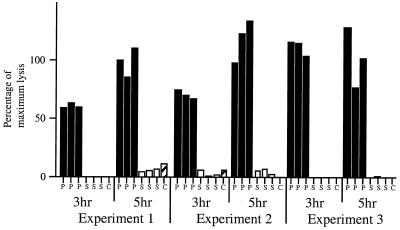
Murine macrophage-like J774.2 cells were split into 96-well plates (approximately 1.5 × 105 cells/well) and maintained in Dulbecco modified Eagle medium supplemented with 3% FCS and 2 mM glutamine (assay buffer). Bacterial inocula (5 × 107 CFU per 100 μl, in assay buffer), generated from overnight cultures of P1/7 and S7c, were added to three experimental wells and incubated at 37°C with 5% carbon dioxide for 3 and 5 h. Relative cytotoxicity was assayed as lactate dehydrogenase (LDH) release as determined by the CytoTox96 kit (Promega). The experiment was repeated on three separate occasions (Fig. 2). The parental strain, P1/7, caused extensive damage to cell monolayers in a time-dependent manner. In comparison, S7c elicited only approximately 4% of maximal LDH release after 5 h of incubation. Bacterial viable counts during the experiment revealed identical numbers of CFU for P1/7 and S7c. These experiments show that S. suis efficiently kills J774.2 cells and that this effect is dependent on the presence of suilysin, which is thus probably the only cytolysin produced by S. suis.
FIG. 2.
Comparison of cytotoxicities of P1/7 and S7c for J774.2 cells. Monolayers of J774. 2 cells (2 × 105 cells) were infected with P1/7 (P) or S7c (S). Relative levels of LDH released at 3 and 5 h were measured and plotted as percentages of the maximum lysis achieved by chemical treatment. Experiments were performed in triplicate for each datum point. Where no bars are present, the lysis was effectively zero. Spontaneous lysis (C) occurred without the addition of bacteria. It is clear that wild-type bacteria cause substantial lysis of the cell monolayer, whereas the mutant lacking suilysin is unable to lyse the cells.
Mouse infection experiments.
Overnight cultures of P1/7 and S7c, grown in Todd-Hewitt broth containing 10% FCS, were diluted in the same medium to achieve 108, 107, 106, and 105 CFU/0.5-ml dose. These 0.5-ml inocula were introduced intraperitoneally into 5-week-old female BALB/c mice, in groups of five mice per inoculum. Mice were monitored over a period of 1 week, during which deaths were recorded and moribund animals were humanely killed. Inoculation of 108 and 107 wild-type bacteria killed 9 of 10 mice and 8 of 10 mice, respectively, over the two experiments, within 48 h, providing 50% lethal doses (9) of log 6.45 and log 6.73, respectively, whereas all mice infected with S7c survived. These data show unequivocally that mutation of suilysin prevents S. suis from killing mice via the intraperitoneal route of infection.
Pig infection experiment.
Pigs, aged between 35 and 40 days, were obtained from Cotswold Pigs Ltd. (Colsterworth Farm, United Kingdom). Fourteen days prior to challenge, animals were screened for the presence of major pathogens and injected with ceftiofur (Excenel; Pharmacia & Upjohn Ltd.) for three consecutive days. Bacteria for challenge were grown overnight in brain heart infusion broth at 37°C and were subcultured in the same medium for 6 h at 37°C. Cultures were diluted in phosphate-buffered saline (pH 7.2), and each animal was challenged by intravenous injection of 1 ml of phosphate-buffered saline containing between 1 × 106 and 5 × 106 CFU of either strain S7c (group 1) or P1/7 (group 2). Animals were monitored for clinical signs of disease every 3 h; piglets showing marked signs of illness were killed for postmortem examination. Surviving animals were killed for postmortem examination 44 h postinfection. At postmortem, a visual inspection was made in order to detect pathological lesions and swabs were taken from heart blood, the lateral ventricle of the brain, the lungs, the serous cavity, and two limbs for bacterial examination. Results (lesion scores, bacteriology, survival times) between groups were analyzed statistically using Student's t test via MINITAB release 12.1 for Windows. Tests were performed assuming equal variance and were repeated assuming unequal variance where necessary.
The results of the challenge experiment are summarized in Table 1. Two pigs infected with S7c and one infected with P1/7 showed signs of clinical disease by 12 h postinfection, with marked lameness, shivering, and vomiting. By 15 h postinfection, three pigs challenged with S7c and all five infected with P1/7 were affected, typical signs being dullness, swollen joints, and dyspnea. Three pigs infected with P1/7 and three infected with S7c required euthanasia commencing 21 h postinfection, due to severe lameness. A fourth pig infected with P1/7 showed signs of meningitis (wide-based stance, rigid posture, ataxia, and muscle tremors). At 44 h postinfection the experiment was terminated, by which time only one pig infected with P1/7 and two pigs infected with S7c were left alive. Mean survival times were not significantly different between groups.
TABLE 1.
Summary of pig infection experiment
| No. of pigs infected | Challenge strain | Cumulative no. of pigs:
|
Clinical signs of surviving animal(s) (pig no.) | Mean ± SD of:
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Showing signs of diseasea at p.i. time
|
Euthanatized at p.i. time
|
||||||||
| 12 h | 15 h | 21 h | 44 h | Survival timeb (h) | Bacterial scorec | Pathological lesion scored | |||
| 5 | S7c | 2 | 3 | 3 | 3 | Subdued, mild lameness (1), Few signs (4) | 37.2 ± 9.4 | 4.2 ± 3.6 | 3.4 ± 2.6 |
| 5 | P1/7 | 1 | 5 | 4 | 4 | Subdued, mild lameness (9) | 35.3 ± 7.3 | 12.8 ± 6.8 | 9.6 ± 1.5 |
Marked lameness, shivering, vomiting, dullness, swollen joints, or dyspnea.
Survival times reflect the time taken for animals to reach predefined clinical end points. Three animals that did not reach these end points were assigned a time of 44 h. Differences between the two groups were not statistically significant.
Cumulative bacterial scores from each pig were obtained and the means were calculated per pig (maximum value, 24). Differences between the two groups were not statistically significant.
Pathological lesions in the peritonea, pleurae, pericardia, lungs, and joints were assessed by an experienced veterinary pathologist and were given scores from 0 (unaffected) to 3 (severely affected). For the joints, the highest value assigned was 4 (very severely affected). Differences between the two groups were statistically significant (P = 0.0018).
S. suis was isolated from all pigs following challenge. Thirty colonies from the five pigs challenged with S7c were tested by PCR for the suilysin gene. In all these colonies only the mutant allele was detected, whereas all colonies isolated from P1/7-infected animals had intact suilysin genes (8). Bacterial numbers were assessed semiquantitatively (from a value of 0 for no growth to a value of 4 for heavy growth). Bacterial growth in vivo was more profuse in tissues from organs of pigs challenged with P1/7 than in those from pigs challenged with S7c, but the differences were not significantly different statistically (Table 1). Bacteriology scores of 3 or 4 from heart blood, serous exudates, lungs, and joints were common in pigs challenged with P1/7, whereas only 3 of 30 sites in pigs challenged with S7c had scores of 3 and no sites had scores of 4. The only animal with marked levels of S. suis in the brain was also the only animal (infected with P1/7) that showed signs of meningitis. S. suis was also isolated from the brains of two animals euthanatized for lameness (one infected with P1/7 and one with S7c), but in each case growth was scant (scored as 1).
The main lesions observed at postmortem examination were serous or purulent arthritis and periarticulitis of main limb joints with pericarditis and pleurisy. Joint lesions were observed in pigs in both groups. Lesions of pericarditis were mainly restricted to pigs challenged with P1/7, with extensive gelatinous pericarditis, restricted pleurisy, and scant accumulations of pus along the margins of the lung lobes being present in the majority of pigs in this group. Mild peritonitis was observed in five pigs, some from each group. The total lesion scores were calculated and were significantly higher in pigs challenged with P1/7 (Table 1). The one pig (infected with P1/7) that showed signs of meningitis also revealed positive histological signs of meningitis. Bacteria were recovered from the brain of a pig infected with S7c, but no histological confirmation of meningitis was apparent.
These experiments strongly indicate that suilysin is not required for infection with S. suis to progress to clinically apparent disease once the bacteria have reached the circulatory system. Some evidence in these limited experiments supports a role for suilysin in increasing the severity of clinical signs and indicates that it may be involved in allowing S. suis colonization of the organs to reach higher levels. These data also indicate that suilysin is not required for infection of the meninges.
It has been suggested that suilysin is required for colonization and for the establishment of the initial stages of infection in pigs (8). S. suis can invade and lyse a monolayer of human laryngeal epithelial cells (HEp-2), with more virulent strains able to adhere to and invade the cells significantly more efficiently than less virulent or avirulent strains. With all strains tested, cytotoxicity was abrogated by the addition of a neutralizing antisuilysin monoclonal antibody, suggesting that suilysin is the only cytotoxin secreted by S. suis. These data correlate well with our observation that S7c is noncytotoxic for J774.2 cells. S. suis also adheres to human brain microvascular endothelial cells (BMEC), a constituent of the blood-brain barrier (BBB) (1). In these experiments only some strains were cytotoxic for these cells, and this was related to the presence of suilysin, with cytotoxicity inhibited by cholesterol and antisuilysin antibodies. Invasion of the BMEC was not observed under any conditions, unlike with other meningitis-causing bacteria, such as group B streptococci, Streptococcus pneumoniae, and Escherichia coli K1. It was proposed that S. suis may pass through the BBB by invading the intracellular junctions of BMEC monolayers and that suilysin may play a role in this (1). In the experiments reported here, the recovery of bacteria from the brain of a pig infected with S7c indicates that S. suis not producing suilysin can still cross the BBB in this species. Thus, suilysin may aid penetration of the BBB, but it is not essential for this process. Additionally, suilysin has been shown not to induce the release of inflammatory mediators from in vitro-cultured macrophages and so is probably not important in the development of clinical signs of meningitis (11). Similarly, pneumolysin does not appear to have a role in the inflammatory response in the cerebrospinal fluid in a rabbit meningitis model which leads to the development of disease attributed to cell wall components (3, 12, 13).
The data in this study do not rule out roles for suilysin at a number of stages in the development of disease. Following colonization of the upper respiratory tract, suilysin may aid entry of S. suis through the epithelium. In these initial studies, we have used the intravenous route of infection in pigs in order to look for major differences in pathogenicity in systemic stages of the disease, but this does not allow investigation of any role for suilysin during colonization and infection from the tonsils, and this may be a location where suilysin contributes to pathogenesis. To investigate this will require an intranasal model of infection, which can be highly variable and thus needs large numbers of animals; this was beyond the scope of the present study.
The comparison between pig and mouse models of infection provides insight into the relevance of using animal models in drawing conclusions regarding pathogenicity. However, it must be stated that direct comparison of the models is difficult to make, since the modes of infection and the infectious doses were different between the models. In addition, lethality assays for sly+ and sly mutant strains of S. suis in the pig model could not be performed for ethical reasons. In summary, these animal infection data leave the precise role of suilysin in the pathogenesis of disease caused by S. suis unclear, with further investigation by extensive experimentation in different pig models required. Future studies will be aimed at identifying whether suilysin is involved in the early events required for colonization and invasion in the initiation of disease.
Acknowledgments
The Wellcome Trust RCDF (051033) awarded to A. G. Allen supported the work performed at the University of Cambridge, and the Institute for Animal Health, Compton, is supported by the BBSRC.
REFERENCES
- 1.Charland N, Nizet V, Rubens C E, Kim K S, Lacouture S, Gottschalk M. Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells. Infect Immun. 2000;68:637–643. doi: 10.1128/iai.68.2.637-643.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feder I, Chengappa M M, Fenwick B, Rider M, Staats J. Partial characterization of Streptococcus suis type 2 hemolysin. J Clin Microbiol. 1994;32:1256–1260. doi: 10.1128/jcm.32.5.1256-1260.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedland I R, Paris M M, Hickey S, Shelton S, Olsen K, Paton J C, McCracken G H. The limited role of pneumolysin in the pathogenesis of pneumococcal meningitis. J Infect Dis. 1995;172:805–809. doi: 10.1093/infdis/172.3.805. [DOI] [PubMed] [Google Scholar]
- 4.Gottschalk M G, Lacouture S, Dubreuil J D. Characterization of Streptococcus suis capsular type 2 haemolysin. Microbiology. 1995;141:189–195. doi: 10.1099/00221287-141-1-189. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs A A, van den Berg A J, Baars J C, Nielsen B, Johannsen L W. Production of suilysin, the thiol-activated haemolysin of Streptococcus suis, by field isolates from diseased pigs. Vet Rec. 1995;137:295–296. doi: 10.1136/vr.137.12.295. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs A A, van den Berg A J, Loeffen P L. Protection of experimentally infected pigs by suilysin, the thiol-activated haemolysin of Streptococcus suis. Vet Rec. 1996;139:225–228. doi: 10.1136/vr.139.10.225. [DOI] [PubMed] [Google Scholar]
- 7.Jacobs A A C, Loeffen P L W, van den Berg A J G, Storm P K. Identification, purification, and characterization of a thiol-activated hemolysin (suilysin) of Streptococcus suis. Infect Immun. 1994;62:1742–1748. doi: 10.1093/benz/9780199773787.article.b00034458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norton P M, Rolph C, Ward P N, Bentley R W, Leigh J A. Epithelial invasion and cell lysis by virulent strains of Streptococcus suis is enhanced by the presence of suilysin. FEMS Immunol Med Microbiol. 1999;26:25–35. doi: 10.1111/j.1574-695X.1999.tb01369.x. [DOI] [PubMed] [Google Scholar]
- 9.Reed L J, Muench H. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 10.Segers R P, Kenter T, de Haan L A, Jacobs A A. Characterisation of the gene encoding suilysin from Streptococcus suis and expression in field strains. FEMS Microbiol Lett. 1998;167:255–261. doi: 10.1111/j.1574-6968.1998.tb13236.x. [DOI] [PubMed] [Google Scholar]
- 11.Segura M, Stankova J, Gottschalk M. Heat-killed Streptococcus suis capsular type 2 strains stimulate tumor necrosis factor alpha and interleukin-6 production by murine macrophages. Infect Immun. 1999;67:4646–4654. doi: 10.1128/iai.67.9.4646-4654.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomasz A, Saukkonen K. The nature of cell wall-derived inflammatory components of pneumococci. Pediatr Infect Dis J. 1989;8:902–903. doi: 10.1097/00006454-198912000-00034. [DOI] [PubMed] [Google Scholar]
- 13.Tuomanen E, Tomasz A, Hengstler B, Zak O. The relative role of bacterial cell wall and capsule in the induction of inflammation in pneumococcal meningitis. J Infect Dis. 1985;151:535–540. doi: 10.1093/infdis/151.3.535. [DOI] [PubMed] [Google Scholar]